- 1Student Research Committee, Baqiyatallah University of Medical Sciences, Tehran, Iran
- 2Health Research Center, Life Style Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran
- 3Chemical Injuries Research Center, Systems Biology and Poisonings Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran
Background: Corona Virus Disease 2019 (COVID-19) has severely impacted global health, resulting in high morbidity and mortality, and overwhelming healthcare systems, particularly in Iran. Understanding reinfection is crucial as it has significant implications for immunity, public health strategies, and vaccine development. This study aims to identify rate and the risk factors associated with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) reinfection and compare the clinical course of initial infection versus reinfection in readmitted COVID-19 patients in Iran.
Methods: This retrospective cohort study was conducted from January 2020 to the end of 2022 in five hospitals in Iran. The study compared demographic and clinical data, vaccination status, and clinical outcomes between patients with reinfection (defined as a positive PCR test for SARS-CoV-2 at least 90 days after the primary admission) and a control group (patients who had an initial confirmed SARS-CoV-2 infection but were not readmitted with a positive PCR test for SARS-CoV-2 at least 90 days after their primary infection). Risk factors for reinfection were evaluated using a regression model. Propensity score matching (PSM) was used to compare post-clinical and laboratory outcomes between the matched case and control groups.
Results: Out of 31,245 patients, 153 (0.49%) experienced reinfections. The reinfection rate was significantly higher during B.1.617.2 and B.1.1.529 variant wave (p < 0.001). After multivariable regression analysis, incomplete vaccination status (OR: 1.68, 95% CI: 1.34–2.31, p = 0.021) and lack of booster vaccination (OR: 2.48, 95% CI: 1.96–3.65, p = 0.001) were the risk factors for reinfection. Furthermore, reinfection was associated with atypical COVID-19 symptoms, and shorter ICU and hospital stays (p < 0.001). The B.1.1.529 variant was significantly more common among reinfected patients (p < 0.001).
Conclusion: SARS-CoV-2 reinfections are more frequently observed during waves of novel variants and are associated with a milder clinical course and shorter hospital stays. Full vaccination and booster doses can effectively reduce the risk of SARS-CoV-2 reinfections.
1 Introduction
Coronavirus Disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has profoundly impacted global health since its emergence in late 2019 (1–4). The pandemic has led to significant morbidity and mortality worldwide, overwhelming healthcare systems and disrupting economies (5, 6). With millions of confirmed cases and deaths, COVID-19 has highlighted the vulnerability of global health infrastructures to emerging infectious diseases (7, 8). The virus’s rapid spread and high transmissibility have necessitated unprecedented public health measures, including lockdowns, social distancing, and mass vaccination campaigns, to mitigate its impact (9–11).
The pandemic has unfolded in multiple waves, each characterized by different variants of the virus, varying levels of transmissibility, and distinct patterns of morbidity and mortality (12). These waves have placed immense pressure on healthcare resources and have been associated with significant fluctuations in case numbers and healthcare demand (13). The emergence of new variants, such as B.1.617.2 and B.1.1.529, has complicated efforts to control the pandemic, with each variant posing unique challenges in terms of transmissibility, vaccine effectiveness, and disease severity (14).
In Iran, the burden of COVID-19 has been substantial, with the country experiencing several waves of infection that have strained its healthcare system. High rates of infection and mortality have been reported, particularly during the peaks of the pandemic (15). The Iranian healthcare system has faced numerous challenges, including shortages of medical supplies, overwhelmed hospitals, and difficulties in implementing public health measures (16). Despite these challenges, efforts have been made to enhance testing, treatment, and vaccination to control the spread of the virus and reduce its impact on the population.
Despite extensive global research on COVID-19, understanding reinfection, particularly its clinical implications and associated variants, remains limited. Given the high burden of COVID-19 in Iran and the evolving nature of the virus, it is crucial to investigate SARS-CoV-2 reinfection in in this specific demographic. The aim of this study was to identify the rate and the risk factors associated with SARS-CoV-2 reinfection and compare the clinical course of initial infection versus reinfection in readmitted COVID-19 patients in Iran.
2 Materials and methods
2.1 Study design
This retrospective cohort study examines the rate, risk factors, and outcomes of SARS-CoV-2 reinfection in the Islamic Republic of Iran during all waves of the COVID-19 pandemic from January 2020 to the end of 2022. Data were collected from five referral hospitals in Tehran, Tabriz, Isfahan, Kerman, and Kermanshah. Sampling was conducted through a consecutive sampling method and the study population includes patients who were diagnosed with COVID-19 through a confirmed positive reverse transcription polymerase chain reaction (RT-PCR) test during their initial hospital admission and subsequently discharged. The inclusion criteria were readmission due to reinfection. Reinfection was defined as a subsequent positive PCR test for SARS-CoV-2 at least 90 days after the initial infection following readmission, based on the World Health Organization (WHO) criteria (17). Patients who did not have a documented reinfection were assumed to be the control group. The exclusion criteria included patients with a positive RT-PCR test for SARS-CoV-2 within 90 days of the initial infection, those hospitalized with clinical symptoms of COVID-19 without a follow-up RT-PCR test, those with incomplete medical records or missing relevant data, and those transferred to hospitals outside the study sites during their treatment.
2.2 Diagnosis of SARS-CoV-2
To determine the infection status, all patients were diagnosed with COVID-19 using RT-PCR tests performed on nasopharyngeal swab samples collected at the time of hospital admission. The RT-PCR assays targeted specific genes of SARS-CoV-2 and followed protocols approved by the national health authority in Iran and the WHO guidelines to ensure high sensitivity and specificity (18). When feasible, whole-genome sequencing was performed in reference laboratories on samples with sufficient viral load, and the variant classification was based on comparison with global reference data (19). In most cases, only the infection status (positive or negative) was available, and the variant determination was performed when appropriate testing resources were available.
2.3 Data collection
Data collection was conducted using a research-made checklist by the principal investigator. Data were collected from the integrated electronic health system of the hospitals and the medial record database. Demographic data, including age, sex, and the presence of underlying medical conditions such as hypertension (HTN), diabetes mellitus (DM), cardiovascular diseases, and cancer, were collected. The vaccination status and the status of receiving the vaccine booster dose of patients was recorded, and patients were classified into two groups: those who were fully vaccinated [received two doses of vaccine (20)] and those who were not fully vaccinated. The clinical symptoms were classified into two categories based on the chief complaint of patients at admission: common symptoms of SARS-CoV-2 infection, including cough, fever, shortness of breath, sore throat, fatigue, and myalgia; and less common symptoms, such as diarrhea, joint pain, and neurological disorders (21). Laboratory parameters, including white blood cell (WBC) count, Interleukin-6 (IL-6) levels, and C-reactive protein (CRP) levels, were also gathered. Moreover, the duration of their intensive care unit (ICU) and hospital stays, as well as their mortality during the admission period, were documented. When available, data on the variant of SARS-CoV-2 responsible for the infection were also collected.
2.4 Statistical analysis
Data were analyzed using SPSS version 25. After calculating the SARS-CoV-2 reinfection rate by dividing the number of reinfection cases by the total number of admitted patients, the quantitative data were presented as mean and standard deviation, while qualitative data were expressed as frequency and percentage. Comparisons were made between the reinfection group and the control (non-reinfection) group. The independent sample t-test and Chi-square test were used to compare quantitative and qualitative data, respectively. Risk factors for reinfection were evaluated through univariable and multivariable regression models. To ensure a balanced comparison between the clinical course and clinical outcomes of the reinfected and non-reinfected groups, propensity score matching (PSM) was conducted based on baseline demographic variables, including age, sex, and underlying disease. The matching was performed in a 1:1 ratio using R software. A p-value less than 0.05 considered as significant.
2.5 Ethical considerations
The study was conducted in accordance with ethical standards and guidelines to ensure the protection and confidentiality of patient information. Informed consent was waived by the ethics committee due to the retrospective nature of the study, which involved the analysis of existing medical records without direct patient interaction. To maintain confidentiality, all patient data were anonymized and stored securely. Access to the data was restricted to the research team members who were directly involved in the study. Approval was obtained from the Ethics Committee of Baqiyatallah University of Medical Sciences (Ethics code: IR.BMSU.REC.1400.159).
3 Results
3.1 Demographic data
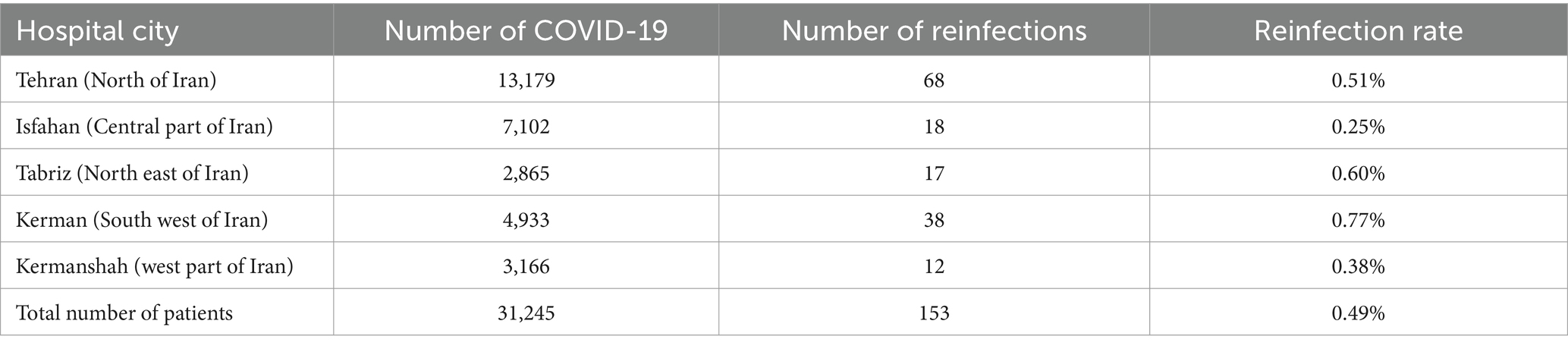
A total of 31,245 patients with confirmed cases of SARS-CoV-2 infection from five hospitals in Tehran, Isfahan, Tabriz, Kerman, and Kermanshah were included in the final analysis to determine the rate of reinfection. Of these patients, 153 (0.49%) experienced reinfections based on the study criteria. Table 1 presents the status of reinfection in different hospitals and regions of Iran. The mean age of patients with reinfection was 54.5 ± 12.5 years, while the mean age in patients without confirmed reinfection was 62.3 ± 13.6 years (p < 0.001). Among the 153 patients, 128 (83.7%) were male and 25 (16.3%) were female. The sex distribution was significantly different between the reinfection group and the control group (p < 0.001). The demographic data are presented in Table 1.
3.2 Clinical, laboratory and outcome data
A total of 112 (73.2%) patients with reinfection reported at least one underlying condition such as diabetes, obesity, pulmonary disease, or cardiovascular disease (p = 0.041). In addition, 53 patients (34.6%) in the reinfection group had a full history of vaccination, while the number of fully vaccinated patients in patients without reinfection was 18,033 patients (57.9%, p < 0.001). Reinfection cases were more likely to present with atypical symptoms, whereas in patients without reinfection, COVID-19 generally manifested with typical symptoms (p < 0.001). In the assessment of laboratory values, patients with only primary infection had significant lower WBC count compared to patients in the reinfection group (8.5 ± 2 vs. 6.5 ± 2; p < 0.001). In addition, CRP and IL-6 levels were also significantly higher in primary SARS-CoV-2 infection (50 ± 20 vs. 30 ± 20; p < 0.001; and 40 ± 18 vs. 25 ± 15; p < 0.001, respectively). Primary SARS-CoV-2 infection resulted in longer ICU admissions compared to reinfections (5 ± 4 vs. 12 ± 10; p < 0.001). Also, patients with reinfection had shorter course of overall hospital stay (7 ± 6 vs. 16 ± 21; p < 0.001). Table 2 presents the clinical data of COVID-19 between the reinfection group and the control group.

Table 2. Clinical, laboratory and outcome data of patients with reinfection of SARS-CoV-2 and the control group.
3.3 Risk factors of reinfection
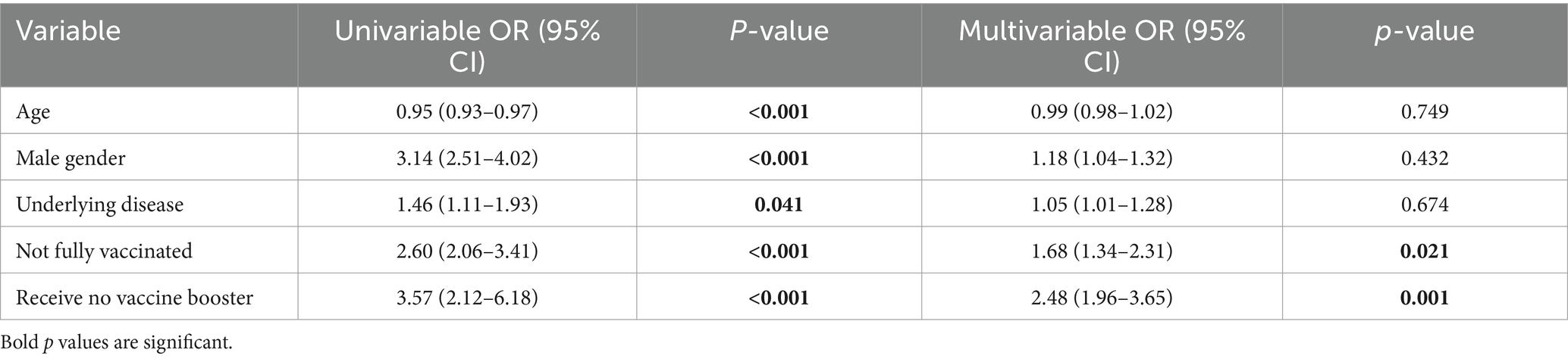
In the univariable analysis, age was found to be a significant factor, with an odds ratio (OR) of 0.95 (95% CI: 0.93–0.97; p < 0.001). Males had significantly higher odds of reinfection, with an OR of 3.14 (95% CI: 2.51–4.02; p < 0.001). The presence of an underlying disease also showed a statistically significant association with reinfection risk, with an OR of 1.46 (95% CI: 1.11–1.93; p = 0.041). Vaccination status was strongly associated with reinfection risk, with an OR of 2.60 (95% CI: 2.06–3.41; p < 0.001). Additionally, vaccine booster status was the most significant predictor in the univariable analysis, with an OR of 3.57 (95% CI: 2.12–6.18; p < 0.001). After adjusting for potential confounders in the multivariable logistic regression model, vaccination remained a significant predictor of reduced reinfection risk (OR: 1.68, 95% CI: 1.34–2.31; p = 0.021). Also, vaccine booster status continued to be the most significant factor associated with reinfection, with those who did not receive a booster having an OR of 2.48 (95% CI: 1.96–3.65; p = 0.001). The results of the regression analysis are presented in Table 3.
3.4 Propensity score matching
After propensity score matching based on age, sex, and also underlying disease, atypical symptoms were significantly higher in patients with SARS-CoV-2 reinfection (26.8% vs. 6%; p < 0.001). In addition, the level of CRP in these patients was significantly lower than in patients in the control group (30 ± 20 vs. 40 ± 15; p < 0.001). Patients in the control group had a longer course of ICU stay (6 ± 3 vs. 5 ± 4; p = 0.014) and the hospital stay was significantly longer in control group (10 ± 5 vs. 7 ± 6; p < 0.001). There were no significant differences in terms of vaccination status, O2 saturation at admission, WBC, IL-6 and also mortality during hospital stay between the two groups (p = 0.702). Table 4 suggested the results after PSM.
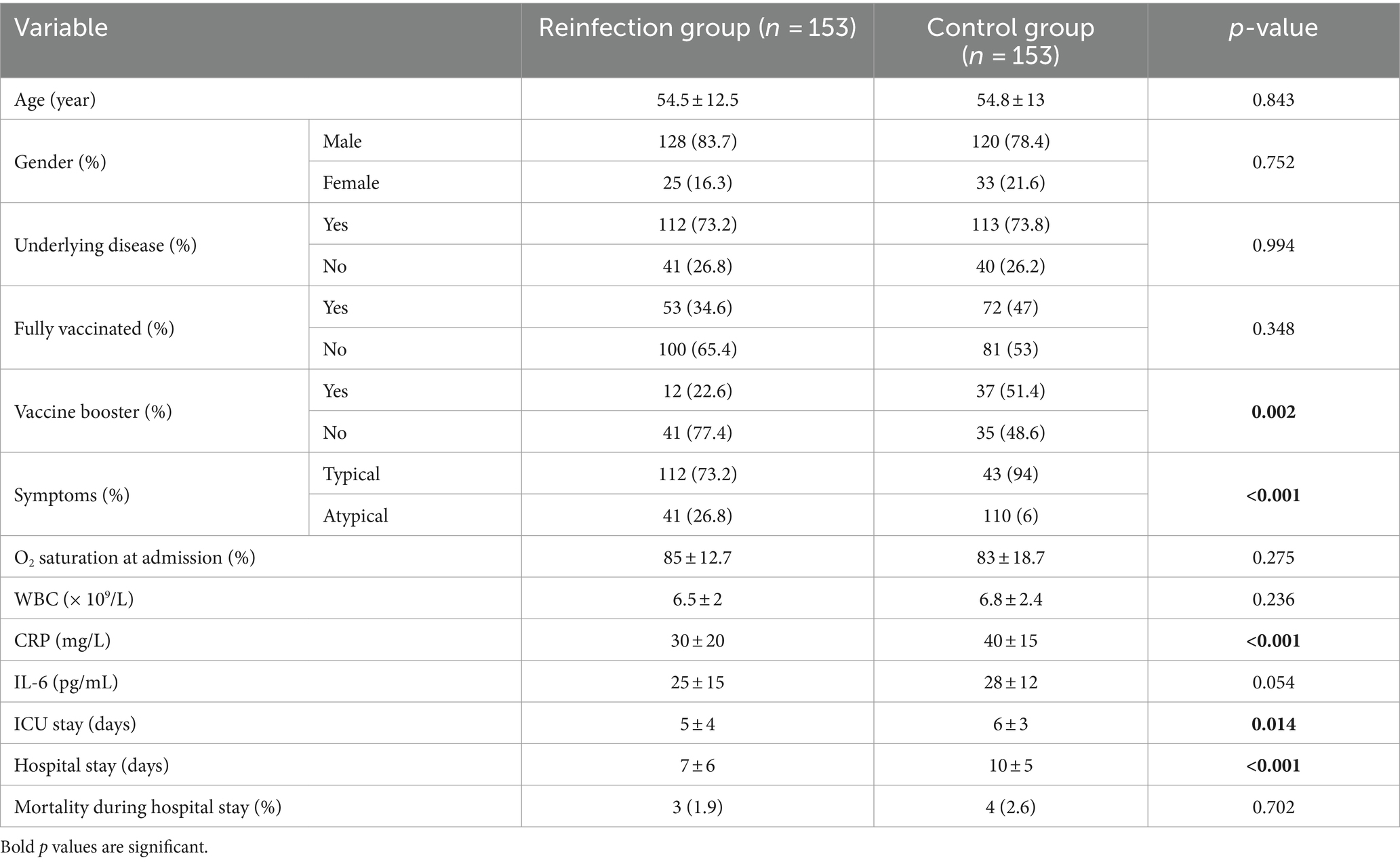
Table 4. Clinical, laboratory and outcome data of patients with reinfection of SARS-CoV-2 and the control group after PSM.
3.5 Different dates and COVID-19 waves
Table 5 and Figure 1 show the distribution of SARS-CoV-2 variants in the reinfection and control groups over three different time periods corresponding to the B.1.1.7, B.1.617.2, and B.1.1.529 waves. During the B.1.1.7 wave (December 2020–June 2021), 10.5% of reinfection cases occurred compared to 19.6% of patients in the control group. In the B.1.617.2 wave (July 2021–November 2021), 33.4% of reinfections occurred compared to 38.8% of patients in the control group. During the B.1.1.529 wave (December 2021 – end of 2022), 56.1% of reinfections were recorded, compared to 41.6% of patients in the control group. The overall distribution of SARS-CoV-2 variants in the reinfected and control groups suggests a shift toward a higher likelihood of reinfection from B.1.1.7 to B.1.1.529 wave. The chi-squared test suggests that reinfections were more likely to occur during periods of new SARS-CoV-2 variants such as the B.1.1.529 and the B.1.617.2 variants (p < 0.001).
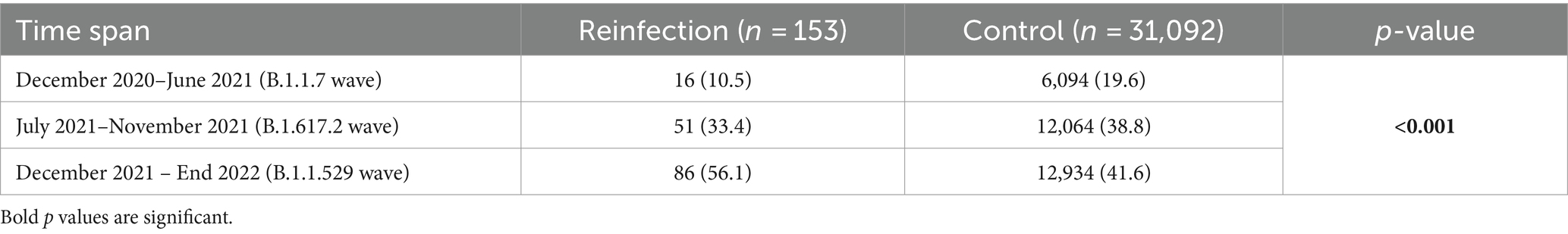
Table 5. Distribution of SARS-CoV-2 variants among reinfection and control group across different waves.
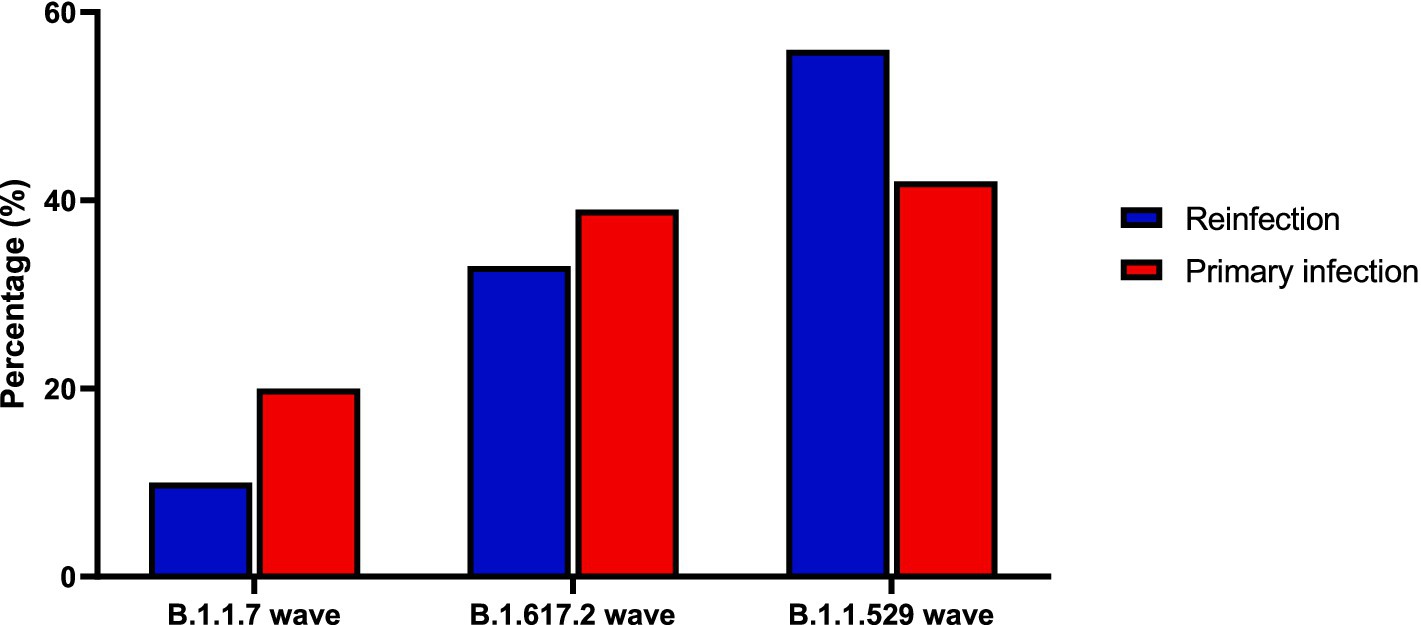
Figure 1. Distribution of SARS-CoV-2 variants among reinfection and control group across different waves.
3.6 Different SARS-CoV-2 variants
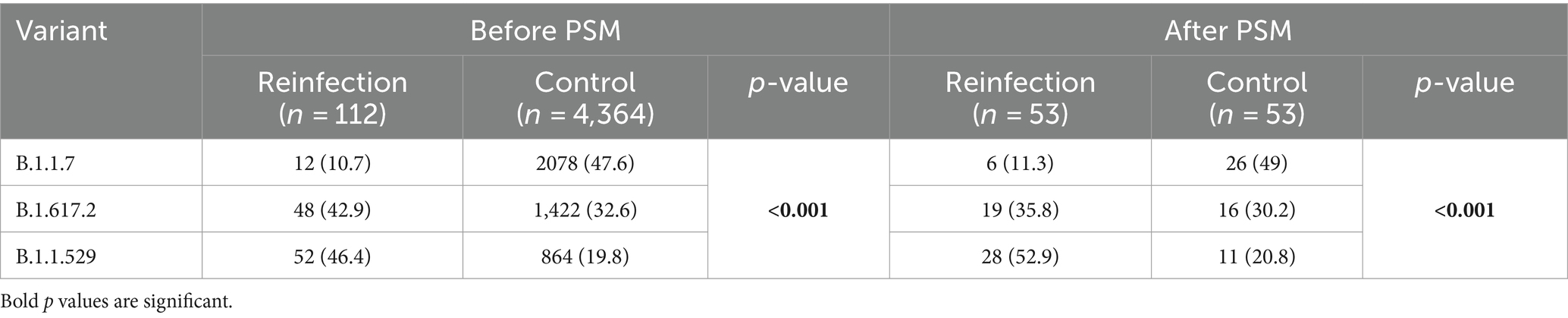
The data for SARS-CoV-2 variants were present in 112 patients (73.2%) in the reinfection group and 4,364 patients (14%) in the control group. It was suggested that patients in the control group had significantly higher distribution of B1.1.7 variant while the reinfection group had more B1.1.529 variants. After propensity score matching based on age, sex, and also underlying disease, 53 patients remained in both groups. Same as the results before PSM, the B.1.617.2 and the B.1.1.529 variants were more prevalent in the reinfection group while the B.1.1.7 variant was more prevalent in the control group (p < 0.001). The results for SARS-CoV-2 variants in both groups are presented in Table 6.
4 Discussion
The results of our study indicate that among the 0.5% of cases that met the WHO criteria for reinfection, these individuals had milder disease manifestations and a milder clinical course than those with primary infections. It has been suggested that reinfection is more pronounced in the new wave of SARS-CoV-2 variants and that full vaccination, especially booster vaccination, may be effective in preventing reinfection. It is noteworthy that the B.1.1.529 variant was more prevalent among those who had experienced reinfection. These findings contribute significantly to our understanding of virus dynamics and inform ongoing efforts in public health strategies, particularly in the context of emerging variants and vaccine efficacy.
It is crucial to distinguish between a reinfection with the SARS-CoV-2 virus that causes the disease and a return of symptoms. These two conditions can be challenging issues in clinical practice (22). The diagnosis of reinfection with SARS-CoV-2 is complex. Reinfection can sometimes be overreported based on clinical symptoms or radiological findings, and in some cases, PCR tests might be negative despite an active infection (23). In such scenarios, patients might experience a relapse of the original infection, or they could have other diseases with symptoms similar to SARS-CoV-2, such as influenza or respiratory syncytial virus (24). In addition, many individuals experiencing reinfection may not seek medical attention or may only visit outpatient clinics, thus not being captured in hospital-based data (25). The current study assumed a reinfection rate of 0.5%. This result is consistent with previous studies in the current literature. In the meta-analysis of 23 studies in 2023, the reinfection rate ranged from 0.1 to 6.8% (26). In addition, in a recent meta-analysis in 2024, the pooled rate of reinfection in 55 studies was estimated to be 0.94% (27). The observed difference in the reported rate of reinfection may be mainly due to differences in patient selection criteria. In our study, we made a concerted effort to carefully select patients in a manner that minimized the likelihood of false positives while adhering to WHO guidelines.
Our results align with previous studies suggesting that reinfections generally present with less severe outcomes compared to primary infections (28). The observation that cases of reinfection had shorter hospital and ICU stays is consistent with other reports indicating that immune responses from prior infections or vaccinations might reduce the severity of subsequent infections (29). Furthermore, the discrepancy in the predominant SARS-CoV-2 variants between reinfection and primary infection cases highlights the virus’s evolving nature and its impact on disease presentation (30). The lower frequency of full vaccination in reinfected individuals compared to the non-reinfected group also underscores the need for continued research into the effectiveness of current vaccines against emerging variants (31). Although this difference was not statistically significant following PSM, it remains critical to continue evaluating vaccine efficacy, particularly in the context of emerging variants. The effectiveness of vaccines against variants has been shown to diminish over time, necessitating booster doses to maintain protective immunity (32). Our study’s findings are consistent with the literature, which indicates that while vaccines remain effective in preventing severe disease, the evolving nature of SARS-CoV-2 variants calls for regular updates to vaccination protocols and booster recommendations (33).
The distribution of SARS-CoV-2 variants in the reinfection and control groups highlighted a clear trend toward a higher proportion of reinfections during waves dominated by newer variants. Reinfections were less frequent during the B.1.1.7 wave. This trend shifted significantly with the emergence of the B.1.617.2 variant and became even more pronounced during the B.1.1.529 wave. The increasing reinfection rate in later waves is consistent with the immune-evading properties of newer variants such as B.1.1.529, which have been shown to have higher transmissibility and reduced vaccine efficacy (34). Studies have shown that B.1.1.529, with its numerous spike protein mutations, is more adept at escaping both natural immunity and vaccine-induced immunity, leading to increased reinfection rates (35). Our analysis also demonstrated that individuals who received a vaccine booster were significantly less likely to experience reinfection, even during the B.1.1.529 wave, highlighting the positive effect of booster doses in enhancing protection against immune-evading variants. This is further supported by studies showing that booster doses can restore vaccine efficacy against variants such as B.1.1.529 (36).
The strength of our study lies in its large, representative sample of over 30,000 confirmed SARS-CoV-2 infection cases from five major hospitals in different regions of Iran, ensuring a robust analysis of reinfection patterns and outcomes. By adhering to WHO criteria for reinfection and focusing on hospitalized patients with confirmed PCR results, we ensured high diagnostic accuracy. The study’s findings of greater susceptibility to reinfection during the B.1.1.529 wave and the protective effect of booster doses are consistent with global research and underscore the importance of booster vaccination against immune-evading variants. In addition, the inclusion of regional data highlights the impact of healthcare disparities in Iran, where differences in access to resources may influence hospitalization and outcomes.
It is important to acknowledge the limitations of our study, despite its robust design. The first limitation is the reliance on PCR testing as the sole method for confirming SARS-CoV-2 infection, which may not capture all cases, particularly those with low viral loads where PCR sensitivity might be reduced. This could potentially lead to an underestimation of reinfection rates. However, by focusing on positive PCR results, we ensured diagnostic certainty and accuracy in identifying true reinfection cases. Another significant challenge encountered in this study was the limited access to diagnostic kits for SARS-CoV-2 variant identification in Iran. Due to sanctions, these kits were frequently unavailable or in short supply, which constrained our capacity to accurately identify specific variants. Additionally, many of the reinfection cases occurred after the introduction of variant-specific diagnostic kits, whereas a large portion of the control cases were from a period when new variants had not yet been identified. Consequently, variant data, particularly in the control group, may have been underrepresented. To address this imbalance and ensure a robust comparison between the two groups, we applied PSM. Another limitation is the lack of detailed data on treatment scenarios. Although there are established guidelines for COVID-19 treatment, the treatment protocols changed over the pandemic time (37). In addition, the administration of management strategies varied depending on the availability of pharmaceuticals and the limitation of resources in different cities and medical centers. The last limitation of our study is the lack of extended follow-up data. Although reinfections generally present with milder symptoms, they may be associated with severe long-term complications such as stroke, myocardial infarction, deep vein thrombosis, or pulmonary embolism (38).
This study recommends prioritizing revaccination campaigns to improve protection against reinfection with SARS-CoV-2, especially in the face of emerging immune-evading variants such as B.1.1.529. Public health strategies should be regularly updated to reflect the evolving nature of the virus, and efforts must be made to improve access to variant-specific diagnostic tools in resource-limited settings. In addition, future research should focus on longer-term follow-up to assess potential complications of reinfection, such as cardiovascular events, to provide a more comprehensive understanding of the long-term effects of SARS-CoV-2.
5 Conclusion
SARS-CoV-2 reinfection generally exhibited milder symptoms and shorter hospital stays than primary infections. Novel SARS-CoV-2 variants were more common among reinfected individuals. Although vaccination can help prevent reinfection, the complex relationship between vaccination and reinfection highlights the need for further research. Future studies are needed to assess the long-term complications of SARS-CoV-2 reinfection.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Baqiyatallah University of Medical Sciences (Ethics code: IR.BMSU.REC.1400.159). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MSh: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. YA: Formal analysis, Methodology, Software, Writing – review & editing. RY: Data curation, Resources, Writing – review & editing. MSa: Methodology, Validation, Writing – review & editing. MI: Investigation, Methodology, Validation, Writing – review & editing. MR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Thanks to guidance and advice from the “Clinical Research Development Unit of Baqiyatallah Hospital”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CRP, C-reactive protein; COVID-19, Coronavirus Disease 2019; DM, diabetes mellitus; HTN, hypertension; ICU, intensive care unit; IL-6, Interleukin-6; OR, odds ratio; PSM, propensity score matching; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; WBC, white blood cell; WHO, World Health Organization.
References
1. Shahrbaf, MA, Tabary, M, and Khaheshi, I. Cardiovascular considerations of Remdesivir and Favipiravir in the treatment of COVID-19. Cardiovasc Haematol Disord Drug Targets. (2021) 21:88–90. doi: 10.2174/1871529X21666210812103535
2. Shahrbaf, MA, Tabary, M, and Khaheshi, I. The right ventricle in COVID-19 patients. Eur Heart J. (2021) 42:559–60. doi: 10.1093/eurheartj/ehaa832
3. Barekat, M, Shahrbaf, MA, Rahi, K, and Vosough, M. Hypertension in COVID-19, a risk factor for infection or a late consequence? Cell J. (2022) 24:424. doi: 10.22074/cellj.2022.8487
4. Robat-Jazi, B, Ghorban, K, Gholami, M, Samizadeh, E, Aghazadeh, Z, Shahrbaf, MA, et al. β-D-mannuronic acid (M2000) and inflammatory cytokines in COVID-19; an in vitro study. Iran J Allergy Asthma Immunol. (2022) 21:677–86. doi: 10.18502/ijaai.v21i6.11528
5. Shahrbaf, MA, Hassan, M, and Vosough, M. COVID-19 and hygiene hypothesis: increment of the inflammatory bowel diseases in next generation? Expert Rev Gastroenterol Hepatol. (2022) 16:1–3. doi: 10.1080/17474124.2022.2020647
6. Masoumbeigi, H, Mirshafiee, A, Ghanizadeh, G, Raei, M, Saffarri, M, Arfaei, RY, et al. Evaluation of the effect of educational interventions on knowledge, attitude, and practice against COVID-19 in a residential complex in Tehran: a prospective cross-sectional study. Med J Islam Repub Iran. (2023) 37:50. doi: 10.47176/mjiri.37.50
7. Tehrani, S, Fekri, S, Demirci, H, Nourizadeh, AM, Kashefizadeh, A, Shahrbaf, MA, et al. Coincidence of candida endophthalmitis, and aspergillus and pneumocystis jirovecii pneumonia in a COVID-19 patient: case report. Ocul Immunol Inflamm. (2023) 31:1291–4. doi: 10.1080/09273948.2023.2188224
8. Zarrabi, M, Shahrbaf, MA, Nouri, M, Shekari, F, Hosseini, S-E, Hashemian, S-MR, et al. Allogenic mesenchymal stromal cells and their extracellular vesicles in COVID-19 induced ARDS: a randomized controlled trial. Stem Cell Res Ther. (2023) 14:169. doi: 10.1186/s13287-023-03402-8
9. Raei, M, Shahrbaf, MA, Salaree, MM, Yaghoubi, M, and Parandeh, A. Prevalence and predictors of burnout among nurses during the COVID-19 pandemic: a survey in teaching hospitals. Work. (2023) 77:1049–57. doi: 10.3233/WOR-220001
10. Saberi-Hamedani, M, Amiri, P, Keramatinia, A, Shahrbaf, MA, and Shekarriz-Foumani, R. The prediction of suicide ideation based on perceived social support, personality traits, and meaning of life in medical students during COVID-19 pandemic: a cross-sectional study. Int J Body Mind Cult. (2023) 10:2345–5802. doi: 10.22122/ijbmc.vi.485
11. Shahrbaf, MA, Nasr, DS, and Langroudi, ZT. COVID-19 and health promoting hospitals in Iran; what do we stand? International journal of preventive medicine. Int J Prev Med. (2022) 13:13–125. doi: 10.4103/ijpvm.ijpvm_492_21
12. El-Shabasy, RM, Nayel, MA, Taher, MM, Abdelmonem, R, Shoueir, KR, and Kenawy, ER. Three waves changes, new variant strains, and vaccination effect against COVID-19 pandemic. Int J Biol Macromol. (2022) 204:161–8. doi: 10.1016/j.ijbiomac.2022.01.118
13. Moynihan, R, Sanders, S, Michaleff, ZA, Scott, AM, Clark, J, To, EJ, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. (2021) 11:e045343. doi: 10.1136/bmjopen-2020-045343
14. Dhama, K, Nainu, F, Frediansyah, A, Yatoo, MI, Mohapatra, RK, Chakraborty, S, et al. Global emerging omicron variant of SARS-CoV-2: impacts, challenges and strategies. J Infect Public Health. (2023) 16:4–14. doi: 10.1016/j.jiph.2022.11.024
15. Heidari, M, Sayfouri, N, and Jafari, H. Consecutive waves of COVID-19 in Iran: various dimensions and probable causes. Disaster Med Public Health Prep. (2022) 17:e136. doi: 10.1017/dmp.2022.45
16. Khankeh, H, Farrokhi, M, Roudini, J, Pourvakhshoori, N, Ahmadi, S, Abbasabadi-Arab, M, et al. Challenges to manage pandemic of coronavirus disease (COVID-19) in Iran with a special situation: a qualitative multi-method study. BMC Public Health. (2021) 21:1919. doi: 10.1186/s12889-021-11973-5
17. Chisale, MRO, Sinyiza, FW, Kaseka, PU, Chimbatata, CS, Mbakaya, BC, Wu, TJ, et al. Coronavirus disease 2019 (COVID-19) reinfection rates in Malawi: a possible tool to guide vaccine prioritisation and immunisation policies. Vaccines. (2023) 11:1185. doi: 10.3390/vaccines11071185
18. Dip, SD, Sarkar, SL, Setu, MAA, Das, PK, Pramanik, MHA, Alam, A, et al. Evaluation of RT-PCR assays for detection of SARS-CoV-2 variants of concern. Sci Rep. (2023) 13:2342. doi: 10.1038/s41598-023-28275-y
19. Ntagereka, PB, Oyola, SO, Baenyi, SP, Rono, GK, Birindwa, AB, Shukuru, DW, et al. Whole-genome sequencing of SARS-CoV-2 reveals diverse mutations in circulating alpha and Delta variants during the first, second, and third waves of COVID-19 in south Kivu, east of the Democratic Republic of the Congo. Int J Infect Dis. (2022) 122:136–43. doi: 10.1016/j.ijid.2022.05.041
20. Seo, WJ, Kang, J, Kang, HK, Park, SH, Koo, HK, Park, HK, et al. Impact of prior vaccination on clinical outcomes of patients with COVID-19. Emerg Microbes Infect. (2022) 11:1316–24. doi: 10.1080/22221751.2022.2069516
21. Adhikari, SP, Meng, S, Wu, Y-J, Mao, Y-P, Ye, R-X, Wang, Q-Z, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. (2020) 9:29. doi: 10.1186/s40249-020-00646-x
22. Raveendran, AV, Jayadevan, R, and Sashidharan, S. Long COVID: an overview. Diabetes Metab Syndr. (2021) 15:869–75. doi: 10.1016/j.dsx.2021.04.007
23. Raveendran, AV . COVID-19 re-infection: diagnostic challenges and proposed diagnostic criteria. Diabetes Metab Syndr. (2021) 15:645–8. doi: 10.1016/j.dsx.2021.02.007
24. Phan, T, Tran, NYK, Gottlieb, T, Siarakas, S, and McKew, G. Evaluation of the influenza and respiratory syncytial virus (RSV) targets in the AusDiagnostics SARS-CoV-2, influenza and RSV 8-well assay: sample pooling increases testing throughput. Pathology. (2022) 54:466–71. doi: 10.1016/j.pathol.2022.02.002
25. Azam, M, Pribadi, FS, Rahadian, A, Saefurrohim, MZ, Dharmawan, Y, Fibriana, AI, et al. Incidence of COVID-19 reinfection: an analysis of outpatient-based data in the United States of America. medRxiv. (2021) 2021:7206. doi: 10.1101/2021.12.07.21267206
26. Nguyen, NN, Nguyen, YN, Hoang, VT, Million, M, and Gautret, P. SARS-CoV-2 reinfection and severity of the disease: a systematic review and Meta-analysis. Viruses. (2023) 15:967. doi: 10.3390/v15040967
27. Chen, Y, Zhu, W, Han, X, Chen, M, Li, X, Huang, H, et al. How does the SARS-CoV-2 reinfection rate change over time? The global evidence from systematic review and meta-analysis. BMC Infect Dis. (2024) 24:339. doi: 10.1186/s12879-024-09225-z
28. Deng, J, Ma, Y, Liu, Q, Du, M, Liu, M, and Liu, J. Severity and outcomes of SARS-CoV-2 reinfection compared with primary infection: a systematic review and Meta-analysis. Int J Environ Res Public Health. (2023) 20:3335. doi: 10.3390/ijerph20043335
29. de La Vega, MA, Polychronopoulou, E, Xiii, A, Ding, Z, Chen, T, Liu, Q, et al. SARS-CoV-2 infection-induced immunity reduces rates of reinfection and hospitalization caused by the Delta or omicron variants. Emerg Microbes Infect. (2023) 12:e2169198. doi: 10.1080/22221751.2023.2169198
30. Manirambona, E, Okesanya, OJ, Olaleke, NO, Oso, TA, and Lucero-Prisno, DE. Evolution and implications of SARS-CoV-2 variants in the post-pandemic era. Discover Public Health. (2024) 21:16. doi: 10.1186/s12982-024-00140-x
31. Gómez-Gonzales, W, Chihuantito-Abal, LA, Gamarra-Bustillos, C, Morón-Valenzuela, J, Zavaleta-Oliver, J, Gomez-Livias, M, et al. Risk factors contributing to reinfection by SARS-CoV-2: a systematic review. Adv Respir Med. (2023) 91:560–70. doi: 10.3390/arm91060041
32. Dadras, O, SeyedAlinaghi, S, Karimi, A, Shojaei, A, Amiri, A, Mahdiabadi, S, et al. COVID-19 Vaccines' protection over time and the need for booster doses; a systematic review. Arch Acad Emerg Med. (2022) 10:e53. doi: 10.22037/aaem.v10i1.1582
33. Hogan, AB, Doohan, P, Wu, SL, Mesa, DO, Toor, J, Watson, OJ, et al. Estimating long-term vaccine effectiveness against SARS-CoV-2 variants: a model-based approach. Nat Commun. (2023) 14:4325. doi: 10.1038/s41467-023-39736-3
34. He, X, Hong, W, Pan, X, Lu, G, and Wei, X. SARS-CoV-2 omicron variant: characteristics and prevention. MedComm. (2021) 2:838–45. doi: 10.1002/mco2.110
35. Pulliam, JRC, van Schalkwyk, C, Govender, N, von Gottberg, A, Cohen, C, Groome, MJ, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of omicron in South Africa. Science. (2022) 376:abn4947. doi: 10.1126/science.abn4947
36. Bar-On, YM, Goldberg, Y, Mandel, M, Bodenheimer, O, Amir, O, Freedman, L, et al. Protection by a fourth dose of BNT162b2 against omicron in Israel. N Engl J Med. (2022) 386:1712–20. doi: 10.1056/NEJMoa2201570
37. Wu, Y, Feng, X, Gong, M, Han, J, Jiao, Y, Li, S, et al. Evolution and major changes of the diagnosis and treatment protocol for COVID-19 patients in China 2020–2023. Health Care Sci. (2023) 2:135–52. doi: 10.1002/hcs2.45
Keywords: COVID-19, SARS-CoV-2, reinfection, vaccination, prevention
Citation: Shahrbaf M, Alimohamadi Y, Yousefi Arfaei R, Salesi M, Izadi M and Raei M (2024) Rate, risk factors, and clinical outcomes of SARS-CoV-2 reinfection vs. primary infection in readmitted COVID-19 patients in Iran: a retrospective cohort study. Front. Public Health. 12:1480805. doi: 10.3389/fpubh.2024.1480805
Edited by:
Peter Bai James, Southern Cross University, AustraliaReviewed by:
Samar Ahmed Amer, Zagazig University, EgyptJia Wei, University of Oxford, United Kingdom
Tetyana Chumachenko, Kharkiv National Medical University, Ukraine
Copyright © 2024 Shahrbaf, Alimohamadi, Yousefi Arfaei, Salesi, Izadi and Raei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Raei, mehdi_r_d@yahoo.com
 Mohammadamin Shahrbaf
Mohammadamin Shahrbaf