REVIEW
Published on 17 Aug 2021
Current Insights and Advancements in Head and Neck Cancer: Emerging Biomarkers and Therapeutics with Cues from Single Cell and 3D Model Omics Profiling

doi 10.3389/fonc.2021.676948
- 7,798 views
- 13 citations
30k
Total downloads
116k
Total views and downloads
REVIEW
Published on 17 Aug 2021

ORIGINAL RESEARCH
Published on 06 Aug 2021

REVIEW
Published on 16 Jul 2021

ORIGINAL RESEARCH
Published on 09 Jul 2021

ORIGINAL RESEARCH
Published on 08 Jul 2021
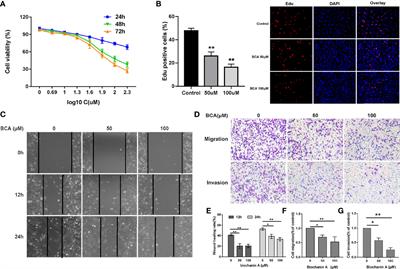
REVIEW
Published on 07 Jul 2021

REVIEW
Published on 01 Jul 2021

REVIEW
Published on 27 May 2021

REVIEW
Published on 07 May 2021
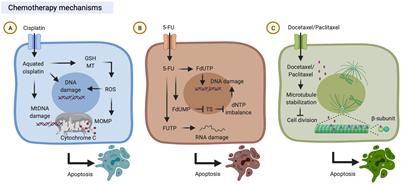
ORIGINAL RESEARCH
Published on 26 Apr 2021

ORIGINAL RESEARCH
Published on 30 Mar 2021

REVIEW
Published on 11 Mar 2021
