- Department of Nursing, College of Medicine and Health Sciences, Debre Markos University, Debre Markos, Ethiopia
Background: Assessing pain in critically ill patients who cannot communicate verbally poses significant challenges. Traditional self-report measures are ineffective for these patients, making the need for reliable observational tools crucial.
Objective: To evaluate the effectiveness, reliability, and clinical applicability of the Critical Care Pain Observation Tool (CPOT) in various intensive care unit (ICU) settings and to explore potential innovations for improving its use and integration into clinical practice.
Methods: A narrative review evaluated the Critical Care Pain Observation Tool (CPOT) for non-communicative ICU patients, comparing it to the Behavioral Pain Scale (BPS) and the FLACC scale. The review assessed CPOT's effectiveness across different ICU settings, identified limitations and challenges, and explored potential enhancements such as electronic scoring, additional physiological indicators, and improved training protocols.
Results: The CPOT has been validated as an effective pain assessment tool for non-verbal ICU patients. It evaluates pain through facial expressions, body movements, muscle tension, and ventilator compliance. The CPOT shows superior sensitivity at 76.5% compared to 62.7% for the BPS and offers a more comprehensive assessment of pain indicators like muscle tension and ventilator compliance than the FLACC scale. Despite its strengths, the CPOT has limitations, including inter-rater variability and challenges in certain patient populations. Barriers to implementation include resource constraints and the need for extensive training.
Conclusion: The Critical Care Pain Observation Tool (CPOT) is a highly effective instrument for assessing pain in non-verbal ICU patients, demonstrating superior accuracy and reliability compared to other tools like the Behavioral Pain Scale (BPS) and FLACC scale. Its detailed approach, covering facial expressions, body movements, muscle tension, and ventilator compliance, offers a detailed measure of pain. However, challenges such as inter-rater variability and limitations in specific patient populations highlight the need for ongoing refinement and research.
Introduction
Accurate pain assessment in critically ill patients, particularly those who are non-verbal, is significant challenge in critical care settings (1). These patients often lack the ability to communicate their discomfort verbally, making effective pain management critical for their overall care and recovery. Inadequate pain assessment can lead to unrecognized suffering, increased stress responses, and prolonged recovery times, ultimately impacting patient outcomes and quality of life (2).
Traditional self-report pain scales, which rely on patient communication, are not applicable for these non-verbal individuals, necessitating the development of reliable observational tools (3). The Critical Care Pain Observation Tool (CPOT) was primarily designed for use in ICU patients who are unable to self-report their pain, particularly those on mechanical ventilation. However, it can also be applied to other non-verbal ICU patients who may not be able to communicate their pain effectively, regardless of whether they are on mechanical ventilation. The tool assesses pain through observable behaviors and physiological indicators, making it versatile for various critically ill patients (2, 4).
CPOT was developed to improve pain assessment accuracy in patients who cannot self-report their discomfort, focusing on four key indicators: facial expression, body movements, muscle tension, and ventilator compliance (5, 6). The tool's development was driven by the need for a more comprehensive approach to pain evaluation in critically ill patients, particularly those who are intubated or deeply sedated (7). Validation studies have demonstrated that CPOT effectively identifies pain with high sensitivity and specificity, making it a valuable asset in critical care environments (6).
Despite its advantages, the application of CPOT in clinical practice is not without challenges. Issues such as inter-rater variability and the tool's performance in specific patient populations, including those with severe neurological impairments, have been noted (8, 9). Moreover, barriers to effective implementation, such as resource constraints and the need for extensive training, can impact the consistent use of CPOT across different ICU settings (10). This narrative review aims to evaluate CPOT's effectiveness, explore its clinical applications, and identify areas for improvement to enhance pain management in non-verbal critical care patients.
Objectives
The primary objective of this review is to identify and evaluate the existing literature on the Critical Care Pain Observation Tool (CPOT) and its applications in assessing pain among non-verbal patients within critical care settings.
Methods
Inclusion and exclusion criteria
This review included only peer-reviewed studies such as randomized controlled trials, observational studies, cohort studies, and systematic reviews that focus on the effectiveness of the Critical Care Pain Observation Tool (CPOT). Eligible studies must involve critically ill patients in intensive care settings who are unable to communicate verbally due to sedation, intubation, or severe neurological impairments resulting from various diseases process. Only studies published in English up to the year 2023 that have obtained ethical approval from relevant institutional review boards were considered. While recent studies are often prioritized for relevance, older studies can provide valuable foundational insights, historical context, or demonstrate the evolution of concepts in the field.
Conversely, the review excluded anecdotal reports, case studies, opinion pieces, editorials, and non-peer-reviewed articles. Studies focusing on patients who can communicate verbally or who are not critically ill, such as those in outpatient settings, were also excluded. Articles that do not directly assess CPOT's effectiveness or lacks relevant pain assessment outcomes were filtered out. Additionally, duplicate publications presenting overlapping data were excluded, as were studies with insufficient sample sizes or incomplete data regarding CPOT's application, reliability, or validity. Lastly, studies primarily focused on other pain assessment tools was not be considered. Similarly, studies lacking full texts and duplicate articles were also excluded. Based on these criteria, a total of 200 articles were retrieved, of which only 25 were included in the review, while 175 articles were excluded according to the specified parameters.
Search databases
To conduct a comprehensive search on a wide range of peer-reviewed literatures, several key databases were utilized, including PubMed, MEDLINE, CINAHL, Web of Science, Cochrane Library, PsycINFO and Google Scholar.
Search terms
A strategic combination of search terms was employed using Boolean operators to refine the search results. The primary search terms were included “Critical Care Pain Observation Tool,” “CPOT,” “pain assessment,” “non-verbal patients,” “pain in critically ill patients,” “behavioral pain assessment,” and “pain evaluation ICU.” This combination is designed to yield comprehensive results focusing on the tool's application in the target population.
Search strategy
The search strategy involved combining search terms with Boolean operators (AND, OR) to ensure relevant literature is captured. For example, the search was structured as follows: (“Critical Care Pain Observation Tool” OR “CPOT”) AND (“non-verbal” OR “non-communicating”) AND (“pain assessment” OR “pain evaluation”). Filters was applied to restrict study types. Additionally, reference lists from included studies were reviewed to identify any further relevant articles that may not have been captured in the initial search.
Synthesis
The final synthesis was summarized the findings to emphasize the role and effectiveness of the CPOT in pain assessment for non-verbal patients in critical care settings. This synthesis was also provided recommendations for practice based on the evidence gathered.
Overview of CPOT
Development and purpose
The Critical Care Pain Observation Tool (CPOT) was developed in response to the need for an effective pain assessment method for patients in the intensive care unit (ICU) who are unable to verbally communicate their pain. The CPOT was first introduced by Payen as a solution to the limitations of self-report tools and to address the challenges of assessing pain in non-communicative patients (11). Its development was based on the recognition that traditional pain assessment methods were inadequate for critically ill patients, who often have altered consciousness or are sedated (12). The CPOT was designed to provide a reliable and objective measure of pain through behavioral and physiological indicators, thus enhancing pain management in ICU settings (4).
The Critical Care Pain Observation Tool (CPOT) is a rigorously validated instrument designed to assess pain in non-communicative critically ill patients. Psychometric evaluations highlight its strong reliability and validity, with high inter-rater reliability (kappa coefficient of 0.80–0.90) and effective differentiation between pain and non-pain states (13, 14). Its construct validity is supported by its correlation with other pain assessment tools, and it exhibits good sensitivity and specificity for diverse patient populations (15). By focusing on observable behaviors rather than self-reported pain, the CPOT meets the complex needs of critically ill patients and ensures accurate pain assessment in critical care settings (7, 16).
Components of CPOT
The CPOT evaluates pain through four distinct behavioral indicators: facial expression, body movements, muscle tension, and ventilator compliance (for intubated patients) or vocalization (for non-intubated patients). Each component is scored from 0 to 2, with the total score ranging from 0 to 8 (2). The following descriptions and Table 1 summarize the CPOT scoring system and its interpretation (17–19).
1. Facial Expression:
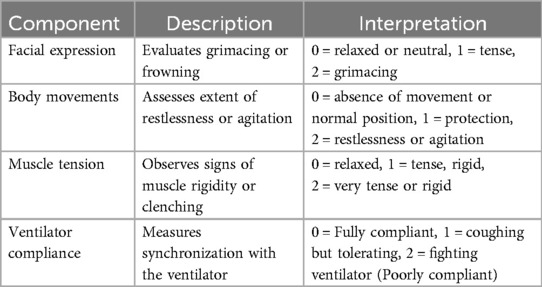
Table 1. The CPOT scoring system and interpretation for non-verbal patients in critical care (2).
Facial expression is assessed by observing the presence and intensity of grimacing or other signs of discomfort on the patient's face. Facial expressions are a key indicator of pain, with evidence showing that grimacing is a reliable marker of pain severity. A score of 0 indicates no signs of pain(relaxed or neutral), while a score of 2 reflects severe facial grimacing (2, 12).
2. Body Movements:
Body movements are evaluated based on the extent of restlessness or agitation. Increased body movements are typically associated with higher pain levels, as restlessness can be a behavioral response to pain. Scores range from 0, indicating no movement or normal position, to 2, indicating constant, severe agitation or restless (2, 5).
3. Muscle Tension:
Muscle tension is observed by noting physical signs such as clenched fists or a rigid posture. Muscle tension is a significant indicator of pain, as increased rigidity can correlate with higher pain intensity. Scores range from 0, with no muscle tension(relaxed), to 2, with severe muscle rigidity or tension (2, 5).
4. Ventilator Compliance:
Ventilator compliance for intubated patients assesses how well the patient synchronizes with the ventilator. Poor compliance or irregular breathing patterns can be indicative of pain or discomfort. This component is crucial for intubated patients, with scores ranging from 0, indicating full compliance, to 2, indicating poor compliance (2, 19, 20).
Interpretation of CPOT scores
Behavioral pain scores should be interpreted differently from the patient's self-report pain intensity scores. More specifically, behavioral scores based on the nurse's observations are associated with the behavioral dimension of pain, and the patient's self-report of pain intensity relates to the sensory dimension of pain (21). Therefore, it is important to know that behavioral pain scales only allow the detection of the presence vs. absence of pain. Generally, a COPT score 0–2 indicates no or minimal pain whereas a CPOT score higher than 2 strongly suggests the presence of pain (2).
Clinical applications of CPOT
Effectiveness in pain assessment
The CPOT has demonstrated robust effectiveness in identifying pain among ICU patients, often outperforming other pain assessment tools in terms of accuracy and reliability (22). Research indicates that the CPOT is highly effective in detecting pain in non-communicative patients, with studies showing that it consistently identifies pain levels with high sensitivity and specificity (23). Compared to other tools such as the Behavioral Pain Scale (BPS) and the FLACC scale, the CPOT has shown superior performance in distinguishing pain from other sources of discomfort, which is crucial in the complex ICU environment (17). A study found that CPOT provides a more detailed and accurate assessment of pain compared to the BPS, particularly in patients with varying levels of sedation (6).
Implementation in different settings
CPOT has been widely implemented across various ICU settings, demonstrating its versatility and adaptability. In general ICU settings, it has been employed successfully to monitor pain in critically ill patients who are unable to communicate verbally, leading to improved pain management practices (5). Its implementation extends to specialized ICUs, such as cardiac and neurocritical care units, where it has been adapted to address specific patient needs and conditions (2). For example, in cardiac ICUs, CPOT has been used to assess pain in patients recovering from major surgeries, while in neurocritical care units, modifications have been made to better accommodate patients with severe neurological impairments (23). These adaptations highlight CPOT's flexibility and effectiveness in diverse clinical environments.
Training and utilization
The integration of CPOT into clinical practice involves structured training programs for healthcare providers to ensure accurate and consistent use of the tool (24). Training typically includes educational sessions and hands-on workshops to familiarize ICU staff with CPOT's components and scoring system (11, 25). The tool's implementation has been associated with significant improvements in pain management, as it enables clinicians to make more informed decisions regarding analgesic interventions (26). Evidence suggests that CPOT facilitates better pain control and enhances overall patient comfort by providing a reliable means of assessing pain in critically ill patients (6). The consistent use of CPOT has also been linked to improved patient outcomes and a reduction in pain-related complications, emphasizing its impact on enhancing care quality in the ICU (27).
Challenges and limitations
Limitations of CPOT
Despite its effectiveness, the CPOT is not without limitations. One notable challenge is related to inter-rater reliability. Studies have shown that while CPOT is generally reliable, there can be variability in scoring between different raters, which may affect the consistency of pain assessments (28, 29). Additionally, the applicability of CPOT can be limited in certain patient populations. For instance, patients with severe neurological conditions or those under deep sedation may present challenges in interpreting some behavioral indicators, such as facial expressions and muscle tension, which may not accurately reflect their pain levels (30). Moreover, in patients with certain conditions, such as those with facial paralysis or neuromuscular disorders or patients under neuromuscular blocking agents, the facial expression component of CPOT may not be a reliable indicator of pain (31, 32). These limitations emphasize the need for ongoing validation and possible adjustments of the tool for specific patient groups.
Barriers to implementation
Implementing CPOT effectively can be hindered by several barriers. One significant challenge is resource constraints, which can limit the availability of training programs and the integration of CPOT into routine clinical practice (33, 34). In some healthcare settings, especially in low-resource environments, the lack of sufficient training and educational resources can impede the effective use of CPOT (35, 36). Additionally, resistance to adopting new tools can be another barrier; healthcare providers may be hesitant to change established pain assessment practices, especially if they are unfamiliar with CPOT or perceive it as cumbersome (37). This resistance can be mitigated through targeted education and demonstrating the tool's benefits, but overcoming initial reluctance can be a significant hurdle (38). Finally, the integration of CPOT into existing electronic health record systems and workflows may require additional adjustments and technical support, which can pose logistical challenges (39).
In conclusion, while the CPOT offers a valuable approach to pain assessment in the ICU, it is essential to address its limitations, such as variability in inter-rater reliability and challenges in certain patient populations. Additionally, overcoming barriers related to resource constraints, training, and resistance to new practices is crucial for the successful implementation and utilization of CPOT in clinical settings.
Comparisons with other tools
Comparison with other pain assessment tools
The Critical Care Pain Observation Tool (CPOT) is frequently compared with other pain assessment tools used in critical care settings, such as the Behavioral Pain Scale (BPS) and the Faces, Legs, Activity, Cry, Consolability (FLACC) scale (40). The BPS, another commonly used tool in ICUs, evaluates pain based on facial expressions, upper limb movements, and compliance with the ventilator, offering a comprehensive assessment of pain through observable behaviors (1). Research has demonstrated that CPOT and BPS are both effective in assessing pain in non-communicative patients, but CPOT generally offers a more comprehensive assessment by including muscle tension as an additional indicator (10, 22).
The FLACC scale, designed primarily for pediatric patients but also used in some adult settings, assesses pain based on facial expression, leg movement, activity, cry, and consolability (41). While the FLACC scale is beneficial for its simplicity and ease of use, CPOT is often preferred in adult critical care environments due to its more nuanced approach, particularly its focus on muscle tension and ventilator compliance, which are critical for assessing pain in intubated patients (42). Studies have indicated that CPOT may offer better sensitivity and specificity in identifying pain in patients who are deeply sedated or mechanically ventilated, where the FLACC scale might fall short (7, 43).
Advantages and disadvantages
CPOT has several advantages over other tools like BPS and FLACC. One key advantage is its inclusion of muscle tension and ventilator compliance as indicators, which can provide a more detailed picture of pain, especially in mechanically ventilated patients (44). This additional detail can enhance pain assessment accuracy in critically ill patients whose pain responses may be less overt or complicated by their medical conditions (45). Additionally, CPOT's focus on observable behaviors and physiological responses helps mitigate the challenges associated with self-reporting tools and provides a reliable method for pain assessment in patients who cannot communicate (24).
However, CPOT is not without its disadvantages. One limitation is that it requires thorough training to ensure accurate and consistent application, which can be resource-intensive (35). Furthermore, while CPOT addresses some of the limitations of the BPS and FLACC, it may still face challenges in certain patient populations, such as those with severe neurological impairments where facial expressions or muscle tension might not accurately reflect pain (40). Additionally, inter-rater variability can be a concern, as with other observational tools, potentially affecting the reliability of pain assessments (46).
In summary, CPOT offers a more comprehensive approach to pain assessment in critical care compared to the BPS and FLACC scale, particularly through its inclusion of muscle tension and ventilator compliance. However, it also has limitations related to training requirements and applicability in certain patient populations, which need to be addressed to maximize its effectiveness in clinical practice.
Potential innovations
There are several potential innovations that could enhance the CPOT and pain assessment practices in critical care. One promising area is the integration of technology to improve the accuracy and ease of CPOT administration. For instance, developing electronic versions of CPOT that incorporate automated scoring and real-time data analysis could reduce the burden on healthcare providers and minimize inter-rater variability (47). Studies have shown that electronic tracking can provide more accurate and timely data, reduce reliance on memory, and minimize biases associated with recalling past events, making it a more reliable option than paper-based records (47). This innovation enhances efficiency and aligns with the growing trend of digital transformation in healthcare, including critical care (48).
Advances in artificial intelligence(AI) and machine learning could further refine CPOT by analyzing patterns in pain indicators, enabling more precise pain assessments and predictive analytics (49). Research indicates that AI algorithms can identify subtle changes in patient data that human observers might miss, potentially leading to more timely interventions (50). By integrating these technologies, healthcare providers could enhance pain management protocols and improve patient outcomes.
Another innovation could involve expanding CPOT's behavioral indicators to include additional physiological indicators, such as blood pressure, heart rate and respiratory rate variability or biomarkers, which may offer a more comprehensive assessment of pain (51). While vital sign values generally increase during painful procedures, their effectiveness in the pain assessment remains questionable (52, 53). However, an integrated review study indicated that incorporating a wider range of physiological indicators could improve the reliability of pain evaluation, fostering a more holistic approach to patient care (54).
Furthermore, incorporating patient feedback mechanisms, where feasible, could improve the tool's responsiveness and accuracy by aligning it more closely with patient experiences and reported pain levels (55). Lastly, enhancing training programs with simulation-based learning and ongoing support could improve the consistency and reliability of CPOT use among clinicians (56).
Limitation of the study
Despite the review's thorough evaluation, several limitations exist. The reliance on existing literature may overlook details in CPOT's use across specialized or diverse patient populations, such as those with severe neurological impairments or in pediatric and geriatric ICUs. Variability in training and implementation practices could affect CPOT's consistency and effectiveness. Additionally, the review does not include firsthand user experiences, potentially missing practical challenges.
Discussion
One study compared the sensitivity of CPOT and BPS in mechanically ventilated patients showed that CPOT is more sensitive (76.5%) than BPS (62.7%). However, Both the CPOT and the BPS demonstrated strong criterion and discriminant validity (57). Additionally, a study on the validity and reliability of the CPOT revealed that its inter-rater reliability (IRR) ranged from fair to almost perfect, indicating that while the instrument's reliability is acceptable, it may vary. Conversely, the discriminant validity (DV) demonstrated a significant difference in mean scores between noxious (painful) and non-noxious (non-painful) procedures (20).
In other studies, the CPOT demonstrated moderate to high inter-rater reliability, as assessed by the Kappa coefficient from two or more raters. The values ranged from 0.79 to 0.94 across three studies (40, 58, 59). This strong agreement among assessors emphasizes CPOT's reliability as an observational tool in critical care settings. The authors noted that the tool's design allows for nuanced assessment through multiple indicators, including facial expressions, body movements, and muscle tension, and either compliance with ventilator (for intubated patients) or vocalization (for non-intubated patients which contribute to its superior performance in pain identification compared to traditional scales.
The Critical Pain Observation Tool (CPOT) provides a more comprehensive approach to pain assessment in critical care settings compared to the Behavioral Pain Scale (BPS) and the FLACC scale. One of the key advantages of CPOT is its incorporation of specific indicators such as muscle tension and ventilator compliance, which are particularly relevant for critically ill patients. By assessing muscle tension, CPOT captures a crucial aspect of pain expression that may not be fully represented in other scales. Additionally, ventilator compliance reflects the patient's ability to respond to pain stimuli while on mechanical ventilation, allowing for a more nuanced understanding of their pain experience. This multidimensional approach enhances the accuracy and effectiveness of pain assessment, ultimately leading to better pain management strategies for non-communicative patients in the ICU (2, 60, 61).
The CPOT has demonstrated considerable versatility and applicability across various ICU settings, specialized units such as cardiac and neurocritical care. In neurocritical care units, for instance, modifications to the tool have been implemented to better accommodate patients with severe neurological impairments. This adaptability underscores CPOT's potential to enhance pain assessment and management in diverse clinical contexts. By tailoring the tool to meet the specific needs of vulnerable populations, CPOT ensures that even those patients who are least able to communicate their pain receive the appropriate care they require. This capacity for customization not only improves pain management outcomes but also contributes to a more patient-centered approach in critical care settings (2, 5, 23).
Conclusion
This review has highlighted the Critical Care Pain Observation Tool (CPOT) as a valuable instrument for pain assessment in critical care settings. The CPOT has demonstrated robust effectiveness in identifying pain among non-communicative ICU patients, often surpassing other tools such as the Behavioral Pain Scale (BPS) and the FLACC scale in terms of accuracy and reliability. Its comprehensive approach, which includes facial expression, body movements, muscle tension, and ventilator compliance, provides a nuanced and reliable measure of pain, especially in mechanically ventilated and deeply sedated patients. Despite its strengths, the CPOT faces limitations such as inter-rater variability and challenges in specific patient populations, which necessitate further refinement and research.
Clinical implications
The clinical implications of using CPOT in the ICU are significant. The tool facilitates more accurate and objective pain assessment, which is crucial for effective pain management in critically ill patients who are unable to communicate their pain verbally. By improving pain detection and management, CPOT enhances patient comfort, potentially reduces pain-related complications, and contributes to overall better patient outcomes. The integration of CPOT into routine practice supports a more systematic approach to pain management, ensuring that pain is adequately assessed and addressed in a vulnerable patient population.
Integration of CPOT into routine practice
Integrating the Critical Care Pain Observation Tool (CPOT) into routine practice in the ICU is essential for optimizing pain management in non-verbal patients. This process requires comprehensive training for ICU staff to ensure familiarity with CPOT's components and scoring system. Developing standardized protocols promotes consistent use, while engaging a multidisciplinary team fosters collaboration in pain assessment. Incorporating CPOT into electronic health records facilitates documentation and real-time monitoring of pain trends. Regular audits and feedback encourage adherence and highlight the tool's impact on care. Involving patients’ families in discussions about pain management provides valuable insights, and a framework for continuous quality improvement ensures ongoing evaluation and adaptation of the tool based on staff and patient feedback.
Recommendations
To optimize the use of the Critical Care Pain Observation Tool (CPOT) in critical care settings, it is crucial to continue research aimed at enhancing its sensitivity and specificity, particularly for diverse patient populations such as those with severe neurological impairments or deep sedation. Future studies should focus on refining CPOT's indicators and evaluating its effectiveness in specialized ICUs like pediatric or geriatric units. Additionally, exploring technological advancements, such as electronic versions with automated scoring, could reduce inter-rater variability. Comprehensive training programs incorporating simulation-based learning are essential to ensure consistent application and improve clinician proficiency.
Author contributions
AA: Conceptualization, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
I extend my gratitude to Debre Markos University, College of Medicine and Health Sciences, for their support, and to our colleagues for their invaluable feedback.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Payen J-F, Bru O, Bosson J-L, Lagrasta A, Novel E, Deschaux I, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. (2001) 29(12):2258–63. doi: 10.1097/00003246-200112000-00004
2. Urden LD, Stacy KM, Lough ME. Critical Care Nursing-e-Book: Diagnosis and Management. St. Louis, Missouri: Elsevier Health Sciences (2017).
3. Puntillo K. Pain assessment and management in the critically ill: wizardry or science? Am J Crit Care. (2003) 12(4):310–6. doi: 10.4037/ajcc2003.12.4.310
4. Stamp R, Tucker L, Tohid H, Gray R. Reliability and validity of the critical-care pain observation tool: a rapid synthesis of evidence. J Nurs Meas. (2018) 26(2):1–2. doi: 10.1891/1061-3749.26.2.378
5. Gélinas C, Fillion L, Puntillo KA, Viens C, Fortier M. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. (2006) 15(4):420–7. doi: 10.4037/ajcc2006.15.4.420
6. Gélinas C. Nurses’ evaluations of the feasibility and the clinical utility of the critical-care pain observation tool. Pain Manag Nurs. (2010) 11(2):115–25. doi: 10.1016/j.pmn.2009.05.002
7. Buttes P, Keal G, Cronin SN, Stocks L, Stout C. Validation of the critical-care pain observation tool in adult critically ill patients. Dimens Crit Care Nurs. (2014) 33(2):78–81. doi: 10.1097/DCC.0000000000000021
8. Echegaray-Benites C, Kapoustina O, Gélinas C. Validation of the use of the critical-care pain observation tool (CPOT) with brain surgery patients in the neurosurgical intensive care unit. Intensive Crit Care Nurs. (2014) 30(5):257–65. doi: 10.1016/j.iccn.2014.04.002
9. Gélinas C, Bérubé M, Puntillo KA, Boitor M, Richard-Lalonde M, Bernard F, et al. Validation of the critical-care pain observation tool-neuro in brain-injured adults in the intensive care unit: a prospective cohort study. Crit Care. (2021) 25:1–15. doi: 10.1186/s13054-021-03561-1
10. Rijkenberg S, Stilma W, Endeman H, Bosman R, Oudemans-van Straaten H. Pain measurement in mechanically ventilated critically ill patients: behavioral pain scale versus critical-care pain observation tool. J Crit Care. (2015) 30(1):167–72. doi: 10.1016/j.jcrc.2014.09.007
11. Storsveen A-M, Hall-Lord M-L. The CPOT–a tool for pain assessment for intensive care patients. Sykepleien (2016) 6:1–4. doi: 10.4220/Sykepleienf.2016.59668en.
12. Gélinas C, Johnston C. Pain assessment in the critically ill ventilated adult: validation of the critical-care pain observation tool and physiologic indicators. Clin J Pain. (2007) 23(6):497–505. doi: 10.1097/AJP.0b013e31806a23fb
13. Ross M, Boitor M, Gélinas C. Validation of the critical-care pain observation tool with seriously ill patients. J Hosp Palliat Nurs. (2016) 18(5):413–20. doi: 10.1097/NJH.0000000000000266
14. Gélinas C, Joffe AM, Szumita PM, Payen J-F, Bérubé M, Shahiri T S, et al. A psychometric analysis update of behavioral pain assessment tools for noncommunicative, critically ill adults. AACN Adv Crit Care. (2019) 30(4):365–87. doi: 10.4037/aacnacc2019952
15. Gélinas C, Puntillo KA, Levin P, Azoulay E. The behavior pain assessment tool for critically ill adults: a validation study in 28 countries. Pain. (2017) 158(5):811–21. doi: 10.1097/j.pain.0000000000000834
16. Dale CM, Prendergast V, Gélinas C, Rose L. Validation of the critical-care pain observation tool (CPOT) for the detection of oral-pharyngeal pain in critically ill adults. J Crit Care. (2018) 48:334–8. doi: 10.1016/j.jcrc.2018.09.024
17. Zhai Y, Cai S, Zhang Y. The diagnostic accuracy of critical care pain observation tool (CPOT) in ICU patients: a systematic review and meta-analysis. J Pain Symptom Manage. (2020) 60(4):847–56.e13. doi: 10.1016/j.jpainsymman.2020.06.006
18. Rafiei M, Ghadami A, Irajpour A, Feizi A. Validation of critical care pain observation tool in patients hospitalized in surgical wards. Iran J Nurs Midwifery Res. (2016) 21(5):464–9. doi: 10.4103/1735-9066.193391
19. Li MM, Ocay DD, Larche CL, Vickers K, Saran N, Ouellet JA, et al. Validation of the critical-care pain observation tool (CPOT) in pediatric patients undergoing orthopedic surgery. Canad J Pain. (2023) 7(1):2156332. doi: 10.1080/24740527.2022.2156332
20. Keane KM. Validity and reliability of the critical care pain observation tool: a replication study. Pain Manag Nurs. (2013) 14(4):e216–e25. doi: 10.1016/j.pmn.2012.01.002
21. Bouajram RH, Sebat CM, Love D, Louie EL, Wilson MD, Duby JJ. Comparison of self-reported and behavioral pain assessment tools in critically ill patients. J Intensive Care Med. (2020) 35(5):453–60. doi: 10.1177/0885066618757450
22. Gomarverdi S, Sedighie L, Seifrabiei MA, Nikooseresht M. Comparison of two pain scales: behavioral pain scale and critical-care pain observation tool during invasive and noninvasive procedures in intensive care unit-admitted patients. Iran J Nurs Midwifery Res. (2019) 24(2):151–5. doi: 10.4103/ijnmr.IJNMR_47_18
23. Gélinas C, Harel F, Fillion L, Puntillo KA, Johnston CC. Sensitivity and specificity of the critical-care pain observation tool for the detection of pain in intubated adults after cardiac surgery. J Pain Symptom Manage. (2009) 37(1):58–67. doi: 10.1016/j.jpainsymman.2007.12.022
24. Gélinas C, Arbour C, Michaud C, Vaillant F, Desjardins S. Implementation of the critical-care pain observation tool on pain assessment/management nursing practices in an intensive care unit with nonverbal critically ill adults: a before and after study. Int J Nurs Stud. (2011) 48(12):1495–504. doi: 10.1016/j.ijnurstu.2011.03.012
25. Siddiqui AS, Ahmed A, Rehman A, Afshan G. Pain assessment in intensive care units of a low-middle income country: impact of the basic educational course. BMC Med Educ. (2023) 23(1):567. doi: 10.1186/s12909-023-04523-7
26. Modanloo M, Mohsenpour A, Rahmani H, Moghaddam S, Khoddam H. Impact of implementing the critical care pain observation tool on nurses’ performance in assessing and managing pain in the critically ill patients. Indian J Crit Care Med. (2019) 23(4):165. doi: 10.5005/jp-journals-10071-23146
27. Kouhi F, Froutan R, Moghaddam AB. Investigating the effect of the CPOT-based pain management program on the pain intensity and dose adjustment of analgesics in mechanically ventilated patients: a randomized clinical trial. Nurs Pract Today. (2023) 10:215–6. doi: 10.18502/npt.v10i3.13430
28. Emsden C, Schäfer UB, Denhaerynck K, Grossmann F, Frei IA, Kirsch M. Validating a pain assessment tool in heterogeneous ICU patients: is it possible? Nurs Crit Care. (2020) 25(1):8–15. doi: 10.1111/nicc.12469
29. Chookalayia H, Heidarzadeh M, Hassanpour-Darghah M, Aghamohammadi-Kalkhoran M, Karimollahi M. The critical care pain observation tool is reliable in non-agitated but not in agitated intubated patients. Intensive Crit Care Nurs. (2018) 44:123–8. doi: 10.1016/j.iccn.2017.07.012
30. Cade CH. Clinical tools for the assessment of pain in sedated critically ill adults. Nurs Crit Care. (2008) 13(6):288–97. doi: 10.1111/j.1478-5153.2008.00294.x
31. Komlakh K, Hatefi M, Soltany B. Comparison of pain score in patients with brain disorders using care pain observation tool (CPOT) and nonverbal pain scale (NVPS). Arch Neurosci. (2022) 9(4):3–4. doi: 10.5812/ans-123099
32. Gélinas C, Arbour C. Behavioral and physiologic indicators during a nociceptive procedure in conscious and unconscious mechanically ventilated adults: similar or different? J Crit Care. (2009) 24(4):628. e7–.e17. doi: 10.1016/j.jcrc.2009.01.013
33. Chaleewong N, Chaiviboontham S, Christensen M. Knowledge, attitudes, and perceived barriers regarding pain assessment and management among Thai critical care nurses: a cross-sectional study. Intensive Crit Care Nurs. (2024) 84:103764. doi: 10.1016/j.iccn.2024.103764
34. Rababa M, Al-Sabbah S, Hayajneh AA, Al-Rawashdeh S. Critical care nurses’ perceived barriers and enablers of pain assessment and management. Pain Manag. (2023) 13(2):105–14. doi: 10.2217/pmt-2022-0075
35. Afshan G, Siddiqui AS. Pain assessment in critically ill patients in low resource countries. Anaesth Pain Intensive Care. (2018) 22:S83–S7. doi: 10.35975/apic.v22i1.1107
37. Franck LS, Bruce E. Putting pain assessment into practice: why is it so painful? Pain Res Manag. (2009) 14(1):13–20. doi: 10.1155/2009/856587
38. Gélinas C, Ross M, Boitor M, Desjardins S, Vaillant F, Michaud C. Nurses’ evaluations of the CPOT use at 12-month post-implementation in the intensive care unit. Nurs Crit Care. (2014) 19(6):272–80. doi: 10.1111/nicc.12084
39. Dorji KD. Implementation and Evaluation of Critical Care Pain Observation Tool (CPOT). California: Touro University California (2019).
40. Chanques G, Pohlman A, Kress JP, Molinari N, De Jong A, Jaber S, et al. Psychometric comparison of three behavioural scales for the assessment of pain in critically ill patients unable to self-report. Crit Care. (2014) 18:1–12. doi: 10.1186/cc14000
41. Malviya S, Voepel-Lewis T, Burke C, Merkel S, Tait AR. The revised FLACC observational pain tool: improved reliability and validity for pain assessment in children with cognitive impairment. Pediatr Anesth. (2006) 16(3):258–65. doi: 10.1111/j.1460-9592.2005.01773.x
42. Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. (1997) 23(3):293–7.9220806
43. Hora T, Alves IGN. Scales for the assessment of pain in the intensive care unit. Systematic review. BrJP. (2020) 3(3):263–74. doi: 10.5935/2595-0118.20200043
44. Vázquez M, Pardavila MI, Lucia M, Aguado Y, Margall M, Asiain MC. Pain assessment in turning procedures for patients with invasive mechanical ventilation. Nurs Crit Care. (2011) 16(4):178–85. doi: 10.1111/j.1478-5153.2011.00436.x
45. Pinheiro A, Marques RMD. Behavioral pain scale and critical care pain observation tool for pain evaluation in orotracheally tubed critical patients. A systematic review of the literature. Rev Bras Ter Intensiva. (2020) 31:571–81. doi: 10.5935/0103-507X.20190070
46. Aktaş YY, Karabulut N. A Turkish version of the critical-care pain observation tool: reliability and validity assessment. J Perianesth Nurs. (2017) 32(4):341–51. doi: 10.1016/j.jopan.2015.12.015
47. Marceau LD, Smith LD, Jamison RN. Electronic pain assessment in clinical practice. Pain Manag. (2011) 1(4):325–36. doi: 10.2217/pmt.11.32
48. Vogt C, Gersch M, Spies C, Bengler K. Digital transformation in healthcare: how the potential of digital health is tackled to transform the care process of intensive care patients across all healthcare sectors. Digit Cases. (2019):343–61. doi: 10.1007/978-3-319-95273-4_18
49. Nerella S, Guan Z, Siegel S, Zhang J, Khezeli K, Bihorac A, et al. AI-enhanced intensive care unit: revolutionizing patient care with pervasive sensing. arXiv Preprint ArXiv. (2023):230306252. doi: 10.48550/arXiv.2303.06252
50. Zhang M, Zhu L, Lin S-Y, Herr K, Chi C-L, Demir I, et al. Using artificial intelligence to improve pain assessment and pain management: a scoping review. J Am Med Inform Assoc. (2023) 30(3):570–87. doi: 10.1093/jamia/ocac231
51. Yetwin AK, Mahrer NE, Bell TS, Gold JI. Heart rate variability biofeedback therapy for children and adolescents with chronic pain: a pilot study. J Pediatr Nurs. (2022) 66:151–9. doi: 10.1016/j.pedn.2022.06.008
52. Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJ, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. (2018) 46(9):e825–e73. doi: 10.1097/CCM.0000000000003299
53. Gélinas C. Pain assessment in the critically ill adult: recent evidence and new trends. Intensive Crit Care Nurs. (2016) 34:1–11. doi: 10.1016/j.iccn.2016.03.001
54. Arbour C, Gélinas C. Behavioral and physiologic indicators of pain in nonverbal patients with a traumatic brain injury: an integrative review. Pain Manag Nurs. (2014) 15(2):506–18. doi: 10.1016/j.pmn.2012.03.004
55. Erdek MA, Pronovost PJ. Improving assessment and treatment of pain in the critically ill. Int J Qual Health Care. (2004) 16(1):59–64. doi: 10.1093/intqhc/mzh010
56. Andrews LB, Barta L. Simulation to teach pharmacist’s patient care planning and assessment of pain and agitation management in critical care. In: Andrews LB, Barta L, editors. International Meeting on Simulation in Healthcare (IMSH 2021) Technical Proceedings. NJ, United States: Rutgers University (2021).
57. Severgnini P, Pelosi P, Contino E, Serafinelli E, Novario R, Chiaranda M. Accuracy of critical care pain observation tool and behavioral pain scale to assess pain in critically ill conscious and unconscious patients: prospective, observational study. J Intensive Care. (2016) 4:1–8. doi: 10.1186/s40560-016-0192-x
58. Nürnberg Damström D, Saboonchi F, Sackey P, Björling G. A preliminary validation of the Swedish version of the critical-care pain observation tool in adults. Acta Anaesthesiol Scand. (2011) 55(4):379–86. doi: 10.1111/j.1399-6576.2010.02376.x
59. Linde SM, Badger JM, Machan JT, Beaudry J, Brucker A, Martin K, et al. Reevaluation of the critical-care pain observation tool in intubated adults after cardiac surgery. Am J Crit Care. (2013) 22(6):491–7. doi: 10.4037/ajcc2013700
60. Sole ML, Klein DG, Moseley MJ. Introduction to Critical Care Nursing E-Book: Introduction to Critical Care Nursing E-Book. St. Louis, Missouri: Elsevier Health Sciences (2020).
Keywords: pain evaluation, non-verbal patient, critical care, critical care pain observation tool, clinical applications
Citation: Afenigus AD (2024) Evaluating pain in non-verbal critical care patients: a narrative review of the critical care pain observation tool and Its clinical applications. Front. Pain Res. 5:1481085. doi: 10.3389/fpain.2024.1481085
Received: 15 August 2024; Accepted: 30 September 2024;
Published: 15 October 2024.
Edited by:
William K. Schmidt, NorthStar Consulting, LLC, United StatesReviewed by:
Dmytro Dmytriiev, National Pirogov Memorial Medical University, UkraineStephen Henry, University of California, Davis, United States
Copyright: © 2024 Afenigus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abebe Dilie Afenigus, YWJleGRtdUBnbWFpbC5jb20=; YWJlYmVfZGlsaWVAZG11LmVkdS5ldA==
 Abebe Dilie Afenigus
Abebe Dilie Afenigus