
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Ecol. Evol., 16 November 2020
Sec. Behavioral and Evolutionary Ecology
Volume 8 - 2020 | https://doi.org/10.3389/fevo.2020.576665
This article is part of the Research TopicBehavioral Adaptations to Life in the CityView all 13 articles
As cities expand to accommodate a growing human population, their impacts to natural ecosystems and the wildlife residing within them increase. Some animals that persist in urban environments demonstrate behaviors distinct from their non-urban counterparts. These potential behavioral changes are the subject of a growing body of research in the areas of wildlife ecology, biology, and conservation. In spite of increasing urban wildlife research, studies focused specifically on changing behavior in urban mammals is limited. We conducted a systematic literature review to synthesize current research on behavior changes in wild urban mammals. We included 83 papers published between 1987 and March 2020. Omnivores were the leading subject of study, closely followed by carnivores and the specific behaviors most widely studied were home range and vigilance. Among the reviewed studies, there were 166 observations of 44 distinct behaviors with 155 occurrences of behavior change relative to conspecifics in non-urban areas. The most commonly studied and observed type of behavior change was alert behavior. Results indicate urban environments drive adaptive responses in behavior including changes in home range and diet preference, shifts in activity budget and vigilance, decreased flight initiation distance, and increased nocturnal activity. Some urban mammal species even demonstrated the ability to modulate behaviors based on environmental cues. Our results highlight the need for long-term wildlife behavior studies across a variety of urban settings to promote successful urban wildlife management and conservation.
By 2050, 68% of the world's 9.7 billion people will be residing in urban areas (United Nations (UN) Department of Economic Social Affairs, 2019a; United Nations (UN) Department of Economic Social Affairs, 2019b). As cities expand to accommodate more people, their impacts to ecosystem processes and biota increase. Urban areas present unique and dynamic challenges for resident wildlife (Lowry et al., 2013; Miranda et al., 2013; Alberti, 2015; Birnie-Gauvin et al., 2016). In response to anthropogenic stressors, urban wildlife may exhibit behaviors differently than their non-urban counterparts (Ditchkoff et al., 2006; Chapman et al., 2012; DeCandia et al., 2019). As learning and behavioral adjustments are the primary ways animals cope with changing environments, the highly modified urban landscape provides a veritable proving ground for the ability of wildlife to adapt (Brown, 2012; Greggor et al., 2016). Decreasing natural habitat—alongside increasing anthropogenic resources—can lead to behavioral shifts in urban wildlife populations that present unique management and conservation challenges (Riley et al., 2010; Bateman and Fleming, 2012; Magle et al., 2019). Efforts to promote urban biodiversity while minimizing human-wildlife conflict will require a comprehensive understanding of what behavior changes are occurring in urban wildlife and how these species are potentially adapting over time.
Although behavior change can occur in wildlife without adaptation, it is helpful to consider behavioral responses, in terms of timescale and permanence, as either regulatory, acclimatory, or developmental (Lopez-Sepulcre and Kokko, 2012; McDonnell and Hahs, 2015). Where behavior changes fall among these three categories of adaptative response can offer insight into the mechanisms of change and whether behaviors may revert to population norms or progress toward permanent adaptation (Dingemanse et al., 2010; McDonnell and Hahs, 2015). Regulatory responses such as changes in alert behavior like harm avoidance or decreased flight initiation distance (FID) often develop within seconds to hours, whereas acclimation (e.g., adjustments in social structures and territoriality) may develop gradually over days and weeks (Bateman and Fleming, 2012; McDonnell and Hahs, 2015). Physiological changes and behavioral syndromes such as neophilia and boldness may indicate more permanent developmental response potentially leading to evolutionary change (Dingemanse et al., 2010; Lopez-Sepulcre and Kokko, 2012; McDonnell and Hahs, 2015). These adaptive responses may complement species survivability in some cases while being detrimental in others (Lopez-Sepulcre and Kokko, 2012; Lowry et al., 2013; Robertson, 2018; Ellington and Gehrt, 2019). As humans continue to alter the habitat and resources available to urban wildlife, knowing how these animals are adapting their behavior is key to understanding how certain species will persist in urban environments (Ryan and Partan, 2014; Soulsbury and White, 2015).
Despite urbanization's significant impact on wildlife, urban wildlife research remains a young and poorly understood field (Birnie-Gauvin et al., 2016; Magle et al., 2019). In their review of urban wildlife research, Magle et al. (2012) found that urban wildlife studies comprised 2% of total publication volume. Although animal behavior is a common research topic and behavioral changes between urban and non-urban conspecifics are somewhat widely studied, mammals have been underrepresented (Magle et al., 2012; Miranda et al., 2013; McDonnell and Hahs, 2015; Schell, 2018). This is somewhat surprising as changes in mammalian behavior can often be precursors to conflict with humans and understanding how mammals use urban areas is an important component of wildlife management (Gehrt and McGraw, 2007; Karelus et al., 2017). Although selective urban pressures can have contrasting effects among mammalian species, it appears that behavioral flexibility among mammals allows them to better adapt to the urban environment (Santini et al., 2019). Generally, mammals are easily disturbed by human activity which drive changes in their behavior that can impact diet, reproduction, stress levels, dial activity, and disease prevalence (Ditchkoff et al., 2006; Birnie-Gauvin et al., 2016). These changes can lead to adaptations that may have important eco-evolutionary consequences. Despite the importance of understanding behavior changes in urban mammals, there has been no comprehensive review of the current primary literature specific to urban mammal behavior.
Following PRISMA guidelines, we conducted a systematic literature review of research pertaining to urban mammal behavior conducted over the past five decades. The aim of this review was to synthesize all research generating significant findings of behavior change in urban mammal populations (population) that were conducted in an urban setting, including those using conspecific and predator decoys, human-interaction, camera trap, trap and release, and/or remote tracking protocols (interventions) to assess behavior change comparative to a non-urban population as defined by each individual study (comparator). Further, we sought to coalesce all research identifying specific behavior change (outcomes) in urban mammal populations, whether these changes were assessed via direct observation or inferred from remotely sensed/spatial data (study designs). Specifically, we were interested in the extent of urban mammal behavior change research and what, taken together, this research reveals in terms of adaption to the urban environment. In answering this research question, we unveil the predominate types of behavioral adjustments observed in urban mammals, which taxa were most studied, the journals that publish these studies, geographically where these studies were conducted, and how these trends might inform future research. Our findings underscore the importance of long-term behavioral studies to fully understand how short-term behavior changes become more permanent adaptations and to better inform urban wildlife management decisions ranging from conservation to human-wildlife co-existence.
To quantify the body of research specific to behavioral change in urban mammals, we conducted a systematic literature review following Pullin et al. (2018) using Web of Science and Google Scholar. We searched Web of Science for papers in the primary literature using the following search terms and Boolean operators: “urban*,”, “city,” “town” OR “metro;” “animal,” “wild*,” OR “mammal;” “beh*;” and “chang*,” “mod*,” “adapt*,” “alter*” OR “evol*.” For Google Scholar, we used multiple combinations of primary search terms (urban, animal, behavior, change, mammal, and wildlife) in various sub-sections (e.g., “in the title,” “anywhere,” “in subject”). Specific search parameters can be found in Supplementary Table 1. We also reviewed citations within each retained paper for additional relevant studies.
We first compared titles to eliminate redundancy from our two searches. We included or excluded papers using pre-defined inclusion/exclusion criteria (Table 1) based on the title and/or abstract. For each study retained, we recorded how data was collected, study region, and the season each study took place. We also recorded species information, behavior studied, whether there was a change in behavior, the direction of effect where appropriate, and the type of adaptation demonstrated by the behavior change. We used the non-evolutionary adaptive responses identified by Ricklefs (1990) to group observed behavior changes into one of three adaptive response categories: regulatory, acclimatory, or developmental (McDonnell and Hahs, 2015).
Our Web of Science search resulted in 640 records, and Google Scholar yielded 136 for a total of 776 records. After removing duplicates, we were left with 744 unique records. After applying inclusion/exclusion criteria, we were left with 65 papers from our database searches. We then reviewed the citations within each retained paper and found an additional 18 papers that met our inclusion criteria for a final total of 83 studies (Supplementary Figure 1). These 83 studies spanned from 1987 to 2020 and represent 8 general publication categories (Figure 1). The studies were predominately published in journals specific to zoology and mammalogy.
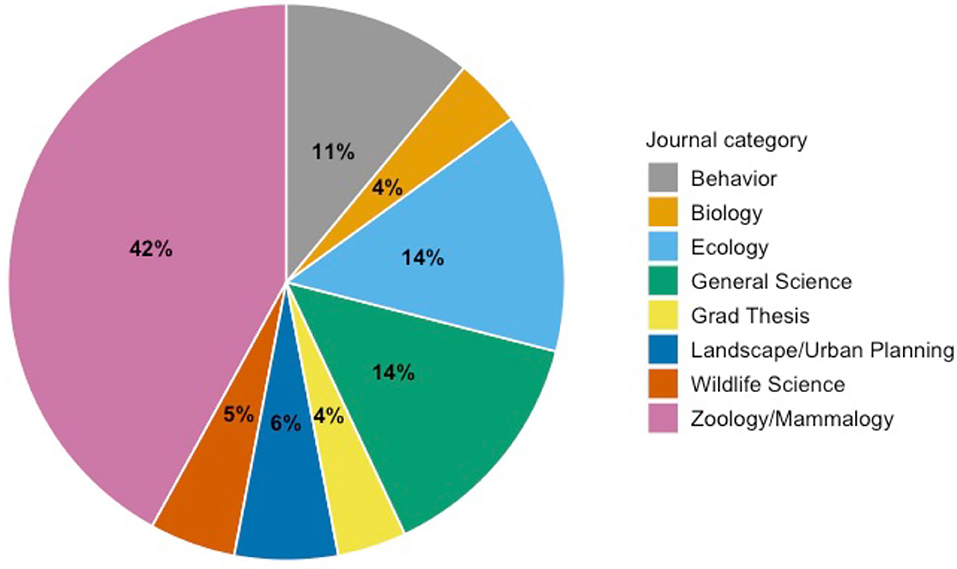
Figure 1. Publication categories of journals that published urban mammal behavior change studies between 1987 and 2020 with percentages of papers in each category.
The region with the greatest number of studies was North America (n = 43, 52%), followed by Europe (n = 17, 20.5%), Australia (n = 9, 11%), Asia (n = 7, 8.5%), Africa (n = 5, 6%), and South America (n = 2, 2%). With respect to diet guilds, 44% of the studies were on omnivores (n = 37), 40% were on carnivores (n = 33), and 16% were on herbivores (n = 13). Every region with the exception of South America had studies from each of these three guilds (Figure 2).
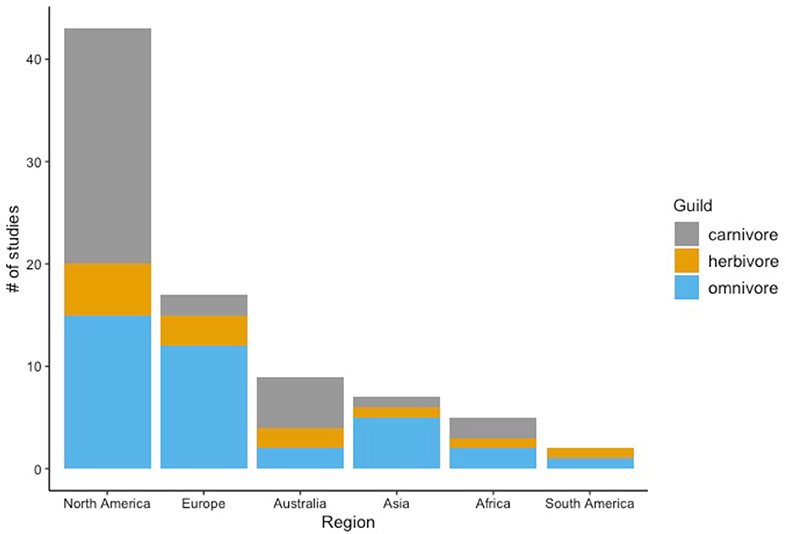
Figure 2. The number of studies that assessed behavioral changes of urban mammals across 6 world continents between 1987 and 2020. Results are categorized by diet guild.
Although most of the 83 studies focused on one species, 3 included observations on 2 or more species. Overall, 45 mammalian species were studied across 10 orders: Carnivora (n = 37 studies, 43%), Rodentia (n = 23 studies, 26%), Primate (n = 7 studies, 8%), Artiodactyla (n = 5 studies, 6%), Chiroptera (n = 4 studies, 5%), Diprotodontia (n = 4 studies, 5%), Lagomorpha (n = 3 studies, 3%), Didelphimorphia (n = 2 studies, 2%), Eulipotyphla (n = 1 study, 1%), and Peramelemorphia (n = 1 study, 1%). The four most studied species were coyote (Canis latrans; n = 12; 27%); eastern gray squirrels (Sciurus carolinensis; n = 5, 11%); Eurasian red squirrels (Sciurus vulgaris, n = 5, 11%); and black bears (Ursus americanusi, n = 5, 11%).
Some studies assessed multiple behaviors, which resulted in 166 observations of 44 different behaviors (Supplementary Table 2). Studied behaviors fell into 8 general types: alert behavior (n = 45; 27.1%), spatial (n = 40, 24.1%), diet preference/foraging/resource use (n = 27, 16.3%), activity budget (n = 22, 13.3%), diel activity (n = 14, 8.4%), behavioral syndrome (n = 9, 5.4%), mating/reproduction (n = 7, 4.2%), and social (n = 2, 1.2%) (Figure 3). With respect to taxa, all orders included at least one spatial behavior study, with the exception of primates (Supplementary Figures 2, 3). Of the two most studied orders, researchers primarily looked at alert behavior in Rodentia (n = 25/45) and spatial behavior in Carnivora (n = 22/40).
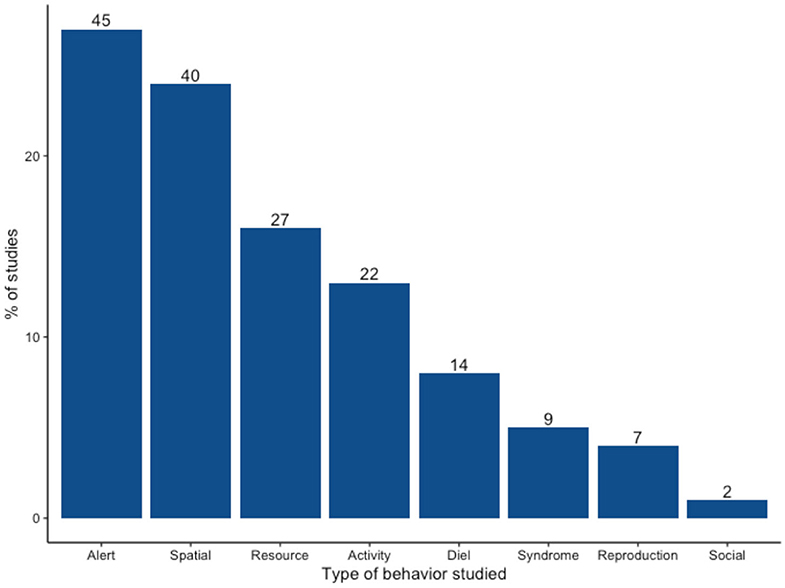
Figure 3. Types of behavior changes studied in urban mammals between 1987 and 2020. Values above bars indicate the total number of observations studied within each behavior type.
Of the 166 studied behaviors, 93% (n = 155) were different from those observed in conspecifics outside the urban setting. In the remaining observations (n = 11; 7%), researchers found no change in behavior when comparing urban and non-urban mammal populations. Behavior changes were observed across all 10 orders (Table 2) and in almost every species studied (n = 41; 91%) with the exception of Merriam's kangaroo rat (Dipodomys merriami) and 3 bats (Lasionycteris noctivagans, Myotis spp., and Eptesicus fuscus). Among the studies that observed changes in behavior, the direction of change was not always consistent, even among species (Supplementary Table 2, Column: “Change”). In addition, some researchers observed multi-directional shifts in behavior in response to varying environmental stimuli (n = 7, 4%).
Acclimation was the most common type of adaptive response (n = 105; 68% of total behavior changes) observed among all taxa in the reviewed studies (Supplementary Table 3). Six of the 8 types of behavior change (activity, diel, diet/resource use, mating/reproduction, social, and spatial) reflect acclimatory response to the urban environment (Figure 4). Of these, decreased home range (n = 19; 18% of total acclimatory responses) was the most frequently observed, followed by increased nocturnality (n = 9; 9%), diet preference changes (n = 9; 9%), and shift in resource selection (n = 9; 9%). All observed changes in alert behavior were categorized as regulatory responses (n = 43; 28% of total behavior changes). The most common regulatory responses were changes in vigilance/caution behavior (n = 11, 4 decreasing, 5 increasing, 2 shifting with no direction noted; 26% of total regulatory behavior changes) and decreased FID (n = 9; 21%). Observations of syndrome behavior in urban mammals indicate developmental response to the urban environment (n = 7; 5% of total behavior changes). The two most prevalent changes in syndrome behavior were increased boldness (n = 3; 43% of total developmental behaviors) and increased exploratory behavior (n = 3; 43%). Together, omnivores, carnivores, and herbivores demonstrate more acclimatory response to the urban environment than regulatory and developmental responses, combined (Figure 5).
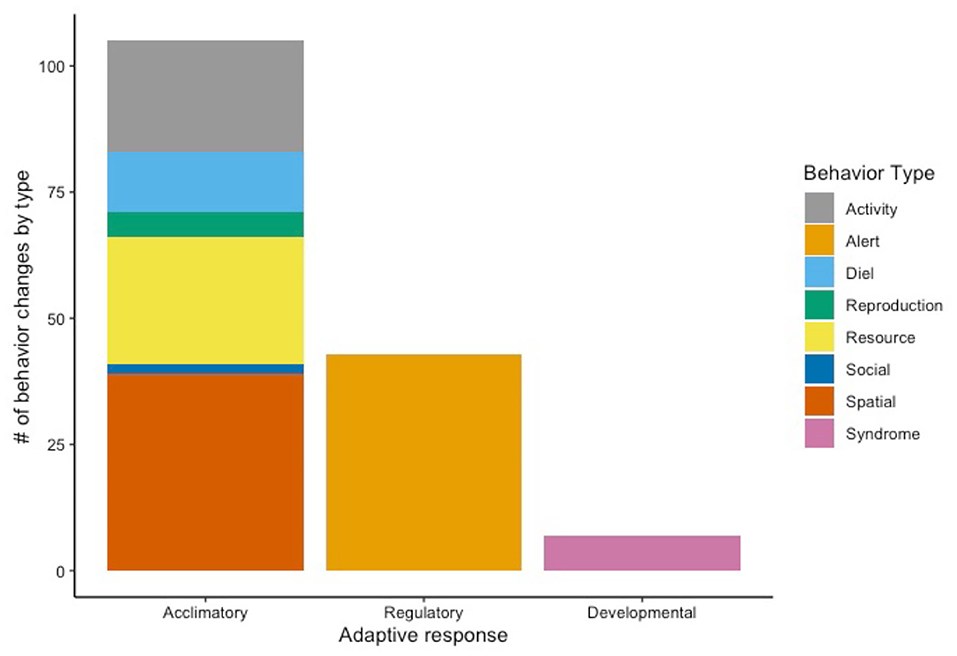
Figure 4. The 8 types of behavior changes observed in urban mammals from 1987 to 2020 categorized by adaptive response.
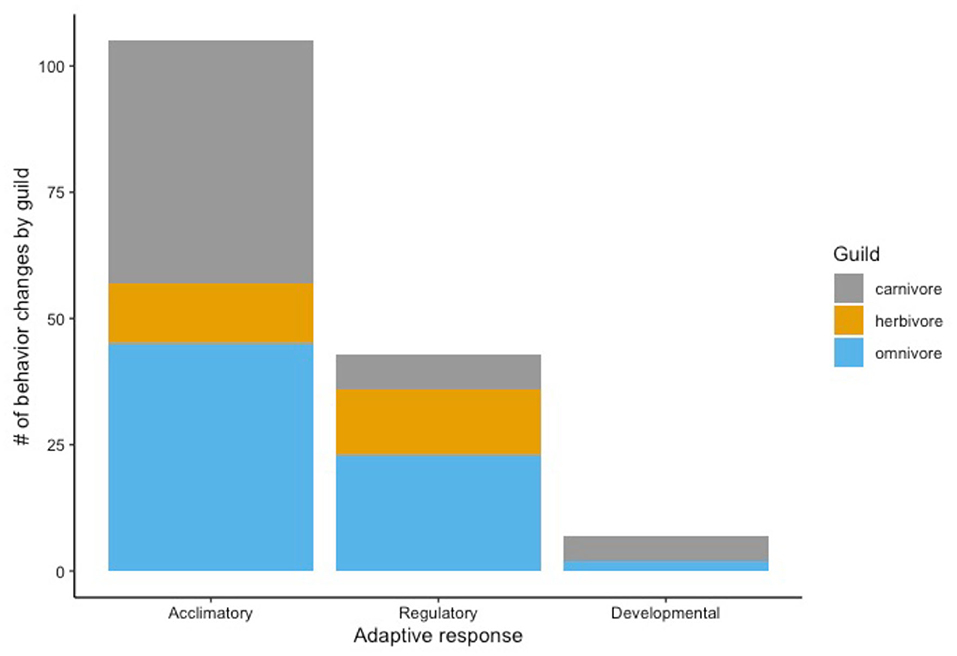
Figure 5. The number of behavior changes by diet guild in urban mammals from 1987 to 2020 categorized by adaptive response.
Our results clearly demonstrate that mammals are responding to the urban environment by changing their behavior. Much less clear is what these changes mean in terms of urban mammalian diversity, survivability, management, and conservation. Although the reported behavior changes reflect various types of adaptive response, the studies do not consistently discuss underlying mechanisms or their potential evolutionary implications. The vast majority of studies documented some degree of behavior change, but findings differed in terms of scale and direction—often depending on region, species, or resource availability. These results suggest there are varying mechanisms behind adaptive behavioral responses in urban mammals and that the nuances of these behavior shifts require further exploration.
In-line with previous reviews, our results indicate that urban wildlife research is an emerging field and just recently gaining attention (Miranda et al., 2013; Magle et al., 2019). We found only 83 studies that explicitly studied mammalian behavior change in urban settings, and 50% of those studies were conducted in the last 5 years. Similarly, a previous review on overall urban wildlife behavior found 9 studies published between 1987 and 2012 that reported changes in alert and syndrome behavior in urban mammals (Miranda et al., 2013). That number increased to 50 in our review. Although this rapid jump is promising, the taxa remains significantly underrepresented, especially when compared to research on avian species in the urban environment (Miranda et al., 2013; Sol et al., 2013; McDonnell and Hahs, 2015). Given the negative impact that urbanization has on mammalian biodiversity (McCleery, 2010), and that the presence of mammals in urban areas often results in conflict with humans (Santini et al., 2019), it is important that future urban wildlife research reflects extant mammal populations in the respective region of study.
Although mammals are an underrepresented taxonomic group in urban wildlife research, our review indicates a greater variety of mammalian species are being studied (n = 45) as compared to previous reviews (Ditchkoff et al., 2006; Miranda et al., 2013; Sol et al., 2013; McDonnell and Hahs, 2015). Although the number of mammalian species has expanded, squirrel (n = 12) and coyote (n = 12) remain the dominate focal species, comprising almost a third of all studies. Likewise, herbivores were not well-represented (n = 31; 19%). Notably, deer (family Cervidae) and raccoon (Procyon lotor) made rare appearances in reviewed studies. Only one species of deer was represented (Odocoileus virginianus) in two studies (Harveson et al., 2007; Gallo et al., 2019) and we found only a single study (Prange et al., 2004) assessing urban racoon behavior change. As deer and raccoons are commonly associated with conflict in urban environment (Curtis and Hadidian, 2010; Hadidian et al., 2010; Westerfield et al., 2019), we were surprised at the apparent lack of interest in their behavior which does not align with on-the-ground management needs (Prange et al., 2003; Urbanek et al., 2011). These findings highlight the persistent gap between animal behavior research and management action, while underscoring the need for urban mammal behavior research that is responsive to management and conservation concern (Caro, 1999; Curtis and Hadidian, 2010; McDonnell and Hahs, 2015; Greggor et al., 2016). Further, increasing taxa representation in this research will establish a foundational understanding of species-specific behavior change which can illuminate the degree and rate of urban-driven behavioral adaptation.
The most common type of adaptive behavioral response observed was acclimatory. This is not surprising as the acclimatory category encompasses behaviors relating to movement, activity, and resource use, all of which were well-represented in the reviewed studies. Urban mammals are widely adapting to the urban environment by acclimating their movement and resource use patterns. In every study assessing home range (n = 25), a change was observed when compared to non-urban populations. The majority (76%) of studies on home range found that these decreased for urban mammals. However, the direction of effect was not consistent, even among the same species. As an example, the home range of urban lesser Asiatic yellow bats varied by sex: the home range of female bats increased in urban areas, while urban males decreased their home range (Atiqah et al., 2015). Conversely, 50% of studies on canids found that coyotes and fox (Vulpes vulpes) decreased their home range while 40% had an increased home range; the other 10% did not demonstrate a change in home range size per se, but a shift in terms of drifting territory or habitat type within the respective range (Doncaster and Macdonald, 1991; Grinder and Krausman, 2001; Gehrt et al., 2009; Grubbs and Krausman, 2009; Rosatte and Allan, 2009; Gese et al., 2012; Poessel et al., 2016; Ellington and Gehrt, 2019). One explanation for inconsistent changes in urban mammal home ranges could be the highly variable nature of urban environments including inconsistent resource availability (Fitzgibbon et al., 2011; Wright et al., 2012; Bateman and Fleming, 2014; Van Helden et al., 2018). Urban mammals may selectively seek out natural prey, even among abundant anthropogenic resources which can drive increased home ranges in some mammals (Newsome et al., 2015). As population densities of mammals tend to be relatively high in urban areas, understanding behaviors that impact movement patterns and resource use can be key to successful management and conservation strategies (Curtis and Hadidian, 2010; Riley et al., 2010).
Although behavioral acclimations reveal much about mammalian adaptation to urban pressures, they do not reflect the full array of immediate behavioral response to urban stimuli, nor longer-term developmental change (McDonnell and Hahs, 2015). As examples, alert response and behavioral syndromes respectively provide insight into regulatory and developmental adaptations, both with important evolutionary implications (Sih et al., 2004; Dingemanse et al., 2010; Réale et al., 2010). Altered anti-predator behavior in the urban environment can significantly alter activity budgets and energy stores (Réale et al., 2010). Consistent behavior modifications across different urban stimuli (i.e., behavioral syndromes such as increased boldness) can likewise impact mortality risk (Luttbeg and Sih, 2010). Each of the three types of adaptation (acclimatory, regulatory, developmental) offer useful clues as to how the urban environment affects mammal populations and how it may drive evolutionary change (Miranda et al., 2013; McDonnell and Hahs, 2015; Greggor et al., 2019). As such, increased research on behaviors that reflect a broader array of regulatory and developmental adaptions will result in a more comprehensive understanding of the mechanisms behind urban mammal behavior change. Taking a collective look across the full temporal range of behavioral adaptation may help predict how urban mammal populations will fare in the face of continued urbanization. Beneficial behavior modifications by founder individuals can lead to increased fitness, whereas other adaptations may decrease survivability, both of which can result in higher-order effects on population dynamics among urban species (Lopez-Sepulcre and Kokko, 2012; Pelletier and Garant, 2012; Alberti, 2015; Birnie-Gauvin et al., 2016; Schell, 2018). Further, urban pressures and other drivers (e.g., anthropogenically provided food) that impact eco-evolutionary feedbacks appear to affect the distribution of behavioral traits (Alberti, 2015; Schell, 2018). Regulatory, acclimatory, and developmental adaptations all have the potential to alter processes that undermine healthy ecosystems and biodiversity, both of which are already fundamentally challenged in the urban environment (Palkovacs and Dalton, 2012; Alberti, 2015; McDonnell and Hahs, 2015). Continued research on how urban mammal behaviors are adapting across all timescales can yield important insights for conservationists, wildlife managers, city planners, and urban residents alike.
Because urban environments present such a dynamic mix of threats, it stands to reason that some changes in mammal behavior are multi-directional and perhaps, fluctuating. A particularly interesting finding among a small number of studies (n = 7/166) is that some mammals demonstrate the ability to modulate adapted responses based on environmental cues. For example, two studies found variation in vigilance levels of individual woodchucks (Marmota monax) based on the intensity of urbanization, possibly reflecting the variable nature of human pressures in highly urbanized areas (Watson, 2010; Lehrer et al., 2012). Likewise, fox squirrels (Sciurus niger) in Texas, USA demonstrated the ability to modulate anti-predator behavior to cope with constant stimuli created by humans in the urban environment (McCleery, 2009). Partan et al. (2010), found that eastern gray squirrels in western Massachusetts, USA modulated their alert behavior by increasing their reliance on visual signals vs. audio signals in noisier environments. A study in New York, United States, found that 90% of urban gray squirrels increased their FID when approached by humans that veered off the sidewalk and looked at them, while squirrels from the same population did not increase FID if approaching humans remained on the sidewalk (Bateman and Fleming, 2014). Likewise, urban Eurasian red squirrels demonstrated the ability to assess risk levels of various approaching objects (e.g., humans and conspecific decoys) and modulated their FID accordingly (Uchida et al., 2019, 2020). Finally, a study on Australian fur seals (Arctocephalus pusillus doriferus) found that seals modulated their alert response based on vessel type and whether or not vessels conformed with mandated approach distance thresholds—indicating that the seals learned the legal distance ships were able to approach (Speakman et al., 2020).
It is not readily apparent from these studies whether the modulations demonstrated are a function of inter-individual differences (behavioral plasticity) or consistent behavioral adaptations in response to repeated urban stimuli but they are all linked to risk assessment which has significant survival, and thus, evolutionary implications (Réale et al., 2010; Lopez-Sepulcre and Kokko, 2012; Bateman and Fleming, 2014). Although these represent a small sample size of reviewed studies, these apparent behavior modulations could begin to explain the behavior change pattern variation among certain urban mammal species. Seemingly, the majority of studies were designed to record discrete behavioral responses to specific stimuli and may have simply missed, or not considered, modulating behaviors. More research should focus on how urban mammals modulate their behaviors in response to variable urban pressures to better inform the drivers of urban-driven evolutionary behavior change. Understanding the mechanisms behind modulating adaptive behaviors, whether behavioral plasticity or contemporary evolution, can provide important insight into urban ecosystem ecology (Palkovacs and Dalton, 2012; Miranda et al., 2013; McDonnell and Hahs, 2015).
In our review, we did not establish a specific definition of “urban.” Instead, we relied on the authors' designation of the research setting as such. This inherently introduces limitations in capturing information about how varying levels of urbanization impact behavior change in mammals. Definitions of “urban” in the reviewed studies, and elsewhere, are broad and may not consistently consider factors such as land use, structures, human population density, and impervious surfaces (McIntyre et al., 2008; Bateman and Fleming, 2012; Alldredge et al., 2019; Ellington and Gehrt, 2019). Thus, we were unable to reliably relate specific features of urbanization to observed changes in behavior.
As raccoon and deer conflict is relatively common in urban settings (Hadidian et al., 2010; Westerfield et al., 2019), it was odd to us that so little of the research focused on these species. Like McDonnell and Hahs (2015), we recommend future urban mammal research focus on delivering specific solutions to conservation and management challenges. Knowing how urban mammals are changing their behavior can improve mitigation strategies and conservation interventions (Caro, 1999; Greggor et al., 2016). Urban mammal researchers should continue to look at a host of behaviors that reflect various types of adaptive response (i.e., regulatory, acclimatory, and developmental) as they may be interrelated or lead to potential adaptative evolution over time (McDonnell and Hahs, 2015). Future research should highlight potential causes for observed adaptive responses to gain a better understanding of the mechanisms underlying urban mammal behavior change (Palkovacs and Dalton, 2012; Greggor et al., 2016). Conducting long-term, parallel studies on specific behaviors across a host of cities could likewise illuminate regional trends and help identify variable mechanisms driving behavior changes in the urban setting (Magle et al., 2019; Santini et al., 2019).
Animal behavior is changing in urban environments and the long-term effects of these changes are unknown. This literature review demonstrates that urban mammals are exhibiting widespread acclimatory behavioral response to urban pressures. Our findings suggest a need to better understand the mechanisms behind urban mammal behavior change and the eco-evolutionary impacts that may result. Although a relatively nascent area of study, urban mammal research requires a shift to align priorities in a way that contributes to the growing body of knowledge on changing behavior while supporting real-time management and conservation efforts. To fully understand changing urban mammal behavior, long-term studies across multiple cities will better inform local wildlife management solutions, establish baselines of species-specific behavior change, and promote the mutually beneficial co-existence of all urban residents.
The original contributions generated for the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
KR and TG conceptualized the study, collected data, and wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to acknowledge Daniel Marzluf for reviewing and coding a subset of journal articles and the George Mason Library staff for going out of their way to swiftly mail copies of interlibrary loan articles and books even when the library was closed during the COVID-19 pandemic.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.576665/full#supplementary-material
Alberti, M. (2015). Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 30, 114–126. doi: 10.1016/j.tree.2014.11.007
Alldredge, M. W., Buderman, F. E., and Blecha, K. A. (2019). Human-Cougar interactions in the wildland-urban interface of Colorado's front range. Ecol. Evol. 9, 10415–10431. doi: 10.1002/ece3.5559
Atiqah, N., Akbar, Z., Syafrinna, Ubaidah, N., and Foo, N. Y. (2015). “Comparison of the ranging behavior of Scotophilus kuhlii (Lesser Asiatic Yellow Bat) in agricultural and urban landscape,” in AIP Conference Proceedings, Vol. 1678 (Bandar Baru Bangi: AIP Publishing LLC), 020026.
Bateman, P. W., and Fleming, P. A. (2012). Big city life: carnivores in urban environments. J. Zool. 287, 1–23. doi: 10.1111/j.1469-7998.2011.00887.x
Bateman, P. W., and Fleming, P. A. (2014). Does human pedestrian behaviour influence risk assessment in a successful mammal urban adapter? J. Zool. 294, 93–98. doi: 10.1111/jzo.12156
Birnie-Gauvin, K., Peiman, K. S., Gallagher, A. J., De Bruijn, R., and Cooke, S. J. (2016). Sublethal consequences of urban life for wild vertebrates. Environ. Rev. 24, 416–425. doi: 10.1139/er-2016-0029
Brown, C. (2012). “Experience and learning in changing environments,” in Behavioural Responses to a Changing World: Mechanisms and Consequences, eds U. Candolin and B. B.M. Wong (Oxford: Oxford University Press), 46–62.
Caro, T. (1999). The behaviour–conservation interface. Trends Ecol. Evol. 14, 366–369. doi: 10.1016/S0169-5347(99)01663-8
Chapman, T., Rymer, T., and Pillay, N. (2012). Behavioural correlates of urbanisation in the cape ground squirrel Xerus inauris. Naturwissenschaften 99, 893–902. doi: 10.1007/s00114-012-0971-8
Curtis, P. D., and Hadidian, J. (2010). “Responding to human-carnivore conflicts in urban areas,” in Urban Carnivores: Ecology, Conflict, and Conservation, eds S. D. Gehrt, S. P. D. Riley, and B. L. Cypher (Baltimore, MA: The Johns Hopkins University Press), 223–232.
DeCandia, A. L., Brzeski, K. E., Heppenheimer, E., Caro, C. V., Camenisch, G., Wandeler, P., et al. (2019). Urban colonization through multiple genetic lenses: the city-fox phenomenon revisited. Ecol. Evol. 9, 2046–2060. doi: 10.1002/ece3.4898
Dingemanse, N. J., Kazem, A. J., Réale, D., and Wright, J. (2010). Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. doi: 10.1016/j.tree.2009.07.013
Ditchkoff, S. S., Saalfeld, S. T., and Gibson, C. J. (2006). Animal behavior in urban ecosystems: modifications due to human-induced stress. Urban Ecosyst. 9, 5–12. doi: 10.1007/s11252-006-3262-3
Doncaster, C. P., and Macdonald, D. W. (1991). Drifting territoriality in the red fox Vulpes vulpes. J. Anim. Ecol. 423–439. doi: 10.2307/5288
Ellington, E. H., and Gehrt, S. D. (2019). Behavioral responses by an apex predator to urbanization. Behav. Ecol. 30, 821–829. doi: 10.1093/beheco/arz019
Fitzgibbon, S. I., Wilson, R. S., and Goldizen, A. W. (2011). The behavioural ecology and population dynamics of a cryptic ground-dwelling mammal in an urban Australian landscape. Austral. Ecol. 36, 722–732. doi: 10.1111/j.1442-9993.2010.02209.x
Gallo, T., Fidino, M., Lehrer, E. W., and Magle, S. (2019). Urbanization alters predator-avoidance behaviours. J. Anim. Ecol. 88, 793–803. doi: 10.1111/1365-2656.12967
Gehrt, S. D., Anchor, C., and White, L. A. (2009). Home range and landscape use of coyotes in a metropolitan landscape: conflict or coexistence? J. Mammal. 90, 1045–1057. doi: 10.1644/08-MAMM-A-277.1
Gehrt, S. D., and McGraw, M. (2007). “Ecology of coyotes in urban landscapes,” in Wildlife Damage Management Conferences Proceedings. Available online at: https://digitalcommons.unl.edu/icwdm_wdmconfproc/63
Gese, E. M., Morey, P. S., and Gehrt, S. D. (2012). Influence of the urban matrix on space use of coyotes in the Chicago metropolitan area. J. Ethol. 30, 413–425. doi: 10.1007/s10164-012-0339-8
Greggor, A. L., Berger-Tal, O., Blumstein, D. T., Angeloni, L., Bessa-Gomes, C., Blackwell, B. F., et al. (2016). Research priorities from animal behaviour for maximising conservation progress. Trends Ecol. Evol. 31, 953–964. doi: 10.1016/j.tree.2016.09.001
Greggor, A. L., Trimmer, P. C., Barrett, B. J., and Sih, A. (2019). Challenges of learning to escape evolutionary traps. Front. Ecol. Evol. 7:408. doi: 10.3389/fevo.2019.00408
Grinder, M. I., and Krausman, P. R. (2001). Home range, habitat use, and nocturnal activity of coyotes in an Urban environment. J. Wildl. Manage. 65, 887–898. doi: 10.2307/3803038
Grubbs, S. E., and Krausman, P. R. (2009). Use of urban landscape by coyotes. Southwest. Nat. 54, 1–12. doi: 10.1894/MLK-05.1
Hadidian, J., Prange, S., Rosatte, R., Riley, S. P. D., and Gehrt, S. D. (2010). “Raccoons (Procyon lotor),” in Urban Carnivores: Ecology, Conflict, and Conservation, eds S. D. Gehrt, S. P. D. Riley, and B. L. Cypher (Baltimore, MA: The Johns Hopkins University Press), 35–47.
Harveson, P. M., Lopez, R. R., Collier, B. A., and Silvy, N. J. (2007). Impacts of urbanization on Florida key deer behavior and population dynamics. Biol. Conserv. 134, 321–331. doi: 10.1016/j.biocon.2006.07.022
Karelus, D. L., McCown, J. W., Scheick, B. K., van de Kerk, M., Bolker, B. M., and Oli, M. K. (2017). Effects of environmental factors and landscape features on movement patterns of Florida black bears. J. Mammal. 98, 1463–1478. doi: 10.1093/jmammal/gyx066
Lehrer, E. W., Schooley, R. L., and Whittington, J. K. (2012). Survival and antipredator behavior of woodchucks (Marmota monax) along an urban-agricultural gradient. Can. J. Zool. 90, 12–21. doi: 10.1139/z11-107
Lopez-Sepulcre, A., and Kokko, H. (2012). “Understanding behavioural responses and their consequences,” in Behavioural Responses to a Changing World: Mechanisms and Consequences, eds U. Candolin and B. B.M. Wong (Oxford: Oxford University Press), 280.
Lowry, H., Lill, A., and Wong, B. B. (2013). Behavioural responses of wildlife to urban environments. Biol. Rev. 88, 537–549. doi: 10.1111/brv.12012
Luttbeg, B., and Sih, A. (2010). Risk, resources and state-dependent adaptive behavioural syndromes. Philos. Trans. R. Soc. B Biol. Sci. 365, 3977–3990. doi: 10.1098/rstb.2010.0207
Magle, S. B., Fidino, M., Lehrer, E. W., Gallo, T., Mulligan, M. P., Ríos, M. J., et al. (2019). Advancing urban wildlife research through a multi-city collaboration. Front. Ecol. Environ. 17, 232–239. doi: 10.1002/fee.2030
Magle, S. B., Hunt, V. M., Vernon, M., and Crooks, K. R. (2012). Urban wildlife research: past, present, and future. Biol. Conserv. 155, 23–32. doi: 10.1016/j.biocon.2012.06.018
McCleery, R. A. (2009). Changes in fox squirrel anti-predator behaviors across the urban–rural gradient. Landsc. Ecol. 24:483–493. doi: 10.1007/s10980-009-9323-2
McDonnell, M. J., and Hahs, A. K. (2015). Adaptation and adaptedness of organisms to urban environments. Annu. Rev. Ecol. Evol. Syst. 46, 261–280. doi: 10.1146/annurev-ecolsys-112414-054258
McIntyre, N. E., Knowles-Yánez, K., and Hope, D. (2008). “Urban ecology as an interdisciplinary field: differences in the use of “urban” between the social and natural sciences,” in Urban Ecology (Boston, MA: Springer), 49–65.
Miranda, A. C., Schielzeth, H., Sonntag, T., and Partecke, J. (2013). Urbanization and its effects on personality traits: a result of microevolution or phenotypic plasticity? Glob. Chang. Biol. 19, 2634–2644. doi: 10.1111/gcb.12258
Newsome, S. D., Garbe, H. M., Wilson, E. C., and Gehrt, S. D. (2015). Individual variation in anthropogenic resource use in an urban carnivore. Oecologia 178, 115–128. doi: 10.1007/s00442-014-3205-2
Palkovacs, E. P., and Dalton, C. M. (2012). “Ecosystem consequences of behavioural plasticity and contemporary evolution,” in Behavioural Responses to a Changing World Mechanisms and Consequences (Oxford: Oxford University Press), 175–189.
Partan, S. R., Fulmer, A. G., Gounard, M. A. M., and Redmond, J. E. (2010). Multimodal alarm behavior in urban and rural gray squirrels studied by means of observation and a mechanical robot. Curr. Zool. 56, 313–326. doi: 10.1093/czoolo/56.3.313
Pelletier, F., and Garant, D. (2012). “Population consequences of individual variation in behaviour,” in Behavioural Responses to a Changing World (Oxford: Oxford University Press), 159−174.
Poessel, S. A., Breck, S. W., and Gese, E. M. (2016). Spatial ecology of coyotes in the denver metropolitan area: influence of the urban matrix. J. Mammal. 97, 1414–1427. doi: 10.1093/jmammal/gyw090
Prange, S., Gehrt, S. D., and Wiggers, E. P. (2003). Demographic factors contributing to high raccoon densities in urban landscapes. J. Wildlife Manag. 67, 324–333. doi: 10.2307/3802774
Prange, S., Gehrt, S. D., and Wiggers, E. P. (2004). Influences of anthropogenic resources on raccoon (Procyon lotor) movements and spatial distribution. J. Mammal. 85, 483–490. doi: 10.1644/BOS-121
Pullin, A., Frampton, G., Livoreil, B., and Petrokofsky, G., (eds.). (2018). Guidelines and Standards for Evidence Synthesis in Environmental Management. Collaboration for Environmental Evidence. Available online at: http://www.environmentalevidence.org/information-for-authors (accessed October 1, 2019).
Réale, D., Dingemanse, N. J., Kazem, A. J., and Wright, J. (2010). Evolutionary and ecological approaches to the study of personality. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 365, 3937–3946. doi: 10.1098/rstb.2010.0222
Riley, S. P. D., Gehrt, S. D., and Cypher, B. L. (2010). “Urban carnivores: final perspectives and future directions,” in Urban Carnivores: Ecology, Conflict, and Conservation, eds S. D. Gehrt, S. P. D. Riley, and B. L. Cypher (Baltimore, MA: The Johns Hopkins University Press), 223−232.
Robertson, K. E. (2018). Boldness Behavior and Chronic Stress in Free-Ranging, Urban Coyotes (Canis latrans). (Ph.D. thesis), The Ohio State University. Available online at: http://rave.ohiolink.edu/etdc/view?acc_num=osu1543529587211372
Rosatte, R., and Allan, M. (2009). The ecology of red foxes, vulpes vulpes. Metropolitan Toronto, Ontario: disease management implications. Canad. Field Nat. 123, 215–220. doi: 10.22621/cfn.v123i3.967
Ryan, A. M., and Partan, S. R. (2014). “Urban wildlife behavior,” in Urban Wildlife Conservation (Boston, MA: Springer), 149–173.
Santini, L., González-Suárez, M., Russo, D., Gonzalez-Voyer, A., von Hardenberg, A., and Ancillotto, L. (2019). One strategy does not fit all: determinants of urban adaptation in mammals. Ecol. Lett. 22, 365–376. doi: 10.1111/ele.13199
Schell, C. J. (2018). Urban evolutionary ecology and the potential benefits of implementing genomics. J. Heredity 109, 138–151. doi: 10.1093/jhered/esy001
Sih, A., Bell, A., and Johnson, J. C. (2004). Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. doi: 10.1016/j.tree.2004.04.009
Sol, D., Lapiedra, O., and González-Lagos, C. (2013). Behavioural adjustments for a life in the city. Anim. Behav. 85, 1101–1112. doi: 10.1016/j.anbehav.2013.01.023
Soulsbury, C. D., and White, P. C. (2015). Human–wildlife interactions in urban areas: a review of conflicts, benefits and opportunities. Wildlife Res. 42, 541–553. doi: 10.1071/WR14229
Speakman, C. N., Johnstone, C. P., and Robb, K. (2020). Increased alertness behavior in Australian fur seals (Arctocephalus pusillus doriferus) at a high vessel traffic haul-out site. Mar. Mamm. Sci. 36, 486–499. doi: 10.1111/mms.12654
Uchida, K., Shimamoto, T., Yanagawa, H., and Koizumi, I. (2020). Comparison of multiple behavioral traits between urban and rural squirrels. Urban Ecosyst. 23, 745–754. doi: 10.1007/s11252-020-00950-2
Uchida, K., Suzuki, K. K., Shimamoto, T., Yanagawa, H., and Koizumi, I. (2019). Decreased vigilance or habituation to humans? mechanisms on increased boldness in urban animals. Behav. Ecol. 30, 1583–1590. doi: 10.1093/beheco/arz117
United Nations (UN) Department of Economic Social Affairs Population Division. (2019a). World Urbanization Prospects: The 2018 Revision (ST/ESA/SER.A/420). Available online at: https://population.un.org/wup/Publications/Files/WUP2018-Report.pdf. (accessed 28 May 2020).
United Nations (UN) Department of Economic Social Affairs Population Division. (2019b). World Population Prospects 2019: Highlights (ST/ESA/SER.A/423). Available online at: https://www.un.org/development/desa/publications/world-population-prospects-2019-highlights.html. (accessed 28 May 2020).
Urbanek, R. E., Allen, K. R., and Nielsen, C. K. (2011). Urban and suburban deer management by state wildlife-conservation agencies. Wildlife Soc. Bull. 35, 310–315. doi: 10.1002/wsb.37
Van Helden, B. E., Speldewinde, P. C., Close, P. G., and Comer, S. J. (2018). Use of urban bushland remnants by the western ringtail possum (Pseudocheirus occidentalis): short-term home-range size and habitat use in Albany, Western Australia. Aust. Mammal. 40:173. doi: 10.1071/AM17026
Watson, E. L. (2010). Effects of urbanization on survival rates, anti-predator behavior, and movements of woodchucks (Marmota monax). Available online at: http://hdl.handle.net/2142/14642
Westerfield, G. D., Shannon, J. M., Duvuvuei, O. V., Decker, T. A., Snow, N. P., Shank, E. D., et al. (2019). Methods for managing human–deer conflicts in urban, suburban, and exurban areas. Hum. Wildlife Inter. Monogr. 3, 1–99. Available online at: https://digitalcommons.usu.edu/hwi_monographs/3/. (Accessed on 16 2020).
Keywords: acclimatory response, adaptive response, FID, home range, nocturnal activity, regulatory response, urban wildlife, behavioral syndrome
Citation: Ritzel K and Gallo T (2020) Behavior Change in Urban Mammals: A Systematic Review. Front. Ecol. Evol. 8:576665. doi: 10.3389/fevo.2020.576665
Received: 26 June 2020; Accepted: 19 October 2020;
Published: 16 November 2020.
Edited by:
Elizabeth Perrault Derryberry, The University of Tennessee, United StatesReviewed by:
Rachel Vera Blakey, UCLA Institute of the Environment and Sustainability, United StatesCopyright © 2020 Ritzel and Gallo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kate Ritzel, kritzel@gmu.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.