- 1Arthritis and Clinical Immunology Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK, United States
- 2Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
- 3Medical Service, Department of Veterans Affairs Medical Center, Oklahoma City, OK, United States
- 4University of Arkansas for Medical Sciences, Little Rock, AK, United States
- 5Department of Plastic and Reconstructive Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 6Genes and Human Diseases Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK, United States
- 7US Department of Veterans Affairs Medical Center, Cincinnati, OH, United States
Objective: Systemic lupus erythematosus (SLE) has a higher prevalence and is more severe in African Americans and Hispanics than in non-Hispanic Whites. To understand the shared and unique genetic risk factors of these populations, an adequate representation of African Americans and Hispanics in clinical and genetic research is indispensable while challenging. The goal of this study was to identify differences in research participation of families of different racial and ethnic backgrounds and the potential causes for the disparities.
Methods: Families were screened for eligibility to the Lupus Family Registry and Repository (LFRR) after self-referral or physician referral. We recorded the sociodemographic characteristics, self-identified race and ethnicity, ACR-SLE criteria, and the reasons given for not completing study participation for all families.
Results: A total of 1,472 families (950 non-Hispanic White, 405 African American, and 117 Hispanic) were screened but only 366 completed study participation (25%). Participation rates and reasons for non-participation varied between racial and ethnic groups. The main reason for African American families to not participate was that subjects critical to the family structure declined participation (OR = 1.6, p = 0.0001), while for White families, the main cause was that purported SLE patients did not meet ACR SLE criteria (OR = 1.81, p < 0.00002). Hispanics were the most likely to complete participation (OR = 4.25, p < 0.0001).
Conclusions: Successful recruitment of patients, families, and specific demographic groups is critical for the study of genetically complex diseases, such as SLE. There are significant disparities in SLE family recruitment across groups of people, likely due to their richly different cultures and environments.
1. Introduction
Systemic lupus erythematosus (SLE) has a higher prevalence and is more severe in African Americans and White Hispanics (Hispanics) than in non-Hispanic Whites (Whites) (1, 2). SLE incidence in African American women is three times that of White women (3) and, while the incidence of SLE in Hispanic women is not precisely known, it is higher than that of White women (4, 5). Similarly, there is ample evidence for different susceptibility genes for SLE between African Americans and Whites or Hispanics (6). Confirming the genetic etiology of some of these differences, a high proportion of Amerindian ancestry correlates with an increased number of risk alleles for SLE(7, 8). Thus, the increased prevalence, worsened severity, and unique genetics of SLE in African Americans and Hispanics make the study of these groups of particular import and interest.
It would be reductive and erroneous, however, to attribute all health disparities in SLE solely to genetic ancestry. Racial and ethnic groups are not monolithic or homogenous, their conditions are influenced by a vast number of factors such as geolocation, socioeconomic status, education, and access to care, amongst others. Any complete inquiry into the source of inequities in health and outcomes must take into account the social and physical environment surrounding a given population (9, 10).
Unfortunately, genetic studies frequently experience difficulties in recruiting, enrolling, and retaining subjects, and such difficulties may be particularly acute in African Americans, perpetuating disparities in research representation. Subjects may not contribute to a study for any number of reasons during the screening and recruiting process: loss of interest, failure to meet study criteria, refusal to fully participate, change of mind, or being unresponsive/unreachable (11). The legacy of the Tuskegee Syphilis Study continues to negatively influence the view of medical research by African Americans (12), who as a group look at medical research, especially genetic research (13), with skepticism and wariness. This knowledge lessens trust in medical research, which in turn produces a barrier to participation in research studies (12, 13). More recently, with increased public awareness of personalized medicine and the role of genetic testing in the clinical setting, the general public's understanding and acceptance of genetic research are improving, including amongst African-Americans, Asian-Americans, and Hispanics (14, 15).
Difficulties faced in recruiting, enrolling and retaining Hispanic subjects vary somewhat from those experienced in the recruitment of African Americans. The degree of cultural assimilation may influence Hispanic families' socioeconomic status, familial and social support, access to health care, and knowledge of genetic studies; thereby, curtailing their participation in such research (16, 17).
For more than 20 years we recruited and enrolled families with two or more SLE patients for genetic studies with a focus on underrepresented populations. The majority of the families that were screened failed to be enrolled. We undertook the present study to determine the differences in enrollment of families among self-identified racial groups. We found important differences in the reasons families do not become part of the study that varied on the basis of race and ethnicity.
2. Materials and methods
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (IRB) of the Oklahoma Medical Research Foundation [IRB #95–12; initial approval 1995]. Informed consent was obtained from all subjects involved in the study before any procedures were initiated.
Study Participants: The data herein described are derived from the Lupus Family Registry and Repository, which has been previously discussed in detail (18), and includes families with 2 or more SLE patients. Candidate families were referred to the study by any of several different sources, e.g.,—physician referral, lupus support groups, family/friends, public media, newsletters, posters, the study website, and the Centers for Medicare and Medicaid Services (CMS) database. Referral sources were tracked in all cases to determine their success rates.
Following the guidelines of the Office of Management and Budget (OMB) Standards, individuals were asked to self-identify their race as American Indian or Alaska Native, Asian, African American, Hawaiian or Pacific Islander, and White, and their ethnicity as Hispanic or non-Hispanic. The vast majority of subjects reporting Hispanic ethnicity self-identified as White. The race and ethnicity of each family was recorded based on the self-reported race and ethnicity of the proband.
After the initial contact, each family underwent three screening phases to determine study eligibility as a multiplex SLE-affected family (i.e., having two or more SLE-affected members). The first phase determined the number of SLE-affected family members and their location within the family tree. Families were eliminated from the study if there was only one SLE-affected member, identical twins as the only SLE-affected, unrelated SLE-affected members, double influence (i.e., an SLE-affected family member with both maternal and paternal SLE-affected relatives), or parent-progeny pair only. The second phase was aimed at determining that the purported SLE patients met the ACR classification criteria (19) and that the family structure was useful for genetic studies. The SLE ACR criteria were confirmed by review and data abstraction from medical records by a physician, physician's assistant, or registered nurse. The families progressed to the third phase if SLE classification criteria were supported by evidence, if they agreed to complete the process, and if the described family structure was useful to the study. This phase had three possible outcomes: 1) families with multiple confirmed SLE cases that were enrolled directly into the study, 2) unconvincing multi-case families that were not pursued further, and 3) families with multiple individuals with <4 ACR criteria that were followed as “Families in Progress.” For the last group, we obtained medical records and verified a minimum of four ACR criteria per affected individual prior to enrollment into the study (Figure 1).
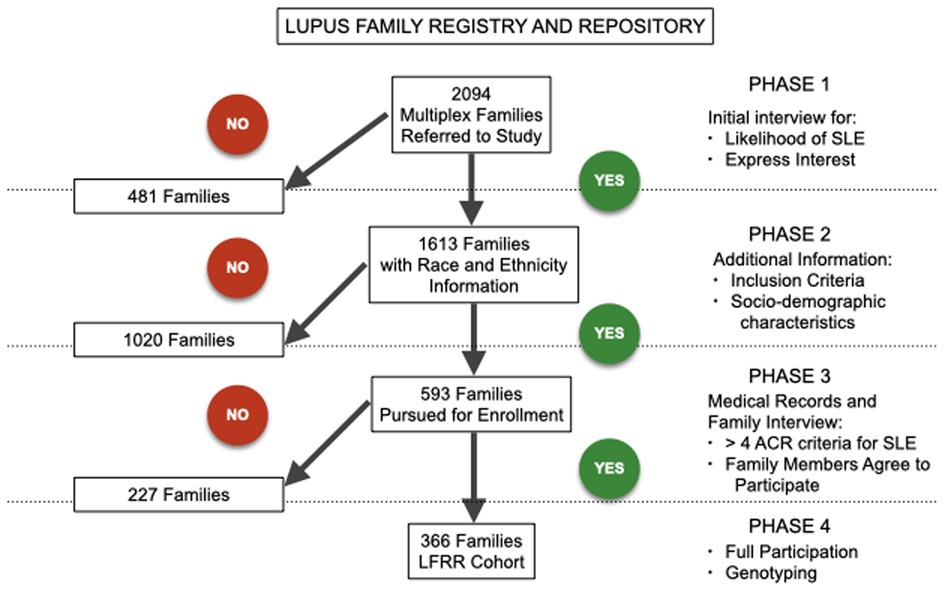
Figure 1. Recruitment flow diagram showing elimination of 1,020 families in the first phase of recruitment with 593 families advancing to the second phase. Eliminated families are shown on the left, while families continuing to the next phase and eventually fully enrolled are shown on the right. A total of 366 families entered the Lupus Family Registry and Repository cohort.
The critical and most difficult stage of participation was the recruitment of the relatives of the proband. This process involved contacting all living individuals who were informative for revealing genetic inheritance between SLE-affected individuals in the family structure, confirming participant interest, obtaining informed consent, sending phlebotomy supplies and paperwork, arranging for the patient's phlebotomy, and delivery of samples to our facility. If family members who directly linked SLE-affected subjects were unavailable, siblings of these individuals were recruited in their stead.
Patients whose families successfully made it through all levels of enrollment were asked to participate in one additional follow-up, in-depth interview approximately 6–12 months after the initial recruiting to assess changes in health status. If the family exited the study at any stage, the reasons for exclusion were recorded for later analysis.
Statistics. Categorical data were assessed by Pearson's Chi-square or Fisher's exact test with 95% confidence intervals (95% CI), while continuous data were assessed by Student's t-test (GraphPad Prism version 7.0 for Mac OS X, GraphPad Software, La Jolla, California, USA). A p-value of ≤0.05 was considered statistically significant.
3. Results
A total of 2,094 families were referred to or contacted the study as potential participants. Of these, 1,613 were pursued long enough to provide a self-identified race and ethnicity. Of this latter group (who form the basis of the present report), 950 (58.9%) were White, 405 (25.1%) were African American and 117 (7.3%) were Hispanic. The remaining 8.7% self-identified either as Native-American (n = 121), Asian/Pacific Islander (n = 14) or other (n = 6), but not enough of these families progressed past phase 1 recruitment to produce a representative sample size.
Considering the final composition of the study, 573 of the 1,613 families, progressed to phase 2 with 326 self-identified as White, 198 as African American, and 49 as Hispanic. In progressing to phase 3 recruitment, 366 families (White n = 202, African American n = 122, and Hispanic n = 42) entered the cohort and subsequently underwent genotyping. The proportion of families that entered the study and completed participation varied by race and ethnicity. The rate of participation for White families was 21.3%, significantly lower than the 30.1% for African American families (OR 0.48, 95% CI 0.32–0.73, p = 0.0004), and 35.9% for Hispanic families (OR 0.63, 95% CI 0.48–0.82, p = 0.0005) (Figure 2A).
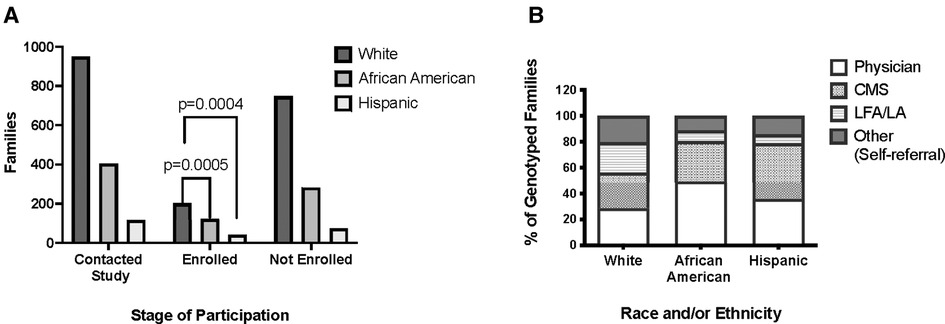
Figure 2. Study participation by race, ethnicity, and referral source. (A) Distribution of families that contacted the study, completed participation, or were excluded from the study by race and ethnicity. (B) Referral sources of families that completed participation in the study and underwent genotyping. White = non-Hispanic White Americans. African-American = non-Hispanic African-Americans. Hispanic = White and Mestizo participants self-described as Hispanic and/or Latino. LFA/LA = Lupus Foundation of American/Lupus Association. CMS = Center for Medicare and Medicaid.
There were also differences between these groups concerning referral source and ultimate enrollment in the cohort. African American families were more likely to make contact with the study through a physician-based source than White (OR 2.70, 95% CI 2.10 to 3.47; p = 1.49 × 10−15) or Hispanic families (OR 1.63, 95% CI 1.06 to 2.51; p = 0.02). Physician referral resulted in higher study completion rates across all ethnicities: 80.3% of African American, 79% of Hispanic, and 56% of White families successfully enrolled. The proportion of White families completing the study that were referred from other sources such as SLE support groups was significantly higher than that of other races or ethnicities (OR 3.22, 95% CI 1.90 to 5.44; p = 4.22 × 10−06 vs. African American families; OR 2.89, 95% CI 1.31 to 6.35; p = 0.007 vs. Hispanic families) (Figure 2B).
We examined the reasons for not pursuing the families that were dropped after Phase1. These included 748 White, 283 African American, and 75 Hispanic families (Figure 3). In contrast to White families, the most common reason African American families were not enrolled was that genetically crucial family members declined to participate. This was the case for 130 (45.9%) of the 283 African American families that did not enter the cohort, compared to 264 (35.2%) of the 748 White families (OR = 1.56, 95% CI 1.18 to 2.06; p = 0.0017). Hispanic families failed to participate for this reason in 38 of 75 (50.7%) families, a rate not significantly different from African Americans but higher than that of Whites (OR = 1.88, 95% CI 1.17 to 3.03; p = 0.009).
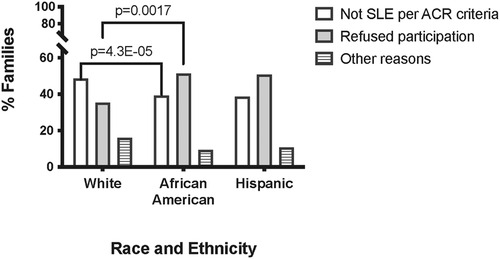
Figure 3. Reasons families did not complete study participation by race and ethnicity. White = non-Hispanic White Americans. African-American = non-Hispanic African-Americans. Hispanic = White and Mestizo participants self-described as Hispanic and/or Latino.
The main reason White families were not enrolled was that we were unable to confirm that the alleged SLE patients met ACR SLE classification criteria. This was the case for 364 (48.7%) White families, in contrast to 99 (35%) African American families (OR 1.76, 95% CI 1.33 to 2.34; p = 4.3 × 10−05). There was no statistically significant difference between White and Hispanic families (38.7%; p = 0.099) or African American and Hispanic families in this regard (p = 0.55) (Figure 3).
4. Discussion
Ensuring adequate representation of African American and Hispanic families in lupus genetics studies is vitally important given their increased incidence and severity of the disease. These results demonstrate that our lupus genetics studies have been successful in reaching African American families with 2 or more SLE patients, with recruitment rates at more than double the 2020 census rate for African Americans (25.1% of cohort vs. 12.4% of national population). This may be due in part to the increased incidence of SLE among African Americans. However, given the prevalence of SLE among African Americans, we appear to be recruiting these families at a lower rate than SLE-affected White families. Conversely, our recruitment of Hispanic SLE families has not been as successful as African American families despite their increased incidence of SLE over Whites: Hispanic recruitment totals only 7.3%, less than half the 2020 census rate of 18.7%. However, in terms of the percentage of families completing participation, the ultimate success is greater with Hispanics than with either African Americans or Whites.
Race as synonymous of biological diversity amongst humans is a flawed concept; what is socially recognized as racial categories encompass genetic ancestry and physical traits, but also culture, language, religion, and identity (20). Human adaptation to selective pressures has influenced genomic diversity (21) and remains an important (if also difficult to define) variable in human health and illness (22). SLE is an example of a disorder with a strong genetic contribution driven by both ancestry-dependent and ancestry-independent risk alleles. Distinguishing the shared vs. ancestry-specific associations is important because an allele identified in one population is likely relevant in others; furthermore, genetic heterogeneity may drive the variability in disease biology and prevalence across populations.
In this study, only White, African American, and Hispanics were frequent enough to analyze. However, our results have important implications for the further study of SLE genetics in American racial and ethnic groups. Namely, recruiting and retaining African Americans into medical research studies is challenging (23, 24). African American families failed to complete participation in the cohort because critical members declined participation. There have been many examples of abuse of human rights in medical research involving African Americans in the United States, of which the most publicized is the Tuskegee Syphilis Study. African Americans are more likely to know of the abuses that occurred in this study than White Americans (25) and their willingness to participate in research is influenced by this knowledge. We find that nearly half of the African American families contributing to our cohort contacted us after a referral from a physician caring for an SLE-affected family member. This was much higher than in the other 2 groups studied. In particular, White families were more likely to come to the study through SLE support and patient organizations. Thus, these results indicate that in order to continue to study the genetics of SLE in African Americans, in whom the disease is more common and severe, we need to enlist the continued support of physicians caring for African American SLE patients.
White families failed to become part of the cohort most often because we could not prove that some of the self-described SLE patients met at least 4 ACR classification criteria to satisfy the definition of the disease (19). Many patients who have incomplete SLE, a few lupus-like symptoms, or a positive ANA, think they have SLE or have been told they have SLE (26). We have recently demonstrated this phenomenon among patients ultimately diagnosed with Sjögren's disease in which a positive ANA was associated with a prior misdiagnosis of SLE (26). Contrary to what we had expected, physician referral was not more efficacious than other sources in referring White families with classifiable SLE.
The third group we studied is Hispanic, which may vary greatly by country of origin and genetic contribution from European, American Indian, or West African ancestors. On the basis of demographic records of our Hispanic families, we found that the majority self-identified as Mexican American (69%, n = 29) or Puerto Rican (19%, n = 8). The remaining five (12%) families self-identified as Honduran (n = 1); Cuban (n = 1); Spanish (n = 1); or of unknown Hispanic origin (n = 2). Hispanic families contributed to the cohort at a significantly higher rate than White or African American families, though the reasons for this were not clear. Level of acculturation influencing Hispanic families' socioeconomic/demographic status (16, 17) may affect knowledge of and/or interest in genetic studies. Bilingual status, which has been employed by other studies as a rough estimate of acculturation, may also influence participation (17). We found that 38.5% of the Hispanic families participating in the final cohort contacted us after a referral from a physician caring for an SLE-affected family member. While we did not directly analyze the effect of our study recruiters who are fluent in Spanish and the use of study and promotional materials in Spanish, we postulate that these are likely important to recruitment success. Similarly, while not as high as the rate among African American families, continued assistance of culturally attuned physicians caring for Hispanic SLE patients will be critical to future recruiting efforts.
The recent social reconning on systemic racism and implicit biases cannot be ignored. Physicians and scientists interpreting a post-racial society as color-blind are neglecting the injustices and inequities that have plagued non-White populations. Acknowledging the harm inflicted by discrimination and racism will be the first step toward nurturing trust in science and modern medicine.
Our findings no doubt reflect the richly varied backgrounds and cultural differences found across the racially diverse population of the United States. It must be recognized that racial and ethnic groups are heterogeneous and dynamic; thus, the interest in participation in genetic studies and biomedical research is reflective of each group's unique history and will require tailored cultural accommodations. Recognizing and understanding these differences is crucial to the continued recruitment of these groups into genetic studies with the ultimate goal of improved shared and ancestry-specific personalized medicine and reduction in disparities.
Significance and innovation
• Genetic studies, in particular those using trans-ancestral mapping, require participants of different racial and ethnic backgrounds. Identifying barriers to the participation of specific groups of people is critical to improving representation in research
• Participation of African American multiplex families with lupus in research is improved when they are referred by trusted physicians but it is difficult to engage extended family members due to health disparities and historical injustices
• Hispanic families are likelier to complete participation than other groups, in particular when provided with culturally appropriate recruiting materials or if they have high levels of assimilation
• Amongst non-Hispanic White families, misdiagnosis or incomplete data of extended family members prevents study completion
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Oklahoma Medical Research Foundation. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
RS, JH, AR—conceived the study, CC, TA, AR—data acquisition, AS, RHS -data analysis, RHS—writing first draft, revising the manuscript—RS, AS, JH. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Institutes of Health, mainly from the National Institute of Arthritis, Musculoskeletal and Skin Diseases [N01AR62277 and P30AR073750] with important contributions from [R0124717, R01AR42460, P20RR020743, R01AR053734, P01AR049084, P20AR046669, RC1AR058554, R01AR043274, AR053483, AI1082714, and U54GM104938] and salary support from the US Department of Veterans Affairs for Dr. Scofield and Dr. Harley.
Acknowledgments
The authors thank additional contributors to the LFRR effort: Gail Bruner, Sharon Johnson, Wes Daniel, Dominique Williams, Sarah Cioli, Anya Grether, Teresa Aberle, Joanne Tesiram, Tiny Powe, Jeanne Morrisey, Summer Frank, Amy Butler, Barbara Leatherwood, Patti Grounds, Jacey Bush, Adriana Rojas-Villarraga, Lina Amezquita, Ryan Parker, Brandon Scharrer, Tamiko Cabatic, Lauren Evans, Michelle Calvo, Nicole Weber, Kay Davis, Sarah Dawson, Kurt Downing, and Neeraj Asundi. We thank the patients and all the other referring health care providers.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Petri M. Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. (2002) 16(5):847–58. doi: 10.1053/berh.2002.0259
2. Fernandez M, Alarcon GS, Calvo-Alen J, Andrade R, McGwin G Jr., Vila LM, et al. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum. (2007) 57(4):576–84. doi: 10.1002/art.22672
3. Bae SC, Fraser P, Liang MH. The epidemiology of systemic lupus erythematosus in populations of African ancestry: a critical review of the “prevalence gradient hypothesis”. Arthritis Rheum. (1998) 41(12):2091–9. doi: 10.1002/1529-0131(199812)41:12%3C2091::AID-ART2%3E3.0.CO;2-D
4. Alarcon GS, Roseman J, Bartolucci AA, Friedman AW, Moulds JM, Goel N, et al. Systemic lupus erythematosus in three ethnic groups: iI. Features predictive of disease activity early in its course. LUMINA study group. Lupus in minority populations, nature versus nurture. Arthritis Rheum. (1998) 41(7):1173–80. doi: 10.1002/1529-0131(199807)41:7%3C1173::AID-ART5%3E3.0.CO;2-A
5. Quintero-Del-Rio AI, Bacino D, Kelly J, Aberle T, Harley JB. Familial systemic lupus erythematosus: a comparison of clinical manifestations and antibody presentation in three ethnic groups. Cell Mol Biol (Noisy-le-Grand). (2001) 47(7):1223–7.11838971
6. Niewold TB. Advances in lupus genetics. Curr Opin Rheumatol. (2015) 27(5):440–7. doi: 10.1097/BOR.0000000000000205
7. Sanchez E, Rasmussen A, Riba L, Acevedo-Vasquez E, Kelly JA, Langefeld CD, et al. Impact of genetic ancestry and sociodemographic status on the clinical expression of systemic lupus erythematosus in American Indian-European populations. Arthritis Rheum. (2012) 64(11):3687–94. doi: 10.1002/art.34650
8. Alarcon-Riquelme ME, Ziegler JT, Molineros J, Howard TD, Moreno-Estrada A, Sanchez-Rodriguez E, et al. Genome-Wide association study in an amerindian ancestry population reveals novel systemic lupus erythematosus risk loci and the role of European admixture. Arthritis & Rheumatology. (2016) 68(4):932–43. doi: 10.1002/art.39504
9. Peschken CA. Health disparities in systemic lupus erythematosus. Rheum Dis Clin North Am. (2020) 46(4):673–83. doi: 10.1016/j.rdc.2020.07.010
10. Gonzalez LA, Ugarte-Gil MF, Pons-Estel GJ, Duran-Barragan S, Toloza S, Burgos PI, et al. Addressing health disparities as a function of ethnicity in systemic lupus erythematosus patients. Lupus. (2022) 31(14):1691–705. doi: 10.1177/09612033221122983
11. Bentley AR, Callier S, Rotimi CN. Diversity and inclusion in genomic research: why the uneven progress? J Community Genet. (2017) 8(4):255–66. doi: 10.1007/s12687-017-0316-6
12. White RM. Sociocultural issues in clinical research: unraveling the tuskegee syphilis study. Arthritis Rheum. (2002) 47(4):457–8. doi: 10.1002/art.10516
13. Schulz A, Caldwell C, Foster S. What are they going to do with the information?” latino/Latina and African American perspectives on the human genome project. Health Educ Behav. (2003) 30(2):151–69. doi: 10.1177/1090198102251026
14. Fisher ER, Pratt R, Esch R, Kocher M, Wilson K, Lee W, et al. The role of race and ethnicity in views toward and participation in genetic studies and precision medicine research in the United States: a systematic review of qualitative and quantitative studies. Mol Genet Genomic Med. (2020) 8(2):e1099. doi: 10.1002/mgg3.1099
15. Scherr CL, Ramesh S, Marshall-Fricker C, Perera MA. A review of African Americans’ beliefs and attitudes about genomic studies: opportunities for message design. Front Genet. (2019) 10:548. doi: 10.3389/fgene.2019.00548
16. Alarcon GS, McGwin G Jr., Bastian HM, Roseman J, Lisse J, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. predictors of early mortality in the LUMINA cohort. LUMINA study group. Arthritis Rheum. (2001) 45(2):191–202. doi: 10.1002/1529-0131(200104)45:2%3C191::AID-ANR173%3E3.0.CO;2-2
17. Alarcon GS, Rodriguez JL, Benavides G Jr., Brooks K, Kurusz H, Reveille JD. Systemic lupus erythematosus in three ethnic groups. V. Acculturation, health-related attitudes and behaviors, and disease activity in hispanic patients from the LUMINA cohort. LUMINA study group. Lupus in minority populations, nature versus nurture. Arthritis Care Res. (1999) 12(4):267–76. doi: 10.1002/1529-0131(199908)12:4%3C267::AID-ART5%3E3.0.CO;2-9
18. Rasmussen A, Sevier S, Kelly JA, Glenn SB, Aberle T, Cooney CM, et al. The lupus family registry and repository. Rheumatology (Oxford, England). (2011) 50(1):47–59. doi: 10.1093/rheumatology/keq302
19. Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. (1997) 40(9):1725. doi: 10.1002/art.1780400928
20. Deyrup A, Graves JL Jr. Racial biology and medical misconceptions. N Engl J Med. (2022) 386(6):501–3. doi: 10.1056/NEJMp2116224
21. Fan S, Hansen ME, Lo Y, Tishkoff SA. Going global by adapting local: a review of recent human adaptation. Science. (2016) 354(6308):54–9. doi: 10.1126/science.aaf5098
22. Witzig R. The medicalization of race: scientific legitimization of a flawed social construct. Ann Intern Med. (1996) 125(8):675–9. doi: 10.7326/0003-4819-125-8-199610150-00008
23. Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. (1999) 14(9):537–46. doi: 10.1046/j.1525-1497.1999.07048.x
24. Shavers VL, Klein WM, Fagan P. Research on race/ethnicity and health care discrimination: where we are and where we need to go. Am J Public Health. (2012) 102(5):930–2. doi: 10.2105/AJPH.2012.300708
25. Shavers VL, Lynch CF, Burmeister LF. Knowledge of the tuskegee study and its impact on the willingness to participate in medical research studies. J Natl Med Assoc. (2000) 92(12):563–72.11202759
Keywords: systemic lupus erythematosus, genetics, familial, recruitment, ethnicity, race
Citation: Scofield R Hal, Sharma R, Aberle T, Cooney Carisa M, Kelly Jennifer A, Harley John B and Rasmussen A (2023) Impact of race and ethnicity on family participation in systemic lupus erythematosus genetic studies. Front. Lupus 1:1100534. doi: 10.3389/flupu.2023.1100534
Received: 16 November 2022; Accepted: 20 March 2023;
Published: 17 April 2023.
Edited by:
Luís Pedro Sousa Inês, Coimbra Hospital and University Center, PortugalReviewed by:
Paula Ramos, Medical University of South Carolina, United StatesRobert George Lahita, Rutgers University, United States
© 2023 Scofield, Sharma, Aberle, Cooney, Kelly, Harley and Rasmussen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R Hal Scofield aGFsLXNjb2ZpZWxkQG9tcmYub3Voc2MuZWR1 Astrid Rasmussen YXN0cmlkLXJhc211c3NlbkBvbXJmLm9yZw==
Specialty Section: This article was submitted to Clinical Research and Treatment in Lupus, a section of the journal Frontiers in Lupus
 R Hal Scofield
R Hal Scofield Rohan Sharma4
Rohan Sharma4 Teresa Aberle
Teresa Aberle Jennifer A Kelly
Jennifer A Kelly John B Harley
John B Harley Astrid Rasmussen
Astrid Rasmussen