- 1Biotechnology Program, Center for Sustainable Development, College of Art and Sciences, Qatar University, Doha, Qatar
- 2High Agronomic Institute of Chott Mariem, Sousse University, Sousse, Tunisia
- 3Laboratory of Biopesticides (LBPES), Center of Biotechnology of Sfax, University of Sfax, Sfax, Tunisia
Introduction: Hydroponic cultivation systems using desalinated groundwater may play pivotal role in reducing freshwater consumption for irrigation. However, reliance on desalination remains unsustainable due to its high cost, energy demand, and the serious environmental impacts of its brine byproducts. Producing a biofertilizer that enables groundwater irrigation in hydroponics by enhancing plant halotolerance and resistance to salt stress offers a promising solution to address freshwater scarcity and low soil quality in arid and semi-arid regions, such as the Arabian Gulf.
Methods: This study investigates the potential of Bacillus spizizenii FMH45 in field experiment to enhance tomato plant production under greenhouse cultivation in hydroponics using directly groundwater for irrigation without desalination.
Results and discussion: Results demonstrated that the FMH45-based biofertilizer (HB45) significantly improved plant physiological parameters under greenhouse conditions. These improvements included a notable increase in shoot elongation (>13%), enhanced SPAD index values (>8%), and significant rises in flower and fruit counts (≃ 11% and 22%, respectively). B. spizizenii HB45 showed significant potential to increase bacterial densities by over 100-fold in various plant organs under saline irrigation and prevent salt infiltration into internal plant tissues. Furthermore, HB45-treatment enhanced the plant oxidative stress response as evidenced by stable catalase activity, an approximately 50% reduction in lipid peroxidation markers such as malondialdehyde (MDA), and a 35% decrease in reactive oxygen species (ROS), including hydrogen peroxide (H2O2). These findings demonstrate that B. spizizenii FMH45 holds significant potential for the development of effective biofertilizers capable of mitigating salt stress while boosting crop productivity. This approach offers a sustainable alternative to desalination-dependent hydroponics, particularly for arid and semi-arid regions, including Qatar.
1 Introduction
Freshwater scarcity is a growing global concern, intensifying each year and posing significant threats to ecosystem balance, food security, and human livelihoods. The agricultural sector is recognized as a water-intensive sector, responsible for consuming nearly 70% of the total water withdrawal volume (Ingrao et al., 2023). This percentage may reach 90%, especially in arid and semi-arid regions like the Gulf Arab Countries (GCC). In Qatar, for instance, agricultural activities account for 91% of available water resources (Alhaj et al., 2017). In the last decade, the use of hydroponic cultivation has emerged as a partial solution to the water scarcity issue. The advantages of these soilless systems include high crop quality and quantity, more efficient water use, which plays a pivotal role in water management, and greater control and efficiency in the productive process (Cardoso et al., 2018; Barone et al., 2019).
However, the adoption of hydroponic systems alone is not enough to resolve water scarcity problems, particularly in regions affected by surface water shortages where there has been a notable shift toward intensive utilization of groundwater resources as an alternative (Levintal et al., 2023). Nevertheless, the predominant portion of the utilized groundwater in these regions is characterized by low quality and high salinity (Mukherjee et al., 2022). Several concerns about using saline water in these cultivations still arise, not only due to the sensitivity of cultivated plants but also because of the accumulation of salt ions on the substrate. The use of native groundwater in irrigation has the potential to dramatically impact soil fertility and plant production (Wang et al., 2023). Salinity reduces the agricultural production of most crops and diminishes economic returns (Nicolas et al., 2023). Moreover, the adoption of the desalination process to generate water suitable for irrigation exhibits various limitations, such as high cost and the environmental impact of the disposed brine generated during the desalination process, which exerts a significant stress on ecosystems and local economies (Khan and Al-Ghouti, 2021). Globally, approximately 142 million m3 of brine are generated daily with 55% of this brine accumulating in the Arabian Gulf, including 1.1 million m3 per day specifically for irrigation, (Jones et al., 2019).
The plant growth promoting halobacteria (PGPH) hold significant potential to enhance plant tolerance to salt stress (Xie et al., 2024). The application of PGPH as a biofertilizer presented many valuable effects, including enhancing plant host growth and adaptation to abiotic or biotic stressors through modulation of plant metabolism, and phytohormone signaling (Mahgoub et al., 2021). Moreover, PGPH colonize the rhizosphere region, form symbiotic partnerships with their hosts, and improve nutrient uptake, hormone production, and resistance to phytopathogens (Glick, 2012; Poria et al., 2022). These bacteria promote plant growth directly by producing hormones, chelating iron, solubilizing minerals for easier uptake of nutrients, and fixing atmospheric nitrogen (Timofeeva et al., 2023). PGPH also adopt certain strategies, such as improving ion selectivity to sustain a higher K+/Na+ ratio, regulating the various antioxidant enzyme levels in plant cells and enhancing osmoprotectors’ accumulation to protect from stress conditions (Yasmin et al., 2020).
Bacillus spizizenii FMH45 has been recognized for its plant growth-promoting (PGP) traits and its ability to mitigate salt stress during the early developmental stages of tomato seedlings (Masmoudi et al., 2019, 2021a). To the best of our knowledge, this study is the first to evaluate the effects of this strain on tomato plant growth and physiological properties up to the fruiting stage, using hydroponic systems irrigated with groundwater. Furthermore, the study investigated its influence on the epiphytic bacterial community and salt infiltration, as well as quantified stress-related biochemical markers, including catalase (CAT), malondialdehyde (MDA), and hydrogen peroxide (H2O2).
2 Materials and methods
2.1 Bacterial strain, growth media and HB45 production
Bacillus spizizenii FMH45 belongs to the bacterial collection of the Biopesticides Laboratory at the Center of Biotechnology of Sfax (CBS), Tunisia (Masmoudi et al., 2019). FMH45 was initially fermented until sporulation in three different media (with and without the addition of 15 g L−1 NaCl) to select the medium yielding the highest spore count. Fresh bacterial cells (Optical density (OD) = 0.01) were inoculated into 500 mL glass flasks containing 100 mL of each medium. Cultures were incubated on a rotary shaker (New Brunswick Innova® 40/40R) at 30°C, 200 rpm until total sporulation. Bacterial cultures were monitored daily for total sporulation, and the harvested spores were quantified and compared using colony-forming unit (CFU mL−1) counting method.
The media screened were: (i) Complex medium: Modified halophilic medium (MH) (g L−1): Peptone, 5; Yeast Extract, 10; Glucose, 1; Sea salt, 5; MgCl2, 3 (Masmoudi et al., 2021b), (ii) Semi-synthetic medium (SSM) (g L−1): Yeast Extract, 0.5; Casein acid hydrolysate, 4.5; (NH4)2SO4, 6; K2HPO4, 1.4; KH2PO4, 1.4; Glucose, 5; MgSO4, 7H2O, 0.161; CaCl2, 2H2O, 0.332; and MnSO4, H2O, 0.006. Glucose and the different salt solutions were prepared and sterilized separately (Sarrafzadeh et al., 2005). (iii): Synthetic medium: G10 medium (g L−1): Artificial sea salt, 10 g; FeSO4, 7H2O, 0.01 g; NH4C1, 2.0 g; K2HPO4, 0.5 g; Glucose, 10 g; and Tris, 12 g. Glucose and phosphate solutions were autoclaved separately (Del Moral et al., 1994). The halobacterial-based biofertilizer HB45 was obtained after mixing the harvested spores produced in the most suitable medium with a sterile saline water solution (15 g L−1 NaCl) containing 2% glycerol and 2% polyvinylpyrrolidone (PVP) (Anith et al., 2016). The HB45 was stored at 4°C for further applications.
2.2 Greenhouse assay and experimental design
The greenhouse assay was conducted in a controlled greenhouse equipped with an automated drip technique and a cooling system, kindly provided by “Agrico Agricultural Development Farm,” a local Qatari agricultural company specializing in organic agriculture. Cherry tomato seedlings, Solanum lycopersicum var. cerasiforme, with four true leaves and uniform size, were initially sown in germination trays and irrigated with freshwater. The seedlings were then transplanted into pots filled with an equal weight of coconut peat for hydroponic cultivation, with one seedling per pot. The first application of HB45 occurred 2 days before initiating the irrigation with native groundwater or diluted groundwater.
The concentrated biofertilizer was mixed with water to achieve a final concentration of 108 spores mL−1. Then, 300 mL of the mixture was added to the substrate at the root level. HB45 was applied biweekly during the cultivation process. The experiment spanned 8 weeks from 28th November 2022 to the end of January 2023 (Figure 1). Greenhouse temperatures were maintained within a range of 24 and 26°C. During this assay, native groundwater (GW) with a salinity of 136.89 mM; and diluted groundwater (DW), with a salinity of 68.44 mM were used for irrigation. Desalinated groundwater via reverse osmosis (RO) with a salinity of 1.71 mM was used as control.
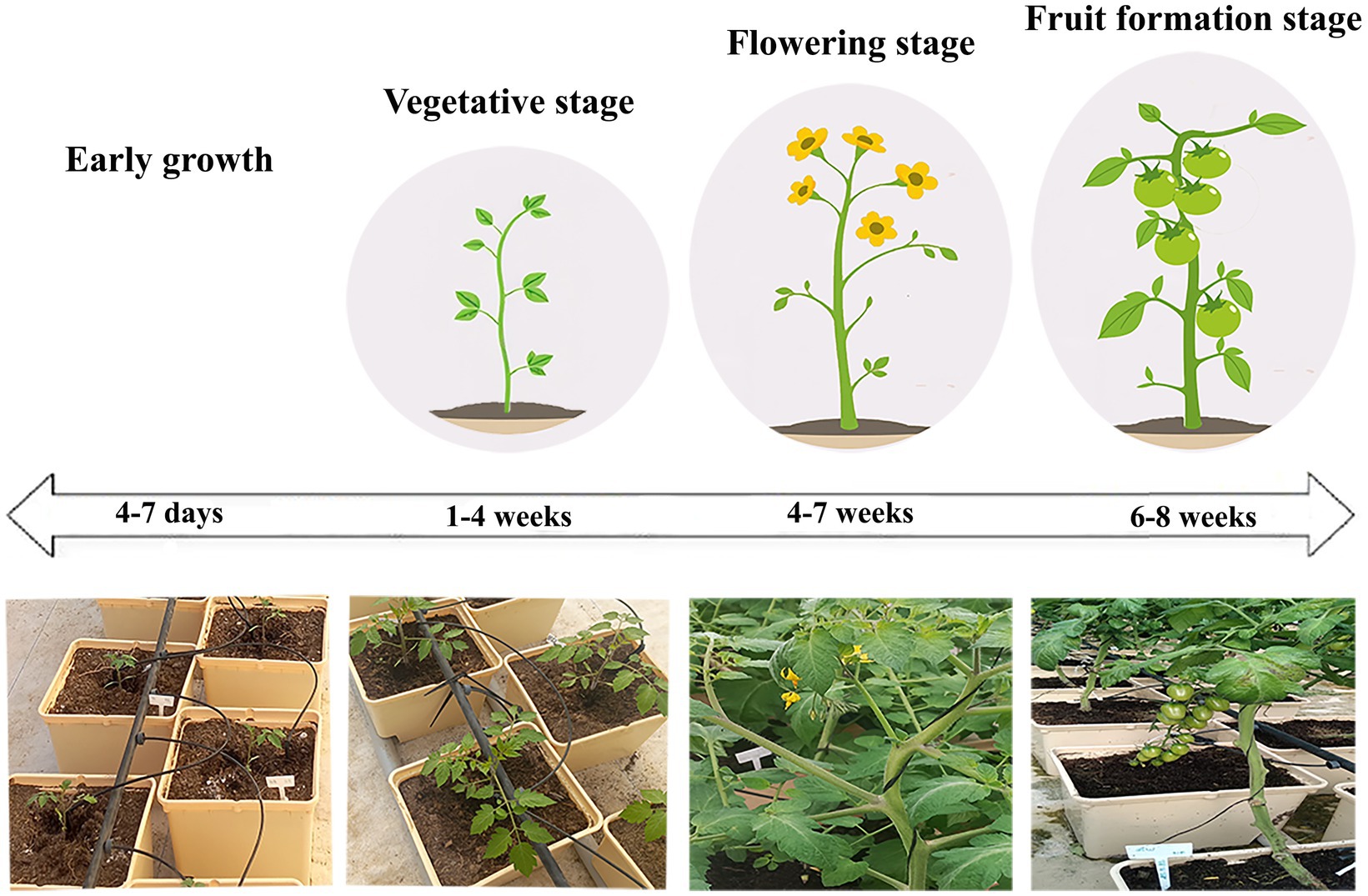
Figure 1. Cultivation of cherry tomato plants in a greenhouse hydroponic system with groundwater irrigation, from early growth to fruit formation stages.
Fertilized (HB45) and non- fertilized (NF) plants were arranged in a factorial design (Table 1) with 15 replicates per treatment as follows: (i) Positive control: NF plants irrigated with RO (RONF) or HB45-treated plants irrigated with RO (ROHB45); (ii) Negative control: NF plants and irrigated with GW (GWNF) or the DW (DWNF); (iii) Treated plants: plants treated with HB45 and irrigated with GW (GWHB45) or the DW (DWHB45).
2.3 Monitoring plant physiological parameters
Various physiological parameters of plant growth were monitored weekly throughout the cultivation period. Randomly selected tomato plants from each treatment group were examined for their height (cm), chlorophyll content, open flower count, trusses number, and fruits’ number.
To assess tomato plant elongation, measurements were recorded from the shoot base, located at the top of the support, to the highest point where fully opened leaves were present. Total chlorophyll content was estimated by measuring the SPAD index of green and healthy tomato leaves using the SPAD-02 chlorophyll meter (Konica Minolta Optics in Osaka, Japan). SPAD index measurements were carried out weekly at the same time, and the average of three measurements on each plant was recorded. Enumeration of open flower number, the number of trusses per plant, and the fruits’ number were conducted manually.
2.4 Total bacterial count in plant substrate and organs
Total bacterial community existing in rhizosphere and colonizing the different plant organs were assessed through colony-forming units (CFU) method. At the beginning of the flowering stage, samples from the different tomato plant compartments, including coconut peat, roots, lower leaves, upper leaves, and flowers were collected in sterile bags. Bacterial count was carried out as follows: First, the soil adhering to each plant root was carefully removed by sterile physiological water. Then, the surfaces of all plant organs including roots, leaves, and flowers, were disinfected using a 3% commercial bleach followed by 3% H2O2 solution. Soil-adhering and disinfected plant tissues were then mixed and homogenized well using Ringer’s solution. Finally, serial dilutions were prepared and plated on HM agar medium and formed colonies where counted.
2.5 Scanning electron microscopy
Samples from various tomato plant organs, including roots, stems, leaves, and flowers, were collected at the beginning of the flowering stage and subjected to scanning electron microscopy (SEM) analysis. The organs were initially dehydrated using a gradual increase in ethanol concentrations, up to 96% ethanol. Subsequently, the dried samples were transferred to formaldehyde dimethyl acetal for 24 h. Following this, the samples underwent critical drying using CO2, were sputter-coated with palladium/gold, and were examined using a Nova™ Nano SEM 50 Series equipped with electron dispersive X-ray spectroscopy (EDX) (Masmoudi et al., 2024).
2.6 Assessment of the total catalase activity (CAT)
The catalase (CAT) production was quantified according to the method described by de Azevedo Neto et al. (2006). Ten milligrams of proteins existing in the enzymatic extract of fresh plant leaves (FM) was mixed with 1 mL phosphate buffer (0.1 M, pH 7). This enzymatic extract was prepared by mixing 1 g of frozen fresh leaf powder with 10 mL ice-cold phosphate buffer (0.1 M, pH 7). The catalase (CAT) reaction was initiated by adding 30 mM of H2O2 and the reduction of absorbance was measured at 240 nm over 60 s using Jenway 7415 Nano Scanning Spectrophotometer. Catalase activity was quantified using a molar extinction coefficient (36 M−1 cm−1) and expressed as millimoles of hydrogen peroxide per second per gram of fresh matter (mmol H2O2 s−1 gFM−1).
2.7 Determination of malondialdehyde contents (MDA)
Lipid peroxidation levels in plant tissues were evaluated through MDA quantification. For such purpose, a reaction mixture composed of 100 mg of fresh leaf powder, 1 mL TCA (0.1%), and 4 mL of TBA was incubated at 95°C for 30 min. After centrifugation, the absorbance was measured using plate reader (Biotek Instruments Synergy 2) at wave length of 532 and 600 nm. The MDA content was quantified using the molar extinction coefficient of 155 mM−1 cm−1 (Masmoudi et al., 2021b).
2.8 Assessment of hydrogen peroxide content (H2O2)
Reactive oxygen species (ROS) existing in plant cells were evaluated through the quantification of H2O2 content (Masmoudi et al., 2021b). Hundred milligrams of fresh leaves powder were initially homogenized with 1 mL Trichloroacetic acid (TCA), then the supernatant was homogenized with phosphate buffer (pH 7) (v:v) and potassium iodide (KI) (v:2v). After dark incubation for 60 min, the reaction absorbance was assessed using a spectrophotometer at 390 nm (Masmoudi et al., 2021b). Standard curve prepared with H2O2 solutions was adopted to estimate hydrogen peroxide content.
2.9 Statistical analysis
Statistical Package for the Social Sciences (SPSS V.11; SPSS Inc., Chicago, IL, USA) was adopted for the analysis of variance. ANOVA analysis using Duncan’s multiple range was used to compare the mean values among treatments at a significance level of 5% (p ≤ 0.05).
3 Results and discussion
3.1 Screening of different media for sporulation of Bacillus Spizizenii FMH45
To optimize spore production for the biofertilizer production, the bacterial strain B. spizizenii FMH45 were initially cultivated in three different media containing varying concentrations of carbon and nitrogen sources, with and without the addition of NaCl. Obtained results showed that media rich in nitrogen sources, specifically MH and MSS, supported the highest spore production densities reaching 2 × 108 spores mL−1 after 72 h of fermentation. However, the synthetic medium G10, rich in glucose which presents the main energy source, resulted in spore production 10 times lower than those achieved in the nitrogen-rich media (MH and SSM) (Figure 2).
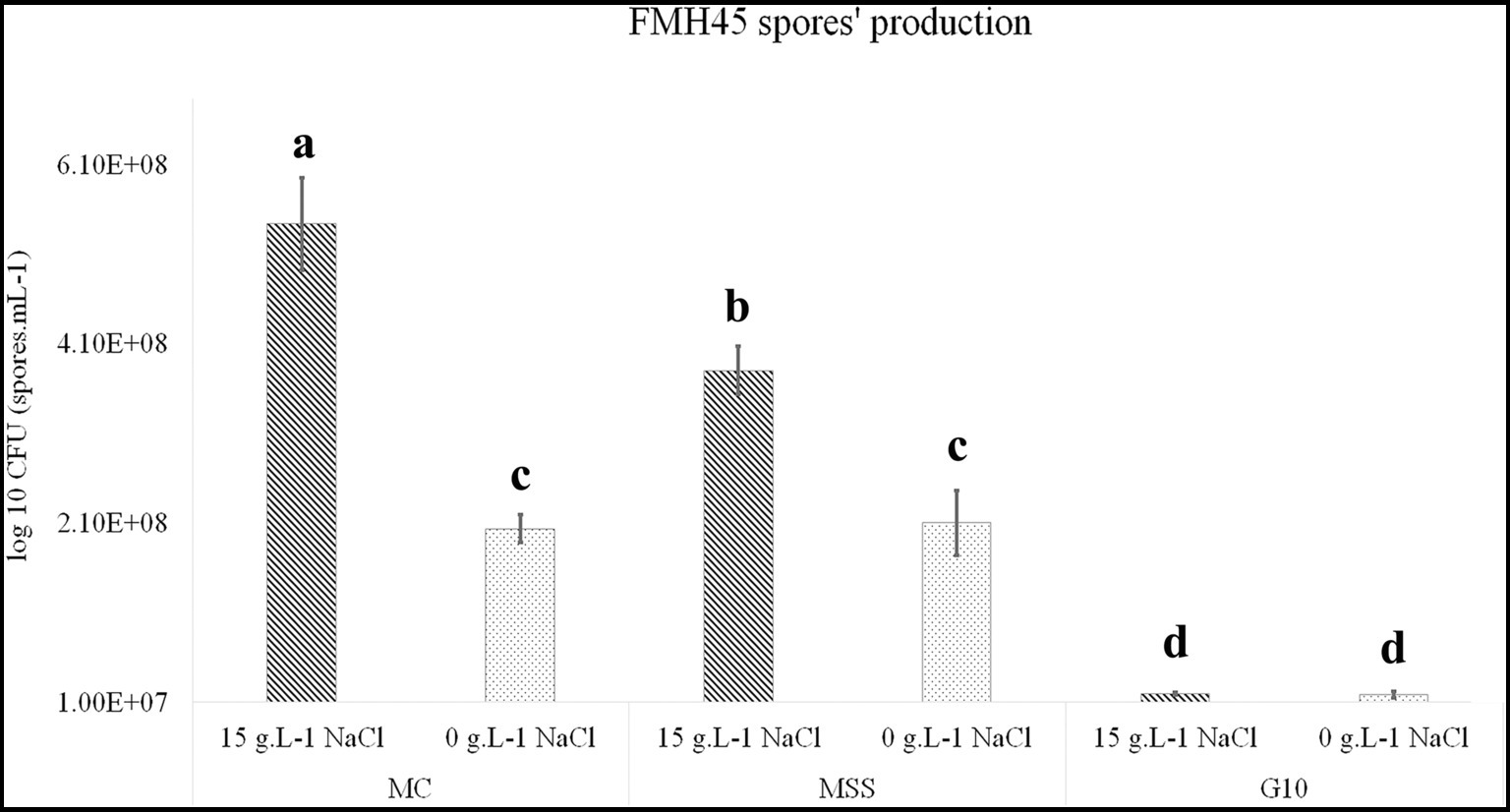
Figure 2. Production of B. spizizenii FMH45 spores in three different culture media (SSM, CM, and G10) with and without addition of NaCl. a–d: bars with the same letters indicate no significant differences between means at the confidence level 99.9% according to the Duncan’s multiple range test (at p ≤ 0.05). Errors bars indicate ± SE. All tests were replicated three times.
These findings suggest that nitrogen availability is a crucial factor in promoting bacterial growth and spore production. In fact, nitrogen sources play a pivotal role in protein synthesis, enzyme production, and cellular metabolism (He et al., 2023) Furthermore, the presence of yeast extract in MH and SSM media, further increases bacteria growth and sporulation processes, as also it is well reported by its richness in vitamins, particularly B vitamins, nucleotides, and growth factors, which are critical for metabolic functions such as DNA replication, energy production, and enzyme activity (Tao et al., 2023).
Moreover, the differences in spore production among the different media can also be linked to the significant difference in glucose concentrations and thereby in carbon sources. Indeed, glucose serves as a readily available carbon source for energy production, which is vital for biomass production and/or primary or secondary metabolite synthesis. However, glucose may exert a repressive effect on cell growth due to carbon catabolite repression, limiting the synthesis of metabolites (Ben Khedher et al., 2014; Singh et al., 2017).
Additionally, the role of inorganic salts should be taken into consideration, particularly magnesium ions (Mg2+), which were present in the MH and SSM media in higher concentrations than in the G10 medium, likely influence spore formation (Bressuire-Isoard et al., 2018). For instance, Posada-Uribe et al. (2015) reported that optimization of glucose and Mg2+ concentrations in a sporulation medium led to a 17 fold increase in spore yield of a B. subtilis EA-CB0575.
Interestingly, the addition of NaCl led to a significant improvement in spore production, both in MH and MSS media, resulting in maximum densities of 5.4 × 108 and 3.8 × 108 spores mL−1, respectively, while no notable variation was recorded in G10 medium. These findings suggest that NaCl plays a key role in stimulating bacterial growth. These results proved the halophilic behavior of B. spizizenii FMH45 and highlighted its requirement for sodium ions to support growth and metabolism (Corral et al., 2019).
Overall, these results highlight the importance of a nutrient-rich medium containing essential nitrogen and NaCl to maximize spores’ production, crucial for effective biofertilizer production.
3.2 Measurement of plant’s physiological parameters
The comparative analysis of untreated plant physiological parameters, such as plant height, chlorophyll content, open flower count, number of trusses per plant, and number of fruits, clearly indicated a high sensitivity to salt stress, with varying degrees of sensitivity depending on the severity of the stress. For instance, after 8 weeks of cultivation, untreated plants subjected to GW irrigation showed significant decreases in studied parameters compared to those irrigated with RO: plant height was reduced by 21%, flower production capacity declined by 13%, and the number of trusses per plant decreased by 30% decrease (Figures 3A,C,D).
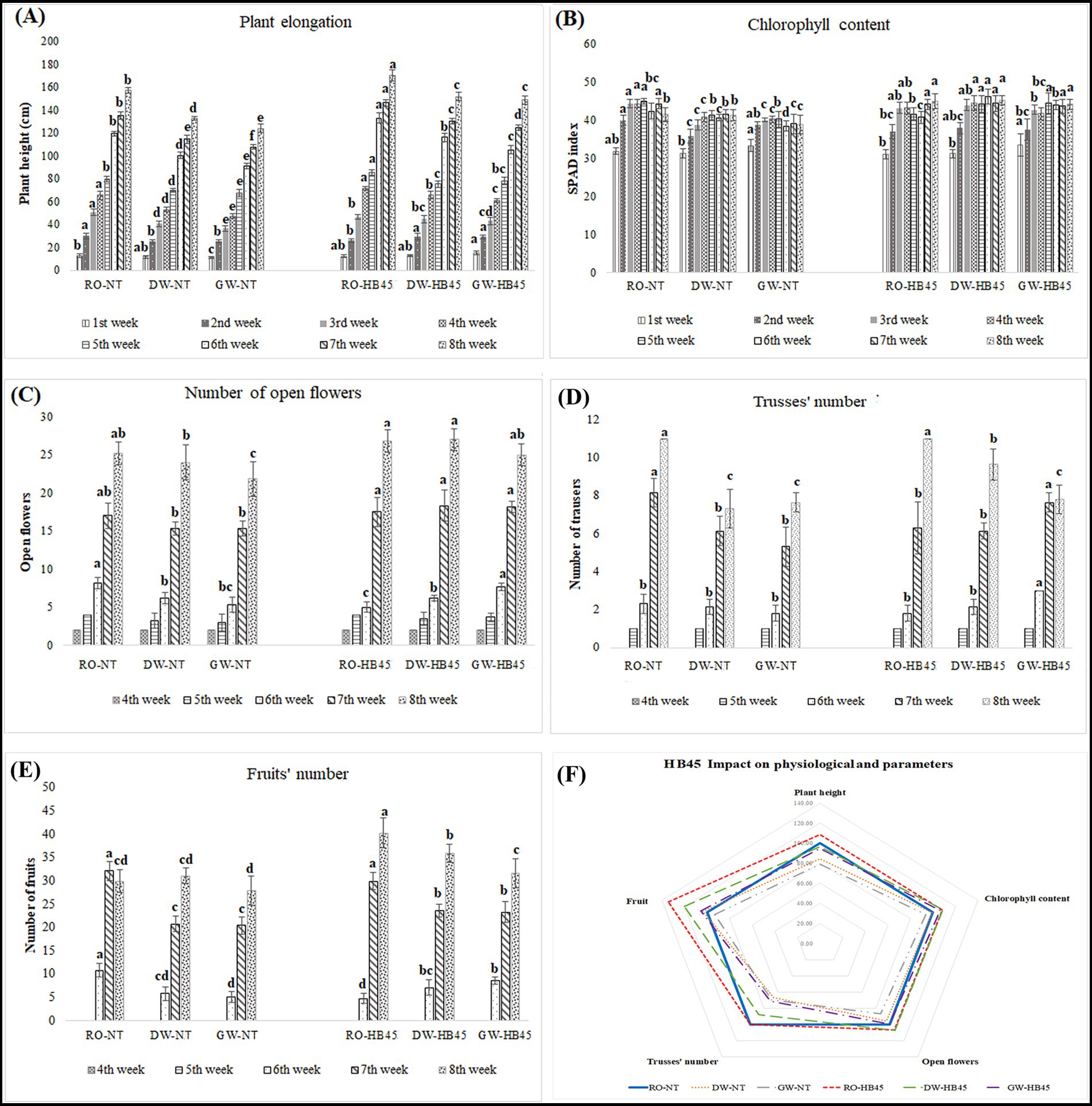
Figure 3. Monitoring of physiological parameters in HB45-treated and non-treated tomato plants cultivated under a hydroponic system and irrigated with RO, DW, and GW. (A) Plant elongation; (B) chlorophyll contents; (C) Count of open flower; (D) Trusses’ number; (E) green-immature fruits’ number; (F) HB45 impact on plant physiological parameters. a–f: bars with the same letters indicate no significant differences between means at the confidence level 99.9% according to the Duncan’s multiple range test (at p ≤ 0.05). Errors bars indicate ± SE. All measurements were taken from five randomly selected tomato plants per treatment.
Interestingly, HB45-treatment exhibited a positive impact on the studied physiological parameters in both salt-stressed and non-stressed plants. For instance, under RO irrigation, and after 8 weeks of cultivation, HB45-treated plants showed a 9% improvement in both plant height and chlorophyll content, and a 35% increase in fruit count (Figures 3A,B,E). When exposed to saline irrigation, HB45-treated plants presented significant improvements compared to non-treated plants under the same stressful conditions.
After DW and GW irrigations, plant elongation increased by 13 and 17%, respectively, chlorophyll content improved by 8 and 12%, open flower count rose by 11 and 12%, the number of trusses increased by 24 and 21%, respectively, and the number of mature green fruits increased by 22% under both DW and GW irrigations. It is important to note that HB45-treated plants irrigated with DW showed no significant difference in growth and reproductive parameters compared to untreated plants not exposed to any stressful conditions (Figure 3).
Salt stress is recognized as one of the most detrimental factors affecting plant growth and reproduction such as photosynthesis, plant height, flower emission and fruit yield (Stegelmeier et al., 2022). For instance, each increase in electrical conductivity (EC) resulted to a 7.2% reduction in tomato yield (Zhang et al., 2016). Several studies have highlighted the potential of Bacillus strains to alleviate the negative impact of salt stress on plant growth. However, there are limited reports on the positive effects of these strains in alleviating salt stress under greenhouse conditions. For example, Elakhdar et al. (2020) demonstrated that B. halotolerans MSR-H4 increased the chlorophyll content of wheat exposed to saline conditions. Similarly, the treatment of rice plants with B. amyloliquefaciens SN13 (B) in greenhouse conditions led to significant improvements in shoot length, root length, and dry weight under salt stress (Nautiyal et al., 2013).
During this study, our findings highlighted the pivotal role played by HB45 in mitigating salt stress on tomato plants in greenhouse conditions. These findings reinforce our earlier work reported by Masmoudi et al. (2021a), where in vivo inoculation with B. spizizenii FMH45 improved plant elongation, chlorophyll content, leaf emission, and fresh and dry weights in tomato seedlings exposed to varying levels of salt stress reaching 141 mM. B. spizizenii still not well reported in alleviating salt stress in plants. For instance, Orhan (2021) demonstrated that B. spizizenii B13 significantly improved total plant weight under salt stress conditions. To the best of our knowledge, this is the first report demonstrating the potential of the halobacterial biofertilizer based on the B. spizizenii strain in alleviating salt stress under greenhouse cultivation.
3.3 Salt impact on the existing bacterial communities
At the beginning of the flowering stage and following 4 weeks of hydroponic cultivation, bacterial densities present in both the non-treated and HB45-treated plants were investigated.
Overall, external colonization of the rhizoplane was significantly greater than internal colonization, and lower colonization of the plant components was more important compared to upper organs. Across the different non-treated plant organs, distinct colony shapes and colors were observed, indicating variability in bacterial populations. The densities of rhizospheric and endospheric bacteria were notably affected by increasing salinity levels. For instance, under normal conditions, the density of existing microflora in the soil and epiphytic bacterial densities on plant roots exceeded 6 Log10 CFU gFM−1. However, under GW irrigation, these densities decreased to 4 Log10 CFU gFM−1. Similar findings were observed in the non-treated internal organs, where endophytic bacterial densities at the root and stem levels declined from 5.5 Log10 CFU gFM−1 in non-saline conditions to 3 Log10 CFU gFM−1 after regular GW irrigation. Moreover, these bacterial community were found inefficient in colonizing upper internal plant tissues, such as leaves and flowers, both in absence and presence of salt stress, with microbial densities not exceeding 3 Log10 CFU gFM−1 in the leaf’s levels (Figure 4A).
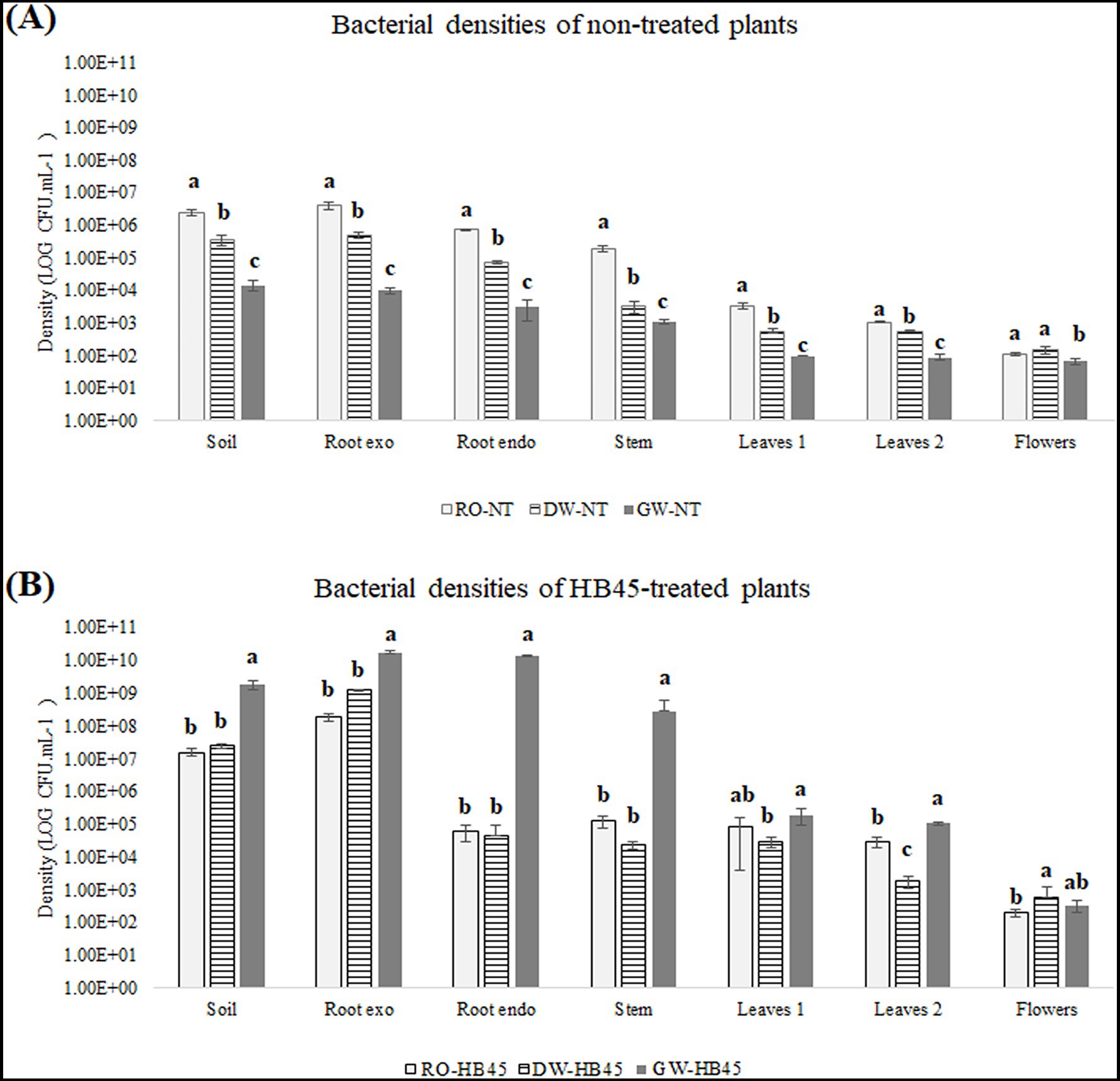
Figure 4. Comparison of bacterial densities in non-treated (A) and HB45-treated tomato plants (B) at plant tissues: rhizoplane, root, stem, leaves and flowers after RO, DW, and GW irrigation. a–c: bars with the same letters indicate no significant differences between means at the confidence level 99.9% according to the Duncan’s multiple range test (at p ≤ 0.05). Errors bars indicate ± SE. All tests were replicated three times.
However, bacterial densities observed after HB45-treatement showed different outcomes. At the peat and root levels different bacterial forms and shape has been depicted with important densities exceeding 7 Log10 CFU gFM−1. However, the dominant endophytic colony shapes and appearances observed across internal plant organs closely resembled those B. spizizenii FMH45 colonies, suggesting the FMH45’s capabilities to penetrate into internal plant organs and colonize the different tissues including roots, stems, and leaves with a considerable CFU count superior to 4.5 Log10 CFU gFM−1 (Figure 4B).
Interestingly, contrary to non-treated plants, bacterial densities in HB45-treated plants remained non-affected when irrigated with saline water, instead, they were present in higher concentrations in most plant organs, with densities exceeding 9 Log10 CFU gFM−1 in external plant organs irrigated with GW and 5 Log10 CFU gFM−1 in internal plant tissues (excluding flowers) exposed to groundwater irrigation (Figure 4B). However, despite their important densities in various internal plant tissues, bacterial counts significantly declined at the flower level, with CFU numbers inferior 2.5 Log10 CFU gFM−1. These results suggest the safety profile of the biofertilizer HB45, as there is no significant bacterial colonization observed in the plant fruits, thus eliminating concerns regarding bacterial presence at the fruit level.
Environmental factors such as pH, temperature, and salt stress have been reported to significantly influence the microbial composition of plants (Sharma et al., 2021) and have been extensively studied for their negative impact on the existing epiphytic microflora such as bacteria and fungi (Hashem et al., 2016). These factors not only alter bacterial phenotypic characteristics related to root colonization capacity (Nabti et al., 2015; Cappellari et al., 2023), but also drive stressed plants to modify their root exudates, which can inhibit the recruitment of rhizobacteria (Ansari et al., 2019). Beneficial bacterial inoculation can also significantly influence the existing microbial community in plants by altering its composition, enhancing beneficial microbial interactions, and sometimes outcompeting native microorganisms for colonization niches (Mawarda et al., 2020). In this study, HB45-treatment under non-saline conditions did not negatively impact the existing microbial community density. In contrast, it helped to improve these densities under salt stress conditions. Similar findings were reported by Hashem et al. (2016), where the endophytic cellulose-producing B. subtilis alleviated the adverse effects of salt on arbuscular mycorrhizal fungi colonization through improving nitrogenase activities and creating more nodules facilitating deeper Rhizobial penetration. The mechanisms by which bacteria stimulate the growth of existing microbial communities remain not well understood. The improvement in rhizospheric structure and the dynamics of the resident bacteriome may be attributed to the ability of plant growth-promoting (PGP) inoculants to synthesize volatile and quorum-sensing molecules, which modulate bacterial interactions within the existing community and with the host plant (Vuolo et al., 2022). For instance, de Carvalho Neta et al. (2024) reported that the synthesis of 1-aminocyclopropane-1-carboxylate (ACC) deaminase and the production of indole-3-acetic acid (IAA) enhance the establishment of mycorrhizae, stimulating root colonization and the formation of arbuscular. Another explanation suggests that the halotolerant capacity of the inoculated bacteria may play a pivotal role in enhancing nutrient availability and increasing organic carbon levels under stress conditions. This has been linked to a greater relative abundance in microbial density, particularly in the rhizosphere (Yaghoubi Khanghahi et al., 2022). These findings are particularly significant, as the studied strain Bacillus spizizenii HB45 has previously been reported for its ability to produce plant growth-promoting (PGP) molecules under saline stress, fostering root development and improving nutrient availability (Masmoudi et al., 2021a). Such traits highlight the non-competitive behavior of HB45 regarding nutritional resources, thereby preserving the natural balance of the plant rhizoplane and protecting it from disruption (Deng et al., 2019). Therefore, FMH45 may exhibit significant potential for its commercial exploitation as an efficient biofertilizer, thanks to its positive interaction with the native soil microbial community and the absence of evidence for the presence of these bacteria in the tomato fruit. This ensures the safe and consistent utilization of this strain on a large scale. However further metagenomic analyses are needed to validate this hypothesis and strengthen our findings.
3.4 Scanning electron microscopy
SEM analysis of the non-treated plants exposed to freshwater irrigation presented well-structured organs with the presence of some bacterial shape mainly at the root levels (Figure 5A). All plant organs displayed clear and well-arranged cells and structures. The flowers showed a unique petal texture with some visible pollen. EDX analysis of these plants identified the most abundant elements as carbon, oxygen, calcium, and potassium in both roots and leaves. However, under saline irrigation SEM analysis of the non-treated plant surfaces and organ cross-sections showed a significant accumulation of salt crystals on the surfaces of the roots and within the internal tissues of leaves and flowers which was confirmed by EDX signals (Figure 5A). These crystals were primarily composed of sodium and chloride, highlighting substantial salt infiltration into internal plant tissues. HB45-treated plants showed markedly different outcomes. Under freshwater or saline irrigation, SEM images revealed enhanced bacterial densities at the root level and within the leaves (Figure 5B). Interestingly, no bacterial strains were detected within the internal tissue of the plant flowers, confirming results obtained during the counting of the bacterial communities. Furthermore, under GW irrigation HB45-treatement significantly reduced the accumulation of salt crystals at the root level, with a complete absence of such crystals in both the leaves and flowers (Figure 5B). SEM–EDX analysis confirmed these findings, Na and Cl signals were only detected in the external root tissue and trace amounts of sodium and chloride were identified in leaves and flowers, with concentrations below 1%. These results indicate that HB45 effectively mitigates salt infiltration into internal plant tissues, limiting the accumulation of harmful salts and preserving the structural integrity of the plant.
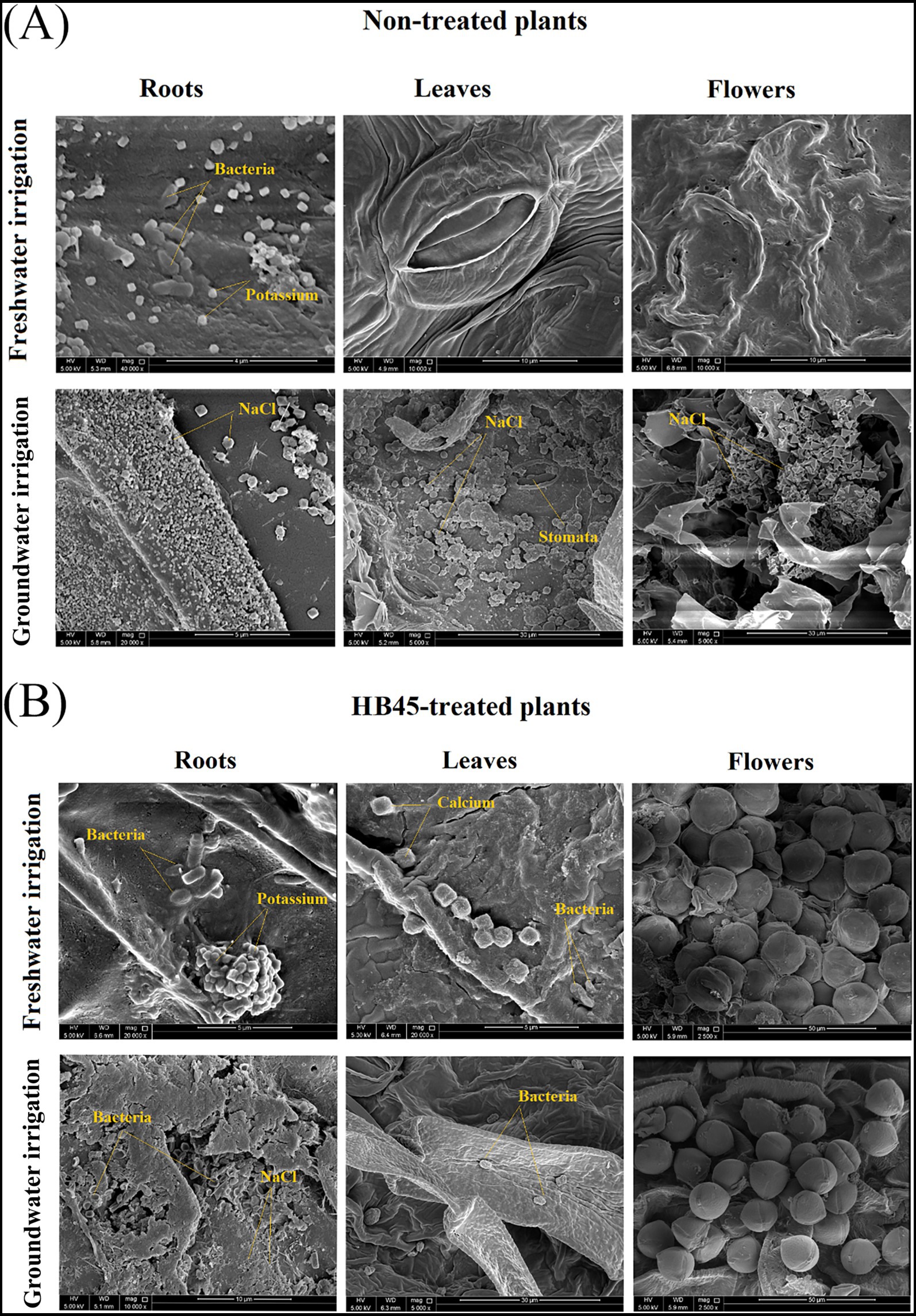
Figure 5. Scanning electron microscopy of the non-treated and the HB45-treated tomato plants cultivated under hydroponic system and exposed to freshwater and Groundwater irrigations. (A) SEM images of the different organs of non-treated plants. (B) SEM images of the different organs of HB45-treated plants. All tests were replicated three times.
The presence of increasing bacterial densities at HB45-treated roots and internal leaf tissues, both in the presence and absence of salt stress, may be attributed to the endophytic potential of B. spizizenii FMH45 linked to its ability to produce several hydrolytic enzymes such as protease, lipase, cellulase, and β-glucanase, even under saline conditions (Masmoudi et al., 2019, 2021a). These enzymes degrade plant cell walls and facilitate bacterial penetration within internal pant tissues. Various prior findings have highlighted the promising endophytic and epiphytic potential of PGPH. For instance, Abo-Kora (2016) used transmission electron microscopy (TEM) to demonstrate the colonization patterns and the endophytic potential of two halotolerant strains Bacillus polymyxa and Azospirillum brasilense in internal organs of Zea mays plants. From their side (Masmoudi et al., 2021b) demonstrated that Bacillus velezensis FMH2 could effectively colonize various plant tissues, even under high salt concentrations reaching 171 mM. Such colonization efficacy and tolerance to saline conditions may be attributed to the capacity of halotolerant bacteria to regulate the accumulation of osmolytes and other stress-related compounds, facilitating osmoadaptation and enhancing bacterial survival and colonization (Sunita et al., 2020). This ability to tolerate saline condition may qualify these microbes to be directly implicated in the regulation of the nutritional and hormonal balances and the induction of systemic plant tolerance to stress (Hashem et al., 2016; Hernández-Canseco et al., 2022). On the other hand, the significant decrease in salt crystal concentrations may be explained by the capabilities of the strain B. spizizenii FMH45 to form biofilm and produce exoplysaccarides (EPS), as demonstrated in our previous report (Masmoudi et al., 2019). Indeed, biofilms are well known for their pivotal role not only in improving bacterial attachment to the root level, but also for their ability to sequester salt ions and preventing their penetration into internal plant tissues (Haque et al., 2022). These results further support the interesting potential of B. spizizenii FMH45 as a promising biofertilizer under salt stress conditions.
3.5 Effect of HB45-treatement on antioxidative response, membrane lipid peroxidation, and reactive oxygen species levels
ROS levels, measured as H2O2 content, antioxidant activity assessed through CAT levels, and lipid peroxidation indicated by MDA contents, were investigated in HB45-treated and non-treated plant tissues irrigated with both fresh and saline water. The highest MDA contents were detected in non-treated plants exposed and non-exposed to saline irrigation (p ≤ 0.05), reflecting a significant membrane leakage and increasing oxidative stress, reaching 19 mmol gFM−1 when irrigated with GW (Figure 6A). HB45-treatement reduced membrane lipid peroxidation by inhibiting MDA generation, with values not crossing 10 mmol gFM−1 in both presence and absence of saline conditions suggesting the absence of oxidative stress within HB45-treated plants tissues (Figure 6A). H2O2 concentrations increased in non-treated plants exposed to saline irrigation, reaching 43 mmol gFM−1 (Figure 6B) and revealing the damaging effects of salinity. However, HB45-treatment significantly reduced H2O2 contents (p ≤ 0.05). For instance, under GW irrigation H2O2 levels decreased by 35% compared to those in non-treated plants (Figure 6B). Under RO irrigation, CAT levels in both HB45-treated and non-treated plants remained relatively stable, at around 65 and 85 mmol s−1 gFM−1, respectively, indicating the absence of oxidative stress on plant cells. However, when salt stress was introduced, CAT production significantly increased under DW irrigation surpassing 160 mmol s−1 gFM−1, showcasing a pronounced oxidative stress response. Conversely, under GW irrigation, CAT levels decreased to reach only 18 mmol s−1 gFM−1, reflecting a severe oxidative stress and a diminished capacity to produce antioxidative compounds. Interestingly, in HB45-treated plants regularly irrigated with DW or GW, CAT production exhibited non-significant fluctuations, suggesting the absence of oxidative stress within plants tissues (Figure 6C).
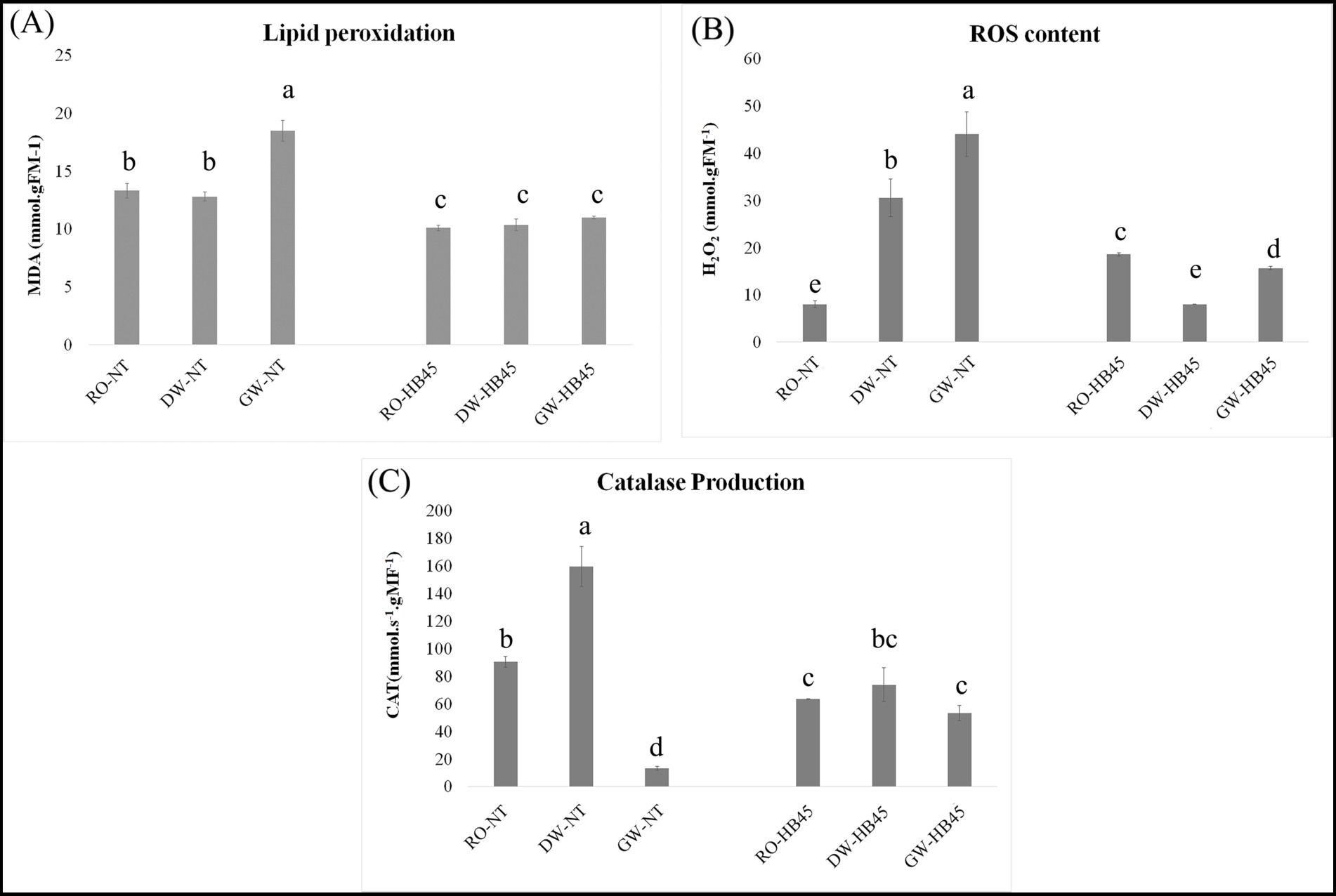
Figure 6. Malondialdehyde (MDA) (A) Hydrogen peroxide (H2O2) (B), and Catalase (CAT) (C), levels in the non-treated and the HB45-treated tomato plants cultivated under hydroponic system and exposed to RO, DW and GW irrigations. a–e: bars with the same letters indicate no significant differences between means at the confidence level 99.9% according to the Duncan’s multiple range test (at p ≤ 0.05). Errors bars indicate ± SE. All tests were replicated three times.
Salt stress is widely reported as an influencing factor that increases ROS levels in plant leaves, induces membrane lipid peroxidation, causes alterations in cell membrane integrity and reduces antioxidant enzyme production evolved in defense mechanisms against oxidative chain reactions This leads to early cell death and plant senescence (Masmoudi et al., 2023; Tiwari et al., 2019).
Halotolerant bacteria may play a crucial role in managing ROS by improving enzymatic and non-enzymatic antioxidant activities (Moncada et al., 2020). Halotolerant Bacillus species have been reported for their ability to mitigate ROS levels through the production of enzymatic metabolites such as peroxidase (POX), catalase (CAT) and superoxide dismutase (SOD). For instance, Masmoudi et al. (2021b) demonstrated the in vivo capability of B. velezensis FMH2 to reduce ROS levels, mitigate lipid peroxidation and enhance POX production in tomato plants exposed to salt stress. Similarly, Sofy et al. (2021) showed that endophytic inoculation of B. subtilis on pea plants decreased oxidative damage and boosted CAT, POX, and SOD production through upregulation of genes encoding these antioxidant enzymes.
HB45-treatement displayed an interesting capability to alleviate oxidative stress in stressed plants. These results confirm earlier findings reported by Masmoudi et al. (2021a), highlighting the interesting in vivo capabilities of B. spizizenii FMH45 to reduce ROS levels, manage lipid peroxidation, and enhance POX production in tomato plants exposed to salt stress. Interestingly, our strain exhibited strong potential under greenhouse cultivation in a hydroponic system, representing a significant advancement toward capability proofing.
This capability ability may be attributed to the interesting endophytic potential of the studied strain, facilitating its penetration into internal plant tissues and stimulating enzymatic and non-enzymatic actions, such as increasing the transcription levels of genes encoding antioxidant enzymes and enhancing ACC deaminase production, in situ, responsible for reducing ethylene concentrations. These mechanisms, among others, enhance plant stress tolerance. However, further investigations are highly required to validate these hypotheses and unveil potential pathways employed by B. spizizenii to alleviate salt stress in tomato plants.
4 Conclusion
This study highlights the potential of HB45 as a sustainable biofertilizer and a promising alternative to costly and environmentally harmful desalination processes. Developed from the halotolerant strain Bacillus spizizenii FMH45, HB45 is designed to reduce reliance on freshwater resources by enabling the use of saline groundwater in hydroponic systems. The biofertilizer demonstrates a strong capacity to alleviate salt stress in tomato plants by enhancing physiological parameters, inhibiting salt infiltration, and boosting antioxidant defense mechanisms. These findings pave the way for the sustainable use of native groundwater, contributing to environmental conservation and improved food security, not only in Qatar and the GCC region but also worldwide. Nonetheless, further research is needed to better understand the mechanisms underlying salt stress tolerance and to assess HB45’s broader applicability across diverse agricultural systems.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
FM: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. IS: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. SBK: Data curation, Methodology, Writing – review & editing. ST: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the QNRF-MME award [MME02-1008-200048] from the Qatar National Research Fund (a member of Qatar Foundation). The findings herein reflect the work and are solely the responsibility of the authors.
Acknowledgments
We thank the team members of the Centre for Sustainable Development, Qatar University, for their continued support. SEM analysis was accomplished in the Central Laboratories unit, Qatar University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abo-Kora, H. A. (2016). Endophytic colonization of maize (Zea mays v.) root plants by PGPRs under salinity stress. Nat. Sci. 14, 34–51. doi: 10.7537/marsnsj14071605
Alhaj, M. A., Mohammed, S., Darwish, M., Hassan, A., and Al-Ghamdi, S. G. (2017). A review of Qatar’s water resources, consumption and virtual water trade. Desalin. Water Treat. 90, 70–85. doi: 10.5004/dwt.2017.21246
Anith, K., Vyshakhi, A. S., Viswanathan, A., Varkey, S., and Aswini, S. (2016). Population dynamics and efficiency of coconut water based liquid formulation of Pseudomonas fluorescens AMB-8. J. Trop. Agric. 54, 184–189. Available at: http://jtropag.kau.in/index.php/ojs2/article/view/381/387
Ansari, F. A., Ahmad, I., and Pichtel, J. (2019). Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl. Soil Ecol. 143, 45–54. doi: 10.1016/j.apsoil.2019.05.023
Barone, V., Puglisi, I., Fragalà, F., Lo Piero, A. R., Giuffrida, F., and Baglieri, A. (2019). Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J. Appl. Phycol. 31, 465–470. doi: 10.1007/s10811-018-1518-y
Ben Khedher, S., Jaoua, S., and Zouari, N. (2014). Overcome of carbon catabolite repression of bioinsecticides production by sporeless Bacillus thuringiensis through adequate fermentation technology. Biotechnol. Res. Int. 2014:698587, 1–8. doi: 10.1155/2014/698587
Bressuire-Isoard, C., Broussolle, V., and Carlin, F. (2018). Sporulation environment influences spore properties in Bacillus: evidence and insights on underlying molecular and physiological mechanisms. FEMS Microbiol. Rev. 42, 614–626. doi: 10.1093/femsre/fuy021
Cappellari, L. D. R., Bogino, P. C., Nievas, F., Giordano, W., and Banchio, E. (2023). Exploring the differential impact of salt stress on root colonization adaptation mechanisms in plant growth-promoting rhizobacteria. Plan. Theory 12:4059. doi: 10.3390/plants12234059
Cardoso, F. B., Martinez, H. E. P., Silva, D., do Carmo Milagres, C., and Barbosa, J. G. (2018). Yield and quality of tomato grown in a hydroponic system, with different planting densities and number of bunches per plant. Pesqui. Agropecuária Trop. 48, 340–349. doi: 10.1590/1983-40632018v4852611
Corral, P., Amoozegar, M. A., and Ventosa, A. (2019). Halophiles and their biomolecules: recent advances and future applications in biomedicine. Mar. Drugs 18:33. doi: 10.3390/md18010033
de Azevedo Neto, A. D., Prisco, J. T., Enéas-Filho, J., De, A. C. E. B., and Gomes-Filho, E. (2006). Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 56, 87–94. doi: 10.1016/j.envexpbot.2005.01.008
de Carvalho Neta, S. J., Araújo, V. L. V. P., Fracetto, F. J. C., da Silva, C. C. G., de Souza, E. R., Silva, W. R., et al. (2024). Growth-promoting bacteria and arbuscular mycorrhizal fungus enhance maize tolerance to saline stress. Microbiol. Res. 284:127708. doi: 10.1016/j.micres.2024.127708
Del Moral, A., Severin, J., Ramos-Cormenzana, A., Truper, H. G., and Galinski, E. A. (1994). Compatible solutes in new moderately halophilic isolates. FEMS Microbiol. Lett. 122, 165–172. doi: 10.1111/j.1574-6968.1994.tb07160.x
Deng, S., Wipf, H. M.-L., Pierroz, G., Raab, T. K., Khanna, R., and Coleman-Derr, D. (2019). A plant growth-promoting microbial soil amendment dynamically alters the strawberry root bacterial microbiome. Sci. Rep. 9:17677. doi: 10.1038/s41598-019-53623-2
Elakhdar, I., Elsakhawy, T., and Abo-Koura, H. A. (2020). Alleviation of salt stress on wheat (Triticum aestivum L.) by plant growth promoting bacteria strains Bacillus halotolerans MSR-H4 and Lelliottia amnigena MSR-M49. J. Adv. Microbiol. 20, 44–58. doi: 10.9734/JAMB/2020/v20i130208
Glick, B. R. (2012). Plant growth‐promoting bacteria: mechanisms and applications. Scientifica. 2012:963401. doi: 10.6064/2012/963401
Haque, M. M., Biswas, M. S., Mosharaf, M. K., Haque, M. A., Islam, M. S., Nahar, K., et al. (2022). Halotolerant biofilm-producing rhizobacteria mitigate seawater-induced salt stress and promote growth of tomato. Sci. Rep. 12:5599. doi: 10.1038/s41598-022-09519-9
Hashem, A., Abd, E. F., Alqarawi, A. A., al-Huqail, A. A., Wirth, S., and Egamberdieva, D. (2016). The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 7:7. doi: 10.3389/fmicb.2016.01089
He, H., Li, Y., Zhang, L., Ding, Z., and Shi, G. (2023). Understanding and application of Bacillus nitrogen regulation: a synthetic biology perspective. J. Adv. Res. 49, 1–14. doi: 10.1016/j.jare.2022.09.003
Hernández-Canseco, J., Bautista-Cruz, A., Sánchez-Mendoza, S., Aquino-Bolaños, T., and Sánchez-Medina, P. S. (2022). Plant growth-promoting halobacteria and their ability to protect crops from abiotic stress: an eco-friendly alternative for saline soils. Agronomy 12:804. doi: 10.3390/agronomy12040804
Ingrao, C., Strippoli, R., Lagioia, G., and Huisingh, D. (2023). Water scarcity in agriculture: an overview of causes, impacts and approaches for reducing the risks. Heliyon 9:e18507. doi: 10.1016/j.heliyon.2023.e18507
Jones, E., Qadir, M., van Vliet, M. T., and Kang SM, S. V. (2019). The state of desalination and brine production: a global outlook. Sci. Total Environ. 657, 1343–1356. doi: 10.1016/j.scitotenv.2018.12.076
Khan, M., and Al-Ghouti, M. A. (2021). DPSIR framework and sustainable approaches of brine management from seawater desalination plants in Qatar. J. Clean. Prod. 319:128485. doi: 10.1016/j.jclepro.2021.128485
Levintal, E., Kniffin, M. L., Ganot, Y., Marwaha, N., Murphy, N. P., and Dahlke, H. E. (2023). Agricultural managed aquifer recharge (ag-MAR)—a method for sustainable groundwater management: a review. Crit. Rev. Environ. Sci. Technol. 53, 291–314. doi: 10.1080/10643389.2022.2050160
Mahgoub, H. A. M., Fouda, A., Eid, A. M., Ewais, E. E.-D., and Hassan, S. E.-D. (2021). Biotechnological application of plant growth-promoting endophytic bacteria isolated from halophytic plants to ameliorate salinity tolerance of Vicia faba L. Plant Biotechnol. Rep. 15, 819–843. doi: 10.1007/s11816-021-00716-y
Masmoudi, F., Abdelmalek, N., Tounsi, S., Dunlap, C. A., and Trigui, M. (2019). Abiotic stress resistance, plant growth promotion and antifungal potential of halotolerant bacteria from a Tunisian solar saltern. Microbiol. Res. 229:126331. doi: 10.1016/j.micres.2019.126331
Masmoudi, F., Al Naimi, L., Trigui, M., Al Safran, M., Tounsi, S., and Saadaoui, I. (2024). Novel thermo-halotolerant bacteria Bacillus cabrialesii native to Qatar desert: enhancing seedlings’ growth, halotolerance, and antifungal defense in tomato. J. Plant Growth Regul. doi: 10.1007/s00344-024-11460-2
Masmoudi, F., Alsafran, M., Jabri, H. A., Hosseini, H., Trigui, M., Sayadi, S., et al. (2023). Halobacteria-based biofertilizers: a promising alternative for enhancing soil fertility and crop productivity under biotic and abiotic stresses—a review. Microorganisms 11:1248. doi: 10.3390/microorganisms11051248
Masmoudi, F., Tounsi, S., Dunlap, C. A., and Trigui, M. (2021a). Halotolerant Bacillus spizizenii FMH45 promoting growth, physiological, and antioxidant parameters of tomato plants exposed to salt stress. Plant Cell Rep. 40, 1199–1213. doi: 10.1007/s00299-021-02702-8
Masmoudi, F., Tounsi, S., Dunlap, C. A., and Trigui, M. (2021b). Endophytic halotolerant Bacillus velezensis FMH2 alleviates salt stress on tomato plants by improving plant growth and altering physiological and antioxidant responses. Plant Physiol. Biochem. 165, 217–227. doi: 10.1016/j.plaphy.2021.05.025
Mawarda, P. C., Le Roux, X., Van Elsas, J. D., and Salles, J. F. (2020). Deliberate introduction of invisible invaders: a critical appraisal of the impact of microbial inoculants on soil microbial communities. Soil Biol. Biochem. 148:107874. doi: 10.1016/j.soilbio.2020.107874
Moncada, A., Vetrano, F., and Miceli, A. (2020). Alleviation of salt stress by plant growth-promoting bacteria in hydroponic leaf lettuce. Agronomy 10:1523. doi: 10.3390/agronomy10101523
Mukherjee, I., Singh, U. K., and Chakma, S. (2022). Evaluation of groundwater quality for irrigation water supply using multi-criteria decision-making techniques and GIS in an agroeconomic tract of lower ganga basin, India. J. Environ. Manage. 309:114691. doi: 10.1016/j.jenvman.2022.114691
Nabti, E., Schmid, M., and Hartmann, A. (2015). “Application of halotolerant bacteria to restore plant growth under salt stress” in Halophiles: Biodiversity and sustainable exploitation. eds. D. K. Maheshwari and M. Saraf (Cham: Springer International Publishing), 235–259.
Nautiyal, C. S., Srivastava, S., Chauhan, P. S., Seem, K., Mishra, A., and Sopory, S. K. (2013). Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 66, 1–9. doi: 10.1016/j.plaphy.2013.01.020
Nicolas, F., Kamai, T., Ben-Gal, A., Ochoa-Brito, J., Daccache, A., Ogunmokun, F., et al. (2023). Assessing salinity impacts on crop yield and economic returns in the Central Valley. Agric. Water Manag. 287:108463. doi: 10.1016/j.agwat.2023.108463
Orhan, F. (2021). Potential of halophilic/halotolerant bacteria in enhancing plant growth under salt stress. Curr. Microbiol. 78, 3708–3719. doi: 10.1007/s00284-021-02637-z
Poria, V., Dębiec-Andrzejewska, K., Fiodor, A., Lyzohub, M., Ajijah, N., Singh, S., et al. (2022). Plant Growth-Promoting Bacteria (PGPB) integrated phytotechnology: A sustainable approach for remediation of marginal lands. Front. Plant Sci. 13:999866.doi: 10.3389/fpls.2022.999866
Posada-Uribe, L. F., Romero-Tabarez, M., and Villegas-Escobar, V. (2015). Effect of medium components and culture conditions in Bacillus subtilis EA-CB0575 spore production. Bioprocess Biosyst. Eng. 38, 1879–1888. doi: 10.1007/s00449-015-1428-1
Sarrafzadeh, M. H., Guiraud, J. P., Lagneau, C., Gaven, B., Carron, A., and Navarro, J.-M. (2005). Growth, sporulation, δ-endotoxins synthesis, and toxicity during culture of Bacillus thuringiensis H14. Curr. Microbiol. 51, 75–81. doi: 10.1007/s00284-005-4463-3
Sharma, P., Kumar, T., Yadav, M., Gill, S. S., and Chauhan, N. S. (2021). Plant-microbe interactions for the sustainable agriculture and food security. Plant Gene 28:100325. doi: 10.1016/j.plgene.2021.100325
Singh, V., Haque, S., Niwas, R., Srivastava, A., Pasupuleti, M., and Tripathi, C. K. M. (2017). Strategies for fermentation medium optimization: an in-depth review. Front. Microbiol. 7:2087. doi: 10.3389/fmicb.2016.02087
Sofy, M. R., Aboseidah, A. A., Heneidak, S. A., and Ahmed, H. R. (2021). ACC deaminase containing endophytic bacteria ameliorate salt stress in Pisum sativum through reduced oxidative damage and induction of antioxidative defense systems. Environ. Sci. Pollut. Res. 28, 40971–40991. doi: 10.1007/s11356-021-13585-3
Stegelmeier, A. A., Rose, D. M., Joris, B. R., and Glick, B. R. (2022). The use of PGPB to promote plant hydroponic growth. Plan. Theory 11:2783. doi: 10.3390/plants11202783
Sunita, K., Mishra, I., Mishra, J., Prakash, J., and Arora, N. K. (2020). Secondary metabolites from halotolerant plant growth promoting rhizobacteria for ameliorating salinity stress in plants. Front. Microbiol. 11:567768. doi: 10.3389/fmicb.2020.567768
Tao, Z., Yuan, H., Liu, M., Liu, Q., Zhang, S., Liu, H., et al. (2023). Yeast extract: characteristics, production, applications and future perspectives. J. Microbiol. Biotechnol. 33, 151–166. doi: 10.4014/jmb.2207.07057
Timofeeva, A. M., Galyamova, M. R., and Sedykh, S. E. (2023). Plant growth-promoting soil bacteria: nitrogen fixation, phosphate solubilization, siderophore production, and other biological activities. Plan. Theory 12:4074. doi: 10.3390/plants12244074
Tiwari, S., Prasad, V., and Lata, C. (2019). “Chapter 3 - Bacillus: plant growth promoting bacteria for sustainable agriculture and environment” in New and future developments in microbial biotechnology and bioengineering. eds. J. S. Singh and D. P. Singh (Elsevier), 43–55.
Vuolo, F., Novello, G., Bona, E., Gorrasi, S., and Gamalero, E. (2022). Impact of plant-beneficial bacterial inocula on the resident bacteriome: current knowledge and future perspectives. Microorganisms 10:2462. doi: 10.3390/microorganisms10122462
Wang, T., Wu, Z., Wang, P., Wu, T., Zhang, Y., Yin, J., et al. (2023). Plant-groundwater interactions in drylands: A review of current research and future perspectives. Agric. For. Meteor. 341:109636. doi: 10.1016/j.agrformet.2023.109636
Xie, X., Gan, L., Wang, C., and He, T. (2024). Salt-tolerant plant growth-promoting bacteria as a versatile tool for combating salt stress in crop plants. Arch. Microbiol. 206:341. doi: 10.1007/s00203-024-04071-8
Yaghoubi Khanghahi, M., Crecchio, C., and Verbruggen, E. (2022). Shifts in the rhizosphere and endosphere colonizing bacterial communities under drought and salinity stress as affected by a biofertilizer consortium. Microb. Ecol. 84, 483–495. doi: 10.1007/s00248-021-01856-y
Yasmin, H., Id, N. S., Jabeen, Z., Nosheen, A., Naz, R., Keyani, R., et al. (2020). Halotolerant rhizobacteria pseudomonas pseudoalcaligenes and Bacillus subtilis mediate systemic tolerance in hydroponically grown soybean (Glycine max L.) against salinity stress. PLoS One 15:e0231348. doi: 10.1371/journal.pone.0231348
Keywords: Bacillus spizizenii, groundwater irrigation, halobacteria-based biofertilizer, hydroponic cultivation system, salt stress, oxidative stress
Citation: Masmoudi F, Saadaoui I, Ben Khedher S and Tounsi S (2025) Bacillus Spizizenii FMH45-based biofertilizer enhances growth and halotolerance of cherry tomato plants under hydroponic cultivation systems. Front. Sustain. Food Syst. 8:1520444. doi: 10.3389/fsufs.2024.1520444
Edited by:
Abdelbasset Lakhdar, University of Sfax, TunisiaReviewed by:
Salah Jellali, Sultan Qaboos University, OmanMokhtar Guerfel, Shaqra University, Saudi Arabia
Copyright © 2025 Masmoudi, Saadaoui, Ben Khedher and Tounsi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Imen Saadaoui, aW1lbi5zYWFkYW91aUBxdS5lZHUucWE=
 Fatma Masmoudi
Fatma Masmoudi Imen Saadaoui
Imen Saadaoui Saoussen Ben Khedher2,3
Saoussen Ben Khedher2,3 Slim Tounsi
Slim Tounsi