- 1Department of Biology, College of Sciences, Taif University, Taif, Saudi Arabia
- 2Department of Food Science and Nutrition, College of Sciences, Taif University, Taif, Saudi Arabia
- 3Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, University of Tabuk, Tabuk, Saudi Arabia
- 4Department of Biological Sciences, College of Science, University of Jeddah, Jeddah, Saudi Arabia
- 5Program of Food Sciences and Nutrition, Turabah University College, Taif University, Taif, Saudi Arabia
- 6Pharmacy Administration Department, Taif Health Cluster, Ministry of Health, Taif, Saudi Arabia
- 7Department of Biochemistry, College of Science, University of Jeddah, Jeddah, Saudi Arabia
- 8Department of Food Science and Human Nutrition, College of Agriculture and Food, Qassim University, Buraydah, Saudi Arabia
- 9Department of Food Science and Nutrition, Alkhurmah University College, Taif University, Taif, Saudi Arabia
- 10Department of Biology, Al Khurma University College, Taif University, Taif, Saudi Arabia
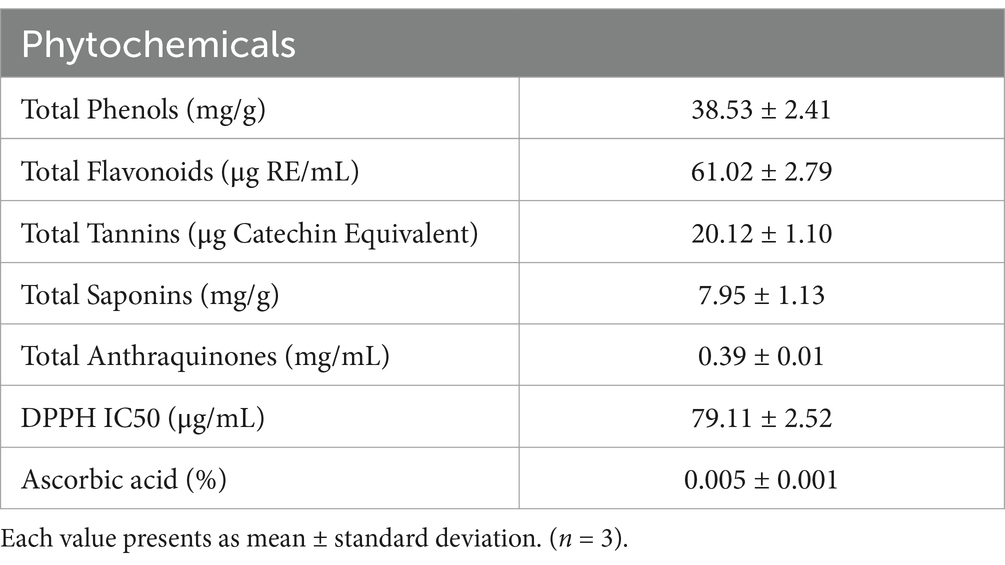
The main objective of the current study was to examine the anti-influenza effects of Aloe vera gel on the Madin–Darby Canine Kidney (MDCK) cell line, as well as its protective efficacy against ultraviolet-C exposure, by studying its biochemical characterization and microbial activities. The lowest IC50 value (4.23 mg/mL) was reported for the treated cells, with a concentration of 8 mg/ML of Aloe vera extract after contamination with the AP/R/8 virus. After 2 h of ultraviolet-C exposure, all cells in the control group and those with single-sided gel application died, while the cells treated with the gel on both sides showed 29.000 viable cells. The lowest inhibition activity was detected against Bacillus subtilis (2.20 mm) at a concentration of 20 μL. In comparison, the highest inhibition activity was observed against Pseudomonas aeruginosa (21.29 mm) at a concentration of 100 μL. The highest values were detected in the Aloe vera gel samples in the following sequence: magnesium (28.43 mg/100 g), phosphorus (2.98 mg/100 g), zinc (143.01 μg/100 g), selenium (137.18 μg/100 g), iron (19.78 μg/100 g), chromium (15.40 μg/100 g), aluminum (12.35 μg/100 g), and vanadium (8.70 μg/100 g). On the other hand, trace elements such as cadmium, cobalt, and nickel showed the lowest concentrations (0.05, 0.12, and 0.23 μg/100 g, respectively). The plant contained high quantities of phenols, flavonoids, tannins, saponins, and anthraquinones, along with high antioxidant activity (1,1-diphenyl-2picrylhydrazyl, DPPH) and ascorbic acid content. Based on these observations, it can be concluded that Aloe vera plants contain variable compounds that may be responsible for their therapeutic and pharmacological uses.
Introduction
Plants serve as primary sources for phytomedicinal compounds (Sánchez et al., 2020). The use of plant-based medicines as alternatives to synthetic therapeutics is supported by their relatively lower rate of adverse reactions and lower cost compared to modern pharmaceuticals (Gokulan et al., 2019). Natural compounds and active pharmaceutical ingredients (APIs) aid in treating infections while promoting overall health (Sun et al., 2020). The natural components can be derived from various plant parts and converted into key ingredients for nutritional supplements, foods, cosmetics, and the correlated pharmaceutical industry, where they serve as potent immunostimulants in the aquaculture field (Mollaei et al., 2020; Asnaashari et al., 2019). It is believed that natural compounds or their semisynthetic counterparts account for 40% of all medicines. Worldwide, consumers have shown a resurgence of interest in herbal therapy. The colorless, mucilaginous fleshy tissue known as Aloe vera gel is extracted from the parenchymatous cells of fresh Aloe vera (Lilliaceae) leaves, which grow in temperate climates (Boumoud et al., 2012). Aloe vera gel is a natural remedy commonly used in traditional medicine as a healing agent, with antitumor, anti-ulcer, anti-aging, antimicrobial, and antioxidant properties. It promotes the healing of radiation damage and is used to treat dermatitis, eczema, psoriasis, insect bites, and stings (Saleem et al., 2022). It also lowers blood sugar and lipid levels, which has positive effects on hepatoprotective actions. Aloe vera contains pain-relieving enzymes such as carboxypeptidase and bradykinase, as well as aloeresin I and dihydrocoumarins, which act as anti-inflammatory agents with immune-modulatory activities (Reynolds and Dweck, 1999). The main active component in Aloe vera is aloin, a small molecular phenolic compound that facilitates alcohol metabolisms, as well as lignin, saponins, sterols, enzymes, and other nutrients (Nicolau-Lapeña et al., 2021). It also demonstrates anti-genotoxic and chemopreventive properties on benzo(α)pyrene-DNA adducts. Previous studies have focused on Aloe vera’s effectiveness against herpes simplex virus type 2 (Zandi et al., 2007) and its potential as an anti-COVID-19 Plant (Mpiana et al., 2020). Acute respiratory infections, such as influenza, are caused by influenza viruses and have a significant worldwide impact. While resistant strains are becoming more common, the use of synthetic antiviral medications and techniques is now limited due to their costly side effects (Arbab et al., 2021). However, the literature on the potential applications of Aloe vera compounds in the prevention of viral illnesses remains limited.
The current study aimed to examine the anti-influenza effects of Aloe vera gel on Madin–Darby Canine Kidney (MDCK) cells and its protective efficacy against ultraviolet-C exposure on human umbilical vein endothelial cells (HUVECs) by investigating its biochemical characteristics and microbial activities.
Materials and methods
Aloe vera leaves
Healthy, mature specimens of Aloe vera leaves were collected from mature plants in April and May 2024 from a garden in Taif City, KSA. Fresh leaves, approximately 50–55 cm in length, with thick stems and a dark green color, were randomly selected and cut by removing the leaf rinds in the early morning for the experimental work. The leaves were transported to Taif University in polyethylene bags to prevent oxidation and microbial contamination.
Preparation of the Aloe vera plant
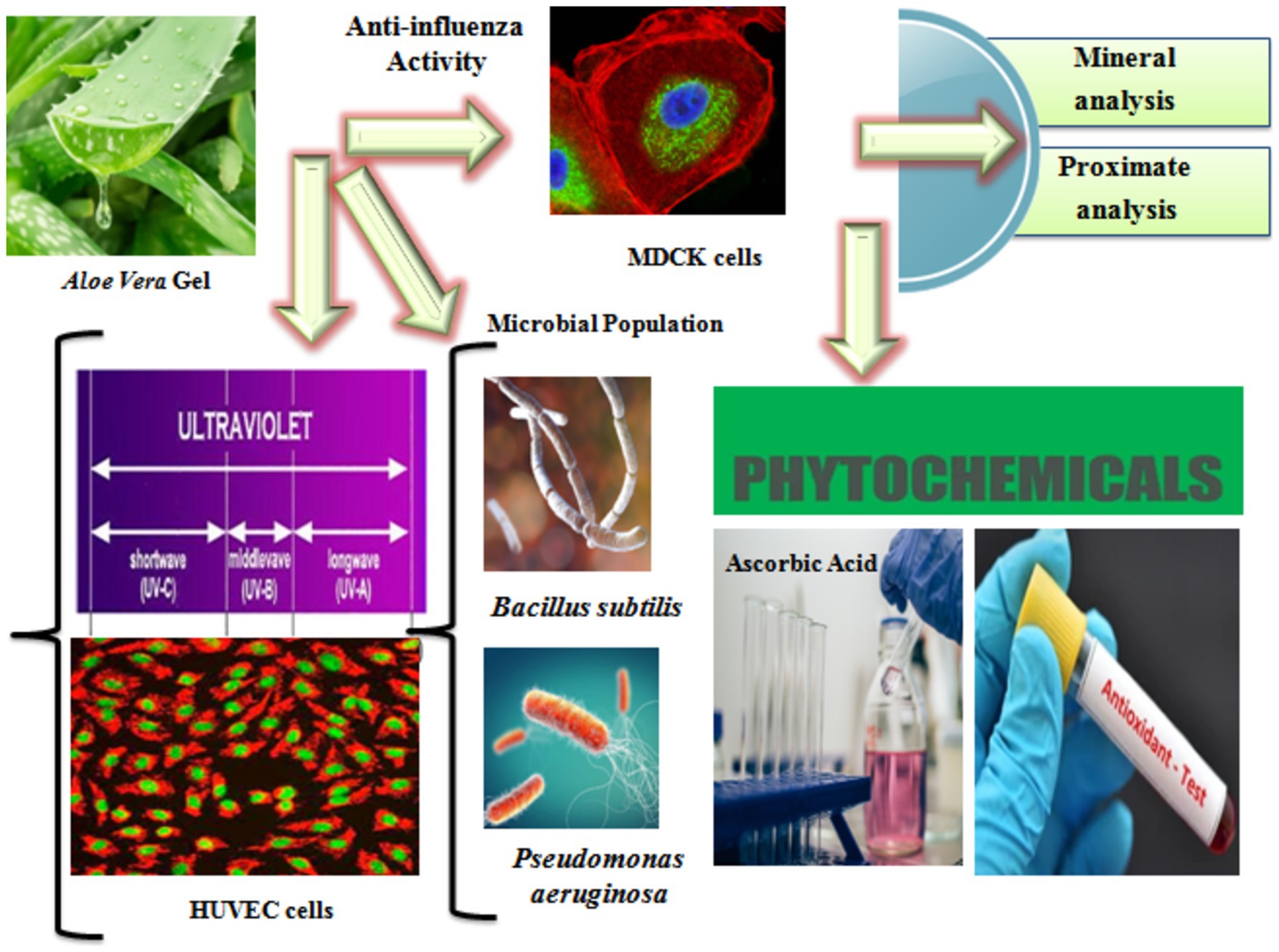
After surface sterilization and washing with distilled water for 5 min to remove any sticking materials, dust, and impurities, the leaves were allowed to dry at room temperature. Using a sterile stainless steel knife, the leaves were manually cut, and the gel portion was retained after the outer green skin was peeled off. The gel was carefully collected using a sterile scalpel and weighed on an electric balance to calculate the yield as a percentage. Water extracts were then prepared for the anti-influenza determinations. First, the gel was frozen at −80°C for 1 h and then freeze-dried using ALPHA 1–4 LSC (Germany) at −50°C and a pressure of 0.010 mbar for 1 day (Qiao et al., 2020). The dried gel was ground using a non-metallic electric grinder and sieved through a300 μm mesh. The Aloe vera powder was stored in a zipper storage bag at −80°C until further use. Figure 1 shows the summary of the experimental study.
Cell culture and determination of the anti-influenza activity
MDCK cells and influenza AP/R/8 virus were obtained from the Shanghai Institute of Biological Sciences, China. Cells (1.5 × 104) were grown and incubated at 37°C for 24 h in a 96-well plate. The influenza virus solution was diluted to 4 × 103 in DMEM containing trypsin–EDTA for the infection, with non-infected MDCK cells used as the control. The MDCK cells were rinsed two times with PBS without the AP/R/8 solution. Approximately 2 g of fresh gel was added to 10 mL of sterile water and then stirred and filtered to remove any residue. Different concentrations of Aloe vera gel, ranging from 0 mg/mL to 8 mg/mL, were prepared, and 10 μL of each concentration was added to 90 μL of AP/R/8 virus solution (TCID50) in a 96-well plate. All samples were analyzed in triplicate and kept in a carbon dioxide incubator with humidity for 48 h. The cells were washed, and cold acetone (70%) was added for 1 h at a temperature of −4°C, according to the method described by Gansukh et al. (2016). The samples were measured using a Synergy HT Microplate Reader (BioTek, USA) at 510 nm for anti-influenza activity, and the results were expressed as IC50 (Al-Eisa et al., 2023a).
Cell culture and determination of the protecting efficiency against exposure to ultraviolet C
The gel was spread on either one or both sides of the cells in the Petri dishes and allowed to dry at ambient temperature. HUVECs were obtained from the Shanghai Institute of Biological Sciences, China. The control consisted of a healthy HUVEC line. DMEM medium was used, supplemented with FBS (10%), L-glutamine, and penicillin–streptomycin, and the cells were incubated at 37°C in a 5% CO2 atmosphere. The cells were washed with PBS, then exposed to Tris/EDTA, and incubated at 37°C in a 5% CO2 atmosphere. Afterward, the cells were separated, centrifuged at 1000 g for 10 min, and counted using trypan blue dye after exposure to ultraviolet C at a wavelength of 254 nm (Kostyuk et al., 2008). The initial cell count recorded 600,000 cells at 0 h. The cells were then exposed to ultraviolet C for different durations (1, 1.5, 2, and 4 h) for the control and for the gel applied to one or both sides.
Determination of the microbial population
The microbial population was applied in vitro using Mueller–Hinton agar as the medium. Two kinds of foodborne bacterial isolates, Bacillus subtilis and Pseudomonas aeruginosa, were obtained from the Agricultural Culture Collection, Egypt. To prepare the plates, 15 milliliters of the medium were added to sterile Petri dishes, allowed to harden for 5 min, and then uniformly swabbed with a 0.1% inoculum suspension before being allowed to dry for another 5 min. The extracts were placed onto 6 mm sterile disks at a concentration of 40 mg/disk. After placing the loaded disks on the surface of the medium and allowing the extract to diffuse for 5 min, the plates were incubated for 24 h at 37°C (Sami et al., 2021e; Sami et al., 2021c). The microbial population was detected in triplicate, and the results were expressed as the zone of inhibition in millimeters (mm).
Determination of the chemical components
For the determination of moisture and total solid contents, the Aloe vera gel samples were freeze-dried at −50°C under a pressure of 0.010 mbar until a constant weight was achieved. Standard assays were performed to evaluate the proximate analysis of ash (Ignition method), protein (Kjeldahl method), fiber (acid and alkaline hydrolysis method), and fat (Soxhlet extraction) contents, according to the assays described by the Association of Official Analytical Chemists (AOAC) (AOAC, 1990). Total polysaccharides in the Aloe vera gel sample were detected according to the assay described by Sami et al. (2019). The phenol-sulfuric assay was used, with glucose as the standard, and absorbance was measured at a wavelength of 490 nm (754 PC, Shanghai, China). Approximately 1 g of the aloe vera gel was mixed with 10 mL of distilled water and shaken regularly for 2 h. The solution was filtered and analyzed using a digital pH meter (Ramdhini and Anggraini, 2024). All chemical components were detected in triplicate, and the results were calculated as percentages, except for the pH value.
Determination of the mineral contents
Approximately 5 g of the Aloe vera gel was ashed at 410–440°C. The white-ash residue was dissolved in a mixture of HNO3, H2SO4, and HClO4 in a ratio of 11:6:3, filtered, and then adjusted to 100 mL with distilled water for mineral evaluations (Sami et al., 2021b). An atomic absorption spectrometer (Perkin Elmer, A Analyst 800) with suitable hollow cathode lamps was used separately for each element. The results were expressed as μg/100 g for Cd, Co, Cu, Ni, Pb, Mn, V, A, Cr, Fe, Se, and Zn and as mg/100 g for Mg and P.
Determination of the phytochemicals, antioxidant activity, and ascorbic acid
Total phenols
The total phenols were estimated spectrophotometrically according to the Folin–Ciocalteu assay, with gallic acid used as a standard at concentrations of 20, 40, 60, 80, and 100 μg/mL to form a calibration curve (Molole et al., 2022). The mixture samples were homogenized, allowed to stand in a dark room at ambient temperature for 2 h, and then absorbed at 765 nm. The results were detected in triplicate and expressed as mg/g.
Total flavonoids
The total flavonoids were estimated using the colorimetric method with the addition of 10% aluminum chloride and 5% sodium nitrite, using a standard solution of rutin at concentrations of 0.2, 0.4, 0.6, 0.8, and 1 mg/mL to form the calibration curve (Sami et al., 2021d). The mixture samples were homogenized for 5 min and kept at ambient temperature for 30 min. The absorbance was measured at a wavelength of 430 nm using a spectrophotometer. The results were detected in triplicate and expressed as μg RE/mL.
Total tannins
The total tannins were estimated using the vanillin–HCl assay (Sami et al., 2021d). Approximately 200 μL of Aloe vera gel in screw-capped tubes was added to 1.5 mL of 4% vanillin in methanol, along with 750 μL of hydrochloric acid, and incubated at 30°C for 10 min in a water bath. The absorbance was measured at a wavelength of 500 nm against catechin monohydrate as a standard. The results were expressed as μg catechin equivalent.
Total saponins
The total saponins were detected according to the colorimetric method described by Parthasarathy et al. (2017). Approximately 0.1 g of the Aloe vera gel was vigorously shaken with distilled water until the formation of a creamy mixture with small bubbles or even persistent froth, indicating the presence of saponins as a standard, and the results were expressed as mg/g.
Total anthraquinones
The total anthraquinones were estimated according to the optical density absorbance (red color reaction). Approximately 0.2 g of Aloe vera gel was mixed with 0.36 mg of anthraquinone, 5% NaOH, and 2% NH3 solutions, and the volume was adjusted to 100 mL. The absorbance was measured against CoCl2 as a standard (Diaz-Munoz et al., 2018).
Radical scavenging activity
The antioxidant activity of the Aloe vera gel was detected using the 1,1-diphenyl-2picrylhydrazyl (DPPH) method, according to its ability to scavenge radicals or donate hydrogen (Rokayya et al., 2013). Approximately 1 mg of the gel was dissolved in 1 mL of MeOH and 150 μL of a DPPH solution with a concentration of 0.1 mM. The mixture was kept in the refrigerator for 30 min, shaken thoroughly, and measured at a wavelength of ~0.800, with an absorbance of 515 nm. DPPH antioxidant activity was detected against MeOH as a standard and calculated as IC50 (μg/mL).
Ascorbic acid
Vitamin C (ascorbic acid) in the Aloe vera gel was detected using the colorimetric method based on the oxidation–reduction reaction of 2,6-dichlorophenol as a redox dye. L-ascorbic acid can be oxidized to L-dehydroascorbic acid, resulting in a rose-pink color in the acidic solution (Cisternas et al., 2014).
Statistical analysis
All experiments were performed in triplicate, and the results were expressed as mean ± standard deviation (SD). The means were compared using one-way ANOVA (version 8.2, SAS, Cary, NC, USA) with a significance level of a p-value of <0.05.
Results and discussion
Effect of the Aloe vera gel on the anti-influenza activity
Herbal medicines derived from natural resources play a significant role in the development of antiviral medications. Novel influenza viruses have pathogenic effects on public health, and there is a need to identify new anti-influenza compounds. Aloe vera gel contains anthraquinone derivatives, which have the potential to exhibit antiviral activities. The control and different concentrations of the Aloe vera gel extracts were evaluated against the influenza AP/R/8 virus, as shown in Figure 2. The highest IC50 value was reported for the control (untreated cells), while the lowest IC50 value (4.23 mg/mL) was reported for the treated cells at a concentration of 8 mg/mL. The lower IC50 value indicated enhanced antiviral activity. The results from using the MDCK cell lines are in agreement with those reported by Rezazadeh et al. (2016) who studied the anti-influenza activity in the Vero cell line. Sydiskis et al. (1991) reported the inhibitory effects of Aloe vera gel against poliovirus, herpes simplex, cytomegalovirus, varicella zoster virus, influenza, rhabdovirus septicemia, papillomavirus, immunodeficiency virus, and both RNA and DNA viruses. Majumder et al. (2019) reported the antiviral effects of Aloe vera on coronavirus and its management in in-vitro and in-vivo studies. Mulu et al. (2015) reported the significant effects of Aloe vera, which are similar to those of the lopinavir/ritonavir drug. The anthraquinone derivatives in the Aloe vera plant, such as aloe-emodin, emodin, and chrysophanol, were the main contributors to the anti-influenza activity (Gansukh et al., 2016).
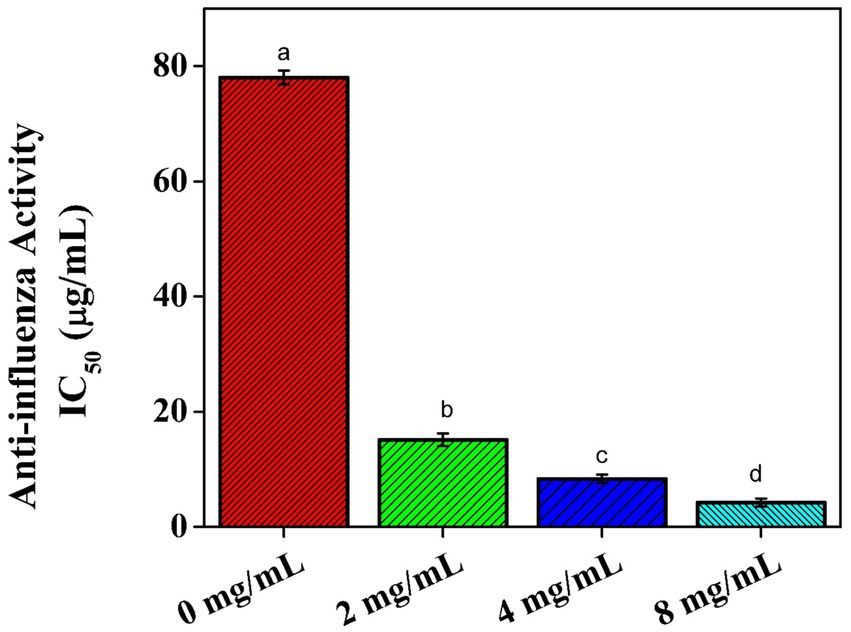
Figure 2. Effect of the Aloe vera gel on the anti-influenza activity of AP/R/8 virus against the MDCK cells.
Effect of the Aloe vera gel against exposure to ultraviolet C
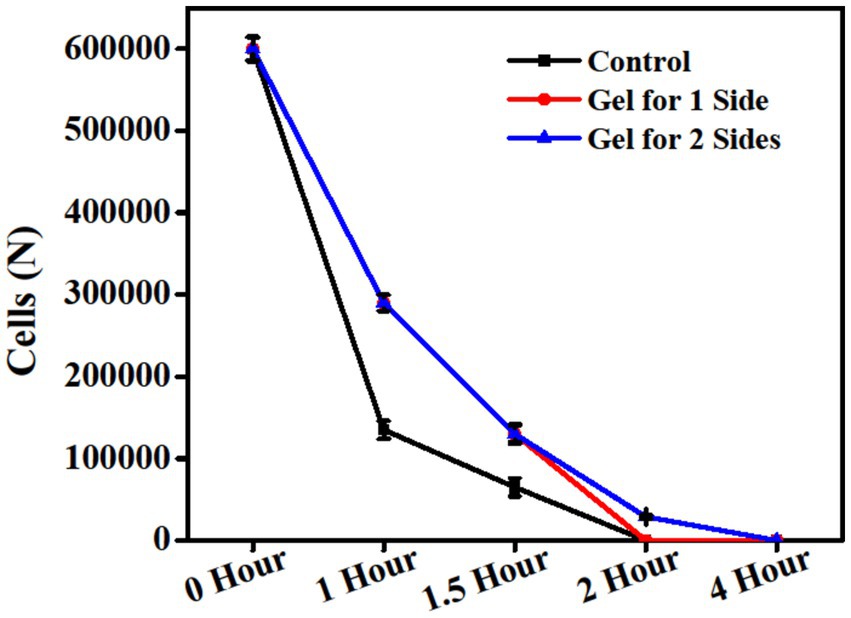
Ultraviolet radiation can be absorbed by nucleic acid, proteins, and cellular membranes and lead to the destruction of chemical bonds and the formation of free radicals (Lekmine et al., 2022). Continuous exposure at a higher rate or for a longer period has negative effects on oxidative free radical-driven reactions and can lead to cell death (Ntourtoglou et al., 2022). Figure 3 presents the differences in the cell counts of the HUVECs among the control, cells treated with the gel applied to one side, and cells treated with the gel applied to both sides after exposure to ultraviolet C for 0, 1, 1.5, 2, and 4 h, respectively. The initial cell counts reported 600.000 viable cells after 1 h of exposure to ultraviolet C. For the cells treated with the gel applied to one side and cells with the gel applied on both sides, 290.000 viable cells were reported. After 1.5 h, the cells treated with the gel applied to one side and cells treated with the gel applied on both sides reported 130.000 viable cells, which represented double the count compared to the control. After exposure to ultraviolet C for 2 h, all cells in the control group and the cells treated with the gel applied to one side died, while the cells treated with the gel applied on both sides reported 29.000 viable cells. Following continuous exposure to ultraviolet C for 4 h, all cells in all groups died. Tariq et al. (2019) reported the protective role of Aloe vera against ultraviolet radiation in the range of 300–400 nm. The two-sided application of the gel under UV-C exposure yielded better results than the single-sided application. This might be due to the high amount of antioxidant components and polyphenol content (Tariq et al., 2019). The results of the current study are in agreement with those of Yao et al. (2016), who found that sterols in Aloe vera gel can help prevent skin photo-aging. Another study by Pugh et al. (2001) applied gamma radiation to animals and treated them with Aloe vera, which demonstrated positive effects on wound healing.
Effect of the Aloe vera gel on the microbial population
The microbial population was detected in the Aloe vera gel extracts at concentrations of 0, 20, 40, 50, and 100 μL, showing resistance to all concentrations against the tested bacterial isolates, such as Bacillus subtilis and Pseudomonas aeruginosa, Figure 4. The biological activity was evaluated by measuring the inhibition zone (mm). The lowest inhibition activity was detected against the Gram-positive bacteria Bacillus subtilis (inhibition zone ≥5 mm), with a value of 2.20 mm at a concentration of 20 μL. The highest inhibition activity among the two foodborne bacterial isolates was detected against the Gram-negative bacteria Pseudomonas aeruginosa (inhibition zone ≥10 mm), with a value of 21.29 mm at a concentration of 100 μL. The current results are in agreement with those of Smith-Palmer et al. (1998), who reported that Gram-positive bacteria are more susceptible to inhibition by natural herbal extracts compared to Gram-negative bacteria. Khattak et al. (2020) reported strong inhibitory effects of Aloe vera gel against several isolates, including Pseudomonas aeruginosa. The high presence of phytochemical compounds in Aloe vera gel is the main reason for its healing properties as it reduces bacterial counts, inhibits bacterial efflux, neutralizes toxins, prevents biofilm formation of the bacteria on wounds, and enhances the effectiveness of certain antibiotics (Kumar et al., 2017).
Figure 4. Effect of the Aloe vera gel on the bacterial isolates: Bacillus subtilis and Pseudomonas aeruginosa.
Effect of the Aloe vera gel on the chemical components and mineral contents
Chemical components
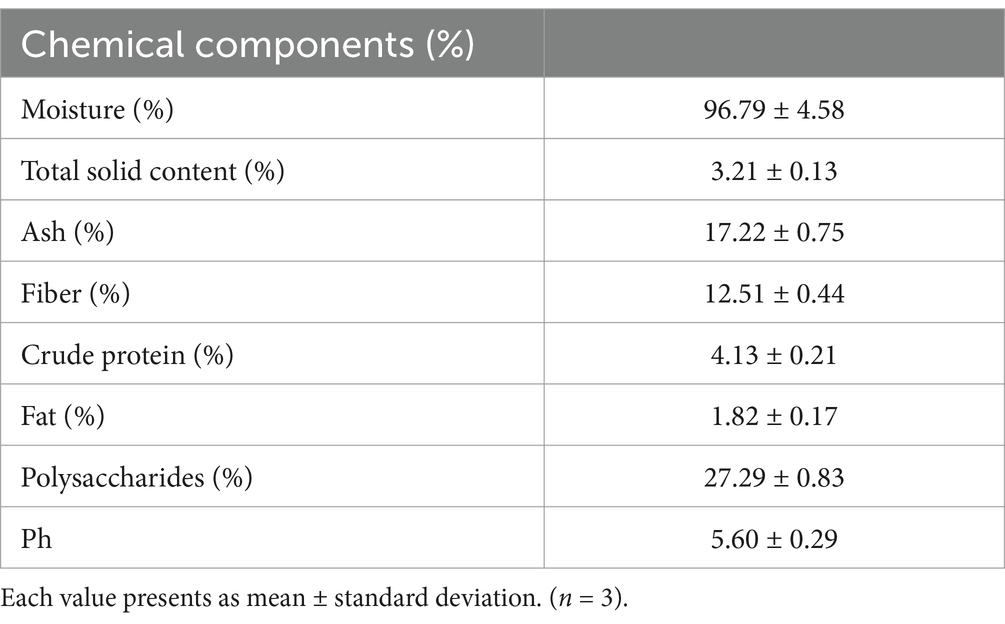
The moisture content was recorded as 96.79%, while the total solid content was recorded as 3.21% after the freeze-drying technique, as shown in Table 1. These results are in agreement with those of Pawar et al. (2023) and Shriwas and Singh (2023) regarding moisture and total solid contents, respectively. The ash content in the current study was 17.22%, indicating that higher ash content is associated with higher mineral content in Aloe vera gel (Femenia et al., 1999). The Aloe vera gel was found rich in fiber (12.51%), with the crude protein content at 4.13%, and the fat content at 1.82%. The results are in agreement with those of Pressman et al. (2019). Polysaccharide content is abundantly found in the leaves (Quezada-Obreque et al., 2017), which was colorimetrically detected using a standard glucose curve and reported as 27.29%. The pH, representing the acidity or alkalinity of an aqueous solution, was reported as 5.6 for the Aloe vera gel sample. The results are in agreement with those of Shinde and Mohite (2024).
Mineral contents
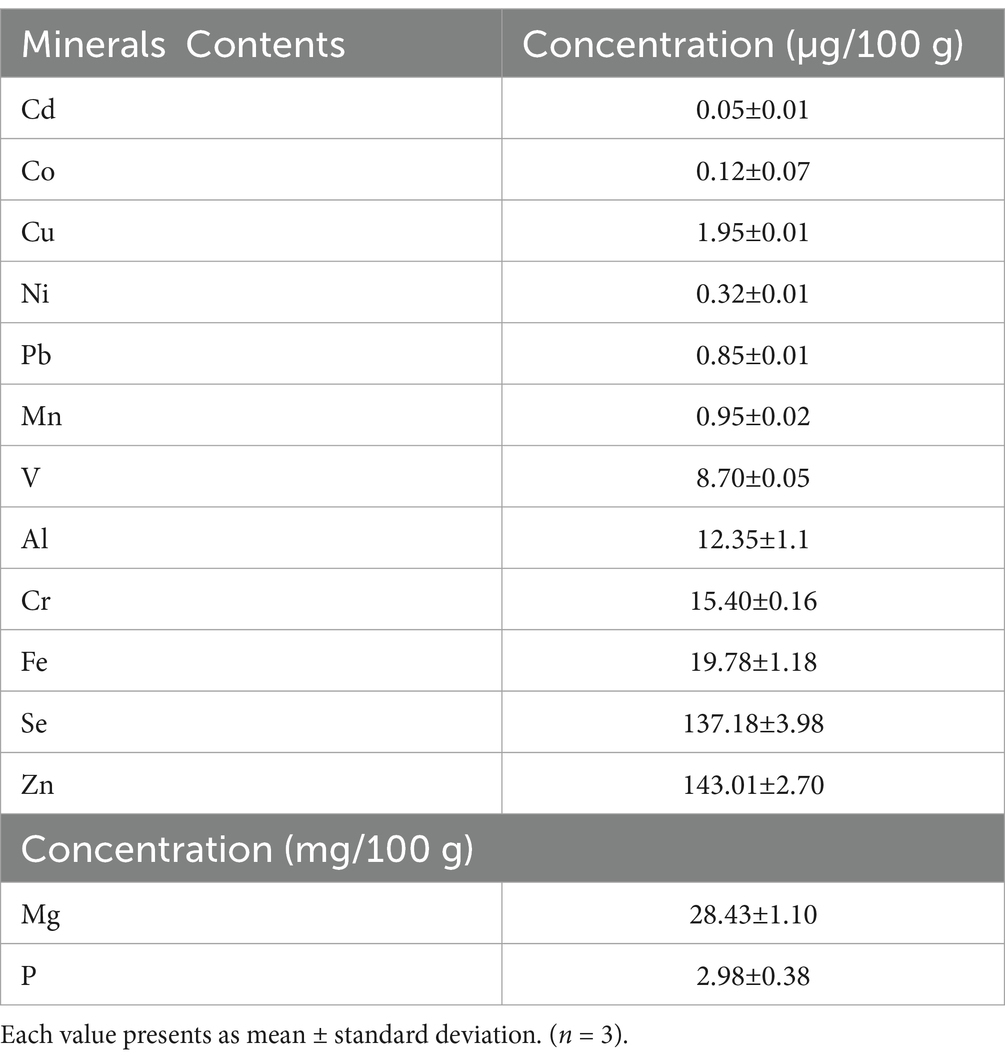
Minerals are considered minor nutrients. In the current study, the presence of 14 elements was detected in the Aloe vera gel samples, as presented in Table 2. Magnesium recorded the highest concentration at 28.43 mg/100 g range, followed by phosphorus at 2.98 mg/100 g. The highest concentrations of micro-minerals detected in the Aloe vera gel samples were zinc (143.01 μg/100 g), selenium (137.18 μg/100 g), iron (19.78 μg/100 g), chromium (15.40 μg/100 g), aluminum (12.35 μg/100 g), and vanadium (8.70 μg/100 g). On the other hand, trace elements such as cadmium, cobalt, and nickel exhibited the lowest values at 0.05, 0.12, and 0.23 μg/100 g, respectively. The results are consistent with those reported by Rajasekaran et al. (2005). Magnesium is essential for healthy bones, fat metabolism, blood pressure regulation, and preventing coronary heart disease. It can help with the electrical conduction of the heart, reduce asthma attacks, release insulin, and improve glucose tolerance in diabetic patients (Sami et al., 2019). Phosphorus is very important for the growth of cells and kidney functions. It plays an essential role in preserving the acid–alkaline balance in our bodies (Sami et al., 2021a). The Aloe vera gel samples also contained trace amounts of zinc, selenium, iron, chromium, aluminum, and vanadium, which may play an important role in various therapeutic aspects, such as immune system support, alleviating nausea, managing diabetes, hemoglobin-oxygen transport, acting as catalytic factors for proteins, facilitating oxidation–reduction reactions, and serving as headache medications (Al-Eisa et al., 2023b). Vanadium may lower glucose levels by mimicking insulin (Wang et al., 2001). Lower levels of nickel can enhance the immunity system and regulate hormonal activities, anemia, osteoporosis, and lipid metabolisms, while copper, detected at 1.95 μg/100 g, is essential for numerous biologically significant enzymes involved in hemoglobin synthesis (Bensaad et al., 2023). Mitra et al. (2023) reported the functional effects of minerals in Aloe vera on enzymes (metalloenzymes) involved in metabolic pathways and their potential antioxidant activities. Mpiana et al. (2020) studied the antiviral activity and highlighted the effective role of zinc against SARS-CoV-1. In addition, the results of the element concentrations in the Aloe vera samples may help determine the appropriate dosage of herbal medications.
Effect of the Aloe vera gel on the phytochemicals, antioxidant activity, and ascorbic acid
The qualitative analysis of the phytochemical components was carried out on the water/ethanolic extract (1:1) of the Aloe vera gel samples in triplicate, and the results are shown in Table 3. The plant was found to contain high quantities of phenols, flavonoids, tannins, saponins, and anthraquinones. The antioxidant activity (DPPH) value, along with the ascorbic acid content, was also detected. These variable compounds may be responsible for their therapeutic and pharmacological uses (Raphael, 2012).
Total phenols
The total phenols in the Aloe vera gel were detected (38.53 mg/g), as shown in Table 3. The results are in agreement with those reported by Jovanović et al. (2017), who used water/ethanol as an extraction medium for total phenols. Several factors can influence the phenolic content, such as aloe species, harvest conditions, climate conditions, plant age, and harvesting methods (Hęś et al., 2019). Ćujić et al. (2016) reported a relationship between polyphenol amounts and an increase in the solid-to-solvent ratio, high temperature, ultrasound-assisted waves extraction assay (>10 W), which results in a higher quantity of polyphenol compounds (2–3 fold increase) and enhanced extraction processes.
Total flavonoids
The total flavonoids in the Aloe vera gel sample were detected (61.02 μg RE/mL), as shown in Table 3. The results are in agreement with those reported by Elbandy et al. (2014), who found that the effect of plant age at harvest can influence total flavonoid quantities, with old plants showing higher values than newly grown ones. Flavonoids are polyphenolic compounds with benzo-γ-pyrone structures and are well-known for their efficient biological and pharmacological activities, particularly against the effects of free radicals (Kumar et al., 2021).
Total tannins
The total tannins in the Aloe vera gel sample were detected (20.12 μg Catechin Equivalent/mL), as shown in Table 3. The results are in agreement with those reported by Kumar et al. (2021), who studied the antioxidant activities of tannins in Aloe vera samples and identified several factors that may influence the detected values of tannins, such as extraction techniques, mediums, geographical origins, and environmental conditions. Tannins are effective for their potential medical applications due to their active roles in antioxidative, antiviral, antitumor, anti-inflammatory, and immunomodulatory activities (Kumari and Jain, 2012).
Total saponins
The average total saponins in the aloe vera gel sample were detected (7.95 mg/g) according to the assay by Parthasarathy et al. (2017). The results are similar to those reported by Mitra et al. (2023), who found 8.34 mg/g. Saponins have potential health benefits for the immune system as they protect the body against cancer and reduce cholesterol, blood lipids, and glucose response levels (Surjushe et al., 2008).
Total anthraquinones
The total anthraquinones in the Aloe vera gel sample were detected (0.39 mg/mL), as shown in Table 3. The results were similar to those reported by Luong et al. (2023), who used ethanol to extract total anthraquinones and salicylic acids, reporting 0.44 and 3.93 mg/mL, respectively. Anthraquinones are essential for health as they act as strong purgatives, promote intestinal peristalsis, invigorate bodily fluid discharge, and provide protection against different diseases such as herpes simplex, cancer, varicella zoster virus, and influenza (Mulu et al., 2015).
Radical scavenging activity
The antioxidant activity, measured as radical scavenging activity, presented an IC50 value of 79.11 μg/mL, as shown in Table 3. Our DPPH radical result is in agreement with that reported by Lotfizadeh et al. (2023). The antioxidant activity is associated with the ethyl acetate fraction and the phenolic nature of the Aloe vera plant (Asamenew et al., 2011). DPPH radical results can vary due to differences in solubility, reactions, and analytical conditions (Li et al., 2021).
Ascorbic acid
Humans need vitamins in small quantities, with few exceptions, as they cannot synthesize most vitamins (Sami et al., 2014). The ascorbic acid content of the aloe vera gel sample was detected (0.005%), as shown in Table 3. Mitra et al. (2023) reported that Aloe vera contains a variety of vitamins, including A, C, E, B1, B2, B6, B9, and B12.
Conclusion
According to the findings, it was demonstrated that Aloe vera gel has significant anti-influenza activity and is effective in protecting cells against ultraviolet-C damage. Aloe vera gel appears to have the potential to be used as a topical UV-C protection solution in cosmetic manufacturing. The synthesized herbal compound, Aloe vera gel, exhibited an inhibitory effect against the Gram-negative bacteria, according to the microbiological examinations. The current research provides a comprehensive summary of the proximate chemical composition and some biochemical activities of Aloe vera gel. A phytochemical profile was identified, which could assist the scientific community in obtaining optimal study results, saving time and costs, and setting up tests under ideal settings. This information can be useful for therapeutic applications and pharmaceutical interest. This investigation should be supported by further scientific studies on the active compounds highlighted in this area of research.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RA-E: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RB: Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. RK: Conceptualization, Formal analysis, Supervision, Visualization, Writing – original draft, Writing – review & editing. SA: Formal analysis, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. DJ: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. AA: Conceptualization, Data curation, Resources, Validation, Writing – original draft, Writing – review & editing. RA: Conceptualization, Data curation, Project administration, Resources, Writing – original draft, Writing – review & editing. AA-Z: Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. SA-D: Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Taif University, Saudi Arabia (Project no. TU-DSPP-2024-10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Eisa, R. A., Ashour, A. A., Helal, M., Aljahani, A. H., Kadi, R. H., Hazzazi, M. S., et al. (2023a). Anticancer effects of honey varieties on human cells by studying some physical parameters, hydrogen peroxide content, catalase, glucose oxidase, and microbial activities. J. Biobaased Mater. Bioenergy 17, 160–166. doi: 10.1166/jbmb.2023.2272
Al-Eisa, R. A., Helal, M., Aljahani, A. H., Sami, R., Banjer, H. J., Algehainy, N. A., et al. (2023b). Ochratoxin A oral mycotoxin and honey dietary intake effects on TNF-α immunology response, lactic acid bacteria microbial louds, β-glucuronidase enzyme activity, some hematological and biochemical parameters on mice. Mater. Express 13, 1203–1211. doi: 10.1166/mex.2023.2462
AOAC, (1990). Official analytical chemists. 15th Edn. Washington, DC: Association of Official Analytical Chemist.
Arbab, S., Ullah, H., Weiwei, W., Wei, X., Ahmad, S. U., Wu, L., et al. (2021). Comparative study of antimicrobial action of aloe vera and antibiotics against different bacterial isolates from skin infection. Vet. Med. Sci. 7, 2061–2067. doi: 10.1002/vms3.488
Asamenew, G., Bisrat, D., Mazumder, A., and Asres, K. (2011). In vitro antimicrobial and antioxidant activities of anthrone and chromone from the latex of Aloe harlana Reynolds. Phytother. Res. 25, 1756–1760. doi: 10.1002/ptr.3482
Asnaashari, S., Delazar, A., Safarzadeh, E., Tabibi, H., Mollaei, S., Rajabi, A., et al. (2019). Phytochemical analysis and various biological activities of the aerial parts of Scrophularia atropatana growing in Iran. Iranian J. Pharmaceutical Res. 18, 1543–1555. doi: 10.22037/ijpr.2019.1100782
Bensaad, M. S., Kahoul, M. A., Khier, M., Mitra, D., Benhoula, M., Banjer, H. J., et al. (2023). An insight-based computational approaches to estimate molecular weight distribution, Allergenicity and immunological aspects, toxicity profile, possible biodegradation, persistence and bioaccumulation factor of four Phyto-compounds. J. Biobaased Mater. Bioenergy 17, 419–432. doi: 10.1166/jbmb.2023.2291
Boumoud, B., Yahiaoui, A. A., Boumoud, T., and Debache, A. (2012). Synthesis and DFT calculations of linear and nonlinear optical responses of novel 2-thioxo-3-N,(4-methylphenyl) thiazolidine-4 one. J. Chem. Pharm. Res. 4, 795–799. doi: 10.1080/17415993.2020.1736073
Cisternas, P., Silva-Alvarez, C., Martínez, F., Fernandez, E., Ferrada, L., Oyarce, K., et al. (2014). The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism. J. Neurochem. 129, 663–671. doi: 10.1111/jnc.12663
Ćujić, N., Šavikin, K., Janković, T., Pljevljakušić, D., Zdunić, G., and Ibrić, S. (2016). Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 194, 135–142. doi: 10.1016/j.foodchem.2015.08.008
Diaz-Munoz, G., Miranda, I. L., Sartori, S. K., De Rezende, D. C., and Diaz, M. A. N. (2018). Anthraquinones: an overview. Stud. Nat. Prod. Chem. 58, 313–338. doi: 10.1016/B978-0-444-64056-7.00011-8
Elbandy, MA, Abed, SM, SSA, Gad, and Abdel-Fadeel, MG. (2014). Aloe vera gel as a functional ingredient and natural preservative in mango nectar. World Journal of Dairy & Food Sciences 9, 191–203. doi: 10.5829/idosi.wjdfs.2014.9.2.1139
Femenia, A., Sánchez, E. S., Simal, S., and Rosselló, C. (1999). Compositional features of polysaccharides from Aloe vera (Aloe barbadensis miller) plant tissues. Carbohydr. Polym. 39, 109–117. doi: 10.1016/S0144-8617(98)00163-5
Gansukh, E., Kazibwe, Z., Pandurangan, M., Judy, G., and Kim, D. H. (2016). Probing the impact of quercetin-7-O-glucoside on influenza virus replication influence. Phytomedicine 23, 958–967. doi: 10.1016/j.phymed.2016.06.001
Gokulan, K., Kolluru, P., Cerniglia, C. E., and Khare, S. (2019). Dose-dependent effects of Aloin on the intestinal bacterial community structure, short chain fatty acids metabolism and intestinal epithelial cell permeability. Front. Microbiol. 10:417233. doi: 10.3389/fmicb.2019.00474
Hęś, M., Dziedzic, K., Górecka, D., Jędrusek-Golińska, A., and Gujska, E. (2019). Aloe vera (L.) Webb.: natural sources of antioxidants–a review. Plant Foods Hum. Nutr. 74, 255–265. doi: 10.1007/s11130-019-00747-5
Jovanović, A. A., Đorđević, V. B., Zdunić, G. M., Pljevljakušić, D. S., Šavikin, K. P., Gođevac, D. M., et al. (2017). Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat-and ultrasound-assisted techniques. Sep. Purif. Technol. 179, 369–380. doi: 10.1016/j.seppur.2017.01.055
Khattak, A. K., Syeda, M. H., and Shahzad, S. M. (2020). General overview of phytochemistry and pharmacological potential of Rheum palmatum (Chinese rhubarb). Innovare J. Ayurvedic Sci. 8, 5–9. doi: 10.22159/ijas.2020.v8i6.39192
Kostyuk, V., Potapovich, A., Suhan, T., De Luca, C., Pressi, G., Dal Toso, R., et al. (2008). Plant polyphenols against UV-C-induced cellular death. Planta Med. 74, 509–514. doi: 10.1055/s-2008-1074499
Kumar, S., Jakhar, D. S., and Singh, R. (2017). Evaluating antimicrobial activity of Aloe vera plant extract in human life. Biomed. J. Sci. Tech Res. 1, 1854–1856. doi: 10.26717/BJSTR.2017.01.000565
Kumar, N., Pratibha, T. P., Khojah, E., Sami, R., and AAM, A.-M. (2021). Chitosan edible films enhanced with pomegranate peel extract: study on physical, biological, thermal, and barrier properties. Materials 14. doi: 10.3390/ma14123305
Kumari, M., and Jain, S. (2012). Tannins: an antinutrient with positive effect to manage diabetes. Res. J. Recent Sci. 2277:2502.
Lekmine, S., Bendjedid, S., Benslama, O., Martín-García, A. I., Boussekine, S., Kadi, K., et al. (2022). Ultrasound-assisted extraction, LC–MS/MS analysis, anticholinesterase, and antioxidant activities of valuable natural metabolites from Astragalus armatus Willd.: in silico molecular docking and in vitro enzymatic studies. Antioxidants 11, 1–15. doi: 10.3390/antiox11102000
Li, Y., Rokayya, S., Jia, F., Nie, X., Xu, J., Elhakem, A., et al. (2021). Shelf-life, quality, safety evaluations of blueberry fruits coated with chitosan nano-material films. Sci. Rep. 11:55. doi: 10.1038/s41598-020-80056-z
Lotfizadeh, V., Mollaei, S., and Hazrati, S. (2023). Biological activities of Aloin-rich extracts obtained from Aloe vera (L.) Burm.f. J. Med. Plants 12, 275–281. doi: 10.22092/JMPB.2021.355897.1395
Luong, T. M. V., Nguyen, T. P. T., Nguyen, L. N. T., Tran, T. T. T., Nguyen, N. T. P., and Mai, C. H. (2023). Extraction of anthraquinone and salicylic acid from Aloe barbadensis miller. Surakarta, Indonesia: IOP Publishing.
Majumder, R., Das, C. K., and Mandal, M. (2019). Lead bioactive compounds of Aloe vera as potential anticancer agent. Pharmacol. Res. 148:104416. doi: 10.1016/j.phrs.2019.104416
Mitra, A., Singh, M., Banga, A., Pandey, J., Tripathi, S. S., and Singh, D. (2023). Bioactive compounds and therapeutic properties of Aloe vera-a review. Plant Science Today. 10, 1–7. doi: 10.14719/pst.1715
Mollaei, S., Hazrati, S., Lotfizadeh, V., Dastan, D., and Asgharian, P. (2020). Phytochemical variation and biological activities of Zosima absinthifolia during various stages of growth. Int. J. Food Prop. 23, 1556–1567. doi: 10.1080/10942912.2020.1818778
Molole, G. J., Gure, A., and Abdissa, N. (2022). Determination of total phenolic content and antioxidant activity of Commiphora mollis (Oliv.). Engl. Resin. BMC Chem. 16, 10–11. doi: 10.1186/s13065-022-00841-x
Mpiana, P. T., Ngbolua, K. N., Tshibangu, D. T., Kilembe, J. T., Gbolo, B. Z., Mwanangombo, D. T., et al. (2020). Aloe vera (L.) Burm. F as a potential anti-Covid-19 plant: a mini-review of its antiviral activity. European. J. Medicinal Plants 31, 86–93. doi: 10.9734/ejmp/2020/v31i830261
Mulu, T., Teshale, F., Gemeda, S., and Sahu, O. (2015). Medicated evaluation of Aloe vera: overview on characteristics and application. World J. Nutr. Health. 3, 1–7. doi: 10.12691/jnh-3-1-1
Nicolau-Lapeña, I., Colàs-Medà, P., Alegre, I., Aguiló-Aguayo, I., Muranyi, P., and Viñas, I. (2021). Aloe vera gel: an update on its use as a functional edible coating to preserve fruits and vegetables. Prog. Org. Coat. 151:106007. doi: 10.1016/j.porgcoat.2020.106007
Ntourtoglou, G., Drosou, F., Chatzimitakos, T., Athanasiadis, V., Bozinou, E., Dourtoglou, V. G., et al. (2022). Combination of pulsed electric field and ultrasound in the extraction of polyphenols and volatile compounds from grape stems. Appl. Sci. 12:6219. doi: 10.3390/app12126219
Parthasarathy, G., Saroja, M., and Venkatachalam, M. (2017). Bio-synthesized nano-formulation of zinc oxide-Aloe vera and to study their characterization and antibacterial activities against multiple pathogens. Int. J. Pharm. Sci. Res. 8:900. doi: 10.13040/IJPSR.0975-8232.8(2).900-07
Pawar, H. M., Shirke, G. D., and Kadam, J. H. (2023). Preparation of Aloe vera powder by different drying methods. Indian J. Ecol. 50, 1333–1337. doi: 10.55362/IJE/2023/4056
Pressman, P., Clemens, R., and Hayes, A. W. (2019). Aloe vera at the frontier of glycobiology and integrative medicine: health implications of an ancient plant. SAGE Open Med. 7:2050312119875921. doi: 10.1177/2050312119875921
Pugh, N., Ross, S. A., ElSohly, M. A., and Pasco, D. S. (2001). Characterization of Aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J. Agric. Food Chem. 49, 1030–1034. doi: 10.1021/jf001036d
Qiao, G. H., Wenxin, D., Zhigang, X., Sami, R., Khojah, E., and Amanullah, S. (2020). Antioxidant and anti-inflammatory capacities of pepper tissues. Italian. J. Food Sci. 32, 1–10. doi: 10.14674/IJFS-1700
Quezada-Obreque, M. P., Salinas, C., Gotteland, M., and Cardemil-Oliva, L. A. (2017). Acemannan and Fructans from Aloe vera (Aloe barbadensis miller) plants as novel prebiotics. J. Agric. Food Chem. 65, 10029–10039. doi: 10.1021/acs.jafc.7b04100
Rajasekaran, S., Sivagnanam, K., and Subramanian, S. (2005). Mineral contents of Aloe vera leaf gel and their role on streptozotocin-induced diabetic rats. Biol. Trace Elem. Res. 108, 185–196. doi: 10.1385/BTER:108:1-3:185
Ramdhini, R. N., and Anggraini, C. S. (2024). Formulation of Aloe Vera (Aloe Vera L.) extract gel as A skin moisturizer with variations of Carbopol 934. Indonesian J. Cosmetics 2, 47–54. doi: 10.4103/0973-1296.117849
Raphael, E. (2012). Phytochemical constituents of some leaves extract of Aloe vera and Azadirachta indica plant species. Global Adv. Res. J. Environment. Sci. Toxicol. 1, 014–017.
Reynolds, T., and Dweck, A. C. (1999). Aloe vera leaf gel: a review update. J. Ethnopharmacol. 68, 3–37. doi: 10.1016/S0378-8741(99)00085-9
Rezazadeh, F., Moshaverinia, M., Motamedifar, M., and Alyaseri, M. (2016). Assessment of anti HSV-1 activity of Aloe vera gel extract: an in vitro study. J. Dent. 17, 49–54
Rokayya, S., Li, C.-J., Zhao, Y., Li, Y., and Sun, C.-H. (2013). Cabbage (Brassica oleracea L. var. capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac. J. Cancer Prev. 14, 6657–6662. doi: 10.7314/APJCP.2013.14.11.6657
Saleem, A., Naureen, I., Naeem, M., Murad, H. S., Maqsood, S., and Tasleem, G. (2022). Aloe vera gel effect on skin and pharmacological properties. Scholars Int. J. Anatomy Physiol. 5, 1–8. doi: 10.36348/sijap.2022.v05i01.001
Sami, R., Alshehry, G., Elgarni, E., and Helal, M. (2021a). Saudi community care awareness food facts, nutrients, immune system and COVID-19 prevention in taif city among different age categories. Afr. J. Food Agric. Nutr. Dev. 21, 17213–17233. doi: 10.18697/ajfand.96.20440
Sami, R., Alshehry, G., Ma, Y., Abdelazez, A., and Benajiba, N. (2019). Evaluation of some specific components existences in okra (Abelmoschus esculentus L.(moench)) cultivated from different areas. J. Food Nutr. Res. 7, 155–161. doi: 10.12691/jfnr-7-2-8
Sami, R., Elhakem, A., Alharbi, M., Almatrafi, M., Benajiba, N., Ahmed Mohamed, T., et al. (2021b). In-vitro evaluation of the antioxidant and anti-inflammatory activity of volatile compounds and minerals in five different onion varieties. Separations 8:57. doi: 10.3390/separations8050057
Sami, R., Khojah, E., Alharbi, M., Am Al-Mushhin, A., Saeed Alkaltham, M., Mohammad Salamatullah, A., et al. (2021c). Functional effects of pomegranate peel extracts on milk: antibacterial measurements, antioxidant activities, and photochemical characterizations. J. Biobaased Mater. Bioenergy 15, 571–579. doi: 10.1166/jbmb.2021.2097
Sami, R., Khojah, E., Mansour, A. M. A., Al-Mushhin, A. A. M., Elhakem, A., El-Sherif, D. M., et al. (2021d). Nutritional values, microbial population and bioactive components of pomegranate (Punica granatum L.) peel extracts. Int. J. Pharmacol. 17, 208–216. doi: 10.3923/ijp.2021.208.216
Sami, R., Li, Y., Qi, B., Wang, S., Zhang, Q., Han, F., et al. (2014). HPLC analysis of water-soluble vitamins (B2, B3, B6, B12, and C) and fat-soluble vitamins (E, K, D, A, and β-carotene) of okra (Abelmoschus esculentus). J. Chem. 2014:831357, 1–6. doi: 10.1155/2014/831357
Sami, R., Soltane, S., and Helal, M. (2021e). Microscopic image segmentation and morphological characterization of novel chitosan/silica nanoparticle/nisin films using antimicrobial technique for blueberry preservation. Membranes 11:303. doi: 10.3390/membranes11050303
Sánchez, M., González-Burgos, E., Iglesias, I., and Gómez-Serranillos, M. P. (2020). Pharmacological update properties of Aloe vera and its major active constituents. Molecules 25:1324. doi: 10.3390/molecules25061324
Shinde, S., and Mohite, S. (2024). Exploring Antipsoriatic potential of Aloe vera gel. Int. J. Drug Delivery Technol. 14, 675–680. doi: 10.25258/ijddt.14.2.10
Shriwas, H. K., and Singh, S. P. (2023). Analytical study of different samples of Aloe Vera juice. J. Ayurveda Integrated Med. Sci. 8, 37–45. doi: 10.21760/jaims.8.4.6
Smith-Palmer, A., Stewart, J., and Fyfe, L. (1998). Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 26, 118–122. doi: 10.1046/j.1472-765X.1998.00303.x
Sun, R., Zhai, R., Ma, C., and Miao, W. (2020). Combination of aloin and metformin enhances the antitumor effect by inhibiting the growth and invasion and inducing apoptosis and autophagy in hepatocellular carcinoma through PI3K/AKT/mTOR pathway. Cancer Med. 9, 1141–1151. doi: 10.1002/cam4.2723
Surjushe, A., Vasani, R., and Saple, D. G. (2008). Aloe vera: a short review. Indian J. Dermatol. 53, 163–166. doi: 10.4103/0019-5154.44785
Sydiskis, R. J., Owen, D. G., Lohr, J. L., Rosler, K. H., and Blomster, R. N. (1991). Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob. Agents Chemother. 35, 2463–2466. doi: 10.1128/AAC.35.12.2463
Tariq, H., Zia, M., Muhammad, S. A., Khan, S. A., Fatima, N., Mannan, A., et al. (2019). Antioxidant, antimicrobial, cytotoxic, and protein kinase inhibition potential in Aloe vera L. Biomed. Res. Int. 2019:6478187. doi: 10.1155/2019/6478187
Wang, J., Yuen, V. G., and McNeill, J. H. (2001). Effect of vanadium on insulin sensitivity and appetite. Metab. Clin. Exp. 50, 667–673. doi: 10.1053/meta.2001.23294
Yao, R., Tanaka, M., Misawa, E., Saito, M., Nabeshima, K., Yamauchi, K., et al. (2016). Daily ingestion of aloe vera gel powder containing aloe sterols prevents skin photoaging in OVX hairless mice. J. Food Sci. 81, H2849–H2857. doi: 10.1111/1750-3841.13527
Keywords: Aloe vera gel, anti-influenza, ultraviolet-C, microbial activities, nutrients, phytochemical analysis
Citation: Al-Eisa RA, Sami R, Bedaiwi RI, Kadi RH, Abushal SA, Johari DM, Alnajeebi AM, Algheshairy RM, Abu-Zaid AA and Al-Dhumri SA (2025) Anti-influenza effects of Aloe vera gel on MDCK cells: protective efficacy against ultraviolet-C exposure and analysis of biochemical characteristics and microbial activities. Front. Sustain. Food Syst. 8:1508809. doi: 10.3389/fsufs.2024.1508809
Edited by:
Raul Avila-Sosa, Benemérita Universidad Autónoma de Puebla, MexicoReviewed by:
Muthukumar Serva Peddha, Central Food Technological Research Institute (CSIR), IndiaJosé Eduardo Lucero-Mejía, Benemérita Universidad Autónoma de Puebla, Mexico
Copyright © 2025 Al-Eisa, Sami, Bedaiwi, Kadi, Abushal, Johari, Alnajeebi, Algheshairy, Abu-Zaid and Al-Dhumri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rokayya Sami, cm9rYXl5YS5kQHR1LmVkdS5zYQ==
 Rasha A. Al-Eisa1
Rasha A. Al-Eisa1 Rokayya Sami
Rokayya Sami Afnan M. Alnajeebi
Afnan M. Alnajeebi