
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 12 May 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.775543
 Elnaz Daneshzad1
Elnaz Daneshzad1 Javad Heshmati2
Javad Heshmati2 Vahid Basirat3
Vahid Basirat3 Seyed-Ali Keshavarz4
Seyed-Ali Keshavarz4 Mostafa Qorbani1,5
Mostafa Qorbani1,5 Bagher Larijani6
Bagher Larijani6 Nick Bellissimo7
Nick Bellissimo7 Leila Azadbakht8,9*
Leila Azadbakht8,9*Background: Some dietary patterns may improve diabetes complications through scavenging oxidants and anti-inflammatory properties. This study evaluated the effect of the Dietary Approaches to Stop Hypertension (DASH) diet on sleep status, mental health, and hormonal changes among Iranian women with type 2 diabetes.
Methods: This randomized controlled trial (RCT) included 66 diabetic women. Participants were randomly divided into the two different diet groups (the DASH diet and control diet; 33 patients in each group) for 3 months. The Pittsburgh Sleep Quality Index and the Depression, Anxiety, and Stress Scale-21 items were used to assess sleep and mental disorders, respectively. Fasting blood sugar, hemoglobin A1c (HbA1c), advanced glycation end products (AGEs), as well as several sex hormones were evaluated at the beginning and the end of the trial.
Results: Anthropometric indices, HbA1c (control: 8.77 ± 0.82 vs. 8.04 ± 1.03; the DASH diet 8.70 ± 1.05 vs.7.41 ± 1.03), and follicle-stimulating hormone (FSH) (control: 72.16 ± 26.02 vs. 68.12 ± 27.63; the DASH diet: 72.99 ± 25.19 vs. 67.43 ± 27.63) significantly decreased over 12 weeks in both the groups (P < .0001). Testosterone, 2-h postprandial glucose (2hPPG), and AGEs significantly decreased over 12 weeks in the DASH diet group. Sleep, depression, and anxiety scores significantly decreased over 12 weeks in the DASH diet group. Night sleep duration significantly increased over 12 weeks in the DASH diet group (P < 0.0001).
Conclusion: A 12-week DASH diet significantly decreases testosterone, 2hPPG, AGEs level, as well as sleep, depression, and anxiety scores in women with type 2 diabetes. However, more RCTs are needed to confirm these findings.
Diabetes is a critical non-communicable disease and a severe worldwide public health problem. The WHO has predicted that diabetes is projected to affect 366 million people by 2030 (1). A complex interaction between genetic predisposition, environmental factors, and lifestyle factors such as inactivity and unhealthy diets can contribute to increased risk of diabetes mellitus (2).
Diabetes is associated with various complications such as micro- and macrovascular disorders, anxiety, depression, and sleep disturbances. Psychological disorders are a major cause of disability and lead to poor social outcomes (3). One-third of people with diabetes experience moderate-to-severe levels of depression, anxiety, or both. Diabetes may also act as a trigger for mental health problems, while mental disorders can interfere with optimal diabetes self-management, leading to poorer glycemic control (4). Moreover, several studies have reported that sleep apnea and sleep deprivation could occur in diabetes (5). On the other hand, sleep deprivation is associated with some other problems and chronic diseases such as cardiometabolic disorders, depression, and elevated mortality rates (6–8). Hyperglycemia increases reactive oxygen species (ROS) in diabetic patients, which cause increased oxidative stress (9). Increasing oxidative stress status and imbalance of oxidative and antioxidative status may lead to psychological distress and sleep disorders (10). Increasing the antioxidant capacity of the diet could be a useful approach to control these severe complications in diabetes. Also, healthy dietary patterns, which are rich in vegetables and low in fat, showed a positive association with longer sleep and good sleep quality (11–13).
Some previous studies have shown that higher consumption of refined grains, sugar-sweetened beverages, saturated fatty acids, and processed meats are associated with psychological disorders. Other studies have shown that consumption of legumes, nuts, vegetables, fruits, and unrefined grains seem to be associated with a reduced risk of depression and anxiety (14, 15). The Dietary Approaches to Stop Hypertension (DASH) diet recommends the high intake of low-fat dairy products, whole grain, fruits and vegetables, and sodium restriction. This diet is a low-glycemic index and low-energy-dense dietary pattern, which is high in unsaturated fatty acids, fiber, and antioxidant components (16). The DASH diet contributes as a source of the healthy food groups containing various nutrients such as magnesium, potassium, and phytoestrogens, which have beneficial effects on glycemic control and insulin sensitivity (17). Studies have shown beneficial effects of the DASH diet on levels of blood glucose and blood lipids among patients with type 2 diabetes mellitus (T2DM) (16). A meta-analysis of randomized clinical trials has shown that the DASH diet can reduce fasting insulin concentration in the intervention group among diabetic patients (17). Most available studies on the effectiveness of the DASH diet have been focused on glycemic and cardiovascular risk factors. There is a cross-sectional study that showed health benefits of the DASH diet score with sleep duration and quality (18). This is the first study that aimed to evaluate the effect of the DASH diet (intervention) on sleep status and mental health (primary outcomes) among Iranian patients with T2DM. Previous studies have shown that depression can affect sex hormones concentration in females and estrogen has a positive effect on mood in middle-aged women (19, 20). Another study showed that lower levels of sex hormone-binding globulin (SHBG) are associated with increasing obstructive sleep apnea (21). Due to limited evidence of sexual hormones and health outcomes, we examined the effect of the DASH diet on changes in sex hormones as secondary outcomes. Sleep and mental disorders are more prevalent among women, especially in older and postmenopausal women (22, 23). Sex-related differences in diabetes prevalence and its associated risk factors have been previously assessed (24, 25) and mental and sleep status can be affected during different phases of menopause. Moreover, it seems that decreases in sex hormones such as estrogen and progesterone are associated with depression, anxiety, and sleep apnea during postmenopausal period (26).
This parallel randomized clinical trial was conducted among 66 postmenopausal females with T2DM who were referred to a diabetes clinic at Tehran University of Medical Sciences (TUMS), Tehran, Iran. This study was conducted from July 2018 to March 2019. This study was approved by the ethics committee of TUMS (Code: 36923). Also, we registered the trial at the Iranian Registry of Clinical Trials (Registration number: IRCT20180312039055N1, Registration date: 20/05/2018; trial ID: 30485). The sample size was calculated based on the following formula using advanced glycation end products (AGEs) variable: (Z1−α/2 + Z1−β)2 + (σ21 + σ22)2/(μ2-μ1)2 = (1.96 + 0.84)2 + (455 + 150)2/(520–276)2 = 30 (27), as well as SHBG: n = (Z1–α/2 + Z1–β)2 + (σ12 + σ22)/(μ2–μ1)2 = (1.96 + 1.28)2 + (18.82 + 21.71)2/(28.80–11.66)2 = 30 (28). Based on this formula, 30 subjects were needed in each group to have adequate power. However, because of dropout, we continued the sampling process to 33 in each group.
Inclusion criteria were as follows: postmenopausal women with T2DM, willing to participate in the trial, history of taking a stabilized dose of oral antidiabetic medication, and no history of taking supplements within 3 months. The exclusion criteria were any change in medical treatments during the trial and if participants did not return to the clinic for follow-up visits. Also, patients who were receiving medical treatment, including antiobesity, anti-inflammatory, or antipsychotics, insulin therapy, hormone therapy, having other clinical diseases such as type 1 diabetes, liver and kidney damage, asthma, malignancy and cancers, gastrointestinal diseases, food allergy, hormonal disorders, pancreatitis, and thyroid dysfunction, were not included in this study. All the patients declared their participation in the project by providing a written informed consent.
After the screening procedure, we used the permuted block randomization method to allocate subjects into the 2 groups (the DASH or control diet; 33 patients in each group). For matching patients, we used stratified randomization based on body mass index (BMI) (normal, overweight, and obese). Hence, the intervention was a diet therapy and we could not perform a concealing procedure. Although participants were not blind to treatment, the laboratory assessors were blinded to group allocation and study hypothesis.
A questionnaire was used for sociodemographic information. Subjects were required to record their 3-day physical activity (PA) every 2 weeks seven times. Finally, PA levels were expressed by the metabolic equivalent hour per day (MET-h/day) (29).
The intervention group (33 patients) received the DASH diet and the other 33 patients received a control diet for 3 months. Both the diets included a macronutrient composition of 50–55% carbohydrates, 15–20% protein, and 30% total fat. The DASH diet was rich in whole grains, low-fat dairy products, vegetables, and fruits. It was low in sweets, refined grains, total fat, cholesterol, and saturated fat. The control diet presented as a usual diet with no recommendation for increasing consumption of whole grains, fish, and nuts, as well as no recommendation for fat intakes restriction. The control group consumed less serving of nuts, whole grains, low-fat dairy, fruits, vegetables, and whole grains compared to the intervention group. Table 1 presents serving sizes of the daily food groups and the main differences between the DASH and control diets based on a 2,000-kcal diet. The amount of sodium was no more than 2,400 mg per day. The prescribed calorie was the same for both the groups. Each intervention group received a list of approved foods with a 7-day menu, as well as an exchange list to ensure diversity of food consumption. We checked the compliance of subjects as follows:
• Dietary records. All the participants were asked to provide seven 3-day food records (every 2 weeks during the trial), which consisted of serving sizes of consumed foods with ingredients. Household measures were used to obtain grams of food consumed (30). Nutritionist IV, which is adapted for Iranian foods, was used to perform nutrient analysis (First Databank Division, The Corporation, San Bruno, California, USA).
• Weekly phone calls.
• Serum level of vitamin C during the first and last visits of this study.
We asked patients in both the groups to sustain their physical activity during this study, which was confirmed through weekly phone calls and reviewing the patients seven 3-day physical activity records.
All the anthropometric measurements were measured at the beginning and end of the trial. Weight was recorded in minimally clothed participants and without footwear using a calibrated digital scale (SECA 803, Germany). Height was measured in a standing position by using an upstretched tape measure, while shoulders were in the normal position. BMI was calculated as weight divided by height squared (kg/m2). Waist circumference (WC) was measured at the narrowest part of the waist between the last rib and the iliac crest over light clothing, using an unscratched anthropometric tape to the nearest 0.1 cm.
Blood samples (10 ml of venous blood) were collected at the beginning and end of this study after a 12-h overnight fasting to determine serum levels of fasting blood sugar (FBS), advanced glycation end products (AGEs), testosterone, sex hormone-binding globulin (SHBG), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and vitamin C. Serum samples were separated after centrifuging at 3,000 rpm for 20 min and stored at −80°C. However, FBS was measured on the day of blood collection by enzymatic colorimetric method using glucose oxidase. Hemoglobin A1c (HbA1c) was measured using the immunoturbidimetric assay in whole blood. ELISA kits were used for measuring AGEs (Crystal day, China), testosterone (PadtanGostar Isar, Iran), SHBG (DiaMetra, Italy), LH (Pishtaz Teb, Iran), FSH (Pishtaz Teb, Iran), and vitamin C (Crystal day, China).
The Depression, Anxiety, and Stress Scale-21 items (DASS-21) was used to assess psychological symptoms (31). This self-reported scale has three subscales (contain 7 questions for each subscale), including depression, anxiety, and stress, which were assessed through 21 questions. Each question was scored from 0 to 3 and scores from each subscale were summed. For depression, a total score of zero to 9 is normal, whereas scores above 10 relate to increasing severity of depression (mild to extremely severe). For anxiety, a total score of zero to 7 is normal and scores above 8 relate to increasing severity of anxiety (mild to extremely severe). For stress, a total score of zero to 14 is normal, whereas scores 15 and above relate to increasing severity of stress (mild to extremely severe). Finally, each subject was classified into either normal or abnormal levels of psychological disorders.
Participant sleep duration was extracted from sociodemographic information. Moreover, all the participants were asked to complete the validated self-report Pittsburgh Sleep Quality Index (PSQI) (32). The PSQI measures the pattern and quality of sleep over the past month and consists of 9 items that explain sleep latency, duration, and efficiency, using sleep medication, sleep disturbances, and daytime dysfunction. These items differentiate poor to good value with a range of 0 to 3 (0, not in the past month; 1, less than once per week; 2, once or twice per week; and 3, three or more times per week). The PSQI scales by a total score of 0 to 21. A score of 5 and above suggests poor sleep quality.
All the statistical analyses were performed using the Statistical Package for Social Sciences (version 16.0; SPSS Incorporation, Chicago, Illinois, USA). The normality of data distribution was assessed by using the Kolmogorov–Smirnov test and also histogram curve. Independent sample t-test was used for determining mean differences between the groups for continuous variables such as age, weight, and body mass index. The chi-squared test was used to determine the distribution of categorical variables between the intervention and control groups. The between-group analysis was determined using repeated measure analysis of covariance (ANCOVA). Adjustments were conducted for baseline values, age, protein intake, socioeconomic status, weight reduction, triglyceride changes, and blood glucose changes in different models. P < .05 was considered as statistically significant.
Data from 66 participants were analyzed and included in the intention-to-treat analysis (n = 66). Therefore, based on recruiting 33 per group rather than 30 per group, the attrition rate was 10%. Figure 1 shows the flow diagram of the trial in this study. Three individuals in the intervention group and 3 individuals in the control group failed to complete the trial. Three subjects in the control group did not desire to complete the trial. In the intervention group, one participant did not desire to finish the trial, 1 participant had a car accident, which led to an inflammatory condition, and 1 participant was excluded due to shifting their medication therapy to insulin therapy. Baseline characteristics in each group are shown in Table 2. The mean age of patients in the DASH diet (57.52 ± 4.99 years) was significantly lower than the control group (60.70 ± 6.33 years) (P =0.027). Also, socioeconomic status score was significantly higher among the DASH diet (19.33 ± 3.76) rather than the control group (15.78 ± 4.05) (P <0.0001). There were no significant differences in waist circumference (the DASH diet: 98.92 ± 7.92 cm vs. the control group: 101.16 ± 8.96 cm) (P = 0.286), BMI (the DASH diet: 29.83 ± 4.25 kg/m2 vs. the control group: 29.31 ± 3.67 kg/m2) (P = 0.594), and physical activity (the DASH diet: 28.91 ± 3.42 MET-h/day vs. the control group: 27.48 ± 2.36 MET-h/day) (P = 0.092) among the intervention and control groups at baseline. As previously shown in Table 1, the intervention group was recommended to consume more vegetables, fruits, grains, especially whole grains and nuts, and to consume lower red and processed meat and fats rather than the control group. These recommendations were applied to find the effect of the DASH diet on primary and secondary outcomes and as shown in Table 3, there was significant difference between the consumed food groups by the intervention and control groups. Dietary intakes of participants during the 3-month intervention period are shown in Table 3. Energy, carbohydrate, and total fat intakes were not significantly different among the DASH diet and the control group (P ≥.05). The DASH diet group consumed more potassium (the DASH diet: 4272.53 ± 210.39 mg vs. the control group: 2355.98 ± 267.03 mg), fiber (the DASH diet: 26.14 ± 2.66 g vs. the control group: 14.61 ± 3.47 g), and vitamin C (the DASH diet: 261.01 ± 53.37 mg vs. the control group: 90.62 ± 35.44 mg) than the control group per day (P < 0.0001).
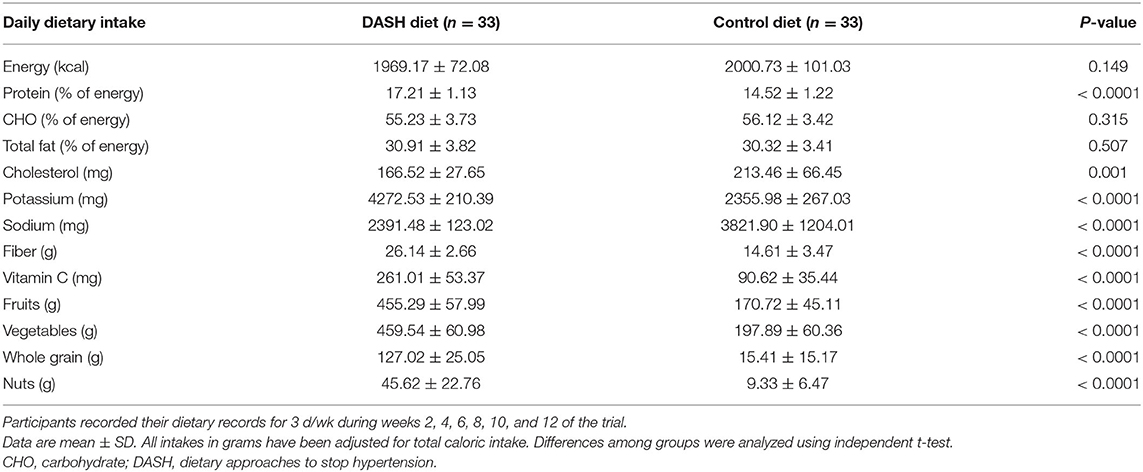
Table 3. Dietary intakes of participants (patients with type 2 diabetes) during 3-month intervention period.
Table 4 indicates the anthropometric indices and biochemical tests at baseline and after 12 weeks of intervention in postmenopausal diabetic patients. Baseline values for weight (the DASH diet: 73.90 ± 11.29 kg vs. the control group: 72.03 ± 10.11 kg), WC (the DASH diet: 98.92 ± 7.92 cm vs. the control group: 101.16 ± 8.96 cm), BMI (the DASH diet: 29.77 ± 4.11 kg/m2 vs. the control group: 29.28 ± 3.61 kg/m2), LH (DASH diet: 23.69 ± 13.23 IU/l vs. the control group: 21.06 ± 12.61 IU/l), FSH (the DASH diet: 72.99 ± 25.19 IU/l vs. the control group: 72.16 ± 26.02 IU/l), SHBG (the DASH diet: 22.22 ± 10.01 nmol/l vs. the control group: 24.96 ± 12.91 nmol/l), testosterone (the DASH diet: 0.83 ± 0.41 nmol/l vs. the control group: 0.79 ± 0.37 nmol/l), FBS (the DASH diet: 153.60 ± 40.52 mg/dl vs. the control group: 155.93 ± 44.19 mg/dl), HbA1c (the DASH diet: 8.70 ± 1.05% vs. the control group: 8.77 ± 0.82%), 2hPPG (the DASH diet: 198.87 ± 60.59 mg/dl vs. the control group: 195.42 ± 60.50 mg/dl), AGEs (the DASH diet: 351.58 ± 215.11 ng/l vs. the control group: 298.34 ± 222.96 ng/l), and serum vitamin C (the DASH diet: 30.58 ± 6.96 ng/l vs. the control group: 29.52 ± 8.34 ng/l) were not significantly different between the intervention and control groups (P > 0.05). At the end of the trial, there was a significant difference among the groups for all the abovementioned factors, except for WC (P = 0.591), LH (P = 0.760), and FSH (P = 0.427). Weight, WC, BMI, HbA1c, and FSH significantly decreased over 12 weeks in both the groups (P < 0.0001). Testosterone, 2hPPG, and AGEs significantly decreased over 12 weeks in the DASH diet group (P < 0.0001). Table 5 depicts sleep, stress, anxiety, and depression scores at baseline and after 12 weeks of intervention in diabetic women.
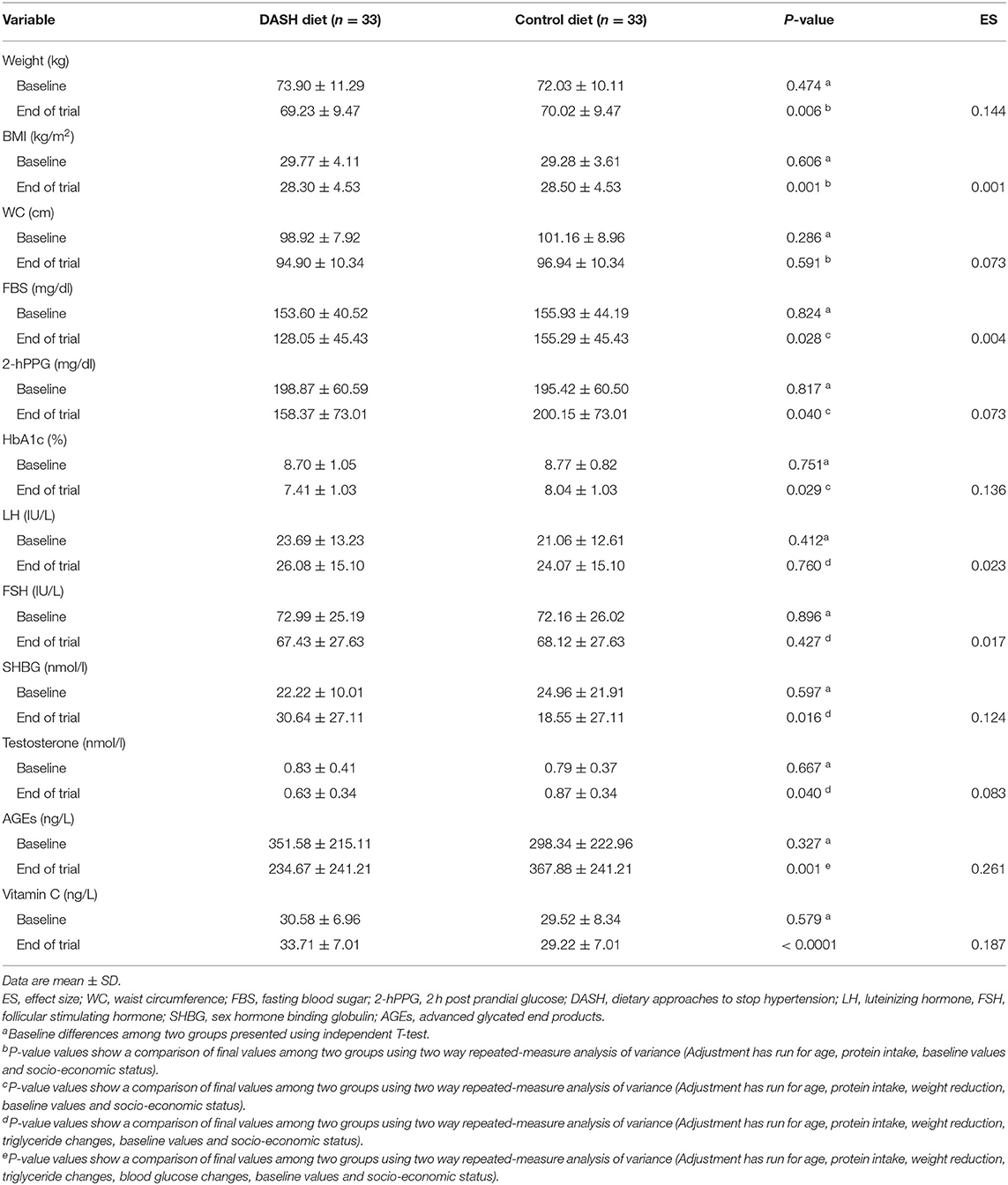
Table 4. Anthropometric indices and biochemical tests at baseline and after 12 weeks of intervention in postmenopausal diabetic patients.
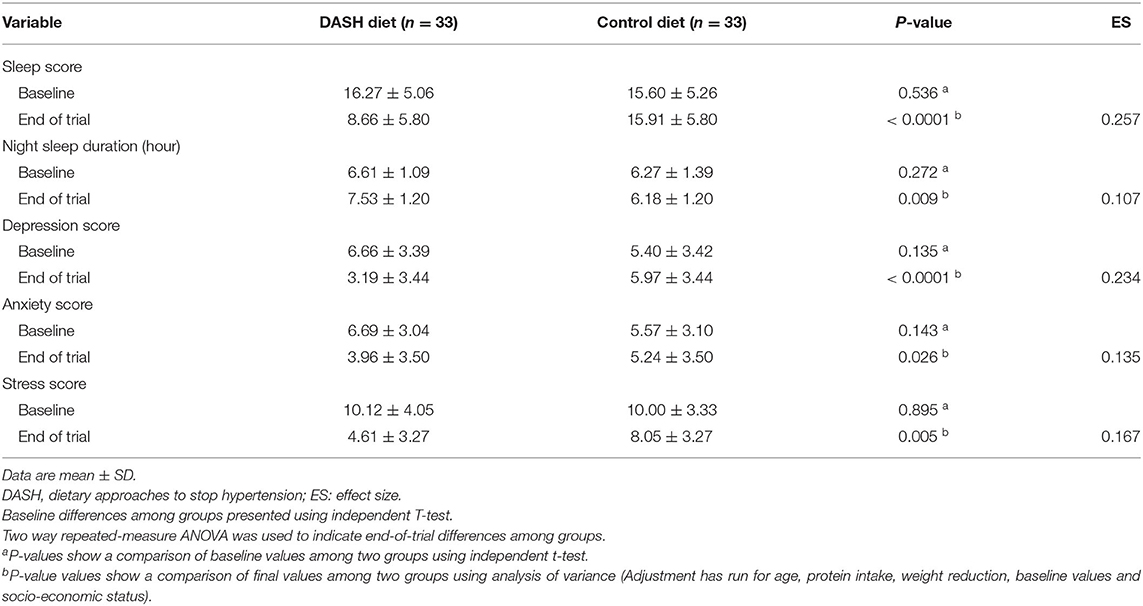
Table 5. Sleep, stress, anxiety, and depression scores at baseline and after 12 weeks of intervention in postmenopausal diabetic patients.
Depression, anxiety, stress, and sleep scores, and night sleep duration were not significantly different between the intervention and control groups (P > 0.05) at the baseline. However, at the end of the trial, there were significant differences between the groups for all of the foregoing factors. The sleep score was lower in the DASH diet group in comparison to control diet (the DASH diet: 8.66 ± 5.80 vs. the control group: 15.91 ± 5.80). Stress score significantly decreased over 12 weeks in both the groups. Moreover, this reduction in the DASH diet group was significant in comparison to the control group (the DASH diet: 4.61 ± 3.27 vs. the control group: 8.05 ± 3.27). Sleep, depression, and anxiety scores significantly decreased over 12 weeks in the DASH diet group. Night sleep duration significantly increased over 12 weeks in the DASH diet group (P < 0.0001).
In this study, we examined the effect of the DASH diet on sleep and psychological status in postmenopausal women with type 2 diabetes. The results suggest that the DASH diet had a beneficial effect on depression, anxiety, and stress, as well as sleep status in type 2 diabetic patients. In a cross-sectional study, Valipour et al. showed that moderate adherence to the DASH diet was negatively associated with depression (33). In this recent cross-sectional study in women with diabetes, patients who adhered to the animal-based dietary patterns were more likely to be poor sleepers or had components of depression, stress, and anxiety (34).
The high antioxidant capacity of the DASH diet leads to reduced oxidative stress, which is related to decreased insulin resistance (35). Moreover, the DASH diet can improve insulin sensitivity independent of weight loss (36). Oxidative stress and inflammation play a role in initiating psychological disorders via altering brain functions (37). The high antioxidant capacity of the DASH diet through consumption of the food groups such as legumes, nuts, vegetables, and fruits can reduce inflammation and oxidative stress and improve brain functioning by affecting synaptic plasticity (38). Healthful-rich plant foods were associated with improved psychological disorders among Iranian women (39). Beezhold et al. showed that a reduction of animal foods and higher consumption of plant-based foods may have beneficial effects on mental health (40). A clinical trial on 75 Iranian women revealed that consumption of high-fiber diets, especially high in whole grains, can improve levels of inflammatory factors and prevent subsequent health outcomes (41).
Vegetables consumed in the DASH diet are good sources of folate and magnesium. These micronutrients play a role in the synthesis of several neurotransmitters, including dopamine, serotonin, and norepinephrine, and may contribute to brain reactions, as well as anti-inflammatory effects (42, 43). Moreover, vitamin D as one of the important ingredients in the DASH diet can improve mood status. A randomized controlled trial of women with T2DM, anxiety, and vitamin D deficiency revealed that vitamin D supplementation for 16 weeks improved mood status (44).
Nuts and beans are the other important foods in the DASH diet, which have beneficial effects through several mechanisms such as ameliorating insulin resistance and improving postmenopausal symptoms. A controlled-feeding study of 50 patients with T2DM showed that daily consumption of cashews for 8 weeks reduced serum insulin (45). In addition, soybeans can promote the expression of estrogen-sensitive genes by binding to estrogen receptors and can reduce hot flashes in postmenopausal women (46). Also, it is well established that sex steroid hormones impact mental health status and mood through neuroprotective and neurodevelopmental processes (47). A cross-sectional study in China found that nut consumption was inversely associated with depression (48). In a large population study of Iranian adults, legumes and nuts consumption were inversely associated with anxiety in adult men. However, there was no significant association between nuts and legumes and anxiety in women. Furthermore, the depressive symptoms were assessed by hospital anxiety and depression scales, not by the DASS-21 (49). Legumes and nuts are high in magnesium content, which is a key nutrient in many brain reactions and metabolism of neurotransmitters. Moreover, they contain fiber, omega-3 fatty acids, folic acid, and other B vitamins, which may have beneficial effects on mood and mental health status (42, 50).
On the other hand, the DASH diet had beneficial effects on anthropometric indices in this study. In line with our results, Fatahi et al. showed that weight-loss diets rich in whole grains, fruits, and vegetables reduced body weight and waist circumference (51). A cross-sectional study in 3,004 adult women showed that anxiety and depression may be related to weight gain (52). However, it should be noted that even after controlling for weight loss, the DASH diet significantly reduced depressive symptoms and sleep status scores.
To the best of our knowledge, there is no study examining the relationship between the adherence to the DASH diet and AGEs. AGEs can be obtained through dietary intakes or can be produced by various reactions such as oxidation of amino acids, lipids, and sugars. AGEs can be generated by oxidative stress even without hyperglycemia. AGEs predispose individuals to inflammation and oxidative stress, which are related to insulin resistance and diabetes and many other chronic diseases such as neurodegenerative diseases, sleep apnea, cardiovascular diseases, and diabetes (53). In this study, the levels of serum AGEs in the patients who adhered to the DASH diet were significantly lower compared to the control group. It was hypothesized that antioxidant capacity of the DASH diet may improve the serum AGEs level. A 3-month calorie-restricted Mediterranean diet in 47 women with overweight and obesity showed a significant reduction in AGEs and insulin resistance (54). Increased intake of antioxidant foods, including vegetables, fruits, nuts, and whole grains and lower intake of red and processed meats, pastries, and sweetened beverages, led to lower intake of AGEs in the Mediterranean diet and may apply to the DASH diet (54).
This study was the first comprehensive study to examining the effect of the DASH diet on several diabetes-related outcomes such as depression, anxiety, stress, sleep status, and the levels of selected sex hormones. However, there are several limitations that should be noted. The reverse causation between diabetes and psychological disorders is unknown. The results are not generalizable to other populations of different ages and gender. Moreover, we did not measure dietary, urinary, or fecal AGEs. Therefore, future studies examining the effect of the DASH diet or other healthy dietary patterns should include a comprehensive assessment of AGEs.
In conclusion, the DASH diet improved the biochemical markers in the intervention group and had beneficial effects on depression, anxiety, and stress scores, as well as sleep status. Further studies are suggested to confirm our results.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Tehran University of Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
ED designed and LA supervised the study. ED conducted the study. ED and MQ performed the statistical analyses. ED, JH, and VB prepared a first draft of the manuscript. LA, S-AK, and BL finalized the manuscript. NB edited and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study is supported by Tehran University of Medical Sciences (grant number: 36923).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank all the patients who participated in this study.
1. Wild S, Roglic G, Green A, Sicree RHK. Global prevalence of diabetes. Diabetes Care. (2004) 27:1047–53. doi: 10.2337/diacare.27.5.1047
2. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. (2017) 15:131. doi: 10.1186/s12916-017-0901-x
4. Balhara YPS. Diabetes and psychiatric disorders. Indian J Endocrinol Metab. (2011) 15:274–83. doi: 10.4103/2230-8210.85579
5. Gupta S, Wang Z. Predictors of sleep disorders among patients with type 2 diabetes mellitus. Diabetes Metab Syndr. (2016) 10:213–20. doi: 10.1016/j.dsx.2016.06.009
6. Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. (2008) 1129:287–304. doi: 10.1196/annals.1417.033
7. Choi JK, Kim MY, Kim JK, Park JK, Oh SS, Koh SB, et al. Association between short sleep duration and high incidence of metabolic syndrome in midlife women. Tohoku J Exp Med. (2011) 225:187–93. doi: 10.1620/tjem.225.187
8. Ji X, Grandner MA, Liu J. The relationship between micronutrient status and sleep patterns: a systematic review. Public Health Nutr. (2017) 20:687–701. doi: 10.1017/S1368980016002603
9. Gupta M, Chari S. Proxidant and antioxidant status in patients of type II diabetes mellitus with IHD. Indian J Clin Biochem. (2006) 21:118–22. doi: 10.1007/BF02912925
10. Lam JC, Tan KC, Lai AY, Lam DC, Ip MS. Increased serum levels of advanced glycation end-products is associated with severity of sleep disordered breathing but not insulin sensitivity in non-diabetic men with obstructive sleep apnoea. Sleep Med. (2012) 13:15–20. doi: 10.1016/j.sleep.2011.07.015
11. Kong F, Li H, Xu G, Ying Y, Gong Q, Zhao J, et al. Association of dietary behaviors and sleep quality: results from the adults chronic diseases and risk factors survey of 2015 in Ningbo, China. Int J Environ Res Public Health. (2018) 15:1823. doi: 10.3390/ijerph15091823
12. Mondin TC, Stuart AL, Williams LJ, Jacka FN, Pasco JA, Ruusunen A. Diet quality, dietary patterns and short sleep duration: a cross-sectional population-based study. Eur J Nutr. (2019) 58:641–51. doi: 10.1007/s00394-018-1655-8
13. Mossavar-Rahmani Y, Jung M, Patel SR, Sotres-Alvarez D, Arens R, Ramos A, et al. Eating behavior by sleep duration in the Hispanic Community Health Study/Study of Latinos. Appetite. (2015) 95:275–84. doi: 10.1016/j.appet.2015.07.014
14. Thielecke F, Jonnalagadda SS. Can whole grain help in weight management? J Clin Gastroenterol. (2014) 48 (Suppl. 1):S70–7. doi: 10.1097/MCG.0000000000000243
15. Hermsdorff HH, Zulet M, Abete I, Martínez JA. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr. (2011) 50:61–9. doi: 10.1007/s00394-010-0115-x
16. Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The dietary approaches to stop hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr. (2011) 141:1083–8. doi: 10.3945/jn.110.136739
17. Shirani F, Salehi-Abargouei A, Azadbakht L. Effects of dietary approaches to stop hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition. (2013) 29:939–47. doi: 10.1016/j.nut.2012.12.021
18. Liang H, Beydoun HA, Hossain S, Maldonado A, Zonderman AB, Fanelli-Kuczmarski MT, et al. Dietary Approaches to Stop Hypertension (DASH) score and its association with sleep quality in a national survey of middle-aged and older men and women. Nutrients. (2020) 12: 1510. doi: 10.3390/nu12051510
19. Lei R, Sun Y, Liao J, Yuan Y, Sun L, Liu Y, et al. Sex hormone levels in females of different ages suffering from depression. BMC Women's health. (2021) 21:215. doi: 10.1186/s12905-021-01350-0
20. Cohen LS, Soares CN, Poitras JR, Prouty J, Alexander AB, Shifren JL. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. (2003) 160:1519–22. doi: 10.1176/appi.ajp.160.8.1519
21. Meston N, Davies RJ, Mullins R, Jenkinson C, Wass JA, Stradling JR. Endocrine effects of nasal continuous positive airway pressure in male patients with obstructive sleep apnoea. J Intern Med. (2003) 254:447–54. doi: 10.1046/j.1365-2796.2003.01212.x
22. Zhang H, Ni J, Yu C, Wu Y, Li J, Liu J, et al. Sex-Based differences in diabetes prevalence and risk factors: a population-based cross-sectional study among low-income adults in China. Front Endocrinol. (2019) 10:658. doi: 10.3389/fendo.2019.00658
23. Albert PR. Why is depression more prevalent in women? J Psychiatry Neurosci. (2015) 40:219–21. doi: 10.1503/jpn.150205
24. Arnetz L, Ekberg NR, Alvarsson M. Sex differences in type 2 diabetes: focus on disease course and outcomes. Diabetes Metab Syndr Obes. (2014) 7:409–20. doi: 10.2147/DMSO.S51301
25. Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav. (2018) 187:20–3. doi: 10.1016/j.physbeh.2017.08.016
26. Jehan S, Masters-Isarilov A, Salifu I, Zizi F, Jean-Louis G, Pandi-Perumal SR, et al. Sleep disorders in postmenopausal women. J Sleep Disord Ther. (2015) 4:212. doi: 10.4172/2167-0277.1000212
27. Tayebinejad N, Neyestani TR, Rashidkhani B, Nikooyeh B, Kalayi A, Shariatzadeh N, et al. The effect of daily consumption of Iranian yogurt drink doogh fortified with vitamin D or vitamin D plus calcium on the serum advanced glycation end products (AGEs) and oxidized LDL concentrations in type 2 diabetes patients: a randomized clinical trial. Iranian-J-Nutr-Sci-Food-Technol. (2012) 7:1–9.
28. Azadi-Yazdi M, Karimi-Zarchi M, Salehi-Abargouei A, Fallahzadeh H, Nadjarzadeh A. Effects of dietary approach to stop hypertension diet on androgens, antioxidant status and body composition in overweight and obese women with polycystic ovary syndrome: a randomised controlled trial. J Hum Nutr Diet. (2017) 30:275–83. doi: 10.1111/jhn.12433
29. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. (2000) 32(Suppl. 9):S498–504. doi: 10.1097/00005768-200009001-00009
30. Ghaffarpour M, Houshiar-Rad AHK. The Manual for Household Measures, Cooking Yields Factors and Edible Portion of Foods. Tehran: Keshaverzi Press (1999).
31. Samani S, B. J. A study on the reliability and validity of the short form of the depression anxiety stress scale (DASS-21). J Soc Sci Humanit. (2007). 26:65–77.
32. Farrahi Moghaddam J, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep Breath. (2012) 16:79–82. doi: 10.1007/s11325-010-0478-5
33. Valipour G, Esmaillzadeh A, Azadbakht L, Afshar H, Hassanzadeh A, Adibi P. Adherence to the DASH diet in relation to psychological profile of Iranian adults. Eur J Nutr. (2017) 56:309–20. doi: 10.1007/s00394-015-1081-0
34. Daneshzad E, Keshavarz SA, Qorbani M, Larijani B, Bellissimo N, Azadbakht L. Association of dietary acid load and plant-based diet index with sleep, stress, anxiety and depression in diabetic women. Br J Nutr. (2020) 123:901–12. doi: 10.1017/S0007114519003179
35. Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension. 2003 41:422–30. doi: 10.1161/01.HYP.0000053450.19998.11
36. Ard JD, Grambow SC, Liu D, Slentz CA, Kraus WE, Svetkey LP. The effect of the PREMIER interventions on insulin sensitivity. Diabetes Care. (2004) 27:340–7. doi: 10.2337/diacare.27.2.340
37. Maes M, Fišar Z, Medina M, Scapagnini G, Nowak G, Berk M. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. and new drug candidates–Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology. (2012) 20:127–50. doi: 10.1007/s10787-011-0111-7
38. Gomez-Pinilla F, Nguyen TT. Natural mood foods: the actions of polyphenols against psychiatric and cognitive disorders. Nutr Neurosci. (2012) 15:127–33. doi: 10.1179/1476830511Y.0000000035
39. Zamani B, Daneshzad E, Siassi F, Guilani B, Bellissimo N, Azadbakht L. Association of plant-based dietary patterns with psychological profile and obesity in Iranian women. Clin Nutr. (2020) 39:1799–808. doi: 10.1016/j.clnu.2019.07.019
40. Beezhold B, Radnitz C, Rinne A, DiMatteo J. Vegans report less stress and anxiety than omnivores. Nutr Neurosci. (2015) 18:289–96. doi: 10.1179/1476830514Y.0000000164
41. Arabzadegan N. Daneshzad E. Effects of dietary whole grain, fruit, and vegetables on weight and inflammatory biomarkers in overweight and obese women. Eat Weight Disor. (2020) 25:1243–51. doi: 10.1007/s40519-019-00757-x
42. Yary T, Aazami S, Soleimannejad K. Dietary intake of magnesium may modulate depression. Biol Trace Elem Res. (2013) 151:324–9. doi: 10.1007/s12011-012-9568-5
43. Fava M, Mischoulon D. Folate in depression: efficacy, safety, differences in formulations, and clinical issues. J Clin Psychiatry. (2009) 70 Suppl 5:12–7. doi: 10.4088/JCP.8157su1c.03
44. Fazelian S, Amani R, Paknahad Z, Kheiri S, Khajehali L. Effect of vitamin D supplement on mood status and inflammation in vitamin D deficient type 2 diabetic women with anxiety: a randomized clinical trial. Int J Prev Med. (2019) 10:17. doi: 10.4103/ijpvm.IJPVM_174_18
45. Darvish Damavandi R, Mousavi SN, Shidfar F, Mohammadi V, Rajab A, Hosseini S, et al. Effects of daily consumption of cashews on oxidative stress and atherogenic indices in patients with type 2 diabetes: a randomized, controlled-feeding trial. Int J Endocrinol Metab. (2019) 17:e70744. doi: 10.5812/ijem.70744
46. Messina M. Soy foods, isoflavones, and the health of postmenopausal women. Am J Clin Nutr. (2014) 100 (Suppl. 1):423s–30. doi: 10.3945/ajcn.113.071464
47. Pinares-Garcia P, Stratikopoulos M, Zagato A, Loke H, Lee J. Sex: a significant risk factor for neurodevelopmental and neurodegenerative disorders. (2018) 8:154. doi: 10.3390/brainsci8080154
48. Su Q, Yu B, He H, Zhang Q, Meng G, Wu H, et al. Nut consumption is associated with depressive symptoms among Chinsese adults. Depress Anxiety. (2016) 33:1065–72. doi: 10.1002/da.22516
49. Anjom-Shoae J, Sadeghi O, Keshteli AH, Afshar H, Esmaillzadeh A, Adibi P. Legume and nut consumption in relation to depression, anxiety and psychological distress in Iranian adults. Eur J Nutr. (2020) 59:3635–45. doi: 10.1007/s00394-020-02197-1
50. Nguyen PH, Grajeda R, Melgar P, Marcinkevage J, DiGirolamo AM, Flores R, et al. Micronutrient supplementation may reduce symptoms of depression in Guatemalan women. Arch Latinoam Nutr. (2009) 59:278–86.
51. Fatahi S, Daneshzad E, Kord-Varkaneh H, Bellissimo N, Brett NR, Azadbakht L. Impact of diets rich in whole grains and fruits and vegetables on cardiovascular risk factors in overweight and obese women: a randomized clinical feeding trial. J Am Coll Nutr. (2018) 37:568–77. doi: 10.1080/07315724.2018.1444520
52. Grundy A, Cotterchio M, Kirsh VA, Kreiger N. Associations between anxiety, depression, antidepressant medication, obesity and weight gain among Canadian women. PLoS ONE. (2014) 9:e99780. doi: 10.1371/journal.pone.0099780
53. Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C, et al. Dietary advanced glycation end products and their role in health and disease. Adv Nutr. (2015) 6:461–73. doi: 10.3945/an.115.008433
Keywords: dietary approches to stop hypertension, depression, anxiety, sleep, diabetes
Citation: Daneshzad E, Heshmati J, Basirat V, Keshavarz S-A, Qorbani M, Larijani B, Bellissimo N and Azadbakht L (2022) The Effect of the Dietary Approaches to Stop Hypertension (DASH) Diet on Sleep, Mental Health, and Hormonal Changes: A Randomized Clinical Trial in Women With Type 2 Diabetes. Front. Nutr. 9:775543. doi: 10.3389/fnut.2022.775543
Received: 14 September 2021; Accepted: 30 March 2022;
Published: 12 May 2022.
Edited by:
Vijaya Juturu, Lonza, SwitzerlandReviewed by:
Pao-Hwa Lin, Duke University Medical Center, United StatesCopyright © 2022 Daneshzad, Heshmati, Basirat, Keshavarz, Qorbani, Larijani, Bellissimo and Azadbakht. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leila Azadbakht, azadbakhtleila@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.