EDITORIAL
Published on 12 Sep 2024
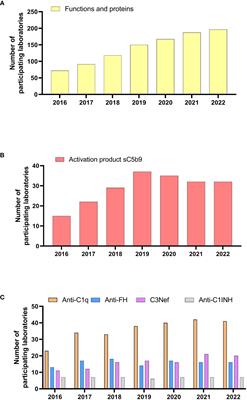
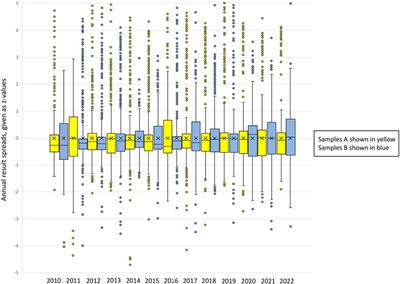
Editorial: Lessons from external quality control in laboratory medicine: important implications for public health!
doi 10.3389/fmolb.2024.1485193
- 415 views
3,555
Total downloads
16k
Total views and downloads
Select the journal/section where you want your idea to be submitted:
EDITORIAL
Published on 12 Sep 2024
HYPOTHESIS AND THEORY
Published on 11 Sep 2024
ORIGINAL RESEARCH
Published on 08 Aug 2024
OPINION
Published on 01 Aug 2024
ORIGINAL RESEARCH
Published on 11 Jul 2024
ORIGINAL RESEARCH
Published on 21 Jun 2024
ORIGINAL RESEARCH
Published on 20 Jun 2024
ORIGINAL RESEARCH
Published on 17 May 2024
ORIGINAL RESEARCH
Published on 01 May 2024
ORIGINAL RESEARCH
Published on 27 Mar 2024
ORIGINAL RESEARCH
Published on 26 Mar 2024
ORIGINAL RESEARCH
Published on 20 Mar 2024

Frontiers in Immunology