- 1Department of Microbiology, School of Bioengineering and Biosciences, Lovely Professional University, Punjab, India
- 2Department of Biomedical Sciences, Institute of Health, Jimma University, Jimma, Ethiopia
- 3Department of Microbiology, Graphic Era Deemed to be University, Dehradun, Uttarakhand, India
The extensive use of antimicrobial growth promoters (AGPs) in livestock has raised global concerns due to increasing antimicrobial resistance (AMR) among pathogenic microbes. This review examines probiotics as a sustainable alternative to AGPs, offering a safer approach for promoting animal growth and health. Probiotics enhance animal productivity and immunity by producing antimicrobial compounds and competing with pathogens for nutrients. In addition, probiotics strengthen the gut barrier and modulate the gut microbiome, facilitating beneficial bacterial growth while suppressing pathogenic species. Studies demonstrate the efficacy of probiotic strains of genera Lactobacillus and Bifidobacterium in inhibiting pathogens such as Clostridium perfringens and Salmonella in livestock. This comprehensive evaluation highlights probiotics' potential to advance sustainable livestock practices, reduce reliance on antibiotics, and mitigate AMR risks, underscoring the need for further research and regulatory considerations for their use in animal husbandry.
1 Introduction
The use of antimicrobial growth promoters (AGPs) has significantly increased following the emergence of various livestock diseases. Despite global efforts to curtail their usage, projections estimate an 8% rise in AGP use between 2020 and 2030 (Mulchandani et al., 2023). While AGPs have been effective in improving livestock production and profits, their overuse has raised alarming concerns about antimicrobial resistance (AMR).1 AMR occurs when pathogenic microbes, frequently exposed to antibiotics, develop resistance mechanisms that render these treatments ineffective. This resistance arises through genetic mutations or horizontal gene transfer, altering microbial gene expression and metabolic pathways. These changes often result in the production of altered proteins and other adaptations, making the pathogens increasingly difficult to combat.
In livestock, several microbes are implicated in AMR, including Moraxella bovis, Moraxella bovoculi, Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni. These pathogens cause significant diseases in cattle, such as infectious bovine keratoconjunctivitis (IBK), bovine respiratory disease (BRD), and thromboembolic meningoencephalitis (TEME), each leading to substantial economic losses. IBK, caused by Moraxella species, results in painful eye conditions, corneal ulcers, and blindness, affecting animal welfare and productivity. BRD, caused by Mannheimia haemolytica and Pasteurella multocida, leads to pneumonia, reduced feed intake, and weight loss. TEME, associated with Histophilus somni, presents with neurological symptoms, fever, and sudden death. These diseases not only compromise animal health but also inflate costs due to treatments and decreased productivity in terms of growth and milk yield. To address AMR and improve livestock health, a comprehensive strategy is essential (Rodrigues et al., 2021). While AGPs have historically played a pivotal role, their scrutiny has grown due to their contribution to resistance development. AGPs (Patyra and Kwiatek, 2023), derived from natural, semi-synthetic, or synthetic sources possess antimicrobial properties that suppress microbial growth and enhance livestock productivity by improving feed efficiency and overall health (Hosain et al., 2021). Their introduction dates back to the 1940s when trials revealed that fermentation by-products of tetracycline production promoted rapid growth in chickens (Stokstad et al., 1949). Initially, this effect was attributed to vitamin B12, but later studies confirmed residual tetracycline as the primary growth-enhancing agent. Since then, AGPs have been integral to industrialized animal husbandry. However, their widespread availability without prescription has exacerbated their overuse, highlighting the urgent need for alternative strategies to ensure sustainable livestock management.
Probiotics have emerged as a safer and effective alternative to antibiotics, offering benefits beyond pathogen control. Unlike antibiotics, probiotics promote health by enhancing gut microbiota composition and function, reducing infection risks, and supporting overall wellbeing. The concept of probiotics is ancient, with early applications in fermented foods such as sour milk and soy sauce dating back to 3000–4000 BC. Modern understanding of probiotics began in 1892 when Doderlein identified their beneficial properties, leading to extensive research into probiotic strains (Schmerold et al., 2023). Probiotics function through two primary mechanisms: bactericidal and bacteriostatic actions. Bactericidal mechanisms involve the direct killing of pathogens via antimicrobial compounds such as bacteriocins, hydrogen peroxide, and organic acids that disrupt bacterial cell membranes. Bacteriostatic activity inhibits pathogen growth by competing for essential nutrients, occupying adhesion sites, and altering environmental factors such as pH to create unfavorable conditions for pathogens (Nataraj and Mallappa, 2021). These mechanisms enable probiotics to effectively reduce pathogenic bacteria populations and prevent infections in livestock.
In addition, probiotics enhance the physiological composition and activity of gut bacteria, aiding digestion, nutrient absorption, and immune modulation (Anjana and Tiwari, 2022). They increase the bioavailability of critical nutrients such as short-chain fatty acids (SCFAs), peptides, and vitamins, while stimulating anti-inflammatory and immune responses. Probiotics also mitigate food intolerances, improve feed conversion rates, and bolster the resilience of livestock against environmental and pathogenic stresses (Redweik et al., 2020). The integration of probiotics into livestock management aligns with the growing demand for sustainable and safe farming practices. By reducing dependency on antibiotics, probiotics not only address AMR but also support animal welfare and productivity. However, their implementation requires careful selection of strains, dose optimization, and monitoring to ensure efficacy and safety.
In this review, we will discuss the role of probiotics as alternatives to antimicrobial growth promoters in livestock, highlighting their mechanisms, benefits, and limitations. Each section provides a focused analysis: The introduction discusses current challenges, and subsequent sections explore probiotics' mechanisms such as direct antagonism and gut modulation, followed by a detailed assessment of targeted pathogens, and conclude with implications for animal growth and regulatory considerations.
2 Mechanisms of antimicrobial action by probiotics
Probiotics, particularly strains of Lactobacillus and other beneficial microorganisms, exert a range of antimicrobial actions that contribute to the health and wellbeing of animals. These mechanisms include competitive exclusion of pathogens, production of antimicrobial compounds such as bacteriocins2 and organic acids, disruption of biofilms, and modulation of the gut microbiota (Table 1). By enhancing the gut barrier function and stimulating immune responses, probiotics not only help in preventing infections but also promote overall animal growth and productivity. Understanding these mechanisms is crucial for optimizing the use of probiotics as a safe and effective alternative to traditional antimicrobial growth promoters in animal husbandry (Santacroce et al., 2019; Anjana and Tiwari, 2022; Nataraj and Mallappa, 2021).
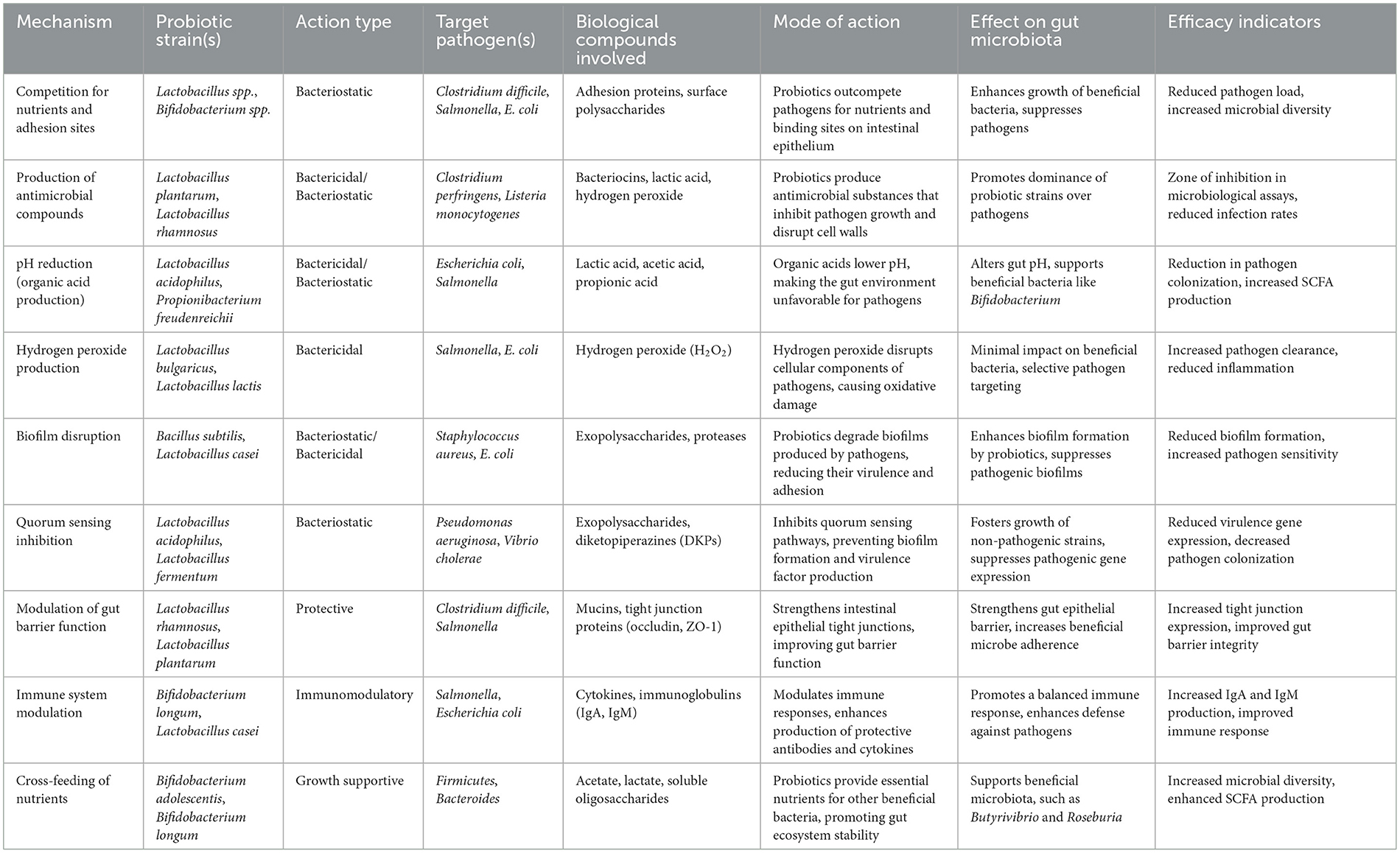
Table 1. Antimicrobial mechanisms of probiotics in animal health (Che et al., 2017; Elmi et al., 2020; Ramlucken et al., 2020; Zhang et al., 2021; Kalia et al., 2022; Wu et al., 2022a,b; Patyra and Kwiatek, 2023).
2.1 Direct antagonism
2.1.1 Competition for nutrients and adhesion sites
In animals, competition among probiotic strains for nutrients and adhesion sites plays a critical role in the inhibition of pathogenic mechanisms. Probiotics act as biological antagonists, vying for the same essential nutrients required by pathogens for growth and colonization. This competition disrupts the nutrient availability for harmful bacteria, impairing their ability to establish infections. In the gut, the interplay between probiotics, pathogens, and commensal microbes is a pivotal determinant of microbial dynamics. Clostridium difficile, a known pathogen, relies on simple sugars as a primary energy source for proliferation. However, probiotic bacteria demonstrate superior efficiency in fermenting key sugars such as sialic acid, glucose, and N-acetylglucosamine within the colon. This metabolic advantage of probiotics suppresses the growth and colonization of C. difficile, thereby mitigating its pathogenic effects (Huang et al., 2019). This nutrient-driven competitive exclusion highlights a critical mechanism by which probiotics contribute to gut health and disease prevention in animals.
Probiotics in animals enhance their beneficial actions by competing for adhesion sites in the gastrointestinal (GI) tract. One hypothesis suggests that bacterial adherence to gut epithelial cells involves targeted interactions between bacterial surface molecules and specific receptors on these cells. This binding process may incorporate electrostatic and hydrophobic mechanisms, facilitated by fatty acids such as lipoic acid and unique biomolecules such as polysaccharides and lectins. Probiotics directly communicate with epithelial surfaces via elements such as DNA, lipoic acid, complex polymers, and polysaccharides. Notably, specific cell surface proteins play a critical role in augmenting bacterial adhesion, enabling their anchoring to the mucous layer (Aleman and Yadav, 2023). For instance, proteomic analysis of Lactobacillus pentosus strains with potential probiotic applications has revealed that the cell wall proteome contains multifunctional proteins. The abundance of these proteins correlates with the strain's unique capacity for mucus adhesion. Adaptation to the GI tract environment further enhances probiotic efficacy. For example, the presence of bile signals bacterial entry into the gut, triggering a reorganization of the bacterial surface proteome. This adaptive response enhances adherence capabilities in the bile-rich environment. Specifically, in Lactobacillus rhamnosus GG, exposure to bile stress increased the abundance of surface-exposed Clp proteins, the chaperone protein DnaK, and enzymes involved in sugar metabolism, collectively improving its adhesion and survival within the GI tract (Lu et al., 2022).
2.1.2 Production of antimicrobial compounds
2.1.2.1 Bacteriocins
Bacteriocins are antimicrobial peptides synthesized by bacterial ribosomes, exerting either bactericidal (killing) or bacteriostatic (growth-inhibiting) effects on pathogens. These peptides can be produced by both Gram-positive and Gram-negative bacteria (Siciliano et al., 2019). Due to their susceptibility to gastrointestinal proteases, bacteriocins typically have limited activity in the digestive system, yet they effectively combat foodborne infections. Their narrow spectrum allows them to target pathogens resistant to common antibiotics, offering an advantage over traditional antimicrobial treatments. Bacteriocins work by interacting with lipid II, a component involved in peptidoglycan synthesis in Gram-positive bacteria, disrupting the plasma membrane to form pores. In Gram-negative bacteria, bacteriocins target DNA or RNA synthesis by penetrating the cell through specific transport proteins. Some bacteriocins, such as LAPs, thiopeptides, and bottromycins, inhibit protein translation by binding to ribosomal components like the elongation factor Tu or aspartyl-tRNA synthetase. In addition, bacteriocins participate in quorum sensing, enhancing their own production and promoting other bacterial species to produce similar peptides, which helps outcompete pathogens by depleting available nutrients and binding sites (Darbandi et al., 2022). Probiotic bacteria that produce bacteriocins offer an effective strategy for pathogen reduction in animals. For instance, Lactobacillus plantarum produces plantaricin, which targets Salmonella and Escherichia coli in poultry, improving gut health and reducing pathogens (Wang et al., 2018). Similarly, Lactobacillus reuteri produces reuterin, inhibiting Staphylococcus aureus in swine (Yehia et al., 2022), while Enterococcus faecium produces enterocin, effective against E. coli in piglets, reducing diarrhea and enhancing growth (Ben Braïek and Smaoui, 2019). In addition, Pediococcus acidilactici produces pediocin, which targets Listeria monocytogenes in ruminants, reducing foodborne infection risks. Bacillus subtilis produces subtilin, effective against Staphylococcus aureus in cattle, reducing mastitis (Teng et al., 2023). Bacillus thuringiensis DPC6431 produces thuricin CD, which eliminates C. difficile isolates in a distal colon model, while preserving commensal microbiota. Bacteriocin-producing lactic acid bacteria (LAB) also show effectiveness against pathogens such as Listeria monocytogenes and enterococci in the intestines. For example, Pediococcus acidilactici UL5 produces pediocin PA-1, demonstrating anti-listerial properties in a mouse model without disrupting natural microbiota (Teng et al., 2023).
2.1.2.2 Organic acids
Probiotic strains such as Acetobacter aceti, Lactobacillus spp., and Propionibacterium spp. produce organic acids such as acetic, lactic, and propionic acids (Anjana and Tiwari, 2022). These organic acids, including short-chain fatty acids (SCFAs), play a crucial role in inhibiting the growth of bacteria and fungi. Acetic acid, at a concentration of 0.2%, can inhibit bacterial growth, particularly at pH levels below 4.5, and is effective against Gram-negative bacteria. Propionic acid, at concentrations of 0.1% to 0.2% and pH 5.0, demonstrates strong antifungal and antibacterial properties, particularly against Gram-negative bacteria and molds. Lactic acid, in concentrations ranging from 1% to 2% and pH above 5, exhibits antibacterial effects, inhibiting both Gram-positive and Gram-negative bacteria, with bactericidal effects on Gram negatives at lower pH. These acids act through a combination of dissociated molecules and undissociated ions, resulting in sublethal damage and promoting bacterial viability loss (Tang et al., 2023). SCFAs, produced by the fermentation of non-digestible carbohydrates (prebiotics), stimulate G-protein-coupled receptors (GPRs) such as GPR41 and GPR43. These receptors are associated with several health benefits, including anti-inflammatory, anti-tumor, and immune-regulating effects, as well as the maintenance of glucose homeostasis and cardiovascular health (Teneva and Denev, 2023). Various probiotics produce organic acids that benefit animal health. For example, Lactobacillus acidophilus generates lactic acid, reducing E. coli and Salmonella in broiler chickens (Elmi et al., 2020). Propionibacterium freudenreichii generates propionic acid, inhibiting Staphylococcus aureus in dairy cattle. Bifidobacterium bifidum produces acetic acid, reducing Salmonella colonization in pigs (Rabah et al., 2017). Lactobacillus casei produces SCFAs that reduce E. coli pathogenicity in poultry, while Bacillus coagulans produces butyric acid, enhancing gut integrity and improving overall health in broilers (Hou et al., 2023).
2.1.2.3 Hydrogen peroxide
Under anaerobic growth conditions, certain bacteria, such as L. bulgaricus, Lactobacillus lactis, Lactobacillus acidophilus, and Lactobacillus johnsonii, can produce hydrogen peroxide (H2O2), which is released into the surrounding medium. Hydrogen peroxide's strong oxidizing properties make it effective against a broad range of microorganisms, including viruses, bacteria, molds, and bacteriophages. Its antimicrobial action stems from its ability to disrupt cellular components through oxidation (Vera-Santander et al., 2023). For example, Lactobacillus johnsonii produces hydrogen peroxide, inhibiting E. coli growth in piglets and reducing infection rates (Xin et al., 2020). Lactobacillus fermentum generates hydrogen peroxide, creating an environment hostile to Staphylococcus aureus in dairy cows, preventing infections. Lactobacillus rhamnosus reduces Salmonella populations in poultry by disrupting their survival mechanisms in the gut (Kunwar, 2024).
2.1.2.4 Other novel metabolites
Probiotic strains also produce other bioactive compounds, such as proteases and exopolysaccharides (EPS), which contribute to antimicrobial properties. Bacillus pumilus produces subtilisin and glutamyl endopeptidase, which effectively degrade biofilms formed by Serratia marcescens, a common hospital-acquired pathogen. These bacteria also produce EPS with antioxidant properties, inhibiting biofilm formation by E. coli and S. aureus (Huang et al., 2023). Lactobacillus crispatus produces bacteriocin-like inhibitory substances (BLIS) that suppress Salmonella in chickens (Ben Braïek and Smaoui, 2019). Bacillus amyloliquefaciens produces surfactin, which disrupts S. aureus biofilms in dairy cattle, preventing mastitis. Streptococcus thermophilus produces EPS that inhibit E. coli adhesion to the gut lining in calves, supporting a healthy microbiota (Sabino et al., 2023). Lactobacillus paracasei produces antimicrobial peptides effective against Salmonella in pigs, controlling bacterial colonization in the intestines (Monteiro et al., 2019).
2.2 Modulation of gut microbiome
Probiotics can positively influence the gut microbiome in animals, promoting the growth of beneficial microorganisms while inhibiting harmful pathogens. The colonization of the intestine by probiotics, particularly lactic acid bacteria (LAB) such as Lactobacillus and Bifidobacterium, is critical for their beneficial effects on animal health. For a probiotic to be effective, it must successfully adhere to the intestinal mucosa and establish a stable presence in the gut. A study on Lactobacillus rhamnosus in mice showed that the number of colonizing strains increased from the proximal to the distal small intestine, with the duodenum, jejunum, and ileum having higher colonization compared to the colon. The intestinal mucus layer, which contains specific O-glycan structures, acts as a receptor for bacterial adhesion, facilitating the colonization of probiotics such as Lactobacillus and Bifidobacterium. Furthermore, adhesion factors such as lipoteichoic acid, peptidoglycan, and S-layer proteins play a crucial role in this process, ensuring that probiotics can firmly attach to the gut lining and resist being flushed out.
Research also highlights the importance of adhesion proteins in enhancing colonization efficiency. For example, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein on the surface of Lactobacillus reuteri ZJ617 significantly boosts the bacteria's adhesion ability, compared to low-adhesion strains such as L. reuteri ZJ615 (Cui et al., 2017). Higher cell membrane permeability and better adhesion to mucin further improve the bacteria's persistence in the intestine. In addition, the formation of probiotic biofilms provides a protective barrier that not only supports the long-term presence of beneficial bacteria but also prevents pathogenic organisms from colonizing the gut. An in vivo study with Lactobacillus casei LC2W demonstrated its ability to inhibit the colonization of Escherichia coli O157:H7 in mice, offering potential for probiotic-based treatments for gut conditions such as colitis (Wang et al., 2019). Therefore, enhancing the colonization ability of probiotics through biofilm formation and adhesion proteins plays a crucial role in maintaining intestinal health and protecting against pathogenic infections.
Lactobacillus rhamnosus helps maintain a balanced gut flora, reducing Salmonella colonization in poultry by outcompeting harmful bacteria for nutrients and space (Villena et al., 2014). Bifidobacterium longum shifts the gut microbiome toward beneficial bacteria, inhibiting E. coli in calves and promoting digestive health. Saccharomyces boulardii modulates the gut microbiota in piglets, reducing Salmonella colonization and improving overall gut health. Lactobacillus casei enhances beneficial microbiota populations in livestock, reducing the risk of Staphylococcus aureus infections (Monteiro et al., 2019; Abou-Kassem et al., 2021). Finally, Bacillus subtilis promotes a balanced microbiome in chickens, decreasing the prevalence of E. coli and supporting a healthier digestive system (Upadhaya et al., 2019).
A study was conducted by Bagarolli et al. which demonstrated mice that were fed a diet high in fat experienced notable changes in their intestinal flora, which were linked to various diseases. The researchers further discovered that when probiotics were administered to obese animals, there was an abatement in the presence of Firmicutes and an rise in Actinobacteria within the intestinal flora. This finding suggests that the administration of probiotics can potentially reverse dysbiosis in the intestinal flora and effectively treat inflammatory responses in mice (Leser and Baker, 2023).
Another mechanism by which probiotics promote the development of valuable bacteria is cross-feeding mechanism. Acetate, lactate, and soluble molecules produced by B. adolescentis through the breakdown of complex carbohydrates can potentially be used as a source of nutrition by other bacteria, especially butyrate-producing species such as Eubacterium hallii, Anaerostipes caccae, and various Roseburia species. This phenomenon is further demonstrated by the cross-feeding interactions observed among different Bifidobacterium species. For example, B. bifidum is typically unable to break down plant-derived glycans such as starch or xylan on its own, but in co-cultures, it can benefit from the simple carbohydrates produced by the extracellular amylase activity of B. adolescentis. In addition, when conventional mice were colonized with a combination of B. adolescentis, B. breve, B. bifidum, and B. longum subsp. infantis, there was a considerable growth in the abundance of each Bifidobacterium species in contrast to mice that were only colonized with one species (Moreno-Muñoz et al., 2024).
Probiotics can inhibit pathogens in animals through ecological shifts in the gut microbiota, promoting a healthy microbial balance that outcompetes harmful pathogens. For instance, Lactobacillus rhamnosus GG has been shown to effectively inhibit the growth of Escherichia coli and Salmonella in the intestines of poultry by enhancing the gut's beneficial bacteria while limiting pathogen colonization. This ecological shift results in a microbiota that is more resistant to pathogen invasion. Similarly, in swine, the administration of Bifidobacterium strains has been reported to reduce the prevalence of Clostridium perfringens, a pathogen responsible for gastrointestinal disorders in pigs. The introduction of probiotics such as Lactobacillus acidophilus and Enterococcus faecium in dairy cows has demonstrated a reduction in the abundance of E. coli and Salmonella species in the gut, thereby improving the overall health and productivity of the animals. These probiotics function by producing antimicrobial substances, modifying the gut pH, and competing for adhesion sites, thus limiting pathogen growth. Moreover, Bifidobacterium animalis has shown potential in reducing pathogen load in calves, illustrating the broader applicability of probiotics in maintaining gut health and preventing disease. These studies highlight how probiotics can induce ecological shifts in the microbiota, reducing pathogen load and improving animal health.
2.2.1 Inhibiting pathogens through ecological shifts
Probiotics can inhibit pathogens in animals through ecological shifts in the gut microbiota, promoting a healthy microbial balance that outcompetes harmful pathogens. For instance, Lactobacillus rhamnosus GG has been shown to effectively inhibit the growth of Escherichia coli and Salmonella in the intestines of poultry by enhancing the gut's beneficial bacteria while limiting pathogen colonization (Kathayat et al., 2022). This ecological shift results in a microbiota that is more resistant to pathogen invasion. Similarly, in swine, the administration of Bifidobacterium strains has been reported to reduce the prevalence of Clostridium perfringens, a pathogen responsible for gastrointestinal disorders in pigs (Zhang et al., 2023). The introduction of probiotics such as Lactobacillus acidophilus and Enterococcus faecium in dairy cows has demonstrated a reduction in the abundance of E. coli and Salmonella species in the gut, thereby improving the overall health and productivity of the animals. These probiotics function by producing antimicrobial substances, modifying the gut pH, and competing for adhesion sites, thus limiting pathogen growth (Monteverde et al., 2017; Smialek et al., 2018). Moreover, Bifidobacterium animalis has shown potential in reducing pathogen load in calves, illustrating the broader applicability of probiotics in maintaining gut health and preventing disease (Du et al., 2023). These studies highlight how probiotics can induce ecological shifts in the microbiota, reducing pathogen load and improving animal health (Kathayat et al., 2022; Du et al., 2023). In addition, research has demonstrated that the presence of certain substances produced by specific strains of Lb. acidophilus, such as extracellular polymeric substances (EPS) and diketopiperazines (DKP), can decrease the expression of biofilm-related genes in E. coli and Pseudomonas aeruginosa (Rabetafika et al., 2023).
2.2.2 Strengthening gut barrier function
The intestinal barrier is crucial for maintaining overall health and mitigating diseases in animals. It serves as a primary defense mechanism, ensuring intestinal homeostasis by protecting against physical, chemical, immune, and microbial threats. Damage or dysfunction in the mucosal layer can compromise these protective functions. Probiotics have emerged as a promising solution to enhance mucosal barrier integrity, reducing the risk of harmful organisms overwhelming the gut environment. The efficacy of the intestinal barrier relies on intestinal epithelial cells (IECs) and their intercellular junction complexes, especially tight junctions (TJs) located on the apical surface of IECs. Probiotics influence the activation of genes and proteins involved in TJ signaling and modulate the balance between cell death (apoptosis) and cell growth (proliferation) of IECs. For example, Lactobacillus acidophilus has demonstrated strain-specific capabilities to improve TJ barrier performance rapidly by forming heterodimeric complexes of Toll-like receptor-2 (TLR-2), specifically TLR-2/TLR-1 and TLR-2/TLR-6. This mechanism helps protect against intestinal inflammation (Gou et al., 2022).
In animals, Lactobacillus plantarum has been shown to enhance the intestinal barrier in pigs, preventing E. coli translocation and supporting gut health. Lactobacillus rhamnosus increases mucus production in poultry, thereby protecting against Salmonella invasion and strengthening gut defense (Sabino et al., 2023). In calves, Bifidobacterium lactis strengthens the epithelial barrier, reducing susceptibility to Staphylococcus aureus infections and promoting overall gut health (Chuang et al., 2022). Similarly, Saccharomyces cerevisiae improves TJ integrity in livestock, limiting E. coli infection by reducing gut permeability (Che et al., 2017). Lactobacillus fermentum enhances the immune response and gut lining resilience, reducing Salmonella infections in broiler chickens and promoting overall gut health (Kalia et al., 2022).
Goblet cells within the intestinal epithelium play a pivotal role in secreting a protective mucus layer composed mainly of mucins, large glycoproteins. This mucus facilitates nutrient absorption, provides attachment points for beneficial bacteria, and forms a barrier against invading microbes (Wu et al., 2022a,b). For instance, Lactobacillus plantarum 12 enhances the chemical barrier of the intestinal mucosa by increasing mucin2 (MUC-2) levels, while certain strains release short-chain fatty acids (SCFAs), which upregulate MUC-2 mRNA expression in cells (Wu et al., 2022a,b).
Extensive evidence underscores the critical role of probiotics in enhancing intestinal function through diverse and well-documented mechanisms. Probiotic bacteria maintain paracellular permeability, fortify the physical mucous layer, stimulate immune system responses, and modulate the composition and activity of resident microbiota, all contributing to optimal intestinal homeostasis (Boirivant and Strober, 2007). These effects have been thoroughly examined in humans, pigs, and chickens, highlighting the central role of probiotic–epithelial barrier interactions in achieving intestinal equilibrium (Cisek and Binek, 2014; Gresse et al., 2017). Lactobacillus spp., a predominant genus in the gastrointestinal tract of humans and animals, has emerged as a cornerstone in probiotic research due to its strain-specific benefits. Studies on strains such as Lactobacillus plantarum MB452, L. casei, L. rhamnosus GG, and L. reuteri I5007 reveal their capacity to influence transepithelial electrical resistance (TER) and epithelial permeability. They also modulate the expression and localization of tight junction (TJ) proteins, directly enhancing intestinal barrier integrity (Patel et al., 2012; Yang et al., 2015).
The immunomodulatory effects of Lactobacillus spp. are equally compelling. For example, Lactobacillus GG and L. rhamnosus CRL1505 promote anti-inflammatory cytokines such as IL-10 and IFN-γ, while strains such as L. reuteri LR1 and L. plantarum 2142 suppress proinflammatory cytokines, including IL-6, IL-8, and TNF-α (Villena et al., 2014; Wang et al., 2016). These actions reduce systemic inflammation and support immune resilience. In addition, L. reuteri I5007 and L. plantarum DSMZ 12028 can stimulate the synthesis of antimicrobial peptides by the intestinal epithelium, enhancing host defenses against pathogens (Liu et al., 2017). The ability of Lactobacillus strains to influence resident microbiota is another key factor in their health-promoting effects. Strains such as L. salivarius UCC118 and L. acidophilus significantly alter the composition and activity of gut microbiota, fostering an environment conducive to intestinal health (Li et al., 2017).
3 Spectrum of targeted pathogens
3.1 Gram-positive pathogens
3.1.1 Clostridium perfringens
The data from the Center for Disease Control and Prevention (CDC)3,4,5 indicate that Clostridium perfringens is a common etiological agent of foodborne diseases, responsible for an estimated 1 million cases annually in the United States (Jiang et al., 2022). C. perfringens exhibits the capacity to form spores, subsequently transitioning into metabolically active bacterial forms that proliferate within contaminated food substrates. Upon ingestion of food contaminated with C. perfringens, the bacterium has the capability to produce a toxin, thereby inducing diarrheal symptoms. Predominantly, individuals afflicted with C. perfringens-related food poisoning manifest symptoms of diarrhea and abdominal discomfort, devoid of emesis (Lone et al., 2021). The onset of symptoms typically occurs within a timeframe spanning from 6 to 24 h post-ingestion of the bacteria. The onset of symptoms is characterized by an acute commencement, typically subsiding within duration of < 24 h (CDC). Different strains of Bifidobacterium and Lactobacillus have demonstrated effectiveness in inhibiting Clostridium perfringens growth through several mechanisms (Ramlucken et al., 2020). Notably, Lactobacillus plantarum strain ATCC 8014 is highly effective against C. perfringens and other pathogenic Clostridium species by producing lactic acid, lowering the environmental pH, generating bacteriocins, and competing for nutrients and adhesion sites, thereby disrupting the pathogen's ability to proliferate and form biofilms (He et al., 2024). Table 2 illustrates various probiotic strains and how they counter the growth of Clostridium perfringens which is validated by diameter of zone of inhibition. More the ZOI, more effective will be that prebiotic strain against C. perfringens.
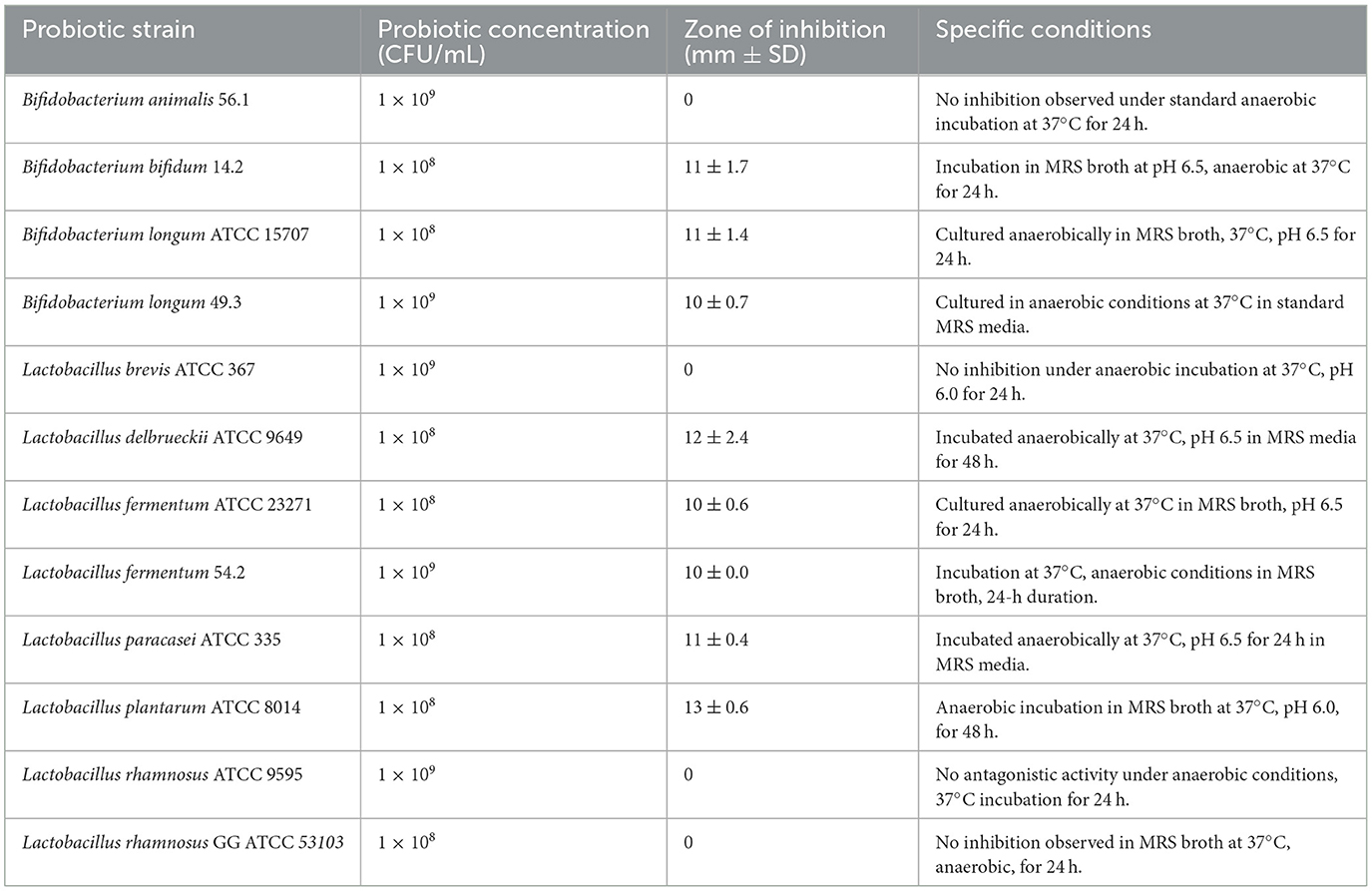
Table 2. Diameter of zone of inhibition produced by various probiotic strains against Clostridium perfringens based on the agar spot test (Monteiro et al., 2019).
3.1.2 Staphylococcus aureus
Staphylococcus aureus is considered a part of normal flora but can cause opportunistic infection which can be considered fatal such as bacteremia or sepsis, endocarditis (heart valves got infected), osteomyelitis (infection in bones), and pneumonia. Also due to its multidrug resistance, it is mandate to use other sources as prophylaxis than antibiotics (CDC), and one of them can be intake of probiotics. Several strains are able to counter the infection of S. aureus, such as Enterococcus durans LAB38, Bacillus, Lactobacillus acidophilus CL1285, and Lactobacillus casei LBC80R. The mechanism of action involved, formation of metabolites (SCFA, ethanol, CO2, sorbic acids), biosurfactants, exopolysaccharides, production of antimicrobial peptides, alteration of the gut microbiota, etc. (Figure 1). Along with these they are induce competition for binding sites, nutritional requirements, inhibition of quorum sensing, reduction in virulence factors formed by S. aureus, bactericidal action by bacteriocins (Nataraj and Mallappa, 2021; Jain et al., 2023).
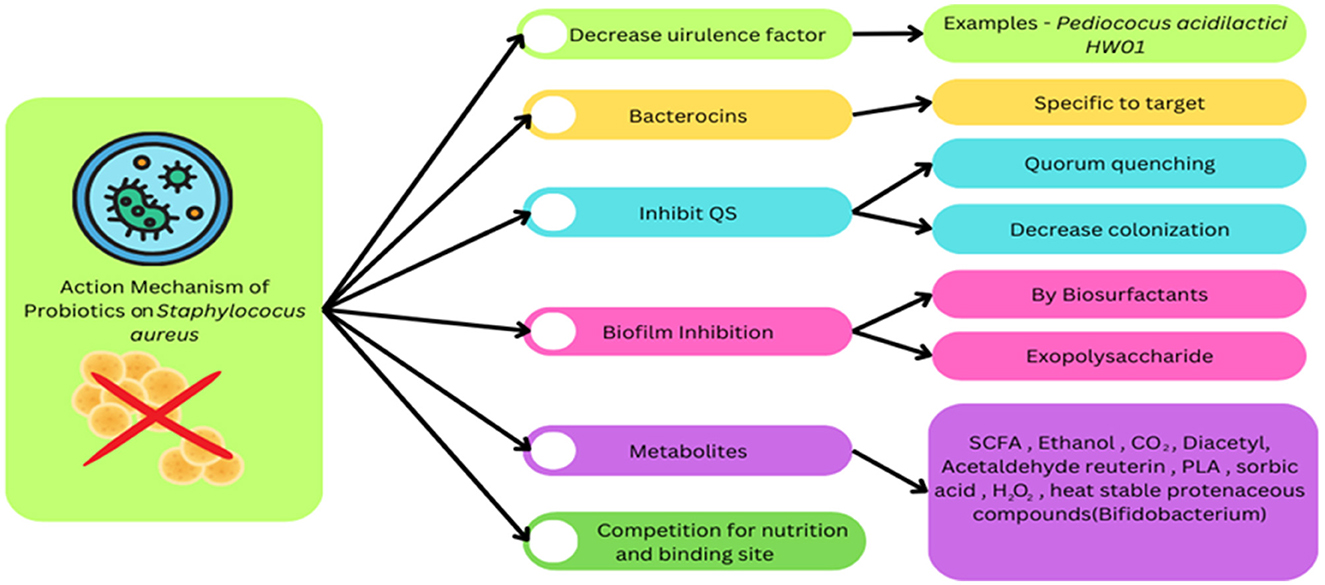
Figure 1. This diagram shows how a generalized probiotic can affect S. aureus by interfering in biofilm production, quorum sensing, and others.
3.2 Gram-negative pathogens
3.2.1 Escherichia coli
Escherichia coli (E. coli) bacteria typically inhabit in the GI tract (animals and humans), primarily the intestines. While the larger proportion of E. coli strains are benign, contributing to the normal functioning of the animals intestinal flora, certain strains possess pathogenic attributes, capable of inducing illness, manifesting as either diarrheal or extraintestinal afflictions. Transmission of diarrheagenic E. coli strains, which encompass six pathotypes associated with diarrheal conditions, occurs through various avenues including intake of water or food that is contaminated, as well as direct contact with infected animals or individuals (CDC).
Infection with E. coli has the potential to compromise the structural integrity of the intestinal tract, resulting in significant impairment of its physiological function. This disturbance extends to the perturbation of the intestinal microbiota equilibrium, consequently precipitating a reduction in immune competence (Braz et al., 2020). Several investigations have suggested that the administration of probiotics can enhance the immune response in calves, as evidenced by heightened serum IgG levels (Smialek et al., 2018). Furthermore, additional research has demonstrated that probiotic supplementation correlates with a decrease in the incidence of calf diarrhea and an elevation in IgM levels by the 14th day, accompanied by increased concentrations of serum IgA, IgM, and IgG by the 28th day (Wu et al., 2022a,b). Moreover, they illustrated that the addition of probiotics (L. acidophilus 3 × 109 CFU/g, B. subtilis 3 × 109 CFU/g, and S. cerevisiae 1 × 109 CFU/g) resulted in enhancements in both the growth metrics and immune reactivity of calves. In line with these observations, our investigation revealed that the concentration of gut secretory immunoglobulin A (SIgA) in jejunum of the calves in the probiotic-supplemented group exhibited a notable increase, reaching 86.48 μg/g (Karamzadeh-Dehaghani et al., 2021).
3.2.2 Salmonella
The transmission of Salmonella typhimurium infection through food continues to be a major public health issue, posing a significant threat even in developed countries (Won and Lee, 2017). Probiotics show promise in the prevention and treatment of Salmonella infections. As an example, studies have demonstrated significant decreases in the growth of S. typhimurium, as well as reduced colonization and severity of disease in mice, when they were exposed to both live and pasteurized Akkermansia muciniphila. Research into the host response to live and pasteurized A. muciniphila has uncovered specific pathways linked to protective effects. Research suggests that the presence of live A. muciniphila in the gut can potentially improve the integrity of the gut barrier. However, when A. muciniphila is pasteurized, it may have the opposite effect by triggering inflammasome activation and the production of cytokines. This can potentially lead to apoptosis and hinder the replication of pathogens.
The mucin layer, which is continuously renewed by goblet cells, is composed of heavily glycosylated and protein-bound molecules. This results in a compact hydrogel that provides a protective barrier for the intestinal epithelium against the presence of native microorganisms. Enhancing the mucin layer may impede bacterial movement and confine them within the mucus matrix. Past studies indicate that expediting the removal of mucin can help in effectively clearing out bacteria during an infection. Research has demonstrated that A. muciniphila can improve the integrity of the gut barrier and increase mucin secretion by goblet cells in conditions such as metabolic disorders or colitis induced by dextran sodium sulfate (DSS). Mice treated with AKK showed heightened expression of Mucin 2 and other tight junction proteins both prior to and following infection. Host cells generate antimicrobial peptides (AMPs), such as RegIII lectins, that serve as natural immune agents in identifying and eradicating harmful bacteria. RegIII lectins have the ability to bind to specific sugars found in Mucin 2. This binding helps to enhance the attachment of harmful lipopolysaccharides and strengthen the protective function of the mucus layer. As a result, they play a crucial role in fighting against bacteria. In addition, the gut microbiota's increased production of short-chain fatty acids (SCFAs) is likely to play a role in preventing colonization. SCFAs have the ability to hinder the growth of harmful pathogens by affecting intracellular pH, reducing the expression of virulence genes, and boosting the ability of macrophages to engulf and destroy them. AKK and pAKK treatment resulted in a notable increase in SCFA production, specifically acetate and propionate, both prior to and following infection. In addition, the AKK group showed an enrichment of Bifidobacteriaceae and Bacteroidetes both before and after antibiotic treatment. This aligns with previous research that has connected traditional probiotics, such as Bifidobacterium, to an increase in the production of short-chain fatty acids (SCFAs). In addition, the presence of certain microbial species has been shown to play a significant role in protecting against S. typhimurium infection by producing high levels of propionate. Therefore, it is possible that A. muciniphila supports host defense through similar mechanisms. The gut microbiota composition was influenced by the administration of both live and pasteurized A. muciniphila (Liu et al., 2023). Figure 2 illustrates the intricate action mechanism of probiotics in countering Salmonella sp. multiplication, elucidating both bactericidal and bacteriostatic effects. The flow chart offers a comprehensive visualization of probiotic intervention against Salmonella proliferation.
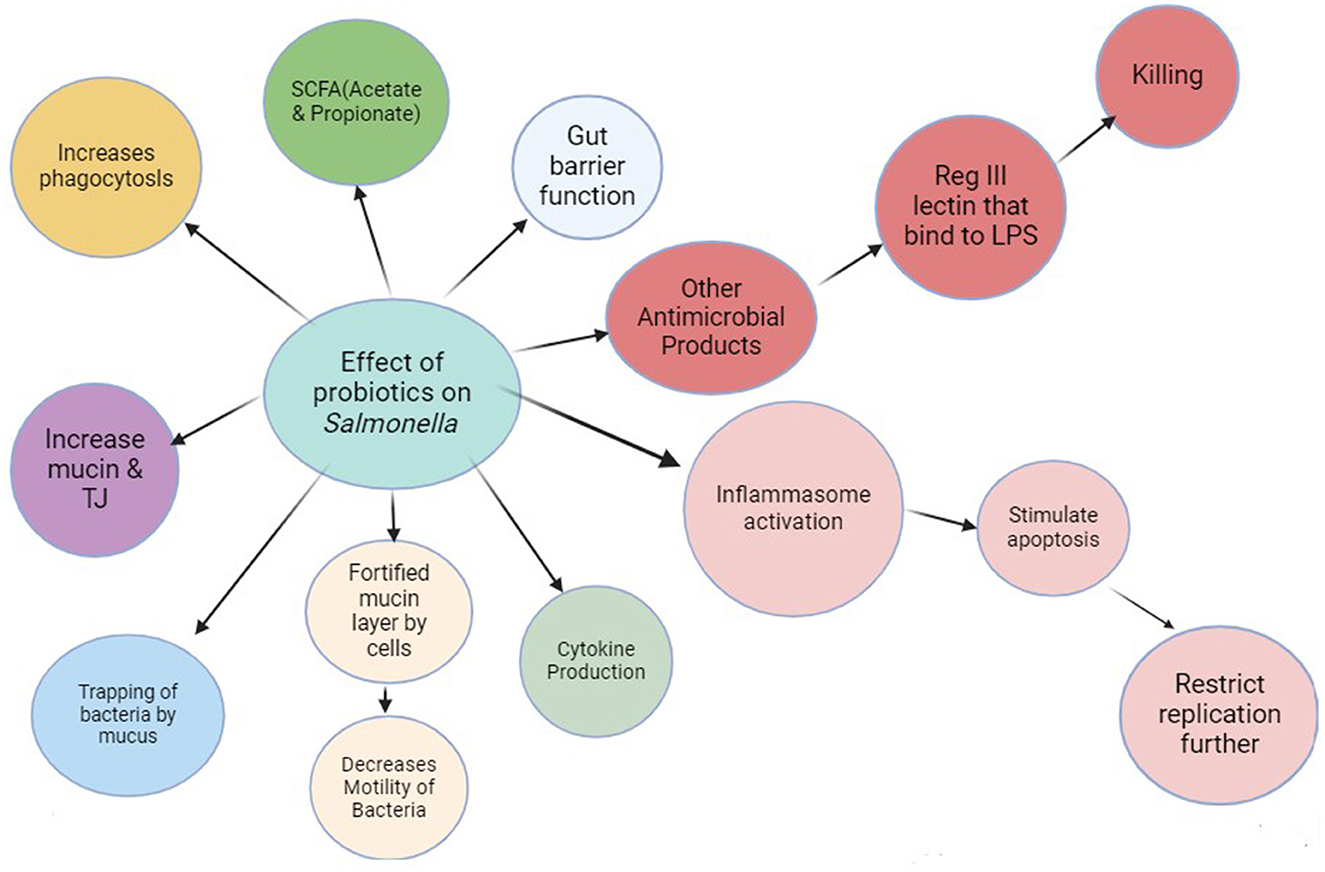
Figure 2. This flow chart depicts how action mechanism of probiotics can counter the multiplication (bactericidal or bacteriostatic) of Salmonella sp.
3.3 Others
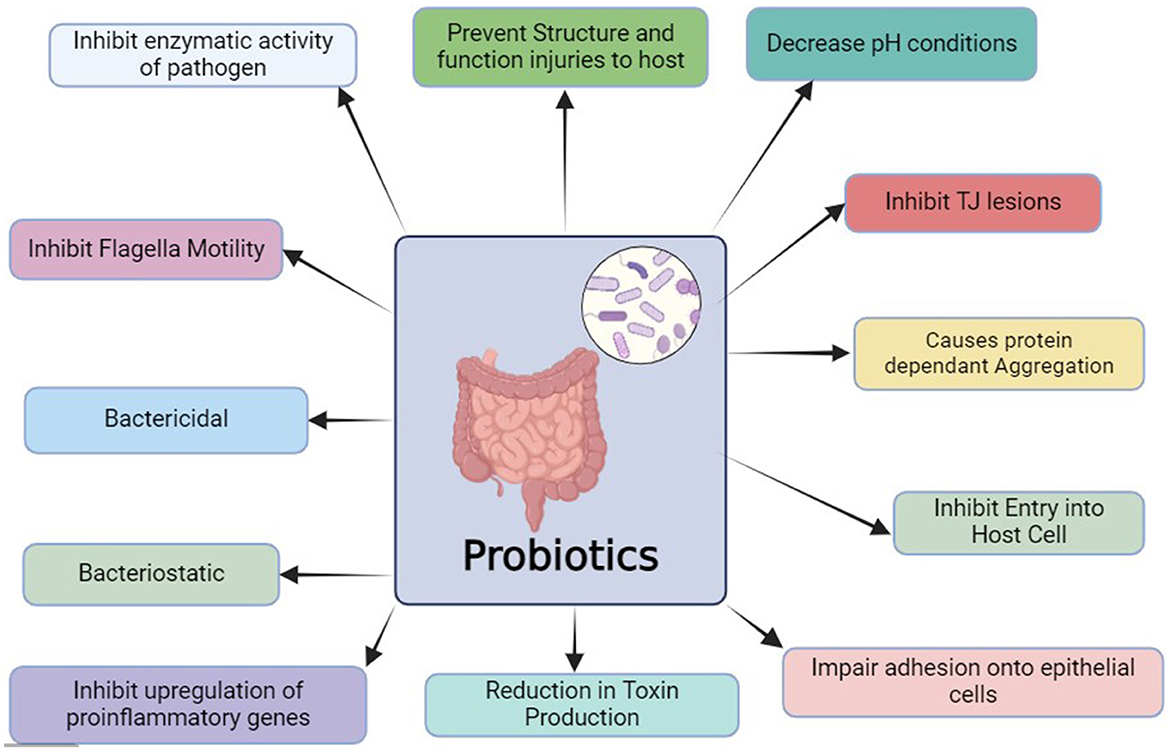
Probiotics can show repertoire effects on pathogenic bacteria leading to inhibit spreading of infection or inhibition in even entry of the same. Some disease causing bacteria such as Helicobacter pylori, enterohemorrhagic Escherichia coli (EHEC), Shigella species, Salmonella species, and Campylobacter species can be encountered effectively either via action of probiotic microbe. Mechanisms may include, either direct action via probiotics or stimulate host body to fight against causative agent (Figure 3), increase in the phagocytic response in the host, production of metabolites which can either change ecology (changer PH conditions) or can act as bactericidal (L. rhamnosus GG, L. johnsonii NCC 533, L. reuteri ATCC 55730, bacteriostatic Lactobacillus strains via production of specific bacteriocins), inhibition of upregulation of proinflammatory genes in host (L. casei DN-114 001 in Shigella infection), impair binding of bacteria to the epithelial layer (L. rhamnosus GG, L. johnsonii NCC 533, L. casei Shirota, L. reuteri ATCC 55730, L. acidophilus LB, etc. in E. coli infection), reduce toxin production (L. rhamnosus GG in E. coli infection), inhibit flagellar motility (L. casei Shirota against S. typhimurium), prevention of structural and function injured to the host, ceasing entry of pathogen into host cell (L. casei Shirota against S. typhimurium), leads to aggregation of pathogen resulting in reduction of spread in the body (L. johnsonii NCC 533 in H. pylori attack), inhibit enzymatic action of the pathogen (L. casei Shirota against H. pylori infection), and enhance tight junctions between the cells (L. rhamnosus GG in E. coli infection) (Villena et al., 2014).
4 Impact on animal growth performance
There are several species of bacterial probiotics known till date, but most popular ones are Lactobacillus, Streptococcus, Lactococcus, and Bifidobacterium, and in case of fungi it can be Saccharomyces cerevisiae and Aspergillus oryzae. Some of them are described below.
Lactobacillus, a Gram-positive bacterium categorized among lactic acid-producing bacteria, predominantly inhabits the mammalian microbiota (Giri et al., 2013; Huang et al., 2022). Numerous species within this genus are commonly utilized as probiotics in various animal consumables, both dairy and non-dairy. Moreover, certain Lactobacillus species, employed as feed supplements, have demonstrated advantageous properties, such as reducing fish mortality, enhancing piglet growth performance, augmenting egg production and quality in poultry, bolstering immune defense mechanisms in fish, and mitigating Salmonella contamination in poultry (Mo et al., 2020; Arsène et al., 2021). Notably, Lactobacillus strains capable of producing active dietary enzymes, including, phytase, amylase, lipase, protease, and protease, present promising probiotic candidates owing to their crucial role in nutrient digestion and absorption. However, it is imperative to acknowledge that certain members of the genus, such as Lactobacillus casei and Lactobacillus rhamnosus, were implicated in bacterial diseases. Consequently, probiotic's usage holds promise for maintaining animal health and serving as a prophylactic measure, albeit with cautious consideration of potential risks (Arsène et al., 2021).
Bifidobacterial strains, well-known for their alleged positive effects on health, are frequently used as probiotics (Esteban-Torres et al., 2021). The benefits encompass protection against infections, regulation of the host immune system, provision of minerals and vitamins, and various other documented favorable effects. Bifidobacteria ferment diverse complex carbohydrates, hence supplying nutrients to the host via their metabolic by-products. Furthermore, an important characteristic of bifidobacterial metabolism is the production of vitamins, including riboflavin (also known as vitamin B2), within the organism itself (Jing et al., 2020). Genomic research has identified genes associated with riboflavin production across the entire Bifidobacterium genus. For instance, researchers have discovered naturally occurring strains of Bifidobacterium longum subsp. infantis ATCC 15697 that produce higher levels of riboflavin. This leads to an increase in the concentration of vitamin B2 in a fecal fermentation system (Monteiro et al., 2019; Abou-Kassem et al., 2021). In addition, these bacteria establish themselves in the gut, protecting against harmful microorganisms by regulating the function of the intestinal lining. Specific taxa of bifidobacteria secrete extracellular layers, such as exopolysaccharide (EPS), which allows them to withstand gastrointestinal obstacles and persist in the stomach for prolonged durations. EPS derived from probiotic bacteria has been associated with beneficial immunomodulatory effects. The role of bifidobacterial EPS in immune cell response has been extensively studied. Moreover, alterations in gut microbiota and their by-products have been linked to several inflammatory and immunological mechanisms connected with the formation of cancer and the growth of tumors. Research has investigated the potential of using Bifidobacterium bifidum JCM 1254 to treat antibiotic-induced dysbiosis. It has been found that Bifidobacterium breve M-16V exhibits long-lasting colonization in the gut after treatment and may contribute to the development of a healthy gut microbiota (Esteban-Torres et al., 2021).
The genus Saccharomyces is an integral component of the gut microbiota. Among the species within this fungal genus, Saccharomyces cerevisiae stands out as the most renowned and widely utilized probiotic strain. Research indicates that S. cerevisiae exhibits beneficial effects, such as enhancing the reproductive performance of sows, augmenting the concentration of immunoglobulin G (IgG) in colostrum and subsequent plasma IgG levels in piglets, improving growth performance, and fostering intestinal health in pigs. Moreover, positive outcomes associated with the utilization of S. cerevisiae have been documented in fish species. For instance, studies have demonstrated its capacity to enhance growth, hematological parameters, antioxidant defenses, and immune responses in Nile tilapia. Furthermore, S. cerevisiae has been shown to bolster the resistance of Nile tilapia against infections caused by the pathogenic fungus A. flavus and enhance the cellular innate immune response in gilthead seabream. In addition, other species within the Saccharomyces genus, such as Saccharomyces carlsbergensis, are also employed as probiotics in animal nutrition (Arsène et al., 2021).
Probiotics show different effect on different type of animal, such as in cattle, poultry, and fishes. A diagram illustrates some of the functions of probiotics in the type of animals and how they are benefitted from them when introduced into their feed.
4.1 Biological mechanisms of probiotics, specifically lactobacillus, in enhancing animal growth
Probiotics, including Lactobacillus species, influence animal growth through a variety of biological mechanisms that contribute to improved health and performance (Khushboo et al., 2023). These mechanisms include enhancing nutrient absorption, reducing pathogen load, and modulating immune and hormonal responses (Redweik et al., 2020).
4.1.1 Enhancing nutrient absorption
Probiotics improve nutrient absorption by promoting a healthier gut environment. Species such as Lactobacillus are known to produce digestive enzymes, such as amylase and protease, which break down complex carbohydrates and proteins into simpler, more absorbable forms. These enzymes facilitate better digestion and nutrient uptake, leading to increased growth rates in animals. In addition, Lactobacillus can stimulate the production of short-chain fatty acids (SCFAs), which are crucial for maintaining gut health. SCFAs, like butyrate, provide energy for intestinal cells and enhance the integrity of the gut barrier, improving nutrient absorption efficiency (Song et al., 2018; Huang et al., 2022). Lactobacillus acidophilus enhances calcium and phosphorus uptake in poultry by promoting an acidic environment conducive to mineral solubilization (Chen et al., 2022). Similarly, Lactobacillus casei ATCC 49178 has been found to enhance protein digestion in pigs by increasing protease activity in the gut (Wang et al., 2019). In ruminants, Lactobacillus plantarum CICC6257 stimulates fiber degradation by producing enzymes that aid cellulose breakdown, enhancing volatile fatty acid production (Reuben et al., 2022). In aquaculture, Lactobacillus delbrueckii promotes fatty acid absorption, improving fish growth performance (Merrifield et al., 2010). In addition, Lactobacillus reuteri has demonstrated improved bioavailability of iron in animal models by reducing gut inflammation and enhancing epithelial transport (Hou et al., 2015).
4.1.2 Reduction of pathogen load
One of the primary benefits of probiotics is their ability to compete with pathogenic bacteria in the gut. Lactobacillus species exert a competitive exclusion effect by occupying binding sites on the intestinal mucosa, limiting the ability of harmful bacteria to colonize the gut. This results in a reduced pathogen load and decreases the likelihood of infections that can hinder growth performance (Redweik et al., 2020; Braz et al., 2020). Moreover, Lactobacillus strains produce antimicrobial compounds such as bacteriocins, lactic acid, and hydrogen peroxide, which inhibit the growth of harmful bacteria. By lowering pathogen levels, these probiotics reduce the energy and immune resources the host would otherwise expend fighting infections, redirecting that energy toward growth and development (Giri et al., 2013). Lactobacillus acidophilus has been shown to reduce Clostridium difficile infections by restoring gut microbiota balance. Similarly, Lactobacillus rhamnosus GG effectively diminishes the load of Salmonella typhimurium in poultry, contributing to food safety (Kizerwetter-Swida and Binek, 2009). Furthermore, Lactobacillus plantarum has demonstrated the ability to inhibit Listeria monocytogenes in fermented food products, enhancing preservation and safety (Behera et al., 2018). These studies highlight the potential of Lactobacillus as a natural biocontrol agent in both clinical and industrial settings, reducing reliance on antibiotics and chemical preservatives. In addition, bacteriocins produced by Lactobacillus, such as nisin and reuterin, possess antimicrobial properties that target specific pathogens without harming beneficial microbes (Griggs and Jacob, 2005; Lutful Kabir, 2009). Competitive exclusion by Lactobacillus involves occupying adhesion sites on epithelial cells, preventing pathogens such as Escherichia coli and Salmonella spp. from colonizing (Corr et al., 2007).
4.1.3 Immune modulation and hormonal stimulation
Probiotics can modulate the immune system, leading to enhanced growth performance. Lactobacillus species stimulate the production of mucosal immunoglobulin A (IgA) and other immune factors that strengthen the gut's defense against pathogens. This improved immunity reduces disease incidence, which can have a positive effect on growth rates. In addition, probiotics may influence hormonal responses related to growth (Lee et al., 2013). They can stimulate the release of growth-related hormones such as insulin-like growth factor 1 (IGF-1), which plays a significant role in promoting muscle growth and development (Du et al., 2018). Enhanced gut health due to probiotic use can also support optimal endocrine function, further contributing to improved growth metrics. Overall, the combination of enhanced nutrient absorption, reduction in pathogen load, and modulation of immune and hormonal responses explains why Lactobacillus and other probiotics are effective in boosting animal growth and performance (Zhang et al., 2021).
4.2 Effect of probiotics on poultry
The forthcoming decade is anticipated to witness a 14% surge in the worldwide consumption of meat proteins by the year 2030, in contrast to the baseline average spanning 2018 to 2020. This escalation is primarily attributed to the rise in both income levels and population numbers (Anee et al., 2021). So, there is a need to increase the yield by providing proper nutrition and disease prevention, for which we can employ the use of probiotics in their diet. Broiler chickens can experience improvements in their immune system, overall growth rate, and also antioxidant levels when they are fed with Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium at a concentration of ~1 percent of their food intake, which corresponds to over 5 × 109 CFU/g (Zhang et al., 2021). In addition, the quality of chicken meat can be enhanced through the use of probiotics. For instance, Bacillus subtilis has been found to have a positive impact on chicken meat quality (Mohammed et al., 2021). Similarly, the growth rate and meat quality of quails can be improved by the administration of Bifidobacterium bifidum and Bacillus toyonensis (Abou-Kassem et al., 2021). Furthermore, the presence of Lactobacillus casei in the diet of broiler chickens can lead to an increase in high-density lipoprotein (HDL) levels and a decrease in low-density lipoprotein (LDL) levels (Sudha et al., 2009). In contrast, probiotics have been acknowledged for their beneficial effects in treating diseases such as salmonellosis in hens. Research has shown that administering 1 × 109 CFU of Lactobacillus plantarum LTC-113 strain to newly hatched chicks through vaccination will successfully protect them from salmonellosis. The particular strain of Lactobacillus plantarum mentioned in the study has the capacity to inhibit the growth of dangerous bacteria in the gut and maintain the proper functioning of tight junction genes in the cells lining the gut. As a result, it enhances the chickens' ability to withstand infections (Wang et al., 2018). Recent research indicates that the combination of probiotics and recombinant attenuated Salmonella vaccine (RASV) can effectively decrease the infection rate of APEC and Salmonella in White Leghorn hens, who are very susceptible to these diseases (Redweik et al., 2020). In addition, the inclusion of Bacillus licheniformis and Bacillus subtilis in feed supplements has been demonstrated to lessen the excretion of E. coli in hens laying eggs (Upadhaya et al., 2019).
4.3 Effect of probiotics on ruminants
Total beef production is projected to rise by 9% in the upcoming decade until 2029, while total milk production is anticipated to grow by 20% during the same timeframe. The increased levels of milk and ruminant meat production are foreseen to primarily stem from the expansion of worldwide cattle herds, which are expected to increase from the current 1.6 billion to almost 1.8 billion by 2028. Probiotics tailored for ruminants encompass a range of direct-fed microbial agents, encompassing yeast species such as Saccharomyces cerevisiae, as well as various bacterial strains including Bacillus, Bifidobacterium, Enterococcus, Lactobacillus, and Propionibacterium. Incorporating S. cerevisiae and Aspergillus oryzae into the diets of dairy cows has been shown to yield significant enhancements in milk production, accompanied by increased concentrations of milk proteins. Similarly, supplementation with strains of Bacillus subtilis as probiotics has been observed to elicit improvements in ruminal fermentation and subsequent milk yield in dairy cows (OECD).6,7 Probiotics are acknowledged as advantageous agents for enhancing milk output in dairy animals. Several specific probiotic bacteria, including as Bacillus subtilis, Saccharomyces cerevisiae, and Enterococcus faecalis, have been recognized as factors that promote higher milk production (Ma et al., 2020). In addition, Bifidobacterium bifidum has demonstrated the capacity to alleviate milk allergy responses (Jing et al., 2020). Furthermore, the use of Bacillus subtilis and Bacillus amyloliquefaciens as probiotics has been associated with improved development and growth of the intestines by increasing the GH/IGF-1 hormone (Du et al., 2018). Probiotics are essential for enhancing rumen fermentation. Several strains of probiotics have been discovered to produce antibacterial substances, which conclusively decrease the presence of zoonotic pathogens and control the creation of ammonia. Rhodopseudomonas palustris, a photosynthetic bacterium, has gained attention as a potential probiotic option in the case of animal feed section of the industry. Chen B. et al. (2020) and Chen Y. Y. et al. (2020) observed that feed additives containing Rhodopseudomonas palustris improved the survival of rumen bacteria, leading to enhanced growth performance and microbial fermentation, ultimately maintaining microbial equilibrium. Moreover, the utilization of Megasphaera elsdenii has demonstrated the ability to increase the generation of butyrate and food intake in newborn calves, indicating its potential in enhancing rumen function and overall calf wellbeing (Muya et al., 2015).
4.4 Effects of probiotics on aquaculture
Aquaculture is a growing industry following substantial rise in 2018, wherein net production, trade, and usage of products reached unprecedented levels, there was a slight decline observed in the global fisheries and aquaculture sector in 2019. However, aquaculture production continued its upward trajectory, expanding by more than 2% (OECD). So, to cater high consumption requirements, production of healthy products is mandatory and one of the approach to this is usage of probiotics instead of harmful antibiotics (Hassoun-Kheir et al., 2020). Bacillus subtilis, derived from the Bacillus genus, is frequently utilized (Olmos et al., 2020). When employed alone, Bacillus probiotics have been shown to effectively fight against a range of hazardous microbes in fish populations, such as Vibrio, Flavobacterium, Aeromonas, Acinetobacter, Clostridium, Pseudomonas, Streptococcus, and white spot syndrome virus (Kuebutornye et al., 2020). In addition, LAB bacteria, specifically Lactococcus lactis (Balcázar et al., 2007) and Lactobacillus plantarum VSG-3 (Giri et al., 2013), are frequently consumed as probiotics in aquaculture. Gram-negative bacteria encompass another crucial category of probiotics employed in fish farming. Another example can be of Saccharomyces cerevisiae which can be beneficial for the health of fishes (Mo et al., 2020; Wu et al., 2020). Figure 4 presents a systematic web diagram showcasing the versatile applications of probiotics in animal husbandry, offering holistic benefits across aquaculture, ruminant farming, and poultry production. It highlights their multifaceted role in enhancing animal health and productivity.
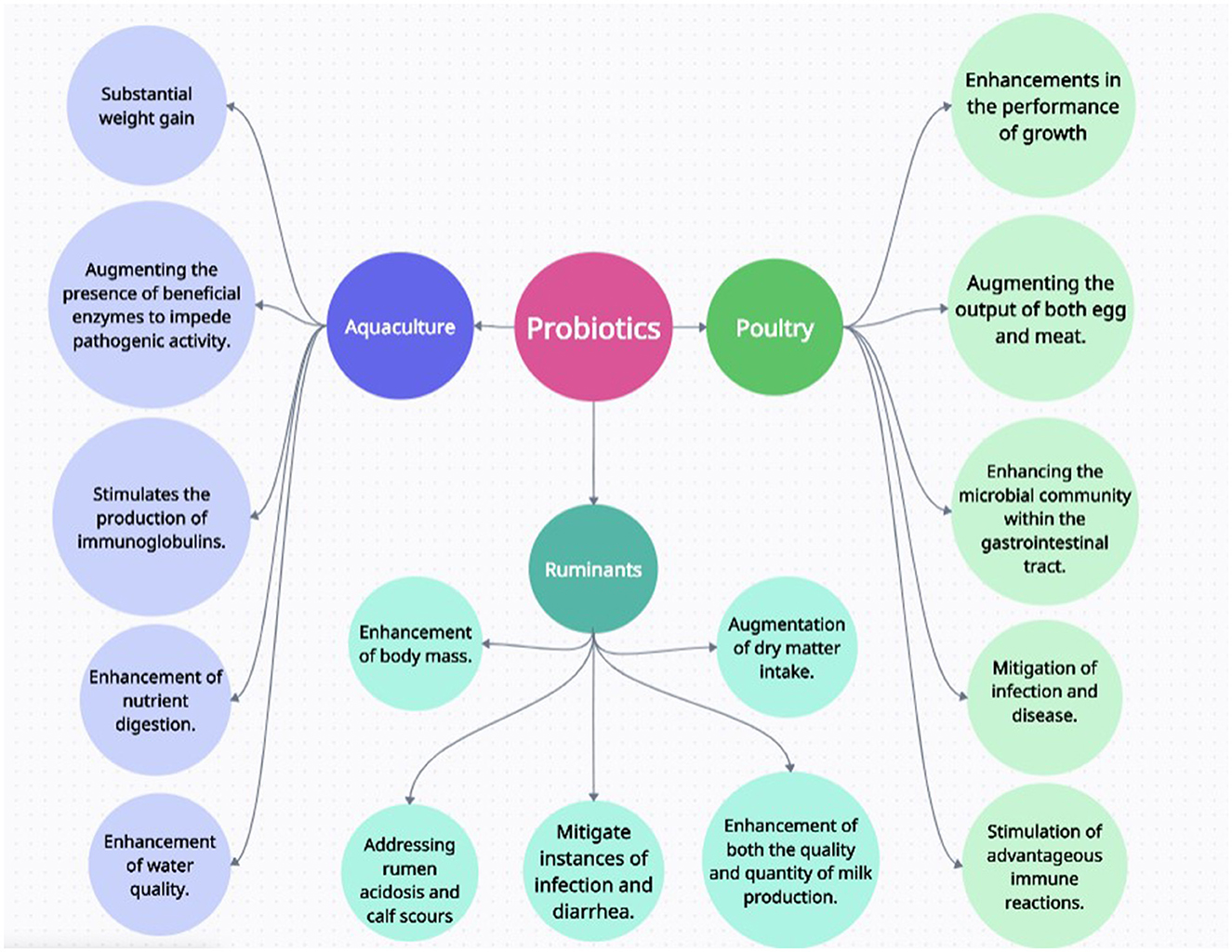
Figure 4. This systematic web diagram illustrates how probiotics is applicable in the animal husbandry and how it is providing holistic benefit in the field of aquaculture (to the left hand side), ruminants (below center), and poultry (to the right hand side).
4.5 Probiotics in enhancing livestock breeding and health
Farm animals are continuously exposed to various environmental stressors, including rearing methods, dietary changes, and housing conditions, which can disrupt the delicate balance of their intestinal ecosystems. Such disturbances heighten the risk of pathogenic infections, directly impacting the health and productivity of livestock. Across all species, maintaining optimal animal health is critical for sustaining efficiency in the production chain (Torres-Rodriquez et al., 2007).
The strategic incorporation of probiotics into animal feed has emerged as a scientifically validated approach to modulating intestinal microbiota. Probiotic strains, administered individually or in combination, significantly enhance feed absorption, nutrient utilization, and weight gain across a range of animals, including turkeys, chickens, piglets, sheep, goats, cattle, and horses. These benefits translate to measurable improvements in the quantity and quality of milk, meat, and eggs. For instance, probiotics have been shown to alleviate issues such as weak limbs in broiler chickens (Samli et al., 2007) and reduce diarrheal episodes in piglets during the critical post-weaning period—a time of heightened susceptibility to nutritional and environmental stressors (Li et al., 2006; Casey et al., 2007).
The efficacy of probiotics in combating diarrheal conditions, a persistent challenge in piglets, has been a focal point in numerous studies (Li et al., 2006; Casey et al., 2007). Furthermore, recombinant probiotics represent an advanced application of genetically modified organisms (GMOs) in animal health, providing targeted benefits without clinical side effects.
4.5.1 Probiotics in swine production
Weaning is a pivotal phase in pig production, characterized by nutritional shifts from milk to plant-based diets and environmental transitions to production farms (Modesto et al., 2009). These changes often impair immunological functions and disrupt intestinal microbiota. Böhmer et al. (2006) demonstrated that supplementing sows' diets with Enterococcus faecium DSM 7134 from late pregnancy through lactation significantly improved feed intake, litter size, and offspring weight. This supplementation also mitigated “starvation sterility” in young sows, attributed to insufficient energy availability during lactation.
Probiotics enhance digestion, particularly cellulolytic processes, and stimulate microbial protein synthesis. Mountzouris et al. (2007) evaluated a probiotic blend containing strains from Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus in broiler chickens, showing growth stimulation comparable to avilamycin, an antibiotic growth promoter. In addition, these probiotics effectively modulated intestinal microbiota composition and activity, highlighting their potential as sustainable alternatives to antibiotics.
4.5.2 Probiotics in ruminants
The use of YEA-SACC-1026 and bacterial strains such as Bacillus licheniformis and Bacillus subtilis has demonstrated significant benefits in ruminants (Kritas et al., 2006). These probiotics, administered during late pregnancy and lactation, improved milk quality (e.g., higher fat and protein content) and enhanced lamb body weight. Similarly, the Bio Plus 2B® probiotic improved sows' blood lipid profiles and milk composition, underscoring its utility in boosting productivity during lactation (Alexopoulos et al., 2004). Yu et al. (1997) explored the effects of adding Aspergillus oryzae culture to steamed corn in cows' diets, observing increases in milk protein content and solids-not-fat (SNF) over a 70-day trial. These results reinforce the potential of probiotics in optimizing milk composition and productivity in dairy cattle.
4.5.3 Probiotics and meat quality
Ceslovas et al. (2005) investigated the impact of probiotics (YEASTURE, MICROBOND) and phytobiotics (YUCCA, QUILLAYA) on pigs' growth and meat quality. Probiotics significantly improved carcass yield, culinary properties, cooking loss reduction, and meat tenderness, outperforming phytobiotics in these aspects. The integration of probiotics into livestock diets represents a scientifically robust and sustainable strategy to enhance animal health, productivity, and product quality. Their application extends beyond mitigating disease risks to improving feed efficiency, growth, and product characteristics. Continued research and tailored formulations will further optimize their efficacy, supporting the transition toward sustainable and resilient animal production systems (Van Immerseel et al., 2006).
5 Safety and regulatory considerations
The assessment of probiotics for safety and efficacy in animals involves a rigorous and multifaceted approach to ensure that the microorganisms provide benefits without posing risks to the host (Merenstein et al., 2023). It is very important to checklist probiotic before being used or administered to any organism, so there are certain criteria to fulfill, like, to properly characterize an isolate, check if that probiotic is safe or not, there should a record of at least one positive clinical trial (Ahire et al., 2024) of the same, and it should retain a specific number in the product during its shelf life (Table 3). These assessments consider factors such as strain specificity, potential adverse effects, and the conditions under which probiotics are used.
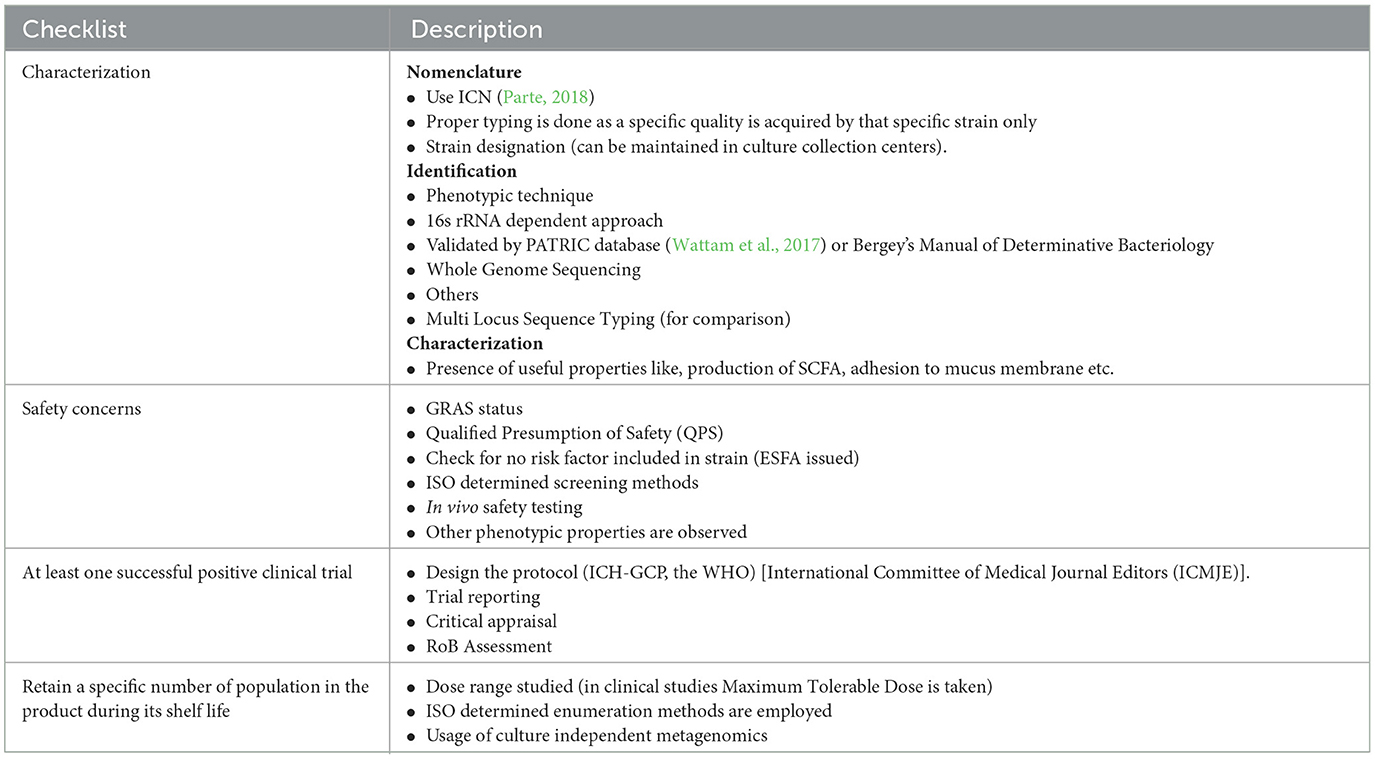
Table 3. This table shows a generalized idea about the steps to be followed to meet the criteria by an organism as probiotic (Wu et al., 2020; Binda et al., 2020; Ahire et al., 2024).
5.1 Selection and characterization of probiotic strains
Probiotic safety begins with the selection of specific strains that have a history of safe use. This involves identifying the strain through genomic sequencing, ensuring that it does not carry harmful genes related to antibiotic resistance, virulence, or toxin production. Characterization of the probiotic strain includes evaluating its potential for beneficial effects, such as improving gut health or immune function, while screening for adverse traits that may pose risks to the host (Haranahalli Nataraj et al., 2024). Strain specificity is crucial as different species and strains of bacteria can have varying effects on different animal species. For instance, a strain that is safe and beneficial for ruminants may not be appropriate for poultry or companion animals. Thus, the choice of strain is closely linked to the target species and intended health outcomes (Hradicka et al., 2023).
5.2 Safety testing in animal models
Before a probiotic is approved for use, it undergoes extensive safety testing in animal models. These studies assess whether the probiotic can survive, colonize, and exert a beneficial effect without causing harm. Key safety endpoints include monitoring for signs of infection, gastrointestinal disturbances, immune responses, and any negative impacts on growth or feed efficiency (Haranahalli Nataraj et al., 2024). Testing often involves evaluating a probiotic's potential to translocate from the gut to other body parts, which could indicate a risk of systemic infection. Animal trials also aim to observe whether the probiotic impacts the animal's native microbiota, potentially disrupting beneficial microbial communities. These trials are designed to mimic the conditions in which the probiotic would be used, considering factors such as dosage, duration of administration, and environmental conditions (Merenstein et al., 2023).
5.3 Monitoring adverse effects and long-term impact
While probiotics are generally considered safe, potential risks must be evaluated, especially for vulnerable populations such as neonatal or immunocompromised animals. Long-term studies are often needed to assess whether probiotics have chronic effects on the animal's health, microbiome composition, or physiological functions. Particular attention is given to the risk of horizontal transfer of antibiotic resistance genes, which could have broader ecological and health implications (Spacova et al., 2023).
5.4 Potential risks associated with probiotics in animals
Despite the benefits, probiotics are not without potential risks. In specific scenarios, probiotics may cause adverse effects in particular animal species or under certain conditions (Rabetafika et al., 2023; da Silva et al., 2024).
5.4.1 Species-specific risks
Some animal species may react differently to the same probiotic strain. For example, strains of Lactobacillus and Bifidobacterium are generally well-tolerated in ruminants but could cause digestive upset in animals with different gut environments.
5.4.2 Immune response
In some cases, probiotics may overstimulate the immune system, leading to inflammation or allergic reactions. This is particularly relevant for immunocompromised animals or those with pre-existing health conditions (Evangelista et al., 2023).
5.4.3 Microbial translocation
There is a risk of probiotics translocating from the gastrointestinal tract to other body sites, potentially leading to systemic infections (Halder et al., 2024). This concern is heightened in animals with compromised intestinal barriers or those receiving probiotics in high doses.
5.4.4 Antibiotic resistance
Probiotic strains must be screened for antibiotic resistance genes. There is a theoretical risk that these genes could be transferred to pathogenic bacteria within the host or the environment, potentially exacerbating antibiotic resistance issues Nataraj and Mallappa, 2021.
The European-Union's ban on antibiotics as growth promoters in animal feed, effective since 1 January 2006, was a critical step to combat antimicrobial resistance (Avicola et al., 2022; Schmerold et al., 2023). Before this ban, antibiotics were widely used at sub-therapeutic levels in animal feed to enhance growth rates and improve feed efficiency. However, extensive scientific research highlighted that this practice contributed significantly to the development of antibiotic-resistant bacteria, posing risks to both animal and human health. The regulation, introduced under Regulation (EC) No 1831/2003, aimed to protect public health by eliminating non-essential antibiotic use in agriculture (Schmerold et al., 2023). It also aligns with the One Health concept, which seeks to balance human, animal, and environmental health. Following the ban, the livestock industry had to adapt to alternative methods for promoting growth and preventing disease. These alternatives include improved animal husbandry practices, enhanced biosecurity measures, and the use of non-antibiotic growth promoters such as probiotics, prebiotics, organic acids, and phytogenics. The EU's policy also sets the stage for stricter regulations on antibiotic usage in veterinary medicine, including the requirement for veterinary oversight for therapeutic applications. This legislation has influenced global practices, with several countries and regions implementing similar restrictions (Nordeus, 2023). The EU ban demonstrated that it is feasible to maintain animal health and productivity without relying on antibiotics for growth promotion. This shift has led to significant advancements in research for sustainable livestock production and antibiotic alternatives, contributing to the broader effort of reducing the spread of antibiotic resistance—a crucial goal for safeguarding future efficacy of antibiotics in both human and veterinary medicine (Schmerold et al., 2023).
Regulatory agencies emphasize the need for probiotics to be thoroughly evaluated for safety, particularly in animal populations intended for food production. Safety assessments often include evaluating the genetic stability of the probiotic strain to ensure that it does not acquire or transfer harmful traits. In addition, adherence to good manufacturing practices (GMP) is critical to prevent contamination with unwanted microorganisms during production (da Silva et al., 2024). Products targeted for specific populations, such as neonatal animals or those in clinical settings, undergo stricter testing to meet higher safety standards (Hradicka et al., 2023). Quality control measures, such as third-party verification of strain purity, potency, and identity, are recommended to ensure the safety and efficacy of probiotics in animal health. The safe and effective use of probiotics in animals relies on a combination of careful strain selection, comprehensive safety testing, and ongoing monitoring for adverse effects. While probiotics hold great promise for enhancing animal health, understanding and mitigating potential risks is essential to ensure they provide benefits without unintended consequences (Merenstein et al., 2023; Haranahalli Nataraj et al., 2024; Ahire et al., 2024).
6 Conclusion
Probiotics represent a promising alternative to traditional antimicrobial growth promoters (AGPs) in animal husbandry, addressing critical concerns such as antimicrobial resistance (AMR) and promoting animal health sustainably. The effectiveness of probiotics hinges on their mechanisms of action, which involve direct antagonism against pathogens, modulation of the gut microbiome, and enhancement of the host's immune system. The ability of probiotics to compete for nutrients and adhesion sites, produce antimicrobial compounds such as bacteriocins and organic acids, and alter the gut's ecological environment underscores their potential to outcompete and inhibit pathogenic bacteria. This direct antagonism has been effective in reducing the prevalence of harmful microbes such as Clostridium perfringens and Salmonella, enhancing overall animal health and productivity.
Furthermore, probiotics positively influence the gut microbiome, fostering beneficial bacteria and inhibiting pathogenic species through ecological shifts. They also reinforce the gut barrier function, crucial for preventing systemic infections and maintaining animal welfare. These attributes collectively contribute to enhanced growth performance and disease resistance across various animal species, including poultry, ruminants, and fish.
Despite the advantages, safety remains a critical aspect in the use of probiotics. The potential risks, such as species-specific adverse reactions, overstimulation of immune responses, microbial translocation, and the horizontal transfer of antibiotic resistance genes, highlight the need for careful strain selection and rigorous testing. Regulatory frameworks ensure that probiotics meet stringent safety standards, particularly in food-producing animals, to minimize risks while maximizing health benefits. The comprehensive assessment and monitoring of probiotics are essential to harness their full potential as a sustainable solution in animal husbandry. The ongoing refinement of safety protocols and regulatory guidelines, combined with advancing research, will be pivotal in expanding the safe use of probiotics, reducing reliance on antibiotics, and mitigating AMR challenges in animal health.
Author contributions
AS: Data curation, Formal analysis, Investigation, Resources, Writing – original draft. TT: Data curation, Validation, Visualization, Writing – review & editing. TM: Data curation, Investigation, Methodology, Validation, Writing – original draft. AB: Data curation, Methodology, Validation, Visualization, Writing – review & editing. AK: Conceptualization, Resources, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors obliged to Graphic era (deemed to be University), Dehradun, and Lovely Professional University, Phagwara, to help me conduct my research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^https://www.avma.org/resources-tools/one-health/antimicrobial-use-and-antimicrobial-resistance/antimicrobial-resistant-pathogens-affecting-animal-health
2. ^https://www.scielo.br/j/cta/a/nnxxYP5VT9C8CyTqCFcWJym/
3. ^https://www.cdc.gov/ecoli/general/index.html
4. ^https://www.cdc.gov/foodsafety/diseases/clostridium-perfringens.html
5. ^https://www.cdc.gov/hai/organisms/staph.html
6. ^https://one.oecd.org/document/TAD/CA/APM/WP(2020)18/FINAL/En/pdf
7. ^https://www.oecd-ilibrary.org/sites/4dd9b3d0-en/index.html?itemId=/content/component/4dd9b3d0-en#section-d1e19686
References
Abou-Kassem, D. E., Elsadek, M. F., Abdel-Moneim, A. E., Mahgoub, S. A., Elaraby, G. M., Taha, A. E., et al. (2021). Growth, carcass characteristics, meat quality, and microbial aspects of growing quail fed diets enriched with two different types of probiotics (Bacillus toyonensis and Bifidobacterium bifidum). Poult. Sci. 100, 84–93. doi: 10.1016/j.psj.2020.04.019
Ahire, J. J., Rohilla, A., Kumar, V., and Tiwari, A. (2024). Quality management of probiotics: Ensuring safety and maximizing health benefits. Curr. Microbiol. 81:1. doi: 10.1007/s00284-023-03526-3
Aleman, R. S., and Yadav, A. (2023). Systematic review of probiotics and their potential for developing functional nondairy foods. Appl. Microbiol. 4, 47–69. doi: 10.3390/applmicrobiol4010004
Alexopoulos, C., Georgoulakis, I. E., Tzivara, A., Kritas, S. K., Siochu, A., and Kyriakis, S. C. (2004). Field evaluation of the efficacy of a probiotic containing Bacillus licheniformis and Bacillus subtilis spores, on the health status and performance of sows and their litters. J. Anim. Physiol. Anim. Nutr. 88, 381–392. doi: 10.1111/j.1439-0396.2004.00492.x
Anee, I. J., Alam, S., Begum, R. A., Shahjahan, R., and Khandaker, A. (2021). The role of probiotics on animal health and nutrition. JoBAZ 82:52. doi: 10.1186/s41936-021-00250-x
Anjana and Tiwari, S. K.. (2022). Bacteriocin-producing probiotic lactic acid bacteria in controlling dysbiosis of the gut microbiota. Front. Cell. Infect. Microbiol. 12:851140. doi: 10.3389/fcimb.2022.851140
Arsène, M. M. J., Davares, A. K. L., Andreevna, S. L., Vladimirovich, E. A., Carime, B. Z., Marouf, R., et al. (2021). The use of probiotics in animal feeding for safe production and as potential alternatives to antibiotics. Vet. world 14, 319–328. doi: 10.14202/vetworld.2021.319-328
Avicola, E. S., Porcino, E. S., America, S., and Welfare, P. (2022). Assessing the results of the EU ban on antibiotic feed additives by hector cervantes, DVM, MS, Dip. ACPV, manager, poultry technical services, phibro animal health corp.-The European Union (EU) banned the use of avoparcin, a widely used antibiotic feed additive in food-producing animals in 1997. The ban was carried out against the advice of the Scientific Committee on Animal Nutrition (1, 22), a panel of experts composed of animal scientists from various EU countries. Health 17:1.
Balcázar, J. L., Rojas-Luna, T., and Cunningham, D. P. (2007). Effect of the addition of four potential probiotic strains on the survival of pacific white shrimp (Litopenaeus vannamei) following immersion challenge with Vibrio parahaemolyticus. J. Invertebr. Pathol. 96, 147–50. doi: 10.1016/j.jip.2007.04.008
Behera, S. S., Ray, R. C., and Zdolec, N. (2018). Lactobacillus plantarum with functional properties: an approach to increase safety and shelf-life of fermented foods. Biomed Res. Int. 2018:9361614. doi: 10.1155/2018/9361614
Ben Braïek, O., and Smaoui, S. (2019). Enterococci: between emerging pathogens and potential probiotics. Biomed Res. Int. 2019:5938210. doi: 10.1155/2019/5938210
Binda, S., Hill, C., Johansen, E., Obis, D., Pot, B., Sanders, M. E., et al. (2020). Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front. Microbiol. 11:1662. doi: 10.3389/fmicb.2020.01662
Böhmer, B. M., Kramer, W., and Roth-Maier, D. A. (2006). Dietary probiotic supplementation and resulting effects on performance, health status and microbial characteristics of primiparous sows. J. Anim. Physiol. Anim. Nutr. 90, 309–315. doi: 10.1111/j.1439-0396.2005.00601.x
Boirivant, M., and Strober, W. (2007). The mechanism of action of probiotics. Curr. Opin. Gastroenterol. 23, 679–692. doi: 10.1097/MOG.0b013e3282f0cffc
Braz, V. S., Melchior, K., and Moreira, C. G. (2020). Escherichia coli as a multifaceted pathogenic and versatile bacterium. Front. Cell Infect. Microbiol. 10:548492. doi: 10.3389/fcimb.2020.548492
Casey, P. G., Gardiner, G. E., Casey, G., Bradshaw, B., Lawlor, P. G., Lynch, P. B., et al. (2007). A 5-strain probiotic combination reduces pathogen shedding and alleviates disease signs in pigs challenged with Salmonella enterica serovar Typhimurium. Appl Environ Microb. 73, 1858–1863. doi: 10.1128/AEM.01840-06
Ceslovas, J., Vigilijus, J., and Almantas, S. (2005). The effect of probiotic and phytobiotics on meat properties and quality in pigs. Vet Zootech. 29, 80–84.
Che, L., Xu, Q., Wu, C., Luo, Y., Huang, X., Zhang, B., et al. (2017). Effects of dietary live yeast supplementation on growth performance, diarrhoea severity, intestinal permeability and immunological parameters of weaned piglets challenged with enterotoxigenic Escherichia coli K88. Br. J. Nutr. 118, 949–958. doi: 10.1017/S0007114517003051
Chen, B., Peng, M., Tong, W., Zhang, Q., and Song, Z. (2020). The quorum quenching bacterium Bacillus licheniformis T-1 protects Zebrafish against Aeromonas hydrophila infection. Probiot. Antimicrob Proteins. 12, 160–171. doi: 10.1007/s12602-018-9495-7
Chen, P., Xu, T., Zhang, C., Tong, X., Shaukat, A., He, Y., et al. (2022). Effects of probiotics and gut microbiota on bone metabolism in chickens: a review. Metabolites 12:1000. doi: 10.3390/metabo12101000
Chen, Y. Y., Wang, Y. L., Wang, W. K., Zhang, Z. W., Si, X. M., Cao, Z. J., et al. (2020). Beneficial effect of Rhodopseudomonas palustris on in vitro rumen digestion and fermentation. Benef. Microbes. 11, 91–99. doi: 10.3920/BM2019.0044
Chuang, S. T., Chen, C. T., Hsieh, J. C., Li, K. Y., Ho, S. T., and Chen, M. J. (2022). Development of next-generation probiotics by investigating the interrelationships between gastrointestinal microbiota and diarrhea in preruminant Holstein calves. Animals 12:695. doi: 10.3390/ani12060695
Cisek, A. A., and Binek, M. (2014). Chicken intestinal microbiota function with a special emphasis on the role of probiotic bacteria. Pol. J. Vet. Sci. 17, 385–394. doi: 10.2478/pjvs-2014-0057
Corr, S. C., Li, Y., Riedel, C. U., O'Toole, P. W., Hill, C., and Gahan, C. G. (2007). Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Nat. Acad. Sci. 104, 7617–7621. doi: 10.1073/pnas.0700440104
Cui, Y., Liu, L., Dou, X., Wang, C., Zhang, W., Gao, K., et al. (2017). Lactobacillus reuteri ZJ617 maintains intestinal integrity via regulating tight junction, autophagy and apoptosis in mice challenged with lipopolysaccharide. Oncotarget 8, 77489–77499. doi: 10.18632/oncotarget.20536
da Silva, T. F., Glória, R. A., Americo, R. D. A., Freitas, M. F., de Jesus, A. D. S., Barroso, L. C. L., et al. (2024). Unlocking the potential of probiotics: a comprehensive review on research, production, and regulation of probiotics. Probiotics Antimicrob. Proteins 16, 1687–1723. doi: 10.1007/s12602-024-10247-x
Darbandi, A., Asadi, A., Mahdizade Ari, M., Ohadi, E., Talebi, M., Halaj Zadeh, M., et al. (2022). Bacteriocins: properties and potential use as antimicrobials. J. Clin. Lab. Anal. 36:e24093. doi: 10.1002/jcla.24093
Du, R., Jiao, S., Dai, Y., An, J., Lv, J., Yan, X., et al. (2018). Probiotic Bacillus amyloliquefaciens C-1 improves growth performance, stimulates GH/IGF-1, and regulates the gut microbiota of growth-retarded beef calves. Front. Microbiol. 9:2006. doi: 10.3389/fmicb.2018.02006
Du, W., Wang, X., Hu, M., Hou, J., Du, Y., Si, W., et al. (2023). Modulating gastrointestinal microbiota to alleviate diarrhea in calves. Front. Microbiol. 14:1181545. doi: 10.3389/fmicb.2023.1181545
Elmi, V. A., Moradi, S., Harsini, S. G., and Rahimi, M. (2020). Effects of Lactobacillus acidophilus and natural antibacterials on growth performance and Salmonella colonization in broiler chickens challenged with Salmonella enteritidis. Livest. Sci. 233:103948. doi: 10.1016/j.livsci.2020.103948
Esteban-Torres, M., Ruiz, L., Lugli, G. A., Ventura, M., Margolles, A., and Van Sinderen, D. (2021). Editorial: Role of bifidobacteria in human and animal health and biotechnological applications. Front. Microbiol. 12:785664. doi: 10.3389/fmicb.2021.785664
Evangelista, A. G., Corrêa, J. A. F., Pinto, A. C. M. S., Gonçalves, F. D. R., and Luciano, F. (2023). Recent advances in the use of bacterial probiotics in animal production. Animal Health Res. Rev. 11, 1–13. doi: 10.1017/S1466252323000063
Giri, S. S., Sukumaran, V., and Oviya, M. (2013). Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol. 34, 660–666. doi: 10.1016/j.fsi.2012.12.008
Gou, H. Z., Zhang, Y. L., Ren, L. F., Li, Z. J., and Zhang, L. (2022). How do intestinal probiotics restore the intestinal barrier? Front. Microbiol. 13:929346. doi: 10.3389/fmicb.2022.929346
Gresse, R., Chaucheyras-Durand, F., Fleury, M. A., Van de Wiele, T., Forano, E., Blanquet-Diot, S., et al. (2017). Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 25, 851–873. doi: 10.1016/j.tim.2017.05.004
Griggs, J. P., and Jacob, J. P. (2005). Alternatives to antibiotics for organic poultry production. J. Appl. Poultry res. 14, 750–756. doi: 10.1093/japr/14.4.750
Halder, N., Sunder, J., De, A. K., Bhattacharya, D., and Joardar, S. N. (2024). Probiotics in poultry: a comprehensive review. J. Basic Appl. Zool. 85:23. doi: 10.1186/s41936-024-00379-5
Haranahalli Nataraj, B., Behare, P. V., Yadav, H., and Srivastava, A. K. (2024). Emerging pre-clinical safety assessments for potential probiotic strains: a review. Crit. Rev. Food Sci. Nutr. 64, 8155–8183. doi: 10.1080/10408398.2023.2197066
Hassoun-Kheir, N., Stabholz, Y., Kreft, J. U., de la Cruz, R., Romalde, J. L., Nesme, J., et al. (2020). Comparison of antibiotic-resistant bacteria and antibiotic resistance genes abundance in hospital and community wastewater: a systematic review. Sci. Total Environ. 743:140804. doi: 10.1016/j.scitotenv.2020.140804
He, G., Long, H., He, J., and Zhu, C. (2024). The immunomodulatory effects and applications of probiotic Lactiplantibacillus plantarum in vaccine development. Probiotics Antimicrob. Proteins 16, 2229–2250. doi: 10.1007/s12602-024-10338-9
Hosain, M. Z., Kabir, S. L., and Kamal, M. M. (2021). Antimicrobial uses for livestock production in developing countries. Veterinary World 14:210. doi: 10.14202/vetworld.2021.210-221
Hou, C., Zeng, X., Yang, F., Liu, H., and Qiao, S. (2015). Study and use of the probiotic Lactobacillus reuteri in pigs: a review. J. Anim. Sci. Biotechnol. 6, 1–8. doi: 10.1186/s40104-015-0014-3
Hou, J., Lian, L., Lu, L., Gu, T., Zeng, T., Chen, L., et al. (2023). Effects of dietary Bacillus coagulans and tributyrin on growth performance, serum antioxidants, intestinal morphology, and cecal microbiota of growing yellow-feathered broilers. Animals 13:3534. doi: 10.3390/ani13223534
Hradicka, P., Adamkova, P., Lenhardt, L., Gancarcikova, S., Iannaccone, S. F., and Demeckova, V. (2023). Addressing safety concerns of long-term probiotic use: in vivo evidence from a rat model. J. Funct. Foods 104, 105521. doi: 10.1016/j.jff.2023.105521
Huang, F., Li, S., Chen, W., Han, Y., Yao, Y., Yang, L., et al. (2023). Postoperative probiotics administration attenuates gastrointestinal complications and gut microbiota dysbiosis caused by chemotherapy in colorectal cancer patients. Nutrients 15:356. doi: 10.3390/nu15020356
Huang, J., Kelly, C. P., Bakirtzi, K., Villafuerte Gálvez, J. A., Lyras, D., Mileto, S. J., et al. (2019). Clostridium difficile toxins induce VEGF-A and vascular permeability to promote disease pathogenesis. Nat. Microbiol. 4, 26–279.
Huang, R., Wu, F., Zhou, Q., Wei, W., Yue, J., Xiao, B., et al. (2022). Lactobacillus and intestinal diseases: Mechanisms of action and clinical applications. Microbiol. Res. 260:127019. doi: 10.1016/j.micres.2022.127019
Jain, M., Stitt, G., Son, L., and Enioutina, E. Y. (2023). Probiotics and their bioproducts: a promising approach for targeting methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Microorganisms 11:2393. doi: 10.3390/microorganisms11102393
Jiang, Z., Su, W., Wen, C., Li, W., Zhang, Y., Gong, T., et al. (2022). Effect of porcine Clostridium perfringens on intestinal barrier, immunity, and quantitative analysis of intestinal bacterial communities in mice. Front. Vet. Sci. 9:881878. doi: 10.3389/fvets.2022.881878
Jing, W., Liu, Q., and Wang, W. (2020). Bifidobacterium bifidum TMC3115 ameliorates milk protein allergy in by affecting gut microbiota: A randomized double-blind control trial. J. Food Biochem. 44:e13489. doi: 10.1111/jfbc.13489
Kalia, V. C., Shim, W. Y., Patel, S. K. S., Gong, C., and Lee, J. K. (2022). Recent developments in antimicrobial growth promoters in chicken health: opportunities and challenges. Sci. Total Environm. 834:155300. doi: 10.1016/j.scitotenv.2022.155300
Karamzadeh-Dehaghani, A., Towhidi, A., Zhandi, M., Mojgani, N., and Fouladi-Nashta, A. (2021). Combined effect of probiotics and specific immunoglobulin Y directed against Escherichia coli on growth performance, diarrhea incidence, and immune system in calves. Animal 15:100124. doi: 10.1016/j.animal.2020.100124
Kathayat, D., Closs Jr, G., Helmy, Y. A., Deblais, L., Srivastava, V., and Rajashekara, G. (2022). In vitro and in vivo evaluation of Lacticaseibacillus rhamnosus GG and Bifidobacterium lactis Bb12 against avian pathogenic Escherichia coli and identification of novel probiotic-derived bioactive peptides. Probiotics Antimicrob. Proteins 14, 1012–1028. doi: 10.1007/s12602-021-09840-1
Khushboo, Karnwal, A., and Malik, T. (2023). Characterization and selection of probiotic lactic acid bacteria from different dietary sources for development of functional foods. Front. Microbiol. 14:1170725. doi: 10.3389/fmicb.2023.1170725
Kizerwetter-Swida, M., and Binek, M. (2009). Protective effect of potentially probiotic Lactobacillus strain on infection with pathogenic bacteria in chickens. Pol. J. Vet. Sci. 12, 15–20.
Kritas, S. K., Govaris, A., Christodoulopouls, G., and Burriel, A. R. (2006). Effect of Bacillus licheniformis and Bacillus subtilis supplementation of Ewe's feed on sheep milk production and young lamb mortality. J. Vet. Med. Ser. 53, 170–173. doi: 10.1111/j.1439-0442.2006.00815.x
Kuebutornye, F. K. A., Abarike, E. D., Lu, Y., Hlordzi, V., Sakyi, M. E., Afriyie, G., et al. (2020). Mechanisms and the role of probiotic Bacillus in mitigating fish pathogens in aquaculture. Fish Physiol. Biochem. 46, 819–841. doi: 10.1007/s10695-019-00754-y
Kunwar, H. (2024). Lactic Acid Bacteria with Antagonistic Properties Towards Dairy Food Microbial Contaminants and Mastitis Pathogens (Doctoral dissertation). Swinburne, Pittsburgh, PA, United States.
Lee, H. W., Cheng, J., Kovbasnjuk, O., Donowitz, M., and Guggino, W. B. (2013). Insulin-like growth factor 1 (IGF-1) enhances the protein expression of CFTR. PLoS One 8:e59992.
Leser, T., and Baker, A. (2023). Bifidobacterium adolescentis - a beneficial microbe. Benef. Microbes 14, 525–551. doi: 10.1163/18762891-20230030
Li, X., Yin, J., Li, D., Chen, X., Zang, J., and Zhou, X. (2006). Dietary supplementation with zinc oxide increases igf-I and igf-I receptor gene expression in the small intestine of weanling piglets. J. Nutr. 136, 1786–1791. doi: 10.1093/jn/136.7.1786
Li, Z., Wang, W., Liu, D., and Guo, Y. (2017). Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS ONE 12:e0188634. doi: 10.1371/journal.pone.0188634
Liu, H., Hou, C., Wang, G., Jia, H., Yu, H., Zeng, X., et al. (2017). Lactobacillus reuteri I5007 modulates intestinal host defense peptide expression in the model of IPEC-J2 cells and neonatal piglets. Nutrients 9:559. doi: 10.3390/nu9060559
Liu, J., Liu, H., Liu, H., Teng, Y., Qin, N., Ren, X., et al. (2023). Live and pasteurized Akkermansia muciniphila decrease susceptibility to Salmonella Typhimurium infection in mice. Journal of advanced research 52, 89–102. doi: 10.1016/j.jare.2023.03.008
Lone, A., Mottawea, W., Ait Chait, Y., and Hammami, R. (2021). Dual inhibition of Salmonella enterica and Clostridium perfringens by new probiotic candidates isolated from chicken intestinal mucosa. Microorganisms 9:166. doi: 10.3390/microorganisms9010166
Lu, Y., Han, S., Zhang, S., Wang, K., Lv, L., McClements, D. J., et al. (2022). The role of probiotic exopolysaccharides in adhesion to mucin in different gastrointestinal conditions. Curr. Res. Food Sci. 5, 581–589.
Lutful Kabir, S. M. (2009). The role of probiotics in the poultry industry. Int. J. Mol. Sci. 10, 3531–3546. doi: 10.3390/ijms10083531
Ma, Z. Z., Cheng, Y. Y., Wang, S. Q., Ge, J. Z., Shi, H. P., and Kou, J. C. (2020). Positive effects of dietary supplementation of three probiotics on milk yield, milk composition and intestinal flora in Sannan dairy goats varied in kind of probiotics. J. Animal Physiol. Animal Nutr. 104, 44–55. doi: 10.1111/jpn.13226
Merenstein, D., Pot, B., Leyer, G., Ouwehand, A. C., Preidis, G. A., Elkins, C., et al. (2023). Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 15:2185034. doi: 10.1080/19490976.2023.2185034
Merrifield, D. L., Dimitroglou, A., Foey, A., Davies, S. J., Baker, R. T., Bøgwald, J., et al. (2010). The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302, 1–18. doi: 10.1016/j.aquaculture.2010.02.007
Mo, W. Y., Choi, W. M., Man, K. Y., and Wong, M. H. (2020). Food waste-based pellets for feeding grass carp (Ctenopharyngodon idellus): adding baker's yeast and enzymes to enhance growth and immunity. Sci. Total Environm. 707:134954. doi: 10.1016/j.scitotenv.2019.134954
Modesto, M., D'Aimmo, M. R., Stefanini, I., Trivisi, P., Fillippi, S. D., Casini, L., et al. (2009). A novel strategy to select Bifidobacterium strains and prebiotics as natural growth promoters in newly weaned pigs. Livest. Sci. 122, 248–258. doi: 10.1016/j.livsci.2008.08.017
Mohammed, A. A., Zaki, R. S., Negm, E. A., Mahmoud, M. A., and Cheng, H. W. (2021). Effects of dietary supplementation of a probiotic (Bacillus subtilis) on bone mass and meat quality of broiler chickens. Poult. Sci. 100:100906. doi: 10.1016/j.psj.2020.11.073
Monteiro, C. R. A. V., do Carmo, M. S., Melo, B. O., Alves, M. S., Dos Santos, C. I., Monteiro, S. G., et al. (2019). In vitro antimicrobial activity and probiotic potential of Bifidobacterium and Lactobacillus against species of Clostridium. Nutrients 11:448. doi: 10.3390/nu11020448
Monteverde, V., Congiu, F., Vazzana, I., Dara, S., di Pietro, S., Piccione, G., et al. (2017). Serum lipid profile modification related to polyunsaturated fatty acid supplementation in thoroughbred horses. J. Appl. Anim. Res. 45, 615–618. doi: 10.1080/09712119.2016.1251439
Moreno-Muñoz, J. A., Ojeda, J. D., and López, J. J. (2024). A probiotic bacterium with activity against the most frequent bacteria and viruses causing pediatric diarrhea: bifidobacterium longum subsp. infantis CECT 7210 (B. infantis IM1®). Microorganisms 12:1183. doi: 10.3390/microorganisms12061183
Mountzouris, K. C., Tsirtsikos, P., Kalamara, E., Nitsch, S., Schatzmayr, G., and Fegeros, K. (2007). Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 86, 309–317. doi: 10.1093/ps/86.2.309
Mulchandani, R., Wang, Y., Gilbert, M., and Van Boeckel, T. P. (2023). Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Global Public Health 3:e0001305. doi: 10.1371/journal.pgph.0001305
Muya, M. C., Nherera, F. V., Miller, K. A., Aperce, C. C., Moshidi, P. M., and Erasmus, L. (2015). Effect of Megasphaera elsdenii NCIMB 41125 dosing on rumen development, volatile fatty acid production and blood β-hydroxybutyrate in neonatal dairy calves. J. Animal Physiol. Animal Nutr. 99, 913–918. doi: 10.1111/jpn.12306
Nataraj, B. H., and Mallappa, R. H. (2021). Antibiotic resistance crisis: an update on antagonistic interactions between probiotics and methicillin-resistant Staphylococcus aureus (MRSA). Curr. Microbiol. 78, 2194–2211. doi: 10.1007/s00284-021-02442-8
Nordeus, K. (2023). “When science is not enough: antibiotic Growth Promoters and the precautionary principle within the European Union,” in From Breeding and Feeding to Medicalization: Animal Farming, Veterinarization and Consumers in Twentieth-Century Western Europe (Belgium: Brepols Publishers), 267–286.
Olmos, J., Acosta, M., Mendoza, G., and Pitones, V. (2020). Bacillus subtilis, an ideal probiotic bacterium to shrimp and fish aquaculture that increase feed digestibility, prevent microbial diseases, and avoid water pollution. Arch. Microbiol. 202, 427–435. doi: 10.1007/s00203-019-01757-2
Parte, A. C. (2018). LPSN - List of prokaryotic names with standing in nomenclature (bacterio.net), 20 years on. Int. J. Syst. Evol. Microbiol. 68, 1825–1829. doi: 10.1099/ijsem.0.002786
Patel, R. M., Myers, L. S., Kurundkar, A. R., Maheshwari, A., Nusrat, A., Lin, P. W., et al. (2012). Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am. J. Pathol. 180, 626–635. doi: 10.1016/j.ajpath.2011.10.025
Patyra, E., and Kwiatek, K. (2023). Insect meals and insect antimicrobial peptides as an alternative for antibiotics and growth promoters in livestock production. Pathogens 12:854. doi: 10.3390/pathogens12060854
Rabah, H., Carmo, F. L. R. D., and Jan, G. (2017). Dairy propionibacteria: versatile probiotics. Microorganisms 5:24. doi: 10.3390/microorganisms5020024
Rabetafika, H. N., Razafindralambo, A., Ebenso, B., and Razafindralambo, H. L. (2023). Probiotics as antibiotic alternatives for human and animal applications. Encyclopedia 3, 561–581. doi: 10.3390/encyclopedia3020040
Ramlucken, U., Ramchuran, S. O., Moonsamy, G., Lalloo, R., and Thantsha, M. S. (2020). A novel Bacillus based multi-strain probiotic improves growth performance and intestinal properties of Clostridium perfringens challenged broilers. Poult. Sci. 99, 331–341. doi: 10.3382/ps/pez496
Redweik, G. A. J., Stromberg, Z. R., Van Goor, A., and Mellata, M. (2020). Protection against avian pathogenic Escherichia coli and Salmonella Kentucky exhibited in chickens given both probiotics and live Salmonella vaccine. Poult. Sci. 99, 752–762. doi: 10.1016/j.psj.2019.10.038
Reuben, R. C., Elghandour, M. M., Alqaisi, O., Cone, J. W., Márquez, O., and Salem, A. Z. (2022). Influence of microbial probiotics on ruminant health and nutrition: sources, mode of action and implications. J. Sci. Food Agric. 102, 1319–1340. doi: 10.1002/jsfa.11643
Rodrigues, G., Maximiano, M. R., and Franco, O. L. (2021). Antimicrobial peptides used as growth promoters in livestock production. Appl. Microbiol. Biotechnol. 105, 7115–7121. doi: 10.1007/s00253-021-11540-3
Sabino, Y. N. V., de Araújo Domingues, K. C., Mathur, H., Gómez-Mascaraque, L. G., Drouin, G., Martínez-Abad, A., et al. (2023). Exopolysaccharides produced by Bacillus spp. inhibit biofilm formation by Staphylococcus aureus strains associated with bovine mastitis. Int. J. Biol. Macromol. 253:126689. doi: 10.1016/j.ijbiomac.2023.126689
Samli, H. E., Senkoylu, N., Koc, F., Kanter, M., and Agma, A. (2007). Effects of Enterococcus faecium and dried whey on broiler performance, gut histomorphology and intestinal microbiota. Arch. Anim. Nutr. 61, 42–49. doi: 10.1080/17450390601106655
Santacroce, L., Charitos, I. A., and Bottalico, L. (2019). A successful history: probiotics and their potential as antimicrobials. Expert Rev. Anti Infect. Ther. 17, 635–645. doi: 10.1080/14787210.2019.1645597
Schmerold, I., van Geijlswijk, I., and Gehring, R. (2023). European regulations on the use of antibiotics in veterinary medicine. Eur. J. Pharmaceut. Sci. 189:106473. doi: 10.1016/j.ejps.2023.106473
Siciliano, R. A., Lippolis, R., and Mazzeo, M. F. (2019). Proteomics for the investigation of surface-exposed proteins in probiotics. Front. Nutr. 6:52. doi: 10.3389/fnut.2019.00052
Smialek, M., Burchardt, S., and Koncicki, A. (2018). The influence of probiotic supplementation in broiler chickens on population and carcass contamination with Campylobacter spp.—Field study. Res. Vet. Sci. 118, 312–316. doi: 10.1016/j.rvsc.2018.03.009
Song, Y., Malmuthuge, N., Steele, M. A., and Guan, L. L. (2018). Shift of hindgut microbiota and microbial short chain fatty acids profiles in dairy calves from birth to pre-weaning. FEMS Microbiol. Ecol. 94:fix179. doi: 10.1093/femsec/fix179
Spacova, I., Binda, S., Ter Haar, J. A., Henoud, S., Legrain-Raspaud, S., Dekker, J., et al. (2023). Comparing technology and regulatory landscape of probiotics as food, dietary supplements and live biotherapeutics. Front. Microbiol. 14:1272754. doi: 10.3389/fmicb.2023.1272754
Stokstad, E. L., Jukes, T. H., Page, P., and Franklin, A. I. (1949). The multiple nature of the animal protein factor. J. Biol. Chem. 180, 647–654. doi: 10.1016/S0021-9258(18)56683-7
Sudha, M. R., Chauhan, P., Dixit, K., and Babu, S. (2009). Probiotics as complementary therapy for hypercholesterolemia. Biol. Med. 1, 1–13.
Tang, H., Huang, W., and Yao, Y. F. (2023). The metabolites of lactic acid bacteria: classification, biosynthesis and modulation of gut microbiota. Microbial. Cell 10, 49–62. doi: 10.15698/mic2023.03.792
Teneva, D., and Denev, P. (2023). Biologically active compounds from probiotic microorganisms and plant extracts used as biopreservatives. Microorganisms 11:1896. doi: 10.3390/microorganisms11081896
Teng, K., Huang, F., Liu, Y., Wang, Y., Xia, T., Yun, F., et al. (2023). Food and gut originated bacteriocins involved in gut microbe-host interactions. Crit. Rev. Microbiol. 49, 515–527. doi: 10.1080/1040841X.2022.2082860
Torres-Rodriquez, A., Donoghue, A. M., Donoghue, D. J., Barton, J. T., Tellez, G., and Hargis, B. M. (2007). Performance and condemnation rate analysis of commercial turkey flocks treated with a Lactobacillus sp.—based probiotic. Poult. Sci. 86, 444–446. doi: 10.1093/ps/86.3.444
Upadhaya, S. D., Rudeaux, F., and Kim, I. H. (2019). Efficacy of dietary Bacillus subtilis and Bacillus licheniformis supplementation continuously in pullet and lay period on egg production, excreta microflora, and egg quality of Hyline-Brown birds. Poult Sci. 98, 4722–4728. doi: 10.3382/ps/pez184
Van Immerseel, F., Russell, J. B., Flythe, M. D., Gantois, I., Timbermont, L., Pasmans, F., et al. (2006). The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. 35, 182–188. doi: 10.1080/03079450600711045
Vera-Santander, V. E., Hernández-Figueroa, R. H., Jiménez-Munguía, M. T., and Mani-López, E. (2023). Health benefits of consuming foods with bacterial probiotics, postbiotics, and their metabolites: a review. Molecules 28:1230. doi: 10.3390/molecules28031230
Villena, J., Chiba, E., Vizoso-Pinto, M. G., Tomosada, Y., Takahashi, T., Ishizuka, T., et al. (2014). Immunobiotic Lactobacillus rhamnosus strains differentially modulate antiviral immune response in porcine intestinal epithelial and antigen presenting cells. BMC Microbiol. 14:126. doi: 10.1186/1471-2180-14-126
Wang, G., Zhang, Y., Song, X., Xia, Y., Lai, P. F., Ai, L., et al. (2019). Lactobacillus casei LC2W can inhibit the colonization of Escherichia coli O157:H7 in vivo and reduce the severity of colitis. Food Funct. 10, 5843–5852. doi: 10.1039/C9FO01390C
Wang, L., Li, L., Lv, Y., Chen, Q., Feng, J., and Zhao, X. (2018). Lactobacillus plantarum restores intestinal permeability disrupted by salmonella infection in newly-hatched chicks. Sci. Reports. 8:2229. doi: 10.1038/s41598-018-20752-z
Wang, Z., Wang, L., Chen, Z., Ma, X., Yang, X., Zhang, J., et al. (2016). In vitro evaluation of swine-derived Lactobacillus reuteri: probiotic properties and effects on intestinal porcine epithelial cells challenged with enterotoxigenic Escherichia coli K88. J. Microbiol. Biotechnol. 26, 1018–1025. doi: 10.4014/jmb.1510.10089
Wattam, A. R., Davis, J. J., Assaf, R., Boisvert, S., Brettin, T., Bun, C., et al. (2017). Improvements to PATRIC, the all-bacterial bioinformatics database and analysis resource center. Nucleic Acids Res. 45, D535–D542. doi: 10.1093/nar/gkw1017
Won, G., and Lee, J. H. (2017). Salmonella Typhimurium, the major causative agent of foodborne illness inactivated by a phage lysis system provides effective protection against lethal challenge by induction of robust cell-mediated immune responses and activation of dendritic cells. Vet. Res. 48:66. doi: 10.1186/s13567-017-0474-x
Wu, R., Shen, J., Tian, D., Yu, J., He, T., Yi, J., et al. (2020). A potential alternative to traditional antibiotics in aquaculture: Yeast glycoprotein exhibits antimicrobial effect in vivo and in vitro on Aeromonas caviae isolated from Carassius auratus gibelio. Vet. Med. Sci. 6, 639–648. doi: 10.1002/vms3.253
Wu, Y., Jha, R., Li, A., Liu, H., Zhang, Z., Zhang, C., et al. (2022a). Probiotics (Lactobacillus plantarum HNU082) supplementation relieves ulcerative colitis by affecting intestinal barrier functions, immunity-related gene expression, gut microbiota, and metabolic pathways in mice. Microbiol. Spect. 10:e0165122. doi: 10.1128/spectrum.01651-22
Wu, Y., Nie, C., Luo, R., Qi, F., Bai, X., Chen, H., et al. (2022b). Effects of multispecies probiotic on intestinal microbiota and mucosal barrier function of neonatal calves infected with E. coli K99. Front. Microbiol. 12:813245. doi: 10.3389/fmicb.2021.813245
Xin, J., Zeng, D., Wang, H., Sun, N., Zhao, Y., Dan, Y., et al. (2020). Probiotic Lactobacillus johnsonii BS15 promotes growth performance, intestinal immunity, and gut microbiota in piglets. Probiotics Antimicrob. Proteins 12, 184–193. doi: 10.1007/s12602-018-9511-y
Yang, F., Wang, A., Zeng, X., Hou, C., Liu, H., Qiao, S., et al. (2015). Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 15:32. doi: 10.1186/s12866-015-0372-1
Yehia, H. M., Alkhuriji, A. F., Savvaidis, I., and Al-Masoud, A. H. (2022). Bactericidal effect of nisin and reuterin on methicillin-resistant Staphylococcus aureus (MRSA) and S. aureus ATCC 25937. Food Sci. Technol. 42:e105321. doi: 10.1590/fst.105321
Yu, P., Huber, J. T., Theurer, C. B., Chen, K. H., Nussio, L. G., Wu, Z., et al. (1997). Effect of steam-flaked or steam-rolled corn with or without Aspergillus oryzae in the diet on performance of dairy cows fed during hot weather. J. Dairy Sci. 80, 3293–3297. doi: 10.3168/jds.S0022-0302(97)76304-5
Zhang, L., Zhang, R., Jia, H., Zhu, Z., and Li, H. (2021). Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens. Open Life Science 16, 311–322. doi: 10.1515/biol-2021-0031
Keywords: antimicrobial growth promoters, probiotics, antimicrobial resistance, gut microbiome modulation, animal growth performance
Citation: Sachdeva A, Tomar T, Malik T, Bains A and Karnwal A (2025) Exploring probiotics as a sustainable alternative to antimicrobial growth promoters: mechanisms and benefits in animal health. Front. Sustain. Food Syst. 8:1523678. doi: 10.3389/fsufs.2024.1523678
Received: 06 November 2024; Accepted: 09 December 2024;
Published: 03 January 2025.
Edited by:
Reza Rastmanesh, American Physical Society, United StatesReviewed by:
Doriana Eurosia Angela Tedesco, University of Milan, ItalyHary Razafindralambo, ProBioLab, Belgium
Copyright © 2025 Sachdeva, Tomar, Malik, Bains and Karnwal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tabarak Malik, bWFsaWtpdHJjQGdtYWlsLmNvbQ==; Arun Karnwal, YXJ1bmthcm53YWxAZ21haWwuY29t
†These authors have contributed equally to this work
 Angel Sachdeva1†
Angel Sachdeva1† Tanu Tomar
Tanu Tomar Tabarak Malik
Tabarak Malik Arun Karnwal
Arun Karnwal