
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 10 March 2025
Sec. Waste Management in Agroecosystems
Volume 9 - 2025 | https://doi.org/10.3389/fsufs.2025.1380876
This article is part of the Research TopicRAMIRAN 2023: Managing Organic Resources in a Changing EnvironmentView all 15 articles
Introduction: Feed additives like humic substances (HS) and probiotics (P) can enhance broiler health, production, welfare, and environmental conditions.
Methods: This study involved 120 one-day-old ROSS 308 broiler chicks divided into three groups for a 39-day fattening period. The first experimental group (HS) received a feed mixture with 0.6% HS; the second group (HS + P) got the same HS concentration along with a probiotic strain (Limosilactobacillus fermentum) in water. The control group (C) was fed a basal diet without additives. The HS mixture was also added to the litter in both the HS and HS + P groups, whereas the C group did not receive any HS. The study examined the effects of HS as litter additives on the physical– chemical properties of poultry litter and its capacity to emit fewer harmful gases. Gas emissions were measured using a plastic chamber connected to a uniTOX instrument, and litter moisture was assessed using AOAC methods.
Results: By days 21, 28, and 35, the moisture levels in the HS and HS + P groups were significantly lower (p < 0.001) compared to the C group. HS maintained a slightly acidic pH in the experimental groups, whereas the C group’s pH became slightly alkaline. The absorption properties of HS and pH stabilization contributed to the rise in NH3 and CO2 emissions to the environment at a lower rate than in the C group (p > 0.05). The second aim was to monitor the influence of HS and P as feed and litter additives on bacterial counts and the antimicrobial resistance of E. coli isolates by the microdilution method. Coliform and fecal coliform counts were significantly lower (p < 0.001) in the litter of the experimental groups on day 35. Minimum inhibitory concentration revealed resistance to ciprofloxacin, tetracycline, ampicillin + sulbactam, and cotrimoxazole among E. coli isolates, with resistance ranging from 5 to 15% across all groups.
Discussion: It is concluded that the effect of the additives used increased litter quality. These findings underscore the importance of incorporating additives into intensive poultry systems, where maintaining litter quality is crucial to reducing disease transmission, improving animal welfare, and increasing production efficiency.
The poultry industry is one of the largest, fastest-growing, and most prosperous industries in the world (Muhammad et al., 2020). However, intensive poultry farming is associated with many adverse environmental impacts, such as the production of harmful gases (carbon dioxide–CO2 and ammonia–NH3) or poultry waste (Kousar et al., 2021). Also, due to the EU ban on the routine farm use of antibiotics as growth promoters and the limited use of prophylaxis and metaphylaxis in EU countries, the interest in alternative additives for animal production has increased. When utilized appropriately, they can improve feed quality and nutrient availability, promote animal health and growth, increase productivity, or improve breeding condition (Ayalew et al., 2022).
Humic substances are complex heterogeneous organic compounds that are naturally formed by the decomposition and transformation of plant and animal matter in the soil, as well as microbial activity. They occur in soil, water, sediment, coal, peat, and other sources. Their composition consists of carbon, hydrogen, oxygen, and nitrogen, with a small proportion of phosphorus and sulfur (Goel and Dhingra, 2021). Based on their solubility differences, HS are divided into three main fractions: humic acid, fulvic acid, and humin. Humin has a high molecular weight. It is insoluble in acids, bases, or water, and its high resistance to biodegradation is an important characteristic. Humin is able to retain water, improve the structure and stability of the material, and is also involved in cation exchange (Gautam et al., 2021). In general, humic acids are polymers of medium molecular weight, with an aromatic core and aliphatic chains with multiple functional groups. Humic acids are insoluble in an acidic environment (pH lower than 2), but they become soluble in an alkaline environment. They also have numerous different mineral elements bound to their molecules, thus playing an important role in ion exchange and metal complexing (Alomar et al., 2023). Fulvic acids are soluble at all pH values. Of all the fractions, they have the lowest molecular weight (Gautam et al., 2021).
The use of HS in the breeding of certain animals, such as broiler chickens, appears more than promising. HS have been shown to have anti-inflammatory, antimicrobial, and anticarcinogenic potential in human and animal populations (Hriciková et al., 2023). Their ability to activate the metabolism of nutrients and water, including their bactericidal action, makes them valuable tools in the treatment of several diseases (Khil’ko et al., 2011). They are also used in the veterinary sector and animal husbandry for their antidiarrheal, analgesic, immunostimulating, and adsorptive properties. Currently, HS are used in the rearing of various types of animals, including poultry, pigs, cattle, goats, rabbits, fish, and others (Domínguez-Negrete et al., 2019).
A large number of experiments have been carried out in order to demonstrate the effect of the use of HS in poultry farming, including broiler chickens, laying hens, quails, and other types of poultry (Bezuglova and Klimenkom, 2022). The addition of HS to feed or water stimulated the growth of broiler chickens and laying hens (Ozturk et al., 2010). Broiler chicken feed containing HS resulted in a significant improvement in the growth rate and viability of chickens exposed to high environmental temperatures (Edmonds et al., 2014). Jaďuttová et al. (2019) observed that supplementation of broiler feed with HS improved feed conversion and resulted in an increased final weight of broilers. The digestibility and usability of feed are improved by humic acids, which also enhance the environment in the gastrointestinal tract of poultry. The number of enterobacteria, including Escherichia coli, decreased (de Lourdes Angeles et al., 2022). HS are responsible for stabilizing the intestinal microbiome and destroying pathogenic bacteria, viruses, and fungi. At the same time, they are capable of buffering and modulating the pH in the intestine (Arif et al., 2019). The addition of HS to the feed for layers had a positive effect on laying performance, egg weight, feed conversion, and eggshell quality. An immunostimulatory effect was also confirmed (Mudroňová et al., 2021). The beneficial effect of administration of HS to broiler feed was observed in the study by Kocabağli et al. (2002). Marcinčáková et al. (2015) concluded that the addition of HS to the feed of the experimental group significantly increased the carcass weight and carcass yield of experimental broilers. Lower consumption of feed was an additional positive. The composition, quality, and sensory properties of the broiler meat were significantly affected by the supplementation of feed with HS. Although there was a decrease in fat content and pH detected, it increased the oxidative stability of the meet during storage (Hudák et al., 2021).
The popularity of probiotics has surged in recent years, particularly for enhancing the growth performance of broilers. Additionally, probiotics help sustain advantageous gastrointestinal microflora and prevent the proliferation of harmful bacteria. They also affect metabolism by boosting the activity of digestive enzymes while diminishing bacterial enzyme activity and ammonia generation, improving digestion and nutrient absorption, as well as stimulating the immune system (Ahfeethah et al., 2023).
There are numerous studies describing the beneficial, neutral, or even detrimental effects of additives on the broiler organism itself (Ahfeethah et al., 2023). However, there is a lack of information and studies conducted that address the issue of the impact of natural additives–humic substances and probiotics–on environmental conditions in general in animal husbandry. Consequently, the first aim of our research was to determine if incorporating HS into poultry litter alters its physical–chemical characteristics or the levels of harmful gases emitted during broiler chicken rearing. The secondary aim was to examine whether HS alone and in conjunction with probiotics (when used together, humic substances and probiotics can have synergistic effects) can influence the survival and quantities of fecal bacteria as well as the antimicrobial resistance of E. coli isolates.
The experiment was carried out on 120 one-day-old ROSS 308 broiler chickens from a commercial hatchery. Two experimental groups and a control group were randomly formed. Each group consisted of 40 broiler chickens, with three replications (13, 13, and 14 broiler chickens per pen). The nutrition, zootechnical measures, environmental conditions, and welfare standards complied with the criteria for chicken fattening. The broilers were reared for 39 days in pens with a concrete floor on which there was a litter of wood shavings. The groups were kept in separate rooms with artificial lighting and ventilation. Up until the seventh day of age, the light intensity remained between 30 and 40 lux. After that, it was lowered to 5–10 lux until the fattening period was over. The air exchange rate in the rooms ranged from 2 to 2.5 m3/h/kg, which meets the requirements for broiler chickens. A negative-pressure ventilation system ensured the ventilation of the chicken house. This system pushed air out of the building to create a pressure differential that pulled fresh air into the chicken house through the inlets spaced evenly around the perimeter of the building. To ensure fresh air was evenly distributed and directed throughout the chicken house, the air velocity in the inlets was maintained at a level of 3.0 m/s. The room temperature was regulated from 32 ± 1.3°C at the beginning to 22 ± 1.5°C at the end of chicken rearing. In the initial week, heat lamps were utilized to provide increased warmth for the chicks. The humidity was maintained at around 65–70%.
Ad libitum access to feed and water was ensured. The chickens were fed conventional mixed feeds: BR1 (since day 1); BR2 (since day 11); and BR3 (since day 28), according to Jaďuttová et al. (2019). The control group (C) was fed a compound regular feed without supplementation during the experiment. The feed mixture given to the first experimental group (HS) included HS at a concentration of 0.6%. In the second experimental group (HS + P), the chickens received a HS together with the feed and a probiotic strain (Limosilactobacillus fermentum; 1 mL/chicken/day) in water. In Table 1, there are listed the components that make up the HS. HS were added to the feed in powder form. Every day, feed and water were prepared separately for each group, and all containers were labeled to prevent confusion. Throughout the experiment, each pen contained a plastic non-mechanical circular fountain along with a non-mechanical feeder. The fountains ensured a steady supply of clean and fresh drinking water for the broilers. The chickens involved in the experiment were not vaccinated or treated with any medication. The health status of broiler chickens was checked every day.
Granular HS was applied (in three doses) by sprinkling on the wood shavings litter used in the pens in which both experimental groups (HS and HS + P) were housed at a concentration of 0.6% and a final dose of 900 g/m2. The rooms with pens were isolated from each other. No humic material was added to the bedding in the control pen. The litter depth was approximately 7 cm in all pens. We used the 0.6% concentration of the specific HS based on results from previous studies where the effects of HS as feed additives were controlled in different concentrations (Hriciková et al., 2024; Hudák et al., 2021; Mudroňová et al., 2021; Bartkovský et al., 2021; Mudroňová et al., 2020; Marcinčáková et al., 2015). In addition, the study examined the impact of HS on reducing emissions in the agricultural environment and mitigating the occurrence of antibiotic resistance in E. coli. The probiotic strain (Limosilactobacillus fermentum) was administered (HS + P) by oral supplementation using a dose of 1 mL/chicken/day in water, following a pilot experiment. The effect of HS and probiotic strain on production parameters and immune system of broilers is reported by Hudec et al. (2024).
Mixed samples of litter (i.e., a combination of bedding materials, excreta, feathers, spilled feed, and water) were collected weekly from each group (days 7, 14, 21, 28, and 35). According to STN ISO 2859-1 (STN EN 2859-1, 1999), litter samples were collected from all four corners and the central area of pens, and thoroughly mixed to obtain a representative material (subsample). A total of five representative samples were obtained from each pen.
Litter moisture content was determined using the AOAC (2000) methodology. Samples were dried at 105°C to a constant weight in an oven. After drying, the samples were cooled in a desiccator and weighed. The following formula was used to calculate the moisture content:
According to the instructions of ISO standard 10390 (STN EN 10390 (838445), 2021), the pH was measured using a digital pH meter (Hach, Loveland, USA) with a glass electrode. Samples of litter were mineralized in a Digesdahl apparatus (Hach, Loveland, USA) to determine the total nitrogen (N) content according to ISO standard 25663 (STN EN 25663, 2000). Steam distillation with NaOH (for NH4–N distillation with modified pH 7.4) and H2SO4 was used for the digestate. The determination of nitrogen (N and NH4–N) included titration with NaOH (Mulvaney, 1996).
Throughout the rearing period, several sensors were placed on the premises to measure and automatically record the measured data at regular intervals. Temperature and relative humidity of the environment were measured and recorded by using a thermo-hygrometer (Testo, Schwarzwalde, Germany) throughout the day. Litter gas emissions were measured using a plastic chamber connected to a measuring instrument with a uniTOX. The measuring sensors were placed during 1 h of gas measurement in a plastic measuring chamber (volume of approximately 1 m3) equipped with a forced fan, according to Anderson et al. (2021). The measuring instruments used detected the emission of CO2 and NH3 in the ambient air in the rooms. UniTOX.CO2 G infrared carbon dioxide detector (Pro-Service, Kraków, Poland), a uniTOX G electrochemical toxic gas detector (Pro-Service, Kraków, Poland), and a portable Multirae instrument (RAE System by Honeywell, CA, USA) were used.
Litter samples from each group were weighed and diluted 1:10 with distilled water. The resulting mixture was then homogenized. A series of 10-fold dilutions were made from the basic suspension, and 1 μL was inoculated onto the surface of Petri dishes containing the following agars: Meat Pepton Agar (HiMedia, Mumbai, India); Endo Agar (HiMedia, Mumbai, India); Slanetz-Bartley Agar (Merck, Darmstadt, Germany) using the streaking technique. Table 2 is a list of the incubation conditions.
Typical E. coli colonies (pink to rose red with metallic sheen) were inoculated from the Endo agar surface onto the Nutrient agar surface and incubated for 24 h at 37°C. The overnight bacterial culture was used to determine phenotypic antibiotic resistance. The minimum inhibitory concentration (MIC) for selected antibiotics for E. coli isolates (30 isolates from each group) was determined by a microdilution colorimetric plate method according to Gattringer et al. (2002) and by the automated diagnostic system Bel-MIDITECH (Bratislava, Slovakia). This diagnostic system consists of the following antibiotics: ampicillin (AMP); ampicillin + sulbactam (SAM); piperacillin + tazobactam (TZP); cefuroxime (CXM); cefotaxime (CTX); ceftazidime (CAZ); cefoperazone + sulbactam (SPZ); cefepime (FEP); ertapenem (ETP); meropenem (MEM); gentamicin (GEN); tobramycin (TOB); amikacin (AMI); tigecycline (TGC); ciprofloxacin (CIP); tetracycline (TET); colistin (COL); cotrimoxazole (COT). The results of the MIC values for each antibiotic were interpreted according to the clinical breakpoints described by The European Committee on Antimicrobial Susceptibility Testing, version 7.0 (EUCAST, 2017).
The obtained data were recorded in Microsoft Excel 2016 (Microsoft Corporation, WA, USA). The results obtained in this experiment were expressed as means of the appropriate units ± standard deviations (SD). Differences in individual physical–chemical and microbiological parameters were analyzed by a Two-Way ANOVA with a post-hoc Tukey test using time and treatment as the main effects. Values of p < 0.05, p < 0.01, and p < 0.001 indicated significant levels of differences between the control and experimental groups. The results were analyzed and evaluated by the software GraphPad Prism 8.3.0 (GraphPad Software Inc., San Diego, CA, USA). Statistical analysis using the Miditech program generated the percentage of antibiotic-resistant E. coli isolates automatically.
Figure 1 displays the litter moisture content during the experiment. At the time of spreading the bedding on the floor of the experimental pens (day 0), the moisture of the bedding was 6.83 ± 1.9% (this value is our control). After the addition of humic substances, the moisture content in the litter increased to the values of 17.80% ± 1.5 (HS) and 17.54% ± 1.3 (HS + P), while most of the moisture came from HS. On day 14, the moisture level in the experimental groups decreased to 12.67% ± 0.9 (p < 0.05) in group HS and to 14.36% ± 0.5 (p < 0.01) in group HS + P. There was only a slight trend toward increasing the moisture content at the end of the experimental period. During the experiment, we detected a gradual rise in moisture content in the control group. On days 21, 28, and 35, we recorded a significant difference (p < 0.001) in both experimental groups in comparison with the control group. The results also showed that the determined litter moisture contents did not exceed the critical range of 35–40%.
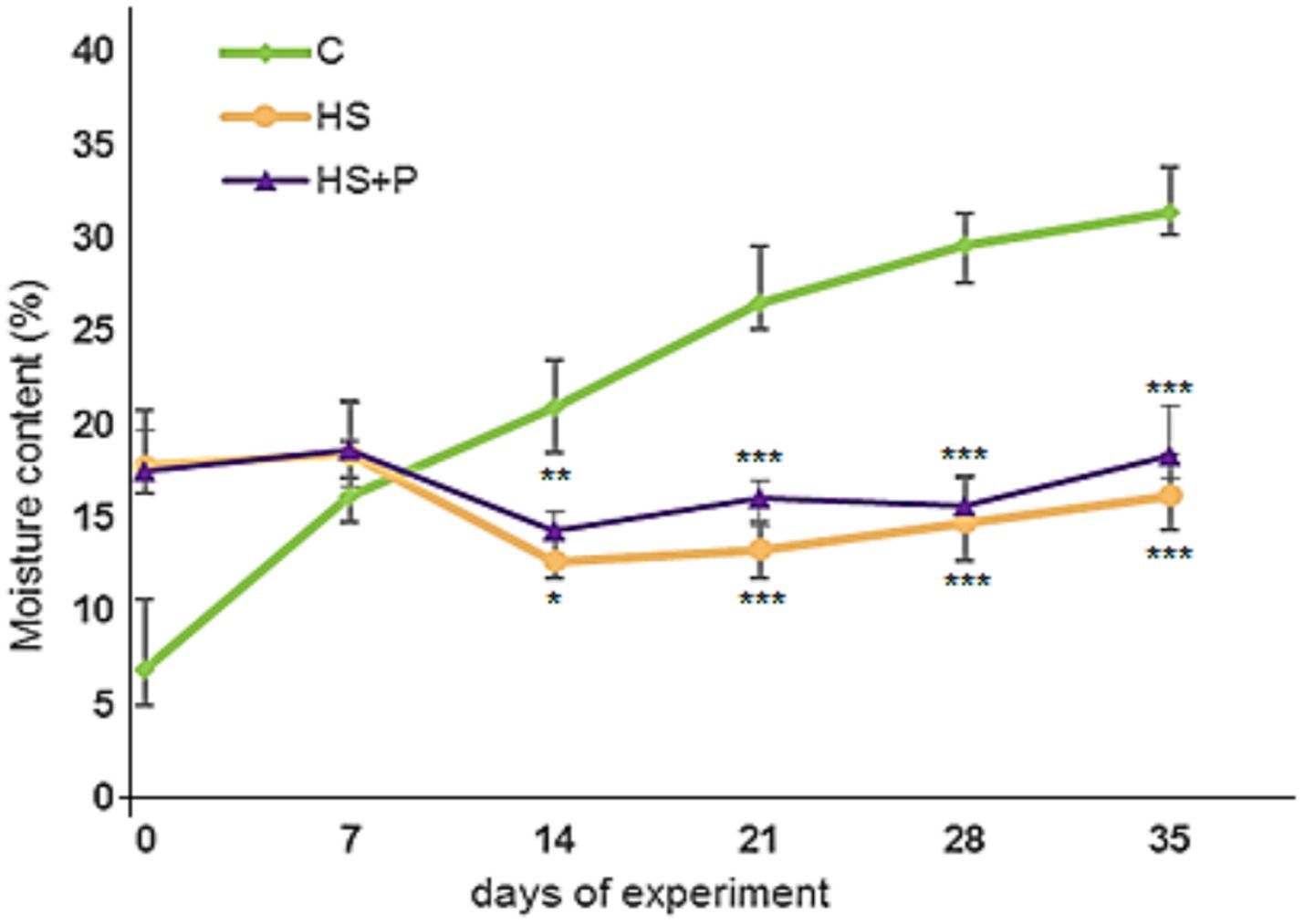
Figure 1. Changes in moisture content in the investigated litters during the experiment including the significance of differences (*p < 0.05; **p < 0.01; ***p < 0.001). C—control group of broilers fed with a diet without supplementation and without litter additives; HS—experimental group of broilers fed with a diet supplemented with humic substances and with litter additives; HS + P—experimental group of broilers fed with a diet supplemented with humic substances and a probiotic strain and with litter additives. An asterisk symbol indicates that the difference between the control and experimental groups (HS and HS + P) is significant at the confidence levels: *p < 0.05; **p < 0.01; ***p < 0.001. Error bars show the SD.
Additional essential physical–chemical parameters of litter are summarized in Table 3.
Before the arrival of the flocks (day 0), the studied litters had a slightly acidic pH reaching the following levels: 5.68 ± 0.09 for HS, 5.78 ± 0.06 for HS + P, and 5.84 ± 0.14 for the control group. The pH value of the litter gradually increased in the control group during the experiment. From an initial value of 6.46 ± 0.15, it increased to 8.11 ± 0.24 on day 35, representing a shift from the slightly acidic to the weakly alkaline region. The pH levels in the litters of the experimental groups with the added HS changed relatively slowly to slightly acidic values. Comparing the experimental groups to the control group revealed no significant differences (p > 0.05) between the impact of time and treatment on pH values. According to our findings, HS could regulate and keep the pH of the litter between the acidic and the neutral level.
We also determined the levels of total N and NH4–N in litter samples. In the control group, total N levels ranged from an initial value of 11.71 g/kg ± 0.08 to 31.87 g/kg ± 0.07 at the end of the experiment. The levels in experimental group HS ranged from 10.13 g/kg ± 0.13 to 30.88 g/kg ± 0.11 g/kg and in the group HS + P from 11.05 g/kg ± 0.04 to 31.12 g/kg ± 0.24. During the fattening period, total N levels increased gradually in all groups, but no statistically significant differences were found between time and treatments (p > 0.05) compared to the control group.
Compared to total N, the levels of NH4–N differed between the groups. The initial concentrations were 3.58 ± 0.03 g/kg in the control group, 3.14 g/kg ± 0.06 in HS and 3.36 g/kg ± 0.11 in HS + P. From day 14, we recorded changes in concentrations between the groups. On day 35, the NH4–N levels in the litter of the control group decreased (2.21 g/kg ± 0.09) when ideal conditions were reached for NH4 conversion to gaseous NH3. In contrast, the levels in the experimental groups were higher (10.04 g/kg ± 0.25 for HS and 9.99 g/kg ± 0.18 for HS + P at the end of the experiment) but without significant effect of time and treatments (p > 0.05).
In general, the measured gas emissions (CO2 and NH3) did not exceed the acceptable recommended limits, which ensured that the animals had suitable living conditions throughout the experiment.
The emissions of CO2 and NH3 showed no significant differences between the groups (Figures 2, 3). It is evident that as the broilers raised the concentrations of both gases increased. The highest concentrations were detected on day 35 of the experiment. The gas concentrations in the experimental groups of broilers housed on litter amended with HS increased gradually but at a lower rate than those in the control group. The measured CO2 concentrations ranged from 850 ppm ± 30 to 2,210 ppm ± 115 in the HS group and from 855 ppm ± 50 to 2,300 ppm ± 130 in the HS + P group, in contrast with the control group, where they ranged from 975 ppm ± 45 to 2,630 ppm ± 155. NH3 emissions from experimental groups ranged from 0.7 ppm ± 0.1 to 3.5 ppm ± 0.12 (HS) and from 0.8 ppm ± 0.6 to 3.7 ppm ± 0.16 (HS + P) compared to 0.9 ppm ± 0.4–5.0 ppm ± 0.34 in the control group. We did not observe a statistically significant effect of time and effect of treatment on CO2 and NH3 concentrations (p > 0.05). No presence of hydrogen sulfide (H2S) in the environment (0 ppm) of all three groups was detected during the experiment. HS added to the bedding materials during the experiment could help absorb the unwanted gases, thus reducing their concentration in the air in the broiler house and creating a more optimal environment for the fattening of broiler chickens. Our findings indicated that HS added to the litter participated in reduced moisture content, ensured a pH lower than 7, and also could reduce the release of harmful gases in experimental groups compared to the control group (although without statistically significant differences).
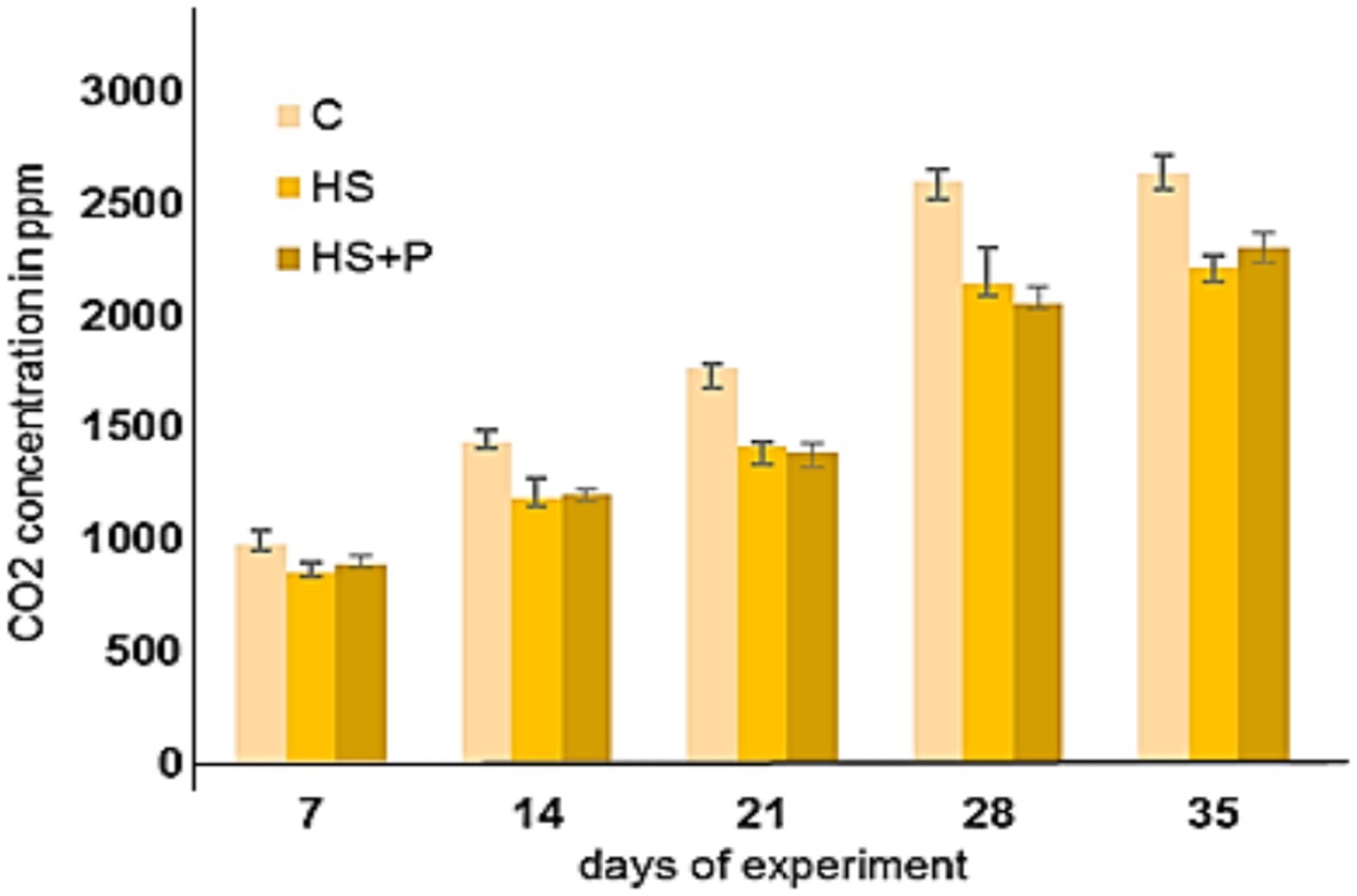
Figure 2. Concentrations of CO2 in the control and experimental groups. C—control group of broilers fed with a diet without supplementation and without litter additives; HS—experimental group of broilers fed with a diet supplemented with humic substances and with litter additives; HS + P—experimental group of broilers fed with a diet supplemented with humic substances and a probiotic strain and with litter additives. Error bars show the SD. No statistically significant differences were found between time and treatments (p > 0.05) compared to the control group.
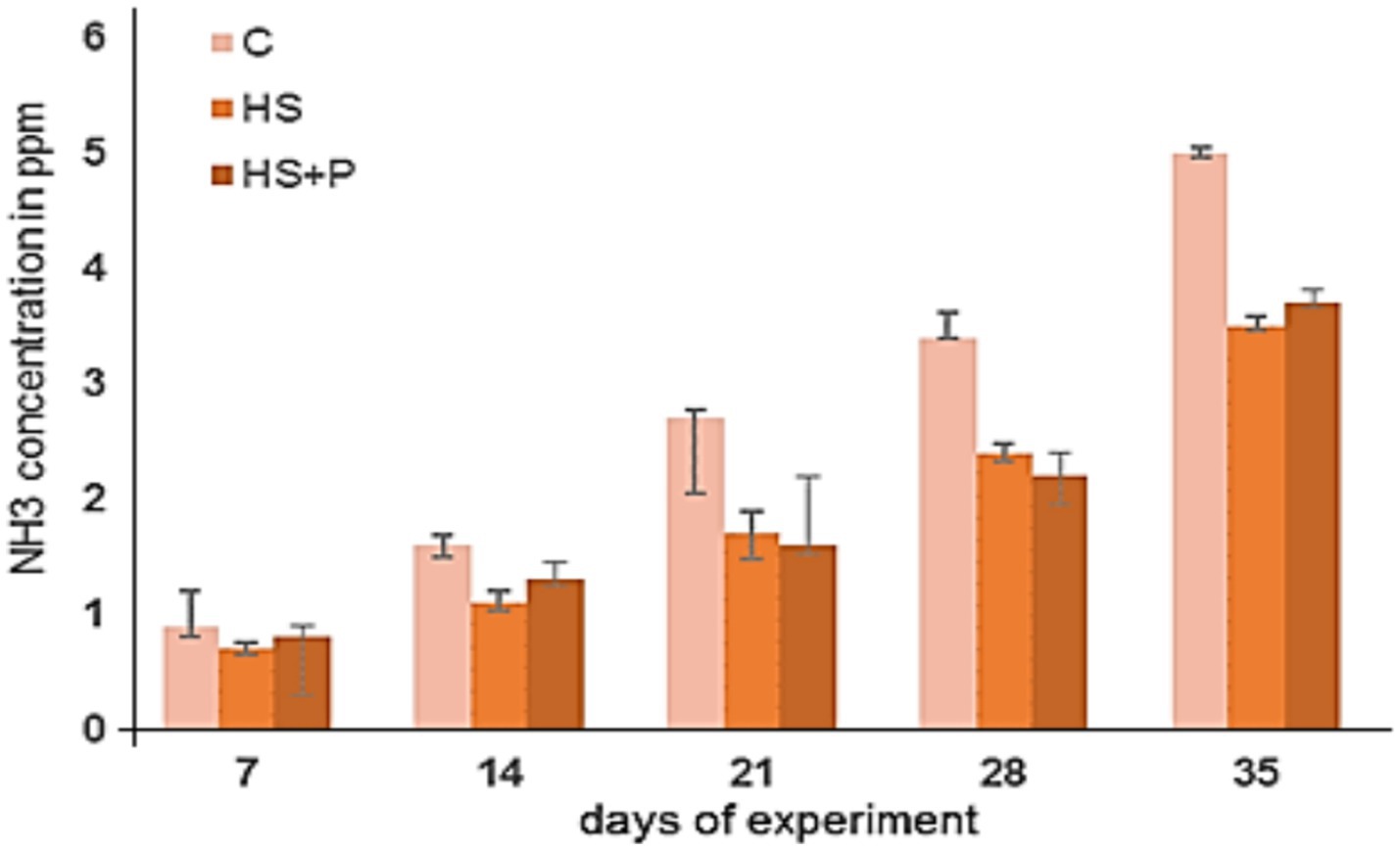
Figure 3. Concentrations of NH3 in the control and experimental groups. C—control group of broilers fed with a diet without supplementation and without litter additives; HS—experimental group of broilers fed with a diet supplemented with humic substances and with litter additives; HS + P—experimental group of broilers fed with a diet supplemented with humic substances and a probiotic strain and with litter additives. Error bars show the SD. No statistically significant differences were found between time and treatments (p > 0.05) compared to the control group.
Microbial contamination of the litter during the fattening of broilers was checked every 7 days. On day 7, total bacterial counts in the litter of evaluated groups ranged from 7.00 to 7.36 log10 CFU/g. In all groups, we observed a no significant increase in total bacterial counts by 2 logs by the end of the experiment. On day 35, we observed that the effect of HS and the combination of HS and the probiotic strain significantly reduced the counts of coliform bacteria (p < 0.01) and fecal coliform bacteria (p < 0.05). There was no statistically significant effect of time and treatment on the total count of bacteria and fecal enterococci (p > 0.05) compared to the control group (Figures 4–6).
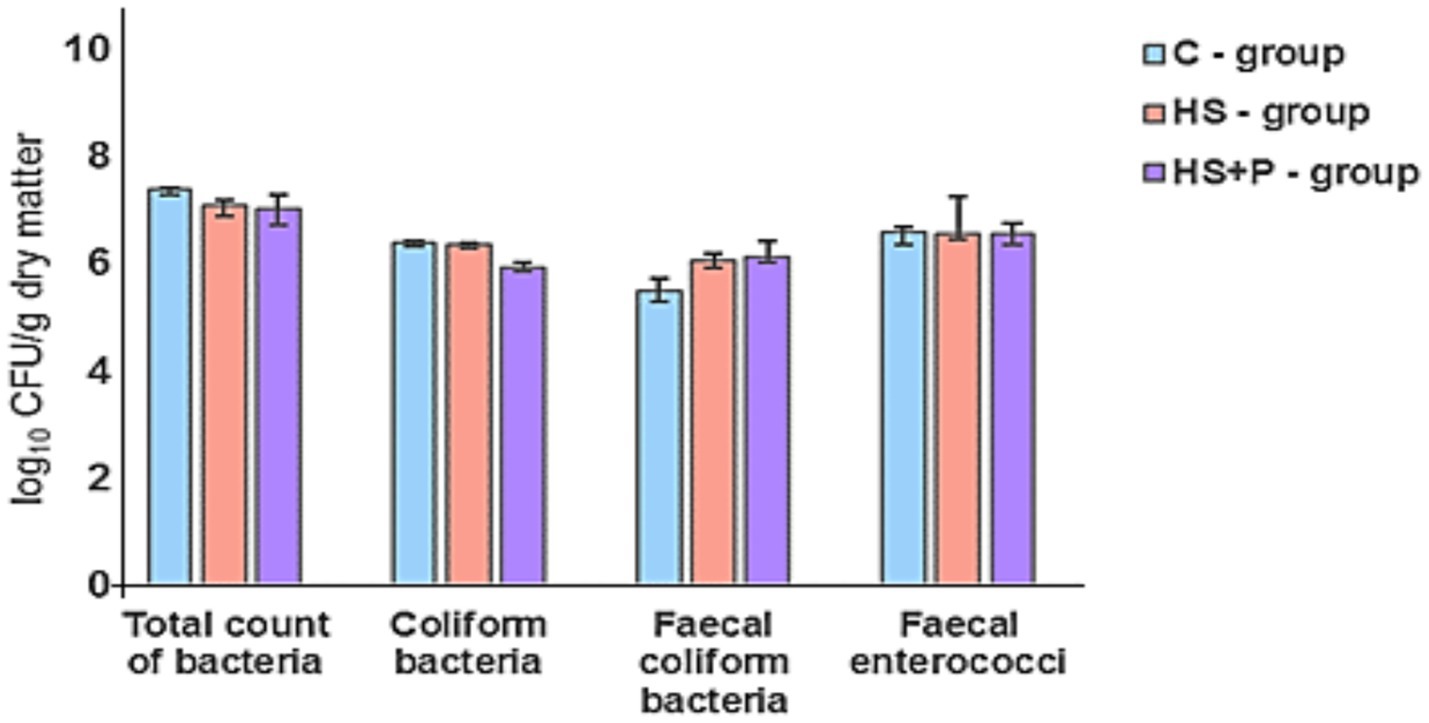
Figure 4. Bacterial contamination (log10) in litter (1 g) detected on day 7. C—control group of broilers fed with a diet without supplementation and without litter additives; HS—experimental group of broilers fed with a diet supplemented with humic substances and with litter additives; HS + P—experimental group of broilers fed with a diet supplemented with humic substances and a probiotic strain and with litter additives. Error bars show the SD. No statistically significant differences were found between time and treatments (p > 0.05) compared to the control group.
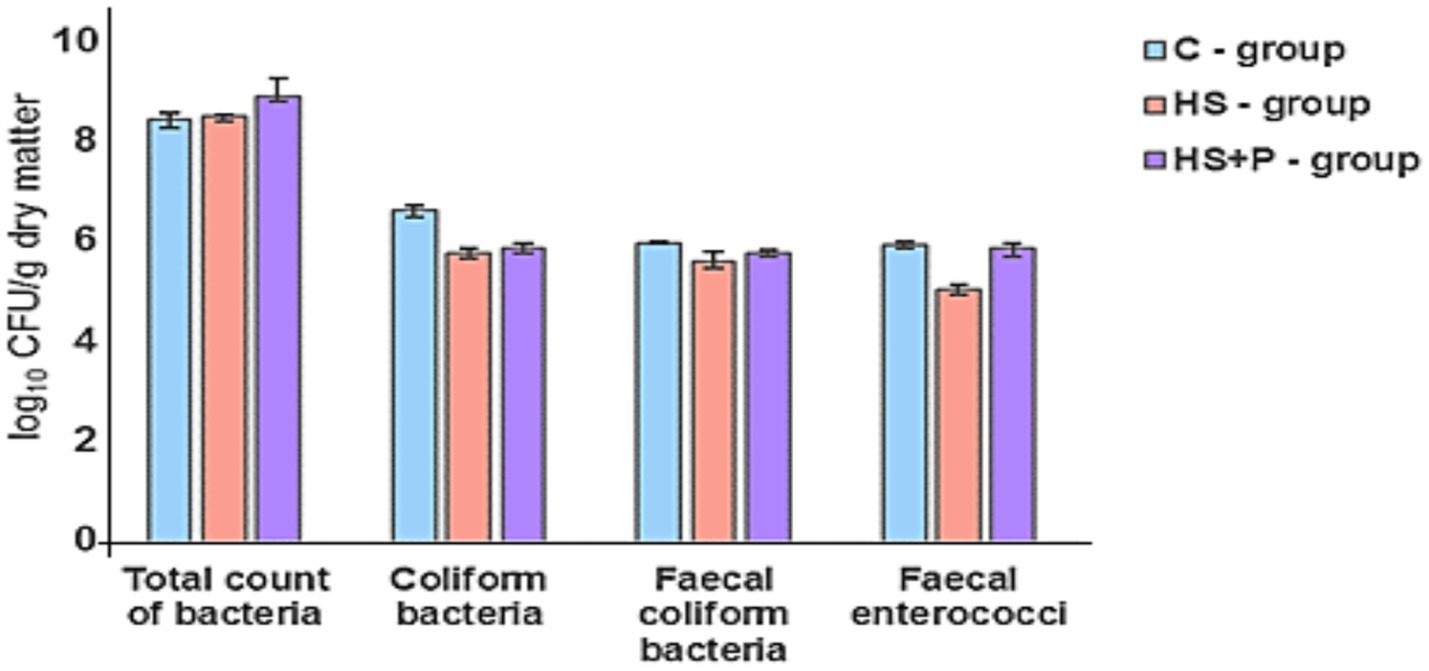
Figure 5. Bacterial contamination (log10) in litter (1 g) detected on day 21. C—control group of broilers fed with a diet without supplementation and without litter additives; HS—experimental group of broilers fed with a diet supplemented with humic substances and with litter additives; HS + P—experimental group of broilers fed with a diet supplemented with humic substances and a probiotic strain and with litter additives. Error bars show the SD. No statistically significant differences were found between time and treatments (p > 0.05) compared to the control group.
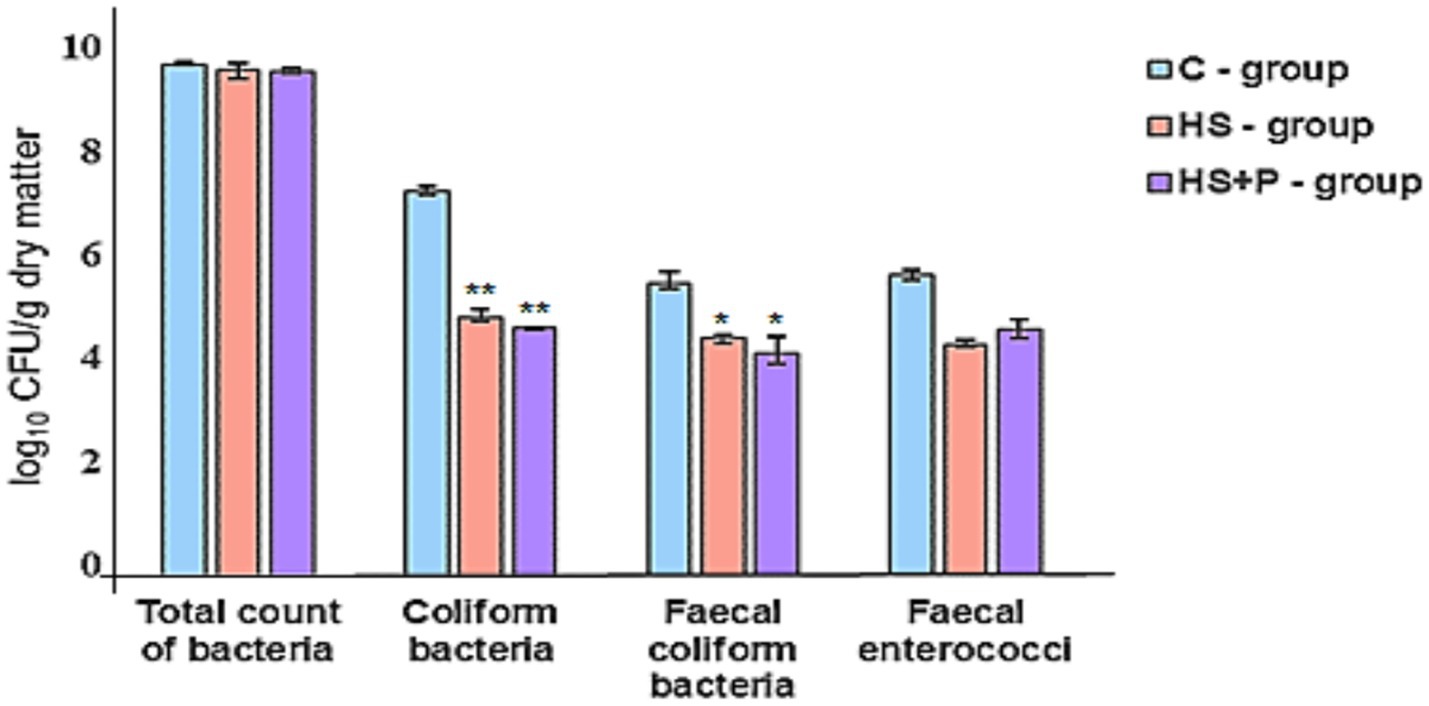
Figure 6. Bacterial contamination (log10) in litter (1 g) detected on day 35. C—control group of broilers fed with a diet without supplementation and without litter additives; HS—experimental group of broilers fed with a diet supplemented with humic substances and with litter additives; HS + P—experimental group of broilers fed with a diet supplemented with humic substances and a probiotic strain and with litter additives. An asterisk symbol indicates that the difference between the control and experimental groups (HS and HS + P) is significant at the confidence levels: *p < 0.05; **p < 0.01. Error bars show the SD.
Ninety E. coli strains isolated from the litter were subjected to antibiotic susceptibility tests (30 isolates from each group). The tests revealed resistance to ciprofloxacin, tetracycline, ampicillin + sulbactam, and cotrimoxazole. Escherichia coli strains were susceptible to TZP, CXM, CTX, CAZ, SPZ, FEP, ETP, MEM, GEN, TOB, AMI, TGC, and COL, and therefore these ATBs are not shown in Figure 7.
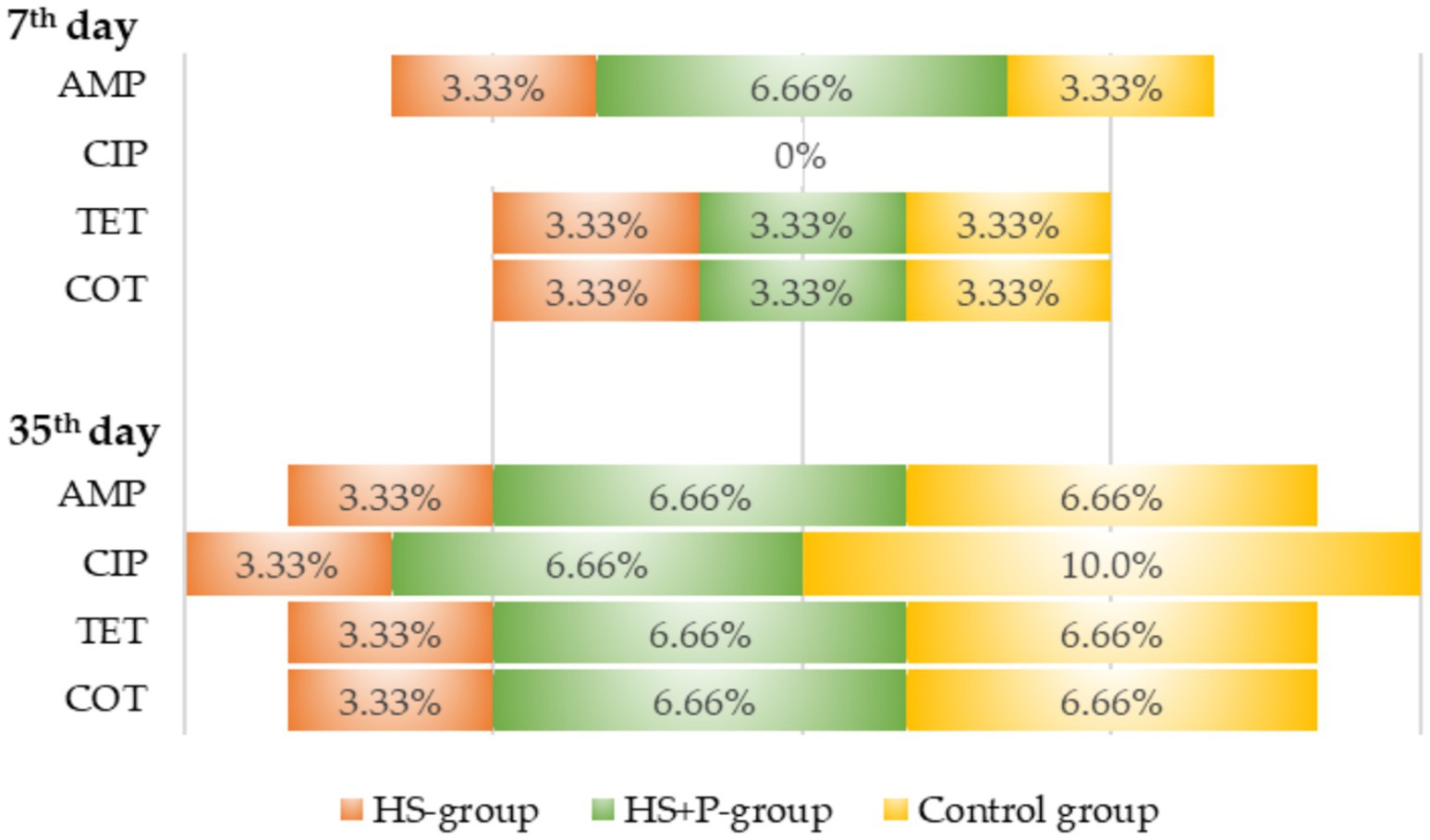
Figure 7. Percentage of antibiotic resistance of E. coli isolates from individual groups. C—control group of broilers fed with a diet without supplementation and without litter additives; HS—experimental group of broilers fed with a diet supplemented with humic substances and with litter additives; HS + P—experimental group of broilers fed with a diet supplemented with humic substances and a probiotic strain and with litter additives.
There were not many noticeable differences in ATB resistance between the experimental groups. The level of resistance ranged from 3.3 to 10.0% of E. coli resistant to ATB. Comparison with the control group allowed us to conclude that the addition of HS and the combination of HS and probiotic strains had no or little effect on the level of antibiotic resistance of E. coli isolates.
The physical–chemical properties of the bedding material can influence the activity of microorganisms and the production of gases. The ideal bedding material should be able to not only absorb moisture but also release moisture quickly. An essential characteristic for evaluation of the litter quality is its capacity to retain and discharge water (Garcês et al., 2013).
Rico-Contreras et al. (2017) classified poultry litter according to moisture content into three groups: 1. dry litter (25%), 2. semi-moist litter (25–35%), and 3. wet litter (35%).
During the experiment, we detected a gradual rise in moisture content in the control group. On days 21, 28, and 35, we recorded a significant decrease (p < 0.001) in both experimental groups (dry litters) in comparison with the control group (semi-moist litter). At the end of the experiment, the control group reached the highest value, which, however, did not exceed the critical threshold of 35%.
When HS are added to poultry litter, they act as a natural material that can retain moisture through adsorption, binding properties, and aeration. HS incorporated into litter can absorb excess moisture and reduce the overall moisture content of the litter. They can bind with molecules of water, hindering their evaporation and thus leading to drier bedding material. By reducing litter moisture, HS can also help improve the airflow and aeration of the poultry house. These factors can contribute to a healthier environment for poultry and reduce the potential for NH3 emissions (Vikram et al., 2022; Fragouli et al., 2023; Bay, 2021; Sharma and Anthal, 2016).
In our experiment, we also found an increase in pH, which represents a shift from the slightly acidic to the weakly alkaline region. On the contrary, in the experimental groups, the pH during rearing was maintained in only a slightly acidic area. Due to the low pH, the growth of microbes responsible for the production of NH3 was inhibited, the activity of decomposition of nitrogenous substances slowed down, and the ammonium ion (NH4+) remained stable, which reduced the concentration of harmful gases in the environment.
The accumulation of chicken feces increases the pH of the litter (Munir et al., 2019). Garcês et al. (2013) found an increase in the pH of wood shaving litter from 6.3 to 8.9 after 35 days of chicken rearing. In other experiments with the addition of peat to the litter for chickens, the pH value increased from 6.53 to 8.13 (Živkov-Baloš et al., 2020) or from 4.0 to 8.1 (Kaukonen et al., 2017).
The pH value of the litter is also an important factor affecting the growth and survival of pathogenic microorganisms (Jie et al., 2022).
Poultry manure is a rich source of nitrogen for the increased intake of amino acids and proteins by feed. Chicken litter also contains a high proportion of many other minerals (C, P, K, Ca, S, Mg, Cu, B, Fe, Zn, Mo, and Mn) and organic matter, which makes it one of the most valuable livestock wastes (Chen and Jiang, 2014; Živkov-Baloš et al., 2020). Nitrogen occurs in chicken litter in several forms, with uric acid being the predominant form (Murakami et al., 2011). The combination of increased litter moisture and high environmental temperatures favors the growth and activity of bacteria that rapidly convert uric acid into NH3 gas (Garcês et al., 2013). The total N content of the litter immediately after the hen has defecated is approximately 60 g/kg (Nakatani, 2002). In general, poultry feces generally contain higher amounts of N than other types of animal manure (Murakami et al., 2011).
Although there was an increasing trend in the total N detected values from day 7 to day 35, there were no significant differences between the control and experimental groups according to the effect of time and treatment. According to the results, HS had no effect on total N concentrations. We believe that it could be explained hypothetically, that litter in the control experiment contains other types of N compounds such as ammonium, nitrite, and nitrate.
The measured NH3 emissions and the calculated NH4–N values were related. In the control group, we confirmed lower values of NH4–N, but high concentrations of gaseous NH3. On the contrary, HS in the litter absorbed and accumulated NH4–N, which resulted in higher values of NH4–N, but we detected lower NH3 emissions in the air of the chicken house. However, no significant differences were found between the control and experimental groups during the fattening period.
The HS in the litter affected the absorption of NH4+ due to environmental factors (humidity, temperature, moisture content, and pH level). NH4+ was not converted into NH3 and released into the atmosphere; instead, it remained as an ionized form of NH3 in the litter.
In poultry litter, the addition of HS led to a decrease in moisture level (p < 0.001). Although statistical significance was not found, HS in the experimental groups appeared to influence pH stabilization (below 7), inhibited NH4+ absorption, and retained NH4+ as a stable ammonium ion compared to the control group, which also contributed to a reduced release of harmful gases into the environment.
The quality of the indoor air in the chicken house largely depends on the litter quality (Živkov-Baloš et al., 2020). Volatile particles also originate from NH3, which arises mainly from the degradation of nitrogenous substances in feces and urine by the activity of microorganisms present in the litter and negatively affects poultry performance (Al-Kerwi et al., 2022). One chicken during fattening is capable of producing approximately 1.05 kg of litter and manure (Moore et al., 2011).
The handling, storage, and processing of poultry manure on poultry farms contribute significantly to the production of greenhouse gas emissions. Environmental temperature (>32°C) and humidity (>70%), litter’s pH (>9), and litter’s moisture (>40%) are the factors that result in increased conversion of NH4+ ions to NH3 gas (Fogaça et al., 2022). In order to reduce and control the emission of harmful gases in the chicken house and to ensure optimum living conditions for the poultry, additives with suitable adsorption properties can be added to the litter (Jie et al., 2022). The addition of HS into bedding material for broilers is able to bind harmful gases (such as NH3, CO2, CH4, or H2S), as well as other compounds dangerous for the organism (Hriciková et al., 2023).
Concentrations of NH3 and CO2 in the air were not exceeded in our experiment with broiler chickens in either group, as stated in Council Directive 2007/43/EC (2007). The emissions of CO2 and NH3 showed no significant differences between the experimental groups by the effect of time and treatment. However, the gas concentrations in both experimental groups (HS and HS + P) increased gradually but at a lower rate than in the control group. HS added to the bedding materials during the experiment probably absorbed the investigated gases (CO2, NH3), thus reducing their concentration in the air of the broiler house (2 ppm difference in NH3).
Adsorption of NH3 by HS is a complex process that involves a combination of physical and chemical interactions. This process is facilitated by the structure and properties of HS, such as their high surface area, porosity, and functional groups. The combination of these factors allows HS to effectively adsorb NH3 molecules, thereby helping to reduce NH3 emissions from broiler litter and improving air quality in the poultry environment. HS have a complex and diverse molecular structure that provides a large surface area for interactions with other molecules. The large surface area allows more opportunities for NH3 molecules to come into contact with HS and bind to them (Fragouli et al., 2023; Song et al., 2019). HS contain functional groups such as carboxyl (–COOH), phenolic (–OH), and amino groups (–NH2), which can attract and bind NH3 molecules through electrostatic interactions and hydrogen bonding, which allows them to act as effective NH3 adsorbents, especially in soil and water systems (Mahler et al., 2021). In the case of NH3, the positively charged ammonium ion (NH4+) can be attracted to the negatively charged functional groups of HS (Zhang et al., 2022). The porous character of HS allows NH3 molecules to diffuse into their internal structure, where they can be temporarily immobilized. This helps to reduce the immediate release of NH3 gas into the environment (Alvarez-Puebla et al., 2005). When the NH3 molecule comes into contact with the surface of the humic substance, it can form chemical bonds with the functional groups present. This adsorption process involves the transfer of electrons between the NH3 molecule and the humic substance, which leads to the binding of the NH3 molecule to the surface of the material (Xi et al., 2018).
Vučemilo et al. (2007) found a significant relationship between increasing air NH3 concentration and the age of animals and humidity. The recorded CO2 emissions were lower in the experimental groups compared to the control group. Miles et al. (2006) reported that CO2 levels in broiler houses increased over time as the broiler chickens grew and breathed. As stated in the Council Directive 2007/43/EC (2007), laying down minimum rules for the protection of chickens kept for meat production, as amended, that in poultry farming, it is imperative to guarantee that air NH3 concentrations of 20 ppm and CO2 concentrations of 3,000 ppm are not exceeded.
The most reliable strategies to reduce the concentration of harmful gases involve regular examination of the conditions of the internal environment in poultry housings, ensuring sufficient air circulation, monitoring the moisture of the litter, and using additives (e.g., humic substances) to prevent the release of gases (Kilic and Yaslioglu, 2014; Bailey et al., 2021).
In general, there is a complex relationship between HS and bacterial populations that can vary depending on the environment and conditions. In addition, HS can positively affect the microflora in the gastrointestinal tract of broilers and in litter. They can act as a source of nutrients and energy, supporting the growth and activity of beneficial bacteria (such as Lactobacillus and Bifidobacterium) and thus improving gut health and overall broiler performance. They can also help to maintain a balanced gut microbiome and reduce the number of pathogens, including Salmonella typhimurium, Enterobacter cloacae, Proteus vulgaris, Pseudomonas aeruginosa, and Staphylococcus spp., by inhibiting adherence in the avian organism (Korsakov et al., 2019; Domínguez-Negrete et al., 2019; Arif et al., 2019). Overall, the use of HS can support healthy intestinal microflora in broilers and contribute to better litter management. In addition, HS can serve as a surface for some bacteria to attach to and help them colonize and form biofilms. These biofilms can protect these bacteria from environmental factors and facilitate communication with other bacterial species (Bogdanov et al., 2022; Rodrigues et al., 2008). HS can stabilize pH levels at either acidic or neutral levels, absorb adverse gases, and significantly lower the moisture content of litter.
Apart from the previously mentioned benefits, probiotics used in chicken farming can also replace harmful bacteria and eliminate them from the digestive system (Bhogoju and Nahashon, 2022). According to Ouwehand et al. (2016), probiotics do not contribute to the spread of antimicrobial resistance and may even reduce it. To what extent this is possible is still under investigation. Coliforms, fecal coliform bacteria and fecal enterococci are bacteria naturally occurring in the gastrointestinal tract of broilers. During the experiment, we observed a positive effect of the additives on the counts of the investigated bacteria. HS and combinations of HS with probiotics suppressed the growth of coliform bacteria, fecal coliform bacteria, and fecal enterococci.
The administered additives had a beneficial effect on the adjustment of microflora in the gastrointestinal tract of broilers and also in litter, compared to the control group. Our findings are consistent with those of Thaxton et al. (2003), who discovered that despite the unsuitable conditions, the mentioned bacteria were reduced but not eliminated. They concluded that a defined population of bacteria formed over time. The bacteria concentration remained stable regardless of the number of housed chickens. Winkler et al. (2017) found an average of less than 7.1 log10 CFU/g of coliform bacteria in litter from 12 poultry farms. They did not record any significant differences in counts between the individual sampling sites. According to Barker et al. (2010), the average coliform counts ranged from 6.37 to 7.17 log10 CFU/g on three broiler farms. Likewise, Terzich et al. (2000) detected average counts of coliform bacteria in litter samples from several countries, ranging from 6.42 to 8.77 log10 CFU/g. The counts of fecal enterococci in the control group (6.85 log10 CFU/g) were similar to the findings by Winkler et al. (2017) and Diarra et al. (2007), who reported a mean value of 6.4 and 7.86 log10 CFU/g. The average counts of fecal enterococci in the litter samples in the experimental groups were lower. Our results are consistent with other studies that observed an increase in the total count of bacteria by at least one or two orders of magnitude during the first 3 weeks of broiler fattening (Gontar et al., 2022; Milanov et al., 2019).
Even though the broiler chickens were not given any antibiotics during the fattening period, we discovered that antibiotic-resistant E. coli isolates were present in their litter. ATB-resistant E. coli isolates in the litter from hatched chicks could originate from the vertical transmission of genetic information from the parents as well as from the environment of the hatchery itself. ATB resistant genes can be transmitted by horizontal gene transfer within flocks and farm staff (Khong et al., 2023). We confirmed resistance to AMP, SAM, CIP, TET, and COT in the E. coli isolates from all investigated groups, with a low difference in results. The percentage of resistance ranged from 3.3 to 10.0%. This suggested that even if the broilers did not receive any drugs during fattening, antibiotic-resistant strains of E. coli may have been spread in the environment.
Another study conducted on a commercial Slovak broiler farm reported similar or higher results concerning the litter of one-week old broilers. The E. coli isolates were resistant to ampicillin (85.3%), enrofloxacin (83.8%), and cephalosporins (ceftiofur – 36.8% and cefquinome – 19.1%) which gradually decreased during their growth (Gregová et al., 2013).
Although there were not evident differences in ATB-resistance of E. coli isolates, HS and probiotics can have a positive effect on gut microflora. The concentrations of coliform bacteria, fecal coliform bacteria, and fecal enterococci were lower in the litter of the HS and HS + P groups.
Another study conducted by our team revealed that 0.8% HS addition to broiler feed had an immunostimulatory effect on phagocytic activity and gut protection (Mudroňová et al., 2020). The 0.6% supplementation of feed with HS positively affected the quality of the produced breast meat (pH and lipid oxidation) (Hudák et al., 2021). The same beneficial effect of humic substance administration during broiler fattening was observed in the study by Kocabağli et al. (2002).
In conclusion, adding humic substances to bedding materials effectively absorbs excess moisture, resulting in a lower overall moisture level in the litter. Although the statistical significance remains unconfirmed, we noticed enhanced physical–chemical properties in the litter, with reduced gas concentrations in groups that received humic substances compared to the control group. Furthermore, the use of humic substances, whether used alone or in conjunction with a probiotic strain, led to a considerable decrease in the counts of coliform and fecal coliform bacteria in the litter. Given these results, further investigation is necessary to examine the potential advantages of these feed additives in intensive farming systems.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The animal study was approved by the Ethical Committee for Animal Care and Use of University of Veterinary Medicine and Pharmacy in Košice (Slovakia) and the State Veterinary and Food Administration of the Slovak Republic under the protocol no. 3040-14-221. The “European Directive on the protection of vertebrate animals used for experimental and other scientific purposes” (EC, 2010) was followed in all aspects of the current study’s procedures. The study was conducted in accordance with the local legislation and institutional requirements.
ND: Conceptualization, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. GG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. TS: Data curation, Methodology, Supervision, Writing – review & editing. SM: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This publication was supported by the Operational program Integrated Infrastructure within the project: Demand-driven research for the sustainable and innovative food, Drive4SIFood 313011V336, co-financed by the European Regional Development Fund. Also, it was supported by Cultural and educational grant agency by Ministry of Education, Science, Research and Sport of the Slovak Republic, project KEGA 001UVLF-4/2022.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahfeethah, F., Elazomi, A., and Kammon, A. (2023). Effect of humic acid and probiotics on immunity of broiler chickens. Open Vet. J. 13, 839–845. doi: 10.5455/OVJ.2023.v13.i7.5
Al-Kerwi, M. S. M., Mardenli, O., Jasim, M. R. M., and Al-Majeed, M. A. (2022). Effects of harmful gases emitted from poultry houses on productive and health performance. IOP Conf. Ser.: Earth Environ. Sci. 1060:012082. doi: 10.1088/1755-1315/1060/1/012082
Alomar, T., Qiblawey, H., Almomani, F., Al-Raoush, R. I., Han, D. S., and Ahmad, N. M. (2023). Recent advances on humic acid removal from wastewater using adsorption process. J. Water Process Eng. 53:103679. doi: 10.1016/j.jwpe.2023.103679
Alvarez-Puebla, R. A., Goulet, P. J. G., and Garrido, J. J. (2005). Characterization of the porous structure of different humic fractions. Colloids Surf. A Physicochem. Eng. Asp. 256, 129–135. doi: 10.1016/j.colsurfa.2004.12.062
Anderson, K., Moore, P. A. Jr., Martin, J., and Ashworth, A. J. (2021). Evaluation of a novel poultry litter amendment on greenhouse gas emissions. Atmos. 12:563. doi: 10.3390/atmos12050563
AOAC (2000). Official methods of analysis. 17th Edn. The Association of Official Analytical Chemists (AOAC): Gaithersburg, USA.
Arif, M., Alagawany, M., Abd El-Hack, M. E., Saeed, M., Arain, M. A., and Elnesr, S. S. (2019). Humic acid as a feed additive in poultry diets: a review. Iran. J. Vet. Res. 20, 167–172. 31656520. doi: 10.22099/ijvr.2019.5345
Ayalew, H., Zhang, H., Wang, J., Wu, S., Qiu, K., Qi, G., et al. (2022). Potential feed additives as antibiotic alternatives in broiler production. Front. Vet. Sci. 9:916473. doi: 10.3389/fvets.2022.916473
Bailey, M. A., Hess, J. B., Krehling, J. T., and Macklin, K. S. (2021). Broiler performance and litter ammonia levels as affected by sulfur added to the bird's diet. J. Appl. Poult. Res. 30:100159. doi: 10.1016/j.japr.2021.100159
Barker, K. J., Purswell, J. L., Davis, J. D., Parker, H. M., Kidd, M. T., McDaniel, C. D., et al. (2010). Distribution of bacteria at different poultry litter depths. Int. J. Poult. Sci. 9, 10–13. doi: 10.3923/ijps.2010.10.13
Bartkovský, M., Marcinčáková, D., Makiš, A., Klempová, T., Semjon, B., Roba, P., et al. (2021). Effect of feeding of 10% prefermented feed on fatty acid profile and oxidation changes in chicken breast meat. MASO Int. J. Food Sci. Technol. 11, 11–15. doi: 10.2478/mjfst-2022-0002
Bay, J. (2021). The powerful features of humic acid fertilizer. Available online at: https://www.linkedin.com/pulse/powerful-features-humic-acid-fertilizer-tim-weng-2c (Accessed March 28, 2021).
Bezuglova, O., and Klimenkom, A. (2022). Application of humic substances in agricultural industry. Agronomy 12:584. doi: 10.3390/agronomy12030584
Bhogoju, S., and Nahashon, S. (2022). Recent advances in probiotic application in animal health and nutrition: a review. Agriculture 12:304. doi: 10.3390/agriculture12020304
Bogdanov, K. I., Kostina, N. V., Plakunov, V. K., and Zhurina, M. V. (2022). Effect of humic acids on biofilm formation on polyethylene surface and its biodegradation by soil bacteria. Eurasian Soil Sc. 55, 474–484. doi: 10.1134/S1064229322040056
Chen, Z., and Jiang, X. (2014). Microbiological safety of chicken litter or chicken litter-based organic fertilizers: a review. Agriculture 4, 1–29. doi: 10.3390/agriculture4010001
de Lourdes Angeles, M., Gómez-Rosales, S., and Téllez-Isaias, G. (2022). “Mechanisms of action of humic substances as growth promoters in animals” in Humus and humic substances – Recent advances. ed. A. Makan (London: IntechOpen).
Diarra, M. S., Silversides, F. G., Diarrassouba, F., Pritchard, J., Masson, L., Brousseau, R., et al. (2007). Impact of feed supplementation with antimicrobial agents on growth performance of broiler chickens, Clostridium perfringens and Enterococcus counts, and antibiotic resistance phenotypes and distribution of antimicrobial resistance determinants in Escherichia coli isolates. Appl. Environ. Microbiol. 73, 6566–6576. doi: 10.1128/AEM.01086-07
Domínguez-Negrete, A., Gómez-Rosales, S., Angeles, M. D. L., López-Hernández, L. H., Reis-de Souza, T. C., López-García, Y., et al. (2019). Effect of the addition of humic substances as growth promoter in broiler chickens under two feeding regimens. Animals 9:1101. doi: 10.3390/ani9121101
EC (2007). Council directive 2007/43/EC of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production. Off. J. Eur. Union. 182, 19–28.
EC (2010). Directive 2010/63/EU of the European Parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union. 276, 33–79.
Edmonds, M. S., Johal, S., and Moreland, S. (2014). Effect of supplemental humic and butyric acid on performance and mortality in broilers raised under various environmental conditions. J. Appl. Poult. Res. 23, 260–267. doi: 10.3382/japr.2013-00901
EUCAST (2017). Guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Version 2.0. Växjö, Sweden: European Committee on Antimicrobial Susceptibility Testing (EUCAST).
Fogaça, I., Ferreira, E., Mehanna, M. N., Saturnino, K. C., and Doni, T. R. D. S. (2022). Ammonia levels during broiler rearing cycle. Arq. Inst. Biol. 89:e00202020. doi: 10.1590/1808-1657000202020
Fragouli, P. G., Roulia, M., and Vassiliadis, A. A. (2023). Macromolecular size and architecture of humic substances used in the dyes’ adsorptive removal from water and soil. Agronomy 13:2926. doi: 10.3390/agronomy13122926
Garcês, A. P. J. T., Afonso, S. M. S., Chilundo, A., and Jairoce, C. T. S. (2013). Evaluation of different litter materials for broiler production in a hot and humid environment: 1. Litter characteristics and quality. J. Appl. Poult. Res. 22, 168–176. doi: 10.3382/japr.2012-00547
Gattringer, R., Niks, M., Ostertag, R., Schwarz, K., Medvedovic, H., Graninger, W., et al. (2002). Evaluation of MIDITECH automated colorimetric MIC reading for antimicrobial susceptibility testing. J. Antimicrob. Chemother. 49, 651–659. doi: 10.1093/jac/49.4.651
Gautam, R. K., Navaratna, D., Muthukumaran, S., Singh, A., Islamuddin,, and More, N. (2021). “Biology associated with soil, plants and environment” in Humic substances. ed. A. Makan (London: IntechOpen).
Goel, P., and Dhingra, M. (2021). “Humic substances: prospects for use in agriculture and medicine” in Humic substances. ed. A. Makan (London: IntechOpen).
Gontar, Ł., Sitarek-Andrzejczyk, M., Kochański, M., Buła, M., Drutowska, A., Zych, D., et al. (2022). Dynamics and diversity of microbial contamination in poultry bedding materials containing parts of medicinal plants. Materials 15:1290. doi: 10.3390/ma15041290
Gregová, G., Venglovský, J., Kmeť, V., and Zábronský, R. (2013). “Microbial environmental contamination and zoonotic risk of antibiotic resistant Escherichia coli in broiler chickens” in In ISAH. Animal hygiene, health and welfare as corner stones of sustainable animal production; 2013 May 5–9 (Nanjing: Nanjing Agricultural University), 109–111.
Hriciková, S., Kožárová, I., Hudáková, N., Reitznerová, A., Nagy, J., and Marcinčák, S. (2023). Humic substances as a versatile intermediary. Life 13:858. doi: 10.3390/life13040858
Hriciková, S., Kožárová, I., Koréneková, B., and Marcinčák, S. (2024). The effect of the supplementation of humic substances and fermented products in the feed on the content of salinomycin residues in poultry tissues. Food Secur. 13:68. doi: 10.3390/foods13010068
Hudák, M., Semjon, B., Marcinčáková, D., Bujňák, L., Naď, P., Koréneková, B., et al. (2021). Effect of broilers chicken diet supplementation with natural and acidified humic substances on quality of produced breast meat. Animals 11:1087. doi: 10.3390/ani11041087
Hudec, E., Mudroňová, D., Marcinčák, S., Bartkovský, M., Makiš, A., Faldyna, M., et al. (2024). The effect of Limosilactobacillus fermentum 2i3 and 0.6% addition of humic substances on production parameters and the immune system of broilers. Poult. Sci. 103:103884. doi: 10.1016/j.psj.2024.103884
Jaďuttová, I., Marcinčáková, D., Bartkovský, M., Semjon, B., Harčárová, M., Nagyová, A., et al. (2019). The effect of dietary humic substances on the fattening performance, carcass yield, blood biochemistry parameters and bone mineral profile of broiler chickens. Acta Vet. Brno 88, 307–313. doi: 10.2754/avb201988030307
Jie, D., Zhang, Z., He, J., Zhou, Y., and Zhu, G. (2022). Impact of waste tea litter on NH3 and CO2 emissions during broiler rearing. Appl. Sci. 12:2559. doi: 10.3390/app12052559
Kaukonen, E., Norring, M., and Valros, A. (2017). Evaluating the effects of bedding materials and elevated platforms on contact dermatitis and plumage cleanliness of commercial broilers and on litter condition in broiler houses. Br. Poult. Sci. 58, 480–489. doi: 10.1080/00071668.2017.1340588
Khil’ko, S. L., Efimova, I. V., and Smirnova, O. V. (2011). Antioxidant properties of humic acids from brown coal. Solid. Fuel. Chem. 45, 367–371. doi: 10.3103/S036152191106005X
Khong, M. J., Snyder, A. M., Magnaterra, A. K., Young, M. M., Barbieri, N. L., and Weimer, S. L. (2023). Antimicrobial resistance profile of Escherichia coli isolated from poultry litter. Poult. Sci. 102:102305. doi: 10.1016/j.psj.2022.102305
Kilic, I., and Yaslioglu, E. (2014). Ammonia and carbon dioxide concentrations in a layer house. Asian Australas. J. Anim. Sci. 27, 1211–1218. doi: 10.5713/ajas.2014.14099
Kocabağli, N., Alp, M., Acar, N., and Kahraman, R. (2002). The effects of dietary humate supplementation on broiler growth and carcass yield. Poult. Sci. 81, 227–230. doi: 10.1093/ps/81.2.227
Korsakov, K. V., Vasiliev, A. A., Moskalenko, S. P., Sivokhina, L. A., Kuznetsov, M. Y., Petrakov, E. S., et al. (2019). Humic acids as the key to high productivity of broiler chickens. Ann. Agri-Bio Res. 24, 294–302.
Kousar, S., Rehman, N., Javed, A., Hussain, A., Naeem, M., Masood, S., et al. (2021). Intensive poultry farming practices influence antibiotic resistance profiles in Pseudomonas aeruginosa inhabiting nearby soils. Infect. Drug. Resist. 14, 4511–4516. doi: 10.2147/IDR.S324055
Mahler, C. F., Svierzoski, N. D. S., and Bernardino, C. A. R. (2021). “Chemical characteristics of humic substances in nature” in Humic substances. ed. A. Makan (London: IntechOpen).
Marcinčáková, D., Mačanga, J., Nagy, J., Marcinčák, S., Popelka, P., Vašková, J., et al. (2015). Effect of supplementation of the diet with humic acids on growth performance and carcass yield of broilers. Folia Vet. 59, 165–168.
Milanov, D., Knežević, S., Vidaković, S., Pajić, M., Živkov-Baloš, M., and Aleksić, N. (2019). Microbial contamination of poultry litter during fattening period. Biotechnol. Anim. Husb. 35, 253–265. doi: 10.2298/BAH1903253M
Miles, D. M., Owens, P. R., and Rowe, D. E. (2006). Spatial variability of litter gaseous flux within a commercial broiler house: ammonia, nitrous oxide, carbon dioxide, and methane. Poult. Sci. 85, 167–172. doi: 10.1093/ps/85.2.167
Moore, P. A. Jr., Miles, D., Burns, R., Pote, D., Berg, K., and Choi, I. H. (2011). Ammonia emission factors from broiler litter in barns, in storage, and after land application. J. Environ. Qual. 40, 1395–1404. doi: 10.2134/jeq2009.0383
Mudroňová, D., Karaffová, V., Pešulová, T., Koščová, J., Maruščáková, I. C., Bartkovský, M., et al. (2020). The effect of humic substances on gut microbiota and immune response of broilers. Food Agric. Immunol. 31, 137–149. doi: 10.1080/09540105.2019.1707780
Mudroňová, D., Karaffová, V., Semjon, B., Naď, P., Koščová, J., Bartkovský, M., et al. (2021). Effects of dietary supplementation of humic substances on production parameters, immune status and gut microbiota of laying hens. Agriculture 11:744. doi: 10.3390/agriculture11080744
Muhammad, J., Khan, S., Su, J. Q., Hesham, A. E. L., Ditta, A., Nawab, J., et al. (2020). Antibiotics in poultry manure and their associated health issues: a systematic review. J. Soils Sediments 20, 486–497. doi: 10.1007/s11368-019-02360-0
Mulvaney, R. L. (1996). “Total nitrogen, nitrogen-inorganic forms” in Methods of soil analysis. Part 3. Chemical methods. ed. D. L. Sparks (Madison, WI: Soil Science Society of America) 1123–1184.
Munir, M. T., Belloncle, C., Irle, M., and Federighi, M. (2019). Wood-based litter in poultry production: a review. Worlds Poult. Sci. J. 75, 5–16. doi: 10.1017/S0043933918000909
Murakami, K., Hara, M., Kondo, T., and Hashimoto, Y. (2011). Increased total nitrogen content of poultry manure by decreasing water content through composting processes. Soil Sci. Plant Nutr. 57, 705–709. doi: 10.1080/00380768.2011.616856
Nakatani, H. (2002). Effect of composting period and season on nitrogen mineralization in poultry manure. Res. Bull. Aichi Agric. Res. Ctr. 34, 239–243. (in Japanese with English abstract)
Ouwehand, A. C., Forssten, S., Hibberd, A. A., Lyra, A., and Stahl, B. (2016). Probiotic approach to prevent antibiotic resistance. Ann. Med. 48, 246–255. doi: 10.3109/07853890.2016.1161232
Ozturk, E., Ocak, N., Coskun, I., Turhan, S., and Erener, G. (2010). Effects of humic substances supplementation provided through drinking water on performance, carcass traits and meat quality of broilers. J. Anim. Physiol. Anim. Nutr. 94, 78–85. doi: 10.1111/j.1439-0396.2008.00886.x
Rico-Contreras, J. O., Aguilar-Lasserre, A. A., Méndez-Contreras, J. M., López-Andrés, J. J., and Cid-Chama, G. (2017). Moisture content prediction in poultry litter using artificial intelligence techniques and Monte Carlo simulation to determine the economic yield from energy use. J. Environ. Manag. 202, 254–267. doi: 10.1016/j.jenvman.2017.07.034
Rodrigues, A. L., Brito, A. G., Janknecht, P., Silva, J., Machado, A. V., and Nogueira, R. (2008). Characterization of biofilm formation on a humic material. JIMB 35, 1269–1276. doi: 10.1007/s10295-008-0424-8
Sharma, A., and Anthal, R. (2016). Humic substances in aquatic ecosystems: a review. Int. J. Innov. Res. Sci. Eng. Technol. 5, 18462–18470. doi: 10.15680/IJIRSET.2016.0510051
Song, M., Song, B., Meng, F., Chen, D., Sun, F., and Wei, Y. (2019). Incorporation of humic acid into biomass derived carbon for enhanced adsorption of phenol. Sci. Rep. 9:19931. doi: 10.1038/s41598-019-56425-8
STN EN 10390 (838445) (2021). Soil, treated biowaste and sludge. Determination of pH. Geneva, Switzerland: ISO (In Slovak).
STN EN 25663 (2000). Determination of Kjeldahl nitrogen. Method after mineralization with selenium. Geneva, Switzerland: ISO (In Slovak).
STN EN 2859-1 (1999). Sampling procedures for inspection by attributes — Part 1: Sampling schemes indexed by acceptance quality limit (AQL) for lot-by-lot inspection. Geneva, Switzerland: ISO.
Terzich, M., Pope, M. J., Cherry, T. E., and Hollinger, J. (2000). Survey of pathogens in poultry litter in the United States. J. Appl. Poult. Res. 9, 287–291. doi: 10.1093/japr/9.3.287
Thaxton, Y. V., Balzli, C. L., and Tankson, J. D. (2003). Relationship of broiler flock numbers to litter microflora. J. Appl. Poult. Res. 12, 81–84. doi: 10.1093/japr/12.1.81
Vikram, N., Sagar, A., Gangwar, C. H., Husain, R., and Kewat, R. N. (2022). “Properties of humic acid substances and their effect in soil quality and plant health” in Humic substances. ed. A. Makan (London: IntechOpen).
Vučemilo, M., Matković, K., Vinković, B., Jakšić, S., Granić, K., and Mas, N. (2007). The effect of animal age on air pollutant concentration in a broiler house. Czeh J. Anim. Sci. 52, 170–174. doi: 10.17221/2318-CJAS
Winkler, S., Coufal, C., Harmel, D., Martin, E., Brooks, J. P., Popham, S., et al. (2017). Within-house spatial distribution of fecal indicator bacteria in poultry litter. J. Environ. Qual. 46, 1003–1009. doi: 10.2134/jeq2017.05.0188
Xi, B., Tang, Z., Jiang, J., Tan, W., Huang, C., Yuan, W., et al. (2018). Responses of the electron transfer capacity of soil humic substances to agricultural land-use types. RCS Adv. 8, 32588–32596. doi: 10.1039/C8RA04278K
Zhang, P., Duan, W., Peng, H., Pan, B., and Xing, B. (2022). Functional biochar and its balanced design. ACS Environ. Au 2, 115–127. doi: 10.1021/acsenvironau.1c00032
Keywords: feed additives, humic substances, broilers, Escherichia coli , gases
Citation: Dančová N, Gregová G, Szabóová T and Marcinčák S (2025) The effects of natural additives on litter condition, microclimate environment and antimicrobial resistance in the broiler chickens rearing. Front. Sustain. Food Syst. 9:1380876. doi: 10.3389/fsufs.2025.1380876
Received: 02 February 2024; Accepted: 24 February 2025;
Published: 10 March 2025.
Edited by:
Fiona Nicholson, Agricultural Development Advisory Service, United KingdomReviewed by:
Biswarup Sen, Independent Researcher, Bengaluru, IndiaCopyright © 2025 Dančová, Gregová, Szabóová and Marcinčák. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriela Gregová, Z2FicmllbGEuZ3JlZ292YUB1dmxmLnNr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.