
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 30 September 2022
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.961394
 Wei-Li Xia1†
Wei-Li Xia1† Xiao-Hui Zhao1†
Xiao-Hui Zhao1† Yuan- Guo1
Yuan- Guo1 Guang-Shao Cao2
Guang-Shao Cao2 Gang Wu3
Gang Wu3 Wei-Jun Fan4
Wei-Jun Fan4 Quan-Jun Yao1
Quan-Jun Yao1 Shi-Jun Xu1
Shi-Jun Xu1 Chen-Yang Guo1
Chen-Yang Guo1 Hong-Tao Hu1*
Hong-Tao Hu1* Hai-Liang Li1*
Hai-Liang Li1*Objective: We evaluated the efficacy and safety of transarterial chemoembolization (TACE) combined with apatinib plus PD-1 inhibitors (TACE-AP) compared with TACE combined with apatinib (TACE-A) in patients with advanced hepatocellular carcinoma (HCC) and to explore the prognostic factors affecting patient survival.
Methods: Data from patients with unresectable HCC who received TACE-AP or TACE-A from December 2018 to June 2021 were collected retrospectively. The main outcome of the study was overall survival (OS) and prognostic factors affecting survival, while the secondary outcomes were progression-free survival (PFS), the objective response rate (ORR), and treatment-related adverse events (TRAEs). Propensity score matching (PSM) analysis was used to reduce patient selection bias, and the random survival forest (RF) model was employed to explore prognostic factors affecting patient survival.
Results: We enrolled 216 patients, including 148 and 68 patients in the TACE-A and TACE-AP groups, respectively. A total of 59 pairs of patients were matched using PSM analysis. Before and after PSM, the OS, PFS, and ORR in the TACE-AP group were significantly higher than in the TACE-A group (before, OS: 22.5 months vs. 12.8 months, P < 0.001; PFS: 6.7 months vs. 4.3 months, P < 0.001; ORR: 63.2% vs. 34.5%, P < 0.001; after, OS: 22.5 months vs. 12.0 months, P < 0.001; PFS: 6.7 months vs. 4.3 months, P < 0.001; ORR: 62.7% vs. 30.5%, P = 0.003). Multivariate Cox regression and RF models before and after PSM analysis revealed that the main prognostic factors affecting survival were tumor number, portal vein tumor thrombus (PVTT) invasion, alpha-fetoprotein (AFP) levels, total bilirubin (TBIL) level, and treatment. There was no significant difference in TRAEs between the two groups (P > 0.05).
Conclusion: Compared with TACE-A, TACE-AP significantly improved OS, PFS, and ORR in patients with advanced HCC. The number of tumors, PVTT invasion, AFP levels, TBIL level, and treatment were significant prognostic factors associated with patient survival. All observed TRAEs were mild and controllable.
Liver cancer is the sixth most common malignancy and the fourth leading cause of tumor-related death worldwide, with close to half of global patients with hepatocellular carcinoma (HCC) being from China (1, 2). HCC is the most common pathologic type of primary liver cancer, with the characteristics of insidious onset and high malignancy. Most patients are diagnosed with HCC in the middle and late stages, contributing to a poor disease prognosis (3, 4).
Transarterial chemoembolization (TACE) is the most effective treatment option for patients with inoperable liver cancer (5). However, previous studies found that the efficacy of TACE attenuates over time and aggravates liver damage. Tumors are in a hypoxic environment after TACE, which can upregulate the expression of angiogenic factors and increase the risk of recurrence and metastasis of HCC (6). These factors result in unsatisfactory long-term efficacy of TACE in patients with HCC. Previous studies showed that the survival rate of patients is greater when combined therapy is used as early as possible after TACE (7).
Apatinib is a small-molecule multi-target tyrosine kinase inhibitor that can inhibit tumor angiogenesis by selectively inhibiting the tyrosine kinase activity of vascular endothelial growth factor receptor 2 (VEGFR-2). Previous studies showed that apatinib combined with TACE can significantly reduce tumor volume, inhibit tumor revascularization, and prolong patient survival (8). Immune checkpoint inhibitors (PD-1 inhibitors), which can reverse immune failure, are effective in the treatment of advanced HCC when used concomitantly with TACE (9). Compared with monotherapy, the combination of TACE, PD-1 inhibitors, and an antiangiogenic agent showed excellent antitumor activity and significantly improved patient survival (10).
Currently, TACE combined with apatinib and PD-1 inhibitors shows great potential for the treatment of advanced HCC (11). However, no control studies of TACE combined with apatinib (TACE-A) and TACE combined with apatinib and PD-1 inhibitors (TACE-AP) have been reported to date. This retrospective multicenter study compared the efficacy and safety of TACE-A and TACE-AP to treat patients with advanced HCC, and we analyzed the prognostic factors affecting patient survival using a random survival forest (RF) model.
Clinical records for patients with HCC treated with TACE-A or TACE-AP from December 2018 to June 2021 were collected and analyzed retrospectively. The study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University and the Henan Cancer Hospital review board (approval number 2019198). The study was retrospective in nature; therefore, all requirements for informed consent were waived, and anonymous analyses of extracted data were performed.
Patients who met the following criteria were included in the study: (1) aged between 18 and 75 years; (2) diagnosed with HCC based on pathological data or using the non-invasive criteria outlined by the American Association for the Study of Liver Diseases; (3) categorized as Barcelona Clinic Liver Cancer (BCLC) tumor stage C; (4) Child-Pugh grade A or B; (5) Eastern Cooperative Oncology Group (ECOG) ≤ 1; and (6) platelet counts ≥ 60 × 109/L. The exclusion criteria were: (1) complete occlusion of the main portal vein or invasion of the superior mesenteric vein; (2) central nervous system metastasis; (3) previous radiotherapy, chemotherapy, targeted therapy, or immunotherapy; (4) other malignant tumors; and (5) the presence of severe complications, including severe cardiac, pulmonary, renal, or coagulation disorders (Figure 1).
As reported previously, the TACE procedure remained consistent across the centers (12). All TACE procedures were performed by at least two experienced interventional radiologists using the traditional femoral artery approach under local anesthesia. 5F RH catheters (Terumo, Tokyo, Japan) were first used for routine angiography, and then microcatheters (Terumo) were used for super-selective arterial catheterization to enter the blood supply branch of the tumor. A mixed solution containing lipiodol (Laboratoire Guerbet, Paris, France) and doxorubicin (Haizheng Pharmaceutical, Taizhou, China) was injected into the tumor-feeding vessels, followed by injection with gelatin sponge particles of 500–700 µm (ALICON Pharmaceutical, Hangzhou, China) to supplement embolization until the blood flow nearly ceased. The dose of doxorubicin was 50–70 mg, while that of lipiodol was 5–20 mL. The individualized doses were adjusted according to the patient’s tumor number and size, liver function, blood vessel distribution, and body surface area.
After three days of TACE, patients in the TACE-A group received oral apatinib (Hengrui Pharmaceutical, Lianyungang, China) at a dose of 250 mg/day, and those in the TACE-AP group received the same dose of apatinib and an intravenous injection of PD-1 inhibitors (200 mg) every three weeks until intolerable toxicity or disease progression. The administered PD-1 inhibitors included sintilimab (Innovent Pharmaceutical, Suzhou, China), tislelizumab (BeiGene Pharmaceutical, Shanghai, China), pembrolizumab (Merck, Kenilworth, NJ, USA), and camrelizumab (Hengrui Pharmaceutical, Lianyungang, China).
When patient adverse events were equal to or greater than grade 3 and determined to be related to apatinib, apatinib was administered every other day, and its administration was suspended if the adverse events continued after the dose adjustment. Nevertheless, the dose was restored to 250 mg/day if the adverse events were relieved or eliminated. If the adverse events were determined to be related to PD-1 inhibitors, the medication was suspended and was not resumed until the adverse events had resolved. Generally, patient follow-up should be performed every 4–6 weeks after TACE. From the extracted data, the follow-up included chest computed tomography (CT) and liver multiphase enhanced magnetic resonance imaging (MRI), routine blood tests, and liver and renal function tests. When MRI results showed recurrence or residual activity of the intrahepatic tumor, TACE should be repeated if the patient has good liver function test results. Herein, patients received apatinib continuously before TACE was repeated, and the treatment was interrupted for three days after TACE was repeated.
The original treatment regimen was discontinued if intolerable toxicity or disease progression occurred during the follow-up period. Following discussions between the multidisciplinary team and according to the patient’s wishes, all patients were treated under the same principles as follows: PD-1 inhibitors (for patients in the TACE-A group), a second-line targeted drug (regorafenib), radiotherapy (including radioactive iodine 125 seed implantation), or best supportive care. The main outcomes of this study were median overall survival (OS) and prognostic factors affecting survival, while the secondary outcomes were median progression-free survival (PFS), objective remission rate (ORR), and treatment-related adverse events (TRAEs).
The last follow-up of the study was conducted in April 2022. OS was defined as the time to death or last follow-up. According to the modified evaluation criteria for solid tumors (mRECIST), the patient’s imaging data were evaluated by two radiologists with intermediate titles or above, and the results were assessed as complete remission (CR), partial remission (PR), stable disease (SD), and disease progression (progressive disease, PD). Portal vein tumor thrombus (PVTT) invasion was stratified according to Cheng’s PVTT classification system: Type I, tumor thrombi involving segmental branches of the portal vein; Type II, tumor thrombi involving the right/left portal vein; and Type III, tumor thrombi involving the main portal vein and trunk. The adverse reaction grades were evaluated using the National Cancer Institute Standard for Adverse Events, version 3.0.
To balance the variables between the TACE-A and TACE-AP groups and to reduce patient selection bias, we performed PSM analysis using a 1:1 ratio to construct a balanced cohort with a caliper value of 0.1. Baseline variables included age, sex, BCLC stage, Child-Pugh grade, metastasis, PVTT, alpha-fetoprotein (AFP) levels, hepatitis B virus infection, and ECOG score, as well as total bilirubin, albumin, alanine aminotransferase, creatinine levels, and tumor size and number.
To avoid sample size loss caused by the PSM analysis, important factors affecting OS were further analyzed by constructing a stochastic survival forest model using variable importance (VIMP) and minimum depth methods. A VIMP value < 0 indicated that the variable reduced the accuracy of the prediction, while a VIMP value > 0 improved the prediction accuracy. The minimum depth method revealed the importance of each variable to the outcome event by calculating the minimum depth when running the final node.
Categorical variables were expressed as percentages, calculated by the chi-squared test, while continuous variables were expressed as means ± standard deviation, calculated by the Student’s t-test. The difference in OS between the two groups was evaluated using the Kaplan-Meier method. The statistical significance of clinical characteristics was assessed by univariate analysis, and statistically significant variables were included in the analysis using multivariate Cox regression models to identify predictors associated with OS. The difference was considered statistically significant when P < 0.05. All analyses were performed using R statistical software (version 4.1.2; R Statistical Computing Foundation, Vienna, Austria; http://www.r-project.org/).
We screened 330 patients, 114 of which were excluded; thus, 216 (148 in the TACE-A group and 68 in the TACE-AP group) were included in this study. The administered PD-1 inhibitors were sintilimab (25 patients; 36.7%), tislelizumab (20 patients; 29.4%), camrelizumab (15 patients; 22.1%), and pembrolizumab (8 patients; 11.8%). Table 1 lists the baseline characteristics of the patients before and after PSM analysis.
At the end of the follow-up period (April 2022), the proportions of patients that reached the study endpoint in the TACE-A and TACE-AP groups were 94.6% (140/148) and 95.6% (65/68), respectively. Before PSM, the median PFS was 4.3 months (95% confidence interval [CI]: 3.9–4.6) and 6.7 months (95% CI: 5.4–7.5), and the median OS was 12.8 months (95% CI: 11.1–14.7) and 22.5 months (95% CI: 16.6–26.9) in the TACE-A and TACE-AP groups, respectively. There was a significant difference in the median PFS and median OS between the two groups (P < 0.001). After PSM, the median PFS was 4.3 months (95% CI: 3.6–4.7) and 6.7 months (95% CI: 5.4–7.4), and the median OS of the two groups was 12.0 months (95% CI: 8.8–15.0) and 22.5 months (95% CI: 16.6–26.0), respectively, and there was a significant difference between the groups (P < 0.001; Figures 2, 3).
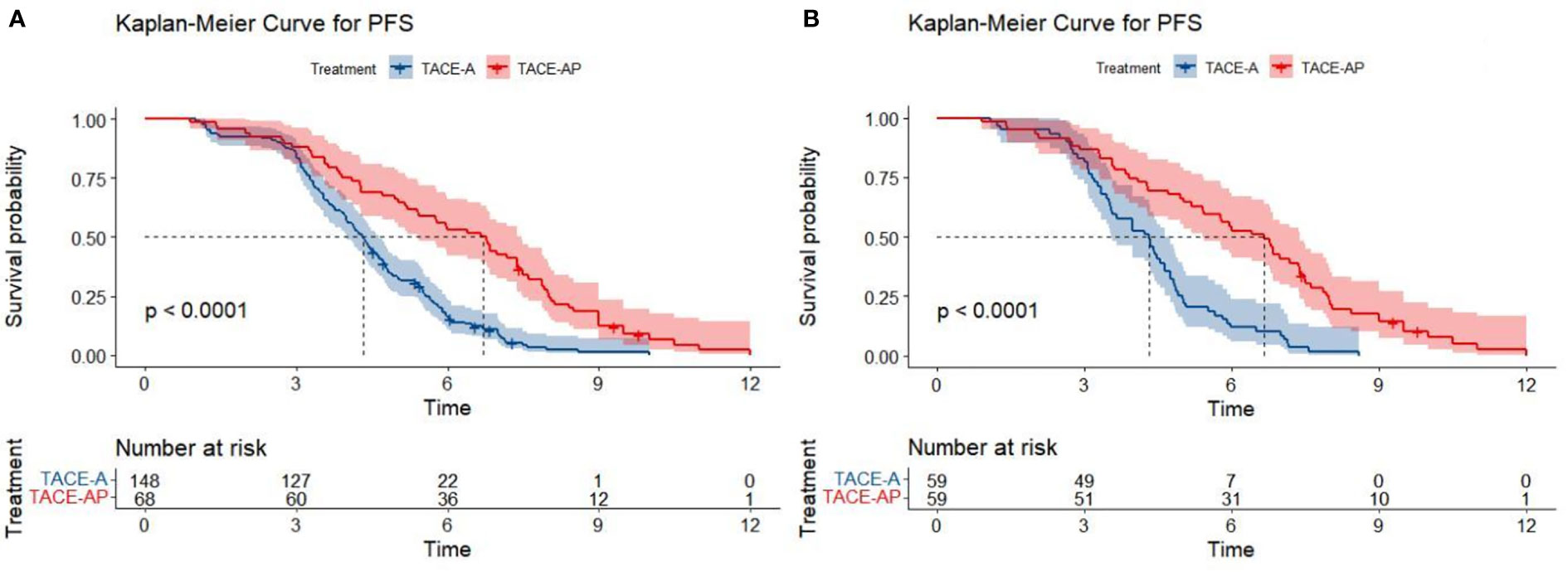
Figure 2 Kaplan-Meier curves for PFS in patients with advanced HCC treated with TACE-A or TACE-AP before (A) and after (B) PSM.
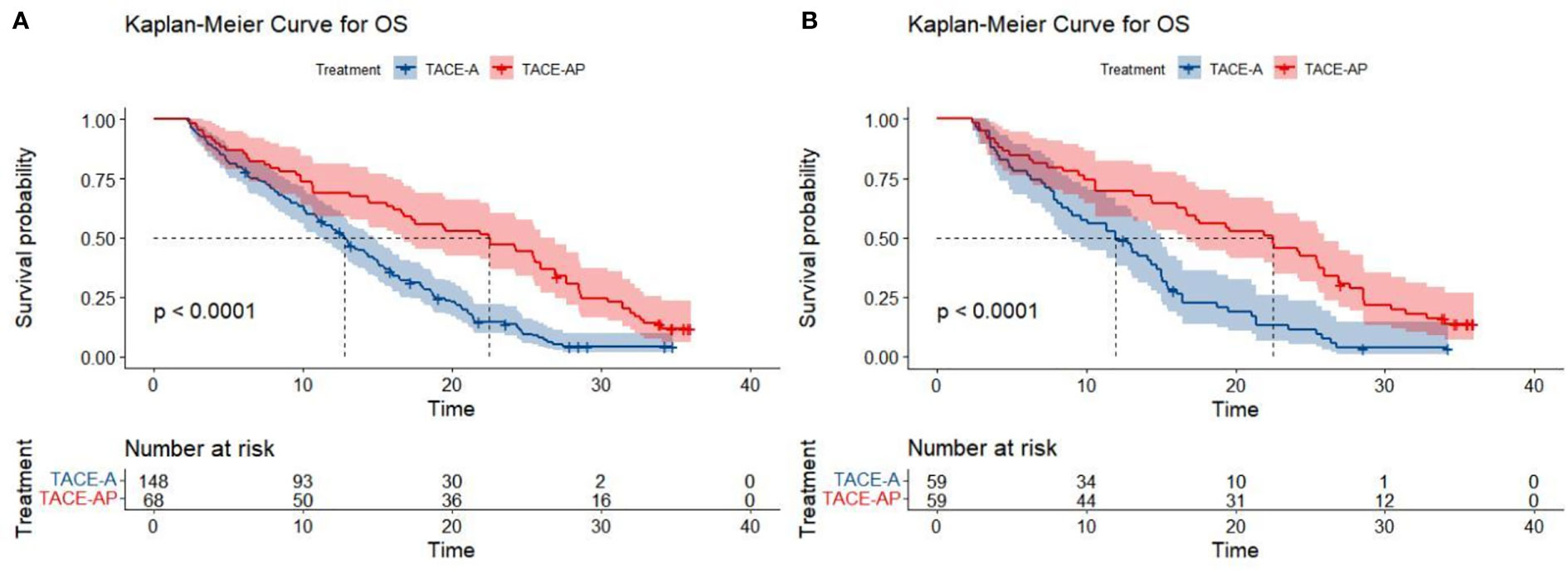
Figure 3 Kaplan-Meier curves for OS in patients with advanced HCC treated with TACE-A or TACE-AP before (A) and after (B) PSM.
Before PSM, the ORR findings of the TACE-A and TACE-AP groups were 34.5% and 63.2%, respectively, with a significant difference between the two groups (P < 0.001). After PSM analysis, the ORR was 62.7% in the TACE-AP group and 30.5% in the TACE-A group, the former being significantly higher than the latter (P = 0.003). The tumor responses of all the patients are listed in Table 2.
Univariate and multivariate Cox proportional hazard regression models were used to identify prognostic factors associated with OS (Table 3). Before PSM, univariate analysis showed that PVTT invasion, tumor number, treatment (TACE-A or TACE-AP), AFP, and total bilirubin (TBIL) level were the prognostic factors affecting OS (P < 0.05). The multivariate Cox analysis showed that distant metastasis (P = 0.048), PVTT (type = III; P = 0.002), AFP ≥ 400 ng/ml (P = 0.025), and multiple tumors (P < 0.001) were independent risk factors for OS, and TACE-AP treatment modality (TACE-A or TACE-AP; P < 0.001) were independent protective factors for OS. After PSM, the univariate analysis showed that PVTT invasion, tumor number, treatment modality, AFP, and TBIL were prognostic factors for OS (P < 0.05). The multivariate analysis showed that metastasis, PVTT invasion, tumor number, TBIL, and treatment modality were independent prognostic factors affecting OS (P < 0.05).
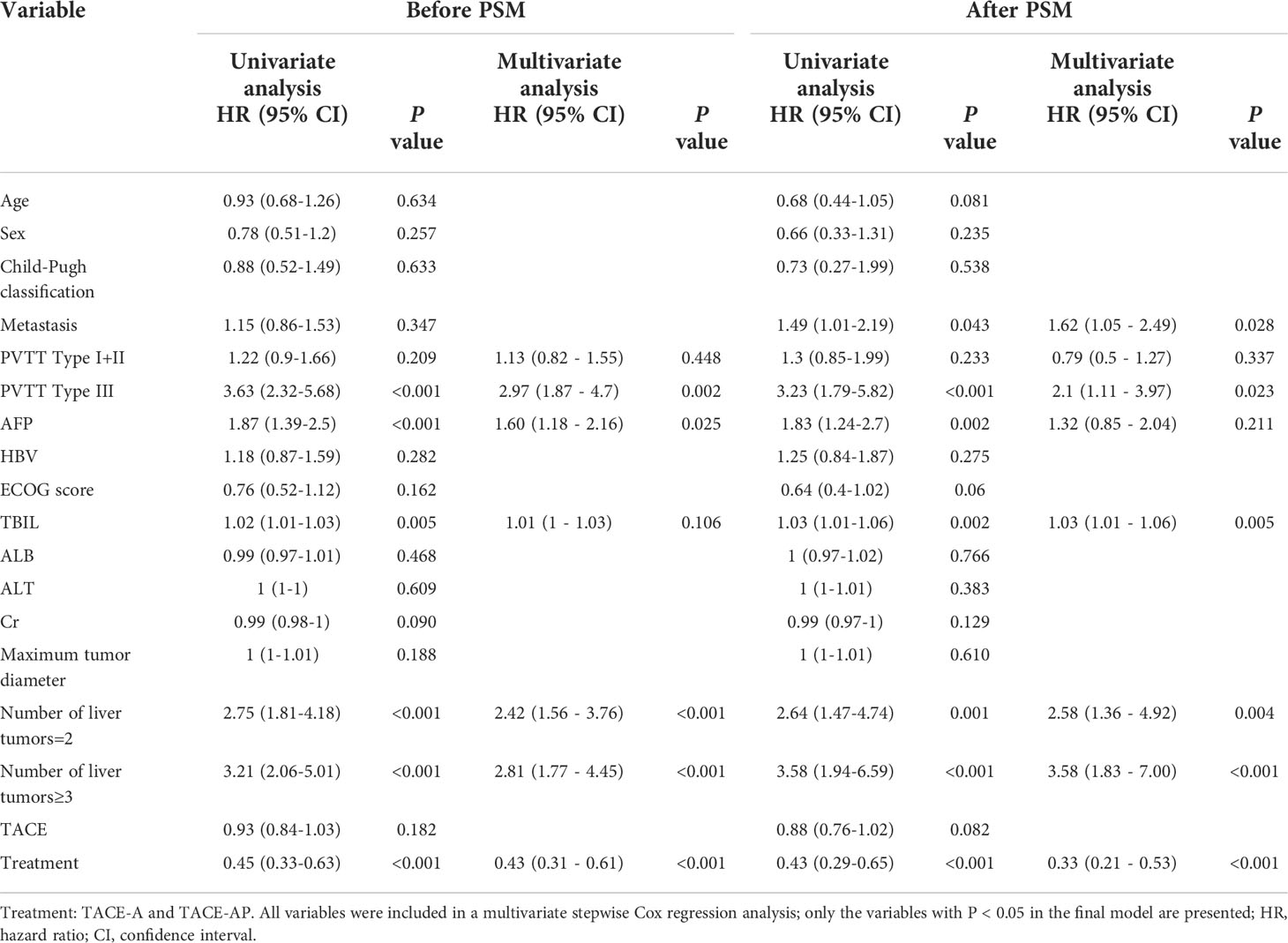
Table 3 Univariate and multivariate Cox proportional hazards regression model analysis of OS before and after PSM.
The RF model was established based on baseline characteristics and treatment methods for 15 related factors using Harrell’s concordance index to calculate model accuracy. The consistency index is a common indicator used to evaluate survival analysis models. The value of the consistency index is positively correlated with the quality of the model. Figure 3 shows a C-index of the model of approximately 0.705.
The model generated 1000 binary survival trees by default. As depicted in Figure 4A, when the number of surviving trees increased to a certain value, the error rate curve leveled off (29.5%). Figure 4B shows the scatter plot of the two methods of the integrated VIMP and minimum depth method. The results show that AFP and TBIL were important variables that simultaneously met both conditions in PVTT invasion, tumor number, treatment (TACE-A or TACE-AP), and albumin.
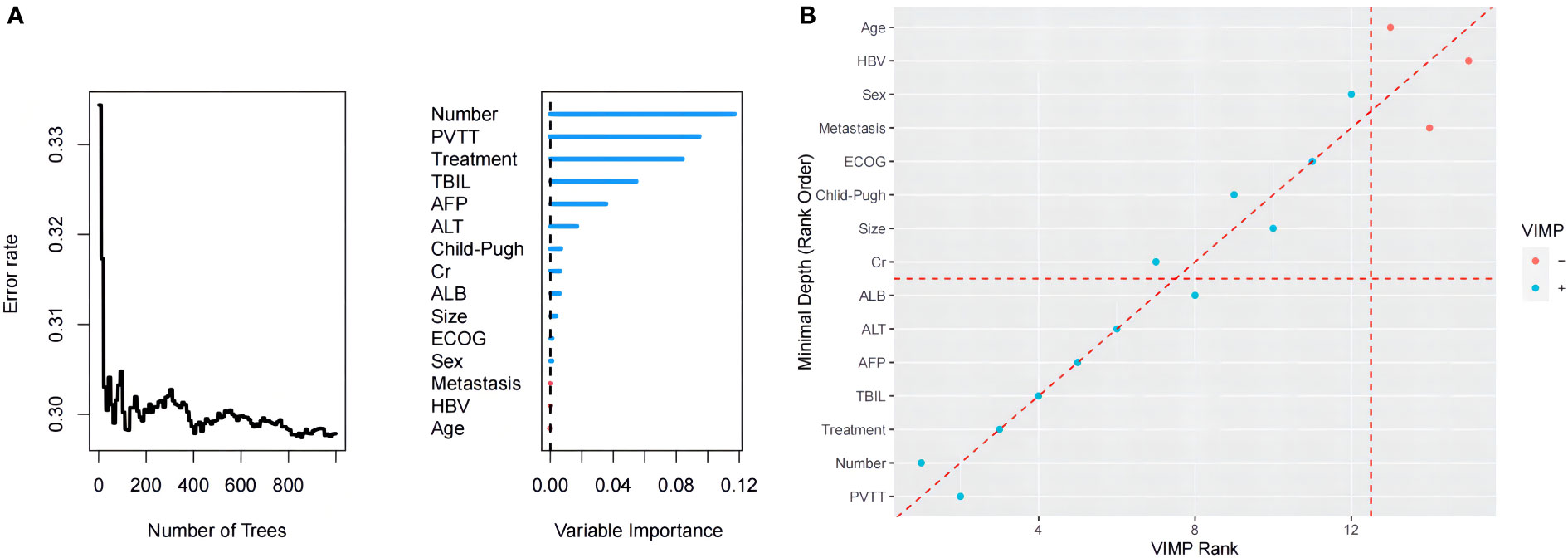
Figure 4 (A) Order of importance of the variables in the RF model for OS. (B) The blue point represents a VIMP value > 0, the red a value < 0; the point on the red diagonal dashed line represents the same ranking of the two methods on the variable, and the point higher than the diagonal dashed line represents its VIMP ranking. Higher points below the diagonal dotted line represent a higher minimum depth ranking.
During the treatment and follow-up periods, the most common TRAEs in the two groups were post-embolization syndrome, including nausea and vomiting, pain, fatigue, and fever, which improved after symptomatic treatment. There was no significant difference in the incidence of TRAEs above grade 3 between the two groups (P > 0.05). All TRAEs were resolved after symptomatic treatment, drug dose reduction, or discontinuation. In the TACE-AP group, three patients (4.41%) developed hyperthyroidism, nine (13.23%) developed hypothyroidism, and one (1.47%) developed reactive cutaneous capillary endothelial proliferation, all of which were graded 1 or 2 and were relieved after symptomatic treatment. Immune-related pneumonia occurred in two cases (2.94%), of which one case reached grade 3, which gradually eased after stopping the PD-1 inhibitor. There were no deaths related to adverse reactions (Table 4).
TACE is one of the most effective treatments for advanced unresectable HCC (5, 13). However, due to disease heterogeneity and the limitations of TACE itself, the long-term effect of TACE is not guaranteed. The TACTICS study revealed that TACE combined with sorafenib significantly improved TACE efficacy. The results of several clinical trials on atlizumab showed that when used in patients with HCC, atlizumab- plus bevacizumab-treated patients had a longer OS and PFS than sorafenib or atlizumab alone. KEYNOTE 524 studies have suggested that pembrolizumab combined with lenvatinib can significantly improve the survival of patients with advanced HCC. These clinical trials confirmed that tyrosine kinase inhibitors combined with PD-1 inhibitors can confer survival benefits to patients with unresectable HCC. These results show that the combination of TACE, targeted therapy, and immunotherapy can improve therapeutic efficacy in patients with unresectable HCC (6, 14–16). A recent RESCUE trial demonstrated that apatinib combined with carilizumab showed better efficacy and safety as a first- or second-line treatment for advanced HCC, and it is worth noting that most patients in that study received local treatment, including TACE, after progression (17). Owing to these reports, we conducted a multicenter retrospective study of TACE combined with apatinib and PD-1 inhibitors.
To our knowledge, this multicenter retrospective study included a larger sample size than did the aforementioned reports. This retrospective study showed that TACE-AP provides a more significant survival benefit than TACE-A in patients with advanced HCC. Zhu et al. (18) reported a median OS of TACE combined with lenvatinib and PD-1 inhibitors of 16.9 months (95% CI: 14.9–18.8), showing that this therapeutic regimen is more effective than TACE combined with lenvatinib, which is similar to our findings. Many studies have shown that TACE combined with targeted therapy and immunotherapy significantly improves the survival of patients with unresectable advanced HCC (19, 20). The most likely reason is that ischemic tumor necrosis due to TACE increases the release of tumor antigens, thereby increasing the expression of PD-1 and PD-L1, which improves tumor recognition (21, 22). The second possible reason is that the tumor microenvironment in advanced HCC is in an immunosuppressed state, leading to T cell dysfunction. Furthermore, the anti-VEGF effect of apatinib reduces immunosuppression in the tumor microenvironment, creating an inflammatory environment more suitable for T cell responses and an improved immunosuppressive state (23). In addition, the therapeutic drug for TACE in these studies was doxorubicin, which may induce and enhance immunogenic cell death and enhance immunity (24, 25). Therefore, the combination of TACE with apatinib and a PD-1 inhibitor may have a synergistic effect that achieves improved clinical efficacy in the treatment of patients with unresectable HCC.
Ju et al. (19) reported a median OS of patients treated with TACE combined with apatinib and camrelizumab of 24.8 months. A previous study by Cao et al. (20) on the treatment of unresectable advanced HCC showed a median OS and PFS of TACE combined with lenvatinib and sintilimab of 23.6 and 13.3 months, respectively. Liu et al. (26) reported a median OS and PFS of TACE combined with lenvatinib and camrelizumab in advanced HCC of 24 and 11.4 months, respectively. The results of TACE combined with targeted therapy were better than those of our study, which may be due to large differences in the baseline characteristics. Our study enrolled a greater number of patients with advanced-stage disease. The proportion of patients with BCLC stage C tumors enrolled in the above-mentioned studies was 76.8%, 75%, and 45%, respectively, but all patients enrolled in our study were BCLC stage C. Another possible reason is that the patients we enrolled had large tumor burdens with a maximum tumor diameter > 7 cm; therefore, it should be noted that the proportion of patients with PVTT invasion was as high as 50.5%, which may have greatly shortened the survival time of these patients. In addition, after the outbreak of COVID-19, due to the local epidemic prevention and control policies, some patients experienced delayed drug therapy, which may affect therapeutic efficacy. Nevertheless, compared with the above retrospective studies, the efficacy of TACE combined with apatinib and PD-1 inhibitors in advanced HCC patients reported in this study cannot be considered lower because of the relatively larger number of patients enrolled. Moreover, we used PSM to analyze the data, which reduced patient selection bias.
The incidence of TRAEs in this study was consistent with the published data (27–30). TACE-AP and TACE-A are safe, and the adverse reactions are tolerable. In this study, the adverse reactions related to embolism mainly included post-embolic syndrome (fever, nausea, vomiting, and pain) and apatinib-related adverse reactions, including hand-foot skin reactions, hypertension, proteinuria, fatigue, hoarseness, oral ulceration, and rash. The occurrences of hyperthyroidism, hypothyroidism, myocarditis, and pneumonia may be related to the use of PD-1 inhibitors. After symptomatic treatment or the temporary interruption of medication, these adverse event-related symptoms were relieved or eliminated. The incidence of TRAEs related to TACE and apatinib was not significantly different between the TACE-A and TACE-AP groups, suggesting that PD-1 inhibitors did not increase the risk of developing adverse effects in patients that underwent TACE. Therefore, our findings suggest that TACE combined with apatinib or TACE combined with apatinib and a PD-1 inhibitor to treat advanced HCC patients is controllable and safe.
This study was retrospective in nature. We achieved a balance in the baseline data of patients in the TACE-A and TACE-AP groups by using PSM analysis. There was a large reduction in sample size in the TACE-A group, which might have led to some key data not being included in the analysis, with analysis based on the differential baseline data lacking credibility for the control study. Therefore, to compensate for these shortcomings, we applied the stochastic survival forest model to further analyze the relevant factors affecting OS. Combined with traditional survival analysis, the final results showed that PVTT invasion, tumor number, AFP, TBIL, and treatment mode were continually the most significant prognostic factors affecting patient survival, consistent with previously reported research (8, 18).
This study has some limitations. First, this was a multicenter retrospective study, and the data were analyzed by PSM; however, potential patient selection bias could not be completely avoided. Second, multiple PD-1 inhibitors were used in this study, and the consistency of drug treatment could not be guaranteed. In addition, some patients did not receive apatinib or PD-1 inhibitors regularly after TACE due to economic or epidemic prevention and control measures. If patients had received combination therapy regularly, they may have obtained better clinical outcomes.
In conclusion, our study shows that, compared with TACE combined with apatinib, TACE combined with apatinib and a PD-1 inhibitor significantly prolonged OS and improved the ORR in patients with advanced HCC. Furthermore, the observed treatment-related reactions were safe and controllable. Nonetheless, these findings should be confirmed by prospective, multicenter, randomized controlled trials.
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by The study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital review board (Approval number 2019198). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conception and design the study: H-LL, H-TH; Provision of study materials or patients: W-LX, G-SC, GW, W-JF, Q-JY. Collection and assembly of data: W-LX, Y-G, X-HZ, S-JX. Data analysis and interpretation: X-HZ, Y-G. Manuscript writing: W-LX. Manuscript reviewing: H-LL, H-TH. Final approval of manuscript: All authors.
This work was supported by The National Natural Science Foundation (82002596), Henan Province Natural Science Foundation (212300410403), Medical Science and Technology Research Project of Henan Province (no. LHGJ20190633), Science and Technology Department of Henan Province (No. 212102310162), Beijing Health Alliance Charitable Foundation (HN-20201017-001), and the Technology Major Project of the Ministry of Science and Technology of China (2018ZX10303502).
Thanks to all patients and medical staff who participated in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Villanueva A. Hepatocellular carcinoma. N Engl J Med (2019) 380:1450–62. doi: 10.1056/NEJMra1713263
3. Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19:541–65. doi: 10.6004/jnccn.2021.0022
4. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer (2020) 9:682–720. doi: 10.1159/000509424
5. de Baere T, Arai Y, Lencioni R, Geschwind JF, Rilling W, Salem R, et al. Treatment of liver tumors with lipiodol TACE: Technical recommendations from experts opinion. Cardiovasc Intervent Radiol (2016) 39:334–43. doi: 10.1007/s00270-015-1208-y
6. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut (2020) 69:1492–501. doi: 10.1136/gutjnl-2019-318934
7. Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-To-Seven criteria and child-pugh a liver function: A proof-Of-Concept study. Cancers (Basel) (2019) 11(8):1084. doi: 10.3390/cancers11081084
8. Kan X, Liang B, Zhou G, Xiong B, Pan F, Ren Y, et al. Transarterial chemoembolization combined with apatinib for advanced hepatocellular carcinoma: A propensity score matching analysis. Front Oncol (2020) 10:970. doi: 10.3389/fonc.2020.00970
9. Guo Y, Ren Y, Chen L, Sun T, Zhang W, Sun B, et al. Transarterial chemoembolization combined with camrelizumab for recurrent hepatocellular carcinoma. BMC Cancer (2022) 22:270. doi: 10.1186/s12885-022-09325-6
10. Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A multicenter retrospective study. J Hepatocell Carcinoma (2021) 8:1233–40. doi: 10.2147/JHC.S332420
11. Ju S, Zhou C, Hu J, Wang Y, Wang C, Liu J, et al. Late combination of transarterial chemoembolization with apatinib and camrelizumab for unresectable hepatocellular carcinoma is superior to early combination. BMC Cancer (2022) 22:335. doi: 10.1186/s12885-022-09451-1
12. Hu HT, Luo JP, Cao GS, Li Z, Jiang M, Guo CY, et al. Hepatocellular carcinoma with portal vein tumor thrombus treated with transarterial chemoembolization and sorafenib vs. (125)Iodine implantation. Front Oncol (2021) 11:806907. doi: 10.3389/fonc.2021.806907
13. Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29:iv238–238iv255. doi: 10.1093/annonc/mdy308
14. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
15. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
16. Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol (2020) 21:808–20. doi: 10.1016/S1470-2045(20)30156-X
17. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer Res (2021) 27:1003–11. doi: 10.1158/1078-0432.CCR-20-2571
18. Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: A retrospective cohort study. Front Immunol (2022) 13:848387. doi: 10.3389/fimmu.2022.848387
19. Ju S, Zhou C, Yang C, Wang C, Liu J, Wang Y, et al. Apatinib plus camrelizumab With/Without chemoembolization for hepatocellular carcinoma: A real-world experience of a single center. Front Oncol (2021) 11:835889. doi: 10.3389/fonc.2021.835889
20. Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: A multicenter retrospective study. Front Oncol (2021) 11:783480. doi: 10.3389/fonc.2021.783480
21. Montasser A, Beaufrère A, Cauchy F, Bouattour M, Soubrane O, Albuquerque M, et al. Transarterial chemoembolisation enhances programmed death-1 and programmed death-ligand 1 expression in hepatocellular carcinoma. Histopathology (2021) 79:36–46. doi: 10.1111/his.14317
22. Cheu JW, Wong CC. Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology (2021) 74:2264–76. doi: 10.1002/hep.31840
23. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: From enhancement to normalization. Cell (2018) 175:313–26. doi: 10.1016/j.cell.2018.09.035
24. Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined locoregional-immunotherapy for liver cancer. J Hepatol (2019) 70:999–1007. doi: 10.1016/j.jhep.2019.01.027
25. Facciorusso A, Bellanti F, Villani R, Salvatore V, Muscatiello N, Piscaglia F, et al. Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: A meta-analysis of randomized trials. United Eur Gastroenterol J (2017) 5:511–8. doi: 10.1177/2050640616673516
26. Liu J, Li Z, Zhang W, Lu H, Sun Z, Wang G, et al. Comprehensive treatment of trans-arterial chemoembolization plus lenvatinib followed by camrelizumab for advanced hepatocellular carcinoma patients. Front Pharmacol (2021) 12:709060. doi: 10.3389/fphar.2021.709060
27. Lan CY, Wang Y, Xiong Y, Li JD, Shen JX, Li YF, et al. Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study. Lancet Oncol (2018) 19:1239–46. doi: 10.1016/S1470-2045(18)30349-8
28. Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol (2013) 31:3219–25. doi: 10.1200/JCO.2013.48.8585
29. Feun LG, Li YY, Wu C, Wangpaichitr M, Jones PD, Richman SP, et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer (2019) 125:3603–14. doi: 10.1002/cncr.32339
Keywords: hepatocellular carcinoma, transarterial chemoembolization, apatinib, PD-1 inhibitors, propensity score matching, random survival forest (RSF)
Citation: Xia W-L, Zhao X-H, Guo Y-, Cao G-S, Wu G, Fan W-J, Yao Q-J, Xu S-J, Guo C-Y, Hu H-T and Li H-L (2022) Transarterial chemoembolization combined with apatinib with or without PD-1 inhibitors in BCLC stage C hepatocellular carcinoma: A multicenter retrospective study. Front. Oncol. 12:961394. doi: 10.3389/fonc.2022.961394
Received: 04 June 2022; Accepted: 16 September 2022;
Published: 30 September 2022.
Edited by:
Zhiyu Xiao, Sun Yat-sen University, ChinaReviewed by:
Weixing Guo, Eastern Hepatobiliary Surgery Hospital, ChinaCopyright © 2022 Xia, Zhao, Guo, Cao, Wu, Fan, Yao, Xu, Guo, Hu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Tao Hu, SHVodWhvbmd0YW9neUAxNjMuY29t; Hai-Liang Li, bGloYWlsaWFuZ2d5QDE2My5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.