- 1Department of Hepatobiliary Surgery, 900TH Hospital of Logistics Support Force, Fuzhou, China
- 2Outpatient Department, Meng chao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, China
Objective: The aim of this study was to evaluate the association between preoperative IL-25 levels and HBV-HCC patient outcomes following liver surgery.
Methods: This study enrolled consecutive HCC patients that had undergone liver surgery from 2008 to 2015. Baseline patient clinical properties were assessed to establish predictors of postoperative overall survival and recurrence-free survival (OS and RFS, respectively) following liver resection. In addition, serum IL-25 levels were assessed via ELISA.
Results: Cox regression analyses revealed IL-25 levels to be independently related to the OS and RFS of 896 HBV-associated HCC patients. An optimal IL-25 cutoff level of 14.9 μg/ml was identified, with 206 patients in this cohort having IL-25 levels above this threshold. Both the OS and RFS of patients with an IL-25 level <14.9 μg/ml were significantly better after liver resection as compared to those of patients with higher preoperative levels of this cytokine (p < 0.05). Cox multivariate regression analyses revealed an IL-25 level ≥ 14.9 μg/L to be an independent predictor of poorer RFS and OS. A combination of IL-25 levels and tumor diameter may be an even more reliable predictor of OS.
Conclusions: IL-25 levels are independent predictors of postoperative survival within HCC patients undergoing liver resection.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors of the digestive system, with high morbidity and mortality (1, 2). Its early diagnosis is crucial for timely treatment and improvement of survival rate (3). Although ultrasound, magnetic resonance imaging (MRI), and other imaging techniques have greatly improved the accuracy of HCC diagnosis, their application is limited due to their high cost, strong invasiveness, and insensitivity to small tumors (3). Therefore, convenient, inexpensive, noninvasive, and reproducible serum biomarkers have played an important role in the diagnosis of HCC (4). Alpha-fetoprotein (AFP) is a widely used biomarker for the diagnosis of liver cancer, but its diagnostic accuracy is limited because it has a high false-negative rate in the detection of small tumors and early tumors. In addition, AFP may be elevated in some benign liver diseases, such as chronic hepatitis and cirrhosis without HCC (5). At present, the application of AFP in early screening of liver cancer has been controversial (5).
Therefore, it is very important to find new biomarkers related to liver cancer, achieve multi-indicator combined detection, improve the accuracy of early diagnosis of liver cancer, and reduce the rate of missed diagnosis. Over the years, other tumor markers for HCC have been proposed, such as Golgi protein 73 (GP73), Glypican-3 (GPC3), and cytokeratin 19 (CK-19) (6–8). GP73 is considered a potential marker of liver cancer, but serum GP73 levels may also be elevated in patients with liver parenchymal tumors. Therefore, GP73 detection is not suitable for distinguishing HCC from benign liver disease (6). Liu et al. found that serum GPC3 level was increased in patients with liver cancer; however, GPC3 was not sensitive to distinguish benign diseases from early liver cancer (7). Previous studies have shown that CK-19 expression is related to the invasive behavior of HCC, such as low differentiation, metastasis, and microvascular invasion, which indicates that CK-19 can be used as an indicator of survival and recurrence of HCC patients (8). However, these markers have not been considered effective enough for clinical use as indicators for HCC diagnosis.
Chronic inflammation is often a main driver of oncogenesis, and suppressing such inflammation can thus slow or arrest the physiological progression of cancer (9, 10). Inflammatory factors have been closely linked to many solid tumor types, including HCC (11), with certain cytokines including interleukin-6 (IL-6) serving as key mediators of systemic immune responses (12). There have been several previous reports demonstrating that the serum levels of inflammatory factors can predict the development or prognosis of many forms of cancer, including HCC (13). In addition, the understanding of the relationship between IL-25 and clinicopathological features, as well as the role of IL-25 in assessing the diagnostic role in HCC, has not been fully investigated. These findings may contribute to a more complete understanding of the significance of IL-25 in HCC. Here, to resolve these controversies, we measured serum IL-25 levels to evaluate the individual and combined diagnostic performance of IL-25 and AFP for HCC. The diagnostic ability of IL-25 for AFP-negative HCC was also evaluated. In addition, we analyzed the relationship between serum IL-25 levels and clinicopathological features in patients with HCC, in order to investigate the value of IL-25 in assessing the progression and prognosis of HCC. Herein, we therefore explored the prognostic relevance of preoperative IL-25 levels among hepatitis B virus (HBV)-associated HCC cases that had undergone liver resection.
Patients and methods
Patients
For this study, HBV-infected patients that had undergone liver transplantation conducted by a single surgical team at the 900th Hospital of Logistics Support Force from January 2008 to June 2015 were retrospectively enrolled. All patients had been diagnosed with HCC as per the European Society for the Study of the Liver (EASL) criteria (14), with pathological examination being used to confirm this diagnosis. Selection criteria for cases that participated in this research cohort were as follows: World Health Organization (WHO) preoperative status = 0–1; Child-Pugh Class A; no macrovascular invasion; no distant metastases; and no preoperative chemotherapy, radiosurgery, radiotherapy, or dermal ethanol injections prior to resection of liver. Patients were HBsAg positive and hepatitis C virus (HCV)-Ab negative. The Hospital Institutional Review Committee of the 900th Hospital of Logistics Support Force confirmed the present research, with cases having given the letter of aware satisfaction.
Follow-up
For the first 2 years after surgery, patients experienced follow-up every 3 months, and every 6 months thereafter. Hospital staff blinded to study objectives conducted all follow-up. All patients were regularly monitored for recurrence using approaches including AFP analyses, chest x-rays, and abdominal USG, MRI, or CT scans that were conducted every 3 months. HCC recurrence was diagnosed using the same criteria as were used to diagnose the primary disease before surgery. Approaches to treating recurrent diseases included TACE, PRFA, and PEI, with the exact procedure being selected based on patient- and tumor-specific factors.
Propensity score matching
To diminish the potential for bias inherent in this retrospective analysis, propensity score matching (PSM) was performed. Specifically, cases with low and high IL-25 were matched via a PSM approach as described previously (15). Covariates included in this PSM model are Ishak’s inflammation, tumor diameter, AFP, AST, HBeAg, HBV-DNA load, encapsulation of tumor, microvascular invasion, tumor count, and the degree of liver resection. Matching was executed at a 1:1 ratio for cases with low and high IL-25 levels as detailed previously (16).
ELISA
Levels of serum IL-25 were measured in HCC patient samples with the Human IL-25 DuoSet ELISA kit (R&D Systems) based on the provided directions.
Statistical studies
All outcomes are given as median (range) or mean ± standard deviation (SD), and were studied by implementing unpaired Student’s t-tests or χ2 assessments as appropriate. The OS and RFS cases were assessed with Kaplan–Meier curves as well as log-rank measurements. Multivariate and univariate methods were used to guide the design of a prognostic nomogram, which was constructed with the “rms” package using R v.3.5.1 (http://www.r-project.org/). This nomogram was assessed based on measurements of the conformity index (C-index), with rcorrp.cens being used to compare the C-index values for this nomogram to those for other nomograms in Hmisc (17). Analyses of the receiver operating characteristic (ROC) curve were implemented to study nomograms and predictors, with p < 0.05 as the threshold of significance.
Results
Baseline patient characteristics
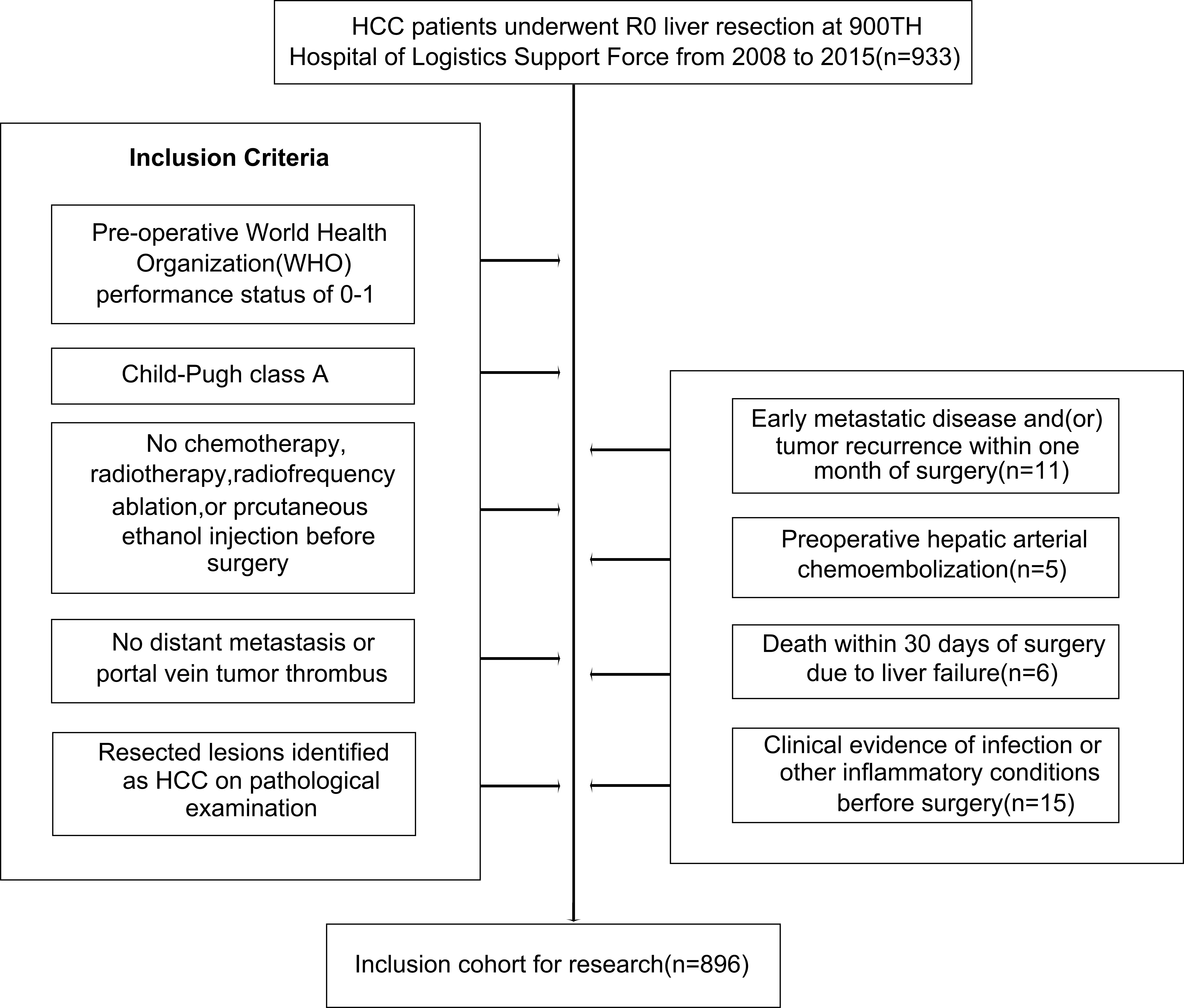
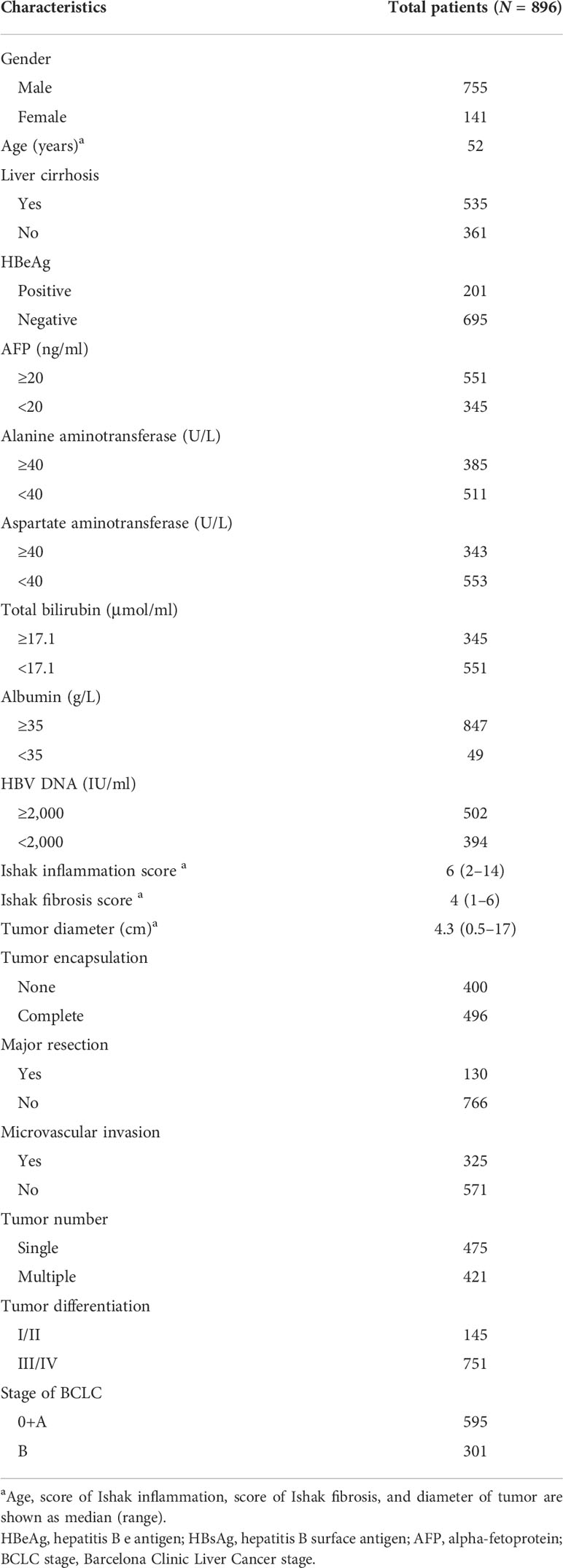
Over the defined study period, 933 patients with HBV-associated HCC underwent liver transplantation for curative purposes, and were registered in the present survey. Of these cases, 37 were excluded for reasons including early metastasis or recurrence within 30 days postoperatively (n = 11), surgery (n = 5), liver failure-related mortality within 30 days postoperatively (n = 6), or clinically detected preoperative infection (n = 15), leaving a cohort of 896 patients eligible for these analyses (Figure 1). These patients exhibited a mean age of 52 years (range: 29–75), and were predominantly male (755 male patients and 141 female patients) as shown in Table 1. All patients were positive for HBeAg and the remaining 695 were negative for HBeAg. All exhibited Child-Pugh A liver function levels, with a median inflammation level of 6 (range: 2–14). A total of 502 cases exhibited HBV DNA levels ≥ 2,000 IU/ml. Primary tumors were a median of 4.3 cm in size (range: 0.5–17 cm), and serum IL-25 levels ranged from 0.25 to 45 µg/L (median: 10.1 μg/L). Under BCLC staging criteria, 595 patients were stage 0 and 301 were stage B. Of these 896 patients, 325 exhibited microvascular, 421 presented with multiple tumors, and 496 exhibited complete tumor encapsulations. In addition, 130 patients underwent major liver resection. Tumors differed markedly in 145 patients (E-S Grades I and II). The median period of follow-up was 41.5 months (range: 9.5–98.5).
IL-25 levels are associated with HCC patient clinicopathological characteristics
Next, ROC curve analyses were used to establish an optimal IL-25 cutoff level capable of differentiating between HCC patient outcomes. The selected cutoff value was 14.9 μg/L, yielding an AUC value of 0.730, a specificity of 0.640, and a sensitivity of 0.757 (Figure 2). In total, 334 and 562 patients were respectively clustered into IL-25-high and -low groups, and there were clear differences in clinical characteristics among these groups (Table 2). Specifically, individuals with high IL-25 levels exhibited higher AFP levels, greater viral loads (≥2,000 IU/ml), and larger tumor sizes (all p < 0.05), indicating that higher IL-25 levels are associated with more advanced HCC. After a PSM analysis, 156 patient pairs were generated (Table 3). Following PSM, clinical characteristics did not differ between these cohorts (p > 0.05).
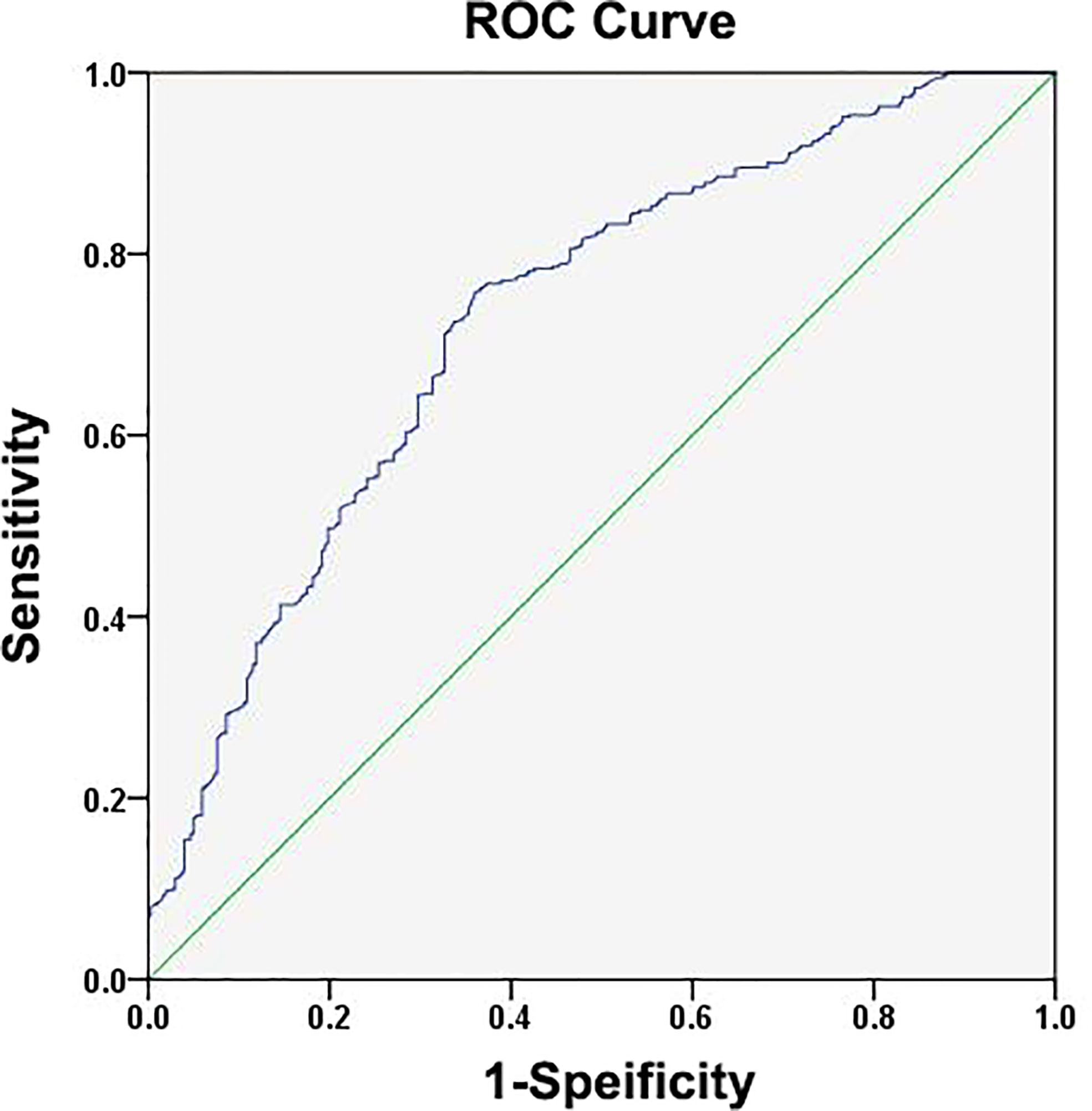
Figure 2 Assessments of the receiver operating characteristic (ROC) curve for the specificity and sensitivity of IL-25 in HCC patients.
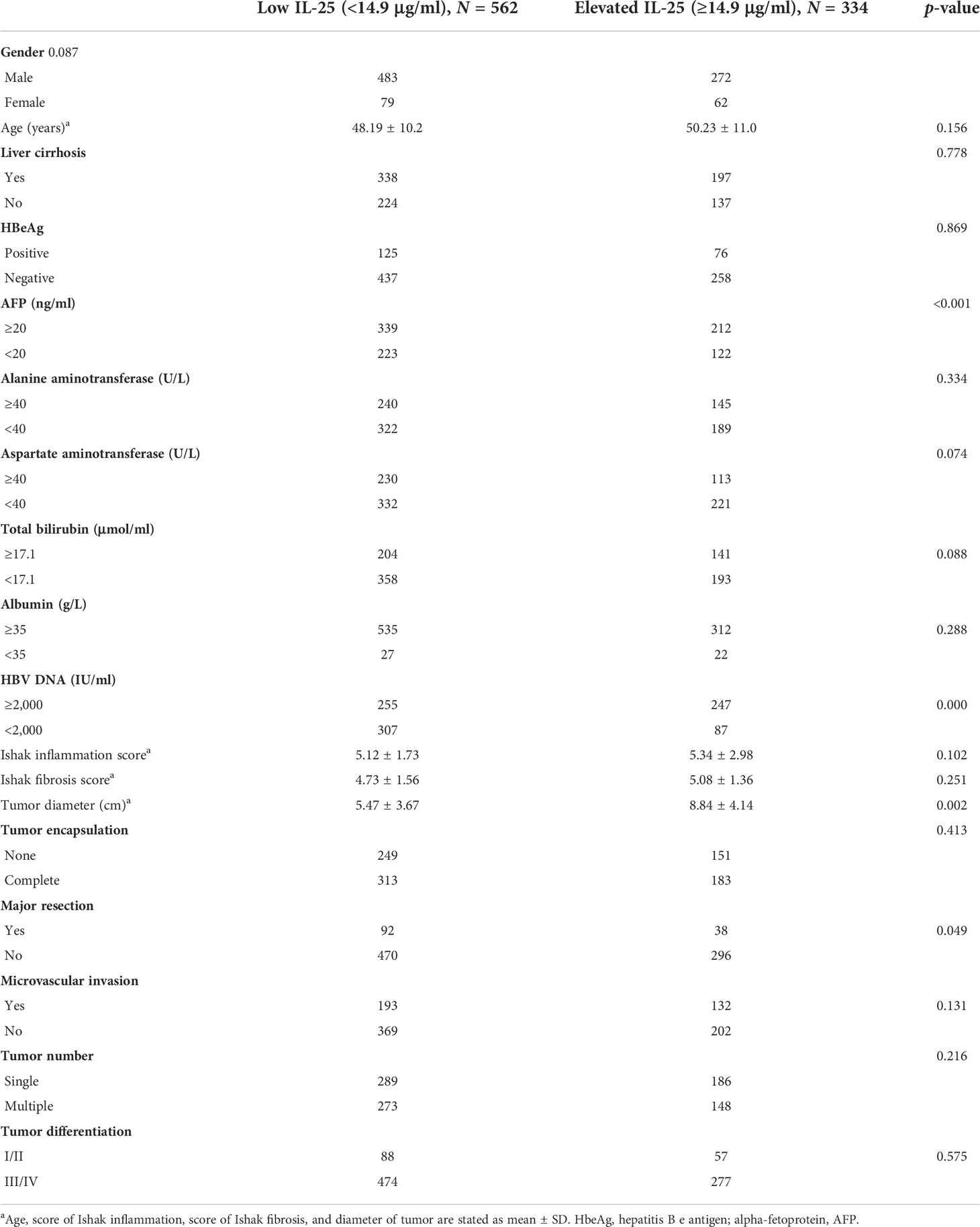
Table 2 HCC patient clinicopathological and demographic characteristics as a function of IL-25 levels.
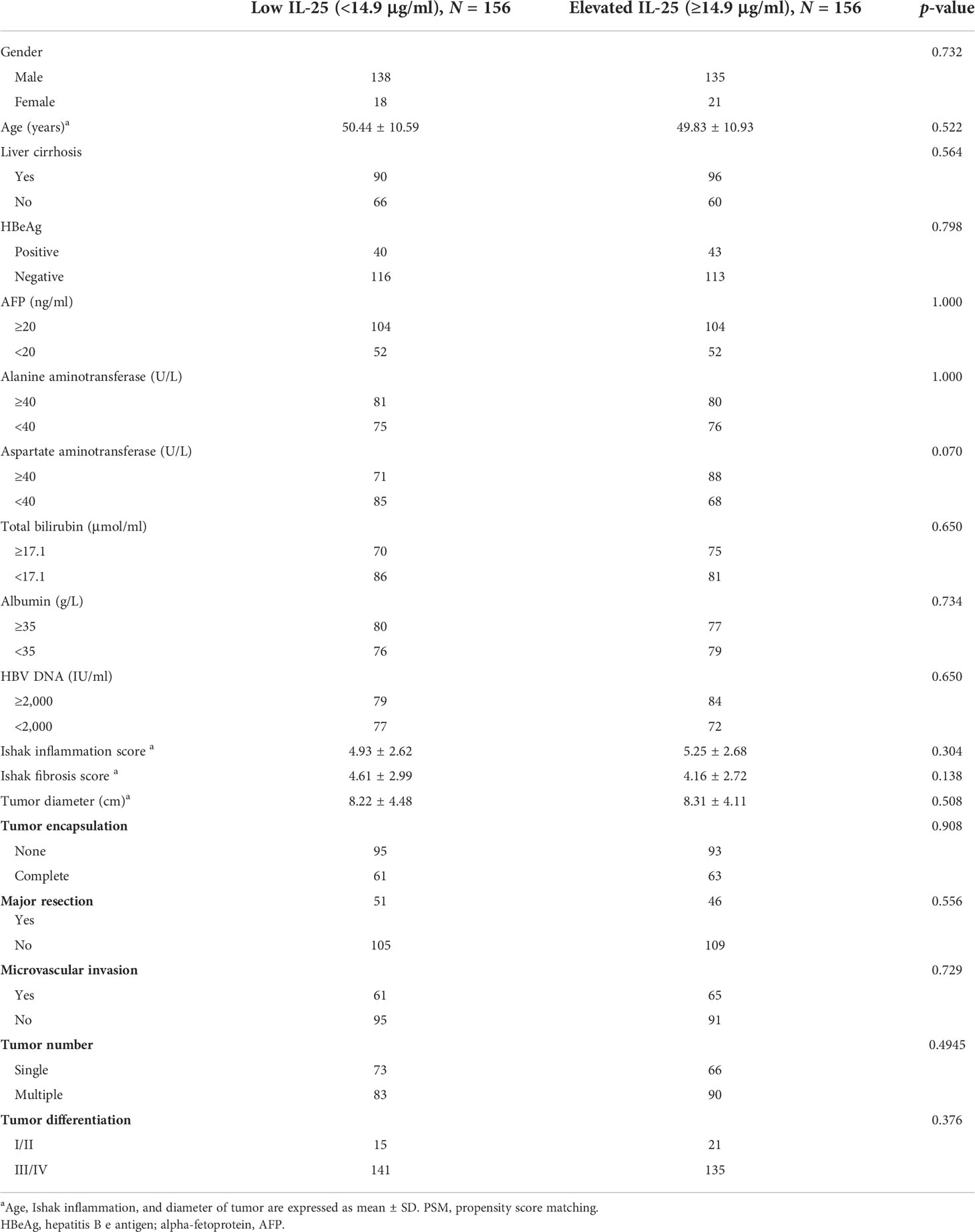
Table 3 HCC patient clinicopathological and demographic characteristics as a function of IL-25 levels after propensity score matching (PSM).
IL-25 levels are correlated with HCC patient prognosis
The 3- and 5-year RFS rates of patients in the group of high IL-25 were detected to be considerably decreased in comparison to those of patients in the low IL-25 group (64.1% and 42.2%, respectively, vs. 90.1% and 78.5%, respectively; p < 0.05). Higher levels of IL-25 were also associated with decreased 3- and 5-year OS relative to lower IL-25 levels (77.3% and 61.8%, respectively, vs. 97.6% and 95.1%, respectively; p < 0.05) (Figures 3A, B).
Following PSM, the 3- and 5-year RFS rates in the IL-25-high group were 61.6% and 42.3%, respectively, whereas they were significantly higher at 89.1% and 76.2%, respectively, in the IL-25-low group (p < 0.05). Similarly, following the PSM, the 3- and 5-year OS of cases with high IL-25 levels were 77.6% and 61.1%, respectively, which were significantly decreased as compared to those of cases with low IL-25 levels (p < 0.05) (Figures 3C, D)
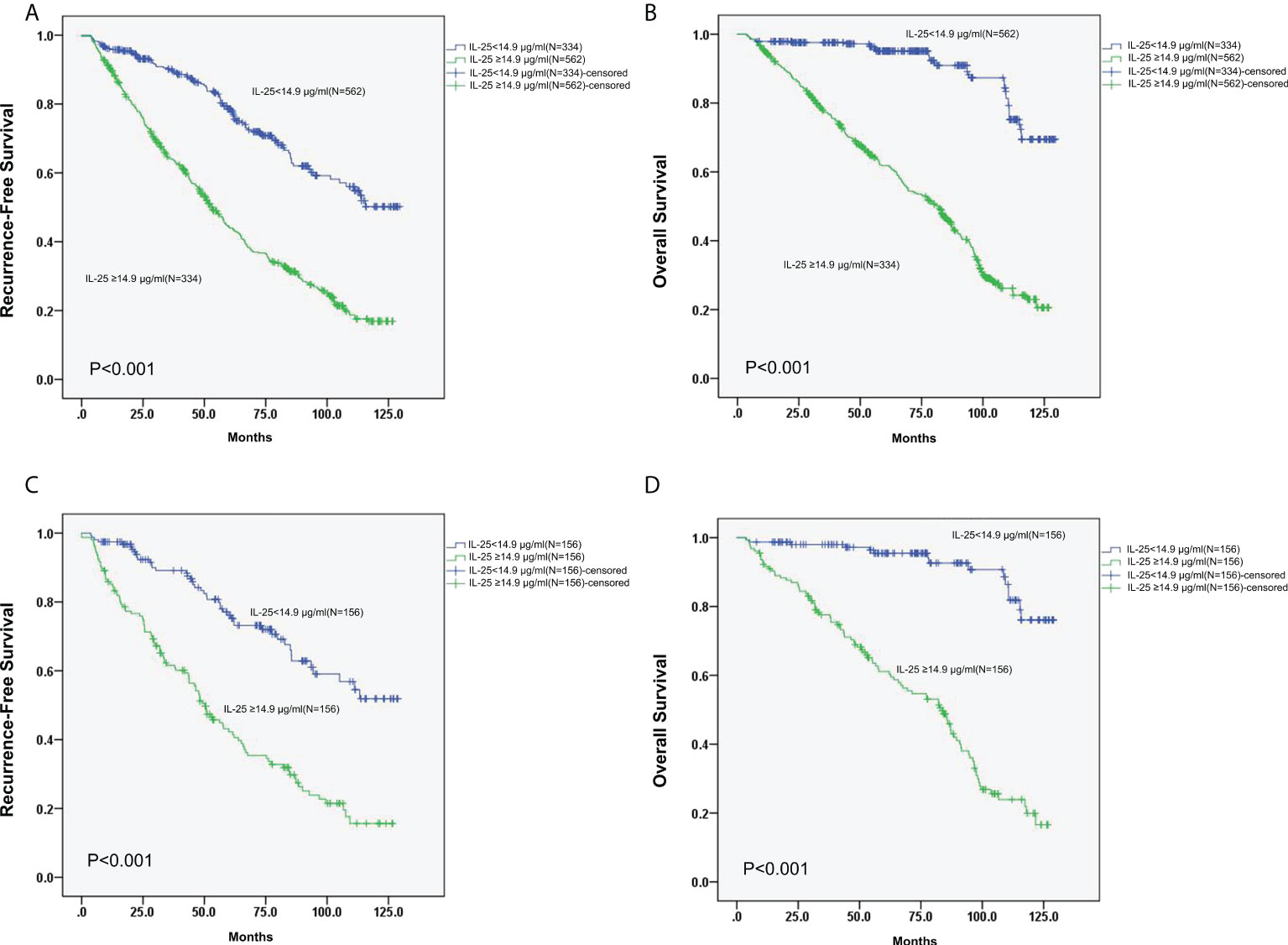
Figure 3 (A, B) RFS (A) and OS (B) curves for all 896 HCC patients with low or high IL-25 levels. (C) Curves of RFS for HCC patients in the PSM cohort with low or high IL-25 levels. (D) Curves of OS for HCC patients in the PSM cohort with low or high IL-25 levels.
Identification of factors related to HCC patient prognosis
Cox regression analyses were next applied to detect risk factors independently correlated with HCC patient RFS and OS. In univariate analyses, HBV DNA levels, IL-25 levels, AFP levels, microvascular invasion, tumor encapsulation, tumor number, tumor differences, tumor scale, and cirrhosis were all independently correlated with a decreased RFS (Table 4), whereas HBV DNA levels, IL-25 levels, AFP levels, tumor encapsulation, and invasion were correlated with worse OS. Subsequently, multivariate approach revealed that HBV DNA levels ≥2,000 IU/ml, IL-25 ≥ 14.9 μg/ml, AFP ≥ 20 ng/ml, a lack of complete encapsulation of tumor, multiple tumors, microvascular invasion, and tumor size ≥ 5 cm were independent predictors of worse patient RFS (Table 4), while HBV DNA levels ≥ 2,000 IU/ml, IL-25 ≥ 14.9 μg/L, AFP ≥ 20 ng/ml, a lack of complete tumor encapsulation, and tumor size ≥ 5 cm were independently predictive of worse OS (Table 5). Additional analyses of Cox regression were conducted to detect independent predictors of OS and RFS in the cohort of PSM (Tables 6, 7). In this group, an elevated IL-25 level (≥14.9 μg/ml) remained independently associated with poorer RFS and OS.
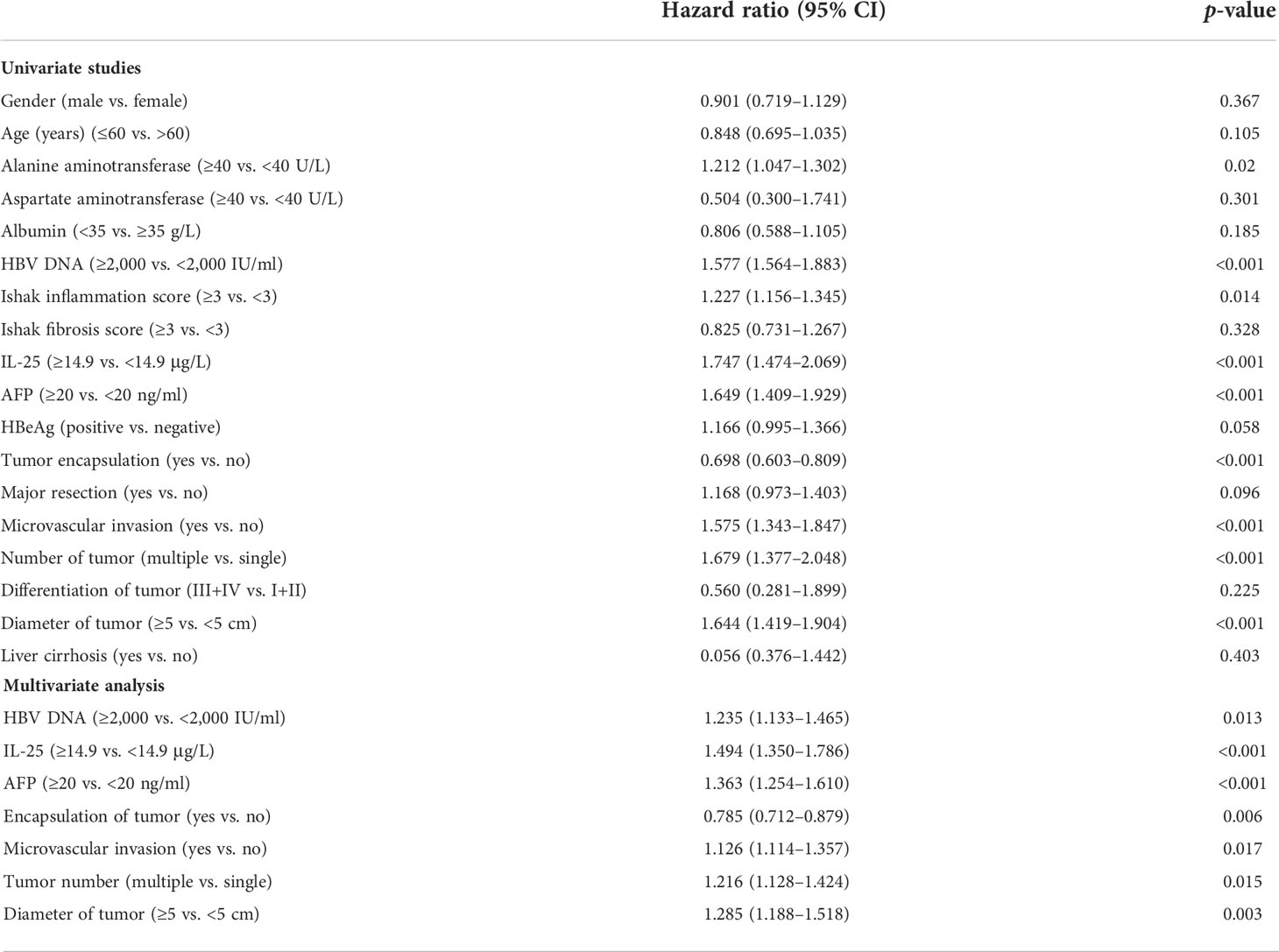
Table 4 Multivariate and univariate studies of factors correlated with HCC patient recurrence-free survival.

Table 5 Multivariate and univariate studies of factors correlated with HCC patient overall survival.
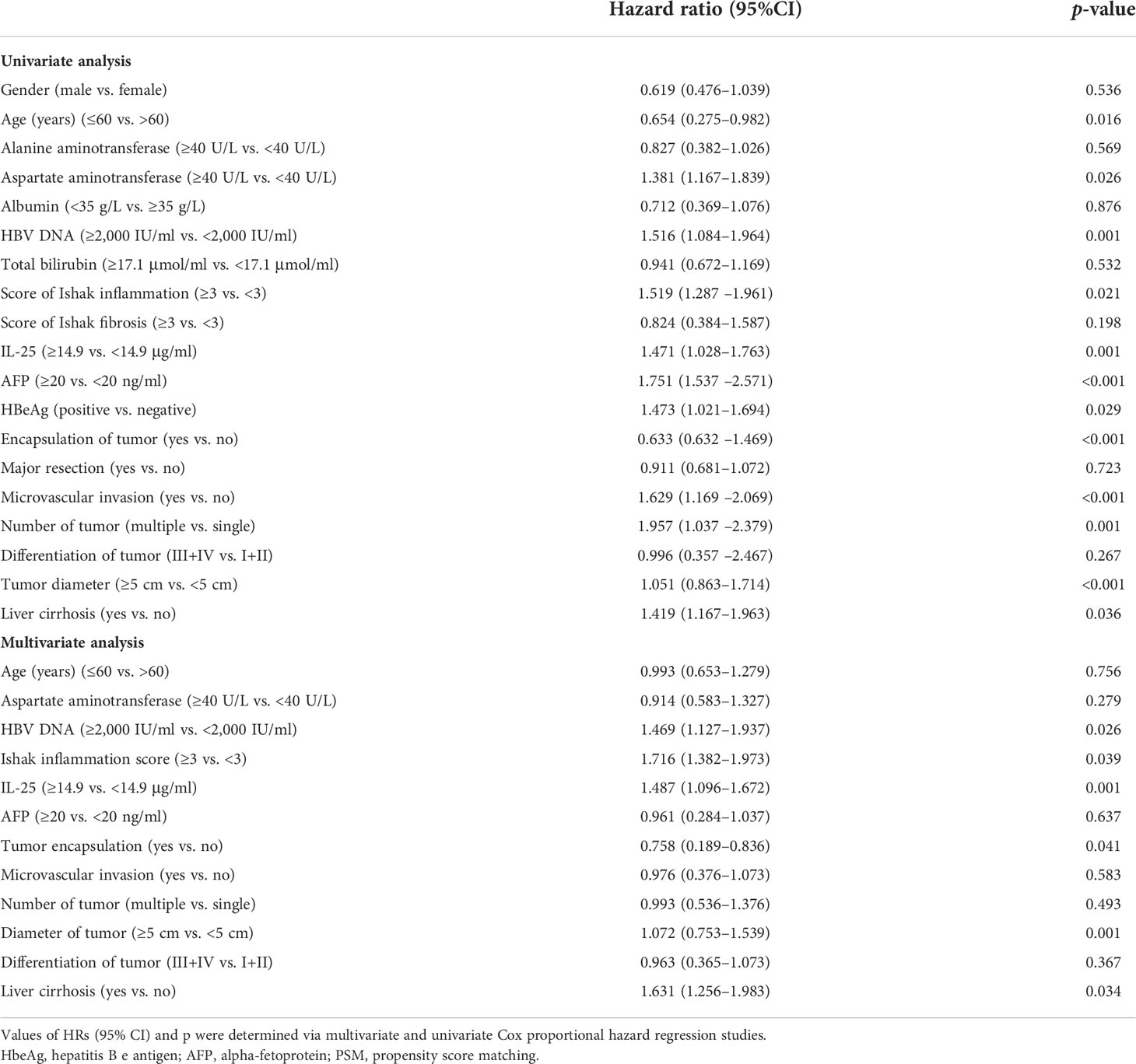
Table 6 Multivariate and univariate studies of factors correlated with HCC patient recurrence-free survival in a propensity score matching (PSM) cohort.
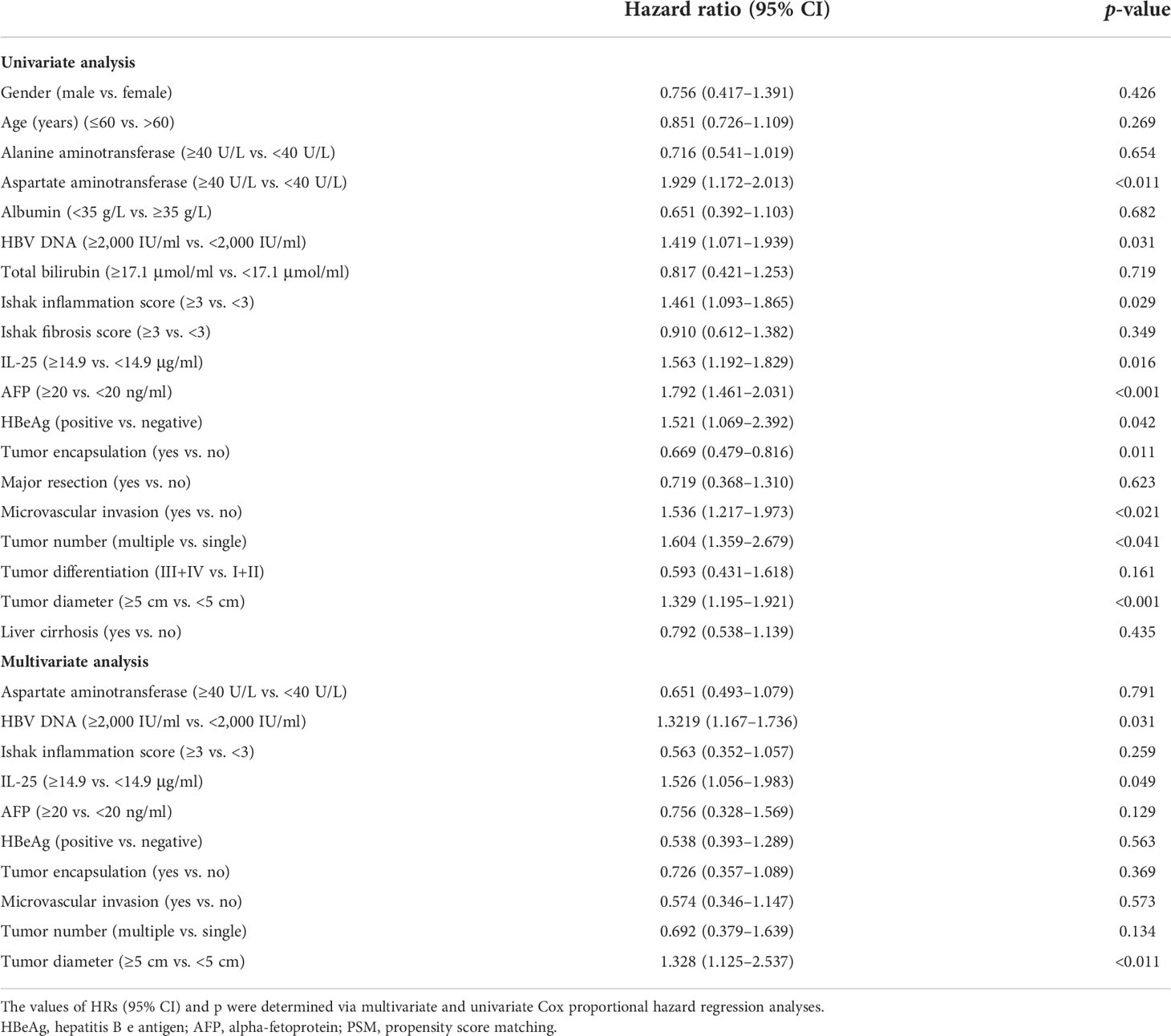
Table 7 Multivariate and univariate analyses of factors correlated with HCC patient overall survival in a propensity score matching (PSM) cohort.
Construction and evaluation of nomograms capable of predicting HCC patient survival outcomes
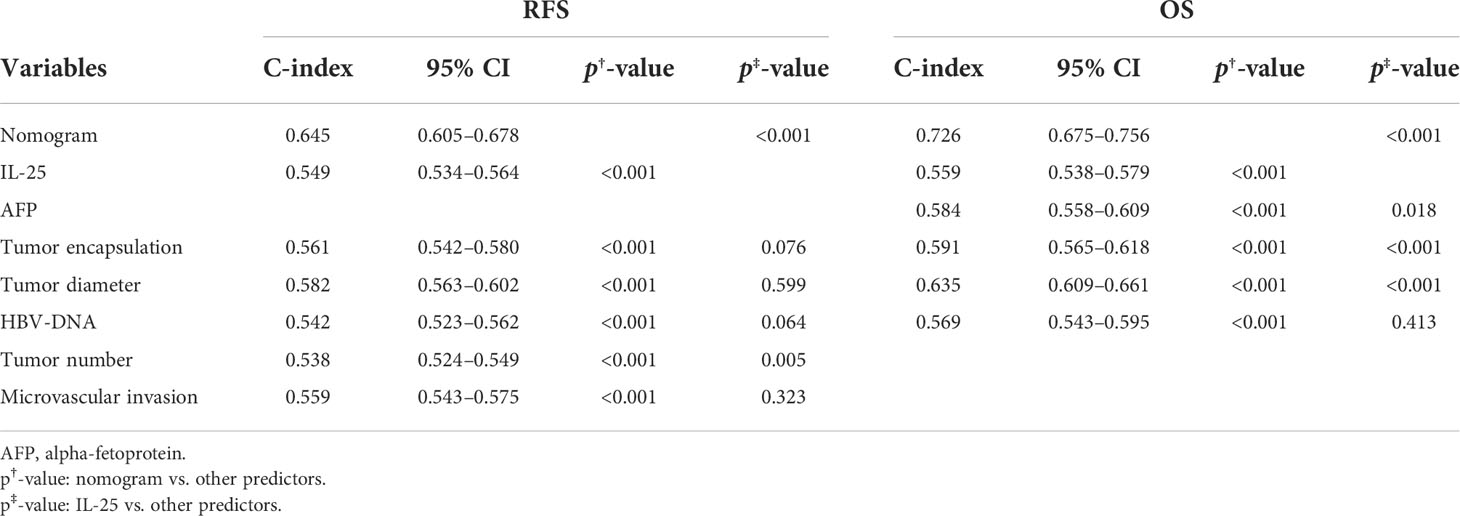
Next, the independent predictors identified in the above multivariate analysis were used to construct nomograms capable of predicting the RFS (Figure 4A) and OS of HCC patients (Figure 4B). The respective values of the C-index for these nomograms of OS and RFS were 0.726 and 0.645. To explore the predictive value of these nomograms, they were next compared with other independent predictors identified above (Table 8). The RFS nomogram C-index value (0.645) was higher than that for HBV DNA (0.542), AFP (0.564), tumor count (0.538), encapsulation of tumor (0.561), IL-25 (0.549), tumor diameter (0.582), and microvascular invasion (0.559) (all p < 0.001). The nomogram C-index value (0.726) for OS was higher than that for IL-25 (0.559), HBV-DNA (0.569), AFP (0.584), encapsulation of tumor (0.591), and diameter of tumor (0.635) (all p < 0.05). These results thus supported the predictive accuracy of these nomograms, with both the RFS and OS nomograms exhibiting AUC values higher than those for other prognostic risk factors (Table 9).
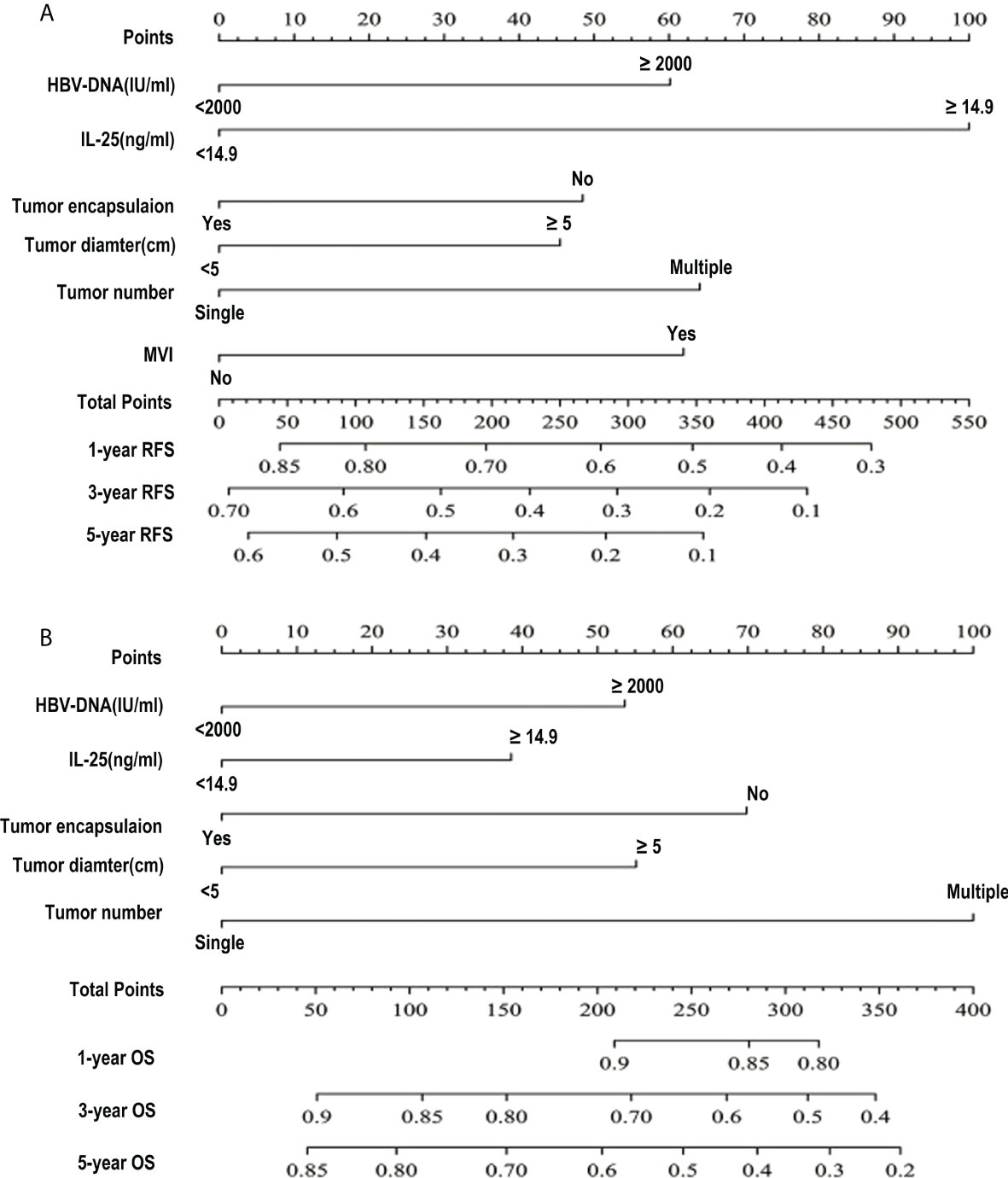
Figure 4 HCC patient survival nomogram. (A, B) A survival nomogram designed to assess HCC patient RFS (A) and OS (B).
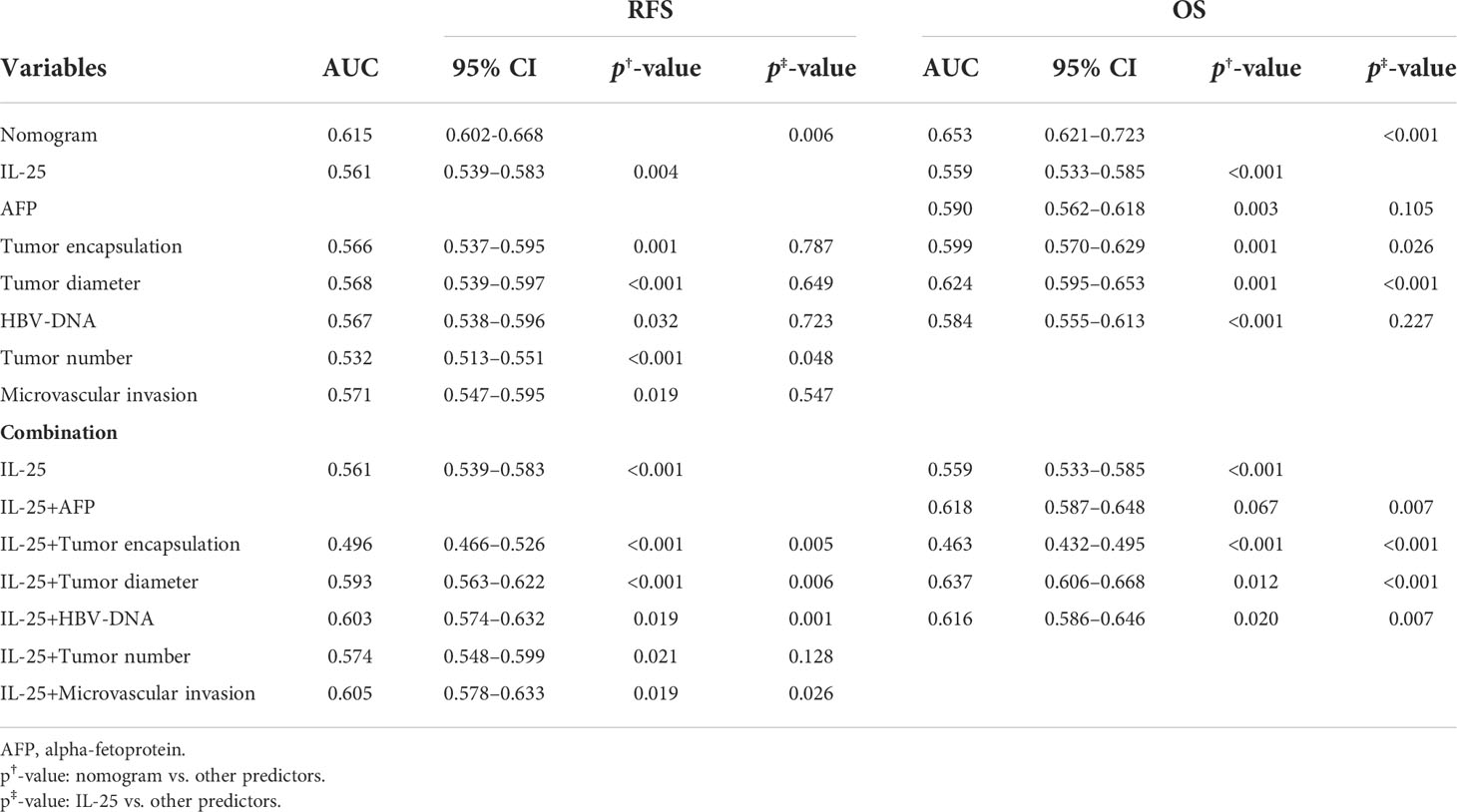
Table 9 ROC curve results pertaining to analyses of the predictors of recurrence-free and overall HCC patient survival.
Assessment of the prognostic value of IL-25 as a predictor of HCC patient survival
For RFS, the C-index value for IL-25 was 0.549, which was considerably greater as compared to that associated with the number of tumors (p < 0.05) (Table 8). In a ROC curve analysis for RFS (Table 9), no differences were observed. The AUC value for IL-25 was higher than that for all other predictors with the exception of tumor number (p < 0.05), and in an analysis of multivariate for predictors associated with patient RFS, the HR for IL-25 was the greatest. As IL-25 exhibited the greatest specific weight of any factor in a predictive nomogram for HCC patient RFS, this suggested that IL-25 is the most robust predictor of RFS in this patient population (Figure 4A). The C-index value for IL-25 when used to predict HCC patient OS was 0.559, which was the lowest of all tested predictors (Table 8) in an analysis of the curve of ROC (Table 9). Furthermore, IL-25 exhibited a lower AUC value than any other predictor analyzed in this study, and consistently possessed the least specific weight in a nomogram used to predict patient OS (Figure 4B). As such, we evaluated combinations of IL-25 and other predictors with the goal of defining the most reliable prognostic combination associated with patient OS (Table 9), revealing that a combination of IL-25 and tumor diameter yielded a greater AUC value than any other combination, thus suggesting that these two parameters may represent a more reliable means of predicting HCC patient OS.
Discussion
The onset and progression of HCC are driven in large part by interactions between nascent tumor cells and the surrounding inflammatory milieu (18–22). There is thus clear value in further elucidating the specific roles played by particular inflammatory mediators during the progression of cancer (9, 10). HCC is a common malignant tumor of the digestive system, characterized by aggressive growth and early metastasis, and is the second leading cause of cancer mortality in China (2, 3). Because the early symptoms are not obvious, many patients with liver cancer are diagnosed as advanced stage (23). Systematic screening of high-risk groups is necessary for early diagnosis. AFP is the most commonly used biomarker for HCC patients, although its sensitivity and specificity are unsatisfactory, especially for early-stage disease (14, 15). Previous studies have shown that the ability of AFP to diagnose liver cancer is relatively poor. Using cutoff values of 17.76 ng/ml and 21.47 ng/ml would result in 60 (35.71%) and 62 (36.90%) of 168 HCC patients being considered negative. Fifteen of 153 healthy controls (9.80%) and 23 of 150 patients with benign liver disease (15.33%) were considered positive, and these inaccuracies supported the inadequacy of AFP as a biomarker (24). Therefore, new and reliable biomarkers are needed to improve the diagnostic level of liver cancer.
IL-25 is an inflammatory IL-17 family cytokine that is best studied as a driver of type 2 immune responses (13, 25–27). In previous reports, IL-25 was shown to perform a central task in the incidence of acute hepatitis (AH), liver fibrosis, and cirrhosis (28–30). As an anti-inflammatory cytokine, IL-25 promotes type 2 cytokine-dependent immunity and limits the production of pro-inflammatory cytokines by inhibiting the expression of type 1 cytokines. Deregulation of IL-25 has been found in many inflammation-related diseases, including helminth parasite infection, inflammatory bowel disease, asthma, severe hepatitis, and NAFLD (31–33). Meanwhile, IL-25 also plays an important role in several human cancers (31–35). However, it is not completely clear whether IL-25 affects the development of HCC. Studies have shown that IL-25 plays a direct role in cancer cells and affects the development of breast cancer (32–34). Previous results showed that IL-25 did not directly affect the growth, apoptosis, or migration of HCC cells. IL-25-induced M2 macrophages attenuated obesity and NAFLD (36). Similarly, Wang et al. reported that IL-25 induces hepatic macrophages to have an M2 phenotype, negatively regulates the pro-inflammatory immune microenvironment, and improves HDF-induced hepatic steatosis (37). Rizzo et al. reported that IL-25-induced alternatively activated macrophages inhibit colitis (38). In addition, Zhujun Jiang et al. reported that inhibition of IL-25 led to a decrease in the incidence rate of type 2 diabetes T cells and macrophages in the primary tumor microenvironment, as well as enhanced breast tumor invasion and subsequent lung metastasis (31). These findings suggest that macrophages are the key targets of IL-25, and the activation of M2 phenotype may be the main pathway by which IL-25 promotes the development of HCC. Herein, we found that elevated preoperative IL-25 levels were correlated with features of more advanced HCC and with poorer clinical outcomes (RFS and OS) within HBV-associated HCC cases following the resection of the liver. Tumor recurrence differed significantly between cases with low and high levels of serum IL-25 determined via a multivariate analysis approach, with elevated preoperative IL-25 levels being independent predictors of decreased OS and RFS in these cases. Importantly, high IL-25 levels functioned as an accurate predictor of long-lasting survival in cases with early-stage disease. While IL-25 levels were the best-identified predictor of RFS in this study, a combination of IL-25 levels and tumor diameter was better able to predict HBV-associated HCC patient OS. The mechanisms behind these effects are not fully understood, but some researchers believe that IL-25-induced dysregulation of intestinal microbiota promotes hepatocellular carcinoma through alternate activation of macrophages in the tumor microenvironment. Together, these results provide clear evidence that preoperative serum IL-25 levels can predict HCC patient prognosis.
The primary limitation of this research is that it was a single-center retrospective analysis, and it is thus susceptible to potential bias with respect to patient selection. Future large-scale multi-center studies validating and expanding upon our results will thus be essential to affirm the clinical relevance of serum IL-25 as a prognostic biomarker in HBV-HCC patients.
Conclusion
This study suggests that serum IL-25 levels may be an independent and useful tumor marker for the diagnosis of liver cancer. IL-25 is still valuable in the diagnosis of AFP-negative HCC and can be used as a supplement to AFP in the diagnosis of HCC. The combined diagnosis of the two markers greatly improves the early diagnostic accuracy of HCC. In addition, IL-25 values are associated with several pathological features that represent tumor aggressiveness and/or poor prognosis. Finally, IL-25 could help in the customized management of cases with risk factors for HCC recurrence after liver resection.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of 900TH Hospital of Logistics Support Force (LLH-20150801). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception and design: S-hC and XW; Administrative support: S-hC and XW; Provision of study materials or patients: S-hC and XW; Collection and assembly of data: All authors; Data analysis and interpretation: All authors; Manuscript writing: All authors; Final approval of manuscript: All authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Dechassa ML, Tryndyak V, de Conti A, Xiao W, Beland FA, Pogribny IP. Identification of chromatin-accessible domains in non-alcoholic steatohepatitis-derived hepatocellular carcinoma. Mol Carcinog (2018) 57(8):978–87. doi: 10.1002/mc.22818
3. Cheng Z, Lei Z, Yang P, Si A, Xiang D, Tang X, et al. Exosome-transmitted p120-catenin suppresses hepatocellular carcinoma progression via STAT3 pathways. Mol Carcinog (2019) 58(8):1389–99. doi: 10.1002/mc.23022
4. Zhang N, Zhang S, Wu W, Lu W, Jiang M, Zheng N, et al. Regorafenib inhibits migration, invasion, and vasculogenic mimicry of hepatocellular carcinoma via targeting ID1-mediated EMT. Mol Carcinog (2021) 60(2):151–63. doi: 10.1002/mc.23279
5. Bao Y, Suvesh M, Li X, Bai X, Li H, Li X, et al. Ebp1 p48 promotes oncogenic properties in hepatocellular carcinoma through p38 MAPK/HIF1α activation and p53 downregulation. Mol Carcinog (2021) 60(4):252–64. doi: 10.1002/mc.23288
6. Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, et al. GP73, a resident golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol (2005) 43(6):1007–12. doi: 10.1016/j.jhep.2005.05.028
7. Xu C, Yan Z, Zhou L, Wang YM. A comparison of glypican-3 with alpha-fetoprotein as a serum marker for hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol (2013) 139(8):1417–24. doi: 10.1007/s00432-013-1458-5
8. Kawai T, Yasuchika K, Ishii T, Katayama H, Yoshitoshi EY, Ogiso S, et al. Keratin 19, a cancer stem cell marker in human hepatocellular carcinoma. Clin Cancer Res (2015) 21(13):3081–91. doi: 10.1158/1078-0432.CCR-14-1936
9. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025
10. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
11. Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res (2015) 35(1):1–16. doi: 10.1089/jir.2014.0026.
12. Cheung YT, Ng T, Shwe M, Ho HK, Foo KM, Cham MT, et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol (2015) 26(7):1446–51. doi: 10.1093/annonc/mdv206
13. Unver N, Delgado O, Zeleke K, Cumpian A, Tang XM, Caetano M, et al. Reduced IL-6 levels and tumor-associated phospho-STAT3 are associated with reduced tumor development in a mouse model of lung cancer chemoprevention with myo-inositol. Int J Cancer (2018) 142(7):1405–17. doi: 10.1002/ijc.31152
14. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. conclusions of the Barcelona-2000 EASL conference. European association for the study of the liver. J Hepatol (2001) 35:421–30. doi: 10.1016/S0168-8278(01)00130-1
15. Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics (1996) 52:249–64. doi: 10.2307/2533160
16. Yang T, Lu JH, Lau WY, Zhang TY, Zhang H, Shen YN, et al. Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma: A propensity score matching analysis. J Hepatol (2016) 64(3):583–93. doi: 10.1016/j.jhep.2015.10.012
17. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol (2013) 31(9):1188–95. doi: 10.1200/JCO.2012.41.5984
18. Yang YM, Kim SY, Seki E. Inflammation and liver cancer: Molecular mechanisms and therapeutic targets. Semin Liver Dis (2019) 39(1):26–42. doi: 10.1055/s-0038-1676806
19. Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol (2014) 816:401–35. doi: 10.1007/978-3-0348-0837-8_16
20. Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol (2018) 19(3):222–32. doi: 10.1038/s41590-018-0044-z
21. Refolo MG, Messa C, Guerra V, Carr BI, D'Alessandro R. Inflammatory mechanisms of HCC development. Cancers (Basel) (2020) 12(3):641. doi: 10.3390/cancers12030641
22. Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol (2015) 21(37):10573–83. doi: 10.3748/wjg.v21.i37.10573
23. Yavari K. Anti-angiogenesis therapy of cancer cells using 153Sm-bevasesomab. Emerg Sci J (2018) 219(4):717–25. doi: 10.28991/esj-2018-01136
24. Kim MN, Kim BK, Kim SU, Park JY, Ahn SH, Han KH, et al. Longitudinal assessment of alpha-fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis. Scand J Gastroenterol (2019) 54:1283–90. doi: 10.1080/00365521.2019.1673478
25. Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X, et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget (2017) 8(13):20741–50. doi: 10.18632/oncotarget.15119
26. Ruzzo A, Catalano V, Canestrari E, Giacomini E, Santini D, Tonini G, et al. Genetic modulation of the interleukin 6 (IL-6) system in patients with advanced gastric cancer: a background for an alternative target therapy. BMC Cancer (2014) 14:357. doi: 10.1186/1471-2407-14-357
27. Dalal V, Kumar R, Kumar S, Sharma A, Kumar L, Sharma JB, et al. Biomarker potential of IL-6 and VEGF-a in ascitic fluid of epithelial ovarian cancer patients. Clin Chim Acta (2018) 482:27–32. doi: 10.1016/j.cca.2018.03.019
28. Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther (2014) 141(2):125–39. doi: 10.1016/j.pharmthera.2013.09.004
29. Caetano MS, Zhang H, Cumpian AM, Gong L, Unver N, Ostrin EJ, et al. IL6 blockade reprograms the lung tumor microenvironment to limit the development and progression of K-ras-Mutant lung cancer. Cancer Res (2016) 76(11):3189–99. doi: 10.1158/0008-5472.CAN-15-2840
30. Ma H, Yan D, Wang Y, Shi W, Liu T, Zhao C, et al. Bazedoxifene exhibits growth suppressive activity by targeting interleukin-6/glycoprotein 130/signal transducer and activator of transcription 3 signaling in hepatocellular carcinoma. Cancer Sci (2019) 110(3):950–61. doi: 10.1111/cas.13940
31. Jiang Z, Chen J, Du X, Cheng H, Wang X, Dong C. IL-25 blockade inhibits metastasis in breast cancer. Protein Cell (2017) 8:191–201. doi: 10.1007/s13238-016-0345-7
32. Mombelli S, Cochaud S, Merrouche Y, Garbar C, Antonicelli F, Laprevotte E, et al. IL-17A and its homologs IL-25/IL-17E recruit the c-RAF/S6 kinase pathway and the generation of pro-oncogenic LMW-e in breast cancer cells. Sci Rep (2015) 5:11874. doi: 10.1038/srep11874
33. Furuta S, Jeng YM, Zhou L, Huang L, Kuhn I, Bissell MJ, et al. IL-25 causes apoptosis of IL-25R-expressing breast cancer cells without toxicity to nonmalignant cells. Sci Transl Med (2011) 3:78ra31. doi: 10.1126/scitranslmed.3001374
34. Younesi V, Nejatollahi F. Induction of anti-proliferative and apoptotic effects by anti-IL-25 receptor single chain antibodies in breast cancer cells. Int Immunopharmacol (2014) 23:624–32. doi: 10.1016/j.intimp.2014.10.015
35. Benatar T, Cao MY, Lee Y, Lightfoot J, Feng N, Gu X, et al. IL-17E, a proinflammatory cytokine, has antitumor efficacy against several tumor types in vivo. Cancer Immunol Immunother (2010) 59:805–17. doi: 10.1007/s00262-009-0802-8
36. Feng J, Li L, Ou Z, Li Q, Gong B, Zhao Z, et al. IL-25 stimulates M2 macrophage polarization and thereby promotes mitochondrial respiratory capacity and lipolysis in adipose tissues against obesity. Cell Mol Immunol (2018) 15493–505. doi: 10.1038/cmi.2016.71
37. Wang AJ, Yang Z, Grinchuk V, Smith A, Qin B, Lu N, et al. IL-25 or IL-17E protects against high-fat diet-induced hepatic steatosis in mice dependent upon IL-13 activation of STAT6. J Immunol (2015) 195:4771–80. doi: 10.4049/jimmunol.1500337
Keywords: IL-25, hepatitis B virus, hepatocellular carcinoma, prognosis, biomarker
Citation: Chen S-h and Wang X (2022) A high preoperative serum IL-25 level is a negative prognosis predictor after liver resection for HBV-HCC. Front. Oncol. 12:858151. doi: 10.3389/fonc.2022.858151
Received: 21 January 2022; Accepted: 10 August 2022;
Published: 02 September 2022.
Edited by:
Massimiliano Berretta, University of Messina, ItalyReviewed by:
Alex Giakoustidis, Aristotle University of Thessaloniki, GreeceLiliana Chemello, University of Padua, Italy
Copyright © 2022 Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Wang, bW91bnRhaW4xOTgzMDEwMUBzaW5hLmNvbQ==
 Shao-hua Chen1
Shao-hua Chen1 Xu Wang
Xu Wang