- 1School of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing, China
- 2Physical and Chemical Inspection Institute, Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, China
1 Introduction
1.1 Salinization of groundwater seriously affects agricultural production and the ecological environment
With the advancement of economic development and population growth, the problem of groundwater salinization in arid and semi-arid regions globally has become increasingly prominent. Studies showed that the salinization issue was particularly severe in coastal areas in China, where the total dissolved solids content in groundwater exceeded 2,000 mg/L in some regions (Zhao et al., 2022). Groundwater salinization is primarily caused by natural factors such as seawater intrusion and evaporative concentration, as well as anthropogenic factors including overexploitation of groundwater, improper agricultural irrigation, industrial wastewater discharge, and defects in urban drainage systems. These factors lead to the accumulation of salts in the soil and groundwater, exacerbating the salinization process. Groundwater salinization is characterized by a significant increase in salts and alkaline substances. This leads to increased water hardness and reduced usability. It has a negative impact on agricultural irrigation and triggers secondary soil salinization, thereby affecting the living environment of plants, animals, and microorganisms (Yang et al., 2022).
1.2 Long-term accumulation of trace contaminants in saline-alkaline water further exacerbates human health risks
As a result of long-term human activities, groundwater salinization areas are often accompanied by trace contaminants such as antibiotics, pesticides, heavy metals (e.g., arsenic and lead) and fluoride. The long-term accumulation of these pollutants poses a significant threat to human health. The persistence of antibiotics can lead to the spread of antibiotic resistance genes in groundwater and facilitate the dissemination of resistance. The accumulation of drug-resistant pathogens is a direct threat to human health. It also disrupts the microbial balance in ecosystems, which affects the natural purification functions of soil and water bodies (Chng et al., 2020). Moreover, high concentrations of fluoride, when people are exposed to them for extended periods, pose health risks such as cardiovascular disease and osteoporosis (Kumar et al., 2020). Therefore, the issue of groundwater salinization and its associated trace pollutants urgently requires attention and must be addressed through integrated management measures.
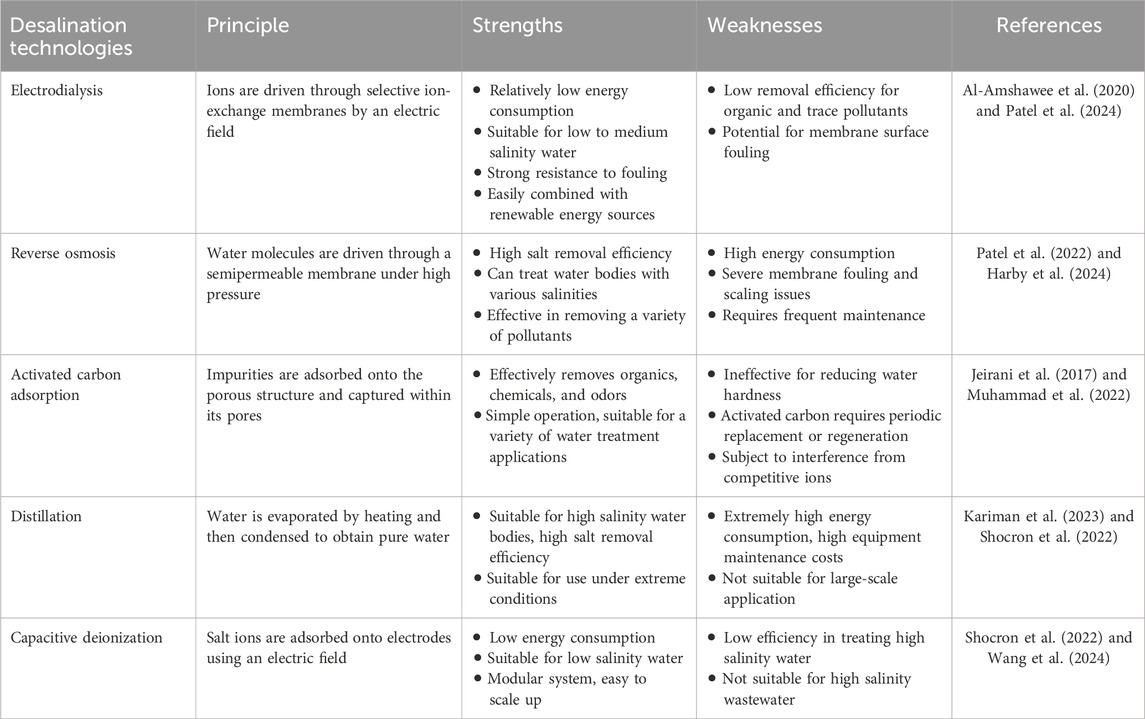
2 Electrodialysis and activated carbon in the spotlight for saline-alkaline water desalination and contaminant remediation
Desalination technologies are a class of treatment methods aimed at removing salts and other contaminants from saline water bodies to obtain fresh water resources. These technologies are widely used in water-scarce areas, especially in coastal arid and semi-arid regions, to meet the needs of drinking water, agricultural water, and industrial water. Common saline-alkaline water desalination technologies and their strengths and weaknesses are shown in Table 1.
2.1 Electrodialysis (ED) technology is an efficient way to desalinate saline-alkaline water
Electrodialysis (ED) is a process that utilizes an electric field to drive the separation of ions through selective membranes, exhibiting high efficiency in desalinating saline-alkaline water. It is particularly effective for treating waters with salinities in the range of 1–10 g/L, significantly reducing the salt content while maintaining a high rate of freshwater recovery (Al-Amshawee et al., 2020). This is of significant importance in alleviating the global freshwater scarcity issue. Compared to other desalination technologies, ED stands out with its resistance to fouling and scaling, compact system size, high flexibility, and particularly lower investment costs for small-scale applications. For instance, ED has reportedly achieved an energy consumption of 1.8 kWh/m3 in seawater desalination, which is lower than that of reverse osmosis systems (3–4 kWh/m3) (Patel et al., 2022). Furthermore, ED can be easily integrated with renewable energy systems, offering new possibilities for sustainable freshwater production (Hopsort et al., 2024).
2.2 Nano-activated carbon (NAC): as a green strategy to capture trace pollutants in saline-alkaline water
Mechanically processed nano-activated carbon (NAC) is recognized as an effective strategy for capturing trace pollutants, including pesticides, dyes, and pharmaceutical residues, in saline-alkaline waters, owing to its high adsorption capacity and environmental compatibility. NAC’s porous structure endows it with a vast surface area and abundant functional groups, facilitating the efficient adsorption of organic pollutants, heavy metal ions, and other harmful substances from water (Muhammad et al., 2022). NAC’s pollutant remediation primarily involves mechanisms such as physical adsorption, which captures a wide range of pollutants, π-π interactions that target specific organic molecules, hydrogen bond formation for selective adsorption, hydrophobic effects that draw out nonpolar contaminants, and ion exchange for metal ion capture (Jeirani et al., 2017). Furthermore, the regenerable nature of NAC offers long-term economic and environmental advantages in water treatment.
3 ED-NAC coupling process as a low-cost sustainable solution for saline-alkaline water treatment
ED demonstrates numerous advantages in treating saline-alkaline water, yet it faces challenges in concurrently removing trace pollutants found in complex water bodies. NAC, utilizing its vast specific surface area and porous structure, successfully eliminates trace pollutants from water through physical and chemical adsorption. However, in water bodies characterized by high salinity and complex organic matter, the adsorption efficiency of NAC can be hindered by competitive ions and organic compounds. Notably, ED has the capacity to enhance the adsorption efficiency of NAC by removing competitive ions from the water and boosting the electrochemical activity of NAC. For example, Altınbaş et al. (2022) demonstrated that the presence of an adsorbent in an Adsorption-ED hybrid system substantially enhanced the removal rate of boron from actual geothermal saline-alkaline water, increasing it from 7.2% to 73.3%. Furthermore, the combined process of chemical precipitation, bipolar membrane ED, and AC adsorption reported by Ye et al. (2022) successfully eliminated heavy metals (removal efficiency >99%), inorganic salts (>92%), and total organic carbon (>58%), while recovering acids and bases from complex soil-washing wastewater. Additionally, the continuous operation of ED increases the regeneration frequency of NAC, alleviating aggregation issues and thereby reducing operational costs. Patel et al. (2024) reported that ED has better economics and recovery compared to reverse osmosis at feed salinities up to 3 g/L. In addition, It has been noted that nano-activated carbon prepared from agricultural waste is cost-effective, with an economic value of about $3/kg, and can be reused without significant loss of adsorption capacity (Muhammad et al., 2022). Thus, the coupled ED-NAC process not only overcomes the limitations of a single method and significantly improves the treatment efficiency of saline-alkaline water but also mitigates environmental impacts and enhances resource recycling.
4 Broad application prospects of ED-NAC coupling process
In the midst of the heightened consciousness surrounding environmental protection and sustainable resource utilization, the ED-NAC coupling process emerges as a highly efficient and eco-friendly solution for addressing pollutants present in saline-alkaline water. To scale it up from the laboratory to real-world engineering applications, we need to adopt the following strategies: Firstly, a detailed analysis of water quality, tailored to the characteristics of saline-alkaline water from various sources, is essential for selecting appropriate process parameters and treatment strategies. Secondly, to ensure economic feasibility, optimization of energy consumption and reduction in the frequency and cost of NAC replacement are crucial. Thirdly, considering the long-term sustainability of the technology is imperative, which encompasses developing efficient material regeneration methods and rational waste treatment solutions. Lastly, ongoing focus on technological innovation and optimization is vital, including the use of sensors and intelligent control systems for real-time monitoring of water quality and automatic adjustment of process parameters based on data feedback, as well as the integration of machine learning algorithms for analyzing historical and real-time data to optimize parameters and operational strategies, ultimately enhancing the overall system performance and efficiency. These efforts will make a more significant contribution to water resource protection, environmental remediation, and the achievement of sustainable development goals.
Author contributions
XJ: Data curation, Investigation, Writing–original draft. HJ: Data curation, Investigation, Writing–original draft. ZH: Resources, Validation, Writing–original draft. CZ: Conceptualization, Supervision, Validation, Writing–review and editing. HC: Conceptualization, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Fundamental Research Funds for the Central Universities (No. 30924010938), the Open Fund for Large Instrumentation of Nanjing University of Science and Technology (2024), Postgraduate Research and Practice Innovation Program of Jiangsu Province (SJCX24_0158), and Chunhui Talent Project of Hebei Provincial Natural Science Foundation (E2023519001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Amshawee, S., Yunus, M. Y. B. M., Azoddein, A. A. M., Hassell, D. G., Dakhil, I. H., and Hasan, H. A. (2020). Electrodialysis desalination for water and wastewater: a review. Chem. Eng. J. 380, 122231. doi:10.1016/j.cej.2019.122231
Altınbaş, B. F., Orak, C., Okten, H. E., and Yüksel, A. (2022). Novel hybrid adsorption-electrodialysis (AdED) system for removal of boron from geothermal brine. ACS Omega 7, 45422–45431. doi:10.1021/acsomega.2c06046
Chng, K. R., Li, C., Bertrand, D., Ng, A. H. Q., Kwah, J. S., Low, H. M., et al. (2020). Cartography of opportunistic pathogens and antibiotic resistance genes in a tertiary hospital environment. Nat. Med. 26, 941–951. doi:10.1038/s41591-020-0894-4
Harby, K., Emad, M., Benghanem, M., Abolibda, T. Z., Almohammadi, K., Aljabri, A., et al. (2024). Reverse osmosis hybridization with other desalination techniques: an overview and opportunities. Desalination 117600. doi:10.1016/j.desal.2024.117600
Hopsort, G., Cacciuttolo, Q., and Pasquier, D. (2024). Electrodialysis as a key operating unit in chemical processes: from lab to pilot scale of latest breakthroughs. Chem. Eng. J. 153111. doi:10.1016/j.cej.2024.153111
Jeirani, Z., Niu, C. H., and Soltan, J. (2017). Adsorption of emerging pollutants on activated carbon. Rev. Chem. Eng. 33, 491–522. doi:10.1515/revce-2016-0027
Kariman, H., Shafieian, A., and Khiadani, M. (2023). Small scale desalination technologies: a comprehensive review. Desalination 116985. doi:10.1016/j.desal.2023.116985
Kumar, M., Goswami, R., Patel, A. K., Srivastava, M., and Das, N. (2020). Scenario, perspectives and mechanism of arsenic and fluoride co-occurrence in the groundwater: a review. Chemosphere 249, 126126. doi:10.1016/j.chemosphere.2020.126126
Muhammad, S., Abdul Khalil, H. P. S., Abd Hamid, S., Albadn, Y. M., Suriani, A. B., Kamaruzzaman, S., et al. (2022). Insights into agricultural-waste-based nano-activated carbon fabrication and modifications for wastewater treatment application. Agriculture 12, 1737. doi:10.3390/agriculture12101737
Patel, C. G., Barad, D., and Swaminathan, J. (2022). Desalination using pressure or electric field? A fundamental comparison of RO and electrodialysis. Desalination 530, 115620. doi:10.1016/j.desal.2022.115620
Patel, S. K., Lee, B., Westerhoff, P., and Elimelech, M. (2024). The potential of electrodialysis as a cost-effective alternative to reverse osmosis for brackish water desalination. Water Res. 250, 121009. doi:10.1016/j.watres.2023.121009
Shocron, A. N., Roth, R. S., Guyes, E. N., Epsztein, R., and Suss, M. E. (2022). Comparison of ion selectivity in electrodialysis and capacitive deionization. Environ. Sci. Technol. Lett. 9, 889–899. doi:10.1021/acs.estlett.2c00551
Wang, H., Liu, Y., Li, Y., Xu, X., Liu, X., Yao, Y., et al. (2024). Tactics for boosting the desalination stability of capacitive deionization. Chem. Eng. J. 153808. doi:10.1016/j.cej.2024.153808
Yang, F., Jia, C., Yang, H., and Yang, X. (2022). Development, hotspots and trend directions of groundwater salinization research in both coastal and inland areas: a bibliometric and visualization analysis from 1970 to 2021. Environ. Sci. Pollut. Res. 29, 67704–67727. doi:10.1007/s11356-022-22134-5
Ye, B., Lan, J., Nong, Z., Qin, C., Ye, M., Liang, J., et al. (2022). Efficiently combined technology of precipitation, bipolar membrane electrodialysis, and adsorption for salt-containing soil washing wastewater treatment. Process Saf. Environ. Prot. 165, 205–216. doi:10.1016/j.psep.2022.07.015
Keywords: electrodialysis, groundwater, emerging contaminants, rapid adsorption, saline environment
Citation: Ji X, Jiang H, Huo Z, Zhu C and Chen H (2024) Electrodialysis coupled with nano-activated carbon (ED-NAC): a promising technology for the removal of trace pollutants in saline-alkaline waters. Front. Environ. Sci. 12:1507095. doi: 10.3389/fenvs.2024.1507095
Received: 07 October 2024; Accepted: 01 November 2024;
Published: 13 November 2024.
Edited by:
Feng Tan, Dalian University of Technology, ChinaReviewed by:
Aileen Orbecido, De La Salle University, PhilippinesChao Ma, Anhui Agricultural University, China
Lin Zhang, Henan University of Science and Technology, China
Copyright © 2024 Ji, Jiang, Huo, Zhu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun Zhu, emh1Y2h1bjIwN0AxMjYuY29t; Haoming Chen, Y2hlbmhhb21pbmc4OUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Xincheng Ji
Xincheng Ji Hanfeng Jiang1†
Hanfeng Jiang1† Haoming Chen
Haoming Chen