- 1Department of Food Science, Foshan Polytechnic, Foshan, China
- 2School of Food Science, Guangdong Pharmaceutical University, Zhongshan, China
- 3School of Health Sciences Research, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand
- 4Department of Histology and Embryology, Guangdong Medical University, Zhanjiang, China
- 5Guangzhou Vocational College of Technology and Business, Guangzhou, China
- 6Guangdong Provincial Key Laboratory of Food Quality and Safety, College of Food Science, South China Agricultural University, Guangzhou, China
- 7Tropical Medicine Institute and South China Chinese Medicine Collaborative Innovation Center, Guangzhou University of Chinese Medicine, Guangzhou, China
Malachite green (MG), a triphenylmethane dye, is used in the aquaculture industry as a disinfectant and insect repellent due to its potent bactericidal and pesticidal properties. However, its use poses potential environmental and health risks. This study analyzed and designed two haptens using computer simulation. Serum data confirmed the feasibility of introducing an arm at the dimethylamine group. Subsequently, a highly selective monoclonal antibody strain was successfully prepared based on the hapten. After optimizing the working conditions of indirect competitive enzyme-linked immunosorbent assay (IC-ELISA), the IC50 value was 0.83 ng/mL, with a detection limit (IC10) of 0.08 ng/mL and a linear range of 0.19–3.52 ng/mL. The developed monoclonal antibody exhibited a crossover rate of less than 0.1% with other similar structures and can be used to establish an immunoassay.
1 Introduction
Malachite green (MG) is a toxic triphenylmethane compound known for its strong bactericidal, fungicidal, and antiparasitic properties, making it one of the most effective drugs used in aquaculture (Hu et al., 2021; Wang et al., 2023). In fish, MG metabolizes into the more toxic leucomalachite green (LMG), which can persist in the organism for extended periods (Teepoo et al., 2020). Upon human ingestion, MG can induce apoptosis, trigger tumor formation, and damage DNA, leading to carcinogenesis (He et al., 2023). This has raised significant concerns globally. Health Canada has prohibited the sale of fish products containing MG or LMG residues exceeding 1 ng/g, a threshold stricter than the European Union’s 2 ng/g standard. Many countries and regions, including China and the United States, have imposed strict bans on its use in aquaculture (Zhou et al., 2019). Despite these regulations, some vendors continue to use MG illegally due to its low cost, effective antimicrobial properties, and the lack of suitable alternatives.
Current detection methods for MG rely primarily on large-scale instrumentation techniques such as liquid chromatography (Faraji et al., 2020), liquid chromatography-mass spectrometry (Chen et al., 2020; Hussain Hakami et al., 2021), ionization mass spectrometry (Xiao et al., 2020), capillary electrophoresis (Pradel and Tong, 2017), and Raman spectroscopy (Su et al., 2024; Zhang et al., 2024). Although these methods can accurately detect trace amounts of MG in aquatic products, they are time-consuming, costly, require specialized personnel, and are unsuitable for rapid on-site screening of large sample volumes. Therefore, there is a growing need for a rapid and highly specific detection method for monitoring MG residue levels. Immunoassay methods, as a novel analytical approach, offer advantages such as low cost, speed, simplicity (Wu et al., 2024), high sensitivity (Wang et al., 2024), and accuracy (Shin et al., 2024), potentially overcoming the limitations of traditional instrumental methods (Abdelhamid et al., 2024; Sayee et al., 2024; Yue et al., 2024). The effectiveness of immunoassays largely depends on obtaining antibodies with high specificity and affinity, which is critically influenced by the structure of the hapten.
Developing specific antibodies for MG has been a significant challenge. Xing et al. (2009) synthesized LMG derivatives with amino groups on the benzene ring as immunogenic haptens, while Oplatowska et al. (2011) used carboxyl-MG as the immunogenic hapten. Both methods successfully produced monoclonal antibodies (mAbs) but exhibited high cross-reactivity with crystal violet (CV). Shen et al. (2011) designed a novel immunogenic hapten with a carboxyl methoxy group directly on the phenyl ring; however, the resulting mAb also showed high cross-reactivity with CV. To address this issue, this study employed computer simulations of existing hapten research to identify key antigenic epitopes of malachite green. A novel hapten strategy was developed, leading to the successful production of highly selective and sensitive mAbs. Optimized using the indirect competitive enzyme-linked immunosorbent assay (IC-ELISA), the detection limit achieved was 0.08 ng/mL, facilitating the establishment of a specific immunoassay method for malachite green.
2 Materials and methods
2.1 Materials and instruments
Methyl aniline, N,N-dimethyl aniline, zinc chloride, 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC), N,N-dimethylformamide (DMF), and N-hydroxysuccinimide (NHS) were obtained from Aladdin Reagent Company. All other reagents used in the study met or exceeded analytical reagent grade standards. Female Balb/c mice were provided by the Guangdong Medical Laboratory Animal Center (SCXK (Yue) 2018-0002) and were subsequently housed and maintained at the South China Agricultural University Animal Center (SYXK (Yue) 2019-0136).
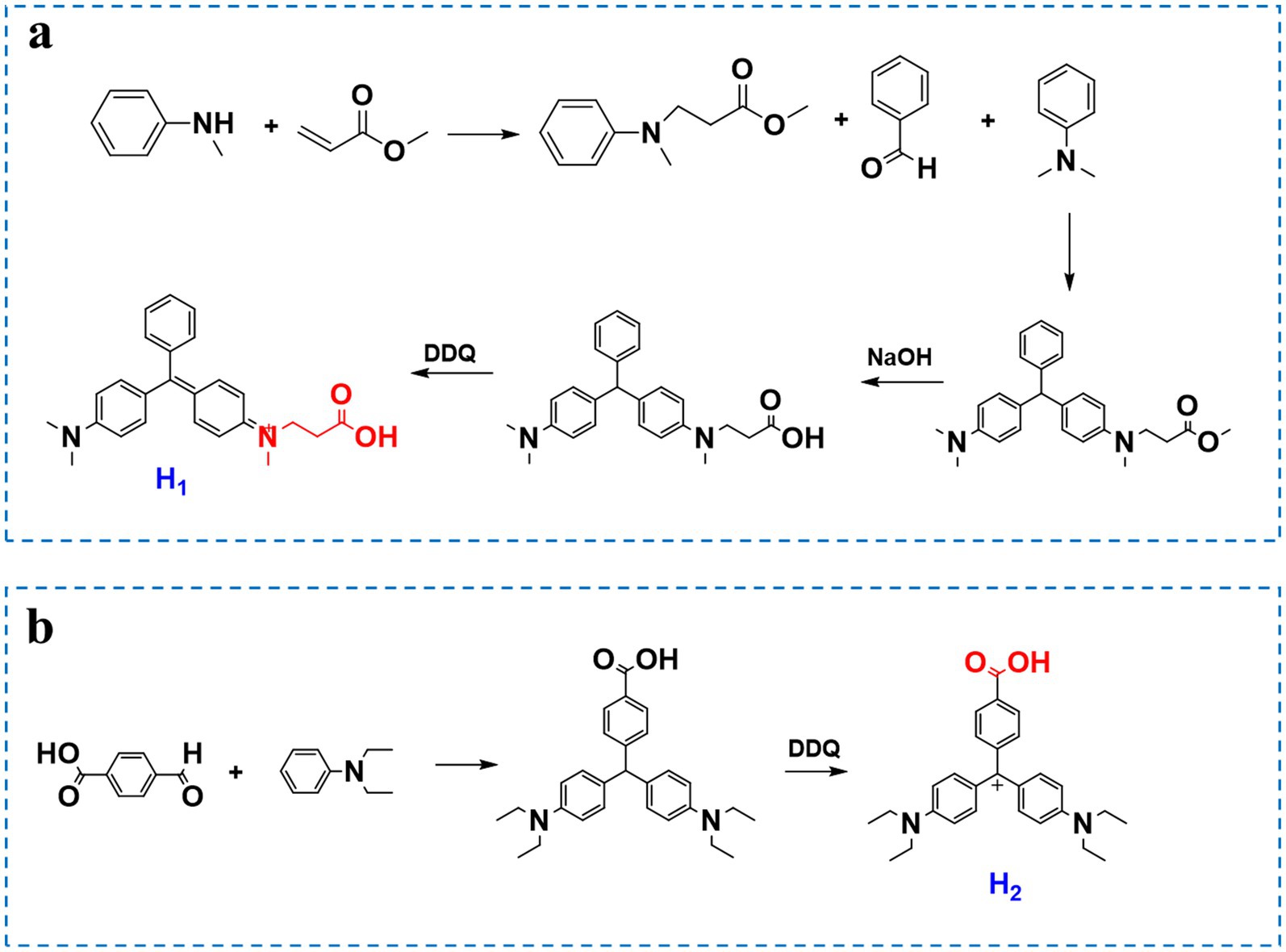
2.2 Synthesis of the MG hapten H1
As shown in Figure 1, Step 1: Add 7 g (6.5 mmol) of N-methylaniline to a 250 mL single-necked flask. Dissolve it completely in 100 mL of anhydrous methanol. Then, add 17 g (19.7 mmol) of methyl acrylate and 3 mL of triethylamine. React in an 85°C oil bath overnight to obtain compound 1 (8.8 g, 70%).
Step 2: Add 100 mL of anhydrous ethanol to dissolve 8.8 g (4.5 mmol) of compound 1 in a 250 mL single-necked flask. Then add 4.6 mL (4.5 mmol) of benzaldehyde, 5.7 mL (4.5 mmol) of N,N-dimethylaniline, and 20 g (13.5 mmol) of zinc chloride. React at 100°C in an oil bath overnight. Concentrate the solution, adjust the pH to 8 with 10% aqueous sodium hydroxide, add an appropriate amount of ethyl acetate, and then pump-filter to obtain compound 2 (2 g, 10%).
Step 3: Dissolve 2 g (4.96 mmol) of compound 2 in 10 mL of methanol in a 100 mL single-necked flask. Add 10 mL of 10% aqueous sodium hydroxide solution and react at 70°C in an oil bath overnight. Concentrate the solution, adjust the pH to 8 with dilute hydrochloric acid, extract with an appropriate amount of ethyl acetate, and purify the residue by column chromatography (PE:EA = 15:1; 10:1; 1:1; EA) to obtain compound 3.
Step 4: Dissolve compound 3 in 20 mL of acetonitrile in a 100 mL single-necked vial. Slowly add 10 mL of an acetonitrile solution with DDQ while stirring. The color of the reaction solution will immediately darken. Stir the reaction for 0.5 h. Perform column chromatography purification to obtain a small amount of the target product MG-H1.
2.3 Synthesis of the MG hapten H2
As shown in Figure 2, Dissolve 1.5 g (10 mmol) of p-carboxybenzaldehyde in 60 mL of anhydrous ethanol in a 250 mL flask. Add 4 g (3 mmol) of zinc chloride and 4 mL (3.15 mmol) of N,N-diethylaniline, and reflux overnight. Prepare a 3:2 mixture of acetonitrile and methanol, then add DDQ to completely dissolve the compound. Weigh compound 4 into a 100 mL single-necked vial, add the mixture, evaporate the solvent, wash off the DDQ with ether, and purify the residue by column chromatography to obtain the green product MG-H2.
2.4 Computer simulation of 3D structures and electrostatic potential maps
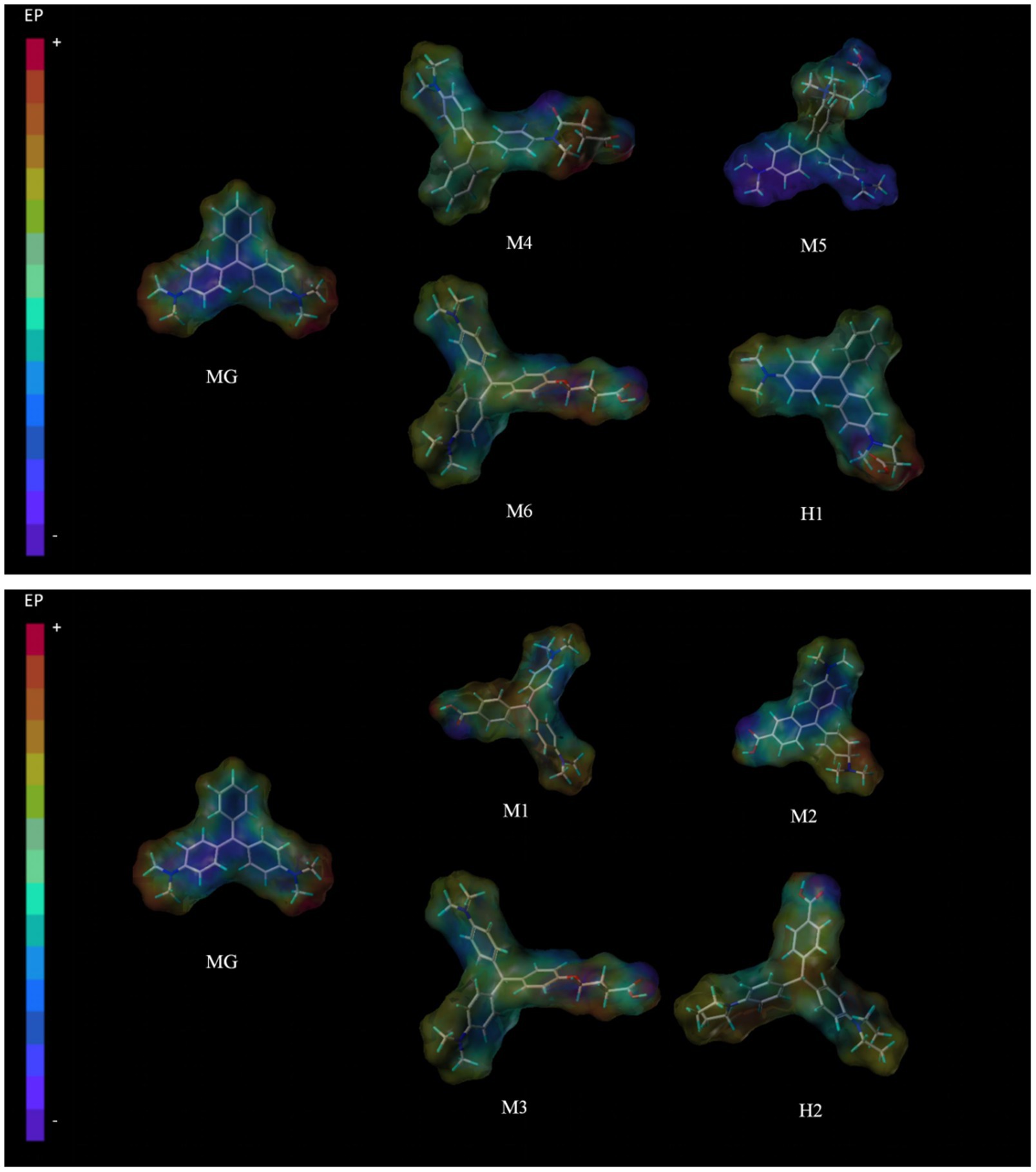
The three-dimensional (3D) energy-minimized structure of the MG hapten was constructed using Sybyl 8.1 (provided by Professor Xin’an Huang from Guangzhou University of Chinese Medicine). Initially, a sketch was created and then geometrically optimized using the standard Tripos force field, incorporating non-bonded interactions and Gasteiger-Hückel charges with an 8 Å cutoff, a termination gradient of 0.005 kcal/(molÅ), and dielectric constants based on previous research. The surface electrostatic potential of the obtained structure was generated using the MOLCAD surface program in Sybyl 8.1 with Gasteiger-Hückel charges.
2.5 Synthesis of immunogens and coating antigens
Immunogens and coating antigens were synthesized using previously established methods. Immunogens were prepared by conjugating haptens with carrier proteins, such as lactoglobulin (LF). Coating antigens were prepared by conjugating all haptens with bovine serum albumin (BSA). The structure of the final conjugates was verified using ultraviolet–visible (UV–vis) spectral data.
2.6 Hybridoma cell line preparation and screening
Female BALB/c mice aged 6–8 weeks were subcutaneously immunized with the immunogen, with four immunizations at 3-week intervals. A mouse exhibiting the highest titer against each immunogen was selected as the spleen cell donor for hybridoma production. Spleen cells from the immunized mouse were fused with SP2/0 myeloma cells, which had been revived 10 days prior to fusion, using 50% PEG 1500 as the fusion agent. The hybridoma cells were distributed in a 96-well cell culture plate and cultured in a medium containing 80% Dulbecco’s Modified Eagle’s Medium (DMEM), 20% Fetal Bovine Serum (FBS), and Hypoxanthine-Aminopterin-Thymidine (HAT). After 2 weeks, the medium was replaced with Hypoxanthine-Thymidine (HT). Cells were incubated in a cell culture incubator (37°C, 5% CO2). After 7 days, antibody titer and binding characteristics in the culture supernatant were tested using the same method as for serum. Cells with high affinity and specificity were subcloned using limited dilution until a stable monoclonal cell line secreting antibodies was obtained.
2.7 Production of mAbs
Animal experiments strictly adhere to the guidelines of Chinese laws and regulations, the studies involving animals were reviewed and approved by the Animal Ethics Committee of South China Agricultural University. The animal immunization and hybridoma preparation process was carried out according to the previously established protocol. Male BALB/c mice aged 10–12 weeks were injected with liquid paraffin. After 1 week, hybridoma cells were injected into the mice. Two weeks later, ascitic fluid was collected from mice with noticeably enlarged abdomens. Proteins were precipitated using ammonium sulfate and then purified using Protein A columns. Protein content was determined by ultraviolet spectrophotometry using the following formula: Protein Concentration/(mg/mL) = [1.45 A280–0.74 A260] × Dilution Factor.
2.8 Optimization and characterization of antibody performance
The IC-ELISA method was used to optimize working conditions, including antigen coating, antibody working concentration, and organic solvent tolerance. After optimization, a competitive IC-ELISA method was employed to establish a standard curve and determine the specificity of mAbs against MG analogs. The cross-reactivity rate was calculated using the formula:
3 Discussion
3.1 Hapten design
The design of haptens is paramount in antibody preparation. Recent hapten designs targeting MG have primarily focused on introducing reactive groups onto the benzene ring without incorporating dimethylamino groups. However, the prevailing trend in recent years has neglected the presence of dimethylamino groups, despite the fact that numerous monoclonal antibodies (mAbs) produced using these designs exhibit significant cross-reactivity with crystal violet, suggesting that the dimethylamino group could be a crucial factor contributing to this cross-reactivity. To validate this hypothesis, we conducted a systematic review of the latest advancements in the field and performed in-depth analyses utilizing computational modeling techniques. As depicted in Figure 2, due to the strong electron-donating property of nitrogen atoms, the structure of MG is highly negatively charged, thereby facilitating the formation of hydrogen bonds. In contrast, crystal violet possesses similar dimethylamino antigenic epitopes, which may undermine the selectivity of the prepared antibodies toward malachite green. Introducing spacer arms onto the benzene ring results in a more positively charged overall structure, disrupting the original high negative charge state. Notably, the charge associated with the central double bonds resembles that of the parent drug, and the incorporation of spacer arms alongside dimethylamino groups has minimal impact on the overall charge distribution. Consequently, we propose a novel hapten design (Hapten 1), which not only incorporates dimethylamino groups to enhance antigen selectivity but also retains the central double bonds to mimic the immunogenicity of the parent drug. Furthermore, to further enhance antibody coating efficiency, we devised another hapten (Hapten 2) featuring exposed dimethylamino groups, thereby strengthening its interaction capabilities with antibodies. This design strategy not only improves the immune recognition efficiency of antigens but also lays a solid foundation for the subsequent development of highly sensitive and selective immunoassay methods.
3.2 Production of mAbs
H1-LF and H2-LF were used as immunogens, and H1-BSA and H2-BSA were used as coating antigens for mouse immunization, respectively. After the fourth immunization, mouse serum was collected to determine antibody titer and affinity for MG (Table 1). Absorbance and corresponding inhibition rates of MG were used as evaluation criteria. For H2-LF, serum from mouse 3 exhibited the strongest recognition of MG at 200 ng/mL, with inhibition rates of 27 and 30% for H1-BSA and H2-BSA, respectively. For H1-LF, serum from mouse 2 showed inhibition rates of 63 and 45% for H2-BSA and H1-BSA, respectively. Both H1 and H2 haptens induced strong immune responses against MG, but H1-LF, enhanced by H2-BSA, demonstrated significant improvements in titer and inhibition rate. Consequently, mouse 2 (H1-LF) was selected for cell fusion experiments.
3.3 Antibody characterization and purification
Following cell fusion, monoclonal antibody M1 was obtained from H2-LF. Protein G affinity chromatography was used for purification due to its strong binding affinity with mammalian IgG. By adjusting the pH of the mobile phase, high-purity mAbs were achieved. The antibody was concentrated using dialysis bags covered with polyethylene glycol 20,000 at the bottom and surface. Observations were made every 30 min until the desired volume was reached. After a 4-h PBS dialysis exchange, the final concentration was determined to be 4.2 mg/mL.
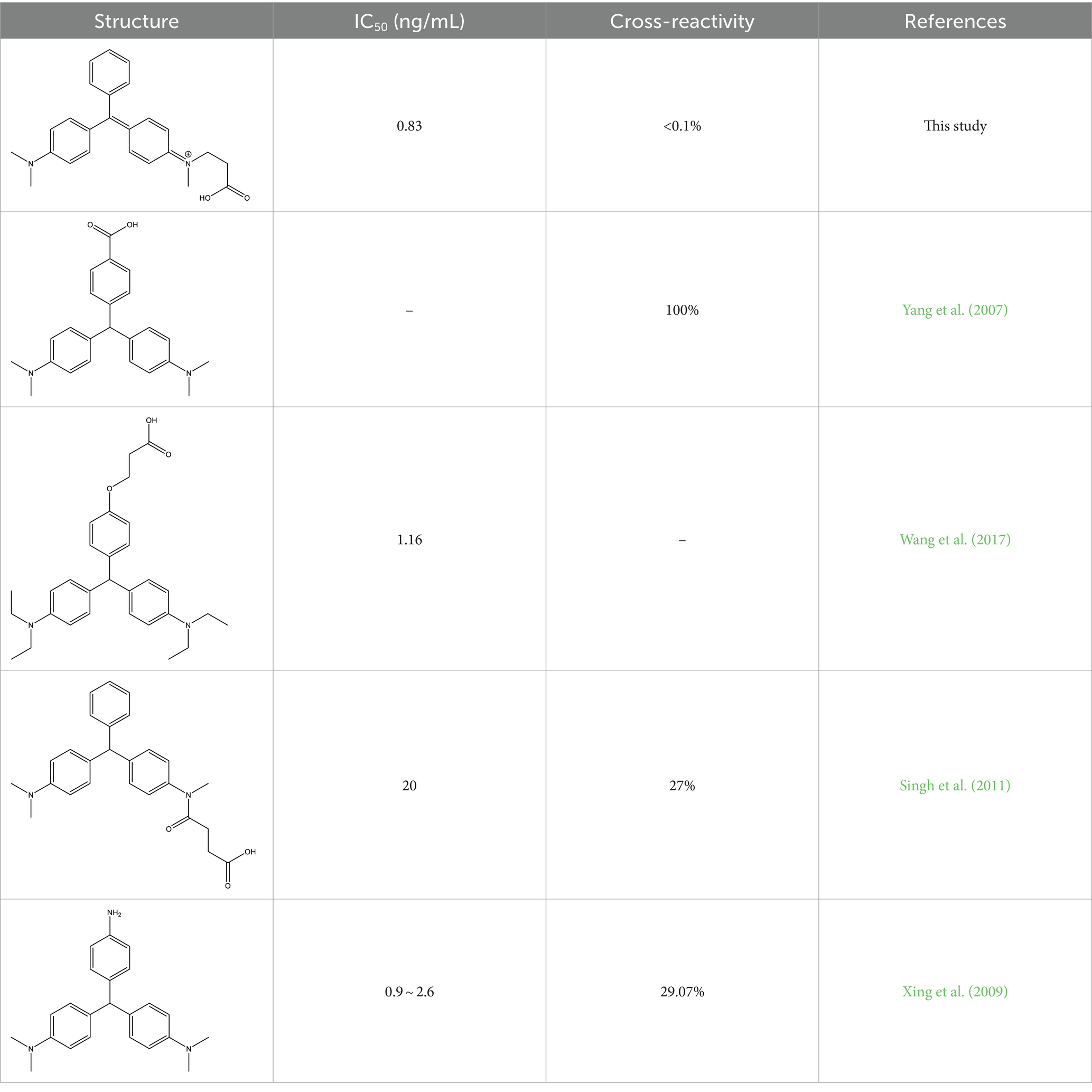
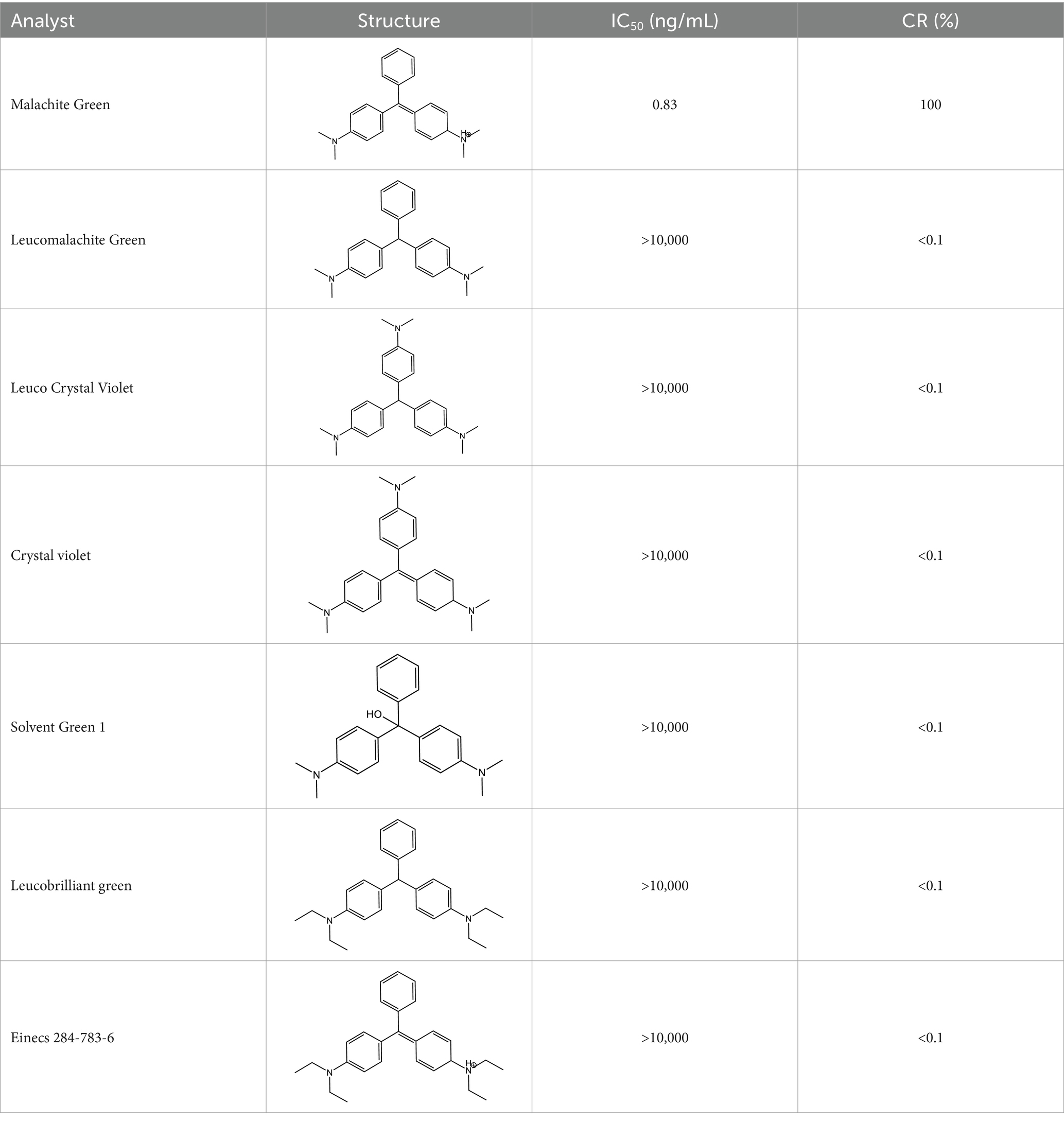
As shown in Table 2, a comparison of the prepared monoclonal antibodies with previous studies revealed that the introduction of an arm onto the benzene ring that never contained a dimethylamino group resulted in a cross-reactivity with CV as high as 100%. Subsequently, the cross-reactivity decreased to 27% when an arm was introduced onto the dimethylamino group. This pattern also confirms that the dimethylamino group is the most crucial antigenic epitope for MG recognition. Based on this finding, this study further introduced a double bond to make the structure closer to MG. The successfully prepared monoclonal antibody not only outperformed previous studies in terms of sensitivity but also in specificity.
3.4 Optimization of antibody performance
Theoretically, a decrease in the concentration of the coated antigen leads to a decrease in its own antibody’s competitive binding ability, while enhancing the competitive binding ability of the drug’s antibody. To maintain stable working conditions, the antibody’s working concentration needs to be adjusted upwards. In this experiment, the checkerboard method was used to optimize the concentrations of coated antigen and antibody. Under other consistent conditions, IC-ELISA was used to compare the IC50 values under different combinations of coated antigen and antibody concentrations. The resulting standard curve had the lowest IC50 value when the coated antigen concentration was 0.25 μg/mL and the antibody concentration was 125 ng/mL. Therefore, a coated antigen concentration of 0.25 μg/mL and an antibody concentration of 125 ng/mL were selected as the optimal working concentrations.
As shown in Figure 3, the buffer had little effect on MG. When the standards were diluted with PBST, the IC50 values were relatively low, indicating that PBST improved the detection sensitivity compared with distilled water. This enhancement may be attributed to the compositional similarity between PBST and the antibody diluent, which maintains the pH and ionic concentration of the buffer system, thus avoiding adverse effects on the antigen–antibody reaction. In contrast, buffers such as distilled water and phosphate buffer (PB) may disrupt this equilibrium and affect detection sensitivity. Therefore, PBST was chosen as the optimal diluent for the standard.
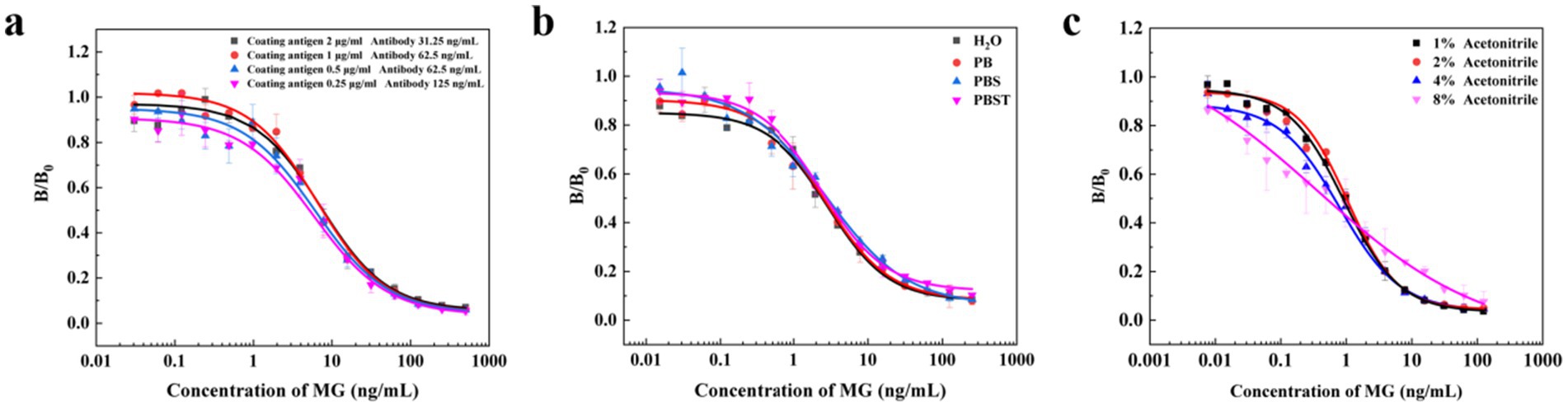
Figure 3. The optimization of working conditions. Effects of coating antigen and antibody concentration (a), buffer (b) and amount of acetonitrile (c).
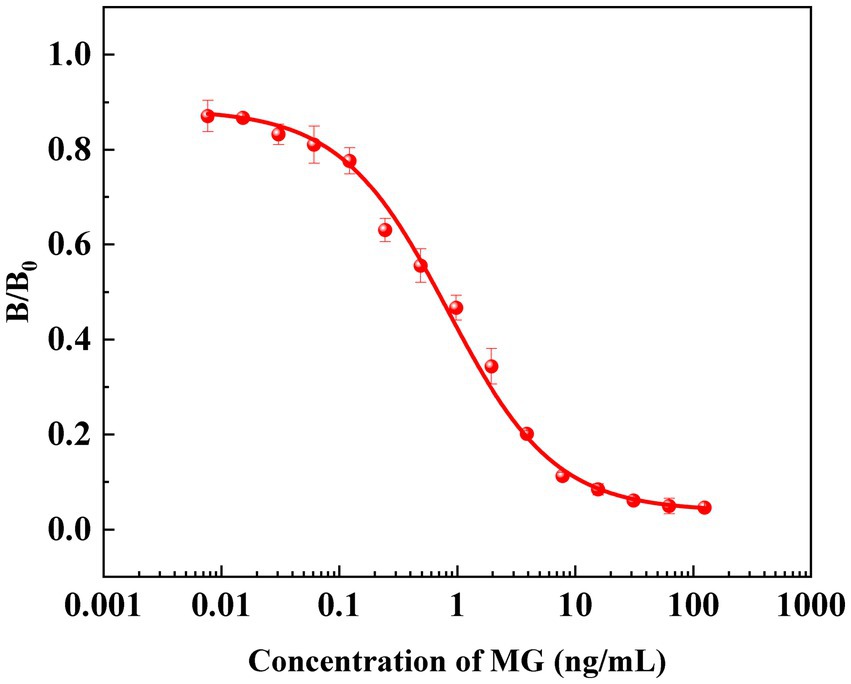
Due to the strong lipophilicity of MG, its standard solution mainly needs to be prepared with acetonitrile as the solvent. In the actual sample detection process, the concentration of organic solvents in the reaction system has an important influence on the biological activity of the antibody, the binding efficiency of antigen and antibody, and the solubility of the drug. Therefore, the concentration of acetonitrile in the PBST buffer solution was optimized to study the organic solvent tolerance of the antibody. The results showed that when the buffer contained a low concentration of acetonitrile, the PBST buffer could not dissolve the MG well, resulting in a higher IC50 value. When the acetonitrile content in the buffer was increased from 1 to 8%, the lowest IC50 ratio was observed at 4% acetonitrile content. However, when the acetonitrile content in the buffer reached 8%, some of the antibodies were inactivated due to the high concentration of organic solvent. Therefore, the PBST buffer containing 4% acetonitrile was selected as the optimal buffer system condition. In summary, after a series of optimization, the IC-ELISA standard curve of MG was established under the optimal experimental conditions: as shown in Figure 4, the IC50 value of MG detected by IC-ELISA was 0.83 ng/mL, and the linear ranges was 0.19–3.52 ng/mL, and IC10 was 0.08 ng/mL.
3.5 Evaluation of antibody specificity
The specificity of the antibodies was evaluated using Malachite Green (MG), Leucomalachite Green (LMG), Crystal violet (CV), Leuco Crystal Violet (LCV), Einecs 284-783-6 (BG) and Leucobrilliant green (LBG) as structural analogs. As shown in Table 3, the cross-reactivity of anti-MG mAb with other structural analogs was below 0.1. This indicates that the design of dimethylamino group effectively improves the selectivity of the antibody, and also proves that dimethylamino group is the key antigenic recognition epitope of malachite green, confirming the feasibility of our strategy. The antibody prepared in this study can be used to further develop highly sensitive and selective immunoassay methods (Figure 4).
4 Conclusion
In this study, two hapten were designed and synthesized, and computer simulations demonstrated that the spacer arm position of MG significantly affects the recognition of MG by the mAb. High-quality mAbs were subsequently prepared using these hapten, showing an IC50 value of 0.83 ng/mL, a detection limit (IC10) of 0.08 ng/mL, and a linear range of 0.19–3.52 ng/mL. This study presents a novel strategy for designing MG hapten, with great potential for application in immunoassay methods.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Animal experiments strictly adhere to the guidelines of Chinese laws and regulations, the studies involving animals were reviewed and approved by the Animal Ethics Committee of South China Agricultural University.
Author contributions
M-FW: Writing – original draft, Writing – review & editing. NX: Writing – original draft. SL: Writing – review & editing. Y-LH: Writing – original draft. M-HW: Writing – review & editing. J-DL: Writing – original draft. R-SC: Writing – original draft. W-MX: Writing – review & editing. Y-JL: Writing – review & editing. H-TL: Writing – review & editing. X-AH: Writing – review & editing. Z-LX: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by GuangDong Basic and Applied Basic Research Foundation (2020A1515110332), Research Platform and Projects of Guangdong Provincial Department of Education in 2023 (20232023ZDZX4117).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelhamid, A. G., Wick, M., and Yousef, A. E. (2024). Production of polyclonal antibodies and development of competitive ELISA for quantification of the Lantibiotic Paenibacillin. Fermentation 10:232. doi: 10.3390/fermentation10050232
Chen, Y., Xia, S., Han, X., and Fu, Z. (2020). Simultaneous determination of malachite green, chloramphenicols, sulfonamides, and fluoroquinolones residues in fish by liquid chromatography-mass spectrometry. J. Anal. Methods Chem. 2020, 1–12. doi: 10.1155/2020/3725618
Faraji, M., Adeli, M., and Noormohammadi, F. (2020). Deep eutectic solvent-based dispersive liquid-liquid micro-extraction for extraction of malachite green and crystal violet in water samples prior their determination using high performance liquid chromatography. Int. J. Environ. An. Ch. 102, 681–689. doi: 10.1080/03067319.2020.1724992
He, J., Mo, P., Luo, Y.-S., Yang, P.-H., and Ghafarifarsani, H. (2023). Strategies for solving the issue of malachite green residues in aquatic products: a review. Aquac. Res. 2023, 1–17. doi: 10.1155/2023/8578570
Hu, Y., Gao, Z., and Luo, J. (2021). Fluorescence detection of malachite green in fish tissue using red emissive Se,N,Cl-doped carbon dots. Food Chem. 335:127677. doi: 10.1016/j.foodchem.2020.127677
Hussain Hakami, A. A., Ahmed, M. A., Khan, M. A., AlOthman, Z. A., Rafatullah, M., Islam, M. A., et al. (2021). Quantitative analysis of malachite green in environmental samples using liquid chromatography-mass spectrometry. Water 13. doi: 10.3390/w13202864
Oplatowska, M., Connolly, L., Stevenson, P., Stead, S., and Elliott, C. T. (2011). Development and validation of a fast monoclonal based disequilibrium enzyme-linked immunosorbent assay for the detection of triphenylmethane dyes and their metabolites in fish. Anal. Chim. Acta 698, 51–60. doi: 10.1016/j.aca.2011.04.047
Pradel, J. S., and Tong, W. G. (2017). Determination of malachite green, crystal violet, brilliant green and methylene blue by micro-cloud-point extraction and nonlinear laser wave-mixing detection interfaced to micellar capillary electrophoresis. Anal. Methods 9, 6411–6419. doi: 10.1039/C7AY01706E
Sayee, R. H., Hosamani, M., Krishnaswamy, N., Shanmuganathan, S., Charan, M. S. S., Sheshagiri, G., et al. (2024). Monoclonal antibody based solid phase competition ELISA to detect FMDV serotype a specific antibodies. J. Virol. Methods 328. doi: 10.1016/j.jviromet.2024.114959
Shen, Y. D., Deng, X. F., Xu, Z. L., Wang, Y., Lei, H. T., Wang, H., et al. (2011). Simultaneous determination of malachite green, brilliant green and crystal violet in grass carp tissues by a broad-specificity indirect competitive enzyme-linked immunosorbent assay. Anal. Chim. Acta 707, 148–154. doi: 10.1016/j.aca.2011.09.006
Shin, J.-H., Lee, M.-J., Kim, Y.-J., Kim, T.-H., Choi, J.-H., and Oh, B.-K. (2024). Development of multi-HRP-conjugated branched PEI/antibody-functionalized gold nanoparticles for ultra-sensitive ELISA. Biochip J. 18, 419–426. doi: 10.1007/s13206-024-00165-z
Singh, G., Koerner, T., Gelinas, J.-M., Abbott, M., Brady, B., Huet, A.-C., et al. (2011). Design and characterization of a direct ELISA for the detection and quantification of leucomalachite green. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 28, 731–739. doi: 10.1080/19440049.2011.567360
Su, P., Wang, H., Liu, T., Li, X., Yu, L., Wang, X., et al. (2024). Adsorption-based preconcentration effect enhanced FRET and PET from rhodamine B to analyte malachite green for ultra-sensitive sensing application. Microchem. J. 203:110840. doi: 10.1016/j.microc.2024.110840
Teepoo, S., Wongtongdee, U., and Phapugrangkul, P. (2020). Development of qualitative and quantitative immunochromatographic strip test assay for rapid and simple detection of leucomalachite green residual in aquatic animals. Food Chem. 320:126613. doi: 10.1016/j.foodchem.2020.126613
Wang, L., He, Z., Guo, Y., Ran, X., Cheng, Y., and He, Z. (2024). A novel quantitative double antigen sandwich ELISA for detecting total antibodies against Candida albicans enolase 1. Eur. J. Clin. Microbiol. Infect. Dis. 43, 1815–1823. doi: 10.1007/s10096-024-04899-4
Wang, X., Yang, T., Chen, X., Fang, L., Yang, Y., Cao, G., et al. (2023). Quantitative detection of malachite green in sediment by a time-resolved immunofluorescence method combined with a portable 3D printing equipment platform. Sci. Total Environ. 855:158897. doi: 10.1016/j.scitotenv.2022.158897
Wang, Y., Yang, J., Shen, Y., Sun, Y., Xiao, Z., Lei, H., et al. (2017). Novel haptens synthesis and development of a monoclonal antibody-based enzyme-linked immunosorbent assay for leuco-malachite green in fish. Food Agric. Immunol. 28, 1460–1476. doi: 10.1080/09540105.2017.1348490
Wu, Q., Ma, Z., Pan, Q., Liu, T., Zhang, Y., Xin, J., et al. (2024). A candidate competitive ELISA based on monoclonal antibody 3A8 for diagnosis of contagious bovine pleuropneumonia. Appl. Microbiol. Biotechnol. 108:290. doi: 10.1007/s00253-024-13127-0
Xiao, X., Chen, C., Deng, J., Wu, J., He, K., Xiang, Z., et al. (2020). Analysis of trace malachite green, crystal violet, and their metabolites in zebrafish by surface-coated probe nanoelectrospray ionization mass spectrometry. Talanta 217:121064. doi: 10.1016/j.talanta.2020.121064
Xing, W., He, L., Yang, H., Sun, C., Li, D., Yang, X., et al. (2009). Development of a sensitive and group-specific polyclonal antibody-based enzyme-linked immunosorbent assay (ELISA) for detection of malachite green and leucomalachite green in water and fish samples. J. Sci. Food Agr. 89, 2165–2173. doi: 10.1002/jsfa.3695
Yang, M.-C., Fang, J.-M., Kuo, T.-F., Wang, D.-M., Huang, Y.-L., Liu, L.-Y., et al. (2007). Production of antibodies for selective detection of malachite green and the related triphenyl methane dyes in fish and fishpond water. J. Agric. Food Chem. 55, 8851–8856. doi: 10.1021/jf071195y
Yue, S., Liu, Y., Zhou, R., Zhan, Z., Kang, L., Huang, L., et al. (2024). A highly sensitive and rapid colloidal gold immunoassay for Puerarin detection. J. Agric. Food Chem. 72, 8817–8822. doi: 10.1021/acs.jafc.4c00644
Zhang, Z., Li, H., Huang, L., Wang, H., Niu, H., Yang, Z., et al. (2024). Rapid identification and quantitative analysis of malachite green in fish via SERS and 1D convolutional neural network. Spectrochim. Acta A Mol. Biomol. Spectrosc. 320:124655. doi: 10.1016/j.saa.2024.124655
Keywords: malachite green, monoclonal antibody, molecular simulation, IC-ELISA, hapten design
Citation: Wu M-F, Xu N, Li S, Huang Y-L, Wu M-H, Li J-D, Chen R-S, Xiong W-M, Li Y-J, Lei H-T, Huang X-A and Xu Z-L (2024) A hapten design strategy to enhance the selectivity of monoclonal antibodies against malachite green. Front. Sustain. Food Syst. 8:1490750. doi: 10.3389/fsufs.2024.1490750
Edited by:
Xiuxiu Dong, Jiangsu University, ChinaReviewed by:
Xixia Liu, Hubei Normal University, ChinaWen Ping Zhao, Shandong University of Technology, China
Copyright © 2024 Wu, Xu, Li, Huang, Wu, Li, Chen, Xiong, Li, Lei, Huang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sha Li, bGlzaGExOTkwMDdAMTYzLmNvbQ==
†These authors share first authorship
 Min-Fu Wu
Min-Fu Wu Nuo Xu2†
Nuo Xu2† Min-Hua Wu
Min-Hua Wu Hong-Tao Lei
Hong-Tao Lei Zhen-Lin Xu
Zhen-Lin Xu