
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 27 July 2022
Sec. Agro-Food Safety
Volume 6 - 2022 | https://doi.org/10.3389/fsufs.2022.874219
This article is part of the Research TopicEmerging Clean Label Approaches to Food SafetyView all 4 articles
 Abraham Joseph Pellissery1
Abraham Joseph Pellissery1 Poonam Gopika Vinayamohan2
Poonam Gopika Vinayamohan2 Jingyi Xue3
Jingyi Xue3 Xinhao Wang3
Xinhao Wang3 Leya Susan Viju1
Leya Susan Viju1 Divya Joseph1
Divya Joseph1 Yangchao Luo3
Yangchao Luo3 Ann M. Donoghue4
Ann M. Donoghue4 Kumar Venkitanarayanan1*
Kumar Venkitanarayanan1*Among the animal derived food products, contamination of poultry eggs, and egg shell surface is one of the major causes for foodborne salmonellosis in the United States. As a means of reducing the pathogen transfer to the internal egg contents, polysaccharide-based coatings containing antimicrobial phytochemicals could potentially serve as a biocontrol strategy for shelled egg products. The current study investigated the efficacy of four GRAS (Generally Recognized as Safe)-status plant-derived compounds, namely, caproic acid (CAO), caprylic acid (CAY), linalool (LIN) and cuminaldehyde (CUM), as pectin-based coating treatments, individually or in combination, for reducing Salmonella Heidelberg (SH) on shell eggs. A three-strain mixture of SH (~8.0 log CFU in 50 μL inoculum) was spot-inoculated on surface sterilized white-shelled eggs. Eggs were evenly coated with either pectin-based treatments of CAO (1%), CAY (1%), LIN (1%) and CUM (1%), individually, or a combination of 4 phytochemicals (COMB- each phytochemical at 0.5% v/v level of inclusion). The treated eggs were stored at 4°C and SH counts were enumerated on days 0, 1, 3, 5, 7, 14, and 21 of storage. The study was replicated thrice, 3 eggs/treatment/day time point, and the data were analyzed using two-way ANOVA with significance tested at p < 0.05. On day 0, pectin-coated control eggs had ~7.6 log CFU of SH/egg. At the end of refrigerated storage (day 21), pectin-based coating of CAO and CAY at 1% level reduced SH by 2.0–2.5 log CFU/egg (P < 0.05) when compared to controls. In addition, the CUM and LIN based coatings produced 3.0 log and 3.9 log reduction, respectively, in SH counts on eggs by day 21 of storage. Among the treatments with phytochemical combinations, COMB1 [pectin (2%) + Caprylic acid, caproic acid and cuminaldehyde (each at 0.5% level)] was found to be most effective, reducing SH counts to 2.5–3.3 log CFU/egg from day 0 through day 14, and by the end of storage period (day 21), a 3.5 log CFU reduction/egg (p < 0.05) compared to untreated controls. Morphological studies of treated eggs using atomic force microscopy (AFM) have shown that the roughness of eggs can be influenced by a combination of various compounds. Results indicate the potential efficacy of the aforesaid phytochemicals in reducing SH on shell eggs; however, further studies investigating their industrial feasibility and effects on sensory attributes of eggs are warranted.
Eggs constitute a nutrient-dense commodity with high biological value that provides consumers with a high quality protein source along with essential minerals, vitamins, and trace elements (Ahnen and Slavin, 2019). In the US, the annual consumption of eggs is ~293 eggs per capita in 2019 and the demand has grown over the last several years (Trejo-Pech and White, 2020). However, for nearly more than three decades, there has been an increase in human salmonellosis outbreaks through consumption of shell egg and its products (Chemaly and Salvat, 2011; Musgrove, 2011; Hessel et al., 2019). Globally, serovars of Salmonella enterica continue to be the single largest egg safety threat resulting in recalls of commercial egg and egg products (Paramithiotis et al., 2017). Non-typhoidal Salmonella enterica serovars derived from poultry products, especially Enteritidis, Heidelberg, and Typhimurium have been implicated in ~50% of the foodborne salmonellosis in the United States (Schoeni et al., 1995; Foley et al., 2011; Chousalkar et al., 2017). Although Salmonella Enteritidis is a predominant serovar isolated from eggshell and egg contents and has been commonly associated with foodborne outbreaks in the US (Martelli and Davies, 2012), more recent egg-associated outbreaks have implicated Salmonella Heidelberg as an emerging concern by virtue of being associated with highly invasive infections and mortality in humans (Park et al., 2012; Kaldhone et al., 2017; Dewi et al., 2021). Recent inferences from quantitative foodborne relatedness (FBR) measures also indicated that of the 20 serotypes of non-typhoidal Salmonella in the US, Salmonella Heidelberg is one among the top three serovars (amongst serovars Saintpaul and Berta) involved in foodborne outbreak-associated Salmonella illnesses (Luvsansharav et al., 2020). In addition, Heidelberg serovar has emerged as a leading cause of documented outbreaks originating form eggs and egg production system. Certain isolates of Salmonella Heidelberg with antibiotic resistance phenotypes are known to cause a highly invasive disease in humans (Kaldhone et al., 2017).
The US-Food and Drug Administration requires shell egg producers to practice washing and refrigeration of poultry eggs to reduce the risk of Salmonella-associated illness and deaths (U.S. Food and Drug Administration, HHS, 2009). Several disinfectants such as chlorine, iodine-based sanitizer, hydrogen peroxide, quaternary compounds, electrolyzed water and ozone, have been used for reducing the microbial load on eggs, however, most of these strategies compounds have limited antimicrobial activity and do not render shell eggs pathogen-free (Upadhyaya et al., 2016). Moreover, there is a discordant purview between the US and the European Union (EU) countries regarding egg washing (Kulshreshtha et al., 2018). Countries such as US, Australia and Japan promote egg washing procedures for commercial egg production, whereas the washing of eggs is currently forbidden within the EU [Regulation (EC) No 589/2008; Kulshreshtha et al., 2018]. On the one hand, egg sanitation through washing with disinfectants may help reduce the microbial load, but on the other hand it is also known to alter egg shell surface and allow greater microbial penetration into the eggs (Gabriela da Silva Pires et al., 2020). Antimicrobial incorporated coatings made from edible, biodegradable polymers for food products are a potential strategy for reducing pathogens on foods and reduce foodborne related outbreaks. Several categories of natural coating agents containing antimicrobials have been applied in various food products such as fruits, vegetables, fish, and poultry products (Jin et al., 2013; Morsy et al., 2015; Xing et al., 2016; Al-Tayyar et al., 2020; Fu et al., 2022; Shahidi and Hossain, 2022). Clean label approaches that utilize antimicrobial coatings composed of natural and environmentally friendly compounds could potentially serve as a biocontrol strategy for controlling foodborne Salmonella on shell egg products by reducing pathogen transfer to the internal egg contents. Additionally, application of coating agents can aid to maintain and preserve the internal egg quality and improve the shelf life of refrigerated eggs (de Almeida et al., 2016).
Phytochemicals represent a group of compounds that have traditionally been used as antimicrobials, flavor enhancers and food preservatives (Wollenweber, 1988). As a natural and environmentally friendly option for a biocontrol agent, phytochemicals have gained acceptance and are preferred for use over chemical disinfectants that raise toxicity concerns and resistance development (Mir et al., 1997; Zhang et al., 2015; Martínez-Suárez et al., 2016; Yang et al., 2016; Wang et al., 2020). A significant amount of research work has been conducted in developing edible composite coatings incorporated with antimicrobial agents as a postharvest food safety measure on a variety of food products such as shelled eggs, dry pet food, and poultry meat products (De Leo et al., 2018; Chen et al., 2019; Shrestha et al., 2019; Wagle et al., 2019; Pires et al., 2020). In the past decade, researchers have identified the potential of using natural and biodegradable antimicrobial coating agents for various food products (Leleu et al., 2011; Jin et al., 2013; Hromiš et al., 2015; Gabriela da Silva Pires et al., 2020). Phytochemicals are secondary metabolites produced by plants and many of them serve as a defense against predation by microorganisms and insects (Upadhyay et al., 2014; Reichling, 2018; Hiruma, 2019). They are chemically categorized as alkaloids, phenolic derivatives, flavonoids, and medium to long chain fatty acids, and possess wide applicability in the food industry and animal agriculture (Desbois, 2012; Ullah et al., 2015; Reichling, 2018). In the current study, we investigated the potential application of GRAS-status, medium chain fatty acids (caprylic acid and caproic acid), a benzaldehyde (cuminaldehyde), and a monoterpenoid (linalool) as pectin-based coating for reducing S. Heidelberg on shell eggs.
Three isolates of Salmonella Heidelberg (SH) (SH-V6FA, SH-1, and SH – poultry origin) were used for this study. Each SH strain was cultured separately in 10 mL of tryptic soy broth (Difco, Becton Dickinson, Sparks, MD) and incubated at 37°C for 24 h. Lawn cultures of the SH isolates were prepared by spread plating one milliliter of overnight culture on Xylose Lysine Desoxycholate agar plates (XLD) (Difco, Becton Dickinson, Sparks, MD) (3 plates per isolate) and incubated at 37°C for 24 h. The lawn cultures from all the XLD plates were collected using sterile disposable inoculation loops, pooled, and resuspended into 20 mL of phosphate buffered saline (PBS; pH 7.0) and sedimented by centrifugation at 2,758 g for 15 min at 4°C. The centrifugation step was repeated twice for obtaining washed bacterial pellets which was finally resuspended again in 20 mL of PBS and used as the inoculum. The three-strain bacterial mix thus derived contained ~10 log CFU/mL as confirmed by plating on XLD agar with incubation at 37°C for 24 h (Upadhyaya et al., 2016). Inoculum preparation using lawn cultures with higher log inoculation levels per egg (~8 log CFU/mL) has enabled for the persistence of Salmonella over the 21 day trial without drastically reducing the bacterial counts to negligible levels.
Pectin coating solution was prepared based on previously established protocol in our laboratory with slight modifications (Ayala-Zavala et al., 2013). Pectin powder from citrus peel was purchased from Sigma Aldrich (St. Louis, MO). The phytochemicals with purity of ≥98% were used in this study; the compounds used for this study were caprylic acid (MP Biomedicals, Solon, OH), caproic acid (MP Biomedicals), cuminaldehyde (Acros Organics, NJ), and linalool (Sigma Aldrich). Two percent pectin coating solutions (in autoclaved deionized water) were prepared with the following phytochemical treatments individually or in combination, i.e., 1% caprylic acid (CAY), 1% caproic acid (CAO), 1% linalool (LIN), 1% cuminaldehyde (CUM), a combination of 0.5% each of caprylic acid, caproic acid and cuminaldehyde (COMB1), a combination of 0.5% each of caprylic acid, caproic acid and linalool (COMB2), and a combination of 0.5% each of caprylic acid, caproic acid, cuminaldehyde and linalool (COMB3). Two percent pectin coating solution without the phytochemicals served as the control for the experiment. The details of the various treatments are provided in Table 1.
Refrigerated, medium sized eggs were surface sterilized using 70% ethanol and air dried in a BSL-2 cabinet under UV light for 1 h. Subsequently, 50 μL of the 3-strain SH mixture was spot inoculated (~8.0 log CFU/egg) at the air sac end of the eggs and allowed to dry for 2 h before subjecting the eggs for the coating treatments.
For bacterial enumeration, based on our preliminary experiments, we observed that tryptic soy agar and XLD yielded similar Salmonella populations on shell eggs after treatments. Moreover, similar findings observed in from our previous publications (Upadhyaya et al., 2013, 2016) related to phytochemical based inactivation of Salmonella on egg shell surface. Therefore, the current experimental study resorted to using only XLD agar plates to enumerate the Salmonella population from eggs treated with the respective pectin-based coating agents.
The dipping-based coating method used in this study was based on previously published protocol with slight modifications (Kim et al., 2008). Inoculated eggs were dip treated in a sterile Whirl-Pak bag (Fisher Scientific, Pittsburgh, PA) containing 80 mL of the prepared coating solution and allowed to stand in the treatment bag for 1 min. The coated eggs were transferred to a sterile rack in the laminar hood and maintained for 2 h at 25°C, followed by transferring the eggs into a new sterile Whirl-Pak bag and stored at 4°C for 21 days. Viable populations of SH on eggs were enumerated on days 0, 1, 3, 5, 7, 14, and 21 of storage. Each bag containing the egg was filled with 50 mL of Dey-Engley neutralizing broth (Remel, Inc., San Diego, CA) and placed on a shaker for 15 min at 250 rpm. Thereafter the broth from sample bags was serially diluted and plated on XLD agar plates (Upadhyaya et al., 2013, 2016). The plates were incubated for 24–48 h before counting the colonies. In addition, 10 mL aliquots of the neutralizing broth from each bag were separately transferred to 100 mL of selenite cysteine broth (Sigma Aldrich) and enriched at 37°C for 48 h. Selenite cysteine broth media favors the growth of Salmonella that was not detectable in the zeroth dilution of the neutralizing broth of each treatment egg. Enrichment negative samples for SH were assigned a value of 0, whereas enrichment positive samples were assigned a value of 1.0 log CFU/egg. The resulting cultures were streaked on XLD agar plates, incubated for 24–48 h and the resultant colonies (representative samples) were confirmed as Salmonella Heidelberg using Salmonella rapid detection kit (Microgen Bioproducts Ltd, Camberley, UK).
Atomic force microscopy has been used to study three-dimensional surface characteristics of a variety of food surfaces (Yang et al., 2005; Athanasiadou et al., 2018; Arzate-Vázquez et al., 2019; Khodabakhshian and Baghbani, 2021). In the current study, the impact of pectin-based phytochemical coatings on egg shell surface roughness was measured using an atomic force microscope (AFM) imaging technique (Tosca 200, Anton Paar, Graz, Austria). In a separate experiment, eggs coated with respective pectin coating treatments (one egg per treatment per day time point) was prepared and stored for day time points of 0 and 7 as previously mentioned. Prior to imaging, the egg shell surface was washed with sterile distilled water in order to wash off the pectin-based coating treatment and to facilitate the imaging of the raw egg shell surface. The washed eggs were dried up using Kimtech wipes (Kimberly-Clark, Irving, TX) and egg shells were cracked to retrieve 2 × 2 mm sized egg shell samples for AFM imaging. The samples were placed on an AFM sample holder and the images were obtained using tapping mode using an AP-ARROW_NCR silicon cantilever with a force constant of 42 N/m and a tip radius <10 nm. For root mean squared (RMS) surface roughness measurement (Rq), the root mean squares of surface measured microscopic peaks and valleys of five equidistant horizontal and vertical directional planes of the amplitude trace AFM images (n = 10) were measured and averaged per egg sample. The RMS surface roughness (Rq) was analyzed using Gwyddion modular program for scanning probe microscopy (SPM) data visualization and analysis (v. 1.87; http://gwyddion.net/ - accessed 16th June, 2022) and the measurements were expressed in micrometers.
Three eggs per treatment at every sampling time point during the 3-week trial were included in all three independent replicated experiments (N = 504, n = 24). The experiment was a completely randomized design have 8 treatments (CON, CAY, CAO, CUM, LIN, COMB1, COMB2, and COMB3) and 7 time points (days 0, 1, 3, 5, 7, 14, 21). In the AFM studies, one egg per pectin-based coating treatment per day time point (day 0 and 7) was used to measure surface roughness of the egg shell surface using atomic force microscopy. From the amplitude trace AFM images, surface roughness measurements of five horizontal and five vertical directional planes per treatment (n = 10) were selected and averaged to quantitate the Root Mean Squared roughness (Rq). The mean difference comparison of the bacterial counts and Rq was performed between the untreated pectin coating group and respective pectin-based phytochemical coating treatments within the respective day time points. The data were analyzed by two-way mixed ANOVA analysis using GraphPad Prism 9.3.1. The level of significance was tested at p < 0.05 with Šídák's multiple comparisons test.
The efficacy of various phytochemical incorporated pectin-based coating treatments in reducing SH counts is shown in Figures 1A–G. Data table underneath each graph represents the mean differences between pectin-coated eggs without phytochemicals (control) and the respective pectin-based phytochemical treatments (CAY, CAO, LIN, CUM, COMB1, COMB2, and COMB3) and the p values for the corresponding day time point. On day 0, eggs having the pectin only coating without phytochemicals (CON) yielded an average of 7.6 log CFU of SH/egg. However, the phytochemical treatments (CAY, CAO, CUM, LIN, COMB1, COMB2, and COMB3) significantly reduced the SH counts on eggs by ~3.5–4.5 log on day 0 when compared to control group (p ≤ 0.05).
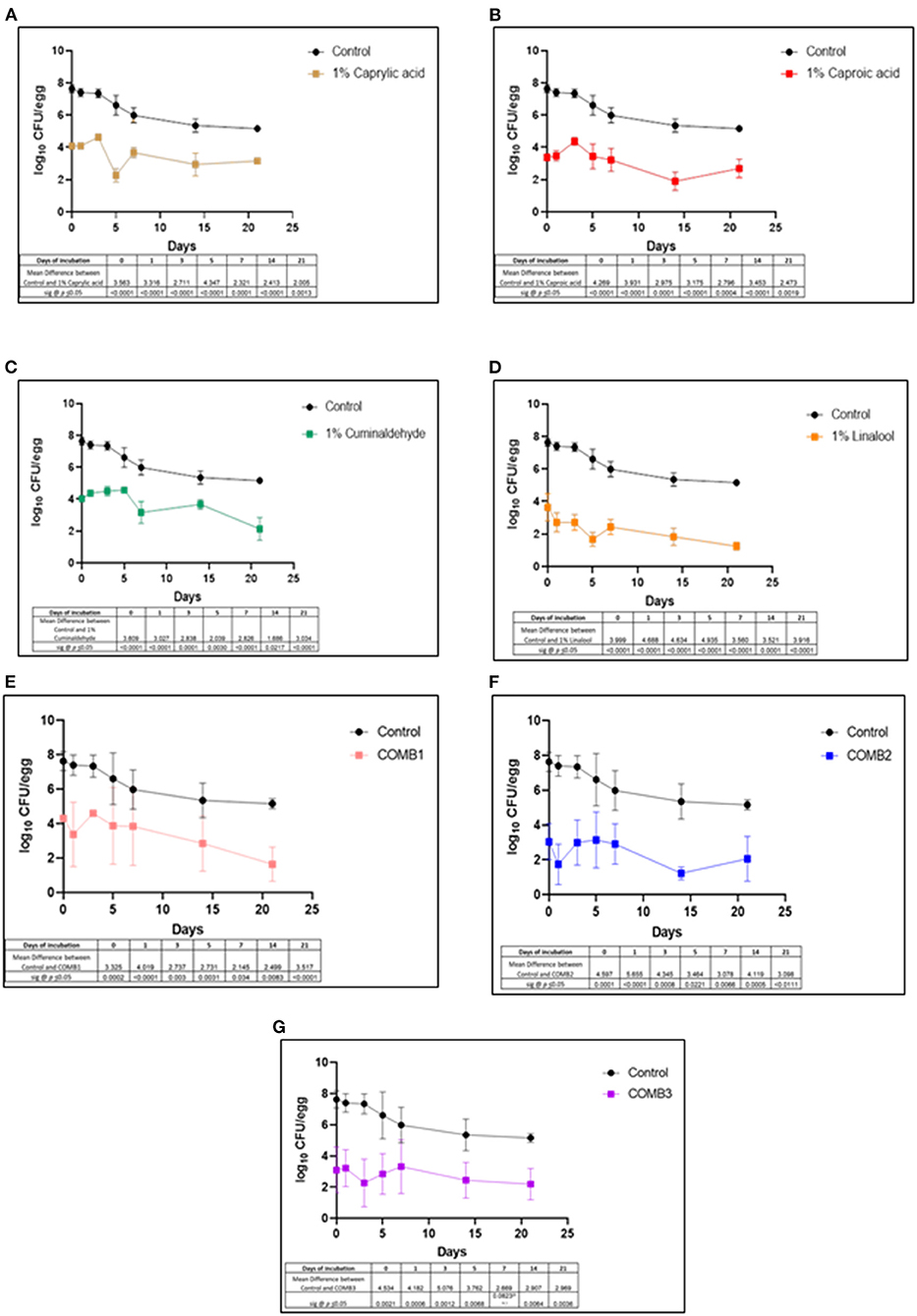
Figure 1. Effect of (A) 1% caprylic acid, (B) 1% caproic acid, (C) 1% cuminaldehyde, (D) 1% linalool, (E) combination of 0.5% each of caprylic acid, caproic acid, and cuminaldehyde, (F) combination of 0.5% each of caprylic acid, caproic acid and linalool, and (G) combination of 0.5% each of caprylic acid, caproic acid, cuminaldehyde and linalool as pectin (2%) based coating in reducing Salmonella Heidelberg on shelled eggs. Pectin (2%) only coated eggs were also included. Three eggs per treatment per sampling point (0, 1, 3, 5, 7, 14, 21 days) were included, and the experiment was replicated three times. Enrichment negative samples for SH were assigned a value of 0. Enrichment positive samples were assigned a value of 1.0 log CFU/egg. The differences between the means were compared at a significance level of 5%. Error bars represent SEM. Data tables below each graph indicate the mean differences in log counts between control and the respective treatment application and the associated p values for each time point.
Until day 3 of storage, the CON eggs had SH counts ranging between 7.3 and 7.6 log CFU/egg, and from day 5 the bacterial counts steadily decreased reaching an average of 5 log CFU/egg by the last day of storage. The phytochemical-coated eggs, however, maintained the reduced bacterial counts per egg from day 0 through the end of storage (p ≤ 0.05). Although the coating treatments did not reduce SH counts to undetectable levels, the percent reduction of SH counts on eggs treated with the various phytochemical treatments equates to ≥99.99% compared to the untreated control eggs across all time points. Of the individual phytochemical coating treatments, CAY and CAO coatings reduced the SH load by ~2.0–2.5 log compared to the control by the last day of storage. However, CUM and LIN coating treatments resulted in ~3.0 and 3.9 log reduction in SH counts, respectively, by the last day of storage. Among the coating treatments having the phytochemical combinations, by day 21, COMB1 brought about the greatest log reduction in SH counts (~3.5 log) followed by COMB2 and COMB3 treatments, both of which produced ~3.0 log reduction (p < 0.05). All eggs with phytochemical coating treatments that yielded no SH colonies through serial dilution and plating were enrichment positive and were assigned a value of 1 log CFU/egg.
The morphology and surface roughness measurements of the eggs examined using AFM are presented in Supplementary Figures 1, 2, and Figure 2, respectively. The images in Supplementary Figures 1, 2 represent the amplitude trace of the egg shell surface morphology. Using these images, the surface roughness was measured and expressed as root mean squared surface roughness (Rq) and the data is provided in Figure 2. On day 0, the surface roughness pectin-based phytochemical treated eggs was not significantly different when compared to the pectin alone coated eggs. However, on day 7, the surface roughness parameters of the CAY and COMB1 treatment groups reduced by 1.76 and 1.85 μm, respectively, when compared to the control (p < 0.05).
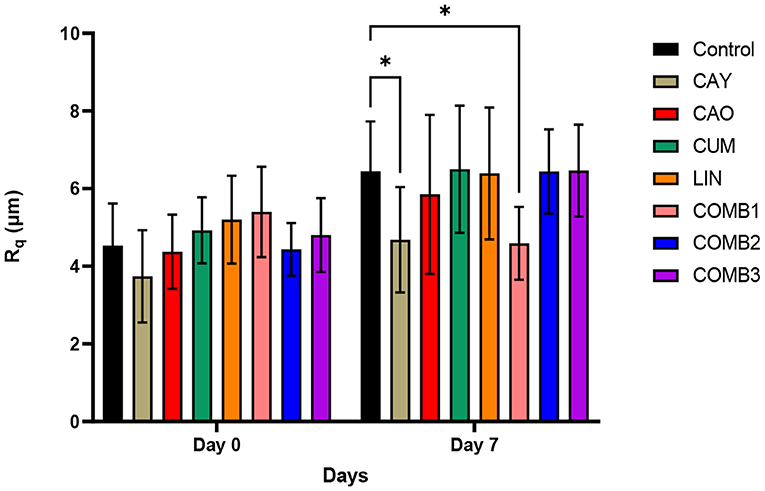
Figure 2. Root mean square surface roughness (Rq) measures from amplitude trace images captured from chicken eggshell by means of AFM. The differences between the means were compared at a significance level of 5%. Error bars represent SEM. The * symbol indicates values of p < 0.05 which are statistically significant difference.
Cleaning and disinfection protocols in commercial egg production are pivotal in controlling outbreaks of non-typhoidal Salmonella, especially S. Heidelberg (Kaldhone et al., 2017). Although washing and rinsing practices are recommended primarily to improve hygiene and bacterial load reduction, these steps can compromise the integrity of the egg shell cuticle. In particular, chemical agents used for shell egg sanitation such as sodium carbonate, cetylpridinium chloride, and sodium hypochlorite, are known to elicit cuticle damage on egg shell (Kulshreshtha et al., 2018). The negative effect produced on the cuticle by these sanitizers potentially provides easy access for pathogens such as non-typhoidal Salmonella strains, thereby leading to the contamination of internal egg contents. Chemicals used to wash eggs are considered potential food additives which should be cost effective, potentially safe to the workers and the environment and be easily incorporated in a HACCP plan (Scott and Swetnam, 1993). Therefore, naturally derived, environmentally friendly coating agents incorporated with antimicrobials represent a viable and sustainable approach for egg disinfection during storage and transport. Moreover, such naturally derived agents outweigh the negative impacts that chemical disinfectants may have on the egg shell cuticle as well as their potential health hazards (Dunnick and Melnick, 1993; Yuan et al., 2022). This study investigated the efficacy of pectin-based coating incorporating caprylic acid (CAY), caproic acid (CAO), cuminaldehyde (CUM), and linalool (LIN), either individually or in combination for reducing S. Heidelberg on refrigerated shell eggs.
Our previous investigations for standardizing egg coating experiments used an inoculation level of 6.0 log CFU of S. Enteritidis per egg (Upadhyaya et al., 2016). However, when SH was inoculated on egg surface at 6.0 log CFU, its population drastically reduced below 4.0 log level even on day 0, and the counts decreased further to undetectable levels by plating by day 3 of refrigerated storage. Therefore, the current study was standardized with an 8.0 log CFU inoculum level per egg to ascertain the inhibitory effect of the coating agents over a 3-week period. The storage time for the current study was set for a period of 21 days since the recommended period of refrigerated storage of raw shelled eggs by the USDA ranges from 3 to 5 weeks (USDA FSIS, 2011). Pectin, a plant derived heteropolysaccharide, is a GRAS status ingredient used in the food industry. It is commonly used as a gelling, stabilizing or thickening agent, and as edible coatings infused with antimicrobials (Espitia et al., 2014). Prior investigations in our laboratory have confirmed that pectin itself as a coating agent on eggs is not inhibitory to the growth of Salmonella (Upadhyaya et al., 2016). Moreover, before enumerating SH load on eggs, the eggs were rinsed in Dey-Engley neutralizing broth to neutralize and help prevent any residual carryover of the antimicrobials to the enumerating agar medium (Singh et al., 2002).
Among the coating treatments containing a single phytochemical, 1% linalool and 1% cuminaldehyde demonstrated greatest reduction in SH populations after day 3 of refrigerated storage, of which linalool showed a greater efficacy than cuminaldehyde (Figures 1C,D). CAY and CAO coating treatments resulted in ~2.0–2.5 log reduction in SH counts by the last day of storage. Among the combination coating treatments, COMB1 and COMB3 showed a significant downward trend in SH counts from day 7 of storage (Figures 1E,G). By day 21 of storage, COMB1 and COMB3 treatments resulted in ~3.50 and 3.0 log reduction in SH counts, respectively (p < 0.05), in comparison to untreated pectin control group. Although the magnitude of SH reduction by the various phytochemical coatings differed, results indicate that all the treatments were able to decrease the pathogen counts at least by 2.0 log CFU/egg during storage (p < 0.5). This level of pathogen reduction is of practical significance since apparently “clean” shell eggs generally have been reported to contain low levels of Salmonella enterica (~2 log CFU/egg) (De Reu et al., 2008). Moreover, the ability of lipid soluble phytochemicals to cause damage to bacterial membrane proteins, depletion of proton motive force, cell leakage and disruption of cytoplasmic constituents (Burt, 2004) along with increased contact time of compounds on the pathogen may have augmented to consistently lower SH population across time points when compared to the untreated control.
The AFM based surface roughness measurement was performed to assess the impact of the pectin-based phytochemical treatments on the egg shell surface. From the pectin coating treatment containing single phytochemical treatments (CAY, CAO, CUM, and LIN) on day 7, the CAY coating reduced the surface roughness parameters when compared to the control. This reduction could possibly be attributed to the interaction of caprylic acid with the calcite present in egg shells that could have resulted in the mild reduction of the microscopic peaks and valleys of the egg shell surface. From the phytochemical combination-based pectin coating treatments, the COMB1 coating also produced a similar reduction in surface roughness. The tandem interactions of caprylic acid, caproic acid, and cuminaldehyde on the egg shell surface may also have contributed to potentiating the reduction of the egg shell surface roughness. Apart from these findings, one beneficial aspect that we were able to observe from this study was that the CAY and COMB1 coatings maintained a surface roughness measure comparable to the mean surface roughness value for the CON eggs on day 0. Moreover, except for the CAY and COMB1 treatments, the rest of the pectin coating treatments seems to have a higher roughness measure. The formation of coating film produced by the pectin-based treatments could have attributed to the surface roughness parameters. Our current observations are in partially in agreement with the research work by Yuan and coworkers. They identified that pectin and chitosan-based coating films of shelled eggs help to construct a better barrier against the negative effect of damaged cuticle subsequent to chicken egg sanitation. In addition, their studies also proved that a combination of sanitation and polysaccharide-based coating films helped reduced the invasion of Salmonella Enteritidis through the chicken egg shell (Yuan et al., 2022). However, with regards to the current study, further investigation related to egg shell stability and quality is required to fully assess the impact of the pectin-based phytochemical coatings.
To conclude, results of this study indicate that the phytochemical treatments, especially, LIN, COMB1 and COMB3, when applied as a pectin-based coating are effective in reducing SH populations on chicken eggs (p < 0.05). The surface roughness increased except for CAY and COMB1, which decreased after 7-day storage. Follow up studies on the shell cuticle stability, egg shell thickness, strength, structural changes, and sensorial quality characteristics of treated eggs are required before recommending their use. In future studies, the potential of nanoemulsions of phytochemicals applied as coating on eggs for enhanced efficacy will also be investigated.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
AP, PV, and KV designed the study. AP, PV, LV, and DJ performed the experiments. JX and XW performed the AFM experiment. AP and PV reviewed and analyzed the data. AP wrote the original manuscript. PV, AD, JX, XW, YL, and KV edited the manuscript. All authors read, commented, and approved the final manuscript.
This research was supported by the USDA-NIFA Award #2017-51300-26815.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2022.874219/full#supplementary-material
Ahnen, R. T., and Slavin, J. L. (2019). “Eggs as part of a healthy eating pattern,” in Steviol Glycosides: Cultivation, Processing, Analysis and Applications in Food, Vol. 14, eds J. Wu (Royal Society of Chemistry), 1–21. doi: 10.1039/9781788013833-00001
Al-Tayyar, N. A., Youssef, A. M., and Al-Hindi, R. R. (2020). Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables: a review. Sustain. Mater. Technol. 26:e00215. doi: 10.1016/j.susmat.2020.e00215
Arzate-Vázquez, I., Méndez-Méndez, J. V., Flores-Johnson, E. A., Nicolás-Bermúdez, J., Chanona-Pérez, J. J., and Santiago-Cortés, E. (2019). Study of the porosity of calcified chicken eggshell using atomic force microscopy and image processing. Micron 118, 50–57. doi: 10.1016/j.micron.2018.12.008
Athanasiadou, D., Jiang, W., Goldbaum, D., Saleem, A., Basu, K., and Pacella, M. S. (2018). Nanostructure, osteopontin, and mechanical properties of calcitic avian eggshell. Sci. Adv. 4:eaar3219. doi: 10.1126/sciadv.aar3219
Ayala-Zavala, J. F., Silva-Espinoza, B. A., Cruz-Valenzuela, M. R., Leyva, J. M., Ortega-Ramírez, L. A., Carrazco-Lugo, D. K., et al. (2013). Pectin-cinnamon leaf oil coatings add antioxidant and antibacterial properties to fresh-cut peach. Flavour Fragr. J. 28, 39–45. doi: 10.1002/ffj.3125
Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in foods–a review. Int. J. Food Microbiol. 94, 223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022
Chemaly, M., and Salvat, G. (2011). “Foodborne disease associated with eggs: Microbial hazards and Salmonella Enteritidis risk assessment,” in Improving the Safety and Quality of Eggs and Egg Products, eds F. V. Immersrseel, Y. Nys, and M. Bain (Cambridge: Woodhead Publishing Limited), 34–45. doi: 10.1533/9780857093929.1.34
Chen, C. H., Yin, H. B., Upadhayay, A., Brown, S., and Venkitanarayanan, K. (2019). Efficacy of plant-derived antimicrobials for controlling Salmonella Schwarzengrund on dry pet food. Int. J. Food Microbiol. 296, 1–7. doi: 10.1016/j.ijfoodmicro.2019.02.007
Chousalkar, K., Gast, R., Martelli, F., and Pande, V. (2017). Review of egg-related salmonellosis and reduction strategies in United States, Australia, United Kingdom and New Zealand. Crit. Rev. Microbiol. 44, 290–303. doi: 10.1080/1040841X.2017.1368998
de Almeida, D. S., Schneider, A. F., Yuri, F. M., Machado, B. D., and Gewehr, C. E. (2016). Métodos de tratamento de casca sobre a qualidade de ovos comerciais. Cienc. Rural 46, 336–341. doi: 10.1590/0103-8478cr20140904
De Leo, R., Quartieri, A., Haghighi, H., Gigliano, S., Bedin, E., and Pulvirenti, A. (2018). Application of pectin-alginate and pectin-alginate-laurolyl arginate ethyl coatings to eliminate Salmonella enteritidis cross contamination in egg shells. J. Food Saf. 38:e12567. doi: 10.1111/jfs.12567
De Reu, K., Messens, W., Heyndrickx, M., Rodenburg, T. B., Uyttendaele, M., and Herman, L. (2008). Bacterial contamination of table eggs and the influence of housing systems. Worlds Poult. Sci. J. 64, 5–19. doi: 10.1017/S0043933907001687
Desbois, P. A. (2012). Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Pat. Antiinfect. Drug Discov. 7, 111–122. doi: 10.2174/157489112801619728
Dewi, G., Manjankattil, S., Peichel, C., Jia, S., Nair, D., Vickers, Z., et al. (2021). Effect of plant-derived antimicrobials against multidrug-resistant Salmonella Heidelberg in ground Turkey. Poult. Sci. 101:101581. doi: 10.1016/j.psj.2021.101581
Dunnick, J. K., and Melnick, R. L. (1993). Assessment of the carcinogenic potential of chlorinated water: experimental studies of chlorine, chloramine, and trihalomethanes. JNCI J. Natl Cancer Inst. 85, 817–822. doi: 10.1093/jnci/85.10.817
Espitia, P. J. P., Du, W. X., Avena-Bustillos, R., de Fátima Ferreira Soares, N., and McHugh, T. H. (2014). Edible films from pectin: physical-mechanical and antimicrobial properties - A review. Food Hydrocolloids 35, 287–296. doi: 10.1016/j.foodhyd.2013.06.005
Foley, S. L., Nayak, R., Hanning, I. B., Johnson, T. J., Han, J., and Ricke, S. C. (2011). Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl. Environ. Microbiol. 77, 4273–4279. doi: 10.1128/AEM.00598-11
Fu, Y., Bhunia, A. K., and Yao, Y. (2022). Alginate-based antimicrobial coating reduces pathogens on alfalfa seeds and sprouts. Food Microbiol. 103:1039543. doi: 10.1016/j.fm.2021.103954
Gabriela da Silva Pires, P., Daniela da Silva Pires, P., Cardinal, K. M., and Bavaresco, C. (2020). The use of coatings in eggs: a systematic review. Trends Food Sci. Technol. 106, 312–321. doi: 10.1016/j.tifs.2020.10.019
Hessel, C. T., de Oliveira Elias, S., Pessoa, J. P., Zanin, L. M., Stedefeldt, E., and Tondo, E. C. (2019). Food safety behavior and handling practices during purchase, preparation, storage and consumption of chicken meat and eggs. Food Res. Int. 125:108631. doi: 10.1016/j.foodres.2019.108631
Hiruma, K. (2019). Roles of plant-derived secondary metabolites during interactions with pathogenic and beneficial microbes under conditions of environmental stress. Microorganisms 7:362. doi: 10.3390/microorganisms7090362
Hromiš, N. M., Lazić, V. L., Markov, S. L., Vaštag, Ž. G., Popović, S. Z., Šuput, D. Z., et al. (2015). Optimization of chitosan biofilm properties by addition of caraway essential oil and beeswax. J. Food Eng. 158, 86–93. doi: 10.1016/j.jfoodeng.2015.01.001
Jin, T. Z., Gurtler, J. B., and Li, S. Q. (2013). Development of antimicrobial coatings for improving the microbiological safety and quality of shell eggs. J. Food Prot. 76, 779–785. doi: 10.4315/0362-028X.JFP-12-460
Kaldhone, P. R., Foley, S. L., and Ricke, S. C. (2017). “Salmonella Heidelberg in Layer Hens and Egg Production: Incidence and Potential Issues,” in Producing Safe Eggs: Microbial Ecology of Salmonella, eds S. C. Ricke and R. K. Gast R.K., (San Diego, CA: Elsevier), 235–256. doi: 10.1016/B978-0-12-802582-6.00012-4
Khodabakhshian, R., and Baghbani, R. (2021). Classification of bananas during ripening using peel roughness analysis—An application of atomic force microscopy to food process. J. Food Process Eng. 44:e13857. doi: 10.1111/jfpe.13857
Kim, S. H., No, H. K., and Prinyawiwatkul, W. (2008). Plasticizer types and coating methods affect quality and shelf life of eggs coated with chitosan. J. Food Sci. 73, S111–S117. doi: 10.1111/j.1750-3841.2007.00650.x
Kulshreshtha, G., Rodriguez-Navarro, A., Sanchez-Rodriguez, E., Diep, T., and Hincke, M. T. (2018). Cuticle and pore plug properties in the table egg. Poult. Sci. 97, 1382–1390. doi: 10.3382/ps/pex409
Leleu, S., Herman, L., Heyndrickx, M., De Reu, K., Michiels, C. W., De Baerdemaeker, J., et al. (2011). Effects on Salmonella shell contamination and trans-shell penetration of coating hens' eggs with chitosan. Int. J. Food Microbiol. 145, 43–48. doi: 10.1016/j.ijfoodmicro.2010.11.023
Luvsansharav, U. O., Vieira, A., Bennett, S., Huang, J., Healy, J. M., Hoekstra, R. M., et al. (2020). Salmonella serotypes: a novel measure of association with foodborne transmission. Foodborne Pathog. Dis. 17, 151–155. doi: 10.1089/fpd.2019.2641
Martelli, F., and Davies, R. H. (2012). Salmonella serovars isolated from table eggs: an overview. Food Res. Int. 45, 745–754. doi: 10.1016/j.foodres.2011.03.054
Martínez-Suárez, J. V., Ortiz, S., and López-Alonso, V. (2016). Potential impact of the resistance to quaternary ammonium disinfectants on the persistence of Listeria monocytogenes in food processing environments. Front. Microbiol. 7:638. doi: 10.3389/fmicb.2016.00638
Mir, J., Morató, J., and Ribas, F. (1997). Resistance to chlorine of freshwater bacterial strains. J. Appl. Microbiol. 82, 7–18. doi: 10.1111/j.1365-2672.1997.tb03292.x
Morsy, M. K., Sharoba, A. M., Khalaf, H. H., El-Tanahy, H. H., and Cutter, C. N. (2015). Efficacy of antimicrobial pullulan-based coating to improve internal quality and shelf-life of chicken eggs during storage. J. Food Sci. 80, M1066–M1074. doi: 10.1111/1750-3841.12855
Musgrove, M. T. (2011). “Microbiology and safety of table eggs,” in Improving the Safety and Quality of Eggs and Egg Products, eds F. V. Immersrseel, Y. Nys, and M. Bain (Cambridge: Woodhead Publishing Limited), 3–33. doi: 10.1533/9780857093929.1.3
Paramithiotis, S., Drosinos, E. H., and Skandamis, P. N. (2017). Food recalls and warnings due to the presence of foodborne pathogens — a focus on fresh fruits, vegetables, dairy and eggs. Curr. Opin. Food Sci. 18, 71–75. doi: 10.1016/j.cofs.2017.11.007
Park, T. S., Kang, K. S., and Han, J. Y. (2012). Current genomic editing approaches in avian transgenesis. Gen. Comp. Endocrinol. 190, 144–148. doi: 10.1016/j.ygcen.2012.11.020
Pires, P. G. S., Leuven, A. F. R., Franceschi, C. H., Machado, G. S., Pires, P. D. S., Moraes, P. O., et al. (2020). Effects of rice protein coating enriched with essential oils on internal quality and shelf life of eggs during room temperature storage. Poult. Sci. 99, 604–611. doi: 10.3382/ps/pez546
Reichling, J. (2018). “Plant-Microbe Interactions and Secondary Metabolites with Antibacterial, Antifungal and Antiviral Properties,” in Annual Plant Reviews Online, ed M. Wink (Oxford: John Wiley and Sons Ltd), 214–347. doi: 10.1002/9781119312994.apr0420
Schoeni, J. L., Glass, K. A., McDermott, J. L., and Wong, A. C. L. (1995). Growth and penetration of Salmonella enteritidis, Salmonella heidelberg and Salmonella typhimurium in eggs. Int. J. Food Microbiol. 24, 385–396. doi: 10.1016/0168-1605(94)00042-5
Scott, T. A., and Swetnam, C. (1993). Screening sanitizing agents and methods of application for hatching eggs I. environmental and user friendliness. J. Appl. Poult. Res. 2, 1–6. doi: 10.1093/japr/2.1.1
Shahidi, F., and Hossain, A. (2022). Preservation of aquatic food using edible films and coatings containing essential oils: a review. Crit. Rev. Food Sci. Nutr. 62, 66–105. doi: 10.1080/10408398.2020.1812048
Shrestha, S., Wagle, B. R., Upadhyay, A., Arsi, K., Upadhyaya, I., Donoghue, D. J., et al. (2019). Edible coatings fortified with carvacrol reduce campylobacter jejunion chicken wingettes and modulate expression of select virulence genes. Front. Microbiol. 10:583. doi: 10.3389/fmicb.2019.00583
Singh, N., Singh, R. K., Bhunia, A. K., and Stroshine, R. L. (2002). Efficacy of chlorine dioxide, ozone, and thyme essential oil or a sequential washing in killing Escherichia coli O157:H7 on lettuce and baby carrots. Lebenson. Wiss. Technol. 35, 720–729. doi: 10.1006/fstl.2002.0933
Trejo-Pech, C. J. O., and White, S. (2020). Capital budgeting analysis of a vertically integrated egg firm: conventional and cage-free egg production. Appl. Econ. Teach. Resour. 2, 34–46. doi: 10.22004/ag.econ.307148
U.S. Food and Drug Administration HHS. (2009). Prevention of Salmonella Enteritidis in shell eggs during production, storage, and transportation. Final rule. Fed Regist. 74, 33029—33101.
Ullah, I., Khan, A. L., Ali, L., Khan, A. R., Waqas, M., Hussain, J., et al. (2015). Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J. Microbiol. 53, 127–133. doi: 10.1007/s12275-015-4632-4
Upadhyay, A., Upadhyaya, I., Kollanoor-Johny, A., and Venkitanarayanan, K. (2014). Combating pathogenic microorganisms using plant-derived antimicrobials: A minireview of the mechanistic basis. Biomed. Res. Int. 2014:761741. doi: 10.1155/2014/761741
Upadhyaya, I., Upadhyay, A., Kollanoor-Johny, A., Baskaran, S. A., Mooyottu, S., Darre, M. J., et al. (2013). Rapid inactivation of Salmonella Enteritidis on shell eggs by plant-derived antimicrobials. Poult. Sci. 92, 3228–3235. doi: 10.3382/ps.2013-03126
Upadhyaya, I., Yin, H. B., Surendran Nair, M., Chen, C. H., Lang, R., Darre, M. J., et al. (2016). Inactivation of Salmonella enteritidis on shell eggs by coating with phytochemicals. Poult. Sci. 95, 2106–2111. doi: 10.3382/ps/pew152
USDA FSIS (2011). Shell Eggs From Farm to Table. Food Saf. Insp. Sevice - US Dep. Agric. 1–10. Available online at: https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/eggs/shell-eggs-farm-table (accessed February 7, 2022).
Wagle, B. R., Upadhyay, A., Shrestha, S., Arsi, K., Upadhyaya, I., Donoghue, A. M., et al. (2019). Pectin or chitosan coating fortified with eugenol reduces Campylobacter jejunion chicken wingettes and modulates expression of critical survival genes. Poult. Sci. 98, 1461–1471. doi: 10.3382/ps/pey505
Wang, Z., Fang, Y., Zhi, S., Simpson, D. J., Gill, A., McMullen, L. M., et al. (2020). The locus of heat resistance confers resistance to chlorine and other oxidizing chemicals in escherichia coli. Appl. Environ. Microbiol. 86:e02123–19. doi: 10.1128/AEM.02123-19
Wollenweber, E. (1988). Occurrence of flavonoid aglycones in medicinal plants. Prog. Clin. Biol. Res. 280, 45–55.
Xing, Y., Xu, Q., Li, X., Chen, C., Ma, L., Li, S., et al. (2016). Chitosan-based coating with antimicrobial agents: preparation, property, mechanism, and application effectiveness on fruits and vegetables. Int. J. Polym Sci. 2016, 1–24. doi: 10.1155/2016/4851730
Yang, H., An, H., Feng, G., and Li, Y. (2005). Visualization and quantitative roughness analysis of peach skin by atomic force microscopy under storage. LWT Food Sci. Technol. 38, 571–577. doi: 10.1016/j.lwt.2004.09.007
Yang, Y., Mikš-Krajnik, M., Zheng, Q., Lee, S. B., Lee, S. C., and Yuk, H. G. (2016). Biofilm formation of Salmonella Enteritidis under food-related environmental stress conditions and its subsequent resistance to chlorine treatment. Food Microbiol. 54, 98–105. doi: 10.1016/j.fm.2015.10.010
Yuan, X., Li, Y., Mo, Q., Zhang, B., Shu, D., Sun, L., et al. (2022). A combined approach using slightly acidic electrolyzed water spraying and chitosan and pectin coating on the quality of the egg cuticle, prevention of bacterial invasion, and extension of shelf life of eggs during storage. Food Chem. 389:133129. doi: 10.1016/j.foodchem.2022.133129
Keywords: Salmonella Heidelberg, phytochemicals, pectin, eggs, coating
Citation: Pellissery AJ, Vinayamohan PG, Xue J, Wang X, Viju LS, Joseph D, Luo Y, Donoghue AM and Venkitanarayanan K (2022) Efficacy of pectin-based caproic acid, caprylic acid, linalool, and cuminaldehyde coatings in reducing Salmonella Heidelberg on chicken eggs. Front. Sustain. Food Syst. 6:874219. doi: 10.3389/fsufs.2022.874219
Received: 11 February 2022; Accepted: 29 June 2022;
Published: 27 July 2022.
Edited by:
Elza Neelima Mathew, University of Massachusetts Medical School, United StatesReviewed by:
Chyer Kim, Virginia State University, United StatesCopyright © 2022 Pellissery, Vinayamohan, Xue, Wang, Viju, Joseph, Luo, Donoghue and Venkitanarayanan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kumar Venkitanarayanan, kumar.venkitanarayanan@uconn.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.