- 1ICAR-Central Plantatsion Crops Research Institute, Kasaragod, Kerala, India
- 2ICAR-Central Plantation Crops Research Institute-Mohitnagar, Jalpaiguri, India
- 3ICAR-Central Plantation Crops Research Institute Centre, Kahikuchi, India
- 4Coconut Development Board, Ministry of Agriculture and Farmers Welfare, Kochi, India
- 5Department of Agriculture Co-operation and Farmers Welfare, Ministry of Agriculture and Farmers Welfare, New Delhi, India
Introduction: Coconut testa, a by-product of the coconut processing industry, is currently underutilised. This study aimed to extract a coconut testa-based food colourant using various organic solvents and physical methods, and to utilise this colourant in food product preparation.
Methods: Different organic solvents, along with various time and temperature combinations, were employed for colourant extraction using both a laboratory-scale water bath and ultrasonication. The colour coordinate values (CIELab) of the testa-derived colourants were measured, and the colourants were screened for various phytochemicals. The in vitro antioxidant potential of the testa colourant was assessed by quantifying total phenolics, and the phytochemical composition, including monomeric anthocyanins, was evaluated.
Results: The study determined the optimal combinations of organic solvents, temperature and time to obtain extracts with maximum antioxidant activity and total phenolic content (TPC). Acidified ethanol-based extracts of testa colourants yielded highest polyphenol content (154.39 ± 2.63 mg GAE/g) and flavonoids content (53.65 ± 0.62 mg QE/g). Similarly, ethanol-based extractants of coconut testa produced high anthocyanin content [823.02 ± 1.81 mg Cy-3-glc equivalents (C3GE)/100 g]. Acidified (0.3 M HCl) solvents at relatively high temperature and time combinations exhibited high antioxidant potential of testa colourant, as measured by CUPRAC, FRAP, and DPPH assays. Following the foam mat drying process of the colourant, a mature coconut water-based jelly was prepared by incorporating the testa colourant extracted with acidified ethanol.
Discussion: This study highlights the biochemical and antioxidant potential of the food colorant derived from coconut testa and explores its suitability for functional food applications. Therefore, coconut testa extract serves a dual purpose: it enhances the aesthetic appeal of food as a colourant and provides significant health-promoting properties due to its high anthocyanin content. Insights from this study could help in promoting the valorization of one of the beneficial by products of coconut industry.
1 Introduction
The food industry uses synthetic colourants to meet consumers’ sensory expectations, restore lost color, enhance existing color, or make food and beverages more appealing. However, these artificial colourants come with negative health and environmental consequences, posing toxicological risks to human health, including the development of allergies and tumours (Mota et al., 2023). On the other hand biocolourants refer to pigments, whether isolated or derived, sourced from nature, including plants, microbes, animals, and minerals. These biocolourants are used to add, preserve, or impact colour to a range of products such as foods, feeds, drugs, or cosmetics. The biocolourant category also encompasses naturally occurring pigments that are chemically synthesised on an industrial scale (Maronpot et al., 2020). Plant-derived natural colorants, are increasingly popular in products like food and beverages (Thakur and Modi, 2022), due to safety concerns related to synthetic colourants and a growing consumer focus on health (Rodriguez-Amaya, 2016). Beyond their role in pigmentation, plant-derived colourants often serve as reservoirs of substances with nutraceutical properties (Shegokar and Mitri, 2012; Weiss et al., 2023).
Natural food colourants are primarily derived from fruits, vegetables, algae, and spices, such as turmeric, beetroot juice, potatoes, sweet potatoes, cabbages, and spirulina. These sources not only provide vibrant colours but also offer health benefits. Numerous natural colorants, rich in anthocyanins, carotenoids (such as beta-carotene from carrots, carrot oil, corn endosperm, and bell pepper Capsicum annuum L.), chlorophylls from alfalfa (Medicago sativa), curcuminoids from turmeric (Curcuma longa L.), betalains from beet (Beta vulgaris L.) powder, and carminic acid from cochineal (Dactylopius coccus) extract, have been successfully commercialised globally (Luzardo-Ocampo et al., 2021). Spirulina (Arthrospira platensis) recently received FDA approval for use in candy and chewing gum (Shkolnikov Lozober et al., 2023). In addition to their sensory attributes, natural pigments often provide additional health benefits, including antioxidant properties and effects such as anticancer, hepatoprotective, antimicrobial, anti-diabetic, and anti-inflammatory activities (Manzoor et al., 2021).
Coconut (Cocos nucifera L.) is a versatile plantation crop that provides a range of products with significant nutritive potential (Ramesh et al., 2021; Ramesh et al., 2023a; Ramesh et al., 2023b). The coconut testa, also known as the mesocarp, is the brown covering of the coconut endosperm or kernel. The testa, a by-product of coconut milk, desiccated coconut powder, and virgin coconut oil production, is typically discarded despite its contribution of a brown colour to these products. In India alone, the confectionery and baking industry consumes around 40,000 tonnes of desiccated coconut annually resulting in an estimated 4,000 tonnes of underutilised testa. India hosts over 150 desiccated coconut powder production units, with a total production capacity of approximately 0.1 million metric tonnes. The production potential for coconut testa in India is estimated to be around 88,000 tonnes (Appaiah et al., 2014). Therefore, between 4,000 and over 80,000 tonnes of testa remain underutilised in India. Biochemical analyses have revealed that testa is rich in nutritive phenolic acids (16) and flavonoids (12), showcasing substantial antioxidant potential and colourant potential (Arivalagan et al., 2018). Although the nutritive potential of coconut testa is known, a large quantum of testa is diverted for the production of animal feed. These underutilised phytonutrients merit the development of appropriate processing technology to harness their benefits for incorporation into various food products (Ramesh et al., 2023a). Consequently, this research aims to explore the utilisation of coconut testa in creating a biocolourant, to examine its biochemical characteristics, more specifically the colour conferring anthocyanin components, and to incorporate the extracted testa-colourant into a food product.
2 Materials and methods
2.1 Chemicals
All phenolic acid and flavonoid reference standards, including gallic acid, quercetin, cyanidin 3 -O-glucoside chloride, along with DPPH (1,1-diphenyl-2-picrylhydrazyl), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), TPTZ [2,4,6-tris(2-pyridyl)-s-triazine], potassium persulphate, and neocuproine (2,9-dimethyl-1,10-phenanthroline), and other reference standards such as anthraquinone, saponin, gallotannin, menthol were purchased from Sigma-Aldrich Co., St. Louis, MO, United States. Analytical grade ethanol, methanol, acetone, glacial acetic acid, sodium acetate, concentrated hydrochloric acid, ferric chloride, ammonium acetate, copper (II) chloride, Folin–Ciocalteu’s phenol reagent, aluminium chloride, citric acid, sodium hydrogen phosphate, sodium azide and sodium carbonate were purchased from Merck KGaA, Darmstadt, Germany. All chemicals were of analytical grade and used as received, without any purification.
2.2 Testa preparation
Coconut fruits (cultivar West Coast Tall) were carefully dehusked at an appropriate stage of maturity, and the coconut kernel was separated from the shell using a coconut de-sheller. The in-house developed testa remover was used to remove the testa, and the resulting testa meal was dried in a mechanical tray dryer at 50°C. Testa remover features a circular wheel covered with emery cloth or sandpaper, which is rotated by an electric motor. The coconut kernel is pressed against the surface of the rotating wheel, either manually or with a fork to remove the testa which is collected at the bottom of the machine.1 Testa samples obtained from various lots of mature coconuts were mixed to ensure that the starting material was representative. After drying, the coconut testa was placed in a cellulose cartridge (Fisher Scientific catalogue number 12-101-100), and petroleum ether (boiling point 60–80°C) was used for a 5-h extraction of fat components in a Soxhlet apparatus. The defatted testa meal was finely ground using a ball mill (Mixer Mill MM400, RETSCH GmbH, Germany), and sifted through a 200-mesh screen to ensure a consistent particle size. This pre-processed testa was then utilised as the substrate for subsequent analyses (Ramesh et al., 2023b) (Figure 1).

Figure 1. Coconut testa powder prior to defatting (A) and defatted coconut testa powder (B). Coconut testa was defatted using petroleum ether to remove the fat and the defatted testa powder was used as substrate for organic solvent and ultrasonication assisted extraction of colourant.
2.3 Organic solvents-based extraction
An amount of 1.0 g of defatted coconut testa powder was combined with 10 mL of suitable solvents in amber-coloured centrifuge tubes. The mixture was then placed on a rotospin shaker (Tarsons Products Pvt. Ltd., Kolkata, India) and subjected to specified periods of agitation at room temperature for extraction. Details of the various organic solvents, treatment durations, and temperature combinations for both direct extraction and ultrasonication-assisted extraction (UAE) are provided in Supplementary Table 1. After extraction with organic solvents and UAE, the extracts were centrifuged, and the resulting supernatant was collected in amber reagent bottles. Filtrates from the three consecutive extractions were combined and used for subsequent analysis.
2.4 Colour measurement
Colour coordinate values (CIELab) of the testa extractants were measured using HUNTERLAB (aperture: 25 mm; Model 45/0, HunterLab Associates Laboratory Inc., Hong Kong, PRC) set to Illuminant D65 and 10° standard observer. Appropriate working standards (white tiles) of the instrument traceable to NIST standards were used to calibrate the instrument prior to measuring the colour of testa extracts. The testa-based extractants were placed in a clean sample cup, ensuring that the samples fully covered the bottom surface of the cup. The sample cup was covered with a white tile and then placed onto the sensor to measure the colour coordinates. The CIELab colour space system is based on a Cartesian representation of three orthogonal axes: L*, a*, and b*. The L* coordinate represents lightness (L* = 0 for black and L* = 100 for white), a* represents the green/red colour component (a* > 0 for red and a* < 0 for green), and b* represents the blue/yellow colour component (b* > 0 for yellow and b* < 0 for blue). The parameters L*, a*, and b* were determined by measuring the transmittance from 380 to 770 nm at 5-nm intervals (HunterLab, 2008).
2.5 Screening for phytochemicals
The phytochemical composition of the testa extractants was analyzed following the methods described by Trease and Evans (1996), Sofowora (1982), and Kazeem et al. (2013). Briefly, 0.5 mL of the extract was mixed with 5 mL of chloroform, and the resulting mixture was shaken for 5 min and filtered. The filtrate was then shaken with equal volume of 10% ammonia solution. A bright pink colour in the aqueous layer indicates the presence of anthraquinones. For flavonoids, a known quantity of testa extract was heated with 10 mL of ethyl acetate over a steam bath for 3 min. The mixture was filtered, and 4 mL of the filtrate was shaken with 1 mL of dilute ammonia solution. The development of yellow colouration indicated the presence of flavonoids. To test for saponins, around 2 mL of testa extract was filtered, and the filtrate was mixed with 5 mL of distilled water, shaken vigorously and observed for a stable persistent froth. The froth was then mixed with a few drops of olive oil, shaken vigorously again, and observed for the formation of an emulsion, which indicates the presence of saponins. For steroids, 0.5 mL of extract was mixed with 2 mL of concentrated sulphuric acid (H2SO4) and 2 mL of acetic anhydride. A colour change from violet to blue or green indicates the presence of steroids. To test for tannins, around 2 mL of testa extract was filtered, and the filtrate was mixed with a few drops of 0.1% ferric chloride. The formation of brownish-green or blue-black coloration indicates the presence of tannins. For terpenoids, 0.5 mL of coconut testa extract was mixed with 2 mL chloroform, followed by slow and careful addition of 3 mL H2SO4 to form an interface layer. A reddish-brown colour at the interface indicates the presence of terpenoids. Appropriate standards were used as controls for all the phytochemical colour reactions.
2.6 Estimation of total phenolics content
The colour extract from the testa was diluted with the same organic solvents used in the extraction process. Specifically, 100 μL of the diluted extract was taken and adjusted to 1 mL. To this, 250 μL of Folin-Ciocalteau Reagent (FCR/Water, 1:1.25 v/v) was added. After a 3-min interval, 500 μL of a 7% Na2CO3 solution was introduced into the mixture. The contents were thoroughly mixed and left to stand for 45 min at room temperature in the dark. The total phenolic content (TPC) was determined by measuring the absorbance at 745 nm using a Shimadzu UV-160 A, UV–Visible recording spectrophotometer (P/N 204–04550, Shimadzu, Tokyo, Japan). A calibration curve was established using gallic acid as a standard, and the results are expressed as milligrammes of gallic acid equivalent (mg GAE) per gramme of dry weight of defatted testa (Arivalagan et al., 2018).
2.7 Estimation of total flavonoid content
The diluted extract was used to determine the total flavonoid content (TFC) by assessing the intensity of the yellow-coloured flavonoid-aluminum complex formed in an alkaline environment (Zhishen et al., 1999). Briefly, 250 μL of extract was mixed with 1.75 mL of distilled water, followed by the addition of 300 μL of 5% NaNO2. The tubes were kept in the dark at room temperature. After 5 min, 300 μL of 10% AlCl3 solution was added, and the mixture was left to stand for another 5 min. Subsequently, 2.5 mL of 1 M NaOH was added. The absorbance was measured after 10 min at 510 nm using a Shimadzu UV-160 A, UV–Visible recording spectrophotometer (P/N 204–04550, Shimadzu, Tokyo, Japan). A calibration curve was generated with quercetin as the standard, and the results are expressed as milligrammes of quercetin equivalents (QE) per gramme of testa samples (Arivalagan et al., 2018).
2.8 Determination of monomeric anthocyanins
The monomeric anthocyanin content in testa extracts was determined using the pH-differential method described by Lee et al. (2005), employing two buffer systems: potassium chloride buffer at pH 1.0 (0.025 M) and sodium acetate buffer at pH 4.5 (0.4 M). The concentrated testa extracts were appropriately diluted, and a 0.1 mL aliquot was transferred to a 10 mL volumetric flask, which was then filled to10 mL with corresponding buffer. The absorbance was measured at 510 and 700 nm. Total anthocyanins were calculated as cyanindin-3-glucoside (C3G) equivalents (C3GE) as follows:
Where A = (A520nm-A700nm) pH 1.0-(A520 nm-A700nm) pH 4.5.
MW (molecular weight) = 449.2 g/mol for cyanidin −3-glucoside (cyd-3 glu).
DF = dilution factor.
1 = path length in cm.
€ = 26,900 molar extinction coefficient in mol−3 for cyanidin−1glucoside (cyd-3-glu).
10^3 = conversion from g to mg.
2.9 Determination of in vitro anti-oxidant potential
The radical scavenging activities of testa extractants were assessed by measuring the reduction in absorbance of DPPH when it came into contact with the testa extractants, following the method described by Brand-Williams et al. (1995) and Arivalagan et al. (2018). The Scavenging Concentration (SC50), indicating the sample concentration required to scavenge 50% of the DPPH●, was determined using Trolox as a positive control, and the results were expressed in micromoles of Trolox equivalents per gramme (μmol TE/g). Additionally, the Ferric Reducing Antioxidant Power (FRAP) and Cupric Ion Reducing Antioxidant Capacity (CUPRAC) assays were conducted following established protocols (Benzie and Strain, 1996; Apak et al., 2004; Arivalagan et al., 2018). Trolox served as the reference, and the outcomes were presented as μmol TE/g of the sample.
2.10 pH stability of colourant
The coconut testa-derived 0.3 M acidified ethanol extract colourants were standardised to an absorbance of 1.50 ± 0.02 at 525 nm. The standardised colourant preparations were subjected to various pH treatments in McIlvaine buffer (Indrawati et al., 2017) with slight modifications. Briefly, buffered solutions of various pH levels were prepared using citric acid (2% w/v), sodium hydrogen phosphate (2% w/v), and sodium azide (0.02% w/v). The preparations were stored in a dark place at both cold storage (4°C) and room temperature (26.0 ± 0.5°C) for 15 days.
2.11 Foam mat drying and incorporation of testa colourant in coconut water jelly
Foam mat drying, an alternative to spray drying, was applied for the production of coconut testa colourant powder using sodium caseinate (5% w/v), maltodextrin (6% w/v) and 0.5% of carboxymethyl cellulose (CMC) following the protocol described by Beegum et al. (2022). A mature coconut water-based jelly, comprising 15% (w/v) refined sugar, 1.65% (w/v) China grass (sea weed, agar), and 2.5% (v/v) lime juice, was prepared by incorporating the testa colourant obtained through acidified ethanol-based extraction using hydrocholoric acid and phosphoric acid (Beegum et al., 2021).
2.12 Statistical analysis
The data was analyzed an unbalanced factorial CRD template. Analysis of variance was performed using R software version 4.0.3 (R Core Team, 2020). Summary statistics and the significance of differences between treatment means and their interactions were determined using the Fisher-LSD test in the agricolae package (De Mendiburu and Simon, 2015). Bartlett’s test of homogeneity of variances was performed to ensure that the distributions of the outcomes in each independent group were comparable. The relationships amongst the studied parameters were further explored using principal component analysis (PCA). The results of PCA were visualised with a biplot constructed between the first two principal components (Dim 1 and Dim 2) using the “factoextra” package in R (Kassambara and Mundt, 2020).
3 Results and discussion
The exploration of natural colourants is a broad and dynamic field of research, driven by the increasing interest in replacing synthetic colourants, which have harmful effects on human health (Thakur and Modi, 2022). In the food industry, carotenoids and anthocyanins are the primary vegetable-derived colourants (Nabi et al., 2023). Anthocyanins are characterised by their antioxidant activity, which helps prevent various health issues such as neuronal and cardiovascular diseases, cancer, and diabetes (Mattioli et al., 2020). Numerous studies have examined the impact of anthocyanins on cancer treatments (Khoo et al., 2017), human nutrition (Duchowicz et al., 2019), and their biological activity (Szymanowska et al., 2015). Given the considerable potential of natural anthocyanins as health-promoting pigments, a growing body of literature addresses various aspects of human health. These include the development of analytical techniques for purification and separation and applications in food (Hu et al., 2014); identification and distribution in plants; monitoring colour and pigment changes; biosynthesis; and quantitative analysis using chromatographic and electrophoretic techniques, and the impact on stress (Rahman et al., 2006; Coklar and Akbulut, 2017) amongst others etc., Various studies have elucidated the biochemical characteristics of anthocyanins derived from a diverse array of plant species, including onions (Zhang et al., 2016), Mahonia aquifolium (Coklar and Akbulut, 2017), Lycium ruthenicum Murray and Nitraria tangutorum (Hu et al., 2014), blue corn (Nankar et al., 2016), potatoes (Heinonen et al., 2016), pomegranates (Ambigaipalan et al., 2017), blueberries (He et al., 2016) mulberries (Espada-Bellido et al., 2017), jamun (Jampani et al., 2014), and others. Hence, this study was formulated with the objective of exploiting the health-promotional biochemical components of coconut testa, the anthocyanins, to develop a food biocolourant. Additionally, unlike existing non-conventional methodologies such as super-critical fluid extraction, pressurised liquid extraction, and microwave or enzyme-assisted extraction, the method described in this work used a laboratory-scale water bath to extract colourant from coconut testa. This highlights the utility, applicability, and scalability of the method for operational ease.
3.1 Features of coconut testa derived colourant
The CIE LAB colour space values of various testa-based colourants obtained using different organic solvents in laboratory-scale water bath and ultrasonication are shown in Supplementary Table 1. Acidified organic solvents in water bath yielded dark brownish or reddish-coloured extracts (Supplementary Figure 1). On the other hand, extracts obtained from the ultrasonication process were more visually appealing, with dark pink, orange colours. Measurement of L*a*b* values of the extractants revealed that L* values were positive, with the highest L* values of 14.03 and 12.73 obtained when extracted with acidified ethanol (acidified with 0.3 M phosphoric acid and 0.1 M citric acid, respectively) in a water bath. Further, comparable L* values in the range of 10.55–11.47 were obtained when the extraction was performed under ultrasonication using acidified ethanol and acetone as solvents. Incorporation of red and purple potatoes derived natural colourants in the preparation of soft drinks showed L* values of around 25.00 suggesting relative blackness of testa based colourants (Sampaio et al., 2021). More positive a* values, corroborated the presence of magenta/red colour in the ethanol-based extracts (both in water bath and ultrasonication process) compared to the solvent acetone. Extraction using 0.3 M HCl-acidified ethanol in the lab water bath showed an increasing trend in the magenta/red colour (increase in a*) with an increase in temperature from 60 to 75° C. Similarly, extracts based on acidified ethanol (0.3 M HCl) as a solvent in ultrasonication process showed upward trend in a* values (7.35, 10.84, and 12.53, respectively, for 60, 90, and 120 min-treatments) with an increase in duration of treatment from 60 to 120 min. However, for a similar treatment profile, acidified acetone-based extracts yielded a maximum a* of 6.24 (for 120 min treatment) suggesting ethanol is a suitable solvent for increased red/magenta colour in the extract. Green-red colour component (a*) of red and purple coloured potato-based soft drinks were high (not <25) compared to the testa-based colourants (Sampaio et al., 2021). Acidified ethanol (0.3 M HCl) showed an increase in the yellow colour of the extracts (b* values ranged from 4.50 to 6.76) until the temperature was 70°C, beyond which a decline in b* (consequently a decrease in yellow) was observed. As previously proven colour of the extract is affected by the temperature profile during extraction process, explaining the decline in yellowness (Aykın-Dinçer et al., 2021). Acidified ethanol (0.3 M phosphoric acid, 0.1 M citric acid, and 0.1 M HCl) yielded relatively high b* values (indicating increased yellowness) in the range of 9.29–12.27, compared to extracts obtained from 0.3 M acidified ethanol or acetone. Similarly, in the ultrasonication process, acidified ethanol (0.3 M HCl) yielded relatively high b* values of 14.64 compared to acidified acetone (0.3 M HCl) with b* values of 13.75 (Supplementary Figure 1). Comparing the CIE L*a*b* results of testa colourant with that of commonly used natural food colourants reveal the need for further in-depth analysis of its phytoconstituents. For instance, depending on the curcumin content, the L* values of turmeric colourant varied from 32.6 to 54.7 (lighter values); a* varied from 17.5 to 39.1 (reddishness); b* from 31.5 to 46.1 (yellowness) as it increased L* and b* and decreased a* values (Singhee and Sarkar, 2022). Similarly, bixin concentration in annatto colourant decreased b*/a* values suggesting yellow colouration (Singhee and Sarkar, 2022). On the application front, extraction of biocolourant from red and purple-fleshed potatoes and their addition in soft drink formulations not only provided stable colourant but also an appreciable sensory profile to the product (Sampaio et al., 2021). Anthocyanin-rich extract of jabuticaba epicarp was incorporated into macaron to yield delphinidin-3-O-glucoside and cyanidin-3-O-glucoside enriched bakery product (Albuquerque et al., 2020).
3.2 Phytochemical composition of testa colourant and anthocyanins
The coconut testa extractants, obtained using various solvents (0.3 M HCL-acidified acetone, ethanol, and 0.5 M phosphoric acid-acidified ethanol), were screened for biochemical constituents, revealing the presence of flavonoids, tannins and anthocyanins whilst anthraquinones, steroids, saponins and terpenoids were conspicuously absent (Supplementary Table 2; Supplementary Figure 2).
Table 1 shows the total anthocyanin content of the testa-based colourants extracted using various organic solvents and ultrasonication assisted extraction (UAE). Extraction of colourant from testa using 0.3 M HCl acidified ethanol for 60 min at 70°C yielded highest anthocyanin content of 723.71 ± 2.19 mg Cy-3-glc equivalents (C3GE) per 100 g, whereas the lowest anthocyanin content was obtained for 0.3 M HCl-acidified ethanol for 60 min at 60°C (714.07 ± 1.26 mg C3GE/100 g). Amongst the extracts derived from the ultrasonication process, acidified-ethanol at 60°C for 90 min yielded an anthocyanin content of 718.20 ± 2.09 mg C3GE/ 100 g. Statistically comparable levels of anthocyanins were obtained with 60 min at 60°C (715.19 ± 1.62 mg C3GE/100 g) in UAE and when 0.3 M acidified-ethanol was used for extraction under two conditions (65°C for 60 min and 60°C for 60 min). Overall, acidified acetone yielded lower anthocyanin content compared to extracts from acidified-ethanol. Hence, to improve the functional components in the coconut testa-based colourant, the use of acidified ethanol as organic solvent, either in water bath or UAE method, is suggested. Additionally, extraction of anthocyanins in conventional solvent extraction and UAE did not show appreciable differences. Similarly, anthocyanin-based colourants from strawberry fruit were incorporated into the development of naturally coloured yoghurt (Benchikh et al., 2021). However, the anthocyanin content was found to be very low (38.04 mg C3GE/100 g FW), suggesting coconut testa as a novel potential source of anthocyanins. A functional drink developed from the colourants of Rubus fruticosus L. and Morus nigra L showed a considerable increase in the content of total anthocyanins (70–90 mg/100 g), revealing unexplored variations in anthocyanins content from plant sources (Vega et al., 2021). On the other hand, incorporation of anthocyanins derived from black goji berry (Lycium ruthenicum) yielded a functional food product (yoghurt) containing total anthocyanin content of 131.6 ± 5.93 mg C3GE/kg (Gamage et al., 2024). Enhanced stability of apigeninidin, an anthocyanin extracted from red sorghum, was observed at high or alkaline pH levels by Akogou et al. (2019). Also, high colour stability (for 30 days) of a soft drink product was observed when anthocyanin-rich natural colourants from coloured potato fleshes were supplemented (Sampaio et al., 2021). A preliminary assessment of the role of pH in determining the stability of anthocyanins derived from coconut testa was performed. It was found that the colourants were stable for 15 days under at cold storage (4°C); however, colourants beyond pH 7.5 showed a slight bluish tinge after 7 days of storage due to degradation of anthocyanins. However, acidified organic solvents have yielded appreciable quantities of anthocyanins from coconut testa, as an increased pH is expected to have an unfavourable effect on the stability of anthocyanin. These results also suggest that further in-depth metabolite profiling studies are required, considering the considerable pH stability of coconut testa-derived colourant over a wide range of pH.
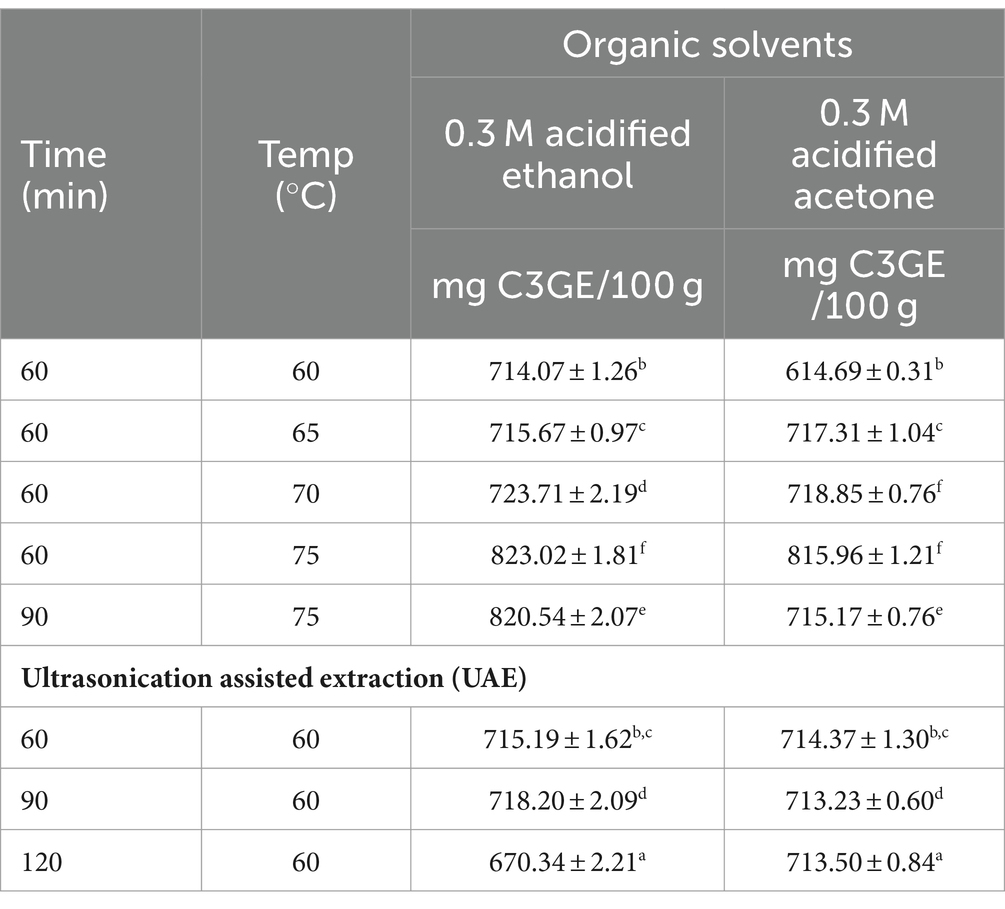
Table 1. Anthocyanin content [expressed as Cy-3-glc equivalents (C3GE)] of coconut testa-based colourant extracted using organic solvents in water bath and ultrasonication based extraction (UAE) (Bartlett test of homogeneity of variances was performed for the data: for 0.3 M acidified ethanol: Bartlett’s K-squared = 3.1018, p = 0.8754; for 0.3 M acidified acetone: Bartlett’s K-squared = 2.3269, p = 0.9396; the same superscript across the column denotes non-significant values).
3.3 Polyphenol and flavonoid contents of testa biocolourant
Total polyphenol content (TPC) of testa extractants is presented in Table 2. The TPC in testa extracts varied from 52.94 ± 2.33 (mg GAE/g) (0.1 M phosphoric acid-acidified ethanol) to 154.39 ± 2.63 mg GAE/g (0.3 M HCl-ethanol). An increase in temperature increased the TPC of extractants, yielding a maximum of 127.24 ± 2.89 mg GAE/g with 0.1 M HCl-acidified ethanol and 154.39 ± 2.63 mg GAE/g using 0.3 M HCl-acidified ethanol. This suggests the role of temperature and acidification of organic solvents in extracting the bound phenolic components in testa. The improvement in the yield of TPC utilizing UAE was also observed with increased acidification of solvents. Thus, the acidification of organic solvents enabled maximum extraction of conjugated phenolic complexes from coconut testa (Arivalagan et al., 2018). Increase in temperature conditions beyond 70° C when acidified-acetone was used as a solvent did not cause any significant change in TPC. On the other hand, acidified-ethanol when used in combination with temperature conditions above 70°C yielded significantly higher TPC (154.39 ± 2.63 mg GAE/g). Similarly, the effect of acidification of organic solvents in obtaining total flavonoid content (TFC) was also evident, as 0.3 M acidified ethanol yielded a maximum TFC of 53.65 ± 0.62 mg QE/g of testa. Overall, the TFC of testa varied from 7.54 ± 0.49 mg QE/g (1% citric acid-acidified ethanol) to 53.65 ± 0.62 mg QE/g (0.3 M HCl-acidified ethanol). Thus, Bartlett’s test of homogeneity of variances between the acidified acetone and acidified ethanol extracts reveal that the latter method yielded statistically significant high TPC and TFC contents across the temperature and time combinations (Table 2). Comparison of TPC between organic solvent-based extraction (OSE) and UAE revealed that the conventional technique of organic solvents yielded higher values (154.39 ± 2.63 mg GAE/g) than UAE (140.54 ± 1.73 mg GAE/g). A similar trend was observed with TFC between OSE and UAE in coconut testa. However, Swer and Chauhan (2019) reported the advantage of enzyme-assisted extraction (EAE) in enhancing the anthocyanin content of Prunus nepalensis L. compared to conventional solvent extraction. It was also observed that EAE further improved phenolic content by releasing it from bound forms.
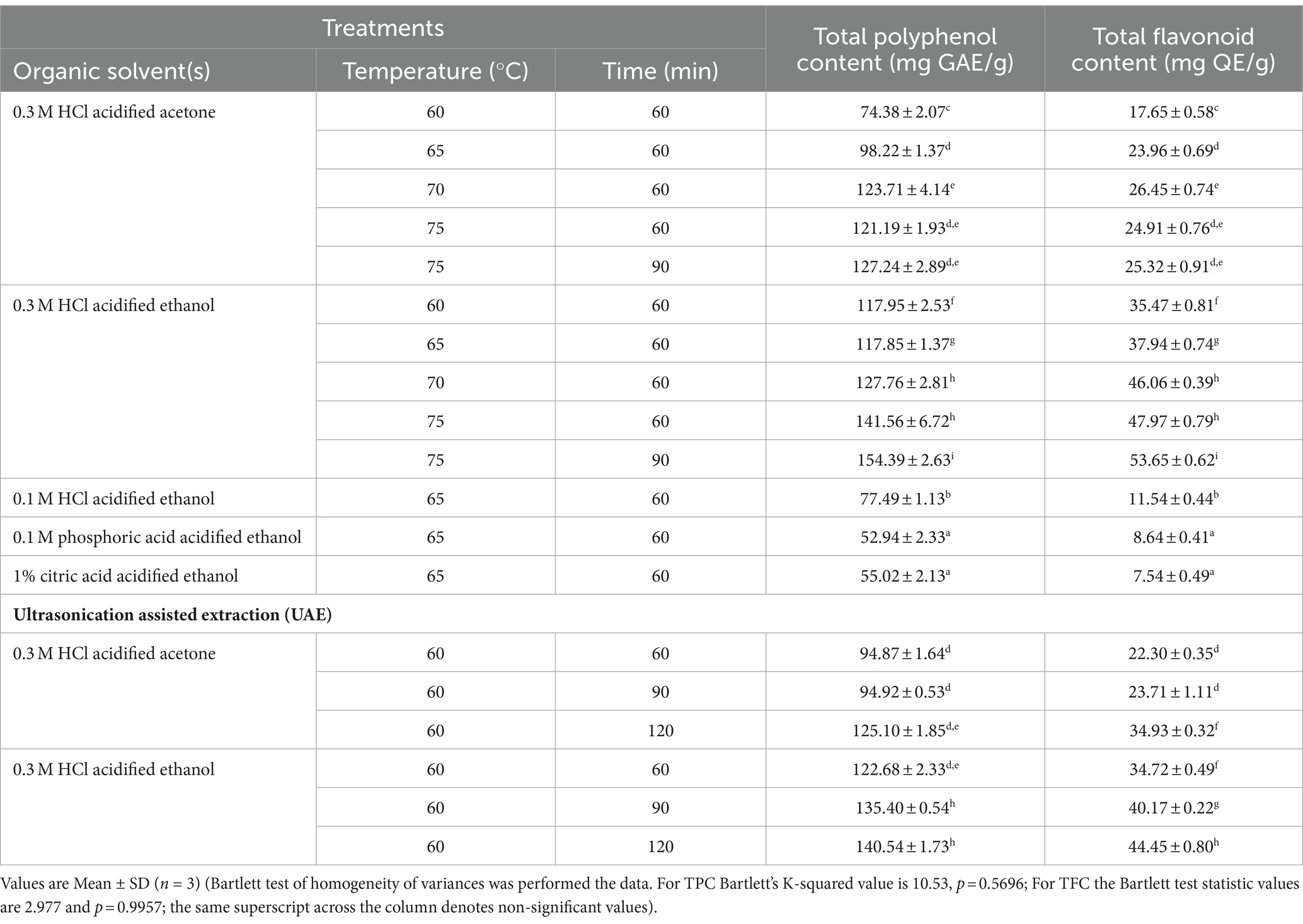
Table 2. Total polyphenol content (TPC) and total flavonoids content (TFC) of testa-based colourant extracts obtained using various organic solvents and ultrasound assisted extraction (UAE).
Acidified ethanol yielded the maximum TPC and TFC in coconut testa-based colorants. Nevertheless, studies have indicated that aqueous ethanol and water are suitable solvents for extracting phenolics from wheat bran (Verma et al., 2008) and sorghum leaves (Agbangnan et al., 2012), respectively. Additionally, 80% acetone acidified with 0.3 M HCl and 80% methanol were found to be suitable for extracting maximum phenolics and flavonoids from coconut testa (Arivalagan et al., 2018). Classical solvent extraction and UAE of bioactives from the corms of saffron (Crocus sativus) also showed comparable quantities of TPC (Swer and Chauhan, 2019; Esmaeelian et al., 2021). However, the flavonoid contents of coconut testa samples, reported herein, are relatively high. Comparing the reported TPC of saffron (Crocus sativus) (89.280 ± 0.88 to 100.396 ± 0.58 mg GAE/100 g dry saffron), the values reported herein are very high (154.39 ± 2.63 mg GAE/g) for defatted dried coconut testa (Esmaeelian et al., 2021). However, such high values of TPC in coconut testa (as much as 167 mg GAE/g) and the resultant antioxidant activities have been reported (Arivalagan et al., 2018). Therefore, coconut testa exemplifies a natural reservoir of diverse phenolic acids and flavonoids, showcasing robust antioxidant capabilities. These inherent compounds offer a promising alternative to synthetic antioxidants in food formulations, establishing themselves as a noteworthy natural source of antioxidant properties.
3.4 Antioxidant profile of testa-colourants
The antioxidant potential of the testa-based colourants is presented in Table 3. The antioxidant potential of the testa-based colourant extractants was assessed for their reducing power using CUPRAC and FRAP assays. Acidified solvents (0.3 M HCl) at relatively high temperature and time conditions exhibited high but comparable CUPRAC values of 323 and 346.4 μmol TE/g. Similarly, the ferric-reducing capability of the extract was found to be high (194.08 and 209.7) when acidified organic solvents were utilised. The antioxidant potential of the testa colour extracts, as measured by DPPH, varied from 171.9 μmol TE/g to 248.6 μmol TE/g. Acidification of organic solvents significantly increased the DPPH activities of the extracts. The DPPH radical scavenging activity of yoghurt prepared from black goji berry-derived anthocyanins demonstrated considerably higher antioxidant potential compared to the functional food product developed from purple sweet potato (Gamage et al., 2024). The trolox equivalent anti-oxidant capacity (TEAC) of black coloured carrot infused yoghurt was in the range of 37.83–50.21 μg TEAC/g suggesting the high anti-oxidant potential of coconut testa derived colourant (Baria et al., 2021).
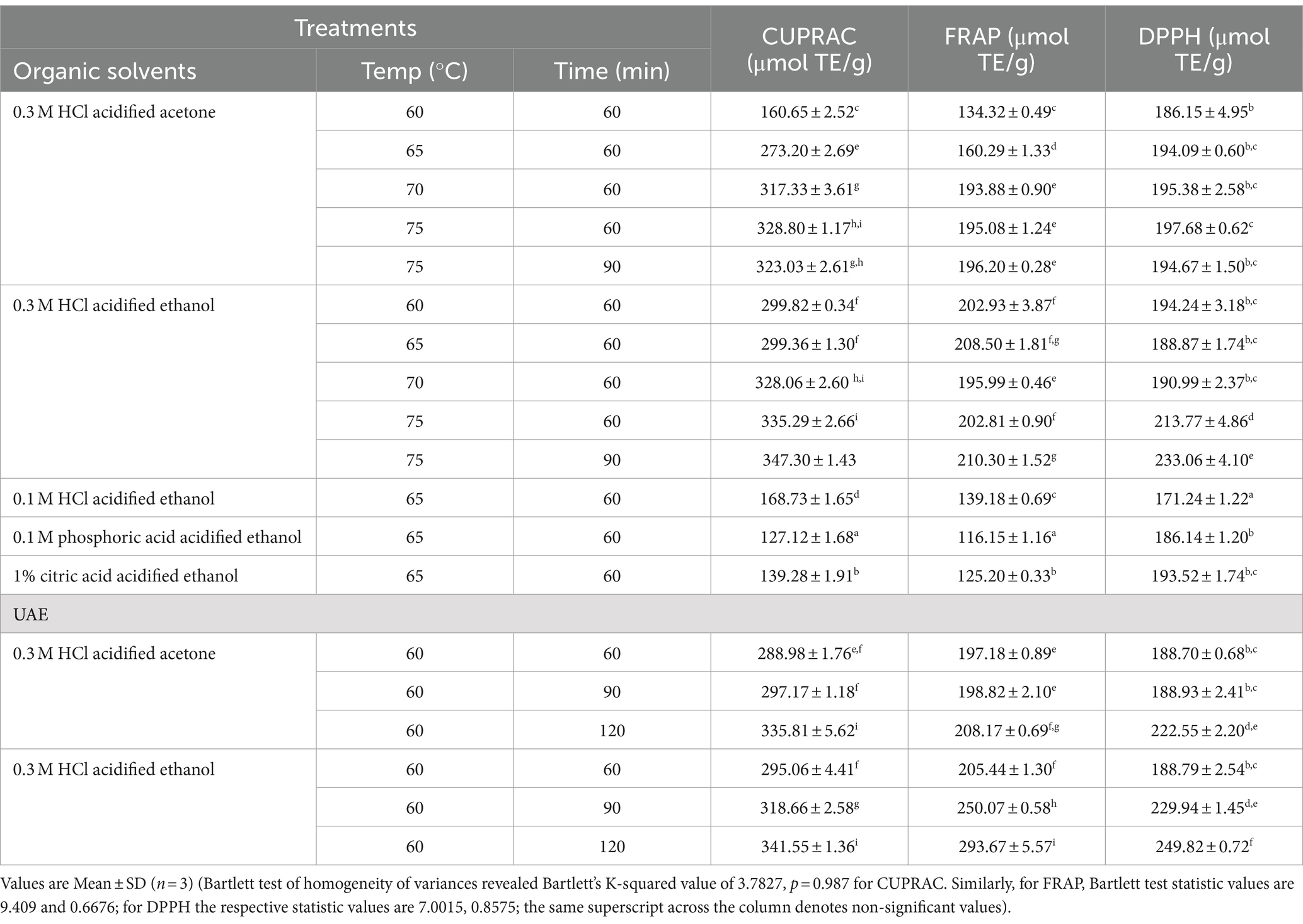
Table 3. Anti-oxidant profile of testa-based colourant extracts obtained using various organic solvents and UAE.
3.5 Principal component analysis
The principal component loadings for testa colourant quality characteristics are shown in Figure 2. Principal component analysis (PCA) was used to study the relationship between the quality characteristics of testa colourant obtained from various solvent treatments. PCA revealed that the initial four principal components defined 100% of the total variance in the testa colourant quality characteristics. The first principal component (PC1) represented 78.7% of total variability, the second principal component (PC2) accounted for 13.4% of total variability, and the third principal component represented 6% of the variability. Figure 3 shows the correlation biplot (score plot) of the testa colourant quality features on the first two principal components. The correlation score plots distinguished between various combinations of organic solvent, time and temperature used in producing the testa colourant. PCA biplot analysis reveals a positive correlation between TPC, TFC, and antioxidant assay values DPPH, CURRAC, and FRAP.
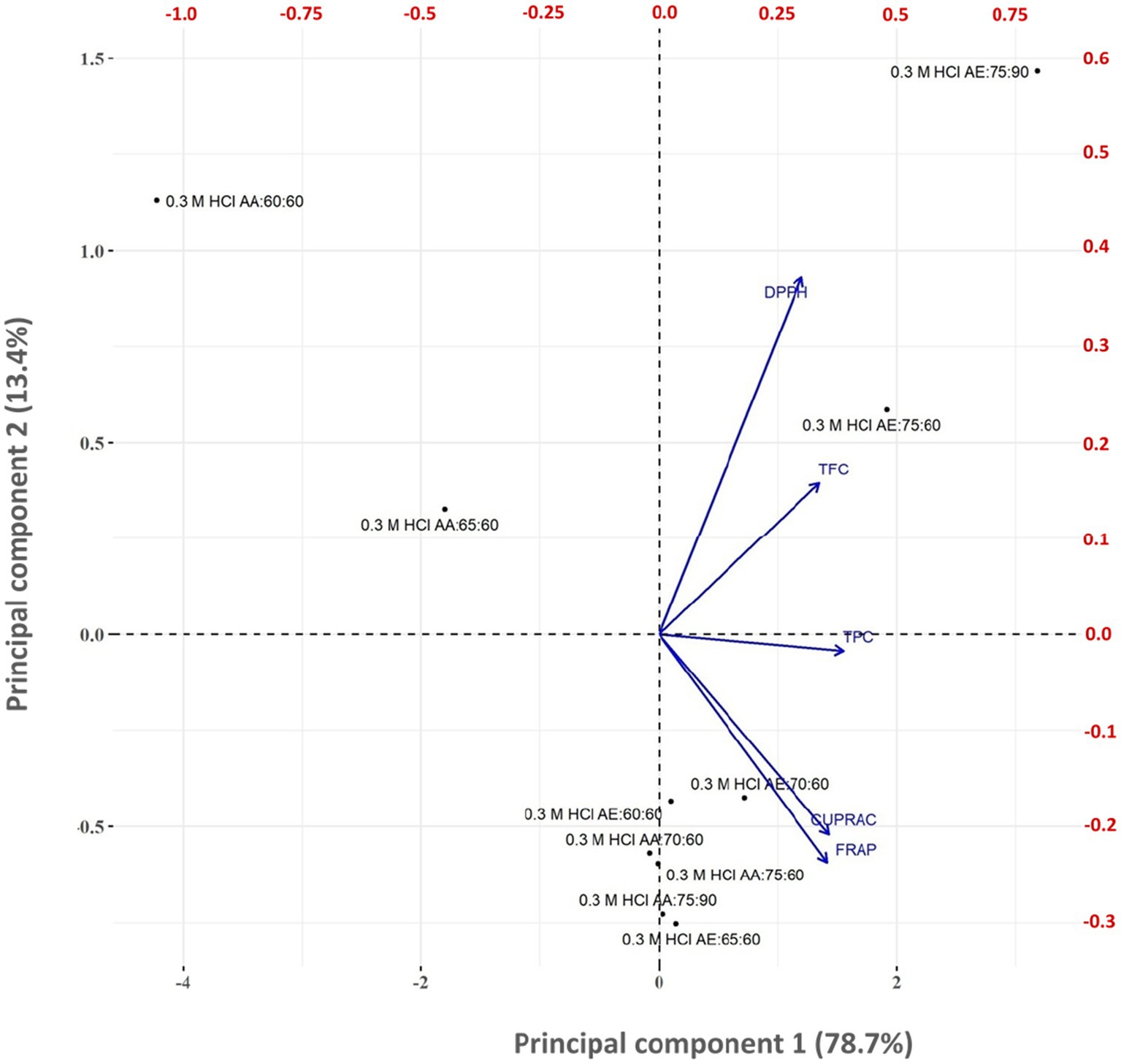
Figure 2. Biplot for principal component-1 (PC1) and principal component-1 (PC2) based on the principal component analysis of quality parameters of testa colourant produced using different organic solvent, time, and temperature combinations.

Figure 3. Incorporation of coconut testa-derived biocolourant in food products (coconut water-based jelly) (A) foam mat drying process used to prepare colourant solids from the testa extractants (B) control jelly without colourant (C) jelly infused with colourant from ethanol extract (D) jelly infused with colourant obtained from ultrasonication assisted extract.
Foam mat-dried colourant was utilised as an ingredient in the production of coconut water-based jelly (Figure 3). Similarly, incorporating C-phycocyanin, a protein-based bioactive compound extracted from cyanobacteria, in ice cream provided a stable blue dye with enhanced antioxidant activity, suggesting utility in terms of colour and biological activities (de Amarante et al., 2020). Bioactives such as betalains from beet root powder have been exploited in the development of a stable chocolate (Baycar et al., 2021). Additionally, apigeninidin extract from sorghum leaf sheaths has been proven to be a bioactive red biocolourant with potential application in fermented foods (Akogou et al., 2019). Plant-derived anthocyanin extracts have multiple functional properties besides their conventional use as food colourants, including anti-microbial properties, anti-oxidant attributes, use as preservatives, and other health-promoting benefits (Arruda et al., 2021). Anthocyanin -rich plants such as red or purple potatoes, red cabbage, black carrots, purple corn, and sweet potatoes predominantly contain acylated anthocyanins that impart high stability during the processing and storage, thereby enhancing the bioavailability of these components (Arruda et al., 2021; Kaur et al., 2024). As discussed above, biochemical characterisation of coconut testa-derived anthocyanins for further use in food products as a functional ingredient at an industrial scale is warranted.
4 Conclusion
The natural colourant extracted from coconut testa is notably rich in anthocyanin, phenolics, exhibiting significant antioxidant potential. The anthocyanin content of testa contributes to its distinct colour. Extraction conditions reveal that ethanol is an effective solvent for enhancing the red/magenta hue in the extract, whilst acidified ethanol (0.3 M HCl) increases the yellow colour of the extracts. Phytochemical screening of the coconut testa colourant shows a substantial presence of anthocyanins, total phenolics, and flavonoids, highlighting its remarkable antioxidant and colourant potential. This colourant has been successfully incorporated into coconut water-based jelly, demonstrating its utility in food product development. Therefore, coconut testa extract serves a dual purpose: it enhances the aesthetic appeal of food as a colourant and provides significant health-promoting properties due to its high anthocyanin content. Several factors have impeded the commercial development of natural pigments thus far. These include the limited range of natural colours approved for food use, the lengthy regulatory approval process for new colorants, their higher cost compared to synthetic alternatives, and the substantial volumes of biomass needed to extract a given amount of natural colorant. However, as stated elsewhere around 4,000 tonnes of underutilised coconut testa in the country offers unique opportunity to exploit this biowaste into a useful food product. The colourants remained stable for 15 days in cold storage. However, after 7 days, those with a pH above 7.5 showed a slight bluish tinge due to the degradation of anthocyanins. These findings indicate the need for further in-depth metabolite profiling studies, given the considerable stability of coconut testa-derived colourant across a wide pH range.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. RP: Formal analysis, Investigation, Validation, Writing – review & editing. PPS: Formal analysis, Investigation, Validation, Writing – review & editing. SS: Formal analysis, Resources, Software, Writing – review & editing. PS: Formal analysis, Validation, Writing – review & editing. KS: Formal analysis, Validation, Writing – review & editing. MM: Formal analysis, Investigation, Resources, Writing – review & editing. MG: Formal analysis, Investigation, Resources, Validation, Writing – review & editing. KH: Conceptualization, Formal analysis, Resources, Supervision, Writing – review & editing. AU: Funding acquisition, Resources, Writing – review & editing. AD: Funding acquisition, Resources, Writing – review & editing. RB: Resources, Funding acquisition, Writing – review & editing. BG: Resources, Funding acquisition, Writing – review & editing. PK: Resources, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the ICAR-Central Plantation Crops Research Institute (ICAR-CPCRI grant no.: 1000766014) and ICAR-NEH fund of ICAR-CPCRI, Kahikuchi and Coconut Development Board funded project (ICAR-CPCRI-1050761159).
Acknowledgments
Authors acknowledge the instrumentation facility rendered by KCAET, Tavanur, Kerala.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2024.1382214/full#supplementary-material
Footnotes
References
Agbangnan, P. D., Tachon, C., Bonin, H., Chrostowka, A., Fouquet, E., and Sohounhloue, D. C. (2012). Phytochemical study of a tinctorial plant of Benin traditional pharmacopoeia: the red sorghum (Sorghum caudatum) of Benin. Sci. Study Res. Chem. Eng. Biotechnol. Food Industry 13:121.
Akogou, F. U., Canoy, T. S., Kayodé, A. P., den Besten, H. M., Linnemann, A. R., and Fogliano, V. (2019). Application of apigeninidin-rich red sorghum biocolorant in a fermented food improves product quality. J. Sci. Food Agric. 99, 2014–2020. doi: 10.1002/jsfa.9427
Albuquerque, B. R., Pinela, J., Barros, L., Oliveira, M. B. P., and Ferreira, I. C. (2020). Anthocyanin-rich extract of jabuticaba epicarp as a natural colorant: optimization of heat-and ultrasound-assisted extractions and application in a bakery product. Food Chem. 316:126364. doi: 10.1016/j.foodchem.2020.126364
Ambigaipalan, P., de Camargo, A. C., and Shahidi, F. (2017). Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI-MSn. Food Chem. 221, 1883–1894. doi: 10.1016/j.foodchem.2016.10.058
Anonymous, Machinery, and Gadgets. (2024). Available at: https://cpcriagribiz.in/home/machinery (Accessed August 1, 2024).
Apak, R., Guclu, K., Ozyurek, M., and Karademir, S. E. (2004). Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 52, 7970–7981. doi: 10.1021/jf048741x
Appaiah, P., Sunil, L., Prasanth Kumar, P. K., and Gopala Krishna, A. G. (2014). Composition of coconut testa, coconut kernel and its oil. J. Am. Oil Chem. Soc. 91, 917–924. doi: 10.1007/s11746-014-2447-9
Arivalagan, M., Roy, T. K., Yasmeen, A. M., Pavithra, K. C., Jwala, P. N., Shivasankara, K. S., et al. (2018). Extraction of phenolic compounds with antioxidant potential from coconut (Cocos nucifera L.) testa and identification of phenolic acids and flavonoids using UPLC coupled with TQD-MS/MS. LWT 92, 116–126. doi: 10.1016/j.lwt.2018.02.024
Arruda, H. S., Silva, E. K., Peixoto Araujo, N. M., Pereira, G. A., Pastore, G. M., and Marostica Junior, M. R. (2021). Anthocyanins recovered from Agri-food by-products using innovative processes: trends, challenges, and perspectives for their application in food systems. Molecules 26:2632. doi: 10.3390/molecules26092632
Aykın-Dinçer, E., Güngör, K. K., Çağlar, E., and Erbaş, M. (2021). The use of beetroot extract and extract powder in sausages as natural food colorant. Int. J. Food Eng. 17, 75–82. doi: 10.1515/ijfe-2019-0052
Baria, B., Singh, A. K., Panjagari, N. R., Arora, S., and Minz, P. S. (2021). Colouring properties and stability of black carrot anthocyanins in yoghurt. J. Food Sci. Technol. 58, 3953–3962. doi: 10.1007/s13197-020-04858-9
Baycar, A., Konar, N. E. V. Z. A. T., Goktas, H., Sagdic, O., and Polat, D. G. (2021). The effects of beetroot powder as a colorant on the color stability and product quality of white compound chocolate and chocolate spread. Food Sci. Technol. 42:66220. doi: 10.1590/fst.66220
Beegum, P. P. S., Manikantan, M. R., Anju, K. B., Vinija, V., Pandiselvam, R., Jayashekhar, S., et al. (2022). Foam mat drying technique in coconut milk: effect of additives on foaming and powder properties and its economic analysis. J. Food Proc. Preserv. 46:e17122. doi: 10.1111/jfpp.17122
Beegum, P. P. S., Pandiselvam, R., Manikantan, M. R., Mathew, A. C., Hebbar, K. B., and Muralidharan, K. (2021). Technologies for value added coconut products. Kudlu: ICAR-CPCRI Publication.
Benchikh, Y., Aissaoui, A., Allouch, R., and Mohellebi, N. (2021). Optimising anthocyanin extraction from strawberry fruits using response surface methodology and application in yoghurt as natural colorants and antioxidants. J. Food Sci. Technol. 58, 1987–1995. doi: 10.1007/s13197-020-04710-0
Benzie, I. E. F., and Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal. Biochem. 239, 70–76. doi: 10.1006/abio.1996.0292
Brand-Williams, W., Cuvelier, M. E., and Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28, 25–30.
Coklar, H., and Akbulut, M. (2017). Anthocyanins and phenolic compounds of Mahonia aquifolium berries and their contributions to antioxidant activity. J. Funct. Foods 35, 166–174. doi: 10.1016/j.jff.2017.05.037
de Amarante, M. C. A., Braga, A. R. C., Sala, L., and Kalil, S. J. (2020). Colour stability and antioxidant activity of C-phycocyanin-added ice creams after in vitro digestion. Food Res. Int. 137:109602. doi: 10.1016/j.foodres.2020.109602
De Mendiburu, F., and Simon, R. (2015). Agricolae-ten years of an open source statistical tool for experiments in breeding, agriculture and biology. Tech. Rep. 3:e1404v1. doi: 10.7287/peerj.preprints.1404v1
Duchowicz, P. R., Szewczuk, N. A., and Pomilio, A. B. (2019). QSAR studies of the antioxidant activity of anthocyanins. J. Food Sci. Technol. 56, 5518–5530. doi: 10.1007/s13197-019-04024-w
Esmaeelian, M., Jahani, M., Feizy, J., and Einafshar, S. (2021). Effects of ultrasound-assisted and direct solvent extraction methods on the antioxidant and antibacterial properties of saffron (Crocus sativus L.) corm extract. Food Anal. Methods 14, 74–87. doi: 10.1007/s12161-020-01855-8
Espada-Bellido, E., Ferreiro-González, M., Carrera, C., Palma, M., Barroso, C. G., and Barbero, G. F. (2017). Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 219, 23–32. doi: 10.1016/j.foodchem.2016.09.122
Gamage, G. C. V., Goh, J. K., and Choo, W. S. (2024). Application of anthocyanins from black goji berry in fermented dairy model food systems: an alternate natural purple color. LWT 198:115975. doi: 10.1016/j.lwt.2024.115975
He, B., Zhang, L.-L., Yue, X.-Y., Liang, J., Jiang, J., Gao, X.-L., et al. (2016). Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 204, 70–76. doi: 10.1016/j.foodchem.2016.02.094
Heinonen, J., Farahmandazad, H., Vuorinen, A., Kallio, H., Yang, B., and Sainio, T. (2016). Extraction and purification of anthocyanins from purple-fleshed potato. Food Bioprod. Process. 99, 136–146. doi: 10.1016/j.fbp.2016.05.004
Hu, N., Zheng, J., Li, W., and Suo, Y. (2014). Isolation, stability, and antioxidant activity of anthocyanins from Lycium ruthenicum Murray and Nitraria tangutorum Bobr of Qinghai-Tibetan plateau. Sep. Sci. Technol. 49, 2897–2906. doi: 10.1080/01496395.2014.943770
HunterLab (2008). CIE L*a*b* color scale. Available at: http://www.hunterlab.com/appnotes/an07_96a.pdf (Accessed December 15, 2023).
Indrawati, R., Lukitasari, D. M., Yuniarti, Y., and Limantara, L. (2017). Encapsulation, properties, and thermal study of red biocolorant from selected plants obtained through physical extraction. Int. J. Chem. Eng. Appl. 8, 371–376. doi: 10.18178/ijcea.2017.8.6.686
Jampani, C., Naik, A., and Raghavarao, K. S. M. S. (2014). Purification of anthocyanins from jamun (Syzygium cumini L.) employing adsorption. Sep. Purif. Technol. 125, 170–178. doi: 10.1016/j.seppur.2014.01.047
Kassambara, A., and Mundt, F. (2020). Factoextra: extract and visualize the results of multivariate data analyses. Available at: https://CRAN.R-project.org/package=factoextra (Accessed August 1, 2023).
Kaur, D., Yousuf, B., and Qadri, O. S. (2024). Syzygium cumini anthocyanins: recent advances in biological activities, extraction, stability, characterisation and utilisation in food systems. Food Prod. Process. Nutr. 6, 1–17. doi: 10.1186/s43014-023-00177-6
Kazeem, M. I., Adamson, J. O., and Ogunwande, I. A. (2013). Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Res. Int. 2013:527570. doi: 10.1155/2013/527570
Khoo, H. E., Azlan, A., Tang, S. T., and Lim, S. M. (2017). Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 61:1361779. doi: 10.1080/16546628.2017.1361779
Lee, J., Durst, R. W., and Wrolstad, R. E. (2005). Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J. AOAC Int. 88, 1269–1278. doi: 10.1093/jaoac/88.5.1269
Luzardo-Ocampo, I., Ramírez-Jiménez, A. K., Yañez, J., Mojica, L., and Luna-Vital, D. A. (2021). Technological applications of natural colorants in food systems: a review. Food Secur. 10:634. doi: 10.3390/foods10030634
Manzoor, M., Singh, J., Gani, A., and Noor, N. (2021). Valorization of natural colors as health-promoting bioactive compounds: phytochemical profile, extraction techniques, and pharmacological perspectives. Food Chem. 362:130141. doi: 10.1016/j.foodchem.2021.1301
Maronpot, R. R., Hayashi, S. M., and Bastaki, M. (2020). Synthetic and natural food colorants. Foods Ingredients J. 225, 100–110.
Mattioli, R., Francioso, A., Mosca, L., and Silva, P. (2020). Anthocyanins: a comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 25:3809. doi: 10.3390/molecules25173809
Mota, I. G. C., Neves, R. A. M. D., Nascimento, S. S. D. C., Maciel, B. L. L., Morais, A. H. D. A., and Passos, T. S. (2023). Artificial dyes: health risks and the need for revision of international regulations. Food Rev. Intl. 39, 1578–1593. doi: 10.1080/87559129.2021.1934694
Nabi, B. G., Mukhtar, K., Ahmed, W., Manzoor, M. F., Ranjha, M. M. A. N., Kieliszek, M., et al. (2023). Natural pigments: anthocyanins, carotenoids, chlorophylls, and betalains as food colorants in food products. Food Biosci. 52:102403. doi: 10.1016/j.fbio.2023.102403
Nankar, A. N., Dungan, B., Paz, N., Sudasinghe, N., Schaub, T., Holguin, F. O., et al. (2016). Quantitative and qualitative evaluation of kernel anthocyanins from southwestern United States blue corn. J. Sci. Food Agric. 96, 4542–4552. doi: 10.1002/jsfa.7671
R Core Team (2020). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Rahman, M. M., Ichiyanagi, T., Komiyama, T., Hatano, Y., and Konishi, T. (2006). Superoxide radical- and peroxynitrite-scavenging activity of anthocyanins; structure-activity relationship and their synergism. Free Radic. Res. 40, 993–1002. doi: 10.1080/10715760600815322
Ramesh, S. V., Krishnan, V., Praveen, S., and Hebbar, K. B. (2021, 2021). Dietary prospects of coconut oil for the prevention and treatment of Alzheimer’s disease (AD): a review of recent evidences. Trends Food Sci. Technol. 112, 201–211. doi: 10.1016/j.tifs.2021.03.046
Ramesh, S. V., Mary, R., Beegum Puthiya, P. S., Pandiselvam, R., Padmanabhan, S., Sathyan, N., et al. (2023b, 2023). Physicochemical characterization and fatty acid profiles of testa oils from various coconut (Cocos nucifera L.) genotypes. J. Sci. Food Agric. 103, 370–379. doi: 10.1002/jsfa.12150
Ramesh, S. V., Pandiselvam, R., Shameena Beegum, P. P., Saravana Kumar, R. M., Manikantan, M. R., and Hebbar, K. B. (2023a). Review of Cocos nucifera L. testa-derived phytonutrients with special reference to phenolics and its potential for encapsulation. J. Food Sci. Technol. 60, 1–10. doi: 10.1007/s13197-021-05310-2
Rodriguez-Amaya, D. B. (2016). Natural food pigments and colorants. Curr. Opin. Food Sci. 7, 20–26. doi: 10.1016/j.cofs.2015.08.004
Sampaio, S. L., Lonchamp, J., Dias, M. I., Liddle, C., Petropoulos, S. A., Glamočlija, J., et al. (2021). Anthocyanin-rich extracts from purple and red potatoes as natural colourants: bioactive properties, application in a soft drink formulation and sensory analysis. Food Chem. 342:128526. doi: 10.1016/j.foodchem.2020.128526
Shegokar, R., and Mitri, K. (2012). Carotenoid lutein: a promising candidate for pharmaceutical and nutraceutical applications. J. Dietary Suppl. 9, 183–210. doi: 10.3109/19390211.2012.708716
Shkolnikov Lozober, H., Okun, Z., Parvari, G., and Shpigelman, A. (2023). The effect of storage and pasteurization (thermal and high-pressure) conditions on the stability of phycocyanobilin and phycobiliproteins. Antioxidants 12:568. doi: 10.3390/antiox12030568
Singhee, D., and Sarkar, A. (2022). “Colorimetric measurement and functional analysis of selective natural colorants applicable for food and textile products” in Colorimetry. ed. I. Nimeroff (London: Intech Open), 128–130.
Sofowora, A. (1982). Medical plants and traditional medicine in Africa. New York, NY: John Wiley and Sons.
Swer, T. L., and Chauhan, K. (2019). Stability studies of enzyme aided anthocyanin extracts from Prunus nepalensis L. LWT 102, 181–189. doi: 10.1016/j.lwt.2018.12.016
Szymanowska, U., Złotek, U., Karaś, M., and Baraniak, B. (2015). Anti-inflammatory and antioxidative activity of anthocyanins from purple basil leaves induced by selected abiotic elicitors. Food Chem. 172, 71–77. doi: 10.1016/j.foodchem.2014.09.043
Thakur, M., and Modi, V. K. (2022). Biocolorants in food: sources, extraction, applications and future prospects. Crit. Rev. Food Sci. Nutr. 64, 4674–4713. doi: 10.1080/10408398.2022.2144997
Vega, E. N., Molina, A. K., Pereira, C., Dias, M. I., Heleno, S. A., Rodrigues, P., et al. (2021). Anthocyanins from Rubus fruticosus L. and Morus nigra L. applied as food colorants: a natural alternative. Plan. Theory 10:1181. doi: 10.3390/plants10061181
Verma, B., Hucl, P., and Chibbar, R. N. (2008). Phenolic content and antioxidant properties of bran in 51 wheat cultivars. Cereal Chem. 85, 544–549. doi: 10.1094/CCHEM-85-4-0544
Weiss, V., Okun, Z., and Shpigelman, A. (2023). Tackling the safety and health effects of food colorants. Food Safety Health. 1, 107–109. doi: 10.1002/fsh3.12015
Zhang, S., Deng, P., Xu, Y., Lu, S., and Wang, J. (2016). Quantification and analysis of anthocyanin and flavonoids compositions, and antioxidant activities in onions with three different colors. Food Chemistry 64, 555–559. doi: 10.1016/S2095-3119(16)61385-0
Keywords: anthocyanins, biocolourant, coconut testa, food processing, phenolics, functional foods, valorization
Citation: Ramesh SV, Pandiselvam R, Shameena Beegum PP, Shil S, Sugatha P, Sharanya K, Manikantan MR, Gopal M, Hebbar KB, Uchoi A, Das A, Bhat R, Gowda BH and Kumar P (2024) Valorization of coconut (Cocos nucifera L.) testa as a biocolourant. Front. Sustain. Food Syst. 8:1382214. doi: 10.3389/fsufs.2024.1382214
Edited by:
Kathleen L. Hefferon, Cornell University, United StatesReviewed by:
Luqman Jameel Rather, Southwest University, ChinaFabian Dayrit, Ateneo de Manila University, Philippines
Muhammad Adnan Hafeez, Superior University, Pakistan
Monika Thakur, Amity University, India
Copyright © 2024 Ramesh, Pandiselvam, Shameena Beegum, Shil, Sugatha, Sharanya, Manikantan, Gopal, Hebbar, Uchoi, Das, Bhat, Gowda and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. V. Ramesh, cmFtZXNoLnN2QGljYXIuZ292Lmlu
 S. V. Ramesh
S. V. Ramesh R. Pandiselvam
R. Pandiselvam P. P. Shameena Beegum1
P. P. Shameena Beegum1 Sandip Shil
Sandip Shil Murali Gopal
Murali Gopal K. B. Hebbar
K. B. Hebbar Anok Uchoi
Anok Uchoi