- 1Department of Minimal-Invasive Intervention, the Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China
- 2Department of Minimally Invasive Interventional Radiology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Cancer for Cancer Medicine, Guangzhou, China
Objective: To explore the relationship between plasma arginase-1 (ARG1) and early transarterial chemoembolization (TACE) refractoriness in patients with hepatocellular carcinoma (HCC) and develop nomograms for predicting early TACE refractoriness.
Methods: A total of 200 patients with HCC, treated with TACE, were included in the study, including 120 in the training set and 80 in the validation set. Pre-treatment enzyme-linked immunosorbent assay was used to detected the plasma ARG1 levels of the patient, and independent predictors of early TACE refractoriness were determined using a multivariate logistic regression model, based on which a predictive model was developed using a nomogram.
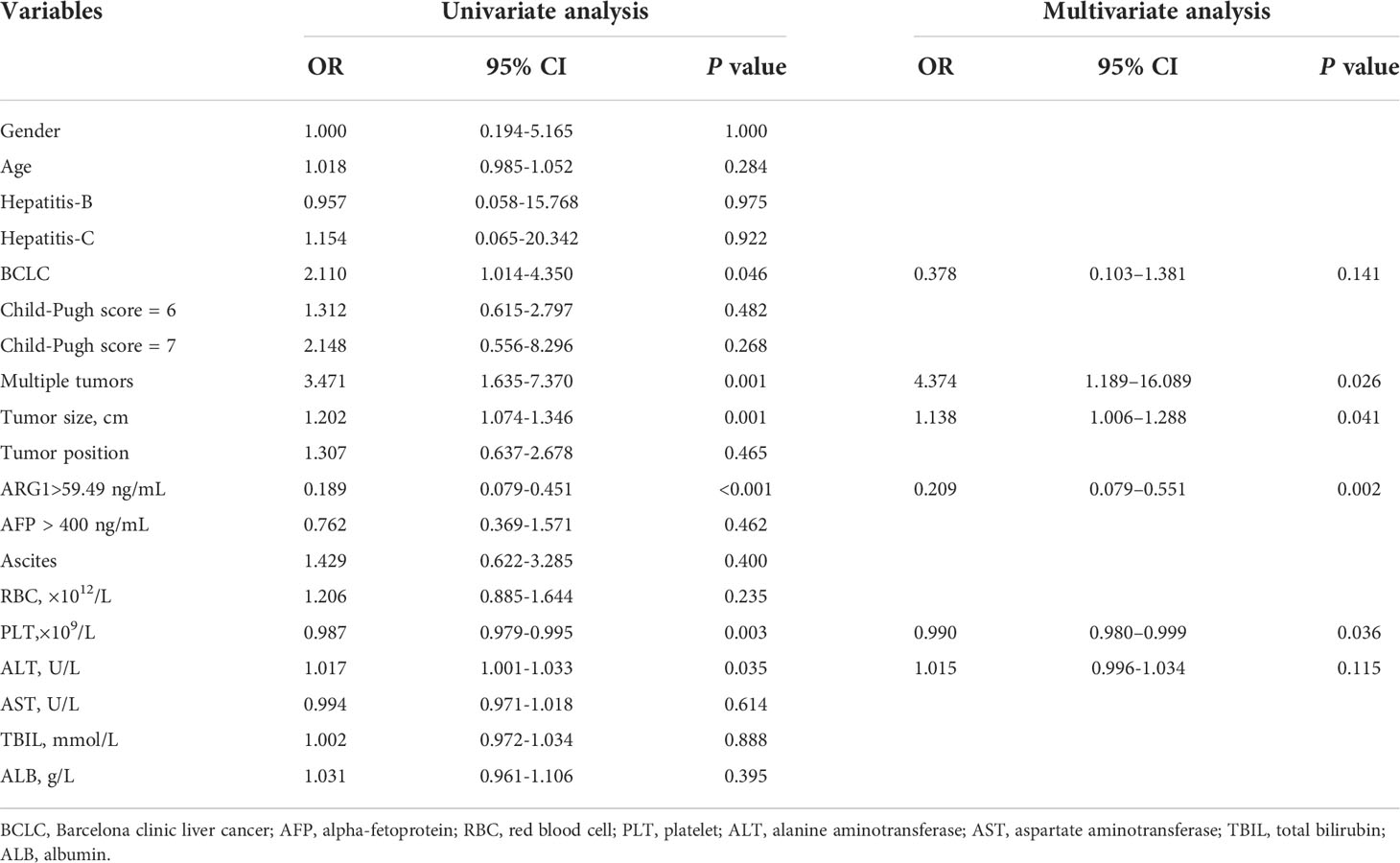
Results: Risk of early TACE refractoriness was negatively correlated with plasma ARG1 levels, and multivariate logistic analysis showed tumour size (OR = 1.138, 95% CI = 1.006-1.288, P = 0.041), multiple tumors (OR=4.374, 95% CI = 1.189-16.089, P = 0.026), platelet count (OR = 0.990, 95% CI = 0.980-0.999, P = 0.036), and plasma ARG1 levels (OR = 0.209, 95% CI = 0.079-0.551, P = 0.002) to be independent prognostic factors for early TACE refractoriness.The AUC value for the nomogram of the training cohort was 0.786 (95% CI = 0.702–0.870), and the validation set AUC value was 0.833 (95% CI = 0.791-0.875).The decision curve analysis suggested that the nomogram had good clinical utility.
Conclusion: High plasma ARG1 expression was associated with a lower incidence of early TACE refractoriness. The nomogram constructed based on four independent prognostic factors could facilitate an individualised prediction of the incidence of early TACE refractoriness.
Introduction
Hepatocellular carcinoma (HCC) is estimated to be one of the most common malignant tumors in the world and one of the most common causes of cancer-related death (1, 2). Intrahepatic metastasis and multi-center origin are the biological characteristics of HCC, which has an insidious onset and a high degree of malignancy (3). According to the Barcelona Clinic Liver Cancer (BCLC) and European Society for Medical Oncology guidelines, transarterial chemoembolization (TACE) is the most important treatment option for patients with unresectable HCC in the early and middle stages (4–6); however, many years of clinical practice has shown proven that repeated TACE can weaken the therapeutic effect and cause TACE refractoriness due to its heterogeneity and the limitations of TACE surgery (7). Studies have found that in cases experiencing TACE refractoriness, the early use of combination therapy confers significant survival benefits and protection of liver function (8). The time required for identifying TACE refractoriness and the adjustment of the corresponding treatment strategy can have various effects on overall survival (OS) in patients with unresectable HCC. Accordingly, early detection of TACE refractoriness and transfer to systemic therapy can have important clinical effects (9).
Arginase-1 (ARG1), an enzyme that converts arginine to urea in the urea cycle, is mainly found in hepatocytes around the hilar of the liver. It can hydrolyze L-arginine, generate urea and L-ornithine, and detoxify ammonia. ARG1 is a more sensitive and specific marker of hepatocyte differentiation compared with other hepatocyte markers. Previous studies have shown that the expression level of ARG1 in liver tissues and paracancerous tissues is significantly higher than that in HCC tissues, and the disease-free survival (DFS) and overall survival (OS) of patients with HCC are related to the expression of ARG1, indicating that ARG1 may play a regulatory role in the occurrence and development of HCC (10, 11).
Previous studies had primarily focused on the relationship between TACE refractoriness and common imaging and laboratory indicators. In this study, by investigating the association between pre-treatment plasma ARG1 levels and early TACE refractoriness in patients with HCC, we aimed to identify patients who may show TACE refractoriness by developing a novel plasma ARG1-based nomogram.
Materials and methods
Patients
The study cohort comprised patients receiving TACE treatment in the Affiliated Cancer Hospital of Zhengzhou University from September 2017 to December 2020. The study was conducted in line with the guidelines of the Declaration of Helsinki and was approved by the Ethics Review Committee of the Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital (approval number: 2016ct004). Due to the retrospective nature of the study, the ethics committee waived the requirement for informed consent. The data was analyzed anonymously. HCC was defined by pathological evidence or diagnosed using the non-invasive criteria of the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases.
The training and validation cohorts were subjected to the same inclusion and exclusion criteria. The inclusion criteria were as follows: (1) Patients should be over 18 years old with at least one measurable target lesion on the liver; (2) Patients should show indications and no contraindication for TACE; (3) TACE performed as monotherapy; (4) Before the first TACE treatment, the status score of Eastern Cooperative Oncology Group performance should be 0; (5) Patients at BCLC stage A or B, on whom radical surgical resection or ablation could not be performed; (6) Patients with good liver function with Child-Pugh A or B grade; and (7) Patients with complete clinical data. The exclusion criteria were as follows: (1) Patients who have received systemic therapy such as radiotherapy, chemotherapy, targeted therapy, and immunotherapy, (2) Patients who have received surgery or radiofrequency ablation within 6 months after the first TACE; (3) Those with severe heart disease, or those with irrecoverable blood clotting disorder or renal dysfunction.
TACE procedure
As reported previously (12), all TACE procedures were performed by at least two experienced interventional radiologists, and TACE was performed through the traditional femoral artery approach under local anesthesia. 5F RH catheters (Terumo, Tokyo, Japan) were first used for routine angiography, and then microcatheters (Terumo, Tokyo, Japan) were used for super selective arterial catheterization to enter the blood supply branch of the tumor. A mixed solution containing lipiodol (Laboratories Guerbet, Paris, France) and doxorubicin (Haizheng Pharmaceutical, Taizhou, China) was administered into the tumor-feeding vessels, followed by the injection of gelatin sponge particles (500 mm-700 mm; ALICON Dr. SCI&TEC Co., Ltd., Hangzhou, China) to supplement embolization until blood flow nearly ceased. The dose of doxorubicin was 50-70 mg and that of lipiodol was 5-20 ml; the specific dose should be adjusted according to the patient’s tumor number and size, liver function, blood vessel distribution, and body surface area.
Definition of TACE refractoriness
As previously reported by the Japan Society of Hepatology (JSH), TACE refractoriness was mainly defined in terms of an insufficient response of the treated tumor or a progressive tumor response, indicated through the following observations: 1) viable lesions more than 50% after two or more TACE; 2) two or more intrahepatic lesions after TACE; 3) an increase in the extent of intrahepatic vascular invasion; 4) occurrence of extrahepatic metastasis; and 5) a continuous increase in the levels of tumor markers, following a temporary decrease in the levels after TACE (13). As had been reported previously, if the patient developed TACE refractoriness within 12 months after the initial TACE, we defined it as early TACE refractoriness (14).
If viable tumors were detected and liver function was adequate, TACE was repeated every 6–8 weeks. Follow-up included check-up for general health condition, liver function tests, routine blood investigations, and contrast-enhanced CT or MRI examination of the liver
ARG1 measurements
Blood samples of the patients were collected before the first TACE; the plasma was then separated by centrifugation at 1000 × g for 10min at 4°C and stored at -80°C until ARG1 analyses. Human plasma ARG1 levels were quantified using an ELISA kit. Measurements were performed according to the manufacturer’s (Enzyme-linked Biotechnology Co., Ltd., Shanghai, China) instructions; the lowest and highest detectable levels of the kit were 0.9375 ng/mL and 30 ng/mL, respectively, and all samples were diluted 3-fold with the sample diluent prior to the experiment. Furthermore, 50 mL of both sample and standard were added to the corresponding wells, followed by the addition of 100 mL of horseradish peroxidase-labeled detection antibody to each well, and incubation at 37°C for 60 min. Thereafter, the liquid was discarded, and each well was washed five times with 350 µL of washing solution; 50 µL each of substrate A and B was added next and the mixture was incubated at 37°C for 15 min in the dark. Next, 50 mL of stop solution was added to each well, and using a plate reader (Thermos Fisher Scientific, China), absorbance was measured at 450 nm, after 15 min.
Statistical analyses
SPSS (version 13.0; SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and a binary logistic regression model was used to incorporate statistically significant variables into the multivariate analysis to identify predictors associated with the development of early TACE refractoriness. Nomograms were developed using R programming (Fundamentals of Statistical Computing version 4.1.2, Vienna, Austria), and the performance of each was evaluated using the consistency index (c-index). The c-index value ranges from 0.5 to 1, where a higher c-index indicates better predictive ability of the model. The value of the c-index ranged from 0.5 to 1. Decision curve analysis (DCA) was used to evaluate the accuracy of the model and net clinical benefits. Statistical significance was set at P < 0.05.
Results
ARG1 expression and cutoff value
A total of 228 patients were enrolled in the study. Till December 2020, 200 (200/228, 87.71%) patients developed TACE refractoriness during follow-up and were included in the analysis, whereas 28 were excluded. The patients were divided into a training set and a validation set according at a ratio of 3:2. The ARG1 levels in the training and validation sets were 45.38 ± 28.07 ng/mL and 45.38 ± 27.68 ng/mL, respectively. There was no significant difference in the expression of ARG1 between the two groups (P=1.000). In the training set, the area under the ROC curve of ARG1 was 0.687 (0.592-0.781), the best cut-off value was 59.49 ng/mL, the sensitivity was 48.3%, and the specificity was 85.0% (Figure 1). The ARG1 expression levels were further used to classify the patients as belonging to the high expression group (>59.49 ng/mL) and the low expression group (<59.49 ng/mL).
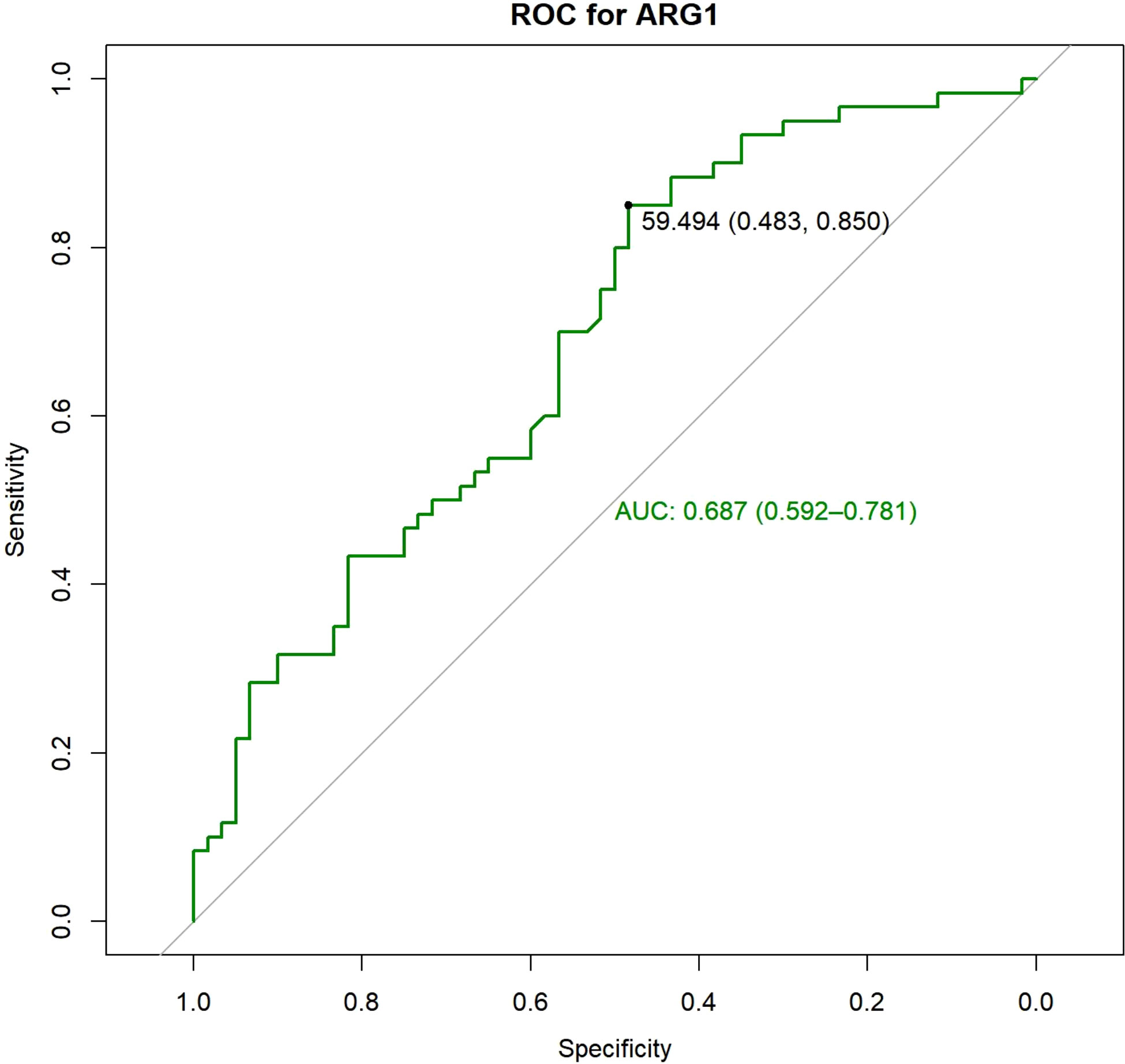
Figure 1 ROC analysis was performed and the best cut-off value was determined to be 59.49 ng/ml, at which both sensitivity and specificity were high.
Baseline characteristics of the patients
There was no statistically significant difference in the baseline data of the training and validation sets of the patients (Table 1). There were 120 patients in the training set, of which 60 patients showed TACE refractoriness within 12 months of follow-up (early TACE refractoriness), indicating an incidence rate of 50%. The incidence of early TACE refractoriness in the validation set was 48.75%, and there was no statistically significant difference between the two groups (P=0.888). All baseline characteristics of patients in training set and validation set were not significantly different (P>0.05).
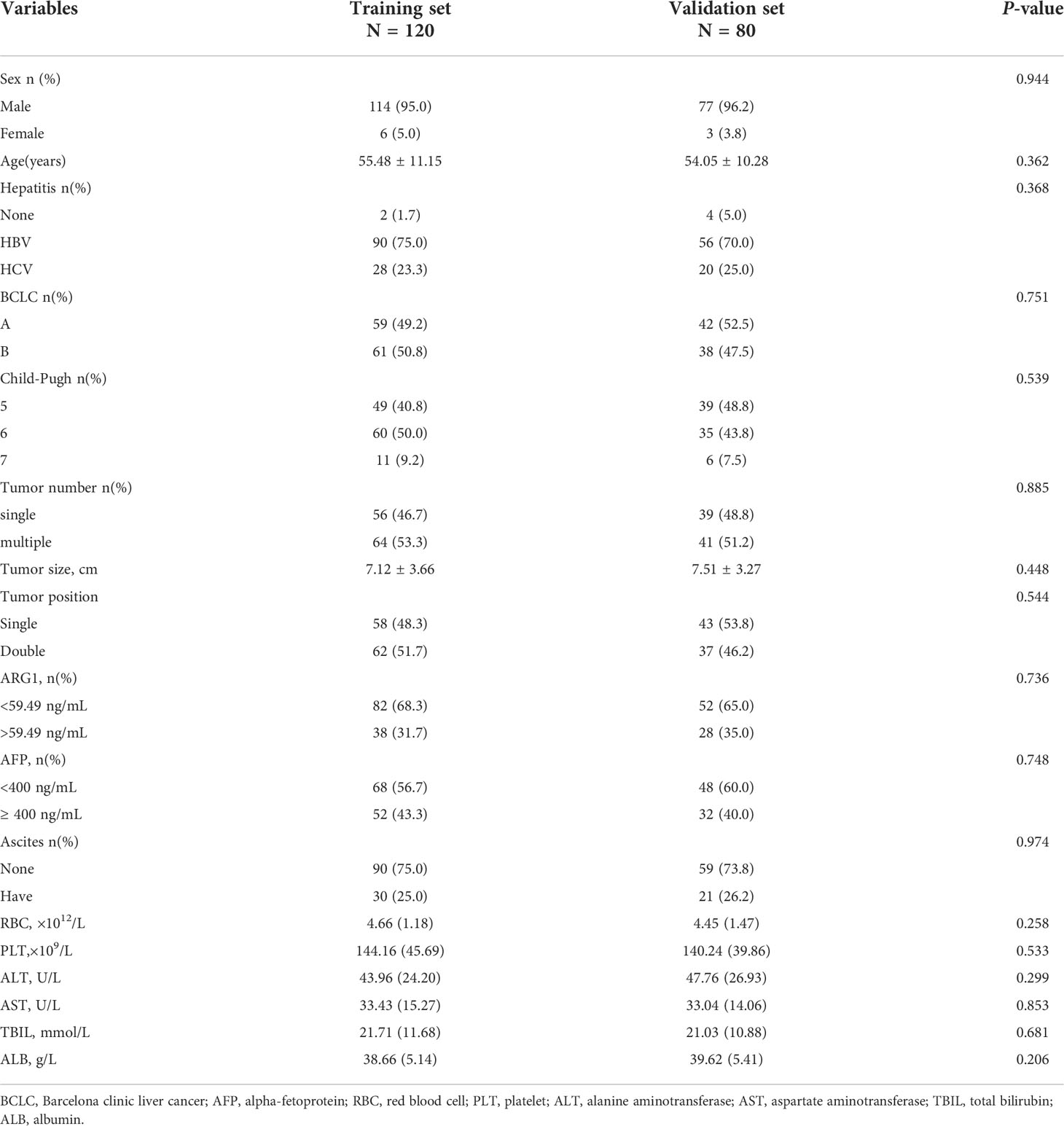
Table 1 Baseline characteristics of patients with TACE refractoriness in training set and validation set.
In the training set, 95% of the patients were male with an average age of 55.48 ± 11.15 years. Viral hepatitis was the main cause of HCC (98.3%), including infection with hepatitis B or C virus. Patients with single tumors accounted for approximately 46.7% of all patients and those with multiple tumors accounted for 53.3%; among the patients with multiple tumors, those with 2–3 tumors accounted for approximately 28.1%, and those with ≥ 4 tumors accounted for approximately 25.2%. The average size of the largest tumor in all patients was 7.12 ± 3.66 cm. According to the BCLC staging criteria, the proportion of patients with stages A and B was 49.2% and 50.8%, respectively. TACE refractoriness was mainly characterized by new intrahepatic lesions (71.7%), vascular invasion (20.0%) and extrahepatic lesions (8.3%).
Prognostic factors affecting patients with early TACE refractoriness
Univariate analysis of the predictors of early TACE refractoriness showed that BCLC stage (OR=2.110, 95%CI=1.014-4.350, P=0.046), tumor size (OR=1.202, 95% CI=1.074-1.346, P=0.001), occurrence of multiple tumors (OR=3.471, 95% CI=1.635-7.370, P=0.001), platelet count (OR =0.987, 95%CI=0.979-0.995,P=0.003), alanine aminotransferase levels (OR=1.017, 95%CI= 1.001-1.033,P=0.035), and high ARG1 levels (OR=0.189, 95% CI=0.079-0.451, P<0.001) were predictors of early TACE refractoriness. Among them, high platelet count and high ARG1 levels were protective factors against early TACE refractoriness. Multivariate analysis showed that tumor size (OR =1.138, 95%CI=1.006–1.288, P=0.041), occurrence of multiple tumors (OR=4.374, 95% CI = 1.189–16.089, P=0.026), platelet count (OR =0.99, 95%CI=0.980–0.999, P=0.036), and high ARG1 levels (OR=0.209, 95%CI=0.079–0.551, P=0.002) were independent predictors of early TACE refractoriness (Table 2).
Establishment of a predictive model for early TACE refractoriness based on plasma ARG1 levels
We established a nomogram based on the significant predictors identified by binary logistic regression analysis of the univariate and multivariate analysis data (Figure 2), including those on tumor size, tumor number, platelet count, and ARG1 expression. The AUC value obtained for the nomogram of the training cohort was 0.786 (95% CI=0.702–0.870), and the validation set AUC value was 0.833 (95% CI=0.791–0.875), indicating a good diagnostic value and some significance for prediction of early TACE refractoriness in individuals.
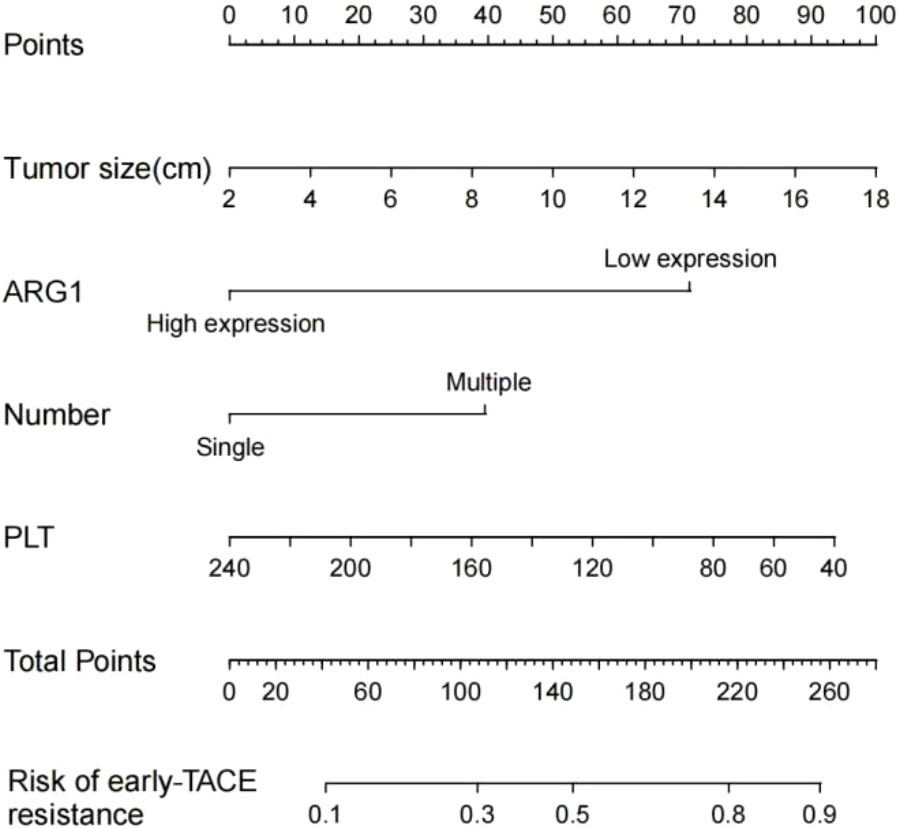
Figure 2 Prognostic nomogram for early TACE refractoriness. Each value of the patient is indicated on the axis with the variable. A line is drawn upward to determine the number of points received by each variable. Then, the total number of points are calculated and a line is drawn downward to determine the prediction probability of early TACE refractoriness.
The DCA result for the nomogram is presented in Figure 3. In the training set, the decision curve demonstrated that if the threshold probability of a patient or physician was between 9% and 75%, using the developed nomogram to predict the incidence of early TACE refractoriness was more beneficial, than when using the treat-all-scheme or treat-none schemes. Therefore, the developed nomogram was more beneficial than the treat-all scheme or the treat-none scheme for decision-making regarding treatment administration. Further, the validation set showed better results.
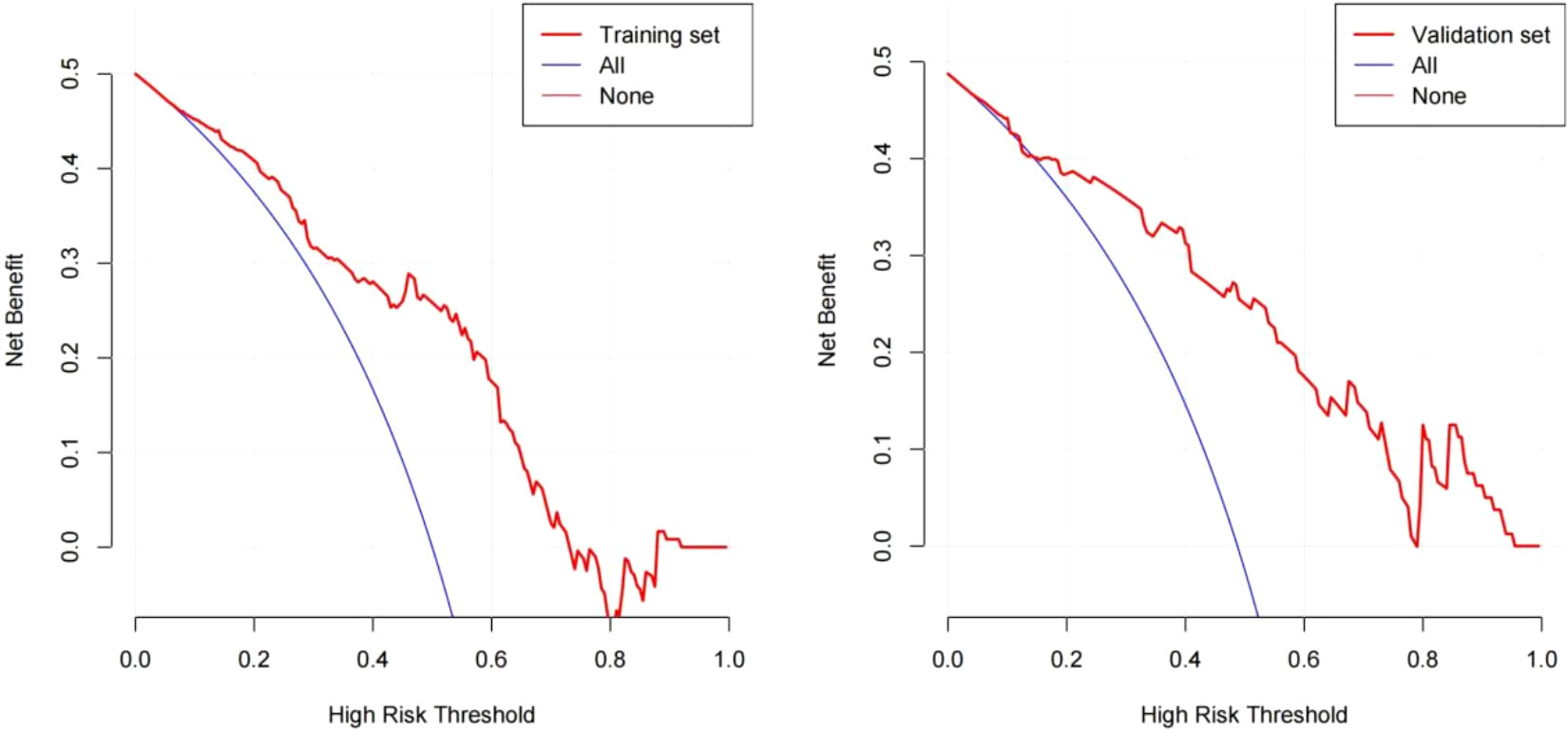
Figure 3 Decision curve analysis for the nomogram based on clinical characteristics. The red polyline represents the nomogram. The horizontal line with an ordinate of 0 represents all negative samples. For these participants, the treat-none scheme was applied. The blue fine line represents all positive samples, and for these participants, the treat-all scheme was applied.
Presentation of a patient with early TACE refractoriness
The clinical data of a 56-year-old female patient with hepatocellular carcinoma has been presented here. The platelet count, tumor size, number of lesions, and plasma ARG1 levels of the patient were 67.7×109/L, approximately 7.42 cm, 2, and 22.6 ng/mL, respectively. The above data were consistent with the nomogram-total score of 220, which corresponds to a probability of early TACE resistance of approximately 82% (Figure 2). The CT image results from March 2020 are shown in Figure 4A; two TACE treatments were performed in March and May 2020. In August 2020, the patient developed a portal vein tumor thrombus (Figure 4B), which indicated disease progression. Furthermore, disease progression occurred 5 months after the patient’s first TACE treatment, suggesting that the patient developed early TACE refractoriness. Predicted results of the nomogram were consistent with the observed condition of the patient.
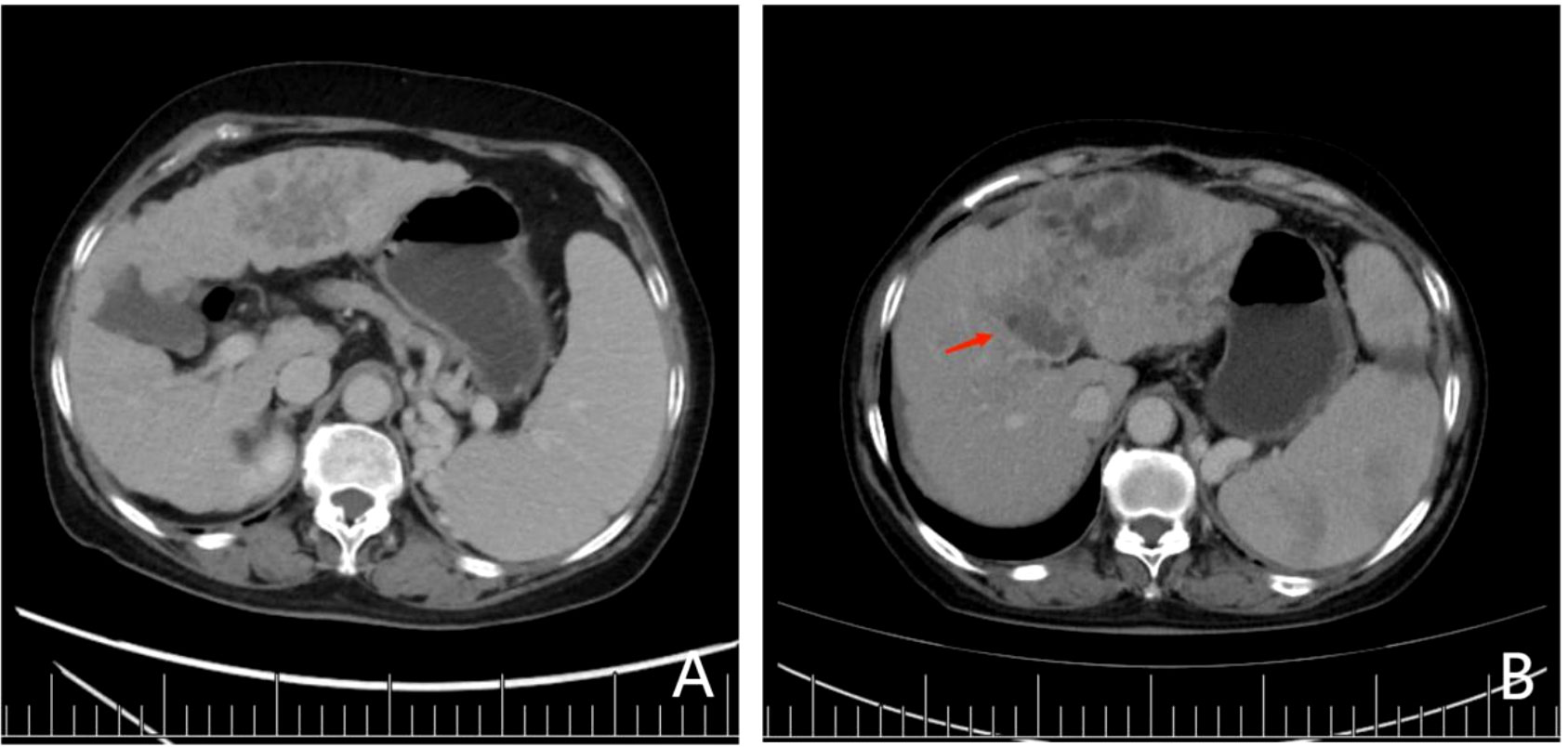
Figure 4 (A) Preoperative contrast-enhanced CT showed two lesions in the patient’s liver with cirrhosis. The largest lesion was 7.42 cm in diameter, located in the left lobe of the liver. In contrast-enhanced CT, lesions in the arterial phase were significantly enhanced, although they were weakened in the delayed phase, which was in accordance with the diagnostic criteria for hepatocellular carcinoma. Portal vein blood flow was unobstructed, and no portal vein tumor thrombus was found. (B) After 2 cycles of TACE treatment, lesions in the left hepatic lobe were enlarged, and tumor thrombus was seen in the portal vein (red arrow).
Discussion
To our knowledge, this was the first study to report the correlation between plasma ARG1 levels and the efficacy of TACE in unresectable HCC patients. This study found that plasma ARG1 expression was an independent predictor of early TACE refractoriness. We developed a nomogram based on ARG1 expression levels, combined with different tumor parameters, to predict the risk of early TACE refractoriness in unresectable HCC patients treated with TACE. Incorporation of ARG1 expression and the associated clinical prognostic factors into an easy-to-use nomogram could facilitate an individualized assessment of the risk of developing early TACE refractoriness preoperatively.
Recent studies have confirmed that ARG1 can be induced in alternately activated (M2) macrophages and is involved in the occurrence and development of tumors, mainly due to the anti-inflammatory response, tumor immunity, tumor proliferation, metastasis, and immunosuppression (15). Therefore, altered expression of ARG1 may lead to changes in the metabolism of the liver tissue and have marked effects on the metabolic and growth statuses of tumor cells. Previous studies have shown that expression of ARG1 was closely related to HCC differentiation, histological type, Edmondson grade, and other indicators that indicate the degree of tumor differentiation. With a decrease in ARG1 expression, the differentiation degree of HCC worsened, suggesting that ARG1 may be a molecular marker for determining the degree of HCC differentiation (10, 16).
Our study found that the average ARG1 level of 60 patients with early TACE refractoriness was 36.55 ng/mL and that of patients without early TACE was 54.22 ng/mL; the former value was significantly lower than the latter, and the difference was statistically significant (P < 0.05). In unresectable HCC patients treated with TACE, the risk of early TACE refractoriness decreased with an increase in ARG1 expression, possibly because the increased ARG1 expression increased the catalysis and consumption of arginine and reduced the amount of arginine in the microenvironment (17). Hepatoma cells are malignant tumor cells with arginine deficiency. Arginine is an essential amino acid. The growth of cancer cells is inhibited in the absence of arginine. A reduction in the levels of amino acids in HCC tissues inhibits the growth and reproduction of tumor cells, improves the efficacy of TACE, and reduces the risk of tumor progression and TACE refractoriness after repeated TACE. If exogenous arginine is administered to the tumor microenvironment to deplete arginine, tumor growth can be inhibited. Currently, the antitumor drug PEGylated recombinant human arginase is being tested in clinical trial including patients with unresectable HCC (18).
However, the effect of ARG1 on patient survival remains controversial. Obiorah et al. reported that patients with high ARG1 expression have a shorter median time to recurrence (19), whereas Mao et al. found that patients with low ARG1 expression in HCC had shorter DFS and OS (11). Our study showed that the expression of plasma ARG1 in unresectable HCC patients is negatively correlated with the probability of early TACE refractoriness, that is, the higher the expression of plasma ARG1, the lower the risk of early TACE refractoriness and the better the prognosis. Our results were similar to Mao’s findings, suggesting that the assessment of plasma ARG1 levels would be helpful in assessing the risk of early TACE refractoriness in patients with HCC treated with TACE.
There is no consensus yet regarding the risk factors affecting TACE refractoriness, and the results of various studies differ widely. By studying the molecular indicators and clinical biochemical indicators, some researchers found that microRNA, TP53 mutation, the M2 isoform of pyruvate kinase expression level, AFP level, and interleukin-8 expression level can affect the outcome of TACE (14, 20–22). Shehta et al. found thrombocytopenia to possibly be an important predictor of tumor recurrence after hepatectomy in HCC patients with cirrhosis, which is similar to our findings (23). However, the pathophysiological mechanism remains to be explored further. The results of our study showed that BCLC stage, tumor size, the presence of multiple tumors, platelet count, and plasma ARG1 level as shown by our univariate analyses may be predictive factors for early TACE refractoriness. However, in multivariate analyses we found that only tumor number, tumor size, and platelet and plasma ARG1 levels were independent predictive factors for early TACE refractoriness. Moreover, our univariate analyses indicated that BCLC and ALT were predictors of early TACE refractoriness. However, our multivariate analyses of these factors instead yielded statistically insignificant associations. One possible explanation for this seeming contradiction is that since tumor size and number were both strongly correlated with BCLC stage, they therefore may also be related to ALT. In other words, we managed to obtain a complete result from analyzing only a single factor. And by eliminating the influences of other factors via our multivariate analyses, we revealed that neither BCLC stage nor ALT independently influence the prediction of early TACE refractoriness.
In recent years, many studies have attempted to establish a predictive model for the prognosis and response to TACE treatment. However, relatively few studies have used TACE refractoriness as an endpoint (24, 25). Current research mainly focuses on predicting postoperative response to TACE, and there are very few studies on the early prediction of TACE refractoriness, most of them being retrospective, focusing on the relationship between a certain index and TACE refractoriness. Furthermore, only a few predictive models integrate multiple clinical indicators. Existing models are mostly based on local samples and lack clinical and external validations (26). In this study, we developed a nomogram based on ARG1 expression level, combined with different tumor parameters, to predict the risk of early TACE refractoriness in patients with unresectable HCC. The logistic regression model included ARG1, tumor size and number, and platelet counts, and the results showed the model c-index to be higher (0.833), indicating that models incorporating ARG1 expression level had higher prediction accuracy. The DCA suggested that the nomogram had good clinical utility. Many previous studies have thoroughly discussed the relationships between tumor size, number, and hepatic physiology-related indicators and TACE efficacy. Increases in either tumor size or number lead to increased |tumor burdens that markedly influence liver cancer treatment outcomes (20, 27–29). Hu et al. retrospectively analyzed the association between TACE refractoriness and various biomarkers; they proposed that the main risk factors for refractory TACE include AFP, some liver function indicators and tumor imaging findings (30). Taken together, these previous results are consistent with our findings.
Our study had some limitations. First, we used the definition of TACE refractoriness provided by the JSH, which is applicable in clinical practice; however, since TACE treatment itself is highly heterogeneous, the concept of “refractoriness” has certain limitations and presently, there is no international consensus regarding its definition. Second, this is a single-center retrospective study with a small sample size, which may lead to potential data selection bias. Therefore, the results of this study may need to be further verified by prospective, multicenter, large sample, randomized controlled trials. Third, this predictive model is temporarily not applicable to patients with advanced HCC or those receiving TACE combined with other treatments. The correlation between ARG1 and TACE refractoriness in these specific patient populations will be the topic of our continued research in this direction.
In conclusion, to our knowledge, we showed for the first time that the expression of plasma ARG1 in unresectable HCC patients is negatively correlated with the probability of early TACE refractoriness. The expression of plasma ARG1 before TACE in patients with unresectable HCC can be used as one of the candidate biomarkers for predicting early TACE refractoriness. A nomogram based on tumor size, tumor number, platelet, and plasma ARG1 levels could help predict the possibility of early TACE refractoriness before TACE treatment to achieve an individualized prediction of early TACE refractoriness in different patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The ethics of the research program has been approved by the Ethics Committee the Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital review board. The ethics committee waived the requirement of written informed consent for participation.
Author contributions
Conceptualization and design the study: H-LL and H-TH; Provision of study materials or patients: W-LX, LZ, D-YZ, Q-JY, and C-YG; Collection and assembly of data: W-LX, YG, X-HZ, and S-JX; Data analysis and interpretation: X-HZ, YG, YZ,LZ, and D-YZ; Manuscript writing: W-LX; Manuscript reviewing: H-LL, H-TH, and W-JF; Final approval of manuscript: All authors.
Funding
This work was supported by The National Natural Science Foundation (82002596), Henan Province Natural Science Foundation (212300410403),Science and Technology Department of Henan Province (No. 212102310162); Medical Science and Technology Research Project of Henan Province (No. LHGJ20190633) and Technology Major Project of the Ministry of Science and Technology of China (2018ZX10303502).
Acknowledgments
Thanks to all patients and medical staff who participated in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Villanueva A. Hepatocellular carcinoma. N Engl J Med (2019) 380:1450–62. doi: 10.1056/NEJMra1713263
3. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol (2019) 16:589–604. doi: 10.1038/s41575-019-0186-y
4. de Baere T, Arai Y, Lencioni R, Geschwind JF, Rilling W, Salem R, et al. Treatment of liver tumors with lipiodol TACE: Technical recommendations from experts opinion. Cardiovasc Intervent Radiol (2016) 39:334–43. doi: 10.1007/s00270-015-1208-y
5. Kim J, Sinn DH, Choi MS, Kang W, Gwak GY, Paik YH, et al. Hepatocellular carcinoma with extrahepatic metastasis: Are there still candidates for transarterial chemoembolization as an initial treatment. PloS One (2019) 14:e0213547. doi: 10.1371/journal.pone.0213547
6. Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29:iv238–238iv255. doi: 10.1093/annonc/mdy308
7. Arizumi T, Minami T, Chishina H, Kono M, Takita M, Yada N, et al. Time to transcatheter arterial chemoembolization refractoriness in patients with hepatocellular carcinoma in kinki criteria stages B1 and B2. Dig Dis (2017) 35:589–97. doi: 10.1159/000480208
8. Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-To-Seven criteria and child-pugh a liver function: A proof-Of-Concept study. Cancers (Basel) (2019) 11 (8): 1084. doi: 10.3390/cancers11081084
9. Ogasawara S, Ooka Y, Koroki K, Maruta S, Kanzaki H, Kanayama K, et al. Switching to systemic therapy after locoregional treatment failure: Definition and best timing. Clin Mol Hepatol (2020) 26:155–62. doi: 10.3350/cmh.2019.0021n
10. You J, Chen W, Chen J, Zheng Q, Dong J, Zhu Y. The oncogenic role of ARG1 in progression and metastasis of hepatocellular carcinoma. BioMed Res Int (2018) 2018:2109865. doi: 10.1155/2018/2109865
11. Mao H, Gao W, Lu G, Fang F, Teng L. Clinicopathological and prognostic implications of arginase expression in hepatocellular carcinoma. Clin Lab (2013) 59:37–43. doi: 10.7754/Clin.Lab.2012.120210
12. Hu HT, Luo JP, Cao GS, Li Z, Jiang M, Guo CY, et al. Hepatocellular carcinoma with portal vein tumor thrombus treated with transarterial chemoembolization and sorafenib vs. (125) iodine implantation. Front Oncol (2021) 11:806907. doi: 10.3389/fonc.2021.806907
13. Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology (2014) 87 (Suppl 1):22–31. doi: 10.1159/000368142
14. Kim SS, Cho HJ, Won JH, Bae JI, Kang DR, Lee JD, et al. Interleukin-8 level as a prognostic marker in patients with hepatitis b virus-associated hepatocellular carcinoma treated with transarterial chemoembolization. Cytokine (2015) 76:449–57. doi: 10.1016/j.cyto.2015.07.001
15. Zhang J, Li Y, Duan Z, Kang J, Chen K, Li G, et al. The effects of the M2a macrophage-induced axonal regeneration of neurons by arginase 1. Biosci Rep (2020) 40 (2). doi: 10.1042/BSR20193031
16. Chrzanowska A, Graboń W, Mielczarek-Puta M, Barańczyk-Kuźma A. Significance of arginase determination in body fluids of patients with hepatocellular carcinoma and liver cirrhosis before and after surgical treatment. Clin Biochem (2014) 47:1056–9. doi: 10.1016/j.clinbiochem.2014.03.019
17. Kakehashi A, Suzuki S, Ishii N, Okuno T, Kuwae Y, Fujioka M, et al. Accumulation of 8-hydroxydeoxyguanosine, l-arginine and glucose metabolites by liver tumor cells are the important characteristic features of metabolic syndrome and non-alcoholic steatohepatitis-associated hepatocarcinogenesis. Int J Mol Sci (2020) 21 (20): 7746. doi: 10.3390/ijms21207746
18. Yau T, Cheng PN, Chan P, Chan W, Chen L, Yuen J, et al. A phase 1 dose-escalating study of pegylated recombinant human arginase 1 (Peg-rhArg1) in patients with advanced hepatocellular carcinoma. Invest N Drugs (2013) 31:99–107. doi: 10.1007/s10637-012-9807-9
19. Obiorah IE, Chahine J, Ko K, Park BU, deGuzman J, Kallakury B. Prognostic implications of arginase and cytokeratin 19 expression in hepatocellular carcinoma after curative hepatectomy: Correlation with recurrence-free survival. Gastroenterol Res (2019) 12:78–87. doi: 10.14740/gr1156
20. Choi J, Lee D, Shim JH, Kim KM, Lim YS, Lee YS, et al. Evaluation of transarterial chemoembolization refractoriness in patients with hepatocellular carcinoma. PloS One (2020) 15:e0229696. doi: 10.1371/journal.pone.0229696
21. Martin SP, Fako V, Dang H, Dominguez DA, Khatib S, Ma L, et al. PKM2 inhibition may reverse therapeutic resistance to transarterial chemoembolization in hepatocellular carcinoma. J Exp Clin Cancer Res (2020) 39:99. doi: 10.1186/s13046-020-01605-y
22. Xue M, Wu Y, Fan W, Guo J, Wei J, Wang H, et al. Prognostic value of TP53 Mutation for transcatheter arterial Chemoembolization Failure/Refractoriness in HBV-related advanced hepatocellular carcinoma. Cancer Res Treat (2020) 52:925–37. doi: 10.4143/crt.2019.533
23. Shehta A, Han HS, Ahn S, Yoon YS, Cho JY, Choi YR. Post-resection recurrence of hepatocellular carcinoma in cirrhotic patients: Is thrombocytopenia a risk factor for recurrence. Surg Oncol (2016) 25:364–9. doi: 10.1016/j.suronc.2016.08.002
24. Chen RX, Gan YH, Ge NL, Chen Y, Ma M, Zhang BH, et al. A new prediction model for prognosis of patients with intermediate-stage HCC after conventional transarterial chemoembolization: an internally validated study. J Cancer (2019) 10:6535–42. doi: 10.7150/jca.34064
25. Jia F, Wu B, Yan R, Li L, Wang K, Han D. Prediction model for intermediate-stage hepatocellular carcinoma response to transarterial chemoembolization. J Magn Reson Imaging (2020) 52:1657–67. doi: 10.1002/jmri.27189
26. Niu XK, He XF. Development of a computed tomography-based radiomics nomogram for prediction of transarterial chemoembolization refractoriness in hepatocellular carcinoma. World J Gastroenterol (2021) 27:189–207. doi: 10.3748/wjg.v27.i2.189
27. Miki I, Murata S, Uchiyama F, Yasui D, Ueda T, Sugihara F, et al. Evaluation of the relationship between hepatocellular carcinoma location and transarterial chemoembolization efficacy. World J Gastroenterol (2017) 23:6437–47. doi: 10.3748/wjg.v23.i35.6437
28. Vesselle G, Quirier-Leleu C, Velasco S, Charier F, Silvain C, Boucebci S, et al. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol (2016) 26:1640–8. doi: 10.1007/s00330-015-3982-y
29. Tao PY, Zhang ZS, Wang TC, Yu MQ, Xiao YD. A predictive model of incomplete response after transarterial chemoembolization for early or intermediate stage of hepatocellular carcinoma: Consideration of hepatic angiographic and cross-sectional imaging. Abdom Radiol (NY) (2021) 46:581–9. doi: 10.1007/s00261-020-02701-5
Keywords: hepatocellular carcinoma, arg1, TACE refractoriness, prognostic factor, predictive models
Citation: Xia W-L, Xu S-J, Guo Y, Zhao X-H, Hu H-T, Zhao Y, Yao Q-J, Zheng L, Zhang D-Y, Guo C-Y, Fan W-J and Li H-L (2022) Plasma arginase-1 as a predictive marker for early transarterial chemoembolization refractoriness in unresectable hepatocellular carcinoma. Front. Oncol. 12:1014653. doi: 10.3389/fonc.2022.1014653
Received: 08 August 2022; Accepted: 22 August 2022;
Published: 20 September 2022.
Edited by:
Zeming Liu, Huazhong University of Science and Technology, ChinaReviewed by:
Shuntao Wang, Huazhong University of Science and Technology, ChinaChenxing Jian, Affiliated Hospital of Putian University, China
Copyright © 2022 Xia, Xu, Guo, Zhao, Hu, Zhao, Yao, Zheng, Zhang, Guo, Fan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-Liang Li, lihailianggy@163.com
 Wei-Li Xia1
Wei-Li Xia1