
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 26 August 2021
Sec. Neurocognitive Aging and Behavior
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.708130
This article is part of the Research TopicAffective, Cognitive and Social Neuroscience: New Knowledge in Normal Aging, Minor and Major Neurocognitive DisordersView all 25 articles
Background: Heart rate fragmentation (HRF), a new non-invasive metric quantifying cardiac neuroautonomic function, is associated with increasing age and cardiovascular disease. Since these are risk factors for cognitive decline and dementia, in the Multi-Ethnic Study of Atherosclerosis (MESA), we investigated whether disrupted cardiac neuroautonomic function, evidenced by increased HRF, would be associated with worse cognitive function assessed concurrently and at a later examination, and with greater cognitive decline.
Methods: HRF was derived from the ECG channel of the polysomnographic recordings obtained in an ancillary study (n = 1,897) conducted in conjunction with MESA exam 5 (2010–2012). Cognitive function was assessed at exam 5 and 6.4 ± 0.5 years later at exam 6 (2016–2018) with tests of global cognitive performance (the Cognitive Abilities Screening Instrument, CASI), processing speed (Digit Symbol Coding, DSC) and working memory (Digit Span). Multivariable regression models were used to quantify the associations between HRF indices and cognitive scores.
Results: The participants’ mean age was 68 ± 9 years (54% female). Higher HRF at baseline was independently associated with lower cognitive scores at both exams 5 and 6. Specifically, in cross-sectional analyses, a one-standard deviation (SD) (13.7%) increase in HRF was associated with a 0.51 (95% CI: 0.17–0.86) points reduction in CASI and a 1.12 (0.34–1.90) points reduction in DSC. Quantitatively similar effects were obtained in longitudinal analyses. A one-SD increase in HRF was associated with a 0.44 (0.03–0.86) and a 1.04 (0.28–1.81) points reduction in CASI and DSC from exams 5 to 6, respectively. HRF added predictive value to the Cardiovascular Risk Factors, Aging, and Incidence of Dementia (CAIDE-APOE-ε4) risk score and to models adjusted for serum concentration of NT-proBNP, an analyte associated with cognitive impairment and dementia.
Conclusion: Increased HRF assessed during sleep was independently associated with diminished cognitive performance (concurrent and future) and with greater cognitive decline. These findings lend support to the links between cardiac neuroautonomic regulation and cognitive function. As a non-invasive, repeatable and inexpensive probe, HRF technology may be useful in monitoring cognitive status, predicting risk of dementia and assessing therapeutic interventions.
Risk assessment of cognitive impairment is a major public health priority, especially given the increasing prevalence of dementia syndromes in the aging population. A key barrier to progress is the lack of reliable, quantitative and non-invasive methods that complement expensive brain imaging technologies, semi-quantitative functional tests of cognitive status as well as emerging biochemical and “omics” probes. Important insights into the pathophysiology of cognitive impairment syndromes derive from increasing evidence linking central nervous system dysfunction with perturbations in autonomic function (Collins et al., 2012; Fanciulli et al., 2013; Meel-van den Abeelen et al., 2013; Tahsili-Fahadan and Geocadin, 2017; Ahmed et al., 2018). The major probes of such interactions have been heart rate variability (HRV) measures. A PubMed search for “(heart rate variability) AND (cognitive function)” yields over 1,000 citations. Despite this large body of work, traditional HRV measures have failed to gain traction in clinical practice as reliable predictors of mild cognitive impairment or of major adverse cardiovascular events (CVEs), which are strong correlates of cognitive decline and dementia syndromes (Zlokovic et al., 2020).
The failure of traditional HRV indices to fulfill their promise as translational, non-invasive probes of cardiac neuroautonomic function is ascribable to its fundamental assumption, one that equates the amplitude of variations in sinus normal-to-normal (NN) intervals with the degree of vagal tone modulation (HRV, 1996). With aging and organic heart disease, physiologic vagal activity almost invariably decreases. In these contexts, a consistent reduction in short-term HRV values would be expected. However, multiple studies (Reardon and Malik, 1996; Bruyne et al., 1999; Stein et al., 2005; Huikuri and Stein, 2012; Drawz et al., 2013; Raman et al., 2017) have reported a counterintuitive increase in the amounts of short-term/high-frequency (0.15–0.4 Hz) variability in high-risk individuals. We (Costa et al., 2017a) have referred to the occurrence of high HRV in settings where highly diminished vagal tone modulation is anticipated as the “HRV paradox.”
Recently, we (Costa et al., 2017a,b) delineated a property of heart rate (HR) dynamics, termed heart rate fragmentation (HRF), which helps resolve this paradox. Heart rate fragmentation metrics quantify variations in NN intervals due to non-respiratory sinus arrhythmia and other anomalous dynamical variants. We previously reported that: (i) HRF monotonically increases with cross-sectional age both in healthy subjects and in those with coronary artery disease (Costa et al., 2017a,b); (ii) increased HRF is associated with both incident major adverse CVEs (Costa et al., 2018) and incident atrial fibrillation (AF) (Costa et al., 2021) in the Multi-Ethnic Study of Atherosclerosis (MESA), and (iii) HRF metrics add significant predictive value to cardiac risk indices such as Framingham (D’Agostino et al., 2008; Costa et al., 2018) and CHARGE-AF1 (Alonso et al., 2013; Costa et al., 2021), in MESA. These findings motivated the present investigation. Our working hypotheses were that disrupted cardiac neuroautonomic function at a baseline time, evidenced by increased degree of HRF, would be independently associated with (1) lower cognitive performance, measured concurrently and at a future time, and (2) greater longitudinal cognitive decline in a cohort of participants in MESA. Secondarily, we hypothesized that HRF metrics would be inversely associated with cognitive test scores in models with serum concentration of NH2-terminal prohormone B-type natriuretic peptide (NT-proBNP), a major cardiovascular (CV) risk factor also reported to be associated with dementia (Ostovaneh et al., 2020), cognitive performance (Ferguson et al., 2018; Ostovaneh et al., 2020) and brain structure and function by MRI (Zonneveld et al., 2017; Ferguson et al., 2018).
We included widely-used HRV metrics in this study to contrast their performances with those of the HRF indices. Within the canonical HRV interpretative framework, in which increased amount of high-frequency variability is a reflection of increased parasympathetic activity, positive cross-sectional and longitudinal relationships would be expected between the HRV indices and the cognitive scores. However, we did not anticipate finding consistent associations due to the fact that MESA is a study of middle-age to older participants, which increases the probability that the observed variability is due to HRF rather than vagal tone modulation.
The MESA study has been previously described in detail (Bild et al., 2002). Briefly, in 2000–2002, 6,814 persons between the ages of 45 and 84 without clinically evident CV disease were recruited at six U.S. field centers. Institutional review boards approved the conduct of the study. Written informed consent was obtained from all participants. MESA fifth examination, conducted in 2010–2012, included 4,656 participants. MESA sixth examination, conducted in 2016–2018, included 3,303 participants, 95% (3,153) of whom had also participated in exam 5.
The HR dynamical indices used here (HRF and HRV) were obtained from an ancillary sleep study conducted in 2010–2013 in conjunction with MESA’s fifth examination. The study included 2,237 (out of 4,656) participants, who underwent in-home overnight polysomnography (PSG) following a standardized protocol (Chen et al., 2015). Two thousand and fifty seven participants had a valid PSG study. Hereafter, we refer to this group as the MESA-Sleep cohort. Compared with the participants who did not undergo the sleep examination, those who did and had a valid study were relatively younger (mean, 68 vs. 71 yr), less likely to be hypertensive (58 vs. 62%), and more likely to be Hispanic (23.5% vs. 19.1%). They had lower coronary artery calcification (CAC) scores (median [interquartile range]: 30 [0–215] vs. 57 [0–338]) and lower blood concentration of NT-proBNP (71 [35–139] vs. 83 [43–181] pg/ml). There were no differences in sex, body mass index, smoking status, diabetes and prevalent CV disease. Similarly, there were no differences in the prevalence of self-report doctor-diagnosed sleep apnea.
The PSG data were scored at a centralized sleep reading center in accordance with published guidelines (Redline et al., 1998, 2007). The electrocardiographic (ECG) channel, sampled at 256 Hz, was processed using Compumedics Somte software (Compumedics LTd., Abbottsville, Australia) for detection and classification of the QRS complexes as normal sinus (N), premature ventricular complexes or premature supraventricular complexes. The automated annotations were reviewed and corrected when necessary by a trained technician. One hundred and sixty participants were excluded due to: poor signal quality (n = 33), electronic pacemaker (n = 14), missing annotations for sleep stage or QRS complexes (n = 14), <2 h of combined sleep periods scored as rapid eye movement (REM) or non-REM (n = 14), in AF at the time of the PSG (n = 22), prevalent dementia as defined below (n = 23), missing information on dementia status (n = 29) and those for whom (n = 11) the last recorded follow-up for AF events was prior to the PSG study. Overall, HR dynamical measures were calculated for 1,897 participants.
In addition to clinical exams, MESA participants were contacted by telephone every nine to twelve months to obtain information about hospital admissions and medical events. For those over age 65 and enrolled in fee-for-service Medicare, claims data were also used to identify diagnosis and procedure codes. Trained personnel abstracted any hospital records suggesting possible CVEs, which were then adjudicated by the MESA Morbidity and Mortality Committee. Non-fatal endpoints in MESA include congestive heart failure, angina, myocardial infarction, percutaneous coronary intervention, coronary bypass grafting or other revascularization procedure, resuscitated cardiac arrest, peripheral arterial disease, stroke (non-hemorrhagic) and transient ischemic attack. Cardiovascular deaths, as adjudicated by committee review, included fatalities directly related to stroke or coronary heart disease. The definition and adjudication of these events have been previously described (Bild et al., 2002; Bluemke et al., 2008; Yeboah et al., 2012). The identification of prevalent AF (Heckbert et al., 2017), including fibrillation and flutter, was based on diagnostic codes from hospital admissions, inpatient and outpatient Medicare claims (for those enrolled in fee-for-service Medicare) and study 12-lead ECGs. Dementia diagnosis was based on death certificates and hospital discharge ICD codes (Fujiyoshi et al., 2017) in addition to usage of acetylcholinesterase inhibitor and other medications used specifically for the treatment of Alzheimer’s disease and related dementias.
In MESA (Fitzpatrick et al., 2015), three different standardized and validated tests were used to quantify cognitive performance: the Cognitive Abilities Screening Instrument (CASI, version 2) (Teng et al., 1994), the digit symbol coding (DSC) (Wechsler, 1997), and the digit span (DS) (Wechsler, 1997). The CASI is a test of global cognitive function that contains 25 items representing nine cognitive domains, including short- and long-term memory, attention, concentration, orientation, language, verbal fluency, visual construction, and abstraction/judgment. Scores from individual items on the CASI were summed to obtain an overall cognitive function score (range 0–100). The DSC (range 0–133) is a subtest of the Wechsler Adult Intelligence Scale III (WAIS-III). Participants are given a random sequence of digits (1–9) and asked to write the correct symbol (e.g., +, >) for each digit based on a legend of digit–symbol pairs. The score is the number of correct symbols written in 120 seconds. The DSC score quantifies processing speed (how quickly simple perceptual or mental operations can be performed). The DS, also a subtest of WAIS-III, consists of two tasks. In one, participants are asked to repeat forward and in the other backward, increasingly longer spans of randomly ordered digits read to them. The scores are based on the total number of correct answers (DS forward: range 0–16; DS backward: range, 0–14). The DS forward and backward measure related but slightly different aspects of memory (Ramsay and Reynolds, 1995; Cullum, 1998; Izawa et al., 2009). The DS forward is assumed to assess mainly attention and short-term auditory memory. The DS backward is assumed to measure working memory subsuming short-term memory and elements of executive control required to manipulate verbal information. Higher CASI, DSC and DS scores are indicative of better cognitive performance.
We excluded participants with missing or invalid cognitive scores due to physical (hearing, vision, motor) impairments that interfere with performance, language barriers, and circumstantial reasons, such as answering a cell phone call during a test. In the case of CASI, tests with a score <20 (n = 12) and those missing responses to more than three questions (n = 9) were also considered invalid. Overall, analyses with exam 5 CASI, DS forward, DS backward and DSC scores included 1,823 (96%), 1,881 (99%), 1,884 (99%) and 1,715 (90%) of the 1,897 participants for whom HR dynamical indices were calculated. Participants in the MESA-Sleep cohort with missing or invalid vs. valid DSC scores were on average two years older, had significantly higher systolic blood pressure (BP) and body mass index (BMI) values. A higher percentage had had a CVE (12.5 vs. 8.6%). Their degree of HRF was significantly higher (PNNSS: 68.3 vs. 65.6%).
Approximately 62% (2,051 out of 3,303) of participants in MESA exam 6 underwent cognitive evaluation. The number of participants with a PSG study and valid cognitive scores at exams 5 and 6 was 971, 1016 and 845 for the CASI, DS and DSC tests, respectively.
Age, sex, race/ethnicity (White, Chinese-American, African-American and Hispanic) smoking status, and medication use were self-reported. Systolic and diastolic BPs were measured three times after the participant rested for five minutes; the means of the last two measurements were used here. Diabetes was defined by a fasting blood glucose ≥126 mg/dL or self-reported use of antidiabetic medication. Antihypertensive medication included beta-blockers, calcium channel blockers, angiotensin converting enzyme inhibitors or receptor blockers, vasodilators and diuretics. Depression was defined as a score of more than 15 points in the Center of Epidemiological Studies Depression scale. Antidepressant medication included tricyclic and non-tricyclic (other than monoamine oxidase inhibitors) medications accompanied or not by antipsychotic medications. Alcohol use (a binary variable: yes/no) was defined as consuming ≥ 1 glass of wine or the equivalent per week. Physical activity, defined as the number of metabolic equivalent minutes per week of intentional moderate and vigorous activity, was quantified using the MESA Typical Week Physical Activity Survey, adapted from the Cross-Cultural Activity Participation Study (Bertoni et al., 2009). APOE isoforms were estimated from single nucleotide polymorphisms rs429358 and rs7412 (Fitzpatrick et al., 2015). Gross family income was dichotomized as below and above $75,000 (top quartile). Sleep maintenance efficiency was defined as the percentage of sleep time asleep after sleep onset. Two measures of sleep structure included in this study were the percentage of time in sleep stages N3 (slow wave sleep [SWS]) and the percentage of time in REM. Sleep disordered breathing was assessed by the apnea hypopnea index (AHI), defined as the average number per hour of sleep of all apneas and hypopneas associated with ≥3% oxygen desaturation or arousal, and two measures of overnight hypoxemia: the percentage of sleep time with pulse oximeter oxygen saturation <90% (% time SpO2 < 90%), and the oxygen desaturation index (ODI), defined as the number of oxygen desaturations ≥ 3% per hour of sleep. The CAC scores, derived from computed tomography imaging using the Agatston method (Agatston et al., 1990), was available for 1,464 (out of 1,897; 77%) participants at exam 5 (Carr et al., 2005). There were no significant differences in demographic (age, sex, race) and CV risk factors (diabetes, pack-years of smoking, hypertensive status, BMI, CV and AF disease prevalence) between those with and without CAC scores. Serum concentration of NT-proBNP measured at exam 5 was available for all but 39 participants in our study.
The ECG channels of the PSG recordings obtained at MESA exam 5 were analyzed for detection and classification of each QRS complex as normal (N) sinus, premature supraventricular or premature ventricular complexes. The sequence of normal-to-normal (NN) intervals between sleep onset and sleep termination were extracted for computation of HRF and traditional HRV indices.
Heart rate fragmentation was quantified by an ensemble of statistical metrics derived from the analysis of NN interval time series, as described in Costa et al. (2017a, 2018). These metrics are based on counts of HR acceleration, deceleration and no-change intervals. Let ti represent the time of occurrence of a given QRS complex and NNi the time interval (ti – ti–1) between consecutive QRS complexes. Heart rate acceleration, deceleration and no-change intervals (in seconds) are defined as ΔNNi ≤ –n/SF, ΔNNi ≥ n/SF and –n/SF < ΔNNi < n/SF, respectively, where ΔNNi = NNi – NNi–1, n is a positive integer (in this case, n = 1) and SF is the sampling frequency (in Hz) of the ECG signal. (Note that HR and ΔNNi values are inversely related.) Sequences of negative (positive) ΔNN intervals are termed accelerative (decelerative) segments. The length of a segment is the number of ΔNN intervals it contains.
We computed three inter-related indices of overall degree of fragmentation (PIP, PNNLS, PNNSS). Briefly, PIP, the percentage of “inflection points,” is defined as the combined percentage of transitions from HR acceleration to HR deceleration and vice-versa (“hard” inflection points), and from HR acceleration/deceleration to no-change in HR and vice-versa (“soft” inflection points). Mathematically, a given NNi interval is an inflection point if ΔNNi+1 ×ΔNNi ≤ 0 and ΔNNi+1 ≠ΔNNi. The overall percentage of ΔNN intervals in long segments, PNNLS, is the number of ΔNN intervals in accelerative/decelerative segments with ≥3 ΔNN intervals over the total number of ΔNN intervals. The percentage of ΔNN intervals in short segments, PNNSS, is the number of ΔNN intervals in accelerative/decelerative segments with <3 ΔNN intervals over the number of ΔNN intervals in accelerative/decelerative segments of any length. More fragmented time series have higher PIP and PNNSS values, and lower PNNLS values.
Traditional time domain HRV indices (HRV, 1996) were calculated from NN interval time series between sleep onset and sleep termination using a 5-min sliding window (without overlap). Windows with <150 beats and/or >75% NN intervals were excluded. The following time domain measures were computed: (1) the average of all NN intervals (AVNN), (2) the mean of the standard deviations (SDs) of NN intervals in all qualified, 5-min window (SDNN), and (3) the root mean square of successive NN interval differences (rMSSD). On the frequency domain, we calculated the metric high-frequency (HF) power defined as the total spectral power of NN intervals between 0.15 and 0.4 Hz. Power spectrum estimates were obtained using the Lomb periodogram method. For each subject, the values from the different windows were averaged. Of note, mean HR is not a measure of variability of the fluctuations in HR. However, AVNN, which is inversely related to mean HR, is generally included in the set of indices that became known as traditional HRV measures. Here, we use the term HRV to refer to the method of time series analysis and HR variability to refer to the amplitude of the fluctuations (variability) in HR. (Mean HR is an HRV measure but not a measure of HR variability.) The source codes for HRV computations are available at www.physionet.org (Goldberger et al., 2000).
Participants with higher HRF, i.e., higher PIP and PNNSS values and lower PNNLS values, at baseline (exam 5) were hypothesized to have (1) lower cognitive scores both at baseline and at exam 6, and (2) larger decreases in cognitive scores from exams 5 to 6.
This presentation includes both cross-sectional and longitudinal analyses. The former used MESA-Sleep (exam 5) data. The latter used MESA-Sleep data, our baseline, and MESA exam 6 data obtained 6.4 ± 0.5 (mean ± SD) years later. (In the longitudinal studies only participants with both cognitive testing at exams 5 and 6 were included.) Continuous variables are summarized as median and interquartile range, unless otherwise indicated. Categorical variables are presented as numbers and percentages. Differences in baseline characteristics between those with and without cognitive testing at exam 6 were evaluated using the χ2 and Mann-Whitney tests for categorical and continuous variables, respectively. Differences in baseline characteristics across quartiles of PNNSS were assessed using the χ2 and the ANOVA tests for categorical and continuous variables, respectively. Variables with skewed distributions (rMSSD, SDNN, HF, weekly amount of moderate and vigorous physical activity, serum concentration of NT-proBNP, pack-years of cigarette smoking, % time SpO2 < 90%, ODI, and CAC score) were transformed using the natural logarithm. In the case of the last three measures, we added 1 to the variables’ values before the logarithmic transformation due to the occurrence of 0 values.
We used multivariable linear regression models to quantify the associations between cognitive test scores and HR dynamical indices. In the text of the manuscript, we show the results for five models. Model 1 was unadjusted. Model 2 was adjusted for the Cardiovascular Risk Factors, Aging, and Incidence of Dementia (CAIDE) Risk Score with APOE-ε4 (CAIDE–APOE-ε4), a validated tool to predict late-life dementia risk (20 years later) (Kivipelto et al., 2006; Exalto et al., 2014). Model 3 was adjusted for age, race/ethnicity, sex, and education level. Model 4, hereafter referred to as “fully adjusted,” included the variables of Model 3 in addition to APOE-ε4, CV risk factors (usage of anti-hypertensive and lipid lowering medications, systolic BP, diabetes mellitus status, total cholesterol, high-density lipoprotein cholesterol, pack-years of cigarette smoking), alcohol consumption, weekly amounts of moderate and vigorous physical activity, depression, usage of antidepressant medications, total gross family income ≥ $75,000, mean HR and prevalent CV and AF events. Model 5, was further adjusted for NT-proBNP (Zonneveld et al., 2017; Ferguson et al., 2018; Nagata et al., 2019; Ostovaneh et al., 2020). Finally, we considered models that included the variables in Model 4 and one of the following variables previously reported to be associated with overall or specific domains of cognitive function: CAC scores (Bos et al., 2015; Deckers et al., 2017), % SWS (Wunderlin et al., 2020) and % REM (Djonlagic et al., 2021), % time SpO2 < 90% (Yaffe et al., 2011; Johnson et al., 2017) and ODI (Johnson et al., 2017).
Continuous independent variables were standardized. Thus, the regression coefficients presented in Tables 3–6 indicate the expected difference in cognitive scores per one-SD increment in the independent variable. Multivariable linear regression models were also used to quantify the associations between the baseline HRF indices and the changes in cognitive test scores from baseline to exam 6 (Δ = exam 6 – exam 5). These analyses were adjusted for the covariates detailed above in addition to baseline cognitive test scores. Of note, the regression of ΔY = Y2– Y1 on both Y1 and X is equivalent to the regression of Y2 on Y1 and X (Werts and Linn, 1970). Standardized regression coefficients (where only the independent variables were standardized) are presented.
The likelihood ratio test was used to evaluate the difference in performance between two nested models. The larger model contained an HRF index in addition to the variables in the base model. A statistically significant result for the likelihood ratio test indicates that the addition of the HRF index to the base model significantly increased its fit, i.e., its predictive value. Statistical significance was set at a p-value <0.05. All p-values reported were two-sided. A p-value <0.1 was considered borderline significant. All analyses were performed using STATA software (version12.0 for Linux).
Participants in the MESA-Sleep cohort compared to those enrolled in exam 5 who did not undergo the sleep examination were relatively younger (mean, 68 vs. 71 years), less likely to be hypertensive (58% vs. 62%), and more likely to be Hispanic (23.5% vs. 19.1%) [19]. They had lower CAC scores (median [inter-quartile range]: 30 [0–215] vs. 57 [0–338]) and higher DSC scores (52 [39–65] vs. 50 [37–62]). There were no differences in sex, BMI, smoking status, diabetes, number of participants with prevalent CV disease, and in the results of the two other cognitive tests, CASI and the DS.
Approximately 50% of the participants in the MESA-Sleep cohort underwent cognitive testing at exam 6. Table 1 summarizes demographic, clinical and HR dynamical characteristics of participants in the MESA-Sleep cohort, and in the subgroups of those with (subgroup A) and without (subgroup B) cognitive testing at exam 6. Participants in subgroup A had higher cognitive test scores at exam 5. They were approximately three years younger and healthier overall.
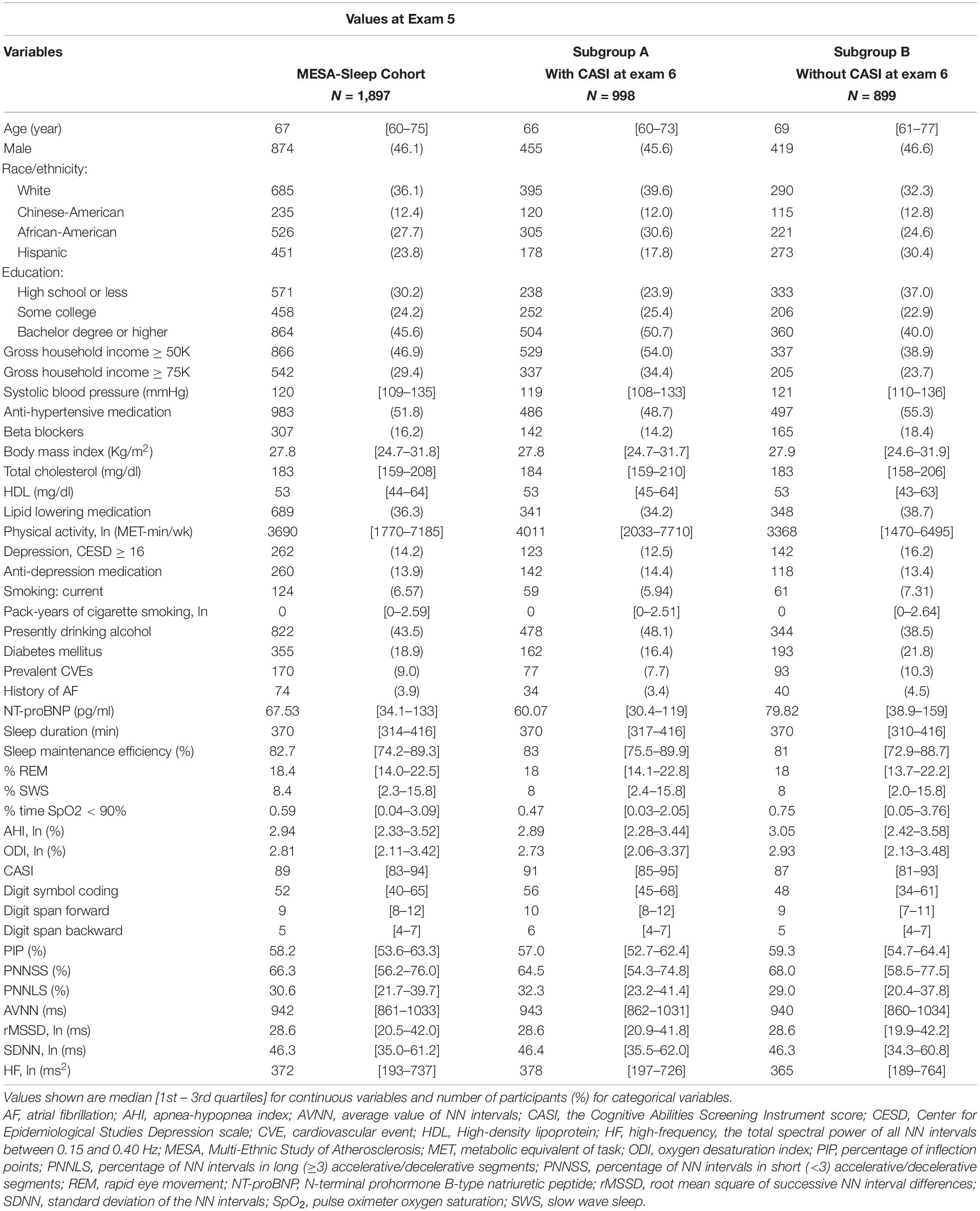
Table 1. Values at exam 5 of demographic, clinical and other characteristics of participants in the study cohort and in the subgroups of those with and without cognitive tests at exam 6.
Table 2 summarizes demographic, clinical and HR dynamical characteristics of the MESA-Sleep cohort per quartiles of the HRF index, PNNSS. Those in the bottom quartiles (less fragmented) were younger, more active and, overall, healthier. Accordingly, they were less likely to be diabetic, to have had a CVE prior to the PSG study or history of AF. A smaller percentage used anti-hypertensive, beta-blocker and/or lipid lowering medications. A higher percentage had attained higher levels of education and belonged to households with higher gross income. Additionally, those with lower HRF had lower NT-proBNP serum concentration, longer sleep duration, higher sleep maintenance efficiency and lower % time SpO2 < 90%. However, they did not differ in terms of sleep structure, AHI and ODI. Participants with lower HRF, had higher CASI, DSC and DS backward scores both at exams 5 and 6.
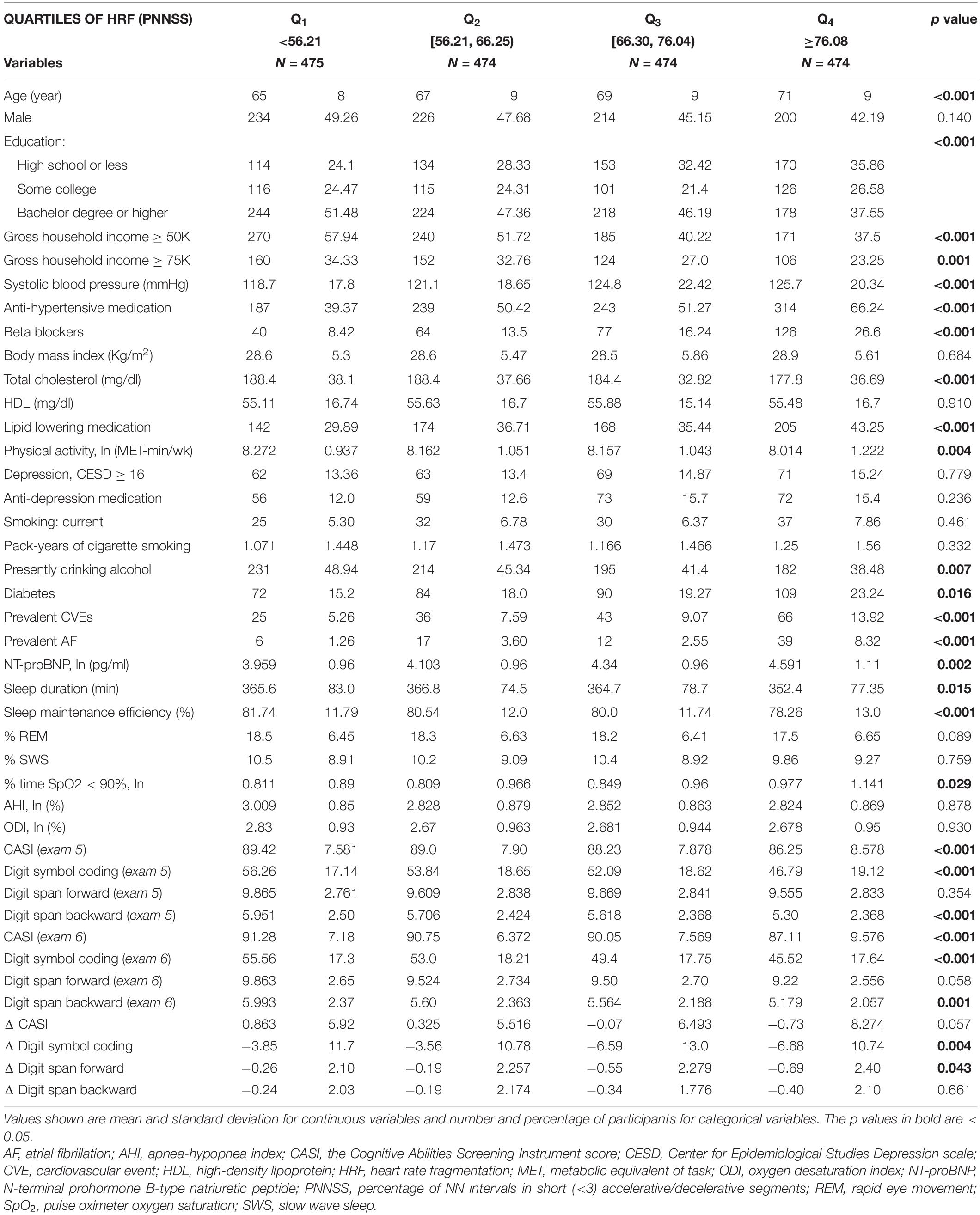
Table 2. Demographic and clinical characteristics of MESA-Sleep study cohort per quartile of the HRF index PNNSS.
Figure 1 shows the relationships of two HRF indices, PIP (A) and PNNSS (B), and of two HRV indices, rMSSD (C) and SDNN (D) with cross-sectional age. As expected, HRF indices monotonically increased with cross-sectional age. The measure whose values increase as fragmentation decreases, PNNLS, monotonically decreased with age (not shown). In contrast, the measures of HR variability, rMSSD and SDNN, exhibited a parabolic relationship with cross-sectional age. Variability was higher both for younger and older participants. A similar relationship was observed for HF power (not shown).
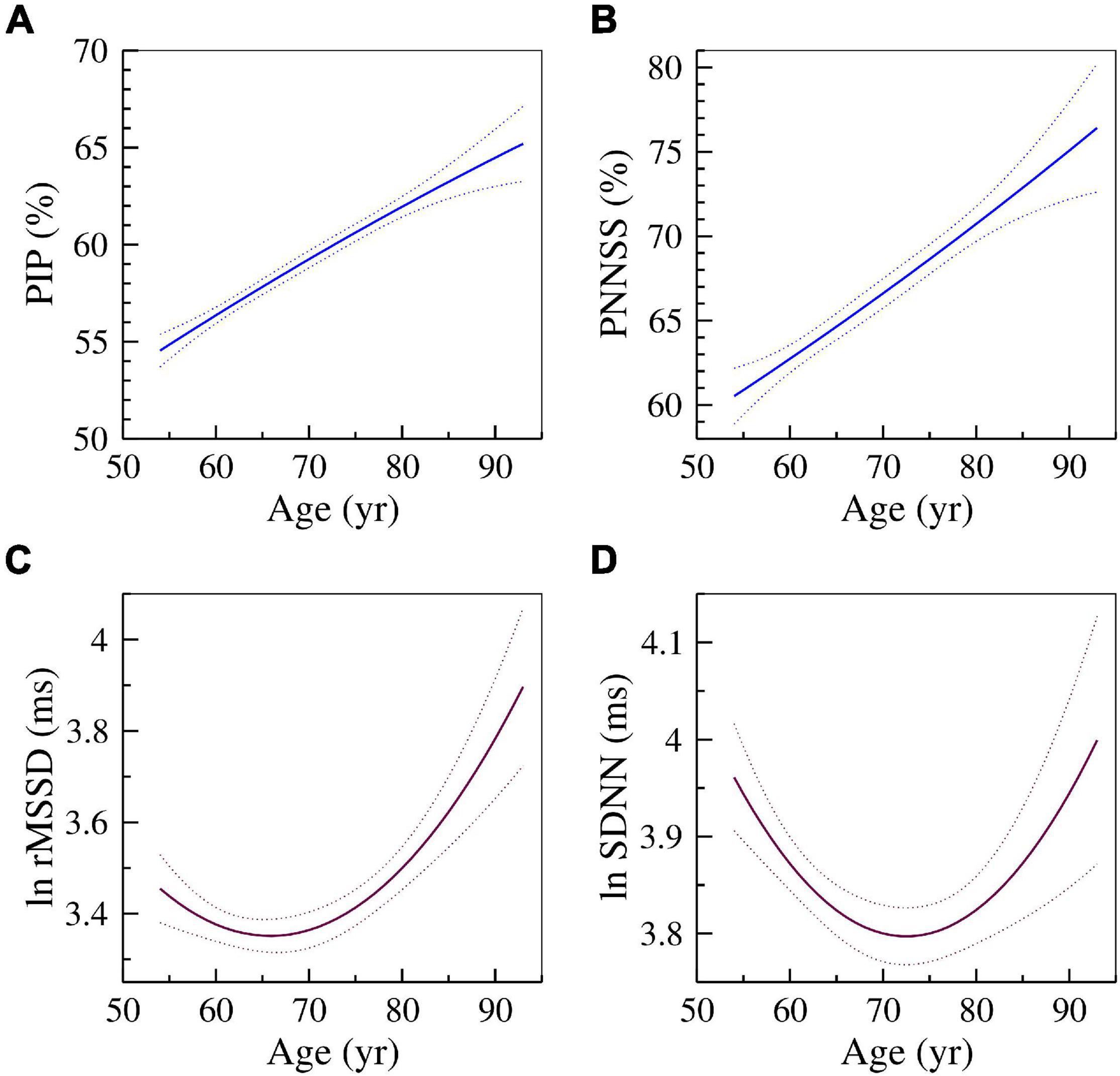
Figure 1. Changes in HRF and HRV with cross-sectional age. The solid and dotted lines are the quadratic fit and 95% confidence intervals obtained from the regressions of the dependent variables (A) PIP, (B) PNNSS, (C) ln rMSSD and (D) ln SDNN on age and age2.
In all models, the three HRF indices were strongly associated with the CASI and the DSC scores (Table 3). The associations were negative for PIP and PNNSS, whose values are higher for more fragmented time series, and positive for PNNLS, whose values are higher for less fragmented (more fluent) time series. Specifically, a one-SD increase in PNNSS (13.7%) was associated with −0.51 (95% CI: −0.86 to −0.17) and −1.12 (−1.90 to −0.34) points decrease in the CASI and DSC scores, respectively, in analyses of Model 4. Heart rate fragmentation indices were more strongly associated with the DS backward than the DS forward scores. The associations of HRF indices with DS backward scores were significant in Models 1–3 and borderline significant in the two most adjusted models. The associations between HRF indices and DS forward scores were only significant in unadjusted analyses and those adjusted for the CAIDE–APOE-ε4 risk index. Further adjusting the previous analyses for NT-proBNP did not qualitatively change any of our results.
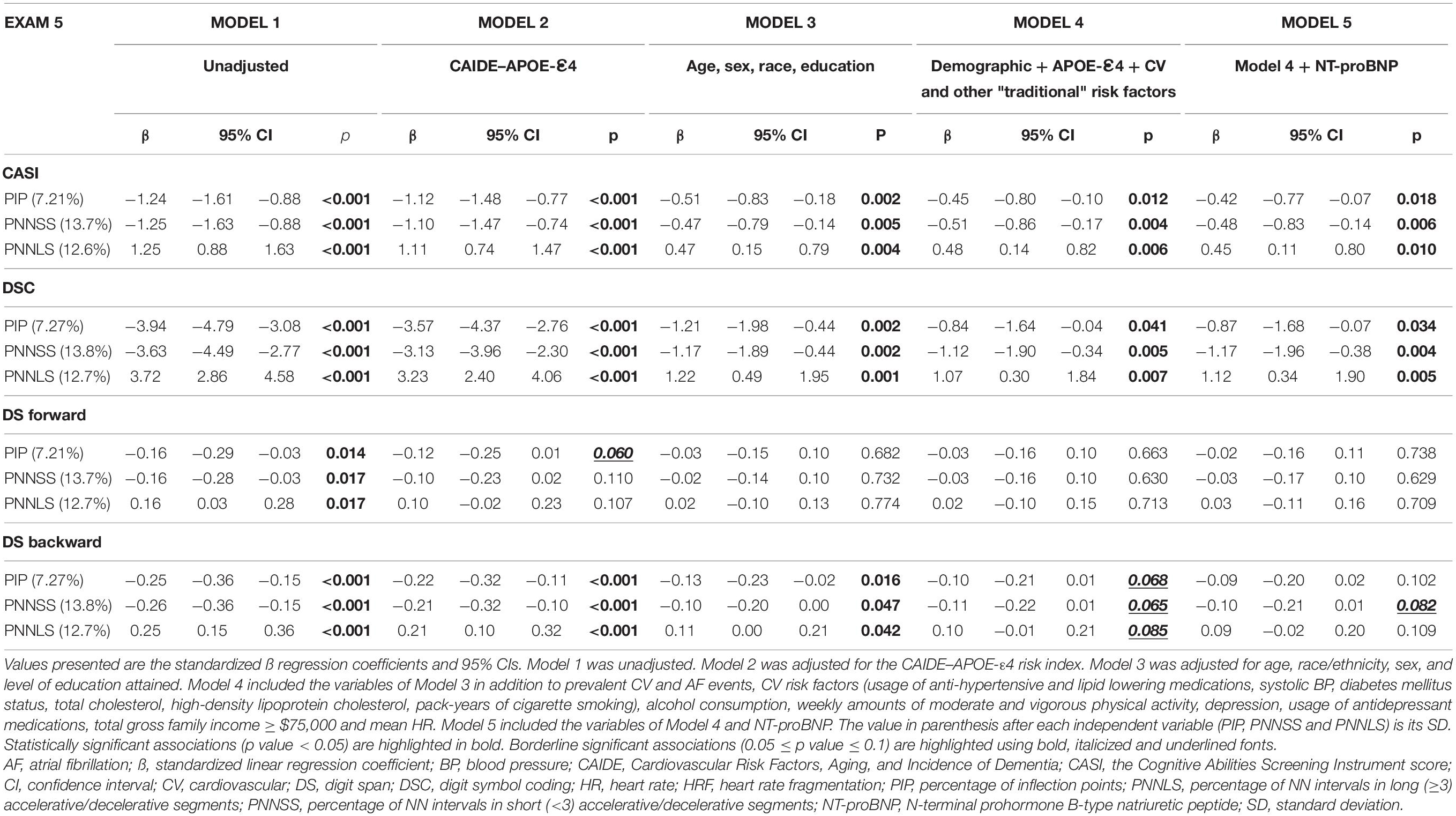
Table 3. Multivariable linear regression analyses of the associations between the HRF indices (PIP, PNNSS and PNNLS) and the cognitive scores (CASI, DSC and DS) measured at exam 5.
The CAIDE–APOE-ε4 risk index attenuated the associations between HRF indices and the cognitive test scores only slightly. In contrast, age, sex, race and education reduced the strength of these associations between 40% and 70%. Additional adjustments had only a relatively small effect. Consistent with these observations is the finding that the adjusted R2 values of Models 4 (0.325) and 5 (0.326) were only 2% higher than that of Model 3 (0.307). Of note, despite including information on the strongest predictors of cognitive performance, namely age, sex and education, the model with the CAIDE–APOE-ε4 risk index had an adjusted R2 of only 0.072 (75% lower than that of Model 3).
In fully adjusted models, AVNN (inversely related to mean HR) was positively associated with CASI (p < 0.10) and the DSC (p < 0.05) scores (Appendix Table 1). None of the variability measures (rMSSD, SDNN and HF) was associated with any of the cognitive scores in the fully adjusted models. There were some significant or borderline significant associations in Models 1–3. However, for both rMSSD and HF power, these associations were negative, i.e., higher variability was associated with lower cognitive scores. Higher SDNN was associated with higher CASI scores solely in analyses adjusted for CAIDE–APOE-ε4.
Heart rate fragmentation indices were associated (Table 4) with the CASI (p < 0.05), DSC (p < 0.05), DS forward (p < 0.1) and DS backward (p < 0.05) scores obtained at exam 6, 6.4 ± 0.5 (mean ± SD) years after exam 5. In fully adjusted analyses, a one-SD increase in PNNSS (13.7%) was associated with −0.61 (95% CI: −1.07 to −0.14), −1.54 (−2.54 to −0.54), −0.14 (−0.31 to 0.02) and −0.17 (−0.31 to −0.04) points decrease in the CASI, DSC, DS forward and DS backward scores, respectively.
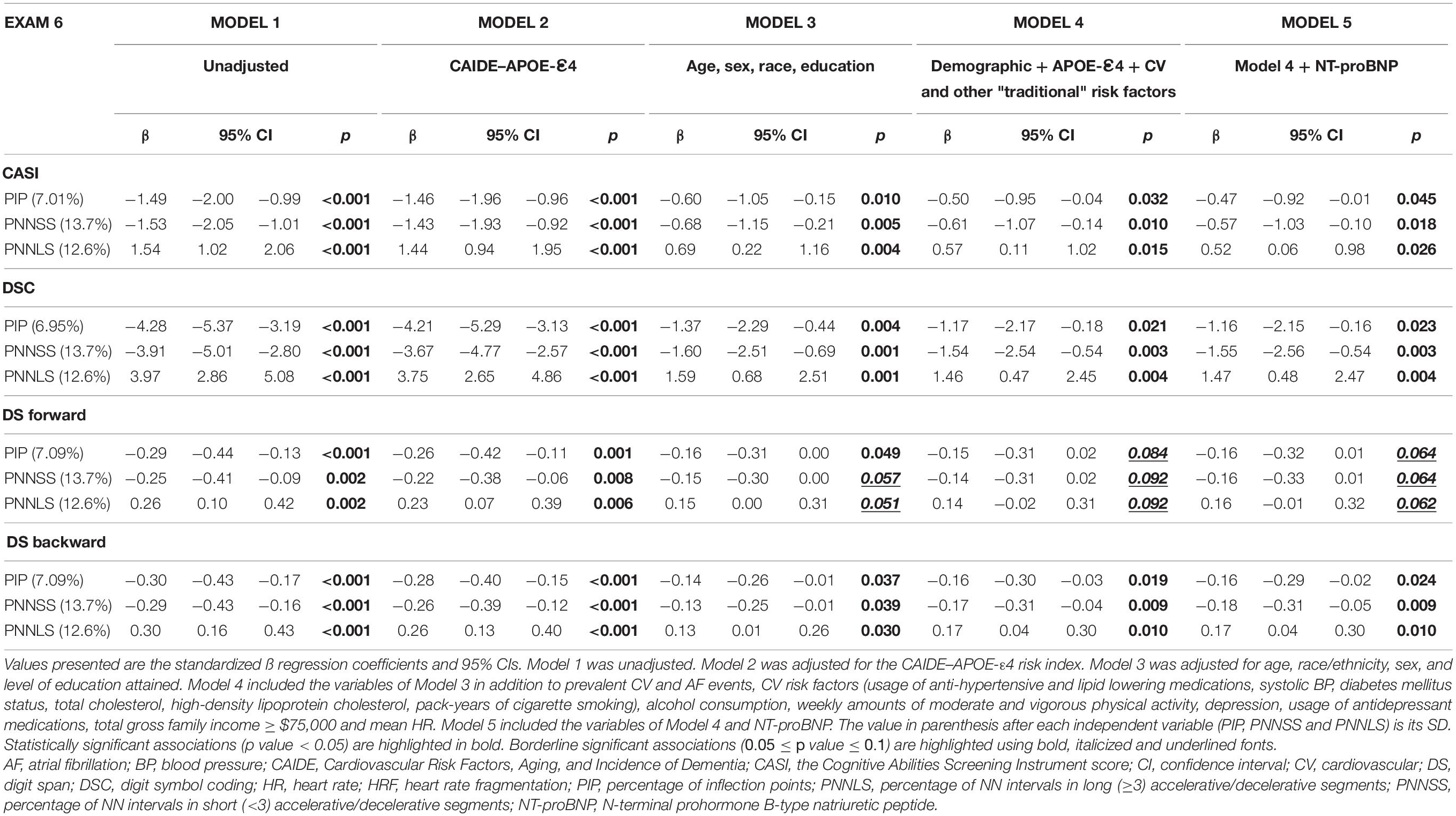
Table 4. Multivariable linear regression analyses of the associations between the HRF indices (PIP, PNNSS and PNNLS) and the cognitive scores (CASI, DSC, DS forward and DS backward) measured at exam 6.
There were relatively few (13 out of 80) significant or borderline significant associations between the HRV indices and the cognitive scores (Appendix Table 2). Notably, in the most adjusted models, there was only one significant association. Slower HR (higher AVNN) was associated with higher DS backward scores.
The HRF indices were strongly associated with the changes in cognitive scores (Table 5). Overall, higher HRF, i.e., higher PIP and PNNSS, was associated with larger decrements in cognitive scores. Similarly, lower HRF (higher degree of HR fluency indicated by higher PNNLS values) was associated with smaller decrements. In fully adjusted analyses, the associations were strongest with the Δ DSC scores and weakest with the Δ DS forward scores.
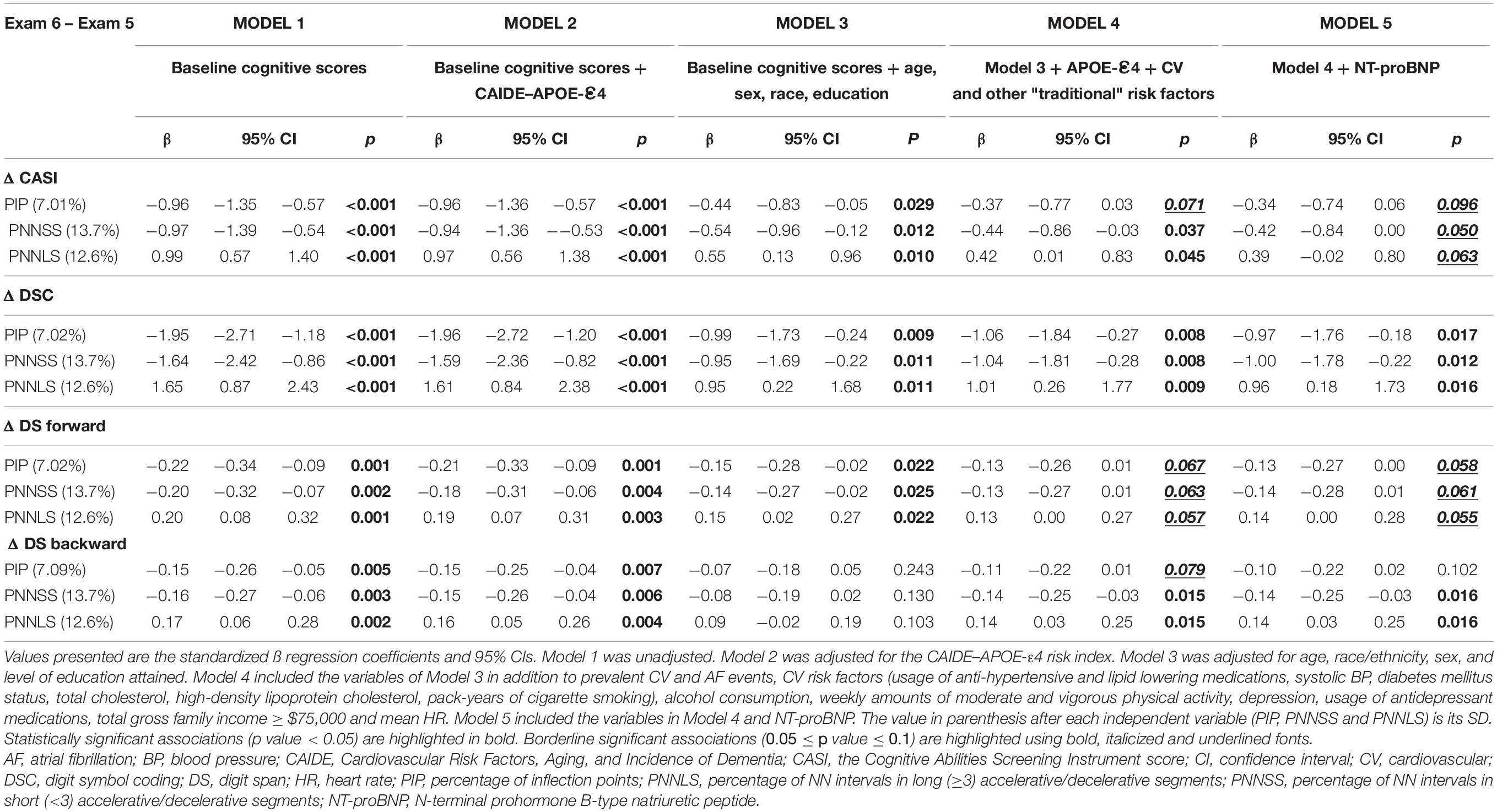
Table 5. Multivariable linear regression analyses of the associations between the HRF indices (PIP, PNNSS and PNNLS) and the changes (exam 6 – exam 5) in cognitive test scores (Δ CASI, Δ DSC, Δ DS forward and Δ DS backward).
In fully adjusted models, a one-SD increase in PNNSS was associated with a decrease of −0.44 (95% CI: −0.86 to −0.03), −1.04 (−1.81 to −0.28), −0.13 (−0.27 to 0.01) and −0.14 (−0.25 to −0.03) points in the CASI, DSC, DS forward and DS backward scores, respectively. Figure 2 depicts the predicted changes in DS backward scores derived from fully adjusted analyses as a function of the participants’ age (left) and baseline PNNSS (right). The representative graphs show that cognitive decline accelerated with the participants’ age as well as with baseline degree of HRF. In the time period between exams 5 and 6, cognitive performance decreased more for older than younger participants. Similarly, in the same time period, cognitive performance decreased more for participants with higher degrees of HRF. Of note, the associations between HRF and the changes in cognitive scores were not dependent on the participants’ age (the p-value for the interaction term of HRF and age was not significant in any model).
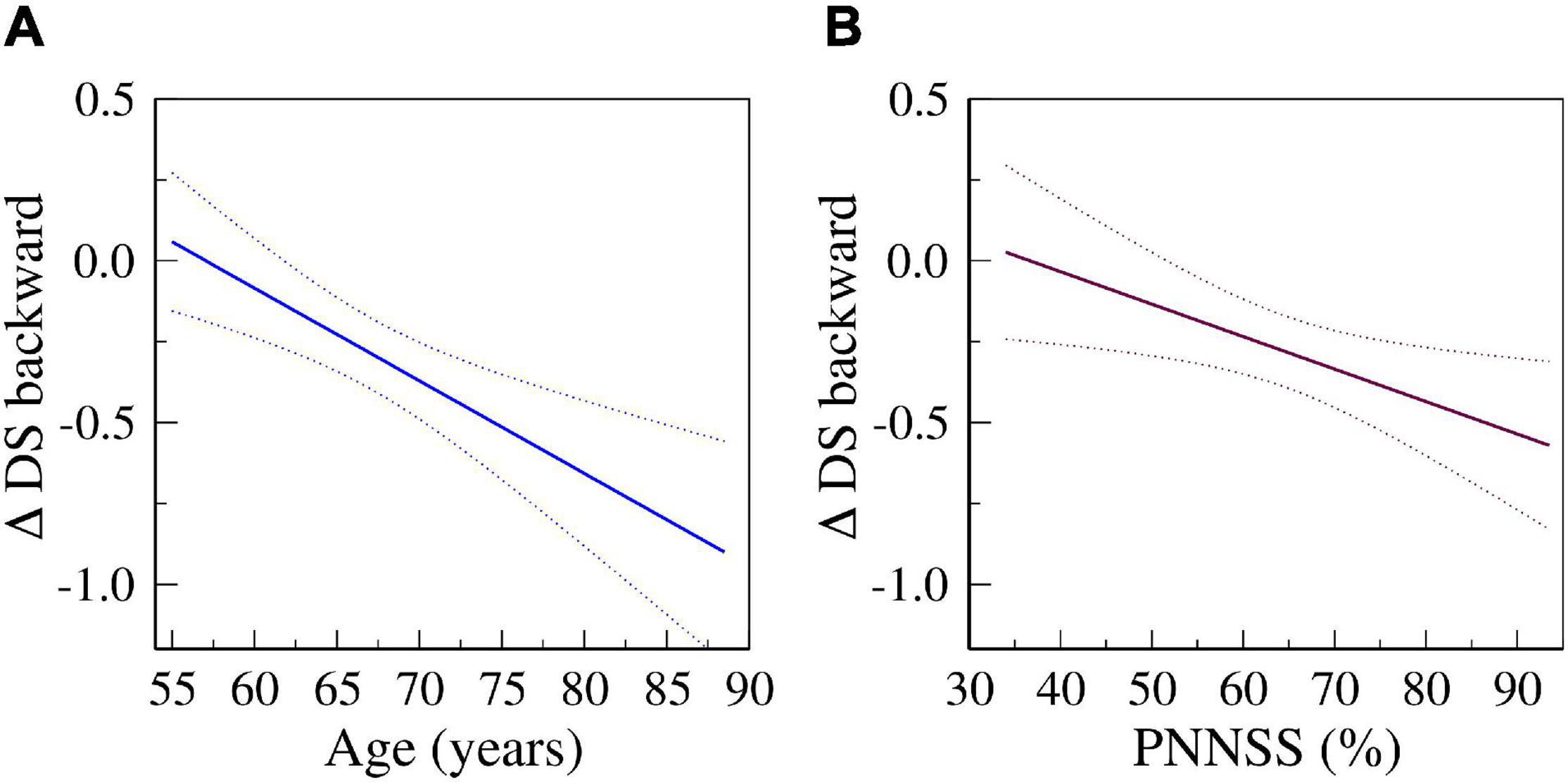
Figure 2. Predicted changes in DS backward scores from exams 5 to 6 as a function of age (A) and of PNNSS values (B) derived from fully adjusted models (Model 4). Solid and dotted lines are the predicted and 95% confidence interval values, respectively.
There were few (5 out of 80) significant or borderline associations between HRV indices and the longitudinal changes in cognitive scores (Appendix Table 3). In fully adjusted analyses, there was only one significant result. Namely, slower HR was associated with smaller decreases in the DS backward scores.
Figure 3 presents a graphical summary of the results of two sets of analyses described above, one adjusted for age, sex, race and education (Model 3, open symbols), and the other “fully” adjusted (Model 4, solid symbols). Specifically, the figure shows the standardized ß coefficients for the associations of PNNSS (blue symbols) and rMSSD (red symbols) with cognitive scores at exams 5 and 6 and the changes in cognitive scores.
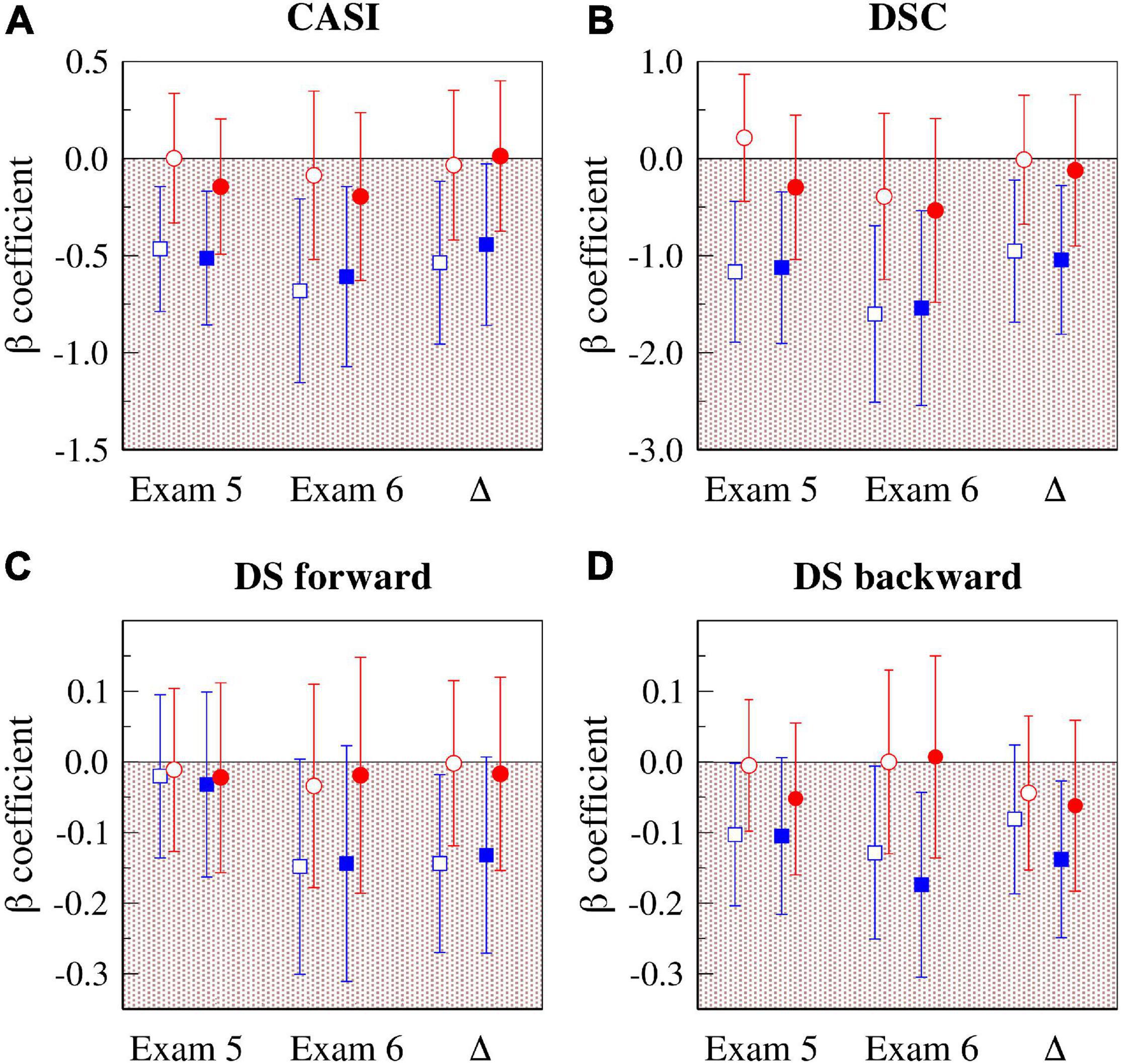
Figure 3. Associations of HR dynamical indices (HRF: PNNSS and HRV: rMSSD) with cognitive test scores at exams 5 and 6, and with their longitudinal changes (Δ = exam 6 – exam 5). The graphs show the standardized ß coefficients for the associations of CASI (A), DSC (B), DS forward (C) and DS backward (D) with PNNSS (blue symbols) and rMSSD (red symbols). The open symbols are the standardized ß coefficients from models adjusted for age, sex, race and education. The solid symbols are the standardized ß coefficients from fully adjusted models (Model 4). The error bars are the 95% CIs. CASI, the Cognitive Abilities Screening Instrument score; DSC, digit symbol coding; DS, digit span; CI, confidence interval; HR, heart rate; HRF, heart rate fragmentation; HRV, heart rate variability; PNNSS, percentage of NN intervals in short (<3) accelerative/decelerative segments; rMSSD, root mean square of successive NN interval differences.
Table 6 shows the regression coefficients for all the variables included in the fully adjusted models. Higher PNNSS was consistently associated (p < 0.05 or p < 0.1) with the lower cognitive scores at exams 5 and 6 and with larger decrements in scores from exams 5 to 6. (The only exception was the cross-sectional association with DS forward.)
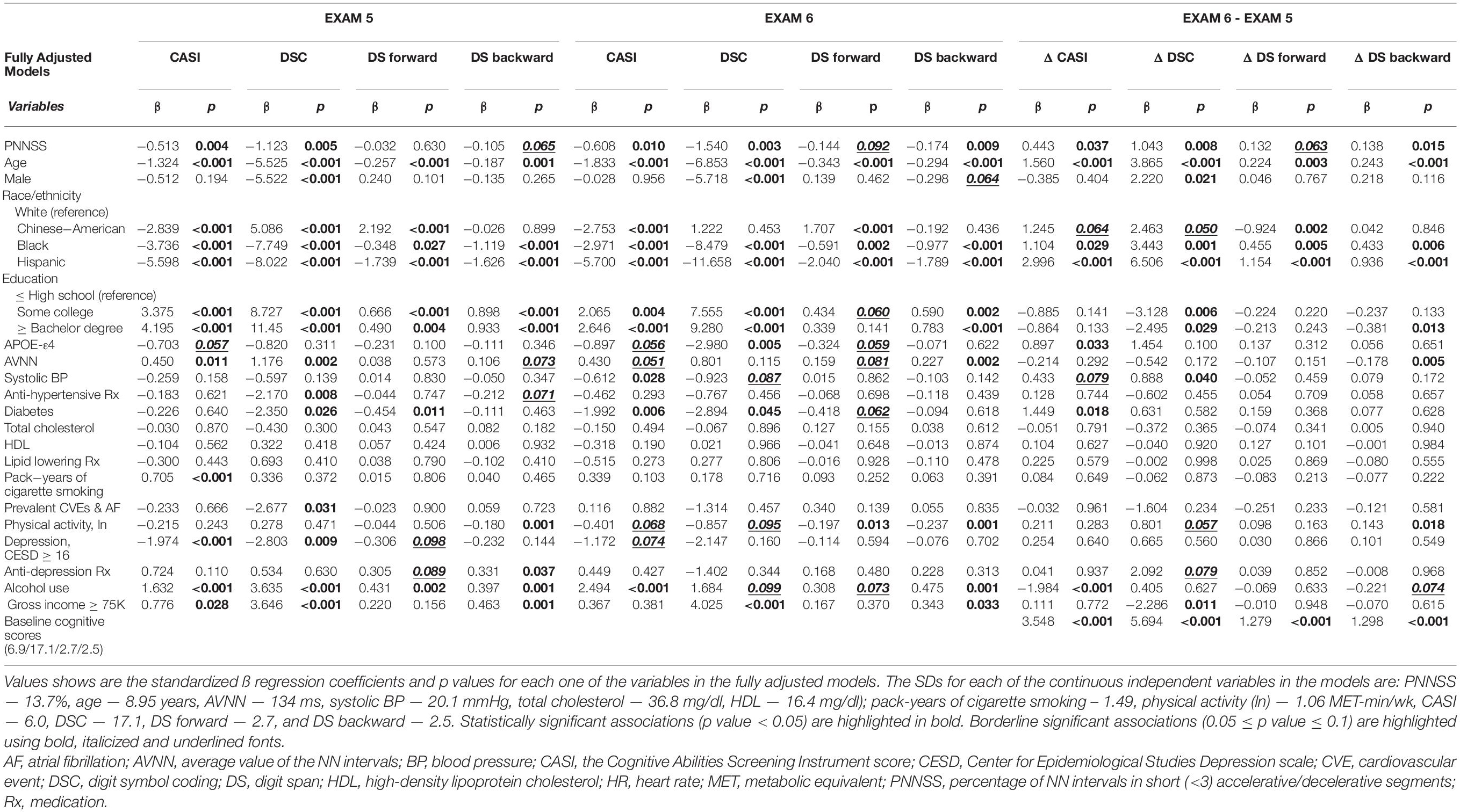
Table 6. Multivariable linear regression models of CASI, DSC, DS forward and backward scores at exams 5 and 6 and of their changes from exams 5 to 6.
Of note, further adjusting the cross-sectional and longitudinal analyses described above (Model 4) for CAC, measures of sleep structure (the percentage of time in REM and the percentage time in SWS), a measure of sleep disorder breathing (AHI), and measures of hypoxemia (ODI and the % of time SpO2 < 90%) did not qualitatively change our results (not shown). Finally, we note that no interactions by sex or race were detected in any of the cross-sectional or the longitudinal analyses previously described.
The present investigation was designed to evaluate cross-sectional and prospective associations between HRF, a newly identified property of cardiac neuroautonomic dynamics, and cognitive function in the large MESA cohort of those who underwent a PSG study. The degree of HRF was assessed at exam 5 (our baseline) using previously described metrics (Costa et al., 2017a,b). Cognitive performance was assessed at this baseline and 6.4 ± 0.5 years later at exam 6, using the CASI, DSC and DS forward and backward tests, which are designed to assess overall cognitive performance, processing speed, short-term memory and working memory, respectively.
Our key findings were: (1) in cross-sectional analyses, HRF was inversely associated with all cognitive scores other than the DS forward scores (higher HRF, lower scores); (2) in prospective analyses, HRF was inversely associated with all cognitive scores; (3) HRF was associated with the changes in all cognitive scores (higher baseline HRF, larger decrement in scores); (4) HRF metrics added predictive value to the CAIDE–APOE-ε4 risk index and to models with a combination of risk factors for cognitive impairment including NT-proBNP serum concentration; (5) slower HR was cross-sectionally associated with the CASI and DSC scores, and prospectively associated with the DS backward scores; and (5) the HRV indices rMSSD, SDNN and HF power were not cross-sectionally or longitudinally associated with cognitive scores.
Overall, our results indicate that an increased degree of HRF was associated with both worse concurrent and future cognitive function. Our analyses also showed that increased baseline HRF was associated with steeper future cognitive decline. For example, in fully adjusted analyses (Figure 2), participants whose HRF at baseline (PNNSS) was 80% vs. 60% lost twice the number of points in the DS backward test from exams 5 to 6 [0.44 (0.27–0.60) vs. 0.23 (0.12–0.35) points]. Similarly, we found that older age was associated with greater cognitive decline – the older the participants the larger the longitudinal decrease in cognitive scores. For example, in fully adjusted analyses (Figure 2), the decrease in DS backward scores from exams 5 to 6 for participants aged 75 vs. 65 was approximately twice as high [0.51 (0.35–0.68) vs. 0.23 (0.11–0.34) points]. The results are consistent with the acceleration of cognitive decline with aging as well as with baseline degree of HRF.
In both cross-sectional and longitudinal analyses, HRF added predictive value to the CAIDE–APOE-ε4 dementia risk index (Kivipelto et al., 2006; Exalto et al., 2014; Rundek et al., 2020). This index was developed to identify individuals at increased risk for dementia. It was validated in a multiethnic population in the United States (Exalto et al., 2014). The risk index is based on age, sex, BMI, systolic BP, total cholesterol, physical activity, educational level and APOE-ε4 status. Notably, the strength of the associations between cognitive scores and HRF indices were minimally attenuated by the inclusion in the models of this widely used risk index for dementia. This finding highlights the independence (“orthogonality”) of the two quantities, HRF from CAIDE-APOE-ε4.
We found that HRF was significantly associated with cognitive performance (higher HRF, worse cognitive performance) independent of a combination of widely acknowledged risk factors of cognitive impairment including demographic (age, sex), socioeconomic (level of education, gross family income), genomic (APOE-ε4 allele), behavioral (smoking, alcohol consumption, degree of physical activity), mental health (depression) variables, and CV risk factors (hypertension, hyperlipidemia, diabetes and CAC). Moreover, HRF added predictive value to models that in addition to the variables mentioned above also included serum concentration of NT-proBNP. This analyte has been reported to be associated with incident dementia in MESA (Ostovaneh et al., 2020) and other studies (Nagata et al., 2019), with MRI measures of cognitive function and structure (Zonneveld et al., 2017; Ferguson et al., 2018), as well as, with overall or some domains of cognitive impairment (Kerola et al., 2010; Ferguson et al., 2018; Gallo et al., 2020).
Our findings are consistent with proposed links between cardiac neuroautonomic regulation and cognitive function (Collins et al., 2012). Although epidemiologic studies such as the present one cannot resolve the complex and likely multifactorial underpinnings of this relationship, the findings are supportive of a number of mechanistic considerations. First, there is evidence that central nervous system dysfunction is closely linked to both clinical atherosclerotic disease and multiple CV risk factors (Newman et al., 2005; Gorelick et al., 2011). Our previous studies (Costa et al., 2018, 2021) in MESA also supported this link. We found that high HRF was predictive of major adverse CVEs and AF, which are themselves associated with advanced cognitive impairment and dementia (Deckers et al., 2017). We adjusted the analyses for common CV risk factors in addition to other potential confounders such as CAC (a marker of sustained, end-stage inflammation and severe vascular disease). Of note, to the extent that CV disease processes are only partially captured by the variables above, the possibility remains that preclinical or clinical CV disease markers still partially mediate the associations between HRF and cognitive performance.
A second and related possibility is that cardiac neuroautonomic impairment affects cognitive performance via its contribution to arterial BP dysregulation, encompassing but not limited to degraded baroreflex sensitivity (Meel-van den Abeelen et al., 2013). The resulting hemodynamic instability may manifest as increased BP fluctuations leading to suboptimal cerebral perfusion (Collins et al., 2012; Wolters et al., 2016; Schaich et al., 2020). Dysregulation of BP has been linked to cerebrovascular disease and related brain pathology (e.g., white matter lesions and lacunar infarctions) (Gómez-Angelats et al., 2004), factors that may further degrade cardiac neuroautonomic control. The result of these interconnected processes may be a vicious cycle whereby impaired cerebral autoregulation begets further cognitive impairments.
A third putative link between cardiac autonomic dysfunction and cognitive impairment is via pathologic alterations in neuro-immunomodulation. The role of the vagus in regulating systemic inflammatory response and tissue damage at multiple anatomic sites has emerged as a major area of investigation (Sartori et al., 2012; Pavlov and Tracey, 2017). The salutary and likely complex effects of vagal activation in suppressing excessive cytokine activation and other mediators of inflammation have been ascribed to a “cholinergic anti-inflammatory” network (Pavlov and Tracey, 2005, 2017; Bonaz et al., 2016). Conversely, abnormally decreased vagal activity, of which increased HRF is a marker, may mediate proinflammatory diatheses affecting the central nervous system. In the most severe cases, these inflammatory processes may contribute to the pathogenesis of Alzheimer’s disease and related dementias (Jessen et al., 2010).
Of further note, evidence that parasympathetic disruption may be initiated and perpetuated by peripheral and central neurodegenerative changes seen with aging, CV disease, as well as subclinical Alzheimer’s disease, suggests that HRF may have additional value as a predictor of incident dementias.
For comparison of performance and translational value, we included traditional HRV metrics in our study. Traditional indices of HR variability and measures of HRF assess different mathematical properties of HR dynamics. The former measures the amplitude of beat-to-beat fluctuations in HR. The latter quantifies the frequency of reversals in acceleration, i.e., of the changes from HR acceleration (when the difference between consecutive NN intervals contracts) to deceleration (expands) and vice-versa. In this study, we found that the amplitude of HR fluctuations, as quantified by traditional HRV metrics, was not consistently associated with cognitive performance. Other large epidemiologic studies have also investigated the associations between HRV indices, as putative measures of neuroautonomic function, and cognitive test scores. The results are difficult to reconcile. Some report positive associations (Britton et al., 2008; Frewen et al., 2013; Mahinrad et al., 2016; Schaich et al., 2020) between selected HRV metrics and specific cognitive test scores; others, like ours, find no consistent associations (Kim et al., 2006; Collins et al., 2012).
These discrepancies are likely due to the limitations of traditional HRV metrics themselves (Costa et al., 2017a; Hayano and Yuda, 2019; Hayano et al., 2020), namely the fact that fragmentation of HR dynamics instead of physiologic vagal tone modulation may inflate the value of HRV metrics. This concern is especially pertinent in populations of middle age to older individuals, such as the MESA cohort. Additionally, differences in study populations, cognitive tests and methods of assessing HRV, which can be based on different metrics and may be derived from short (typically 10 s) or long (typically multiple hours) ECG recordings, may also contribute to the differences in findings.
As discussed in detail in references (Costa et al., 2017a, 2018, 2021; Costa and Goldberger, 2019) the two major sources of short-term cardiac interbeat interval variability, namely parasympathetic activity (vagal tone modulation) and HRF, are not distinguishable using traditional HRV measures. This limitation undermines the reliability and translational value of these metrics (Hayano and Yuda, 2019). The graphs of the cross-sectional relationships of rMSSD and SDNN with participants’ age (Figure 1) illustrate this major limitation. Vagal tone modulation is known to decrease with aging. If the amplitude of HR fluctuation quantified by rMSSD and SDNN were reliable measures of vagal tone modulation, they should also decrease with cross-sectional age. Instead, we found non-monotonic (U-shaped) relationships of rMSSD and SDNN with age (Figure 1). Similar results were obtained for HF power (not shown). The U-shaped relationships between HRV metrics and age have been noted by others (Almeida-Santos et al., 2016; Hayano et al., 2020). In contrast, the HFR metrics, PIP and PNNSS, increased monotonically (and linearly) with cross-sectional age (Figure 1). (The PNNLS index [whose values decrease as fragmentation increases] monotonically decreased with cross-sectional age [not shown].) Consistent with a previous MESA study (Schaich et al., 2020), we found that slower HR, a global marker of relative parasympathetic predominance, was associated with higher cognitive performance.
In an era of ECG wearables technology, a dynamical risk marker such as HRF may be especially attractive given the fact that it is computationally inexpensive and it can be continuously updated. In this context, HRF may also have important applications in monitoring and prediction of the safety and efficacy of therapeutic interventions: those that increase HRF will potentially be of concern. The results presented here in conjunction with those reported elsewhere (showing that increased HRF was a precursor of incident major adverse CV (Costa et al., 2018) and AF (Costa et al., 2021) events), suggest that HRF may help define populations that would benefit from early initiation of therapeutic interventions aimed at delaying/reversing cognitive decline and/or preventing adverse CVEs, themselves associated with worse cognition.
In MESA, assessment of cognitive function is limited to three standard tests, CASI, DSC and DS, which only probe selected cognitive domains. Another limitation is the fact that despite adjusting our analyses for demographic, socioeconomic and behavioral variables, in addition to socioeconomic status, traditional CV risk factors and APOE-ε4 allele carriage, the possibility of residual confounding could not be excluded. However, we did try to minimize such possibility by further adjusting the models for CAC, NT-proBNP, sleep structure and hypoxemia variables, which have themselves been associated with incident CV and AF events and/or cognitive decline. We note that the participants who underwent cognitive testing at exam 6 (only 53% of those in our MESA-Sleep study cohort) were on average three years younger and overall healthier than those who did not. Furthermore, at exam 5, participants who would be re-tested at exam 6 performed significantly better in all cognitive tests. Despite this selection bias and the lower statistical power for the longitudinal analyses compared to the cross-sectional ones, our results were still significant. Our findings will need to be validated in other populations.
In MESA, higher degree of HRF, a marker of cardiac neuroautonomic impairment, was cross-sectionally and prospectively associated with worse cognitive performance. Furthermore, cognitive decline; accelerated with age as well as baseline degree of HRF (the higher HRF at baseline, the greater the loss of cognitive performance during follow-up). The potential translational utility of these findings is enhanced by the observation that HRF added predictive value to well-known risk factors of cognitive decline, such as age and subclinical CV risk markers.
The data analyzed in this study is subject to the following licenses/restrictions: MESA data can be accessed after submitting a manuscript proposal to MESA Publications & Presentations Committee and obtaining its approval to conduct the research work. Requests to access these datasets should be directed to Karen Hansen, aGFuc2VuazNAdS53YXNoaW5ndG9uLmVkdQ==.
Institutional review boards approved the conduct of the study. Written informed consent was obtained from all participants.
MC, SH, and AG: conception and research design. SR (PSG), SH, and TH (cognitive scores): data acquisition. MC: data analysis and preparation of tables and figures. MC and AG: manuscript drafting. All authors: interpretation of results and manuscript editing.
The MESA study was supported by contracts HHSN26820150 0003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, 75N92020D00001, 75N92020D00002, 75N92020D00003, 75N92020D00005, 75N92020D00006, and 75N92020D00007, from the National Heart, Lung, and Blood Institute, by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The MESA Sleep ancillary study was supported through R01HL098433. The MESA Atrial Fibrillation ancillary study was supported through R01HL127659. Coronary calcification data were obtained in part under STAR research assistance agreements, No. RD831697 (MESA Air) and RD83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency. The authors gratefully acknowledge support from NIH grants R35HL135818 (SR). R01HL144510 (SH, MC, and AG), R01EB030362 and R01EB030362–14S1 (AG and MC).
SR reports consulting fees from Apnimed Inc., Jazz Pharma and Eisai Inc., unrelated to this work, grant funding from Jazz Pharma unrelated to this work and receipt of loaned equipment from Nox Medical Inc. and Philips Respironics for use in an NIH trial.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.708130/full#supplementary-material
Supplementary Table 1 | Multivariable linear regression analyses of the associations between the HRV indices (AVNN, rMSSD, SDNN and HF power) and the cognitive scores (CASI, DSC and DS) measured at exam 5.
Supplementary Table 2 | Multivariable linear regression analyses of the associations between the HRV indices (AVNN, rMSSD, SDNN and HF power) and the cognitive scores (CASI, DSC and DS) measured at exam 6.
Supplementary Table 3 | Multivariable linear regression analyses of the associations between the HRV indices (AVNN, rMSSD, SDNN and HF) and the changes (exam 6 – exam 5) in cognitive scores (Δ CASI, Δ DSC, Δ DS forward and Δ DS backward).
AF, atrial fibrillation; AVNN, average of all NN intervals; BMI, body mass index; BP, blood pressure; CAC, coronary artery calcification; CAIDE, Cardiovascular Risk Factors, Aging, and Incidence of Dementia; CASI, Cognitive Abilities Screening Instrument; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; CV, cardiovascular; CVE, cardiovascular event; DS, digit span; DSC, digit symbol coding; ECG, electrocardiogram/electrocardiographic; HF, high-frequency power, the total spectral power of all NN intervals between 0.15 and 0.40 Hz; HR, heart rate; HRF, heart rate fragmentation metric, the percentage of Δ NN that belong to long (≥ 3 Δ NN) accelerative and decelerative segments; HRV, heart rate variability; MESA, Multi-Ethnic Study of Atherosclerosis; N, normal sinus beat; NN, normal-to-normal sinus interbeat interval; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; ODI, oxygen desaturation index; PIP, percentage of inflection points; PNNLS, percentage of Δ NN (≥ 3) intervals in long accelerative/decelerative segments; PNNSS, percentage of Δ NN intervals in short (< 3) accelerative/decelerative segments; PSG, polysomnographic study; REM, rapid eye movement; rMSSD, the root mean square of successive NN interval differences; SD, standard deviation; SDNN, mean of the SDs of NN intervals in all 5-min segments; SpO2, pulse oximeter oxygen saturation; SWS, slow wave sleep; WAIS-III, Wechsler Adult Intelligence Scale III.
Agatston, A. S., Janowitz, W. R., Hildner, F. J., Zusmer, N. R., Viamonte, M. Jr., and Detrano, R. (1990). Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 15, 827–832. doi: 10.1016/0735-1097(90)90282-t
Ahmed, R. M., Ke, Y. D., Vucic, S., Ittner, L. M., Seeley, W., Hodges, J. R., et al. (2018). Physiological changes in neurodegeneration - mechanistic insights and clinical utility. Nat. Rev. Neurol. 14, 259–271. doi: 10.1038/nrneurol.2018.23
Almeida-Santos, M. A., Barreto-Filho, J. A., Oliveira, J. L., Reis, F. P., da Cunha Oliveira, C. C., and Sousa, A. C. (2016). Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch. Gerontol. Geriatr. 63, 1–8. doi: 10.1016/j.archger.2015.11.011
Alonso, A., Krijthe, B. P., Aspelund, T., Stepas, K. A., Pencina, M. J., Moser, C. B., et al. (2013). Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J. Am. Heart Assoc. 2:e000102. doi: 10.1161/JAHA.112.000102
Bertoni, A. G., Whitt-Glover, M. C., Chung, H., Le, K. Y., Barr, R. G., Mahesh, M., et al. (2009). The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am. J. Epidemiol. 169, 444–454. doi: 10.1093/aje/kwn350
Bild, D. E., Bluemke, D. A., Burke, G. L., Detrano, R., Diez Roux, A. V., Folsom, A. R., et al. (2002). Multi-Ethnic Study of Atherosclerosis: objectives and design. Am. J. Epidemiol. 156, 871–881. doi: 10.1093/aje/kwf113
Bluemke, D. A., Kronmal, R. A., Lima, J. A., Liu, K., Olson, J., Burke, G. L., et al. (2008). The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J. Am. Coll. Cardiol. 52, 2148–2155. doi: 10.1016/j.jacc.2008
Bonaz, B., Sinniger, V., and Pellissier, S. (2016). Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J. Physiol. 594, 5781–5790. doi: 10.1113/JP271539
Bos, D., Vernooij, M. W., de Bruijn, R. F., Koudstaal, P. J., Hofman, A., Franco, O. H., et al. (2015). Atherosclerotic calcification is related to a higher risk of dementia and cognitive decline. Alzheimers Dement 11, 639.e–647.e. doi: 10.1016/j.jalz.2014.05.1758
Britton, A., Singh-Manoux, A., Hnatkova, K., Malik, M., Marmot, M. G., and Shipley, M. (2008). The association between heart rate variability and cognitive impairment in middle-aged men and women. The Whitehall II cohort study. Neuroepidemiology 31, 115–121. doi: 10.1159/00014825
Bruyne, M. C., Kors, J. A., Hoes, A. W., Klootwijk, P., Dekker, J. M., Hofman, A., et al. (1999). Both decreased and increased heart rate variability on the standard 10-second electrocardiogram predict cardiac mortality in the elderly: the Rotterdam Study. Am. J. Epidemiol. 150, 1282–1288. doi: 10.1093/oxfordjournals.aje.a009959
Carr, J. J., Nelson, J. C., Wong, N. D., McNitt-Gray, M., Arad, Y., Jacobs, D. R., et al. (2005). Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 234, 35–43. doi: 10.1148/radiol.2341040439
Chen, X., Wang, R., Zee, P., Lutsey, P. L., Javaheri, S., Alcántara, C., et al. (2015). Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 38, 877–888. doi: 10.5665/sleep.4732
Collins, O., Dillon, S., Finucane, C., Lawlor, B., and Kenny, R. A. (2012). Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol. Aging 33, 2324–2333. doi: 10.1016/j.neurobiolaging.2011.11.017
Costa, M. D., and Goldberger, A. L. (2019). Heart rate fragmentation: using cardiac pacemaker dynamics to probe the pace of biological aging. Am. J. Physiol. Heart. Circ. Physiol. 316, H1341–H1344. doi: 10.1152/ajpheart.00110.2019
Costa, M. D., Davis, R. B., and Goldberger, A. L. (2017a). Heart rate fragmentation: a new approach to the analysis of cardiac interbeat interval dynamics. Front. Physiol. 8:255. doi: 10.3389/fphys.2017.00255
Costa, M. D., Davis, R. B., and Goldberger, A. L. (2017b). Heart rate fragmentation: a symbolic dynamical approach. Front. Physiol. 8:827. doi: 10.3389/fphys.2017.00827
Costa, M. D., Redline, S., Davis, R. B., Heckbert, S. R., Soliman, E. Z., and Goldberger, A. L. (2018). Heart rate fragmentation as a novel biomarker of adverse cardiovascular events: the Multi-Ethnic Study of Atherosclerosis. Front. Physiol. 9:1117. doi: 10.3389/fphys.2018.01117
Costa, M. D., Redline, S., Soliman, E. Z., Goldberger, A. L., and Heckbert, S. R. (2021). Fragmented sinoatrial dynamics in the prediction of atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Am. J. Physiol. Heart. Circ. Physiol. 320, H256–H271. doi: 10.1152/ajpheart00421.2020
Cullum, C. M. (1998). “4.11 - Neuropsychological Assessment of Adults,” in Comprehensive Clinical Psychology, eds A. S. Bellack and M. Hersen (Oxford: Pergamon), 303–347. doi: 10.1016/B0080-4270(73)00227-3
D’Agostino, R. B. Sr., Vasan, R. S., Pencina, M. J., Wolf, P. A., Cobain, M., Massaro, J. M., et al. (2008). General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117, 743–753. doi: 10.1161/CIRCULATIONAHA.107.699579
Deckers, K., Schievink, S., Rodriquez, M., van Oostenbrugge, R. J., van Boxtel, M., Verhey, F., et al. (2017). Coronary heart disease and risk for cognitive impairment or dementia: Systematic review and meta-analysis. PLoS One 12:e0184244. doi: 10.1371/journal.pone.0184244
Djonlagic, I., Mariani, S., Fitzpatrick, A. L., Van Der Klei, V., Johnson, D. A., Wood, A. C., et al. (2021). Macro and micro sleep architecture and cognitive performance in older adults. Nat. Hum. Behav. 5, 123–145. doi: 10.1038/s41562-020-00964-y
Drawz, P. E., Babineau, D. C., Brecklin, C., He, J., Kallem, R. R., Soliman, E. Z., et al. (2013). Heart rate variability is a predictor of mortality in chronic kidney disease: a report from the CRIC Study. Am. J. Nephrol. 38, 517–528. doi: 10.1159/000357200
Exalto, L. G., Quesenberry, C. P., Barnes, D., Kivipelto, M., Biessels, G. J., and Whitmer, R. A. (2014). Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement 10, 562–570. doi: 10.1016/j.jalz.2013.05.1772
Fanciulli, A., Strano, S., Colosimo, C., Caltagirone, C., Spalletta, G., and Pontieri, F. E. (2013). The potential prognostic role of cardiovascular autonomic failure in α-synucleinopathies. Eur. J. Neurol. 20, 231–235. doi: 10.1111/j.1468-1331.2012.03819.x
Ferguson, I. T., Elbejjani, M., Sabayan, B., Jacobs, D. R. Jr., Meirelles, O., Sanchez, O. A., et al. (2018). N-terminal pro-brain natriuretic peptide and associations with brain magnetic resonance imaging (MRI) features in middle age: The CARDIA Brain MRI Study. Front. Neurol. 9:307. doi: 10.3389/fneur.2018.00307
Fitzpatrick, A. L., Rapp, S. R., Luchsinger, J., Hill-Briggs, F., Alonso, A., Gottesman, R., et al. (2015). Sociodemographic correlates of cognition in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Geriatr. Psychiatry 23, 684–697. doi: 10.1016/j.jagp.2015.01.003
Frewen, J., Finucane, C., Savva, G. M., Boyle, G., Coen, R. F., and Kenny, R. A. (2013). Cognitive function is associated with impaired heart rate variability in ageing adults: the Irish longitudinal study on ageing wave one results. Clin Auton. Res. 23, 313–323. doi: 10.1007/s10286-013-0214-x
Fujiyoshi, A., Jacobs, D. R. Jr., Alonso, A., Luchsinger, J. A., Rapp, S. R., and Duprez, D. A. (2017). Validity of death certificate and hospital discharge ICD codes for dementia diagnosis: The Multi-Ethnic Study of Atherosclerosis. Alzheimer. Dis. Assoc. Disord. 31, 168–172. doi: 10.1097/WAD.0000000000000164
Gallo, G., Bianchi, F., Cotugno, M., Volpe, M., and Rubattu, S. (2020). Natriuretic Peptides, Cognitive Impairment and Dementia: An Intriguing Pathogenic Link with Implications in Hypertension. J. Clin. Med. 9:2265. doi: 10.3390/jcm9072265
Goldberger, A. L., Amaral, L. A., Glass, L., Hausdorff, J. M., Ivanov, P. C., Mark, R. G., et al. (2000). PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 101, E215–E220. doi: 10.1161/01.cir.101.23.e215
Gómez-Angelats, E., de La Sierra, A., Sierra, C., Parati, G., Mancia, G., and Coca, A. (2004). Blood pressure variability and silent cerebral damage in essential hypertension. Am. J. Hypertens. 17, 696–700. doi: 10.1016/j.amjhyper.2004.05.002
Gorelick, P. B., Scuteri, A., Black, S. E., Decarli, C., Greenberg, S. M., Iadecola, C., et al. (2011). Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 2672–2713. doi: 10.1161/STR.0b013e3182299496
Hayano, J., and Yuda, E. (2019). Pitfalls of assessment of autonomic function by heart rate variability. J. Physiol. Anthropol. 38:3. doi: 10.1186/s40101-019-0193-2
Hayano, J., Kisohara, M., Ueda, N., and Yuda, E. (2020). Impact of heart rate fragmentation on the assessment of heart rate variability. Appl. Sci. 10:3314. doi: 10.3390/app10093314
Heckbert, S. R., Wiggins, K. L., Blackshear, C., Yang, Y., Ding, J., Liu, J., et al. (2017). Pericardial fat volume and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis and Jackson Heart Study. Obesity 25, 1115–1121. doi: 10.1002/oby.21835
HRV (1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93, 1043–1065. doi: 10.1093/oxfordjournals.eurheartj.a014868
Huikuri, H. V., and Stein, P. K. (2012). Clinical application of heart rate variability after acute myocardial infarction. Front. Physiol. 3:41. doi: 10.3389/fphys.2012.00041
Izawa, Y., Urakami, K., Kojima, T., and Ohama, E. (2009). Wechsler Adult Intelligence Scale, 3rd Edition (WAIS-III): usefulness in the early detection of Alzheimer’s disease. Yonago Acta Med. 52, 11–20.
Jessen, F., Kaduszkiewicz, H., Daerr, M., Bickel, H., Pentzek, M., Riedel-Heller, S., et al. (2010). Anticholinergic drug use and risk for dementia: target for dementia prevention. Eur Arch Psychiatry Clin Neurosci. 260, 111–115. doi: 10.1007/s00406-010-0156-4
Johnson, D. A., Lane, J., Wang, R., Reid, M., Djonlagic, I., Fitzpatrick, A. L., et al. (2017). Greater cognitive deficits with sleep-disordered breathing among individuals with genetic susceptibility to Alzheimer’s disease. The Multi-Ethnic Study of Atherosclerosis. Ann. Am. Thorac. Soc. 14, 1697–1705. doi: 10.1513/AnnalsATS.201701-052OC
Kerola, T., Nieminen, T., Hartikainen, S., Sulkava, R., Vuolteenaho, O., and Kettunen, R. (2010). B-type natriuretic peptide as a predictor of declining cognitive function and dementia–a cohort study of an elderly general population with a 5-year follow-up. Ann. Med. 2, 207–215. doi: 10.3109/07853891003652542
Kim, D. H., Lipsitz, L. A., Ferrucci, L., Varadhan, R., Guralnik, J. M., Carlson, M. C., et al. (2006). Association between reduced heart rate variability and cognitive impairment in older disabled women in the community: Women’s Health and Aging Study I. J. Am. Geriatr. Soc. 54, 1751–1757. doi: 10.1111/j.1532-5415.2006.00940.x
Kivipelto, M., Ngandu, T., Laatikainen, T., Winblad, B., Soininen, H., and Tuomilehto, J. (2006). Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurol. 5, 735–741. doi: 10.1016/S1474-4422(06)70537-3
Mahinrad, S., Jukema, J. W., van Heemst, D., Macfarlane, P. W., Clark, E. N., de Craen, A. J., et al. (2016). 10-Second heart rate variability and cognitive function in old age. Neurology 86, 1120–1127. doi: 10.1212/WNL.0000000000002499
Meel-van den Abeelen, A. S., Lagro, J., Gommer, E. D., Reulen, J. P., and Claassen, J. A. (2013). Baroreflex function is reduced in Alzheimer’s disease: a candidate biomarker? Neurobiol. Aging. 34, 1170–1176. doi: 10.1016/j.neurobiolaging.2012.10.010
Nagata, T., Ohara, T., Hata, J., Sakata, S., Furuta, Y., Yoshida, D., et al. (2019). NT-proBNP and risk of dementia in a general Japanese elderly population: The Hisayama Study. J. Am. Heart Assoc. 8:e011652. doi: 10.1161/JAHA.118.011652
Newman, A. B., Fitzpatrick, A. L., Lopez, O., Jackson, S., Lyketsos, C., Jagust, W., et al. (2005). Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J. Am. Geriatr. Soc. 53, 1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x
Ostovaneh, M. R., Moazzami, K., Yoneyama, K., A Venkatesh, B., Heckbert, S. R., Wu, C. O., et al. (2020). Change in NT-proBNP (N-Terminal Pro-B-Type Natriuretic Peptide) level and risk of dementia in Multi-Ethnic Study of Atherosclerosis (MESA). Hypertension 75, 316–323. doi: 10.1161/HYPERTENSIONAHA
Pavlov, V. A., and Tracey, K. J. (2005). The cholinergic anti-inflammatory pathway. Brain Behav. Immun. 19, 493–499. doi: 10.1016/j.bbi.2005.03.015
Pavlov, V. A., and Tracey, K. J. (2017). Neural regulation of immunity: molecular mechanisms and clinical translation. Nat. Neurosci. 20:156. doi: 10.1038/nn.4477
Raman, D., Kaffashi, F., Lui, L. Y., Sauer, W. H., Redline, S., Stone, P., et al. (2017). Polysomnographic heart rate variability indices and atrial ectopy associated with incident atrial fibrillation risk in older community-dwelling men. JACC. Clin. Electrophysiol. 3, 451–460. doi: 10.1016/j.jacep.2016.09.001
Ramsay, M. C., and Reynolds, C. R. (1995). Separate digits tests: a brief history, a literature review, and a reexamination of the factor structure of the Test of Memory and Learning (TOMAL) Neuropsychol. Rev. 5, 51–171. doi: 10.1007/BF02214760
Reardon, M., and Malik, M. (1996). Changes in heart rate variability with age. Pacing Clin. Electrophysiol. 19, 1863–1866. doi: 10.1111/j.1540-8159.1996.tb03241.x
Redline, S., Budhiraja, R., Kapur, V., Marcus, C. L., Mateika, J. H., Mehra, R., et al. (2007). The scoring of respiratory events in sleep: reliability and validity. J. Clin. Sleep Med. 3, 169–200. doi: 10.5664/jcsm.26818
Redline, S., Sanders, M. H., Lind, B. K., Quan, S. F., Gottlieb, D. J., Bonekat, W. H., et al. (1998). Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. SHHS Res. Group. Sleep 21, 759–767.
Rundek, T., Gardener, H., Dias Saporta, A. S., Loewenstein, D. A., Duara, R., Wright, C. B., et al. (2020). Global Vascular Risk Score and CAIDE Dementia Risk Score predict cognitive function in the Northern Manhattan Study. J. Alzheimers Dis. 73, 1221–1231. doi: 10.3233/JAD-190925
Sartori, A. C., Vance, D. E., Slater, L. Z., and Crowe, M. (2012). The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. J. Neurosci. Nurs. 44, 206–217. doi: 10.1097/JNN.0b013e3182527690
Schaich, C. L., Malaver, D., Chen, H., Shaltout, H. A., Zeki, Al Hazzouri, A., et al. (2020). Association of heart rate variability with cognitive performance: The Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 9:e013827. doi: 10.1161/JAHA.119.013827
Stein, P. K., Domitrovich, P. P., Hui, N., Rautaharju, P., and Gottdiener, J. (2005). Sometimes higher heart rate variability is not better heart rate variability: results of graphical and nonlinear analyses. J. Cardiovasc. Electrophysiol. 16, 954–959. doi: 10.1111/j.1540-8167.2005.40788.x
Tahsili-Fahadan, P., and Geocadin, R. G. (2017). Heart–brain axis: effects of neurologic injury on cardiovascular function. Circ. Res. 120, 559–572. doi: 10.1161/CIRCRESAHA.116
Teng, E. L., Hasegawa, K., Homma, A., Imai, Y., Larson, E., Graves, A., et al. (1994). The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int. Psychogeriatr. 6, 45–62. doi: 10.1017/s1041610294001602
Wechsler, D. (1997). Wechsler Adult Intelligence Scale. Third Edition. San Antonio, TX: Harcourt Assessment.
Werts, C. E., and Linn, R. L. (1970). A general linear model for studying growth. Psychol. Bull. 73, 17–22. doi: 10.1037/h0028330
Wolters, F. J., Mattace-Raso, F. U. S., Koudstaal, P. J., Hofman, A., Ikram, M. A., and Heart Brain, et al. (2016). Orthostatic hypotension and the long-term risk of dementia: a population-based study. PLoS Med. 13:e1002143. doi: 10.1371/journal.pmed.1002143
Wunderlin, M., Züst, M. A., Fehér, K. D., Klöppel, S., and Nissen, C. (2020). The role of slow wave sleep in the development of dementia and its potential for preventative interventions. Psychiatry Res. Neuroimaging 306:111178. doi: 10.1016/j.pscychresns.2020.111178
Yaffe, K., Laffan, A. M., Harrison, S. L., Redline, S., Spira, A. P., Ensrud, K. E., et al. (2011). Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 306, 613–619. doi: 10.1001/jama.2011.1115
Yeboah, J., McClelland, R. L., Polonsky, T. S., Burke, G. L., Sibley, C. T., O’Leary, D., et al. (2012). Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 308, 788–795. doi: 10.1001/jama.2012.9624
Zlokovic, B. V., Gottesman, R. F., Bernstein, K. E., Seshadri, S., McKee, A., Snyder, H., et al. (2020). Vascular contributions to cognitive impairment and dementia (VCID): A report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement 16, 1714–1733. doi: 10.1002/alz.12157
Keywords: aging, autonomic nervous system, cognitive status, dementia, heart rate fragmentation and heart rate variability
Citation: Costa MD, Redline S, Hughes TM, Heckbert SR and Goldberger AL (2021) Prediction of Cognitive Decline Using Heart Rate Fragmentation Analysis: The Multi-Ethnic Study of Atherosclerosis. Front. Aging Neurosci. 13:708130. doi: 10.3389/fnagi.2021.708130
Received: 11 May 2021; Accepted: 06 July 2021;
Published: 26 August 2021.
Edited by:
Sara Palermo, Carlo Besta Neurological Institute (IRCCS), ItalyReviewed by:
Akira Fujiyoshi, Wakayama Medical University, JapanCopyright © 2021 Costa, Redline, Hughes, Heckbert and Goldberger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madalena D. Costa, bWNvc3RhM0BiaWRtYy5oYXJ2YXJkLmVkdQ==
†These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.