
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 19 February 2025
Sec. Nutrition and Sustainable Diets
Volume 9 - 2025 | https://doi.org/10.3389/fsufs.2025.1509423
 Ashiq Hussain1
Ashiq Hussain1 Aziz Mouhaddach2*
Aziz Mouhaddach2* Ibtissame Khalid3
Ibtissame Khalid3 Tusneem Kausar1
Tusneem Kausar1 Mohamed Bouhrim4*
Mohamed Bouhrim4* Abdelaaty A. Shahat5
Abdelaaty A. Shahat5 Omar M. Noman5
Omar M. Noman5 Bruno Eto6
Bruno Eto6 Shazia Yaqub1
Shazia Yaqub1 Rizwan Arshad7
Rizwan Arshad7 Humna Farooq8
Humna Farooq8 Nida Firdous9
Nida Firdous9 Muhammad Bilal10
Muhammad Bilal10Introduction: New alternate food and pharma sources, halophyte plants have long been displaying exceptional nutritional and functional qualities.
Methods: Present investigations were carried out to explore three important halophyte herbs; Anabasis articulata, Lycium shawii, and Zilla spinosa,harvested from deserts, for phytochemical composition and potential food application.
Results and discussion: Plant leaves, converted into powders showed significant amounts of ash, fiber, and important minerals as contents (%) of ash and fiber in Anabasis articulata, Lycium shawii, and Zilla spinosa were 6.39 and 6.79, 8.19 and 9.14, and 4.35 and 8.87, respectively, while Mg, Zn, Cu and Mn were significantly higher in Anabasis articulata, with values 52.40, 50.59, 16.33 and 48.25 mg/kg, respectively, whereas Zilla spinosa presented Fe as 120.95 mg/kg, highest from all. Total phenolic contents (45.92 mg gallic acid equivalent/g), total flavonoid contents (11.31 mg quercetin equivalent/g) and total antioxidant activity (22.85 mg Trolox/g) were found highest in ethanolic extract of Lycium shawii, while in remaining two plants these values were also prominent. Extracts of these three halophytes leaves exhibited prominent antimicrobial activities, as calculated zone of inhibitions (mm) were comparable to reference drugs. Incorporation of powders of all three halophytes resulted in significant increment in ash fiber and minerals (Mg, Zn, Fe, Cu and Mn) content of biscuits but with slight decrement in moisture, fat and protein. Formulated biscuits were also found boosted in total phenolic and flavonoid contents with elevated antioxidant activity as a result of incorporation of halophytes powders. Assessment of biscuits revealed that 5% levels of all halophyte’s powders produced acceptable product in terms of color, taste, texture and overall acceptability. Thus, halophyte plant powders and extracts could be used as new and sustainable source for food and pharma products.
Since international conflicts, health invasions, and catastrophic climate change are causing the world’s hunger crisis to worsen, it is imperative that we carefully consider alternative food sources, such as halophyte plants, which can thrive in the most unfavorable conditions, including salinity levels higher than seawater, while still yielding large amounts of food and exhibiting remarkable nutritional and functional qualities (Oron et al., 2023; Gupta, 2025). Halophytes, the unusual plants, are found practically everywhere in the world and can survive in a variety of environments, including salt marshes and saline deserts. Halophytes have evolved a range of unique defense mechanisms to maintain homeostasis in these harsh settings, including the significant synthesis of several secondary metabolites with strong biological activity such as antioxidant and antibacterial, as is the case for polyphenols (Cheeseman, 2016; Patel and Ghane, 2021; Lokhande, 2025). Water scarcity and soil degradation brought on by recent global shifts have prompted the development of alternate agricultural practices (Duarte et al., 2022). In addition to serving as a base of nutrients, halophytes also contribute to the extension of therapeutic agents. There are many possible uses of halophytes in various fields of research and development; hence, these plants must be properly investigated to get maximum (Jamshidi-Kia et al., 2017; Saleem et al., 2021). The phytochemicals found in halophytes have various physiological and therapeutic effects such as antioxidant, antibacterial, antidiabetic, anti-constitutive, antiviral, and neuroprotective potentials which provide a comprehensive summary of these activities and so used by humans (Beshah et al., 2020; Nazir et al., 2020a; Nazir et al., 2021).
High salinity, arid climate, lack of water and nutrients, and habitat-related impacts have highlighted the significance of halophytic herbs in the domains of drug development and complementary medicine. In order to survive in these difficult climatic conditions and protect themselves against bacterial infection, excessive oxidative stress, and approach of grazing animals, plants must maintain higher levels of chemicals with defensive activities (Ishtiyaq et al., 2021). Salt-tolerant species known as halophytes that grow in these areas are rich in fiber and medicinal characteristics. Certain halophytes have a unique mechanism for tolerating salinity called osmotic adjustment. These plants store amino acids, organic acids, carbohydrates, and inorganic ions (Ghafar et al., 2022). Halophytes have a lot of promise as currency crops for things such as medicine, food, and other uses. These salty locale plants can replace traditional crops and thrive in soils with high salt concentrations. There are 728 different halophyte species known to exist in Southwest Asia. These are members of 68 households. Families such as Chenopodiaceae, Poaceae, Leguminosae (Papiliondeae), Asteraceae, and Cyperaceae account for the bulk of species. The most varieties and genera are found in the Chenopodiaceae family. Poaceae, which has more families but fewer species, surpasses it. Approximately half of the halophyte taxa (and families) known to exist worldwide are found in this area. Here, 115 halophyte species are assessed as food, and 331 are assessed as fodder. More than 100 taxa of halophytes are used as livestock feed in Iran, Afghanistan, Iraq, and Pakistan. In Southwest Asia, more than 40 taxa of halophytes are employed as sustenance (Öztürk et al., 2019; Lokhande, 2025).
Importance of using these medicinal plants extracts as food and beverage additives as well as in the cosmetics industry is rising nowadays, as an outcome of customers’ mounting interest in these extracts and the fact that these are safer than synthetic chemicals/oils and without side effects (Ibrahim et al., 2017). It is a need of time to investigate the chemical makeup of many therapeutic plants. Plant-based medications are used throughout the world presently. Therefore, it is critical to discover alternative solutions for the healing of specific illnesses that do not require lengthened therapy and may be healed by the utilization of herbal products (Tariq et al., 2018). Traditional medicine in the Arabian Peninsula uses Lycium shawii Roem. and Schult. (Solanaceae), a local shrub, to treat mouth sores, backaches, coughs, constipation, stomachaches, and a few types of animal fevers. The anti-trypanosomal, anti-inflammatory, hepatoprotective, anti-plasmodial, hypoglycemic, and cytotoxic properties of Lycium shawii extracts have also been employed (Rehman et al., 2016; Rehman et al., 2018; Rehman et al., 2020). Several phenolics and flavonoids were identified from leaves and stems of Lycium shawii, which have been found involved in antioxidant activities (Ahmed et al., 2017). Zilla spinosa is a member of the 3,709 species and 375 genera-strong family Cruciferae, also known as the Brassicaceae. Zilla spinosa, one of the many medicinal plants of the Brassicaceae family, has a variety of historical use in medicine. Because of its broad use in conventional medicine, it is common throughout the Asia region and well-known to local residents (Ullah et al., 2018). Different phytochemicals showing nutraceutical properties are abundantly present in these halophyte plants (Al-Qahtani et al., 2020; Suleiman and Ateeg, 2020; Ullah et al., 2020). One of the desert plants, Anabasis articulata, a member of the Chenopodiaceae family, is known as “ramet” in Arabic also known as Eshnanor Ajrem. Anabasis articulata stems, roots, and leaves are frequently applied in old-style medication to extravagance kidney infections, fever, diabetes, and skin conditions (Abdulsahib et al., 2016). Anabasis articulata’s phytochemical components revealed the presence of a number of active substances that may have multiple pharmacological effects. Tannins, triterpenoids, flavonoids, coumarins, phenolics, alkaloids, anthraquinones, iridoids, glycosides, carbohydrates, unsaturated sterols, triterpenoids, saponins, and glycosides have all been found in Anabasis articulata (Abdulsahib et al., 2016; Al-Joufi et al., 2022). The antioxidant and free radical scavenging functions of plants are produced by these secondary metabolisms (Belyagoubi-Benhammou et al., 2019; Benzineb et al., 2019; Al-Joufi et al., 2022).
Industrial applications of halophytes have different examples in the recent past with few experimental studies, such as production of industrial oil from Salvadora persica (Reddy et al., 2008), antioxidant studies of four different halophytes (Oueslati et al., 2012), medicinal bioactive ingredients and salt response mechanism of Limonium bicolor (Wang et al., 2016), potential industrial uses of 21 different halophytes plants (Lopes et al., 2016), industrial natural products with anti-aging potential from Armeria pungens (Rodrigues et al., 2018), extraction of polyphenols from Limonium algarvense (Rodrigues et al., 2020), cultivation of Salicornia bigelovii for green biomass (Christiansen et al., 2021), salt stress tolerance of Lycium ruthenicum (Mo et al., 2022), anticancer bioactives (Pourabdollah-Kaleybar et al., 2025), and transforming heavy metals in the soil into non-toxic compounds (Bhusare et al., 2025). Halophyte plants have been found unexploited source of nutraceuticals, while their extracts and isolated bioactive compounds have been found applications in the pharmacological fields. Polyphenols, carotenoids, chlorophylls, enzymes, and vitamins have been found abundantly in these salt loving plants (Oueslati et al., 2012; Rehman et al., 2019; Hulkko et al., 2022), whereas their withstanding power in the abiotic stress has provided these plants these loads of antioxidant bioactives (He et al., 2018). The use of halophytes has been remained limited to feed and pharma industry, just because of the no interest or unfamiliarity by the food producers, but chemical and biochemical profiling of halophyte plants and plant parts might prove helpful, exploring the use of these halophytes’ powders and extracts as ingredients of pharma foods. For that reason, first time in this study three unfamiliar and underutilized halophyte plants were nominated and used as source of food industry ingredient. By keeping in view that above facts and figures about the medical importance of halophytic plants, fewer studies about their phytochemical composition, and uses in food formulations, the purpose of these trials was to explore the three medicinal halophytes (Anabasis articulata, Lycium shawii, and Zilla spinosa) for physicochemical and phytochemical analysis by developing powders and ethanolic extracts, and potential food application of plant powders in the form of biscuits developed at various replacement levels.
Plant materials Anabasis articulata (Forssk.) Moq., Lycium shawii Roem. & Schult., and Zilla spinosa (L.) Prantl were collected from Cholistan Desert of southern Punjab, Pakistan. Collected halophytic plant species were washed properly using running household water and then by double distilled water. Plants collected in the field were identified and authenticated in the Department of Botany and Department of Biological Sciences, Sargodha University, Punjab, Pakistan. Different taxonomists from Karakorum University and the literature that is currently accessible were consulted to identify and name the plant species as earlier done by Hameed et al. (2020), especially following the flora of halophytes in Pakistan deserts. Plant identification code numbers were as follows: Anabasis articulata, Taxonomy ID: 996498; Lycium shawii, Taxonomy ID: 43455; and Zilla spinosa, Taxonomy ID: 127626.
The Sigma-Aldrich chemicals company provided all the reagent-grade chemicals used in this study project. Glassware and accessories for analyses were available in scientific laboratory of University of Sargodha, Pakistan. Raw materials for the production of food items were bought at local markets in Pakistan’s District Sargodha. Different strains of microorganisms for antimicrobial activities were attained from Microbiological Research Center, Pakistan.
Any foreign material that was adhered to the plants was removed, and leaves were sorted for drying. The stem, roots, and any blossoms were cut off, and the leaves were then thoroughly washed with distilled water once again. The leaves were placed in stainless steel containers, followed by drying at 65°C in hot air-based oven (DHG-9023A, Malaysia) by succeeding the process adopted by Hussain et al. (2021), with mandatory changes. The leaves were dried till constant weight was obtained. The dried leaves were grinded into powder through grinder (PK-1200, Pakistan), which was sieved with 100-mesh sieve and kept in airtight jars for further analysis. Halophyte powders for analysis ahead were kept under normal conditions of laboratory in airtight bags.
The Hussain et al. (2022a) approach was followed for the production of the biscuits with various replacement levels (5 and 10%) of plant powders, with a minor change. To put it simply, ingredients were measured, combined to make batter to produce sheets. By using molds, shaping and cutting of biscuits was done and then set-in stainless steel trays. The biscuits were baked at 180°C by placing in commercially used oven for at least 20 min. Biscuits were prepared by using wheat flour and had two different replacement amounts of 5 and 10% for each plant powder, with 100% white flour biscuits serving as the control. The prepared biscuits were maintained at ambient temperatures in polythene bags for subsequent analysis.
The biscuits, which contained powders from three halophytic plants in various proportion, were evaluated olfactorily by applying a 9-point hedonic assessment scale that was earlier adopted by Tsikritzi et al. (2014). Briefly stated, a group of 40 specialists having average age of 45 and representation from both genders were given ratings on a scale of 1 to 9, with 1 denoting an extreme dislike and 9 denoting an extreme like. Different treatment biscuits with special codes were served to the specialists, along with bottles of distilled water for mouth washing and counterbalancing after each test. Calculations and analyses were performed on the acquired data.
To prepare the extracts from plant powders, methodology provided by Hussain et al. (2021) was adopted. Briefly explaining, each plant powder was separately soaked in 80% ethanol for 48 h with shaking at 50–100 rpm. Then, it was filtered by passing through Whatman filter paper No. 1. After filtering, the powder was separated. The process was repeated in triplicate for each powder type, and all the filtrate was gathered and dried using a rotary evaporator. Then, the extracts were stored at proper refrigeration temperature and used for further phytochemical analysis (TPC, TFC, antioxidant potential, antimicrobial assay). Schematic diagram for development of powders, extracts, and analyses of extracts can be seen drawn as Figure 1.
To determine the amount of crude protein, fat, ash, fiber, and moisture in plant sample’s powders and developed biscuits, respective methods of AACC (2000) were used. Contents of moisture by following method No. 44-15A, protein contents by adopting method No. 46–12, fat contents by following method No. 30–10, fiber contents through method No. 32–10, and ash contents by adopting method No. 8–01 of AACC (2000) were determined. These materials’ contents were reported as percentages, with each percentage indicating the mean of three replicates of each sample.
Ethanolic (80%) extracts of plants leave’s powders and formulated biscuits were also used to determine polyphenolic contents including total phenolic content (TPC) and total flavonoid content (TFC), and at the end the total antioxidant activity (TAA).
Total phenolic contents in each plant extract and formulated biscuits were calculated using the Folin–Ciocalteu technique by following the procedure given by Suleiman and Ateeg (2020), with required changes. Briefly describing, the plant extracts were combined with 2.5 mL of 0.2 N Folin–Ciocalteu reagent and stirred for 5 min before 2.0 mL of a 7.5% aqueous sodium carbonate solution was added. To allow for color development, the reactants were then incubated for at least for 90 min at temperature 30°C. Then at 760 nm UV/Vis spectrophotometer was used to record absorbance. To create a calibration curve, same process was repeated for the solutions of gallic acid in ethanol as standards (0, 0.01, 0.02, 0.04, 0.06, 0.08, 0.1, and 0.2 mg/mL). The regression equation resultant from the typical curve was used to determine the TPC. The results of the calculations were given as mg GAE/g of the plant extracts’ dry weight.
The TFC in the three selected plants ethanolic extracts, and then plant powders incorporated nutritional biscuits were calculated using aluminum chloride assay that was slightly improved from the one described by Ordonez et al. (2006). Briefly describing, in a test tube, 2 mL of a 2% aluminum chloride solution was combined with 2 mL of the plant extracts (0.1 mg/mL in ethanol). Test tube was incubated at 3°C for 60 min for color development. At wavelength 415 nm, the absorbance was determined. The calibration curve was created using a variety of quercetin standards (0.01–0.1 mg/mL in ethanol), and it was examined in the same way as the plant extracts. The regression equation generated from the quercetin standard curve was used to compute the TFC. In terms of dry weight of the plant extracts, the values are represented as mg QE/g.
A modified version of the assay for the determination of TAA of halophytic plants and formulated biscuits ethanolic extracts was followed as described by Re et al. (1999), with slight changes. Initially, 2.45 mM potassium persulfate and 7 mM ABTS+ were combined in a 1:1 (v/v) ratio. The combination was maintained at room temperature and in the dark for 6 h. A workable solution with an absorbance value of 0.70 0.02 at 734 nm was then created by diluting an aliquot in 75 mM phosphate buffer (pH = 7.4). Following a 30-min incubation period at 30°C, 20 mL of the sample (10 mg/mL, dm) was combined with 200 mL of the ABTS working solution, and the absorbance at 730 nm was measured. The results were given in units of Eq Trolox/g of sample (dm). As a benchmark for antioxidants, Trolox was utilized. For use as a stock standard, Trolox (2.5 mM) was prepared in 5 mM phosphate buffered saline, pH 7.4.
The following pathogenic microbes were used as test organisms that include bacteria; gram positive (Bacillus cereus, ATCC 14579), gram negative (Escherichia coli, ATCC 11229); and fungi (Aspergillus niger, ATCC 1015 and Candida albicans, 10231).
Agar well diffusion technique was adopted to assess the plant extracts for inhibitory effects against the test microorganisms. The plant extracts’ inhibitory potential against particular human pathogens was assayed. In tubes containing LB broth medium, all indicator organisms were cultivated by inoculating loop-full samples and incubating for 24 h at 37°C. Then, 100 μL of test bacteria was added with micropipette in 250 mL of Luke warm sterile nutrient agar media discretely. Before pouring on selected petri plate, it was appropriately mixed. After solidification of agar media, a sterile borer measuring 5 mm was used to create wells in an agar plate. After that, 100 microliters of plant extract were added in each well. Before incubation, it was given enough time for effective absorption. In mm, the zone of inhibition was calculated by succeeding the guiding principle given the method adopted by Yaqub et al. (2019), by using reference antifungal drug Penicillium.
The agar well diffusion method, as described by Khaing (2011), was adopted to test the antifungal activity of plants extracted in 80% ethanol. Initially, two fungal strains, namely, Candida albicans, and Aspergillus niger, were grown in shaking water bath in their corresponding broths at 25–30°C. After incubation, strains were harvested, and fugal suspensions (100 μL) were obtained through swab on a particular media termed Sabouraud Dextrose Agar (SDA). By using sterile borer, 5 mm wells were produced on agar plates. These wells were filled with 100 μL of plant ethanol extracts. After filling, petri plates were inoculated for 24 h at 37°C, and the inhibition zones of microorganisms were measured, while Diflozon was used as standard antifungal drug.
To report the results as means of triplicate values along with standard deviations, triplicate analyses were done for each trial. The one-way ANOVA was used for the statistical analysis. To distinguish between the mean values, Duncan’s multiple-range test was applied at a level of significance p ≤ 0.05. Guidelines from the procedures of Steel et al. (1997) were taken for the statistical analyses.
Data presented in Table 1 show the mean values of different parameters of proximate composition of three halophytic plants. From these results, it can be seen that Lycium shawii has maximum nutritional contents as compared to other two plants. The contents of protein (6.24%), fat (2.04%), ash (8.19%), and fiber (9.14%) were significantly higher (p≤ 0.05) in Lycium shawii as compared to Anabasis articulata and Zilla spinosa. It can also be seen from Table 1 that Zilla spinosa powder has maximum moisture contents (6.86%). From the chemical composition of these three selected halophytic plant powders, it was evident that these plants are good sources of ash and fiber contents. Specifically, it is well-known that halophyte plants are high in ash and nutritional fiber. High ash contents of halophytes, in particular, are closely linked with salty environment and capability of plants to absorb minerals for osmotic adjustment (Custodio et al., 2021). Current research results were also witnessed, when Duarte et al. (2022) assessed the nutrient content of six halophytes grown in different salt ponds and watered by fresh estuarine water. These halophytes were edible and had high levels of ash, fiber, protein, and other bioactive contents. Toumi et al. (2022) reported total ash as 46% and total fiber as 28% of the total dry matter of a halophytic plant powder, and also reliable quantities of protein and fat contents, empowering the findings of current study. Al-Qahtani et al. (2020) examined effect of region, seasonal variation, and light (morning and day) on the chemical composition of Zilla spinosa plant and reported similar proximate composition. The findings, similar to current study, were also observed when El-Amier et al. (2022) determined proximate composition of five halophyte plants from three different families and reported higher contents of ash, fiber, and protein in halophytes powders. The current study results are also similar to the results from the study of Ahmed et al. (2017), when they investigated leaves and stems of Lycium shawii for chemical composition and reported higher contents of ash and fiber in stem portion as compared to leaves. Experiments regarding calculations of seasonal variation in proximate composition of this halophytic plant provided noteworthy variance in the outcomes, which might be owing to the availability and scarcity of water in deserts. Mayouf and Arbouche (2014) investigated three halophyte shrubs for chemical composition, due to their importance and use in feed, and reported high contents of dry matter (90.60%), ash (10.26%), fiber (3.35%), and crude protein (17.13%) in Anabasis articulata, which proved the significance of this halophytic shrub in food and feed industry to meet the nutritional requirements of animals and humans.
Current study revealed the varied composition of minerals in three halophytes as the results are shown in Table 2, from where it is found that considerable amount of minerals was found in all of these halophytes. Zilla spinosa had significantly higher (p ≤ 0.05) amount of iron (120.95 mg/kg), while Lycium shawii had minimum iron contents (28.59 mg/kg). When compared, it was observed that maximum quantity of magnesium (52.40 mg/kg), zinc (19.36 mg/kg), copper (16.33 mg/kg), and manganese (48.25 mg/kg) was found in Anabasis articulata. From the results of mineral analyses, it was easy to conclude that these halophytic plants were loaded with essential minerals, which probably was due to the accumulation of minerals in these plants, due to lesser moisture contents and high salt percentage in the environment (Custodio et al., 2021). Due to high amounts of iron and zinc, the nutritional and medicinal importance of these halophytes may be increased in the scientific community. Even though the soil and water’s composition, the plants’ state of development, and the time between harvesting and harvesting all affect the halophyte plants’ mineral composition, sufficient amount of macro and micro minerals have been found in halophyte plant (Toumi et al., 2022). Mohammed et al. (2021) selected some halophyte plants from the Saudi Arabia deserts and performed elemental analysis, the results were much similar to the findings of current research work as all these plants were found sufficient in minerals, as data showed higher contents of Fe in Zilla spinosa, whereas contents of Zn were higher in Anabasis articulata. Contents of Mg, Cu, and Mn were also present in all selected plants with much greater values of Mg and relatively lower values of Cu and Mn. During the macronutrient study, it was reported by Al-Qahtani et al. (2020) that mineral contents (Ca, Mg, K, and P) of Zilla spinosa were significantly affected by area of sampling, season, and time of sampling. Moreover, similar to current study, the quantities of Cu, Mn, Fe, Zn, and Na were also reported during the micronutrient analysis. In another similar study, the mineral amounts of two distinct developing inhabitant halophytes (natural occurrence and domestically irrigated) of Sarcocornia ambigua were assessed by Bertin et al. (2016), using plasma mass spectrometry, inductively coupled. Potassium (K) (19–24 μg/g) was the mineral found in the greatest concentrations across all samples, followed by magnesium (Mg) (8.6–14 μg/g) and calcium (Ca) (2.6–4.0 μg/g). The bioaccessibility of the trace elements vanadium (V), chromium (Cr), cobalt (Co), copper (Cu), and lithium (Li) was determined to be greater than 50% of the total concentration. That study witnessed not only the presence of important minerals in the halophytes but also high bioaccessibility during in vitro digestion. The presence of sufficient minerals in halophytes powders was also reported in the findings of El-Amier et al. (2022), when five halophyte plants were investigated for mineral contents. Investigations of Mayouf and Arbouche (2014) about halophytic Anabasis articulata revealed the nutritional importance of this shrub due to the presence of high ash and mineral contents. In a controlled study, Duarte et al. (2022) reported that halophytes contain a number of mineral elements that are typically rare in contemporary human diets. Halophytes demonstrated a high nutritional value when compared with conventional veggies, such as spinach, supporting food application of these underutilized plants, with condensed form of nutrients.
Table 3 shows results about the polyphenols and antioxidant potential of 80% ethanolic extracts of Anabasis articulata, Lycium shawii, and Zilla spinosa. Each extract’s TPC was found to be significantly (p ≤ 0.05) different. Lycium Shawii had the highest TPC concentration (45.92 mg GAE/g) among the three halophytes, while Anabasis Articulata and Zilla Spinosa had lower TPC values. From the present study, it was discovered that Lycium shawii has a maximum total flavonoid content (11.31 mg QE/g). Lycium shawii demonstrated the highest antioxidant potential of 22.85 mg Trolox/g due to significantly (p ≤ 0.05) greater TPC and TFC. A positive association among phenolic, flavonoids, and antioxidant activity was evident from the presented results, which proved the fact that phenolic and flavonoid compounds have positive role in scavenging free radicals. Phenolic substances are water-soluble antioxidants that are essential in removing reactive oxygen species from the environment. A similar halophytic study investigated the elevated phenolic compound accumulation and antioxidant capacity of halophyte species located in Pakistan. Upon identification, carotenoids, chlorophyll, and ascorbic acid were found to be the active components in these plants (Ghafar et al., 2022). Exploring the nutritional importance of under controlled halophytes, Duarte et al. (2022) investigated six species, and all contained a significant amount of concentrated phenolic and flavonoids as antioxidant compounds, just as has been found in the current results. Saleem et al. (2021) explored the phytochemical profile of a related halophyte plant and reported that ethanol extracts were found to have greater phenolic and flavonoid contents, which is consistent with a higher level of radical scavenging capability. Similarly, UHPLC profiling led to the preliminary identification of 11 different secondary metabolites. El-Amier et al. (2022) reported TPC 17.1 to 41.83 mg/g dw and TFC 4.52 to 8.23 mg/g dw in five different halophytes from three different families. Experimental study also provided significant scavenging activities of these plant extracts, very comparable to the ascorbic acid as standard. In vitro antioxidant activity of another salt loving plant Mesembryanthemum edule L. was reported by Falleh et al. (2011), owing to high polyphenol contents present in leaf, stem, and shoot of plant. In a similar study, Mohammed et al. (2021) investigated four similar halophyte plants, namely, Anabasis articulata, Lycium shawii, Zilla spinosa, and Rumex vesicarius, from deserts of Saudi Arabia, to explore phytochemical variations and performed phytochemical analysis. Lycium shawii contained the highest amounts of flavonoids and phenolics, and maximum antioxidant activity was shown by Lycium shawii. The presence of phenolics and flavonoids contributes toward antioxidant capacities of plants extracts, as another study by Ullah et al. (2018) strengthened this fact, when extracts (methanolic and n-hexane) of two important halophytic plants Hammada elegans and Zilla spinosa at dose of 250 𝜇g/mL demonstrated substantial antioxidant actions of 38.16, 35.65, and 31.18%, respectively. Ullah et al. (2020) again prepared Zilla spinosa extracts in butanol and chloroform and studied antioxidant activity by using DPPH assay and found consistent results. Halophyte plants are regarded as a significant source of bioactive compounds with numerous biotechnological uses, such as antioxidants. According to an experimental investigation, Limonium algarvense, especially its blooms, are auspicious spring of biologically active antioxidants with prospective uses in a variety of industries, including the agro-food industry (Rodrigues et al., 2015; Rodrigues et al., 2020).
A similar study by Alghanem et al. (2018) on phytochemical composition of important halophytic plant revealed that Zilla spinosa had quantities of alkaloids, flavonoids, and phenolics as 17.56 mg/g, 11.22 mg/g, and 28.22 mg/g dehydrated weight, respectively. Relation of these bioactives to scavenge free radicals could prove very beneficial toward estimation of antioxidant capacity of this important medicinal plant as high amount of phenolics and flavonoids was noticed in Zilla spinosa extracts. Suleiman and Ateeg (2020) reported that phytochemical screening of Zilla spinosa root extracts exhibited high phytochemicals (phenols, flavonoids, alkaloids, glycosides, saponins, and triterpenoids) as compared with the aerial parts of plant, which proved that each part of this halophytic plant is heavily loaded with bioactives. Study reported that extracts from roots and aerial parts both showed strong antioxidant capacity against various tests: DPPH, H2O2, and FRAP. Abdulsahib et al. (2016) reported that methanol extracts of halophyte Anabasis articulata stems have antioxidant agents and further revealed free radical scavenging activity by DPPH assay. In a separate investigation, Benzineb et al. (2019) made extracts (dichloromethane, methanol, and ethyl acetate) of aerial parts of Anabasis articulata and assessed TPC, TFC, and DPPH free radical scavenging activities. The methanolic extracts had the highest concentration of polyphenols and flavonoids, measuring 230.00 ± 46 mg/g gallic acid equivalent and 9.50 ± 31 mg/g quercetin equivalents, respectively. The methanolic fraction displayed the same antioxidant capacity using both methods and a comparatively high level of polyphenols. The phenolic substances from Anabasis articulata have potent antioxidant effects that make it effective as a pharma plant for a number of ailments prevention. Stems and roots of Anabasis articulata were extracted using different solvents, and contents of phenolics, flavonoids, carotenoids, and tannins were found higher in stem portion as compared with roots. Similarly, antioxidant capacity and DPPH activity was calculated, which was found directly correlated with the number and amount of these bioactives (Benhammou et al., 2013). Recent research has supported this claim that antioxidant activity has strong relation with polyphenols. The antioxidant capacity is influenced by the total amount of phenolic hydroxyl groups and where they are located on the aromatic core (Bourgou et al., 2008; Benhammou et al., 2013).
Al-Joufi et al. (2022) prepared methanolic extracts of Anabasis articulata and estimated the TPC and TFC and found similar outcomes. By using the DPPH free radical scavenging potential assay, Al-Joufi et al. (2022) also evaluated the antioxidant activities of Anabasis articulata and found that the flavonoids and phenolics are abundant in this plant. Because flavonoids and phenolics have benzene rings in structural makeup, these bioactives are excellent free radical scavengers, effectively scavenging the free radicals, ABTS, and DPPH. Investigations about phytochemicals present in stem and leaves of Lycium shawii by Ahmed et al. (2017) provided significant evidence for the presence of phenolics, flavonoids, and other bioactives in this halophytic plant. Rehman et al. (2018) reported 14 different classes of phytochemicals from two varieties of Lycium shawii, exhibiting a positive correlation with the current outcomes. Castañeda-Loaiza et al. (2020) reported that phenolic bioactives of a halophyte were found involved in strong antioxidant activities, which witnessed the use of halophyte plants and plant extracts as a source of medicinal ingredients.
Table 4 displays the findings of the antimicrobial activities of ethanolic extracts of three halophytes: Anabasis articulata, Lycium shawii, and Zilla spinosa against tested organisms. All extracts significantly inhibited the bacterial and fungal growth, while maximum inhibition was observed against bacteria Bacillus cereus (14.47 mm) and yeast Candida albicans (10.33 mm) by Zilla spinosa extract. Escherichia coli was maximally inhibited by extracts of Anabasis articulata. Lycium shawii showed maximum inhibition (16.30 mm) against Aspergillus niger, whereas highest zone of inhibitions was exhibited by the reference antibacterial and antifungal drugs, against bacterial and fungal species, respectively. Both conventional medicine and present-day medications have employed several medicinal plants to treat a diversity of disorders. Many studies demonstrate the efficacy of various extracts from medicinal plants as antibacterial and antifungal agents (Ibrahim et al., 2017; Hussain et al., 2024). Present findings were found consistent when Mohammed et al. (2021) developed hydroethanolic extracts of four halophyte plants and used these extracts to assess antimicrobial potential through agar well diffusion method. The results revealed that extracts of these halophyte plants displayed prominent antimicrobial activities against nominated bacterial and fungal species as maximum bacterial inhibition was observed against Bacillus cereus by Lycium shawii while Aspergillus niger ATCC 6275 was maximally inhibited by Zilla spinosa. In another similar study, Mohammed et al. (2022) checked antimicrobial activities of the Lycium shawii extracts using the agar well diffusion method against Streptococcus mutans, Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae, and the results revealed that extracts of this halophyte plant can be used as antibacterial agents. Alghanem et al. (2018) prepared five different types of extracts of Zilla spinosa and checked antimicrobial activities against five test bacteria and found that Staphylococcus aureus was the most inhibited bacteria followed by Streptococcus pyogenes, Klebsiella pneumonia, and Bacillus subtilis. Methylene chloride extract does not affect both Klebsiella pneumoniae and Escherichia coli but prevents others. Similarly, Staphylococcus aureus, Streptococcus pyogenes, and Bacillus subtilis were inhibited by ethyl acetate extracts. With the exception of Escherichia coli and Klebsiella pneumonia, all bacteria were suppressed by the acetone. All microorganisms with varied inhibition zones were inhibited by methyl alcohol extract, except for Bacillus subtilis. These results, however, were consistent with the present study findings. Alghanem et al. (2018) also checked the antifungal potential of five different types of extracts of Zilla spinosa against four test fungi, namely, Aspergillus niger, Aspergillus fumigatus, Candida albicans, and Mucor spp. Acetone extract showed the highest antifungal activity (18.8 mm) against Mucor spp., while Aspergillus niger was not inhibited by any extract. Suleiman and Ateeg (2020) prepared ethanolic and methanolic extracts of Zilla Spinosa (roots and aerial part) and performed antimicrobial assay against bacterial and fungal strains, to assess the medicinal potential of halophyte. The highest inhibitory activity was observed from methanol extract of aerial parts against Staphylococcus aureus (26.5 mm). Each extract that was examined demonstrated strong antifungal action against Candida albicans. Methanol extracts (aerial part) showed the greatest inhibition, with an inhibition zone of 12.6 mm. Overall, the findings supported the plant’s efficacy due to its strong antioxidant and antibacterial properties. In a similar study about exploration of phytochemical and medicinal potential of halophytic plant Anabasis articulata, Benzineb et al. (2019) prepared extracts of aerial parts of Anabasis articulata and performed antibacterial potential assay of Anabasis articulata extracts against three indicator bacteria: Pseudomonas aeruginosa ATCC 14028, Salmonella Typhimurium ATCC 9027, and Bacillus subtilis ATCC 6633, by disk diffusion method and found that methanolic extracts (25 mg/L) have maximum antibacterial potential of 13 mm against Pseudomonas aeruginosa ATCC 14028, confirming the medicinal properties of the phytochemicals it contained. Antimicrobial potential of Anabasis articulata was also justified by the studies of Al-Joufi et al. (2022) when the agar disk diffusion method was used to evaluate the antibacterial activity of methanolic crude extract of Anabasis articulata against Shigella dysentery (diarrhea causing pathogen), Escherichia coli, and Salmonella Typhi. Significant zone of inhibitions from halophyte plant extracts was exhibited strengthening the fact that plant may be used as a source for isolating antibacterial chemicals due to its substantial antibacterial properties. The identified chemicals of halophytes such as polyphenols, peptides, tannins, polysaccharides, and saponins have been shown in previous studies to have antimicrobial properties (Nazir et al., 2018; Nazir et al., 2020b; Nazir et al., 2021). Saatchi et al. (2014) reported that essential oil of medicinal herbs acts as preservative because of retarding mold growth; therefore, essential oils from the halophyte plants may act as antimicrobial agents.
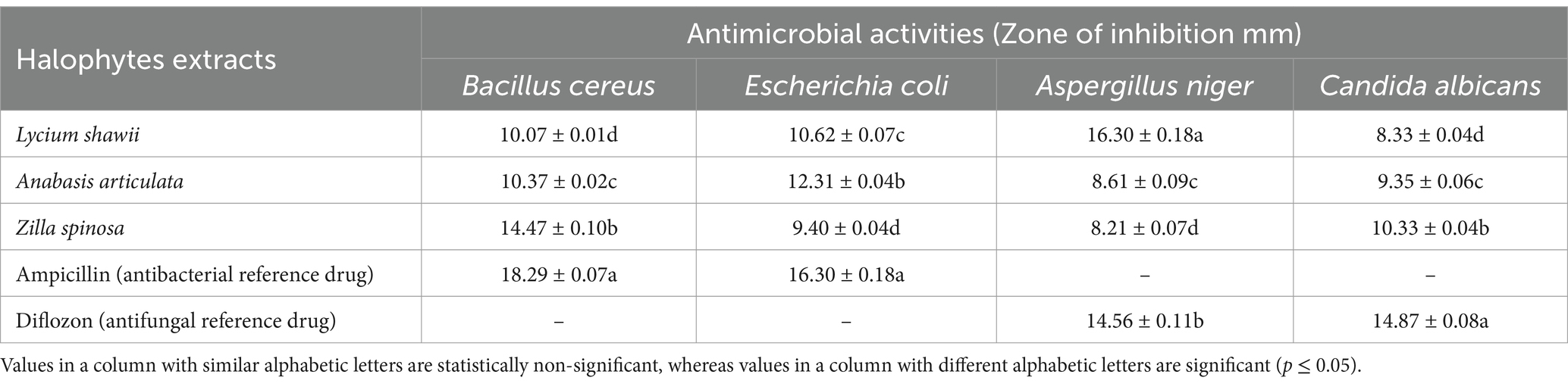
Table 4. Antimicrobial activities of selected halophytes against selected bacterial and fungal species.
The use of halophytes in the culinary innovations in the form of powders could bring the new range of food products in the markets, which could provide the consumers more range of nutrients (Ngxabi et al., 2025). The mean values of proximate analysis results of biscuits prepared with 100% wheat flour and biscuits manufactured with varied concentrations (5 and 10%) of dried leaf powder from three halophytes, Lycium shawii, Anabasis articulata, and Zilla spinosa are shown in Table 5. From these findings, it was clear that biscuits made with powdered Lycium shawii leaves had significantly higher (p ≤ 0.05) moisture, protein, fat, fiber, and ash contents as compared to the biscuits made with powdered Anabasis articulata and Zilla spinosa. When biscuits prepared with plant leave powder in proportions of 5 and 10% were compared, it was found that maximum moisture (7.68%), fat (25.12%), and protein contents (6.83%) were found in the biscuits made with 5% Lycium shawii powder while minimum moisture, protein, and fiber contents were observed in biscuits prepared with Anabasis articulata powder. The maximum quantity of ash and fiber contents as 3.23 and 4.88%, respectively, was observed in the biscuits prepared with 10% Lycium shawii powder while minimum ash (1.36%) and fat (24.30%) contents were found in the biscuits made with Zilla spinosa 5 and 10%, respectively. No specific scientific literature was found about the use of these halophytic medicinal plant powders or extracts in the food formulations, which might be due the unawareness and uninterest of people about these alternate food sources. The results of this study could prove very beneficial in exploring the new use of these underutilized halophytic plants for functional and medicinal food formulations. For comparing and witnessing the findings of current research work, citations from previous scientific reports related to addition, fortification, replacement, and incorporation of non-wheat flours from fruits, vegetables, herbs, plants, and crops in wheat flour for the development of bakery products have been discussed ahead. Lee et al. (2010) did research to examine the qualities of bread with saltwort powder added in amounts of 0, 3, 5, and 7% based on the weight of wheat flour. The findings showed that the 7% group had the least amount of water and the most carbon and protein. No sample’s fat level varied significantly from any other sample. Alam et al. (2014) prepared herbal biscuits by adding tulsi and Moringa in different concentrations. The nutritional quality assessment of these herbal plants showed that theses herbs increased the quantity of protein, fat, ash, and fiber in biscuits, while carbohydrate contents were reduced as concentration of herbs was increased in biscuits. Hussain et al. (2022b) developed nutritional biscuits by incorporation of powders from peel, flesh, and seeds of selected vegetables and compared the values with control (100% wheat flour biscuits). Incorporation of plant material-based powders in bakery products results in increment in ash and fiber contents, which was witnessed by the findings of Hussain et al. (2023) during development of lemon pomace incorporated biscuits.
Table 6 displays the average values of the findings after the analysis of mineral content of biscuits made with 100% wheat flour and biscuits made with varying amounts (5 and 10%) of dried leaf powder from the three halophytes: Anabasis articulata, Lycium shawii, and Zilla spinosa. With regard to magnesium, zinc, copper, and manganese content, it was evident from these results that biscuits made with powdered Anabasis articulata leaves had significantly higher (p ≤ 0.05) levels of minerals than those made with powdered Lycium shawii and Zilla spinosa. Biscuits prepared with 10% Zilla spinosa powder have highest iron contents of 11.82%. The maximum magnesium (19.83 mg/100 g), zinc (7.45 mg/100 g), copper (2.57 mg/100 g), and manganese (6.70 mg/100 g) contents were found in the biscuits made with 10% Anabasis articulata powder, while biscuits containing 5% Lycium shawii powder have minimum manganese and iron contents. Hussain et al. (2022a) determined Zn and Fe contents in 100% wheat flour biscuits and reported values not much different from the present study. Bhol et al. (2016) investigated the effect of bagasse powder of pomegranate whole fruit on the mineral contents of the bread. An increase in the minerals was observed for the 15% substitution level of pomegranate whole fruit bagasse powder in bread. High ash contents in non-wheat flours, especially that of peels and pomaces from fruits, vegetables, and plants, have been found positively related to the high content of minerals in incorporated products. Increase in important minerals of bakery products as a result of incorporation of herbal plants powders was also supported when Yadav et al. (2022) developed biscuits by combining Moringa leaf powder and flower powder. The quantity of mineral contents was increased in Moringa flower and Moringa leave powder supplemented biscuits with a noteworthy increase in iron (from 0.47% in control up to 2.62–3.35%) and calcium (from 26.25% in control up to 98.7–115%) in formulated biscuits supplemented with Moringa powders. Khalil et al. (2015) measured the concentrations of four micro and four macro minerals in seeds chicory and milk thistle, comparing with wheat flour. According to the findings, chicory and milk thistle seeds appear to be high in calcium (880 mg/100 g) and phosphorus (750 mg/100 g) but low in salt (40 and 60 mg/100 g, respectively). The data also showed that the chicory seed had high concentrations of iron, zinc, and manganese (397.16, 4.48, and 7.30 mg/100 g, respectively), and milk thistle had 65.50, 3.76, and 2.21 mg/100 g, respectively, of these trace elements. Refined wheat flour had the lowest concentrations of minerals when compared with others. Therefore, incorporation of these powders in wheat flour to develop different food items can provide mineral rich products.
Table 7 displays the findings of the analysis of TPC, TFC, and TAA of biscuits made with 100% wheat flour and biscuits made with varying amounts (5 and 10%) of dried leaf powder from three halophytes: Anabasis articulata, Lycium shawii, and Zilla spinosa. The findings demonstrated that 10% powdered Lycium shawii containing biscuits have the highest TPC (24.32 mg GAE/g), TFC (10.49 mg QE/g), and TAA (13.70 mg Trolox/g). The biscuits produced with 5% Anabasis articulata powder had the least amount of TPC (14.11 mg GAE/g), and as a result, it had the least amount of antioxidant activity (9.03 mg Trolox/g). The lowest TFC was found in the biscuits made with 5% Zilla spinosa, with a value 6.93 mg QE/g. From these results, it was also evident that control biscuits were lowest in the phytochemicals, with minimum antioxidant capacity, but addition of halophytic plant powders significantly rose these parameters, due to excellent phytochemical profiles of these herbs. Bakery products developed with 100% straight grade wheat flour normally lack high concentrations of phytochemicals, thus limiting the use as antioxidant sources. Hussain et al. (2022c) performed phytochemical analysis of different formulations of biscuits and determined TPC, TFC, and DPPH free radical scavenging activities, and the results for control biscuits were very much similar to the findings of current research work. Bhol et al. (2016) determined the quality characteristics, total phenols, and antioxidant activities of bread fortified with pomegranate bagasse powder and were capable in modifying the antioxidant capacity of formulated breads to high level. In another similar study, Rowayshed et al. (2015) added various concentrations of extracts from potato peel in biscuits and reported that potato peel exhibits very robust antioxidant potential as compared with synthetic antioxidants, which was due to polyphenols presence. The biscuits with 0.5 and 1% potato peel showed acceptable organoleptic properties. The use of herbal plants, extracts, and essential oils from plants, as source of bioactives, has several examples in past studies as Saatchi et al. (2014) extracted essential oils of herbs lemon balm, camel thorn, and ajwain, and these essential oils showed more antioxidant properties as compared with synthetic antioxidants hence could be used in baking industry to prevent fat rancidity and imparting flavor to product. In another similar study, Tesby et al. (2018) explored the effect of adding herbs (thyme, cumin, and anise) in different concentrations to different bakery products (Arabic bread, cressina, and Patton sale samples). They noted that acrylamide formation was reduced due to the phenolics and flavonoids that have antioxidant potential. Supportive results were also observed when Král et al. (2021) verified the effect of spice and herbs (cloves, cinnamon, peppermint, and grape flour) addition in different concentrations on polyphenols contents to biscuits. With the inclusion of spices and herbs, the antioxidant capacity of all samples increased. Clove samples at a 10% concentration showed the highest antioxidant capability. The herbs and indigenous spices used have a high polyphenol content and, consequently, a high antioxidant capability by nature. Studies of Khalil et al. (2015) also provided scientific evidences related to the present study as was reported that given the concerns about the use of synthetic antioxidants in food additives, herbal plant seeds can be regarded as a useful source of natural antioxidants. Another very important medicinal plant loaded with phytochemicals was tested during a similar study, when Yadav et al. (2022) prepared biscuits by adding of powder from leaf and flowers of Moringa. The sample with the highest flower content had the highest TPC content (72.60 mg GAE/100 g dry extract), whereas flavonoid content in herbal biscuits varied from 308.08 to 347.04 mg/100 g quercetin equivalent. Bajaj et al. (2016) blended three varieties of peppermint and spearmint (powder, extract, and pure menthol) to create herbal cookies. Their results persuaded the scientific community that herbaceous plants are rich in antioxidant bioactives.
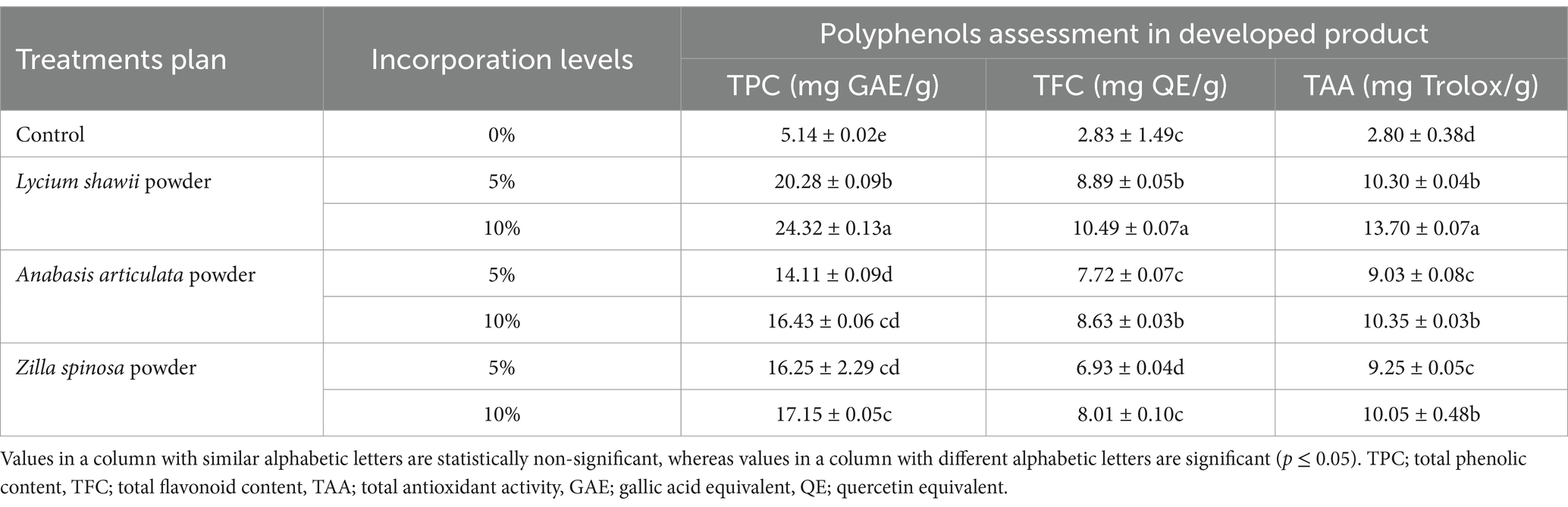
Table 7. Analysis of the total phenolic contents, total flavonoid contents, and antioxidant activity of developed products.
In Table 8, the findings from the sensory evaluation of biscuits prepared with 100% wheat flour and biscuits made with varying levels (5 and 10%) of dried leaf powder from three halophytes: Anabasis articulata, Lycium shawii, and Zilla spinosa, are presented. In accordance with the findings, color, flavor, and texture of biscuits containing 5% powdered Lycium shawii have the highest score, while flavor and texture of biscuits containing Anabasis articulata powder were least liked. The taste of biscuits prepared with 5% Zilla spinosa powder was maximally liked as it got 7.40 score. When overall acceptability was compared, it was observed that the biscuits containing 5% Lycium shawii powder get maximum acceptability with 7.20 score on hedonic scale, while biscuits with 10% Zilla spinosa powder were least acceptable. Utilization of halophytes powders in bakery items was found in the studies of Toumi et al. (2022), when common table salt was replaced with Salicornia ramosissima powder at different levels and observed effect on nutritional and sensory qualities of breads. Using medium to high amounts of Salicornia powder instead of sodium chloride, to make low-sodium doughs and breads that satisfy a healthier dietary sodium intake, while preserving technological superiority attributes has been demonstrated for the first time in an industrial application study. In a similar study, Lee et al. (2010) reported that 3% saltwort powder proved best combination to develop good quality bread with acceptable sensory properties. Development of nutritive biscuits by combination of lemon pomace powder was found acceptable, when level of replacement was up to 5%, as reported by Hussain et al. (2023), suggesting the enrichment of bakery products from powders of different plant-based materials, as source of bioactives. During enrichment of wheat flour with non-wheat flour, compromised sensory features of developed products are obvious, which occur due to interaction among the ingredients such as fat, fiber, protein, and other enzymes, affecting the rheological and baking properties of the dough. To cover these drawbacks, appropriate selection of level of replacement of these plant-based powders is very crucial to develop good quality acceptable bakery goods.
The present study, for the first time, explored and utilized three underutilized and unexplored halophyte plants for screening of nutritional and bioactive contents and for utilization at various levels to develop bakery product. Proximate analysis results showed that three halophyte plant powders were good source of ash and fiber and moderate source of fat and protein. Anabasis articulata powder was found to be significantly high (p ≤ 0.05) source of Mg, Zn, Cu, and Mn, while Zilla spinosa powder showed high Fe content. When ethanolic extracts of these three halophyte plant powders were explored and compared for bioactive contents, the Lycium shawii extracts showed significantly high (p ≤ 0.05) TPC, TFC, and TAA. These halophytic plants’ stronger antioxidant and antibacterial activities were discovered to be connected with high phenolic and flavonoid contents. The higher heights of TPC, TFC, and antioxidant capacity in Lycium shawii supported the significance of bioactive content as biologically energetic plant components. When these plant powders were utilized at various levels replacing wheat flour to develop biscuits, positive results were seen as nutritional and bioactive contents of the biscuits were found to be increased. However, a 5% level of replacement of these plant powders was found suitable in developing biscuits with high sensor scores nearly comparable to the control. The results may indicate that ethanolic extracts include pharmacologically potent substances that can be employed as a natural preservative for food or cosmetic products.
The current study found that the halophytes it looked at have a significant number of nutritious components and minerals. The current results showed that the studied dominating halophytes could be used as eco-friendly, green natural resources for bioactive chemicals or as alternatives for feed production. For the purpose of assessing the safety and viability of the investigated halophytes as alternative food and medicine for humans, further experimental research is advised. These activities were associated with high levels of total phenolics and flavonoids, which may suggest that the ethanolic extracts of three particular halophytes contain compounds with antioxidant properties that can be used as natural preservatives for food or cosmetic products. Further quantification and identification may also be helpful for the development of pharmaceutical foods and medicines.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
AH: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. AM: Conceptualization, Methodology, Writing – original draft. IK: Conceptualization, Data curation, Formal analysis, Writing – original draft. TK: Conceptualization, Data curation, Formal analysis, Writing – original draft. MoB: Conceptualization, Data curation, Formal analysis, Writing – review & editing. AS: Funding acquisition, Validation, Visualization, Writing – review & editing. ON: Funding acquisition, Validation, Visualization, Writing – review & editing. BE: Validation, Visualization, Writing – review & editing. SY: Formal analysis, Investigation, Visualization, Writing – original draft. RA: Data curation, Formal analysis, Visualization, Writing – original draft. HF: Formal analysis, Investigation, Visualization, Writing – original draft. NF: Supervision, Validation, Writing – review & editing. MuB: Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Researchers Supporting Project number. (RSPD2025R1057), King Saud University, Riyadh, Saudi Arabia.
The authors expressed their appreciation to the Researchers Supporting Project (RSPD2025R1057), King Saud University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AACC (2000). Approved methods committee. Approved methods of the American association of cereal chemists, vol. 1. St. Paul, MN: AACC.
Abdulsahib, K., Abdulkareem, A., Ban Jumaa, Q., and Hayder, S. (2016). Antiangiogenesis and antioxidant effect of Anabasis articulata stems extracts. Int. J. Pharma. Sci. Rev. Res. 41, 88–94.
Ahmed, F. A., Abdallah, N. M., Ezz, M. K., and El-Azab, M. M. (2017). Seasonal variations and identification of biologically active constituents of Lycium shawii plant roem. & shult. (family Solanaceae). Int. J. Innov. Sci. Eng. Technol. 4, 2348–7968.
Alam, M., Alam, M., Hakim, M., Huq, A. O., and Moktadir, S. G. (2014). Development of fiber enriched herbal biscuits: a preliminary study on sensory evaluation and chemical composition. Int. J. Nutr. Food Sci. 3, 246–250. doi: 10.11648/j.ijnfs.20140304.13
Alghanem, S., Al-hadithy, O. N., and Milad, M. (2018). Regular article antimicrobial activity and phytochemical characterization from Zilla spinosa. J. Med. Bot. 2, 24–27. doi: 10.25081/jmb.2018.v2.987
Al-Joufi, F. A., Jan, M., Zahoor, M., Nazir, N., Naz, S., Talha, M., et al. (2022). Anabasis articulata (Forssk.) Moq: A good source of phytochemicals with antibacterial, antioxidant, and antidiabetic potential. Molecules 27:3526. doi: 10.3390/molecules27113526
Al-Qahtani, H., Alfarhan, A. H., and Al-Othman, Z. M. (2020). Changes in chemical composition of Zilla spinosa Forssk. Medicinal plants grown in Saudi Arabia in response to spatial and seasonal variations. Saudi. J. Biol. Sci. 27, 2756–2769. doi: 10.1016/j.sjbs.2020.06.035
Bajaj, S., Urooj, A., and Prabhasankar, P. (2016). Antioxidative properties of mint (Mentha spicata L.) and its application in biscuits. Curr. Res. Nutr. Food Sci. J. 4, 209–216. doi: 10.12944/CRNFSJ.4.3.07
Belyagoubi-Benhammou, N., Belyagoubi, L., Gismondi, A., Di Marco, G., Canini, A., and Atik Bekkara, F. (2019). GC/MS analysis, and antioxidant and antimicrobial activities of alkaloids extracted by polar and apolar solvents from the stems of Anabasis articulata. Med. Chem. Res. 28, 754–767. doi: 10.1007/s00044-019-02332-6
Benhammou, N., Ghambaza, N., Benabdelkader, S., Atik-Bekkara, F., and Panovska, F. K. (2013). Phytochemicals and antioxidant properties of extracts from the root and stems of Anabasis articulata. Int. Food Res. J. 20, 2057–2063.
Benzineb, E., Kambouche, N., Hamiani, A., Bellahouel, S., Zitouni, H., and Toumi, H. (2019). Phenolics compounds and biological activity of leaves of Anabasis articulata, an Algerian medicinal plant. Int. J. Pharm. Res. Allied Sci. 8, 1–5.
Bertin, R. L., Maltez, H. F., de Gois, J. S., Borges, D. L., Borges, G. D. S. C., Gonzaga, L. V., et al. (2016). Mineral composition and bioaccessibility in Sarcocornia ambigua using ICP-MS. J. Food Comp. Anal. 47, 45–51. doi: 10.1016/j.jfca.2015.12.009
Beshah, F., Hunde, Y., Getachew, M., Bachheti, R. K., Husen, A., and Bachheti, A. (2020). Ethnopharmacological, phytochemistry and other potential applications of Dodonaea genus: a comprehensive review. Curr. Res. Biotechnol. 2, 103–119. doi: 10.1016/j.crbiot.2020.09.002
Bhol, S., Lanka, D., and Bosco, S. J. D. (2016). Quality characteristics and antioxidant properties of breads incorporated with pomegranate whole fruit bagasse. J. Food Sci. Technol. 53, 1717–1721. doi: 10.1007/s13197-015-2085-8
Bhusare, B. P., Nikalje, G. C., Sanap, R. R., and Kale, A. D. (2025). “Potential role of halophytes in environmental clean-up” in Physiology of halophytes. eds. N. M. Desai and G. C. Nikalje (Florida: Apple Academic Press), 417–429.
Bourgou, S., Ksouri, R., Bellila, A., Skandrani, I., Falleh, H., and Marzouk, B. (2008). Phenolic composition and biological activities of Tunisian Nigella sativa L. shoots and roots. Comptes Rendus Biol. 331, 48–55. doi: 10.1016/j.crvi.2007.11.001
Castañeda-Loaiza, V., Oliveira, M., Santos, T., Schüler, L., Lima, A. R., Gama, F., et al. (2020). Wild vs cultivated halophytes: nutritional and functional differences. Food Chem. 333:127536. doi: 10.1016/j.foodchem.2020.127536
Cheeseman, J. (2016). “Food security in the face of salinity, drought, climate change, and population growth” in Halophytes for food security in dry lands. ed. J. Cheeseman (Cambridge, MA: Academic Press), 111–123.
Christiansen, A. H., Lyra, D. A., and Jørgensen, H. (2021). Increasing the value of Salicornia bigelovii green biomass grown in a desert environment through biorefining. Ind. Crop. Prod. 160:113105. doi: 10.1016/j.indcrop.2020.113105
Custodio, L., Rodrigues, M. J., Pereira, C. G., Castañeda-Loaiza, V., Fernandes, E., Standing, D., et al. (2021). A review on Sarcocornia species: Ethnopharmacology, nutritional properties, phytochemistry, biological activities and propagation. Food Secur. 10:2778. doi: 10.3390/foods10112778
Duarte, B., Feijão, E., Pinto, M. V., Matos, A. R., Silva, A., Figueiredo, A., et al. (2022). Nutritional valuation and food safety of endemic mediterranean halophytes species cultivated in abandoned salt pans under a natural irrigation scheme. Estuar. Coast. Shelf Sci. 265:107733. doi: 10.1016/j.ecss.2021.107733
Öztürk, M., Altay, V., and Güvensen, A. (2019). Ecophysiology, abiotic stress responses and utilization of halophytes. Berlin: Springer, 235–257.
El-Amier, Y. A., Soufan, W., Almutairi, K. F., Zaghloul, N. S., and Abd-ElGawad, A. M. (2022). Proximate composition, bioactive compounds, and antioxidant potential of wild halophytes grown in coastal salt marsh habitats. Molecules 27:28. doi: 10.3390/molecules27010028
Falleh, H., Ksouri, R., Medini, F., Guyot, S., Abdelly, C., and Magné, C. (2011). Antioxidant activity and phenolic composition of the medicinal and edible halophyte Mesembryanthemum edule L. Ind. Crop. Prod. 34, 1066–1071. doi: 10.1016/j.indcrop.2011.03.018
Ghafar, M. A., Akram, N. A., Gul, B., and Pirasteh-Anosheh, H. A. D. I. (2022). Physio-biochemical analyses of selected halophytes from the saline regions of Pakistan and their potential for biosaline agriculture in arid environments. Pak. J. Bot. 54, 1697–1706. doi: 10.30848/PJB2022-5(29)
Gupta, V. (2025). “Metabolomic studies of halophytes under salinity stress” in Physiology of halophytes. ed. V. Gupta (Florida: Apple Academic Press), 1–37.
Hameed, A., Zafar, M., Ullah, R., Shahat, A. A., Ahmad, M., Cheema, S. I., et al. (2020). Systematic significance of pollen morphology and foliar epidermal anatomy of medicinal plants using SEM and LM techniques. Microsc. Res. Tech. 83, 1007–1022. doi: 10.1002/jemt.23493
He, M., He, C. Q., and Ding, N. Z. (2018). Abiotic stresses: general defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 9:1771. doi: 10.3389/fpls.2018.01771
Hulkko, L. S., Chaturvedi, T., and Thomsen, M. H. (2022). Extraction and quantification of chlorophylls, carotenoids, phenolic compounds, and vitamins from halophyte biomasses. Appl. Sci. 12:840. doi: 10.3390/app12020840
Hussain, A., Batool, A., Yaqub, S., Iqbal, A., Kauser, S., Arif, M. R., et al. (2024). Effects of spray drying and ultrasonic assisted extraction on the phytochemicals, antioxidant and antimicrobial activities of strawberry fruit. Food Chem. Adv. 5:100755. doi: 10.1016/j.focha.2024.100755
Hussain, A., Kausar, T., Din, A., Murtaza, M. A., Jamil, M. A., Noreen, S., et al. (2021). Determination of total phenolic, flavonoid, carotenoid, and mineral contents in peel, flesh, and seeds of pumpkin (Cucurbita maxima). J. Food Process. Preserv. 45:e15542. doi: 10.1111/jfpp.15542
Hussain, A., Kausar, T., Majeed, M. A., Aslam, J., Imtiaz, M., Haroon, H., et al. (2022a). Development of nutritional biscuits for children, rich in Fe and Zn, by incorporation of pumpkin (Cucurbita maxima) seeds powder; a healthy pharma food in current post COVID 19 period. Pure Appl. Biol. 12, 392–403. doi: 10.19045/bspab.2023.120042
Hussain, A., Kausar, T., Murtaza, M. A., Jamil, M. A., Iqbal, M. A., Majeed, M. A., et al. (2022b). Production, characterization, food application and biological study of powder of pumpkin (Cucurbita maxima) parts (peel, flesh and seeds). Pure Appl. Biol. 12, 48–60. doi: 10.19045/bspab.2023.120006
Hussain, A., Kausar, T., Sehar, S., Sarwar, A., Ashraf, A. H., Jamil, M. A., et al. (2022c). Determination of total phenolics, flavonoids, carotenoids, β-carotene and DPPH free radical scavenging activity of biscuits developed with different replacement levels of pumpkin (Cucurbita maxima) peel, flesh and seeds powders. Turk. J. Agric. Food Sci. Technol. 10, 1506–1514. doi: 10.24925/turjaf.v10i8.1506-1514.5129
Hussain, A., Kauser, T., Aslam, J., Quddoos, M. Y., Ali, A., Kauser, S., et al. (2023). Comparison of different techno-functional properties of raw lemon pomace and lemon pomace powder, and development of nutritional biscuits by incorporation of lemon pomace powder. Caraka Tani: J. Sust. Agric. 38, 176–192. doi: 10.20961/carakatani.v38i1.67769
Ibrahim, M. H., Chee Kong, Y., and Mohd Zain, N. A. (2017). Effect of cadmium and copper exposure on growth, secondary metabolites and antioxidant activity in the medicinal plant Sambung Nyawa (Gynura procumbens (Lour.) Merr). Molecules 22:1623. doi: 10.3390/molecules22101623
Ishtiyaq, S., Kumar, H., Varun, M., Ogunkunle, C. O., and Paul, M. S. (2021). “Role of secondary metabolites in salt and heavy metal stress mitigation by halophytic plants: an overview” in Handbook of bioremediation. ed. S. Ishtiyaq (Amsterdam, Netherlands: Elsevier), 307–327.
Jamshidi-Kia, F., Lorigooini, Z., and Amini-Khoei, H. (2017). Medicinal plants: past history and future perspective. J. Herb. Med. Pharmacol. 7, 1–7. doi: 10.15171/jhp.2018.01
Khaing, T. A. (2011). Evaluation of the antifungal and antioxidant activities of the leaf extract of Aloe vera (Aloe barbadensis miller). World Acad. Sci. Eng. Technol. 75, 610–612.
Khalil, M. M., Abouraya, M. A., Ibrahim, F. Y., AbdELrasool, E., and AbdElall, E. E. (2015). Chemical and technological studies on some medicinal plants. J. Food Dairy Sci. 6, 119–136. doi: 10.21608/jfds.2015.48537
Král, O., Javůrková, Z., Dordevic, D., Pospiech, M., Jančíková, S., Fursova, K., et al. (2021). Biscuits polyphenol content fortification through herbs and grape seed flour addition. PRO 9:1455. doi: 10.3390/pr9081455
Lee, Y. S., Hong, G. J., Kim, W. M., and Shin, M. K. (2010). Quality characteristics of bread with added saltwort powder (Salicornia herbacea L.). J. East Asian Soc. Diet. Life. 20, 706–712.
Lokhande, V. H. (2025). “Halophytes: Prospectives and perspectives for sustainable, eco-friendly environmental and agricultural development” in Physiology of halophytes. ed. V. H. Lokhande (Florida: Apple Academic Press), 261–288.
Lopes, A., Rodrigues, M. J., Pereira, C., Oliveira, M., Barreira, L., Varela, J., et al. (2016). Natural products from extreme marine environments: searching for potential industrial uses within extremophile plants. Ind. Crop. Prod. 94, 299–307. doi: 10.1016/j.indcrop.2016.08.040
Mayouf, R., and Arbouche, F. (2014). Chemical composition and relative feed value of three Mediterranean fodder shrubs. Afr. J. of Agric. Res. 9, 746–749. doi: 10.5897/AJAR2013.7805
Mo, S., Biao, A., Wang, Z., Lin, S., Yang, T., Pan, L., et al. (2022). Spatio transcriptome uncover novel insight into the Lycium ruthenicum seedling tolerant to salt stress. Ind. Crop. Prod. 177:114502. doi: 10.1016/j.indcrop.2021.114502
Mohammed, H. A., Ali, H. M., Qureshi, K. A., Alsharidah, M., Kandil, Y. I., Said, R., et al. (2021). Comparative phytochemical profile and biological activity of four major medicinal halophytes from Qassim flora. Plan. Theory 10:2208. doi: 10.3390/plants10102208
Mohammed, A. E., Ameen, F., Aabed, K., Suliman, R. S., Alghamdi, S. S., Safhi, F. A., et al. (2022). In-silico predicting as a tool to develop plant-based biomedicines and nanoparticles: Lycium shawii metabolites. Biomed. Pharmacother. 150:113008. doi: 10.1016/j.biopha.2022.113008
Nazir, N., Khalil, A. A. K., Nisar, M., Zahoor, M., and Ahmad, S. (2020a). HPLC-UV characterization, anticholinesterase, and free radical-scavenging activities of Rosa moschata Herrm. Leaves and fruits methanolic extracts. Braz. J. Bot. 43, 523–530. doi: 10.1007/s40415-020-00635-2
Nazir, N., Zahoor, M., Nisar, M., Karim, N., Latif, A., Ahmad, S., et al. (2020b). Evaluation of neuroprotective and anti-amnesic effects of Elaeagnus umbellata Thunb. On scopolamine-induced memory impairment in mice. BMC Complemt. Med. Ther. 20, 143–117. doi: 10.1186/s12906-020-02942-3
Nazir, N., Zahoor, M., Nisar, M., Khan, I., Karim, N., Abdel-Halim, H., et al. (2018). Phytochemical analysis and antidiabetic potential of Elaeagnus umbellata (Thunb.) in streptozotocin-induced diabetic rats: pharmacological and computational approach. BMC Complement. Alt. Med. 18, 1–16. doi: 10.1186/s12906-018-2381-8
Nazir, N., Zahoor, M., Nisar, M., Khan, I., Ullah, R., and Alotaibi, A. (2021). Antioxidants isolated from Elaeagnus umbellata (thunb.) protect against bacterial infections and diabetes in streptozotocin-induced diabetic rat model. Molecules 26:4464. doi: 10.3390/molecules26154464
Ngxabi, S., Jimoh, M. O., Sogoni, A., Laubscher, C. P., and Kambizi, L. (2025). Nutraceutical, agricultural, and economic potential of Trachyandra ciliata (wild cabbage), an underutilized halophyte from the Western Cape Province, South Africa. Food Sec. Nutr. 1, 273–290.
Ordonez, A. A. L., Gomez, J. D., and Vattuone, M. A. (2006). Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 97, 452–458. doi: 10.1016/j.foodchem.2005.05.024
Oron, G., Appelbaum, S., and Guy, O. (2023). Reuse of brine from inland desalination plants with duckweed, fish and halophytes toward increased food production and improved environmental control. Desalination 549:116317. doi: 10.1016/j.desal.2022.116317
Oueslati, S., Trabelsi, N., Boulaaba, M., Legault, J., Abdelly, C., and Ksouri, R. (2012). Evaluation of antioxidant activities of the edible and medicinal Suaeda species and related phenolic compounds. Ind. Crop. Prod. 36, 513–518. doi: 10.1016/j.indcrop.2011.10.006
Patel, S. B., and Ghane, S. G. (2021). Phyto-constituents profiling of Luffa echinata and in vitro assessment of antioxidant, anti-diabetic, anticancer and anti-acetylcholine esterase activities. Saudi J. Biol. Sci. 28, 3835–3846. doi: 10.1016/j.sjbs.2021.03.050
Pourabdollah-Kaleybar, V., Pourabdollah-Kaleybar, P., Eskandani, M., and Nazemiyeh, H. (2025). Toxicity of bioactive compounds from Halocnemum strobilaceum against A549 lung cancer cells. Toxicon 253:108186. doi: 10.1016/j.toxicon.2024.108186
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237. doi: 10.1016/S0891-5849(98)00315-3
Reddy, M. P., Shah, M. T., and Patolia, J. S. (2008). Salvadora persica, a potential species for industrial oil production in semiarid saline and alkali soils. Ind. Crop. Prod. 28, 273–278. doi: 10.1016/j.indcrop.2008.03.001
Rehman, N. U., Alsabahi, J. N., Bakht, N., Khan, A., and Al-Harrasi, A. (2020). Chemical constituents and biological activities of the oil from Lycium shawii STEM. Chem. Nat. Comp. 56, 1156–1158. doi: 10.1007/s10600-020-03254-1
Rehman, N. U., Hussain, H., Al-Riyami, S. A., Csuk, R., Khiat, M., Abbas, G., et al. (2016). Lyciumaside and lyciumate: a new diacylglycoside and sesquiterpene lactone from Lycium shawii. Helv. Chim. Acta 99, 632–635. doi: 10.1002/hlca.201600066
Rehman, N. U., Hussain, H., Al-Riyami, S. A., Green, I., and Al-Harrasi, A. (2018). Chemical constituents isolated from Lycium shawii and their chemotaxonomic significance. Rec. Nat. Prod. 12, 380–384. doi: 10.25135/rnp.34.17.09.160
Rehman, N. U., Khan, A., Al-Harrasi, A., Khiat, M., Hussain, H., Wadood, A., et al. (2019). Natural urease inhibitors from Aloe vera resin and Lycium shawii and their structural-activity relationship and molecular docking study. Bioorg. Chem. 88:102955. doi: 10.1016/j.bioorg.2019.102955
Rodrigues, M. J., Monteiro, I., Castañeda-Loaiza, V., Placines, C., Oliveira, M. C., Reis, C., et al. (2020). Growth performance, in vitro antioxidant properties and chemical composition of the halophyte Limonium algarvense Erben are strongly influenced by the irrigation salinity. Ind. Crop. Prod. 143:111930. doi: 10.1016/j.indcrop.2019.111930
Rodrigues, M. J., Pereira, C. A., Oliveira, M., Neng, N. R., Nogueira, J. M., Zengin, G., et al. (2018). Sea rose (Armeria pungens (link) Hoffmanns. & link) as a potential source of innovative industrial products for anti-ageing applications. Ind. Crop. Prod. 121, 250–257. doi: 10.1016/j.indcrop.2018.05.018
Rodrigues, M. J., Soszynski, A., Martins, A., Rauter, A. P., Neng, N. R., Nogueira, J. M., et al. (2015). Unravelling the antioxidant potential and the phenolic composition of different anatomical organs of the marine halophyte Limonium algarvense. Ind. Crop. Prod. 77, 315–322. doi: 10.1016/j.indcrop.2015.08.061
Rowayshed, G., Sharaf, A. M., El-Faham, S. Y., Ashour, M., and Zaky, A. A. (2015). Utilization of potato peels extract as source of phytochemicals in biscuits. J. Basic Appl. Res. Int. 8, 190–201.
Saatchi, A., Kadivar, M., Soleimanian Zad, S., and Abaee, M. S. (2014). Application of some antifungal and antioxidant compounds extracted from some herbs to be used in cakes as bio preservatives. J. Agric. Sci. Technol. 16, 561–568.
Saleem, H., Khurshid, U., Sarfraz, M., Tousif, M. I., Alamri, A., Anwar, S., et al. (2021). A comprehensive phytochemical, biological, toxicological and molecular docking evaluation of Suaeda fruticosa (L.) Forssk.: an edible halophyte medicinal plant. Food Chem. Toxicol. 154:112348. doi: 10.1016/j.fct.2021.112348
Steel, R., Torrie, J., and Dickey, D. (1997). Principles and procedures of statistics A biometrical approach. 3rd Edn. New York, USA: McGraw Hill Book Company Inc., 334–381.
Suleiman, M. H., and Ateeg, A. A. (2020). Antimicrobial and antioxidant activities of different extracts from different parts of Zilla spinosa (L.) prantl. Evidence-based complement. Alt. Med 2020:433. doi: 10.1155/2020/6690433
Tariq, A., Adnan, M., Iqbal, A., Sadia, S., Fan, Y., Nazar, A., et al. (2018). Ethnopharmacology and toxicology of Pakistani medicinal plants used to treat gynecological complaints and sexually transmitted infections. S. Afr. J. Bot. 114, 132–149. doi: 10.1016/j.sajb.2017.11.004
Tesby, M. R. L., Neveen, F. A., Neveen, A., and Nashwa, M. Y. (2018). Effect of thyme, cumin and anise on the formation of acrylamide in some bakery products. Alexandria J. Agric. Sci. 63, 183–192. doi: 10.21608/alexja.2018.81839
Toumi, O., Conte, P., da Silva, A. M. G. M., Barroca, M. J., and Fadda, C. (2022). Use of response surface methodology to investigate the effect of sodium chloride substitution with Salicornia ramosissima powder in common wheat dough and bread. J. Funct. Food. 99:105349. doi: 10.1016/j.jff.2022.105349
Tsikritzi, R., Moynihan, P. J., Gosney, M. A., Allen, V. J., and Methven, L. (2014). The effect of macro-and micro-nutrient fortification of biscuits on their sensory properties and on hedonic liking of older people. J. Sci. Food Agric. 94, 2040–2048. doi: 10.1002/jsfa.6522
Ullah, R., Abdelaaty, A. S., Alqahtani, A. S., Almarfadi, O. M., Alharbi, M. S. M., Ahamad, S. R., et al. (2020). Anti-inflammatory, antipyretic and analgesic potential of Zilla spinosa in animal model. Ind. J. Animal Res. 54, 889–894. doi: 10.18805/ijar.B-1183
Ullah, R., Alsaid, M. S., Shahat, A. A., Naser, A. A., Al-Mishari, A. A., Adnan, M., et al. (2018). Antioxidant and hepatoprotective effects of methanolic extracts of Zilla spinosa and Hammada elegans against carbon tetrachloride induced hepatotoxicity in rats. Open Chem. 16, 133–140. doi: 10.1515/chem-2018-0021
Wang, L., Li, W., Ma, L., Chen, J., Lü, H., and Jian, T. (2016). Salt stress changes chemical composition in Limonium bicolor (bag.) Kuntze, a medicinal halophytic plant. Ind. Crop. Prod. 84, 248–253. doi: 10.1016/j.indcrop.2016.01.050
Yadav, K. C., Bhattarai, S., Shiwakoti, L. D., Paudel, S., Subedi, M., Pant, B. R., et al. (2022). Sensorial and chemical analysis of biscuits prepared by incorporating Moringa flower powder and leaf powder. Int. J. Food Prop. 25, 894–906. doi: 10.1080/10942912.2022.2069807
Keywords: halophytes, Anabasis articulata , Lycium shawii , Zilla spinosa , phenolics, flavonoids, antioxidants
Citation: Hussain A, Mouhaddach A, Khalid I, Kausar T, Bouhrim M, Shahat AA, Noman OM, Eto B, Yaqub S, Arshad R, Farooq H, Firdous N and Bilal M (2025) Nutritional, bioactive, and antimicrobial analysis of powders and ethanolic extracts of three important halophyte plants (Anabasis articulata, Lycium shawii, and Zilla spinosa), and their application in bakery product. Front. Sustain. Food Syst. 9:1509423. doi: 10.3389/fsufs.2025.1509423
Received: 10 October 2024; Accepted: 03 February 2025;
Published: 19 February 2025.
Edited by:
Kathleen L. Hefferon, Cornell University, United StatesReviewed by:
Aymen Souid, Institute of Agricultural Biology and Biotechnology, National Research Council (CNR), ItalyCopyright © 2025 Hussain, Mouhaddach, Khalid, Kausar, Bouhrim, Shahat, Noman, Eto, Yaqub, Arshad, Farooq, Firdous and Bilal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Bouhrim, bS5ib3VocmltQHVzbXMubWE=; Aziz Mouhaddach, bW91aGFkZGFjaC5hQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.