
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 28 July 2022
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.946361
Background: An increasing prevalence of cognitive disorders warrants comprehensive systematic reviews on the effect of diet on cognitive health. Studies have suggested that the Mediterranean (MeDi) diet has protective effects against metabolic diseases. However, comprehensive systematic reviews on the effect of the MeDi diet on the cognitive decline are limited. We investigated whether adherence to the MeDi diet could lower the risk of the cognitive disorder or improve cognitive function in older adults.
Methods: In this systematic review and meta-analysis, PubMed, Web of Science, PsycINFO, Scopus, and Cochrane databases were searched from inception to June 2021. Cohort studies and randomized controlled trials (RCTs) were included. The effect sizes were estimated as log risk ratios and standard mean differences (SMDs) with 95% confidence intervals (CIs). The Newcastle–Ottawa score and Cochrane Collaboration’s tool were used to assess the risk of bias in cohort studies and RCTs, respectively.
Results: Of the 1,687 screened studies, 31 cohort studies and five RCTs met the eligibility criteria for qualitative analysis; 26 cohort studies and two RCTs were included in the meta-analysis. In the cohort studies, high adherence to the MeDi diet was associated with lower risk of mild cognitive impairment (MCI) [risk ratio (RR) = 0.75 (0.66–0.86)], and Alzheimer’s disease (AD) [RR = 0.71 (0.56–0.89)]. In the RCTs, high adherence to the MeDi diet was associated with better episodic [SMD = 0.20 (0.09–0.30)] and working memories [SMD = 0.17 (0.01–0.32)] than lowest group.
Conclusion: Adherence to the MeDi diet may reduce the risk of MCI and AD. However, other associations with cognitive outcomes (global cognition, working memory, and episodic memory) remain open to interpretation. Overall, the MeDi diet is recommended to prevent or delay cognitive disorders and improve cognitive function. Further, long-term RCTs are warranted to strengthen the evidence.
Systematic review registration: [https://www.crd.york.ac.uk], identifier [CRD42021276801].
Mild cognitive impairment (MCI) is defined as a cognitive decline greater than that expected for the age and education level of the individual while not interfering with activities of daily living (1). MCI is a stage in the progression from normal cognitive function to dementia (2). Globally, dementia is the seventh most common cause of death and the most common cause of illness in older adults. According to the WHO, there are currently more than 55 million confirmed cases of dementia worldwide, and the number of new cases is increasing at a rate of 10 million per year. In addition to this, the number of people with dementia is projected to grow to 78 million by 2030 and 139 million by 2050. This is owing to the increasing proportion of older people worldwide. The most commonly diagnosed form of dementia is Alzheimer’s disease (AD), which accounts for approximately 60–70% of cases (3). According to the Alzheimer’s Association, the number of deaths from AD increased by 145% from 2000 to 2019 (4). Moreover, during the coronavirus disease pandemic, deaths owing to AD and dementia have increased by 16% (5). AD is an irreversible degenerative brain disease (6), and currently, there is no cure for dementia (3). Therefore, determining whether cognitive impairment can be prevented or delayed by dietary modification is important.
The Mediterranean (MeDi) diet is a dietary pattern that has been followed by the Mediterranean Basin countries since the early 1960s (7) and is mainly based on abundant plant-based consumption with food that is minimally processed, seasonal, fresh, and locally grown. Fresh fruit is consumed every day, and olive oil is the main source of fat. Additionally, low-to-moderate amounts of fish and seafood, poultry, and dairy products are consumed daily. A regular but moderate amount of wine is also consumed, along with 0–4 eggs that are consumed per week. Sweets containing sugar or honey and red meat are sparingly consumed (8).
The association between the MeDi diet and increased longevity and reduced mortality and morbidity from certain cancers and other nutrition-related diseases has been widely studied (9–12). However, whether adherence to the MeDi diet can prevent or delay the risk of cognitive disorders and improve cognitive function remains understudied. While some epidemiological studies did not show a relationship (13–15), others have shown positive associations between the MeDi diet and the prevention of cognitive disorders and improvement in cognitive function (16–19). Although several systematic reviews focusing on cognitive disorders or cognitive function have been published (20–25), there is no systematic review to quantitatively evaluate the association between cognitive disorders and cognitive function from both prospective studies and RCTs simultaneously. In addition, although previous reviews conducted meta-analyses, they did not conduct any further analysis to investigate the high heterogeneity source, which might lead to results bias. Therefore, we performed a systematic review and meta-analysis of both cohort studies and randomized controlled trials (RCTs) to comprehensively analyze the association between adherence to the MeDi diet and cognitive disorders (i.e., MCI, dementia, and AD) and cognitive functions (i.e., attention, episodic memory, executive function, global cognition, processing speed, and working memory). Furthermore, we conducted subgroup and meta-regression analyses to identify whether a wide range of characteristics contributed to the differences in the results of the cohort studies.
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (26). A protocol was designed and registered in the PROSPERO database (PROSPERO 2021 CRD42021276801). We searched five electronic databases: PubMed, Web of Science, PsycINFO, Scopus, and Cochrane, for articles published before June 2021. The search terms used were “Mediterranean diet,” “mild cognitive impairment,” “dementia,” “Alzheimer’s disease,” and “cognitive function,” and only English articles were included. Two authors independently screened the titles and abstracts of the research papers. After this, they read the full texts to identify potentially eligible studies. The PICOS criteria are shown in Table 1; see Supplementary Table 1 for the detailed search methodology.
Studies were selected and included based on the following inclusion criteria:
1. Studies that were published in English, with no restrictions on the study sample size or the participants’ age, sex, or health status.
2. Studies that were RCTs or prospective observational studies and investigated the relationship between adherence to the MeDi diet and cognitive function (including attention, episodic memory, executive function, global cognition, processing speed, or working memory) or the risk of cognitive disorders (including MCI, dementia, or AD).
3. If two studies used the same cohort database, both were included.
The exclusion criteria were as follows:
1. Studies that did not adhere to the MeDi diet. For example, studies with adherence to a particular national dietary pattern or a healthy eating index.
2. Studies that included participants with cognitive disorders or abnormal cognitive functioning at baseline.
3. Case-control studies, cross-sectional studies, systematic reviews, narrative reviews, conference reports, or letters.
Two authors (JF and L-JT) independently extracted data using the same extraction method. In the case of a dispute, a third author (SS) helped reach a consensus. The data on the last name of the first author, publication year, the health status of participants, follow-up duration, baseline age, percentage of men, sample size, dietary assessment method, MeDi diet assessment method, cognitive assessment methods, cognitive domains measured, and results were extracted from cohort studies. For the RCTs, data on the last name of the first author, publication year, country, population selection, follow-up duration, intervention group, placebo group, dietary assessment methods, and the MeDi diet assessment method, the baseline age and number of participants, outcome test methods, and results were extracted. Two reviewers independently assessed cognitive disorders and cognitive function of cohort studies and cognitive function of RCTs. Cognitive disorders included MCI, dementia, and AD, which were based on the battery of neuropsychological test or diagnosis criteria. Cognitive function included attention, episodic memory, executive function, global cognition, processing speed, and working memory, which were tested by the Mini-Mental State Examination (MMSE) or other common cognitive function tests [e.g., Telephone Interview for Cognitive Status (TICS), the Digit Span-Backward Test (DST)].
Two independent reviewers evaluated the quality of the cohort studies and RCTs. The Newcastle–Ottawa score (NOS) was used to evaluate the quality of the prospective cohort studies (27). Scores of ≥ 7 were considered high-quality scores, and scores of ≤ 4 were considered low-quality scores. The Cochrane Collaboration tool was used to evaluate the risk of bias in RCTs (28). The deviation from risk assessment criteria for each factor was divided into three levels—“high,” “low,” and “unclear” risk of bias.
We performed a meta-analysis on three cognitive disorders and six cognitive domains. From the cohort studies, we extracted risk ratios (RRs) [hazard ratio (HRs) or odds ratio (ORs)] with 95% confidence intervals (CIs) for cognitive disorders (MCI, dementia, and AD) and effect sizes and standard errors for cognitive function (episodic memory, global cognition, and working memory). Owing to the asymmetry of the RRs, these values were log-transformed (base 10) (29). From RCTs, we extracted changed means and changed standard deviation (SD) for cognitive function (attention, episodic memory, executive function, global cognition, processing speed, and working memory). If the results of the original manuscript contained only the means or SD of the baseline and final groups, the changed mean was calculated by subtracting the final mean from the baseline mean. Changed SD was obtained using the following equation, where the correlation coefficient R was set at 0.5 (30).
If the study reported 95% CI instead of SD, we used the following formula:
All statistical analyses were performed in STATA MP 17.0 and the Review Manager 5.4. A two-tailed P value of < 0.05 was considered statistically significant. The heterogeneity test in this review was examined using the Cochran’s Q test and quantified using the I2 statistic. For medium and low heterogeneity (I2 of < 50%), fixed-effects models were used, while random-effects models were used for high heterogeneity (I2 of ≥ 75%) (31, 32). Moreover, potential sources of heterogeneity were examined using meta-regression and subgroup analysis based on covariates such as study location: (1) Mediterranean region or (2) non-Mediterranean region; publication year: (1) after 2015 or (2) before 2015; duration of follow-up: (1) ≥ 5 years or (2) < 5 years; method of assessing dietary intake: (1) food frequency questionnaire (FFQ) or (2) others; and study quality: (1) scores = 9, (2) scores = 8, or (3) scores = 7 (30, 33, 34). Publication bias was assessed based on at least 10 studies and was quantitatively assessed using Egger’s test, Begg’s test, and funnel plots (35).
A total of 1,687 studies in PubMed, Web of Science, PsycINFO, Scopus, and Cochrane databases were identified. Of these, 820 studies were excluded after searching the keywords and reviewing the studies by their titles to assess relevance and eligibility. The abstracts of 103 studies were reviewed, after which the full texts of 47 studies were assessed. After excluding irrelevant studies and including reviews and cross-sectional studies, 31 cohort studies and five RCTs were included in the qualitative assessment. Finally, 26 cohort studies and two RCTs that met the criteria were included in the meta-analysis (Figure 1).
In total, 31 cohort studies and five RCTs were included in the review. Characteristics of prospective studies and RCTs are summarized in Tables 2 and 3, respectively. Nine cohort studies were conducted in the Mediterranean countries of France, Greece, Spain, and Italy (15, 36–43), while the other 22 studies were from China, the United States of America, Australia, Sweden, the United Kingdom, and Singapore (13, 14, 16–19, 44–60). Most studies (n = 25) were women dominant, with five studies including only women and three studies including only men (19, 41, 47). The sample sizes varied from 200 to 27,842 participants. Additionally, follow-up median or mean durations ranged from 2.2 to 26 years, and baseline ages ranged from 45 to 92 years. The cut-off value selected by most of the studies was over 65 years (n = 13). The majority of studies used an FFQ to assess the MeDi diet (n = 26), and two of these studies used both an FFQ and a 24-h dietary recall to assess diets (15, 16). Among the studies using an FFQ, two used the Council of Victoria FFQ and Women’s Health Initiative FFQ, while the others used the food groups’ semi-quantitative FFQ (14, 45). Moreover, the MeDi diet was primarily scored on a 0–9 scoring range (n = 25), while 1 study used a 0–15 MeDi diet score, defined by Morris et al. (55, 61) and 5 studies used a 0–55 MeDi diet score, defined by Panagiotakos et al. (17, 36, 46, 56, 57). Cognitive function was assessed using a large number of tests to quantify the cognitive domains of global cognition, episodic memory, working memory, processing speed, executive function, and attention. The most common and widely used test was MMSE (n = 19), which included tests on attention, language, memory, visual-spatial skills, and orientation. Owing to individual differences in research, studies used different cognitive function tests, including the DST, TICS, Benton Visual Retention Test (BVRT).
Five RCTs were eligible for inclusion, all of which had a parallel design and included cognitively stable participants (62–66). Of the five RCTs, two were from Australia with follow-up times of only 0.5 years and an average participant age of over 70 years (62, 63). The other three RCTs were from Spain, with a follow-up duration range of 3–6.5 years and an average participant age of 67 years (65–67). The earliest and most recent trials were conducted in 2011 (65) and 2020 (62), respectively. Of the trials conducted in Spain, intervention groups were subdivided into the following two groups: those receiving a free allotment of extra virgin olive oil (EVOO) or unprocessed mixed nuts. To ensure the accuracy and rigor of the MeDi diet, participants received a collection of recipes or intensive education. As for the control group, participants were asked to maintain their current lifestyle or advised to adhere to a low-fat diet. In the five RCTs, there were 883 participants in the experimental groups and 396 participants in the control groups. Several methods were used to assess cognitive function, including the MMSE, Rey Auditory Verbal Learning Test, and Color Trail Test.
Of the 31 cohort studies, 93.6% achieved high-quality NOS scores (n = 29), and only two studies had scores of 6 and were, thus, regarded as having a high risk of bias (Supplementary Table 2 shows the NOS grades of the 31 cohort studies). Regarding selection bias, all five RCTs mentioned random sequence generation, and four trials were conducted with allocation concealment (Supplementary Figure 1 summarizes the risk of bias in the five RCTs). All five trials had a high risk of performance bias, without participant blinding, and three trials were blinded to outcome assessment. Four of these trials had a low risk of bias in incomplete outcome data, as it had been properly addressed. One trial, however, had a high risk of attrition bias. All five RCTs were free from reporting and other biases.
A forest plot revealed the relationship between adherence to the MeDi diet and three domains of cognitive function in 14 cohort studies (Figure 2). Pooled results did not show significant associations with global cognition when compared with the lowest group (SMD = 0.03; 95% CI: 0.00–0.07; I2 = 87.5%, P < 0.000). No significant associations were found with episodic memory (SMD = 0.02; 95% CI: -0.03–0.06; I2 = 66.0%, P = 0.012) or working memory (SMD = 0.05; 95% CI: -0.02–0.13; I2 = 56.5%, P = 0.129).
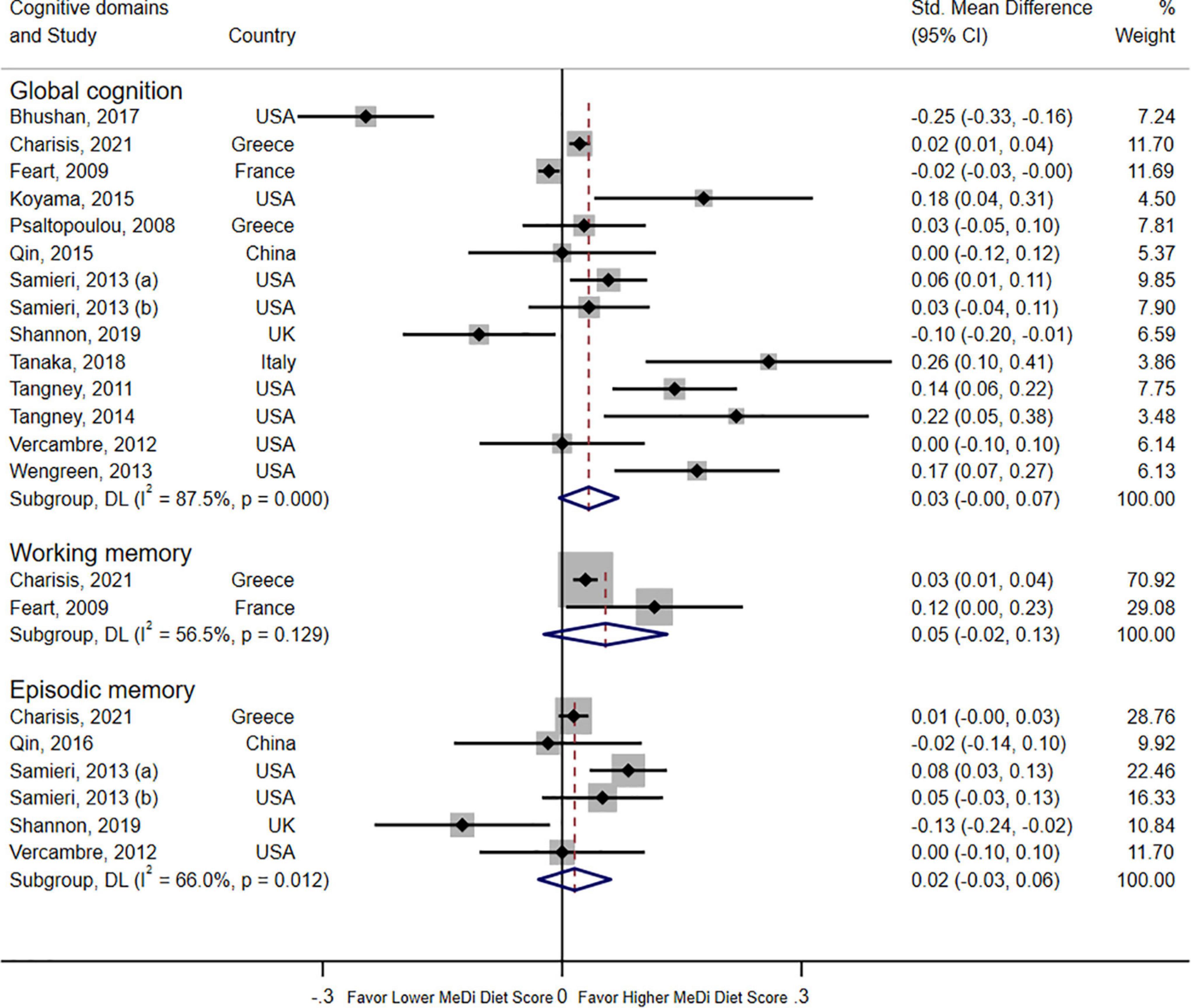
Figure 2. Forest plot for cohort studies with a standardized mean difference (std. mean difference) and 95% CIs showing the associations between the Mediterranean diet score and three domains of cognition function (global cognition, episodic memory, and working memory).
The forest plot shown in Figure 3 displays the relationship between adherence to the MeDi diet and three types of cognitive disorders in 17 cohort studies. Pooled results of high adherence to the MeDi diet showed a positive association with reduced risk of MCI (RR = 0.75; 95% CI: 0.66–0.86; I2 = 63.7%, P = 0.002). Moreover, pooled results indicated that high adherence to the MeDi diet was not associated with reduced risk of dementia (RR = 0.85; 95% CI: 0.59–1.23, P = 0.399; I2 = 61.3%, P = 0.024). The pooled analysis, however, indicated that the MeDi diet could reduce the risk of AD by 29% (RR = 0.71; 95% CI: 0.56–0.89; I2 = 52.3%, P = 0.063).
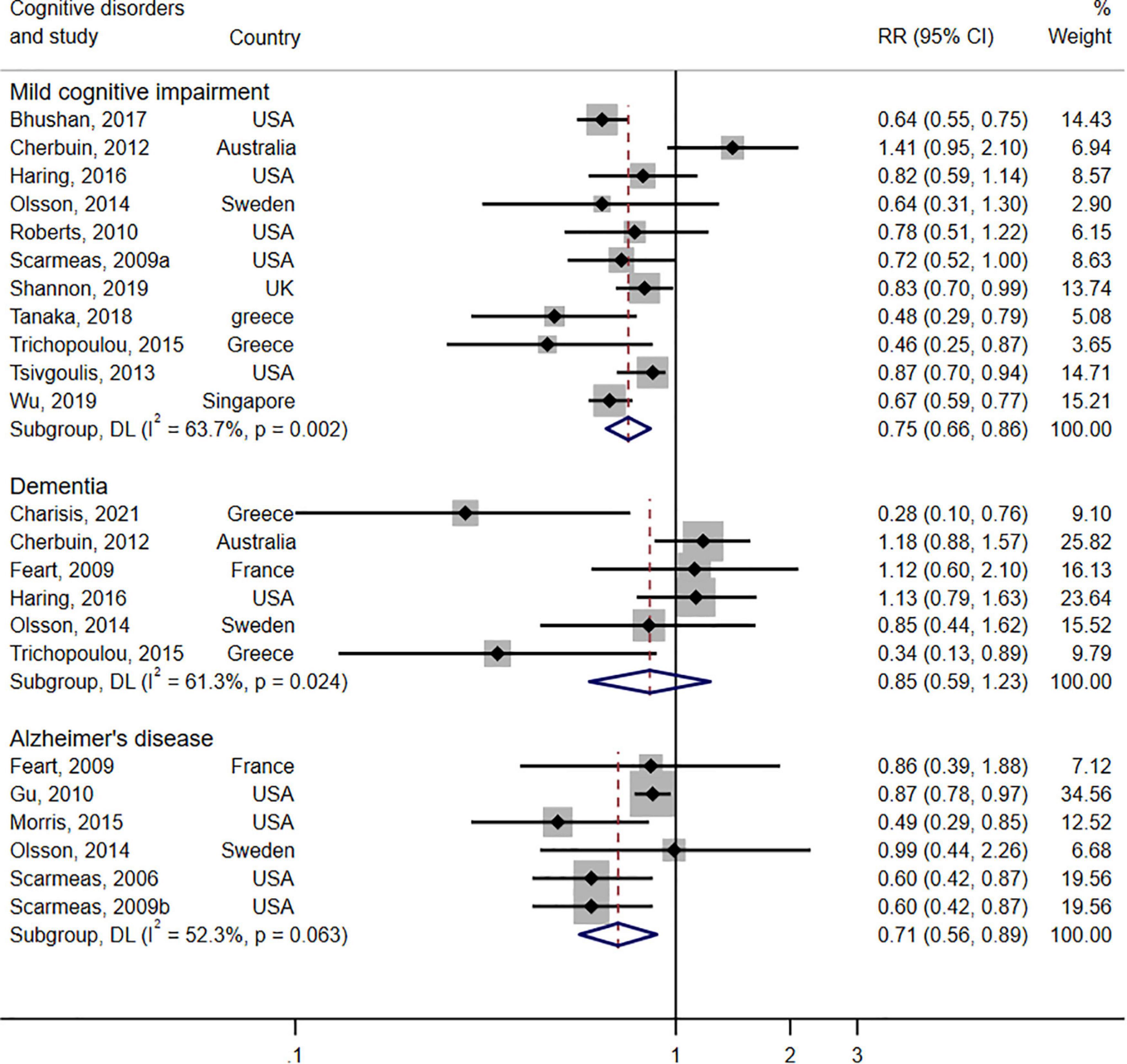
Figure 3. Forest plot for prospective studies of the risk ratio and 95% confidence intervals showing forest plot for the associations between the Mediterranean diet score and the risk of three types of cognitive disorders (mild cognitive impairment, dementia, Alzheimer’s disease).
Two RCTs examined the effects on cognitive function based on six types of cognitive domains (Figure 4). The pooled results indicated that the MeDi diet could strengthen working memory (SMD = 0.17; 95% CI: 0.01–0.32, P = 0.033; I2 = 11.8%, P = 0.339), episodic memory (SMD = 0.20; 95% CI: 0.09–0.30, P < 0.000; I2 = 19.0%, P = 0.285), and global cognition (SMD = 0.19; 95% CI: 0.00–0.39, P = 0.046; I2 = 0.0%, P = 0.460) as compared with the control group. On the contrary, the MeDi diet showed an adverse effect on attention (SMD = -0.41; 95% CI: -0.67–0.16, P = 0.001; I2 = 0.0%, P = 0.417) and was not associated with executive function and processing speed (P = 0.374, P = 0.625, respectively); both parameters showed no between-study heterogeneity (I2 = 9.4%, I2 = 0.0%, respectively).
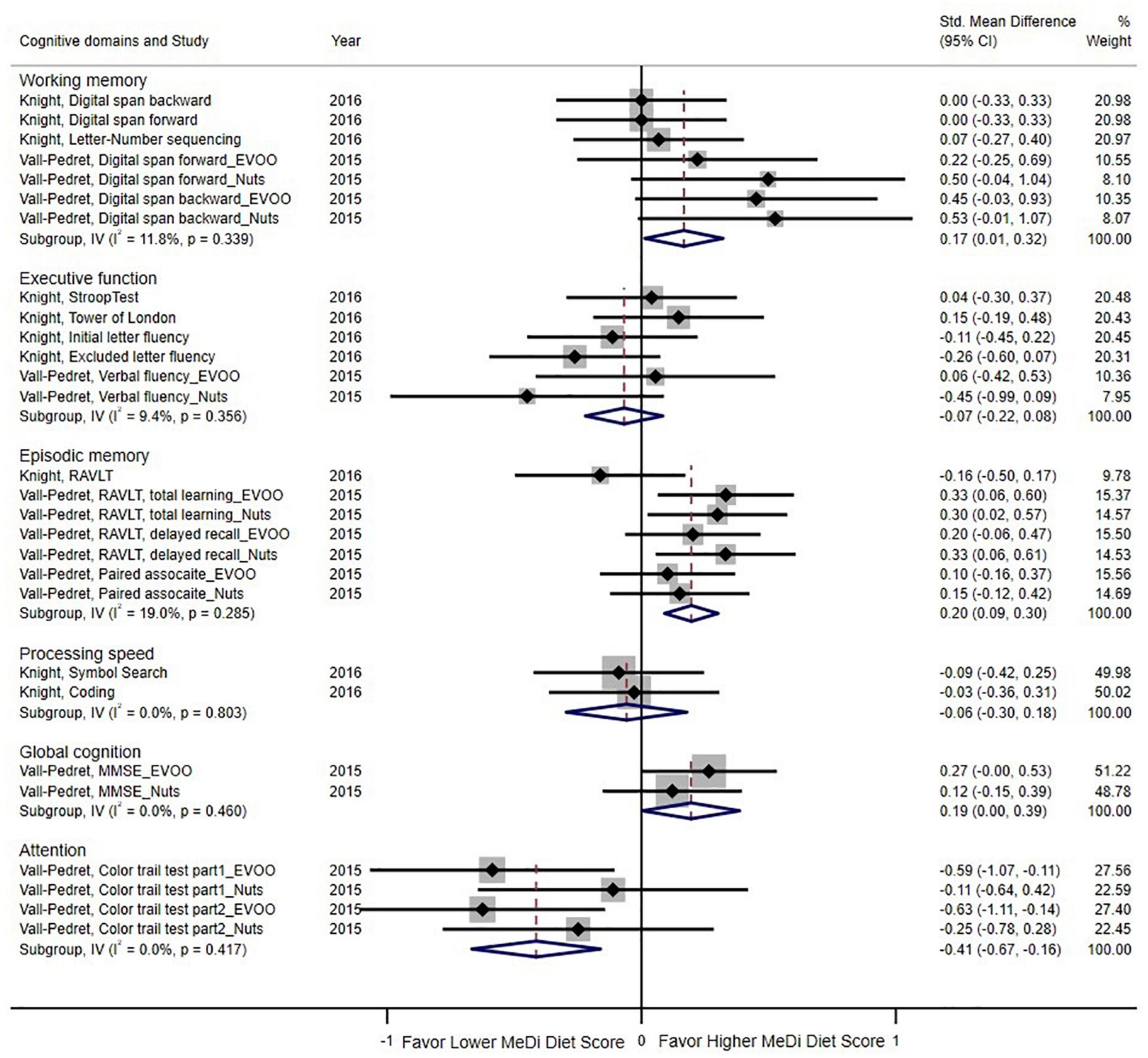
Figure 4. Forest plot for randomized controlled trials of standardized mean difference (std. mean difference) and 95% confidence intervals showing forest plot for the associations between the Mediterranean diet score and cognition function by cognitive domains (working memory, executive function, episodic memory, processing speed, global function, attention). MMSE, Mini-Mental State Examination; EVOO, Extra virgin olive oil; RAVLT, Rey Auditory Verbal Learning Test.
Subgroup and meta-regression analyses were performed in the cohort studies on the following covariates: study location, study published year, duration of follow-up, dietary intake assessment method, and study quality. However, owing to the limited number of studies (<10), the meta-regression was only conducted for global cognition and MCI risk (Supplementary Tables 3, 5).
Regarding the relationship between the MeDi diet and global cognition, no covariates were found to affect heterogeneity except for the dietary assessment method (P < 0.000; Supplementary Table 3). A subgroup analysis of different assessment methods showed that studies using methods other than an FFQ had a negative association with global cognition (SMD = -0.02, 95% CI: -0.03–0.00; I2 = 0.0%). Pooled results of meta regression analyses revealed that study location, study published year, and duration of follow-up affect heterogeneity (P < 0.05) in terms of the MeDi diet and MCI risk, whereas the exposure assessment method and study quality do not affect heterogeneity (P = 0.307; P = 0.059; Supplementary Table 5). Subgroup analysis found no heterogeneity among Mediterranean region studies (I2 = 0.0%), while high adherence to the MeDi diet was associated with lower risk of MCI (RR = 0.57; 95% CI: 0.32–0.70).
The relationship between the MeDi diet and episodic memory or dementia was examined in the subgroup analysis (Supplementary Tables 4, 6). Pooled results revealed a positive association between high MeDi diet score and episodic memory in studies conducted before 2015 with low heterogeneity (SMD = 0.06; 95% CI: 0.02–0.10; I2 = 8.4%) and studies with follow up duration < 5 years without heterogeneity (SMD = 0.02; 95% CI: 0.00–0.03; I2 = 0.0%; Supplementary Table 4). For dementia, study location, study published year, and exposure assessment method affected heterogeneity. For the Non-Mediterranean region, studies conducted before 2015 and exposure assessments conducted using other method, there was no heterogeneity (All I2 = 0.0%; Supplementary Table 6).
Owing to the limitations in evidence (requiring more than 10 studies), publication bias was only investigated for cohort studies that analyzed the associations between the MeDi diet and global cognition and MCI. No publication bias was found (Supplementary Figure 2 shows the funnel plot), and both Egger’s and Begg’s tests showed no publication bias in the relationship between adherence to the MeDi diet and global cognition in the cohort studies (Egger’s test: P = 0.330; Begg’s test: P = 0.443). A funnel plot of the relationship between the MeDi diet score and MCI risk in cohort studies was designed (Supplementary Figure 3 shows the funnel plot), and no publication bias was found. Both Egger’s and Begg’s tests showed similar results (Egger’s test: P = 0.968; Begg’s test: P = 0.876).
This systematic review and meta-analysis qualitatively analyzed 31 cohort studies and five RCTs and quantitatively analyzed 26 cohort studies and two RCTs. Pooled results of the RCTs indicated that adherence to the MeDi diet could increase global cognition, episodic memory, and working memory but may reduce attention. The main findings from the prospective studies indicated that high adherence to the MeDi diet could reduce MCI and AD risks. However, no significant associations between adherence to the MeDi diet and cognitive function or dementia were found in the cohort studies.
Our results on cognitive function from the prospective studies are in partial agreement with those of previous studies. Loughrey et al. conducted the first systematic review that investigated the relationship between the MeDi diet and cognitive function (delayed recall, episodic memory, global memory, and working memory) among healthy older adults (24). Their conclusions were similar to our findings, and the differences were mostly not significant, with a small effect size. However, there was high heterogeneity in the effect size. Several possible explanations were given for the contradiction. First, this could have been due to different MeDi diet scoring methods used in the studies. Similarly, when the same MeDi diet score was used, there were individual differences as it was not possible to ensure that every participant strictly adhered to the MeDi diet. Moreover, the current MeDi diet differs from the traditional MeDi diet owing to social, economic, geographical, cultural, and educational factors (68). In addition, differences in cooking methods may have had an impact on the bioavailability of nutrients which could indirectly affect cognitive function. The impact of cooking method varies. For example, gently fried can enhance glucose metabolism which may be linked to increased cognitive function, and it does not destroy dietary phenolic compounds as compared to frying (69). Studies have found that high-temperature cooking such as frying produces acrylamide, and dietary exposure to acrylamide is associated with cognitive function decline (70). Loughrey et al. inferred that the MeDi diet was beneficial in improving global cognition, which is contrary to our findings (24). This may be due to the use of the MMSE scale in the included studies, which may not be sensitive to cognitive changes in healthy populations, according to Gluhm et al. (71). Secondly, some of the populations included in the studies were relatively young (approximately 40 years). Therefore, the effects of the MeDi diet on cognitive changes may have been highly confounded by other factors.
In 2021, a meta-analysis indicated that the results on the relationship between the MeDi diet and cognitive disorders were similar to those in our study. In particular, high adherence to the MeDi diet was beneficial to lower the risk of MCI and AD (22). The Spanish team included 22 studies in the qualitative analysis and 11 studies in the meta-analysis and concluded that the MeDi diet could lower the risk of MCI (RR = 0.91, 95% CI: 0.85–0.97) and AD (RR = 0.89, 95% CI: 0.84–0.93) (22). Before this, Wu et al. and Singh et al. both reached similar conclusions (20, 21), indicating that higher adherence to the MeDi diet could reduce MCI incidence by 17 and 27%, respectively, and AD incidence by 40 and 36%, respectively. Many meta-analyses have demonstrated the effects of representative food groups or potentially beneficial nutrients in the MeDi diet on cognitive health. The MeDi diet typically includes daily consumption of vegetables, fruits, whole grains, and moderate consumption of fish and red wine, as well as partaking in exercise (8). Intake of foods, such as fruit, vegetables, fish, and cereals, as well as nutrients, including vitamins and omega 3, can reduce mild and even severe cognitive impairment (72–75). The MeDi diet may also reduce cognitive decline by reducing oxidative stress (76, 77). Furthermore, in 2004, Chrysohoou et al. conducted the Attica study and found that adherence to the MeDi diet could lower C-reactive protein (CRP) and interleukin levels, thus protecting cognitive health (78, 79). Gu et al. showed similar results in 2010, indicating that high adherence to the MeDi diet could lower high-sensitivity CRP levels, thereby reducing the risk of AD by 34% (18). Additionally, there is some evidence that olive oil plays a key role in the MeDi diet and may be protective against AD risk, especially in ApoE4 carriers (70, 80). Therefore, it may be important to modulate pathways affected by genetic risk factors (i.e., ApoE 4), as ApoE is the most important susceptibility locus and a non-modifiable genetic risk factor for AD (64, 81). Many systematic reviews uncovered no association between high MeDi diet score and dementia (21, 23). A meta-analysis conducted by Wu et al. indicated that adherence to the MeDi diet was not related to dementia risk (RR = 1.07, 95% CI: 0.81–1.42) (21), which is consistent with our results. A possible explanation for this is that the effect of the MeDi diet on dementia may be in delaying the onset of dementia, which would take at least 5 years, if not 10+ years, to reveal (82).
Regarding the relationship between the MeDi diet and cognitive function in RCTs, the earliest meta-analysis of RCTs on this topic was presented by Loughrey et al. in 2017. They showed that high adherence to the MeDi diet could strengthen delayed recall, global cognition, and working memory, but no such association with attention was found (24). In 2018, Radd-Vagenas et al. also conducted a systematic review and meta-analysis of RCTs and reported the effects of the MeDi diet on seven cognitive domains (global cognition, attention, verbal and visual memory, working memory, processing speed, and executive function) (83). However, part of the results was inconsistent with our conclusion, as we found that high adherence to the MeDi diet had an adverse effect on attention. This discrepancy was likely owing to the limited evidence, as our review only analyzed the RCTs conducted by Vall-Pedret et al. and Knight et al. (63, 66). Therefore, future clinical studies are undoubtedly needed to obtain more convincing results. Secondly, since the Mediterranean diet is a dietary pattern rather than a single diet or diet group, it is possible that one of the diet groups considered as Mediterranean had a negative impact on cognitive function but was masked by the effects of other groups. For example, studies have shown that meat consumption is associated with poorer cognitive function (84). Animal models have revealed that these meat products contain a large amount of saturated fatty acids, trans fatty acids, conjugated linoleic acid, and other substances, which may adversely affect the central nervous system and impact cognitive function (85).
Of note, the pooled results of the included cohort studies and RCTs in our study were not the same, which may have been due to the insufficient number of included studies. Second, differences in the assessment of the MeDi diet also led to differences in the results. Third, the follow-up period was extremely short, as the RCT conducted by Knight et al. lasted for only 6 months (63), while cognitive function needs time to develop detectable change. Lastly, there was a lack of standardized tests for cognitive health to measure changes in cognitive function.
The strength of this paper primarily lies in the following four factors: First, only cohort studies and RCTs were included in this review, and the results were discussed and analyzed separately. Second, this review strictly followed the PRISMA guidelines in the review process, and each step was carefully checked and examined. Third, a meta-regression analysis was conducted to determine the source of heterogeneity. Finally, when performing data extraction, we attempted to contact the authors to obtain accurate raw data. However, there were also limitations to our study. First, the MeDi diet assessment scores and cognitive function testing methods were different, which may have caused bias in the results. Additionally, differences in the dietary assessment methods may also limit comparability and increase error. Third, the follow-up duration of some cohort studies may have been too short to account for changes in cognitive function. Finally, we could not control for dietary differences due to regional variations. Although all studies adhered to the MeDi diet, it was thought that it would be easier and more effective for people from MeDi regions to follow the MeDi diet than for people in other regions.
In summary, this review provided significant evidence that adherence to the MeDi diet could lower the risk of MCI and AD, whereas adherence to the MeDi diet was not related to dementia and other specific cognitive function domains (global cognition, working memory, and episodic memory) in the cohort studies. Across the RCTs, high adherence to the MeDi diet was positively associated with global cognition, working memory, and episodic memory. However, a negative association between the MeDi diet and attention was found. Overall, the MeDi diet is recommended to prevent or delay cognitive disorders and improve cognitive function. These results reinforce further clinical trials on the association between the MeDi diet and cognitive health, with longer follow-up time, especially on attention. Besides that, studies focus on cooking methods, cooking frequency in the MeDi diet was suggested to conduct as well.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
JF and L-JT: literature review and data extraction, data synthesis and statistical analysis, manuscript drafting. SS and JL: manuscript critical revision. All authors approved the final version to be submitted.
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1F1A1074279). MSIT: Ministry of Science and ICT.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.946361/full#supplementary-material
1. Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet. (2006) 367:1262–70. doi: 10.1016/S0140-6736(06)68542-5
2. Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. (2001) 58:1985–92. doi: 10.1001/archneur.58.12.1985
3. WHO. Dementia. (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed November 29, 2021).
4. Monica Moore M, Díaz-Santos M, Vossel K. Alzheimer’s disease facts and figures. Chicago, IL: Alzheimer’s Association (2022).
5. Khazanchi R, Evans CT, Marcelin JR. Racism, not race, drives inequity across the COVID-19 continuum. JAMA Netw Open. (2020) 3:e2019933. doi: 10.1001/jamanetworkopen.2020.19933
6. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. (2011) 377:1019–31. doi: 10.1016/S0140-6736(10)61349-9
7. Trichopoulou A, Lagiou P. Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutr Rev. (1997) 55:383–9. doi: 10.1111/j.1753-4887.1997.tb01578.x
8. Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. (1995) 61:1402S–6S. doi: 10.1093/ajcn/61.6.1402S
9. Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. (2017) 9:1063. doi: 10.3390/nu9101063
10. Rosato V, Temple NJ, La Vecchia C, Castellan G, Tavani A, Guercio V. Mediterranean diet and cardiovascular disease: a systematic review and meta-analysis of observational studies. Eur J Nutr. (2019) 58:173–91. doi: 10.1007/s00394-017-1582-0
11. Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. (2015) 5:e008222. doi: 10.1136/bmjopen-2015-008222
12. Cowell OR, Mistry N, Deighton K, Matu J, Griffiths A, Minihane AM, et al. Effects of a Mediterranean diet on blood pressure: a systematic review and meta-analysis of randomized controlled trials and observational studies. J Hypertens. (2021) 39:729–39. doi: 10.1097/HJH.0000000000002667
13. Cherbuin N, Anstey KJ. The Mediterranean diet is not related to cognitive change in a large prospective investigation: the PATH through life study. Am J Geriatr Psychiatry. (2012) 20:635–9. doi: 10.1097/JGP.0b013e31823032a9
14. Haring B, Wu C, Mossavar-Rahmani Y, Snetselaar L, Brunner R, Wallace RB, et al. No association between dietary patterns and risk for cognitive decline in older women with 9-year follow-up: data from the women’s health initiative memory study. J Acad Nutr Diet. (2016) 116:921–30.e1. doi: 10.1016/j.jand.2015.12.017
15. Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. (2009) 302:638–48. doi: 10.1001/jama.2009.1146
16. Wu J, Song X, Chen GC, Neelakantan N, van Dam RM, Feng L, et al. Dietary pattern in midlife and cognitive impairment in late life: a prospective study in Chinese adults. Am J Clin Nutr. (2019) 110:912–20. doi: 10.1093/ajcn/nqz150
17. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. (2015) 11:1007–14. doi: 10.1016/j.jalz.2014.11.009
18. Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer’s disease. J Alzheimers Dis. (2010) 22:483–92. doi: 10.3233/JAD-2010-100897
19. Bhushan A, Fondell E, Ascherio A, Yuan C, Grodstein F, Willett W. Adherence to Mediterranean diet and subjective cognitive function in men. Eur J Epidemiol. (2018) 33:223–34. doi: 10.1007/s10654-017-0330-3
20. Singh B, Parsaik AK, Mielke MM, Erwin PJ, Knopman DS, Petersen RC, et al. Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. (2014) 39:271–82. doi: 10.3233/JAD-130830
21. Wu L, Sun D. Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci Rep. (2017) 7:41317. doi: 10.1038/srep41317
22. Garcia-Casares N, Fuentes PG, Barbancho MA, Lopez-Gigosos R, Garcia-Rodriguez A, Gutierrez-Bedmar M. Alzheimer’s disease, mild cognitive impairment and Mediterranean diet. A systematic review and dose-response meta-analysis. J. Clin. Med. (2021) 10:4642. doi: 10.3390/jcm10204642
23. Limongi F, Siviero P, Bozanic A, Noale M, Veronese N, Maggi S. The effect of adherence to the mediterranean diet on late-life cognitive disorders: a systematic review. J Am Med Dir Assoc. (2020) 21:1402–9. doi: 10.1016/j.jamda.2020.08.020
24. Loughrey DG, Lavecchia S, Brennan S, Lawlor BA, Kelly ME. The impact of the Mediterranean diet on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Adv Nutr. (2017) 8:571–86. doi: 10.3945/an.117.015495
25. Aridi YS, Walker JL, Wright ORL. The association between the Mediterranean dietary pattern and cognitive health: a systematic review. Nutrients. (2017) 9:674. doi: 10.3390/nu9070674
26. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
27. Wells GA, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. Ottawa, ON: University of Ottawa (2022).
28. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
29. Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. (1998) 280:1690–1. doi: 10.1001/jama.280.19.1690
30. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons (2019).
31. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
32. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
33. Sterne JAC. Meta-analysis in Stata: An updated collection from the Stata Journal. College Station, TX: StataCorp LP (2009).
34. Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. (2002) 21:1559–73. doi: 10.1002/sim.1187
35. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
36. Charisis S, Ntanasi E, Yannakoulia M, Anastasiou CA, Kosmidis MH, Dardiotis E, et al. Mediterranean diet and risk for dementia and cognitive decline in a Mediterranean population. J Am Geriatr Soc. (2021) 69:1548–59. doi: 10.1111/jgs.17072
37. Tanaka T, Talegawkar SA, Jin Y, Colpo M, Ferrucci L, Bandinelli S. Adherence to a Mediterranean diet protects from cognitive decline in the invecchiare in Chianti study of aging. Nutrients. (2018) 10:2007. doi: 10.3390/nu10122007
38. Trichopoulou A, Kyrozis A, Rossi M, Katsoulis M, Trichopoulos D, La Vecchia C, et al. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur J Nutr. (2015) 54:1311–21. doi: 10.1007/s00394-014-0811-z
39. Galbete C, Toledo E, Toledo JB, Bes-Rastrollo M, Buil-Cosiales P, Marti A, et al. Mediterranean diet and cognitive function: the SUN project. J Nutr Health Aging. (2015) 19:305–12. doi: 10.1007/s12603-015-0441-z
40. Gallucci M, Mazzuco S, Ongaro F, Di Giorgi E, Mecocci P, Cesari M, et al. Body mass index, lifestyles, physical performance and cognitive decline: the “Treviso Longeva (TRELONG)” study. J Nutr Health Aging. (2013) 17:378–84. doi: 10.1007/s12603-012-0397-1
41. Lutski M, Weinstein G, Ben-Zvi S, Goldbourt U, Tanne D. Adherence to Mediterranean diet and subsequent cognitive decline in men with cardiovascular disease. Nutr. Neurosci. (2020) 21:1402–9. doi: 10.1080/1028415X.2020.1715049
42. Kesse-Guyot E, Andreeva VA, Lassale C, Ferry M, Jeandel C, Hercberg S, et al. Mediterranean diet and cognitive function: a French study. Am J Clin Nutr. (2013) 97:369–76. doi: 10.3945/ajcn.112.047993
43. Psaltopoulou T, Kyrozis A, Stathopoulos P, Trichopoulos D, Vassilopoulos D, Trichopoulou A. Diet, physical activity and cognitive impairment among elders: the EPIC-Greece cohort (European prospective investigation into cancer and nutrition). Public Health Nutr. (2008) 11:1054–62. doi: 10.1017/S1368980007001607
44. Berendsen AM, Kang JH, Feskens EJM, de Groot C, Grodstein F, van de Rest O. Association of long-term adherence to the MIND diet with cognitive function and cognitive decline in American women. J Nutr Health Aging. (2018) 22:222–9. doi: 10.1007/s12603-017-0909-0
45. Gardener SL, Rainey-Smith SR, Barnes MB, Sohrabi HR, Weinborn M, Lim YY, et al. Dietary patterns and cognitive decline in an Australian study of ageing. Mol Psychiatry. (2015) 20:860–6. doi: 10.1038/mp.2014.79
46. Koyama A, Houston DK, Simonsick EM, Lee JS, Ayonayon HN, Shahar DR, et al. Association between the Mediterranean diet and cognitive decline in a biracial population. J Gerontol A Biol Sci Med Sci. (2015) 70:354–9. doi: 10.1093/gerona/glu097
47. Olsson E, Karlstrom B, Kilander L, Byberg L, Cederholm T, Sjogren P. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J Alzheimers Dis. (2015) 43:109–19. doi: 10.3233/JAD-140867
48. Qin B, Adair LS, Plassman BL, Batis C, Edwards LJ, Popkin BM, et al. Dietary patterns and cognitive decline among Chinese older adults. Epidemiology. (2015) 26:758–68. doi: 10.1097/EDE.0000000000000338
49. Roberts RO, Geda YE, Cerhan JR, Knopman DS, Cha RH, Christianson TJ, et al. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement Geriatr Cogn Disord. (2010) 29:413–23. doi: 10.1159/000305099
50. Samieri C, Okereke OI, E Devore E, Grodstein F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr. (2013) 143:493–9. doi: 10.3945/jn.112.169896
51. Samieri C, Grodstein F, Rosner BA, Kang JH, Cook NR, Manson JE, et al. Mediterranean diet and cognitive function in older age. Epidemiology. (2013) 24:490–9. doi: 10.1097/EDE.0b013e318294a065
52. Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. (2009) 66:216–25. doi: 10.1001/archneurol.2008.536
53. Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. (2009) 302:627–37. doi: 10.1001/jama.2009.1144
54. Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. (2006) 59:912–21. doi: 10.1002/ana.20854
55. Shannon OM, Stephan BCM, Granic A, Lentjes M, Hayat S, Mulligan A, et al. Mediterranean diet adherence and cognitive function in older UK adults: the European prospective investigation into cancer and nutrition-norfolk (EPIC-Norfolk) study. Am J Clin Nutr. (2019) 110:938–48. doi: 10.1093/ajcn/nqz114
56. Tangney CC, Li H, Wang YM, Barnes L, Schneider JA, Bennett DA, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. (2014) 83:1410–6. doi: 10.1212/Wnl.0000000000000884
57. Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr. (2011) 93:601–7. doi: 10.3945/ajcn.110.007369
58. Tsivgoulis G, Judd S, Letter AJ, Alexandrov AV, Howard G, Nahab F, et al. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology. (2013) 80:1684–92. doi: 10.1212/WNL.0b013e3182904f69
59. Vercambre MN, Grodstein F, Berr C, Kang JH. Mediterranean diet and cognitive decline in women with cardiovascular disease or risk factors. J Acad Nutr Diet. (2012) 112:816–23. doi: 10.1016/j.jand.2012.02.023
60. Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran C, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. (2013) 98:1263–71. doi: 10.3945/ajcn.112.051276
61. Morris L, Bhatnagar D. The Mediterranean diet. Curr Opin Lipidol. (2016) 27:89–91. doi: 10.1097/MOL.0000000000000266
62. Hardman RJ, Meyer D, Kennedy G, Macpherson H, Scholey AB, Pipingas A. Findings of a pilot study investigating the effects of Mediterranean diet and aerobic exercise on cognition in cognitively healthy older people living independently within aged-care facilities: the lifestyle intervention in independent living aged care (LIILAC) study. Curr Dev Nutr. (2020) 4:nzaa077. doi: 10.1093/cdn/nzaa077
63. Knight A, Bryan J, Wilson C, Hodgson JM, Davis CR, Murphy KJ. The Mediterranean diet and cognitive function among healthy older adults in a 6-month randomised controlled trial: the MedLey study. Nutrients. (2016) 8:579. doi: 10.3390/nu8090579
64. Martinez-Lapiscina EH, Galbete C, Corella D, Toledo E, Buil-Cosiales P, Salas-Salvado J, et al. Genotype patterns at CLU, CR1, PICALM and APOE, cognition and Mediterranean diet: the PREDIMED-NAVARRA trial. Genes Nutr. (2014) 9:393. doi: 10.1007/s12263-014-0393-7
65. Sanchez-Villegas A, Galbete C, Martinez-Gonzalez MA, Martinez JA, Razquin C, Salas-Salvado J, et al. The effect of the Mediterranean diet on plasma brain-derived neurotrophic factor (BDNF) levels: the PREDIMED-NAVARRA randomized trial. Nutr Neurosci. (2011) 14:195–201. doi: 10.1179/1476830511Y.0000000011
66. Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martinez-Gonzalez MA, et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med. (2015) 175:1094–103. doi: 10.1001/jamainternmed.2015.1668
67. Martinez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvado J, San Julian B, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. (2013) 84:1318–25. doi: 10.1136/jnnp-2012-304792
68. Zbeida M, Goldsmith R, Shimony T, Vardi H, Naggan L, Shahar DR. Mediterranean diet and functional indicators among older adults in non-Mediterranean and Mediterranean countries. J Nutr Health Aging. (2014) 18:411–8. doi: 10.1007/s12603-014-0003-9
69. Bakker L, Ramakers I, van Boxtel MPJ, Schram MT, Stehouwer CDA, van der Kallen CJH, et al. Associations between plasma kynurenines and cognitive function in individuals with normal glucose metabolism, prediabetes and type 2 diabetes: the Maastricht study. Diabetologia. (2021) 64:2445–57. doi: 10.1007/s00125-021-05521-4
70. Liu ZM, Tse LA, Chen B, Wu S, Chan D, Kowk T, et al. Dietary acrylamide exposure was associated with mild cognition decline among non-smoking Chinese elderly men. Sci Rep. (2017) 7:6395. doi: 10.1038/s41598-017-06813-9
71. Gluhm S, Goldstein J, Loc K, Colt A, Liew CV, Corey-Bloom J. Cognitive performance on the mini-mental state examination and the montreal cognitive assessment across the healthy adult lifespan. Cogn Behav Neurol. (2013) 26:1–5. doi: 10.1097/WNN.0b013e31828b7d26
72. Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr. (2016) 103:330–40. doi: 10.3945/ajcn.115.124081
73. Mottaghi T, Amirabdollahian F, Haghighatdoost F. Fruit and vegetable intake and cognitive impairment: a systematic review and meta-analysis of observational studies. Eur J Clin Nutr. (2018) 72:1336–44. doi: 10.1038/s41430-017-0005-x
74. Solfrizzi V, Custodero C, Lozupone M, Imbimbo BP, Valiani V, Agosti P, et al. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer’s disease and late-life cognitive disorders: a systematic review. J Alzheimers Dis. (2017) 59:815–49. doi: 10.3233/JAD-170248
75. Marti Del Moral A, Fortique F. Omega-3 fatty acids and cognitive decline: a systematic review. Nutr Hosp. (2019) 36:939–49. doi: 10.20960/nh.02496
76. Butterfield D, Castegna A, Pocernich C, Drake J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J Nutr Biochem. (2002) 13:444. doi: 10.1016/s0955-2863(02)00205-x
77. Mecocci P. Oxidative stress in mild cognitive impairment and Alzheimer disease: a continuum. J Alzheimers Dis. (2004) 6:159–63. doi: 10.3233/jad-2004-6207
78. Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: the ATTICA study. J Am Coll Cardiol. (2004) 44:152–8. doi: 10.1016/j.jacc.2004.03.039
79. Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol. (2005) 4:371–80. doi: 10.1016/S1474-4422(05)70099-5
80. Norwitz NG, Saif N, Ariza IE, Isaacson RS. Precision nutrition for Alzheimer’s prevention in ApoE4 carriers. Nutrients. (2021) 13:1362. doi: 10.3390/nu13041362
82. Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, et al. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol. (2008) 64:492–8. doi: 10.1002/ana.21509
83. Radd-Vagenas S, Duffy SL, Naismith SL, Brew BJ, Flood VM, Fiatarone Singh MA. Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am J Clin Nutr. (2018) 107:389–404. doi: 10.1093/ajcn/nqx070
84. Titova OE, Ax E, Brooks SJ, Sjogren P, Cederholm T, Kilander L, et al. Mediterranean diet habits in older individuals: associations with cognitive functioning and brain volumes. Exp Gerontol. (2013) 48:1443–8. doi: 10.1016/j.exger.2013.10.002
Keywords: cognitive function, mild cognitive impairment, dementia, Alzheiemer’s disease, Mediterranean diet (MD)
Citation: Fu J, Tan L-J, Lee JE and Shin S (2022) Association between the mediterranean diet and cognitive health among healthy adults: A systematic review and meta-analysis. Front. Nutr. 9:946361. doi: 10.3389/fnut.2022.946361
Received: 17 May 2022; Accepted: 12 July 2022;
Published: 28 July 2022.
Edited by:
Ioannis Zabetakis, University of Limerick, IrelandReviewed by:
Mary Yannakoulia, Harokopio University, GreeceCopyright © 2022 Fu, Tan, Lee and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sangah Shin, ivory8320@cau.ac.kr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.