
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 18 December 2020
Sec. Marine Megafauna
Volume 7 - 2020 | https://doi.org/10.3389/fmars.2020.601277
This article is part of the Research Topic Whale-watching Impacts: Science, Human Dimensions and Management View all 15 articles
Ecotourism focused on whales and dolphins has become a popular activity and an important source of revenue for many countries. Whale watching is vital to supporting conservation efforts and provides numerous benefits to local communities including educational opportunities and job creation. However, the sustainability of whale-based ecotourism depends on the behavior and health of whale populations and it is crucial that ecotourism industries consider the impact of their activities on whale behavior. To address this statement, we collected behavioral data (e.g., change in swimming direction, frequency of breaching, slap behaviors, diving, and spy hops) from humpback whales (Megaptera novaeangliae) in the marine protected area of Las Perlas Archipelago off the Pacific coast of Panama. The goal was to determine if tourist vessel presence had an influence on whale behaviors. We conducted this study during the humpback whale breeding season from August through September 2019. Based on 47 behavioral observations, we found that higher boat density corresponded with humpback whales’ frequency of direction changes, which based on previous literature is believed to be a sign of disturbance. Alternatively, no changes in behavior were observed with varying boat density. This result is important given Panamanian regulations first implemented in 2007 by Resolution AMD/ARAP No. 01, 2007 prohibit whale-based tourism from disturbing whales, which is explicitly measured by changes in whale behavior. Because there is no systematic monitoring of whale watching activity to enforce the regulations, there is currently little compliance from tour operators and tourists. The integration of animal behavior research into management planning should result in more effective regulation and compliance of such conservation policies.
Wildlife-based ecotourism, which includes whale watching, is identified as, “tourism based on encounters with non-domesticated animals…[which] can occur in either an animal’s natural environment or in captivity” (Higginbottom, 2004). It provides economic benefits to many countries around the world (Hoyt, 2001; O’Connor et al., 2009; Guidino et al., 2020; Wiener et al., 2020) and has increased drastically in popularity over the past 50 years. Globally, over 13 million tourists take trips to view cetaceans each year, generating over $2 billion US dollars in revenue across 119 countries (Hoyt and Hvenegaard, 2002; O’Connor et al., 2009; Stoeckl et al., 2010; Guidino et al., 2020; Wiener et al., 2020). Whale watching industries are rapidly growing in developing countries such as Cambodia, Laos, Nicaragua, and Panama (Hoyt, 2001; O’Connor et al., 2009). While ecotourism activities have many economical, educational, and ecological benefits relating to conservation, there have been many published studies that conversely question the benefits of this form of ecotourism, proposing that these activities may be harming the wildlife involved (Parsons, 2012; Leslie et al., 2015; Larson et al., 2016). Therefore, these activities deserve a higher level of scrutiny and monitoring to ensure their ongoing sustainability and economic, community, educational, etc., contributions (Stamation et al., 2007; The International Ecotourism Society, 2015).
The main contribution of whale watching is that it provides a stable financial alternative to the traditionally consumptive use of whales through hunting or “whaling.” The growing influx of tourists coming to watch whales provides the revenue required to support local communities and cultures while simultaneously supporting whale conservation efforts (Wearing et al., 2014). Whale watching focused on humpback whales (Megaptera novaeangliae) was also found to be effective in encouraging natural resource protection and patronization of local businesses, especially when tour guides take care to disseminate conservation messages to tourists (Peake et al., 2009). In addition, through specific platforms such as whale watching, the public has the opportunity to come in contact with often endangered or threatened wildlife and learn about conservation efforts (García-Cegarra and Pacheco, 2017). This practice falls under “responsible” whale watching guidelines, which is defined as an environmental and economical use of whales that is sustainable, promotes whale conservation, and education while simultaneously supporting local communities (O’Connor et al., 2009). This is especially relevant given that the rapid urbanization of society is prompting people to desire opportunities to “reconnect” themselves with nature (Curtin and Kragh, 2014).
The growing popularity of this industry also has drawbacks especially in developing countries, where regulatory frameworks are often lacking leading to a higher likelihood that animal welfare and safety regulations will be ignored (Sitar et al., 2016; Kassamali-Fox et al., 2020). Tour boat operators may intentionally or inadvertently utilize locations that experience little enforcement or actively violate the rules of responsible whale watching in the desire to attract more clients, ultimately leading to detrimental impacts on whale welfare (Corbelli, 2006; Parsons, 2012; Kessler and Harcourt, 2013; Sitar et al., 2016). Accordingly, the International Whaling Commission (IWC) established measures to protect whales and published guidelines for responsible whale watching in 1997 (International Whaling Commission, 1997). Unfortunately, with little enforcement in some countries, there are still high levels of detrimental whale interactions with non-consumptive whale-watching vessels (Parsons, 2012).
During encounters when high numbers of vessels are present, whales will often exhibit a high frequency of behavioral shifts, such as direction changes, which are reflective of avoidance tactics employed when whales encounter predators (Frid and Dill, 2002; Williams et al., 2002). These behaviors, combined with the high-speeds and unpredictable approach angles often displayed by vessels, greatly increase the risk of collision (Guzman et al., 2013). In addition, whales must expend extra energy to avoid boats, while decreasing the occurrence of necessary survival activities (e.g., nursing, foraging, and reproduction; Morete et al., 2007; Stamation et al., 2010; Schaffar et al., 2013; Fournet et al., 2018; Fiori et al., 2019). This is especially true in breeding areas that are primarily frequented by mother and calf groups because they are more susceptible to whale watching disturbances (Morete et al., 2007; García-Cegarra et al., 2019). One study suggested that long-term disruption of routine behaviors caused by high levels of negative boat and whale interactions could have lasting reproductive impacts on humpback whale populations (Braithwaite et al., 2015), while others highlight potential impacts due to the inability for whales to effectively communicate due to “acoustic masking” from loud boat sounds (Rossi-Santos, 2016; Erbe et al., 2018, p. 290). This could result in reduced success when finding a mate in breeding areas, locating food in feeding areas, and further expended energy to increase call volume or duration (Foote et al., 2004; Fournet et al., 2018; Putland et al., 2018).
Given these issues and mediating factors, the marine and coastal areas of Panama are ideal locations for observing the whale–vessel interactions and potential repercussions. Panama is a popular tourist destination due to the presence of the Panama Canal, which only serves to increase daily levels of vessel traffic through its service as an important commercial trade route. Humpback whales from the southeast Pacific population migrate from their feeding grounds in Chile and Antarctica to the tropical areas along the Pacific coasts of Central America for the breeding season (Rasmussen et al., 2007; Acevedo et al., 2017). The season extends from June to October, and sometimes December, with peaks in August and September (Guzman et al., 2015). The humpback whale population of this archipelago is estimated to be around 1,000 individuals, with about 25–50 calves born annually (Guzman et al., 2015). This population of humpback whales is identified as Breeding Stock G (International Whaling Commission, 1998), which is one of the seven “stocks” inhabiting oceans in the southern hemisphere. This specific population undertakes one of the longest migration distances (Stone et al., 1990; Acevedo et al., 2017) of more than 16,000 km roundtrip from the feeding grounds to the breeding grounds (Rasmussen et al., 2007; Félix and Guzmán, 2014; De Weerdt et al., 2020), with the entire stock swimming along 9,000 km of coastline (Félix et al., 2011). Female and calf pairs tend to remain closer to shore (Glockner and Venus, 1983; Bruce et al., 2014; Oña et al., 2017) while adults prefer more direct routes in deeper waters (Félix and Haase, 2005; Rasmussen et al., 2012; Félix and Guzmán, 2014; Guzman and Félix, 2017). Mother–calf pair preference for coastal waters poses higher risks of vessel collision and entanglement in gillnets, as fishermen and commercial ships share these waters (Félix and Guzmán, 2014). Although whales are migratory for the majority of the year, they congregate in waters less than 200 m deep for their annual breeding season. Hence, the shallow waters of the Pacific of Panama are ideal for mother humpback whales giving birth, due to the lack of competitive males pursuing the females, ocean turbulence, and predators (Flórez-González et al., 1994; Corkeron and Connor, 1999; Darling and Nicklin, 2002; Cartwright and Sullivan, 2009; Craig et al., 2014; Pitman et al., 2015). The objective of this study is to determine if tour boat number and mode of approach to whales elicit changes in their behavior frequencies in the Las Perlas Archipelago in Panama. We hypothesize that whales will decrease the frequency of certain behaviors as indicators of disturbance when boat presence increases and when boat captains are not complying with regulations.
This study was conducted in the Las Perlas Archipelago (8.41°N, 79.02°W) in the Gulf of Panama, which lies about 60 km southeast of Panama City (Figure 1). The archipelago comprises 250 basaltic rock islands and islets spread over 1,688 km, making it the fourth-largest coastal marine protected area of Panama (ADM/ARAP No. 1 from 13 February 2007; Guzman et al., 2008). We recorded behavioral responses of humpback whales in the Las Perlas Archipelago between 18 August and 6 September 2019, with observational sessions consisting of both land-based and boat-based visual behavioral studies, from a lookout point on Contadora Island (the largest inhabited island) and a ∼9.75 m whale-watching vessel, respectively. 720 total minutes were recorded, with 135 min from land-based surveys and 585 min from boat-based surveys, throughout the 16 days of research. Our boat, unlike other boats, took extreme precautions to minimize our potential disturbance to the whales by following the mandated regulations such as keeping a 250 m distance, having a certified boat operator with a permit for commercial operations, and limiting observation times to 30 min per group.
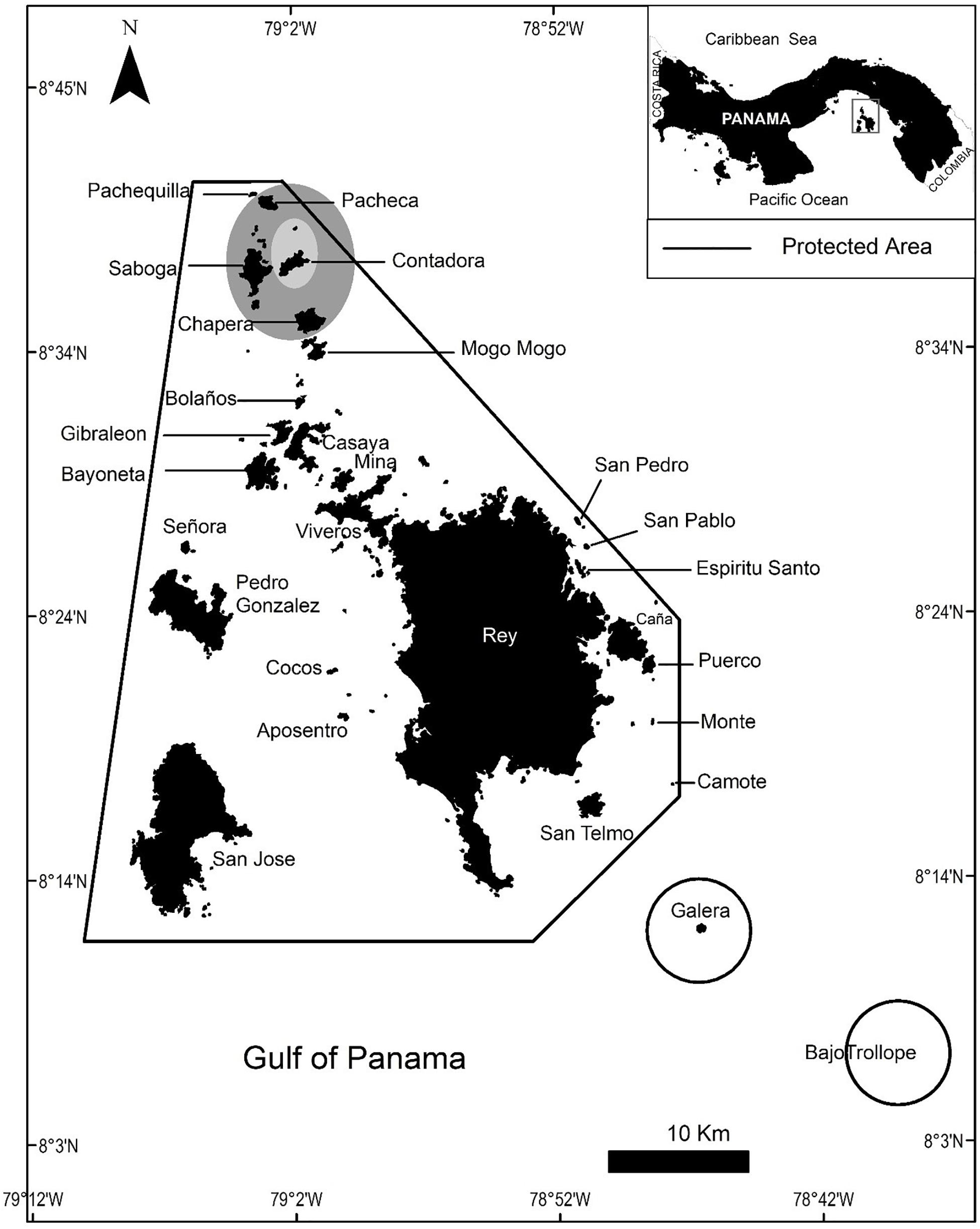
Figure 1. Map showing the study area in Las Perlas archipelago in Panama. The solid lines indicate the limits of the protected area. The shaded area is the core of the fieldwork and data collection, but qualitative observations extended to the outer areas.
Data were collected using a whale group-follow protocol, as the presence of competitive groups (i.e., a female surrounded by one or several males displaying active behaviors, such as breaching, striking, charging, and trumpeting), made it difficult to follow specific individuals (Mann, 1999). Therefore, a focal follow method was used. Variables in the data included four group types (e.g., competitive, mother–calf pair and escort, pair, and lone whale), group size, behaviors (see Table 1), Global Positioning System (GPS) coordinates, whale direction changes, and the number of boats within approximately 300 m of the whales. The distance was visually measured for both boat and land surveys by the researcher using Chapera island as a referential point. We then estimated how far the whale was between the boat or Contadora (the lookout point) and Chapera. Diving behavior was measured by the number of fluke dives that occurred within 15 min by a focal whale (including adults and calves). Other recorded variables were measured to characterize the environmental conditions during the survey which included the Beaufort wind scale and cloud cover (Spencer et al., 2006).

Table 1. Description of behavior categories for humpback whale behaviors (based on descriptions by: Glockner-Ferrari and Ferrari (1984) and Gabriele (1992) (adapted from Bauer, 1986; Helweg, 1989; Corkeron, 1995; Darling and Nicklin, 2002).
A mother and calf pair consisted of a large whale (assumed to be the mother) together with a small individual one-third the length of the adult, the calf (Chittleborough, 1958; Cartwright and Sullivan, 2009). A lone whale was a single individual traveling without any other individuals observed within 50 m, while pairs contained two adult whales traveling in the same direction. Meanwhile, whales were considered a group (or “pod”) if three or more individuals were moving in the same direction and less than 50 m from each other. These groups were considered competitive if three or more adult humpback whales were within 50 m of each other, exhibiting high energy behavior. The composition of these groups usually consisted of a single female (with or without calf), with one or more males that are showing a high frequency of surface behavior and physical contact with each other (Herman et al., 2007).
During boat-based surveys, observation began once humpback whales were spotted within 300 m of the research vessel, which gave a clear view for researchers to collect data. Land-based study sessions began once a whale was spotted within approximately 3 miles (4.8 km) of the lookout point given the increased visibility and the use of Outland X 10x42 binoculars. For both land and boat surveys, once whales were spotted, one researcher tracked the GPS coordination and direction change of humpback whales. The GPS coordinates were subjectively estimated by the researcher judging the whale’s distance between the two islands of Contadora (the lookout point) and the island of Chapera in viewpoint of the land-based studies. Researchers pinpointed the estimated location of the whale on the device’s map using a mobile device’s GPS application and recorded the coordinates. A change in direction was visually measured by considering the location and forward positioning of whales when surfacing. If the whale group surfaced in a different location and in a different facing direction than their original position, it indicated a direction change. The second researcher tracked all 15 behaviors from Table 1 as well as group type, group size, Beaufort wind scale, number of boats, and cloud cover. If more than one group was spotted during a study session, the group closest to the observer was tracked. Studies occurred in good weather conditions (Beaufort wind scale < 5) but were obstructed in severe weather conditions (Beaufort scale > 5; Cloud cover = 100%), if whale sightings were lost, or if the whale group split during the observation session. If a whale or pod were spotted, then every 15 min, observations of weather conditions, and a scan of behaviors (e.g., scan sample) were conducted and then recorded. We did not record the frequency of behaviors that occurred continuously over 15 min.
While counting the number of vessels, boats were only included in a session if it was observed to be clearly following the humpback whale group and if they were within 300 m of the whale. Observation sessions that included zero boats present were considered controls. Since the lack of boat presence served as the control variable, these observations could only be conducted during land-based studies to avoid inadvertent effects from the research vessel. Many behavioral events, such as singing, trumpeting, side flukes, and colliding, were omitted from the study given their low or absent sample sizes. Others were combined into a single category because of low individual incidence. For example, head slaps, tail slaps, and pectoral fin slaps were all combined into “slap behaviors.”
We used a Chi-squared goodness of fit test to measure the influence of boat density on whale behavior. We used this method due to its established ability to test the relationship between behaviors and boat presence (Bagdonavicius and Nikulin, 2011). To normalize the data, we proportionally measured the behavioral observations using a linear regression hypothesis test. Individual whales may express different behavioral responses when faced with a disturbance. Thus, to determine if group type was a significant predictor of a whale’s behavior, we applied a Kruskal–Wallis and a post hoc pairwise Wilcoxon test to assess which sets of groups had a significantly different number of direction changes from each other (Pohlert, 2014). Results are reported as mean ± standard error followed by the p-value. Finally, we used a regression model featuring a Pearson’s product-moment test to test the strength or weakness between the relationship between direction changes and the number of vessels. Such a test is essential for drawing a best-fit line through the two variables (direction change and vessel numbers) and examining how far off the variables are from the regression line (Benesty et al., 2009). Both boat and land-based studies were included in every analytical test. We performed all statistical analyses in R version 3.6.0 (R Core Team, 2017) and Microsoft Excel (2003).
Between August and September 2019, we recorded 47 behavioral sessions. Groups with mother and calf pairs and mother–calf and escort groups were pooled for analysis due to the low sample size of mother–calf and escort groups (three groups total). These 47 samples consisted of 24 mother and calf pairs and escort sightings (51%), 11 competitive group sightings (23%), seven lone whale sightings (15%), and five paired adult sightings (11%). The average number of individuals in a pod was 2.57 with a range of one individual to a maximum of eight individual whales in the study. Thereafter, we compared the explanatory variable (number of boats) and two dependent variables (direction change and behavior). Mother–calf and escort pods made up 50% of the samples involving boats but were rarely observed during controlled samples, making up only 14% of the data, respectively.
We collected behavioral observations in both the absence and presence of vessels. Overall, the Chi-squared goodness of fit test indicated a significant difference among all behaviors (X2 = 57.1147, p < 0.001). In the presence of vessels, breaching gradually increased as boat numbers grew, then significantly declined with more than three boats present (2%; Figure 2). This decline in breaching often occurred when boats were chasing whales. Humpback whales were most often seen executing slap behaviors (e.g., pectoral fin slaps, tail slaps, and head slaps) during sampling with zero boats present (55%). Alternatively, the frequency of diving behavior (e.g., tail up-dives) varied widely among different levels of boat presence (36% of dives occurred in sessions with two boats, 29% with four or more boats, 28% with zero boats, 5% with one boat, and 2% with three boats), but there was no discernable pattern related to the number of vessels. While spy hops/head rises were rarely seen, they only occurred during situations when boats were present.
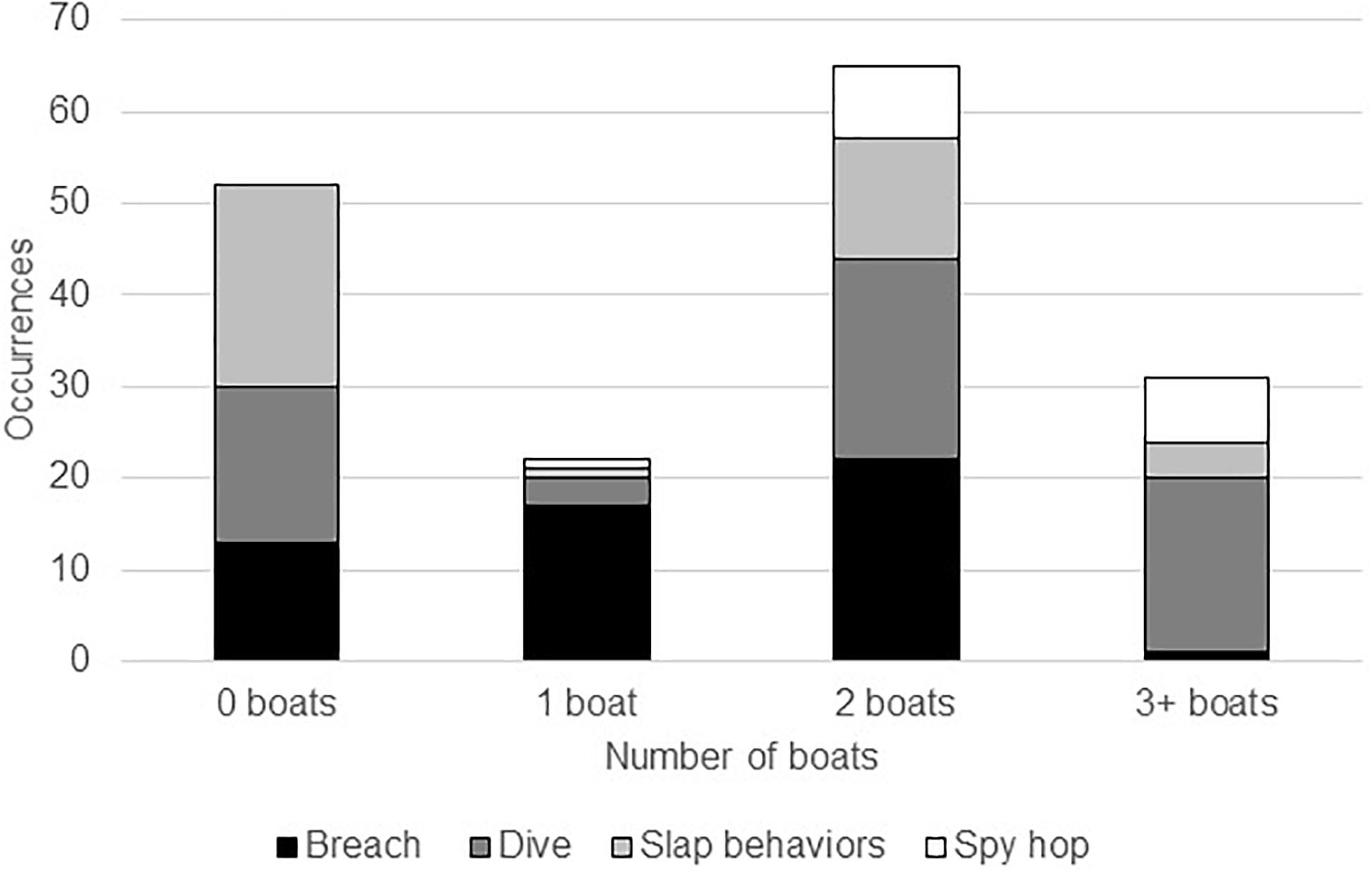
Figure 2. Megaptera novaeangliae. Total number of categorized whale behavior occurrences observed during varying boat presence while conducting group-follow behavioral samples.
More than 62% of behaviors were observed in eleven competitive groups ranging from three to eight individuals within each pod. Mother–calf and escort groups provided 20% of the behavioral data samples, five pair groups provided 16%, and seven lone whale samples accounted for only 2% of the behavioral data. Breaching was the only behavior that was not predominately expressed by competitive whale groups. Competitive groups made up 23% of the breaching while other non-competitive groups made up the other 77%. This occurred primarily with paired groups (40%), followed by mother–calf and escort pods (36%) and lone whales (<2%). In addition, a linear regression model (Figure 3) capturing the proportion of behavioral transitions presented no clear indication of significance of change with varying boat numbers (Breach: R2 = 0.42, p > 0.05; Dive: R2 = 0.09, p > 0.05; Slap: R2 = 0.13, p > 0.05; Spy hop: R2 = 0.34, p > 0.05).
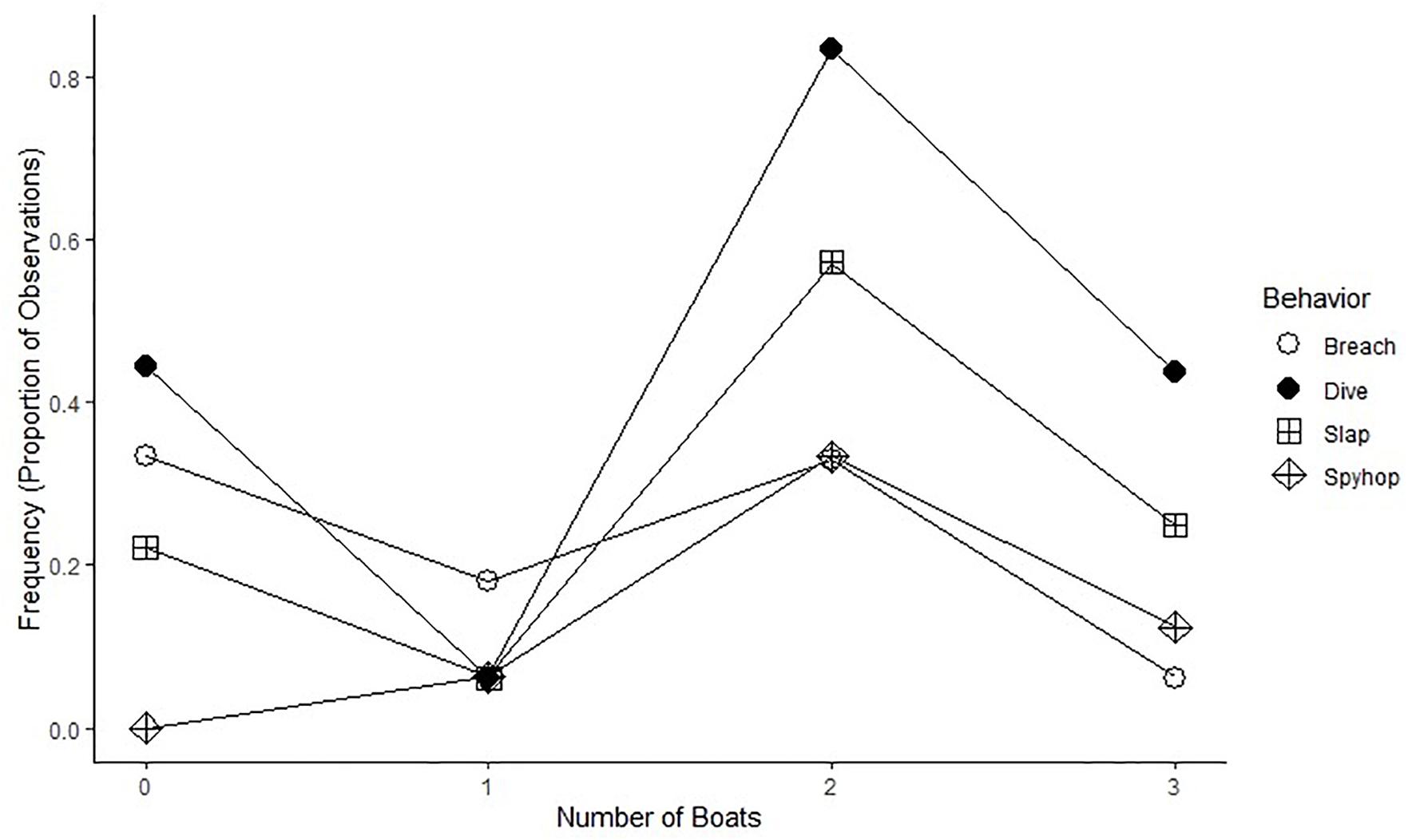
Figure 3. The frequency of behavioral observations as a proportion of the total numbers of observations for each boat type.
We conducted a post hoc pairwise Wilcoxon test (Figure 4) to assess which group types exhibited a significantly different number of direction changes from each other. Of the comparisons across all whale group types, the pair versus competitive group type and pair versus calf group type were the only pairwise comparisons rejected (Z = 1.68, 16 ± 0.474, p < 0.05; Z = 1.68, 29 ± 0.486, p < 0.05, respectively). The rest of the pair-wise comparisons, therefore, supported the null hypothesis, which assumes little to no difference occurred among the number of direction changes for each group type.
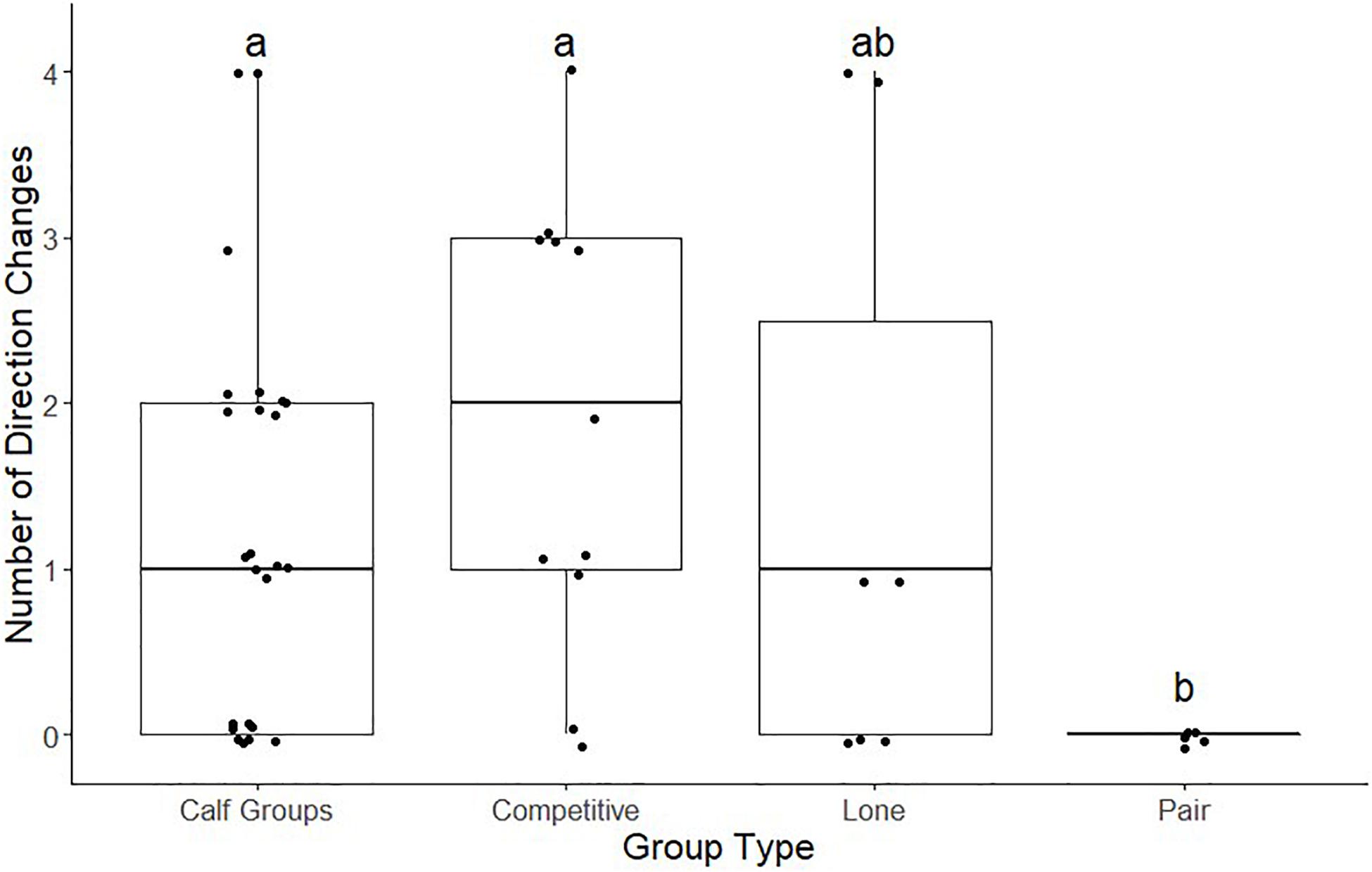
Figure 4. Megaptera novaeangliae. Box plot comparing the number of direction changes of four different whale group types (competitive groups, lone groups, calf groups, and pairs). Groups with shared letters are not significantly different.
As the number of vessels during an observational session increased, the number of whale direction changes increased. Using a linear regression model, direction change, and the number of vessels also produced a positive relationship (R2 = 0.38, p < 0.001; Figure 5). Few direction changes occurred when zero boats were present, with only one competitive group changing direction (once) during a controlled observation. However, the number of observations within each treatment was unequal. Observation sessions with one boat occurred most frequently (34%) followed by four or more boats (22%), zero boats (20%), two boats (15%), and three boats (9%). Mother–calf and escort groups had the most amount of direction changes, with 26 total direction alterations (see Figure 5). This result may be attributed to calf-mother-escort groups being the most observed group type (51% of samples).
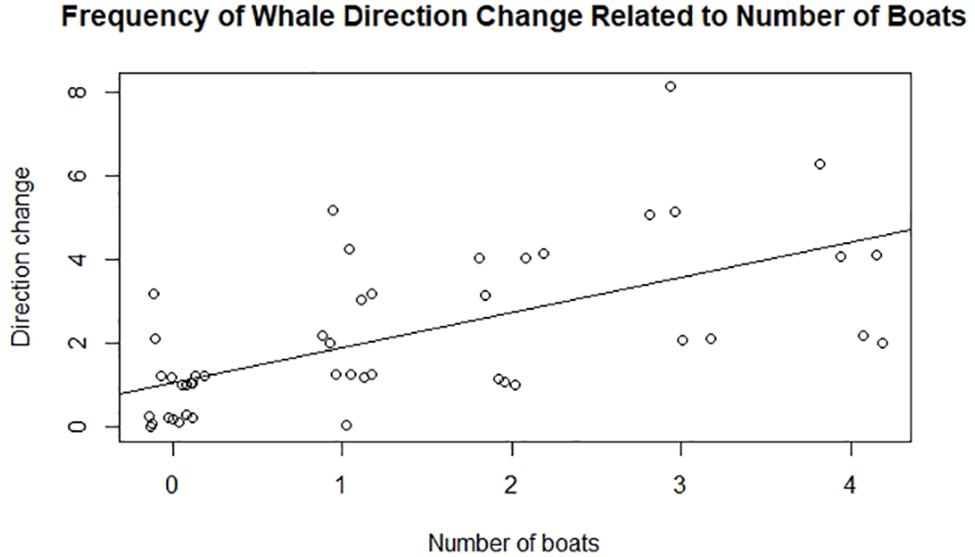
Figure 5. Megaptera novaeangliae. Observed correlation between the number of boats and the total number of direction changes while conducting group-follow behavioral samples.
The number of boats present had varying effects on the four behavior events measured during our study: breaching, diving, slap behaviors, and spy hop. It is theorized that breaching represents a communicative tactic caused by whales slapping their bodies on the surface when vocalization is obstructed (Whitehead, 1985; Dunlop et al., 2010; Kavanagh et al., 2017). While numerous factors can hinder vocalization such as high wind speeds, rain, and vessel noise, whale communication in this environment could have been blocked by higher levels of vessel noise, leading to an increase in the observed breaching behavior (Whitehead, 1985; Richardson et al., 1995; Moore et al., 2012; Cholewiak et al., 2018; Gabriele et al., 2018).
This is reflected in the results as the increase in vessel number appeared to be associated with higher incidences of breaching. However, the breaching frequency decreased once vessel numbers exceeded two boats. It is also possible that crowding from multiple boats limited surface area, restricting available space for whales to breach. Whale breaching has also been theorized to be a form of play, especially when exhibited by calves (Whitehead, 1985), which could explain the relatively high involvement of calf pairs in breaching. Alternatively, breaching may also be a tactic for male humpback whales to display their physical abilities when seeking a mate, which explains the high breach count that also occurred in pairs and competitive groups (Whitehead, 1985; Darling and Nicklin, 2002; Pacheco et al., 2013). Unfortunately, due to boats being more inclined to violate the existing whale-watching regulations, the high levels of whale-chasing exhibited by vessels could negatively influence the stress level of the whales, reducing their breaching. These behaviors could eventually be replaced with an increase in avoidance behaviors, such as direction change and longer dive times (Stamation et al., 2010).
The number of slap behaviors increased when the number of boats declined. This observation supports the theory that if a whale is close to another group, they will communicate through slapping behavior (Shapiro, 2008). Whales of both sexes slap their fins to communicate or gain attention when seeking a mate. Females specifically use this slapping tactic since they do not sing (Deakos, 2002; Herman et al., 2007). This behavior was especially evident in this study as Panama is a hotspot for breeding humpback whales, with increased competitive behaviors exhibited between males and females.
Competitive groups had the highest incidences for three of the four observed behaviors: slap behaviors, spy hopping, and diving. However, these results may differ from other studies conducted at different times of the year because whale behavior changes dramatically during breeding seasons (Corkeron, 1995; Stamation et al., 2010; Schaffar et al., 2013).
Tail slaps are the most common surface behavior observed, most likely because they are not associated with high energetic costs (Noren et al., 2009; Segre et al., 2020). Therefore, humpback whales may only resort to breaching when noise pollution (such as that caused by high vessel presence) increases, as the sound of breaching travels much farther than the noise of a tail or pectoral fin slap. Schuler et al. (2019) attribute the change in surface behavior to the disparity between the weight and surface area of a whale’s tail versus that of their body. Researchers of previous studies also found an increase in vessel number to cause humpback whales exhibiting surface behaviors to switch from surface activity to traveling. This may have long-term effects on individuals and groups because of the high energy expenditure of reacting to the boats (Schuler et al., 2019; Table 2).
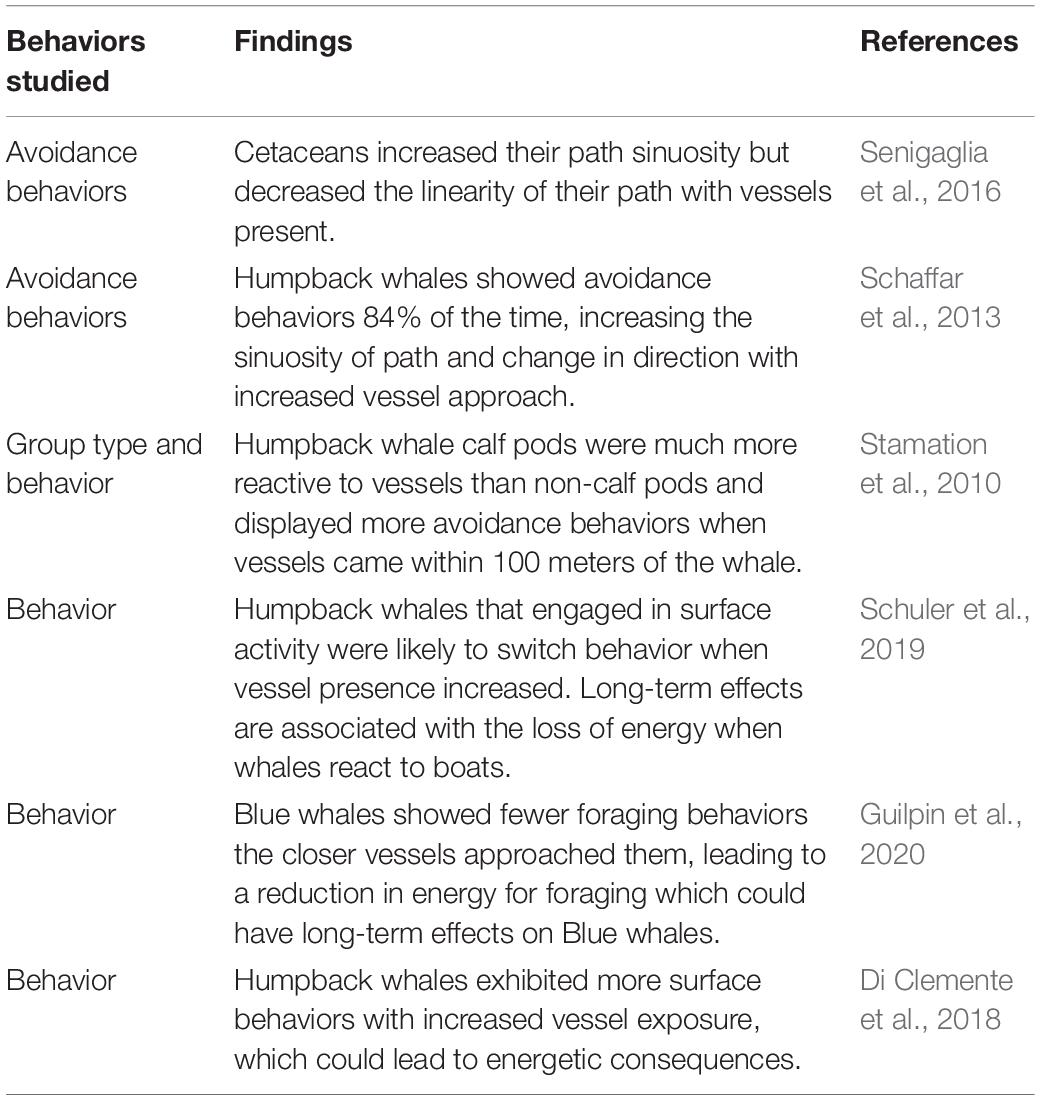
Table 2. Categories of behaviors used in this study from prior studies on the impacts of whale watching on orcas and humpback whale behavior.
Spy hops or head raises occurred less frequently than the other documented behaviors, but more occurred during sessions of high boat presence. This could suggest that spy hops ensue when whales wish to view activities above the surface (Galvin, 2006). In this study, as spy-hopping only occurred when vessels were present, the whales were most likely curious about the vessels following them, thereby supporting this hypothesis.
The linear regression model displayed no clear indication of different behavior event frequencies when correlated with varying vessel numbers. This result could be an indication of habituation to anthropogenic presence and noise, which poses additional risks (Richardson et al., 1995; Stone and Yoshinaga, 2000), as well as the range of different types of vessels which vary in their acoustic volume. With Panama being one of the central ports in the global cargo-shipping network, higher levels of vessel traffic will likely only increase the risk of whale-vessel collision (Kaluza et al., 2010; Guzman et al., 2013). While the risk of collision with larger ships has been reduced due to the passage of the Gulf of Panama Traffic Separation Scheme in 2014 (CITE), the lack of regulation enforcement among non-commercial ships means potentially hazardous collisions between cetaceans and vessels (Panama Maritime Authority, 2014).
This is an observational study and we did not experimentally manipulate boat numbers. Thus, the number of samples differs between boat numbers. Further studies are required to confirm if humpback whales in the Las Perlas Archipelago display the same behavioral responses that whale groups exhibit in published studies (Darling and Nicklin, 2002; Williams et al., 2002; Lusseau and Bejder, 2007; Morete et al., 2007; Stamation et al., 2010; Schaffar et al., 2013; Fiori et al., 2019; Schuler et al., 2019).
We suggest that the whale group type was not a significant predictor of the number of direction changes exhibited. The significant difference between pairs versus competitive groups and pairs versus groups found in Las Perlas Archipelago may be the result of competitive groups being in a setting where they must be vigilant and always watching their competitors; however, this attentive attribute may cause them to exhibit stress-based behaviors to avoid boats. The concern of energy costs is especially relevant with regard to calf groups since it seems whale watching vessels prefer following calf groups due to their playful behavior. As calves may feel more threatened by boats than other group types, Panamanian regulations have extra laws to protect calves, given their vulnerable state. We saw a significant difference even with a small sample size, but a greater number of samples would clarify the extent of the relationship. Nevertheless, these results support findings from previous whale behavioral reports. In one study, group type was included as an explanatory variable to predict dive time, swim speed, and directness index and found the relationship between group type and the other variables had no significance and did not lead to a better fitting model (Schaffar et al., 2013). Unfortunately, we were unable to measure changes in swim speed in this study. Another study found pods with calves exhibit higher levels of activity compared to non-calf pods (Stamation et al., 2010). This supports the results of our study, which showed groups with calves executing the largest number of direction changes. However, due to the small sample size of this study, no other conclusions can be confidently derived regarding whether whale behavior can be predicted by the whale group type.
The results of this study displayed a positive correlation between the direction change and the number of vessels present, with most of these changes being exhibited by pod groups containing calves (Figure 5). Calves are especially vulnerable to increased vessel presence due to the higher likelihood of vessel collision, less knowledge of vessel movement, and decreased ability to partake in essential behaviors such as feeding, nursing, and learning how to care for themselves (Scheidat et al., 2004; Stamation et al., 2010). This may explain why calf groups had the highest sum of direction changes compared to all other whale pod types since vessels had a higher preference for chasing whale groups with calves. Results from this study show clear indications of behavioral change being a consequence of increased vessel presence, violating Panama’s regulation that prohibits vessels from “chang[ing] the behavior of cetaceans” (sensu Carlson, 2010).
Previous research suggests that the proximity of boats is a robust predictor of the number of directional changes a whale might exhibit (Schaffar et al., 2013). As direction change is a tactic humpback whales use to avoid predators, this avoidance behavior may also be utilized when faced with a boat, which could be viewed as a perceived threat (Schaffar et al., 2013; Table 2). Several researchers suggest direction changes are also related to stress and could indicate an increased level of physiological disturbance (Kruse, 1991; Beale, 2007; Schaffar et al., 2013; Schuler et al., 2019; see Table 2). Thus, while avoidance behavior may ensure self and group preservation, it also comes at a physiological cost to the organism. Not only can increased levels of stress negatively impact an organism’s health, but it can also inhibit normal whale behavior and interactions, which can disrupt social interactions (mother–calf pair in particular), mating, and foraging (Beale, 2007; Lusseau et al., 2009).
Due to concerns about whale interactions with vessels, Panama initially passed Resolution Decree ADM/ARAP No. 1 on 13 February 2007, to control the level of vessel disturbance on cetaceans to conserve their populations. Regulations from this decree require operators to have a permit for commercial operations, have a maximum of two whale-watching vessels per group, take extra care when calves are present, maintain a 250 m distance from the whales, and limited observation times to 30 min per group or no more than 15 min when calves are involved, and obey the restriction of individuals from entering the water with whales, to prevent altering the behaviors of cetaceans (sensu Carlson, 2010).
Lack of enforcement from the Panamanian government has elicited the reiteration and repeated implementation of these policies every year. While boat operators may be aware of the policies currently in place, there is little structural enforcement to ensure regulatory compliance. Better enforcement protocols must, therefore, be enacted to better ensure vessels are abiding by Panama’s regulations. To reduce vessels from violating whale watching regulations, a satellite-based monitoring system should be implemented to track the activities of these vessels. This technology has already been shown to be successful in fisheries management plans and has alleviated illegal, unreported, and unregulated (IUU) fishing (Schmidt, 2005). Alternatively, lack of compliance may also be the result of poor communication from the government concerning the regulations, leaving local whale watching companies and boat operators with a lack of knowledge about the existence of these laws (Sitar et al., 2016, 2017).
In summary, Panama has strict whale watching operation regulations that are not being followed or enforced in the Las Perlas Archipelago. At multiple times throughout this study, we observed all laws pertaining to vessel regulations being broken at least once by boats. The on-going lack of regulation enforcement may result in more audacious decisions from boat operators in the future, leading to harmful or even lethal collisions with adult whales and calves. At the very least, these results show increased changes in whale behavior when vessels are present, which is illegal according to Panamanian protocols (sensu Carlson, 2010). Thus, it is highly recommended that both boat operators and tourists be educated about regulations and the importance of abiding by the law. While the purpose of this study was not to propose the eradication of whale watching, it was to highlight the potential harm being done due to the lack of compliance with responsible whale watching protocols. Responsible whale watching develops an interdependent relationship between people and whales: people gain from the ecosystem services provided by whales and economic income from this tourism industry, while whales benefit from less stress from vessels and indirectly from tour guides expanding environmental awareness and enlightening tourists about environmental or conservation issues. Continued whale research, monitoring, and modeling efforts in Panama must be implemented to better inform management decisions regarding stricter regulatory and enforcement protocols that are vital to minimize disturbance on this vulnerable population of humpback whales.
Although our study is limited to the short-term impacts of boat vessel presence on humpback whale behavior, long-term changes in behavior may indirectly lower reproduction rates (Lusseau and Bejder, 2007). This can occur through drowned out vocalizations and a reduction in the success of whales finding mates due to vessels altering whale group dynamics and travel direction (Weilgart, 2013). Additionally, whale health can be negatively affected due to chronic levels of stress, increases in energy expenditures, and discontinuation of essential behaviors such as feeding, resting, nursing, etc. (Parsons, 2012). Constant changes in behavior and less concentration on survival activities could result in eventual population declines over time, as groups with calves are the most vulnerable, especially as whale watching vessels prefer following calf groups for their charismatic physical characteristics and playful behaviors. Due to this increased vulnerability, Panamanian regulations need to contain extra provisions to ensure the protection of whale calves (sensu Carlson, 2010).
However, behavior and stress may not necessarily be coupled in a way that can be easily observed. This was evident in this study when some whales did not appear to change their behaviors despite increased levels of vessel interaction. It has been proven that animals may not exhibit avoidance behaviors, but nevertheless experience high levels of stress hormones (Schuler et al., 2019). For this reason, additional physiological studies are recommended. Previous studies have shown that biopsy samples of cortisol found within blubber samples can provide measurements of stress levels over several weeks to a month, which would provide insight into how stress levels may fluctuate throughout an entire whale watching season as the number of tourist boats changes (Noren and Mocklin, 2012; Teerlink et al., 2018). Alternative cortisol collection methods including fecal (Wasser et al., 2000; Rolland et al., 2005; Hunt et al., 2006; Burgess et al., 2013) and blowhole spray have also been shown to provide more acute measurements of stress related to vessel presence.
Future behavioral research should also include the use of more accurate measuring methods and tools. For example, the use of a theodolite tool would produce accurate distance measurements between vessels and whales, which is essential for understanding if distance impacts whale behaviors. Unmanned aerial systems (UAS) (drones) would allow for the collection of more accurate behavioral samples via less invasive observational methods (Torres et al., 2018). Visual observations could also be maintained more consistently by drones due to optimal viewing angles, as traditional vessel-based observations can only be made while the whale is surfacing. Additional social surveys should be collected from tourists, local communities, and boat operators to help us understand their impression of the whale watching industry and whether whale watching is, generally, of value.
In Las Perlas Archipelago, whale watching generates income for local communities. It also creates employment opportunities and provides ecosystem services to tourists, residents, and boat operators. However, if disturbances to these whales continue unabated, it may lead to the eventual abandonment of the Archipelago by the population, as has occurred in other popular whale-watching locations elsewhere in the world (Dean et al., 1985). The satisfaction of tourists is vital to the ongoing sustainability of the Panamanian ecotourism industry, as a report by the World Bank in 2005 found that two-thirds of all visitors to Panama were motivated to visit the country due to environmental or ecotourism reasons and income from international tourists totaled 7% of the GDP (World Bank, 2005, p. 9). Decreases in tourist motivation to partake in ecotourism activities such as whale watching potentially cause the industry to suffer, thereby affecting the Panamanian economy. This is already somewhat evidenced by the drastic decrease in tourism caused by the COVID-19 pandemic. Understanding the dynamic changes in human well-being and animal population viability are critical for establishing effective wildlife conservation strategies. Variations in socioeconomic factors that benefit the local communities can motivate more people to protect and care about whales. It is therefore important to consider the coupled nature of ecological and socio-economic systems to understand the impacts of wildlife tourism on both humans and nature.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Smithsonian Tropical Research Institute and the Institutional Animal Care and Use Committee.
AA is the primary author and this research is a component of her M.S. thesis paper. HG, BP, KS, and LG contributed to the overall idea, writing, research, data collection, and analysis of this paper. All authors contributed to the article and approved the submitted version.
Funding for this project was provided by the partnership between Arizona State University and the Smithsonian Tropical Research Institute.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Special thanks to our funders, research assistants, reviewers, boat captains, and colleagues that supported and refined our research and writing. We thank the government of Panama for support and providing the research permit SE/A-79-18 from the Ministerio de Ambiente.
Acevedo, J., Aguayo-Lobo, A., Allen, J., Botero-Acosta, N., Cepella, J., Castro, C., et al. (2017). Migratory preferences of humpback whales between feeding and breeding grounds in the eastern South Pacific. Mar. Mam. Sci. 33, 1035–1052. doi: 10.1111/mms.12423
Bagdonavicius, V., and Nikulin, M. S. (2011). Chi-squared goodness-of-fit test for right-censored data. Int. J. Appl. Math. Stat. 24, 1–11. doi: 10.1002/9781119427605.ch1
Bauer, G. B. (1986). The Behavior of Humpback Whales in Hawaii and Modifications of Behavior Induced by Human Interventions. [Dissertation]. (Honolulu: University of Hawaii).
Beale, C. (2007). The behavioral ecology of disturbance responses. Int. J. Comp. Psychol. 20, 111–120.
Benesty, J., Chen, J., Huang, Y., and Cohen, I. (2009). “Pearson correlation coefficient,” in Noise Reduction in Speech Processing (Berlin: Springer), 1–4. doi: 10.1007/978-3-642-00296-0_5
Braithwaite, J., Meeuwig, J., and Hipsey, M. (2015). Optimal migration energetics of humpback whales and the implications of disturbance. Conserv. Physiol. 3:cov001. doi: 10.1093/conphys/cov001
Bruce, E., Albright, L., Sheehan, S., and Blewitt, M. (2014). Distribution patterns of migrating humpback whales (Megaptera novaeangliae) in Jervis Bay, Australia: a spatial analysis using geographical citizen science data. Appl. Geogr. 54, 83–95. doi: 10.1016/j.apgeog.2014.06.014
Burgess, E., Blanshard, W. H., Barnes, A. D., Gilchrist, S., Keeley, T., Chua, J., et al. (2013). Reproductive hormone monitoring of dugongs in captivity: detecting the onset of sexual maturity in a cryptic marine mammal. Anim. Reprod. Sci. 140, 255–267. doi: 10.1016/j.anireprosci.2013.06.005
Carlson, C. (2010). A Review of Whale Watching Guidelines and Regulations around the World. Report for the International Fund for Animal Welfare. Yarmouth Port: International Fund for Animal Welfare.
Cartwright, R., and Sullivan, M. (2009). Associations with multiple male groups increase the energy expenditure of humpback whale (Megaptera novaeangliae) female and calf pairs on the breeding grounds. Behaviour 146, 1573–1600. doi: 10.1163/156853909X458377
Chittleborough, R. (1958). The breeding cycle of the female humpback whale, Megaptera nodosa (Bonnaterre). Aust. J. Mar. Freshw. Res. 9, 1–18. doi: 10.1071/MF9580001
Cholewiak, D., Clark, C. W., Ponirakis, D., Frankel, A., Hatch, L. T., Risch, D., et al. (2018). Communicating amidst the noise: modeling the aggregate influence of ambient and vessel noise on baleen whale communication space in a national marine sanctuary. Endang. Species Res. 36, 59–75. doi: 10.3354/esr00875
Corbelli, C. (2006). An Evaluation of the Impact of Commercial Whale Watching on Humpback Whales, Megaptera novaengliae, in Newfoundland and Labrador, and of the Effectiveness of a Voluntary Code of Conduct as a Management Strategy. St. John’s, NL: Memorial University of Newfoundland.
Corkeron, P. (1995). Humpback whales Megaptera novaeangliae in Hervey Bay, Queensland: behaviour and responses to whale-watching vessels. Can. J. Zool. 73, 1290–1299. doi: 10.1139/z95-153
Corkeron, P. J., and Connor, R. C. (1999). Why do baleen whales migrate? Mar. Mam. Sci. 15, 1228–1245. doi: 10.1111/j.1748-7692.1999.tb00887.x
Craig, A., Herman, L., Pack, A., and Waterman, J. (2014). Habitat segregation by female humpback whales in Hawaiian waters: avoidance of males? Behaviour 151, 613–631. doi: 10.1163/1568539X-00003151
Curtin, S., and Kragh, G. (2014). Wildlife tourism: reconnecting people with nature. Hum. Dimens. Wildl. 19, 545–554. doi: 10.1080/10871209.2014.921957
Darling, J. D., and Nicklin, C. (2002). Characterization of Behavior of Humpback Whales in Hawaiian Waters. Hawaiian Islands Humpback Whale National Marine Sanctuary: Division of Aquatic Resources. Honolulu: Department of Land and Natural Resources.
De Weerdt, J., Ramos, E. A., and Cheeseman, T. (2020). Northernmost records of Southern Hemisphere humpback whales (Megaptera novaeangliae) migrating from the Antarctic Peninsula to the Pacific coast of Nicaragua. Mar. Mam. Sci. 36, 1015–1021. doi: 10.1111/mms.12677
Deakos, M. H. (2002). Humpback Whale (Megaptera novaeangliae) Communication: The Context and Potential Functions of Pec-Slapping Behavior on the Hawaiian Wintering Grounds. Doctoral dissertation, University of Hawaii at Manoa, Honolulu.
Dean, F. C., Jurasz, C. M., Palmer, V. P., Curby, C. H., and Thomas, D. L. (1985). Analysis of Humpback Whale (Megaptera Novaeangliae) Blow Interval Data/Glacier Bay Alaska, 1976-1979. Fairbanks, AK: US National Park Service.
Di Clemente, J., Christiansen, F., Pirotta, E., Steckler, D., Wahlberg, M., and Pearson, H. C. (2018). Effects of whale watching on the activity budgets of humpback whales, Megaptera novaeangliae (Borowski, 1781), on a feeding ground. Aquat. Conserv. 28, 810–820. doi: 10.1002/aqc.2909
Dunlop, R. A., Cato, D. H., and Noad, M. J. (2010). Your attention please: increasing ambient noise levels elicits a change in communication behaviour in humpback whales (Megaptera novaeangliae). Proc. R. Soc. B. 277, 2521–2529. doi: 10.1098/rspb.2009.2319
Erbe, C., Dunlop, R., and Dolman, S. (2018). The effects of ship noise on marine mammals. Front. Mar. Sci. 6:606. doi: 10.3389/fmars.2019.00606
Félix, F., Castro, C., Laake, J., Haase, B., and Scheidat, M. (2011). Abundance and survival estimates of the southeastern Pacific humpback whale stock from 1991–2006 photoidentification surveys in Ecuador. J. Cetacean Res. Manag. 3, 301–307. doi: 10.47536/jcrm.vi.303
Félix, F., and Guzmán, H. M. (2014). Satellite tracking and sighting data analyses of southeast pacific humpback whales (Megaptera novaeangliae): is the migratory route coastal or oceanic? Aquat. Mamm. 40, 329–340. doi: 10.1578/AM.40.4.2014.329
Félix, F., and Haase, B. (2005). Distribution of humpback whales along the coast of Ecuador and management implications. J. Cetacean Res. Manag. 7, 21–31.
Fiori, L., Martinez, E., Orams, M. B., and Bollard, B. (2019). Effects of whale-based tourism in Vava’u, Kingdom of Tonga: Behavioural responses of humpback whales to vessel and swimming tourism activities. PLoS One 14:e0219364. doi: 10.1371/journal.pone.0219364
Flórez-González, L., Capella, J. J., and Rosebaum, H. C. (1994). Attack of killer whales (Orcinus orca) on humpback whales (Megaptera novaeangliae) on a South American Pacific breeding ground. Mar. Mam. Sci. 10, 218–222. doi: 10.1111/j.1748-7692.1994.tb00264.x
Foote, A. D., Osborne, R. W., and Hoelzel, A. R. (2004). Environment: whale-call response to masking boat noise. Nature 428:910. doi: 10.1038/428910a
Fournet, M. E. H., Matthews, L. P., Gabriele, C. M., Haver, S., Mellinger, D. K., and Klinck, H. (2018). Humpback whales Megaptera novaeangliae alter calling behavior in response to natural sounds and vessel noise. Mar. Ecol. Prog. 607, 251–268. doi: 10.3354/meps12784
Frid, A., and Dill, L. M. (2002). Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 6:11. doi: 10.5751/ES-00404-060111
Gabriele, C. M. (1992). The Behavior and Residence Characteristics of Reproductive Classes of Humpback Whales (Megaptera novaeangliae) in the Hawaiian Islands. [Master’s thesis]. (Honolulu: University of Hawaii).
Gabriele, C. M., Ponirakis, D., Clark, C. W., Womble, J., and Vanselow, P. B. S. (2018). Underwater acoustic ecology metrics in an Alaska marine protected area reveal marine mammal communication masking and management alternatives. Front. Mar. Sci. 5:17. doi: 10.3389/fmars.2018.00270
Galvin, C. (2006). Surface-piercing activities of the humpback Whale, Megaptera, related to parasites and mechanics. Eos 87:52.
García-Cegarra, A. M., and Pacheco, A. S. (2017). Whale-watching trips in Peru lead to increases in tourist knowledge, pro-conservation intentions and tourist concern for the impacts of whale-watching on humpback whales. Aquat. Conserv. Mar. Freshw. Ecosyst. 27, 1011–1020. doi: 10.1002/aqc.2754
García-Cegarra, A. M., Villagra, D., Gallardo, D. I., and Pacheco, A. S. (2019). Statistical dependence for detecting whale-watching effects on humpback whales. J. Wild Manag. 83, 467–477. doi: 10.1002/jwmg.21602
Glockner-Ferrari, D. A., and Ferrari, M. J. (1984). Reproduction in Humpback Whales, Megaptera novaeangliae, in Hawaiian Waters. Rep. Int. Whal. Comm. 237–242.
Glockner, A. D., and Venus, S. C. (1983). Identification, Growth Rate and Behavior of Humpback Whale (Megaptera novaeangliae) Cows and Calves in the Waters of Maui. Hawaii: Westview Press, 223–258.
Guidino, C., Campbell, E., Alcorta, B., Gonzalez Borasca, V., Mangel, J., Pacheco, A. S., et al. (2020). Whale Watching in Northern Peru: an economic boom? Tour. Mar. Environ. 15, 1–10. doi: 10.3727/154427320x15819596320544
Guilpin, M., Lesage, V., McQuinn, I., Brosset, P., Doniol-Valcroze, T., Jeanniard-du-Dot, T., et al. (2020). Repeated vessel interactions and climate- or fishery-driven changes in prey density limit energy acquisition by foraging blue whales. Front. Mar. Sci. 7:626. doi: 10.3389/fmars.2020.00626
Guzman, H. M., Benfield, S., Breedy, O., and Mair, J. M. (2008). Broadening reef protection across the Marine Conservation Corridor of the Eastern Tropical Pacific: distribution and diversity of reefs in Las Perlas Archipelago, Panama. Environ. Conserv. 35, 46–54. doi: 10.1017/S0376892908004542
Guzman, H. M., Condit, R., Perez, B., Capella, J., and Stevick, P. (2015). Population size and migratory connectivity of humpback whales wintering in Las Perlas Archipelago. Panama. Mar. Mamm. Sci. 31, 90–105. doi: 10.1111/mms.12136
Guzman, H. M., and Félix, F. (2017). Movements and habitat use by Southeast Pacific humpback whales (Megaptera novaeangliae) satellite tracked at two breeding sites. Aquat. Mamm. 43, 139–155. doi: 10.1578/AM.43.2.2017.139
Guzman, H. M., Gomez, C. G., Guevara, C. A., and Kleivane, L. (2013). Potential vessel collisions with southern hemisphere humpback whales wintering off Pacific Panama. Mar. Mamm. Sci. 29, 629–642. doi: 10.1111/j.1748-7692.2012.00605.x
Helweg, D. A. (1989). Daily and Seasonal Patterns of Behavior and Abundance of Humpback Whales (Megaptera novaeangliae) in Hawaiian Waters. [Master’s thesis]. (Honolulu: University of Hawaii).
Herman, E. Y., Herman, L. M., Pack, A. A., Marshall, G. J., Shepard, M., and Bakhtiari, M. (2007). When whales collide: crittercam offers insight into the competitive behavior of humpback whales on their Hawaiian wintering grounds. Mar. Technol. Soc. J. 41, 35–43. doi: 10.4031/002533207787441971
Higginbottom, K. (2004). Wildlife Tourism: Impacts, Management and Planning. Gold Coast, QLD: Common Ground Publishing in association with the Cooperative Research Centre for Sustainable Tourism, 1–14.
Hoyt, E. (2001). Whale Watching 2001: Worldwide Tourism – Numbers, Expenditures and Expanding Socio-Economic Benefits. Yarmouth Port, MA: International Fund for Animal Welfare, 157.
Hoyt, E., and Hvenegaard, G. T. (2002). A review of whale-watching and whaling with applications for the Caribbean. Coast Manag. 30, 381–399. doi: 10.1080/089207502900273
Hunt, K. E., Rolland, R. M., Kraus, S. D., and Wasser, S. K. (2006). Analysis of fecal glucocorticoids in the North Atlantic right whale (Eubalaena glacialis). Gen. Comp. Endocrinol. 148, 260–272. doi: 10.1016/j.ygcen.2006.03.012
International Whaling Commission (1997). Report of the whale watching working group. Rep. Int. Whaling Comm. 47, 250–256.
International Whaling Commission (1998). Report of the scientific committee, annex G. Report of the sub-committee on the comprehensive assessment of southern hemisphere humpback whales. Rep. Int. Whaling Comm. 48, 170–182.
Kaluza, P., Kolzsch, A., Gastner, M. T., and Blasius, B. (2010). The complex network of global cargo ship movements. Interface 7, 1093–1103. doi: 10.1098/rsif.2009.0495
Kassamali-Fox, A., Christiansen, F., May-Collado, L., Ramos, E. A., and Kaplin, B. (2020). Tour boats affect the activity patterns of bottlenose dolphins (Tursiops truncatus) in Bocas del Toro, Panama. PeerJ 8:e8804. doi: 10.7717/peerj.8804
Kavanagh, A. S., Owen, K., Williamson, M. J., Blomberg, S. P., Noad, M. J., Goldizen, A. W., et al. (2017). Evidence for the functions of surface-active behaviors in humpback whales (Megaptera novaeangliae). Mar. Mamm. Sci. 33, 313–334. doi: 10.1111/mms.12374
Kessler, M. M., and Harcourt, R. (2013). Whale watching regulation compliance trends and the implications for management off Sydney, Australia. Mar. Policy 42, 14–19. doi: 10.1016/j.marpol.2013.01.016
Kruse, S. (1991). “The interactions between killer whales and boats in Johnstone strait, B.C,” in Dolphin Societies: Discoveries and Puzzles, eds K. Pryor, and K. S. Norris (Berkeley, CA: University of California Press), 149–160.
Larson, C. L., Reed, S. E., Merenlender, A. M., and Crooks, K. R. (2016). Effects of recreation on animals revealed as widespread through a global systematic review. PLoS One 11:e0167259. doi: 10.1371/journal.pone.0167259
Leslie, H. M., Basurto, X., Nenadovic, M., Sievanen, L., Cavanaugh, K. C., Cota-Neito, J. J., et al. (2015). Operationalizing the social-ecological systems framework to assess. Proc. Natl. Acad. Sci. U.S.A. 112, 5979–5984. doi: 10.1073/pnas.1414640112
Lusseau, D., Bain, D., Williams, R., and Smith, J. C. (2009). Vessel traffic disrupts the foraging behavior of Southern Resident Killer whales Orcinus orca. Endanger Spec. Res. 6, 211–221. doi: 10.3354/ESR00154
Lusseau, D., and Bejder, L. (2007). The long-term consequences of short-term responses to disturbance experiences from whale watching impact assessment. Int. J. Comp. Psychol. 20, 228–236.
Mann, J. (1999). Behavioral sampling methods for cetaceans: A review and critique. Mar. Mam. Sci. 15, 102–122. doi: 10.1111/j.1748-7692.1999.tb00784.x
Moore, S. E., Randall, R. R., Southall, B. L., Ragen, T. J., Suydam, R. S., and Clark, C. W. (2012). A framework for assessing the effects of anthropogenic sound on marine mammals in a rapidly changing Arctic. Biomed. Sci. 62, 289–295. doi: 10.1525/bio.2012.62.3.10
Morete, M. E., Bisi, T. L., and Rosso, S. (2007). 241 Mother and calf humpback whale responses to vessels around the Abrolhos Archipelago, Bahia, Brazil. J. Cetacean Res. Manag. 9, 241–248.
Noren, D. P., Johnson, A. H., Rehder, D., and Larson, A. (2009). Close approaches by vessels elicit surface-active behaviors by Southern Resident Killer whales. Endanger Species Res. 8, 179–192. doi: 10.3354/esr00205
Noren, D. P., and Mocklin, J. (2012). Review of cetacean biopsy techniques: Factors contributing to successful sample collection and physiological and behavioral impacts. Mar. Mam. Sci. 28, 154–199. doi: 10.1111/j.1748-7692.2011.00469.x
O’Connor, S., Campbell, R., Cortez, H., and Knowles, T. (2009). Whale watching worldwide: tourism numbers, expenditures and expanding economic benefits. Int. Fund Anim. Welfare 21, 38–46.
Oña, J., Garland, E. C., and Denkinger, J. (2017). Southeastern Pacific humpback whales (Megaptera novaeangliae) and their breeding grounds: distribution and habitat preference of singers and social groups off the coast of Ecuador. Mar. Mam. Sci. 33, 219–235. doi: 10.1111/mms.12365
Pacheco, A. S., Silva, S., Alcorta, B., Balducci, N., Guidino, C., Llapapasca, M. A., et al. (2013). Aerial behavior of humpback whales Megaptera novaeangliae at the southern limit of the southeast Pacific breeding area. Rev. Biol. Mar. Oceanogr. 48, 185–191. doi: 10.3856/vol39-issue1-fulltext-20
Panama Maritime Authority (2014). Implementation of New Ships’ Routeing System in Panama. Available online at: https://panamashipregistry.com/wp-content/uploads/2020/08/MMC-304-Nov.-2014.pdf (accessed October 11, 2020).
Parsons, E. C. M. (2012). The negative impacts of whale-watching. Mar. Biol. 2012, 807294. doi: 10.1155/2012/807294
Peake, S., Innes, P., and Dyer, P. (2009). Ecotourism and conservation: factors influencing effective conservation messages. J. Sustain. Tour. 17, 107–127. doi: 10.1080/09669580802276000
Pitman, R. L., Totterdell, J. A., Fearnbach, H., Ballance, L. T., Durban, J. W., and Kemps, H. (2015). Whale killers: prevalence and ecological implications of killer whale predation on humpback whale calves off Western Australia. Mar. Mamm. Sci. 31, 629–657. doi: 10.1111/mms.12182
Pohlert, T. (2014). The pairwise multiple comparison of mean ranks package (PMCMR). R Package 27:10.
Putland, R. L., Merchant, N. D., Farcas, A., and Radford, C. A. (2018). Vessel noise cuts down communication space for vocalizing fish and marine mammals. Glob. Chang. Biol. 1, 1708–1721. doi: 10.1111/gcb.13996
Rasmussen, K., Calambokidis, J., and Steiger, G. H. (2012). Distribution and migratory destinations of humpback whales off the Pacific coast of Central America during the boreal winters of 1996–2003. Mar. Mamm. Sci. 28, E267–E279. doi: 10.1111/j.1748-7692.2011.00529.x
Rasmussen, K., Palacios, D. M., Calambokidis, J., Saborio, M. T., Dalla Rosa, L., Secchi, E. R., et al. (2007). Southern hemisphere humpback whales wintering off Central America: Insights from water temperature into the longest mammalian migration. Mar. Mamm. Sci. 33, 1035–1052. doi: 10.1111/mms.12423
Richardson, W. J., Greene, C. R., Malme, C. I., and Thomson, D. H. (1995). Marine Mammals and Noise. San Diego, CA: Academic Press. doi: 10.1016/B978-0-08-057303-8.50006-9
Rolland, R. M., Hunt, K. E., Kraus, S. D., and Wasser, S. K. (2005). Assessing reproductive status of right whales (Eubalaena glacialis) using fecal hormone metabolites. Gen. Comp. Endocrinol. 142, 308–317. doi: 10.1016/j.ygcen.2005.02.002
Rossi-Santos, M. (2016). Whale-watching noise effects on the behavior of humpback whales (Megaptera novaeangliae) in the Brazilian breeding ground. Proc. Meet. Acoust. 27:040003. doi: 10.1121/2.0000271
Schaffar, A., Madon, B., Garrigue, C., and Constantine, R. (2013). Behavioral effects of whale-watching activities on an endangered population of humpback whales wintering in New Caledonia. Endanger Species Res. 19, 245–254. doi: 10.3354/esr00466
Scheidat, M., Castro, C., Gonzalez, J., and Williams, R. (2004). Behavioural responses of humpback whales (Megaptera novaeangliae) to whale watching boats near Isla de la Plata, Machalilla National Park, Ecuador. J. Cetacean Res. Manag. 6, 63–68. doi: 10.1111/j.1748-7692.2009.00320.x
Schmidt, C. C. (2005). Economic drivers of illegal, unreported and unregulated (IUU) fishing. Int. J. Mar. Coast. Law. 20, 479–507. doi: 10.1016/j.marpol.2005.09.008
Schuler, A. R., Piwetz, S., Di Clemente, J., Steckler, D., Mueter, F., and Pearson, H. C. (2019). Humpback whale movements and behavior in response to whale-watching vessels in Juneau, AK. Front. Mar. Sci. 6:710. doi: 10.3389/fmars.2019.00710
Segre, P. S., Potvin, J., Cade, D. E., Calambokidis, J., Di Clemente, J., Fish, F. E., et al. (2020). Energetic and physical limitations on the breaching performance of large whales. eLife 9:e51760. doi: 10.7554/eLife.51760
Senigaglia, V., Christiansen, F., Bejder, L., Gendron, D., Lundquist, D., Noren, D. P., et al. (2016). Meta-analyses of whale-watching impact studies: Comparisons of cetacean responses to disturbance. Mar. Ecol. Prog. 542, 251–263. doi: 10.3354/meps11497
Shapiro, A. D. (2008). Orchestration: The Movement and Vocal Behavior of Free-Ranging Norwegian Killer Whales (Orcinus orca). Cambridge, MA: Massachusetts Institute of Technology, doi: 10.1575/1912/2421
Sitar, A., May-Collado, L., Wright, A., Peters-Burton, E., Rockwood, L., and Parsons, E. (2016). Boat operators in Bocas del Toro, Panama display low levels of compliance with national whale-watching regulations. Mar. Policy 68, 221–228. doi: 10.1016/j.marpol.2016.03.011
Sitar, A., May-Collado, L., Wright, A., Peters-Burton, E., Rockwood, L., and Parsons, E. (2017). Opinions and perspectives of the dolphin watching boat operators in Bocas del Toro, Panama. Tour. Mar. Environ. 12, 79–94. doi: 10.3727/154427316X14820977775343
Spencer, L., Shah, M., and Guha, R. (2006). Determining scale and sea state from water video. IEEE Trans. Image Process. 15, 1525–1535. doi: 10.1109/TIP.2006.871102
Stamation, K. A., Croft, D. B., Shaughnessy, P. D., Waples, K. A., and Briggs, S. V. (2007). Educational and conservation value of whale watching. Tour. Mar. Environ. 4, 41–55. doi: 10.3727/154427307784835660
Stamation, K. A., Croft, D. B., Shaughnessy, P. D., Waples, K. A., and Briggs, S. V. (2010). Behavioral responses of humpback whales (Megaptera novaeangliae) to whale-watching vessels on the southeastern coast of Australia. Mar. Mamm. Sci. 26, 98–122. doi: 10.1111/j.1748-7692.2009.00320.x
Stoeckl, N., Birtles, A., Farr, M., Mangott, A., Curnock, M., and Valentine, P. (2010). Live-aboard dive boats in the Great Barrier Reef: regional economic impact and the relative values of their target marine species. Tour. Econ. 16, 995–1018. doi: 10.5367/te.2010.0005
Stone, G. S., Flórez-González, L., and Katona, S. (1990). Whale migration record. Nature. 346, 705. doi: 10.1038/346705a0
Stone, G. S., and Yoshinaga, A. (2000). Hector’s dolphin Cephalorhynchus hectori calf mortalities may indicate new risks from boat traffic and habituation. Pac. Conserv. Biol. 6, 162–170. doi: 10.1071/PC000162
Teerlink, S., Horstmann, L., and Witteveen, B. (2018). Humpback whale (Megaptera novaeangliae) blubber steroid hormone concentration to evaluate chronic stress reponse from whale-watching vessels. Aquat. Mamm. 44, 411–425. doi: 10.1578/am.44.4.2018.411
The International Ecotourism Society (2015). What Is Ecotourism?. Available online at: ecotourism.org/what-is-ecotourism/ (accessed March 26, 2020).
Torres, L. G., Nieukirk, S. L., Lemos, L., and Chandler, T. E. (2018). Drone Up! Quantifying whale behavior from a new perspective improves observational capacity. Front. Mar. Sci. 5:319. doi: 10.3389/fmars.2018.00319
Wasser, S. K., Hunt, K. E., Brown, J. L., Cooper, K., Crockett, C. M., Bechert, U., et al. (2000). A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen. Comp. Endocrinol. 120, 260–275. doi: 10.1006/gcen.2000.7557
Wearing, S. L., Cunningham, P. A., Schweinsberg, S., and Jobberns, C. (2014). Whale watching as ecotourism: how sustainable is it? Cosmopolitan Civil Soc. 6, 38–55. doi: 10.5130/ccs.v6i1.3714
Weilgart, L. (2013). “A review of the impacts of seismic airgun surveys on marine life,” in CBD Expert Workshop on Underwater Noise and its Impacts on Marine and Coastal Biodiversity (London: Marine and Coastal Biodiversity).
Wiener, C., Bejder, L., Joohnston, D., Fawcett, L., and Wilkinson, P. (2020). Cashing in on spinners: revenue estimates of wild dolphin-swim tourism in the Hawaiian Islands. Front. Mar. Sci. 7:60. doi: 10.3389/fmars.2020.00660
Williams, R., Bain, D., Ford, J., and Trites, A. (2002). Behavioural responses of killer whales to a ‘leapfrogging’ vessel. J. Cetacean Res. Manag. 4, 305–310.
Keywords: ecotourism, Megaptera novaeangliae, disturbance, stress, behavioral ecology, animal welfare, wildlife-ecotourism
Citation: Amrein AM, Guzman HM, Surrey KC, Polidoro B and Gerber LR (2020) Impacts of Whale Watching on the Behavior of Humpback Whales (Megaptera novaeangliae) in the Coast of Panama. Front. Mar. Sci. 7:601277. doi: 10.3389/fmars.2020.601277
Received: 31 August 2020; Accepted: 23 November 2020;
Published: 18 December 2020.
Edited by:
Aldo S. Pacheco, National University of San Marcos, PeruReviewed by:
Laura J. May-Collado, University of Vermont, United StatesCopyright © 2020 Amrein, Guzman, Surrey, Polidoro and Gerber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arielle M. Amrein, YXJpZWxsZWFtcmVpbkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.