
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 04 April 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1124367
This article is part of the Research TopicAdvances in Diabetic Kidney Disease: Pathophysiology, Clinical Characteristics, and Future DirectionsView all 10 articles
 Tadesse Asmamaw Dejenie1*
Tadesse Asmamaw Dejenie1* Endeshaw Chekol Abebe2
Endeshaw Chekol Abebe2 Misganaw Asmamaw Mengstie2
Misganaw Asmamaw Mengstie2 Mohammed Abdu Seid3
Mohammed Abdu Seid3 Natnael Atnafu Gebeyehu4
Natnael Atnafu Gebeyehu4 Getachew Asmare Adella5
Getachew Asmare Adella5 Gizchew Ambaw Kassie6
Gizchew Ambaw Kassie6 Amanuel Yosef Gebrekidan7
Amanuel Yosef Gebrekidan7 Molalegn Mesele Gesese4
Molalegn Mesele Gesese4 Kirubel Dagnaw Tegegne8
Kirubel Dagnaw Tegegne8 Denekew Tenaw Anley9
Denekew Tenaw Anley9 Sefineh Fenta Feleke10
Sefineh Fenta Feleke10 Melkamu Aderajew Zemene9
Melkamu Aderajew Zemene9 Anteneh Mengist Dessie9
Anteneh Mengist Dessie9 Natnael Moges11
Natnael Moges11 Yenealem Solomon Kebede12
Yenealem Solomon Kebede12 Berihun Bantie13
Berihun Bantie13 Dagnew Getnet Adugna14
Dagnew Getnet Adugna14Background: Diabetic nephropathy is a leading cause of end-stage renal disease. The diagnostic markers of nephropathy, including the presence of albuminuria and/or a reduced estimated glomerular filtration rate, are not clinically ideal, and most of them are raised after a significant reduction in renal function. Therefore, it is crucial to seek more sensitive and non-invasive biomarkers for the diagnosis of diabetic nephropathy.
Objective of the study: This study aimed to investigate the serum cystatin C levels and dyslipidemia for the detection of diabetic nephropathy in patients with type 2 diabetes mellitus.
Methodology: A hospital-based comparative cross-sectional study was conducted from December 2021 to August 2022 in Tikur, Anbessa specialized teaching hospital with a sample size of 140 patients with type2 diabetes mellitus. Socio-demographic data was collected using a structured questionnaire, and 5 mL of blood was collected from each participant following overnight fasting for biochemical analyses.
Results: In type 2 diabetes patients with nephropathy, we found significant lipoprotein abnormalities and an increase in serum cystatin C (P < 0.001) compared to those without nephropathy. Serum cystatin C, systolic blood pressure, fasting blood glucose, total cholesterol, triglyceride, low density lipoprotein, very low-density lipoprotein, high density lipoprotein, and duration of diabetes were identified as being significantly associated with diabetic nephropathy (P < 0.05) in multivariable logistic regression analysis. The mean values of total cholesterol levels, triglyceride levels, and high-density lipoprotein cholesterol levels were also found to be significantly higher (P < 0.05) in females as compared to male type-2 diabetic patients. The fasting blood glucose levels and lipid profiles of the participants were found to be significantly associated with serum cystatin C levels.
Conclusion: The present study found significant serum cystatin C and lipoprotein abnormalities in T2DM patients with diabetic nephropathy when compared with those without diabetic nephropathy, and these lipoprotein abnormalities were significantly associated with serum cystatin C levels.
Diabetes mellitus (DM) is a common metabolic disorder resulting from insufficient insulin secretion or insulin action that leads to elevated blood glucose levels (1). The metabolic disorders associated with DM cause secondary pathophysiological changes in multiple organ systems caused by acute and chronic complications (2). Diabetic nephropathy is one of the major chronic microvascular complications of diabetic and a leading cause of end-stage renal disease (ESRD) (3), and it is predominantly due to type 2 diabetes mellitus (T2DM) (4). Hyperglycemia is the main driving force behind the development of chronic complications of diabetic, including DKD (5). There are hypotheses that have been widely supported by scholars about how hyperglycemia causes diabetic complications (including DN), which are the polyol pathway, the hexosamine pathway (HBP), the production of AGEs, and PKC. It stimulates the resident and non-resident renal cells to produce humoral mediators and cytokines that can lead to functional and phenotypic changes in renal cells and tissues, interference with cell growth, interacting proteins, advanced glycation end products (AGEs), etc., ultimately resulting in glomerular and tubular damage and the onset of kidney disease. Therefore, poor blood glucose control is a particularly important risk factor for the development of DN.
T2DM Patients with diabetic nephropathy are often associated with abnormal lipid metabolism and lipid deposition (6). This altered lipid profile is termed “diabetic dyslipidemia,” which includes high plasma levels of total cholesterol, very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and triglycerides (TG) and a lower concentration of high-density lipoprotein (HDL). Several studies suggest that diabetic dyslipidemia plays a major role in the pathogenetic development of diabetic nephropathy in T2DM patients (7, 8). However, this has not been well studied in most of the sub-Saharan African countries, including Ethiopia.
The diagnostic markers of nephropathy, including the presence of albuminuria and/or a reduced estimated glomerular filtration rate (eGFR), are not clinically ideal, and most of them are raised after a significant reduction in renal function (9, 10). Kidney biopsy is the gold standard for differentiating diabetic nephropathy from non-diabetic nephropathy (11), but it is invasive and not suitable for routine clinical practice. Therefore, it is crucial to investigate the pathogenesis and seek more sensitive and non-invasive biomarkers for the diagnosis of diabetic nephropathy.
Human cystatin C is a member of the cystatin superfamily (inhibitors of cysteine proteinases) and a nonglycosylated basic protein with a low molecular mass (13 kD) that is freely filtered by the glomerulus and produced by all nucleated cells (12, 13). Studies have reported that unlike serum creatinine, serum cystatin C is not influenced by age, sex, muscle mass, or dietary intake (14, 15). It is currently under investigation as a promising marker and a replacement for serum creatinine and other renal markers for the early detection of renal impairment (16). Recent studies showed that cystatin C is a more sensitive serum marker for renal function assessment compared to serum creatinine (15, 17). Increased cystatin C and disorders of lipid metabolism are associated with diabetic nephropathy in T2DM patients (18). Therefore, this study aimed to explore the clinical significance of serum cystatin C and lipid profile abnormalities for the differential diagnosis of diabetic nephropathy and non-diabetic nephropathy in patients with T2DM.
A hospital-based comparative cross-sectional study was conducted from December 2021 to August 2022 in Tikur, Anbessa specialized teaching hospital, Addis Ababa, Ethiopia, with the aim of evaluating the serum cystatin C and lipid profiles as markers of diabetic nephropathy in known type 2 diabetic patients who were on regular follow-up at the diabetic clinic of Tikur, Anbessa specialized hospital. A total of 140 eligible T2DM patients who had follow-ups at the diabetic center of Tikur Anbesa specialized hospital during the study period were recruited for this study. We classified the T2DM participants into those with and without nephropathy. The first group included a total of 83 T2DM patients without nephropathy, and the second group included 57 T2DM patients with nephropathy diagnosed by physicians. We excluded patients who had any of the other complications of diabetic, medical or surgical problems, were pregnant women, or had taken lipid-lowering drugs from the study.
A structured questionnaire was used to collect socio-demographic data, including age, sex, address, history, and duration of diabetes. Anthropometric measurements such as weight, height, body mass index (BMI), and blood pressure (BP) were determined using appropriate standardized devices. The height and weight of the participants were measured following standard protocols. Then, BMI was calculated via weight in kilograms divided by height in meters squared (BMI = Kg/m2). Furthermore, blood pressure was measured using a digital automatic BP monitor apparatus for three consecutive times (five minutes apart), and the average values for both diastolic and systolic BP were considered for this study.
Maintaining all aseptic precautions, 5 mL of blood was collected following overnight fasting from the ante-capital vein of each volunteer participant by two trained laboratory technicians for biochemical analyses. The blood sample was collected using an appropriate test tube and transferred to a clean, dry serum separator tube. Then it was centrifuged at 3000 revolutions per minute (rpm) at room temperature (20 to 25°C). A pure serum sample was separated and placed in the nunc tube, where it was stored at -20 until biochemical analysis was done.
Fasting blood glucose (FBG) level, serum TC, HDL cholesterol, and TG levels were determined using Dimension EXL 200 System chemistry analyzer through enzymatic method from the serum sample using commercially available kit of auto analyzer. Serum creatinine and blood urea nitrogen (BUN) were also estimated by the enzymatic method. LDL cholesterol and VLDL cholesterol concentrations were calculated using the Friedewald formula (19). The cystatin C levels of serum were measured by a turbidimetric immunoassay test. All biochemical investigations were done on a fully automated analyzer at the National Reference Laboratory for Clinical Chemistry, Ethiopian Public Health Institute (EPHI).
Data was entered and analyzed using SPSS version 25. Categorical variables were presented in frequency and percentages. Continuous variables including demographics, clinical and biomedical data were expressed as mean ± standard deviation. An independent sample t-test or Mann–Whitney U test was used for the comparison of variables as appropriate. An adjusted logistic regression and multiple linear regression models were employed to examine the association of predictor variables with diabetic nephropathy. All statistical tests were two-tailed, and p-values <0.05 at a 95% confidence interval (CI), were taken as statistically significant.
A total of 140 T2DM patients were recruited for this study (83 without diabetic nephropathy and 53 with diabetic nephropathy). The mean age of all patients was 45.2 ± 13.2 years. From all the subjects involved in the study, 74 (52.9%) were males and the rest 66 (47.1%) were females. Majority of T2DM patients 116(82.9%) were living in urban areas. In all patients, the mean ± SD of BMI was 26.3 ± 3.7 kg/m2. The mean ± SD of the duration of patients with T2DM was 13.3 ± 8.6 years with the maximum and minimum duration of 36 years and 1year respectively. In T2DM patients with diabetic nephropathy, we found that the TG, TC, LDL, and VLDL cholesterol levels were significantly increased, whereas HDL cholesterol levels were significantly lower (P < 0.001) in those patients. The detailed basic characteristics of the study participants are depicted in Table 1.
Out of 140 T2DM patients, 85 (60.7%) used oral medication, 25 (17.86%) used insulin injection, and 30 (21.4%) used both oral medication and insulin injection. After adjusting for all possible confounders using multivariable logistic regression analysis serum cystatin C (P <0.001), SBP (P = 0.02), FBG (P =0.001), TC (P <0.01), TG (P <0.001), LDL (P <0.001), VLDL (P <0.001), HDL (P<0.061) and duration of T2DM (P =0.04) were identified to be significantly associated with diabetic nephropathy. Increasing serum cystatin C levels by one unit was associated with 1.41 times (AOR: 1.41, 95% CI: 1.09, 2.77) higher odds of having diabetic nephropathy. For a unit increase in the duration of T2DM, there was a 1.32 times (AOR: 1.32, 95% CI: 1.11, 2.87) greater likelihood of having diabetic nephropathy. The odds of T2DM patients with nephropathy significantly increased per a unit rise in SBP, FBG, TC, TG, LDL, and VLDL levels (Table 2).
An independent sample t-test showed that the mean values of TC (P = 0.021), TG (P = 0.011), and HDL cholesterol levels (P = 0.01) were significantly higher in females as compared to male type-2 diabetic patients, but significantly lower in serum creatinine levels (P = 0.03) as compared to males (Table 3).
In the multiple linear regression analyses, the SBP, FPG, TC, TG, HDL, LDL, and VLDL of the T2DM participants were significantly associated with serum cystatin C levels (Table 4).
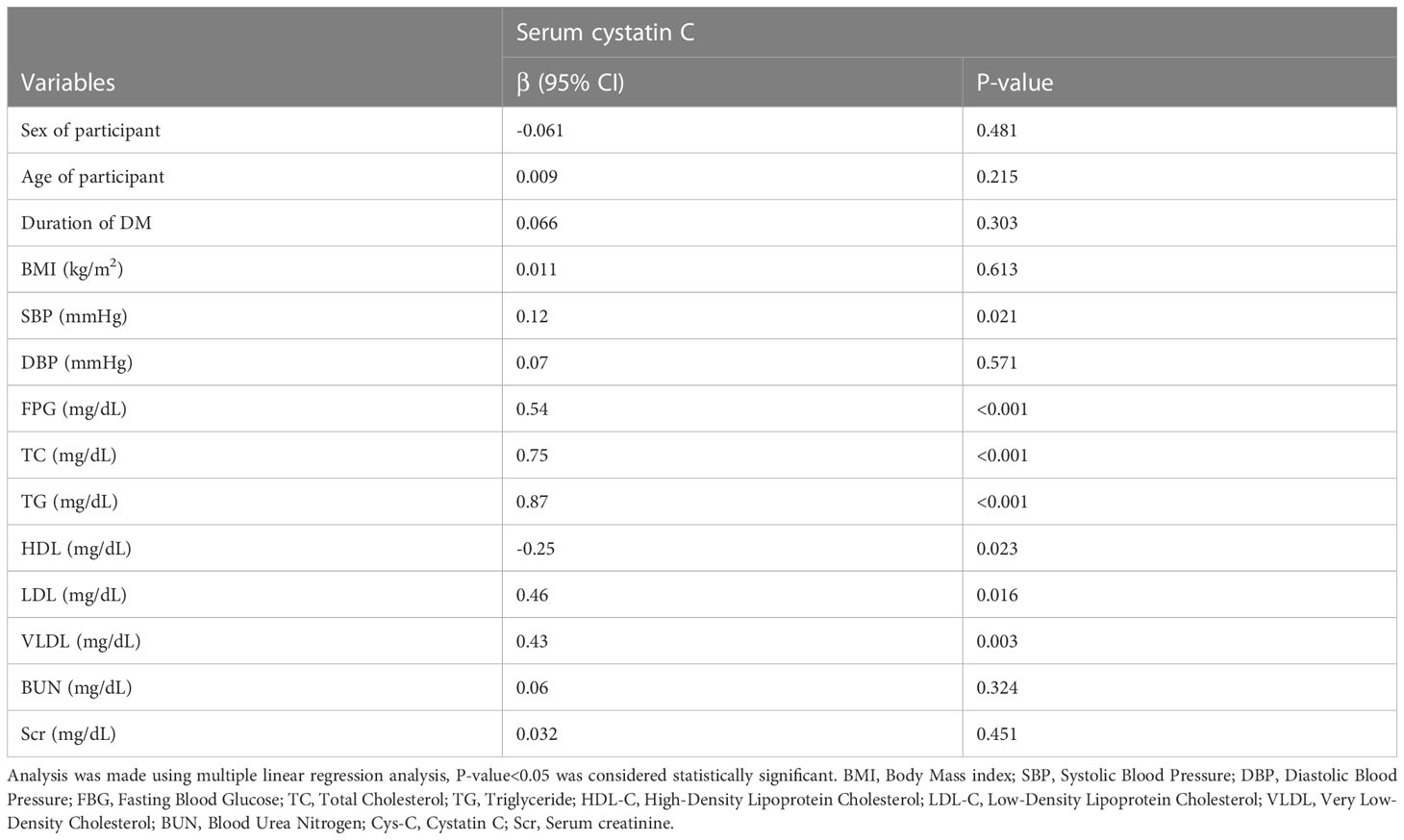
Table 4 Multiple linear regression analysis of serum cystatin C with clinical and biomedical parameters in type 2 diabetic patients.
This study aimed to evaluate serum cystatin C and lipid profiles for assessing the potential predictor markers of diabetic nephropathy in patients with T2DM. High serum creatinine and low cytostatin C levels were found in males, whereas low creatinine and high cystatin C levels were found in females. It is well known that the creatinine level is highly affected by muscle mass, which means that males are more muscular than females. But the cystatin C difference needs further study as it is a promising marker currently under investigation.
The study found significantly higher serum cystatin C levels and SBP and lipid profile abnormalities in type 2 diabetics with nephropathy compared to those without nephropathy. These findings are similar to other previous studies (18, 20). In contrast to these findings, the study conducted by Jing Wei et al. revealed that the serum parameters, including HDL and LDL, were not significantly different between T2DM patients with nephropathy and those without diabetic nephropathy (21). However, in the case of serum cystatin C levels, TC, and TG, this study agreed with the findings of the present study, and unlike our finding, it found a significant difference in serum creatinine in type 2 diabetics with nephropathy compared to those without nephropathy.
There is growing evidence that abnormalities in lipid metabolism contribute to renal disease progression (6). In T2DM patients with diabetic nephropathy, we found that HDL cholesterol levels were significantly low in T2DM patient with nephropathy and other parameters of lipoproteins were significantly higher. Numerous experiments studies explained the mechanism as the hyperlipidemia can cause glomerulosclerosis and tubulointerstitial sclerosis by stimulating resident renal cells to produce fibrotic cytokines and chemokines, leading to infiltration of monocytes or macrophages into glomerular tissue to promote extracellular matrix deposition and have shown that lipid accumulation, lipid toxicity and lipid metabolism disorders are related to diabetic kidney damage (6, 22).
Several studies found that the levels of serum cystatin C rose earlier than creatinine (22, 23). In this study we found that the cystatin C level of the type 2 DM is increased significantly and associated with lipoproteins in T2DM patients diagnosed nephropathy. This showed that the serum cystatin C level is related to subclinical tubular impairment and can be an earlier marker of renal disease in diabetic patients before the onset of other renal markers. This is in agreement with some other earlier studies (23, 24).
In the multivariate regression analysis, we found that the serum cystatin C level is significantly associated with FBG, TC, TG, HDL, LDL, and VLDL (P < 0.001). However, it is insignificantly associated with serum creatinine, duration of DM, BMI, BUN, and the age of the participant. This was in line with study conducted by Kachhawa et al. (18).
This study also revealed the mean values of TC, and TG were significantly higher (p < 0.05) in females as compared to male type-2 diabetic patients, but low in HDL and serum creatinine levels as compared to males. Another study conducted by Radzeviciene et al., support these results on type one diabetic patients (19, 25).
We found that serum cystatin C is not significantly affected by sex, unlike serum creatinine, and a significant difference was found between T2DM patients with nephropathy and those without nephropathy. The present study also found significant lipoprotein abnormalities in T2DM patients with diabetic nephropathy when compared with those without diabetic nephropathy, and these lipoprotein abnormalities were significantly associated with serum cystatin C levels.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Addis Ababa University Department of Ethics and Research Committee (DRERC). The patients/participants provided their written informed consent to participate in this study.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, data acquisition, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing for important intellectual content. All authors have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
We would like to express our great gratitude to AAU and EPHI for their material and equipment support for the research work. It is our pleasure to express our heartfelt gratitude to the doctors and nurses who collaborated on data collection and blood sample collection at the TASH Internal Medicine Department’s diabetic clinic. Without their support, it would have been difficult to find all these participants for the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DM, Diabetes mellitus, T2DM, type 2 diabetes mellitus; ESRD, end-stage renal disease, very VLDL, low-density lipoprotein; LDL, low-density lipoprotein, TG, triglycerides; HDL, high-density lipoprotein, TC, total cholesterol, eGFR, estimated glomerular filtration rate; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; rmp, revolutions per minute; FBG, fasting blood glucose; BUN, blood urea nitrogen; EPHI, Ethiopian Public Health Institute; SPSS, Statistical Package for Social Sciences, CI, confidence interval; DRERC, Department of Ethics and Research Committee; Cys-C, Cystatin C; Scr; Serum creatinine, AOR, adjusted odds ratio; SD, standard deviation.
1. Asmamaw T, Genet S, Menon M, Tarekegn G, Chekol E, Geto Z, et al. Early detection of renal impairment among patients with type 2 diabetes mellitus through evaluation of serum cystatin c in comparison with serum creatinine levels: A cross-sectional study. Diabetes Metab Syndrome Obesity: Targets Ther (2020) 13:4727–35. doi: 10.2147/DMSO.S279949
2. Zhou B, Zou H, Xu G. Clinical utility of serum cystatin c in predicting diabetic nephropathy among patients with diabetes mellitus: a meta-analysis. Kidney Blood Pressure Res (2016) 41(6):919–28. doi: 10.1159/000452593
3. Zhang XX, Kong J, Yun K. Prevalence of diabetic nephropathy among patients with type 2 diabetes mellitus in China: A meta-analysis of observational studies. J Diabetes Res (2020) 2020:2315607. doi: 10.1155/2020/2315607
4. Shahwan MJ, Gacem SA, Zaidi SK. Prevalence of diabetic nephropathy and associated risk factors among type 2 diabetes mellitus patients in ramallah, Palestine. Diabetes Metab Syndrome (2019) 13(2):1491–6. doi: 10.1016/j.dsx.2019.02.017
5. Amorim RG, Guedes GDS, Vasconcelos SML, Santos JCF. Kidney disease in diabetes mellitus: Cross-linking between hyperglycemia, redox imbalance and inflammation. Arq Bras Cardiol (2019) 112(5):577–87. doi: 10.5935/abc.20190077
6. Chen SC, Tseng CH. Dyslipidemia, kidney disease, and cardiovascular disease in diabetic patients. Rev Diabetic Studies: RDS (2013) 10(2-3):88–100. doi: 10.1900/RDS.2013.10.88
7. Hager MR, Narla AD, Tannock LR. Dyslipidemia in patients with chronic kidney disease. Rev Endocrine Metab Disord (2017) 18(1):29–40. doi: 10.1007/s11154-016-9402-z
8. Opazo-Ríos L, Mas S, Marín-Royo G, Mezzano S, Gómez-Guerrero C, Moreno JA, et al. Lipotoxicity and diabetic nephropathy: Novel mechanistic insights and therapeutic opportunities. Int J Mol Sci (2020) 21(7):2632. doi: 10.3390/ijms21072632
9. Thipsawat S. Early detection of diabetic nephropathy in patient with type 2 diabetes mellitus: A review of the literature. Diabetes Vasc Dis Res (2021) 18(6):14791641211058856. doi: 10.1177/14791641211058856
10. Pugliese G, Penno G, Natali A, Barutta F, Di Paolo S, Reboldi G, et al. Diabetic kidney disease: new clinical and therapeutic issues. joint position statement of the Italian diabetes society and the Italian society of nephrology on “The natural history of diabetic kidney disease and treatment of hyperglycemia in patients with type 2 diabetes and impaired renal function”. J Nephrol (2020) 33(1):9–35. doi: 10.1007/s40620-019-00650-x
11. Zhang W, Liu X, Dong Z, Wang Q, Pei Z, Chen Y, et al. New diagnostic model for the differentiation of diabetic nephropathy from non-diabetic nephropathy in Chinese patients. Front Endocrinol (2022) 13:913021. doi: 10.3389/fendo.2022.913021
12. Sapkota S, Khatiwada S, Shrestha S, Baral N, Maskey R, Majhi S, et al. Diagnostic accuracy of serum cystatin c for early recognition of nephropathy in type 2 diabetes mellitus. Int J Nephrol (2021) 2021. doi: 10.1155/2021/8884126
13. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin c. New Engl J Med (2012) 367(1):20–9. doi: 10.1056/NEJMoa1114248
14. Tangri N, Stevens LA, Schmid CH, Zhang YL, Beck GJ, Greene T, et al. Changes in dietary protein intake has no effect on serum cystatin c levels independent of the glomerular filtration rate. Kidney Int (2011) 79(4):471–7. doi: 10.1038/ki.2010.431
15. Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin c. Clin J Am Soc Nephrol (2008) 3(2):348–54. doi: 10.2215/CJN.02870707
16. Arceo ES, Dizon GA, Tiongco REG. Serum cystatin c as an early marker of nephropathy among type 2 diabetics: A meta-analysis. Diabetes Metab Syndrome (2019) 13(6):3093–7. doi: 10.1016/j.dsx.2019.11.007
17. Amer AH, Haridas N. Early diagnostic markers in diabetic nephropathy patients. J Clin Diagn Res (2018) 12(11). doi: 10.7860/JCDR/2018/36574.12203
18. Knopfholz J, Disserol CC, Pierin AJ, Schirr FL, Streisky L, Takito LL, et al. Validation of the friedewald formula in patients with metabolic syndrome. Cholesterol (2014) 2014:261878. doi: 10.1155/2014/261878
19. Stankute I, Radzeviciene L, Monstaviciene A, Dobrovolskiene R, Danyte E, Verkauskiene R. Serum cystatin C as a biomarker for early diabetic kidney disease and dyslipidemia in young type 1 diabetes patients. Medicine (Kaunas, Lithuania) (2022) 58(2):218 doi: 10.3390/medicina58020218
20. Wei J, Wang B, Shen FJ, Zhang TT, Duan Z, Zhou DM. Diagnostic value of triglyceride and cystatin c ratio in diabetic kidney disease: a retrospective and prospective cohort study based on renal biopsy. BMC Nephrol (2022) 23(1):270. doi: 10.1186/s12882-022-02888-3
21. Su W, Cao R, He YC, Guan YF, Ruan XZ. Crosstalk of hyperglycemia and dyslipidemia in diabetic kidney disease. Kidney Dis (2017) 3(4):171–80. doi: 10.1159/000479874
22. Murty MS, Sharma UK, Pandey VB, Kankare SB. Serum cystatin c as a marker of renal function in detection of early acute kidney injury. Indian J Nephrol (2013) 23(3):180–3. doi: 10.4103/0971-4065.111840
23. Woo KS, Choi JL, Kim BR, Kim JE, Han JY. Clinical usefulness of serum cystatin c as a marker of renal function. Diabetes Metab J (2014) 38(4):278–84. doi: 10.4093/dmj.2014.38.4.278
24. Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes: a review of early natural history, pathogenesis, and diagnosis. Diabetes/metabol Res Rev (2017) 33(2). doi: 10.1002/dmrr.2841
25. Nayak BS, Butcher DM, Bujhawan S, Chang D, Chang S, Cabral-Samaroo D, et al. Association of low serum creatinine, abnormal lipid profile, gender, age and ethnicity with type 2 diabetes mellitus in Trinidad and Tobago. Diabetes Res Clin Pract (2011) 91(3):342–7. doi: 10.1016/j.diabres.2010.12.017
Keywords: diabetes, diabetic nephropathy, serum cystatin C, serum creatinine, dyslipidemia
Citation: Dejenie TA, Abebe EC, Mengstie MA, Seid MA, Gebeyehu NA, Adella GA, Kassie GA, Gebrekidan AY, Gesese MM, Tegegne KD, Anley DT, Feleke SF, Zemene MA, Dessie AM, Moges N, Kebede YS, Bantie B and Adugna DG (2023) Dyslipidemia and serum cystatin C levels as biomarker of diabetic nephropathy in patients with type 2 diabetes mellitus. Front. Endocrinol. 14:1124367. doi: 10.3389/fendo.2023.1124367
Received: 15 December 2022; Accepted: 22 March 2023;
Published: 04 April 2023.
Edited by:
Saleem Aladaileh, University of Hafr Al Batin, Saudi ArabiaReviewed by:
Belayhun Temesgen, Hawassa University, EthiopiaCopyright © 2023 Dejenie, Abebe, Mengstie, Seid, Gebeyehu, Adella, Kassie, Gebrekidan, Gesese, Tegegne, Anley, Feleke, Zemene, Dessie, Moges, Kebede, Bantie and Adugna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tadesse Asmamaw Dejenie, as24tadesse@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.