- 1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China
Objective: This study was conducted in order to compare the efficacy and safety of transarterial chemoembolization (TACE) plus apatinib plus camrelizumab (TACE+AC) and apatinib plus camrelizumab (AC) in the treatment of unresectable hepatocellular carcinoma (HCC) in a real-world setting.
Methods: In this single-center retrospective study, the data of patients with unresectable HCC who had received TACE+AC or AC treatment during March 2017 to May 2021 were assessed. Patients in the AC group received intravenous administration of camrelizumab 200 mg every 3 weeks and oral apatinib 250 mg/day treatment. Patients in the TACE+AC group received the same dose of camrelizumab and apatinib 1 week after TACE. The primary endpoint of the study was overall survival (OS), objective response rate (ORR), disease control rate (DCR), and adverse events (AEs) as the secondary endpoints.
Results: A total of 108 patients were enrolled in the study. There were 52 patients in the AC group and 56 patients in TACE+AC group. Median OS was significantly longer in the TACE+AC group than in the AC group (24.8 vs. 13.1 months; P = 0.005). Patients in the TACE+AC group achieved a higher ORR [24 (42.9%) vs. 9 (17.3%), P = 0.004] than those in the AC group. Patients in the TACE+AC group also achieved a higher disease control rate (DCR) [48 (85.7%) vs. 30 (57.7%), P = 0.001] than patients in the AC group. There was no significant difference in the incidence of AEs related to apatinib and camrelizumab between the two groups, except for gastrointestinal reaction (P > 0.05, all; P < 0.05, gastrointestinal reaction).
Conclusion: TACE plus apatinib plus camrelizumab significantly improved OS, ORR, and DCR over apatinib plus camrelizumab in patients with unresectable HCC. AEs were tolerable and manageable.
Introduction
Liver cancer is the sixth most common cancer and the fourth leading cancer-related cause of death worldwide (1). Hepatocellular carcinoma (HCC) is the main histological subtype of liver cancer and accounts for about 90% of cases (2). Since the onset of HCC is insidious, most patients present with advanced stage and have a poor prognosis (3, 4).
Transcatheter therapy that included transarterial embolization (TAE) and transarterial chemoembolization (TACE) has been used for unresectable hepatic tumors (5, 6). Compared with TAE, TACE can increase the intertumor concentration of the chemotherapeutic agent. Doxorubicin is the preferred and most common chemotherapeutic agent used in TACE and it is considered an immunogenic drug, potentiating immunogenic cell death (6, 7). TACE is widely used in the treatment of intermediate and advanced HCC, especially unresectable hepatocellular carcinoma (8, 9). However, TACE leads to hypoxia in tumor tissue, resulting in upregulation of hypoxia inducible factor 1α (HIF-1α), which in turn induces the expression of vascular endothelial growth factor (VEGF) and increases tumor angiogenesis, thus promoting tumor growth and metastasis (10). Preclinical models have shown that the combination of anti-angiogenic therapy with TACE reduced tumor volume and vessel density and prolonged survival compared with TACE alone (11).
Apatinib is a novel oral small molecule VEGFR tyrosine kinase inhibitor, which can highly selectively inhibit the tyrosine kinase activity of VEGFR-2 and reduce tumor neoangiogenesis. A prospective, randomized, multicenter phase II clinical trial of apatinib for the first-line treatment of advanced HCC showed that apatinib improved patient survival and that adverse events were tolerable (12). Qiu et al. (13) suggested that TACE in combination with apatinib for unresectable HCC could improve the efficacy compared with TACE alone.
The identification of immune checkpoint molecules has provided a theoretical basis for the development of immunotherapy for HCC, such as programmed cell death-1 (PD-1) and programmed cell death-ligand 1 (PD-L1) (14). Tumor vascular abnormalities are the characteristic of most solid tumors and contribute to immune escape. Combining anti-angiogenic drugs with immunotherapy can improve the effectiveness of immunotherapy and improve patient prognosis (15). IMbrave150 showed that atezolizumab combined with bevacizumab had significantly better overall survival and progression-free survival than sorafenib in patients with unresectable HCC (3). The RESCUE trial also demonstrated the benefits of anti-angiogenic drugs in combination with immunotherapy in advanced HCC (16). Camrelizumab in combination with apatinib has a safety and efficacy profile, with objective response rate (ORR) rates of 34.3% and 22.5% for first-line and second-line treatment, respectively (16). More than half of the patients in both the IMbrave150 trial and the RESCUE trial had received local or interventional therapy (3, 16). Due to the fact that TACE can induce immunogenic tumor cell death, TACE combined with immunotherapy can enhance the efficacy (7, 17).
Apatinib in combination with camrelizumab improves clinical benefit in patients with advanced HCC, whereas TACE combined with apatinib plus camrelizumab for unresectable HCC patients has not yet been reported (16). We conducted this real-world study to explore the safety and efficacy of TACE in combination with camrelizumab plus apatinib compared with camrelizumab plus apatinib alone in patients with unresectable HCC.
Materials and Methods
Patients
The data analysis for this study was based on real-world data. This retrospective study was approved by the Institutional Ethics Review Board of Wuhan Union Hospital and was conducted in accordance with the Declaration of Helsinki. Written informed consent was waived, as this study was retrospective. From March 2017 to May 2021, a total of 153 HCC patients who underwent apatinib (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) plus camrelizumab (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) (AC) treatment were screened at our hospital. These patients were classified into TACE + apatinib + camrelizumab (TACE+AC) group and apatinib + camrelizumab (AC) group according to whether they had received TACE treatment or not. Patients in the TACE+AC group were from the departments of intervention, oncology and surgery, while patients in the AC group were from the departments of oncology and surgery. The main inclusion criteria were as follows: 1) diagnosed as unresectable HCC based on imaging techniques and/or biopsy according to the American Association for the Study of the Liver Diseases (AASLD) (18); 2) at least one measurable target lesion, including multinodular HCC; 3) Barcelona Clinic Liver Cancer (BCLC) tumor stage B or C; 4) Child–Pugh stage A or B7; 5) normal renal function, normal coagulation, or correction by appropriate treatment; 6) Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 or 1 within 1 week before treatment; and 7) the TACE+AC group received at least one cycle of TACE combined with apatinib plus camrelizumab and the AC group received at least one dose of apatinib–camrelizumab. The exclusion criteria included the following: 1) diffuse HCC or tumor burden exceeding 70% of the whole liver; 2) complete obstruction of the main portal vein by tumor thrombi; 3) ECOG PS >1; 4) Child–Pugh stage C; 5) severe coagulation disorders, renal dysfunction, and cardiopulmonary dysfunction which cannot be corrected; and 6) incomplete data.
Treatment Protocol
The TACE procedure was performed as described by Wang et al. (19) Patients are treated with TACE under local anesthesia by interventional radiologists with over 10 years of experience. Proactive measures were taken to minimize the amount of absorbed radiant dose to the operators and patients during the operation (20). The tip of a 5-French (F) catheter (COOK) was inserted into the celiac trunk artery and superior mesenteric artery sequentially for high-pressure angiograph through the femoral artery so that the tumor feed artery could be identified. Then a 2.7-F microcatheter (Terumo, Tokyo, Japan) was super-selected into the tumor feed artery for embolization. The embolization modality was determined by the interventional physicians and patients, including the following: 1) different diameter drug-eluting bead (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) loaded with 60 mg doxorubicin (DOX); and 2) the iodine oil–DOX emulsion, a water-in-oil type of chemoembolization, which was prepared by using doxorubicin mixed with lipiodol (21). For drug-eluting bead (DEB)-TACE, the operator slowly injected the drug-eluting bead through the microcatheter until the tumor staining disappeared. For conventional TACE (cTACE), the operator slowly injected iodine oil–DOX emulsion through the microcatheter, followed by embolization of the vascular trunk with absorbable gelatin sponge particles until the tumor staining disappeared. The TACE process was repeated when the tumor still had a blood supply on review using dynamic CT or MRI and a Child–Pugh classification of A or B. Two experienced radiologists evaluated the CT or MRI images of the patient to assess whether the tumor still had an arterial blood supply (22).
Patients in the AC group received intravenous administration of camrelizumab 200 mg every 3 weeks and oral apatinib 250 mg/day treatment. Patients in the TACE+AC group received the same dose of camrelizumab and apatinib 1 week after TACE. Apatinib was suspended 3 days before the following TACE procedure. The dose of camrelizumab and apatinib in patients who experienced adverse events (AEs) due to those agents was reduced, suspended, or discontinued.
Study Endpoints
The primary endpoint was overall survival (OS). OS was defined from the date of the first TACE therapy to the date of death arising from any cause or the date of last contact in the TACE+AC group. In the AC group, OS was defined from date of the first combination therapy to the date of death arising from any cause or the date of last contact. Secondary endpoints of this study included ORR, disease control rate (DCR), and AEs. Tumor response was evaluated by two experienced radiologists using the modified Response Evaluation Criteria in Solid Tumors (mRECIST, version 1.1), including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). ORR was defined as CR+PR, and DCR was defined as CR+PR+SD. AEs were assessed based on the Common Terminology Criteria for Adverse Events (CTCAE, version 4.03).
Statistical Methods
All data were analyzed using SPSS 21.0 statistical software (IBM Corp., Armonk, NY, USA). All the continuous variables were reported as means ± standard deviation (SD) or median (minimum, maximum), and categorical variables were expressed as numbers (percentages). Continuous data were compared using Student’s t-test, and categorical data were compared using the chi-squared or Fisher’s exact test. Survival curve was estimated by the Kaplan–Meier method, from which median OS was calculated, followed by comparison using the log-rank test. Factors affecting OS were predicted by multifactorial Cox regression analysis. A two-sided P-value <0.05 was considered significant.
Results
Patient Demographics
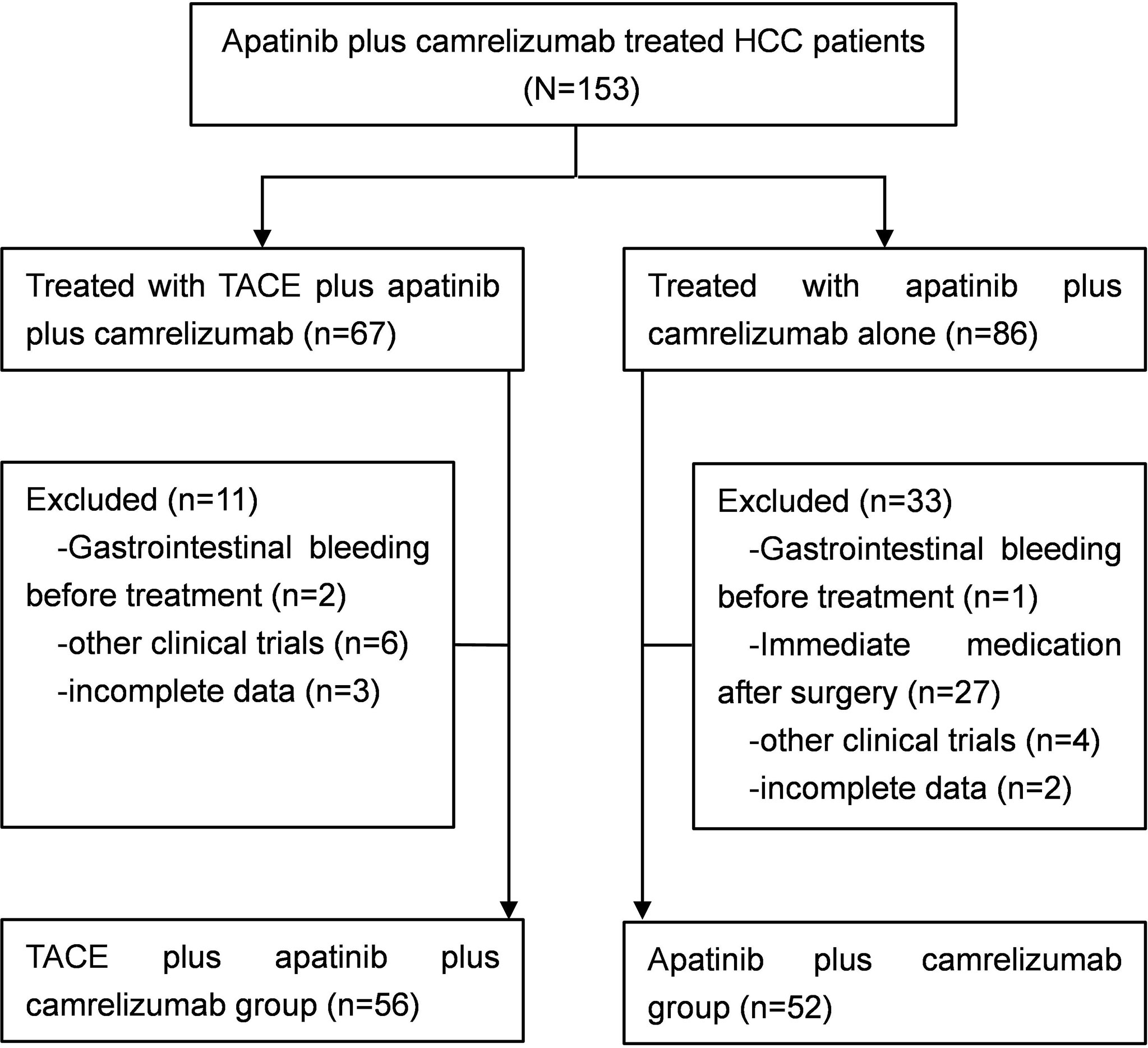
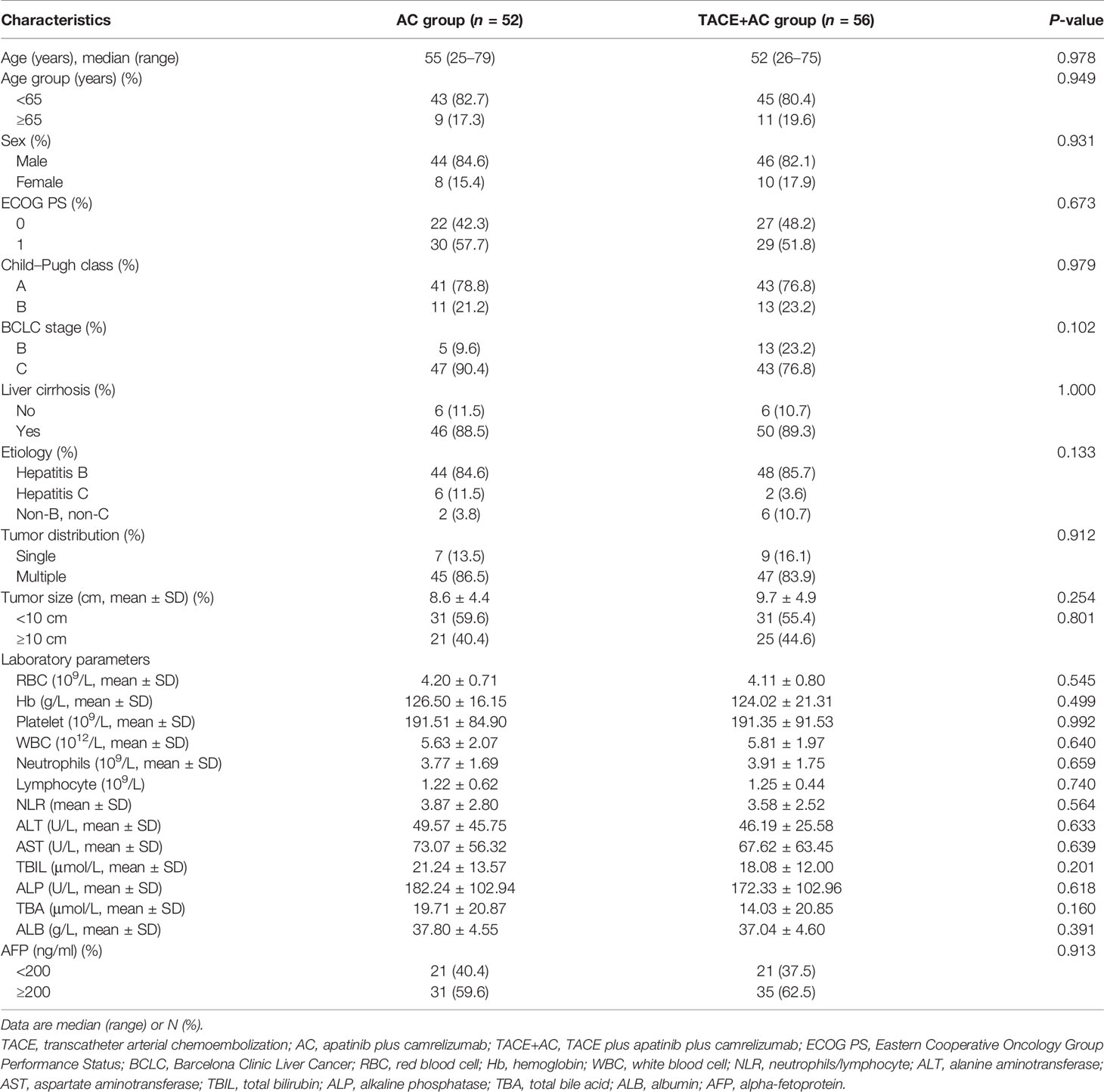
Between March 2017 and May 2021, a total of 108 patients with unresectable HCC were enrolled in this study. Fifty-six patients underwent TACE combined with apatinib plus camrelizumab treatment in the TACE+AC group, while 52 patients underwent apatinib plus camrelizumab alone in the AC group (Figure 1). The two groups were comparable in terms of age, age group, sex, ECOG PS, Child–Pugh class, BCLC stage, liver cirrhosis, etiology, tumor distribution, tumor size, and laboratory parameters. The baseline characteristics were well balanced among the treatment groups, as shown in Table 1. The follow-up ended on October 1, 2021. Median follow-up time was 13.5 months.
Efficacy
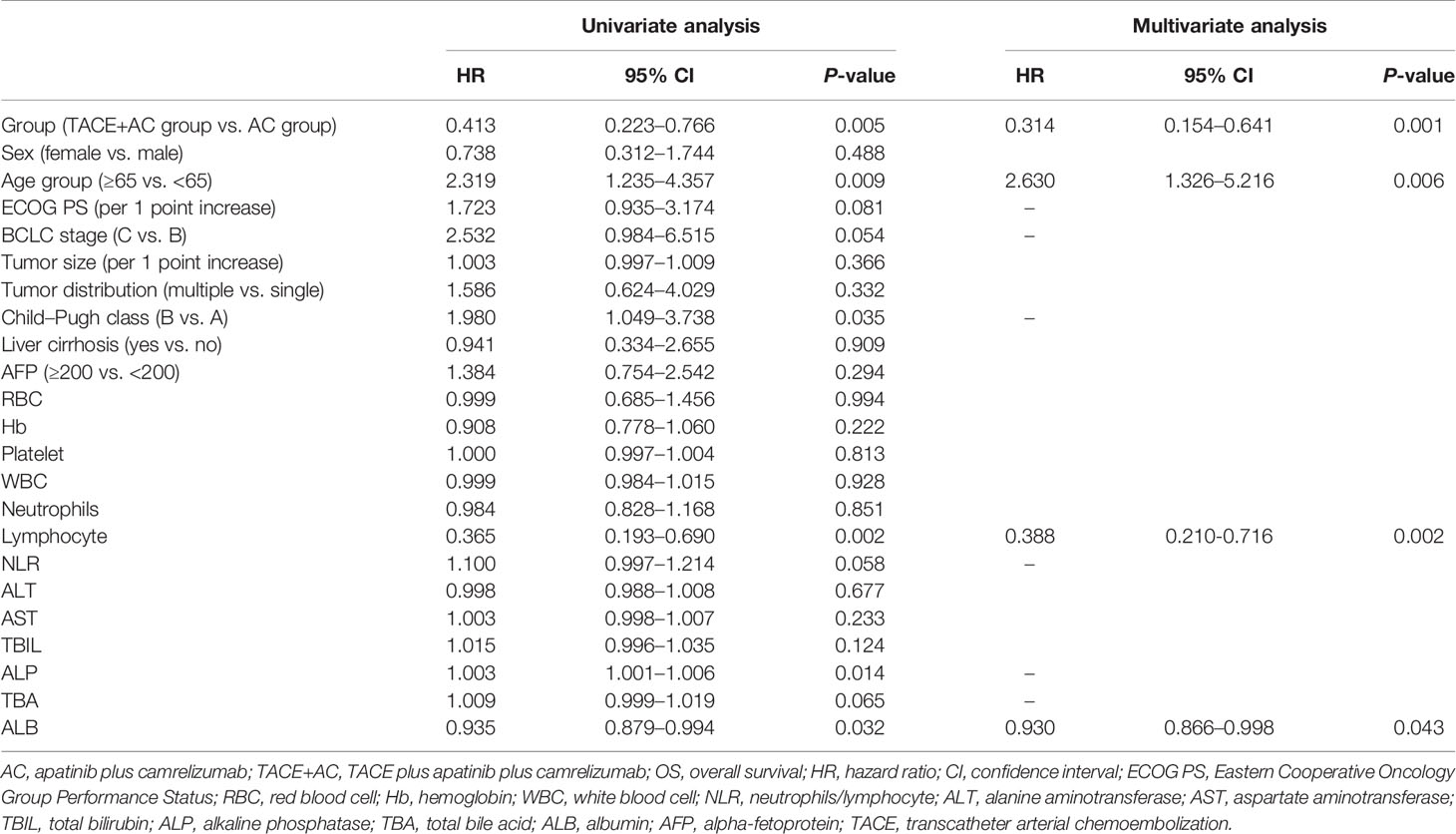
Patients in the TACE+AC group had a median OS of 24.8 months (95% CI = 10.9–38.6) compared with 13.1 months (95% CI = 4.6–21.5) for those in the AC group (HR 0.41, 95% CI = 0.22–0.77, P = 0.005; Figure 2). Multivariable Cox regression showed that the independent risk factors for OS were group (HR = 0.314, 95% CI = 0.154–0.641, P < 0.001), age group (HR = 2.630, 95% CI = 1.326–5.216, P = 0.006), lymphocyte (HR = 0.388, 95% CI = 0.210–0.716, P = 0.002), and ALB (HR = 0.930, 95% CI = 0.866–0.998, P = 0.043) (Table 2). The subgroup analyses of OS are presented in Figure 3. TACE+AC provided a clinical benefit for OS in these subgroups as follows: age less than 65 years, ECOG PS score of 1, Child–Pugh classification of B, AFP greater than or equal to 200 ng/ml, male, liver cirrhosis, and hepatitis B infected.
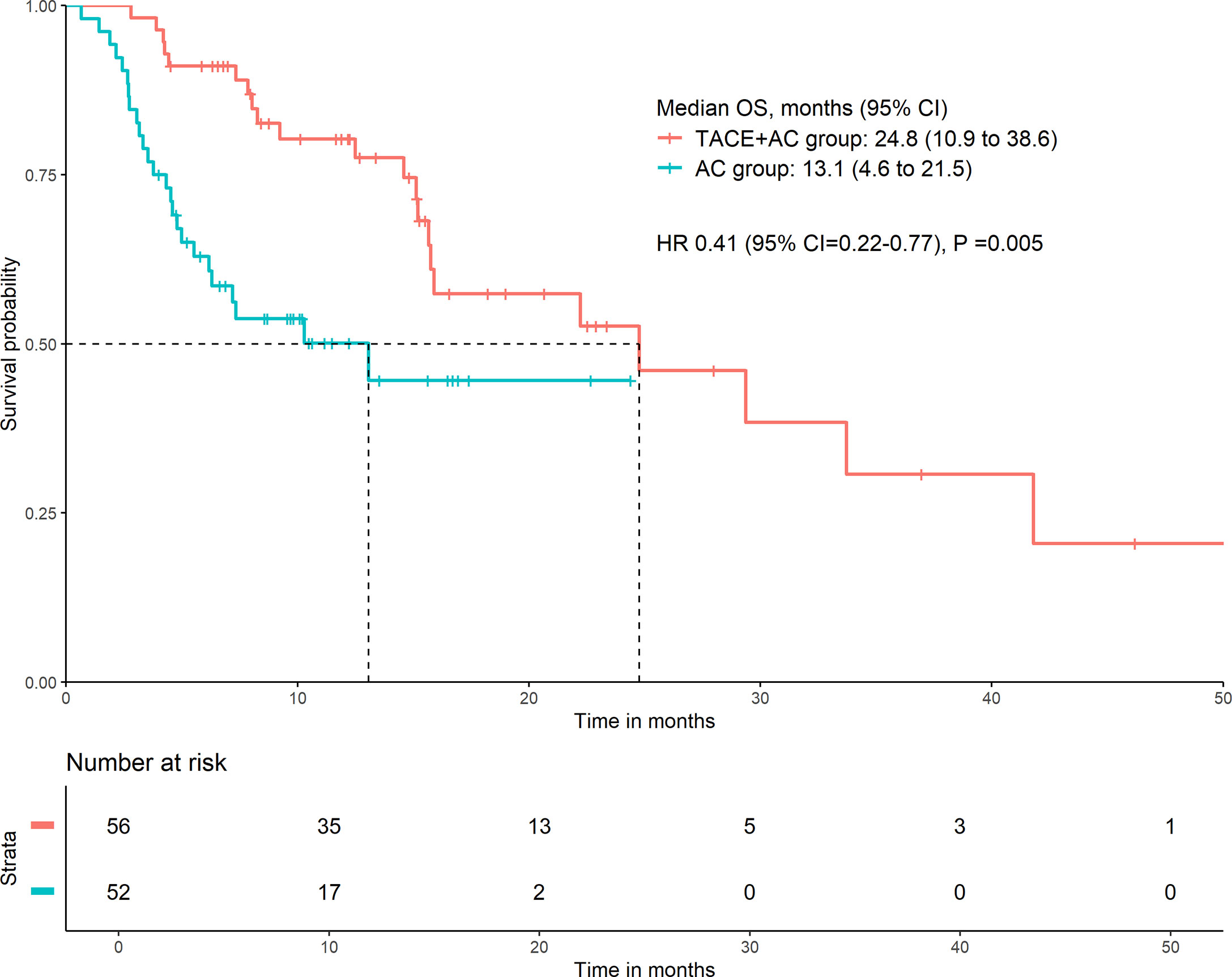
Figure 2 Kaplan–Meier curve for OS. OS, overall survival; HR, hazard ratio; CI, confidence interval; TACE+AC, TACE plus apatinib plus camrelizumab; AC, apatinib plus camrelizumab; TACE, transcatheter arterial chemoembolization.
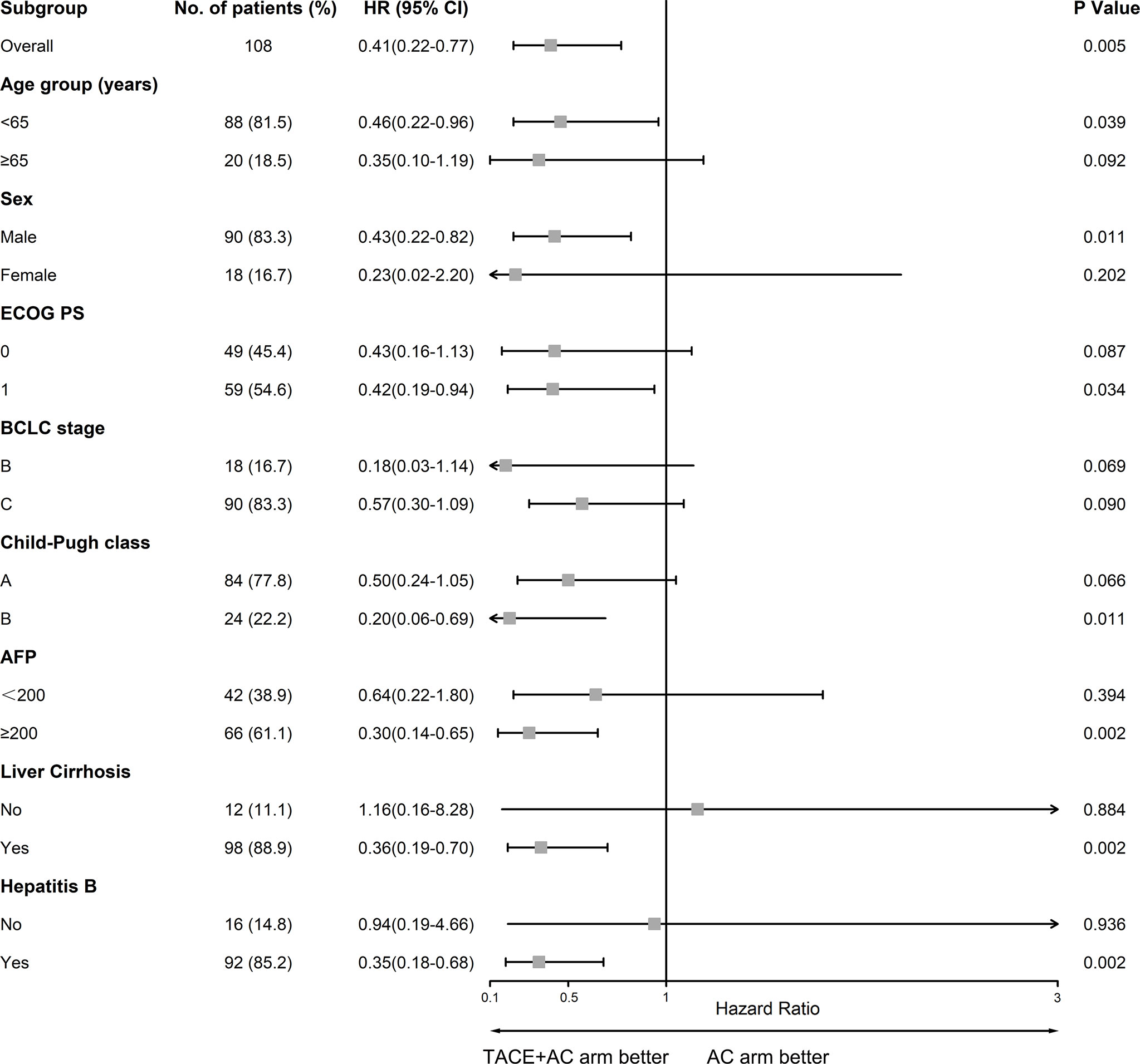
Figure 3 Forest plot of OS in subgroups of patients treated with TACE plus apatinib plus camrelizumab and apatinib plus camrelizumab. TACE, transcatheter arterial chemoembolization; ECOG PS, Eastern Cooperative Oncology Group Performance Status; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein.
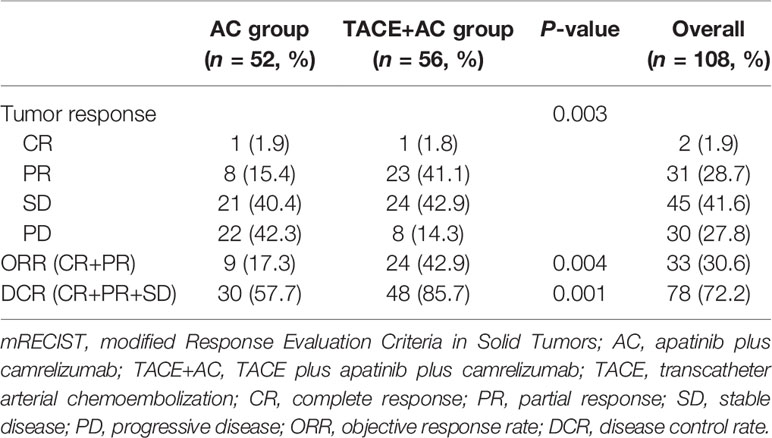
The tumor responses of the two groups of patients are shown in Table 3. On the basis of mRECIST 1.1, there was a significant difference between the two groups of patients in terms of tumor response (P = 0.003). Patients in the TACE+AC group achieved a higher ORR [24 (42.9%) vs. 9 (17.3%), P = 0.004] than those in the AC group. Patients in the TACE+AC group also achieved a higher DCR [48 (85.7%) vs. 30 (57.7%), P = 0.001] than patients in the AC group.
Safety
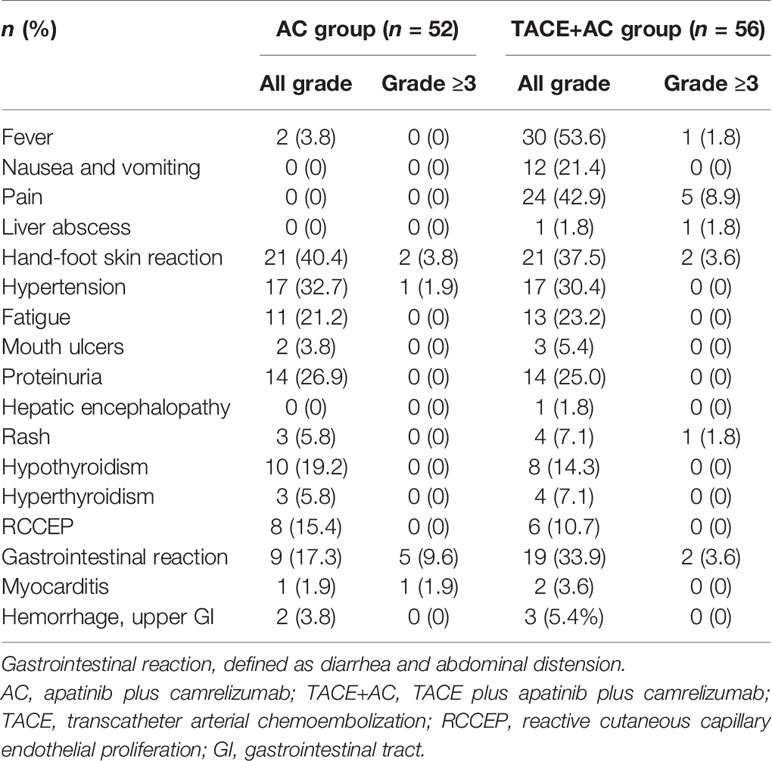
No treatment-related deaths were observed (Table 4). The most common AEs associated with apatinib and camrelizumab observed in the AC group were hand-foot skin reaction (40.4%), hypertension (32.7%), proteinuria (26.9%), abnormal thyroid function [hypothyroidism (19.2%) and hyperthyroidism (5.8%)], and gastrointestinal reaction (defined as diarrhea and abdominal distension) (17.3%). Typical AEs related to TACE included fever (53.6%), nausea and vomiting (21.4%), pain (42.9%), and gastrointestinal reaction (33.9%). Because TACE treatment was available in the TACE+AC group, there was no significant difference in the incidence of AEs related to apatinib and camrelizumab between the two groups, except for gastrointestinal reaction (P > 0.05, all; P < 0.05, gastrointestinal reaction). One patient in the TACE+AC group suffered liver abscess and recovered after tube drainage and anti-infection treatment. According to CTCAE 4.03, most AEs were classified as grades 1–2, including 5 patients who experienced mouth ulcers [2 (3.8%] in the AC group, 3 (5.4%) in the TACE+AC group, P > 0.05], 1 patient who developed hepatic encephalopathy in the TACE+AC group, 7 patients who had rash [3 (5.8%) in the AC group, 4 (7.1%) in the TACE+AC group, P > 0.05], 14 patients who had reactive cutaneous capillary endothelial proliferation (RCCEP) [8 (15.4%) in the AC group, 6 (10.7%) in the TACE+AC group, P > 0.05], 3 patients who were observed to have myocarditis [1 (1.9%) in the AC group, 2 (3.6%) in the TACE+AC group, P > 0.05], and 5 patients who had hemorrhage and upper gastrointestinal tract [2 (3.8%) in the AC group, 3 (5.4%) in the TACE+AC group, P > 0.05]. One patient in the AC group developed grade 3 immune myocarditis and then discontinued the subsequent use of camrelizumab. All patients were corrected after symptomatic treatment.
Discussion
Treatment options for unresectable HCC have rapidly evolved in recent years. Treatment options including TACE, multikinase inhibitors, and immune checkpoint inhibitors have been applied to patients with unresectable HCC (8). However, the efficacy of monotherapy still needs to be improved, and combination therapy could provide additional clinical benefit to patients (3, 4, 15, 23). The IMbrave150 trial showed that atezolizumab plus bevacizumab prolongs progression-free survival (PFS) more than sorafenib patients with unresectable HCC, the median PFS in the atezolizumab plus bevacizumab group was 6.8 months, and in the sorafenib group, it was 4.3 months. The hazard ratio of death for atezumab–bevacizumab was 0.58 (95% CI = 0.42–0.79) compared with sorafenib (3). The RESCUE trial observed that PFS was 5.7 and 5.5 months for first- and second-line treatment of unresectable HCC with apatinib in combination with camrelizumab, respectively (16). In the IMbrave150 trial, 48% of patients in the atezumab–bevacizumab group had received prior local therapy, while more than 60% of patients in the RESCUE trial had received prior interventional therapy (3, 16).
This real-world retrospective study showed significantly better overall survival and tumor response rate outcomes with TACE plus apatinib plus camrelizumab than with apatinib plus camrelizumab in patients with unresectable HCC. Cao et al. (24) reported TACE combined with lenvatinib and sintilimab for unresectable HCC with a median OS of 23.6 months and ORR of 46.7%, which is similar to our results. These suggest that TACE combined with targeted therapy plus immunotherapy is a promising treatment option in unresectable HCC. One possible reason is that TACE can reduce tumor burden by inducing tumor necrosis through cytotoxic effect and tumor tissue ischemia (25). Another possible reason is the use of doxorubicin in the TACE+AC group during TACE treatment. Doxorubicin, a chemotherapeutic reagent widely used in TACE treatment, could induce and potentiate immunogenic cell death (7). Third, tumor tissue necrosis after TACE treatment causes tumor tissue to release tumor antigens and enables increased expression of PD-1 and PD-L1, improving tumor recognizability (26). At the same time, TACE also induces immunogenic cell death, which could stimulate an immune response in the peripheral system that may be further amplified by immune checkpoint blockade (7).
In this study, the ORR according to mRECIST 1.1 was significantly higher in patients in the TACE+AC group than in the AC group (42.9% vs. 17.3%). The ORR of the TACE+AC group was comparable to the results of the RESCUE trial (42.9% vs. 45.7%), but the ORR of the AC group was lower than that of the RESCUE trial (17.3% vs. 45.7%) (16). The possible reasons were that a higher percentage of patients in the AC group had a Child–Pugh classification of B compared with the RESCUE study (21.2% vs. 0%), and none of the patients in the AC group had been treated with interventional therapy (0% vs. 62.9%). The ORR in the TACE+AC was comparable to the RESCUE study probably because 23.2% of the patients had poor liver function levels. This might indirectly demonstrate the advantage of TACE combined with apatinib plus camrelizumab. The ORR of the TACE+AC group was better than or comparable to the results of the IMbrave150 trial (atezolizumab plus bevacizumab: ORR = 33.2%), the phase 1b KEYNOTE-524 trial (lenvatinib plus pembrolizumab: ORR = 46.0%), and the ORIENT-32 trial (sintilimab plus bevacizumab: ORR = 24%), whereas the ORR for the AC group was lower than the results of those studies (3, 4, 27). It also might be attributable to the difference in those study populations. Compared with the AC group, the IMbrave150 trial had a higher proportion of patients who had received local therapy (48%) and a lower proportion of patients with hepatitis B (49%) and poor liver function (Child–Pugh B = 0%), the KEYNOTE-524 trial had a lower percentage of patients with hepatitis B (19%) and poor liver function (Child–Pugh B = 2%), and 66% of patients in the ORIENT-32 trial had received TACE. Poor liver function and hepatitis B-associated HCC might have a poor prognosis after tumor treatment (27). We also argued that TACE improved the clinical benefit of these patients possibly.
Subanalyses of OS were performed based on various factors. TACE+AC provided a higher OS in some subgroup analyses. Other subgroup analyses including patients older than 65 years (18.5%), female (16.7%), without liver cirrhosis (11.1%), and without hepatitis B infection (14.8%) had wide 95% CI ranges, which we considered might be caused by the small sample size. By Cox multivariate regression analysis, we found that group, age group, lymphocyte absolute value, and albumin level were independent risk factors affecting the prognosis of patients in this study.
This study suggested that both TACE+AC and AC were tolerable and had a well-managed safety profile. Since postembolization syndrome (fever, nausea and vomiting, and pain) and liver abscess were likely associated with TACE, the incidence of both of the TACE+AC group was higher than that of the AC group. Hand-foot skin reaction, hypertension, fatigue, mouth ulcers, proteinuria, hepatic encephalopathy, and rash were likely associated with aptinib, while the occurrence of abnormal thyroid function, RCCEP, and myocarditis were likely related with camrelizumab, and the incidence of these AEs was generally consistent between the two groups. Gastrointestinal reaction was thought to be possibly caused by the combination therapy, with a higher incidence in the TACE+AC group. No new or unexpected toxic effects happened (16, 28).
Upper gastrointestinal bleeding may occur in HCC patients in combination with chronic hepatitis B cirrhosis. A total of 85.2% of patients had hepatitis B infection and 88.9% had cirrhosis in this study. This study showed that the incidence of upper gastrointestinal tract hemorrhage was 4.6% (5/108), consisting of 2 (3.8%) patients in the AC group and 3 (5.4%) patients in the TACE+AC group, which was similar to the results in the RESCUE study, and none was considered treatment related (16). One patient developed hepatic encephalopathy, which may be related to the damage of the blood–brain barrier due to the damage of brain endothelial cells by apatinib (28). In addition, for TACE-based combination therapy, it is necessary that interventional radiological procedures are performed by experienced radiologists to reduce stochastic and non-stochastic risks from X-ray exposure and to minimize the increase of X-ray-induced complications (20). Although these patients recovered after treatment, we still need to delve into the specific causes of these AEs and screen the population suitable for this treatment option.
Tovoli et al. (29) reported that better overall survival was associated with longer treatment cycles and less permanent discontinuation due to toxicity. Moreover, the occurrence of adverse events can also predict patient prognosis. Granito et al. (30) showed that post-treatment transaminase elevation predicted the objective response to TACE. The results regarding the management of adverse events with TACE+AC and its relationship with prognosis will be reported in our subsequent study.
There were several limitations in this study. First, this study was a single-center retrospective study with a small population, which might reduce the statistical power of the study. Second, some patients in the TACE+AC group did not receive apatinib plus camrelizumab therapy after their first TACE treatment because of the financial difficulties of their families and the high price of the drugs. If they had received combination therapy earlier, they might have achieved a higher clinical benefit. Third, in TACE+AC group, the TACE treatments included cTACE and DEB-TACE, with a consequent bias of different treatments. Finally, the follow-up time was relatively short and endpoint events had not been observed in some patients. Therefore, further prospective studies or randomized controlled trials with larger sample sizes are needed to validate these results in the future.
In summary, this study showed that TACE plus apatinib plus camrelizumab demonstrated superior efficacy to apatinib plus camrelizumab for patients with unresectable HCC. Both treatments were safe and well tolerated. It provides a strong guidance for the treatment of intermediate and advanced HCC.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Wuhan Union Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Planning and conducting the study: BX and SJ. Collecting the data: SJ, CY, YW, WY, CZ, YB, CW, and SH. Interpreting the data: YB, JL, CY, YC, YW, and TL. Drafting the manuscript: SJ, CZ, and CY. Revising the manuscript: BX. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by grants from the National Natural Science Foundation of China (81873917).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Llovet JM, Kelley RK, Villanueva A, Singal AG, Finn RS. Hepatocellular Carcinoma. Nat Rev Dis Primers (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
3. Finn RS, Qin S, Ikeda M, Galle PR, Cheng AL. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. New Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
4. Finn RS, Ikeda M, Zhu AX, Sung MW, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/JCO.20.00808
5. Kishore SA, Bajwa R, Madoff DC. Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update. Cancers (2020) 12(4):791. doi: 10.3390/cancers12040791
6. Facciorusso A, Bellanti F, Villani R, Salvatore V, Muscatiello N, Piscaglia F, et al. Transarterial Chemoembolization vs Bland Embolization in Hepatocellular Carcinoma: A Meta-Analysis of Randomized Trials. UEG J (2017) 5:511–8. doi: 10.1177/2050640616673516
7. Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined Locoregional-Immunotherapy for Liver Cancer. J Hepatol (2019) 70(5):999–1007. doi: 10.1016/j.jhep.2019.01.027
8. Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, et al. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
9. Zhou J, Sun H, Wang Z, Cong W, Fan J. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer (2020) 9(6):682–720. doi: 10.1159/000509424
10. Kong J, Kong J, Pan B, Ke S, Dong S, Li X, et al. Insufficient Radiofrequency Ablation Promotes Angiogenesis of Residual Hepatocellular Carcinoma via HIF-1α/VEGFA. PloS One (2012) 7:e37266 doi: 10.1371/journal.pone.0037266
11. Jiang H, Meng Q, Tan H, Pan S, Sun B, Xu R, et al. Antiangiogenic Therapy Enhances the Efficacy of Transcatheter Arterial Embolization for Hepatocellular Carcinomas. Int J Cancer (2007) 121(2):416–24. doi: 10.1002/ijc.22655
12. Qin S. Apatinib in Chinese Patients With Advanced Hepatocellular Carcinoma: A Phase II Randomized, Open-Label Trial. Asco Meeting Abstracts (2014) 32(15_suppl):4019. doi: 10.1200/jco.2014.32.15_suppl.4019
13. Qiu Z, Shen L, Chen S, Qi H, Cao F, Xie L. Efficacy Of Apatinib In Transcatheter Arterial Chemoembolization (TACE) Refractory Intermediate And Advanced-Stage Hepatocellular Carcinoma:A Propensity Score Matching Analysis. Cancer Manage Res (2019) 11:9321–30. doi: 10.2147/CMAR.S223271
14. Kudo M. Immune Checkpoint Inhibition in Hepatocellular Carcinoma: Basics and Ongoing Clinical Trials. Oncology (2017) 92 Suppl 1(1):50. doi: 10.1159/000451016
15. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat Rev Clin Oncol (2018) 15(5):325–40. doi: 10.1038/nrclinonc.2018.29
16. Xu J, Shen J, Gu S, Zhang Y, Wang QR. Camrelizumab in Combination With Apatinib in Patients With Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-Label, Phase II Trial. Clin Cancer Res (2020) 27(4):1003–11. doi: 10.1158/1078-0432.CCR-20-2571
17. Greten TF, Sangro B. Targets for Immunotherapy of Liver Cancer. J Hepatol (2017) S0168-8278(17)32287-0. doi: 10.1016/j.jhep.2017.09.007
18. Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatology (2011) 53(3):1020. doi: 10.1002/hep.24199
19. Wang Y, Zhou C, Liu J, Shi Q, Huang S, Yang C, et al. Increased Liquefactive Necrosis Formation After Transarterial Chemoembolization Combined With Molecular Targeted Agents Plus Immune Checkpoint Inhibitors for Hepatocellular Carcinoma. Cancer Manage Res (2021) 13:6935–41. doi: 10.2147/CMAR.S328812
20. Compagnone G, Giampalma E, Domenichelli S, Renzulli M, Golfieri R. Calculation of Conversion Factors for Effective Dose for Various Interventional Radiology Procedures. Med Phys (2012) 39(5):2491–8. doi: 10.1118/1.3702457
21. Renzulli M, Peta G, Vasuri F, Marasco G, Caretti D, Bartalena L, et al. Standardization of Conventional Chemoembolization for Hepatocellular Carcinoma. Ann Hepatol (2021) 22:100278. doi: 10.1016/j.aohep.2020.10.006
22. Tovoli F, Renzulli M, Negrini G, Brocchi S, Ferrarini A, Andreone A, et al. Inter-Operator Variability and Source of Errors in Tumour Response Assessment for Hepatocellular Carcinoma Treated With Sorafenib. Eur Radiol (2018) 28(9):3611–20. doi: 10.1007/s00330-018-5393-3
23. Lee WS, Yang H, Hong JC, Chan K. Combination of Anti-Angiogenic Therapy and Immune Checkpoint Blockade Normalizes Vascular-Immune Crosstalk to Potentiate Cancer Immunity. Exp Mol Med (2020) 52(9):1475–85. doi: 10.1038/s12276-020-00500-y
24. Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, et al. The Efficacy of TACE Combined With Lenvatinib Plus Sintilimab in Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Study. Front Oncol (2021) 11:783480. doi: 10.3389/fonc.2021.783480
25. Raoul J-L, Forner A, Bolondi L, Cheung TT, Kloekner R. Updated Use of TACE for Hepatocellular Carcinoma Treatment: How and When to Use It Based on Clinical Evidence. Cancer Treat Rev (2018) 72:28–36. doi: 10.1016/j.ctrv.2018.11.002
26. Montasser A, Beaufrère A, Cauchy F, Bouattour M, Soubrane O, Albuquerque M, et al. Transarterial Chemoembolization Enhances Programmed Death 1 and Programmed Death-Ligand 1 Expression in Hepatocellular Carcinoma. Histopathology (2021) 79(1):36–46. doi: 10.1111/his.14317
27. Ren Z, Xu J, Bai Y, Xu A, Fan J. Sintilimab Plus a Bevacizumab Biosimilar (IBI305) Versus Sorafenib in Unresectable Hepatocellular Carcinoma (ORIENT-32): A Randomised, Open-Label, Phase 2–3 Study. Lancet Oncol (2021) 22(7):977–90. doi: 10.1016/S1470-2045(21)00252-7
28. Liu Y, Chen X, Gao X, Chen J, Chen J. Apatinib-Induced Hyperammonemic Encephalopathy. J Oncol Pharm Practice: Off Publ Int Soc Oncol Pharm Practitioners (2020) 26(2):465–70. doi: 10.1177/1078155219846253
29. Tovoli F, Ielasi L, Casadei-Gardini A, Granito A, Foschi FG, Rovesti G, et al. Management of Adverse Events With Tailored Sorafenib Dosing Prolongs Survival of Hepatocellular Carcinoma Patients. J Hepatol (2019) 71(6):1175–83. doi: 10.1016/j.jhep.2019.08.015
Keywords: targeted therapy, immunotherapy, transarterial chemoembolization, hepatocellular carcinoma, comprehensive therapy
Citation: Ju S, Zhou C, Yang C, Wang C, Liu J, Wang Y, Huang S, Li T, Chen Y, Bai Y, Yao W and Xiong B (2022) Apatinib Plus Camrelizumab With/Without Chemoembolization for Hepatocellular Carcinoma: A Real-World Experience of a Single Center. Front. Oncol. 11:835889. doi: 10.3389/fonc.2021.835889
Received: 15 December 2021; Accepted: 29 December 2021;
Published: 31 January 2022.
Edited by:
Antonio Facciorusso, University of Foggia, ItalyReviewed by:
Matteo Renzulli, Università di Bologna, ItalyAlessandro Granito, University of Bologna, Italy
Copyright © 2022 Ju, Zhou, Yang, Wang, Liu, Wang, Huang, Li, Chen, Bai, Yao and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Xiong, herr_xiong@126.com; orcid.org/0000-0002-7795-7041
†These authors have contributed equally to this work
 Shuguang Ju1,2†
Shuguang Ju1,2† Chaoyang Wang
Chaoyang Wang Jiacheng Liu
Jiacheng Liu Yingliang Wang
Yingliang Wang Songjiang Huang
Songjiang Huang Yaowei Bai
Yaowei Bai Bin Xiong
Bin Xiong