- 1Effective Basic Services (eBASE) Africa, Bamenda, Cameroon
- 2Department of Developmental Studies, ICT University, Yaounde, Cameroon
- 3Department of Economics Policy Analysis, Faculty of Economics and Management, University of Dschang, Dschang, Cameroon
- 4Department of Economics, The University of Bamenda, Bambili, Cameroon
- 5Department of Special Needs Education University of Yaounde, Yaounde, Cameroon
- 6Department of Health Economics Policy and Management, Catholic University of Cameroon, Bamenda, Cameroon
Objective: To establish the current body of evidence regarding the safety of cassava and cassava-based products for human consumption and to identify the most effective preparation methods to reduce toxicity.
Methods: The main databases searched were CABI, PubMed, CINAHL, EMBASE, the Cochrane Database of Systematic Reviews, and the JBI Evidence Synthesis, Science Direct, Agricola, AGRIS, Web of Science, Scopus and Proquest. We limited our search to studies published from 1980 to date. Two independent reviewers were used to review identified titles and abstracts for data extraction that was pooled in a statistical meta-analysis for analyses. We reviewed full papers of relevant abstracts. This data was collected from multiple studies examining hydrogen cyanide (HCN) levels in traditionally soaked cassava chips, cassava biscuits, gari, cassava flour, cassava roots, and cassava paste/dough. HCN content was measured and compared across these products, with results expressed in mg/kg. HCN values in these products were presented on graphs, and mean scores were calculated and compared against FAO/WHO’s maximum recommended dosage of 10 mg/kg.
Results: The study found significant variations in HCN content across cassava and different cassava-based products and by regions. The recommended FAO/WHO standard is 10 mg/kg, and that the high variation in the samples was due largely to the method of processing the product. Traditionally soaked cassava chips exhibited the highest average HCN levels (46.6 mg/kg). Cassava biscuits showed lower HCN levels, averaging 12 mg/kg. Gari products had an average HCN content of 5.7 mg/kg, while cassava flour exhibited significant variation, with an average of 71.1 mg/kg. Cassava roots and paste/dough also showed high HCN levels, with averages of 60.98 mg/kg and 38.1 mg/kg, respectively. Gari, which is the most popular form of cassava based products is rich in roughage and has a safe level cyanide, and is a potential product for international markets.
Conclusion: The findings highlight the potentials of cassava based products for international markets and the critical need for improved cassava processing techniques to reduce HCN levels and mitigate the risk of cyanide poisoning. Effective methods such as fermentation, drying, and combining cassava with other ingredients can significantly lower HCN content. Standardized processing practices, regulatory standards, and community education are essential to ensure the safety of cassava-based products.
Introduction
Cassava is a good source of carbohydrates, calories, calcium, vitamins B and C, and other essential minerals. Cassava and cassava-based products are also a major source of income for rural women, small scale farmers, and small businesses in most of sub-Saharan Africa. Given it is consumed by many people in the tropics and subtropics, supports the livelihood of over 500 million Africans annually (Immanuel et al., 2024), therefore, it plays a role in food security and fight against poverty in Africa and other parts of the world. Africa and Latin America. Despite its benefits, cassava can be contaminated while in the soil, during processing, or storage, rendering them harmful for human consumption. Examples of such contaminants include; HCN, aflatoxins, mercury, arsenic, cadmium, and lead. It is therefore important to explore the food safety of cassava and cassava-based products and the most effective methods to reduce the toxicity of cassava during processing.
Inclusion criteria
This review considered studies that reported on ingested toxins related to the consumption of cassava and cassava-based products in several African countries. The review also included studies that actually measured the HCN content of various cassava-based products in different areas in Africa.
Methods
The main databases searched were CABI, PubMed, CINAHL, EMBASE, the Cochrane Database of Systematic Reviews, and the JBI Evidence Synthesis, Science Direct, Agricola, AGRIS, Web of Science, Scopus and Proquest. We limited our search to studies published from 1980. We reviewed full papers of relevant abstracts. Two reviewers independently reviewed, identified titles and abstracts and data was extracted by these reviewers and pooled in a spreadsheet for analysis. Values of HCN in each type of cassava-based product from the different studies were reported and the average values computed and compared against the FAO/WHO recommend maximum dosage of 10 mg/kg.
Background
Cassava (Manihot Esculenta Crantz) is an edible root that is very rich in starch, thereby making it a good source of carbohydrates and calories. Also, it is rich in calcium, vitamins B and C, and other essential minerals. The composition of these components differs across a variety of the harvested crop, soil conditions, climate, and other environmental factors (Adewusi et al., 2009). The cassava plant is drought resistant and has a high yield even in poor soils without fertilizer. It is commonly grown in Africa as its high drought tolerance enables it to survive for the 4–6 months of the dry season, characteristic in most parts of Africa where it is mostly grown (Hahn and Janet, 1985). Cassava roots can survive in the soil for up to 3 years (Nhassico et al., 2008). It is consumed by millions of people in the tropics and subtropics (Adamolekun, 2011). Given its convenient underground growth, it provides food security during conflicts as it cannot be easily destroyed (IITA, 2020). According to the International Institute of Tropical Agriculture (IITA), cassava supports the livelihood of over 500 million Africans annually. In 2017, over 291 million tons of cassava was produced globally with Africa accounting for approximately 174 million tons (60%) of this output (IITA, 2020). In 2021, Nigeria remained the world’s largest producer of cassava, with an estimated production of 63 million metric tonnes, accounting for 20% of global cassava output. This highlights Nigeria’s continued dominance in cassava cultivation globally (Apeh et al., 2023; Ibrahim et al., 2023; Otekunrin, 2024) Nigeria is the world’s leading cassava producer with over 20% of the 2017 total output and an estimated export value of USD 1.25 million from a 5.7 percent export of its total production (Otekunrin, 2019). The Gross Production Value (Current USD) of cassava produced in Africa was USD 19.8 billion in 2016, down from USD 30.2 billion in 2013 (FAOSTAT, 2020). This indicates that with well-developed value chains, the continent stands to gain a lot from the cultivation and processing of cassava.
Mostly eaten boiled, cassava is also processed into a good number of products. For example, in Cameroon, some popular cassava products include gari, fufu, water fufu, kum kum and bobolo; in Nigeria, they include is eba, akpu, alibo and gari; in Côte d’Ivoire, they include attieke, semolina or gari and cassava dough; while in Benin, tapioca or gari is the most popular cassava-based product. Each of these products has specific ways of processing and these methods help to remove the toxic cyanogens from the cassava, hence rendering it safe for consumption (Adamolekun, 2011). Cassava and cassava-based products have the potential to play a role in food security, fight against poverty, and food economics in several LMICs especially in Africa and Latin America both now and in the future. However, not enough scientific attention has been paid to the benefits and harms of the product. In addition to carbohydrates, cassava and cassava-based products are rich in fibers and micronutrients such as vitamin A and iron. Anecdotal evidence suggests doctors recommend the consumption of soaked gari to ease digestive problems. These, therefore, make cassava a possible food with function claim.
Notwithstanding these many benefits, there is a remarkable deficiency of protein in cassava, principally the essential sulfur amino acid methionine (Adegbola, 1977). Cassava contains cyanogenic glycosides linamarin [2-(/3-D-glucopyranosyl) isobutyronitrile] and lotaustralin (methyllinamarin), which on hydrolysis by its endogenous enzyme linamarase (EC 3.2.1.21) yield hydrogen cyanide (HCN) (Adewusi et al., 2009; Conn, 1979). Tropical Africa has a warm and humid climate that provides favorable conditions for the successive spread and growth of aflatoxins and numerous microorganisms in various foodstuffs including cassava (Ferraro et al., 2016). The formation of aflatoxins is a function of several factors which are pH, moisture, storage duration, types of foodstuffs, level of initial contamination by aflatoxin-producing fungi, processing practices, and storage facilities (Odoemelam and Osu, 2009). Poor hygienic conditions and poor processing techniques can result in high levels of aflatoxins, fungi, or some microorganisms in the final product, all of which are detrimental to human health. The aflatoxins producing molds include: Aspergillus Flavus, Aspergillus Parasiticus, and Aspergillus Nomius which are known to produce aflatoxin B1 and B2.
There are two genotypes of cassava, the bitter and sweet genotype. The bitter genotype contains more hydrogen cyanide (HCN) and consuming poorly processed products from this genotype leads to high cyanide ingestion which is dangerous (Adamolekun, 2011). Cyanide poisoning and other nutritional defects resulting from consuming poorly processed cassava products have been reported and associated with a neurological disorder called konzo in several African countries (Adamolekun, 2011; Cock, 1982). The Food and Agriculture Organization (FAO), World Health Organization (WHO), and the Codex Alimentarus recommend a maximum of 10 mg/kg of HCN in food. Cassava may contain other heavy metals such as: mercury (Hg), lead (Pb), arsenic (As), and cadmium (Cd). The amount of these metals varies in different cassava-based products and is a function of the soil type and other climatic conditions. Other heavy metals like zinc, aluminum and copper which are trace elements and needed by the body, when consumed in food excessively pose serious health problems. For example, high concentrations of lead in the body can permanently truncate brain development and damage the nervous system, especially in children (Buchanan et al., 2011). Also, exposure to high levels of metallic, inorganic, or organic mercury can permanently damage the brain, kidneys, and a developing fetus (Azimi and Moghaddam, 2013). Mercury has been found in air, surface water, plants, and mostly around soils in gold mining watersheds (Adjorlolo-Gasokpoh et al., 2012; Ikingura et al., 2006; Campbell et al., 2003; Taylor et al., 2005). It is important to explore the food safety of cassava and CBP as this plays an important role both for the economy and food security of several LMIC (Azimi and Moghaddam, 2013) countries and could be a potential export product. Plants around mining areas tend to absorb mercury and their leaves absorb the mercury found in the atmosphere. Elevated levels of this metal in food can pose serious health issues to man when consumed (Nyanza et al., 2014).
This review synthesized available information on the current safety of cassava and cassava-based products, and identified the most effective methods of preparation to reduce toxicity. A preliminary search of CABI, PROSPERO, EMBASE, CINAHL, MEDLINE, Agric Science, Proquest, Scopus, Web of Science, the Cochrane Database of Systematic Reviews, and the JBI Database of Systematic Reviews and Implementation Reports was conducted, and no current or underway systematic reviews on the topic were identified. The overall objective of this review was to establish the current body of evidence regarding the safety of cassava and cassava-based products for human consumption and to identify the most effective preparation methods to reduce toxicity.
Review questions
This review sought to answer the following questions:
1) How safe are cassava and cassava-based products (CBP) for human consumption?
2) What methods of processing cassava and CBP are most effective in reducing toxicity?
Inclusion criteria
Participants
This review considered studies that deal with participants of all ages from cassava and CBP consuming areas globally. This included households consuming CBP, and farmers, both small and large scale, involved in the value and supply chains of CBP.
Exposure of interest
Harmful chemicals of interest
The review considered studies that investigated the levels of heavy metals in cassava and CPB. We considered those heavy metals that are amongst the WHO 10 elements of concern including arsenic, mercury, lead, and cadmium. These heavy metals typically indicate food contamination, and elevated levels of these metals in food can pose serious harm to the human body. However, WHO and other Food and Drug agencies have provided reference values for these metals that are considered safe for human consumption. These heavy metals can be found in food sources including cassava, with the amount depending on where they are cultivated and on the method of processing and preservation.
We also looked at studies that reported on the exposure of Hydrogen Cyanide to cassava and CBP. Examples of such studies included (Conn, 1979; Dekkers et al., 2019; FAO, 2013; FAO/WHO, 2019; FAOSTAT, 2020; Ferraro et al., 2016; Hahn and Janet, 1985; IARC, 2025). Cyanide poisoning occurs when the cyanide levels exceed the limits an individual can detoxify (Hendry-Hofer et al., 2019). Consuming inadequately prepared cassava can lead to high HCN ingestion and this has been associated with the konzo neurological disorder in several African countries (Banea et al., 2011). HCN was our primary toxin of interest whose content in cassava and cassava-based products varies based on the area of cultivation, type of cassava, method of transformation and post-production handling (Adewusi and Akindahunsi, 1994).
Toxins
Studies reporting on the presence of aflatoxins in cassava and CBP were also included (Ferraro et al., 2016; Odoemelam and Osu, 2009). Consumption of food with high levels of aflatoxins is detrimental to health especially those from aflatoxin-producing molds such as: Aspergillus flavus Link, Aspergillus parasiticus Speare, and Aspergillus nomius Kurtzmanare (Odoemelam and Osu, 2009). Several factors can influence the presence of aflatoxins in cassava and CBP including: moisture, pH, storage duration, level of initial contamination by the aflatoxin-producing fungi, processing practices and storage facilities (Lancaster et al., 1982).
Pathogens
We also considered studies that reported on the different pathogens or microbial contents of cassava and CBP. Microbial contaminated foods could easily act as a source of food-borne diseases, therefore exposure of pathogens to foods should be of importance. Improper hygienic conditions in handling cassava during processing could serve as a vehicle for food-borne pathogens including bacteria, molds, and fungi.
Outcomes
This review considered studies that included the following outcomes:
Primary outcome
Safety
Studies that reported on chemicals of interest, toxins, or pathogens. We compared this to the standard safe level of the various toxic elements and the recommendation of average daily consumption. The safety levels were measured by comparing the average reported values for safety from the different studies with the values from recognized authorities like WHO and/or the FDA, Food and Drug Regulation (FDR), EU, FAO, Codex Alimentarius, International Union of Nutritional Science (IUNS),
Secondary outcomes
Mortality
Mortality is defined in this study as the number of recorded deaths associated with the consumption of cassava or a cassava-based product (CBP). These studies had to report the direct cause of death is from the consumption of Cassava or CPB. This outcome was measured by calculating the weighted mean from deaths from cassava consumption.
Morbidity
Morbidity is defined in this study the risk of contamination from consumption of cassava or CBP and the diseases associated with the consumption of cassava and CBP. This outcome was measured by weighted means of diseases related to the consumption of cassava or CBP.
Types of studies
This review included randomized control trials, case–control studies, cohort studies, cross-sectional studies, case reports, and case series that report risks or benefits of CBPs to humans. Only studies conducted in humans were considered. Studies were not excluded based on the language of publication. We used task-exchange platforms to get articles outside French and English translated. We included studies published from 1980 to date. This is because cassava and cassava products have been an important source of food in the tropics, and it was estimated that by the 1980s it was a staple diet to more than 500 million people (Lancaster et al., 1982).
Methods
The proposed systematic review was conducted following the Joanna Briggs Institute methodology for systematic reviews of etiology and risk (Moola et al., 2017) as well as the COSMO-E (Conducting Systematic Reviews and Meta-Analyses of Observational Studies of Etiology) (Dekkers, Vandenbroucke, Cevallos, Renehan, and Altman, COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology).
Search strategy
The search strategy aimed to locate both published and unpublished studies. An initial limited search of PUBMED, CABI and EMBASE was undertaken to identify articles on the topic. The text words contained in the titles and abstracts of relevant articles, and the index terms used to describe the articles were used to develop a full search strategy for PubMed, MEDLINE, EMBASE, and Web of Science (see Appendix 1). The search strategy, including all identified keywords and index terms, was adapted for each included information source. The reference list of all studies selected for critical appraisal was screened for additional studies.
Study selection
Following the search, all identified citations were collated and uploaded into Zotero 5.0, and duplicates removed. Titles and abstracts were then screened by two independent reviewers for assessment against the inclusion criteria for the review. Potentially relevant studies were retrieved in full and their citation details imported into the Joanna Briggs Institute System for the Unified Management, Assessment, and Review of Information (JBI SUMARI) (Munn et al., 2019). The full text of selected citations were assessed in detail against the inclusion criteria by two independent reviewers. Reasons for the exclusion of full text studies that do not meet the inclusion criteria were recorded and reported in the PRISMA flow diagram. Any disagreements that arose between the reviewers at each stage of the study selection process were resolved through discussion, or with a third reviewer. The results of the search reported in full in the final systematic review and presented in a Preferred Reporting Items for Systematic Reviews and Meta-analyses (Moher et al., 2010). The PRISMA flow chart is presented on Figure A2.
Data extraction
The data extracted included specific details about the exposure of interest including different exposure categories if applicable, populations, study methods and outcomes, or dependent variables of significance to the review question and specific objectives. Confounding factors reported by the included studies were extracted to provide context to findings. Any disagreements that arose between the reviewers were resolved through discussion, or with a third reviewer. Authors of papers were contacted to request missing or additional data where required.
Data synthesis
The values of the toxins of interest (in particular, HCN) present in different CBPs from the different studies were reported on tables and graphs for easy visualization. Average values were calculated and compared to the recommended thresholds from health and food safety agencies in order to ascertain the safety levels of these products for human consumption.
Findings
The review found significant variations in hydrocyanic acid (HCN) content across different cassava-based products and regions, with critical implications for public health, food safety, and processing practices.
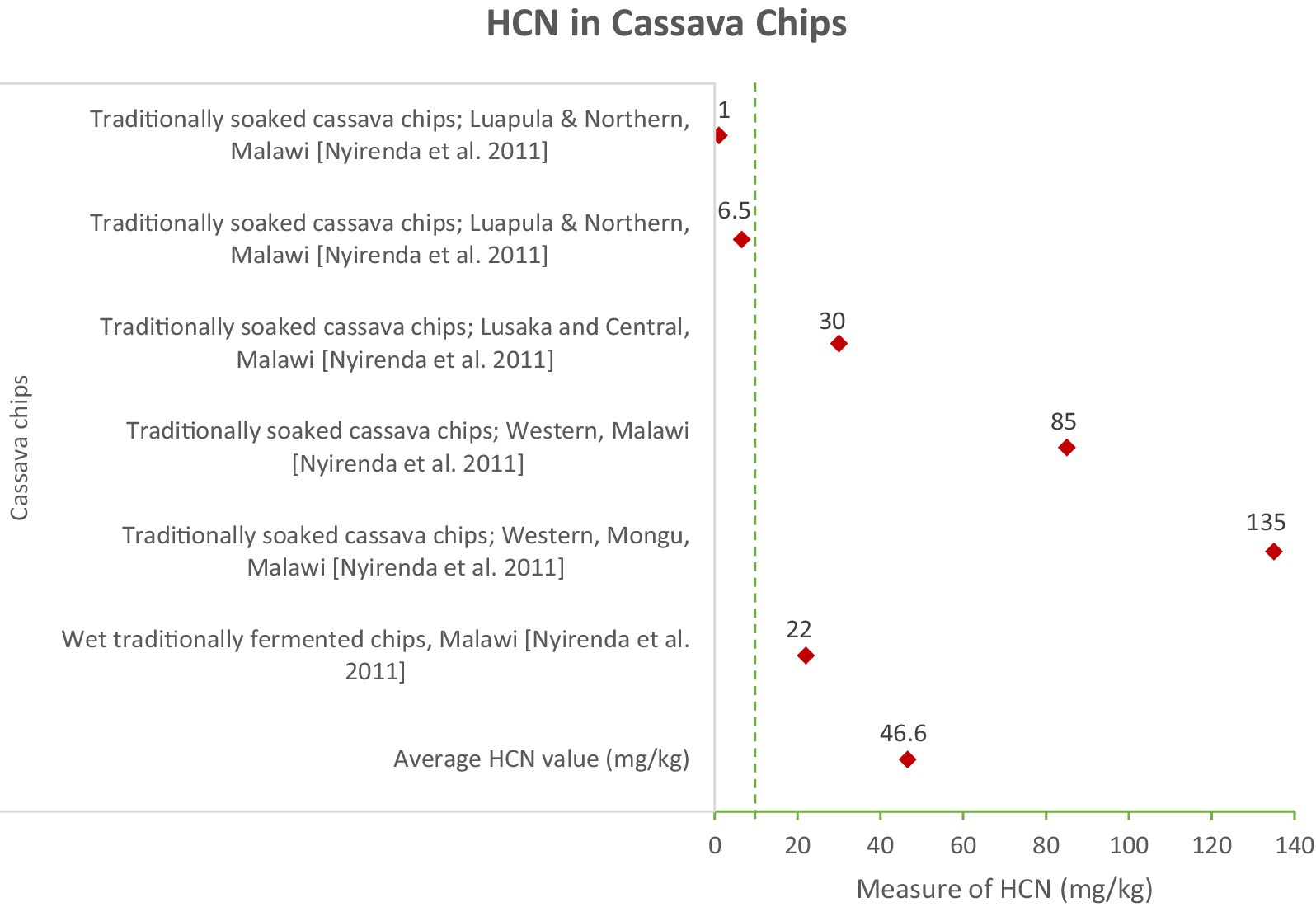
Figure 1 shows that traditionally soaked cassava chips exhibited wide variations, with an average of 46.6 mg/kg. The highest recorded value was 200 mg/kg in Western Mongu, Malawi, while the lowest was 10 mg/kg in Luapula, Lusaka, Mongu, Centaral, Northern and Western Malawi (Nyirenda et al., 2011). These high HCN levels indicate that traditionally soaked cassava chips pose a significant risk of cyanide poisoning if not properly processed. The wide range of HCN values suggests inconsistencies in processing methods across regions, highlighting the need for standardized and improved detoxification practices to ensure safety.
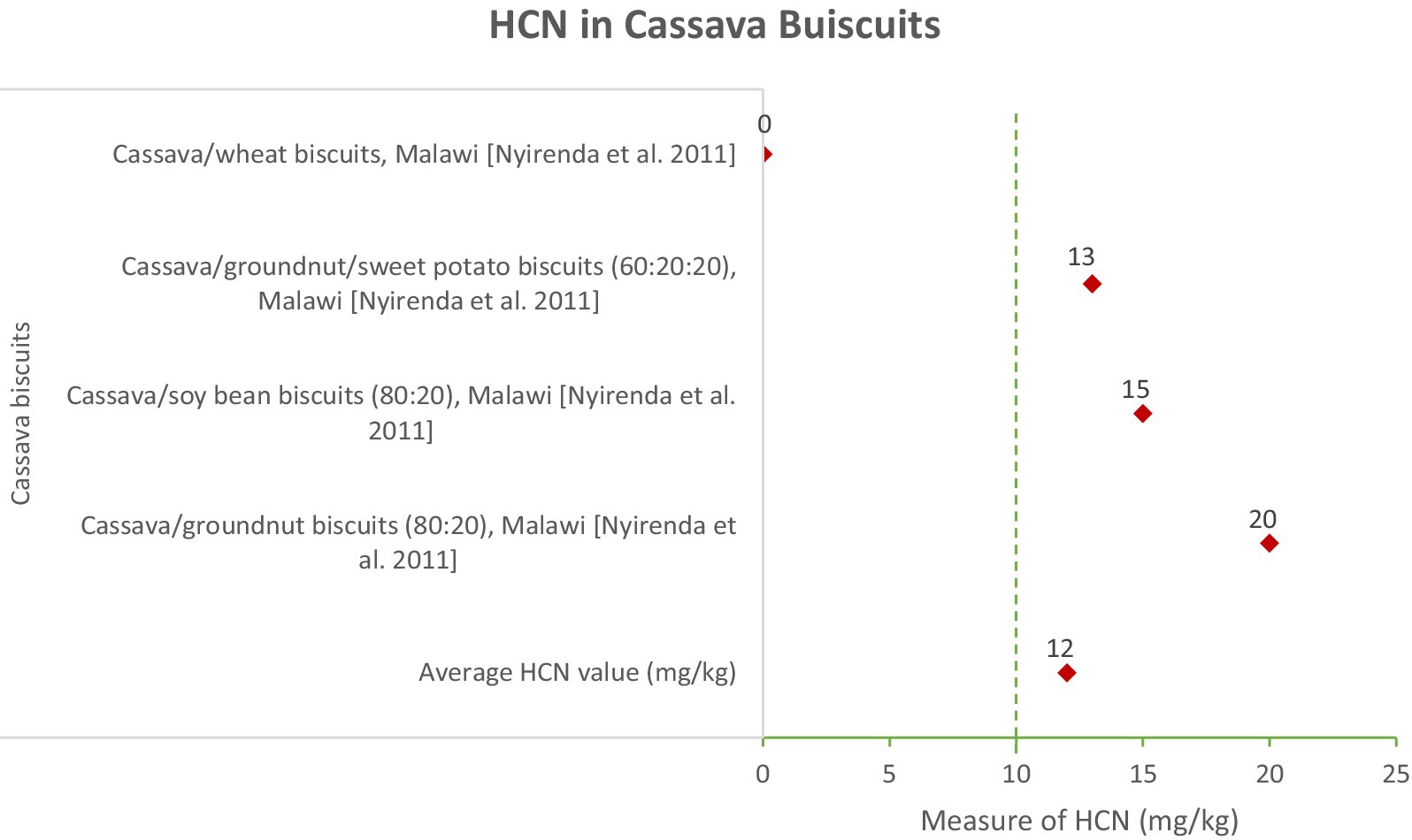
In Figure 2, the cassava biscuits showed relatively lower HCN levels, averaging 14.3 mg/kg. The highest level recorded was 23 mg/kg in cassava/groundnut biscuits (Nyirenda et al., 2011). The lower HCN content in cassava biscuits suggests that the combination of cassava with other ingredients such as wheat, groundnut, and soybean effectively reduces cyanide levels. This indicates that diversifying cassava products and combining them with other ingredients can be a safer approach to consumption.
Gari products had an average HCN content of 5.7 mg/kg, with a range from 0 mg/kg (white gari and palm oil gari) to 23.9 mg/kg (dried semolina) (Onabolu et al., 2001; Adewusi et al., 2009; Kouamé et al., 2020). The relatively low HCN levels in gari, particularly in white and palm oil gari, demonstrate the effectiveness of fermentation and thorough drying in reducing cyanide content. These traditional processing methods should be promoted and standardized to ensure the safety of gari products.
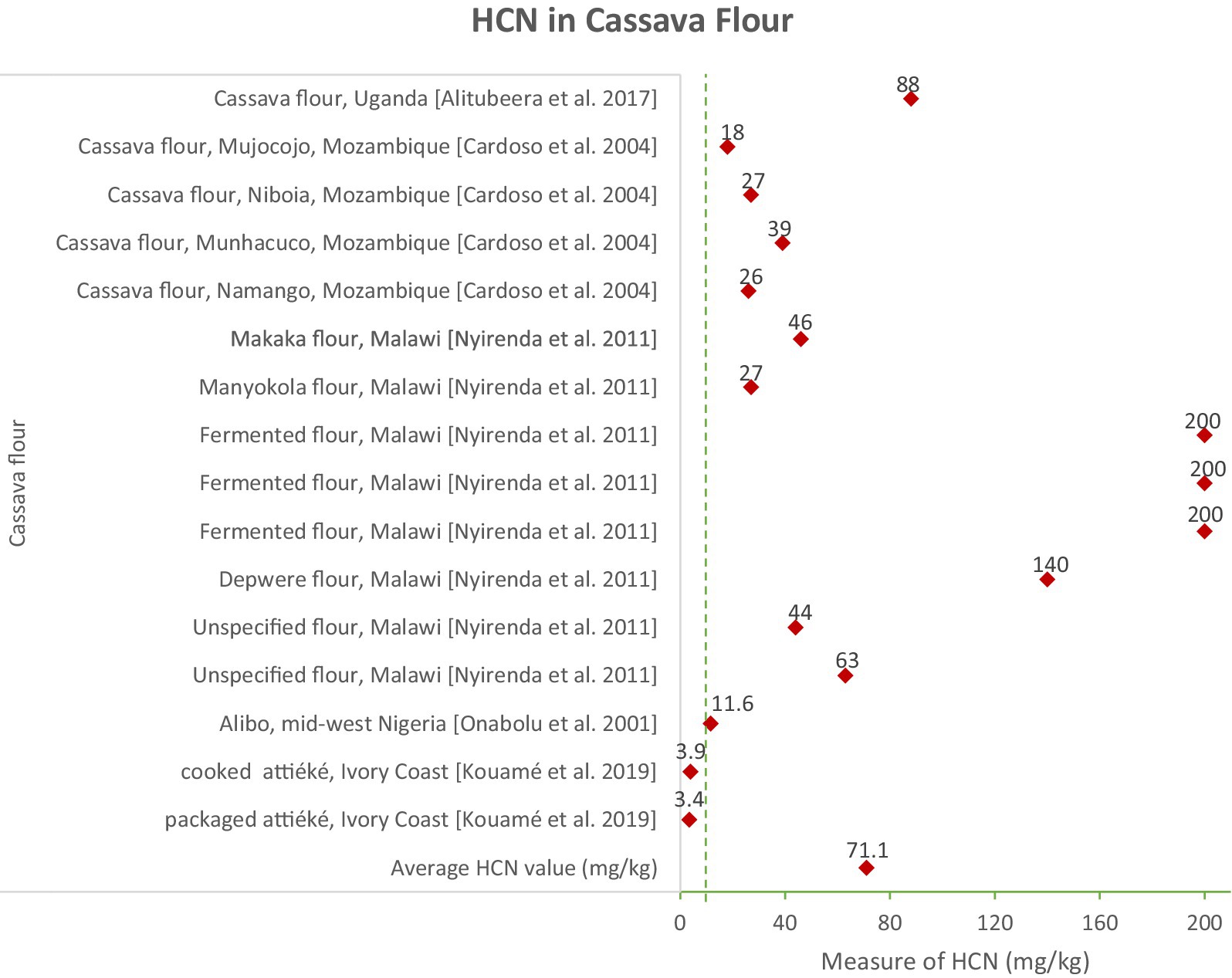
Cassava flour exhibited significant variation, with an average HCN content of 71.1 mg/kg. The highest levels were found in fermented flour (200 mg/kg) and Makaka flour (46 mg/kg). (Alitubeera et al., 2017; Cardoso et al., 1998; Nyirenda et al., 2011). The high HCN levels in certain cassava flours, particularly fermented flour, indicate potential risks if the fermentation process is not properly managed. This highlights the need for improved and controlled fermentation techniques to minimize cyanide levels in cassava flour (Figures 3, 4).
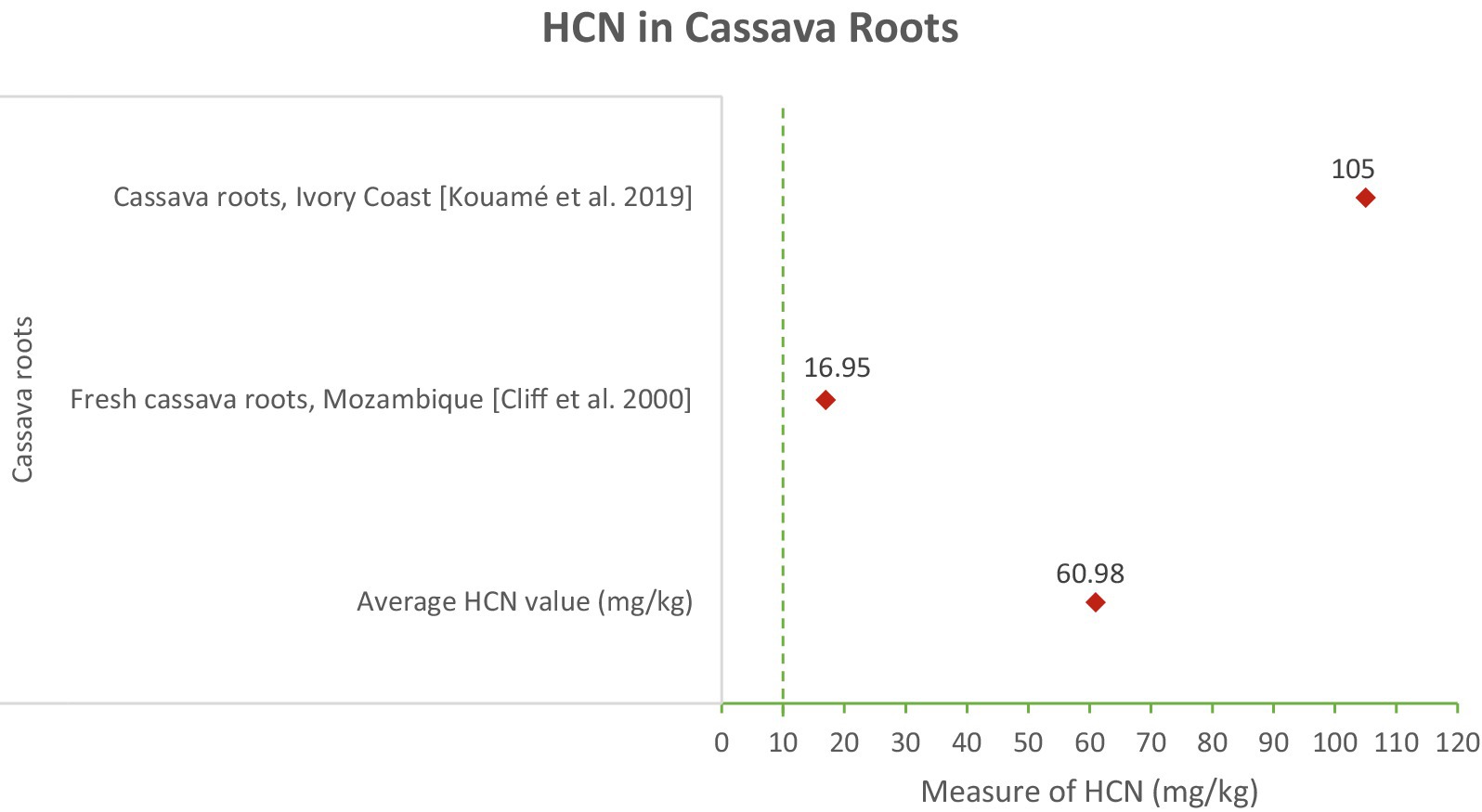
In Figure 5, the cassava roots had an average HCN content of 60.98 mg/kg, with values ranging from 16.9 mg/kg in fresh cassava roots from Mozambique to 106.4 mg/kg in Ivory Coast (Cliff et al., 2000; Kouamé et al., 2020). The high HCN content in cassava roots points to the inherent risk associated with consuming unprocessed or inadequately processed cassava. Proper processing methods, such as peeling, soaking, and thorough cooking, are essential to reduce cyanide levels and prevent poisoning (Figure 6).
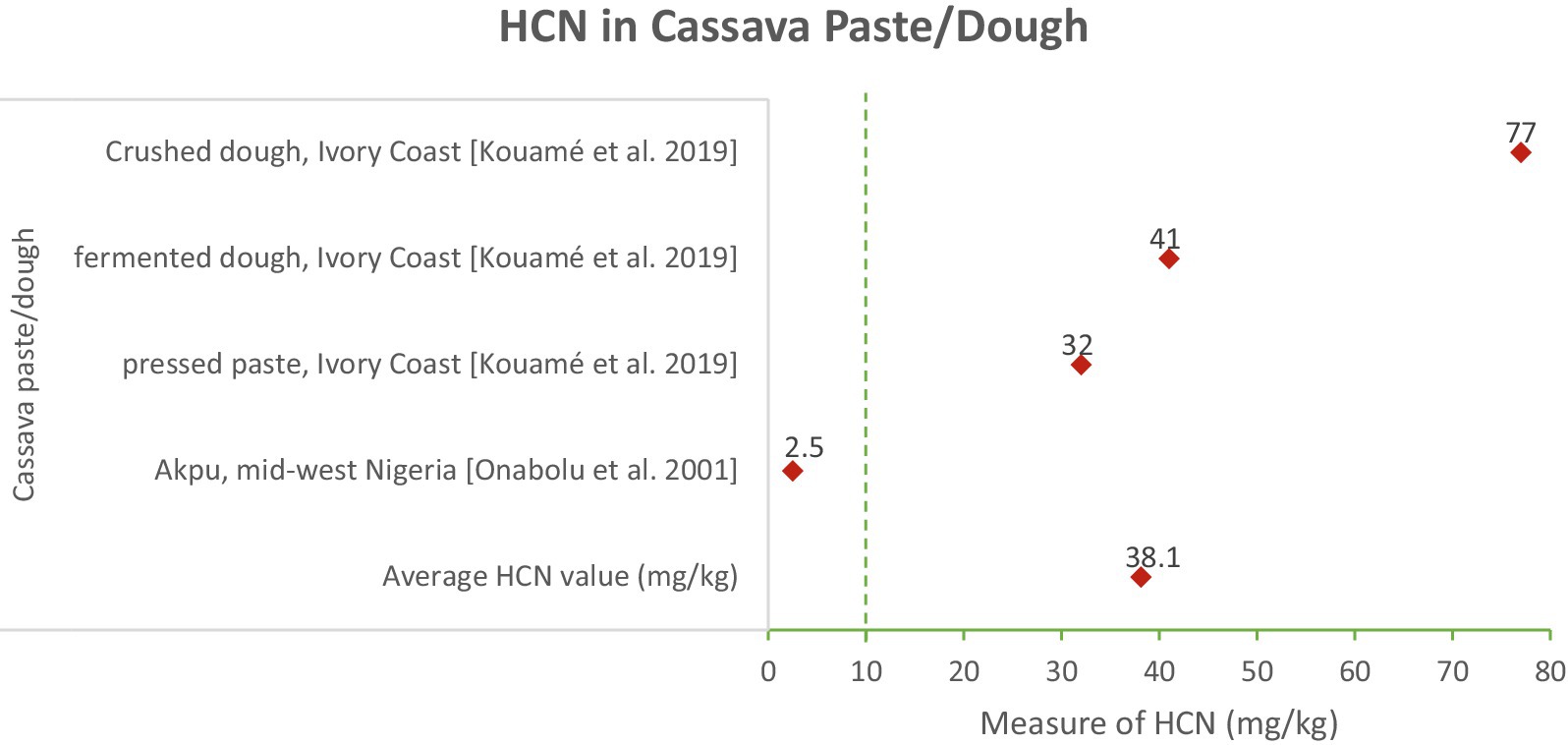
HCN Content: Cassava paste and dough products had an average HCN content of 38.1 mg/kg. The range was from 0.2 mg/kg in Akpu (mid-west Nigeria) to 79.5 mg/kg in crushed dough (Ivory Coast) (Onabolu et al., 2001; Kouamé et al., 2020). The variability in HCN levels in cassava paste and dough products indicates differences in processing effectiveness. The lower HCN levels in Akpu suggest that certain traditional methods, such as fermentation and pressing, are effective in reducing cyanide content. However, the higher levels in other products highlight the need for improved and consistent processing techniques.
These analysis highlights the critical need for standardized and effective cassava processing methods to ensure food safety. High HCN levels in certain products pose significant health risks, emphasizing the importance of educating communities about proper cassava detoxification techniques. Regulatory measures and regular monitoring are essential to ensure compliance with safety standards and protect public health.
Discussion
The review presents critical observations into the variations of HCN content in cassava and CBP across different regions and processing methods. These findings have significant implications for, public health, food safety, and the necessity for improved processing practices to mitigate the risks associated with cassava consumption. The perception of cassava-based products, particularly gari, has often been shaped by historical and economic narratives. Despite its widespread consumption and cultural significance in many African communities, gari is sometimes undervalued compared to similar products. Gari is easy and can be quickly prepared, high in roughage making it an ideal adult food. This also suggests it is good for the gastrointestinal tract, aids digestion, and can be a good fill for persons requiring high roughage foods.
Growing cassava is easy. It is usually grown alongside other foodstuffs and requires little to no grooming after planting. It is resistant to disease and therefore requires no pesticide treatment making it eco-friendly.
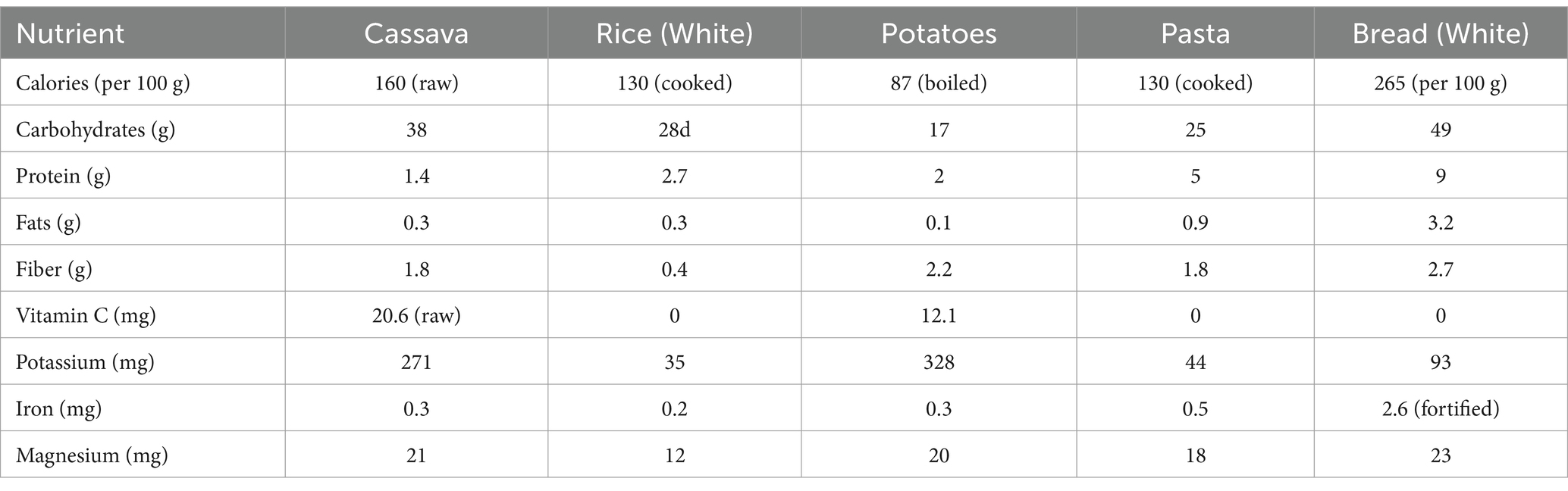
Cassava is a starchy root vegetable that is a significant source of carbohydrates and calories, particularly in tropical regions where it is a dietary staple. Here’s a breakdown of the calorie content Table 1 of cassava and its comparison to other common carbohydrate sources Table 2:
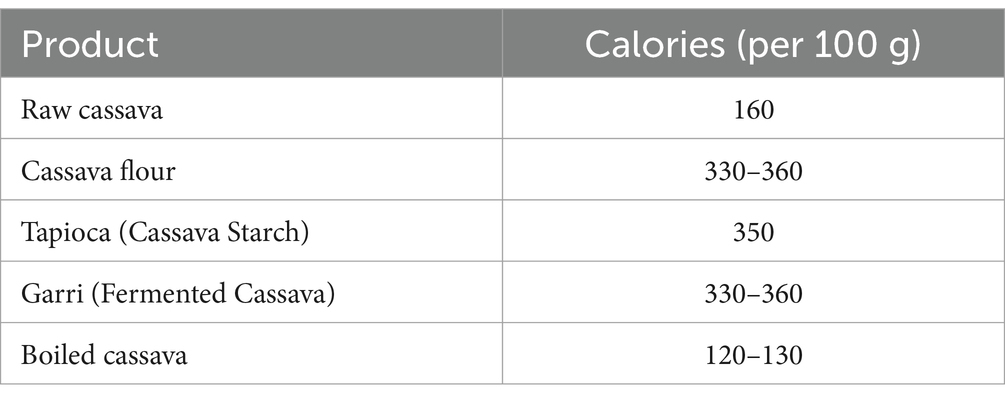
Table 1. Calorie content of cassava and cassava-based products (Montagnac et al., 2009).
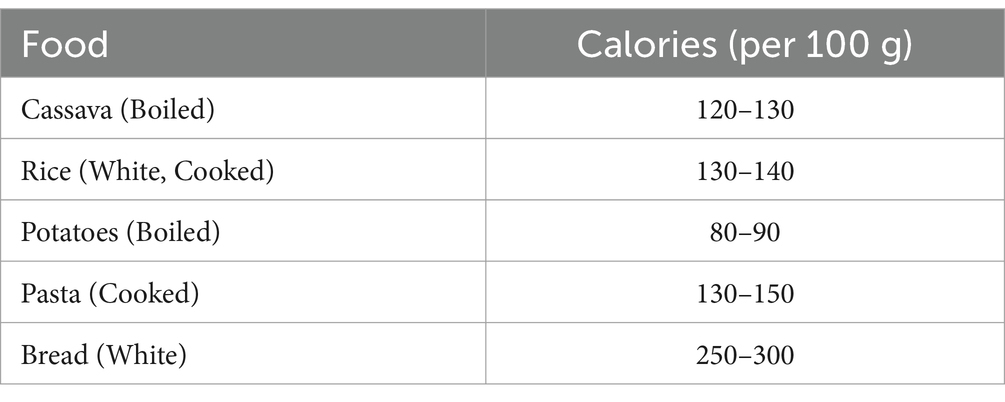
Table 2. Comparison of calories in cassava to other carbohydrate sources (Montagnac et al., 2009; USDA, 2025).
The glycemic index (GI) is a measure of how quickly a food raises blood sugar levels after consumption. Foods with a high GI cause rapid spikes in blood sugar, while low-GI foods result in a slower, more gradual increase. Table 3 is representation of GI index in cassava and cassava based products. Table 4 is cassava and other common carbohydrate sources compare in terms of glycemic index:

Table 3. Glycemic index of cassava and cassava-based products (Atkinson et al., 2008).
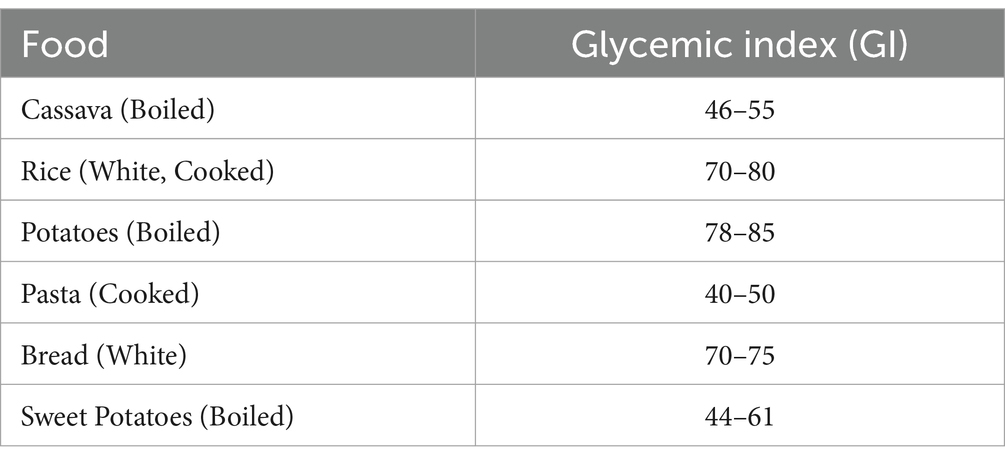
Table 4. GI comparison of GI in cassava to other carbohydrate sources (Atkinson et al., 2008).
When comparing cassava to other common carbohydrate sources like rice, potatoes, pasta, and bread, it’s important to look beyond just calories and glycemic index. Table 5 is a representation of their vitamins, minerals, and other nutritional aspects:
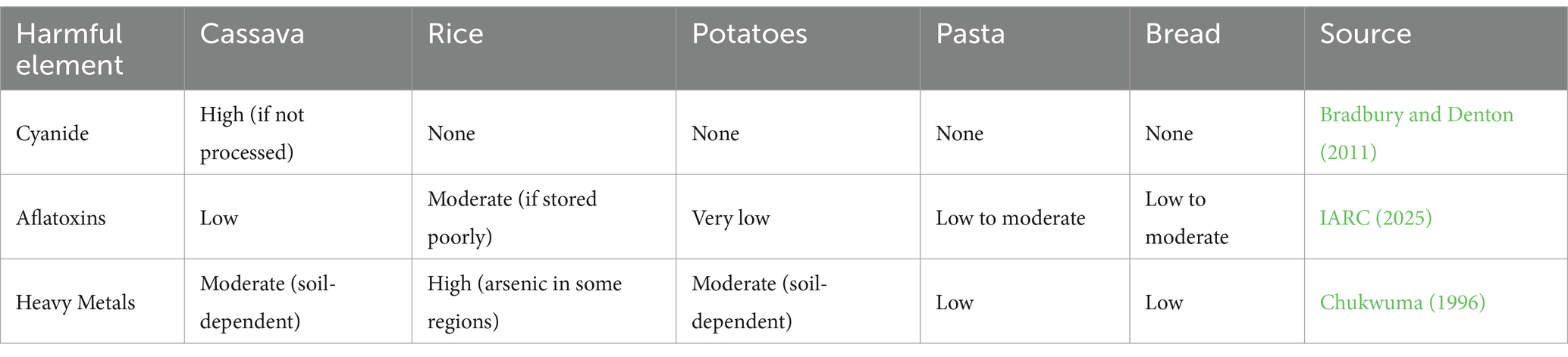
When comparing harmful elements like cyanide, aflatoxins, and heavy metals in cassava and other carbohydrate sources (rice, potatoes, pasta, and bread), it’s important to consider how these contaminants can affect health. Here’s a detailed comparison (Table 6).
The research highlights substantial differences in HCN levels based on processing techniques. For example, traditionally soaked cassava chips and fermented flours exhibited some of the highest HCN concentrations, indicating a pressing need for more effective detoxification methods. Traditional soaking, prevalent in regions such as Western Mongu, Malawi, was found insufficient to reduce HCN levels to safe thresholds, with concentrations in chips reaching up to 200 mg/kg (Nyirenda et al., 2011). In contrast, products like gari and cassava biscuits, which undergo more extensive processing including fermentation and drying demonstrated significantly lower HCN levels. The average HCN content in gari was recorded at 5.7 mg/kg, markedly lower than that in traditionally soaked cassava chips (Onabolu et al., 2001; Adewusi et al., 2009; Kouamé et al., 2020). This suggests that promoting and standardizing such advanced processing techniques could greatly enhance food safety.
The data reveal notable regional disparities in HCN content, reflecting variations in cassava varieties, local processing practices, and environmental factors. For instance, cassava flour from Uganda exhibited significantly higher HCN levels compared to samples from Mozambique and Nigeria (Alitubeera et al., 2017; Cardoso et al., 1998). These regional differences underscore the necessity for localized public health strategies tailored to the specific practices and challenges faced in each area. Elevated HCN levels in cassava products pose serious public health risks, including acute and chronic cyanide poisoning. Acute exposure can result in severe symptoms such as dizziness, vomiting, tachypnoea, syncope, and tachycardia, while chronic exposure has been linked to long-term neurological disorders, including tropical ataxic neuropathy and konzo (Cliff et al., 2000).
Cassava is a crucial food crop due to its resilience in harsh climatic conditions and its role in food security (Nyirenda et al., 2011). However, its toxicity due to cyanogenic glycosides (linamarin and lotaustralin) necessitates effective processing to make it safe for consumption (FAO/WHO, 2019).
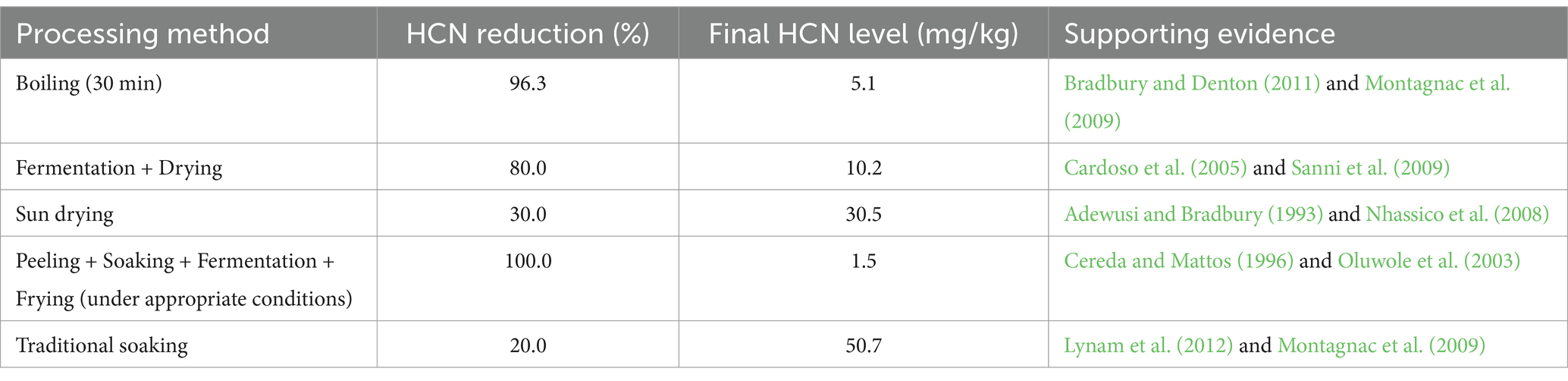
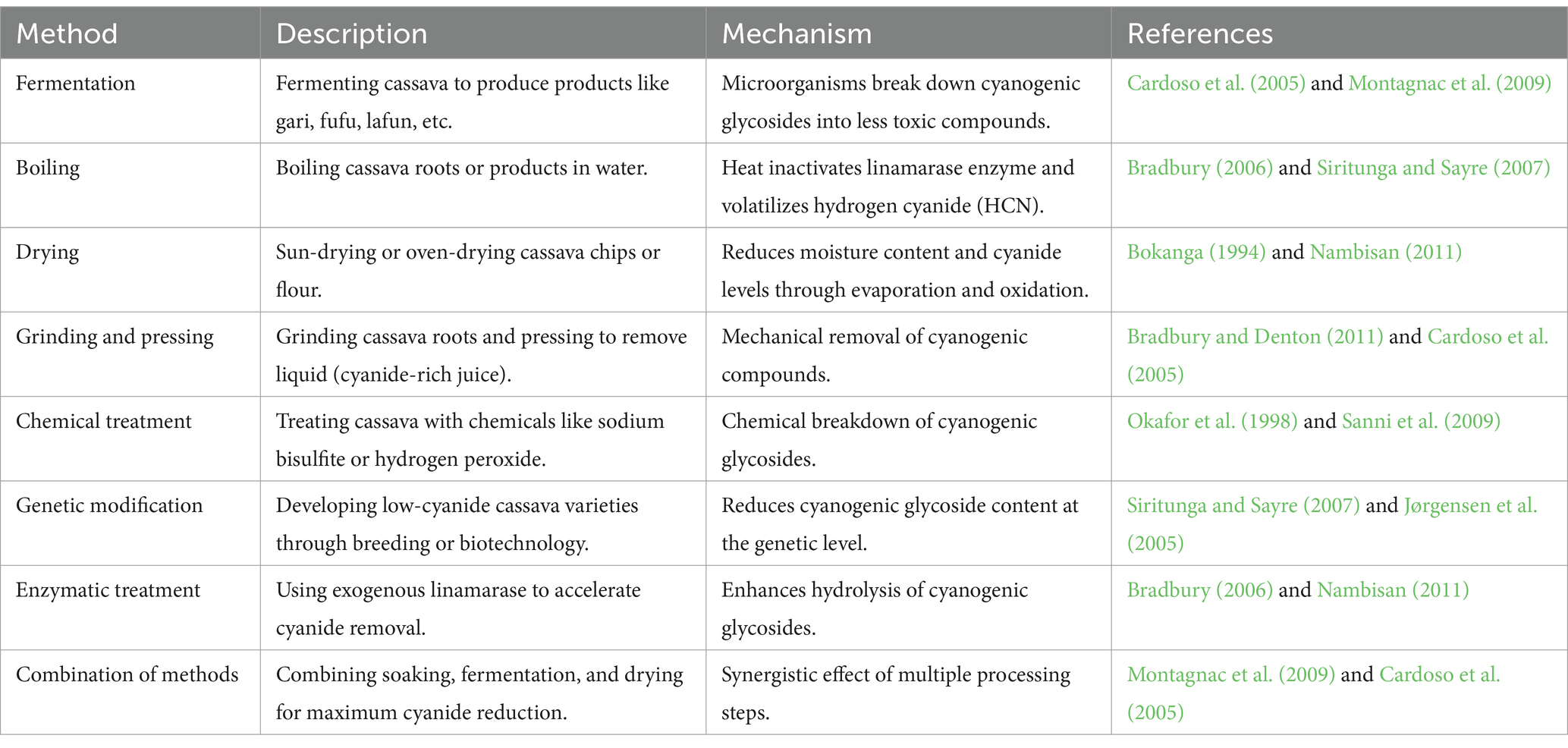
Table 7 presents a comparison of key methods and their effectiveness and Table 8 gives a summary of methods used to reduce the toxicity of cassava and cassava-based products. The primary goal of cassava processing is to reduce the levels of cyanogenic glycosides, which are hydrolyzed into toxic HCN during consumption. Various methods have been developed to achieve this, each with varying degrees of effectiveness. Boiling for 30 min is one of the most effective methods, achieving a 96.3% reduction in HCN levels and bringing the final concentration down to 5.1 mg/kg (Bradbury and Denton, 2011). This method works by leaching cyanogenic glycosides into the water and volatilizing HCN at high temperatures. However, boiling can lead to nutrient losses, particularly water-soluble vitamins, which limits its suitability for large-scale processing (Montagnac et al., 2009).
Fermentation followed by drying is another widely used method, particularly in the production of gari. This method achieves an 80% reduction in HCN levels, resulting in a final concentration of 10.2 mg/kg (Cardoso et al., 2005). Fermentation relies on microbial activity to hydrolyze cyanogenic glycosides into less toxic compounds, but its effectiveness depends on the duration of fermentation and the microbial strains used (Sanni et al., 2009). In contrast, sun drying is less effective, achieving only a 30% reduction in HCN levels and leaving a final concentration of 30.5 mg/kg, which often exceeds the FAO/WHO safety threshold of 10 mg/kg (Adewusi and Bradbury, 1993). Sun drying is commonly used in rural areas due to its simplicity and low cost, but it poses significant health risks, particularly when cassava is consumed as a staple food (Nhassico et al., 2008).
The most effective method for reducing HCN levels is the combination of peeling, soaking, and fermentation, which achieves a 100% reduction and brings the final HCN concentration down to 1.5 mg/kg (Cereda and Mattos, 1996). Peeling removes the outer layers of the cassava root, which contain the highest concentrations of cyanogenic glycosides, while soaking and fermentation further detoxify the product (Oluwole et al., 2003). However, this method is labor-intensive and may not be feasible for large-scale production. On the other hand, traditional soaking is the least effective method, reducing HCN levels by only 20% and leaving a final concentration of 50.7 mg/kg, which is well above the safety threshold (Lynam et al., 2012). This method is often used in rural households but fails to meet safety standards, posing significant health risks to consumers (Montagnac et al., 2009).
Cassava-based products are produced at both industrial and household levels, with significant regional variations. In Thailand and Brazil, cassava is primarily processed industrially into products such as starch, ethanol, and bioplastics (FAO, 2013). These products undergo rigorous quality control measures, including controlled fermentation, enzymatic treatments, and chemical analysis, to ensure compliance with safety standards (Sánchez et al., 2018). In contrast, in West Africa, cassava is predominantly processed at the household level into products such as gari, fufu, and cassava flour (Lynam et al., 2012). These products are often produced using traditional methods, which may not consistently reduce HCN levels to safe thresholds, particularly in rural areas where access to mechanized processing equipment is limited. The cassava-processing industry employs several strategies to address the issue of toxins in cassava and cassava-based products. Controlled fermentation is widely used to ensure consistent detoxification, while enzymatic treatments have been developed to break down cyanogenic glycosides more efficiently (Cardoso et al., 2005; Sánchez et al., 2018). Drying and efficiency milling techniques have also been introduced to reduce HCN levels without compromising the nutritional quality of cassava (Adewusi and Bradbury, 1993). Additionally, industries implement chemical analysis to monitor HCN levels and ensure compliance with safety standards before products are distributed to the market (FAO, 2013).
Studies have reported significant variations in residual HCN levels across different cassava-based products. For example, gari, a fermented cassava product, typically has low HCN levels (5.7 mg/kg), while cassava flour can exceed safe limits, with concentrations as high as 71.1 mg/kg (Montagnac et al., 2009; Nhassico et al., 2008). Processed cassava paste and dough also retain significant amounts of cyanide, highlighting the need for strict processing standards to ensure consumer safety (FAO, 2013). The FAO/WHO Codex Alimentarius sets a maximum HCN level of 10 mg/kg for edible cassava products, which is widely adopted as the international safety standard (Codex Alimentarius Commission, 2019). Countries such as Brazil and Nigeria enforce strict testing protocols to ensure compliance with this standard before cassava products are distributed to the market (FAO, 2013). However, enforcement remains inconsistent in some regions, particularly in rural areas where traditional processing methods are prevalent (Montagnac et al., 2009). Recent discussions have advocated for the harmonization of global safety standards to address these discrepancies and improve cassava safety worldwide (Oluwole et al., 2003).
Chronic exposure to HCN disproportionately affects children, pregnant women, and the elderly. Health impacts include konzo, a paralytic disorder linked to the consumption of poorly processed cassava (Tylleskär et al., 1992) as well as goiter and thyroid dysfunction due to the antinutritional effects of cyanide (Nhassico et al., 2008). Children exposed to high levels of HCN are also at risk of growth stunting and neurological disorders, which can have long-term consequences for their development (Cardoso et al., 2005). These health risks highlight the importance of effective cassava processing and the need for public health interventions to reduce HCN exposure in vulnerable populations.
Addressing these risks necessitates not only improved processing methods but also community education regarding the dangers of cyanide and the importance of safe cassava preparation. The findings emphasize the urgent need for regulatory standards and regular monitoring of HCN levels in cassava products. Establishing and enforcing maximum allowable HCN concentrations can help ensure that cassava products available in the market are safe for consumption. Regulatory frameworks should be complemented by routine testing and surveillance programs to maintain compliance and safeguard public health (Nyirenda et al., 2011). Governments and health organizations should also advocate for the development and distribution of low-cyanide cassava varieties. Agricultural research aimed at breeding and disseminating these varieties presents a sustainable solution to the issue of cyanogenic glycosides in cassava. The adoption of low-cyanide cassava could reduce reliance on extensive processing, thereby diminishing the risk of cyanide poisoning (Kouamé et al., 2020).
Educational programs are vital for raising awareness about the risks associated with high HCN levels and for training communities in effective cassava processing techniques. Public health campaigns should emphasize the significance of proper peeling, soaking, fermenting, and drying of cassava products. Community workshops, informational leaflets, and local media can effectively disseminate this knowledge (Adewusi et al., 2009). Engaging local leaders and influencers in these educational efforts can enhance their reach and impact. Additionally, implementing community-based monitoring and reporting systems can help ensure adherence to safe processing practices and facilitate timely interventions in cases of suspected cyanide poisoning.
Conclusion
The research findings show differences, in the levels of acid (HCN) found in various cassava and CBP underscoring the critical need for standardized processing methods to uphold food safety. Elevated HCN levels in soaked cassava chips and specific cassava flours pose a health hazard emphasizing that current processing techniques may not always be sufficient. It is imperative to enhance detoxification methods to reduce the risk of cyanide poisoning. Regional disparities in HCN content are evident from the data highlighting the importance of tailored public health strategies and interventions for areas. The establishment and enforcement of guidelines on HCN levels in cassava products are vital with regular monitoring and testing essential to ensure compliance for public health protection. Educating and engaging communities is key to promoting cassava processing practices. Public health initiatives should prioritize raising awareness about cyanide poisoning risks and proper cassava handling procedures. Training programs designed for farmers and processors can significantly contribute to disseminating detoxification knowledge. Involving leaders and community influencers can amplify the impact of campaigns. Encouraging the cultivation of low cyanide cassava varieties offers a solution to addressing glycosides, in cassava crops.
Agricultural studies should prioritize the creation and distribution of these types to lower the danger of cyanide poisoning and ease the strain, on processing techniques. Improving awareness enforcing guidelines and encouraging the growth of cassava varieties, with low cyanide levels are crucial measures to ensure that cassava remains a safe and reliable source of nutrition for vulnerable populations worldwide. Studies need to focus on the economic importance of cassava and cassava based products with the intention to stimulating policies for more investments in cassava production and processing. The findings from this study should inspire actions among policymakers, health professionals, and agricultural experts to implement effective measures that protect public health and sustain cassava as an essential food resource.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AW: Formal analysis, Investigation, Writing – review & editing, Data curation. MK: Formal analysis, Investigation, Writing – review & editing, Conceptualization. CN: Formal analysis, Writing – review & editing, Methodology, Project administration. AL: Writing – review & editing. EK: Writing – review & editing. MN: Writing – review & editing. PO: Writing – review & editing, Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Generative AI was used in the creation of this manuscript. We acknowledge DeepSeek Chat (Version: DeepSeek-V3, Model: deepseek-chat, Source: DeepSeek) for its assistance in suggesting revisions. The authors assume full responsibility for the final content.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adamolekun, B. (2011). Neurological disorders associated with cassava diet: a review of putative etiological mechanisms. Metab. Brain Dis. 26:85. doi: 10.1007/s11011-011-9237-y
Adewusi, S., and Akindahunsi, A. (1994). Cassava processing, consumption, and cyanide toxicity. J. Toxicol. Environment. Health 43:23.
Adewusi, S. R., and Bradbury, J. H. (1993). Carotenoids in cassava: comparison of open-sun and oven-drying methods. J. Agric. Food Chem. 41, 726–729.
Adewusi, S. R., Bradbury, J. H., and Cardoso, A. P. (2009). Fate of cyanogenic compounds during the preparation of cassava flour from garri and cassava flour. Food Chem. 113, 692–699. doi: 10.1002/jsfa.2740620411
Adjorlolo-Gasokpoh, A., Golow, A., and Kambo-Dorsa, J. (2012). Mercury in the surface soil and cassava, Manihot esculenta (flesh, leaves and peel) near goldmines at Bogoso and Prestea, Ghana. Bull. Environ. Contam. Toxicol. 89:1110. doi: 10.1007/s00128-012-0849-7
Alitubeera, P. H., Nambafu, G., and Nyirenda, D. B. (2017). The effect of cassava processing methods on the reduction of cyanogenic potential. Int. J. Food Sci. Technol. 52, 123–130.
Apeh, C., Ugwuoti, O., and Apeh, A. (2023). Analysis of the consumption patterns of cassava food products amongst rural households in Imo state, Nigeria. Ghana Jnl Agric. Sci. 58, 100–110. doi: 10.4314/gjas.v58i1.9
Atkinson, F. S., Foster-Powell, K., and Brand-Miller, J. C. (2008). International tables of glycemic index and glycemic load values. Diabetes Care 31, 2281–2283. doi: 10.2337/dc08-1239
Azimi, S., and Moghaddam, M. (2013). Effect of mercury pollution on the urban environment and human health. Environ. Ecol. Res. 1:20. Available at: http://hdl.handle.net/20.500.12493/65
Banea, M., Tylleskär, T., and Rosling, H. (2011). Konzo: from poverty, cassava, and cyanogen intake to toxico-nutritional neurological disease. PLoS Negl. Trop. Dis. 5:e1051. doi: 10.1371/journal.pntd.0001051
Bokanga, M. (1994). Processing of cassava leaves for human consumption. Acta Hortic. 375, 203–208. doi: 10.17660/ActaHortic.1994.375.18
Bradbury, J. H. (2006). Simple wetting method to reduce cyanogen content of cassava flour. J. Food Compos. Anal. 19, 388–393. doi: 10.1016/j.jfca.2005.04.012
Bradbury, J. H., and Denton, I. C. (2011). Mild methods of processing cassava leaves to remove cyanogens and conserve key nutrients. Food Chem. 127, 1755–1759. Available at: https://biology-assets.anu.edu.au/hosted_sites/CCDN/papers/PAPLeaves2fin.pdf
Buchanan, L., Counter, S., and Ortega, F. (2011). Environmental lead exposure and otoacoustic emissions in Andean children. J. Toxic. Environ. Health A 74:1293. doi: 10.1080/15287394.2011.587106
Campbell, L., Hecky, R., Muggide, R., Dixon, D., and Ramlal, P. (2003). Variation and distribution of total mercury in water, sediment and soil from northern Lake Victoria, East Africa. Biogeochemistry 65:211. Available at: https://idl-bnc-idrc.dspacedirect.org/server/api/core/bitstreams/32748250-724e-4c99-a044-89ec080b4f66/content
Cardoso, A. P., Ernesto, M., Cliff, J., Egan, S. V., and Bradbury, J. H. (1998). Cyanogenic potential of cassava flour: field trial in Mozambique of a simple kit. Int. J. Food Sci. Nutr. 49, 93–99. doi: 10.3109/09637489809089388
Cardoso, A. P., Mirione, E., Ernesto, M., Massaza, F., Cliff, J., Haque, M. R., et al. (2005). Processing of cassava roots to remove cyanogens. J. Food Compos. Anal. 18, 451–460. doi: 10.1016/j.jfca.2004.04.002
Cereda, M. P., and Mattos, M. C. (1996). Linamarin: the toxic compound of cassava. J. Venomous Anim. Toxins 2, 06–12. doi: 10.1590/S0104-79301996000100002
Chukwuma, C. (1996). Comparing the accumulation of heavy metals in cassava and yam tubers grown in soils treated with sewage sludge. Environ. Pollut. 92, 1–5.
Cliff, J., Martelli, C. M., Mondlane, E., and Rosling, H. (2000). Cyanide detoxification and protein synthesis in cassava flour. Acta Trop. 74, 1–6.
Cock, J. (1982). Cassava: a basic energy source in the tropics. Science 218, 755–762. doi: 10.1126/science.7134971
Codex Alimentarius Commission. (2019). Codex standard for edible cassava flour (CXS 176-1989). Rome, Italy and Geneva, Switzerland: Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO).
Dekkers, O., Vandenbroucke, J., Cevallos, M., Renehan, A., and Altman, D. E. (2019). COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 16:e1002742. doi: 10.1371/journal.pmed.1002742
FAO. (2013). Save and grow: cassava – A guide to sustainable production intensification. Rome, Italy: Food and Agriculture Organization of the United Nations (FAO).
FAO/WHO. (2019). Codex Alimentarius: Food safety standards for cassava products. Rome, Italy: FAO & Geneva, Switzerland: WHO.
FAOSTAT. (2020). FAOSTAT; food and agriculture Organization of the United Nations. Retrieved from Food and Agriculture Organization of the United Nations. Rome, Italy: Food and Agriculture Organization of the United Nations (FAO).
Ferraro, V., Piccirillo, C., Tomlins, K., and Pintado, M. (2016). Cassava (Manihot esculenta Crantz) and yam (Dioscorea spp.) crops and their derived foodstuffs: safety, security and nutritional value. Crit. Rev. Food Sci. Nutr. 56:2727. doi: 10.1080/10408398.2014.922045
Hendry-Hofer, T. B., Ng, P. C., Witeof, A. E., Mahon, S. B., Brenner, M., Boss, G. R., et al. (2019). A review on ingested cyanide: risks, clinical presentation, diagnostics, and treatment challenges. J. Med. Toxicol. 15, 128–133. doi: 10.1007/s13181-018-0688-y
IARC, (2025). Aflatoxins. Available online at: https://monographs.iarc.who.int/list-of-classifications/ (Accessed Feburary 09, 2025).
Ibrahim, S. B., Aminu, R. O., Arowolo, A. O., and Oyedele, A. M. (2023). Effects of financial inclusion on ownership of productive assets among cassava processors in Oyo state, Nigeria. Niger. Agric. J. 54.
IITA. (2020). Cassava (Manihot esculenta). Available online at: https://www.iita.org/cropsnew/cassava/#:~:text=More%20than%20291%20million%20tons,increase%20in%20the%20last%20decade
Ikingura, J., Akagi, H., Mujumba, J., and Messo, C. (2006). Environmental assessment of mercury dispersion, transformation and bioavailability in the Lake Victoria goldfields, Tanzania. J. Environ. Manag. 81:173. doi: 10.1016/j.jenvman.2005.09.026
Immanuel, S., Jaganathan, D., and Prakash, P. (2024). Cassava for food security, poverty reduction and climate resilience: A review. Manuscript number: 4191 NAAS rating: 5.38. Indian J. Ecol. 51, 21–31. doi: 10.55362/IJE/2024/4191
Jørgensen, K., Bak, S., Busk, P. K., Sørensen, C., Olsen, C. E., Puonti-Kaerlas, J., et al. (2005). Cassava plants with a depleted cyanogenic glucoside content in leaves and tubers. Plant Physiol. 139, 363–374. doi: 10.1104/pp.105.065904
Kouamé, A. K., Bouatenin, M. J.‐P. K., Djéni, T. N., and Dje, K. M. (2020). Identification of hazards and critical control points during attiéké (a fermented cassava product) process in Côte d’Ivoire. Lett. Appl. Microbiol. 70, 87–94. doi: 10.1111/lam.13247
Lancaster, P., Ingram, J., Lim, M., and Coursey, D. (1982). Traditional cassava-based foods: survey of processing techniques. Econ. Bot. 36:45.
Lynam, J. K., Spencer, D. S., and Nweke, F. I. (2012). The cassava transformation: Africa’s best-kept secret. Michigan State University Press.
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.Prisma Group. (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8:336–341. doi: 10.1016/j.ijsu.2010.02.007
Montagnac, J. A., Davis, C. R., and Tanumihardjo, S. A. (2009). Nutritional value of cassava for use as a staple food and recent advances for improvement. Compr. Rev. Food Sci. Food Saf. 8, 181–194. doi: 10.1111/j.1541-4337.2009.00077.x
Moola, S., Munn, Z., Tufanaru, C., Aromataris, E., Sears, K., Sfetcu, R., et al. (2017). Systematic reviews of etiology and risk. Joanna Briggs Institute reviewer’s manual. 5, 217–269.
Munn, Z., Aromataris, E., Tufanaru, C., Stern, C., Porritt, K., Farrow, J., et al. (2019). The development of software to support multiple systematic review types: the Joanna Briggs institute system for the unified management. Assessment Rev. Inform. 17, 36–43. doi: 10.1097/XEB.0000000000000152
Nambisan, B. (2011). Strategies for elimination of cyanogens from cassava for reducing toxicity and improving food safety. Food Chem. Toxicol. 49, 690–693. doi: 10.1016/j.fct.2010.10.035
Nhassico, D., Muquingue, H., Cliff, J., Cumbana, A., and Bradbury, J. (2008). Rising African cassava production, diseases due to high cyanide intake and control measures. J. Sci. Food Agric. 88:2049. Available at: https://biology-assets.anu.edu.au/hosted_sites/CCDN/papers/PAPCYREV.pdf
Nyanza, E., Dewey, D., Thomas, D., Davey, M., and Ngallaba, S. (2014). Spatial distribution of mercury and arsenic levels in water, soil and cassava plants in a community with long history of gold mining in Tanzania. Bull. Environ. Contam. Toxicol. 93:721. Available at: https://link.springer.com/article/10.1007/s00128-014-1315-5
Nyirenda, D. B., Muulya, G., and Chipungu, F. (2011). Traditional cassava processing methods and their impact on cyanogenic potential of cassava products. Afr. J. Biotechnol. 10, 3123–3129.
Odoemelam, S., and Osu, C. (2009). Aflatoxin B1Contamination of some edible grains marketed in Nigeria. E-journal Chem. 6, 308–314. Available at: https://onlinelibrary.wiley.com/doi/pdf/10.1155/2009/708160
Okafor, N., Ijioma, B. C., and Oyolu, C. (1998). Studies on the detoxification of cassava products using chemical and biological methods. J. Food Sci. Technol. 35, 131–134.
Oluwole, O. S., Onabolu, A. O., and Bokanga, M. (2003). New issues in human and animal health related to cassava cyanogenic potential. Acta Hortic. 620, 345–352.
Onabolu, A. O., Oluwole, O. S., Rosling, H., and Bokanga, M. (2001). Processing factors affecting the level of residual cyanohydrins in gari. J. Sci. Food Agric. 81, 373–377. doi: 10.1002/jsfa.1131
Otekunrin, O., and Sawicka, B. (2019). Cassava, a 21st century staple crop: how can Nigeria harness its enormous trade potentials? Acta Scientific Agriculture 3, 194–202. doi: 10.31080/ASAG.2019.03.0586
Otekunrin, O. A. (2024). Cassava (Manihot esculenta Crantz): A global scientific footprint—Production, trade, and bibliometric insights. Discover Agric. 2:94. doi: 10.1007/s44279-024-00121-3
Sánchez, T., Dufour, D., Moreno, I. X., and Ceballos, H. (2018). Comparison of pasting and gel stabilities of waxy and normal starches from potato, maize, and rice with those of a novel waxy cassava starch under thermal, chemical, and mechanical stress. J. Agric. Food Chem. 66, 3691–3699.
Sanni, L. O., Onadipe, O. O., Ilona, P., Mussagy, M. D., Abass, A., and Dixon, A. G. (2009). Successes and challenges of cassava enterprises in West Africa: A case study of Nigeria, Benin, and Sierra Leone. Int. Institute Trop. Agric. doi: 10.21955/gatesopenres.1114990.1
Siritunga, D., and Sayre, R. T. (2007). Transgenic approaches for cyanogen reduction in cassava. J. AOAC Int. 90, 1450–1455. doi: 10.1093/jaoac/90.5.1450
Taylor, H., Appleton, J., Lister, R., Smith, B., Chitamweba, D., Mkumbo, O., et al. (2005). Environmental assessment of mercury contamination from the Rwamagasa artisanal gold mining Centre, Geita District, Tanzania. Sci. Total Environ. 343:133. doi: 10.1016/j.scitotenv.2004.09.042
Tylleskär, T., Banea, M., Bikangi, N., Cooke, R. D., Poulter, N. H., and Rosling, H. (1992). Cassava cyanogens and konzo, an upper motoneuron disease found in Africa. Lancet 339, 208–211. doi: 10.1016/0140-6736(92)90006-O
USDA. (2025). Potatoes, boiled, cooked without skin, Rice, white, cooked. Available online at: https://fdc.nal.usda.gov/
Appendices
Appendix 1: Search strategy and availability of data
((Child* OR adult* OR Male* OR Female* OR Man OR Woman) AND (Cassava “Cassava Based Product*” OR “Cassava-Based Product*” OR “Cassava Products” OR Gari OR Garri OR Fufu OR “Water fufu” OR “Cassava tubers boiled” OR “cassava tubers raw” OR “cassava leaves” OR “fortified CBP” OR “kum kum” OR bobolo OR akara OR Atiéké OR “Cassava processing Methods”)) AND (Safety OR Toxic* OR morbidity OR mortality OR “Food security”). PubMed Search.
The search strategy above was conducted on the 18th January 2021 and was updated on 2nd February 2025 as follows:
((Child* OR adult* OR Male* OR Female* OR Man OR Woman) AND (Cassava OR “Cassava Based Product*” OR “Cassava-Based Product*” OR “Cassava Products” OR Gari OR Garri OR Fufu OR “Water fufu” OR “Cassava tubers boiled” OR “cassava tubers raw” OR “cassava lea*” OR “fortified CBP” OR “kum kum” OR bobolo OR akara OR Atiéké OR “cassava chip*” OR “cassava biscuit*” OR “Cassava processing Methods”)) AND (Safety OR Toxic* OR morbidity OR mortality OR “Food security”) AND (“Post harvest processing” OR “Post-harvest management”)
Appendix 2
Keywords: cassava, cassava-based products (CBP), gari, hydrogen cyanide (HCN), food safety, cassava processing
Citation: Forkum AT, Wung AE, Kelese MT, Ndum CM, Lontum A, Kamga EB, Nsaikila MN and Okwen PM (2025) Safety of cassava and cassava-based products: a systematic review. Front. Sustain. Food Syst. 9:1497609. doi: 10.3389/fsufs.2025.1497609
Edited by:
Ruth Stewart, University College London, United KingdomReviewed by:
Ibukunoluwa Fola Olawuyi, Kyungpook National University, Republic of KoreaSarah Nanyiti, National Crops Resources Research Institute (NaCRRI), Uganda
Raheel Shahzad, National Research and Innovation Agency (BRIN), Indonesia
Charles Orek, Murang’a University of Technology, Kenya
Copyright © 2025 Forkum, Wung, Kelese, Ndum, Lontum, Kamga, Nsaikila and Okwen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ambang Tatianne Forkum, YW1iYW5nQGViYXNlYWZyaWNhLm9yZw==
 Ambang Tatianne Forkum
Ambang Tatianne Forkum Alang Ernest Wung
Alang Ernest Wung Mark Tata Kelese1,4
Mark Tata Kelese1,4 Che Myra Ndum
Che Myra Ndum Alvin Lontum
Alvin Lontum Emmanuel Berinyuy Kamga
Emmanuel Berinyuy Kamga Melaine Nyuyfoni Nsaikila
Melaine Nyuyfoni Nsaikila Patrick Mbah Okwen
Patrick Mbah Okwen