- 1College of Agronomy, Inner Mongolia Agricultural University, Hohhot, China
- 2Inner Mongolia Academy of Agricultural and Animal Husbandry Sciences, Hohhot, China
- 3College of Life Sciences, Inner Mongolia University, Hohhot, China
In potato production, relatively low phosphorus use efficiency (PUE) leads to excessive phosphorus (P) fertilizer application in many regions, resulting in increasingly environmental risks. Consequently, an increasing number of researchers have started to explore the ways to improve the PUE. The symbiosis between arbuscular mycorrhizal fungi (AMF) and crop roots enhances P uptake. However, the effectiveness of AMF inoculation under field conditions depends on the environment and agronomic managements. In Inner Mongolia, China, few field experiments have been conducted on AMF inoculation in potato production. This is mainly due to low estimates of AMF colonization attributed to fungicide use in seed tuber treatments and soil mechanical disturbance caused by ridging. This study aimed to test whether inoculation with AMF after ridging at the seedling stage could improve AMF colonization in potatoes, thereby enhancing P uptake and tuber yield. Field experiments were conducted in Inner Mongolia to compare the effects of AMF inoculation after ridging at seedling stage versus inoculating seed potato with AMF during sowing, and to investigate the potential of reducing the P application rate through inoculation with AMF in potato production. The AMF colonization rate, soil hyphal density, P uptake, plant growth and tuber yield of potatoes under different treatments were measured. The results showed that compared with AMF inoculation at sowing, inoculation after ridging at the seedling stage significantly increased AMF colonization by 8 percentage points. This led to a significant improvement in P uptake and potato growth, ultimately resulting in a yield increase of approximately 6%. Further findings showed that reducing P application by 25% from the conventional rate (160 kg P2O5 ha−1) led to significant yield loss. Whereas with AMF inoculation at the seedling stage, yield levels were maintained and the partial factor productivity of P fertilizer (PFP) was increased by an average of 39%. In conclusion, this study reveals that AMF inoculation after ridging can mitigate the negative impacts of fungicides in seed tuber treatment and ridging-caused soil disturbance on AMF colonization. It highlights importance of inoculation timing for achieving higher AMF population density. Moreover, the study demonstrates that the developed AMF inoculation enables a reduction of P fertilizer application in potato production. This provides a viable approach to enhance PUE and promote sustainable potato production in areas such as Inner Mongolia. It carries significant agronomic and environmental implications.
1 Introduction
As Phosphorus (P) is relatively immobile in the soil and soluble P fertilizers added to soil become fixed over time (Hinsinger, 2001; Richardson et al., 2009), crops only utilize less than 30% of P fertilizer in the first year after P application (Syers et al., 2008; Rosen et al., 2014; Fernandes et al., 2015). Thus, an increasing number of researches has been attracted to improving the P use efficiency (PUE) in crop production systems.
Potato (Solanum tuberosum L.) ranks the fourth most commonly grown crop in the world, after corn (Zea mays L.), wheat (Triticum aestivum L.), and rice (Oryza sativa L.). Its annual planting area is approximately 21.37 million hectares globally (FAO, 2023). Adequate soil P availability is critical for plant development, tuber formation and tuber maturity enhancement (Jenkins and Ali, 2000; Rosen and Bierman Peter, 2008). On the other hand, P deficiency significantly reduces the tuber number per plant and impairs tuber quality (Hopkins et al., 2010;Rosen et al., 2014; Fernandes et al., 2015). However, the biological characteristics of potatoes, such as shallow rooting system and lower root density (Iwama, 2008; Thornton et al., 2014; Wu et al., 2022), result in low PUE of potatoes. Potato farmers subsequently use more P fertilizer for potatoes than for other crops to ensure an ideal yield (Rosen et al., 2014; Fan, 2019). For instance, more than 180 kg P2O5 ha−1 is usually applied in the potato production in Inner Mongolia of China (Qin et al., 2021). This leads to a further decrease in PUE, an increase in production price, and continuous soil P accumulation. The loss of farmland P is a crucial factor contributing to water body pollution. Eutrophication can be triggered when the aqueous P concentration is as low as 0.02 mg P/L (Correll, 1998). As a result, the continuous accumulation of soil P can lead to substantial environmental pollution via surface runoff, soil erosion, and the leaching of soil P (Liu et al., 2023). Thus, improving P use efficiency is particularly important in potato production (Rosen et al., 2014; Fernandes and Soratto, 2016; Soratto et al., 2020). The symbiosis between arbuscular mycorrhizal fungi (AMF) and plant roots is a well-known beneficial interaction in the soil system (Cely et al., 2016). The hair-like hyphae extend extensively into the soil matrix far beyond the reach of the root system. It significantly expands the root area and functions as efficient conduits to enhance plant nutrient acquisition, particularly phosphorus (Smith and Smith, 2012; Mai et al., 2019). Therefore, the symbiotic associations of potato roots with arbuscular mycorrhizal fungi (AMF) have received growing attention for improving P uptake efficiency (Douds et al., 2007; Hopkins et al., 2014; Hijri, 2016; Carrara et al., 2023). However, it is crucial to note that the effectiveness of AMF symbiosis in enhancing P uptake can be influenced by various factors (Smith and Smith, 2011). In some crop production scenarios, there are marked positive responses to AMF inoculation in terms of P uptake, while others show no response (Duan et al., 2011; Klironomos, 2003; Smith and Smith, 2011). Although numerous pot experiments using radioisotopes (32P and 33P) to directly track and quantify P uptake via AMF have demonstrated very significant contributions in crops (Li et al., 2006; Grace et al., 2009; Smith and Smith, 2011), the effects of inoculation with AMF under field conditions are determined by various factors, including the environment, management, and methods of inoculation (Kabir, 2005; Koyama et al., 2019; Soti et al., 2023; Ghorui et al., 2024). Thus, the effects of AMF inoculation need to be evaluated on a case-by-case basis. In some potato production regions, inoculation was performed via the spraying of a suspension of AMF spores onto potato seeds (Hijri, 2016) or placing the inoculum directly beneath the seed potato (Douds et al., 2007). Nevertheless, no research on inoculation of AMF on potatoes under field condition has been reported in semiarid regions, such as Inner Mongolia (one of the largest potato-planting provinces in China).
Local agricultural practices, such as treatment of seed tubers with fungicides and ridging during emergence (Fan, 2019), present significant challenges to the establishment of AMF. Ridging, while beneficial for tuber development, physically disrupts the established hyphal networks in the soil. Fungicides applied to seed tubers, which are crucial for protecting against soil-borne diseases, inhibit the germination and growth of AMF spores. These factors collectively explain the absence of research on AMF inoculation in potatoes under field conditions in the region. We hypothesize that inoculation with AMF after ridging at seedling stage may avoid soil disturbance by ridging and mitigate the negative effects on AMF colonization caused by the fungicides used in seed potato treatment, subsequently resulting in positive outcomes.
The objectives of this study were to: 1. Conduct experiments in Inner Mongolia to compare the effects of inoculation with AMF after ridging at seedling stage versus inoculating seed potato with AMF during sowing; 2. Investigate the potential of reducing the P application rate through inoculation with AMF in potato production. Key parameters measured included the AMF colonization rate, soil hyphal density, P uptake, plant growth and tuber yield of potatoes under different treatments.
2 Materials and methods
2.1 Experiment 1
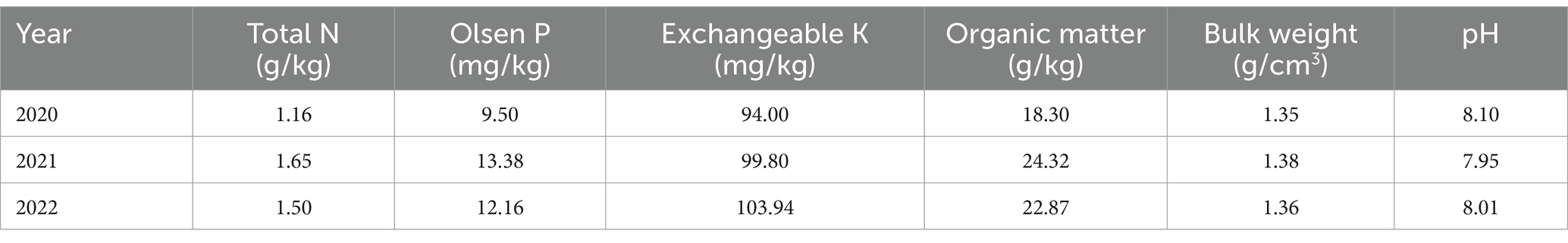
Experiment 1 was conducted in 2020 in Chayouzhongqi, Inner Mongolia (41°30′N, 112°64′E). The soil texture is sandy loam, and other physicochemical properties of the soil are shown in Table 1. The region has a temperate continental monsoon climate, with an annual average temperature of 1.3°C and an annual average precipitation of ~300 mm; therefore, irrigation is necessary for potato production.
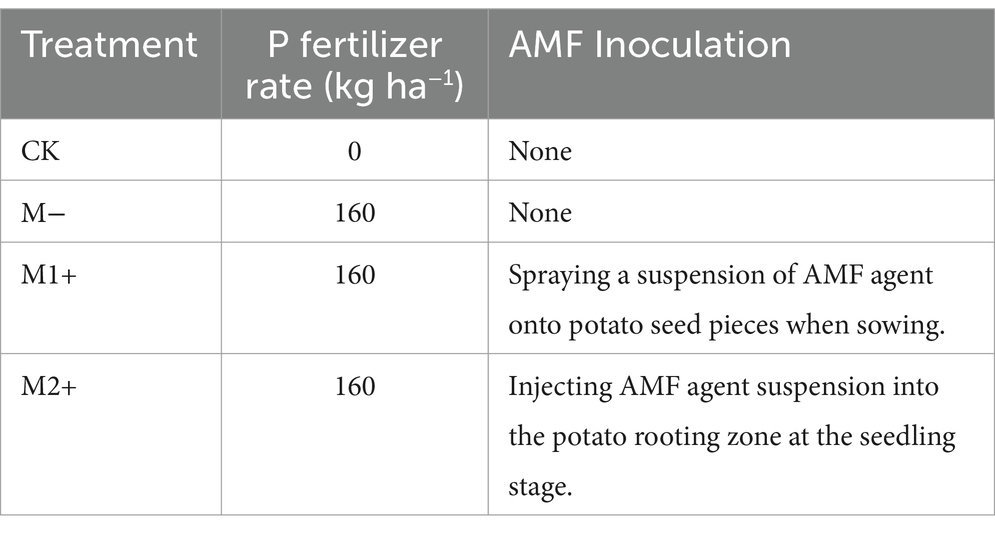
Four treatments were designed, as shown in Table 2. The experiment adopted a randomized complete block design with three replicates. Each plot covered 90 m2 and contained potato plants (cultivar Kexin-1) in rows 90 cm apart, with 20 cm between plants within a row. A drip irrigation system was used in the experiment. No P was applied in control treatment (CK). In contrast, 160 kg P2O5 ha−1 was applied for each of the other treatments. The P source was monoammonium phosphate (61% P2O5). For each treatment, 300 kg N ha−1 as control-released urea and 300 kg K2O ha−1 as potassium sulfate were applied. The total amounts of N, P, and K fertilizers were applied as a basal dose through broadcast application during the sowing. The potatoes were sown on May 3 and were harvested on September 7. A commercial AMF inoculum with Rhizophagus intraradices (Nanjing Cuijingyuan Biotechnology Co., Ltd., China) was used for the M1+ and M2+ treatments. For the M1+ treatment, AMF inoculation was carried out by applying a 225 g L−1 inoculum suspension to seed potato tubers. A total of 5 L of the inoculum suspension was used for the seed tubers in each plot, resulting in an application rate of 2.25 g inoculum (containing approximately 100 spores) per seed tuber. For M2+, inoculation was conducted on Day 8 after emergence through injecting a 11.25 g L−1 inoculum suspension into the soil. The injection points were 20 cm beneath the soil surface, on both sides of each seedling, 10 cm away from the seedling. Each injection hole received 0.1 L of the liquid suspension, so that each plant was inoculated with 2.25 g of the inoculum. M− represents the treatment without AMF inoculation, CK represents the treatment which there is neither AMF inoculation nor the application of P fertilizer. In all treatments, the seed tubers were treated with fungicides in the manner commonly used in local potato cultivation (Fan, 2019).
2.2 Experiment 2
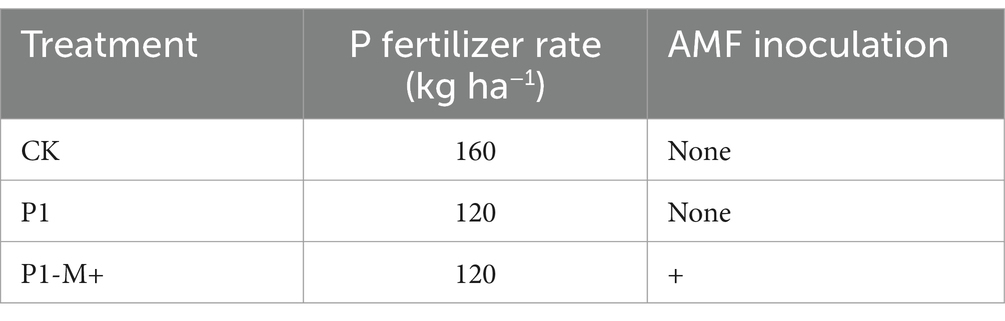
Experiment 2 was conducted in 2021 and 2022 at the same area as in Experiment 1. As shown in Table 3, three treatments were designed: conventional P application rate or farmer practice mode, denoted as CK; A reduction of 25% in P application rate, denoted as P1; a reduction of 25% in P application rate with inoculating AMF at seedling stage, denoted as P1-M+. The AMF inoculation method used M2+ as in Experiment 1. The experiment adopted a randomized complete block design with three replicates. Each plot covered 90 m2 and contained potato plants (cultivar Kexin-1) in rows 90 cm apart, with 20 cm between plants within a row. The nitrogen and potassium fertilizer rates used were the same as those used in Experiment 1. The potatoes were sown on May 3 in 2021 and May 4 in 2022, and were harvested on September 7 and September 3 in 2021 and 2022, respectively.
2.3 Sampling and measurements
2.3.1 Plant dry weight, leaf area index, phosphorus concentration, and mycorrhizal colonization rate
At the tuber formation stage and tuber bulking stage of potatoes each year, three plants were randomly sampled from each plot and then separated according to their roots, stems, leaves, and tubers. The fresh mass of each plant part was measured, and leaf area was measured using a plant leaf scanner (LA-S, Hangzhou, China). The leaf area index (LAI) was measured according to LAI = total leaf area/land area (Cui et al., 2020). After the fresh weight of each part was recorded, half of the root samples were kept in a refrigerator for mycorrhizal colonization rate counting, and all other plant samples were subsequently dried at 80°C to a constant weight for subsequent weighing and P nutrient analysis. Each dry sample was ground and sieved through a 0.25 mm sieve. Subsequently, it was digested in a mixture of H2SO4 and H2O2. After digestion, the total P concentration was determined spectrophotometrically via a continuous flow analyzer system (SKALAR SAN++, Netherlands). When counting the mycorrhizal colonization rate, 30 roots were randomly selected from each treatment, then they were cut into 1 cm segments. Subsequently, the segments were cleaned with 10% (w/v) KOH solution at 90°C for 1 h. After staining the root samples with trypan blue, the observation of the samples and the counting of mycorrhizal colonization were conducted under a microscope employing the grid-cross method (Phillips and Hayman, 1970).
2.3.2 Soil hyphal length density
During the tuber bulking stage, soil samples were collected at five vertical depths (0–10, 10–20, 20–30, 30–40, and 40–50 cm) 0, 3.75, and 7.5 cm apart from the drip tape emitter; five sites were randomly sampled in each plot. After being air dried, the soil samples were passed through a 1 mm sieve for determination of the soil AMF density. The 1 mm sieve was selected primarily because it can retain root residues and sand particles, while allowing AMF hyphae, which have a diameter ranging from 2 to 20 micrometers, to pass through unhindered. This minimizes interference during microscopic observation (Wang et al., 2021). External mycorrhizal hyphae were extracted from six 5 g portions of air-dried substrate by the membrane filter technique. The hyphal length was measured using the gridline intercept method at a magnification of 200×. Subsequently, the measured value was converted into soil hyphal length density, following the method described by Zhang et al. (2018).
2.3.3 Tuber yield
At the end of each experiment, potatoes in 30 m2 of each plot were manually harvested, and the tuber yields were calculated as t ha−1.
2.4 Calculation and statistics
2.4.1 Partial factor productivity of P fertilizer (PFPP)
The partial factor productivity of P fertilizer (PFPP) provides an intuitive assessment of its contribution to crop yield, thereby enabling the evaluation of its application effectiveness. PFPP was calculated via the following formula:
where YP is the potato yield (kg ha−1) and AP is the applied P fertilizer (kg ha−1).
2.4.2 Hyphal length density (HLD)
The hyphal length density (HLD) can be used to understand the growth status, reproduction rate, and distribution characteristics of soil arbuscular mycorrhizal fungi, so as to assess the ecological function of AMF. It was calculated via the following formula:
2.4.3 Statistical methods
Data analysis was performed via SPSS 25.0 software (Version 25.0; SPSS, Inc., Chicago, IL, USA). The plots were created via Surfer 13.0 (Golden Software, Inc., Golden) and Origin 2021 (Origin Lab, Northampton, MA, USA). ANOVA and Tukey’s test were used to evaluate the significance of results at level of p < 0.05.
3 Results
3.1 Responses of potato growth and phosphorus uptake to mycorrhizal agent inoculation time
3.1.1 Mycorrhizal colonization rate
For Experiment 1, the mycorrhizal colonization rates of potatoes at different inoculation times were measured at the tuber formation stage and tuber bulking stage. Compared with inoculation with the AMF agent at sowing (M1+), inoculation with the AMF agent at the seedling stage (M2+) resulted in 11 and 13 percentage points greater mycorrhizal colonization rates (p < 0.05) at the tuber formation stage and tuber bulking stage, respectively. The mycorrhizal colonization rates under no P fertilizer application (CK) were the lowest, ranging from 14 to 16% after tuber formation. P application (with an amount of 160 kg P2O5 ha−1) without AMF inoculation (M−) resulted in higher mycorrhizal infection rates than did the CK at both growth stages (p < 0.05), which ranged from 17 to 23%. However, no significant difference was observed between M− and M1+ (p > 0.05) (Figure 1). Figure 1 also shows that the mycorrhizal colonization rates increased from the tuber formation stage to the tuber bulking stage for all the treatments.
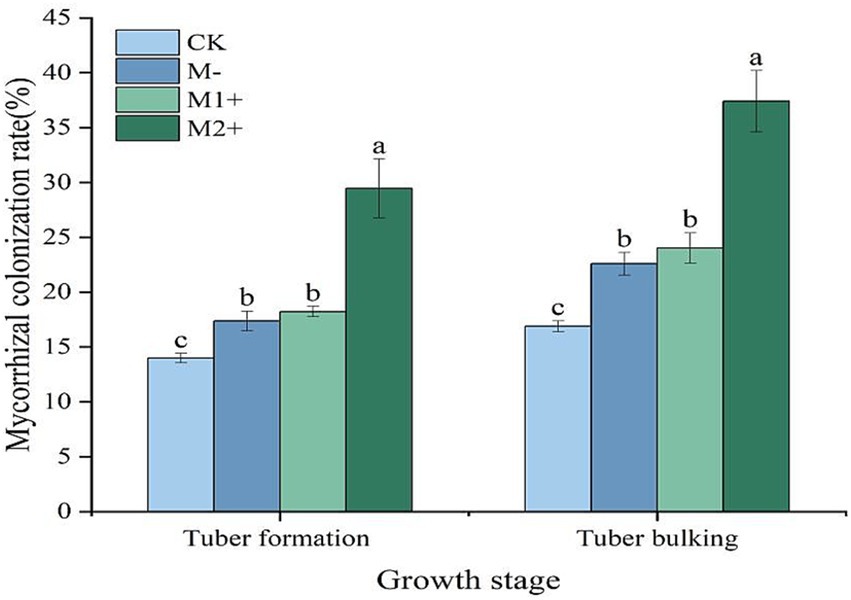
Figure 1. Colonization rate of arbuscular mycorrhizae in potatoes at different growth stages (Exp. 1). Different letters denote significant differences among treatments within the same growth stage (p < 0.05).
3.1.2 Leaf area index
The leaf area index (LAI) increased with potato growth, reached a maximum at the starch accumulation stage, and then decreased. Under CK (no P treatment), the LAI was always the lowest during potato growth. At the seedling stage and tuber formation stage, no difference was observed among the other three treatments (p > 0.05), whereas from the tuber bulking stage to preharvest, the LAI under M2+ was significantly greater than that under M1+ or M− (p < 0.05), which reached 7.38 on average at the starch accumulation stage, approximately 11% greater than that under M1+. No significant difference in the LAI was detected between M1+ and M− during potato growth (p > 0.05) (Figure 2).
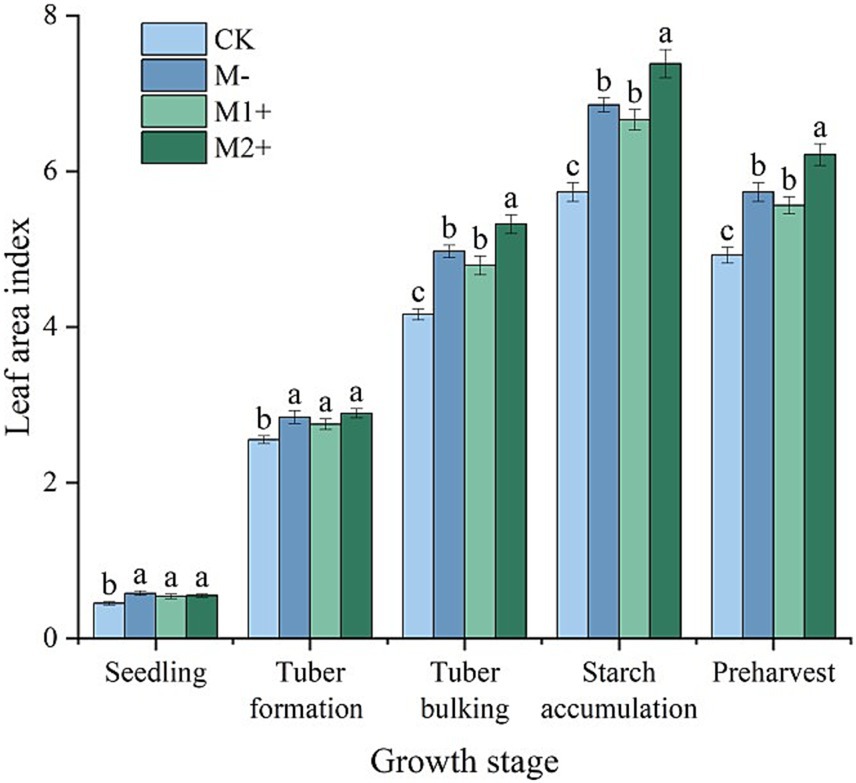
Figure 2. Leaf area indices of potatoes at different growth stages in Exp. 1. Different small letters denote significant differences among treatments at the same growth stage (p < 0.05).
3.1.3 Plant dry weight
Figure 3 illustrates the temporal dynamics of plant dry weight (DW) throughout potato growth, with values progressively increasing to reach maximum levels at the preharvest stage. Throughout the growth cycle, the CK consistently exhibited the lowest DW values. During the seedling stage and tuber formation stage, no significant difference was observed among M−, M1+, and M2+ (p > 0.05), whereas from the tuber bulking stage to preharvest, the DW under M2+ was significantly greater than that under M1+ or M− (p < 0.05). Notably, no statistically significant difference was detected between M1+ and M− in DW at any growth stage (p > 0.05). These DW patterns showed strong correspondence with the mycorrhizal infection rates (Figure 1) and were consistent with the treatment-induced variations in LAI shown in Figure 2.
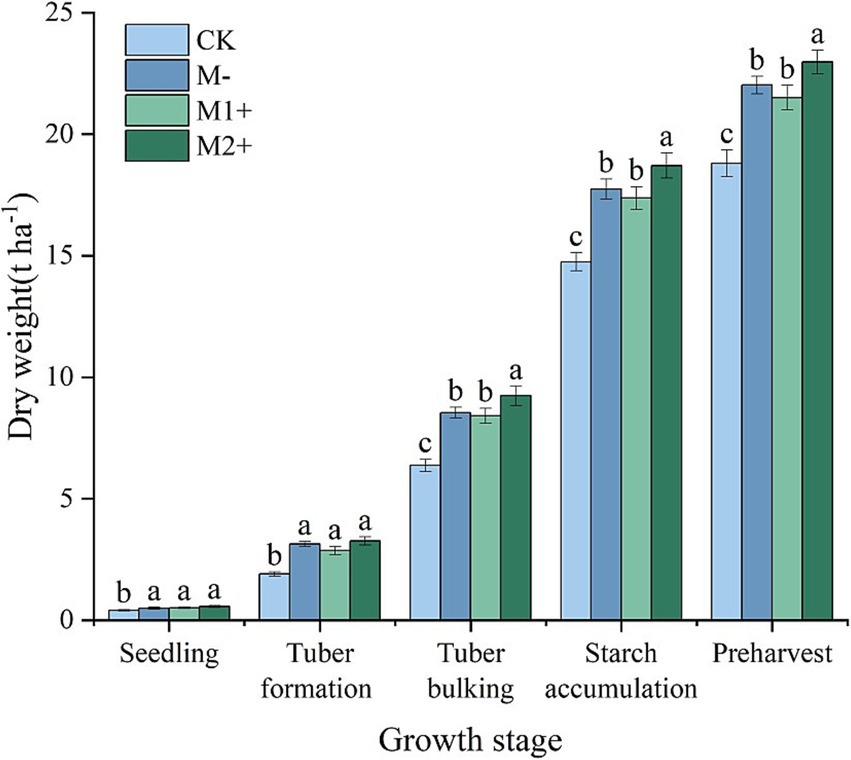
Figure 3. Dry weight of potatoes at different growth stages in Exp. 1. Different small letters denote significant differences among treatments at the same growth stage (p < 0.05).
3.1.4 Tuber yield
As shown in Table 4, tuber yields ranged from 51 to 61 tha−1. Among all treatments, the tuber yield under M2+ was the highest, reaching approximately 61 kg ha−1. It exceeded the yield under M1+ by approximately 6%. This increase in tuber yield is closely related to the elevated mycorrhizal colonization rate (Figure 1), the further expansion of the photosynthetic area (Figure 2) and enhanced plant growth (Figure 3). M1+ did not result in a significantly higher yield than did M− (p > 0.05). The yield under CK was the lowest.
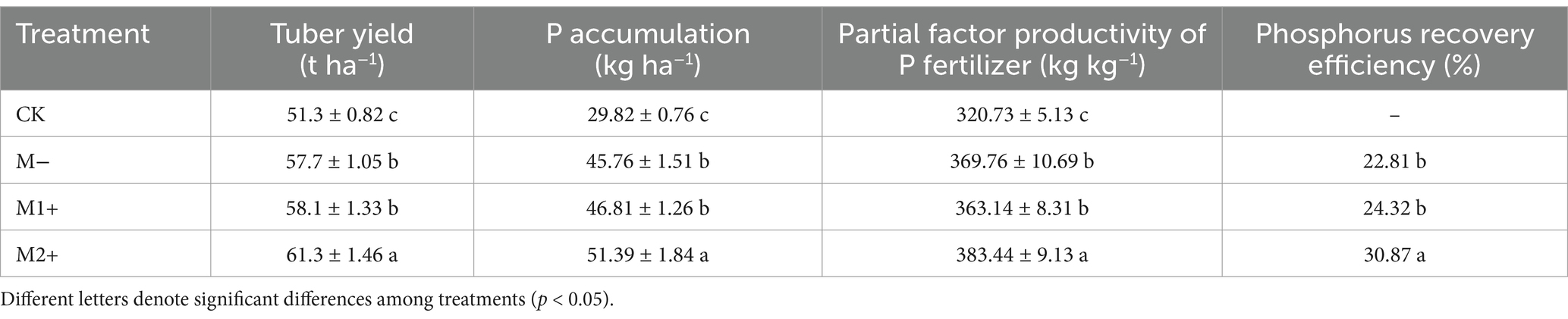
Table 4. Tuber yield, P accumulation and phosphorus use efficiency under different treatments in Exp. 1.
3.1.5 Phosphorus accumulation and phosphorus use efficiency
Table 4 also shows that plant P accumulation under M2+ reached approximately 51 kg ha−1 at preharvest, which was 10% greater than that under M1+ (p < 0.05). However, no significant difference was detected between M1+ and M− in terms of P accumulation (p > 0.05). As the mycorrhizal colonization rate is a crucial parameter for assessing the effectiveness of AMF inoculation, it indicates the degree to which the AMF hyphae expand the P absorption of plant roots. Thus, the enhanced plant P accumulation under M2+ can be speculated obviously to associate with the significantly increased mycorrhizal colonization rate, as shown in Figure 1. The P accumulation patterns in plants reflected the underlying mechanisms driving treatment-induced differences in DW and tuber yield formation (Figure 3, Table 4). The P fertilizer recovery rate under M2+ was much greater than that under any of the other treatments (p < 0.05), reaching approximately 31%. However, the P fertilizer recovery rate under M1+ was not significantly greater than that under M− (p > 0.05). The partial factor productivity of P fertilizer (PFP) exhibited a similar trend: the PFP under M2+ was 20 kg kg−1 greater than that under M1+, and the PFP under CK was the lowest.
3.2 Potato growth, tuber yield and P use efficiency under conditions of AMF inoculation and reduced P fertilizer application (Experiment 2)
3.2.1 Tuber yield and phosphorus use efficiency
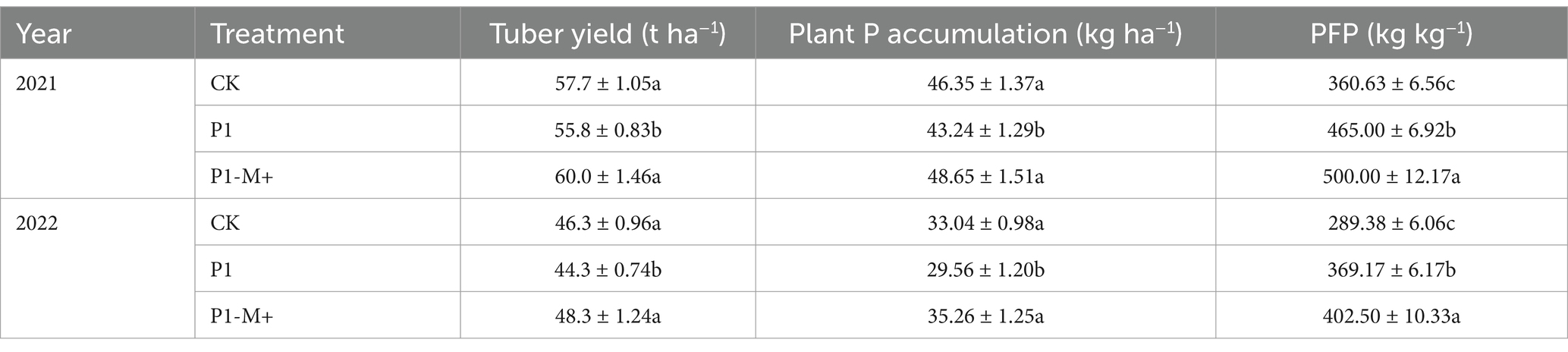
In Experiment 2, compared with the conventional P recommendation treatment (CK, 160 kg P2O5 ha−1), reducing the P fertilizer application rate by 25% (P1, 120 kg P2O5 ha−1) significantly decreased the tuber yield (p < 0.05). On average over 2 years, it was approximately 2 t ha−1 lower than that of the CK. Under P1-M+, i.e., inoculation with AMF with the application of 120 kg P2O5 ha−1, the tuber yield was not only greater than that under P1 but also greater than that under CK (p < 0.05), and consistent results were obtained in both experimental years (Table 5).
Table 5 also shows that, compared with the CK treatment, P1 reduced plant P accumulation by 7–11% in 2 years, whereas P1-M+ significantly increased plant P accumulation (p < 0.05), which was 13 and 19% greater than that of P1 in 2021 and 2022, respectively. In the P1-M+ treatment, the PFP ranged from 400 to 500 kg kg−1, which was, on average, 8% greater than that in P1 and 38% greater than that in CK (p < 0.05).
3.2.2 Colonization rate of arbuscular mycorrhizae in potato
Table 6 shows that the colonization rate of arbuscular mycorrhizae in potato plants under P1-M+ was significantly greater than that under CK or P1 (p < 0.05). At the tuber formation stage, it was on average 10 or 13 percentage points higher than that of P1 or CK, respectively. At the tuber bulking stage, the colonization rates of arbuscular mycorrhizae in potatoes in all the treatments increased significantly (p < 0.05), and those in P1-M+ increased more; on average, the rates were 14 and 17 percentage points higher than those in P1 and CK, respectively. Compared with CK, P1 resulted in higher rates of arbuscular mycorrhizal colonization in potato plants (p < 0.05). The results were consistent over 2 years.
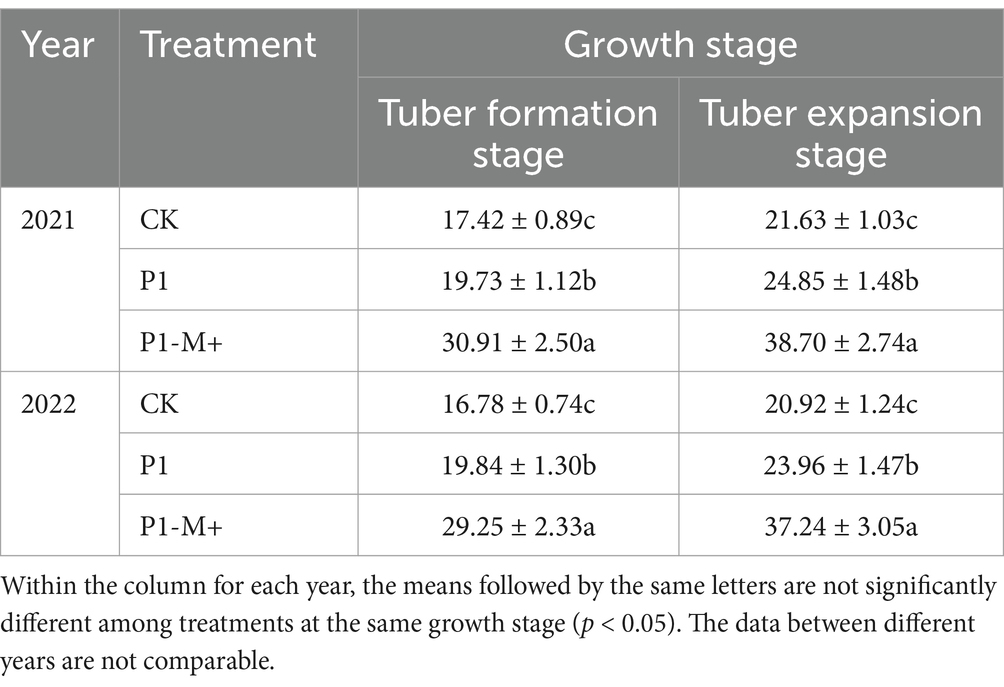
Table 6. Colonization rates of arbuscular mycorrhizae in potatoes (%) at different growth stages in Exp. 2.
Figure 4 presents the spatial distribution of mycorrhizal hyphae in different soil layers at the tuber bulking stage, which is presented as the hyphal length density (HLD, cm g−1). In the 0–20 cm soil layer, the HLD under P1-M+ was 26–36% greater than that under P1 (p < 0.05). The HLD in the 20–40 cm soil layer decreased compared with that in the 0–20 cm soil layer for all the treatments, while the HLD under P1-M+ was still greater than those under P1 and CK in this layer (p < 0.05). The HLD under CK was the lowest in each soil layer. Similar results were observed in the 2 years. The variations in the colonization rate of arbuscular mycorrhizae (Table 6) and the hyphal length density across different treatments (Figure 4) were consistent with the plant P accumulation (Table 5).
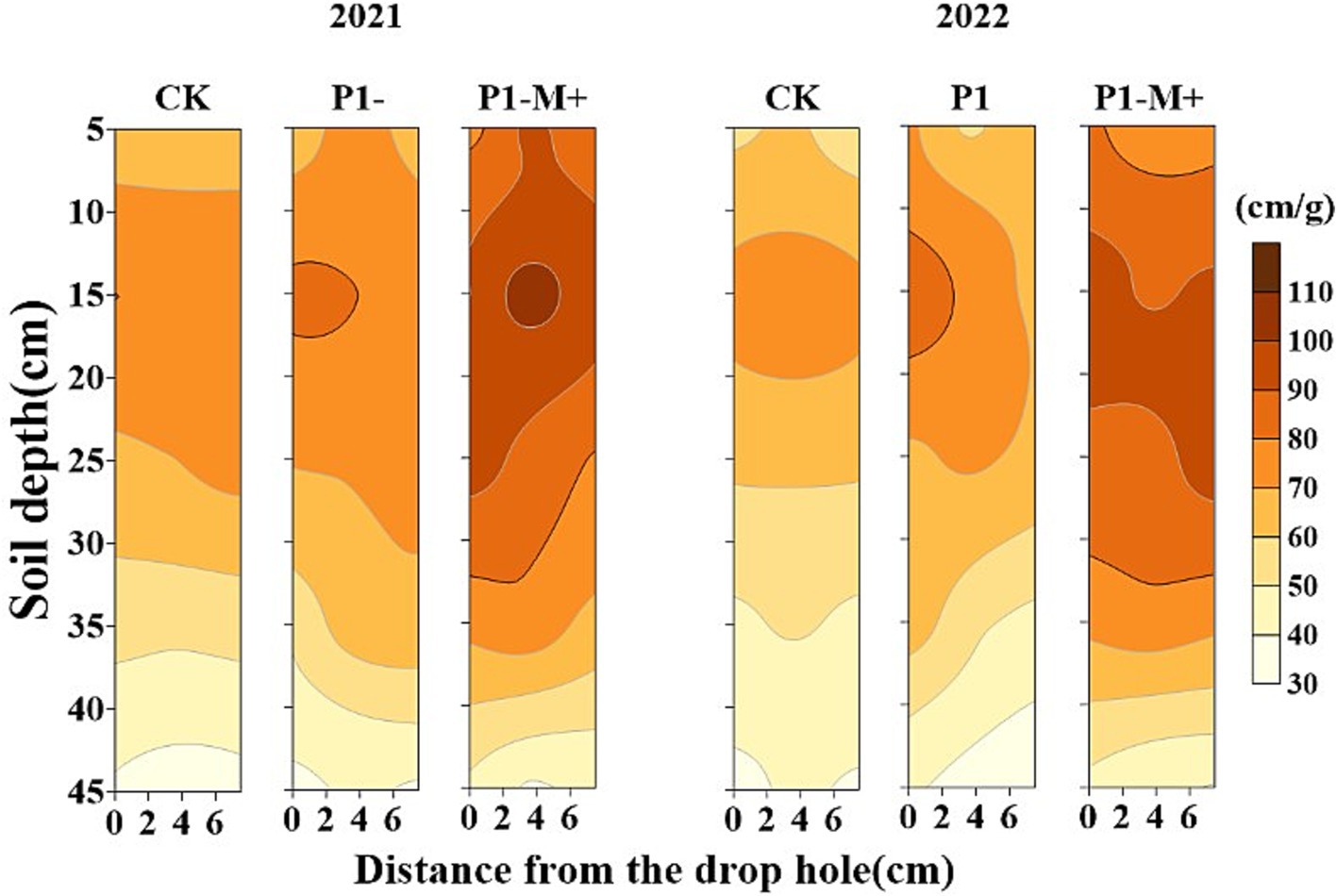
Figure 4. Spatial distribution of mycorrhizal hyphal length density at the tuber bulking stage in Exp. 2.
3.2.3 Leaf area index and plant dry weight
At the seedling stage, no difference was detected among the treatments in terms of the leaf area index (LAI) (p > 0.05), whereas from the tuber formation stage to the starch accumulation stage, the LAI under P1-M+ or CK was greater than that under P1 (p < 0.05), and no significant difference was detected between CK and P1-M+ (p > 0.05). The second-year results showed similar trends (Table 7).
At the seedling stage, the plant dry weight (DW) under the different treatments did not significantly differ (p > 0.05), but from the tuber formation stage to preharvest, the DW under P1-M+ or CK was greater than that under P1 (p < 0.05). From the tuber bulking stage, the DW under P1-M+ was significantly greater than that under CK (p < 0.05), being, on average, 3% greater than that under CK (Figure 5).
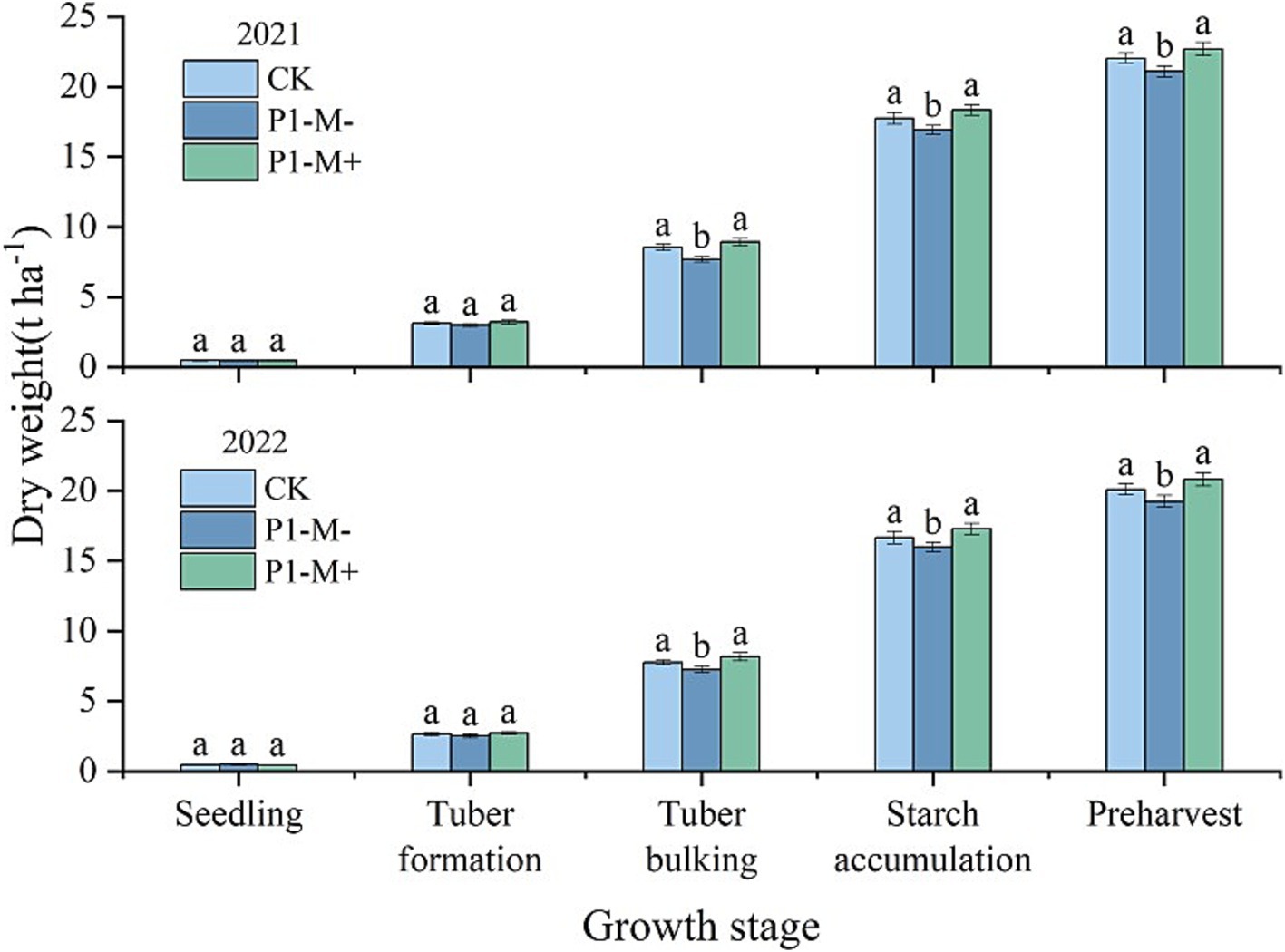
Figure 5. Dry weight of potatoes at different growth stages in Exp. 2. Different small letters denote significant differences among treatments at the same growth stage (p < 0.05).
3.2.4 Correlation between potato performance indicators and the mycorrhizal parameters
Figure 6 shows that colonization rate of arbuscular mycorrhizae, hyphal length density, plant P accumulation, plant dry weight and tuber yield were all positively correlated with each other (p < 0.05). The plant LAI showed significant correlation with both plant dry weight and tuber yield, while no significant correlation between plant LAI and mycorrhizal parameters was observed (p > 0.05). The partial productivity of P fertilizer showed significantly positive correlations (p < 0.05) with both colonization rate of arbuscular mycorrhizae and hyphal length density, but no significant relationship was observed with other indicators (p > 0.05).
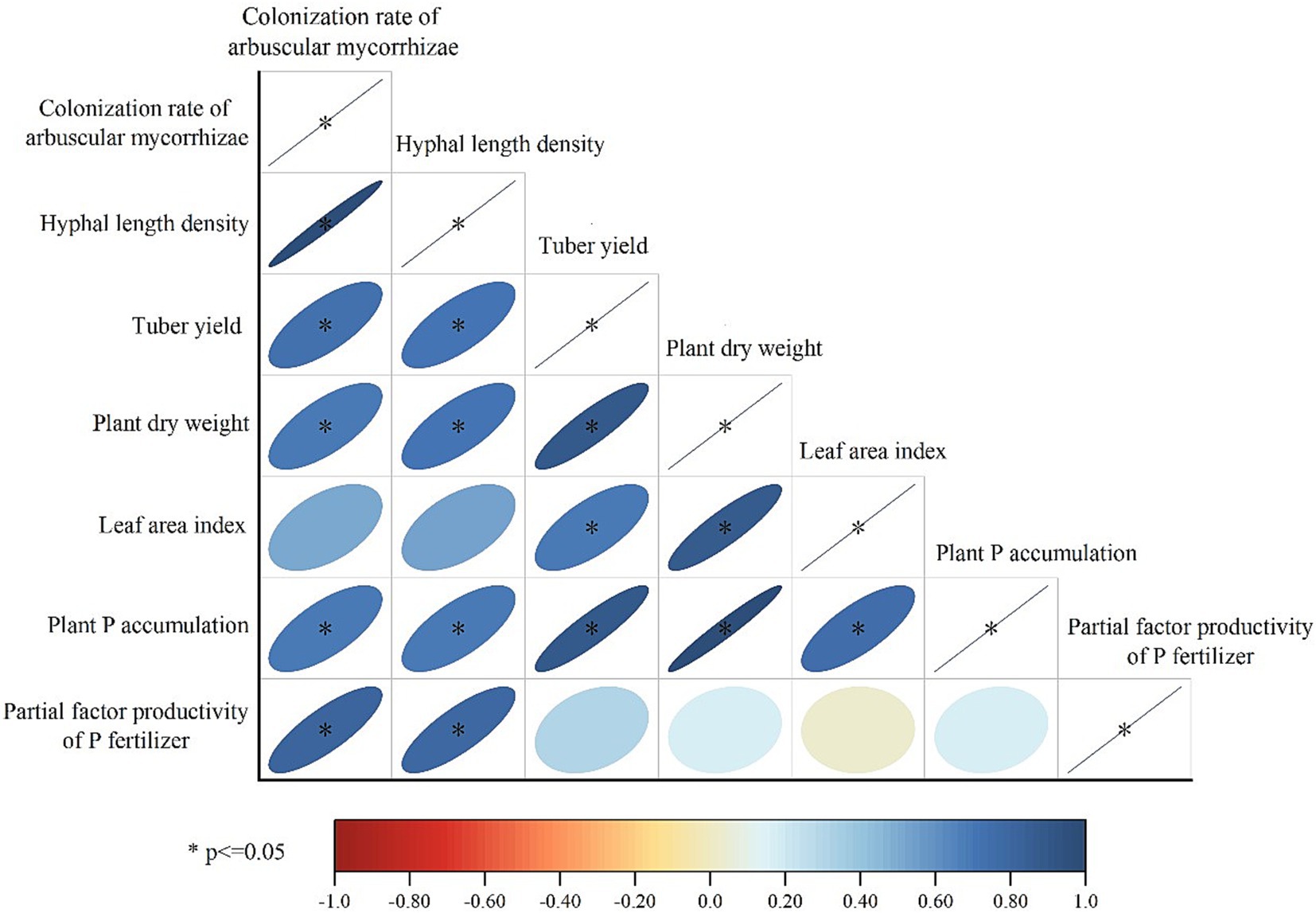
Figure 6. Correlations among potato performance indicators and the mycorrhizal parameters (Pearson correlation).
4 Discussion
Despite the well-documented potential of AMF to enhance P uptake and improve crop yields (Cely et al., 2016; Duan et al., 2025) and its long-standing commercial availability as inoculants, their field application in potato production systems, such as those in Inner Mongolia, remains unexplored. This implementation gap primarily results from two region-specific agricultural practices: the extensive use of fungicides in seed tuber treatment and early-stages ridging operation. Both of which are believed to impede the establishment of high AMF population densities. The results of this research (Figures 1, 3; Table 4) substantiate the previous assumption. They demonstrate that conventional sowing-time AMF inoculation fails to establish an efficient AMF hyphal network during the crucial growth stages of potatoes (Figure 1). Therefore, in potato production systems in Inner Mongolia and similar regions, applying AMF during sowing may not be an appropriate strategy.
Contrary to the ineffectiveness of sowing-time inoculation, our study clearly demonstrates that AMF inoculation at the seedling stage, following ridging operations, increased tuber yield by approximately 6% (Table 4). These yield improvements are strongly correlated with increased mycorrhizal colonization rates (Figures 1, 4, 6). AMF hyphae can penetrate soil pores that are otherwise inaccessible to plant roots, thereby enhancing P absorption. The mycorrhizal colonization rate reflects the degree to which AMF hyphae expand the P absorption of plant roots (Smith and Smith, 2011), making it a critical parameter for assessing AMF inoculation efficacy (Adeyemi et al., 2019; Ul Haq et al., 2022). Given that P in soil is relatively immobile (Hinsinger, 2001; Richardson et al., 2009), the ~13% higher mycorrhizal colonization rates for seedling-stage inoculation to sowing-time inoculation (Figure 1; Table 6) suggests that seedling-stage inoculation can significantly expand the exploring area for soil P. This is strongly evidenced by the ~10% higher plant P accumulation and ~7% greater P fertilizer recovery rate under seedling-stage inoculation than sowing-time inoculation (Tables 4, 5). The enhanced P acquisition further promotes plant growth, manifested as higher plant LAI and plant DW at the critical growth stages of potato (Figures 2, 3, 5; Table 7). These improvements in LAI and plant growth at the critical growth stages of potato provide a solid foundation for the increased tuber yield (as shown Tables 4, 5). Therefore, in potato production systems such as those in Inner Mongolia, inoculating potatoes with AMF agents after ridging at the seedling stage is a viable option.
The optimal inoculation timing identified in this study significantly differ from conventional practices used elsewhere, such as spraying spore suspension on seed tubers or direct placement of inoculum beneath seed tubers at sowing (Douds et al., 2007; Hijri, 2016; Susiana et al., 2019). They highlight the need for region-specific inoculation strategies within specific agroecosystems. This finding is of particular significance for potato production systems in Inner Mongolia and similar regions.
Our study further demonstrates the potential for reducing P fertilizer application rates in potato production when combined with optimized AMF inoculation. The results regarding yield and PFP under condition of reducing P application by 25% with seedling-stage AMF inoculation (Table 5) complement recent reports in other crops (Mai et al., 2019; Das et al., 2022; de Souza Buzo et al., 2022). Moreover, this study provides the first comprehensive evidence for reducing P fertilizer application rate in potato production system in Inner Mongolia’s specific agricultural context.
The effectiveness of AMF is highly dependent on soil temperature, with optimal colonization typically occurring in moderately warm soils (15–25°C) (Smith and Smith, 2011). Soil temperatures consistently below 10°C will significantly delayed spore germination and hyphae expansion, leading to a reduction in the mycorrhizal colonization rate. In the potato production region of Inner Mongolia, soil temperature at the 0–10 cm layer is typically below 10°C before early May. Therefore, inoculation at sowing time may encounter challenges due to relatively low soil temperatures. This study demonstrates that the mycorrhizal colonization rates increased with potato growth, and were significantly higher at the tuber bulking stage than the tuber formation stage (Figure 1; Table 6), confirming the impact of temperature on the effectiveness of AMF. More importantly, it implies that inoculation should be carried out as soon as possible after ridging to make full use of heat resources, thereby ensuring a sufficient AMF infection rate and providing sufficient time for the hyphae to function.
In addition to environmental conditions and management, factors identified as significant for a favorable outcome of AMF inoculation include potato genotype (Vosatka and Gryndler, 2000), and the AMF strain (Davies et al., 2005). The current research focused on a single potato cultivar and AMF strain, suggesting the need for future studies examining different potato genotypes and AMF strain combinations. Additionally, the potential carryover effects of AMF inoculation in Inner Mongolia’s typical potato–wheat–wheat rotation system remain unexplored, representing an important area for future research.
5 Conclusion
This study demonstrates that seedling-stage AMF inoculation, achieved through soil injection, significantly improves mycorrhizal colonization rate (by ~13 percentage points), P uptake (~10% greater), and potato yields (6–8% increase) compared to conventional sowing-time application. Importantly, combining this optimized AMF inoculation with a 25% reduction in P fertilizer application maintains tuber yields while increasing PFP by 39% relative to conventional practices. These results reveal that seedling-stage AMF inoculation can mitigate the negative effects of fungicides in seed tuber treatment and ridging-caused soil disturbance on AMF colonization, highlighting the importance of inoculation time for high AMF population density. The developed AMF inoculation offers significant potential for P fertilizer reduction in potato production, which is crucial for improving P fertilizer use efficiency in sustainable potato production in regions like Inner Mongolia. Thus, it is of both agronomic and environmental significance.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XL: Data curation, Formal analysis, Investigation, Writing – original draft. JY: Data curation, Formal analysis, Investigation, Writing – original draft. JW: Writing – original draft, Conceptualization. YQ: Conceptualization, Writing – original draft, Funding acquisition, Methodology. XS: Methodology, Writing – original draft, Data curation, Formal analysis. KL: Data curation, Formal analysis, Methodology, Writing – original draft. LJ: Methodology, Writing – review & editing. MF: Writing – review & editing, Conceptualization, Resources, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R&D Program of China [2022YFD1900302-03], and the National Natural Science Foundation of China [32060724].
Acknowledgments
We acknowledge support for this work from the Innovation Team of Inner Mongolia Agricultural University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adeyemi, N. O., Atayese, M. O., Olubode, A. A., and Akan, M. E. (2019). Effect of commercial arbuscular mycorrhizal fungi inoculant on growth and yield of soybean under controlled and natural field conditions. J. Plant Nutr. 43, 487–499. doi: 10.1080/01904167.2019.1685101
Carrara, J. E., Reddivari, L., and Heller, W. P. (2023). Inoculation of black turtle beans (Phaseolus vulgaris) with mycorrhizal fungi increases the nutritional quality of seeds. Plant Environ. Interact. (Hoboken, N.J.) 5:e10128. doi: 10.1002/pei3.10128
Cely, M. V. T., de Oliveira, A. G., de Freitas, V. F., de Luca, M. B., Barazetti, A. R., dos Santos, I. M. O., et al. (2016). Inoculant of Arbuscular Mycorrhizal Fungi (Rhizophagus clarus) increase yield of soybean and cotton under field conditions. Front. Microbiol. 7:720. doi: 10.3389/fmicb.2016.00720
Correll, D. L. (1998). The role of phosphorus in the eutrophication of receiving waters: a review. J. Environ. Qual. 27, 261–266. doi: 10.2134/jeq1998.00472425002700020004x
Cui, S., Qin, Y., Yu, J., Shi, X., and Fan, M. (2020). Improving tuber yield and phosphorus use efficiency using split phosphorus application to potatoes in Inner Mongolia. Am. J. Potato Res. 97, 318–324. doi: 10.1007/s12230-020-09783-3
Das, D., Ullah, H., Himanshu, S. K., Tisarum, R., Cha-Um, S., and Datta, A. (2022). Arbuscular mycorrhizal fungal inoculation and phosphorus application improve growth, physiological traits, and grain yield of rice under alternate wetting and drying irrigation. J. Plant Physiol. 278:153829. doi: 10.1016/j.jplph.2022.153829
Davies, F. T., Calderon, C. M., and Huaman, Z. (2005). Influence of arbuscular mycorrhizae indigenous to Peru and a flavonoid on growth, yield, and leaf elemental concentration of ‘Yungay’ potatoes. HortScience 40, 381–385. doi: 10.21273/HORTSCI.40.2.381
de Souza Buzo, F., Satin Mortinho, E., Lobo Santana, T. L., Militão Garcia, I., de Carvalho, C. L. M., and Filho, M. C. M. T. (2022). Bean nutrition and development in the function of reduced phosphorus doses and inoculation with arbuscular mycorrhizal fungus. J. Plant Nutr. 45, 1942–1952. doi: 10.1080/01904167.2022.2043372
Douds, D. D., Nagahashi, G., Reider, C., and Hepperly, P. R. (2007). Inoculation with Arbuscular Mycorrhizal Fungi increases the yield of potatoes in a high P soil. Biol. Agric. Hortic. 25, 67–78. doi: 10.1080/01448765.2007.10823209
Duan, T., Facelli, E., Smith, S. E., Smith, F. A., and Nan, Z. (2011). Differential effects of soil disturbance and plant residue retention on function of arbuscular mycorrhizal (AM) symbiosis are not reflected in colonization of roots or hyphal development in soil. Soil Biol. Biochem. 43, 571–578. doi: 10.1016/j.soilbio.2010.11.024
Duan, S., Huo, Y., Tian, Y., Yan, W., George, T. S., Huang, C., et al. (2025). The interplay of direct and mycorrhizal pathways for plants to efficiently acquire phosphorus from soil. Front. Agric. Sci. Eng. 56. doi: 10.15302/J-FASE-2024589
Fan, M. (2019). Physiology of potato mineral nutrition and nutrient management (in Chinese). Beijing: China Agricultural Press.
FAO. (2023). Forestry data, FAOSTAT Database, Food and Agriculture Organization of the United Nations, Rome, Italy. Available at: https://www.fao.org/faostat/en/#data/QCL. (Accessed July 01, 2024).
Fernandes, A. M., and Soratto, R. P. (2016). Response of potato cultivars to phosphate fertilization in tropical soils with different phosphorus availabilities. Potato Res. 59, 259–278. doi: 10.1007/s11540-016-9330-z
Fernandes, A. M., Soratto, R. P., and Pilon, C. (2015). Soil phosphorus increases dry matter and nutrient accumulation and allocation in potato cultivars. Am. J. Potato Res. 92, 117–127. doi: 10.1007/s12230-014-9422-8
Ghorui, M., Chowdhury, S., Balu, P., and Burla, S. (2024). Arbuscular Mycorrhizal inoculants and its regulatory landscape. Heliyon 10:e30359. doi: 10.1016/j.heliyon.2024.e30359
Grace, E. J., Smith, F. A., and Smith, S. E. (2009). “Deciphering the arbuscular mycorrhizal pathway of P uptake in non responsive plants species” in Mycorrhizas: functional processes and ecological Impact. eds. C. Azcón-Aguilar, J. M. Barea, S. Gianinazzi, and V. Gianinazzi-Pearson (Berlin Heidelberg: Springer), 89–106.
Hijri, M. (2016). Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 26, 209–214. doi: 10.1007/s00572-015-0661-4
Hinsinger, P. (2001). Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237, 173–195. doi: 10.1023/A:1013351617532
Hopkins, B. G., Ellsworth, J. W., Bowen, T. R., Cook, A. G., Stephens, S. C., Jolley, V. D., et al. (2010). Phosphorus fertilizer timing for russet Burbank potato grown in calcareous soil. J. Plant Nutr. 33, 529–540. doi: 10.1080/01904160903506266
Hopkins, B. G., Horneck, D. A., and MacGuidwin, A. E. (2014). Improving phosphorus use efficiency through potato rhizosphere modification and extension. Am. J. Potato Res. 91, 161–174. doi: 10.1007/s12230-014-9370-3
Iwama, K. (2008). Physiology of the potato: new insights into root system and repercussions for crop management. Potato Res. 51, 333–353. doi: 10.1007/s11540-008-9120-3
Jenkins, P. D., and Ali, H. (2000). Phosphate supply and progeny tuber numbers in potato crops. Ann. Appl. Biol. 136, 41–46. doi: 10.1111/j.1744-7348.2000.tb00007.x
Kabir, Z. (2005). Tillage or no-tillage: impact on mycorrhizae. Can. J. Plant Sci. 85, 23–29. doi: 10.4141/P03-160
Klironomos, J. N. (2003). Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84, 2292–2301. doi: 10.1890/02-0413
Koyama, T., Adachi, K., and Suzuki, T. (2019). Response of soybean plants to two inoculation methods with arbuscular mycorrhizal fungus of Glomus sp. strain R-10 under field condition. Plant Prod. Sci. 22, 215–219. doi: 10.1080/1343943X.2018.1548287
Li, H. Y., Smith, S. E., Holloway, R. E., Zhu, Y. G., and Smith, F. A. (2006). Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses. New Phytol. 172, 536–543. doi: 10.1111/j.1469-8137.2006.01846.x
Liu, X., Ma, Z., Qin, Y., Shi, X., Yu, J., Jia, L., et al. (2023). Phosphorus leaching during potato production in coarse soil. Int. J. Plant Prod. 17, 795–802. doi: 10.1007/s42106-023-00271-2
Mai, W., Xue, X., Feng, G., Yang, R., and Tian, C. (2019). Arbuscular mycorrhizal fungi – 15-fold enlargement of the soil volume of cotton roots for phosphorus uptake in intensive planting conditions. Eur. J. Soil Biol. 90, 31–35. doi: 10.1016/j.ejsobi.2018.12.002
Phillips, J. M., and Hayman, D. S. (1970). Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158–IN18. doi: 10.1016/S0007-1536(70)80110-3
Qin, Y., Fan, M., Cui, X., Tian, Y., Xing, H., and Jia, L. (2021). Technical regulation of phosphate fertilizer application reduction and efficiency enhancement for potato under drip irrigation in Inner Mongolia. Phosphate Comp. Fertil. (In Chinese) 36:3. doi: 10.3969/j.issn.1007-6220.2021.02.006
Richardson, A. E., Barea, J. M., McNeill, A. M., and Prigent-Combaret, C. (2009). Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321, 305–339. doi: 10.1007/s11104-009-9895-2
Rosen, C. J., and Bierman Peter, M. (2008). Potato yield and tuber set as affected by phosphorus fertilization. Am. J. Potato Res. 85, 110–120. doi: 10.1007/s12230-008-9001-y
Rosen, C. J., Kelling, K. A., Stark, J. C., and Porter, G. A. (2014). Optimizing phosphorus fertilizer management in potato production. Am. J. Potato Res. 91, 145–160. doi: 10.1007/s12230-014-9371-2
Smith, S. E., and Smith, F. A. (2011). Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 62, 227–250. doi: 10.1146/annurev-arplant-042110-103846
Smith, S. E., and Smith, F. A. (2012). Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 104, 1–13. doi: 10.3852/11-229
Soratto, R. P., Sandana, P., Fernandes, A. M., Martins, J. D., and Job, A. L. (2020). Testing critical phosphorus dilution curves for potato cropped in tropical Oxisols of southeastern Brazil. Eur. J. Agron. 115:126020. doi: 10.1016/j.eja.2020.126020
Soti, P., Kariyat, R., and Racelis, A. (2023). Effective farm management promotes native AMF and benefit organic farming systems. Agric. Ecosyst. Environ. 342:108240. doi: 10.1016/j.agee.2022.108240
Susiana, P., Rukmi, I., and Jannah, S. N. (2019). Applications of mycorrhiza on potato growth and productivity. J. Phys. Conf. Ser. 1217:012143. doi: 10.1088/1742-6596/1217/1/012143
Syers, J. K., Johnston, A. E., and Curtin, D. (2008). Efficiency of soil and fertilizer phosphorus use. FAO Fertil. Plant Nutr. Bull. 18, 5–50. Available at: https://openknowledge.fao.org/handle/20.500.14283/a1595e
Thornton, M. K., Novy, R. G., and Stark, J. C. (2014). Improving phosphorus use efficiency in the future. Am. J. Potato Res. 91, 175–179. doi: 10.1007/s12230-014-9369-9
Ul Haq, J., Sharif, M., Akbar, W. A., Rahim, H. U., Mian, I. A., and Ahmad, S. (2022). Arbuscular mycorrhiza fungi integrated with single super phosphate improve wheat-nitrogen-phosphorus acquisition, yield, root infection activity, and spore density in alkaline-calcareous soil. Gesunde Pflanzen 75, 539–548. doi: 10.1007/s10343-022-00718-y
Vosatka, M., and Gryndler, M. (2000). Response of micropropagated potatoes transplanted to peat media to post-vitro inoculation with arbuscular mycorrhizal fungi and soil bacteria. Appl. Soil Ecol. 15, 145–152. doi: 10.1016/S0929-1393(00)00090-1
Wang, S. Y., Wei, H., Chen, K. Y., Dong, Q., Ji, J. M., and Zhang, J. (2021). Practical methods for arbuscular mycorrhizal fungal spore density, hyphal density and colonization rate of AMF. Bio 101:e2104253. doi: 10.21769/BioProtoc.2104253
Wu, L., Li, L., Ma, Z., and Fan, M. (2022). Improving potato yield, water productivity and nitrogen use efficiency by managing irrigation based on potato root distribution. Int. J. Plant Prod. 16, 547–555. doi: 10.1007/s42106-022-00205-4
Keywords: AMF infection rate, potato growth, soil mycorrhizal hyphal density, phosphorus uptake, tuber yield
Citation: Liu X, Yu J, Wei J, Qin Y, Shi X, Liu K, Jia L and Fan M (2025) Inoculation with arbuscular mycorrhizal fungi at the seedling stage of potatoes improves phosphorus use efficiency. Front. Sustain. Food Syst. 9:1546032. doi: 10.3389/fsufs.2025.1546032
Edited by:
Mohamed Trigui, Institut Préparatoire aux Etudes d’Ingénieur de Sfax (IPEIS), TunisiaReviewed by:
Abd El-Nasser S. Al Borki, University of Benghazi, LibyaFatma Masmoudi, Qatar University, Qatar
Copyright © 2025 Liu, Yu, Wei, Qin, Shi, Liu, Jia and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonglin Qin, cXlsMzMzOUAxNjMuY29t; Mingshou Fan, Zm1zd2hAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Xiaoyu Liu
Xiaoyu Liu Jing Yu1†
Jing Yu1† Jie Wei
Jie Wei Yonglin Qin
Yonglin Qin Liguo Jia
Liguo Jia Mingshou Fan
Mingshou Fan