- 1Department of Pediatrics, Washington University School of Medicine in St. Louis, St. Louis, MO, United States
- 2Lupus Genetic Group, Department of Medicine, University of Southern California, Los Angeles, CA, United States
- 3Department of Pathology and Immunology, Washington University School of Medicine in St. Louis, St. Louis, MO, United States
The leukocyte NADPH oxidase 2 (NOX2) generates superoxide, and derivative reactive oxygen species play important roles in both host defense and immunoregulation. The rs13306575 genetic variant, resulting in an Arginine395→Tryptophan (R395W) substitution in the NOX2 NCF2 subunit, is associated with an increased risk of lupus in patients of Hispanic-American or of Korean ancestry. Arginine395 resides within the NCF2 PB1 domain and participates in a constitutive high-affinity interaction with the NOX2 NCF4 subunit to stabilize their expression. However, whether this variant impacts NCF2 function and NOX2 activity is unknown. To answer this question, mice expressing NCF2-R395W were generated using CRISPR/Cas9. NCF2 and NCF4 expression were reduced by twofold in neutrophils, macrophages, and dendritic cells homozygous for NCF2-R395W. Moreover, following stimulation with soluble or particulate stimuli, reactive oxygen species production at the plasma membrane and within cells was reduced in all three myeloid lineages expressing NCF2-R395W. Additional studies on Ncf2+/− mice, which have a reduced expression of wild-type NCF2 but not of NCF4, suggest that the reduced expression of both NCF2 and NCF4 contributes to the diminished NOX2 activity in NCF2-R395 mice. These results establish that the lupus-associated rs13306575 p.R395W allele is a functional hypomorph. The findings add to growing evidence implicating deficient NOX2 activity in the pathogenesis of lupus.
Introduction
The leukocyte NADPH oxidase 2 (NOX2) is a multi-subunit phagosome and plasma-membrane associated electron transferase expressed primarily in myeloid leukocytes and generates superoxide, the precursor to reactive oxygen species (ROS) that play crucial roles in microbial killing and in immunoregulation (1, 2). The redox center of NOX2 is flavocytochrome b558, a membrane-spanning heterodimer comprised of CYBB (also known as gp91phox), which harbors an NADPH-binding flavoprotein domain and two heme groups, and CYBA (p22phox), which mediates the docking of a trimeric cytosolic complex of regulatory subunits, NCF1 (p47phox), NCF2 (p67phox), and NCF4 (p40phox) (3–5). NCF1 and NCF4 are adaptor proteins that facilitate NOX2 assembly and also regulate its activity via membrane lipids. In contrast, NCF2 is a critical catalytic component and interacts with flavocytochrome b558 to initiate NOX2 activity. NCF2 also binds to Rac-GTP, another essential component of active NOX2. Even in resting cells, NCF2 is constitutively associated with NCF4 via a high-affinity interaction involving their C-terminal PB1 (phox and Bem1) domains that bind in a front-to-back arrangement (6). The absence of either NCF2 or NCF4 markedly reduces the cellular levels of its partner, suggesting that these proteins stabilize each other (7–11). NCF2 is also tethered to NCF1 via an SH3 domain and proline-rich region (PRR) interaction. Following phosphorylation-induced conformational changes in response to soluble or particulate stimuli, the NCF1–NCF2–NCF4 complex, along with Rac1/2, translocates to flavocytochrome b558 to activate the transfer of electrons from NADPH in the cytosol across the membrane to molecular oxygen (3–5, 9).
Both complete and partial defects in NOX2 are associated with human immune disorders (1, 2). The primary immunodeficiency chronic granulomatous disease (CGD) results from inactivating X-linked or recessive mutations in either X-linked CYBB or any of the autosomal genes CYBA, NCF1, NCF2, or NCF4. The absence of NOX2-derived ROS results in recurrent bacterial and fungal infections as well as aberrant inflammation, reflecting a dual importance for microbial killing and for redox regulation of immune responses. In addition, variants in NCF1, NCF2, or NCF4 that partially reduce NOX2 activity, while not associated with infections, are linked to autoimmune and inflammatory disorders (1, 2). Indeed, polymorphisms in NCF1 or NCF2 are now recognized as major genetic risk factors for systemic lupus erythematosus (SLE) (2). The underlying mechanisms by which reduced or absent NOX2-derived ROS promote inflammation and autoimmunity remain incompletely understood, but they are likely to be diverse, given the pleiotropic effects of ROS on cellular pathways, and may also involve different myeloid cell types (1, 2, 12).
Two non-synonymous coding variants in NCF2, both involving highly conserved residues in its PB1 domain, have been linked to lupus. The first reported was rs17849592, a p.H389Q variant associated with childhood and adult–onset SLE in patients of European-American ancestry (13, 14). H389 does not contact NCF4 and is also located at a far-off distance from NCF2 domains that bind to RAC or NCF1. Instead, in silico modeling showed that p.H389Q alters the contact between NCF2 and the guanine nucleotide exchange factor, VAV, which activates RAC downstream of immunoreceptors, accompanied by a twofold reduction in NOX2 activity (14, 15). The rs13306575 NCF2 variant p.R395W, which arises independently, is associated with lupus in Hispanic-Americans and Koreans (16, 17). This allele was identified in as many as 20% of Hispanic Americans with lupus compared with 10% of case controls, with an odds ratio of 5.5 (17). The crystal structure of the NCF2/NCF4 PB1 heterodimer revealed that Arg-395 established a salt link with the C-terminal amino acid of NCF4 to stabilize their PB1–PB1 interaction and GST pull-down binding studies showed that the R395W substitution reduced heterodimerization with NCF4 by half (6). Hence, a weakened interaction between NCF2-R395W and NCF4 could affect their stability within cells. Moreover, in silico modeling showed that NCF2 Arg-395 also helped stabilize a quaternary NCF2-NCF4 -VAV-RAC complex (17), which could contribute to optimizing NOX2 activity. NCF2-R395W was first reported in a CGD patient of Mexican ancestry with biallelic NCF2 mutations and absent NCF2 expression (18). However, the NCF2-R395W allele in this patient additionally harbored an in-frame deletion of nine coding nucleotides in the N-terminus of NCF2 (18), and therefore, the impact of R395W alone on NCF2 expression and NOX2 activity could not be assessed.
Characterizing the functional impact of a lupus-predisposing variant on NOX2 activity is crucial to understanding whether its effect is mediated by impaired ROS production or instead another causal event that is simply linked to the variant. Thus, the goal of the current study was to examine whether the NCF2 variant p.R395W had a deleterious effect on NCF2 expression and NOX2 activity in myeloid leukocytes. As NOX2 subunit genes are highly conserved across species (19, 20), including humans and rodents, we used gene editing with CRISPR/Cas9 to generate mice with the NCF2-R395W variant, assessed the expression of the NCF2-R395W and NCF4 proteins in neutrophils, macrophages, and dendritic cells, and measured ROS generation upon cell stimulation. The results establish that the lupus-associated NCF2-R395W allele is a hypomorph, associated with an approximately twofold reduction in NCF2 and NCF4 levels and in ROS production.
Methods
Mice
Mice were maintained in specific pathogen-free conditions and all experiments were approved by the Institutional Animal Care and Use Committee at Washington University in St. Louis. Age-matched male and female mice with ages ranging between 10 and 20 weeks were used. These included wild-type (WT) C57BL/6J purchased from Jackson Laboratory or bred in-house and mice either heterozygous (Ncf2+/−) or homozygous (Ncf2−/−) for an inactivated Ncf2 allele (21) bred in-house. In addition, we studied newly generated NCF2-R395W mice.
Generation of mice with NCF2-R395W point mutation by CRISPR/Cas9 genome editing
Changing the codon for NCF2 amino acid 395 from arginine (CGT) to tryptophan (TGG) was engineered using CRISPR/Cas9, performed by the Hope Center Transgenic Vectors Core for design and testing of the guide and the Mouse Genetics Core. Mouse transgenesis experiments and microinjections were performed similar to those described in (22, 23). B6/CBAJ mice (Jackson Laboratory) were superovulated by an intraperitoneal injection of five IU pregnant mare serum gonadotropin (MilliporeSigma), followed 48 h later by five IU human chorionic gonadotropin (MilliporeSigma). Mouse zygotes were obtained by mating B6/CBAJ males with superovulated B6/CBAJ females at a 1:1 ratio. One-cell fertilized embryos were microinjected with an RNP containing 12 µg of Cas9 protein, 2 µg of guide RNA, and 100 pmol (∼5 μg) of a single-stranded oligodeoxynucleotide (ssODN) complex as a donor template, prepared in an RNase-free Tris buffer (pH7.4) with 0.1 mM EDTA. The guide RNA sequence was 5′ tccctaccagCTACCGGCGT 3′ and the ssODN donor DNA sequence was 5′ggtgggtcttgtctgatgcagaggactggctatg
ggatgttttttgtgttgatactttctgccttggtccttactcatcatccctaccagCTACCGGtG
gCGGGACAGCCACGAGCTTCTGCTCCTGTCCGAAGAAAGC
ATGAAGGATGCCTGGGGCCAAGTGAAAAACTACTGCCTG
ACTCTGTGGTGTGAGCATACGG 3′.
Offspring were screened by allele-specific PCR (Supplementary Table S1) to identify founder mice harboring the TGG codon. Founders were bred to C57BL/6J mice (Jackson Laboratory), and the F1 offspring were screened by Sanger sequencing to identify mice heterozygous for germline transmission of the NCF2-R395W allele. NCF2-R395W heterozygous mice were backcrossed to C57BL/6J mice for six generations and the colony was then maintained by intercrossing NCF2-R395W heterozygotes.
Preparation of neutrophils, bone-marrow-derived macrophages (BMDM), bone marrow-derived dendritic cells (BMDC), and resident peritoneal macrophages
Marrow was flushed from mouse femurs and tibias in α-minimum essential medium (MEM) (ThermoFisher Scientific). To prepare neutrophils, red blood cells were removed by hypotonic lysis, and marrow was incubated in α-MEM with 2% heat-activated fetal calf serum (FCS) (MilliporeSigma) in a 10-cm tissue culture dish for 1 hour at 37°C in 5% CO2. Non-adherent neutrophils were collected for ROS assays and for cell lysates. The level of purity was ≥70%. For BMDM, marrow was incubated in α-MEM with 20% heat-inactivated FCS for 2 hours in non-tissue-culture-treated plates, and adherent cells were harvested and cultured for 7 days with 25 ng/ml recombinant M-CSF (Peprotech), with the media changed on day 3 (24). On day 7, BMDM were lifted with PBS with 1 mM EDTA and 0.1% BSA and re-plated in 96-well plates at 0.1 × 106 cells/wells for ROS assays or 6-well plates at 1 × 106 cells/wells for cell lysates, performed 1 day after re-plating. For BMDC, red cells were lysed with an ACK lysing buffer (Lonza Group) and cells plated at 0.3 × 106 cells/ml in IMDM with 10% heat-inactivated FCS, 2 mM L-glutamine, 1 mM sodium pyruvate, 1× Penicillin/Streptomycin, 1× MEM non-essential amino acids, and 30 ng/ml recombinant GM-CSF (Peprotech). Floating cells were collected on day 8 and re-plated for ROS assays or cell lysates 1 day later, as done for BMDM.
To isolate resident peritoneal macrophages, peritoneal cells were harvested by lavage with 10 ml PBS containing 2 mM EDTA. After washing with PBS, the cells were plated in a 10 cm tissue culture dish with α-MEM containing 10% FCS for 2 h at 37 °C. After removing non-adherent cells, adherent cells were lifted with PBS containing 2 mM EDTA (10 min, 37°C, gentle scraping), plated into 6-well plates at 1 × 106 cells per well with 2 ml DMEM containing 10% heat-inactivated FCS, and harvested after 1 day for lysates.
Western blot analysis of NOX2 subunit expression
NOX2 subunit expression was analyzed as described (25–27). Lysates were prepared from murine neutrophils, BMDM, BMDC, and resident peritoneal macrophages with a lysis buffer containing 2.5 mM diisopropylfluorophosphate (MilliporeSigma) (for neutrophils), a 1× protease inhibitor mixture (Roche Diagnostics) (for all cell types), and 30 μg loaded for SDS-PAGE and immunoblotting using ECL detection. Mouse monoclonal anti-NOX2/gp91phox (clone 54.1, ab80897), rabbit polyclonal anti-p22phox (ab191512), rabbit polyclonal anti-p67phox (ab175293), goat polyclonal anti-p47phox (ab795), and HRP-conjugated anti-β-actin were obtained from Abcam. Rabbit polyclonal anti-p40phox (07-503) was procured from Upstate Biotechnology, Inc. Densitometry was quantified using ImageJ software (NIH).
Analysis of NOX2 activity
NOX2 activity was assayed in cells plated in 96-well white plates (Costar) and prewarmed to 37°C as described (25–27). Reactive oxygen species were detected with a SpectraMax L Luminometer (Molecular Devices) using luminol with HRP in the absence or presence of superoxide dismutase to measure total ROS or intracellular ROS, respectively. Neutrophils were stimulated with either buffer alone, 200 ng/ml phorbol myristate acetate (PMA), 10 μM fMLF, 100 nM leukotriene B4 (LTB4), serum opsonized zymosan (SOZ) (ratio 5:1 cell), or IgG-coated latex beads (ratio 100:1 cell). BMDM or BMDC was either left untreated or stimulated with 200 ng/ml PMA, SOZ (ratio 5:1 cell), or IgG-coated latex beads (ratio 100:1 cell). After adding particulate stimuli, the plates were spun at 300 g for 1 min at room temperature. Chemiluminescence was recorded as relative light units (RLU) over time. Oxidant production was calculated by using the area under the curve (AUC) and presented as a ratio of the WT response.
Statistics
Prism 9.0 (GraphPad Software Inc.) was used in statistical analyses. Statistical significance was assessed by using Student’s t-test. A value of P < 0.05 was considered significant.
Results
Generation of NCF2-R395W mice
To investigate the effects of the NCF2-R395W substitution, we used CRISPR/Cas9 for genome-editing single-cell mouse embryos, replacing the Ncf2 CGT codon for arginine-395 with a TGG codon for tryptophan (Figures 1A,B) (22, 23). The offspring of the founder gene-edited mice were backcrossed to C57BL6/J to establish a colony of NCF2-R395W mice. NCF2-R395W mice appeared healthy, as might be expected in the C57BL6/J strain, which does not have a genetic predisposition to spontaneously develop lupus. Littermates homozygous for either WT NCF2 or NCF2-R395W (referred to as NCF2-R395W mice) were used for making comparisons of NOX2 subunit expression and NOX2 activity.
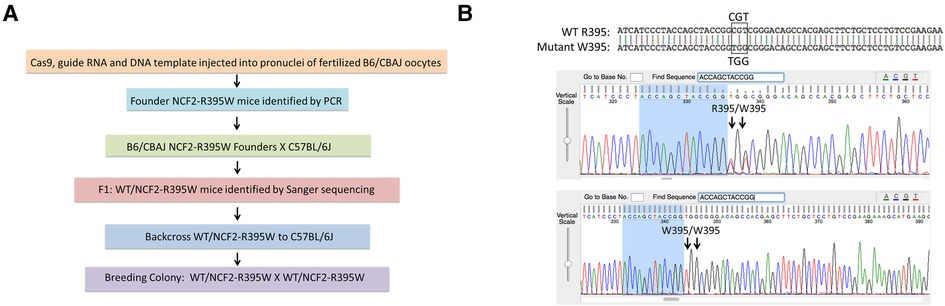
Figure 1. Generation of NCF2-R395W mice. (A) Generation of mice with NCF2R395W point mutation by CRISPR/Cas9 genome editing. (B) Top row: alignment of sequences obtained from mice heterozygous for the WT NCF2-R395 and NCF2-W395 alleles. Middle row: Sanger sequencing data showing both arginine (CGT) and tryptophan (TGG) codons in mice heterozygous for the two alleles. Bottom row: Sanger sequencing data showing only tryptophan (TGG) codon in mice homozygous for the NCF2-W395 allele.
Reduced expression of NOX2 subunits NCF2 and NCF4 in NCF2-R395W myeloid cells
Cell lysates prepared from neutrophils, bone marrow–derived macrophages, bone marrow–derived dendritic cells, and resident peritoneal macrophages were analyzed by immunoblotting (Figure 2). The expressions of both NCF2 and NCF4 were significantly reduced by at least twofold in each of the cell types prepared from NCF2-R395W mice compared with WT. There was also a modest (≈25%) reduction of CYBB in NCF2-R395W resident peritoneal macrophages and of NCF1 in NCF2-R395W neutrophils, each of which reached statistical significance. The consistent reduction in NCF2-R395W and NCF4 in multiple myeloid lineages highlights the importance of the PB1–PB1 domain interaction to stabilize the expression of these two NOX2 subunits.
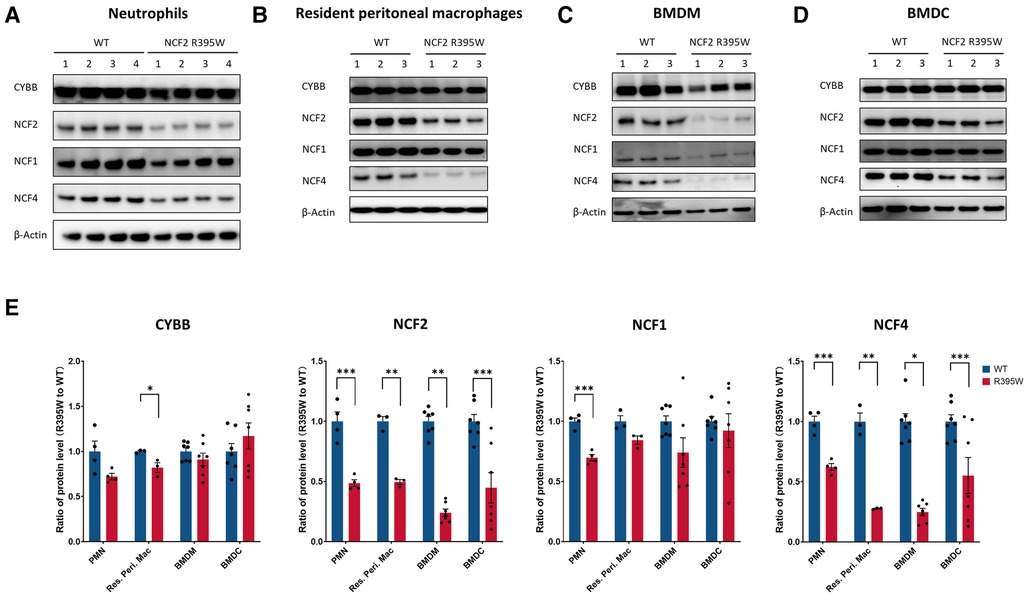
Figure 2. NOX2 subunit expression in myeloid cells from NCF2-R395W mice. Representative immunoblots for CYBB, NCF2, NCF1, and NCF4 (A–D) and densitometry (E) for different populations of myeloid cells, as indicated, from WT mice and littermate mice homozygous for murine NCF2-R395W. β-Actin as an internal control. (E) Densitometric analysis and protein levels are represented as mean ratio values quantified from the protein bands of CYBB, NCF2, NCF2, or NCF4 vs. β-actin compared with WT. PMN, n = 4; resident peritoneal macrophage, n = 3; BMDM, n = 7; BMDC, n = 7. Data are means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 were obtained by using Student’s t-test.
Reduced NOX2 activity in NCF2-R395W myeloid cells
NOX2 activity was measured using chemiluminescence-based detection of ROS following cell stimulation. CGD leukocytes, including those with inactivating mutations in the gene encoding NCF2 (21), lack NOX2 activity. NCF2-R395W neutrophils activated either with the soluble agonists fMLF, LTB4, or PMA or with SOZ or IgG-opsonized beads, particulate stimuli that can be phagocytosed, showed a significant reduction, ranging from ≈25% to 50%, in both total ROS and intracellular ROS (Figures 3A,B). The production of both total and intracellular ROS was also significantly decreased in NCF2-R395W macrophages and dendritic cells when stimulated with PMA, SOZ, or IgG-opsonized beads (Figures 3C,D). Thus, ROS production is consistently diminished by as much as twofold in stimulated NCF2-R395W myeloid cells.
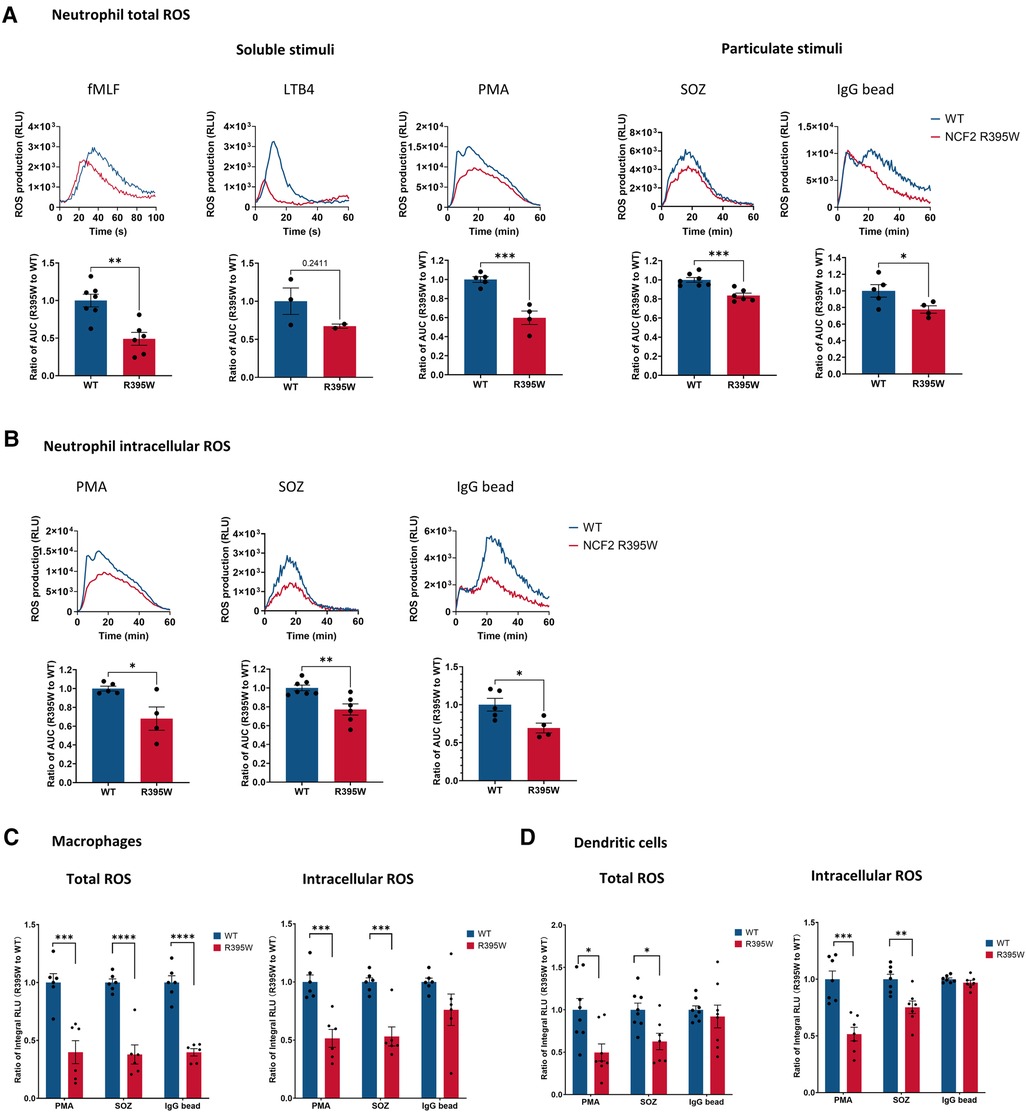
Figure 3. ROS production in myeloid cells from NCF2-R395W mice. (A) Neutrophil (PMN) ROS production was induced by using 10 μM fMLF, 100 nM LTB4, 200 ng/ml PMA, SOZ (the SOZ to cell ratio is 5:1), and IgG-coated latex beads (the IgG bead to cell ratio is 100:1) in neutrophils in the absence of superoxide dismutase, and total oxidant production recorded as relative light units (RLU), as shown in the kinetic tracings (upper row). Total oxidant production was calculated by using the area under the curve (AUC). The AUC of NCF2-R395W neutrophils was normalized with the AUC of littermate WT neutrophils. (B) Neutrophil ROS production was induced by using 200 ng/ml PMA, SOZ (the SOZ to cell ratio is 5:1), and IgG-coated latex beads (the IgG bead to cell ratio is 100:1) in the presence of superoxide dismutase to assess intracellular ROS. Kinetic tracings (upper row) and the AUC of NCF2-R395W neutrophils (lower row) as normalized with the AUC of littermate WT neutrophils. fMLF, 0–99 sec, LTB4, 4–30 sec, PMA, 1–40 min, SOZ, 0–60 min, and IgG bead, 0–60 min. (A) fMLF, n ≥ 6, obtained from three independent experiments. LTB4, n ≥ 2, obtained from one experiment. (A–B) PMA, IgG beads, n ≥ 4, obtained from two independent experiments. SOZ, n ≥ 6, obtained from three independent experiments. (C–D) Total and intracellular ROS production was measured in the absence and presence of superoxide dismutase, following stimulation with 200 ng/ml PMA, SOZ (the SOZ to cell ratio is 5:1), or IgG-coated latex beads (the IgG bead to cell ratio is 100:1) (C) BMDM and (D) BMDC prepared from WT and littermate NCF2-R395W mice. Integral RLU (0–60 min) was calculated as total oxidant production. The integral RLU of NCF2-R395W BMDM or BMDC was normalized with the integral RLU of WT BMDM or BMDC. BMDM, n ≥ 6, obtained from three independent experiments. BMDC, n ≥ 7, obtained from three independent experiments. Data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 were obtained by using Student’s t-test.
Ncf2+/− mice have reduced neutrophil expression of WT NCF2 and reduced ROS production
To parse out the effects of the R395W substitution on NOX2 activity and expression levels of NCF2 and NCF4, we examined the impact of the reduced expression of WT NCF2 alone using Ncf2+/− mice. These mice are heterozygous for a WT Ncf2 allele and a null allele with a disruption of Ncf2 Exon3, and our prior studies in the NZM.2328 strain showed a twofold reduction in NCF2 levels and NOX2 activity in Ncf2+/− neutrophils, macrophages, and dendritic cells (21). In this study, we found that NCF2 levels in neutrophils from C57BL/6J Ncf2+/− mice were decreased by ≈30% from WT neutrophils. (Figure 4A). NCF4 levels in Ncf2−/− neutrophils, which have no detectable NCF2 protein, were reduced by twofold (Figure 4A), as also reported for CGD patients lacking NCF2 (7, 10), However, NCF4 levels in Ncf2+/− neutrophils were not significantly decreased (Figure 4A). Total ROS production was significantly lower in Ncf2+/− neutrophils stimulated with fMLF, PMA, and IgG-opsonized beads, but not in SOZ, and intracellular ROS production was significantly affected only in fMLF (Figure 4B). Thus, reduced levels of WT NCF2 limited ROS production by activated C57BL/6J neutrophils, but not to the extent seen with NCF2-R395W. This suggests that the diminished NOX2 activity in NCF2-R395W cells reflects more than simply the lower levels of the NCF2-R395W subunit and is at least, in part, related to reduced NCF4 levels in NCF2-R395W cells (Figure 2).
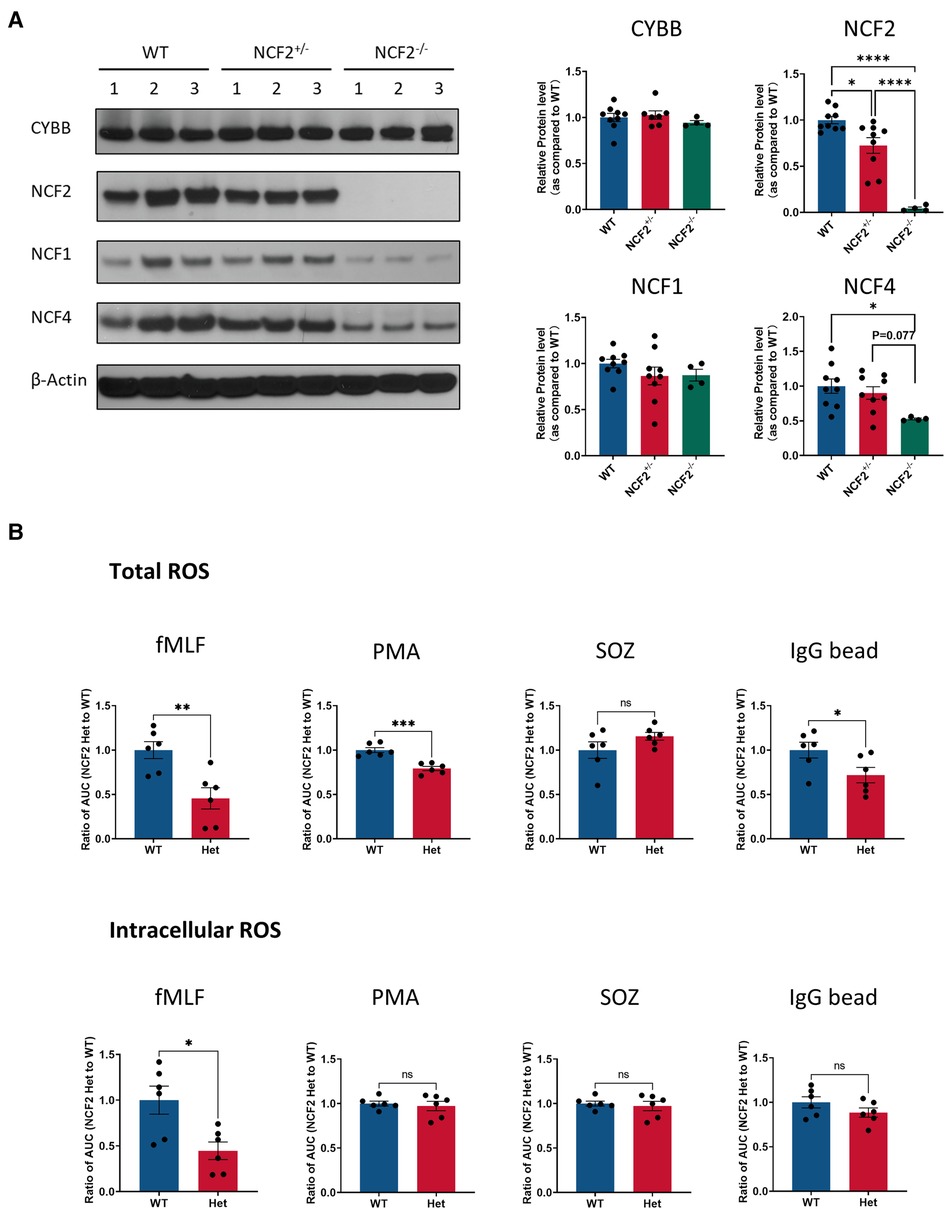
Figure 4. NOX2 subunit expression and ROS production in neutrophils with a genetic deletion of Ncf2. (A) Left, representative immunoblots for the expression of CYBB, NCF2, NCF1, and NCF4 in WT, Ncf2+/− (NCF2 Het), and Ncf2−/− (NCF2 KO) mice. β-Actin as an internal control. Right, densitometric analysis and protein levels are represented as mean ratio values quantified from the protein bands of CYBB, NCF2, NCF2, or NCF4 vs. β-actin compared with WT. n ≥ 4 obtained from more than two independent experiments. Data are means ± SEM.*P < 0.05 and **P < 0.01 were obtained by using Student’s t-test. (B) Total and intracellular ROS production was measured in the absence and presence of superoxide dismutase after stimulation with 200 ng/ml PMA, SOZ (the SOZ to cell ratio is 5:1), or IgG-coated latex beads (the IgG bead to cell ratio is 100:1) in neutrophils. Oxidant production was recorded as relative light units (RLU). Total oxidant production was calculated by using the area under the curve (AUC). The AUC of NCF2+/− neutrophils was normalized with the AUC of WT neutrophils. n = 6 was obtained from two independent experiments. Data are means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 were obtained by using Student’s t-test.
Discussion
NCF2 is an essential catalytic component of an assembled NOX2 oxidase complex, which together with RAC, directly activates flavocytochrome b558 to initiate electron transfer for superoxide production. NCF2 interacts with multiple other NOX2 components (3–5, 9), including a constitutive PB1 domain–mediated association with NCF4. This study establishes that an R395W substitution in the NCF2 PB1 domain, which is a strong genetic risk factor for lupus in Hispanic Americans and Koreans (16, 17), is a hypomorphic variant, impacting the expression of both NCF2 and NCF4 as well as NOX2 activity.
Since the time it was discovered that NCF2 was present in a stable complex with NCF4 in neutrophils, it was recognized that their association affected the cellular levels of these two NOX2 subunits. Studies in humans and mice lacking NCF2 or NCF4 due to genetic defects in the corresponding genes showed markedly reduced levels of the other partner (7–11), suggesting that the PB1-domain-mediated association between these two proteins is important for their stable expression. In this study, we found that the NCF2-R395W substitution significantly reduced the levels of both NCF2-R395W and its NCF4 partner in neutrophils, macrophages, and dendritic cells prepared from genetically engineered mice. NCF2 Arg-395 is highly conserved across all species and identical between humans and mice, as is NCF4 Pro-339, which contacts NCF2 Arg-395 in a salt linkage (6, 17). The tryptophan substitution does not allow this residue to form a hydrogen bond with NCF4 Pro-339 to stabilize the PB1 domain-mediated association between NCF2 and NCF4 (6), and in vitro pull-down assays showed that this substitution reduced the amount of NCF4 PB1 captured by the NCF2 PB1 domain by ≈ twofold (6). These results highlight the importance of Arg395 in full-length NCF2 for maintaining the cellular levels of NCF2 and NCF4 in multiple types of myeloid leukocytes. These data also show that NCF2–NCF4 interaction is important for their mutual stability in macrophages and dendritic cells, extending prior studies in neutrophils (7–11). These findings are consistent with a long-standing concept that proteins found in multiprotein complexes are often subject to post-translational degradation of any unassembled or “orphaned” subunits, which may help maintain optimal stoichiometry within the cell; however, the underlying mechanisms are currently poorly understood, and therefore, an active field of study is the need of the hour (28, 29).
The production of both total and intracellular ROS upon cell activation with various stimuli was also significantly reduced in myeloid leukocytes prepared from mice homozygous for NCF2-R395W. Our current and previous studies (21) on Ncf2+/− mice indicate that reducing cellular levels of WT NCF2 is itself sufficient to diminish ROS production following stimulation. However, we found that NCF2-R395W had an overall greater impact on NOX2 activity, including intracellular ROS production. This could, in part, reflect the reduced NCF4 levels in NCF2-R395W neutrophils, which are unaffected in Ncf2+/−, as NCF4 plays a key role in intracellular NOX2 activity via its binding site for phosphatidylinositol-3-phosphate, a regulatory lipid found on intracellular membranes (1, 5). The importance of NCF4 levels for maintaining NOX2 activity is also highlighted in a prior study that reported that Ncf4+/− cells haploinsufficient in NCF4 had a partial reduction in NOX2 activity (30). In silico modeling suggested that NCF2-R395 could also help stabilize a quaternary NCF2-NCF4-VAV1-RAC association that may be important for optimal NOX2 activity (17). Taken together, we infer that the diminished total and intracellular ROS production by myeloid cells expressing the NCF2-R395W variant is a consequence of the reduced expression of NCF2 and NCF4 and may also reflect altered NCF2 interactions with VAV1 and Rac.
The risk of developing autoimmune disorders is influenced by the effects of variants in multiple genes associated with immune regulation, which for lupus, includes a strong genetic association with hypomorphic mutations in genes encoding the NCF1 and NCF2 NOX2 subunits (2). These findings are supported by studies in animal models with other genetic risk factors for lupus, showing a more rapid onset or increased severity of disease where NOX2 activity is partially reduced (21, 31–33). Understanding how deficient NOX2 ROS production contributes to the pathogenesis of lupus remains an important challenge. This is likely a consequence of diverse mechanisms, as NOX2-derived ROS impact multiple cellular pathways involved in immune regulation and limiting inflammation. These include redox-modulated signal transduction, antigen processing, and interactions among immune cells (1, 2, 12). This work establishes that the R395W substitution in the lupus-associated NCF2 rs13306575 variant impacts the levels of both NCF2 and NCF4 as well NOX2 activity and provides a new animal model for performing further studies of this NCF2 variant.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee at Washington University in St. Louis.
Author contributions
ZS, DGY, RAI, and AB designed and performed the experiments and analyzed data; ZS, DGY, and RAI prepared the figures; COJ and MCD designed the research and wrote the manuscript with inputs from ZS, DGY, and AB. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the National Institutes of Health (NIH), National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR072212 to C.O.J and M.C.D.), and the Children's Discovery Institute of Washington University and St. Louis Children's Hospital (M.C.D.).
Acknowledgments
The authors thank Tina McGrath for assistance with manuscript preparation, Sourav Bhattacharya for helpful discussions, Renate Lewis in the Hope Center Transgenic Vectors Core for design and testing of gene-editing reagents, and Mia Wallace in the Mouse Genetics Core at Washington University in St. Louis School of Medicine for the generation of gene-edited mice and support with animal care.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/flupu.2023.1186641/full#supplementary-material.
References
1. Dinauer MC. Inflammatory consequences of inherited disorders affecting neutrophil function. Blood. (2019) 133(20):2130–9. doi: 10.1182/blood-2018-11-844563
2. Zhong J, Olsson LM, Urbonaviciute V, Yang M, Backdahl L, Holmdahl R. Association of NOX2 subunits genetic variants with autoimmune diseases. Free Radic Biol Med. (2018) 125:72–80. doi: 10.1016/j.freeradbiomed.2018.03.005
3. Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. (2004) 122(4):277–91. doi: 10.1007/s00418-004-0679-8
4. Vermot A, Petit-Hartlein I, Smith SME, Fieschi F. NADPH Oxidases (NOX): an overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants (Basel). (2021) 10(6):890. doi: 10.3390/antiox10060890
5. Nunes P, Demaurex N, Dinauer MC. Regulation of the NADPH oxidase and associated ion fluxes during phagocytosis. Traffic. (2013) 14(11):1118–31. doi: 10.1111/tra.12115
6. Wilson MI, Gill DJ, Perisic O, Quinn MT, Williams RL. PB1 domain-mediated heterodimerization in NADPH oxidase and signaling complexes of atypical protein kinase C with Par6 and p62. Mol Cell. (2003) 12(1):39–50. doi: 10.1016/S1097-2765(03)00246-6
7. Wientjes FB, Hsuan JJ, Totty NF, Segal AW. P40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains. Biochem J. (1993) 296(Pt 3):557–61. doi: 10.1042/bj2960557
8. Tsunawaki S, Mizunari H, Nagata M, Tatsuzawa O, Kuratsuji T. A novel cytosolic component, p40phox, of respiratory burst oxidase associates with p67phox and is absent in patients with chronic granulomatous disease who lack p67phox. Biochem Biophys Res Commun. (1994) 199(3):1378–87. doi: 10.1006/bbrc.1994.1383
9. Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. (2005) 386(Pt 3):401–16. doi: 10.1042/BJ20041835
10. Dusi S, Donini M, Rossi F. Mechanisms of NADPH oxidase activation: translocation of p40phox, Rac1 and Rac2 from the cytosol to the membranes in human neutrophils lacking p47phox or p67phox. Biochem J. (1996) 314(Pt 2):409–12. doi: 10.1042/bj3140409
11. Ellson CD, Davidson K, Ferguson GJ, O'Connor R, Stephens LR, Hawkins PT. Neutrophils from p40phox-/- mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J Exp Med. (2006) 203(8):1927–37. doi: 10.1084/jem.20052069
12. Hoffmann MH, Griffiths HR. The dual role of reactive oxygen Species in autoimmune and inflammatory diseases: evidence from preclinical models. Free Radic Biol Med. (2018) 125:62–71. doi: 10.1016/j.freeradbiomed.2018.03.016
13. Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. (2009) 41(11):1228–33. doi: 10.1038/ng.468
14. Jacob CO, Eisenstein M, Dinauer MC, Ming W, Liu Q, John S, et al. Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc Natl Acad Sci U S A. (2012) 109(2):E59–67. doi: 10.1073/pnas.1113251108
15. Ming W, Li S, Billadeau DD, Quilliam LA, Dinauer MC. The rac effector p67phox regulates phagocyte NADPH oxidase by stimulating Vav1 guanine nucleotide exchange activity. Mol Cell Biol. (2007) 27(1):312–23. doi: 10.1128/MCB.00985-06
16. Kim-Howard X, Sun C, Molineros JE, Maiti AK, Chandru H, Adler A, et al. Allelic heterogeneity in NCF2 associated with systemic lupus erythematosus (SLE) susceptibility across four ethnic populations. Hum Mol Genet. (2014) 23(6):1656–68. doi: 10.1093/hmg/ddt532
17. Armstrong DL, Eisenstein M, Zidovetzki R, Jacob CO. Systemic lupus erythematosus-associated neutrophil cytosolic factor 2 mutation affects the structure of NADPH oxidase complex. J Biol Chem. (2015) 290(20):12595–602. doi: 10.1074/jbc.M115.639021
18. Patino PJ, Rae J, Noack D, Erickson R, Ding J, de Olarte DG, et al. Molecular characterization of autosomal recessive chronic granulomatous disease caused by a defect of the nicotinamide adenine dinucleotide phosphate (reduced form) oxidase component p67-phox. Blood. (1999) 94(7):2505–14. doi: 10.1182/blood.V94.7.2505.419k10_2505_2514
19. Kawahara T, Quinn MT, Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (nox/duox) family of enzymes. BMC Evol Biol. (2007) 7:109. doi: 10.1186/1471-2148-7-109
20. Kawahara T, Lambeth JD. Molecular evolution of phox-related regulatory subunits for NADPH oxidase enzymes. BMC Evol Biol. (2007) 7:178. doi: 10.1186/1471-2148-7-178
21. Jacob CO, Yu N, Yoo DG, Perez-Zapata LJ, Barbu EA, Kaplan MJ, et al. Haploinsufficiency of NADPH oxidase subunit neutrophil cytosolic factor 2 is sufficient to accelerate full-blown lupus in NZM 2328 mice. Arthritis Rheumatol. (2017) 69(8):1647–60. doi: 10.1002/art.40141
22. Sentmanat MF, White JM, Kouranova E, Cui X. Highly reliable creation of floxed alleles by electroporating single-cell embryos. BMC Biol. (2022) 20(1):31. doi: 10.1186/s12915-021-01223-w
23. Harms DW, Quadros RM, Seruggia D, Ohtsuka M, Takahashi G, Montoliu L, et al. Mouse genome editing using the CRISPR/cas system. Curr Protoc Hum Genet. (2014) 83(15):7 1–27. doi: 10.1002/0471142905.hg1507s83
24. Casbon AJ, Allen LA, Dunn KW, Dinauer MC. Macrophage NADPH oxidase flavocytochrome B localizes to the plasma membrane and Rab11-positive recycling endosomes. J Immunol. (2009) 182(4):2325–39. doi: 10.4049/jimmunol.0803476
25. Li XJ, Marchal CC, Stull ND, Stahelin RV, Dinauer MC. P47phox phox homology domain regulates plasma membrane but not phagosome neutrophil NADPH oxidase activation. J Biol Chem. (2010) 285(45):35169–79. doi: 10.1074/jbc.M110.164475
26. Yoo DG, Paracatu LC, Xu E, Lin X, Dinauer MC. NADPH Oxidase limits collaborative pattern-recognition receptor signaling to regulate neutrophil cytokine production in response to fungal pathogen-associated molecular patterns. J Immunol. (2021) 207(3):923–37. doi: 10.4049/jimmunol.2001298
27. Bagaitkar J, Barbu EA, Perez-Zapata LJ, Austin A, Huang G, Pallat S, et al. PI(3)P-p40phox binding regulates NADPH oxidase activation in mouse macrophages and magnitude of inflammatory responses in vivo. J Leukoc Biol. (2017) 101(2):449–57. doi: 10.1189/jlb.3AB0316-139R
28. Taggart JC, Zauber H, Selbach M, Li GW, McShane E. Keeping the proportions of protein Complex components in check. Cell Syst. (2020) 10(2):125–32. doi: 10.1016/j.cels.2020.01.004
29. Juszkiewicz S, Hedge RS. Quality control of orphaned proteins. Mol Cell. (2018) 71(3):443–57. doi: 10.1016/j.molcel.2018.07.001
30. Graham DB, Becker CE, Doan A, Goel G, Villablanca EJ, Knights D, et al. Functional genomics identifies negative regulatory nodes controlling phagocyte oxidative burst. Nat Commun. (2015) 6:7838. doi: 10.1038/ncomms8838
31. Winter S, Hultqvist Hopkins M, Laulund F, Holmdahl R. A reduction in intracellular reactive oxygen Species due to a mutation in NCF4 promotes autoimmune arthritis in mice. Antioxid Redox Signal. (2016) 25(18):983–96. doi: 10.1089/ars.2016.6675
32. Meng Y, Ma J, Yao C, Ye Z, Ding H, Liu C, et al. The NCF1 variant p.R90H aggravates autoimmunity by facilitating the activation of plasmacytoid dendritic cells. J Clin Invest. (2022) 132(16):e153619. doi: 10.1172/JCI153619
Keywords: systemic lupus erythematosus, neutrophil, macrophage, dendritic cell, PB1 domain, NOX2
Citation: Song Z, Yoo DG, Idol RA, Barbu EA, Jacob CO and Dinauer MC (2023) Lupus-associated NCF2 variant p.R395W in the NADPH oxidase 2 complex results in a reduced production of reactive oxygen species by myeloid cells. Front. Lupus 1:1186641. doi: 10.3389/flupu.2023.1186641
Received: 15 March 2023; Accepted: 27 April 2023;
Published: 19 May 2023.
Edited by:
Antonio La Cava, University of California, Los Angeles, United StatesReviewed by:
Klaus Tenbrock, RWTH Aachen University, GermanyJoseph M. Reynolds, Rosalind Franklin University of Medicine and Science, United States
© 2023 Song, Yoo, Idol, Barbu, Jacob and Dinauer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mary C. Dinauer mdinauer@wustl.edu
†These authors have contributed equally to this work and share first authorship
 Zhimin Song
Zhimin Song