ORIGINAL RESEARCH
Published on 30 Jun 2022
Estrogen Receptor and Claudin-6 Might Play Vital Roles for Long-Term Prognosis in Patients With Luminal A Breast Cancer Who Underwent Neoadjuvant Chemotherapy

doi 10.3389/fonc.2022.630065
- 919 views
71k
Total downloads
249k
Total views and downloads
ORIGINAL RESEARCH
Published on 30 Jun 2022

SYSTEMATIC REVIEW
Published on 04 Apr 2022
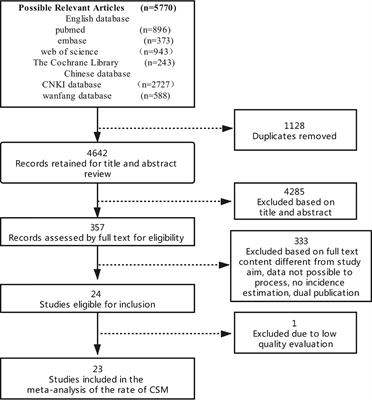
ORIGINAL RESEARCH
Published on 04 Apr 2022

ORIGINAL RESEARCH
Published on 25 Feb 2022
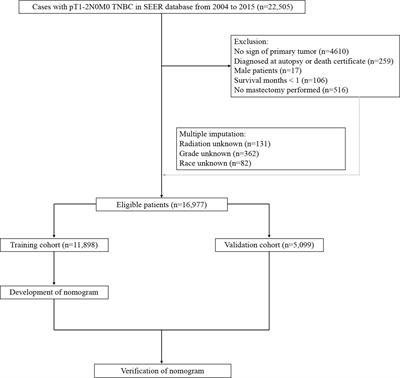
ORIGINAL RESEARCH
Published on 03 Jan 2022

ORIGINAL RESEARCH
Published on 06 Dec 2021

ORIGINAL RESEARCH
Published on 01 Dec 2021

SYSTEMATIC REVIEW
Published on 26 Nov 2021

ORIGINAL RESEARCH
Published on 09 Nov 2021

ORIGINAL RESEARCH
Published on 03 Nov 2021

ORIGINAL RESEARCH
Published on 03 Nov 2021

ORIGINAL RESEARCH
Published on 08 Oct 2021
