- Key Laboratory for Forest Resources Conservation and Utilization in the Southwest Mountains of China, Ministry of Education, Southwest Forestry University, Kunming, China
Paris polyphylla var. yunnanensis is one of the famous Chinese herbs, in which two saponins (polyphyllin II and polyphyllin VII) have anticancer effects. The endangerment of Paris polyphylla var. yunnanensis, makes the study of optimizing the extraction of polyphyllin II and polyphyllin VII from the leaves of Paris polyphylla var. yunnanensis more important. The study established and optimized the process of ultrasound-assisted extraction for polyphyllin II and polyphyllin VII using the Box Behnken Design method of response surface methodology. The results showed that the optimum extraction conditions for polyphyllin II and polyphyllin VII are ethanol concentration of 73 and 70%, extraction temperature of 43 and 50°C, and number of extraction 3, respectively. Under the above conditions, the contents of polyphyllin II and polyphyllin VII were measured to be 6.427 and 19.015 mg/g (DW). The results showed that the experimental model fitted well, and the response surface methodology (RSM) was feasible to optimize the extraction process of polyphyllin II and polyphyllin VII from Paris polyphylla var. yunnanensis leaves. This method provides an effective approach for the comprehensive development and utilization of non-medicinal parts of Paris polyphylla var. yunnanensis.
1 Introduction
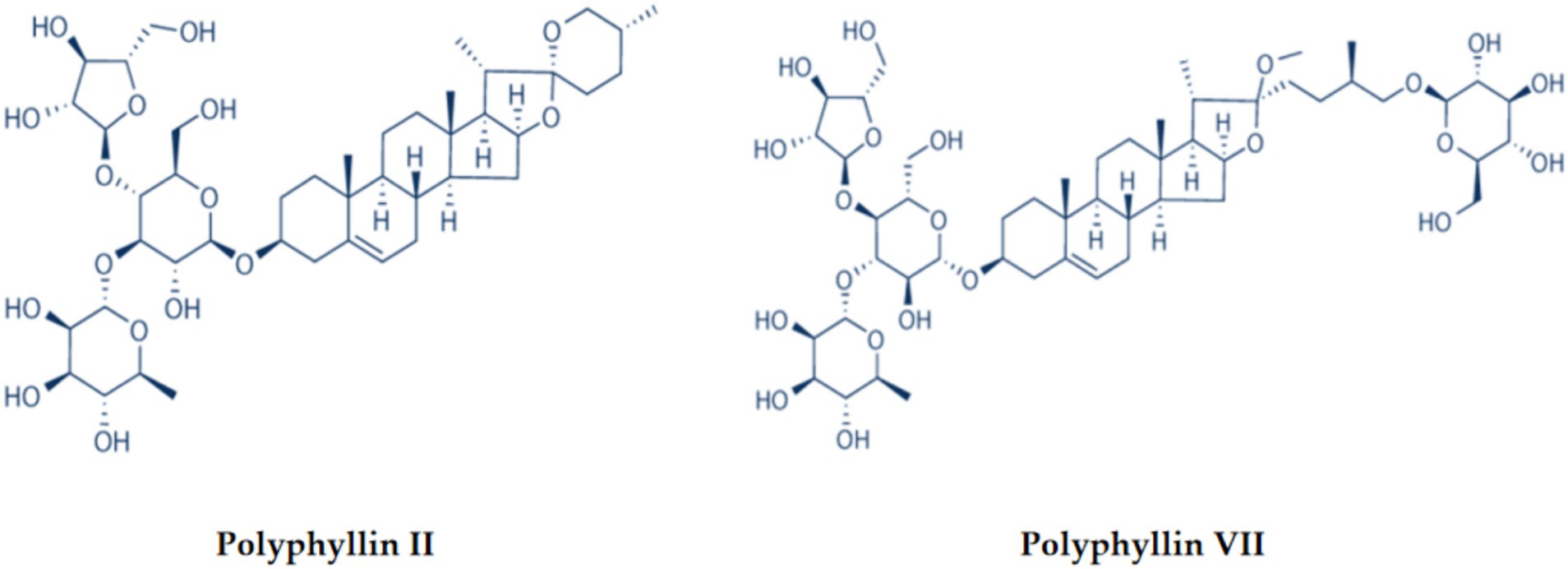
The rhizome of Paris polyphylla var. yunnanensis (P. polyphylla var. yunnanensis) is the raw material of traditional Chinese medicine – Paridis Rhizoma. P. polyphylla var. yunnanensis is mainly distributed in the Gaoligong Mountain Area of Tengchong, Southwest Yunnan and Sichuan Province of China, and the north of Myanmar, including evergreen broad-leaved forest, coniferous forest, and broad-leaved mixed forest at an altitude of 600–2,300 m. It usually grows in hillside shade and shrubs (Wu, 2020). Studies have found that the main medicinal components of Paridis Rhizoma are steroidal saponins (Negi et al., 2014; Thapa et al., 2022). Polyphyllin II and polyphyllin VII (Figure 1) are critical steroidal saponins with anti-cancer, anti-tumour, anti-inflammatory, and analgesic effects (Ding et al., 2021; Liu et al., 2021; Yan et al., 2023). Recent studies have shown that polyphyllin II can physiologically induce apoptosis and treat lung cancer as an adjuvant drug (Wang et al., 2019; Yang et al., 2021; Pang et al., 2023). Polyphyllin VII can inhibit tumour cell proliferation, invasion, and migration, induce tumour cell apoptosis, and reverse tumour drug resistance through multiple ways and mechanisms (Chen et al., 2016; Hsieh et al., 2016). With the discovery of the pharmacological effects of P. polyphylla var. yunnanensis, the demand for Paridis Rhizoma as a medicinal material is increasing. The wild P. polyphylla var. yunnanensis was excavated wantonly, so the growth environment was damaged, and P. polyphylla var. yunnanensis was on the verge of extinction (Cunningham et al., 2018; Shicai et al., 2020). Simultaneously, due to exacting growth conditions, long growth cycles, and immature artificial cultivation technology, Paridis Rhizoma supply is insufficient (Song et al., 2015; Wang and Li, 2018; Yue et al., 2021). In recent years, studies have reported that polyphyllins are mainly synthesized in chloroplasts, transported down through stems, and accumulated in roots, which proves that leaves are important organs for the biosynthesis of steroid saponins. Therefore leaves contain medicinal components similar to those in the rhizoma (Qin et al., 2018; Liang et al., 2019; Guo et al., 2021). However, there is nearly no utilization for the leaves of P. polyphylla var. yunnanensis (Guo et al., 2008; Deb et al., 2015).
The effective chemical components contained in medicinal plants are very complex. How to maximize the extraction of effective ingredients from medicinal plants is crucial to further research on medicinal ingredients, and improve the quality of medicinal materials and clinical efficacy. Therefore, it is of great significance to find suitable extraction methods and optimize the extraction process for the full utilization of medicinal materials. Compared with traditional extraction methods such as boiling, cold soaking and Soxhlet extraction, ultrasound-assisted extraction (UAE) has shown greater advantages in the extraction of effective components of medicinal plants due to its high efficiency, high speed, low temperature and other characteristics (Luo et al., 2010; Lee et al., 2013; Cheok et al., 2014). The response surface methodology (RSM) is a method integrating mathematics and statistics. It seeks the best result through regression equation analysis, solves multivariable problems, and evaluates the nonlinear relationship between indicators and factors. It is easy to use and has good condition predictability. At present, it is often used in the research of extraction of plant components (Heleno et al., 2016; Aydar, 2018; Kumar et al., 2021). Meng et al. (2015) used the central design-response surface method to optimize the ultrasonic extraction of total saponin from the stems and leaves of P. polyphylla var. yunnanensis; Ju et al. (2015) used the central composite de-sign-response surface method to optimize the reflux extraction of total saponins from P. polyphylla var. yunnanensis, however, there are fewer reports on the extraction of single saponin components from P. polyphylla var. yunnanensis. Therefore, based on the single-factor experiment, the ultrasonic extraction for polyphyllin II and polyphyllin VII from the leaves of P. polyphylla var. yunnanensis was optimized using response surface methodology (RSM) in this study. The extraction efficiency of these two saponins is improved, and using the non-medicinal part of P. polyphylla var. yunnanensis alleviates the resource shortage. Finally, it laid a foundation for the comprehensive utilization of P. polyphylla var. yunnanensis.
2 Results and discussion
2.1 Analysis of high-performance liquid chromatography (HPLC)
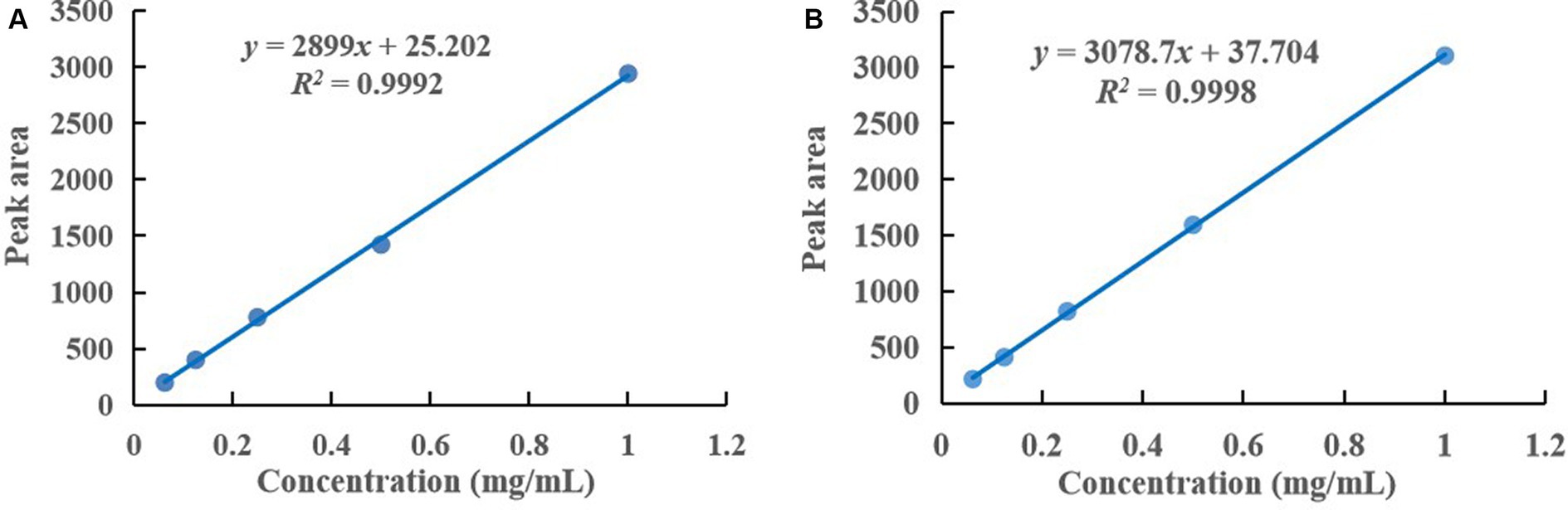
According to the HPLC analysis, a linear regression was performed with the concentration as the horizontal coordinate (X, mg/mL) and the peak area as the vertical coordinate (Y), and the equations of the standard curves obtained as follows: y = 2,899x + 25.202 (R2 = 0.9992) for polyphyllin II and y = 3078.7x + 37.704 (R2 = 0.9998) for polyphyllin VII were shown in Figure 2. They showed a good linearity over the range of 0.0625–1 mg/mL.
2.2 Single-factor experiment
Extracting bioactive secondary metabolites from medicinal plants is influenced by factors such as extraction method, solid–liquid ratio, time, temperature, solvent, and others (Cheok et al., 2014; Meng et al., 2015; Rodrigues et al., 2017; He et al., 2022). Therefore, in order to improve the extraction efficiency of medicinal components, this study investigated the main factors affecting polyphyllin II and polyphyllin VII content in the leaves of P. polyphylla var. yunnanensis. The results are shown in Figure 3.
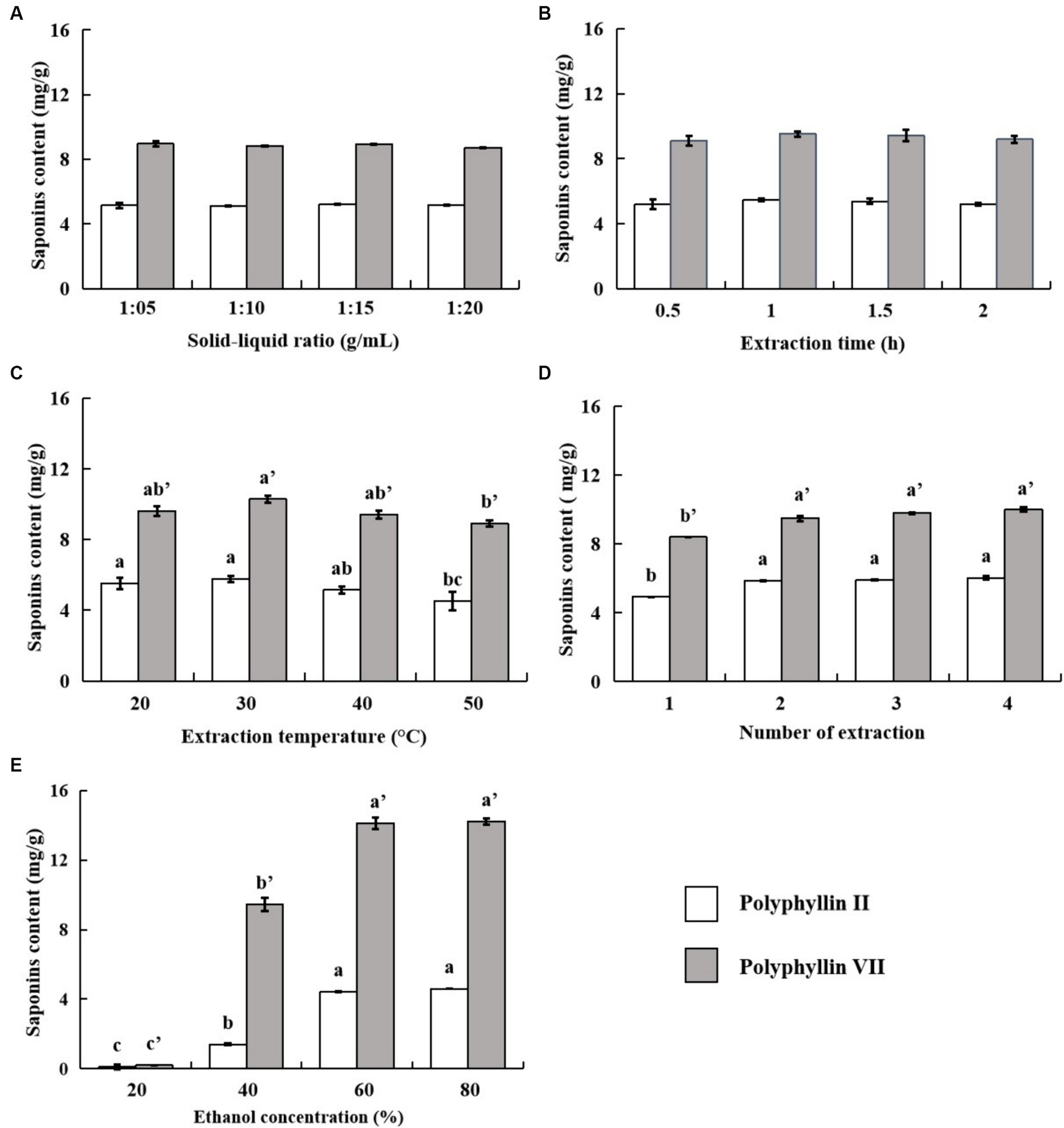
Figure 3. Effects of solid–liquid ratio (A), extraction time (B), extraction temperature (C), number of extraction (D) and ethanol concentration (E) on the extraction content of polyphyllin II and polyphyllin VII. a, b and c represent significant differences among treatments for polyphyllin II; a’, b’ and c’ represent significant differences among treatments for polyphyllin VII (p < 0.05), n = 3.
2.2.1 Effect of solid–liquid ratio on the content of polyphyllin II and polyphyllin VII
Increasing the solid–liquid ratio allows better solubilization of the target components into the solvent, and the amount of extraction solvent has an important effect on the extraction content of saponins (Meng et al., 2015). Therefore, the influence of the solid–liquid ratio on the content of the two saponins is investigated. The results are shown in Figure 3A. There is no significant difference among the treatments for polyphyllin II and polyphyllin VII. The two saponins are effectively extracted into the solvent, and the solid–liquid ratio of 1:5 (g/mL) with less material consumption is appropriate.
2.2.2 Effect of extraction time on the content of polyphyllin II and polyphyllin VII
The increase in extraction time is conducive to the full extraction of active components, but the cost of time and energy also increases. Excessive ultrasonic time may also lead to the degradation of target components (Sarvin et al., 2018; He et al., 2022). The effect of extraction time on the contents of the two saponins was examined, and the results, shown in Figure 3B, indicated no significant changes in the contents of polyphyllin II and polyphyllin VII. The reason for this phenomenon is that the solvent produces cavitation bubbles under the action of ultrasound, causing the plant cell wall to weaken, thus improving the extraction efficiency of the active compounds and making the two saponins completely extracted from the solvent in a relatively short time (Rodrigues et al., 2017). In summary, considering the energy problem, it is more appropriate when the extraction time is 0.5 h.
2.2.3 Effect of extraction temperature on the content of polyphyllin II and polyphyllin VII
Temperature can not only accelerate the dissolution rate of saponins but also can affect the efficiency of UAE. Therefore, it is necessary to choose the appropriate extraction temperature according to the corresponding target compounds to achieve the highest extraction rate (Sarvin et al., 2018). The influences of temperature on the contents of the two saponins were investigated. The results are shown in Figure 3C. The extraction temperature has a significant impact on the extraction of polyphyllin II and polyphyllin VII. As the temperature increased, the content of polyphyllin II did not change significantly between 20 and 30°C, while the content of polyphyllin VII increased significantly and reached the maximum extracted content. As the temperature continued to increase up to 50°C, the contents of the two saponins gradually decreased, which may be due to the degradation of saponins, resulting in the reduction of the extraction content of the two saponins (Shen et al., 2014). Therefore, the extraction temperature in subsequent response surface experiments was chosen to be 30–50°C.
2.2.4 Effect of the number of extraction on the content of polyphyllin II and polyphyllin VII
The more number of extraction, the more solvents are used and the more energy is consumed (Chen et al., 2012), so it is important to choose the most appropriate number of extraction. The effect of the number of extraction on the content of the two saponins is investigated. The results are shown in Figure 3D. The contents of both polyphyllin II and polyphyllin VII increased significantly with the number of extraction increasing from 1 to 2. However, there was no significant difference in the contents of the two saponins number of extraction 2, 3 and 4. It indicated that the two saponins had been fully extracted through twice extraction. Therefore, the number of extraction in subsequent response surface experiments was chosen to be 1–3.
2.2.5 Effect of ethanol concentration on the content of polyphyllin II and polyphyllin VII
The concentration of the extraction solvent affects the solubility of the solvent to the saponins, which is one of the most important factors affecting the extraction rate of steroidal saponins (Shamprasad et al., 2022). The experiment investigated the influence of different ethanol concentrations on the contents of the two saponins. The results are shown in Figure 3E. Both saponins can hardly be extracted with 20% ethanol. With the increase of ethanol concentration (below 80%), the contents of polyphyllin II and polyphyllin VII increase significantly (p < 0.05). However, there was no significant difference between the contents of both saponins extracted with 60 and 80% ethanol, the content of both saponins increased significantly, probably due to the higher polarity of the extracted solution at higher water content, resulting in the insolubility of low-polarity saponins. After 60% ethanol is increased to 80% ethanol, the content of the two saponins does not decrease significantly, indicating that the ethanol with too high concentration is not suitable for the extraction of the two saponins. To sum up, the content of two saponins is significantly higher in the ethanol concentration range of 40–80% ethanol. Therefore, ethanol concentration was chosen to be 40–80% in subsequent response surface experiments.
2.3 Establishment of RSM
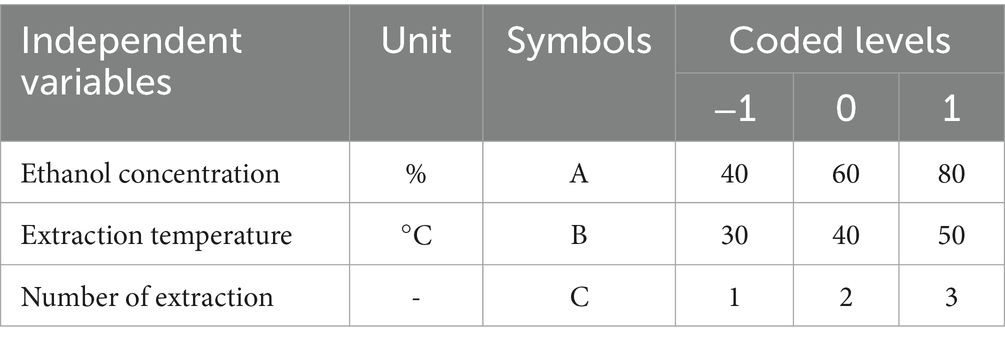
The true value and coding level of the independent variable in the response surface method Box–Behnken design (BBD) method are shown in Table 1. Seventeen sets of experiments were obtained by response surface experimental design with five centroids (0, 0, 0) to estimate the pure error, and the results obtained after the experiments are shown in Table 2.
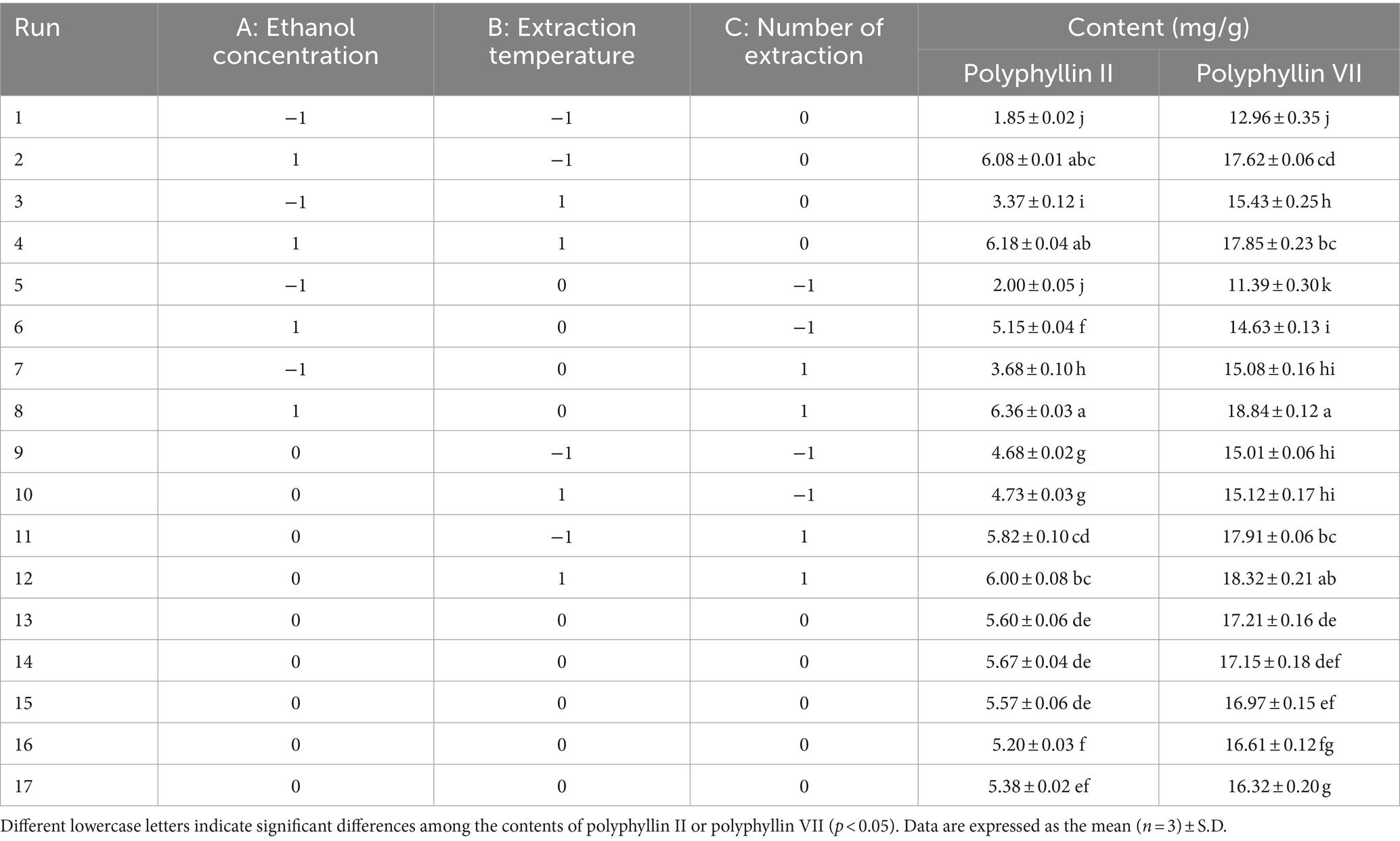
Table 2. Box–Behnken experimental design and results for the content of polyphyllin II and polyphyllin VII.
2.4 Model fitting and statistical analysis
The distributions of the predicted and actual contents for both polyphyllin II (Figure 4A) and polyphyllin VII (Figure 4B) are close to the lines, suggesting that the model fits well. The RSM-optimized UAE process is a good process for extracting steroid saponins from medicinal plants, and other researchers have also made similar reports and have been widely applied (Hadidi et al., 2020; Khoang et al., 2022).
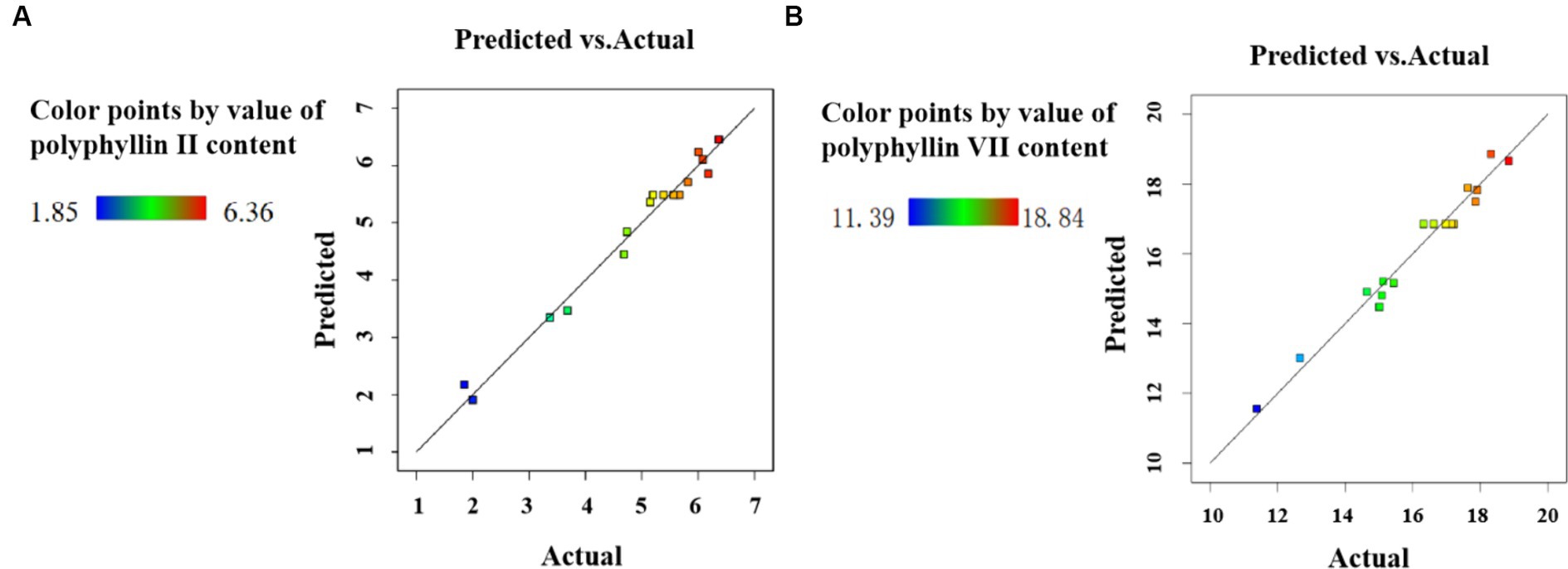
Figure 4. Predicted value and actual value of the contents for polyphyllin II (A) and polyphyllin VII (B).
The quadratic multiple regression model between the contents of polyphyllin II and polyphyllin VII and various factors is obtained after multiple regression fitting analyses of the BBD experimental results, as shown in Table 3. The influence of each factor on the response value is directly reflected in each coefficient in the equation. Within the scope of experimental design, the absolute values of the partial regression coefficients of the dependent variables A, B, and C for polyphyllin II and polyphyllin VII are A > C > B respectively, indicating that the most significant factor affecting the content of polyphyllin II and polyphyllin VII is ethanol concentration, followed by number of extraction and extraction temperature. A higher statistical correlation coefficient (R2 > 90%) indicates a good fit between experimental values with those obtained by the models (Kefi et al., 2022). The coefficients of determination (R2) of the quadratic multiple regression models vary between 0.9805 and 0.9726, and values of predicted coefficients of determination vary from 0.9374 to 0.9553 for polyphyllin II and polyphyllin VII, respectively (Table 3). The lack of fit shows a close agreement that exists between the experimental results and the theoretical values predicted by the quadratic multiple regression model (Carasek and Pawliszyn, 2006; Chávez-Moreno et al., 2018; Zhang et al., 2020).
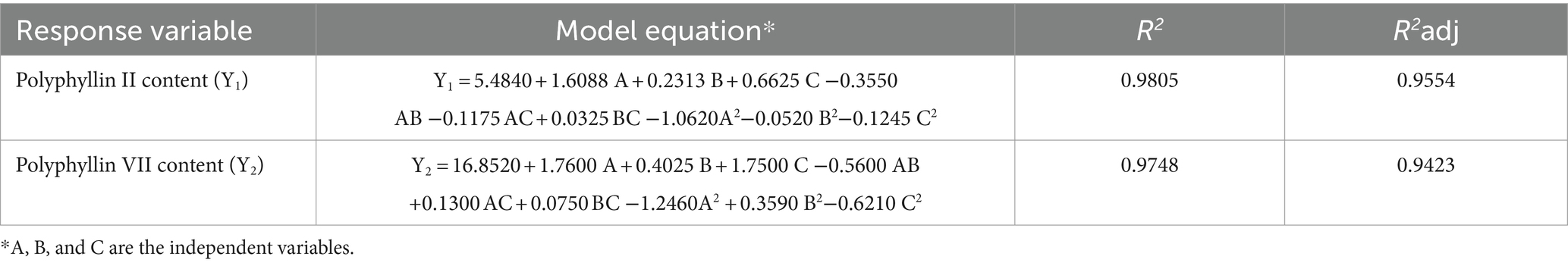
Table 3. Second-order polynomial equation for the relationship between the response variable and the test variable.
The analysis of variance (ANOVA) for each of the factors concerning polyphyllin II and polyphyllin VII contents was obtained by the analysis of the Design Expert 12.0 software. The p-value representing the significance of the coefficient is important for understanding the interaction patterns between variables, and a value below 0.05 (0.01 or 0.0001) indicates that the test parameter is significant (highly significant or extremely significant) at a significance level of 5% (1% or 0.01%) (Zhong et al., 2016). It can be concluded from Table 4 that the polyphyllin II model is extremely significant (F = 39.0700, p < 0.0001), and the lack of fit in the model is not significant (F = 4.11, p = 0.1027 > 0.0500), indicating that the regression equation does not demonstrate lack of fit, i.e., there are no significant influence factors other factors except those factors considered in this experiment, and the regression model can fit the true response value (Zhang et al., 2022). It can be observed that the linear term of ethanol concentration (p < 0.0001) had a higher effect on the polyphyllin II content than that of the number of extraction (p = 0.0004). The two-level interaction between ethanol concentration and extraction temperature had a significant effect on the polyphyllin II content. The quadratic term of ethanol concentration (p = 0.0001) had a highly significant effect on the polyphyllin II content.
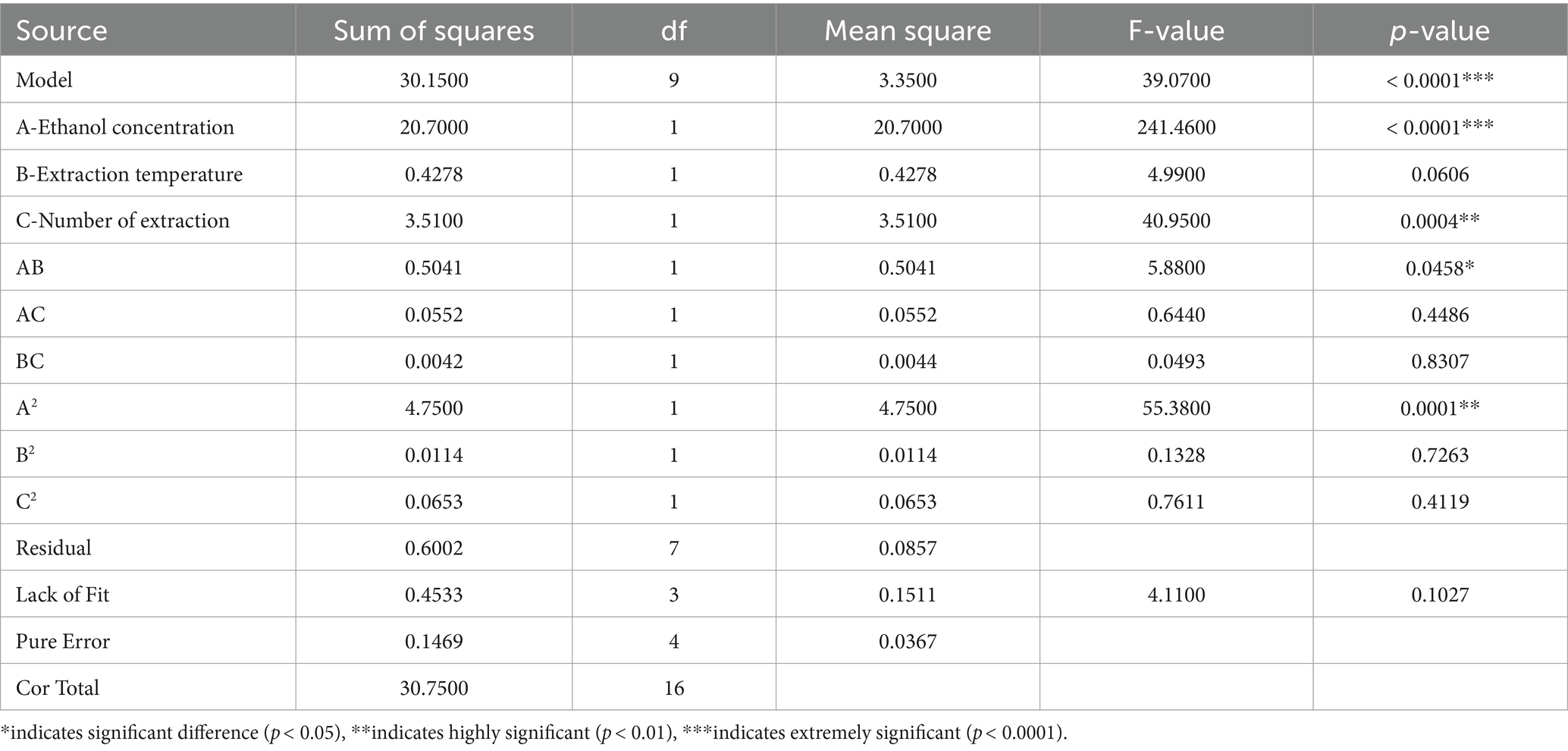
Table 4. Analysis of variance for the fitted quadratic polynomial model of extraction of polyphyllin II.
In Table 5, the F-value of the polyphyllin VII model is extremely significant (F = 30.04, p < 0.0001), and the lack of Fit (F = 2.33, p = 0.2160) was insignificant, indicating that the experimental model has a high fit with the measured results. It can be observed that the linear term of ethanol concentration (p < 0.0001) and number of extraction (p < 0.0001) had a higher effect on the polyphyllin VII content than that of extraction temperature (p = 0.0473). The two-level interaction had no significant effect on the polyphyllin VII content. The quadratic term of ethanol concentration (p = 0.0010) and number of extraction (p = 0.0311) had a significant effect on the polyphyllin VII content.
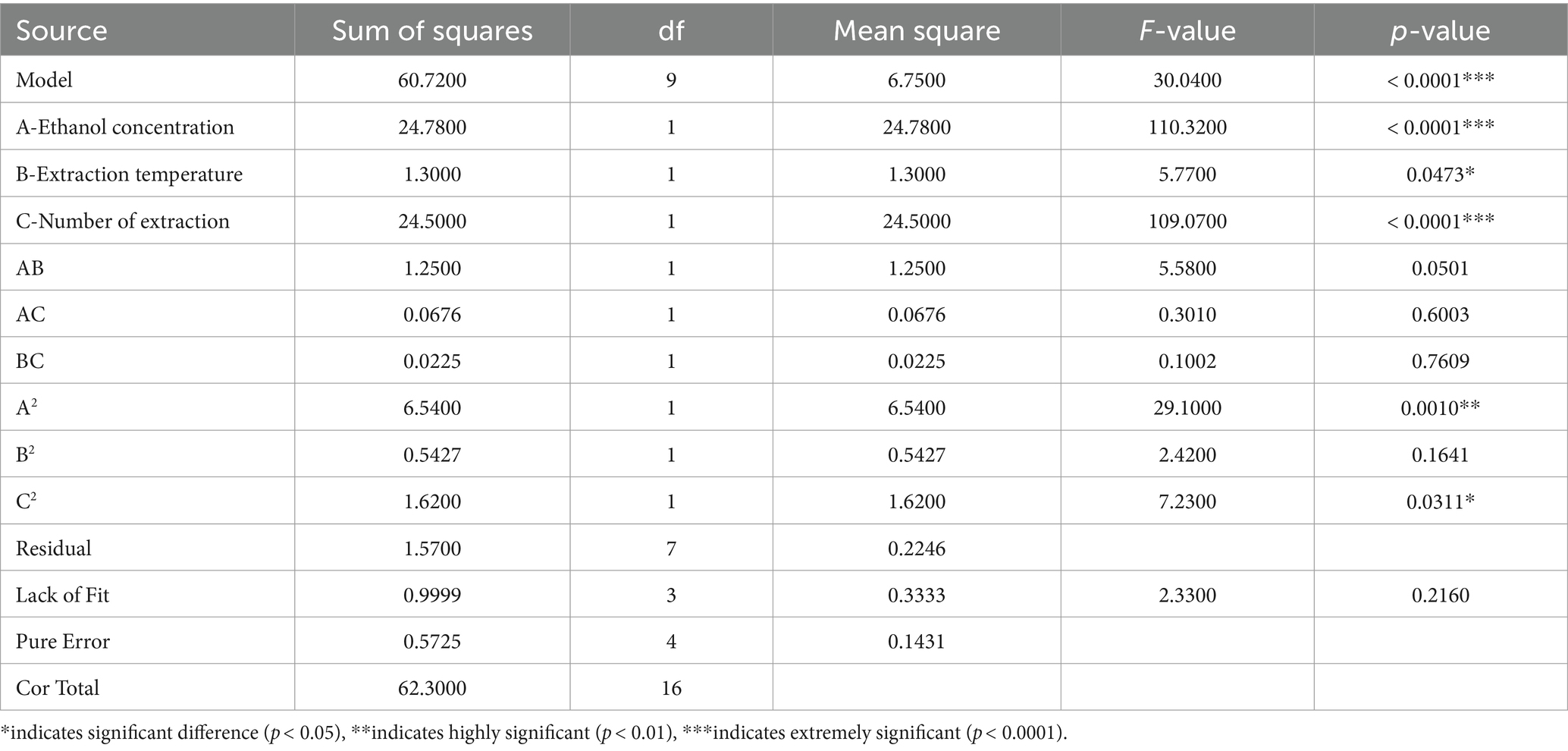
Table 5. Analysis of variance for the fitted quadratic polynomial model of extraction of polyphyllin VII.
2.5 Response surface interaction analysis of polyphyllin II and polyphyllin VII
Three-dimensional (3D) response surface plots and two-dimensional (2D) isopleth plots are useful tools to visually represent the behavior of each variable at different experimental levels, as well as the type of interaction between two variables to determine the optimal conditions for each factor when the response values are at their maximum. These plots are commonly used in experimental design and analysis (Lee et al., 2013; He et al., 2022). The Figures 5A, 6A show that there are convex surfaces with steep slopes and the isopleths have an elliptical shape. This indicates that there are very high response values present and that the interaction between ethanol concentration and extraction temperature has a significant impact on the extraction content of two saponins. The contour lines of the ethanol concentration axis are dense, which indicates that the ethanol concentration has a significant effect on the content of two saponins. As the concentration of ethanol increases from 40 to 80%, the extraction of two saponins initially increases to the highest level and then decreases. This could be because steroidal saponins are usually present in a complex mixture with a higher polarity than ethanol. Therefore, if the ethanol concentration is too high or too low, it can reduce solubility, leading to a decrease in the extraction of the two saponins (Sahu et al., 2008). The sparse isopleths of the extraction temperature axis indicate that the effect of ethanol concentration on two saponins is greater than the extraction temperature. Figures 5B, 6B show steeper surfaces and large slopes, which indicate an interaction between ethanol concentration and the number of extraction. The isopleths are dense along the axes, indicating that the content of two saponins is significantly affected by the ethanol concentration and number of extraction. As shown in Figures 5C, 6C 3D surfaces have small slopes and the 2D isopleth plots are sparse, so the interaction between the two factors does not have a significant effect on the content of the two saponins. However, axial isopleths of the number of extraction are dense, so the number of extraction has a greater effect on the content of the two saponins than the extraction temperature. Simultaneously the extraction temperature axial isopleths are dense in Figure 6C, indicating that the extraction temperature has a significant effect on the content of polyphyllin VII. The solubility of polyphyllin VII increased with increasing extraction temperature due to cell wall disruption, solvent penetration into the plant matrix, and higher mass transfer rates. The analysis of the response surface plot above was consistent with the findings of the ANOVA results.
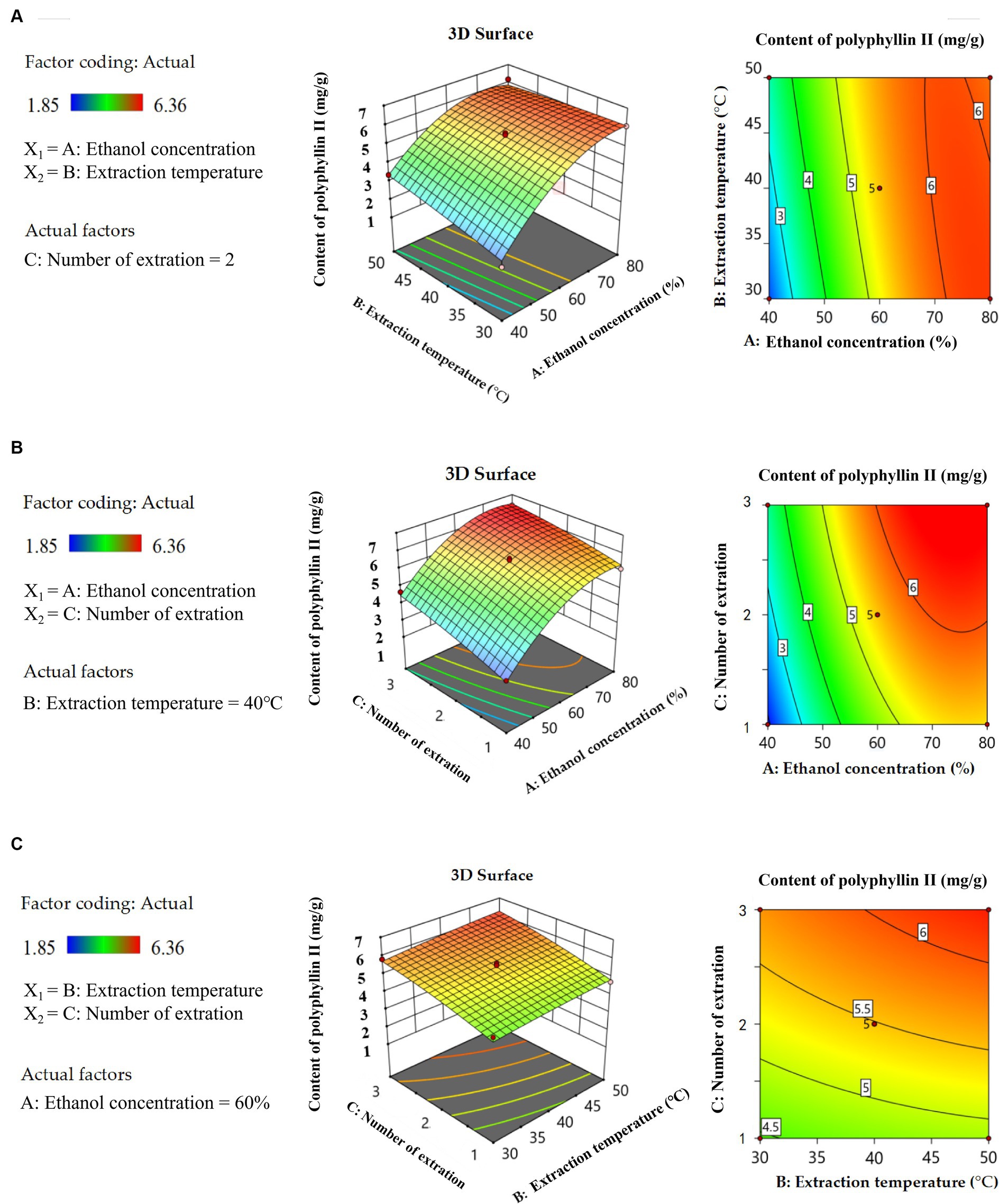
Figure 5. 3D response surface curve plots and 2D isopleth plots of the influence of the interaction between ethanol concentration and extraction temperature (A), ethanol concentration and number of extraction (B), and extraction temperature and number of extraction (C) on the content of polyphyllin II.
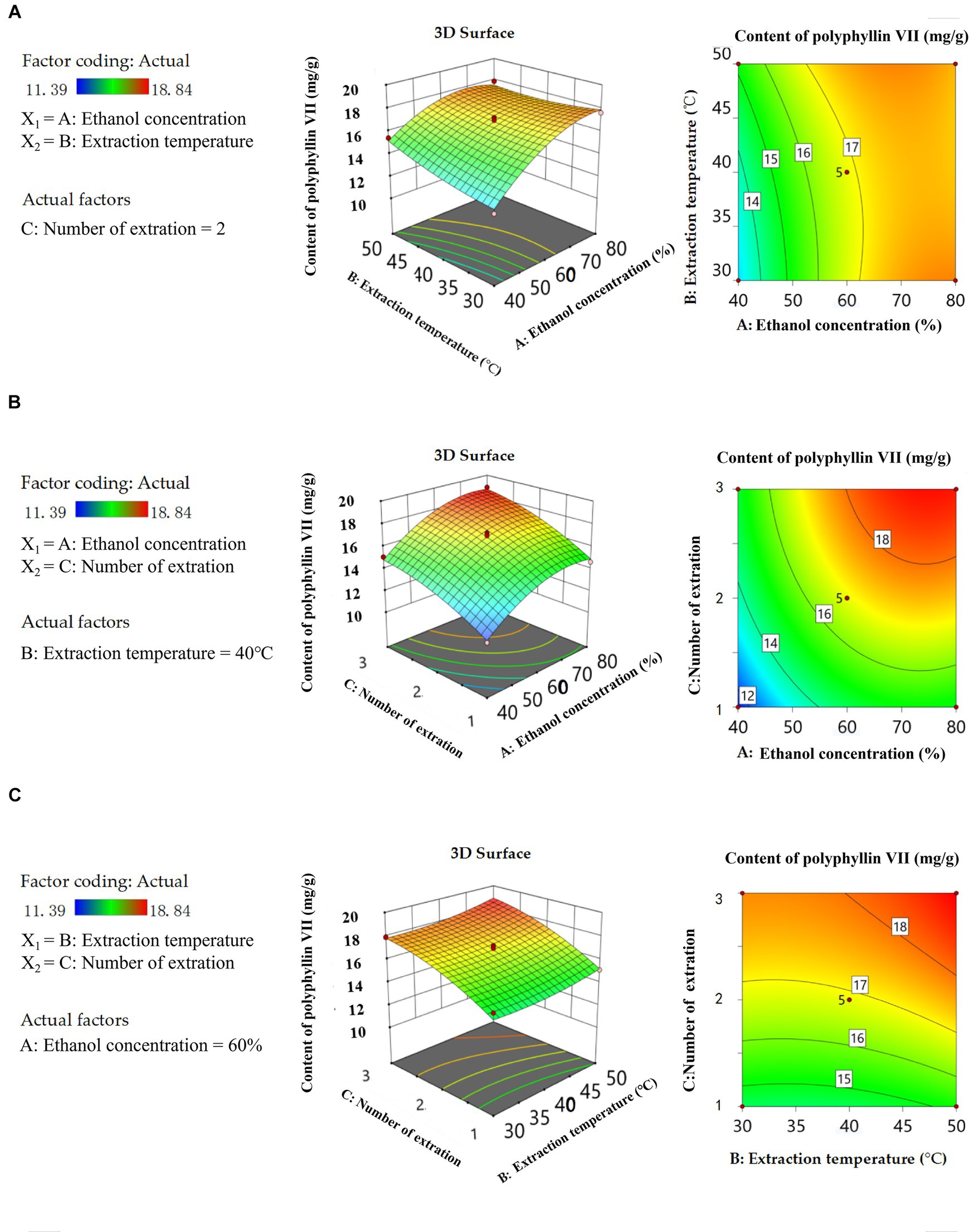
Figure 6. 3D response surface curve plots and 2D isopleth plots of the influence of the interaction between ethanol concentration and extraction temperature (A), ethanol concentration and number of extraction (B), extraction temperature and number of extraction (C) on the content of polyphyllin VII.
2.6 Verification of the models
Predictive model analysis showed that the optimal extraction processes for polyphyllin II and polyphyllin VII were ethanol concentrations of 72.9659 and 70.663%, extraction temperatures of 43.218 and 50.000°C, and number of extraction 3, respectively. The theoretical predicted values of extraction were 6.548 and 19.172 mg/g (DW), respectively. Considering the practical feasibility, the extraction processes of polyphyllin II and polyphyllin VII were adjusted to ethanol concentrations of 73 and 70%, extraction temperatures of 43 and 50°C, and the number of extraction 3, respectively. Under the adjusted process conditions, after five parallel experiments, the content of polyphyllin II was 6.427 mg/g (DW, RSD of 1.40%), with a deviation of 1.84%; and the content of polyphyllin VII was 19.015 mg/g (DW, RSD of 1.97%), with a deviation of 0.82%.
Currently, due to the scarcity and high demand for Paridis Rhizoma, many scholars have optimized the extraction of effective medicinal components from the rhizomes of P. polyphylla var. yunnanensis to alleviate the situation. Tian et al. (2024) extracted polyphyllins from Paris polyphylla var. chinensis rhizomes by using ultrasonic-assisted extraction technology, and the final optimized process was a liquid–solid ratio of 41.72 mL/g, extraction temperature of 55.97°C, extraction time of 30.21 min, and the total polyphyllins extraction yield was 52.56 mg/g. Liu et al. (2022) also used ultrasonic-assisted extraction technology to extract the polyphyllins from Paris polyphylla Smith var. chinensis rhizomes. However, there has been no study on the extraction process optimization of P. polyphylla var. yunnanensis leaves by using ultrasound-assisted extraction technology. Therefore this study was carried out to optimize the extraction process of saponins from non-medicinal parts, leaves of P. polyphylla var. yunnanensis. The utilization of P. polyphylla var. yunnanensis was improved.
3 Materials and methods
3.1 Materials and chemicals
Seedings of P. polyphylla var. yunnanensis (7 to 8 years old) were purchased from Yunnan Kunming Jiange Herbal Cultivation Co., Ltd., The fresh leaves of the P. polyphylla var. yunnanensis seedings were dried to constant weight at 50°C in the thermostatic blast drying oven (DHG-9145A, Shanghai Yiheng Technology Co., Ltd., Shanghai, China) and were crushed with a grinder. The leaf powder used in this study was passed through a 60-mesh sieve.
Polyphyllin II and polyphyllin VII standards with 98% purity were purchased from Shanghai NatureStandard Bio-Technology Co., Ltd., (Shanghai, China). Chromato-graphic acetonitrile (Beijing Bailingwei Technology Co., Ltd., Beijing, China) was used for HPLC analysis. Ultrapure water was made by a water purifier (PP010XXM1, ELGA, United Kingdom). All solvents prepared for HPLC were filtered through a syringe filter (0.22 μm pore size) before use. Ethanol was of analytical grade and purchased from Guangdong Guanghua Technology Co., Ltd., (Guangdong, China).
3.2 Ultrasound-assisted extraction of polyphyllin II and polyphyllin VII
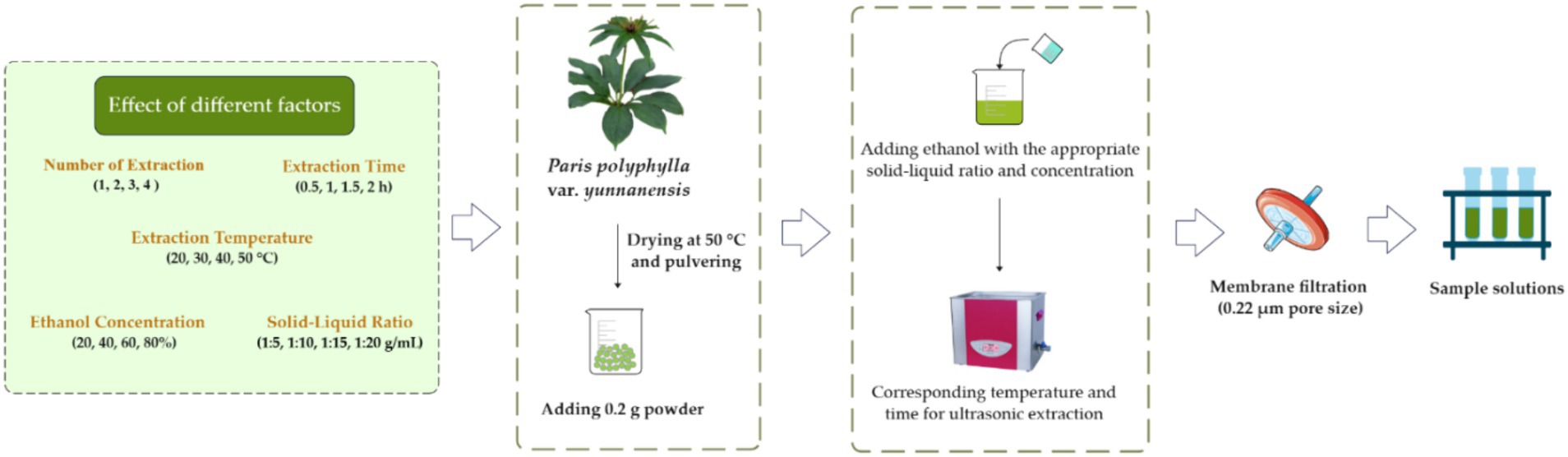
Polyphyllin II and polyphyllin VII were extracted from the leaf of P. polyphylla var. yunnanensis by UAE, which was performed by using ethanol as the extraction solvent at the given ethanol concentration, solid–liquid ratio, extraction time, extraction temperature and number of extraction. That is to say, a 0.2 g sample powder was extracted by 2 mL of 75% ethanol at 30°C in an ultrasonic bath at 53 kHz (SK5210HP, Shanghai Kudos Ultrasonic Instrument Co., Ltd., Shanghai, China) for 0.5 h, and the process was repeated three times. Subsequently, the extracted solutions were combined and filtered through a syringe filter (0.22 μm pore size) for HPLC analysis. When a single-factor experiment was carried out, other factors were set as above condition. The single-factor experiments were carried out on five factors: solid–liquid ratio (1:5, 1:10, 1:15, 1:20 g/mL), extraction time (0.5, 1, 1.5, 2 h), extraction temperature (20, 30, 40, 50°C), number of extraction (1, 2, 3, 4) and ethanol concentration (20, 40, 60, 80%). Each treatment consisted of three repetitions. The single-factor experiment scheme for the extraction of polyphyllin II and polyphyllin VII is shown in Figure 7.
3.3 Response surface methodology (RSM)
BBD is one of the most commonly used design methods in RSM, which can evaluate multiple independent variables and even their interactions (Tirado-Kulieva et al., 2021). Through the regression model obtained, the extracted content of two saponins can be predicted when the factors within the design range are in different combinations at different levels. Based on the single-factor experiment, three important factors, namely, ethanol concentration (A), extraction temperature (B) and number of extraction (C), had a significant influence on the extraction content of two saponins. These three factors with three levels were used for the RSM-BBD experiment design.
A second-order polynomial model was developed to determine the regression coefficients and the significance of the model and the dependent variable (p < 0.05) was tested by ANOVA after the content of the two saponins was determined according to the experimental design. Then, 2D isopleth plots and 3D response surface curve plots were drawn to analyze the effect of the interaction of the factors on the response values. Finally, the optimal ultrasonic extraction process parameters were determined and validated using the response surface prediction model.
3.4 Quantification of polyphyllin II and polyphyllin VII by HPLC
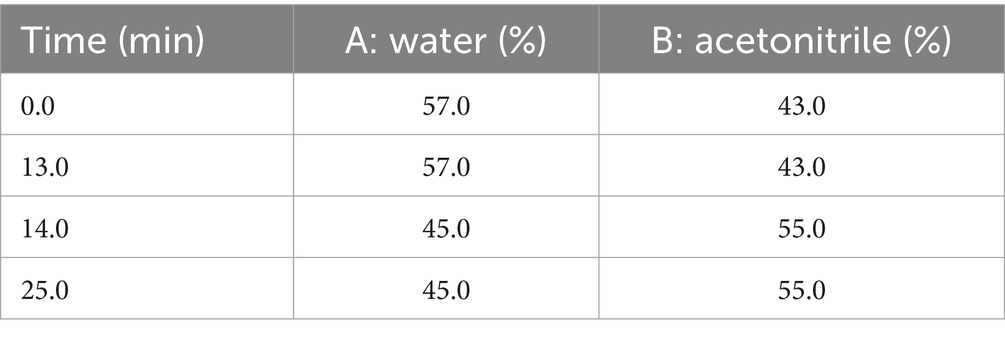
An Agilent high-performance liquid chromatography system (Agilent Technologies, Santa Clara, CA, United States), consisting of a manual injector, a quarternary pump, a DAD UV detector and an EC-C18 column (250 mm × 4.6 mm; 4 μm) was used for the determination of polyphyllin II and polyphyllin VII. The mobile phase consisted of A (water) and B (acetonitrile) using the gradient elution method (Table 6) at a flow rate of 1 mL/min. The column temperature was maintained at 30°C, the detection wavelength was set to 203 nm, and the injection volume was 10 μL. A series of standard solutions of five concentrations, 0.0625 mg/mL, 0.125 mg/mL, 0.25 mg/mL, 0.5 mg/mL and 1.0 mg/mL, were prepared by diluting the mixed standard solution with chromatographic methanol (Beijing Bailingwei Technology Co., Ltd., Beijing, China) for the determination of the standard curves.
3.5 Statistical analysis
The data obtained in the experiments were counted using Excel software (2013, Microsoft Office) and then subjected to T-test and one-way ANOVA using IBM SPSS Statistics 25.0 (Statistical Product Service Solutions, United States). The statistical significance level was set at p < 0.05. Design Expert 12.0 software (Stat Ease, Inc., Minneapolis, MN, United States) was used for experimental design, ANOVA, modelling and prediction optimization.
4 Conclusion
On the basis of a single-factor experiment, three extraction factors (ethanol concentration, extraction temperature, and number of extraction) were optimized for the extraction process of polyphyllin II and polyphyllin VII from P. polyphylla var. yunnanensis leaves using the BBD method of the RSM in this study. The optimal extraction process for polyphyllin II and polyphyllin VII are ethanol concentration of 73 and 70%, extraction temperature of 43 and 50°C, and number of extraction 3, respectively. Under the above conditions, the contents of polyphyllin II and polyphyllin VII were measured to be 6.427 and 19.015 mg/g (DW). The predicted values by response surface methodology were basically consistent with the actual results, and the model fit was good. Therefore, the RSM can be applied to optimize the extraction process of polyphyllin II and polyphyllin VII from P. polyphylla var. yunnanensis leaves. This method provides an effective approach for the comprehensive development and utilization of non-medicinal parts of P. polyphylla var. yunnanensis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XG: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation. QQ: Writing – review & editing, Visualization, Validation, Investigation, Formal analysis. YJ: Writing – review & editing, Visualization, Validation, Investigation, Formal analysis. HL: Writing – original draft, Visualization, Validation, Investigation. KG: Writing – original draft, Visualization, Validation, Investigation. ZZ: Writing – original draft, Visualization, Validation, Investigation. PL: Writing – review & editing, Project administration. AL: Writing – review & editing, Resources, Project administration, Funding acquisition. RS: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Project of the Yunnan Provincial Department of Education Science Research Fund (2022 J0503), the Opening Project of Key Laboratory for Forest Resources Conservation and Utilization in the Southwest Mountains of China, Ministry of Education (KLESWFU201903 and KLESWFU-202010), Yunnan Fundamental Research Projects (202201 AU070072 and 202301AT070216), Foundation of Yunnan Agricultural Basic Research (202101BD070001-033), and the Scientific Research Fund Project of Southwest Forestry University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aydar, A. Y. (2018). “Utilization of response surface methodology in optimization of extraction of plant materials” in Statistical approaches with emphasis on design of experiments applied to chemical processes. ed. V. Silva, InTech. 157–169.
Carasek, E., and Pawliszyn, J. (2006). Screening of tropical fruit volatile compounds using solid-phase microextraction (SPME) fibers and internally cooled SPME fiber. J. Agric. Food Chem. 54, 8688–8696. doi: 10.1021/jf0613942
Chávez-Moreno, C. A., Hinojosa-Reyes, L., Ruiz-Ruiz, E. J., Hernández-Ramírez, A., and Guzmán-Mar, J. L. (2018). Optimization of solid-phase extraction of parabens and benzophenones in water samples using a combination of Plakett-Burman and box-Behnken designs. J. Sep. Sci. 41, 4488–4497. doi: 10.1002/jssc.201800796
Chen, J. C., Hsieh, M. J., Chen, C. J., Lin, J. T., Lo, Y. S., Chuang, Y. C., et al. (2016). Polyphyllin G induce apoptosis and autophagy in human nasopharyngeal cancer cells by modulation of AKT and mitogen-activated protein kinase pathways in vitro and in vivo. Oncotarget 7, 70276–70289. doi: 10.18632/oncotarget.11839
Chen, R., Li, Y., Dong, H., Liu, Z., Li, S., Yang, S., et al. (2012). Optimization of ultrasonic extraction process of polysaccharides from Ornithogalum Caudatum Ait and evaluation of its biological activities. Ultrason. Sonochem. 19, 1160–1168. doi: 10.1016/j.ultsonch.2012.03.008
Cheok, C. Y., Salman, H. A. K., and Sulaiman, R. (2014). Extraction and quantification of saponins: a review. Food Res. Int. 59, 16–40. doi: 10.1016/j.foodres.2014.01.057
Cunningham, A. B., Brinckmann, J. A., Bi, Y. F., Pei, S. J., Schippmann, U., and Luo, P. (2018). Paris in the spring: a review of the trade, conservation and opportunities in the shift from wild harvest to cultivation of Paris polyphylla (Trilliaceae). J. Ethnopharmacol. 222, 208–216. doi: 10.1016/j.jep.2018.04.048
Deb, C. R., Jamir, S. L., and Jamir, N. S. (2015). Studies on vegetative and reproductive ecology of Paris polyphylla smith: a vulnerable medicinal plant. Am. J. Plant Sci. 6, 2561–2568. doi: 10.4236/ajps.2015.616258
Ding, Y. G., Zhao, Y. L., Zhang, J., Zuo, Z. T., Zhang, Q. Z., and Wang, Y. Z. (2021). The traditional uses, phytochemistry, and pharmacological properties of Paris L. (Liliaceae): a review. J. Ethnopharmacol. 278:114293. doi: 10.1016/j.jep.2021.114293
Guo, L., Su, J., Deng, B. W., Yu, Z. Y., Kang, L. P., Zhao, Z. H., et al. (2008). Active pharmaceutical ingredients and mechanisms underlying phasic myometrial contractions stimulated with the saponin extract from Paris polyphylla Sm. Var. yunnanensis used for abnormal uterine bleeding. Hum. Reprod. 23, 964–971. doi: 10.1093/humrep/den001
Guo, S. Y., Yin, Y., Lei, T., Shi, Y. H., Gao, W., Zhang, X. N., et al. (2021). A cycloartenol synthase from the steroidal saponin biosynthesis pathway of Paris polyphylla. J. Asian Nat. Prod. Res. 23, 353–362. doi: 10.1080/10286020.2020.1730331
Hadidi, M., Ibarz, A., and Pagan, J. (2020). Optimisation and kinetic study of the ultrasonic-assisted extraction of total saponins from alfalfa (Medicago sativa) and its bioaccessibility using the response surface methodology. Food Chem. 309:125786. doi: 10.1016/j.foodchem.2019.125786
He, S., Wang, X., Chen, J., Li, X., Gu, W., Zhang, F., et al. (2022). Optimization of the ultrasonic-assisted extraction Technology of Steroidal Saponins from Polygonatum kingianum Collett & Hemsl and evaluating its quality planted in different areas. Molecules 27:1463. doi: 10.3390/molecules27051463
Heleno, S. A., Diz, P., Prieto, M. A., Barros, L., Rodrigues, A., Barreiro, M. F., et al. (2016). Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 197, 1054–1063. doi: 10.1016/j.foodchem.2015.11.108
Hsieh, M. J., Chien, S. Y., Lin, J. T., Yang, S. F., and Chen, M. K. (2016). Polyphyllin G induces apoptosis and autophagy cell death in human oral cancer cells. Phytomedicine 23, 1545–1554. doi: 10.1016/j.phymed.2016.09.004
Ju, J., Zhu, Z., and Du, Z. (2015). Optimization of the extraction Technology of Saponins from Paris polyphylla with central composite De-sign-response surface method. Chin. Pharm. 28, 3967–3969.
Kefi, B. B., Baccouri, S., Torkhani, R., Koumba, S., Martin, P., and M’Hamdi, N. (2022). Application of response surface methodology to optimize solid-phase extraction of benzoic acid and Sorbic acid from food drinks. Food Secur. 11:1257. doi: 10.3390/foods11091257
Khoang, L. T., Huyen, H. T. T., Chung, H. V., Duy, L. X., Toan, T. Q., Bich, H. T., et al. (2022). Optimization of Total Saponin extraction from Polyscias fruticosa roots using the ultrasonic-assisted method and response surface methodology. PRO 10:2034. doi: 10.3390/pr10102034
Kumar, M., Dahuja, A., Tiwari, S., Punia, S., Tak, Y., Amarowicz, R., et al. (2021). Recent trends in extraction of plant bioactives using green technologies: a review. Food Chem. 353:129431. doi: 10.1016/j.foodchem.2021.129431
Lee, L. S., Lee, N., Kim, Y. H., Lee, C. H., Hong, S. P., Jeon, Y. W., et al. (2013). Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules 18, 13530–13545. doi: 10.3390/molecules181113530
Liang, M. Y., Wang, Y. Z., Qiao, X., Lu, Y. W., Chen, M. H., Li, P., et al. (2019). Structural characterisation and discrimination of the aerial parts of Paris polyphylla var. yunnanensis and Paris polyphylla var. chinensis by UHPLC-QTOF-MS coupled with multivariate data analysis. Phytochem. Anal. 30, 437–446. doi: 10.1002/pca.2826
Liu, F., Li, L., Tian, X., Zhang, D., Sun, W., and Jiang, S. (2021). Chemical constituents and pharmacological activities of steroid saponins isolated from rhizoma paridis. J. Chem. 2021, 1–7. doi: 10.1155/2021/1442906
Liu, J., Lin, Z., Kong, W., Zhang, C., Yuan, Q., Fu, Y., et al. (2022). Ultrasonic-assisted extraction-synergistic deep eutectic solvents for green and efficient incremental extraction of Paris polyphylla saponins. J. Mol. Liq. 368:120644. doi: 10.1016/j.molliq.2022.120644
Luo, Y., Liu, S., and Huang, S. (2010). Ultrasonic extraction of total saponins from Paris polyphylla smith by orthogonal design. Pract. Pharm. Clin. Remed. 13, 185–187.
Meng, W., Xin-heng, X., Jun-long, L., Shi-an, S., Jing, L., and Chun-bang, D. (2015). Extraction and antioxidant activity of Total Saponins from stems and leaves of Paris polyphylla var. yunnanensis (Franch.) hand. Mazz. Nat. Product Res. Dev. 27:1794. doi: 10.16333/j.1001-6880.2015.10.020
Negi, J. S., Bisht, V. K., Bhandari, A. K., Bhatt, V. P., Singh, P., and Singh, N. (2014). Paris polyphylla: chemical and biological prospectives. Anti Cancer Agents Med. Chem. 14, 833–839. doi: 10.2174/1871520614666140611101040
Pang, D., Yang, C., Li, C., Zou, Y., Feng, B., Li, L., et al. (2023). Correction: Polyphyllin II inhibits liver cancer cell proliferation, migration and invasion through downregulated cofilin activity and the AKT/NF-κB pathway. Biol. Open 12:bio059765. doi: 10.1242/bio.059765
Qin, X. J., Ni, W., Chen, C. X., and Liu, H. Y. (2018). Seeing the light: shifting from wild rhizomes to extraction of active ingredients from above-ground parts of Paris polyphylla var. yunnanensis. J. Ethnopharmacol. 224, 134–139. doi: 10.1016/j.jep.2018.05.028
Rodrigues, G. D. M., Mello, B. T. F. D., dos Santos Garcia, V. A., and Silva, C. D. (2017). Ultrasound-assisted extraction of oil from macauba pulp using alcoholic solvents. J. Food Process Eng. 40:e12530. doi: 10.1111/jfpe.12530
Sahu, N. P., Banerjee, S., Mondal, N. B., and Mandal, D. (2008). Steroidal saponins. Fortschr. Chem. Org. Naturst. 89, 45–141. doi: 10.1007/978-3-211-74019-4_2
Sarvin, B., Stekolshchikova, E., Rodin, I., Stavrianidi, A., and Shpigun, O. (2018). Optimization and comparison of different techniques for complete extraction of saponins from T. terrestris. J. Appl. Res. Med. Aromat. Plants 8, 75–82. doi: 10.1016/j.jarmap.2017.12.002
Shamprasad, B. R., Subramani, R., Subramaniam, S., and Sivasubramanian, A. (2022). Environmentally benign, ultrasonication assisted, sustainable valorization for commercially important nutraceutical-Daucosterol from the heartwood of invasive Prosopis juliflora (Sw.) DC. Sustain. Chem. Pharm. 29:100810. doi: 10.1016/j.scp.2022.100810
Shen, S., Chen, D., Li, X., Li, T., Yuan, M., Zhou, Y., et al. (2014). Optimization of extraction process and antioxidant activity of polysaccharides from leaves of Paris polyphylla. Carbohydr. Polym. 104, 80–86. doi: 10.1016/j.carbpol.2014.01.006
Shicai, W. U., Guo, L. I., Qiping, W. A. N. G., Wenli, Y. A. N. G., Xuemei, C. H. E. N., Jian, F. E. N. G., et al. (2020). Investigation on the resources of Paris Polyphylla var. yunnanensis. Med. Plant 11:27.
Song, Y., Li, M. F., Xu, J., Zhao, Z., and Chen, N. Z. (2015). Polymorphic microsatellite markers in the traditional Chinese medicinal plant Paris polyphylla var. yunnanensis. Genet. Mol. Res. 14, 9939–9942. doi: 10.4238/2015.August.19.29
Thapa, C. B., Paudel, M. R., Bhattarai, H. D., Pant, K. K., Devkota, H. P., Adhikari, Y. P., et al. (2022). Bioactive secondary metabolites in Paris polyphylla Sm. and their biological activities: a review. Heliyon 8:e08982. doi: 10.1016/j.heliyon.2022.e08982
Tian, X., Liu, J., Jiang, L., Kong, W., Fu, Y., Qin, L., et al. (2024). Efficient extraction and optimization procedures of polyphyllins from Paris polyphylla var chinensis by deep eutectic solvent coupled with ultrasonic-assisted extraction. Microchem. J. 196:109692. doi: 10.1016/j.microc.2023.109692
Tirado-Kulieva, V. A., Sánchez-Chero, M., Yarlequé, M. V., Aguilar, G. F. V., Carrión-Barco, G., and Santa Cruz, A. G. Y. (2021). An overview on the use of response surface methodology to model and optimize extraction processes in the food industry. Curr. Res. Nutr. Food Sci. J. 9, 745–754. doi: 10.12944/CRNFSJ.9.3.03
Wang, W., Dong, X., You, L., Sai, N., Leng, X., Yang, C., et al. (2019). Apoptosis in HepaRG and HL-7702 cells inducted by polyphyllin II through caspases activation and cell-cycle arrest. J. Cell. Physiol. 234, 7078–7089. doi: 10.1002/jcp.27462
Wang, Y. Z., and Li, P. (2018). Effect of cultivation years on saponins in Paris Polyphylla var. yunnanensis using ultra-high liquid chromatography-tandem mass spectrometry and Fourier transform infrared spectroscopy. Plant Growth Regul. 84, 373–381. doi: 10.1007/s10725-017-0348-2
Wu, Y. Y. (2020). A comparative study on the fingerprint of Paris polyphylla var. Polyphylla and Paris polyphylla var. yunnanensis from different origins in Dali and Seven Main steroidal Saponins by HPLC. Chin. Med. Sci. J. 55, 875–882. doi: 10.11669/cpj.2020.11.004
Yan, X. X., Zhao, Y. Q., He, Y., Disayathanoowat, T., Pandith, H., Inta, A., et al. (2023). Cytotoxic and pro-apoptotic effects of botanical drugs derived from the indigenous cultivated medicinal plant Paris polyphylla var. yunnanensis. Front. Pharmacol. 14:1100825. doi: 10.3389/fphar.2023.1100825
Yang, M. Z., Zhang, B. B., Huang, J. C., Bai, X. Y., Liang, Z. Q., Yi, X., et al. (2021). Network pharmacology reveals Polyphyllin II as one hit of Nano Chinese medicine monomers against nasopharyngeal carcinoma. Bioinorg. Chem. Appl. 2021, 9959634–9959610. doi: 10.1155/2021/9959634
Yue, J., Li, Z., Zuo, Z., Zhao, Y., Zhang, J., and Wang, Y. (2021). Study on the identification and evaluation of growth years for Paris polyphylla var. yunnanensis using deep learning combined with 2DCOS. Spectrochim. Acta A Mol. Biomol. Spectrosc. 261:120033. doi: 10.1016/j.saa.2021.120033
Zhang, X., Wang, C., Wang, L., Chen, S., and Xu, Y. (2020). Optimization and validation of a head space solid-phase microextraction-arrow gas chromatography-mass spectrometry method using central composite design for determination of aroma compounds in Chinese liquor (baijiu). J. Chromatogr. A 1610:460584. doi: 10.1016/j.chroma.2019.460584
Zhang, Y., Zhao, Z., Meng, H., Li, W., and Wang, S. (2022). Ultrasonic extraction and separation of Taxanes from Taxus cuspidata optimized by response surface methodology. Separations 9:193. doi: 10.3390/separations9080193
Keywords: Paris polyphylla var. yunnanensis, saponins, ultrasound-assisted extraction, response surface methodology (RSM), optimization of the extraction process
Citation: Guo X, Qiao Q, Jin Y, Lei H, Guo K, Zhao Z, Li P, Liu A and Sun R (2024) Optimization of ultrasound-assisted extraction of two saponins from Paris polyphylla var. yunnanensis leaves using response surface methodology. Front. Sustain. Food Syst. 8:1424285. doi: 10.3389/fsufs.2024.1424285
Edited by:
Muzaffar Hasan, Central Institute of Agricultural Engineering (ICAR), IndiaReviewed by:
Mohd Harun, Indian Agricultural Statistics Research Institute, IndiaNewlove Afoakwah, University for Development Studies, Ghana
Copyright © 2024 Guo, Qiao, Jin, Lei, Guo, Zhao, Li, Liu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aizhong Liu, bGl1YWl6aG9uZ0BtYWlsLmtpYi5hYy5jbg==; Rui Sun, c3VucnVpQHN3ZnUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Xianming Guo
Xianming Guo Qing Qiao†
Qing Qiao† Yutian Jin
Yutian Jin Ping Li
Ping Li Aizhong Liu
Aizhong Liu Rui Sun
Rui Sun