- 1Department of Animal Science, Kwame Nkrumah University of Science and Technology (KNUST), PMB, University Post Office, Kumasi, Ghana
- 2Faculty of Biological Sciences, University of Leeds, Leeds, United Kingdom
- 3School of Food Science and Nutrition, University of Leeds, Leeds, United Kingdom
- 4Department of Chemistry and Physics, Sokoine University of Agriculture, Morogoro, Tanzania
- 5Department of Consumer and Food Sciences, University of Pretoria, Hatfield, South Africa
In Low-Income Food Deficit Countries (LIFDCs), there is a growing demand for ruminant livestock products due to population growth, urbanization, and rising incomes. However, smallholder farmers in these regions face constant challenges in securing reliable year-round feed supply, which affects animal performance and the ability to meet increasing demand for animal products. This comprehensive review thus explores the potential of fruit by-products, specifically cashew apples, papayas, and mangoes, which are often discarded and contribute to environmental pollution but can be valuable resources for livestock farmers. The review examines the current state of small ruminant livestock production in LIFDCs, particularly in Sub-Saharan Africa and adopts a systems thinking approach to consider using cashew apple, papaya, and mango by-products as a potential feed source. Small ruminant livestock production is highlighted for efficiently converting nutrient-rich food waste from fruits like cashew apples, papayas, and mangoes into valuable milk and meat products. The review also addresses the environmental aspect, pointing out potential greenhouse gas emissions resulting from improper disposal of fruit wastes and the urgent need to convert them into animal feeds. It provides data on processing, preservation techniques, chemical composition, and the limited available information on the impact of these fruit by-products on feed intake, growth, carcass quality, methane emissions, and overall well-being of small ruminants. Challenges related to the storage and feeding of these by-products are also discussed. Despite limited data and conflicting evidence, the review strongly advocates using cashew apples, papaya, and mango by-products as vital feed resources for small ruminants. It emphasizes the need for further research to determine their nutritional value in local contexts, establish optimal inclusion levels, and devise strategies for prolonging shelf life. This effort holds promise for addressing food deficits and enhancing food security in LIFDCs where these challenges are most acute.
1 Introduction
Small ruminants play a crucial role in African food systems, providing high-quality protein, generating income, and serving as economic assets for rural communities. Additionally, they contribute to environmental sustainability by transforming crop residues and food waste, unfit for human consumption, into valuable products like milk and meat (Pulina et al., 2017; Wadhwa et al., 2015; Thornton, 2010). Demand for small ruminant products in Low-Income Food Deficit Countries (LIFDCs) in Sub-Saharan Africa (SSA) is rising rapidly, driven by population growth, urbanization, and increasing incomes (Pulina et al., 2017; Thornton, 2010). Meeting this demand is critical to addressing widespread protein and iron deficiencies prevalent in SSA, as well as supporting rural economies through income generation and job creation. However, projections indicate that ruminant product output must increase by 60–70% by 2050 to meet this demand, particularly in LIFDCs (Pulina et al., 2017; Thornton, 2010).
In semi-arid regions, small ruminants are better adapted to harsh climatic conditions, such as drought, high temperatures, and limited rainfall, making them integral to local livelihoods. Despite their resilience, their productivity is constrained by year-round feed shortages, which limit the quality and quantity of available feed. This prevents small ruminants from reaching their full genetic potential (Adzitey, 2013; Arowolo and He, 2018; Ates et al., 2018), undermining their contributions to food security and rural livelihoods. Addressing these feed shortages requires cost-effective, environmentally sustainable feed options that safeguard animal health and productivity.
The integration of agro-industrial by-products (AIBPs) into livestock diets offers a promising solution (Romelle Jones et al., 2023; Yafetto et al., 2023). By-products from mango, cashew apple, and papaya processing are particularly promising due to their abundance in SSA, where high fruit processing volumes and significant post-harvest losses generate millions of tonnes of waste annually (Aluko et al., 2023; Owino and Ambuko, 2021; Evans and Ballen, 2012; Barve et al., 2020; Millogo et al., 2024; Tesfaye, 2017; Magama et al., 2022; Tapsoba et al., 2022; Van Walraven and Stark, 2023). While some organic matter could be reintegrated into soils to improve soil health, excessive volumes pose environmental challenges, such as soil and water pollution, and require alternative solutions (Richard et al., 2018; Zahid and Khedkar, 2021).
Valorizing these by-products as livestock feed provides a dual solution: addressing feed scarcity and managing waste sustainably. Although preventing food losses through improved handling, storage, and distribution remains essential, some level of waste is unavoidable, particularly the inedible portions of fruits such as peels, seeds, and pomace. These by-products are rich in nutrients and bioactive compounds, making them suitable for inclusion in small ruminant diets. Studies show that mango peels, cashew apple pomace, and papaya seeds can enhance feed value and productivity, particularly in regions where traditional feed options are limited (Mirzaei-Aghsaghali and Maheri-Sis, 2008; Gupta et al., 2022; Jahurul et al., 2015).
This review explores the potential of mango, cashew apple, and papaya by-products as sustainable feed resources for small ruminants in semi-arid regions of LIFDCs, focusing on their role in improving food and nutrition security in vulnerable communities. To advance this field, the review moves beyond summarizing existing studies to propose inferences and hypotheses and guide future research, including:
1. Optimizing feed efficiency through the incorporation of fruit by-products to improve rumen fermentation dynamics and nutrient utilization.
2. Exploring the potential of bioactive compounds in reducing methane emissions, contributing to environmental sustainability by altering ruminal fermentation pathways.
3. Assessing the economic benefits valorization strategies for smallholder farmers, including reduced feed costs, improved productivity, and income stability.
By adopting a systems-thinking approach, this review provides a comprehensive framework for leveraging fruit by-products as sustainable feed resources, addressing the interconnected challenges of feed scarcity, environmental sustainability, and economic development in LIFDCs.
2 Methodology
This review draws upon peer-reviewed journal articles, books, and other published materials sourced from databases such as Google Scholar, PubMed Central, and Scopus. It synthesizes the literature using a systems-thinking approach, with a focus on fruit by-product valorization, feed scarcity, and livestock productivity in LIFDCs. The review prioritizes studies relevant to Sub-Saharan Africa, particularly those addressing small ruminant production, nutrient composition, and environmental implications.
3 The context of livestock production in LIFDCs and its relevance in Sub-Saharan Africa
In LIFDCs, particularly in SSA, livestock production is a cornerstone of agricultural systems, primarily driven by smallholder farmers (Amejo et al., 2018; Erdaw, 2023). These farmers play a critical role in meeting the region’s growing demand for meat and dairy while providing income, sustenance, and nutrition for rural households (Erdaw, 2023; Fraval et al., 2019; Ransom and Stagner, 2020). Livestock serves as an economic asset, diversifying income sources and improving livelihoods (Neudert et al., 2020). However, the sector faces significant challenges, particularly due to the region’s heavy reliance on rain-fed agriculture (Descheemaeker et al., 2016; Thornton et al., 1934). Climate change-induced shifts in rainfall patterns and rising temperatures threaten forage availability and water resources, increasing the vulnerability of livestock systems and exacerbating food insecurity (Hidosa and Guyo, 2017; Amwata et al., 2016; Assan, 2022; Omotoso et al., 2023).
Smallholder farmers also grapple with limited access to essential resources and services. Constraints in acquiring quality feed, veterinary care, and improved livestock breeds, coupled with restricted access to credit and financial services, hinder investments and the adoption of improved practices (Kongolo and Dlamini, 2012; Langyintuo, 2020; Okpeku et al., 2019). Poor infrastructure, such as inadequate road networks, limits market access for livestock products (Chekol, 2021; Merkel, 2019; Sehar and Oyekale, 2022). Additionally, livestock diseases, including Foot-and-Mouth Disease (FMD) and African Swine Fever, cause significant economic losses, with FMD alone costing Zambia over $1.6 billion in export revenue (Armson et al., 2020; Campbell et al., 2021; Sinkala et al., 2014).
Despite these challenges, SSA holds considerable potential for improving livestock production. The region is endowed with extensive grazing lands and diverse livestock genetic resources (Seré, 2020). Sustainable intensification through improved feeding, breeding, and animal husbandry practices can significantly enhance productivity and resilience (Erdaw, 2023; Herrero et al., 2013; Rao et al., 2005). Integrating crop-livestock systems, such as agroforestry and mixed farming, provides additional pathways for climate-resilient agriculture (Rao et al., 2005; Sekaran et al., 2021; Powell et al., 2004; Brewer and Gaudin, 2020; Baiyeri et al., 2019). Moreover, rising domestic and international demand for animal-source foods driven by urbanization and income growth offers new market opportunities for smallholder farmers, fostering economic empowerment and poverty reduction (Erdaw, 2023; Steinfeld, 2003).
In summary, livestock production in SSA operates within a complex interplay of challenges and opportunities, including climate change, resource limitations, inadequate veterinary services, and market constraints. Addressing these barriers and leveraging the sector’s potential can drive poverty reduction, food security, and sustainable development in the region.
3.1 The problem of inadequate feed supply and its implication for small ruminant production
Feed scarcity is a major challenge in LIFDCs, significantly affecting livestock productivity, health, and the livelihoods of smallholder farmers (Adzitey, 2013; Arowolo and He, 2018; Ates et al., 2018). This issue is particularly acute in SSA, home to nearly 50% of LIFDCs, where seasonal feed shortages during the dry season exacerbate undernutrition and reduce livestock performance (Desta and Oba, 2004). Natural pastures and crop residues, the primary feed resources, decline sharply in quantity and quality during this period, creating a “Nutritional Feed Gap” that threatens food security and livelihoods (Cooke et al., 2024; Duguma and Janssens, 2021; Tolera et al., 2000). Contributing factors include limited availability and high costs of nutritious feed ingredients, such as grains and forages, compounded by droughts that often result in substantial livestock losses (Cooke et al., 2024; Lamidi and Ologbose, 2014).
Farmers adopt coping strategies such as adjusting feed resources, purchasing in bulk, or reducing herd sizes, but these measures often strain household productivity, especially for women and children (Duguma and Janssens, 2021; Tangka and Jabbar, 2005). Feed scarcity also drives seasonal price fluctuations in feed markets, further burdening smallholder farmers (Ayantunde et al., 2022).
Climate change exacerbates this challenge by reducing forage availability and quality, threatening livestock productivity and essential protein sources like meat, milk, and eggs, thereby intensifying malnutrition and poverty (Hidosa and Guyo, 2017; Abebe, 2017; Thompson et al., 2010; Balehegn et al., 2020; Smith et al., 2013).
Addressing feed scarcity requires sustainable feed production methods (Musundire et al., 2021), increased farmer knowledge (Balehegn et al., 2020), investment in research and development, and strengthening veterinary services (Duguma and Janssens, 2021). One promising solution is the valorization of fruit by-products as ruminant feed in LIFDCs. These by-products, generated during fruit processing—such as mango peels and pomace from juice production, cashew apples from nut harvesting, and papaya peels and seeds from fresh consumption or puree processing—are rich in nutrients and bioactive compounds. Incorporating these by-products into ruminant diets can enhance livestock productivity, reduce reliance on conventional feed ingredients, and address waste management challenges. This approach not only tackles feed scarcity but also improves food security, poverty reduction, and sustainable development in LIFDCs.
3.2 Environmental impact of disposing cashew apple, papaya, and mango wastes in landfills and their contribution to greenhouse gas emissions
When fruit by-products such as cashew apple, papaya, and mango are not repurposed as livestock feed and are instead disposed of in landfills, they present significant environmental challenges. These wastes, rich in organic matter and moisture, decompose anaerobically in landfills, releasing methane (CH₄)—a potent greenhouse gas with a global warming potential 27.9 times greater than that of carbon dioxide (CO₂) (Williams, 2008; Sunil and Tapan, 2008). This process contributes to greenhouse gas emissions, soil and water contamination and, ultimately, climate change (Richard et al., 2018; Zahid and Khedkar, 2021). Methane emissions from landfills play a considerable role in global warming, accounting for approximately one-fifth of anthropogenic climate impact (Groffman et al., 2010).
Globally, landfills emit an estimated 30–70 million tons of CH₄ annually, representing 6–18% of global methane emissions, with levels expected to rise in developing countries due to increasing waste generation (Rena et al., 2020; Robinson et al., 2003; Bingemer and Crutzen, 1987). Beyond methane, the high moisture content in cashew apple, papaya, and mango wastes may contribute to the production of leachate—a toxic liquid by-product of landfill decomposition (Venna et al., 2021; Chinwendu et al., 2019; Sharma et al., 2020). Leachate can contain heavy metals and pathogens, posing serious risks to soil, groundwater, and surrounding ecosystems (Iravanian and Ravari, 2020; Baderna et al., 2019). The presence of high concentrations of N and P in leachate can contribute to eutrophication if it enters water bodies, potentially causing algal blooms that disrupt aquatic ecosystems. Due to their rapid decomposition and high moisture content, these fruit wastes contribute disproportionately to methane emissions and leachate production, making them particularly problematic in landfills.
Repurposing fruit wastes within a circular economy framework provides a sustainable alternative that reduces the environmental footprint and enables productive reuse (Leong and Chang, 2022). By diverting these by-products as livestock feed, the demand for conventional feed is lowered while providing additional nutritional benefits (Leong and Chang, 2022; Tayengwa and Mapiye, 2018). Cashew, papaya, and mango by-products are rich in cellulose, polyunsaturated fatty acids, and phytochemicals, all of which can improve animal nutrition and productivity (Tayengwa and Mapiye, 2018; Jalal et al., 2023). Studies show that incorporating fruit by-products in ruminant diets improves digestibility, milk yield, and antioxidant levels in meat (Jalal et al., 2023; Sahoo et al., 2021). Transforming these fruit wastes into valuable feed resources aligns with circular economy principles, promoting sustainable agriculture, decreasing landfill dependency, and mitigating environmental impacts.
3.3 Systems thinking perspective of using fruit by-products as a sustainable solution to inadequate small ruminant feeds in LIFDCs
A systems thinking approach provides a holistic framework for addressing feed scarcity for small ruminants in LIFDCs. The integration of fruit by-products into livestock systems (Figure 1) provides a sustainable solution to seasonal feed shortages caused by dry seasons and erratic rainfall, which limit conventional feed availability and reduce livestock productivity.
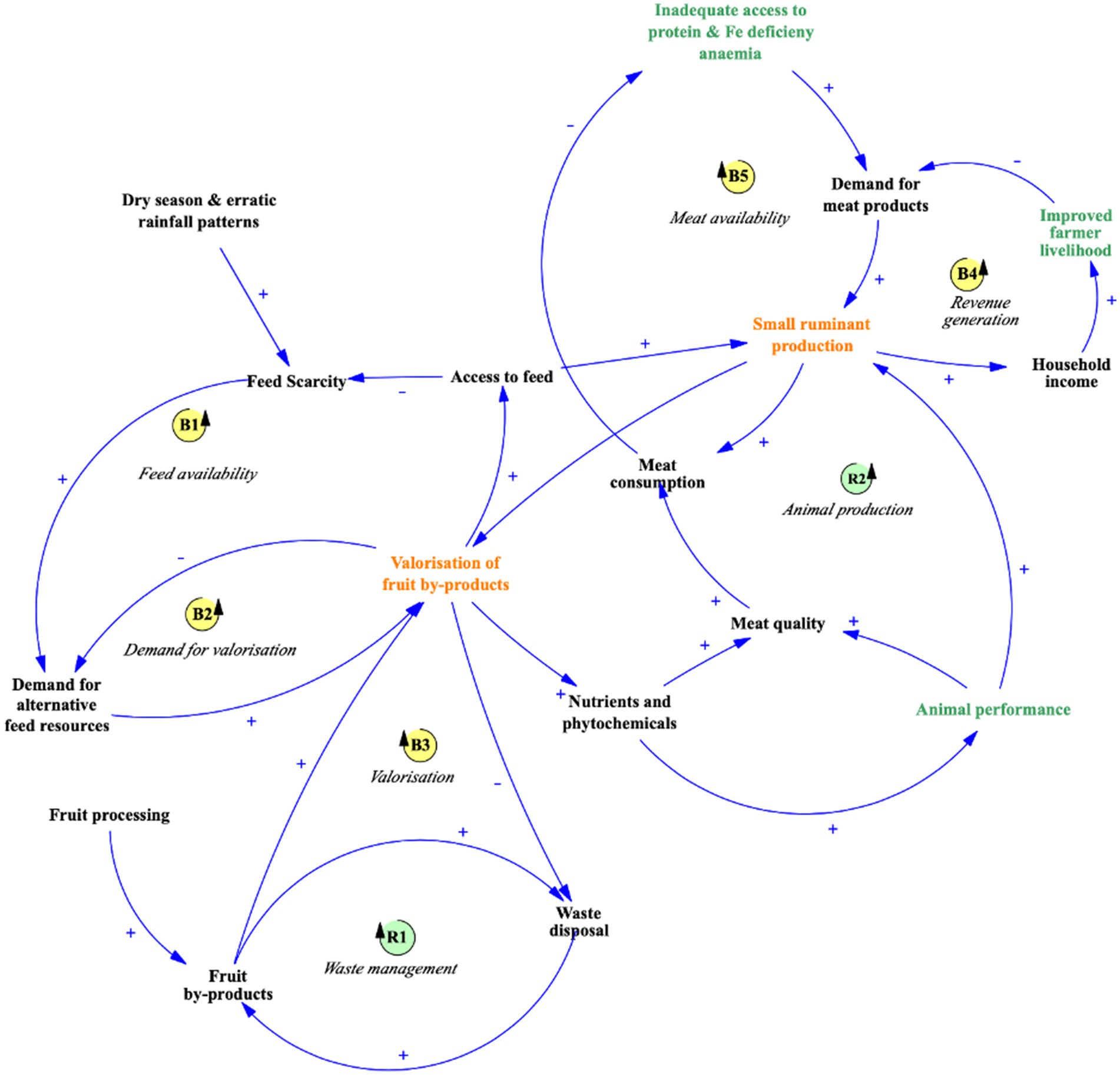
Figure 1. A causal loop diagram depicting the beneficial role of fruit by-products in the meat industry value chain. The arrows represent causality, whereas the polarity indicators, + and −, represent positive and negative correlation. B stands for balancing system loops, and R stands for reinforcing loops.
Valorizing fruit by-products—transforming waste into nutrient-rich feed—presents an efficient way to address feed scarcity and manage agricultural waste. By-products like mango peels, cashew apple pomace, and papaya seeds, which are often discarded, can be repurposed as valuable feed resources rich in nutrients and bioactive compounds. This process supports consistent feed availability during periods of scarcity, as shown in Balancing Loop (B1) in Figure 1. Improved feed access enhances small ruminant productivity and health, resulting in higher meat quality and availability (Mayberry et al., 2018). This increase helps meet the growing demand for animal-sourced foods, driven by efforts to address protein and iron deficiencies in LIFDCs (Fairweather-Tait, 2023).
Integrating fruit by-products also creates a positive feedback loop, as depicted in Reinforcing Loop (R2) in Figure 1. This loop demonstrates how reduced waste and improved livestock productivity contribute to both economic and environmental sustainability. However, barriers such as limited processing infrastructure and low farmer awareness hinder widespread adoption. Policies that promote fruit by-product use—through subsidies, farmer education programs, and investments in preservation infrastructure—are critical for scaling up these practices.
The valorization of fruit by-products addresses feed scarcity, enhances meat quality, and improves household incomes while supporting environmental sustainability. Figure 1 encapsulates this interconnected system, illustrating how integrating fruit by-products into small ruminant production fosters mutual benefits for farmers, livestock, and the environment.
4 Overview of mango, cashew, and papaya by-products
Mango, cashew, and papaya by-products are particularly promising options for alternative livestock feed due to their abundance, nutrient content, and environmental benefits. These examples are drawn from their economic importance and substantial production volumes in tropical regions, especially in Low-Income Food-Deficit Countries (LIFDCs), where they contribute significantly to agricultural outputs. For instance, mango production generates 35–55% by-products as waste (Tesfaye, 2017), with potential quantities reaching 16,180 tons in some areas of sub-Saharan Africa (Millogo et al., 2024). Similarly, cashew nut production produces 36.9 million tons of cashew apple waste annually (Van Walraven and Stark, 2023), and papaya processing generates up to 20% of the fruit’s weight as by-products (Shaheen et al., 2023). These by-products—such as mango peels and seeds, cashew apple pomace, and papaya peels—offer a practical solution to feed scarcity faced by smallholder farmers in these regions. Rich in fiber, antioxidants, and essential nutrients, these by-products can enhance ruminant diets, with potential to improve animal performance and product quality. However, studies on their use in ruminant feed, particularly for small ruminants, remain limited, leaving gaps in understanding their effects on animal health and production. Incorporating such by-products into livestock diets also aligns with environmental goals, as it reduces landfill waste and supports a circular economy by transforming agricultural waste into a valuable resource.
4.1 Mango by-product
Mango (Mangifera indica) is the second most traded tropical fruit globally (Mwaurah et al., 2020). However, mango processing for juices, jams, and desserts utilizes only the pulp, leaving the seed and peel as major by-products. The seed, comprising the seed coat and kernel, accounts for 24–60% of the fruit mass, while the peel (exocarp) constitutes 7–24% (Mwaurah et al., 2020; Marçal and Pintado, 2021). Mango seed kernels contain approximately 15% oil, comparable to cottonseed and soybean oil (18–20%) (Mwaurah et al., 2020). The extracted oil has low free fatty acid and peroxide values, requiring minimal processing for use (Owino and Ambuko, 2021).
Mango peels, on the other hand, are rich in energy, dietary fibre, carbohydrates, protein, and lipids (Garcia-Amezquita et al., 2018; Marcillo-Parra et al., 2021). They are also a concentrated source of bioactive compounds, including anthocyanins, carotenoids, flavonoids, and polyphenols, which are known for their antioxidant activity and therapeutic properties (Ranganath et al., 2018; López-Cobo et al., 2017; Barreto et al., 2008; Serna-Cock et al., 2016; Asif et al., 2016; Dorta et al., 2012). Notably, mango peels contain higher polyphenol levels than mango pulp, making them a valuable feed resource for enhancing the nutritional quality of low-quality fodder, forage, and pastures fed to small ruminants.
4.2 Cashew by-product
Cashew (Anacardium occidentale) is a pseudocarp (false fruit) non-climacteric fruit grown in parts of South America, Asia, and most West African countries. Primarily cultivated for its nut, cashew ranks third globally after almonds and walnuts (Rajkumar and Ganesan, 2021; Preethi et al., 2021). The nut represents only about 10% of the fruit, leaving approximately 3 million tonnes of cashew apples discarded annually in sub-Saharan Africa after nut harvesting (Ahaotu and Ihekoronye, 2019; Aidoo et al., 2022; Deenanath et al., 2015). In Ghana, the cashew harvest season aligns with the dry season, a period of low forage availability and high feed costs, making cashew apples a valuable but underutilized feed resource for addressing seasonal feed shortages. However, their strong astringency, perishability, and limited processing infrastructure often lead to disposal (Akyereko et al., 2022).
Nutritionally, cashew apples are rich in sugars (fructose, glucose, and sucrose) ranging from 7.28 to 9.41%, providing a readily available energy source for livestock (Rithy et al., 2022). They are also high in vitamin C (200–300 mg/100 g), which is about five times that of citrus fruits and ten times that of pineapples (Preethi et al., 2019; Lowor and Agyente-Badu, 2009). This high vitamin C content, combined with phenolic compounds (221–325 mg GAE/100 mL), contributes to their strong antioxidant capacity (Rithy et al., 2022; Figueroa-Valencia et al., 2019). Notably, as the fruit ripens, phenolic content decreases while vitamin C levels and antioxidant capacity increase, potentially enhancing the health benefits for livestock (Gordon et al., 2012).
The primary flavonoids in cashew apples are myricetin and quercetin derivatives, known for their antioxidant properties (Gordon et al., 2012). Beyond antioxidants, cashew apples and their residues (pomace or bagasse) contain cellulose, hemicellulose, pectin, protein, carbohydrates, and essential minerals like calcium, phosphorus, and iron. For this review, “cashew by-product” refers to discarded cashew apple, pomace, or bagasse.
4.3 Papaya by-products
Papaya (Carica papaya) is a perennial herbaceous plant belonging to the family Caricaceae. The fruit, commonly called papua or pawpaw, is popular in almost all tropical countries. The global papaya production reached 13 million tonnes in 2017, with Nigeria being the leading producer in SSA countries (Altendorf, 2019). Papaya is most often consumed in the raw state or processed into jams, candy and other value-added products. The papaya plant is interesting because nearly every part of it, including the roots, leaves, peels, latex, flowers, fruits, and seeds, has nutritional and therapeutic value; thus, it earned the names “tree of health” and “fruit of long life” (Ali et al., 2011). Two important by-products of papaya are the seeds and peels, which represent up to 8.5 and 12%, respectively, of the total papaya fruit weight (Abdel-Hay et al., 2022).
5 Nutrient and bioactive profiles of mango, cashew apple, papaya by-products
5.1 Nutrient composition of cashew pomace, mango, and papaya peels
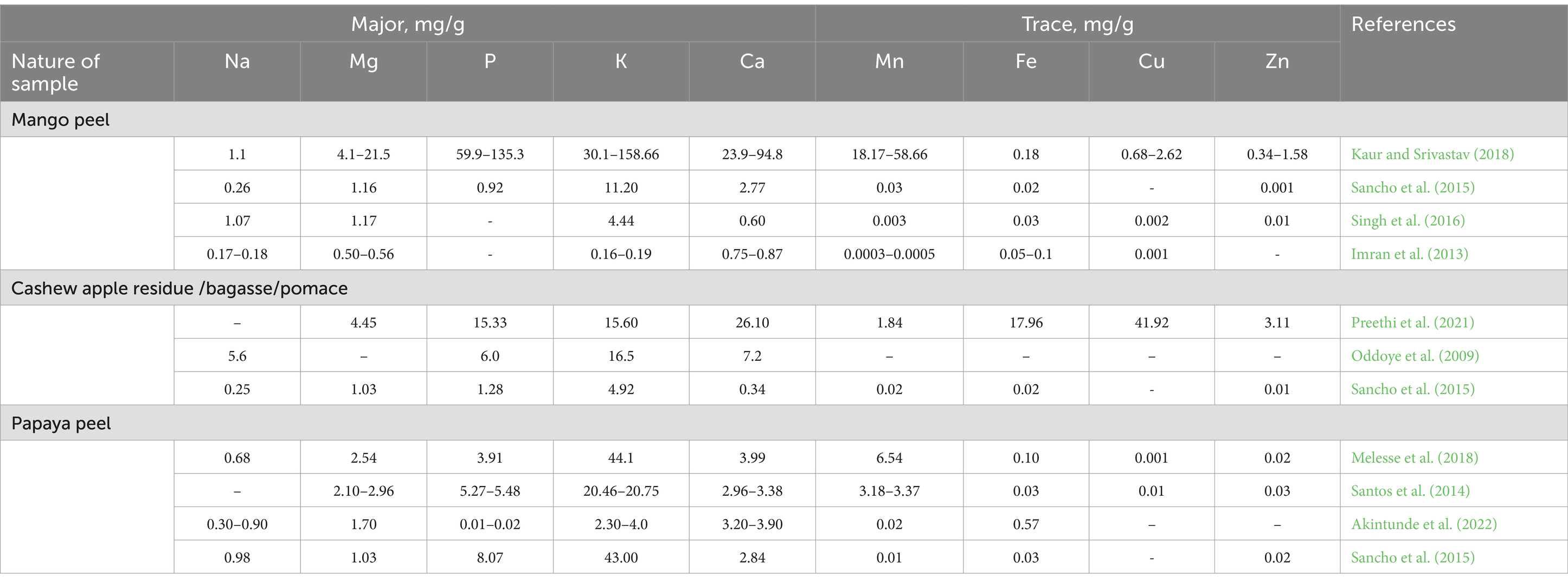
The crude protein (CP) content of mango peels ranges from 26.5–91 g/kg dry matter (DM), while cashew pomace ranges from 54.5–187 g/kg DM and up to 128–240 g/kg DM in certain varieties (Table 1). Papaya peels contain higher CP levels, exceeding 110–130 g/kg DM, sufficient for maintenance and moderate growth in small ruminants (Asaolu et al., 2011). However, mango peels and some varieties of cashew pomace require supplementation with other feed resources to meet the minimum CP requirement of 80 g/kg DM necessary for optimal rumen microbial activity (Asaolu et al., 2012). Insufficient protein reduces the efficiency with which growing animals utilize metabolizable energy (Ranjhan, 2001).
Mango peels have lower fat content than cashew pomace and papaya peels (Table 1), though all fall within the recommended range (<80 g/kg DM). Moderate dietary oil supplementation, up to 5% of dry matter intake, generally does not impair nutrient intake or digestibility, though higher levels can inhibit fiber digestion by suppressing cellulolytic bacteria (Cosgrove et al., 2008; Maia et al., 2012; Ibrahim et al., 2021). It is critical to limit dietary oil as ether extract (EE) levels above 5% of total energy intake may negatively affect carbohydrate digestion in the rumen (Bauman et al., 2003).
Fiber analysis of these by-products is limited, as shown in Table 1. Ensiled cashew pomace generally exhibits higher neutral detergent fiber (NDF) and acid detergent fiber (ADF) values compared to mango and papaya peels, which fall within ranges of 118–237 g/kg DM and 60.2–205 g/kg DM, respectively. These differences underscore the importance of further research to characterize fiber content and optimize their use in ruminant diets.
While limited information is available on the mineral composition of cashew pomace, mango, and papaya peels (Table 2), their calcium, phosphorus, and magnesium content generally align with the recommended maintenance needs of ruminants. For example, these by-products can support daily intakes of 15.4 mg Ca/kg, 16 mg P/kg, and 12–16 mg Mg/kg body weight, respectively (NRC, 2007; Bakshi and Wadhwa, 2013). However, the bioavailability of these minerals varies depending on their form and concentration. Minerals bound in complexes such as oxalates, phytates, or tannin-mineral complexes may have reduced availability, impacting nutrient utilization in the rumen. While rumen microbes can release certain minerals, such as phosphate from phytate complexes, others, like oxalates, remain less bioavailable, limiting absorption and utilization.
Additionally, these by-products, while rich in minerals, may fall short in key amino acids such as methionine and cysteine, which are essential for maintenance in ruminants (NRC, 2016). Addressing this limitation requires further research into their amino acid profiles and strategies to balance diets accordingly. The nutritional composition of fruit by-products is highly variable, influenced by factors such as fruit cultivar, maturity stage, soil conditions, production site, and processing methods (Alañón et al., 2019; Dorta et al., 2014). These variations highlight the need for thorough characterization of fruit by-products to optimize their use in small ruminant diets, improving nutrient utilization and feed efficiency.
The addition of cashew apple, mango, and papaya by-products to ruminant diets enhances nutritional balance by improving key feed characteristics such as dry matter (DM) content, acidity, and nutrient availability in silages and total mixed rations (Table 3). These by-products help maintain feed quality and consistency, promoting better digestion and nutrient absorption, even when partially replacing traditional feed ingredients. They also contribute to higher crude protein (CP) levels, making them useful for protein supplementation. For instance, dehydrated cashew apple and bagasse increase CP, enhancing protein availability, while mango meal and peel silage elevate CP when used as corn substitutes in mixed rations (Araújo et al., 2022; Guerra-Rivas et al., 2017; Pereira et al., 2013; Aung et al., 2024). However, these increases should be balanced to avoid excess nitrogen, which may reduce feed efficiency.
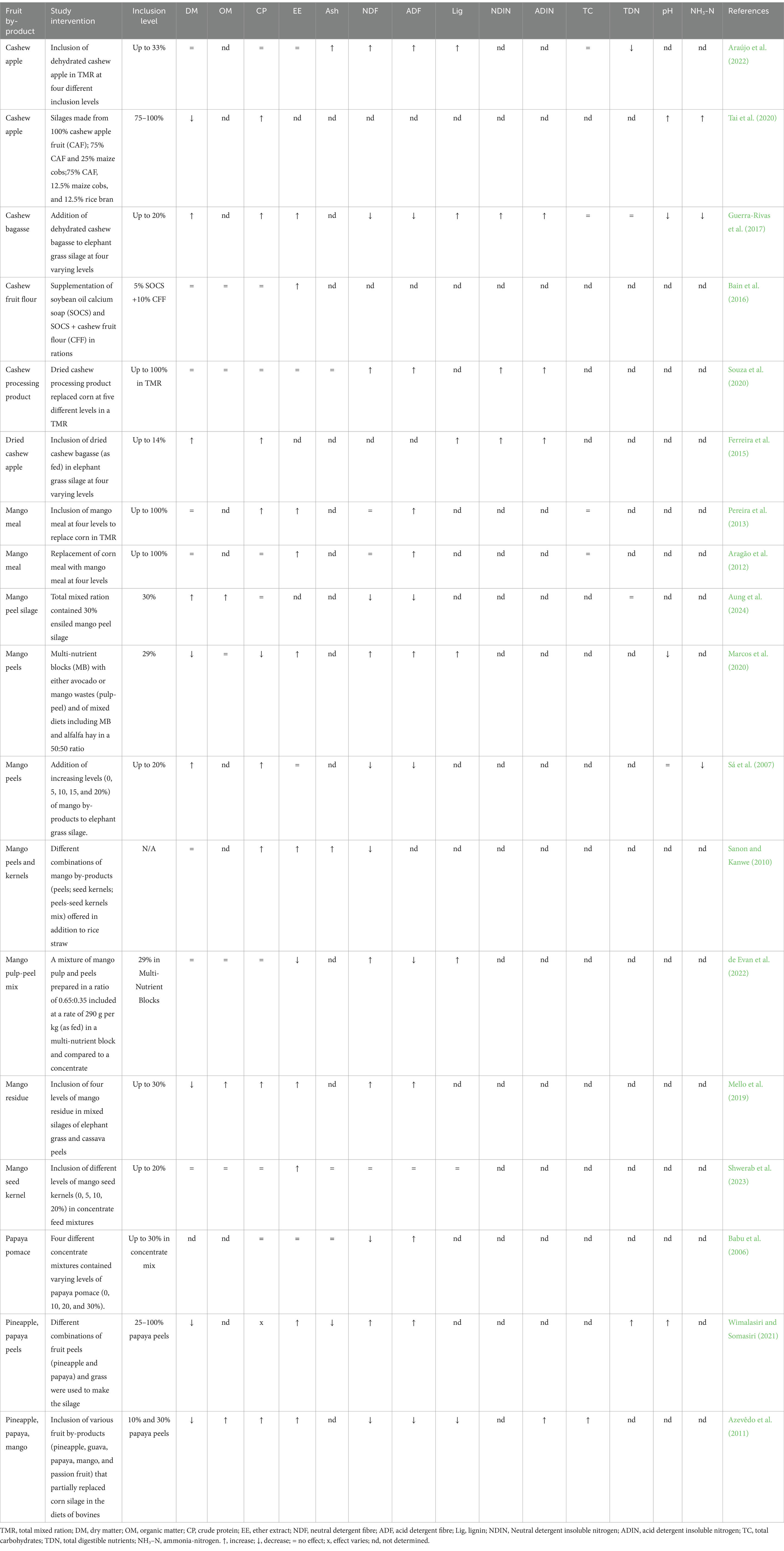
Table 3. Effects of cashew, mango and papaya by-products containing phytonutrients on the chemical and physicochemical composition of ruminant diets.
By-products also raise ether extract (EE) levels, increasing the diet’s energy density. Cashew and mango by-products, such as cashew bagasse and mango meal, provide an energy source that meets high-demand requirements in ruminants, although excess fat should be avoided to prevent impaired fiber digestion (Pereira et al., 2013; Aragão et al., 2012; Bain et al., 2016). Effects on fiber fractions vary: cashew bagasse generally lowers neutral detergent fiber (NDF) and acid detergent fiber (ADF), improving digestibility, while mango meal may occasionally increase ADF, affecting fiber quality (Guerra-Rivas et al., 2017; Aung et al., 2024). Balancing fiber content is essential to support rumen function and digestion.
These by-products also influence rumen stability. Cashew apple silages combined with maize cobs and rice bran increase NH₃-N, enhancing microbial protein synthesis, while mango peel in multi-nutrient blocks lowers pH and NH₃-N, creating a stable rumen environment (Aung et al., 2024; Ferreira et al., 2015). Their inclusion in ruminant diets enhances protein and energy content while stabilizing key parameters like DM and pH. By-products such as mango pulp-peel mixes and papaya-based silages improve feed stability, palatability, and nutrient consistency (Sanon and Kanwe, 2010; Shwerab et al., 2023; Wimalasiri and Somasiri, 2021; Marcos et al., 2020; Tai et al., 2020).
Incorporating cashew, mango, and papaya by-products into ruminant diets offers a sustainable and cost-effective alternative to traditional feed resources. When balanced appropriately, these by-products optimize feed efficiency, maintain rumen health, and promote environmental sustainability in livestock systems.
5.2 Bioactive composition of mango peel, cashew apple, and papaya peel for potential use in small ruminant feed
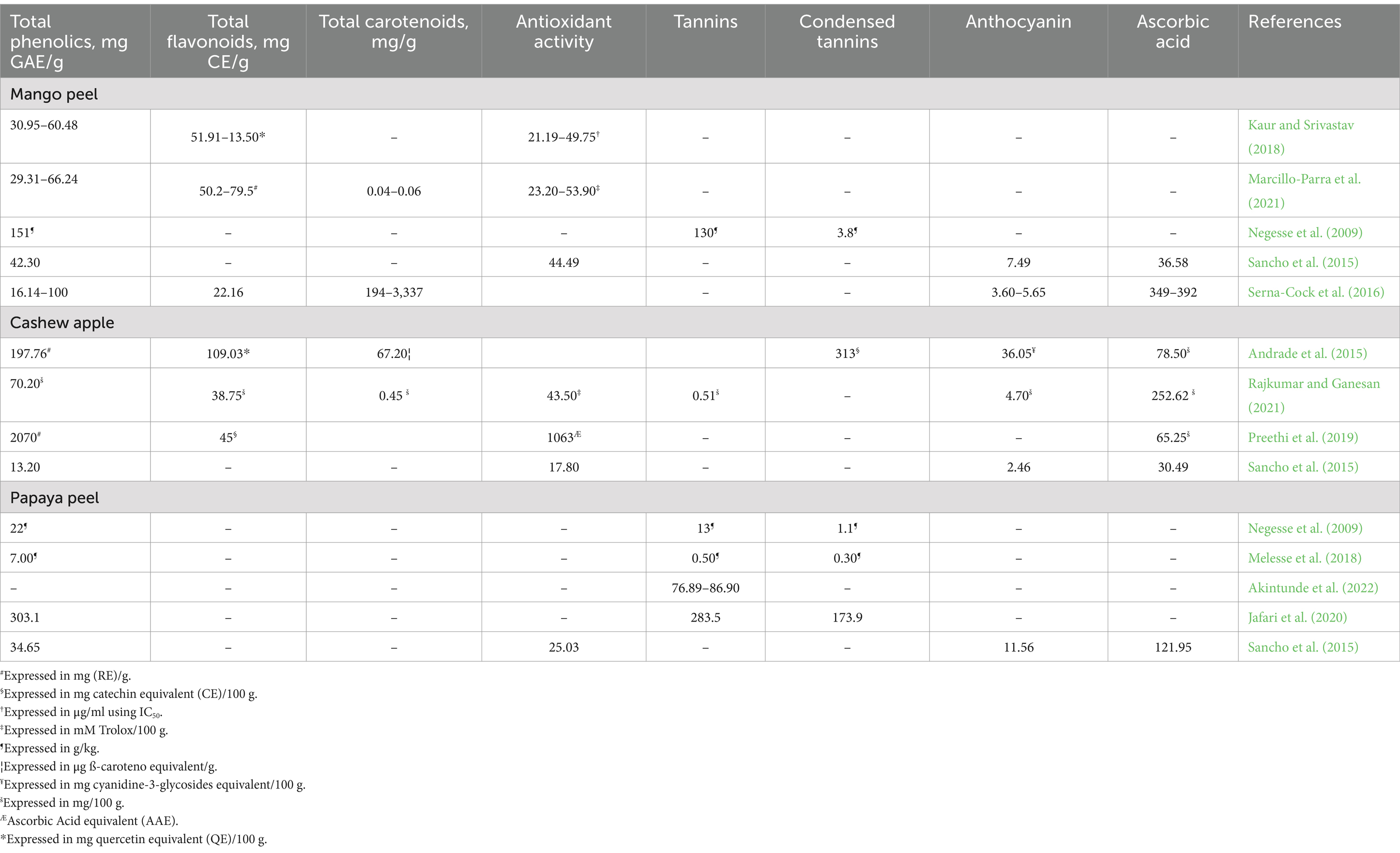
The bioactive profiles of mango peel, cashew apple, and papaya peel present unique nutritional properties that could support the health and productivity of small ruminants, especially in feed-scarce regions. Cashew apple is particularly rich in phenolic compounds, ranging from 13.20 to 2070 mg GAE/g, as well as in flavonoids (up to 109.03 mg CE/g) and ascorbic acid (up to 1,063 mg AAE/g) (Table 4). These high antioxidant levels could enhance immune function in small ruminants by protecting against oxidative stress, a common challenge in arid and nutrient-poor grazing environments (Chauhan et al., 2014; Paul and Dey, 2015). Additionally, cashew apple contains adequate levels of carotenoids, which may support immune health and reduce inflammation (Paul and Dey, 2015; Oh et al., 2017), and it has moderate tannin and anthocyanin contents that could offer antimicrobial benefits (Pathak, 2013; Huang et al., 2018; Min and Solaiman, 2018). This robust bioactive profile suggests that cashew apples could improve overall health and resilience in small ruminants, potentially reducing reliance on medical interventions.
Mango peel has promising bioactive properties, with moderate phenolic content (16.14–100 mg GAE/g) and flavonoids ranging from 22.16 to 79.5 mg CE/g (Table 3). Its antioxidant activity, reported between 21.19 and 53.90 mM Trolox/100 g, is comparable to cashew apple, which could help mitigate oxidative stress in small ruminants. Additionally, mango peel provides ascorbic acid in the range of 349–392 mg/kg, offering a reasonable source of vitamin C. Although its carotenoid content is lower than cashew apple and papaya peel, mango peel’s antioxidant and anti-inflammatory properties could still support animal health, particularly during dry seasons when access to high-quality forage is limited. Mango peels also contain tannins (3.8–7.49 mg CE/g), which might contribute to gastrointestinal health by reducing parasite load, a common issue in small ruminants. Additionally, at appropriate concentrations, tannins can bind to dietary protein, protecting it from rumen microbial degradation and increasing the availability of protein for absorption in the animal’s lower digestive tract.
In contrast, papaya peel has a lower phenolic and flavonoid content but is exceptionally high in carotenoids (76.89–86.90 mg/g) (Table 4), making it a valuable source of vitamin A precursors. This high carotenoid concentration could enhance vision, immune function, and reproductive health in small ruminants. Papaya peel also exhibits substantial antioxidant activity, reaching up to 283.5 mg GAE/g, which may further protect against oxidative stress and support health in challenging environmental conditions. Although lower in ascorbic acid (13–252.62 mg/kg), papaya peel’s unique bioactive profile makes it a good supplement.
6 Influence of feeding mango, cashew apple, and papaya by-products on small ruminant nutrition
6.1 Influence on voluntary intake
Incorporating cashew, mango, and papaya by-products into ruminant diets presents promising opportunities to enhance nutrient intake while supporting sustainable agricultural practices. Given that nutrient intake, especially dry matter (DM), is crucial for ruminant growth and development (Weiss, 2015), these by-products are commonly used in total mixed rations (TMR) or silage, replacing conventional forages. Cashew apple pomace and mango by-products, in particular, have been included in ruminant diets at levels ranging from 100 g/kg DM to 600 g/kg of feed, and their effects on intake vary depending on inclusion levels and specific by-product type.
Research indicates that including cashew apple pomace and mango by-products at lower levels (up to 300 g/kg DM) tends to improve DM, crude protein (CP), neutral detergent fiber (NDF), and acid detergent fiber (ADF) intake in ruminants (Ferreira et al., 2015; Sanon and Kanwe, 2010; Table 5). The increased intake at these levels may result from enhanced silage fermentation, which reduces the moisture content and minimizes rumen distension, thus encouraging greater consumption. These by-products, when ensiled with forage, undergo a reduction in overall moisture content, supporting better fermentation and producing a more palatable, digestible feed that ruminants are more likely to consume. Ensiling fruit by-products alone may not achieve similar outcomes due to their high moisture content and limited structural fiber, which can negatively affect fermentation quality.
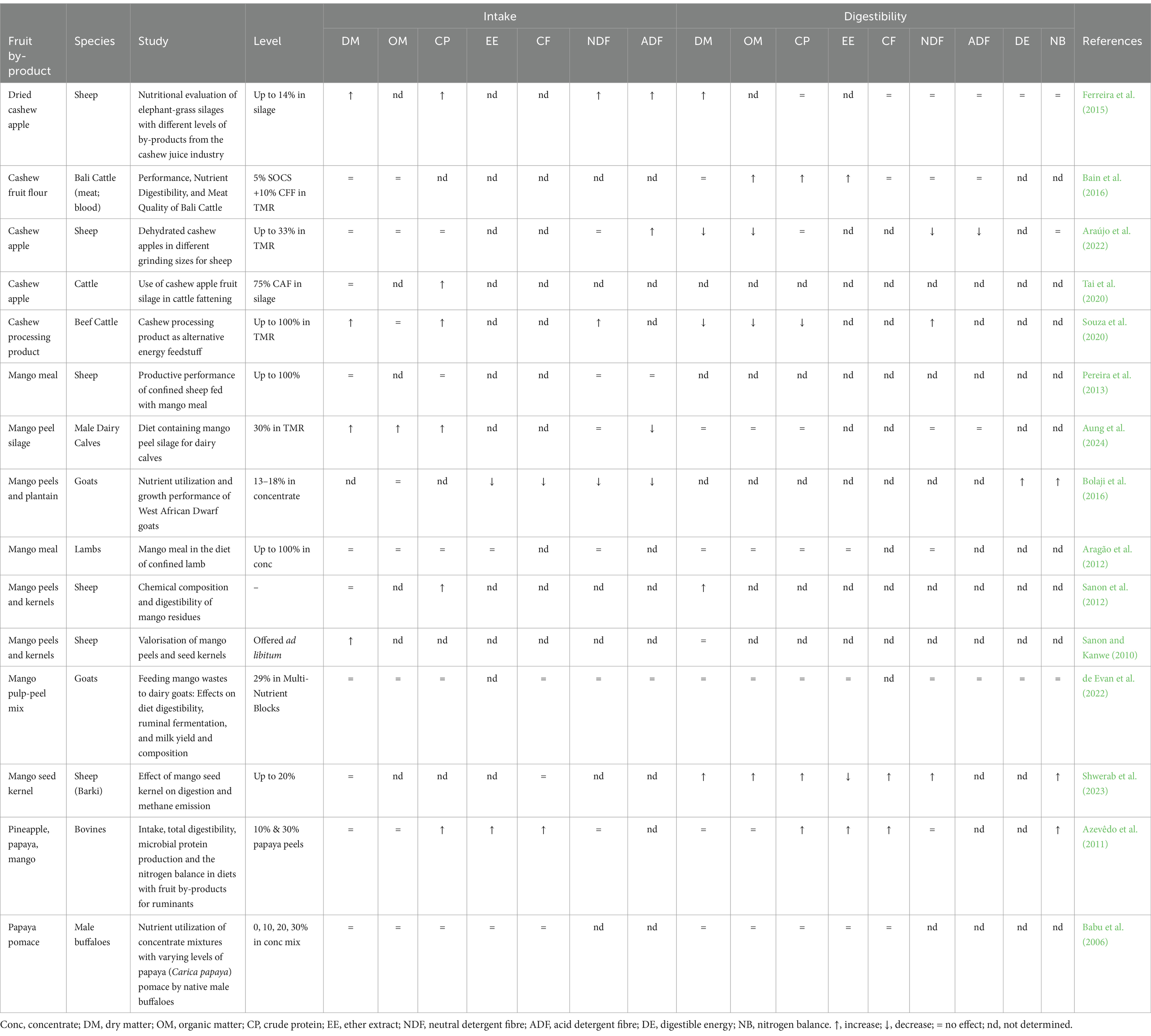
Table 5. Effects of cashew, mango and papaya by-products containing phytonutrients on nutrient intake and digestibility in ruminants.
In Ghana, small ruminants primarily graze on Napier grass, Bracharia, and Elephant grass. However, during the dry season, pasture availability declines, leading to nutrient deficiencies and reduced growth performance. Cashew apples, abundant during this period, offer an underutilized feed resource that can be preserved through ensiling. Feeding trials show that incorporating ensiled cashew apple and grass at inclusion levels of 20–30% improves diet quality, supports growth performance, and alleviates seasonal feed shortages.
Ensiling cashew apple with grass at these inclusion levels yields crude protein (CP) values of 7.00–8.32% and crude fiber (CF) content of 19.49–26.68%, improving digestibility and supporting higher average daily gain (Tompkins and Adger, 2004) and feed conversion efficiency (FCE). This makes cashew apple silage an effective dry-season ration, enhancing protein intake, reducing fiber content, and optimizing rumen fermentation for better nutrient utilization.
Valorizing cashew apples for livestock feed enhances small ruminant productivity, reduces waste, and promotes sustainability. Integrating these seasonal by-products into livestock systems strengthens smallholder resilience and aligns with circular bioeconomy practices.
At higher inclusion levels of cashew apple pomace with grass silage (above 300 g/kg DM), a decrease in DM intake in ruminants has been reported (Souza et al., 2020; Table 5). This reduction can be attributed to the increased concentration of condensed tannins, or proanthocyanins, which are primary phenolic compounds found in cashew apple pomace (Michodjehoun-Mestres et al., 2009). Tannins in high concentrations—exceeding 50 g/kg DM—may hinder feed intake by forming complexes with proteins (that do not dissociate in the abomasum), resulting in a dry, astringent mouth-feel that animals find unpleasant (Naumann et al., 2017). This astringency discourages consumption, but ruminants have developed adaptive strategies for tannin-rich feeds. Ruminants like sheep and goats produce proline-rich proteins in their saliva that bind to tannins, forming tannin-proline-rich-protein complexes that reduce astringency and facilitate higher feed intake (Huang et al., 2018). Goats, for instance, consistently produce these proteins, while sheep do so specifically when consuming tannin-rich diets. These adaptive mechanisms underscore ruminants’ resilience and ability to manage diets with moderate tannin levels.
Furthermore, these by-products contain beneficial phytonutrients, including antioxidants, which offer additional health benefits and can improve the quality of animal products. The antioxidant properties may enhance the health status of the animals, providing an added advantage beyond mere nutrient intake.
As shown in Table 5, the effects of cashew, mango, and papaya by-products on nutrient intake vary depending on the inclusion level, species, and specific by-product type. Moderate incorporation of these by-products improves DM and, either directly or indirectly, CP intake, provided the inclusion levels are carefully managed. Excessive amounts may deter intake due to tannin-related astringency, but the inherent adaptive strategies in ruminants help mitigate these effects. By balancing inclusion rates, livestock farmers can leverage these by-products to enhance productivity, animal health, and sustainable feeding practices.
6.2 Influence on nutrient digestibility
The incorporation of cashew apples, mango, and papaya by-products in ruminant diets has garnered attention for its potential benefits in nutrient utilization. However, digestibility outcomes vary widely, with numerous studies indicating that increased inclusion of these by-products, particularly above certain levels, can lead to declines in dry matter (DM), crude protein (CP), and neutral detergent fiber (NDF) digestibility (Table 5). Cashew by-products exhibit mixed effects on digestibility. Studies show that moderate inclusion (e.g., up to 14% in TMR) can improve DM and CP digestibility in ruminants, as seen with dried cashew apples in sheep diets (Ferreira et al., 2015). However, digestibility often decreases at higher levels, especially above 110 g/kg of feed. For instance, Rêgo, Neiva (Rêgo et al., 2010) and Souza, Moraes (Souza et al., 2020) reported that when cashew apple pomace is included at high levels in cattle diets, DM, CP, and NDF digestibility decreases.
This decline is attributed mainly to the presence of condensed tannins and other polyphenols, which form hydrogen bonds with proteins, carbohydrates, and fats, creating complexes that resist degradation in the rumen’s typical pH range of 5.7–6.7 (Naumann et al., 2017). However, these same tannins and polyphenols also have antioxidant properties that could offer health benefits by reducing oxidative stress and supporting immune function in ruminants. The antioxidant effects may be particularly beneficial at moderate inclusion levels, where they can enhance animal health without significantly impairing digestibility. Only when the feed reaches the abomasum (pH < 3.5) or duodenum (pH ~6) do these nutrient complexes dissociate, meaning fewer nutrients are available for absorption in the rumen, thus reducing overall digestibility. Balancing inclusion levels is therefore critical to maximize the antioxidant benefits of these by-products while minimizing any negative effects on nutrient availability.
Mango by-products, like mango meal and peels, show variable digestibility. At moderate inclusion levels, mango peels can enhance DM, OM, and CP digestibility due to their fermentable sugars, which stimulate rumen microbes (Aung et al., 2024). However, higher levels (above 110 g/kg of feed) tend to reduce digestibility, especially of fiber fractions like NDF. This reduction is partly due to tannins in mango peels, which exert antimicrobial effects on cellulolytic bacteria and protozoa—key contributors to fiber and protein breakdown in the rumen (Vasta et al., 2019; Christensen et al., 2017). Reduced protozoa populations, though not primary rumen microbes, can negatively impact digestibility as they play a complementary role in macronutrient degradation (Choudhury et al., 2015). Additionally, tannins may form complexes with proteins and other macronutrients, further limiting their availability for microbial utilization. Balancing inclusion levels is critical to maximize the benefits of mango by-products while minimizing negative impacts on nutrient digestibility.
Papaya by-products, particularly peels and pomace, demonstrate more consistent digestibility than cashew apple and mango. At moderate inclusion levels (10–30%), papaya peels improve CP and EE digestibility due to their nutrient composition, which supports balanced rumen fermentation (Azevêdo et al., 2011). However, at levels exceeding 11% (110 g/kg DM), diminished DM, CP, and NDF digestibility may occur, potentially due to higher tannin concentrations forming stable protein-polyphenol complexes. Naumann, Tedeschi (Naumann et al., 2017) noted increased fecal nitrogen excretion at higher tannin levels as an indicator of inhibited protein digestibility.
Cashew apple, mango, and papaya by-products can enhance digestibility in ruminants at moderate inclusion levels, but higher inclusions often yield diminishing or adverse effects on digestibility. The threshold appears to be around 110 g/kg, above which polyphenols and tannins exert pronounced effects on nutrient utilization, forming nutrient-binding complexes and reducing microbial populations essential for fiber and protein breakdown. This microbial reduction impacts cellulolytic bacteria and protozoa, subsequently lowering fiber, protein, and fat digestibility (Vasta et al., 2019; Choudhury et al., 2015).
6.2.1 Recommended inclusion levels of fruit by-products in ruminant diets
Careful attention to inclusion levels is therefore critical in harnessing the digestibility benefits of these by-products in sustainable ruminant diets. Given the nutritional composition and digestibility characteristics of mango, cashew apple, and papaya by-products, their strategic incorporation into ruminant feeding systems presents a viable approach to enhancing animal performance while mitigating food waste and promoting circular economy principles.
To provide clearer guidance, the following recommended inclusion levels are proposed for different ruminant categories:
i. Pre-weaned and Growing Ruminants: Diets can include up to 20–30% mango peel silage in total mixed rations (TMR) to enhance dry matter intake (DMI), organic matter (OM) and crude protein (CP) digestibility in dairy calves (Aung et al., 2024). Similarly, up to 30% papaya pomace in concentrate mixtures supports nutrient utilization in growing buffaloes (Babu et al., 2006). Higher inclusion levels should be managed carefully to avoid tannin-induced protein-binding effects, which could reduce protein availability.
ii. Fattening and Finishing Ruminants: Up to 33% cashew apple residue in TMR has shown no adverse effects on digestibility while improving neutral detergent fiber (NDF) digestibility, which is crucial for efficient fiber utilization (Araújo et al., 2022). Papaya peels, included at levels between 10 and 30%, have been found to enhance CP and ether extract (EE) digestibility in bovines (Azevêdo et al., 2011). Additionally, mango meal can replace maize meal entirely in lamb and sheep diets without compromising performance, making it a viable energy source (Pereira et al., 2013; Aragão et al., 2012).
iii. Pregnant and Lactating Ruminants: Up to 30% mango peel or papaya pomace in multi-nutrient blocks has demonstrated improvements in digestibility without negatively affecting rumen fermentation, making them suitable dietary supplements for lactating animals (de Evan et al., 2022). Mango peels and kernels, when offered ad libitum, have also been reported to enhance nitrogen retention in sheep, optimizing protein utilization in dairy production systems (Sanon and Kanwe, 2010). Additionally, cashew apple silage at levels up to 75% in TMR can support increased protein intake and energy balance in pregnant and lactating animals (Tai et al., 2020).
iv. Mature Ruminants: These animals exhibit higher tolerance levels for fruit by-products, allowing mango meal to replace maize up to 100% in TMR (Pereira et al., 2013; Aragão et al., 2012) and cashew processing products to be incorporated at similar levels (Souza et al., 2020). Such inclusion rates provide cost-effective energy sources while ensuring adequate fiber intake.
During periods of forage scarcity, particularly in dry seasons, high-fiber diets incorporating fruit by-products can serve as essential nutritional interventions. The use of cashew apple silage combined with maize cobs at inclusion levels of 75–100% has been shown to enhance crude protein and dry matter intake, providing a viable roughage alternative during seasonal feed shortages (Tai et al., 2020). Similarly, the inclusion of mango peel at up to 20% in elephant grass silage has been observed to maintain digestibility while offering a fiber-rich alternative to conventional forages (Sá et al., 2007).
The incorporation of mango, cashew apple, and papaya by-products presents a viable strategy for improving ruminant nutrition while promoting sustainability. To maximize their benefits, future research should focus on refining optimal inclusion levels, evaluating their interactions with conventional feed ingredients, and assessing their long-term effects on animal health and productivity. A well-balanced integration of these by-products into ruminant diets can enhance both nutritional efficiency and environmental sustainability.
6.3 Influence on rumen fermentation parameters
Limited research exists on the effects of cashew apple, mango, and papaya by-products in ruminant diets, particularly regarding rumen fermentation parameters (Table 6). However, existing studies show promising results, with rumen pH values generally within the optimal range of 5.5–6.9, supporting efficient fiber and protein breakdown by rumen microflora (Shwerab et al., 2023; Omer et al., 2019). Cashew by-products have minimal effects on fermentation, with high inclusion levels slightly increasing pH and ammonia but having little impact on other markers (Tai et al., 2020). Mango by-products, including peel and meal, boost total volatile fatty acids (TVFA) and reduce methane emissions in sheep and goats, improving nutrient utilization and environmental sustainability (Aung et al., 2024; de Evan et al., 2022; Jafari et al., 2020). Papaya by-products, particularly pomace, reduce ammonia levels and increase TVFA, enhancing nitrogen retention and fermentation efficiency without altering rumen pH (Babu et al., 2006). Replacing corn with ripe mango waste in lamb diets stabilized fermentation while lowering protozoal counts, suggesting benefits for microbial modulation (Espinoza-Sánchez et al., 2020).
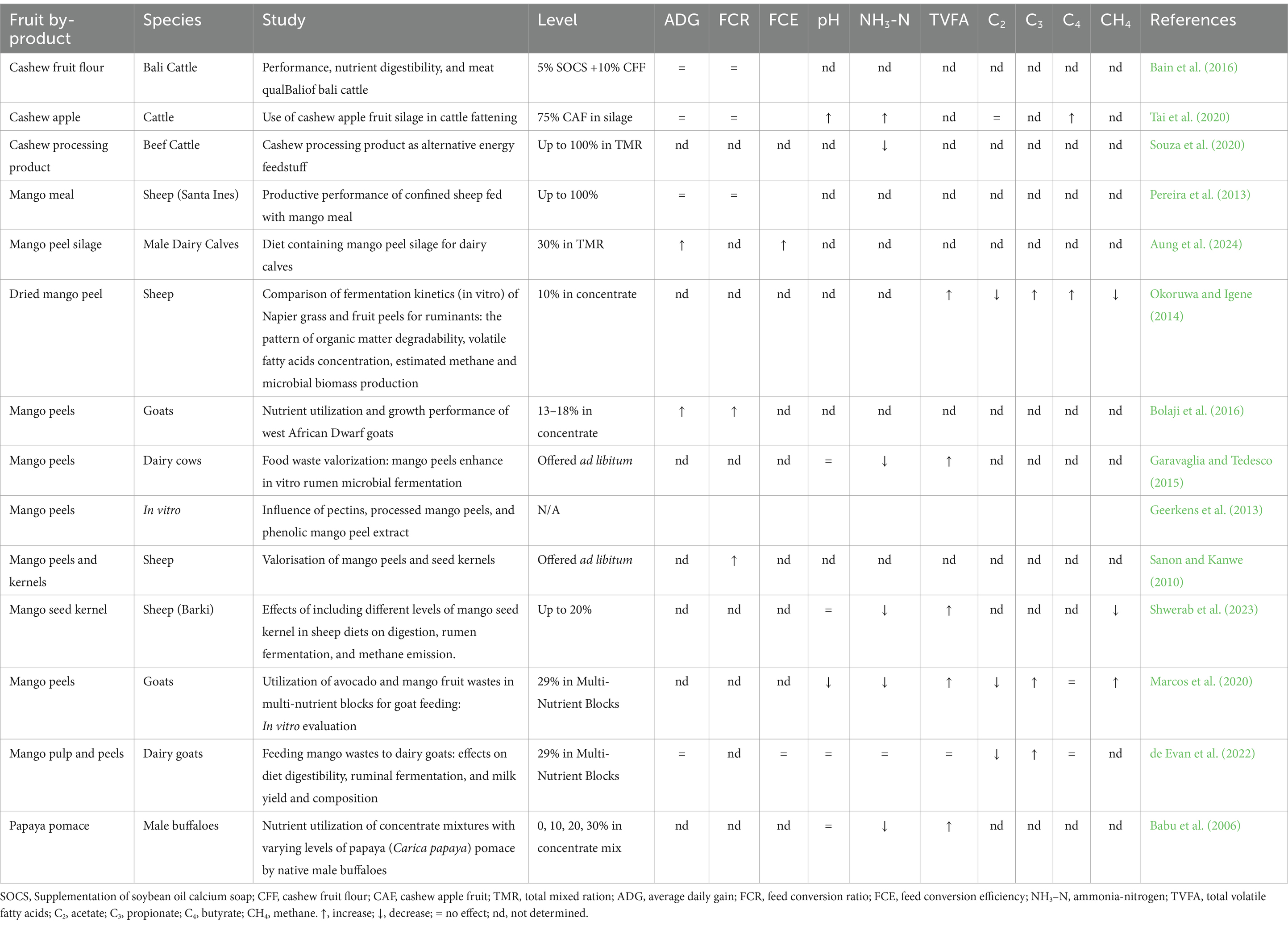
Table 6. Effects of cashew, mango and papaya by-products containing phytonutrients on growth performance, rumen fermentation end-products, and methane production.
Acetate and propionate, the primary volatile fatty acids (VFAs) produced in the rumen, play critical roles in energy utilization. Acetate supports fat synthesis, while propionate serves as the main glucose precursor, promoting growth and lactation. By-products rich in fermentable carbohydrates, such as mango pulp-peel mix, tend to increase propionate production relative to acetate (Table 5). For instance, mango pulp-peel mix at 290 g/kg in goat diets resulted in propionate levels of 12.8 mol/100 mol VFA and acetate levels of 68.6 mol/100 mol, lowering the acetate-to-propionate ratio, which supports efficient glucose synthesis (de Evan et al., 2022). This shift improves energy utilization and reduces hydrogen availability for methane synthesis, potentially lowering methane emissions (Wanapat et al., 2024; Van der Walt and Linington, 1989).
Butyrate production is generally enhanced by the inclusion of these by-products, supporting energy supply and rumen epithelial health, which benefits overall animal productivity. For example, mango pulp-peel mix in goats led to a butyrate concentration of 14.0 mol/100 mol VFA, contributing to improved energy distribution and a potentially well-balanced VFA profile, depending on the relative concentrations of acetate and propionate (de Evan et al., 2022). Cashew apple silage has also been associated with increased butyrate concentration in the rumen, where it aids in efficient microbial protein synthesis and nitrogen utilization in ruminants (Tai et al., 2020). The production of butyrate during the ensiling process may not directly affect microbial activity in the rumen but reflects fermentation quality, which influences the silage’s overall nutritional value.
Ammonia-nitrogen (NH3–N) is a critical parameter in rumen fermentation, as NH3–N levels directly influence microbial protein synthesis. Ideal NH₃–N concentrations for optimal microbial growth in the rumen typically range from 12 to 15 mg/100 mL, but this depends on the availability of fermentable organic matter (FOM) and the rate of its fermentation. These factors influence the energy available for microbes to convert NH₃–N into microbial protein (Shwerab et al., 2023). Mango by-products, particularly mango seed kernel, have been shown to support this range effectively. For instance, mango seed kernel included at levels of 50, 100, and 200 g/kg in sheep diets produced NH3–N concentrations of 13.23, 12.42, and 12.81 mg/100 mL, respectively (Shwerab et al., 2023). Similarly, an inclusion level of 225 g/kg of mango seed kernel in sheep diets resulted in an NH3–N concentration of 15.74 mg/100 mL, within the optimal range to support microbial growth (Omer et al., 2019).
The effectiveness of mango-based diets in promoting nitrogen retention may be due to their moderate protein content combined with bioactive compounds that influence rumen function. Mango by-products, especially seed kernels, contain polyphenols that can reduce the rate of protein degradation in the rumen, leading to a more gradual release of ammonia. This slower degradation may help maintain NH₃–N within the ideal range, allowing for steady microbial protein synthesis without excessive nitrogen loss. Furthermore, low-protein diets have been shown to promote nitrogen retention efficiency through enhanced renal urea reabsorption and the utilization of hydrogen by rumen microbes for the synthesis of methane or microbial biomass in goats (Zhang et al., 2023), which complements the use of mango-based diets. This synergy suggests that mango-based diets which complements the use of mango-based diets. This synergy suggests that mango-based diets are particularly effective not only in maintaining NH3–N within optimal levels for microbial growth but also in enhancing nitrogen retention and microbial protein synthesis, ultimately reducing nitrogen waste (de Evan et al., 2022). The combination of mango seed kernel’s protein content and polyphenolic compounds supports these processes, making mango-based diets a practical choice for optimizing nitrogen utilization and microbial efficiency in ruminants.
The sampling procedure significantly impacts fermentation measurements, affecting pH, VFA, and NH3–N concentrations. Rumen fluid collected through an oral stomach tube typically shows higher pH values (e.g., 7.19 in goats fed with mango pulp-peel mix) and lower VFA and NH3–N concentrations compared to rumen cannula sampling, although VFA proportions generally remain consistent (Ramos-Morales et al., 2014; Terré et al., 2013; de Assis Lage et al., 2020; Shen et al., 2012). Such differences emphasize the importance of standardized sampling to obtain accurate fermentation data.
6.4 Effects on methane production
Ruminant livestock are major contributors to global methane emissions, accounting for 35–40% of anthropogenic methane through enteric fermentation and manure. These emissions are a significant portion of agricultural methane output, alongside other major sources such as paddy rice production (IPCC, 2007; Aluwong et al., 2011; Charmley et al., 2008). Methane from enteric fermentation in ruminants is a natural by-product of their digestion, with cattle being the largest emitters, followed by sheep and goats (Broucek, 2014; Gupta et al., 2018; Cottle et al., 2011). In South Africa, enteric methane emissions exceeded 1171.56 Gg annually from 1990 to 2014, primarily from non-dairy cattle, while small ruminants contributed 15.6% of total emissions (Du Toit et al., 2013; Moeletsi et al., 2017). Methane emissions in developing regions have surged, driven by increasing livestock numbers, with emissions from African cattle, goats, and sheep projected to grow from 7.8 million tons in 2000 to 11.1 million tons by 2030 (Aluwong et al., 2011).
Methane emissions in developing regions have surged, driven by increasing livestock numbers, with emissions from African cattle, goats, and sheep projected to grow from 7.8 million tons in 2000 to 11.1 million tons by 2030 (Mitsumori and Sun, 2008; Morgavi et al., 2010; Baker, 1999). This specialized group of microbes, present in various anaerobic environments, including the rumen, fills a crucial ecological niche by utilizing H2 and CO2 (Moss et al., 2000). Methane emissions from ruminants and other anaerobic settings are major contributors to global warming (Moss et al., 2000). In rumen fermentation, acetate and butyrate promote methane production, whereas propionate formation competes for hydrogen, reducing methane output (Moss et al., 2000; Bica et al., 2022). Feed additives and diet composition can also influence rumen metabolite profiles, potentially altering methane emissions by modifying microbial activity within the rumen (Bica et al., 2022; Palangi and Lackner, 2022). Feeds that increase acetate and carbon dioxide production, such as Napier grass, lead to higher methane emissions and a loss of feed energy, making them less efficient for animal production (McDonald et al., 2011). In contrast, by-products like mango and papaya peels show the potential to reduce methane emissions in ruminant diets due to their bioactive properties, which can inhibit methanogenic archaea and improve rumen fermentation efficiency (Jafari et al., 2020; Okoruwa and Igene, 2014; Geerkens et al., 2013).
Research supports the methane-reducing effects of fruit by-products (Table 5). Okoruwa and Igene (Okoruwa and Igene, 2014) observed lower methane production from mango and papaya peels than from Napier grass, likely due to their influence on volatile fatty acid (VFA) pathways, particularly by redirecting hydrogen toward propionate production rather than methane. Bioactive compounds in mango peels, including phenolics and tannins, have selective antimicrobial properties (Asif et al., 2016), potentially targeting methane-producing archaea without significantly inhibiting other microbial populations. Similarly, papaya waste extracts and seeds contain bioactive compounds that may support fermentation efficiency by maintaining a balanced microbial ecosystem while reducing methane emissions (Sharma et al., 2020). Geerkens, Schweiggert (Geerkens et al., 2013) found that phenolic extracts from mango peels reduced methane emissions by 9% in hay-based diets, and a 20% inclusion of mango seed kernel in a corn-based diet reduced emissions by up to 40% in sheep, likely due to its high tannin content (Shwerab et al., 2023).
Although cashew by-products have been less extensively studied, their bioactive composition suggests potential impacts on methane production, though results are mixed. Studies with cattle indicate that methane emissions remain primarily unchanged with cashew by-product inclusion, suggesting limited effects on methane mitigation in some contexts (Souza et al., 2020). However, additional research could clarify their role in methane reduction, particularly in combination with other dietary factors.
7 Influence of feeding mango, cashew, and papaya by-products on small ruminant growth performance and health
7.1 Effects on growth and feed conversion
The influence of mango and cashew by-products on the growth performance and carcass traits of sheep and goats is multifaceted and contingent upon the inclusion level and processing methods employed. Studies (Table 6) indicate that moderate inclusion levels (15–30%) of by-products, like dried mango pulp, can enhance or maintain average daily gain (Tompkins and Adger, 2004; Tompkins and Adger, 2004) and feed conversion ratio (FCR) in sheep, serving as effective partial substitutes for conventional concentrates without compromising growth performance, particularly when traditional feed sources are limiting (Aung et al., 2024). Additionally, moderate levels do not negatively impact carcass weight or dressing percentage, making them cost-effective feed alternatives under conditions of feed scarcity (Table 5). Mango by-products, particularly mango peel silage and meal, consistently improve ADG in dairy calves and goats at levels up to 30% in total mixed rations, likely due to nutrient density and fiber that enhance rumen fermentation (Aung et al., 2024).
Cashew by-products, such as cashew fruit flour and apple silage, generally stabilize ADG without significant growth gains, while papaya by-products, like papaya pomace, support growth without notable improvement (Bain et al., 2016; Tai et al., 2020; Babu et al., 2006). Mango by-products also enhance FCR, likely due to bioactive compounds that support rumen function, though high inclusion levels (>30%) or tannin-rich, unprocessed by-products like mango peels may reduce FCR and ADG by inhibiting nutrient absorption. Similarly, highly fibrous by-products like papaya peels may reduce digestibility, requiring higher feed intake and worsening FCR (Babu et al., 2006).
Mango by-products emerge as the most promising for improving ADG and FCR, likely due to their high carbohydrate and fiber content, along with bioactive compounds that enhance rumen microbial activity and fermentation efficiency. The sugars and easily fermentable carbohydrates in mango by-products provide a readily available energy source that supports optimal microbial growth and protein synthesis in the rumen, improving nutrient uptake and overall efficiency compared to conventional forage or low-energy by-products. In comparison, cashew and papaya by-products serve more as maintenance feeds, providing stable growth support without significant efficiency gains. This suggests mango by-products are particularly valuable in enhancing growth outcomes, while cashew apple and papaya are reliable for supporting stable performance.
7.2 Effects on health
The introduction of mango, cashew, and papaya by-products into the diets of small ruminants offers a spectrum of potential health benefits, though accompanying drawbacks necessitate consideration. Mango and papaya by-products have shown antiparasitic properties that benefit ruminants when ingested (Guil-Guerrero et al., 2016). For instance, mango peel extract has been found to effectively reduce fecal egg counts in goats, likely due to its antioxidant, anti-inflammatory, and antibacterial phytochemicals, which support rumen health and production parameters (Prasetyo et al., 2024; Umamahesh et al., 2020). Mango peel powder has also been studied as a feed additive for promoting rumen health and reducing methane emissions, offering potential benefits for both livestock productivity and environmental sustainability (Okoruwa and Igene, 2014; Garavaglia and Tedesco, 2015; Cañaveral-Martínez et al., 2023). Papaya seeds and peels, similarly, exhibit potent effects against gastrointestinal nematodes such as Haemonchus contortus. This antiparasitic effect is primarily due to cysteine proteinases found in papaya latex, which disrupt parasite metabolism. Additionally, the carotenoids and polyphenols in papaya by-products enhance immune function and provide antioxidant benefits in dairy cows (Abouzed et al., 2019; Buttle et al., 2011). Nutritionally, both ripe and unripe papaya peels offer essential vitamins, minerals, and energy, enriching livestock diets and supporting overall health (Akintunde et al., 2022).
Cashew apple fiber has also shown promise as an anthelmintic agent against Haemonchus contortus in sheep, likely due to its phenolic compounds exhibiting antiparasitic effects (Lopes et al., 2018). Another possible explanation is the increased post-ruminal flow of protein, resulting from tannins complexing with dietary protein and protecting it from rumen degradation. This improved protein status may enable the animal to mount more effective immune responses. While these by-products have substantial benefits, caution is warranted with cashew apples, as they have been associated with toxicosis in ruminants, particularly cattle. This toxicity is likely linked to the presence of anacardic acid and related phenolic compounds, which are known to cause gastrointestinal irritation and other adverse effects in livestock when consumed in large quantities. Reports from Brazil document symptoms resembling alcoholic intoxication, such as lethargy and staggering, due to ethanol production during fermentation in the rumen (Assis et al., 2009; Filho and Soto-Blanco, 2012). Although these effects are typically reversible and non-lethal, the risk suggests carefully managing cashew apple levels. High levels of tannins in unripe mango peels can reduce feed intake, nutrient absorption, and digestibility, which lowers feed conversion efficiency in ruminants (Falusi et al., 2017; Frutos et al., 2004). Excessively fibrous by-products, may also reduce digestibility, necessitating higher intake to meet nutrient requirements (Mirzaei-Aghsaghali and Maheri-Sis, 2008). Further research into optimal inclusion levels and processing methods will help maximize these benefits and mitigate associated risks.
7.3 Effects on meat quality
The quality of meat from small ruminants fed mango, cashew, and papaya by-products is crucial for consumer acceptance and market value. Nonetheless, research on the effects of mango, cashew, and papaya by-products on carcass and meat quality in ruminants is limited but promising. In ruminants, mango meal can replace corn in lamb diets without negatively impacting carcass traits, though it may affect specific cuts, such as brisket and hindquarter weights (Neto et al., 2014). Cashew by-products, including dehydrated cashew bagasse (DCB), have also been tested in feedlot lambs, where replacing up to 24% of forage sorghum with DCB maintained the physical and chemical quality of the meat, with an 8% replacement yielding lower lipid content (Barreto et al., 2022). Similarly, sun-dried cashew pulp can be incorporated into West African Dwarf (WAD) goat diets at up to 30% without adverse effects on carcass characteristics or internal organs, providing a cost-effective feed alternative and helping reduce environmental waste (Okpanachi et al., 2016).
Research on alternative feed ingredients demonstrated promising outcomes for carcass quality and cost reduction in non-ruminants. For instance, mango-based diets produced leaner carcasses in pigs than traditional feeds (Kiendrebeogo et al., 2018). In poultry, papaya leaf powder did not negatively affect carcass quality (Laihad et al., 2019), and mango fruit waste was successfully incorporated up to 20% in broiler diets without impacting nutrient intake or growth performance (Emshaw et al., 2012). Incorporation of cashew by-products into poultry diets yielded mixed results. Cashew apple waste slightly reduced broiler performance (Swain et al., 2007), possibly due to its high fiber and tannin content, which may limit nutrient digestibility and energy availability. However, it did not affect carcass traits, suggesting that the composition of cashew waste primarily influences growth efficiency rather than final body composition (Swain et al., 2007). In contrast, dehydrated cashew apple meal, when included up to 25%, enhanced weight gain and meat-to-bone ratio, likely because dehydration reduces moisture content and concentrates nutrients, improving energy intake and overall feed efficiency (Ramos et al., 2006). For growing rabbits, dried cashew apple was included up to 30% in diets without adverse effects on performance, as rabbits are generally more tolerant of high-fibre diets, which align well with their natural digestive physiology (Fanimo et al., 2003).
Fruit by-products rich in polyphenols, such as grape pomace and citrus pulp, have shown potential to enhance the fatty acid profile and antioxidant activity in ruminant meat and milk without significantly impacting production (Correddu et al., 2023; Correddu et al., 2020). These by-products can also reduce lipid oxidation in meat during storage (de Evan et al., 2022) and improve meat color stability (Priolo and Vasta, 2007). Although condensed tannins in these by-products are poorly bioavailable, they may exert antioxidant effects through both direct and indirect mechanisms (Soldado et al., 2021). Additionally, tannins can influence ruminal biohydrogenation, increasing beneficial fatty acids such as conjugated linoleic acid and omega-3 fatty acids in milk and meat (Frutos et al., 2020). However, the impact of tannins on fatty acid profiles may vary depending on factors like dosage and animal species (Ponnampalam et al., 2024). Incorporating these by-products into ruminant diets presents a sustainable approach to enhancing product quality while reducing feed costs (Vastolo et al., 2022). Mango and cashew by-products have shown potential for enhancing ruminant meat quality, particularly by improving lipid profiles and antioxidant properties due to their polyphenolic content (Guil-Guerrero et al., 2016; Ponnampalam et al., 2024). However, direct studies examining these effects in ruminants are still limited. Similarly, while papaya by-products are rich in carotenoids and phenolics, specific evidence of their impact when included in ruminant diets on meat quality remains unavailable (Guil-Guerrero et al., 2016; Da Silva et al., 2014). The potential benefits of all three by-products, especially regarding their impact on meat quality, lipid profiles, and antioxidant properties, warrant further research to confirm their effects in ruminant diets.
8 Methods or techniques for preserving fruit by-products for feed
The nutritional quality of fruit by-products (FBPs) can be preserved or enhanced through various physical, chemical, and biological treatments, making them more effective as livestock feed (Balehegn et al., 2022). Physical treatments—such as chopping, pelleting, drying, and densification—reduce bulkiness, improving ease of handling and feed intake. Densification refers to the compaction of by-products into dense pellets or blocks to increase their bulk density, facilitating transport and storage. Chemical treatments use acids or alkalis to break down fibrous structures, enhancing digestibility and nutrient availability. Biological treatments, often involving specific microorganisms, promote beneficial fermentation that reduces anti-nutritional compounds while increasing protein content. However, fermentation typically reduces gross energy content due to energy losses during microbial metabolism.
Due to their high moisture content and bulk (>800 g/kg), fruit by-products require preservation methods to extend shelf life (Zhang et al., 2017). The two primary techniques—drying and ensiling—are widely used. Sun-drying is common for reducing moisture and preventing microbial spoilage (Gan et al., 2016) but can lead to nutrient losses, particularly in heat-sensitive vitamins and antioxidants, diminishing antioxidant and antimicrobial capacities (Chikwanha et al., 2018; Klava et al., 2018). Rapid drying to low moisture levels (~0.7 water activity) is essential to prevent mold and mycotoxin growth, particularly in humid conditions (Chiewchan et al., 2015; Chulze, 2010).
Ensiling, in contrast, preserves proteins and carbohydrates by promoting lactic acid fermentation under anaerobic conditions, creating an acidic environment that inhibits mould and mycotoxin growth. Although Tayengwa and Mapiye (Tayengwa and Mapiye, 2018) speculated that ensiling could negatively impact FBP quality and the environment, their study did not directly investigate these effects. On the contrary, other research indicates that ensiling improves FBP nutritional profiles (Wimalasiri and Somasiri, 2021; Santos et al., 2019) and is particularly beneficial during wet seasons when sunlight is insufficient for effective drying.
8.1 Effect of preservation technique on the quality of by-product
The choice of preservation technique significantly affects the quality of mango, cashew, and papaya by-products. While sun-drying is a practical and accessible preservation method, it can reduce levels of heat-sensitive nutrients, particularly vitamins C and E and carotenoids (Nagle et al., 2011; Kamiloglu et al., 2016; Ndawula et al., 2004). Rapid drying prevents mould and mycotoxin contamination, especially in humid conditions (Chiewchan et al., 2015). In contrast, ensiling has been shown to improve the quality of FBPs by preserving nutrients and enhancing palatability through fermentation, as demonstrated in studies on mango residues, persimmon peel, and grape pomace (Guzmán et al., 2010; Fitri et al., 2020; Mousa et al., 2019). Additionally, ensiling effectively reduces anti-nutritional factors, such as tannins in mango peels, mainly when additives like molasses and urea are included. (Couto Filho et al., 2007; Guzmán et al., 2012; Sruamsiri and Silman, 2009). For instance, ensiling mango residues with maize stover, molasses, and urea produces a stable feed with favorable fermentative and chemical characteristics suitable for animal consumption (Guzmán et al., 2010, 2012).
Ensiling also offers environmental benefits by potentially reducing methane emissions from ruminants due to changes in fibrous fractions during fermentation (Mousa et al., 2019). For mango residues, the ensiling process stabilizes fermentative characteristics within 14–21 days, after which fermentation activity slows significantly (Guzmán et al., 2010, 2012). While ensiling can improve protein solubility, excessive solubility may lead to rapid rumen degradation and increased nitrogen loss. However, if proteins form complexes with tannins present in the ensiled crop, solubility decreases, slowing protein digestion in the rumen and improving nitrogen capture efficiency. Incorporating Lactobacillus plantarum can enhance nutrient utilization and reduce methane emissions from ruminants (Zhang et al., 2022; Chen et al., 2022). Combining hydrolysable and condensed tannins at low doses can improve nitrogen utilization efficiency and mitigate methane production without adversely affecting ruminal fermentation (Chen et al., 2022). These ensiling techniques offer potential environmental benefits by reducing methane emissions from ruminants while improving feed quality, making them promising strategies for sustainable animal production (Zhang et al., 2022; Chen et al., 2022).
While dried by-products may have a coarser texture and reduced palatability, this can be mitigated by grinding or mixing with other feed ingredients. By understanding the impacts of different preservation techniques, producers can make informed decisions to optimize the nutritional value, safety, and palatability of these valuable feed resources, ensuring they meet quality standards and livestock dietary needs.
8.2 Problems associated with the storage and feeding of cashew, papaya and mango by-products
Using cashew, papaya, and mango by-products as feed offers benefits but comes with storage and feeding challenges. Their high moisture content can lead to rapid spoilage if not properly dried, increasing mold growth risks (Musundire et al., 2021). Inadequate storage may also cause unwanted secondary fermentation, reducing the nutritional value and making the feed less palatable due to odors. Proper storage, such as airtight containers, can mitigate these issues (Balehegn et al., 2020). The high sugar content in these by-products attracts insects and rodents, making pest control essential (Musundire et al., 2021). Nutritionally, while rich in certain nutrients, these by-products may lack others, requiring balanced rations to ensure animals receive complete nutrition (Balehegn et al., 2020). Livestock may initially reject these feeds due to unfamiliar tastes; gradual introduction can help (Musundire et al., 2021).
Additionally, some fruit by-products contain anti-nutritional factors, like tannins, which can interfere with nutrient absorption, necessitating further research and mitigation strategies (Balehegn et al., 2020). Seasonal variability in supply poses additional challenges, requiring farmers to plan and establish reliable sourcing networks (Musundire et al., 2021). Quality control during storage and processing is essential to prevent contamination and ensure livestock health, requiring collaborative support from agricultural, livestock, and governmental sectors to optimize the use of fruit by-products in livestock production.
8.3 Co-feeding strategies and nutrient balancing
Strategies for optimizing mango, cashew, and papaya by-products in ruminant diets focus on improved feed intake, nutrient utilization, and animal performance. Co-feeding strategies show the benefits of ensiling these by-products with low-quality roughages and additives to enhance digestibility and nutrient content (Table 7). For instance, ensiling mango peels with rice straw and legumes boosts dry matter and fiber digestibility in cattle, while papaya peels ensiled with pangola grass show improved fermentation characteristics and nutritional quality (Sruamsiri and Silman, 2009; Sánchez-Santillán et al., 2021). Cashew by-products, when ensiled with cassava peels or incorporated into broiler diets, have demonstrated improved nutrient intake and growth in both ruminants and poultry (Ferreira et al., 2015; Venkatramana et al., 2020).
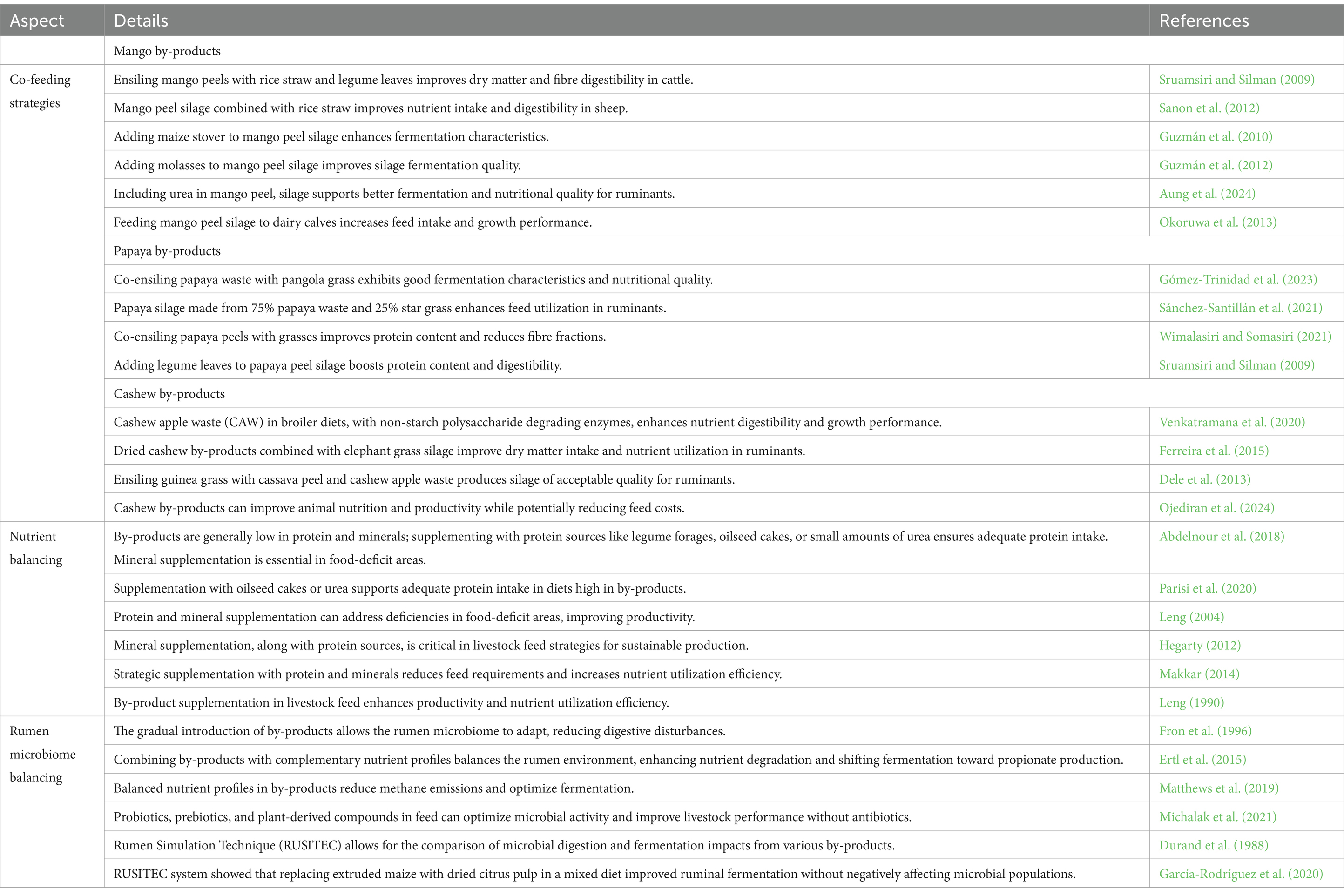
Table 7. Co-feeding strategies and nutrient balancing for ruminants using mango, cashew apple, and papaya by-products.
Nutrient balancing is crucial for optimizing livestock productivity, especially in low-income food-deficit regions where by-products are often low in protein and minerals (Table 7). In these regions, limited access to diverse and high-quality feed resources exacerbates the challenge, making effective nutrient balancing essential to mitigate deficiencies and support livestock health and performance. Supplementation with protein-rich sources like legumes, oilseed cakes, or small amounts of urea can enhance protein intake, and mineral additions may address specific deficiencies (Abdelnour et al., 2018; Leng, 2004). These practices improve livestock productivity and nutrient utilization efficiency, supporting sustainable production (Leng, 1990; Makkar, 2014).
Finally, maintaining a stable rumen environment is essential for effective digestion (Table 7). Gradually introducing these by-products helps rumen microbes adapt to dietary changes, minimizing digestive issues and supporting a stable rumen pH, which is crucial for microbial activity and fibre digestion (Fron et al., 1996). Adding complementary by-products or feed additives, such as probiotics, supports microbial activity, improves nutrient breakdown, and can even reduce methane emissions (Ertl et al., 2015; Smith et al., 2020). Together, these co-feeding and nutrient-balancing strategies promote a more sustainable, efficient approach to livestock feeding in developing areas, maximizing the potential of fruit by-products as a valuable feed resource.
9 Policy implications for promoting the use of fruit by-products as livestock feed
To ensure the widespread adoption of fruit by-products as alternative feed resources in LIFDCs, targeted policies and interventions are needed at the policy, infrastructure, and community levels. These measures aim to increase the utilization of fruit by-products, enhance feed availability, reduce waste, and improve livestock productivity. Key strategies include:
1. Incentivizing Valorization Through Subsidies: Governments can provide financial incentives or subsidies to fruit processors and farmers who engage in valorizing by-products into livestock feed. This could include grants for equipment used in drying, ensiling, or processing fruit by-products.
2. Establishing Regulations for Waste Diversion: Legislation mandating the diversion of fruit processing waste from landfills to animal feed production could significantly reduce environmental pollution. These policies should include guidelines for the safe handling and processing of by-products to ensure their suitability for livestock consumption.
3. Improving Infrastructure for Preservation and Distribution: Adequate storage and transport infrastructure are critical for ensuring that fruit by-products retain their nutritional quality and reach livestock farmers in remote areas. Governments and private stakeholders should invest in affordable preservation technologies, such as silos for ensiling and solar dryers for moisture removal.
4. Strengthening Extension Services: Agricultural extension officers must be trained to educate smallholder farmers about the benefits of using fruit by-products as feed, along with guidance on how to process and balance these by-products in livestock diets. Community-level demonstration projects can also build farmer confidence in adopting these methods.
5. Encouraging Research and Innovation: Funding research to optimize preservation methods, assess regional variations in by-product composition, and determine optimal inclusion levels for livestock is essential. Policies should also promote collaborations between universities, research institutions, and industry stakeholders to create innovative solutions.
6. Fostering Public-Private Partnerships (PPPs): Collaborations between governments, NGOs, and private companies can accelerate the adoption of fruit by-product valorization strategies. For instance, fruit processing industries could partner with cooperatives to supply by-products to livestock farmers, creating mutually beneficial value chains.
7. Incorporating Circular Economy Principles: Policymakers should encourage integrating circular economy models, where fruit by-products are not only seen as waste but as valuable inputs in livestock systems. This could include public awareness campaigns on sustainable waste management practices and their environmental and economic benefits.
By addressing these areas, the adoption of fruit by-products as livestock feed can move from isolated case studies to widespread practice, contributing to food security, environmental sustainability, and rural economic growth in LIFDCs. Such policy interventions could serve as blueprints for other regions facing similar challenges.
10 Concluding remarks
Valorizing fruit by-products such as mango peels, cashew apple pomace, and papaya seeds offers a transformative solution to feed scarcity in small ruminant systems within LIFDCs. These by-products improve feed efficiency, enhance livestock productivity, and mitigate environmental waste, aligning with sustainability and circular bioeconomy goals. Integrating fruit by-products into livestock diets supports smallholder resilience by reducing feed costs and improving meat quality. Bioactive compounds such as tannins also offer potential for methane mitigation, further contributing to climate resilience.
Despite these benefits, challenges such as nutrient variability, inadequate preservation infrastructure, and limited farmer awareness must be addressed. Targeted investments, policy interventions, and research innovations are essential to scale up adoption and maximize impact. By addressing these interconnected challenges, this approach can contribute to sustainable livestock production, improved food security, and rural economic development in vulnerable regions.
Author contributions
AA-J: Writing – original draft, Writing – review & editing, Conceptualization, Data curation. SI: Writing – original draft. PS: Writing – original draft. SG: Writing – original draft. HG: Writing – review & editing. CB: Writing – review & editing. FM: Writing – review & editing. ME: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Food Systems Research Network for Africa (FSNet-Africa). FSNet-Africa was funded by the Global Challenges Research Fund (GCRF) as a Research Excellence project under a partnership between UK Research and Innovation (UKRI) and the African Research Universities Alliance (ARUA). FSNet-Africa is a flagship project in the ARUA Centre of Excellence in Sustainable Food Systems (ARUA-SFS), which is hosted by the University of Pretoria (South Africa) in collaboration with the University of Nairobi (Kenya) and the University of Ghana (Ghana) (grant no. ES/T015128/1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer EP declared a shared affiliation with the author(s) (HG and CB) to the handling editor at the time of review.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Hay, M., Farooq, A., and Kamran, F. (2022). “Papaya wastes and by-products: chemistry, processing, and utilization” in Handbook of fruit wastes and by-products (CRC Press), 175–192.
Abdelnour, S. A., Abd El-Hack, M. E., and Ragni, M. (2018). The efficacy of high-protein tropical forages as alternative protein sources for chickens: a review. Agriculture 8:86. doi: 10.3390/agriculture8060086
Abebe, K. (2017). Effect of climate change on nutritional supply to livestock production. Acad. Res. J. Agric. Sci. Res. 5, 98–106. doi: 10.14662/ARJASR2016.056
Abouzed, T. K., Sadek, K. M., Ayoub, M. M., Saleh, E. A., Nasr, S. M., El-Sayed, Y. S., et al. (2019). Papaya extract upregulates the immune and antioxidants-related genes, and proteins expression in milk somatic cells of Friesian dairy cows. J. Anim. Physiol. Anim. Nutr. 103, 407–415. doi: 10.1111/jpn.13032
Ahaotu, E., and Ihekoronye, B. (2019). Environmental, ecological and anti-nutritional factors for cashew utilization in rabbit production–a review. JIRCAS J. Sci. Pap. 6, 8–22.
Aidoo, R., Kwofie, E. M., and Ngadi, M. O. (2022). Circularity of cashew apples: examining the product-process pathways, techno-functional, nutritional/Phytomolecular qualities for food applications. ACS Food Sci. Technol. 2, 1051–1066. doi: 10.1021/acsfoodscitech.2c00093
Akintunde, A. O., Kolu, P., Akintunde, I. A., Adewole, S. A., Akinboye, O. E., Afodu, O. J., et al. (2022). Evaluation of the nutritive values of Carica Papaya fruit peels as a potential ingredient in livestock nutrition. Anim. Prod. 24, 104–113. doi: 10.20884/1.jap.2022.24.2.129
Akyereko, Y. G., Wireko-Manu, F. D., Alemawor, F., and Adzanyo, M. (2022). Cashew apples in Ghana: stakeholders’ knowledge, perception, and utilization. Int. J. Food Sci. 2022, 1–10. doi: 10.1155/2022/2749234
Alañón, M. E., Palomo, I., Rodríguez, L., Fuentes, E., Arráez-Román, D., and Segura-Carretero, A. (2019). Antiplatelet activity of natural bioactive extracts from mango (Mangifera Indica L.) and its by-products. Antioxidants 8:517. doi: 10.3390/antiox8110517
Ali, A., Devarajan, S., Waly, M., Essa, M. M., and Rahman, M. (2011). Nutritional and medicinal value of papaya (Carica papaya L.). Nat. Produ. Bioact. Comp. Dis. Prevent., 34–42.
Aluko, A., Makule, E., and Kassim, N. (2023). Underutilized cashew apple fruit: its utility and development as a Sou rce of nutrients and value added products in Tanzania. Curr. Res. Nutr. Food Sci. 11, 719–734. doi: 10.12944/CRNFSJ.11.2.22
Aluwong, T., Wuyep, P., and Allam, L. (2011). Livestock-environment interactions: methane emissions from ruminants. Afr. J. Biotechnol. 10, 1265–1269.
Amejo, A. G., Gebere, Y. M., and Kassa, H. (2018). Integrating crop and livestock in smallholder production systems for f ood security and poverty reduction in sub-Saharan Africa. Afr. J. Agric. Res. 13, 1272–1282. doi: 10.5897/AJAR2018.13020
Amwata, D. A., Nyariki, D., and Musimba, N. K. R. (2016). Factors influencing pastoral and Agropastoral household vulnerability to food insecurity in the drylands of Kenya: a case study of Kajiado a nd Makueni counties. J. Int. Dev. 28, 771–787. doi: 10.1002/jid.3123
Andrade, R. A. M. S., Maciel, M., Santos, A., and Melo, E. (2015). Optimization of the extraction process of polyphenols from cashew apple agro-industrial residues. Food Sci. Technol. 35, 354–360. doi: 10.1590/1678-457X.6585
Aragão, A. S. L., Pereira, L. G. R., Chizzotti, M. L., Voltolini, T. V., Azevêdo, J. A. G., Barbosa, L. D., et al. (2012). Farelo de manga na dieta de cordeiros em confinamento. Arq. Bras. Med. Vet. Zoot. 64, 967–973. doi: 10.1590/S0102-09352012000400025
Araújo, G. G. L. D., Araújo, C. D. A., Gois, G. C., Magalhães, A. L. R., Novaes, J. J. D. S., Rodrigues, J. M. D. C. D. S., et al. (2022). Fermentation profile, aerobic stability, chemical and mineral composition of silages of mango combined with cocoa pod husk meal. Braz. J. Vet. Res. Anim. Sci. 59:e194267. doi: 10.11606/issn.1678-4456.bjvras.2022.194267
Araújo, A. R., Costa, J. B., Rogério, M. C. P., Carneiro, M. D. S. D. S., Muniz, L. C., Fontenele, R. M., et al. (2022). Dehydrated cashew apple in different grinding sizes to sheep. Acta Sci. Anim. Sci. 44:e54398. doi: 10.4025/actascianimsci.v44i1.54398
Armah, I. N. A. (2008) The effect of starter-grower pigs fed diets containing varying levels of dried cashew (Anarcadium occidentale l.) pulp (DCP). A thesis submitted to the School of Graduate Studies Kwame Nkrumah University of Science and Technology, Kumasi, in partial fulfilment of the requirement for the award of the degree, Master of Science in Animal Nutrition. Accessed: https://ir.knust.edu.gh/items/1161ae61-242d-4750-bdce-a5be0a60f351
Armson, B., Ekiri, A. B., Alafiatayo, R., and Cook, A. J. (2020). Small ruminant production in Tanzania, Uganda, and Ethiopia: a systematic review of constraints and potential solutions. Vet. Sci. 8:5. doi: 10.3390/vetsci8010005
Arowolo, M. A., and He, J. (2018). Use of probiotics and botanical extracts to improve ruminant production in the tropics: a review. Anim. Nutr. 4, 241–249. doi: 10.1016/j.aninu.2018.04.010
Asaolu, V., Binuomote, R., Akinlade, J., Aderinola, O., and Oyelami, O. (2012). Intake and growth performance of west African dwarf goats fed Moringa oleifera, Gliricidia sepium and Leucaena leucocephala dried leaves as supplements to cassava peels. J. Biolo. Agric. Healthc. 2, 76–88.
Asaolu, V., Binuomote, R., Akinlade, J., Oyelami, O., and Kolapo, K. (2011). Utilization of Moringa oleifera fodder combinations with Leucaena leucocephala and Gliricidia sepium fodders by west African dwarf goats. Int. J. Agric. Res. 6, 607–619. doi: 10.3923/ijar.2011.607.619
Asif, A., Farooq, U., Akram, K., Hayat, Z., Shafi, A., Sarfraz, F., et al. (2016). Therapeutic potentials of bioactive compounds from mango fruit wastes. Trends Food Sci. Technol. 53, 102–112. doi: 10.1016/j.tifs.2016.05.004
Assan, N. (2022). Climate change impact on small-scale animal agriculture: livestock wat er & food security in Africa. Trends J. Sci. Re 1, 13–39. doi: 10.31586/ujfs.2022.541
Assis, T. S., Medeiros, R. M. T., Araújo, J. A., Dantas, A. F. M., and Riet-Correa, F. (2009). Intoxicações por plantas em ruminantes e equídeos no Sertão Paraibano. Pesqui. Vet. Bras. 29, 919–924. doi: 10.1590/S0100-736X2009001100010
Ates, S., Cicek, H., Bell, L. W., Norman, H. C., Mayberry, D. E., Kassam, S., et al., (2018) editors. Sustainable development of smallholder crop-livestock farming in devel oping countries. IOP Conference Series: Earth and Environmental Science 2018; The 4th International Conference on Sustainable Agriculture and Environment (4th ICSAE), 10–12 August 2017 Surakarta, Indonesia: IOP Publishing Ltd.
Aung, K. T., Win, K. S., Mu, K. S., Aung, M., and Kyawt, Y. Y. (2024). A diet containing mango peel silage impacts upon feed intake, energy supply and growth performances of male dairy calves. Anim. Open Space 3:100069. doi: 10.1016/j.anopes.2024.100069
Ayantunde, A. A., Adamou, K., Seybou, G., and Moumouni, O. (2022). Livestock feed markets across seasons in periurban areas of Niger: seller and buyer profiles, feed price and quality. Rev. Elev. Med. Vet. Pays Trop. 75, 47–54. doi: 10.19182/remvt.36921
Azevêdo, J. A. G., Valadares Filho, S. C., Pina, D. S., Detmann, E., Valadares, R. F. D., Pereira, L. G. R., et al. (2011). Consumo, digestibilidade total, produção de proteína microbiana e balanço de nitrogênio em dietas com subprodutos de frutas para ruminantes. Rev. Bras. Zootec. 40, 1052–1060. doi: 10.1590/S1516-35982011000500017
Babu, A. R., Rao, D. S., and Parthasarathy, M. (2006). Nutrient utilization of concentrate mixtures with varying levels of papaya (Carica papaya) pomace by native male buffaloes. Anim. Nutr. Feed. Technol. 6, 159–166.
Baderna, D., Caloni, F., and Benfenati, E. (2019). Investigating landfill leachate toxicity in vitro: a review of cell mo dels and endpoints. Environ. Int. 122, 21–30. doi: 10.1016/j.envint.2018.11.024
Bain, A., Astuti, D. A., Suharti, S., Arman, C., and Wiryawan, K. G. (2016). Performance, nutrient digestibility, and meat quality of Bali cattle fed a ration supplemented with soybean oil calcium soap and cashew fruit flour. Media Petern. 39, 180–188. doi: 10.5398/medpet.2016.39.3.180
Baiyeri, P. K., Foleng, H. N., Machebe, N. S., and Nwobodo, C. E. (2019). “Crop-livestock interaction for sustainable agriculture” in Innovations in sustainable agriculture. eds. M. Farooq and M. Pisante (Cham: Springer International Publishing), 557–582.
Baker, S. (1999). Rumen methanogens, and inhibition of methanogenesis. Aust. J. Agric. Res. 50, 1293–1298. doi: 10.1071/AR99005
Bakshi, M. P. S., and Wadhwa, M. (2013). Nutritional evaluation of cannery and fruit wastes as livestock feed. Indian J. Anim. Sci. 83, 1198–1202. doi: 10.56093/ijans.v83i11.34778
Balehegn, M., Ayantunde, A., Amole, T., Njarui, D., Nkosi, B. D., Müller, F. L., et al. (2022). Forage conservation in sub-Saharan Africa: review of experiences, challenges, and opportunities. Agron. J. 114, 75–99. doi: 10.1002/agj2.20954
Balehegn, M., Duncan, A., Tolera, A., Ayantunde, A. A., Issa, S., Karimou, M., et al. (2020). Improving adoption of technologies and interventions for increasing supply of quality livestock feed in low- and middle-income countries. Glob. Food Sec. 26:100372. doi: 10.1016/j.gfs.2020.100372
Barreto, H. F. M., Assis, A. P. P. D., Lima, R. N. D., Soares, E. C. A., Sousa, Ê. S. D., Moura, A. A. C., et al. (2022). Physical and chemical characteristics of meat from lambs fed sorghum silage with cashew bagasse. Anais da Academia Brasileira de Ciências 94:e20200425. doi: 10.1590/0001-3765202220200425
Barreto, J. C., Trevisan, M. T. S., Hull, W. E., Erben, G., De Brito, E. S., Pfundstein, B., et al. (2008). Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and Peel of mango (Mangifera indical.). J. Agric. Food Chem. 56, 5599–5610. doi: 10.1021/jf800738r
Barve, K., Vaishali, A., Amit, A., and Barve, K. (2020). Anacardium occidentale by-product: a review on sustainable application and added-value. J. Food Nutr. Metabolism 1–6. doi: 10.31487/j.JFNM.2020.01.04
Bauman, D., Perfield, J., De Veth, M., and Lock, A., New perspectives on lipid digestion and metabolism in ruminants. Proceedings of Cornell Nutrition Conference (2003): Cornell University New York, NY.
Bica, R., Palarea-Albaladejo, J., Lima, J., Uhrin, D., Miller, G. A., Bowen, J., et al. (2022). Methane emissions and rumen metabolite concentrations in cattle fed two different silages. Sci. Rep. 12:5441. doi: 10.1038/s41598-022-09108-w
Bingemer, H. G., and Crutzen, P. J. (1987). The production of methane from solid wastes. J. Geophys. Res. 92, 2181–2187. doi: 10.1029/JD092iD02p02181
Boateng, M., Amoah, K. O., Atuahene, P. Y., Frimpong, Y. O., Okai, D. B., and Osei, G. (2021). Effects of dried cashew (Anacardium occidentale L.) apple meal (DCAM) on the growth performance and internal organs of albino rats. Ghana. J. Agric. Sci. 56, 14–21. doi: 10.4314/gjas.v56i2.2
Bolaji, T. A., Ebinabor, G. E., and Okorare, B. K. (2016). Nutrient utilization and growth performance of West African Dwarf goats fed with elephant grass or different proportions of plantain and mango peels. Int. J. Plant Anim. Sci. 4, 123–130.
Brewer, K. M., and Gaudin, A. C. M. (2020). Potential of crop-livestock integration to enhance carbon sequestration and agroecosystem functioning in semi-arid croplands. Soil Biol. Biochem. 149:107936. doi: 10.1016/j.soilbio.2020.107936
Broucek, J. (2014). Production of methane emissions from ruminant husbandry: a review. J. Environ. Prot. 5, 1482–1493. doi: 10.4236/jep.2014.515141
Buttle, D. J., Behnke, J. M., Bartley, Y., Elsheikha, H. M., Bartley, D. J., Garnett, M. C., et al. (2011). Oral dosing with papaya latex is an effective anthelmintic treatment for sheep infected with Haemonchus contortus. Parasit. Vectors 4, 1–11. doi: 10.1186/1756-3305-4-36
Campbell, Z., Coleman, P., Guest, A., Kushwaha, P., Ramuthivheli, T., Osebe, T., et al. (2021). Prioritizing smallholder animal health needs in East Africa, West Africa, and South Asia using three approaches: literature review, expert workshops, and practitioner surveys. Prev. Vet. Med. 189:105279. doi: 10.1016/j.prevetmed.2021.105279
Cañaveral-Martínez, U. R., Sánchez-Santillán, P., Torres-Salado, N., Hernández-Sánchez, D., Herrera-Pérez, J., and Ayala-Monter, M. A. (2023). Effect of waste mango silage on the in vitro gas production, in situ digestibility, intake, apparent digestibility, and ruminal characteristics in calf diets. Vet. World 16, 421–430. doi: 10.14202/vetworld.2023.421-430
Charmley, E., Stephens, M., and Kennedy, P. (2008). Predicting livestock productivity and methane emissions in northern Australia: development of a bio-economic modelling approach. Aust. J. Exp. Agric. 48, 109–113. doi: 10.1071/EA07264
Chauhan, S. S., Celi, P., Ponnampalam, E. N., Leury, B. J., Liu, F., and Dunshea, F. R. (2014). Antioxidant dynamics in the live animal and implications for ruminant health and product (meat/Milk) quality: role of vitamin E and selenium. Anim. Prod. Sci. 54, 1525–1536. doi: 10.1071/AN14334
Chekol, G. A. (2021). Factors affecting the marketing of livestock in south Omo zone: the case of hammer woreda. Glob. J. Bus. Econ. Manag. 11, 119–131.
Chen, L., Bao, X., Guo, G., Huo, W., Li, Q., Xu, Q., et al. (2022). Evaluation of gallnut tannin and Lactobacillus plantarum as natural modifiers for alfalfa silage: ensiling characteristics, in vitro ruminal methane production, fermentation profile and microbiota. J. Appl. Microbiol. 132, 907–918. doi: 10.1111/jam.15246
Chiewchan, N., Mujumdar, A. S., and Devahastin, S. (2015). Application of drying technology to control aflatoxins in foods and feeds: a review. Dry. Technol. 33, 1700–1707. doi: 10.1080/07373937.2015.1068795
Chikwanha, O. C., Raffrenato, E., Opara, U. L., Fawole, O. A., Setati, M. E., Muchenje, V., et al. (2018). Impact of dehydration on retention of bioactive profile and biological activities of different grape (Vitis vinifera L.) pomace varieties. Anim. Feed Sci. Technol. 244, 116–127. doi: 10.1016/j.anifeedsci.2018.08.006
Chinwendu, A. O., Catherine, A., Bassey, A. E., and Okon, E. (2019). The potential of biogas production from fruit wastes (Watermelon, Mang o and Pawpaw). World Journal of Advanced Research and Reviews 1, 52–65. doi: 10.30574/wjarr.2019.1.3.0026
Choudhury, P. K., Salem, A. Z. M., Jena, R., Kumar, S., Singh, R., and Puniya, A. K. (2015). “Rumen Microbiology: An Overview” in Rumen Microbiology: From Evolution to Revolution. eds. A. Puniya, R. Singh, and D. Kamra (New Delhi: Springer). doi: 10.1007/978-81-322-2401-3_1
Christensen, R. G., Eun, J. S., Yang, S. Y., Min, B. R., and MacAdam, J. W. (2017). In vitro effects of birdsfoot trefoil (Lotus corniculatus L.) pasture on ruminal fermentation, microbial population, and methane production. Prof. Anim. Sci. 33, 451–460. doi: 10.15232/pas.2016-01558
Chulze, S. N. (2010). Strategies to reduce mycotoxin levels in maize during storage: a review. Food Addit. Contam. A 27, 651–657.
Cooke, A. S., Machekano, H., Gwiriri, L. C., Tinsley, J. H. I., Silva, G., Nyamukondiwa, C., et al. (2024). The Nutritional Feed Gap: A Review of Seasonal Variation in Ruminant Forage Availability and Quality in Southern Africa. Food Security 17, 73–100. doi: 10.1007/s12571-024-01509-1
Correddu, F., Caratzu, M. F., Lunesu, M. F., Carta, S., Pulina, G., and Nudda, A. (2023). Grape, pomegranate, olive, and tomato by-products fed to dairy ruminants improve milk fatty acid profile without depressing milk production. Food Secur. 12:865. doi: 10.3390/foods12040865
Correddu, F., Lunesu, M. F., Buffa, G., Atzori, A. S., Nudda, A., Battacone, G., et al. (2020). Can agro-industrial by-products rich in polyphenols be advantageously used in the feeding and nutrition of dairy small ruminants? Animals 10:131. doi: 10.3390/ani10010131
Cosgrove, G., Waghorn, G., Anderson, C., Peters, J., Smith, A., Molano, G., et al. (2008). The effect of oils fed to sheep on methane production and digestion of ryegrass pasture. Aust. J. Exp. Agric. 48, 189–192. doi: 10.1071/EA07279
Cottle, D. J., Nolan, J. V., and Wiedemann, S. G. (2011). Ruminant enteric methane mitigation: a review. Anim. Prod. Sci. 51, 491–514. doi: 10.1071/AN10163
Couto Filho, C. C. C., Silva Filho, J. C., Neiva Júnior, A. P., Freitas, R. T. F., Souza, R. M., and Nunes, J. A. R. (2007). Qualidade da silagem de resíduo de manga com diferentes aditivos. Ciênc. Agrotecnol. 31, 1537–1544. doi: 10.1590/S1413-70542007000500040
Da Silva, L. M. R., De Figueiredo, E. A. T., Ricardo, N. M. P. S., Vieira, I. G. P., De Figueiredo, R. W., Brasil, I. M., et al. (2014). Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 143, 398–404. doi: 10.1016/j.foodchem.2013.08.001
de Assis Lage, C. F., Räisänen, S. E., Melgar, A., Nedelkov, K., Chen, X., Oh, J., et al. (2020). Comparison of two sampling techniques for evaluating ruminal fermentation and microbiota in the planktonic phase of rumen digesta in dairy cows. Front. Microbiol. 11:618032. doi: 10.3389/fmicb.2020.618032
de Evan, T., Carro, M. D., Fernández Yepes, J. E., Haro, A., Arbesú, L., Romero-Huelva, M., et al. (2022). Feeding mango wastes to dairy goats: effects on diet digestibility, ruminal fermentation, and milk yield and composition. Anim. Feed Sci. Technol. 286:115252. doi: 10.1016/j.anifeedsci.2022.115252
Deenanath, E. D., Rumbold, K., Daramola, M., Falcon, R., and Iyuke, S. (2015). Evaluation of physicochemical properties of South African cashew apple juice as a biofuel feedstock. Scientifica 2015, 1–9. doi: 10.1155/2015/764196
Dele, P., Jolaosho, A., Arigbede, O., Ojo, V., Amole, T., Okukenu, O., et al. (2013). Chemical composition and in vitro gas production of silage from guinea grass, cassava peel and cashew apple waste at different periods of ensilage. Pak. J. Biol. Sci. 16, 1801–1805. doi: 10.3923/pjbs.2013.1801.1805
Descheemaeker, K., Oosting, S. J., Homann-Kee Tui, S., Masikati, P., Falconnier, G. N., and Giller, K. E. (2016). Climate change adaptation and mitigation in smallholder crop–livestock systems in sub-Saharan Africa: a call for integrated impact assessments. Reg. Environ. Chang. 16, 2331–2343. doi: 10.1007/s10113-016-0957-8
Desta, Z. H., and Oba, G. (2004). Feed scarcity and livestock mortality in enset farming systems in the bale highlands of southern Ethiopia. Outlook Agric. 33, 277–280. doi: 10.5367/0000000042664792
Dorta, E., González, M., Lobo, M. G., Sánchez-Moreno, C., and de Ancos, B. (2014). Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC-ESI-QTOF-MS and multivariate analysis for use as a food ingredient. Food Res. Int. 57, 51–60. doi: 10.1016/j.foodres.2014.01.012
Dorta, E., Lobo, M. G., and Gonzalez, M. (2012). Reutilization of mango byproducts: study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 77, C80–C88. doi: 10.1111/j.1750-3841.2011.02477.x
Du Toit, C. J. L., Van Niekerk, W. A., and Meissner, H. (2013). Direct greenhouse gas emissions of the South African small stock sectors. South Afr. J. Anim. Sci. 43, 340–361. doi: 10.4314/sajas.v43i3.8
Duguma, B., and Janssens, G. P. (2021). Assessment of livestock feed resources and coping strategies with dry season feed scarcity in mixed crop–livestock farming systems around the gilgel gibe catchment, Southwest Ethiopia. Sustain. For. 13:10713. doi: 10.3390/su131910713
Durand, M., Dumay, C., Beaumatin, P., and Morel, M. T. (1988). Use of the rumen simulation technique (RUSITEC) to compare microbial digestion of various by-products. Anim. Feed Sci. Technol. 21, 197–204. doi: 10.1016/0377-8401(88)90101-0
Emshaw, Y., Melesse, A., and Assefa, G. (2012). The effect of dietary inclusion of mango (Magnifera indica L.) fruit waste on feed intake, growth and feed efficiency of Cobb-500 broiler chickens. Ethiop. J. Agric. Sci. 22, 73–83.
Erdaw, M. M. (2023). International Journal of Agricultural Sustainability, 21:, 2247776, doi: 10.1080/14735903.2023.2247776
Ertl, P., Knaus, W., Metzler-Zebeli, B., Klevenhusen, F., Khiaosa-Ard, R., and Zebeli, Q. (2015). Substitution of common concentrates with by-products modulated ruminal fermentation, nutrient degradation, and microbial community composition in vitro. J. Dairy Sci. 98, 4762–4771. doi: 10.3168/jds.2014-9063
Espinoza-Sánchez, J., Sánchez-Santillán, P., Torres-Salado, N., Ayala-Monter, M. A., Herrera-Pérez, J., and Magadan-Olmedo, F. (2020). Inclusion of ripe mango as a source of energy in diets for creole lambs in the dry tropics. Trop. Anim. Health Prod. 52, 3519–3526. doi: 10.1007/s11250-020-02386-4
Evans, E. A., and Ballen, F. H. (2012). “An Overview of Global Papaya Production, Trade, and Consumption: FE913 FE913, 9 2012”. EDIS 2012, vol. 9. Gainesville, FL. doi: 10.32473/edis-fe913-2012
Fairweather-Tait, S. (2023). The role of meat in iron nutrition of vulnerable groups of the UK population. Front. Anim. Sci. 4:1142252. doi: 10.3389/fanim.2023.1142252
Falusi, V., Adesina, I., Aladejimokun, A., and Elehinafe, T. (2017). Phytochemical screening and antibacterial activity of methanolic extracts of ripe and unripe peels of mango (Mangifera indica L.). J. Appl. Life Sci. Int. 14, 1–7. doi: 10.9734/JALSI/2017/36713
Fanimo, A., Oduguwa, O., Alade, A., Ogunnaike, T., and Adesehinwa, A. (2003). Growth performance, nutrient digestibility and carcass characteristic of growing rabbits fed cashew apple waste. Livest. Res. Rural. Dev. 15, 1–8.
Ferreira, A. C. H., Rodriguez, N. M., Neiva, J. N. M., Pimentel, P. G., Gomes, S. P., Campos, W. E., et al. (2015). Nutritional evaluation of elephant-grass silages with different levels of by-products from the cashew juice industry. Rev. Bras. Zootec. 44, 434–442. doi: 10.1590/S1806-92902015001200004
Figueroa-Valencia, M., Rosales-Martinez, P., Santoyo-Tepole, F., Ramos-Monroy, O., García-Ochoa, F., Hernández-Botello, M., et al. (2019). Antioxidant properties of red and yellow varieties of cashew apple, nut and husk (AnacardiumOccidentaleL.) harvested in Mexico. J. Antioxid. Activ. 1, 19–32.
Filho, M. R. R., and Soto-Blanco, B. (2012). Poisoning by cashew apple (Anacardium occidentale L.) in cattle. Acta Sci. Vet. 40, 1–5.
Fitri, A., Obitsu, T., Sugino, T., and Jayanegara, A. (2020). Ensiling of total mixed ration containing persimmon peel: evaluation of chemical composition and in vitro rumen fermentation profiles. Anim. Sci. J. 91:e13403. doi: 10.1111/asj.13403
Fraval, S., Hammond, J., Bogard, J. R., Ng’endo, M., van Etten, J., Herrero, M., et al. (2019). Food access deficiencies in sub-saharan Africa: Prevalence and implications for agricultural interventions. Front. Sustain. Food Syst. 3:104. doi: 10.3389/fsufs.2019.00104
Fron, M., Madeira, H., Richards, C., and Morrison, M. (1996). The impact of feeding condensed distillers byproducts on rumen microbiology and metabolism. Anim. Feed Sci. Technol. 61, 235–245. doi: 10.1016/0377-8401(95)00943-4
Frutos, P., Hervás, G., Giráldez, F. J., and Mantecón, A. R. (2004). Review. Tannins and ruminant nutrition. Span. J. Agric. Res. 2, 191–202. doi: 10.5424/sjar/2004022-73
Frutos, P., Hervás, G., Natalello, A., Luciano, G., Fondevila, M., Priolo, A., et al. (2020). Ability of tannins to modulate ruminal lipid metabolism and milk and meat fatty acid profiles. Anim. Feed Sci. Technol. 269:114623. doi: 10.1016/j.anifeedsci.2020.114623
Gan, H., Charters, E., Driscoll, R., and Srzednicki, G. (2016). Effects of drying and blanching on the retention of bioactive compounds in ginger and turmeric. Horticulturae 3:13. doi: 10.3390/horticulturae3010013
Garavaglia, L., and Tedesco, D. (2015). Food waste valorization: mango peels enhance in vitro rumen microbial fermentation. Animal Science and Production Association (ASPA) 21st Congress, Milano, Italy, June 9-12, 2015: Italian Journal of Animal Science, 14, 133–134.
Garcia-Amezquita, L. E., Tejada-Ortigoza, V., Serna-Saldivar, S. O., and Welti-Chanes, J. (2018). Dietary fiber concentrates from fruit and vegetable by-products: processing, modification, and application as functional ingredients. Food Bioprocess Technol. 11, 1439–1463. doi: 10.1007/s11947-018-2117-2
García-Rodríguez, J., Saro, C., Mateos, I., González, J. S., Carro, M. D., and Ranilla, M. J. (2020). Effects of replacing extruded maize by dried citrus pulp in a mixed diet on ruminal fermentation, methane production, and microbial populations in rusitec fermenters. Animals 10:1316. doi: 10.3390/ani10081316
Geerkens, C. H., Schweiggert, R. M., Steingass, H., Boguhn, J., Rodehutscord, M., and Carle, R. (2013). Influence of apple and Citrus Pectins, processed mango peels, a phenolic mango peel extract, and gallic acid as potential feed supplements on in vitro Total gas production and rumen Methanogenesis. J. Agric. Food Chem. 61, 5727–5737. doi: 10.1021/jf401544v
Gómez-Trinidad, M., Sánchez-Santillán, P., Herrera-Pérez, J., García-Balbuena, A., and Nuñez-Martínez, G. (2023). Evaluación química, nutricional in vitro e in situ de ensilado de papa ya de desecho y pasto pangola. Rev. MVZ Córdoba 28:e2883. doi: 10.21897/rmvz.2883
Gordon, A., Friedrich, M., Da Matta, V. M., Moura, C. F. H., and Marx, F. (2012). Changes in phenolic composition, ascorbic acid and antioxidant capacity in cashew apple (Anacardium occidentale L.) during ripening. Fruits 67, 267–276. doi: 10.1051/fruits/2012023
Groffman, P., Amstel, A. V., Reay, D., and Smith, P. (2010). Methane and climate. Change: Routledge.
Guerra-Rivas, C., Gallardo, B., Mantecón, Á. R., Del Álamo-Sanza, M., and Manso, T. (2017). Evaluation of grape pomace from red wine by-product as feed for sheep. J. Sci. Food Agric. 97, 1885–1893. doi: 10.1002/jsfa.7991
Guil-Guerrero, J. L., Ramos, L., Moreno, C., Zúñiga-Paredes, J. C., Carlosama-Yepez, M., and Ruales, P. (2016). Plant foods by-products as sources of health-promoting agents for animal production: a review focusing on the tropics. Agron. J. 108, 1759–1774. doi: 10.2134/agronj2015.0555
Gupta, A. K., Gurjar, P. S., Beer, K., Pongener, A., Ravi, S. C., Singh, S., et al. (2022). A review on valorization of different byproducts of mango (Mangifera indica L.) for functional food and human health. Food Biosci. 48:101783. doi: 10.1016/j.fbio.2022.101783
Gupta, M., Mondal, T., Lokesha, E., Singh, A., Ashwin, K., Perween, S., et al. (2018). Methane emission by ruminants and its measurement-a review. Int. J. Curr. Microbiol. App. Sci. 7, 3120–3126. doi: 10.20546/ijcmas.2018.707.364
Guzmán, O., Lemus, C., Bugarín, J., Bonilla, J., and Ly, J. (2010). Ensilado de residuos de mango (Mangifera indica L.) para la alimentación animal. Características fermentativas. Rev Comput Prod Porc 17, 218–224.
Guzmán, O., Lemus, C., Martínez, S., Bonilla, J., Plasencia, A., and Ly, J. (2012). Chemical characteristics of silages of mango (Mangifera indica L.) byproducts for animal feeding. Cuban J Agric Sci 46, 369–374.
Hegarty, R. S. (2012). Livestock nutrition – a perspective on future needs in a resource-chal lenged planet. Anim. Prod. Sci. 52:406. doi: 10.1071/AN11346
Herrero, M., Grace, D., Njuki, J., Johnson, N., Enahoro, D., Silvestri, S., et al. (2013). The roles of livestock in developing countries. Animal 7, 3–18. doi: 10.1017/S1751731112001954
Hidosa, D., and Guyo, M. (2017). Climate Change Effects on Livestock Feed Resources: A Review. Journal of Fisheries & Livestock Production. 5:259. doi: 10.4172/2332-2608.1000259
Huang, Q., Liu, X., Zhao, G., Hu, T., and Wang, Y. (2018). Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 4, 137–150. doi: 10.1016/j.aninu.2017.09.004
Ibrahim, N. A., Alimon, A. R., Yaakub, H., Samsudin, A. A., Candyrine, S. C. L., Wan Mohamed, W. N., et al. (2021). Effects of vegetable oil supplementation on rumen fermentation and microbial population in ruminant: a review. Trop. Anim. Health Prod. 53, 1–11. doi: 10.1007/s11250-021-02863-4
Imran, M., Butt, M. S., Anjum, F. M., and Sultan, J. I. (2013). Chemical profiling of different mango peel varieties. Pak. J. Nutr. 12, 934–942. doi: 10.3923/pjn.2013.934.942
IPCC (2007). Climate Change 2007: Migitation of Climate Change: Working Group III Contribution to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Summary for Policymakers and Technical Summary. USA.: Cambridge University Press, Cambridge, United Kingdom and New York, NY.
Iravanian, A., and Ravari, S. O. (2020). Types of Contamination in Landfills and Effects on The Environment: A Review Study. In IOP Conference Series: Earth and Environmental Science, 1st International Conference on Energetics, Civil and Agricultural Engineering, Tashkent, Uzbekistan, 14-16 October 2020; Vol. 614, p 012083. doi: 10.1088/1755-1315/614/1/012083
Jafari, S., Ebrahimi, M., Goh, Y. M., and Rajion, M. A. (2020). Tropical fruit peels as potential modifiers of rumen fermentation characteristics in goats: in vitro and in situ evaluations. Animal Production Science 61, 132–138. doi: 10.1071/AN20059
Jahurul, M. H. A., Zaidul, I. S. M., Ghafoor, K., Al-Juhaimi, F. Y., Nyam, K. L., Norulaini, N. A. N., et al. (2015). Mango (Mangifera indica L.) by-products and their valuable components: a review. Food Chem. 183, 173–180. doi: 10.1016/j.foodchem.2015.03.046
Jalal, H., Giammarco, M., Lanzoni, L., Akram, M. Z., Mammi, L. M. E., Vignola, G., et al. (2023). Potential of Fruits and Vegetable By-Products as an Alternative Feed Source for Sustainable Ruminant Nutrition and Production: A Review. Agriculture 13:286. doi: 10.3390/agriculture13020286
Kamiloglu, S., Toydemir, G., Boyacioğlu, D., Beekwilder, J., Hall, R. D., and Çapanoğlu, E. (2016). A review on the effect of drying on antioxidant potential of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 56, S110–S129. doi: 10.1080/10408398.2015.1045969
Kaur, B., and Srivastav, P. P. (2018). Effect of cryogenic grinding on chemical and morphological characteristics of mango (Mangifera indica L.) peel powder. J. Food Process. Preserv. 42:e13583. doi: 10.1111/jfpp.13583
Kiendrebeogo, T., Logténé, Y. M., and Kaboré-Zoungrana, C.-Y. (2018). Effets de rations à base de déchets de mangue sur les performances pondérales et la qualité de la carcasse de porcs Korhogo en croissance au Burkina Faso. J. Appl. Biosci. 129, 13039–13049. doi: 10.4314/jab.v129i1.7
Klava, D., Kampuse, S., Tomsone, L., Kince, T., and Ozola, L. (2018). Effect of drying technologies on bioactive compounds maintenance in pumpkin by-products. Agronomy Research 16, 1728–1741. doi: 10.15159/AR.18.156
Kongolo, M., and Dlamini, D. (2012). Small-scale livestock farming in developing areas of Swaziland and South Africa. Afrrev Stech. 1, 100–111.
Laihad, J., Leke, J., and Nangoy, F. (2019). A Dietary Inclusion Made of Papaya (Carica papaya L.) Leaf Powder in Feeding toward the Performance and Carcass Quality of Local Chickens. The 4th Animal Production International Seminar Malang, Indonesia, 24-27 October 2019: IOP Conference Series: Earth and Environmental Science, IOP Publishing Ltd, Bristol, United Kingdom.
Lamidi, A., and Ologbose, F. (2014). Dry season feeds and feeding: a threat to sustainable ruminant animal production in Nigeria. J. Agric. Soc. Res. 14, 17–30.
Langyintuo, A. (2020). “Smallholder farmers’ access to inputs and finance in Africa” in The role of smallholder farms in food and nutrition security. eds. I. S. G. Y. Paloma, L. Riesgo, and K. Louhichi (Springer Nature Switzerland), 133–152. doi: 10.1007/978-3-030-42148-9
Leng, R. A. (1990). Factors affecting the utilization of ‘poor-quality’ forages by ruminants particularly under tropical conditions. Nutr. Res. Rev. 3, 277–303. doi: 10.1079/NRR19900016
Leng, R. A. (2004). “Requirements for protein meals for ruminant meat production in developing countries” in Protein Sources for the Animal Feed Industry: Proceedings of the FAO Expert Consultation and Workshop, Bangkok, 29 April - 3 May 2002 (Food and Agriculture Organization of the United Nations), 225–254.
Leong, Y. K., and Chang, J. S. (2022). Valorization of fruit wastes for circular bioeconomy: current advances, challenges, and opportunities. Bioresour. Technol. 359:127459. doi: 10.1016/j.biortech.2022.127459
Lopes, L. G., Silva, M. H., Figueiredo, A., Canuto, K. M., Brito, E. S., Ribeiro, P. R. V., et al. (2018). The intake of dry cashew apple fiber reduced fecal egg counts in Haemonchus contortus-infected sheep. Exp. Parasitol. 195, 38–43. doi: 10.1016/j.exppara.2018.10.004
López-Cobo, A., Verardo, V., Diaz-de-Cerio, E., Segura-Carretero, A., Fernández-Gutiérrez, A., and Gómez-Caravaca, A. M. (2017). Use of HPLC- and GC-QTOF to determine hydrophilic and lipophilic phenols in mango fruit (Mangifera indica L.) and its by-products. Food Res. Int. 100, 423–434. doi: 10.1016/j.foodres.2017.02.008
Lowor, S., and Agyente-Badu, C. (2009). Mineral and proximate composition of cashew apple (Anarcadium occidentale L.) juice from Northern Savannah, Forest and Coastal Savannah regions in Ghana. American Journal of Food Technology 4, 154–161. doi: 10.3923/ajft.2009.154.161
Magama, P., Chiyanzu, I., and Mulopo, J. (2022). A systematic review of sustainable fruit and vegetable waste recycling alternatives and possibilities for anaerobic biorefinery. Bioresour. Technol. Rep. 18:101031. doi: 10.1016/j.biteb.2022.101031
Maia, M. O., Susin, I., Ferreira, E. M., Nolli, C. P., Gentil, R. S., Pires, A. V., et al. (2012). Intake, nutrient apparent digestibility and ruminal constituents of sheep fed diets with canola, sunflower or castor oils. Rev. Bras. Zootec. 41, 2350–2356. doi: 10.1590/S1516-35982012001100008
Makkar, H. (2014). Sustainable increase in livestock productivity in developing countries through efficient utilisation of feed resources. Cuban Journal of Agricultural Science 48, 55–58. Available at: https://www.cjascience.com/index.php/CJAS/article/view/427
Marçal, S., and Pintado, M. (2021). Mango peels as food ingredient / additive: nutritional value, processing, safety and applications. Trends Food Sci. Technol. 114, 472–489. doi: 10.1016/j.tifs.2021.06.012
Marcillo-Parra, V., Anaguano, M., Molina, M., Tupuna-Yerovi, D. S., and Ruales, J. (2021). Characterization and quantification of bioactive compounds and antioxidant activity in three different varieties of mango (Mangifera indica L.) peel from the Ecuadorian region using HPLC-UV/VIS and UPLC-PDA. NFS J. 23, 1–7. doi: 10.1016/j.nfs.2021.02.001
Marcos, C. N., Carro, M. D., Fernández-Yepes, J. E., Arbesu, L., and Molina-Alcaide, E. (2020). Utilization of avocado and mango fruit wastes in multi-nutrient blocks for goats feeding: in vitro evaluation. Animals 10:2279. doi: 10.3390/ani10122279
Matthews, C., Crispie, F., Lewis, E., Reid, M., O’Toole, P. W., and Cotter, P. D. (2019). The rumen microbiome: a crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 10, 115–132. doi: 10.1080/19490976.2018.1505176
Mayberry, D., Ash, A., Prestwidge, D., and Herrero, M. (2018). Closing yield gaps in smallholder goat production systems in Ethiopia and India. Livest. Sci. 214, 238–244. doi: 10.1016/j.livsci.2018.06.015
McDonald, P., Edwards, R. A., Greenhalgh, J. F. D., Morgan, C. A., Sinclair, L. A., and Wilkinson, R. G. (2011). Animal nutrition. 7th Edn. Essex: Pearson Education Publishers.
Melesse, A., Steingass, H., Schollenberger, M., and Rodehutscord, M. (2018). Component composition, in vitro gas and methane production profiles of fruit by-products and leaves of root crops. J. Agric. Sci. 156, 949–958. doi: 10.1017/S0021859618000928
Mello, M. S., Silva, J. D. L., Ferreira, A. C. S., Santos, R. F., Ferreira, M. D. A., Siqueira, M., et al. (2019). Fermentation losses and chemical composition in mixed silages of elephant grass and cassava peels with mango residue. Boletim de Indústria Animal 76, 1–9. doi: 10.17523/bia.2019.v76.e1451
Merkel, R. (2019). Smallholder livestock commercialization. WARTAZOA Indonesian Bull. Anim. Vet. Sci. 29, 43–50. doi: 10.14334/wartazoa.v29i1.1952
Michalak, M., Wojnarowski, K., Cholewińska, P., Szeligowska, N., Bawej, M., and Pacoń, J. (2021). Selected alternative feed additives used to manipulate the rumen microbiome. Animals 11:1542. doi: 10.3390/ani11061542
Michodjehoun-Mestres, L., Souquet, J.-M., Fulcrand, H., Meudec, E., Reynes, M., and Brillouet, J.-M. (2009). Characterisation of highly polymerised prodelphinidins from skin and flesh of four cashew apple (Anacardium occidentale L.) genotypes. Food Chem. 114, 989–995. doi: 10.1016/j.foodchem.2008.10.052
Millogo, D. X., Kiendrebeogo, T., Komdombo, S. R., Toguyeni, A., Dossa, L. H., Youssofoumopate, L., et al. (2024). Breeding practices and use of by-products derived from mango and cassava in cattle and sheep fattening in Western Burkina Faso. Open Access Libr. J. 11, 1–22. doi: 10.4236/oalib.1111228
Min, B. R., and Solaiman, S. G. (2018). Comparative aspects of plant tannins on digestive physiology, nutrition and microbial community changes in sheep and goats: a review. J. Anim. Physiol. Anim. Nutr. 102, 1181–1193. doi: 10.1111/jpn.12938
Mirzaei-Aghsaghali, A., and Maheri-Sis, N. (2008). Nutritive value of some agro-industrial by-products for ruminants - a review. World J. Zool. 3, 40–46.
Mitsumori, M., and Sun, W. (2008). Control of rumen microbial fermentation for mitigating methane emissions from the rumen. Asian Australas. J. Anim. Sci. 21, 144–154. doi: 10.5713/ajas.2008.r01
Moeletsi, M. E., Tongwane, M. I., and Tsubo, M. (2017). Enteric methane emissions estimate for livestock in South Africa for 1990–2014. Atmos. 8:69. doi: 10.3390/atmos8050069
Morgavi, D. P., Forano, E., Martin, C., and Newbold, C. J. (2010). Microbial ecosystem and methanogenesis in ruminants. Animal 4, 1024–1036. doi: 10.1017/S1751731110000546
Moss, A. R., Jouany, J.-P., and Newbold, J. (2000). Methane production by ruminants: its contribution to global warming. Annales de zootechnie 49, 231–253. doi: 10.1051/animres:2000119
Mousa, S. A., Malik, P. K., Kolte, A. P., Bhatta, R., Kasuga, S., and Uyeno, Y. (2019). Evaluation of in vitro ruminal fermentation of ensiled fruit byproducts and their potential for feed use. Asian Australas. J. Anim. Sci. 32, 103–109. doi: 10.5713/ajas.18.0282
Musundire, R., Ngonyama, D., Chemura, A., Ngadze, R. T., Jackson, J., Matanda, M. J., et al. (2021). Stewardship of wild and farmed edible insects as food and feed in sub-Saharan Africa: a perspective. Fronti. Vet. Sci. 8:601386. doi: 10.3389/fvets.2021.601386
Mwaurah, P. W., Kumar, S., Kumar, N., Panghal, A., Attkan, A. K., Singh, V. K., et al. (2020). Physicochemical characteristics, bioactive compounds and industrial applications of mango kernel and its products: a review. Compr. Rev. Food Sci. Food Saf. 19, 2421–2446. doi: 10.1111/1541-4337.12598
Nagle, M., Jankowsky, B., Peña Pineda, K., Ríos, L., Jäger, M., and Müller, J. (2011). “Evaluation of heat-sensitive micronutrients in fresh, sun-dried and solar-dried Capsicum varieties grown in Peru” in Conference on International Research on Food Security, Natural Resource Management and Rural Development (Tropentag 2011), Bonn, Germany, 5–7 October 2011. University of Hohenheim.
Naumann, H. D., Tedeschi, L. O., Zeller, W. E., and Huntley, N. F. (2017). The role of condensed tannins in ruminant animal production: advances, limitations and future directions. Rev. Bras. Zootec. 46, 929–949. doi: 10.1590/s1806-92902017001200009
Ndawula, J., Kabasa, J., and Byaruhanga, Y. (2004). Alterations in fruit and vegetable β-carotene and vitamin C content caused by open-sun drying, visqueen-covered and polyethylene-covered solar-dryers. Afr. Health Sci. 4, 125–130
Negesse, T., Makkar, H. P. S., and Becker, K. (2009). Nutritive value of some non-conventional feed resources of Ethiopia determined by chemical analyses and an in vitro gas method. Anim. Feed Sci. Technol. 154, 204–217. doi: 10.1016/j.anifeedsci.2009.09.010
Neto, J. B. M., Pereira, L. G. R., Chizzotti, M. L., Yamamoto, S. M., Aragão, A. S., and Mascioli, A. S. (2014). Componentes constituintes e não constituintes da carcaça de cordeiros Santa Inês alimentados com farelo de manga em substituição ao milho. Sem Cien Agrar. 35, 437–448. doi: 10.5433/1679-0359.2014v35n1p437
Neudert, R., Allahverdiyeva, N., Mammadov, N., Didebulidze, A., and Beckmann, V. (2020). Diversification of livestock-keeping smallholders in mountainous rural regions of Azerbaijan and Georgia. Land 9:267. doi: 10.3390/land9080267
NRC (2007). Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids. Washington, DC: The National Academies Press. doi: 10.17226/11654
NRC (2016). Nutrient requirements of beef cattle. 8th Edn. Washington, DC: National Academies Press.
Oddoye, E. O. K., Takrama, J. F., Anchirina, V., and Agyente-Badu, K. (2009). Effects on performance of growing pigs fed diets containing different levels of dried cashew pulp. Trop. Anim. Health Prod. 41, 1577–1581. doi: 10.1007/s11250-009-9349-0
Oh, J. I., Wall, E. H., Bravo, D., and Hristov, A. N. (2017). Host-mediated effects of phytonutrients in ruminants: a review. J. Dairy Sci. 100, 5974–5983. doi: 10.3168/jds.2016-12341
Ojediran, T., Akande, O., and Emiola, A. (2024). Cashew (Anacardium Occidentale L.) products and byproducts: nutrient C onstituents and nutritional benefits in livestock diets. Hayv. Bilim. Ürünleri Dergisi 7, 42–62. doi: 10.51970/jasp.1350311
Okoruwa, M., Adewumi, M., and Njidda, A. (2013). Nutrient utilization and growth performance of west African dwarf goats fed with elephant grass or different proportions of plantain and mango peels. World J. Agric. Sci. 1, 194–202.
Okoruwa, M., and Igene, F. (2014). Comparison of fermentation kinetics (in vitro) of napier grass and fruit peels for ruminants: the pattern of organic matter degradability, volatile fatty acid concentration estimated methane and microbial biomass production. J. Agric. Vet. Sci. 7, 21–28.
Okpanachi, U., Ayoade, J. A., and Tuleun, C. D. (2016). Carcass Characteristics, Internal Organs and Economics of Feeding Sun-Dried Yellow Cashew Pulp Based Diets to West African Dwarf Goats. Animal and Veterinary Sciences 4, 1–6. doi: 10.11648/j.avs.s.2016040301.11
Okpeku, M., Ogah, D. M., and Adeleke, M. A. (2019). A review of challenges to genetic improvement of indigenous livestock for improved food production in Nigeria. Afr. J. Food Agric. Nutr. Dev. 19, 13959–13978. doi: 10.18697/ajfand.84.BLFB1021
Omer, H. A. A., Tawila, M. A., Gad, S. M., and Abdel-Magid, S. S. (2019). Mango (Mangifera indica) seed kernels as untraditional source of energy in Rahmani sheep rations. Bull. Natl. Res. Cent. 43:176. doi: 10.1186/s42269-019-0241-4
Omotoso, A. B., Letsoalo, S., Olagunju, K. O., Tshwene, C. S., and Omotayo, A. O. (2023). Climate change and variability in sub-Saharan Africa: a systematic rev iew of trends and impacts on agriculture. J. Clean. Prod. 414:137487. doi: 10.1016/j.jclepro.2023.137487
Owino, W. O., and Ambuko, J. L. (2021). Mango fruit processing: options for small-scale processors in developing countries. Agriculture 11:1105. doi: 10.3390/agriculture11111105
Palangi, V., and Lackner, M. (2022). Management of enteric methane emissions in ruminants using feed additives: a review. Animals 12:3452. doi: 10.3390/ani12243452
Parisi, G., Tulli, F., Fortina, R., Marino, R., Bani, P., Dalle Zotte, A., et al. (2020). Protein hunger of the feed sector: the alternatives offered by the plant world. Ital. J. Anim. Sci. 19, 1204–1225. doi: 10.1080/1828051X.2020.1827993
Pathak, A. K. (2013). Potential of Using Condensed Tannins to Control Gastrointestinal Nematodes and Improve Small Ruminant Performance. International Journal of Molecular Veterinary Research 3, 36–50. doi: 10.5376/ijmvr.2013.03.0008
Paul, S. S., and Dey, A. (2015). Nutrition in health and immune function of ruminants. The Indian Journal of Animal Sciences 85, 103–112. doi: 10.56093/ijans.v85i2.46557
Pereira, L. G. R., Aragão, A. L. S., Santos, R. D., Azevêdo, J. A. G., Neves, A. L. A., Ferreira, A. L., et al. (2013). Desempenho produtivo de ovinos em confinamento alimentados com farelo de manga. Arqu. Bras. Med. Vet. Zoot. 65, 675–680. doi: 10.1590/S0102-09352013000300009
Ponnampalam, E. N., Kearns, M., Kiani, A., Santhiravel, S., Vahmani, P., Prache, S., et al. (2024). Enrichment of ruminant meats with health enhancing fatty acids and antioxidants: feed-based effects on nutritional value and human health aspects–invited review. Front. Anim. Sci. 5:1329346. doi: 10.3389/fanim.2024.1329346
Powell, J. M., Pearson, R. A., and Hiernaux, P. H. (2004). Crop–livestock interactions in the west African drylands. Agron. J. 96, 469–483. doi: 10.2134/agronj2004.4690
Prasetyo, E. N., Rokana, E., Baihaqi, Z. A., and Samudi, S. (2024). Anthelmintic effects of Podang mango (Mangifera indica) fruit peel waste extract through in vivo application on Indonesian Etawa goat production and health. Vet. World 17, 1291–1298. doi: 10.14202/vetworld.2024.1291-1298
Preethi, P., Mangalassery, S., Shradha, K., Pandiselvam, R., Manikantan, M. R., Reddy, S. V. R., et al. (2021). Cashew apple pomace powder enriched the proximate, mineral, functional and structural properties of cereal based extrudates. LWT 139:110539. doi: 10.1016/j.lwt.2020.110539
Preethi, P., Rajkumar, A., Shamsudheen, M., and Nayak, M. (2019). Prospects of cashew apple-A Compilation Report, Technical Bulletin No. 2/2019. ICAR-Directorate of Cashew Research, Puttur, Karnataka, India, 28.
Priolo, Q., and Vasta, V. (2007). Effects of tannin-containing diets on small ruminant meat quality. Ital. J. Anim. Sci. 6, 527–530. doi: 10.4081/ijas.2007.1s.527
Pulina, G., Francesconi, A. H. D., Stefanon, B., Sevi, A., Calamari, L., Lacetera, N., et al. (2017). Sustainable ruminant production to help feed the planet. Ital. J. Anim. Sci. 16, 140–171. doi: 10.1080/1828051X.2016.1260500
Rajkumar, H., and Ganesan, N. D. (2021). Effects of freeze‐drying process on the production of cashew apple powder: Determination of bioactive compounds and fruit powder properties. Journal of Food Processing and Preservation 45:e15466. doi: 10.1111/jfpp.15466
Ramos, L. S. N., Lopes, J. B., Figueirêdo, A. V., Freitas, A. C., Farias, L. A., Santos, L. S., et al. (2006). Polpa de caju em rações para frangos de corte na fase final: desempenho e características de carcaça. Rev. Bras. Zootec. 35, 804–810. doi: 10.1590/S1516-35982006000300024
Ramos-Morales, E., Arco-Pérez, A., Martín-García, A. I., Yáñez-Ruiz, D. R., Frutos, P., and Hervás, G. (2014). Use of stomach tubing as an alternative to rumen cannulation to study ruminal fermentation and microbiota in sheep and goats. Anim. Feed Sci. Technol. 198, 57–66. doi: 10.1016/j.anifeedsci.2014.09.016
Ranganath, K. G., Shivashankara, K. S., Roy, T. K., Dinesh, M. R., Geetha, G. A., Pavithra, K. C., et al. (2018). Profiling of anthocyanins and carotenoids in fruit peel of different colored mango cultivars. J. Food Sci. Technol. 55, 4566–4577. doi: 10.1007/s13197-018-3392-7
Ranjhan, S. K. (ed.). (2001). Animal nutrition in the tropics. (New Delhi, India: Vikas Publishing House Pvt. Ltd.).
Ransom, E. P., and Stagner, F. (2020). “Gender and livestock production” in Routledge Handbook of Gender and Agriculture. eds. C. E. Sachs, L. Jensen, P. Castellanos, and K. Sexsmith. 1st Edition ed (Routledge), 126–136.
Rao, P. P., Birthal, P. S., and Ndjeunga, J. (2005). Crop-livestock economies in the semi-arid tropics: facts, trends and o utlook. Patancheru: International Crops Research Institute for the Semi-Arid Tropics (ICRISAT).
Rêgo, M. M. T., Neiva, J. N. M., Rêgo, A. C. D., Cândido, M. J. D., Alves, A. A., and Lôbo, R. N. B. (2010). Intake, nutrients digestibility and nitrogen balance of elephant grass silages with mango by-product addition. Rev. Bras. Zootec. 39, 74–80. doi: 10.1590/S1516-35982010000100010
Rena, A. S., Chavan, D., Aiman, S., and Kumar, S. (2020). Reducing greenhouse gas emission from waste landfill. Encycl. Renew. Sustain. Mater., 685–701. doi: 10.1016/B978-0-12-803581-8.11022-7
Richard, E. N., Mayo, A. A., and Kimwaga, R. (2018). Characteristics and Anaerobic Digestion of Fruits Wastes from Ubungo-U rafiki Market in Dar Es Salaam. Tanzania Journal of Engineering and Technology 37, 33–46.
Rithy, S., Chim, C., Ousa, S., Phanna, L., Puthearith, T., et al. (2022). Physicochemical and Antioxidant Properties in Varieties of Cashew Apple (Aacardium Occidetale L.) Adv in food tech& pub health: AFTPH-110. doi: 10.46715/aftph2022.04.1000110
Robinson, A., Sewell, G. C., Wu, S., Damodaran, N., and Kalas-Adams, N. (2003). Landfill data from China: addressing information needs for methane rec OVERY : Environmental Protection Agency, US.
Romelle Jones, K., Karuppusamy, S., and Sundaram, V. (2023). Unraveling the promise of agroindustrial byproducts as alternative fee d source for sustainable rabbit meat production. Emerg. Anim. Spec. 10:100044. doi: 10.1016/j.eas.2024.100044
Sá, C. R. L., Neiva, J. N. M., Gonçalves, J. S., Cavalcante, M. A. B., and Lôbo, R. N. B. (2007). Chemical composition and fermentative characteristics of elephant grass (Pennisetum purpureum Schum.) silages added of increasing levels of mango (Mangifera indica L.) byproducts. Revista Ciência Agronômica 38, 199–203.
Sahoo, A., Sarkar, S., Lal, B., Kumawat, P., Sharma, S., and De, K. (2021). Utilization of fruit and vegetable waste as an alternative feed resour ce for sustainable and eco-friendly sheep farming. Waste Manag. 128, 232–242. doi: 10.1016/j.wasman.2021.04.050
Sánchez-Santillán, P., Soriano Marcial, L. A., Saavedra Jiménez, L. A., and Torres Salado, N. (2021). Características de calidad, químicas y fermentativas in vitro de ensil ados de papaya (Carica papaya L) de desecho y heno de pasto estrella (Cynodon nlemfluensis). Tropical and Subtropical Agroecosystems 25:12. doi: 10.56369/tsaes.3863
Sancho, S. D. O., Da Silva, A. R. A., Dantas, A. N. D. S., Magalhães, T. A., Lopes, G. S., Rodrigues, S., et al. (2015). Characterization of the industrial residues of seven fruits and prospection of their potential application as food supplements. J. Chem. 2015, 1–8. doi: 10.1155/2015/264284
Sanon, H., and Kanwe, A. (2010). Valorisation of mango peels and seed kernels in animal feeding: nutritive value and voluntary feed intake by sheep. Adv. Anim. Biosci. 1, 445–446. doi: 10.1017/S2040470010000695
Sanon, H. O., Kanwe, A. B., Millogo, A., and Ledin, I. (2012). Chemical composition, digestibility, and voluntary feed intake of mango residues by sheep. Trop. Anim. Health Prod. 45, 665–669. doi: 10.1007/s11250-012-0275-1
Santos, C. M. D., Abreu, C. M. P. D., Freire, J. M., Queiroz, E. D. R., and Mendonça, M. M. (2014). Chemical characterization of the flour of peel and seed from two papaya cultivars. Food Sci. Technol. 34, 353–357. doi: 10.1590/fst.2014.0048
Santos, R., Silva, J., Ferreira, A., Mello, M., Ferreira, M., Siqueira, M., et al. (2019). Fermentation losses and chemical composition of Calotropis procera ensiling with mango and tomato waste. Livest. Res. Rural. Dev. 31:101. http://lrrd.cipav.org.co/lrrd31/7/silva31101.html
Sehar, M., and Oyekale, A. S. (2022). Effect of livestock farmers’ access to formal markets on marketing inefficiency in Mpumalanga province, South Africa. Afr. J. Sci. Technol. Innov. Dev. 14, 225–233. doi: 10.1080/20421338.2020.1823610
Sekaran, U., Lai, L., Ussiri, D. A. N., Kumar, S., and Clay, S. (2021). Role of integrated crop-livestock systems in improving agriculture production and addressing food security – a review. J. Agric. Food Res. 5:100190. doi: 10.1016/j.jafr.2021.100190
Seré, C. (2020). Investing Sustainably in African Livestock Development: Opportunities and Trade-Offs SSRN Electronic Journal, University of Bonn, Center for Development Research (ZEF) Genscherallee 3 53113 Bonn, Germany. Report No.: 1556-5068. doi: 10.2139/ssrn.3688901
Serna-Cock, L., García-Gonzales, E., and Torres-León, C. (2016). Agro-industrial potential of the mango peel based on its nutritional and functional properties. Food Rev. Intl. 32, 364–376. doi: 10.1080/87559129.2015.1094815
Shaheen, S., Galanakis, C. M., and Farag, M. A. (2023). Carica papaya biowaste valorization: biorefinery advances and extraction optimization. Food Rev. Intl. 39, 4745–4760. doi: 10.1080/87559129.2022.2057527
Sharma, A., Bachheti, A., Sharma, P., Bachheti, R. K., and Husen, A. (2020). Phytochemistry, pharmacological activities, nanoparticle fabrication, commercial products and waste utilization of Carica papaya L.: a comprehensive review. Curr. Res. Biotechnol. 2, 145–160. doi: 10.1016/j.crbiot.2020.11.001
Sharma, P., Gaur, V., Sirohi, R., Larroche, C., Kim, S. H., and Pandey, A. (2020). Valorization of cashew nut processing residues for industrial applications 152, 112550. doi: 10.1016/j.indcrop.2020.112550
Shen, J., Chai, Z., Song, L., Liu, J., and Wu, Y. (2012). Insertion depth of oral stomach tubes may affect the fermentation parameters of ruminal fluid collected in dairy cows. J. Dairy Sci. 95, 5978–5984. doi: 10.3168/jds.2012-5499
Shwerab, A., Hassan, A., Khalel, M., Yacout, M., Abdel-Salam, A., El-Nobi, H., et al. (2023). The effect of using different levels of mango seed kernel on digestion coefficients, rumen fermentation and methane emission in sheep diet. Egypt. J. Nutr. Feeds 26, 223–234. doi: 10.21608/ejnf.2023.332847
Singh, J. P., Kaur, A., Shevkani, K., and Singh, N. (2016). Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J. Food Sci. Technol. 53, 4056–4066. doi: 10.1007/s13197-016-2412-8
Sinkala, Y., Simuunza, M., Pfeiffer, D., Munang'andu, H. M., Mulumba, M., Kasanga, C., et al. (2014). Challenges and economic implications in the control of foot and mouth disease in sub-saharan Africa: lessons from the zambian experience. Vet. Med. Int. 2014:373921, 1–12. doi: 10.1155/2014/373921
Smith, J., Sones, K., Grace, D., MacMillan, S., Tarawali, S., and Herrero, M. (2013). Beyond milk, meat, and eggs: role of livestock in food and nutrition security. Anim. Front. 3, 6–13. doi: 10.2527/af.2013-0002
Smith, P. E., Waters, S. M., Kenny, D. A., Boland, T. M., Heffernan, J., and Kelly, A. K. (2020). Replacing barley and soybean meal with by-products, in a pasture based diet, alters daily methane output and the rumen microbial community in vitro using the rumen simulation technique (RUSITEC). Front. Microbiol. 11:1614. doi: 10.3389/fmicb.2020.01614
Soldado, D., Bessa, R. J., and Jerónimo, E. (2021). Condensed tannins as antioxidants in ruminants—effectiveness and action mechanisms to improve animal antioxidant status and oxidative stability of products. Animals 11:3243. doi: 10.3390/ani11113243
Souza, H. A., Moraes, E. H. B. K., Oliveira, A. S., Batista, E. D., Santos, K. R., Sousa, J. N., et al. (2020). Cashew processing product as alternative energy feedstuff for grazing beef cattle under tropical conditions. Livest. Sci. 236:104022. doi: 10.1016/j.livsci.2020.104022
Sruamsiri, S., and Silman, P. (2009). Nutritive value and nutrient digestibility of ensiled mango by-products. Maejo Int. J. Sci. Technol. 3, 371–378.
Steinfeld, H. (2003). Economic constraints on production and consumption of animal source foods for nutrition in developing countries. J. Nutr. 133, 4054S–4061S. doi: 10.1093/jn/133.11.4054S
Sunil, K., and Tapan, C. (2008). A special grouping of papers on landfill gas modeling and management. J. Air Waste Manag. Assoc. 58:602.
Swain, B., Sundaram, R., and Barbuddhe, S. (2007). Effect of feeding cashew apple waste replacing maize on the performance of broilers. Indian J. Poultry Sci. 42, 208–210.
Tai, V. A., Tuan, B. Q., Van, T. T. T., and Trach, N. X. (2020). Use of cashew apple fruit silage in the cattle fattening diet. Livest. Res. Rural. Dev. 32:5.
Tangka, F. K., and Jabbar, M. A. (2005). “Implications of feed scarcity for gender roles in ruminant livestock production” in Coping with Feed Scarcity in Smallholder Livestock Systems in Developing Countries., June 14-17 1999. 1999 Nairobi, Kenya. Animal Sciences Group,Wageningen UR, the Netherlands, University of Reading, UK, Swiss Federal Institute of Technology, Zurich, Switzerland, and ILRI, Nairobi, Kenya. eds. A. A. Ayatunde, S. Fernandez-Rivera, and G. Mccrabb, 287–296.
Tapsoba, L. D., Kiemde, S. M., Lamond, B. F., and Lépine, J. (2022). On the potential of packaging for reducing fruit and vegetable losses in sub-Saharan Africa. Food Secur. 11:952. doi: 10.3390/foods11070952
Tayengwa, T., and Mapiye, C. (2018). Citrus and winery wastes: promising dietary supplements for sustainable ruminant animal nutrition, health, production, and meat quality. Sustain. For. 10:3718. doi: 10.3390/su10103718
Terré, M., Castells, L., Fàbregas, F., and Bach, A. (2013). Short communication: Comparison of pH, volatile fatty acids, and microbiome of rumen samples from preweaned calves obtained via cannula or stomach tube. Journal of Dairy Science 96, 5290–5294. doi: 10.3168/jds.2012-5921
Tesfaye, T. (2017). Valorisation of mango fruit by-products: physicochemical characterisation and future prospect. Chem. Process Eng. Res. 50, 22–34.
Thompson, H. E., Berrang-Ford, L., and Ford, J. D. (2010). Climate change and food security in sub-Saharan Africa: a systematic literature review. Sustain. For. 2, 2719–2733. doi: 10.3390/su2082719
Thornton, P. K. (2010). Livestock production: recent trends, future prospects. Philos. Trans. R. Soc. B Biol. Sci. 365, 2853–2867. doi: 10.1098/rstb.2010.0134
Thornton, P. K., Jones, P. G., Ericksen, P. J., and Challinor, A. J. (1934). Agriculture and food systems in sub-Saharan Africa in a 4°C world. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 369, 117–136. doi: 10.1098/rsta.2010.0246
Tolera, A., Merkel, R. C., Goetsch, A. L., Sahlu, T., and Negesse, T. (2000). “Nutritional constraints and future prospects for goat production in East Africa” in Proceedings of the opportunities and challenges of enhancing goat production in East Africa, Debub University, Awassa, Ethiopia, November 10-12, 2000. eds. R. C. Merkel, G. Abebe, and A. L. Goetsch (E (Kika) de la Garza institute for goat research, Langston University, Langston, OK), 43–57.
Tompkins, E. L., and Adger, W. N. (2004). Does Adaptive Management of Natural Resources Enhance Resilience to Climate Change? Ecology and Society 9, 10–24. Available at: http://www.jstor.org/stable/26267677
Umamahesh, K., Gandhi, A. D., and Reddy, O. V. S. (2020). Ethnopharmacological Applications of mango (Mangifera indica L.) peel - A Review. Current Pharmaceutical Biotechnology 24, 1298–1303. doi: 10.2174/1389201021666200420075759
Van der Walt, J., and Linington, M. (1989). A review of energy metabolism in producing ruminants. Part 1: metabolism of energy substrates. J. S. Afr. Vet. Assoc. 60, 223–227
Van Walraven, N., and Stark, A. H. (2023). From food waste to functional component: Cashew apple pomace. Critical Reviews in Food Science and Nutrition 64, 7101–7117. doi: 10.1080/10408398.2023.2180616
Vasta, V., Daghio, M., Cappucci, A., Buccioni, A., Serra, A., Viti, C., et al. (2019). Invited review: plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: experimental evidence and methodological approaches. J. Dairy Sci. 102, 3781–3804. doi: 10.3168/jds.2018-14985
Vastolo, A., Calabrò, S., and Cutrignelli, M. I. (2022). A review on the use of agro-industrial CO-products in animals’ diets. Ital. J. Anim. Sci. 21, 577–594. doi: 10.1080/1828051X.2022.2039562
Venkatramana, P., Senthil, M. S., Chacko, B., Patki, H. S., Shyama, K., and Sunanda, C. (2020). Effects of feeding cashew apple waste with or without supplementation of non-starch polysaccharide degrading enzymes on nutrient digestibili ty and growth performance of broilers. Ind J. Anim. Nutr. 37:67. doi: 10.5958/2231-6744.2020.00012.2
Venna, S., Sharma, H. B., Reddy, P. H. P., Chowdhury, S., and Dubey, B. K. (2021). Landfill leachate as an alternative moisture source for hydrothermal carbonization of municipal solid wastes to solid biofuels. Bioresource Technology 320:124410. doi: 10.1016/j.biortech.2020.124410
Wadhwa, M., Bakshi, M. P., and Makkar, H. P. (2015). Waste to worth: fruit wastes and by-products as animal feed. CAB Reviews. 10, 1–26. doi: 10.1079/PAVSNNR201510031
Wanapat, M., Suriyapha, C., Dagaew, G., Prachumchai, R., Phupaboon, S., Sommai, S., et al. (2024). Journal of agriculture and food research. J. Agric. Food Res. 17:101234. doi: 10.1016/j.jafr.2024.101234
Weiss, B. (2015). Optimizing and evaluating dry matter intake of dairy cows. WCDS Adv. Dairy Technol. 27, 189–200.
Wimalasiri, S., and Somasiri, S. C. (2021). “Ensiled Fruit Peels of Pineapple (Ananas comosus) and Papaya (Carica papaya) as an Animal Feed” in 2nd International Conference on Agriculture, Food Security and Safety, Department of Studies in Food Science & Nutrition (India: University of Mysore). iConferences (Pvt) Ltd, 29-43. doi: 10.32789/agrofood.2021.1003
Yafetto, L., Odamtten, G. T., and Wiafe-Kwagyan, M. (2023). Valorization of agro-industrial wastes into animal feed through microb ial fermentation: a review of the global and Ghanaian case. Heliyon. 9:e14814. doi: 10.1016/j.heliyon.2023.e14814
Yang, J., Tan, H., and Cai, Y. (2016). Characteristics of lactic acid bacteria isolates and their effect on silage fermentation of fruit residues. J. Dairy Sci. 99, 5325–5334. doi: 10.3168/jds.2016-10952
Zahid, A., and Khedkar, R. (2021). Valorisation of fruit & vegetable wastes: a review. Curr. Nutr. Food Sci. 18, 315–328. doi: 10.2174/1573401317666210913095237
Zhang, X. M., Chen, W. X., Yan, Q. X., Wang, C., Lin, B., Yi, S. Y., et al. (2023). Low-protein diet promotes nitrogen retention efficiency via enhanced renal urea reabsorption and microbial hydrogen incorporation in the rumen of goats. Anim. Feed Sci. Technol. 305:115762. doi: 10.1016/j.anifeedsci.2023.115762
Zhang, N., Hoadley, A., Patel, J., Lim, S., and Ce, L. (2017). Sustainable options for the utilization of solid residues from wine production. Waste Manag. 60, 173–183. doi: 10.1016/j.wasman.2017.01.006
Zhang, X., Ke, W., Ding, Z., Xu, D., Wang, M., Chen, M., et al. (2022). Microbial mechanisms of using feruloyl esterase-producing Lactobacillus plantarum A1 and grape pomace to improve fermentation quality and mitigate ruminal methane emission of ensiled alfalfa for cleaner animal production. J. Environ. Manag. 308:114637. doi: 10.1016/j.jenvman.2022.114637
Keywords: valorisation, fruit by-products, small ruminant, circular economy, systems thinking, sustainability, food security
Citation: Anim-Jnr AS, Ishaq SBY, Sasu P, Gyimah S, Greathead HMR, Boesch C, Mabiki FP and Emmambux MN (2025) Valorising mango, cashew apple, and papaya by-products for sustainable small ruminant production in low-income food deficit countries—a review. Front. Sustain. Food Syst. 9:1529837. doi: 10.3389/fsufs.2025.1529837
Edited by:
Andrew John Dougill, University of York, United KingdomReviewed by:
Gerardo Mendez-Zamora, Autonomous University of Nuevo León, MexicoTița Ovidiu, Lucian Blaga University of Sibiu, Romania
Effie Papargyropoulou, University of Leeds, United Kingdom
Copyright © 2025 Anim-Jnr, Ishaq, Sasu, Gyimah, Greathead, Boesch, Mabiki and Emmambux. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antoinette Simpah Anim-Jnr, YXNhbmltam5yQGtudXN0LmVkdS5naA==
 Antoinette Simpah Anim-Jnr
Antoinette Simpah Anim-Jnr Salma Binta Yusif Ishaq
Salma Binta Yusif Ishaq Prince Sasu
Prince Sasu Sadat Gyimah
Sadat Gyimah Henry Michael Rivers Greathead
Henry Michael Rivers Greathead Christine Boesch
Christine Boesch Faith Philemon Mabiki
Faith Philemon Mabiki Mohammad Naushad Emmambux5
Mohammad Naushad Emmambux5