
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Vet. Sci. , 27 February 2025
Sec. Veterinary Infectious Diseases
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1472658
 Zhenwen He1,2,3
Zhenwen He1,2,3 Dingyu Liu1,4
Dingyu Liu1,4 Baoling Liu1
Baoling Liu1 Pian Zhang1
Pian Zhang1 Xiaohu Wang1
Xiaohu Wang1 Gang Wang1
Gang Wang1 Yuan Huang1
Yuan Huang1 Jing Chen1
Jing Chen1 Rujian Cai1*
Rujian Cai1*Introduction: Hepatitis E virus (HEV) is a major cause of acute hepatitis in humans and recognized as a zoonotic pathogen, with swine serving as a primary reservoir. Despite substantial research, comprehensive analysis encompassing regional variations and pig growth stages within China, as well as the influence of recent biosecurity measures on HEV prevalence, remains limited. In this study, we aim to assess the prevalence and risk factors associated with swine HEV in China.
Methods: A thorough review of HEV infection studies was conducted using six databases: China National Knowledge Infrastructure, Wanfang, Wipro, Centre for Agriculture and Biosciences International, PubMed, and ScienceDirect, covering publications from January 1, 2004 to December 31, 2023. Eighty-seven studies investigating the seroprevalence of swine HEV IgG antibodies and HEV RNA detection rates were included. A rigorous meta-analysis and quality assessment followed.
Results: The combined seroprevalence of swine HEV IgG antibodies was 58.0% (95% confidence interval [CI]: 52.0–65.0). The seroprevalence from 2019 to 2023 was lower (27.4, 95% CI: 26.3–28.2) than that in other years. The seroprevalence was higher in sows (67.2, 95% CI: 55.8–78.7) than in suckling, nursery, and fattening pigs. The detection rate of HEV RNA was 13.0% (95% CI: 11.0–15.0), with fattening pigs showing a significantly higher positivity rate (16.9, 95% CI: 13.2–20.7) than sows and suckling pigs. HEV RNA detection was significantly lower in bile (8.3, 95% CI: 6.3–10.3) than in feces and liver.
Discussion: This study highlights the widespread presence of HEV in pig farms across China, with prevalence strongly linked to pig growth stage, study year, and sample type. The findings underscore the importance of pig growth stage, sample type, and recent biosecurity measures in controlling HEV prevalence, providing actionable insights for improving biosecurity practices in pig farms.
Hepatitis E virus (HEV) is a major cause of acute hepatitis (1). It is a single-stranded positive-sense RNA virus from the Hepeviridae family, classified into eight genotypes (HEV-1 to HEV-8). HEV-1 and HEV-2 primarily infect humans, while HEV-3 and HEV-4 are found in various animals and are transmitted zoonotically (2). HEV infection is typically mild, presenting symptoms such as jaundice, malaise, fever, nausea, and vomiting, with most individuals recovering spontaneously within a few weeks. However, it can be more severe in pregnant women and immunocompromised individuals (3).
According to the World Health Organization, HEV represents a significant health risk, particularly in developing countries where poor sanitation and contaminated drinking water contribute to the virus spread (4). In recent years, an increasing number of indigenous HEV infections have been reported in developed countries, often linked to zoonotic transmission of HEV-3 and HEV-4 through the consumption of raw pig products and undercooked wild boar meat (5). HEV-3 and HEV-4 RNAs have been detected in the global pork supply chain, with pigs playing a key role in transmitting these genotypes to humans (6).
In China, where pork is a dietary staple, swine HEV poses a substantial public health risk, with seroprevalence among pigs ranging from 1.8 to 73% (7). Notably, outbreaks have been linked to the consumption of HEV-contaminated pig products (8). Another outbreak in Qingdao in July 2018 was also likely related to the consumption of HEV-contaminated pig livers (9). A study in Yunnan Province found that HEV gene sequences from 243 pig samples were highly homologous to human isolates (10), consistent with findings from other studies in China (11). Therefore, the prevalence of swine HEV in China poses a substantial threat to public health, necessitating ongoing monitoring of its risk to humans. Although many studies have examined swine HEV prevalence, a comprehensive synthesis is lacking. This study conducts a meta-analysis to evaluate swine HEV prevalence in China from 2004 to 2023, exploring factors influencing anti-HEV IgG and HEV RNA detection. This analysis aims to provide essential data and a theoretical basis for the prevention and control of swine HEV in China.
This review focuses on cross-sectional studies that investigated swine HEV prevalence in China. These studies were included because they provide a snapshot of HEV prevalence at a specific point in time, which aligns with the aim of this meta-analysis to summarize and evaluate the epidemiological data on swine HEV in China.
The studies included in this review focused on pigs as the study population, specifically examining the presence of HEV in swine populations across different regions of China.
In this study, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (12) to search for relevant literature on swine HEV epidemiological investigations in China.
We conducted searches across six databases: China National Knowledge Infrastructure, Wanfang, Wipro, Centre for Agriculture and Biosciences International (CABI), PubMed, and ScienceDirect. The search was limited to literature published from January 1, 2004, to December 31, 2023, in both Chinese and English. For English-language databases (CABI, PubMed, and ScienceDirect), we used the search terms: “swine,” “boar,” “pig,” “survey,” “prevalence,” “occurrence,” “Hepatitis E,” “virus,” “HEV,” and “China,” combined with Boolean operators “AND” and “OR.” In the Chinese databases, we used the keywords “pig,” “Hepatitis E” (Chinese abbreviation), “Hepatitis E” (Chinese full name), and “HEV.” The full search strategy is outlined in Table 1.
We excluded duplicate review articles and screened the remaining studies according to the following inclusion criteria: [1] study subjects were pigs; [2] the study investigated swine HEV prevalence in China; [3] the study reported the number of pigs examined and the number that tested positive; [4] the sampling period was between 2004 and 2023; and [5] the study design was cross-sectional.
Data were extracted and cross-checked by two independent researchers. Any disagreements were resolved through discussion with the authors. The following information was extracted from eligible studies: first author, study year, geographic area, number of pigs at each growth stage, sample type, sampling site, total number of pigs tested, and number of positive cases. This data was compiled into a database using Microsoft Excel (version 2017).
The quality of the literature was assessed using a scoring system (13) based on five key questions, each scored 1 point: [1] Was the sample randomized in the study? [2] Were the sampling times accurately recorded? [3] Were the sampling locations representative? [4] Was the sample type described in detail? [5] Was the testing method accurately described? Papers were categorized as high-quality (4–5 points), medium-quality (2–3 points), or low-quality (1–2 points) based on their scores.
The primary outcomes of interest were the prevalence rates of anti-HEV IgG antibodies and HEV RNA in pigs. These were defined as the proportion of positive samples relative to the total number of samples.
Meta-analyses were conducted separately to determine the detection rates of anti-HEV IgG antibodies and HEV RNA in pigs. If a study reported multiple prevalence data points (i.e., from different pig herds or sample types), each data point was treated as a separate record. The analyses were performed using Stata version 16.0 software. To approximate a normal distribution, the Freeman–Tukey double inverse chord transformation was applied (14). A random-effects model was employed to assess significant heterogeneity in the HEV positivity rate, with effect sizes expressed as 95% confidence intervals. Heterogeneity among studies was evaluated using I2 statistics. If I2 was greater than 50% and p < 0.05, indicating significant heterogeneity, a random-effects model was used. If I2 was less than 50% and p > 0.05, indicating less heterogeneity, a fixed-effects model was applied. Forest plots were generated for the overall meta-analysis, and funnel plots, along with Egger’s test, were used to assess publication bias. Sensitivity analyses were performed to evaluate the stability of the results. Subgroup and meta-regression analyses were conducted to identify sources of heterogeneity and analyze risk factors for HEV infection.
The risk factors examined included sampling area, pig growth stage, year of study, sampling site, sample type, and paper quality. Sampling areas were classified into seven regions based on administrative divisions in China: Northeast, North, East, South, Central, Northwest, and Southwest. Pig growth stages were classified as lactating pigs (<1 month of age), nursery pigs (1–3 months), fattening pigs (3–6 months), sows (>6 months), and breeding gilts (>6 months). The studies were grouped into three phases based on publication years: 2004–2010, 2011–2018, and 2019–2023. Sampling sites included large-scale farms, free-range farms, and slaughterhouses. Sample types included serum, feces, pig liver, and bile. Paper quality was categorized as high, medium, or low based on the literature quality scores.
A total of 2,215 articles were retrieved from six databases. Figure 1 illustrates the study selection process, starting with the initial 2,215 articles retrieved from six databases, followed by screening for duplicates, titles, abstracts, and full texts. Ultimately, 87 studies were included in the meta-analysis. After removing duplicates and screening titles, abstracts, and full texts, 87 eligible studies were included in this meta-analysis. Among these, 68 studies were used to analyze the rate of anti-HEV IgG antibodies, and 27 studies were used to analyze the rate of HEV RNA in pigs. The selection process is shown in Figure 1. All studies used a cross-sectional design. Based on our quality criteria, 41 studies were classified as high quality (4–5 points), 45 as medium quality (2–3 points), and 11 as low quality (0–1 point). The risk of bias was assessed based on a standard quality assessment tool [e.g., the Newcastle-Ottawa Scale for cross-sectional studies]. Studies were categorized into high, medium, or low quality based on criteria such as randomization, sample representativeness, and the adequacy of reporting. Tables 2, 3 provide detailed information on the studies regarding anti-HEV IgG antibodies and HEV RNA detection rates in pigs, respectively, included in the analysis. A detailed summary of study characteristics, including geographical distribution, sample sizes, and demographic details of the swine populations, is presented in Tables 2, 3. For example, studies were conducted across diverse regions in China, including [list of regions], with sample sizes ranging from [X] to [Y].
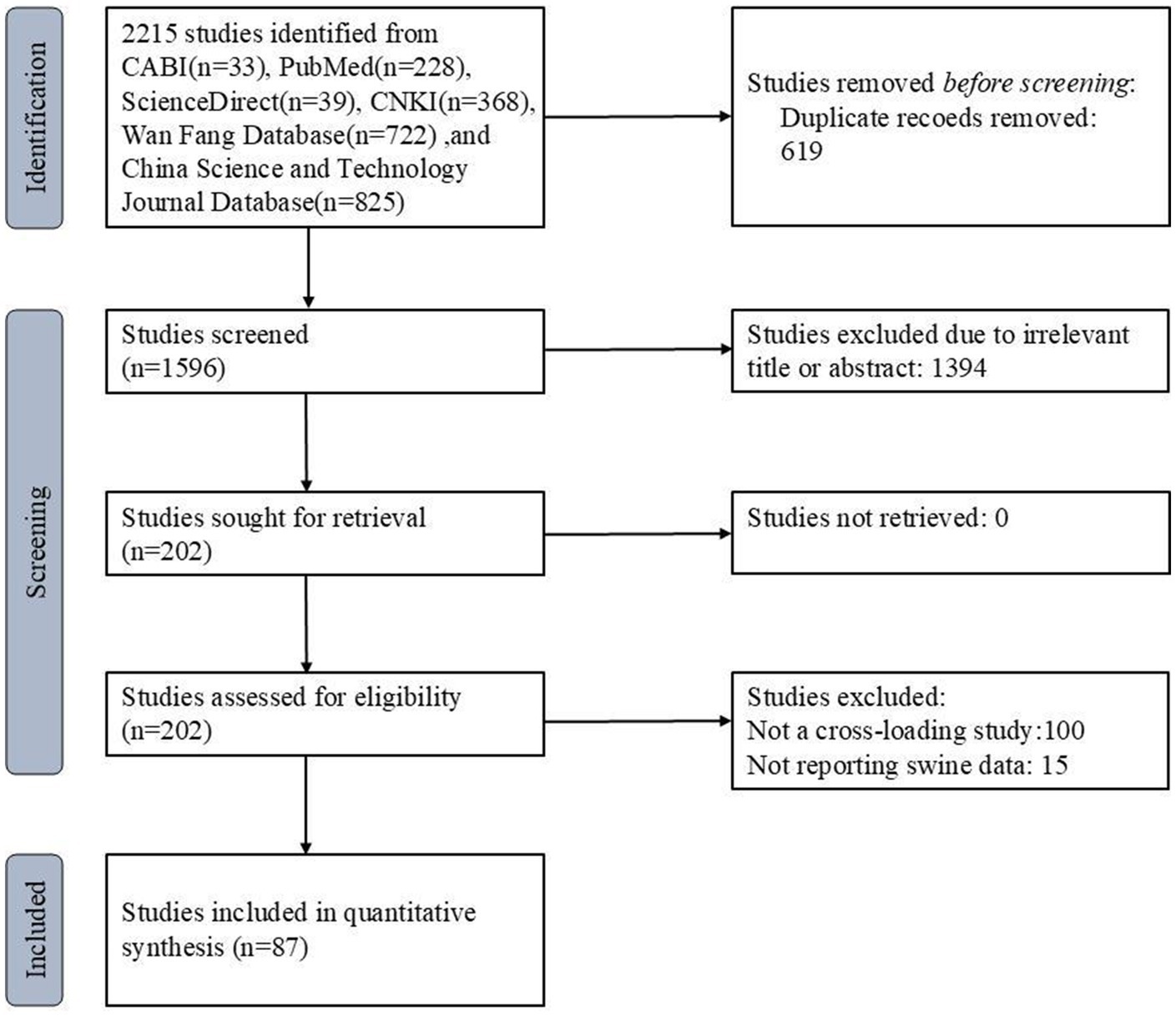
Figure 1. PRISMA flow diagram of the systematic review from the initial search and screening to the final selection of publications included in the study.
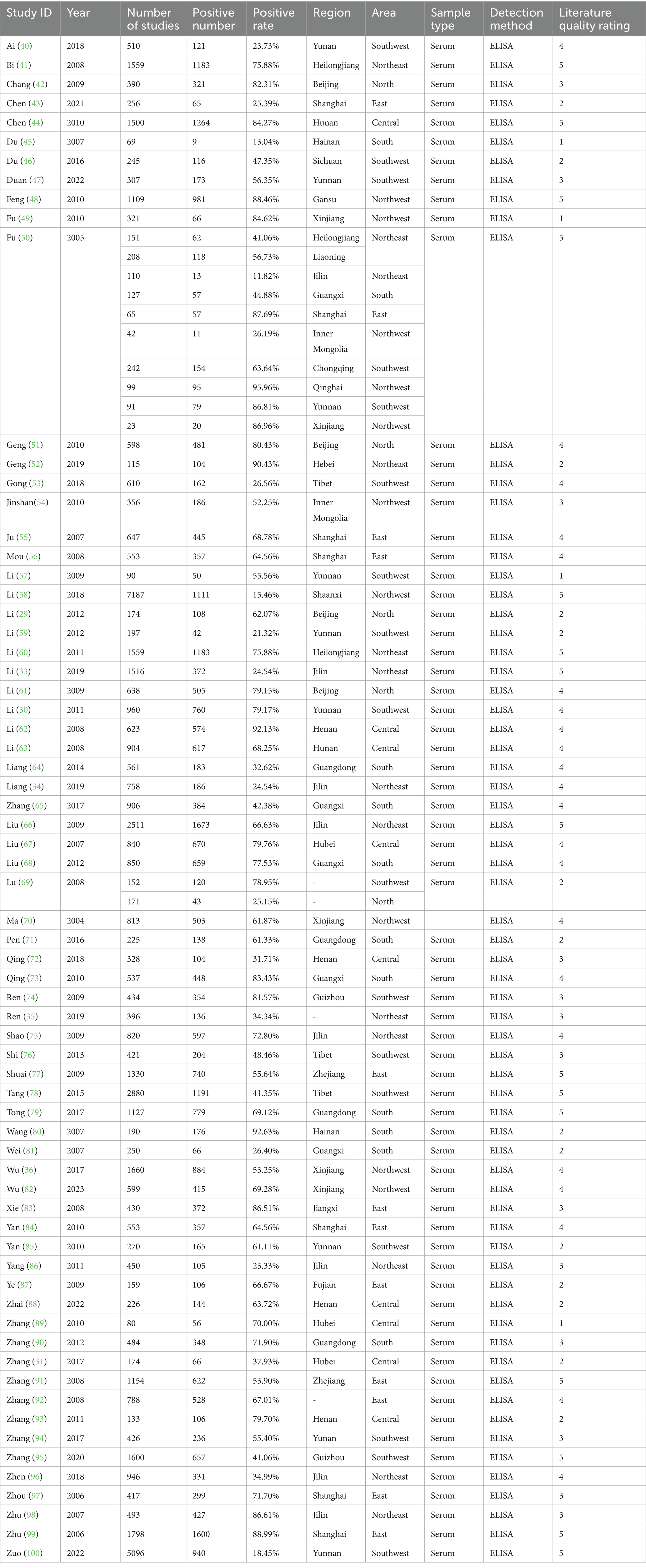
Table 2. Information on the positive rate of serum antibodies to IgG in swine HEV included in the analysis
The heterogeneity test results for studies analyzing anti-HEV IgG antibodies were I2 =99.72% and p < 0.001. For studies analyzing the RNA detection rate of swine HEV, the heterogeneity test results were I2 = 82.70%, p < 0.001, indicating significant heterogeneity in the selected studies. Therefore, a random-effects model was applied for the meta-analysis (Figures 2, 3). Funnel plot analysis revealed that the studies included in the analysis (represented as dots) had a largely symmetrical distribution within the funnel plot, indicating no significant publication bias (Figures 4, 5). Egger’s test results for anti-HEV IgG antibodies were p = 0.5527 (p > 0.05), and for HEV RNA detection rates, the p-value was 0.0572 (p > 0.05), indicating no significant publication bias. Sensitivity analysis showed that the combined effect sizes remained within the 95% CI when each study was excluded individually, demonstrating the stability and reliability of the meta-analysis results (Figures 6, 7).

Figure 2. Forest plot of serum antibody IgG positivity rate of swine hepatitis E virus in studies conducted in China.
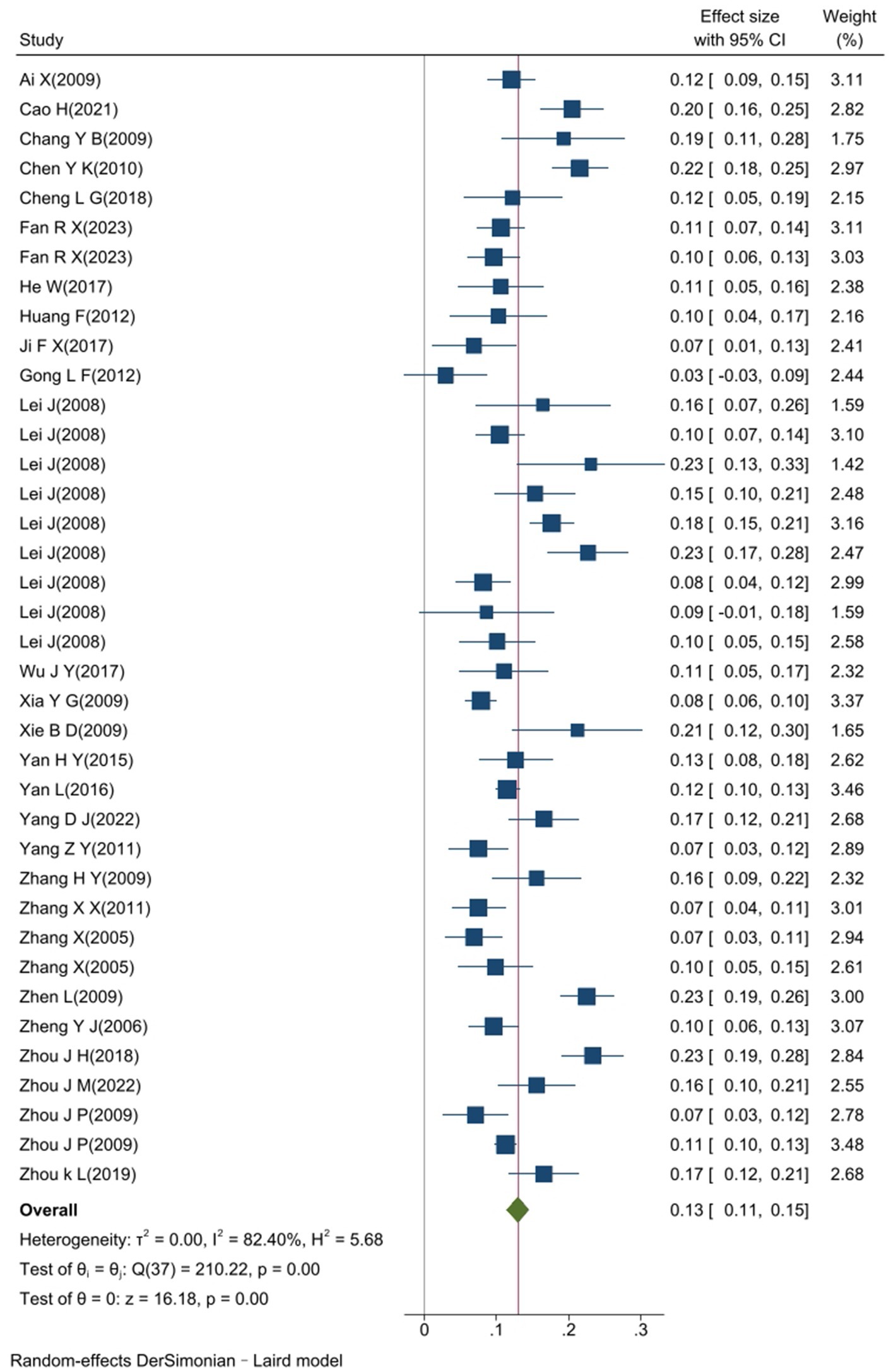
Figure 3. Forest plot of the RNA detection rate of swine hepatitis E virus in a study conducted in China.
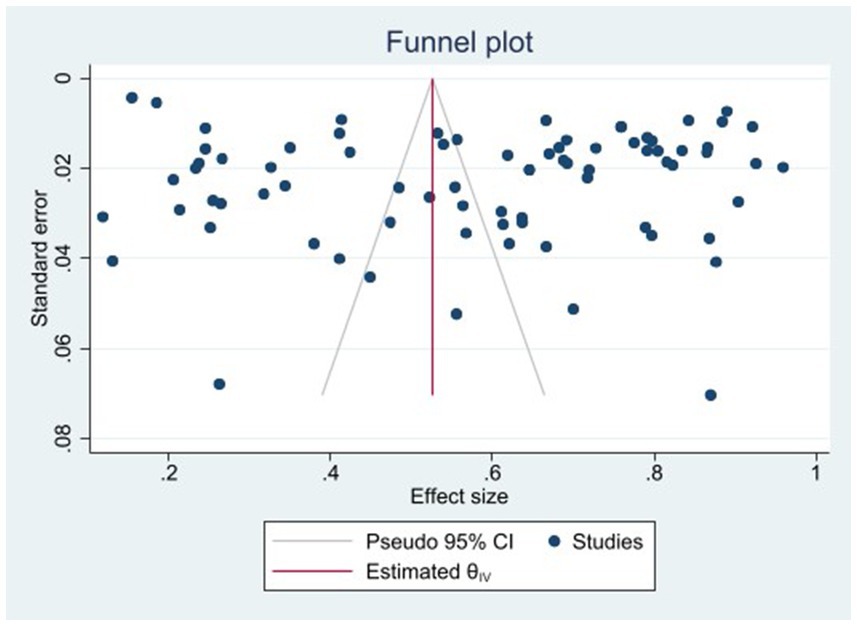
Figure 4. Funnel plot of serum antibody IgG positivity rate of swine hepatitis E virus in the literature.
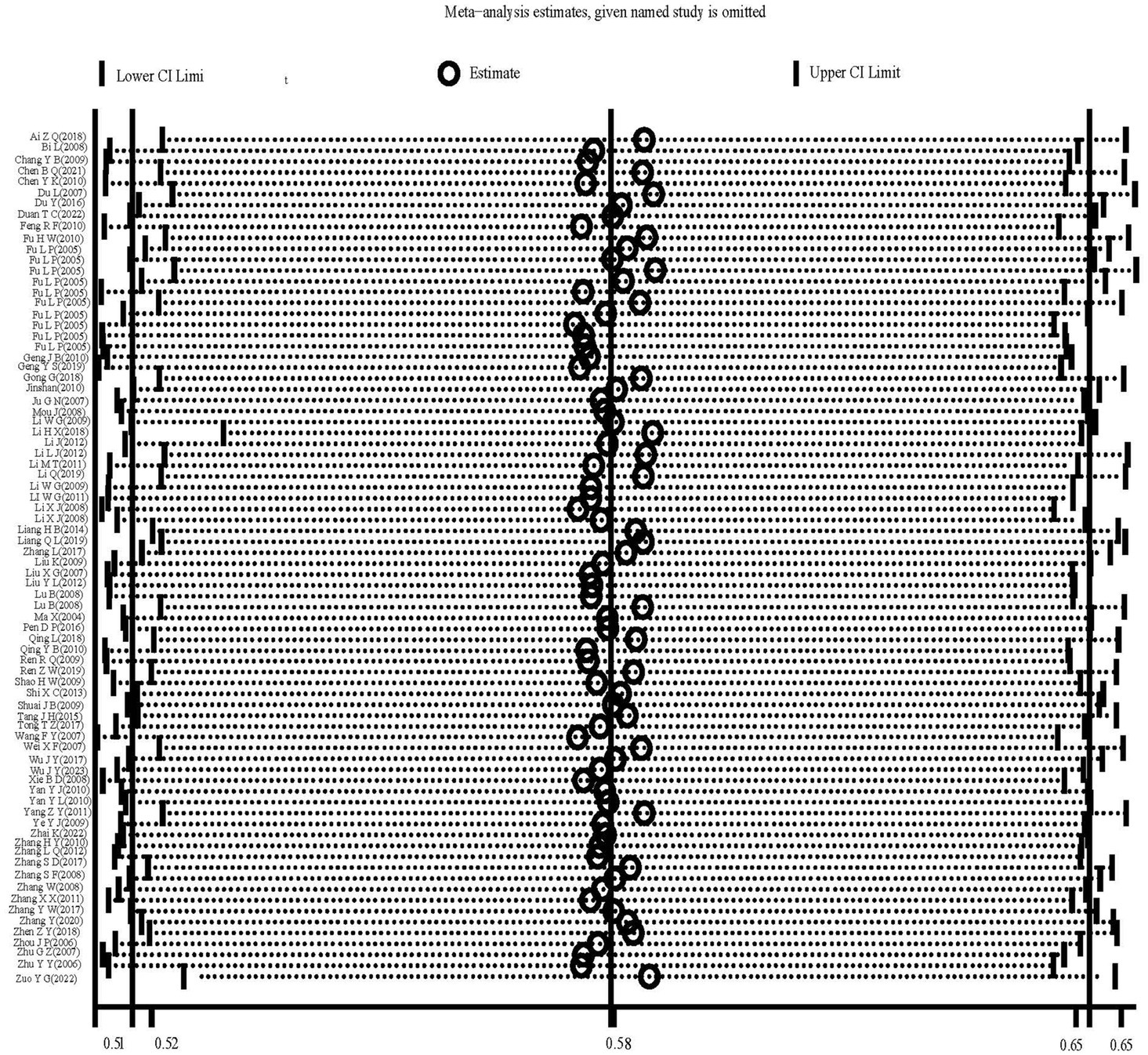
Figure 6. Sensitivity analysis of serum antibody IgG positivity rate of swine hepatitis E virus in the literature.
The overall positive rate for anti-HEV IgG antibodies was 58.0% (95% CI: 52.0, 65.0). No significant difference was observed between different provinces or cities (p = 0.612), as shown in Figure 8. The highest positive rate was observed in Qinghai Province at 96.0% (95% CI: 92.1, 99.8), followed by Hebei Province at 90.4% (95% CI: 85.4, 95.8), and Gansu Province at 88.5% (95% CI: 86.6, 90.3). Lower positive rates were found in Yunnan Province at 30.7% (95% CI: 29.6, 31.7) and Shaanxi Province at 15.5% (95% CI: 14.6, 16.3). The synthesized findings suggest substantial regional variation in the prevalence of anti-HEV IgG antibodies, with higher rates observed in [specific provinces]. These differences could reflect regional differences in farming practices, environmental factors, or pig management systems. No significant differences were found across different study years, indicating that the HEV prevalence rates have remained relatively stable over the past two decades.
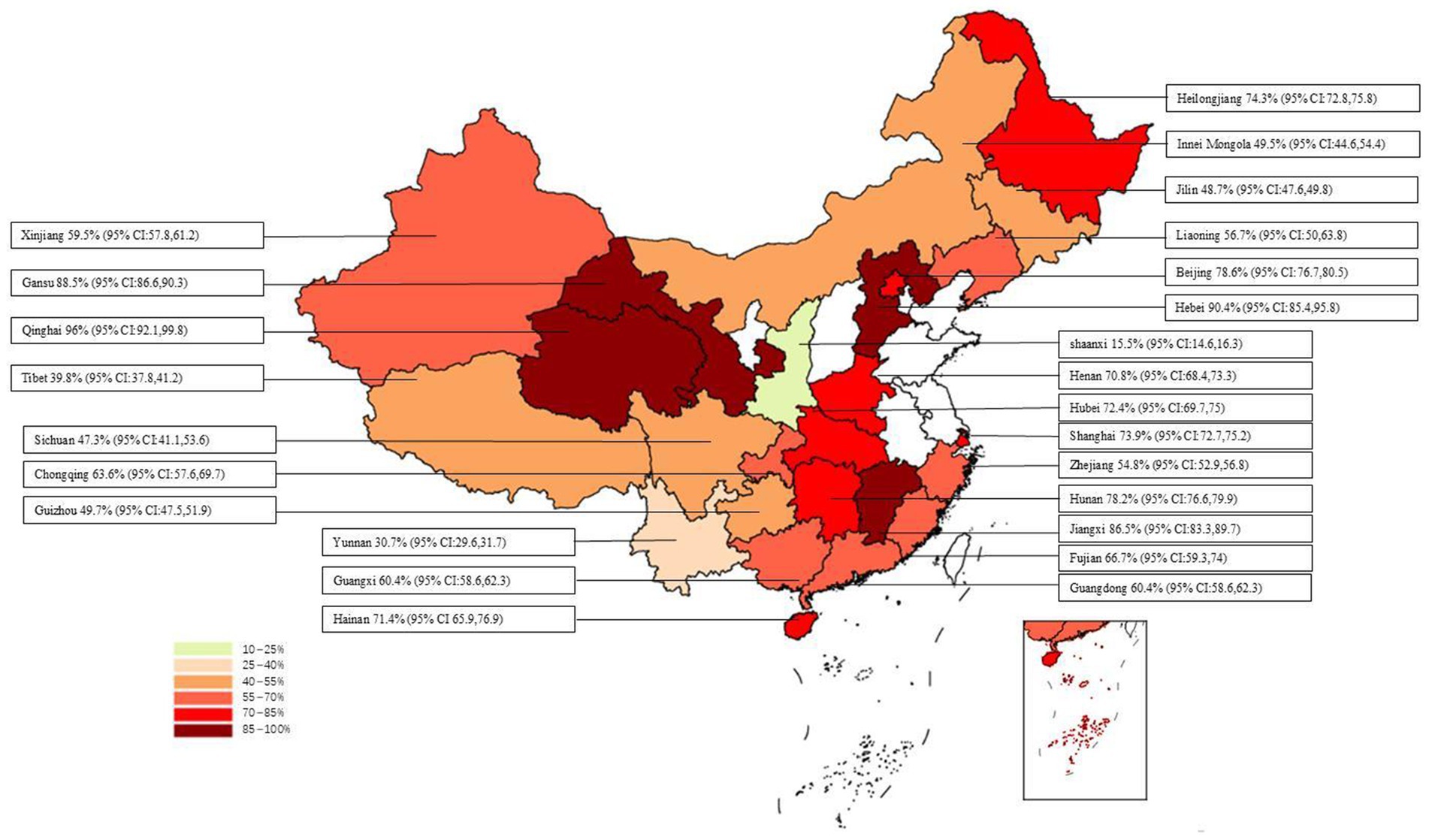
Figure 8. Positive rate of serum antibodies to IgG to swine HEV in different provinces/cities in China.
The overall detection rate of swine HEV RNA was 13.0% (95% CI: 11.0, 15.0), and no significant difference was observed among different provinces or cities (p = 0.120), as shown in Figure 9. The highest detection rate was observed in Gansu Province at 23.3% (95% CI: 19.1, 27.6), followed by Hunan Province at 21.6% (95% CI: 17.8, 25.3), and Jiangxi Province at 21.3% (95% CI: 12.3, 30.2). Lower detection rates were found in Henan Province at 7.5% (95% CI: 3.8, 11.2), Jilin Province at 7.5% (95% CI: 3.4,11.6), and Guangdong Province at 6.8% (95% CI: 1.1, 12.6).
The sources of heterogeneity in the included studies on anti-HEV IgG antibodies were analyzed across five subgroups: sampling area, pig growth stage, sampling site, sampling year, and literature quality (Table 4). The results showed significant differences in positivity rates among different growth stages (p = 0.009, p < 0.05). Sows had the highest positivity rate at 67.2% (95% CI: 55.8, 78.7), followed by fattening pigs at 59.5% (95% CI: 52.6, 66.4). Nursery pigs had a positivity rate of 49.5% (95% CI: 41.2, 57.8), breeding gilts had a rate of 45.0% (95% CI: 27.9, 62.1), and lactating pigs had the lowest rate at 34.3% (95% CI: 22.8, 45.8). Significant differences were observed between study years (p < 0.05). The highest positivity rate was found in studies from 2004 to 2010 at 72.0% (95% CI: 71.5, 72.6), followed by 40.5% (95% CI: 39.6, 41.2) in studies from 2011 to 2018, and the lowest rate was 27.4% (95% CI: 26.3, 28.2) in studies from 2019 onward. The sampling area, sampling site, and literature quality did not significantly contribute to the differences in anti-HEV IgG antibody rates (p > 0.05).
The studies on swine HEV RNA detection rates were analyzed using six subgroups: sampling area, pig growth stage, sample type, sampling site, sampling year, and literature quality (Table 5). The results were consistent with those for anti-HEV IgG antibodies, with pig growth stage being a significant factor (p = 0.006, p < 0.05). The highest detection rate of HEV RNA was 16.9% (95% CI: 13.2, 20.7) in fattening pigs, followed by 15.7% (95% CI: 11.9, 19.6) in nursery pigs. The detection rate of HEV RNA in sows and lactating pigs was significantly lower, at 8.4% (95% CI: 4.2, 12.3) and 6.9% (95% CI: 4.1, 9.7), respectively. Significant differences in HEV RNA detection rates were observed between sample types, with lower detection rates found in bile (8.3%) than in feces (14.6%) and liver (13.0%). The sampling area, sampling site, study year, and literature quality did not significantly influence the differences in HEV RNA detection rates (p > 0.05). Additionally, the analysis revealed that fattening pigs had the highest detection rate, likely reflecting their higher exposure to environmental risk factors.
In this study, we found that pig growth stage significantly influenced both the positivity rates of anti-HEV IgG antibodies and detection rates of HEV RNA in pigs, consistent with the findings of previous studies (15–17). As pigs enter the nursery stage approximately 1 month of age, maternal antibodies disappear, increasing their susceptibility to HEV and subsequently raising infection rates. This transition leads to a noticeable increase in both anti-HEV IgG antibody positivity and HEV RNA detection rates. In our study, anti-HEV IgG antibody positivity increased from 34.3% (95% CI:22.8,45.8) in lactating pigs to 49.5% (95% CI: 41.2, 57.8) in nursery pigs. Similarly, HEV RNA detection rates increased from 6.9% (95% CI: 4.1, 9.7) to 15.7% (95% CI: 11.9, 19.6). Serum IgG antibodies can persist for extended periods, which explains the higher positivity rates in older pigs. The duration of HEV viremia in pigs is estimated to be 2–3 weeks, after which the virus is gradually cleared following the production of serum antibodies. Consequently, pigs older than 6 months have typically experienced infection and cleared the HEV virus from their bodies (18). In this study, the HEV RNA detection rate reached a maximum of 16.9% (95% CI: 13.2, 20.7) at the fattening stage, whereas it decreased to a minimum of 8.4% (95% CL: 4.2, 12.5) in sows older than 6 months, which is consistent with previously reported results. The influence of the pig growth stage on HEV seropositivity was significant, with the highest positivity rates observed in pigs at the nursery stage. This aligns with the results of previous studies that showed increased vulnerability to HEV infection as maternal antibodies wane (15–17). Similarly, biosecurity measures introduced post-2019 appear to have substantially reduced the overall HEV prevalence in pig populations. These findings suggest that the combination of the growth stage transition and enhanced biosecurity practices plays a critical role in determining the HEV infection rates across different stages of pig development.
The global prevalence of IgG antibodies against porcine HEV varies between 20 and 100% (19). In Europe and the United States, most pig infections are attributed to HEV genotype 3, with reported HEV antibody positivity rates ranging from 40 to 88% in some countries (2). For instance, in Italy, the HEV antibody positivity rate in slaughtered pigs is 76.8% (20); in Bulgaria, it ranges from 40.0 to 60.3% (21); in Corsica, France, the rate in free-range pigs is 88% (22); and in the United States, the national average HEV antibody positivity rate in pigs is 40% (23). In comparison, our analysis suggests that the HEV antibody positivity rates in pigs in Europe and the United States tend to be higher than those observed in China. In Asia, HEV genotype 4 predominates, with HEV antibody positivity rates ranging from 35 to 73% (2). In Mongolia, the HEV antibody positivity rate in pigs is 35.5% (24), similar to the findings from China; in Japan, the rate in wild boars is between 5.0 and 15.3% (25), whereas in South Korea, the overall prevalence of anti-HEV antibodies in pigs is 14.8% (26), both of which are lower than those reported in China. By contrast, in Vietnam, the HEV antibody positivity rate in pigs is 58.5% (27), and in India, it is 65.0% (28), both higher than those observed in China. Overall, the HEV antibody positivity rate in pigs in China appears to be moderate when compared to that in other countries in Asia.
Epidemiological studies on swine HEV across various regions have yielded inconsistent results. For instance, Li et al. (29) tested pig sera from the suburbs of Beijing and found an anti-HEV IgG antibody positivity rate of 62.1%. Similarly, Li et al. (30) tested pig sera from farms in Yunnan Province and reported a positivity rate of 79.1%. In contrast, Zhang et al. (31) conducted testing on sera from pig farms in Anlu City, Hubei Province, and found a lower positivity rate of 38.5%. Despite differences in study methodologies, most reports suggest that the HEV antibody positivity rate in pigs across China is moderate, with variation primarily linked to genotype differences rather than geographical factors. This is consistent with our study’s findings, which showed no significant geographic variation in HEV prevalence despite regional differences in climate and farming practices. However, our study did not observe significant differences in the serum anti-HEV IgG antibody positivity or HEV RNA detection rates across pigs from different regions. This suggests that geographic factors are not significant determinants of HEV prevalence in the pig population. While geographic and climatic differences are often considered potential contributors to variations in HEV prevalence, they do not appear to be the main factors in this case. Instead, differences in the genotypes of HEV strains prevalent in each region are likely more influential (32). In this study, all HEV genotypes identified in pigs were genotype 4, with subtype 4a being the predominant form. Some studies also detected subtypes 4b and 4d. The consistent presence of similar HEV genotypes across regions may help explain why our study did not find significant geographic variation in HEV prevalence.
At the end of the fattening stage, approximately 6 months of age, pigs are typically sent to the slaughterhouse. It is reasonable to expect that the anti-HEV IgG antibody positivity rate in slaughterhouse pigs would align with that observed in pigs at the fattening stage on the farm, which our study confirmed. As indicated in our earlier analysis, pigs older than 6 months have generally experienced HEV infection, and the virus has been cleared from their bodies. This suggests that the likelihood of slaughterhouse pigs carrying HEV is relatively low. However, despite the significantly lower detection rate of HEV RNA in slaughterhouse pigs than in pigs on farms and in free-range settings, a detection rate of 10.1% (95% CI: 8.5, 11.8) still indicates the possibility of HEV-positive pigs entering slaughterhouses. Given that slaughterhouses are the initial point in pork production, HEV carried by these pigs could potentially lead to human infections via a foodborne route. Therefore, ongoing surveillance of HEV in slaughterhouse pigs is crucial to assess the risk of HEV transmission through pork products. Additionally, the presence of HEV in slaughterhouse pigs and wild boar populations highlights a significant public health risk, particularly through the consumption of undercooked pork and wild boar meat. Therefore, public health initiatives must focus on education about safe cooking practices and regulations on meat handling to prevent zoonotic transmission.
In this study, the HEV RNA detection rate in pig bile was significantly lower than that in pig liver and feces, which differs from the findings in some previous studies. This discrepancy may be attributed to the fact that the bile samples included in our analysis were exclusively sourced from slaughterhouses, whereas most fecal samples were obtained from pig farms. Previous analyses have shown that the HEV RNA detection rate in pigs from slaughterhouses is significantly lower than that in pigs from farms, which could explain why the RNA positivity rate in bile is even lower than that in fecal samples. In our analysis, fecal samples exhibited the highest HEV RNA detection rate, suggesting that fecal shedding plays a critical role in HEV transmission within pig populations. This supports the hypothesis that HEV spreads through the fecal–oral route, as contaminated feces facilitate environmental dissemination and subsequent transmission among pigs. Based on these findings, we recommend that pig farms prioritize manure management and environmental disinfection to interrupt the fecal-oral transmission of HEV within pig populations. Additionally, the HEV RNA detection rate in pig liver was 13.0%, underscoring the high risk of HEV carriage in this organ. This finding highlights the significant association between the consumption of undercooked liver and human HEV infection. To address this risk, monitoring programs should be developed across different regions to assess infection risks, and public awareness campaigns should be intensified to educate the public on the dangers of consuming undercooked liver and other raw pork products.
We categorized the included studies by research year and found a significant decline in the IgG antibody positivity rate in pig serum after 2019. African swine fever (ASF) began to emerge in China in 2018, drawing significant attention from both the industry and government. In 2019, the General Office of the State Council issued the “Opinions on Strengthening the Prevention and Control of ASF,” mandating pig farms to strengthen cleaning and disinfection, manage personnel and vehicle access, and prohibit feeding pigs with food waste. Furthermore, live pig transportation across the country was progressively restricted, and stringent measures were implemented to combat meat product smuggling. These actions were crucial in preventing the spread of ASF and also had a significant impact on the transmission of other pathogens. Consequently, we believe the enhanced biosecurity measures implemented in pig farms after 2019 reduced the risk of HEV transmission from the environment. Strengthening personnel and vehicle management, along with limiting live pig movement, helped reduce the risk of introducing external HEV strains, thereby significantly lowering HEV prevalence in pig populations. This underscores the importance of biosecurity measures—such as environmental disinfection, control of personnel, vehicles, and materials, and prohibition of food waste use in feeding pigs—in reducing HEV infection risk in pig populations.
In the studies included in our analysis, the majority focused on domestic pigs, with only four studies (33–36) reporting data on serum IgG antibody detection in wild boars, which showed a positivity rate of 38.0% (95% CI: 14.0, 63.0). Although the serum IgG antibody positivity rate in wild boars is lower than that in domestic pigs, wild boars play a crucial role in the transmission of HEV (37). Thus, the prevalence of HEV in wild boars in China should not be overlooked. Several studies have identified risk factors for human HEV infection, suggesting that the virus is transmitted through the consumption of wild boar meat. A case–control study in Germany found that a significant number of local HEV cases were linked to the consumption of undercooked wild boar meat and offal (38). Serological studies have also highlighted that direct contact with infected wild boars increases the risk of HEV infection (39). Consequently, it is essential for regions to monitor wild boar populations and manage their disease risks effectively to prevent zoonotic transmission of HEV to humans or livestock. In Japan, the implementation of livestock health management standards in 2021 required farms in wild boar habitats to install protective fences, limiting contact between farm pigs and wild boars. This measure is likely a key factor in the significant decrease in HEV incidence among pigs in Japan in recent years (25). This management approach could serve as a valuable model for reducing interactions between wild boars, livestock, and humans, thereby curbing the transmission of HEV.
Our study has several limitations. First, articles were sourced from only six databases, which may have excluded relevant literature from other databases. While we included studies from six major databases, our exclusion of studies published in non-English or non-Chinese languages could have led to a selection bias, potentially overlooking important regional findings. Second, the study focused on articles in English and Chinese, potentially overlooking studies published in other languages. Third, some of the studies had small sample sizes, which could have impacted the reliability of the overall estimates. Fourth, the risk factors we extracted, such as sample type and site, may not have been fully comprehensive, limiting our ability to analyze other factors that could influence anti-HEV IgG antibody positivity rates and HEV RNA detection. Fifth, despite including 87 eligible studies, research on swine HEV infection in certain regions of China remains insufficient. As such, future research should aim to better characterize and quantify these regional risk factors.
HEV seropositivity in pigs in China is notably high, with widespread infection across pig farms in all regions. Implementing biosecurity measures on pig farms may significantly contribute to the prevention and control of HEV, and tailored interventions in different regions could help reduce local infection rates. In conclusion, our findings underscore the importance of biosecurity measures in reducing HEV prevalence in pig populations. Given the potential for human transmission through pork products, enhanced surveillance programs at slaughterhouses, coupled with stricter biosecurity protocols on farms, are crucial in mitigating the public health risks associated with HEV. Further research should focus on understanding the effectiveness of region-specific interventions in controlling HEV transmission. Consequently, effective management and monitoring strategies are essential to mitigate HEV infection in pigs, thereby safeguarding public health. Moreover, pig slaughterhouses, as critical points of pork production, are also contaminated with HEV, presenting a potential risk to human health. Routine monitoring of slaughterhouses is crucial to assess the risk of HEV transmission to humans. Future research should focus on characterizing the genotype-specific transmission patterns of HEV and assess the long-term impact of biosecurity interventions. Additionally, studies evaluating the role of wild boars in HEV transmission to both pigs and humans are urgently needed to develop targeted control strategies. Therefore, comprehensive and ongoing surveillance programs should be established nationwide to gather more epidemiological data on swine HEV infections.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
ZH: Writing – original draft, Writing – review & editing. DL: Writing – review & editing. BL: Writing – review & editing. PZ: Writing – review & editing. XW: Writing – review & editing. GW: Writing – review & editing. YH: Writing – review & editing. JC: Writing – review & editing. RC: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Science and Technology Plan Projects of Guangdong Province (grant number 2023B0202010014) 2021, the Yingde City Science and Technology Program Projects, Scientific and Technological Support and Technical Demonstration of Pig Industrial Park in Yun’an District, Yunfu City (grant number K06-005), 2022, and the Pig Intelligent Animal Disease Prevention and Treatment System (grant number K11-006), 2022.
We would like to thank Editage (www.editage.cn) for their assistance with English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Liu, DY, He, ZW, Liu, BL, Wang, G, Wang, XH, Tang, C, et al. Advances in research on hepatitis E virus. China Anim Husb Vet Med. (2024) 51:736–47. doi: 10.16431/j.cnki.1671-7236.2024.02.030
2. He, ZW, Liu, DY, Liu, BL, Zhang, P, Wang, XH, Wang, G, et al. Research progress on cross-species transmission and prevention and control techniques of hepatitis E virus. China Anim Husb Vet Med. (2024) 51:2605–20. doi: 10.16431/j.cnki.1671-7236.2024.06.035
3. Lin, S, and Zhang, YJ. Advances in hepatitis e virus biology and pathogenesis. Viruses. (2021) 13:267. doi: 10.3390/v13020267
4. Xiang, Z, He, XL, Zhu, CW, Yang, JJ, Huang, L, Jiang, C, et al. Animal models of hepatitis e infection: advances and challenges. Hepatobiliary Pancreat Dis Int. (2024) 23:171–80. doi: 10.1016/j.hbpd.2023.10.001
5. Kamar, N, Izopet, J, Pavio, N, Aggarwal, R, Labrique, A, Wedemeyer, H, et al. Hepatitis e virus infection. Nat Rev Dis Primers. (2017) 3:17086. doi: 10.1038/nrdp.2017.86
6. Pallerla, SR, Harms, D, Johne, R, Todt, D, Steinmann, E, Schemmerer, M, et al. Hepatitis e virus infection: circulation, molecular epidemiology, and impact on global health. Pathogens. (2020) 9:856. doi: 10.3390/pathogens9100856
7. Zhu, H, Xueli, LI, Liu, M, Niu, X, Shi, T, and Geng, Y. Prevalence of hepatitis E virus in pigs in China and the risks of HEV transmission through pork consumption. J Med Pest Control. (2021) 37:366–9. doi: 10.7629/yxdwfz202104014
8. Deng, Z, Long, S, Li, F, Xiang, X, Shixiong, H, Zhang, H, et al. Investigation of the first outbreak of hepatitis E in Hunan Province. J Pub Health Prev Med. (2016) 27:92–5.
9. Han, Y, Liang, J, Pan, B, Ren, X, Zhao, C, Song, Q, et al. Epidemiological investigation on 31 cases of hepatitis E outbreak, Qingdao city. Prev Med Trib. (2022) 28:204–6.
10. Yang, G, Wang, G, Song, C, Cai, Y, Xin, W, Chai, J, et al. Genetic evolution analysis on the epidemic strain of porcine hepatitis E virus in Lincang City, Yunnan Province. China Anim Health Inspect. (2023) 40:28–33. doi: 10.3969/j.issn.1005-944X.2023.08.006
11. Geng, J, Wang, L, Wang, X, Fu, H, Bu, Q, Liu, P, et al. Potential risk of zoonotic transmission from young swine to human: seroepidemiological and genetic characterization of hepatitis e virus in human and various animals in Beijing, China. J Viral Hepat. (2011) 18:e583–90. doi: 10.1111/j.1365-2893.2011.01472.x
12. Arya, S, Kaji, AH, and Boermeester, MA. Prisma reporting guidelines for meta-analyses and systematic reviews. JAMA Surg. (2021) 156:789–90. doi: 10.1001/jamasurg.2021.0546
13. Ran, X, Chen, X, Wang, M, Cheng, J, Ni, H, Zhang, X, et al. Correction to: brucellosis seroprevalence in ovine and caprine flocks in China during 2000-2018: a systematic review and meta-analysis. BMC Vet Res. (2019) 15:18. doi: 10.1186/s12917-018-1749-9
14. Barendregt, JJ, Doi, SA, Lee, YY, Norman, RE, and Vos, T. Meta-analysis of prevalence. J Epidemiol Community Health. (2013) 67:974–8. doi: 10.1136/jech-2013-203104
15. Di Pasquale, S, De Santis, P, La Rosa, G, Di Domenico, K, Iaconelli, M, Micarelli, G, et al. Quantification and genetic diversity of hepatitis E virus in wild boar (sus scrofa) hunted for domestic consumption in Central Italy. Food Microbiol. (2019) 82:194–201. doi: 10.1016/j.fm.2019.02.005
16. Fanelli, A, Tizzani, P, and Buonavoglia, D. A systematic review and meta-analysis of hepatitis E virus (HEV) in wild boars. Res Vet Sci. (2021) 142:54–69. doi: 10.1016/j.rvsc.2021.11.015
17. Turlewicz-Podbielska, H, Augustyniak, A, Wojciechowski, J, and Pomorska-Mól, M. Hepatitis E virus in livestock—update on its epidemiology and risk of infection to humans. Animals (Basel). (2023) 13:3239. doi: 10.3390/ani13203239
18. Ianiro, G, Monini, M, De Sabato, L, Chelli, E, Cerini, N, Ostanello, F, et al. Dynamic of hepatitis E virus (HEV) shedding in pigs. Animals (Basel). (2022) 12:1063. doi: 10.3390/ani12091063
19. Salines, M, Andraud, M, and Rose, N. From the epidemiology of hepatitis E virus (HEV) within the swine reservoir to public health risk mitigation strategies: a comprehensive review. Vet Res. (2017) 48:31. doi: 10.1186/s13567-017-0436-3
20. Chelli, E, Suffredini, E, De Santis, P, De Medici, D, Di Bella, S, D’Amato, S, et al. Hepatitis E virus occurrence in pigs slaughtered in Italy. Animals (Basel). (2021) 11:277. doi: 10.3390/ani11020277
21. Palombieri, A, Tsachev, I, Sarchese, V, Fruci, P, Di Profio, F, Pepovich, R, et al. A molecular study on hepatitis E virus (hev) in pigs in Bulgaria. Vet Sci. (2021) 8:267. doi: 10.3390/vetsci8110267
22. Pellerin, M, Trabucco, B, Capai, L, Laval, M, Maestrini, O, Jori, F, et al. Low prevalence of hepatitis E virus in the liver of corsican pigs slaughtered after 12 months despite high antibody seroprevalence. Transbound Emerg Dis. (2022) 69:e2706–18. doi: 10.1111/tbed.14621
23. Sooryanarain, H, Heffron, CL, Hill, DE, Fredericks, J, Rosenthal, BM, Werre, SR, et al. Hepatitis E virus in pigs from slaughterhouses, United States, 2017-2019. Emerg Infect Dis. (2020) 26:354–7. doi: 10.3201/eid2602.191348
24. Batmagnai, E, Boldbaatar, B, Sodbayasgalan, A, Kato-Mori, Y, and Hagiwara, K. Hepatitis E virus (HEV) spreads from pigs and sheep in mongolia. Animals (Basel). (2023) 13:10.
25. Takahashi, M, Nishizawa, T, Nishizono, A, Kawakami, M, Sato, Y, Kawakami, K, et al. Recent decline in hepatitis E virus prevalence among wild boars in Japan: probably due to countermeasures implemented in response to outbreaks of classical swine fever virus infection. Virus Res. (2024) 348:199438. doi: 10.1016/j.virusres.2024.199438
26. Choi, I, Kwon, H, Shin, N, and Yoo, HS. Identification of swine hepatitis E virus (HEV) and prevalence of anti-hev antibodies in swine and human populations in Korea. J Clin Microbiol. (2003) 41:3602–8. doi: 10.1128/JCM.41.8.3602-3608.2003
27. Lee, HS, Dao, DT, Bui, VN, Bui, NA, Le, TD, Nguyen-Viet, H, et al. Prevalence and phylogenetic analysis of hepatitis E virus in pigs in Vietnam. BMC Vet Res. (2020) 16:333. doi: 10.1186/s12917-020-02537-7
28. Bnsal, M, Kaur, S, Deka, D, Singh, R, and Gill, J. Seroepidemiology and molecular characterization of hepatitis E virus infection in swine and occupationally exposed workers in Punjab, India. Zoonoses Public Health. (2017) 64:662–72. doi: 10.1111/zph.12363
29. Li, J, Liu, B, Yang, J, Tian, D, Ling, Y, and He, C. Primary epidemiological survey on the infection of hepatitis E virus in swine in Beijing suburbs. Chin J Vet Med. (2012) 48:6–9. doi: 10.3969/j.issn.0529-6005.2012.06.002
30. Li, W, Shu, X, Pu, Y, Bi, J, Yang, G, and Yin, G. Seroprevalence and molecular detection of hepatitis e virus in Yunnan province, China. Arch Virol. (2011) 156:1989–95. doi: 10.1007/s00705-011-1089-6
31. Zhang, S, Shu, Y, Peng, J, Zhou, D, Xie, J, and Zhang, L. Seroepidemiological study of swine hepatitis E in Anlu City. Hubei Province Pract Prevent Med. (2017) 24:88–9. doi: 10.3969/j.issn.1006-3110.2017.01.027
32. Lu, YH, Qian, HZ, Hu, AQ, Qin, X, Jiang, QW, and Zheng, YJ. Seasonal pattern of hepatitis E virus prevalence in swine in two different geographical areas of China. Epidemiol Infect. (2013) 141:2403–9. doi: 10.1017/S0950268813000113
33. Liang, QL, Nie, LB, Zou, Y, Hou, JL, Chen, XQ, Bai, MJ, et al. Serological evidence of hepatitis e virus and influenza a virus infection in farmed wild boars in China. Acta Trop. (2019) 192:87–90. doi: 10.1016/j.actatropica.2019.02.004
34. Liang, Q. Sero-epidemiological studies of influenza a, hepatitis E, Mycoplasmal pneumoniae and Haemophilus parasuis disease in wild boar. Jilin Province: Jilin Agricultural University (2019).
35. Ren, Z, Ding, M, Peng, P, Cai, J, Zhang, B, Changchun, T, et al. Molecular epidemiology investigation of hepatitis E virus in wild boars and grazing hybrid wild boars in border of Northeast China. Chin J Vet Sci. (2019) 39:695–701. doi: 10.16303/j.cnki.1005-4545.2019.04.16
36. Wu, JY. Epidemiology research of hepatitis E among people and animals in southern area of Xinjiang. Hubei Province: Huazhong Agricultural University (2017).
37. Sato, Y, Sato, H, Naka, K, Furuya, S, Tsukiji, H, Kitagawa, K, et al. A nationwide survey of hepatitis E virus (HEV) infection in wild boars in Japan: identification of boar hev strains of genotypes 3 and 4 and unrecognized genotypes. Arch Virol. (2011) 156:1345–58. doi: 10.1007/s00705-011-0988-x
38. Wichmann, O, Schimanski, S, Koch, J, Kohler, M, Rothe, C, Plentz, A, et al. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis. (2008) 198:1732–41. doi: 10.1086/593211
39. Carpentier, A, Chaussade, H, Rigaud, E, Rodriguez, J, Berthault, C, Boue, F, et al. High hepatitis E virus seroprevalence in forestry workers and in wild boars in France. J Clin Microbiol. (2012) 50:2888–93. doi: 10.1128/JCM.00989-12
40. Ai, Z, Li, L, Li, Y, Min, M, and Shen, Y. Epidemiological investigation on hepatitis E virus infection in pigs fed in Dali. J Dali Univ. (2018) 3:63–6. doi: 10.3969/j.issn.2096-2266.2018.10.015
41. Bi, L. Epidemiological investigation of hepatitis E virus infection and establishment of early diagnosis system in the Heilongjiang Province. Jilin Province: Changchun University of Science and Technology (2008).
42. Chang, Y, Wang, L, Geng, J, Zhu, Y, Fu, H, Ren, F, et al. Zoonotic risk of hepatitis e virus (hev): a study of hev infection in animals and humans in suburbs of Beijing. Hepatol Res. (2009) 39:1153–8. doi: 10.1111/j.1872-034X.2009.00558.x
43. Chen, B, Dong, S, Zhou, J, Si, F, Xie, C, Li, Z, et al. Serological investigation of hepatitis E in a pig farm in a suburban area of Shanghai. Shanghai J Anim Husb Vet Med. (2021) 2:25–8.
44. Chen, Y, Chen, T, Sun, Z, Xiang, J, and Zeng, S. Epidemiological investigation of swine hepatitis E in Hunan. Swine Prod. (2010). 1:65–7. doi: 10.3969/j.issn.1002-1957.2010.01.029
45. Du, L, Li, BY, Liao, QZ, Wang, FY, Zhang, LN, Wang, Y, et al. Survey of acute infection of hepatitis E virus in swine in cltles of Haikou and Sanya. China Trop Med. (2007) 7:2350. doi: 10.3969/j.issn.1009-9727.2007.12.085
46. Du, N. Investigation and analysis of swine hepatitis E virus infection in Panxi area. Livestock Poultry Indust. (2016) 7:74–5. doi: 10.3969/j.issn.1008-0414.2016.07.050
47. Duan, T, Yang, G, Qiongzhen, S, and Zhang, H. Seroepidemiological investigation of swine hepatitis E in Chuxiong, Yunnan Province. Graziery Vet Sci. (2022) 4:1–3.
48. Feng, Y, Ma, Z, Qiao, Z, Feng, Y, Zhao, C, and Wang, Y. HEV sero-epidemiology and genotypes among pigs in Lanzhou city. Chin J Public Health. (2010) 26:715–6. doi: 10.11847/zgggws2010-26-06-30
49. Fu, H, Li, L, Zhu, Y, Wang, L, Geng, J, Chang, Y, et al. Hepatitis e virus infection among animals and humans in Xinjiang, China: possibility of swine to human transmission of sporadic hepatitis e in an endemic area. Am J Trop Med Hyg. (2010) 82:961–6. doi: 10.4269/ajtmh.2010.09-0689
50. Fu, LP, Xiang, WH, Wang, XJ, Wei, LL, Zhao, YJ, Yu, M, et al. Prevalence of antibodies to swine hepatitis E virus in China and analysis of a novel HEV ORF2 partial sequences in swine. Chin J Prev Vet Med. (2005) 27:119–23. doi: 10.3969/j.issn.1008-0589.2005.02.010
51. Geng, J, Hongwei, F, Wang, L, Wang, X, Guan, J, Chang, Y, et al. Hepatitis E virus (HEV) genotype and the prevalence of anti-HEV in 8 species of animals in the suburbs of Beijing. Chin J Epidemiol. (2010) 31:47–50. doi: 10.3760/cma.j.issn.0254-6450.2010.01.012
52. Geng, Y, Zhao, C, Guo, T, Xu, Y, Wang, X, Huang, W, et al. Detection of hepatitis e virus in raw pork and pig viscera as food in HeBei province of China. Foodborne Pathog Dis. (2019) 16:325–30. doi: 10.1089/fpd.2018.2572
53. Ga, Gong, Yifei, Wang, Cuomu, Yixi, and Sizhu, Suolang. Serological epidemiological investigation on hepatitis E virus in Tibetan pigs in some areas of Tibet. China Anim Health Inspect (2018) 35:14–16. doi: 10.3969/j.issn.1005-944X.2018.06.005
54. Jinshan, J, Manglai, D, Takahashi, M, Nagashima, S, and Okamoto, H. Molecular and serological survey of hepatitis e virus infection among domestic pigs in Inner mongolia, China. Arch Virol. (2010) 155:1217–26. doi: 10.1007/s00705-010-0706-0
55. Gongne, J. Study on epidemic situation of hepatitis E virus in different species of animals in Shanghai area. Shanghai: Shanghai Jiaotong University (2007).
56. Mou, J. Construction of ELISA detection of swine anti-HEV antibody and survey of its molecular epidemiology in Shanghai. Jiangsu Province: Nanjing Agricultural University (2008).
57. Li, W, Li, J, Yang, G, Shu, P, and Ying, G. A seroepidemiological survey on swine hepatitis E virus infection in Yunnan Province. J Yunnan Agric Univ. (2009) 24:917–9. doi: 10.3969/j.issn.1004-390X.2009.06.027
58. Li, H, Wu, J, Sheng, Y, Lu, Q, Liu, B, Chen, Y, et al. Prevalence of hepatitis e virus (hev) infection in various pig farms from Shaanxi province, China: first detection of hev rna in pig semen. Transbound Emerg Dis. (2019) 66:72–82. doi: 10.1111/tbed.12966
59. Li, L, Shen, Y, Ai, Z, Wang, T, Zhang, W, Li, D, et al. Seroepidemiology study of HAV, HBV and HEV infection in swines in Dali. Sichuan J Physiol Sci. (2012) 34:103–5. doi: 10.3969/j.issn.1671-3885.2012.03.003
60. Li, M, Wang, Q, and Yuanhua, Y. Detection of hepatitis E virus and serum antibodies against hepatitis E virus in livestock in Heilongjiang Province. J Anhui Agric. (2011) 39:22335–40. doi: 10.3969/j.issn.0517-6611.2011.36.055
61. Li, W, She, R, Wei, H, Zhao, J, Wang, Y, Sun, Q, et al. Prevalence of hepatitis e virus in swine under different breeding environment and abattoir in Beijing, China. Vet Microbiol. (2009) 133:75–83. doi: 10.1016/j.vetmic.2008.06.026
62. Song, A, Li, X, Fan, J, Wang, Y, Zhang, J, and Zhao, C. Epidemiological survey on the infection of hepatitis E virus among pigs in Henan province. Chin J Exp Clin Virol. (2008) 22:24–6. doi: 10.3760/cma.j.issn.1003-9279.2008.01.009
63. Li, X, Zhao, C, Harrison, TJ, Song, A, Fan, J, Zhang, J, et al. Investigation of hepatitis e virus infection in swine from Hunan province, China. J Med Virol. (2008) 80:1391–6. doi: 10.1002/jmv.21246
64. Liang, H, Su, S, Deng, S, Gu, H, Ji, F, Wang, L, et al. The prevalence of hepatitis e virus infections among swine, swine farmers and the general population in Guangdong Province, China. PLoS One. (2014) 9:e88106. doi: 10.1371/journal.pone.0088106
65. Zhang, L, Li, K, Huang, S, Liu, D, Rehman, MU, Lan, Y, et al. Seroprevalence and risk factors associated with hepatitis E virus infections among people and pigs in Tibet, China. Acta Trop. (2017) 172:102–6. doi: 10.1016/j.actatropica.2017.04.033
66. Liu, K. Epidemiological investigation and analysis of zoonotic hepatitis E virus. Jilin Province: Changchun University of Science and Technology (2009).
67. Liu, X, Li, X, Chen, P, Song, A, Luo, Y, Fan, J, et al. Investigation of infection with hepatitis E virus among pigs and different personnels in Hubei. Chin Front Health Quarant. (2007) 5:273–7.
68. Liu, Y, Lai, L, Liao, F, Qianlian, S, Li, B, Xiong, M, et al. Investigation on serological epidemiology of hepatitis E in swine, chicken, cattle and sheep in Guangxi. J Southern Agric. (2012) 43:103–6. doi: 10.3969/j:issn.2095-1191.2012.01.103
69. Lu, B. The epidemiological research of HEV infections among swine and human being in west and north of China. Hunan Province: Hunan Agricultural University (2008).
70. Ma, X, Chengping, L, and Meng, J. Epidemic investigation of swine hepatitis E virus infection in Xinjiang region. Virol Sin. (2004) 3:79–81.
71. Peng, D, Weichang, L, Zhang, J, Xie, Z, and Zhang, X. Serological investigation of swine hepatitis E in Dongguan slaughterhouse. Chin J Anim Husb Vet Med. (2016) 7:27–8. doi: 10.3969/J.ISSN.1671-6027.2016.07.015
72. Qin, L. Seroepidemiological investigation and study of swine hepatitis E in Suiping County. Vet Orient. (2018) 8:211.
73. Qin, Y, Zhao, W, Xiao, A, Liang, J, Liang, B, He, Y, et al. Investigation on serological epidemiology of swine hepatitis E in Guangxi. Guangxi Agric Sci. (2010) 41:383–5. doi: 10.3969/j.issn.2095-1191.2010.04.024
74. Ren, R, Zhang, D, Wen, Z, Pan, S, and Dai, R. Serological investigation of swine hepatitis E virus in Guizhou Province. Guizhou J Anim Husb Vet Med. (2009) 33:19–20. doi: 10.3969/j.issn.1007-1474.2009.05.008
75. Shao, H. Epidemiological investigation of hepatitis E virus in Changchun. J Shandong Educ Instit. (2009) 24:93–5. doi: 10.3969/j.issn.1008-2816.2009.04.028
76. Shi, X, Zhang, X, Cong, W, Ciren, D, Zhu, X, Zhou, E, et al. Seroepidemiological investigation of Tibetan porcine hepatitis E virus in Tibet. Prog Vet Med. (2013) 34:85–8.
77. Shuai, J, Zhang, X, Jingjing, X, Chen, N, and Fang, W. Sero-prevalence of hepatitis E virus infections in pigs from Zhejiang Province during2005-2008. Acta Vet Zootechnica Sinica. (2009) 40:1037–42. doi: 10.3321/j.issn:0366-6964.2009.07.011
78. Tang, J, Chen, X, Cuomu, W, Cidan, L, and Ma, X. Seroepidemiological investigation of Tibetan swine hepatitis E virus along the Lalin highway. Swine Production. (2015):102–4. doi: 10.3969/j.issn.1002-1957.2015.05.039
79. Tong, T, Liu, X, Chunping, LI, Zhou, Y, Ding, N, Luo, Z, et al. EpidemIological investigation of swine hepatitis in Guangdong. Chin J Anim Infect Dis. (2017) 25:13–8.
80. Wang, F, Li, B, Wang, Y, Li, D, Li, X, Shen, M, et al. Investigation of swine hepatitis E virus infection in Haikou and Sanya area. China Trop Med. (2007) 6:897–900. doi: 10.3969/j.issn.1009-9727.2007.06.017
81. Xian-fei, WEI, Jing-rui, LIANG, and Rong-lan, TANG. Serum epidemiological survey of hepatitis E virus infection among swine, rats and dogs in Guangxi. Chin J Public Health. (2007) 2:228–9. doi: 10.3321/j.issn:1001-0580.2007.02.058
82. Wu, JY, Meng, XX, Wei, YR, Bolati, H, Lau, E, and Yang, XY. Prevalence of hepatitis E virus (hev) in feral and farmed wild boars in Xinjiang, Northwest China. Viruses. (2022) 15:78. doi: 10.3390/v15010078
83. Xie, B, He, H, Meng, J, Guan, Y, and Jie, L. Seroepidemiological investigation of swine hepatitis E in some areas of Jiangxi Province. Chin J Vet Med. (2008):41–2. doi: 10.3969/j.issn.0529-6005.2008.10.020
84. Yan, Y. Epidemiological study of animal-derived hepatitis E in Shanghai. Shanghai: Shanghai Jiao Tong University (2008).
85. Yan, Y, Gao, H, Wengui Li, CHEN, Chen, L, Gao, L, and Zhao, R. Serosurvey of swine hepatitis E virus infection in Kunming region. Prog Vet Med. (2010) 31:89–91. doi: 10.3969/j.issn.1007-5038.2010.06.022
86. Yang, Z. Epidemiology analysis of hepatitis E in Jilin city of Jilin province. Jilin Province: Jilin University (2011).
87. Ye, Y, Li, F, Cao, B, Yang, D, Liu, M, Zhao, Z, et al. Serological survey of swine hepatitis E in Fujian Province. Fujian J Anim Husb Vet Med. (2009) 31:17–8. doi: 10.3969/j.issn.1003-4331.2009.06.007
88. Zhai, K. Seroepidemiological investigation of swine hepatitis E in Luoyang area. Livestock Poultry Indust. (2022) 33:24–6.
89. Zhang, HY, Chen, DS, Zhang, Y, Wu, YQ, He, QG, and Liu, ZF. Epidemiological survey of hepatitis E virus in industralized swine herds. Acta Vet Zoot Sinica. (2010) 41:1268–73.
90. Zhang, LQ, Liang, HB, Wang, H, Gao, LZ, Wang, DG, You, J, et al. Epidemiological investigation of swine hepatitis E. China Anim Husb Vet Med. (2012) 39:215–9. doi: 10.3969/j.issn.1671-7236.2012.08.052
91. Zhang, Xiaofeng, Shuai, Jiangbing, Huang, Qiang, Zhu, Zhenjiang, Yu, Zhaofeng, Xu, Sensheng, et al. (2008). Porcine hepatitis E virus infection and its molecular epidemiology in Zhejiang Province. In: 2nd National Symposium on the PCCrevention and Contron of Zoonoses. Taizhou, Jiangsu, China.
92. Zhang, W, Shen, Q, Mou, J, Gong, G, Yang, Z, Cui, L, et al. Hepatitis E virus infection among domestic animals in eastern China. Zoonoses Public Health. (2008) 55:291–8. doi: 10.1111/j.1863-2378.2008.01136.x
93. Zhang, XX, Xu, F, Liang, ZP, and Qiao, GH. Detection and sequence analysis of the swine HEV in Henan. J Henan Agric Univ. (2011) 45:302–6.
94. Zhang, YW, Xu, TY, Zheng, H, Bi, R, Zeng, H, and Li, JT. Investigation and analysis of HEV infection in pig sources. Anim Breeding Feed. (2017) 6:64–5. doi: 10.3969/j.issn.1671-427X.2017.06.032
95. Zhang, Y, Ding, MD, Xie, JB, Qiu, MS, Zhou, LY, Deng, F, et al. Epidemiologic investigation of swine hepatitis E virus among different economic areas in Sichuan Province. Livestock Poultry Indust. (2020) 31:4–6.
96. Zheng, Z. Serological investigation of four important diseases in pigs in Jilin Province. Jilin Province: Jilin Agricultural University (2018).
97. Zhou, J, Sun, Q, Liu, P, Xue, X, Li, K, Jun, L, et al. Serological investigation on hepatitis E of several animal species in Shanghai. Prog Vet Med. (2006) 27:85–8. doi: 10.3969/j.issn.1007-5038.2006.12.021
98. Zhu, G, Liu, T, Sun, Z, Yonggang, Q, Wang, T, and Jin, N. Prevalence of hepatitis E virus in animals in Changchun area, China. Chinese journal of. Biologicals. (2007) 8:570–4. doi: 10.3969/j.issn.1004-5503.2007.08.005
99. Zhu, Y, Li, Y, Zhang, A, Zhou, Y, Shen, R, and Shen, W. Investigation on HEV infection in swine and analysis with partial sequences of swine HEV virus in Shanghai. Shanghai J Prev Med. (2006) 3:103–5. doi: 10.3969/j.issn.1004-9231.2006.03.001
100. Zuo, Y, Li, Y, and Li, J. Pathology of swine hepatitis E in Wenshan prefecture, Yunnan Province. Swine Prod. (2022) 2:117–8. doi: 10.3969/j.issn.1002-1957.2022.02.039
101. AI, X. The investigation of hepatitis E virus infection and etiology study in the epidemiological region of Jiangsu. Jiangsu Province: Southeast University (2009).
102. Hui, CAO, Danjiao, YANG, Min, Z, Ping, L, Chaohui, Z, Cheng, T, et al. Detection and genetic evolution of hepatitis E virus in Tibetan pigs in Sichuan. Acta Vet Zoot Sinica. (2021) 52:2599–608. doi: 10.11843/j.issn.0366-6964.2021.09.023
103. Chen, L. Epidemic characteristics of swine HE in some areas of Heilongjiang Province and pathogenetil characteristics of HE infected rats. Heilongjiang Province: Northeast Agricultural University (2018).
104. Fan, RX. Epidemiological investigation of swine hepatitis E in Tai’an. Shandong Province: Shandong Agricultural University (2023).
105. He, W, Yao, J, Qin, YF, Lin, SY, Zou, L, Huang, JB, et al. Analysis of swine hepatitis E virus genotype in Nanning City of Guangxi from 2015 to 2016. Prog Vet Med. (2017) 38:5–10.
106. Huang, F, Yu, WH, Ma, TW, Jing, SR, and Zeng, WK. Molecuiar epidemiology of swine in a village of Yunnan. Chin J Zoonoses. (2012) 28:274–7. doi: 10.3969/j.issn.1002-2694.2012.03.019
107. Ji, FX, Wang, H, Fu, XL, Wang, LF, Bai, XL, Chen, Y, et al. Molecular epidemiology investigation of swine hepatitis E in some areas of Guangdong. Chin J Vet Sci. (2017) 37:1268–73. doi: 10.16303/j.cnki.1005-4545.2017.07.12
108. Gong, LF, Liu, J, Han, WQ, Cui, WH, Sun, ZL, and Jian, M. The coastal areas of Yantai human and swine hepatitis E virus genotyping analysis. Chin J Exp Clin Virol. (2012) 26:31–3. doi: 10.3760/cma.j.issn.1003-9279.2012.01.011
109. Xia, YG, Lu, YH, Hu, AQ, Qin, X, Dong, XJ, Zhu, JF, et al. Study on the prevalence and genotype of hepatitis E virus among commercial swine population in eastern and southern China. Chin J Epidemiol. (2010) 31:791–4. doi: 10.3760/cma.j.issn.0254-6450.2010.07.016
110. Xie, BD, He, HJ, and Pan, JF. Detection of hepatitis E virus in swine feces from Jiangxi pig farms by RT-nPCR. Chin J Vet Med. (2009) 45:12–4. doi: 10.3969/j.issn.0529-6005.2009.01.004
111. Yan, HY, Zhang, J, Gao, LB, Yin, GF, Shu, XH, Yang, GH, et al. Prevalence of swine hepatitis E in the selected pig farms in Yunnan Province. Anim Husb Vet Med. (2015) 47:10–3.
112. Yan, L. Establishment of fluorescent quantitative PCR assay for swine hepatitis E virus and molecular epidemiology in Yancheng region. Heilongjiang Province: Heilongjiang Bayi Agricultural University (2016).
113. Yang, DJ, Zhang, M, He, ZW, Yang, S, Chen, TY, Wang, YQ, et al. Molecular epidemiology of hepatitis E virus in Tibetan pigs in Ganzi prefecture, Sichuan Province. Chin J Vet Med. (2022) 58:6–11.
114. Zhang, X, Zhen, YJ, Wang, FD, Gao, MY, Zhu, JF, and Jiang, QW. Detection and analysis of partial sequences isolated from swine in rural area of Zhejiang Province. Fudan University. J Med Sci. (2005) 5:586–9. doi: 10.3969/j.issn.1672-8467.2005.05.021
115. Li, Z, Yu, S, Dong, S, Zhu, Y, Si, F, Shen, S, et al. Reduced prevalence of genotype 3 HEV in Shanghai pig farms and hypothetical homeostasis of porcine HEV reservoir. Vet Microbiol. (2009) 137:184–9. doi: 10.1016/j.vetmic.2008.12.013
116. Zheng, Y, Ge, S, Zhang, J, Guo, Q, Ng, MH, Wang, F, et al. Swine as a principal reservoir of hepatitis E virus that infects humans in eastern China. J Infect Dis. (2006) 193:1643–9. doi: 10.1086/504293
117. Zhou, JH, Wang, YN, Li, XR, Liu, YS, Pan, Q, and Lan, X. Prevalence and genetic features of hepatitis e virus in swine, in Gansu, China. Acta Virol. (2018) 62:196–201. doi: 10.4149/av_2018_212
118. Zhou, JM, Chen, G, Si, FS, Yu, RS, Chen, BQ, Xie, CF, et al. Molecular epidemiology investigation of swine hepatitis E in suburb of Shanghai. Acta Agric Shanghai. (2022) 38:93–7. doi: 10.15955/j.issn1000-3924.2022.03.17
119. Zhou, JQ, Liu, PH, Zhang, WY, Liu, J, Ju, HB, Shen, LP, et al. Investigation on swine hepatitis E virus in Shanghai area. J Shanghai Jiaotong Univ. (2009) 27:346–9. doi: 10.3969/j.issn.1671-9964.2009.04.005
Keywords: hepatitis E virus, swine, China, prevalence, meta-analysis, systematic review, China swine hepatitis, epidemiological studies
Citation: He Z, Liu D, Liu B, Zhang P, Wang X, Wang G, Huang Y, Chen J and Cai R (2025) Prevalence of hepatitis E virus in swine in China: a systematic review with meta-analysis (2004–2023). Front. Vet. Sci. 11:1472658. doi: 10.3389/fvets.2024.1472658
Received: 29 July 2024; Accepted: 30 December 2024;
Published: 27 February 2025.
Edited by:
Vassilis Papatsiros, University of Thessaly, GreeceReviewed by:
Elisabetta Razzuoli, Experimental Zooprophylactic Institute for Piedmont, Liguria and Valle d’Aosta (IZSPLVA), ItalyCopyright © 2025 He, Liu, Liu, Zhang, Wang, Wang, Huang, Chen and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rujian Cai, cairujian@gdaas.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.