- 1Discipline of Horticultural Science, School of Agricultural, Earth and Environmental Sciences, University of KwaZulu-Natal, Scottsville, South Africa
- 2Plant Science Laboratory, Cranfield University, Bedfordshire, United Kingdom
- 3Department of Food Systems and Development, Faculty of Natural and Agricultural Sciences, University of Free State, Bloemfontein, South Africa
Edible coatings play a critical role in reducing postharvest losses during storage and supply chain of horticultural commodities. The present study evaluated the efficacy of different concentrations of moringa leaf extract (MLE) combined with carboxymethyl cellulose (CMC) edible coating in preserving the quality and extending the shelf life of “Hass” avocado. Fruit were harvested at different stages of maturity and evaluated by dry matter content. Different concentrations of moringa (8 and 16%) extracted with chilled ethanol (100%) and functionalized with CMC (5%), were used to treat the fruit. Treated fruit were then stored at 5.5 ± 1°C and 90 ± 5% RH for 28 days plus an additional 7 days at 23°C. The changes in physicochemical and biochemical fruit attributes were evaluated at weekly intervals. The application of moringa and CMC-based edible coatings preserved the phenolics, flavonoids, and antioxidant activity of “Hass” avocado. The treatments significantly (p < 0.05) reduced the loss of weight and firmness. Furthermore, treated fruits were found to have a delayed color change and reduction in sugar concentration, particularly mannoheptulose, compared to the control treatment. Therefore, edible coatings prepared by combining CMC and MLE could be the best alternative for substituting the currently used health-compromising synthetic chemicals.
Introduction
Avocado (Persea americana Mill.) is one of the most economically essential fruits from the Lauraceae family. This fruit is mainly produced in tropical and subtropical regions (Eça et al., 2014). The consumption of avocado is steadily increasing because of its health-related benefits, which makes it considered a “superfruit” (Sivakumar et al., 2021). Its mesocarp tissue consists of bioactive phytochemicals, such as sterols, vitamin E, and carotenoids, that provide antioxidants and radical scavenging activities (Bill et al., 2014). However, this fruit is highly perishable and prone to microbial spoilage, contributing significantly to postharvest losses. Reducing avocado postharvest losses could allow the world avocado market to reach its primary target market value of about US$21.56 billion by 2026, as highlighted by Sivakumar et al. (2021).
Most avocado production in countries such as Spain, Chile, Israel, and South Africa is exported to distant overseas markets, mainly Europe (Kassim et al., 2013). Usually, it takes about 21 or more days to transport the fruits from South Africa to the target market overseas. Generally, fruit maturity at harvest significantly influences its postharvest storage life and quality, impacting the marketing, handling, and transporting decisions (Kader, 1999). Therefore, the harvesting decision must accommodate the transporting period and make the marketing flexible (Magwaza and Tesfay, 2015). Most commercial producers use dry matter content, moisture content, and mesocarp oil content to determine the maturity of avocados at harvest (Magwaza and Tesfay, 2015; Rivera et al., 2017). Among these maturity indices, dry matter is preferable due to its cost-effectiveness and less time required, making this technique even more convenient (Blakey et al., 2012).
Due to the climacteric nature of avocados, they produce more ethylene and continue to ripen during storage. This compromises the shelf life and makes it difficult to market this fruit, especially to international markets with a long transit period. The avocado industry highly depends on various synthetic edible films and coatings after harvesting and before storage to maintain the quality and extend the shelf life (Liu et al., 2020). The need to develop eco-friendly treatments to replace synthetic fungicides has been raised by researchers for decades. This is due to health-related concerns caused by the application of chemical-based treatments. Besides their environmental unfriendliness and high residues in the fruit’s edible portion, most pathogens have also developed resistance against some of these fungicides (Sivakumar and Bautista-Baños, 2014; Romanazzi et al., 2018; Sivakumar et al., 2021).
Polysaccharide coating materials have gained popularity for their application in fresh produce because of their characteristics, such as exceptionally high stability and solubility (Panahirad et al., 2021). Coatings from polysaccharides are the most convenient due to their easy accessibility, non-toxicity, and cost-effectiveness (Singh et al., 2019). Among cellulose derivatives, carboxymethyl cellulose (CMC) is the most commonly used commercial derivative, with greater production and applications in the food sector (Dhall, 2016). This is due to its easy accessibility because of its reasonable price, and it is a nontoxic polysaccharide, thus safe for human consumption.
Moringa oleifera Lam has recently drawn more research attention for its use in postharvest quality preservation. This owes to the exceptional performance of edible coatings containing moringa extract in suppressing fruit postharvest diseases, thereby preserving the fruit quality and extending its shelf life (Tesfay and Magwaza, 2017; Tesfay et al., 2017). Most developed countries have opted to use fresh organic products in food preservatives, which necessitates continued research aiming to develop or improve organic postharvest treatments. This study, therefore, evaluated the effect of moringa leaf extract and carboxymethyl cellulose edible coating on the quality and shelf life of “Hass” avocados.
Materials and methods
Preparation of moringa leaf extracts
Fresh moringa leaf powder was obtained from the Agricultural Research Council (ARC), located in Pretoria, South Africa. Moringa extracts were prepared following a blend of modified methods previously described by Tesfay et al. (2016) and Addo et al. (2022), using chilled 100% ethanol, which was firstly refrigerated at −20°C overnight before immediately use. Different moringa extracts were prepared, 8% (g/v) and 16% (g/v). Briefly, 160 and 80 g of moringa leaf powder were separately extracted with 1 L of ethanol for 2 h with constant agitation to extract the free polyphenols. The extract was passed through a 150 μm sieve. After filtering the extract, 1 L of 50% acidified ethanol was added to the crude, followed by heating at 90°C in a water bath for 1 h to extract the membrane-bound polyphenols. The extract was collected and stored at ambient temperature to prepare the coating solutions.
Preparation of coating solution
To prepare the coating solution, 50 g of CMC powder was dissolved in 1 L of the prepared moringa solution to obtain 5% CMC, based on the preliminary study (unpublished). The solution was heated to 51 0\u00B0C with constant stirring until the powder was dissolved. The resulting solution was used to treat avocado fruit.
Application of treatments and storage
The “Hass” avocados used in this study were supplied by Westfalia Fruit (Pty) Ltd. commercial farm located in Howick, South Africa. The fruit were harvested at different maturity stages, determined by dry matter content (DM), which was found to be 25, 27, and 30% for fruit harvested in July, maturity1 (M1); August (M2), and October (M3), respectively, in the year of 2022. From each maturity stage, a total of 250 fresh avocado fruit, free from mechanical damage and diseases, were assigned into three treatments: Control, CMC + 8% MLE, and CMC + 16% MLE. Each treatment was assigned 50 fruits and replicated five times, with each replicate having 10 fruits. Just before cold storage, a sum of five fruits was sampled to assess the fruit status at harvest and as a reference. Before the application of treatments, all fruits were first washed with distilled water to avoid any potential contamination. The fruits were dipped into their assigned treatment for 1 min, whereas the control was only washed with distilled water; no treatment was applied. Following treatments, fruits were allowed to dry at room temperature, placed in labeled open boxes, and kept at 5.5 ± 1°C and 90 ± 5% relative humidity (RH) for 28 days. After 28 days of cold storage, the fruits were transferred to room temperature (± 23°C) at the laboratory shelf-life benches for 7 days. The changes in fruit quality were observed at weekly intervals throughout the 35-day storage period.
Evaluation of postharvest fruit quality
Fruit firmness
Fruit firmness was measured using a whole-fruit compression analysis described by Jeong and Huber (2004). In this analysis, firmness was measured on unpeeled fruit using a Texture Analyzer (Instron3345 Universal Testing machine, Buck, United Kingdom) fitted with a probe of 5 cm in diameter and 100 N load cell. The probe was allowed to establish zero-force contact with the equatorial region of the fruit before it was driven with a crosshead speed of 5 mm/s. The deformation force was recorded at 10 mm deformation depth on three opposite sides of the equatorial region of each fruit. The data was automatically loaded on the Easy-Match-QC software, and the firmness was recorded in newtons (N) as the maximum force required for mesocarp tissue failure.
Fruit weight loss percentage
The fruit weight was measured using a digital weighing scale (RADWAG Wagi Electronic Inc., Poland) and determined as weight loss percentage using Equation 1:
Fruit color
Avocado fruit color was determined on five fruits per treatment using a CR 400 Chromameter (Minolta Co., Ltd., Osaka, Japan). The readings were taken at the same portion of the fruit’s pericarp throughout the experiment. First, the Chromameter was calibrated against a standard white tile. The values for L*, a*, and b* were recorded, where the L* value represented the lightness, the a* value represented the redness (positive) or greenness (negative), and the b* value represented the yellowness (positive) or blueness (negative). The value for Hue angle (H*) was also recorded.
Total phenolic content
The determination of phenolic compounds was performed following a slightly modified Folin-Ciocalteau method previously described by Milbury et al. (2006). Briefly, 0.5 g of freeze-dried avocado mesocarp was extracted with 15 mL ethanol (70% v/v), followed by shaking the mixture at room temperature for 10 min. The solution was then centrifuged for 10 min at 10,000 rpm and 4°C. Thereafter, 0.5 mL of clear extract was pipetted into a test tube, followed by adding 2 mL of 7.5% sodium carbonate. The mixture was allowed to rest for 3 min before adding 2.5 mL of Folin-Ciocalteau reagent (0.2 N). The resulting solution was then heated at 45°C in the ultrasonic water bath for 15 min. The solution was then allowed to cool in water before measuring the absorbance at 765 nm using a UV-1800 Spectrophotometer (Shimadzu Scientific Instruments INC., Columbia, United States) against ethanol as blank. Garlic acid was used to prepare the standard calibration curve, and the total phenolic content was determined and expressed as Gallic Acid Equivalent (GAE) mg/g DM.
Total flavonoid content
The total flavonoid (TF) concentration was determined following the method previously described by Obeng et al. (2020), with slight modifications. Briefly, 60 μL of avocado extract aliquot was mixed with 2 mL of distilled water, then 150 μL of 5% (w/v) sodium nitrite (NaNO2) was added. The solution was allowed to settle for 5 min before adding 0.8 mL of 10% (w/v) aluminum chloride. The mixture was then allowed to settle for another 5 min, and 2 mL of 1.0 M sodium hydroxide (NaOH) was added, followed by vortexing for 30 s. The absorbance was measured at 510 nm against the blank using a UV-1800 Spectrophotometer (Shimadzu Scientific Instruments Inc., Columbia, United States), and the determined total flavonoids were expressed as Quercetin Equivalents (QTE) mg/g DM. The calibration curve was prepared by preparing quercetin solutions at concentrations of 10 to 100 μg/mL in ethanol.
2,2’ Diphenyl-1-picrylhydrazyl antioxidant assay
The DPPH assay was used to estimate avocado mesocarp tissue’s free radical scavenging ability following the modified method previously described by Fan et al. (2022). Briefly, 260 μL of freshly prepared methanolic DPPH reagent (0.1 mM) was added into 40 μL of sample in a cuvette and incubated for 30 min in a dark at room temperature. The absorbance was read at 517 nm against the ethanol as blank using a UV-1800 Spectrophotometer (Shimadzu Scientific Instruments INC., Columbia, USA), and the DPPH scavenging capability was calculated using Equation 2:
Where Ac = absorbance of control; At = absorbance of the extract.
Determination of sugars (mannoheptulose and perseitol)
Determination and quantification of soluble sugars were based on the slightly modified method previously described by Tesfay and Magwaza (2017). Briefly, 0.1 g of freeze-dried mesocarp was added to 10 mL of 80% v/v ethanol/H2O and homogenized for 1 min using Ultraturrax. The resulting mixture was then incubated for 1 h at 80°C in an ultrasonic water bath, followed by storing the samples in a refrigerator at 4°C overnight to facilitate the release of soluble sugars. The samples were then centrifuged at 10,000 rpm and 4°C for 15 min and, thereafter, filtered through glass wool. The filtrates were dried overnight under a vacuum in a GenVac® concentrator (SP Scientific, Genevac Ltd., Suffolk, United Kingdom). Dried samples were reconstituted with 2 mL of ultra-pure water and filtered through a 0.4 μL nylon syringe filter into high-performance liquid chromatography (HPLC) vials. Thereafter, HPLC (Shimadzu, Kyoto, Japan) equipped with a refractive index detector was used to determine the sugars. Different sugars, mannoheptulose, and perseitol, were determined by co-elution with their standards and their concentrations calculated using a standard curve for each sugar.
Statistical analysis
The collected data were subjected to the analysis of variance (ANOVA) using GenStat statistical software (GenStat 20th Edition, VSN International Ltd., United Kingdom). The mean separation was performed using Duncan’s Multiple Range Test (DMRT) at 5% significance level. The Principal Component Analysis (PCA) biplot was performed using R-statistical software.
Results and discussion
Fruit firmness
Fruit firmness is one of the key quality attributes influencing consumer purchase decision and determining the shelf life and market value of most fresh fruits. The firmness of fruit is mainly affected by different factors such as harvest maturity, relative humidity, and storage temperature. This study showed a significant change in fruit firmness for both the treated and untreated avocado fruit during the 28 days of cold storage (5°C) and 7 days of shelf life at room temperature (23°C) (Table 1). As expected, a significant firmness loss was observed after 28 days of storage when the fruits were transferred to 23°C. Generally, firmness loss occurs due to water loss, mainly regulated by the temperature (Paniagua et al., 2013). The results demonstrated a significant effect of the interaction between harvest time and storage period (p < 0.001) on fruit firmness loss during the storage period. Both, the treatments and storage time had a significant effect (p < 0.05) on the firmness of avocado. As expected, all fruits showed firmness loss during the storage period regardless of the harvest time and treatments; however, the loss was severe in untreated fruits (Table 1). At the end of the storage period, the untreated fruits recorded lower firmness than all the treated fruits, which were 6.59, 7.91, and 6.81 N for maturity 1, 2, and 3, respectively. According to the ripening standards described by Jeong and Huber (2004) for avocado fruits, based on the whole fruit compression analysis, the untreated fruits were over-ripe (<10 N) and no longer suitable for markets. Briefly, these standards classify fruits as ripened and ready for consumption when the whole fruit compression attains values ranging between 10 and 20 N. The firmness declines to below 10 N on over-ripened fruit (Jeong and Huber, 2004). Although no sensory evaluations were conducted, the results from this study are aligned with these classifications based on the observations and statistical analysis.
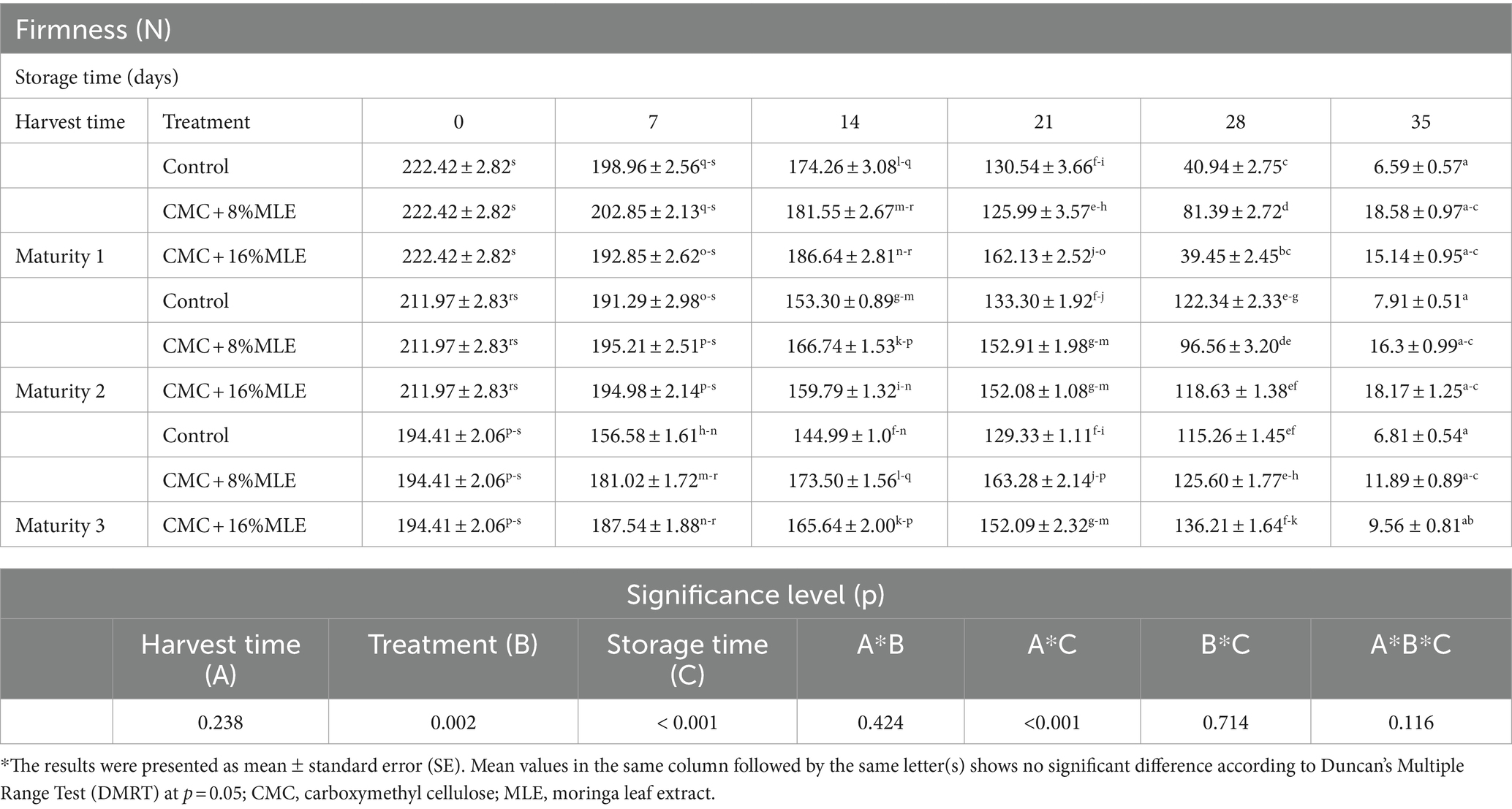
Table 1. The effect of CMC and different MLE concentrations on the firmness (N) of “Hass” avocadoes harvested at different maturity stages during 28 days cold storage at ±5°C followed by 7 days shelf life at ±23°C.
Different concentrations of MLE in combination with CMC delayed firmness loss depending on the harvest time; however, the CMC + 8%MLE treatment was most effective than the other treatments. Given that all the untreated fruit had compression values of less than 10 N at the end of the storage period, this indicates that MLE and CMC composite coating could delay the fruit ripening, thereby delaying the rate of fruit softening. Based on these results, the different concentrations (8 and 16%) of chilled MLE and CMC used in this study potentially delayed changes that take place in different components, such as cell wall structure weakening, hydrolysis of cellulose and hemicellulose, loss of membrane integrity, and depolymerization of pectin and starch, thereby delaying firmness loss (Yaman and Bayoιndιrlι, 2002). Combining 8% chilled MLE and CMC delayed firmness loss on fruit harvested at maturity 1 and 3, whereas increasing the concentration to 16% resulted in reduced firmness loss on fruit harvested at maturity 2 stage, although no significant difference was observed between the two MLE concentrations. The effectiveness of these treatments could be due to the presence of CMC. The carboxylic group in CMC’s chemical structure results in hydrogen bonding inside the coating matrix and between the coating and the fruit peel, resulting in preserved firmness (Panahirad et al., 2019). This positive effect may also be attributed to reduced enzyme activities, including pectin-methylesterase, which contributed to delayed fruit ripening. Pectin methylesterase is a major enzyme that depolymerizes pectin substances (Payasi et al., 2009). This also implies that the coatings could serve as a gas barrier, as the enzymatic activities are reduced by low oxygen and high carbon dioxide concentrations, which ultimately retain the fruit firmness (Payasi et al., 2009). Similarly, Kubheka et al. (2021) reported a reduced firmness loss in “Maluma” avocado treated with 1% CMC and moringa leaf extract.
Fruit weight loss (%)
Weight loss is mainly caused by the water loss during metabolic processes such as transpiration and respiration, and its rate depends on the storage environment (Abebe et al., 2017). This loss of water takes place through stomatal openings and skin cracks. The storage temperature and relative humidity impact the fruit weight loss due to the effect caused by the differences in vapor pressure between the fruit and the atmosphere (Wróblewska-Krepsztul et al., 2018). This was evident when the fruit from all the treatments showed the highest weight loss during the last week, from day 28 to 35, when the fruits were transferred to ambient conditions (± 23°C), compared to cold storage (Figure 1). The interaction between coatings, storage period, and harvest time significantly affected the fruit weight (p < 0.05). All fruits suffered a weight loss throughout the storage time; however, the untreated fruit suffered the most, especially after cold storage, at ambient temperature. All the evaluated edible coating treatments resulted in a lower weight loss percentage than the control for all harvests. The CMC and MLE treatments preserved the fresh weight of treated fruits throughout the evaluated 28 days of cold storage at 5 ± 1°C and 7 days of shelf life at ±23°C. However, CMC + 16% MLE was most effective, followed by CMC + 8% MLE than the control treatments. This can be attributed to the hydrophilic nature of these treatments. It can be argued that the treatments inhibited the transfer of water between the fruit and the atmosphere by forming a semipermeable layer that acted as a barrier between the fruit and the environment, covering the fruit surface and protecting it from mechanical injury and, therefore, reducing desiccation (Khorram et al., 2017). These results agree with those of Tesfay et al. (2017), who reported a reduced weight loss in avocado fruits treated with CMC combined with moringa leaf or seed extract. Similar results were also reported by Kubheka et al. (2021), where the CMC (1%) incorporated with moringa reduced the avocado weight loss throughout the 21 days of cold storage and 7 days of shelf life. Another study conducted by Zhang et al. (2019) reported that Osmunda japonica-CMC coatings significantly reduced the water loss in tomato fruit compared to untreated fruit. The fruit weight loss, caused by water loss, may also result in changes in the whole fruit texture and flavor (Ballesteros et al., 2022), and eventually, the fruit starts to decay as the loss gets severe, which was evident in this study.
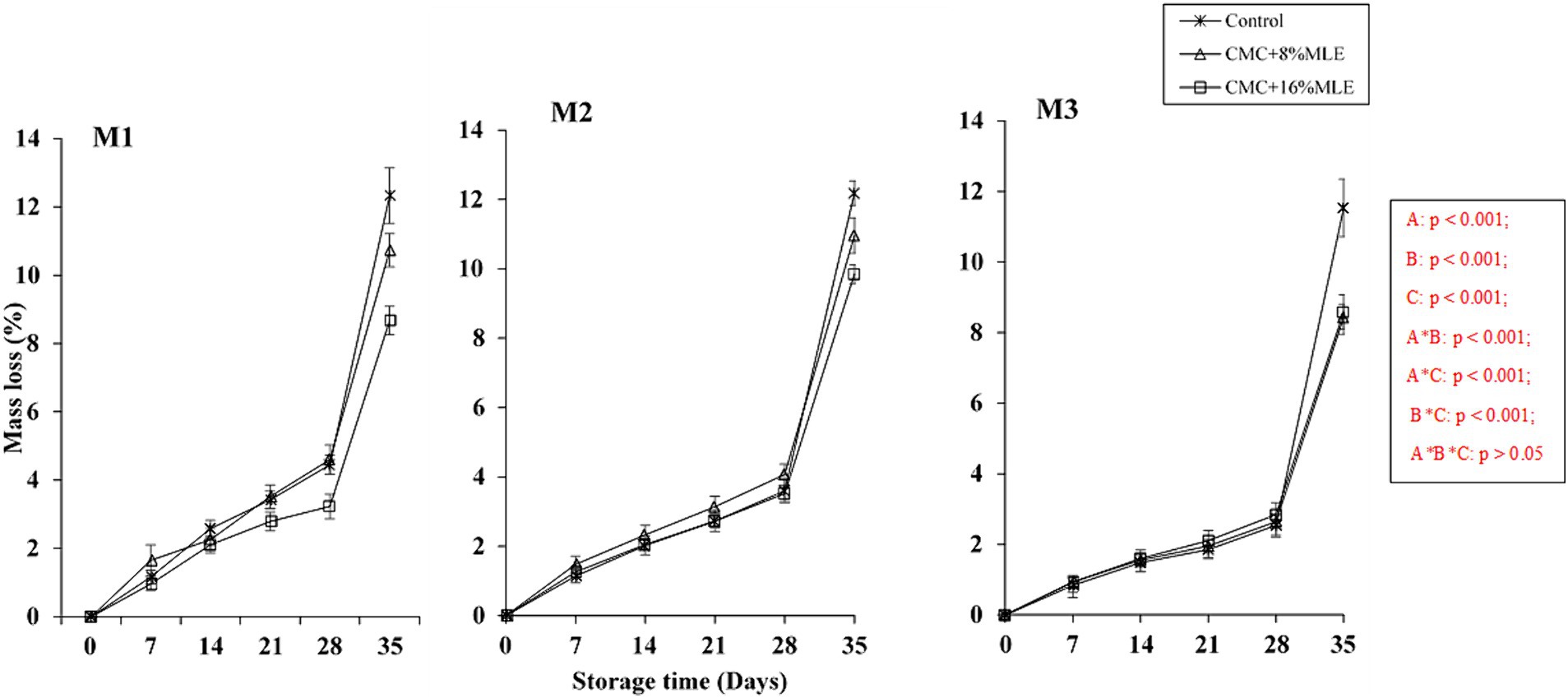
Figure 1. Weight loss of “Hass” avocado fruit harvested at different maturity stages (M1, M2, and M3) as influenced by CMC and different MLE concentrations during 28 days of cold storage and 7 days of shelf life. *The vertical bars represent standard error (SE) at n = 5; CMC, carboxymethyl cellulose; MLE, moringa leaf extract.
Fruit color
Fruit color is the best indicator for the ripening stage in avocadoes, particularly the “Hass” cultivar. This cultivar is characterized by the ripening process that is accompanied by the color change from green to purple or black. In this study, the changes in avocado fruit color were found to be significantly affected by the interaction between harvest time and storage period (p < 0.001), particularly the changes in the a*, b*, and L* values. The study showed a decrease in yellowness (b*), lightness (L*), and hue angle (h0) and an increase in greenness (a*) values during storage as the fruit ripens regardless of the treatments and harvest time (Table 2). The observed increase in a* value from negative to positive indicates color reduction from greener to red with fruit ripening, which was clearly expected in this study. However, there was a notable delayed color change, especially in treated fruits, which could indicate that the composite edible coating of MLE and CMC potentially delays the transition of chloroplasts into chromoplasts that contain yellow and red pigments, thereby inhibiting color change and enzymatic browning (Sharma et al., 2019). The color change was negligible during the cold storage period, with significant changes observed between days 28 and 35. These observations agree with Mwelase et al. (2022), who also reported the influence of temperature on avocado fruit color. Similarly, the higher temperature accelerated the avocado color change compared to cold storage.
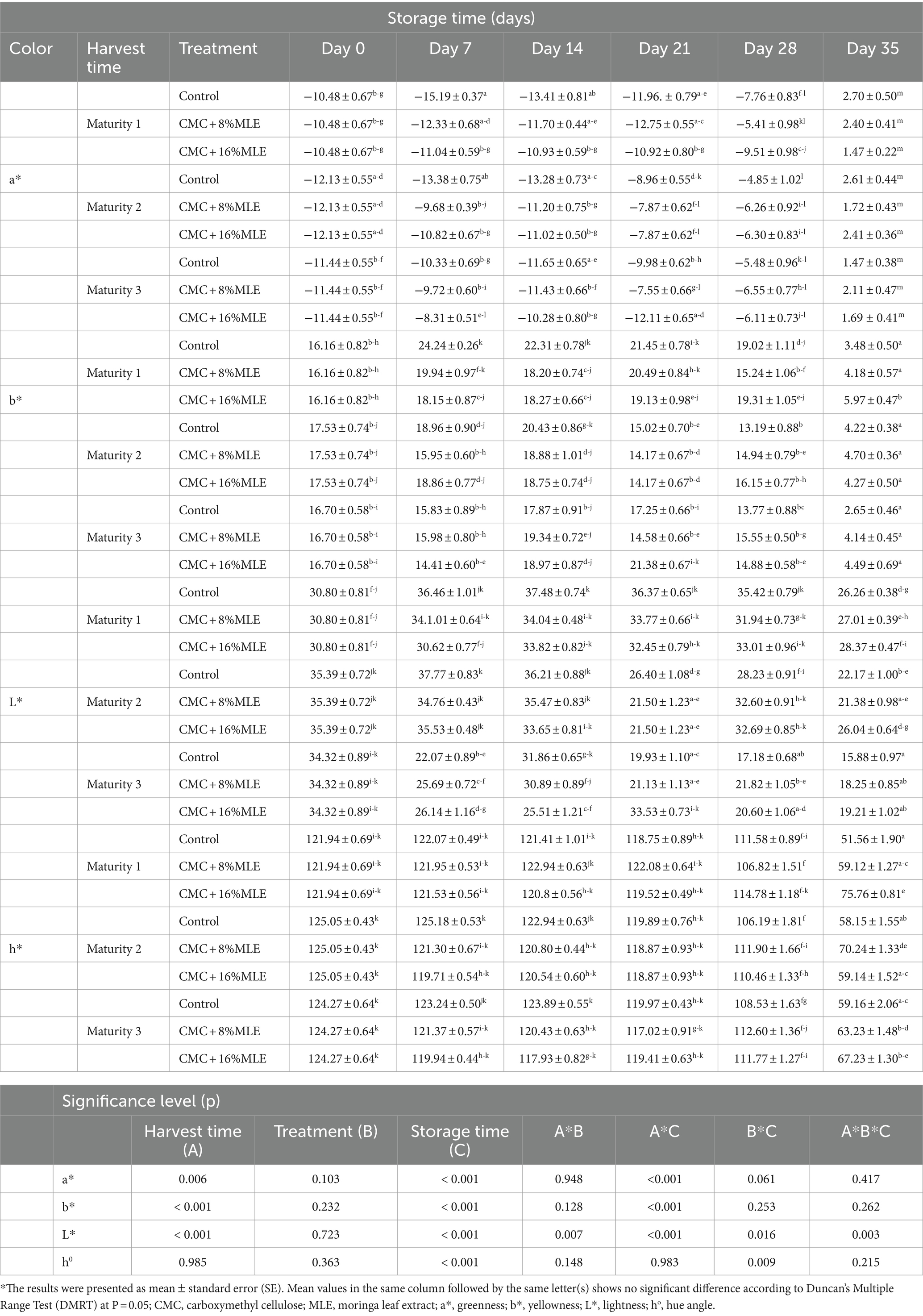
Table 2. The effect of CMC and MLE composite coating on the exocarp color of “Hass” avocado fruit harvested at three maturities during 28 days of cold storage and 7 days shelf-life.
The correlation between the a*, b*, L*, and h0 values in this study is in line with Handayani et al. (2018), who reported an inverse relationship between the a* and b* values on avocados treated with cassava peel edible coating. There was a significant effect (p < 0.05) of treatments and storage time on L* and h*; however, a sharp decline was observed between days 28 and 35 at room temperature. This delayed color change observed in coated fruits could be linked to the effect of the coatings in modifying the fruit’s atmosphere. Edible coatings slow the respiration rate and ethylene accumulation, the ripening hormone (Ali et al., 2011). The results for the L* values and visual judgments also indicated that the temperature, especially in cold storage, was suitable for storing avocado without causing chilling injury, which causes the darkening of fruit pulp (Careli-Gondim et al., 2020).
Total phenolics
Phenolics are produced in fruit tissues as secondary metabolites that activate antioxidants against oxidative stress (Peretto et al., 2017). These phytochemicals have a crucial role in the sensory and nutritional properties of the produce. The storage time, conditions, and stress severity affect the secondary metabolites in fruit. While storage period and harvest time showed a significant effect (p < 0.001), no statistically significant difference (p > 0.05) existed between treatments on the total phenolic compounds. The changes in total phenolics followed the same trend for all harvest times (Figure 2). Early harvested fruit showed a decline in phenolic content for the first 7 days of cold storage; after that, slight changes occurred depending on treatments. The decline in phenolics could be caused by the stress induced by the cold storage. These observed changes in phenolics may also result from applying edible coatings. Edible coatings have been previously reported to influence the production of phenolic compounds by modifying the produce metabolism, producing abiotic stress on produce (Dávila-Aviña et al., 2014). There were no remarkable differences in the fruit’s phenolic content throughout the storage period between the treatments, especially in mid- and late-harvested fruits. This indicates that, besides the potential of CMC and MLE coating to extend the shelf life, they can also retain the fruit phenolic concentration. This corroborates with Maringgal et al. (2020), who demonstrated that edible coatings help preserve the phytonutrients in fruit. Moreover, these results validate that edible coatings modify the internal atmosphere by serving as selective barriers to O2 and CO2, reducing respiration rate and delaying phenolic changes (Awad et al., 2017). The findings from this study are consistent with those presented by Chiabrando and Giacalone (2015), where the decrease in phenolic content and antioxidant capacity in blueberries was delayed by applying polysaccharide (chitosan) coatings. It is, however, important to mention that CMC, in combination with 8% MLE, resulted in a slightly increased phenolic content than all the other treatments at the end of the storage period, particularly in mid- and late-harvested fruits. This indicates that the coatings were able to delay fruit senescence, which results in disrupted cell structure, thereby resulting in reduced phenolics (Riaz et al., 2021). Consequently, the observed differences may have resulted from a higher respiration rate in untreated fruits associated with the breakdown of total phenols (Nair et al., 2018).

Figure 2. The effect of CMC and Moringa-based edible coatings on the changes in phenolic content of “Hass” avocado fruit harvested at maturity M1, M2, and M3 during 28 days of cold storage and 7 days of shelf-life. *The vertical bars represent standard error (SE) at n = 3; CMC, carboxymethyl cellulose; MLE, moringa leaf extract.
Total flavonoids content
Flavonoids are secondary metabolites that resemble variable phenolic structures and are involved in coloring many fruits, vegetables, and flowers. This phytochemical also provides health benefits such as anti-cancer, anti-inflammatory, and antioxidant properties (Zahedi et al., 2019). In the present study, total flavonoids were significantly (p < 0.001) affected by the storage period and harvest time (Figure 3). However, the coating treatments did not affect flavonoid concentration (p > 0.05). Cordenunsi et al. (2005) reported that storage conditions influence the concentration of flavonoids. The total flavonoid concentration was similar for all the treatments and harvesting times; however, the untreated fruits from the early harvest showed an increased flavonoid content after 35 days of storage period. These results are comparable to those of Panahirad et al. (2019), wherein plums treated with 0.5% CMC-based edible coating resulted in higher flavonoid content. In contrast, those treated with concentrations above 0.5% (1 and 1.5%) had less content than the untreated fruits. Langa (2018) also reported a rapid increase of flavonoids in untreated papaya fruit compared to those treated with CMC + moringa leaf or seed extract. Although the results presented in this study are inconsistent, a progressive decline in total flavonoids during the 28 days of cold storage was, however, observed. This was followed by a slight increase during the 7 days of shelf life, especially in early and mid-harvested fruits. Similarly, Ballesteros et al. (2022) reported an increase in flavonoid content in goldenberries stored at 20°C and 65% relative humidity for 12 days, irrespective of CMC-based coatings; this was, however, inverse for fruits stored for 28 days at 4°C and 95% RH. These results show that storage conditions influence flavonoids. In addition, cold storage tends to decrease, while high temperatures increase the flavonoids.
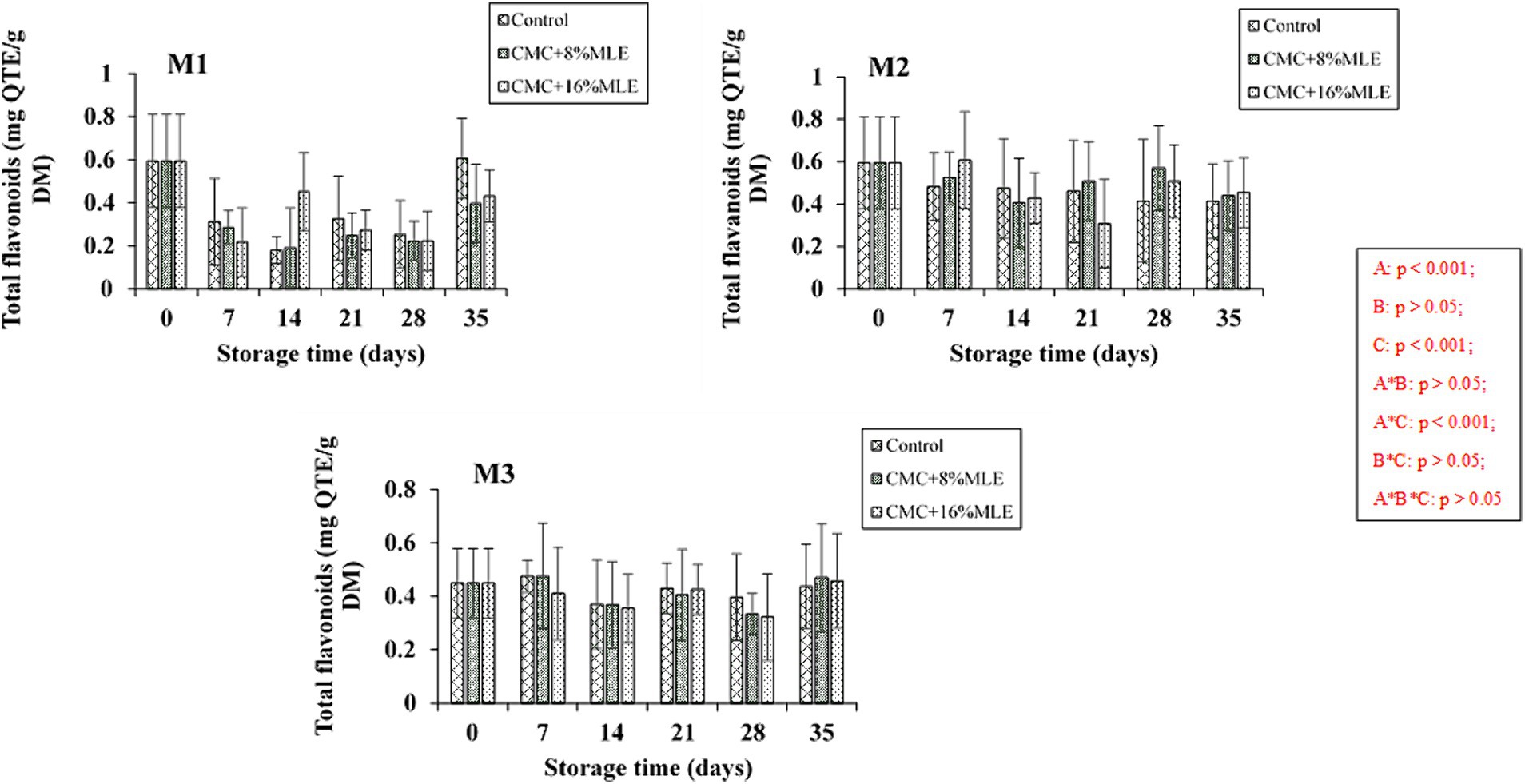
Figure 3. The effect of CMC and Moringa-based edible coatings on the changes in flavonoid content of “Hass” avocado fruit harvested at maturity M1, M2, and M3 during 28 days of cold storage and 7 days of shelf-life. *The vertical bars represent standard error (SE) at n = 3; CMC, carboxymethyl cellulose; MLE, moringa leaf extract.
2,2’ Diphenyl-1-picrylhydrazyl antioxidant assay
Antioxidant activity is a very important parameter that determines the health-related benefits and is usually determined using different methods, including the DPPH radical scavenging assay. This technique is one of the most popular methods to measure antioxidant activity due to its accuracy and convenience. The fruit’s antioxidant properties are greatly influenced by the presence of various secondary metabolites, including flavonoids and phenolics (Maringgal et al., 2020). This study showed a significant effect of the storage period and harvest time (p < 0.001) on the antioxidant activity of avocados. Figure 4 shows an inconsistent trend in the DPPH radical scavenging activity over time during the cold storage period and a decrease at ambient temperature, regardless of the harvest time. Although there was no significant difference between treatments (p > 0.05), the reduction in antioxidants was more pronounced in uncoated fruit than in MLE and CMC-coated avocados, which may indicate the positive effect of these composite coatings. The high decline in antioxidant activity in untreated fruits could be attributed to the fast rate of ripening, which is associated with fruit senescence and decay (Wang and Gao, 2013). The trend displayed by the antioxidant activity in this study contradicts the results by Kumar et al. (2021) on bell peppers treated with chitosan-pullulan composite coating and stored for 18 days at 4°C. These authors reported a decreasing trend in antioxidant activity. However, the present study aligns with Fernando et al. (2014), who reported an increase in antioxidant activities as the banana ripens and declines with senescence. Another study by Thakur et al. (2018) revealed the same trend: the scavenging activity in uncoated plums declined with ripening. Zahedi et al. (2019) also reported that chitosan-coated “Langra” mango fruit had higher antioxidant activities than control after 24 days, at 15 ± 2 and 85–90% RH storage conditions. The authors further stated that this may result from edible coatings forming a protective barrier on the fruit surface, which reduces the decline in antioxidant activity, nutrient loss, and water evaporation. This could show the potential of the MLE and CMC used in this study in retaining the scavenging activity of avocado fruit at 5 and 23°C. Usually, the bioactive compounds in the fruit have an impact on its antioxidant activity (Maftoonazad and Ramaswamy, 2005). This was supported by the results of this study, where the trend between phenolics and flavonoids showed an inverse relationship with antioxidant capacity, which could be associated with changes in these compounds (Awad et al., 2017).
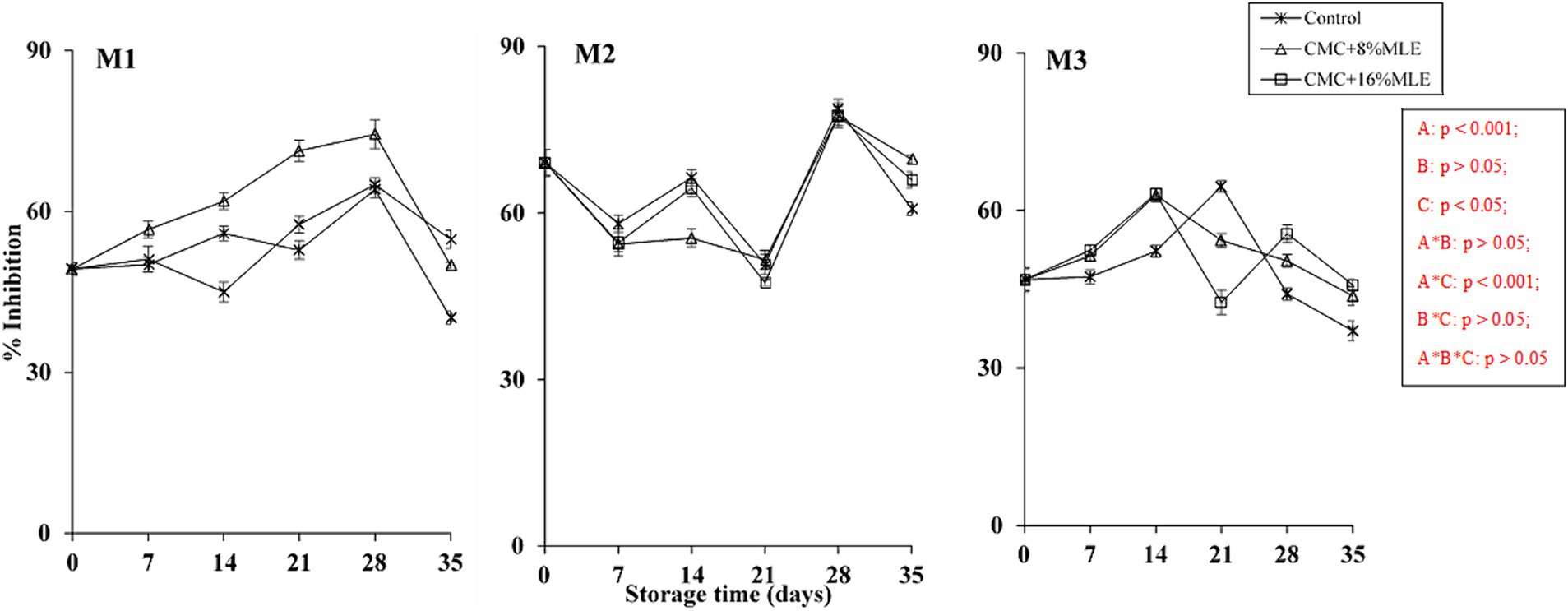
Figure 4. The effect of CMC and Moringa-based edible coatings on antioxidant activity of “Hass” avocado fruit harvested at maturity M1, M2, and M3 during 28 days of cold storage and 7 days of shelf-life. *The vertical bars represent standard error (SE) at n = 3; CMC, carboxymethyl cellulose; MLE, moringa leaf extract.
Sugars (mannoheptulose and perseitol)
Sugar content in avocado fruit is a critical quality indicator (Le et al., 2021). Among other fruits, avocado consists of unique sugars, such as perseitol, d-manno-heptulose (reducing sugar), and the seven-carbon sugar alcohol. Avocados produce D-mannoheptulose and perseitol in higher concentrations than other sugars, such as hexoses (Tesfay and Magwaza, 2017). Generally, the ripening of this fruit is associated with increased glucose and fructose and decreased D-mannoheptulose and perseitol concentrations. It is still in the best interest to evaluate the duration that fruit can be stored and still have optimal sugar concentrations without losing its quality. Figures 5, 6 show the changes in sugar content, mainly mannoheptulose and perseitol, respectively, of CMC-moringa-coated avocado fruit at 7-day intervals during the storage period. The initial sugar concentrations before treatment were determined to be 19.67, 34.04, and 47.34 mg/g DW for mannoheptulose and 15.37, 21.77, and 17.2 mg/g DW for perseitol and for early, mid, and late harvested fruits, respectively. The results showed a significant effect (p > 0.001) of the storage period on the concentration of mannoheptulose and perseitol. The concentrations for these two sugars showed a progressive decline throughout the storage period. In the present study, the untreated fruit had less mannoheptulose concentrations of 4.85, 4.28, and 5.54 mg/g DW for the early, mid, and late harvested fruits, respectively, than fruits coated with different concentrations of MLE and 5% CMC. This indicates a 75.3, 87.4, and 88.3% reduction from the initial concentrations for the early, mid, and late-harvested fruits, respectively. Similar to mannoheptulose, the most reduction in perseitol concentrations was observed in untreated fruits for the early and mid-harvested fruit. The reduction in these C7 sugars is due to their high contribution to the total carbohydrate concentration compared to the 6-carbon (C6) sugars (sucrose, starch, and hexose), with perseitol being dominant (Liu et al., 2002). This reduction validates the assertion by Wolstenholme (2013) that the C7 sugars concentration depends on the ripening stage of the avocado, and its reduction can go above 80% and, in some cultivars, can be depleted. In addition, it was previously reported that the ripening and its associated physiological processes, such as increased ethylene production and respiration, do not occur until the C7 sugars drop below a threshold (20 mg/g DW) (Liu et al., 2002). This could indicate that the C7 sugars are metabolized during the ripening or are the main ones that control the ripening process (Landahl et al., 2009; Blakey et al., 2012). The trend observed in this study is similar to that reported by Shezi et al. (2020) for “Hass” avocado fruit harvested inside and outside the canopy during storage. Moreover, these results are comparable to those reported by Tesfay and Magwaza (2017), who observed a decrease in soluble sugars in “Fuerte” and “Hass” avocados treated with CMC and chitosan based on moringa extracts. Similarly, Kubheka et al. (2021) reported a higher D-mannoheptulose in “Maluma” avocado fruit treated with 1% CMC and MLE. Overall, based on these findings, it was clearly observed that treating fruit with CMC (5%) and MLE is beneficial in minimizing the reduction in C7 sugars.
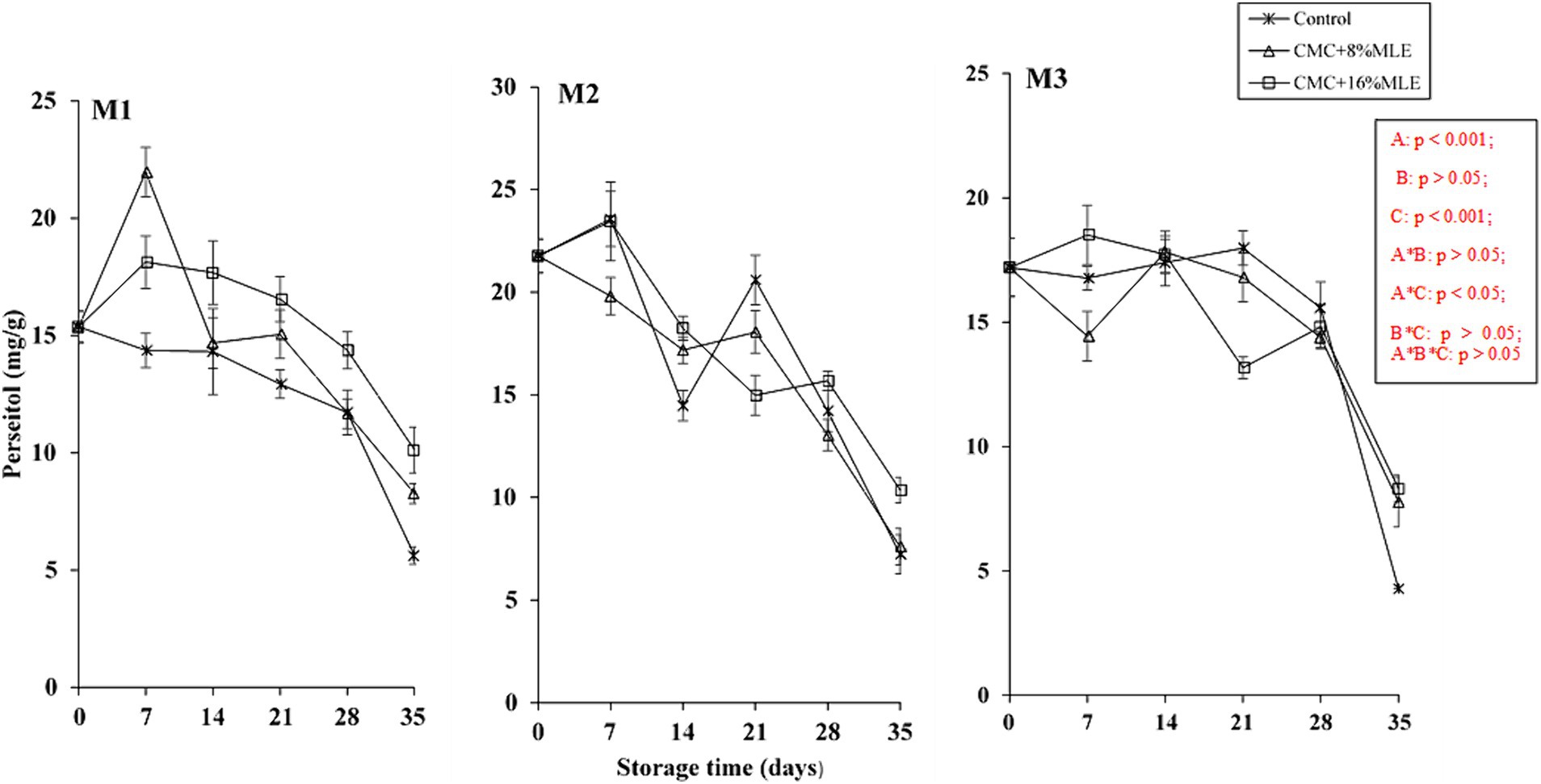
Figure 5. The effect of CMC and MLE-based edible coatings on perseitol of “Hass” avocado harvested at maturity M1, M2, and M3 during 28 days of cold storage and 7 days of shelf-life. *The vertical bars represent standard error (SE) at n = 3; CMC, carboxymethyl cellulose; MLE, moringa leaf extract.
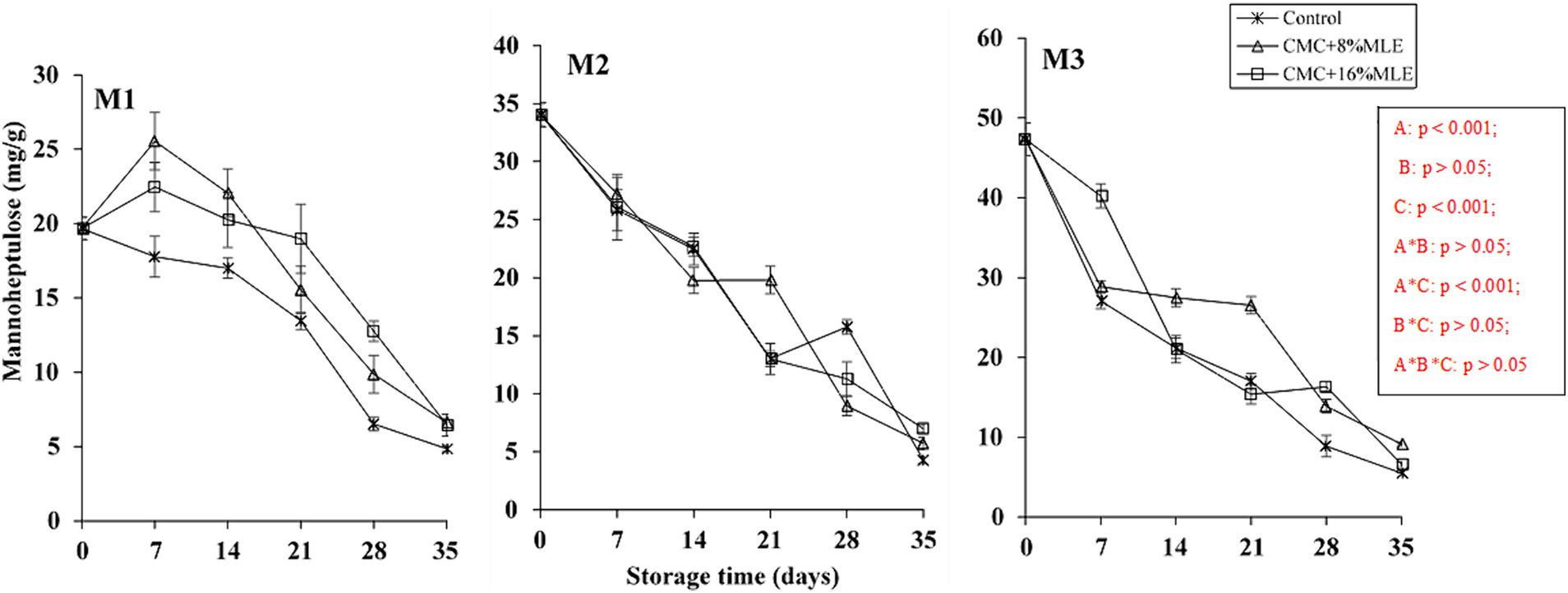
Figure 6. The effect of CMC and MLE-based edible coatings on mannoheptulose of “Hass” avocado harvested at maturity M1, M2, and M3 during 28 days of cold storage and 7 days of shelf-life. *The vertical bars represent standard error (SE) at n = 3; CMC, carboxymethyl cellulose; MLE, moringa leaf extract.
Principal component analysis
The Principal Component Analysis (PCA) was used in this study to determine the most effective coating treatment at day 35 and the best harvesting time. The PCA biplot presented a variability of 67.9%, with the first principal component (Dim1) contributing 41.9% and the second principal component (Dim2) contributing 23.3% of the variation. It was observed that perseitol, yellowness, firmness, hue value, and weight loss contributed more to describing the variation between coating treatments and harvest times (Figure 7). These parameters had a significant and strong positive correlation contributing to (Dim1), besides the weight loss, which showed a negative correlation. The late harvesting period had a lower contribution to the PCA and exhibited poor correlation with most fruit quality measured parameters; therefore, it cannot be recommended, despite the fruit’s high mannoheptulose content. From the generated PCA, it can be commended that harvesting fruits at their early maturity stages and treating them with CMC combined with 16% MLE resulted in fruit with higher hue, yellowness, perseitol, and firmness values; however, they had very low TFC. Treating fruits with CMC and 8% exhibited a mostly neutral effect. Furthermore, fruits harvested at their mid-stage of maturity and treated with CMC and 16% or 8% MLE showed a high antioxidant status, lightness, and total phenolics.
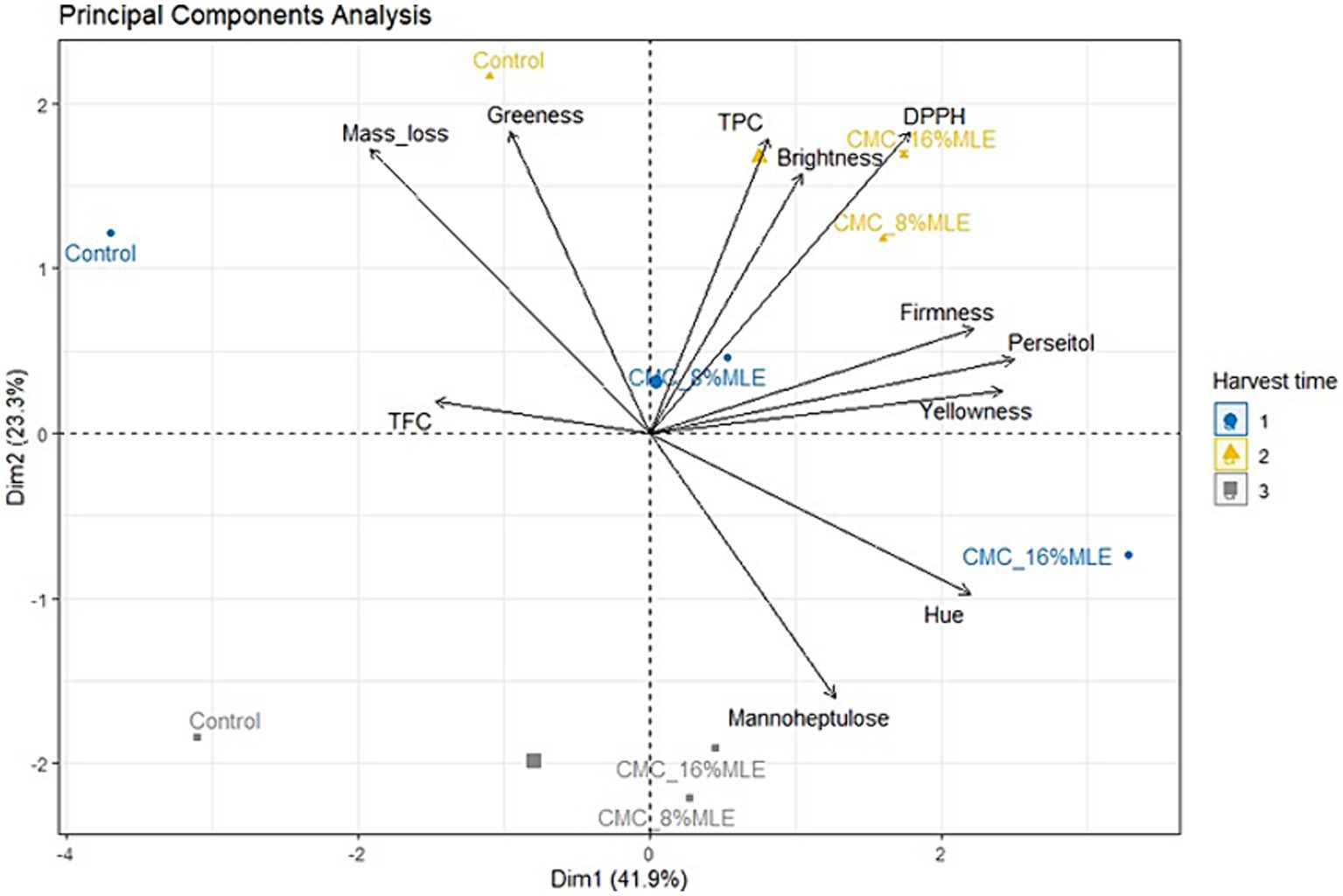
Figure 7. Principal Component Analysis (PCA)-biplot illustrating the correlation between the harvest time, coating treatments, and evaluated parameters at 35 day of storage. CMC, carboxymethyl cellulose; MLE, moringa leaf extract; TPC, total phenolic compounds; TFC, total flavonoids content; DPPH, 2,2’ Diphenyl-1-picrylhydrazyl.
Conclusion
Based on the results of the current study, it can be concluded that CMC and MLE were effective in conserving the postharvest quality of “Hass” avocado fruit during a storage period of 35 days. Different moringa-based treatments successfully inhibited firmness and weight loss, consequently extending fruit shelf life. The coated fruit also had a reduced reduction in soluble sugars. This is an indication that CMC and moringa-based edible coatings could be the best alternative for substituting the currently used chemicals and costly preservative techniques for extending avocado shelf life. This research has also shown the ability of the used coatings to extend the shelf life of avocados without compromising the nutritional quality. From the PCA results, increasing the concentration of moringa resulted in better treatment work efficacy, especially in early and mid-harvested fruits. Notwithstanding, future research could benefit from investigating whether the 16% moringa concentration is the optimum or whether further increasing the concentration could have a more positive effect on the fruit quality. However, from the perspective of easy accessibility and application, these two concentrations are recommended. This is the most effective, environmentally friendly, and affordable technique that could benefit farmers. Most importantly, harvesting avocado fruit at their early to mid-maturity stages is recommended for prolonged quality retention.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SN: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. ST: Conceptualization, Funding acquisition, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing. LM: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing. AM: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Financial support for this study was provided by the National Research Foundation of South Africa (NRF Reference: MND210526603960) and South African Avocado Growers’ Association.
Acknowledgments
The authors are grateful to Thokozani Nkosi for providing technical assistance in the laboratory.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abebe, Z., Tola, Y. B., and Mohammed, A. (2017). Effects of edible coating materials and stages of maturity at harvest on storage life and quality of tomato (Lycopersicon Esculentum mill.) fruits. Afr. J. Agric. Res. 12, 550–565. doi: 10.5897/AJAR2016.11648
Addo, P. W., Sagili, S. U. K. R., Bilodeau, S. E., Gladu-Gallant, F.-A., MacKenzie, D. A., Bates, J., et al. (2022). Cold ethanol extraction of cannabinoids and terpenes from Cannabis using response surface methodology: optimization and comparative study. Molecules 27:8780. doi: 10.3390/molecules27248780
Ali, A., Muhammad, M. T. M., Sijam, K., and Siddiqui, Y. (2011). Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 124, 620–626. doi: 10.1016/j.foodchem.2010.06.085
Awad, M. A., Al-Qurashi, A. D., Mohamed, S. A., and El-Shishtawy, R. M. (2017). Quality and biochemical changes of ‘Hindi-Besennara’mangoes during shelf life as affected by chitosan, gallic acid and chitosan gallate. J. Food Sci. Technol. 54, 4139–4148. doi: 10.1007/s13197-017-2762-x
Ballesteros, L. F., Teixeira, J. A., and Cerqueira, M. A. (2022). Active carboxymethyl cellulose-based edible coatings for the extension of fresh goldenberries shelf-life. Horticulturae 8:936. doi: 10.3390/horticulturae8100936
Bill, M., Sivakumar, D., Thompson, A. K., and Korsten, L. (2014). Avocado fruit quality management during the postharvest supply chain. Food Rev. Intl. 30, 169–202. doi: 10.1080/87559129.2014.907304
Blakey, R., Tesfay, S., Bertling, I., and Bower, J. (2012). Changes in sugars, total protein, and oil in ‘Hass’ avocado (Persea americana mill.) fruit during ripening. J. Hortic. Sci. Biotechnol. 87, 381–387. doi: 10.1080/14620316.2012.11512880
Careli-Gondim, Í., Mesquita, T. C., Vilas Boas, E. V., Caliari, M., and Soares Júnior, M. S. (2020). The effect of active coating and refrigerated storage on the quality of avocado cultivar, quintal. J. Food Sci. Technol. 57, 143–151. doi: 10.1007/s13197-019-04039-3
Chiabrando, V., and Giacalone, G. (2015). Anthocyanins, phenolics and antioxidant capacity after fresh storage of blueberry treated with edible coatings. Int. J. Food Sci. Nutr. 66, 248–253. doi: 10.3109/09637486.2014.986075
Cordenunsi, B. R., Genovese, M. I., Oliveira do Nascimento, J. R., Aymoto Hassimotto, N. M., José dos Santos, R., and Lajolo, F. M. (2005). Effects of temperature on the chemical composition and antioxidant activity of three strawberry cultivars. Food Chem. 91, 113–121. doi: 10.1016/j.foodchem.2004.05.054
Dávila-Aviña, J. E., Villa-Rodríguez, J. A., Villegas-Ochoa, M. A., Tortoledo-Ortiz, O., Olivas, G. I., Ayala-Zavala, J. F., et al. (2014). Effect of edible coatings on bioactive compounds and antioxidant capacity of tomatoes at different maturity stages. J. Food Sci. Technol. 51, 2706–2712. doi: 10.1007/s13197-012-0771-3
Dhall, R. K. (2016). “Application of edible films and coatings on fruits and vegetables” in Edible films and coatings. Eds. Atul Tiwari, Anthony Galanis, Mark D. Soucek (India: Punjab Agricultural University, Ludhiana, Punjab), 381–408.
Eça, K. S., Sartori, T., and Menegalli, F. C. (2014). Films and edible coatings containing antioxidants-a review. Braz. J. Food Technol. 17, 98–112. doi: 10.1590/bjft.2014.017
Fan, S., Qi, Y., Shi, L., Giovani, M., Zaki, N. A. A., Guo, S., et al. (2022). Screening of phenolic compounds in rejected avocado and determination of their antioxidant potential. PRO 10:1747. doi: 10.3390/pr10091747
Fernando, H., Srilaong, V., Pongprasert, N., Boonyaritthongchai, P., and Jitareerat, P. (2014). Changes in antioxidant properties and chemical composition during ripening in banana variety’Hom thong’ (AAA group) and’Khai’ (AA group). Int. Food Res. J. 21:749.
Handayani, M., Karlina, S., Sugiarti, Y., and Cakrawati, D. (2018). Application of edible coating from cassava peel–bay leaf on avocado. J. Phys. Conf. Series 1013:012168. doi: 10.1088/1742-6596/1013/1/012168
Jeong, J., and Huber, D. J. (2004). Suppression of avocado (Persea americana mill.) fruit softening and changes in cell wall matrix polysaccharides and enzyme activities: differential responses to 1-MCP and delayed ethylene application. J. Am. Soc. Hortic. Sci. 129, 752–759. doi: 10.21273/JASHS.129.5.0752
Kader, A. A. (1999). Fruit maturity, ripening, and quality relationships. Acta Hortic. 485, 203–208. doi: 10.17660/ActaHortic.1999.485.27
Kassim, A., Workneh, T., and Bezuidenhout, C. (2013). A review on postharvest handling of avocado fruit. Afr. J. Agric. Res. 8, 2385–2402. doi: 10.5897/AJAR12.1248
Khorram, F., Ramezanian, A., and Hosseini, S. M. H. (2017). Effect of different edible coatings on postharvest quality of ‘Kinnow’mandarin. J. Food Meas. Charact. 11, 1827–1833. doi: 10.1007/s11694-017-9564-8
Kubheka, S. F., Tesfay, S. Z., Mditshwa, A., and Magwaza, L. S. (2021). Efficacy of carboxymethyl cellulose and gum arabic edible coatings in combination with moringa leaf extract in improving postharvest quality of ‘Maluma’ avocado fruit. Acta Hortic, 1306:293–300. doi: 10.17660/ActaHortic.2021.1306.37
Kumar, N., Ojha, A., Upadhyay, A., Singh, R., and Kumar, S. (2021). Effect of active chitosan-pullulan composite edible coating enrich with pomegranate peel extract on the storage quality of green bell pepper. LWT Food Sci. Technol. 138:110435. doi: 10.1016/j.lwt.2020.110435
Landahl, S., Meyer, M. D., and Terry, L. A. (2009). Spatial and temporal analysis of textural and biochemical changes of imported avocado cv. Hass during fruit ripening. J. Agric. Food Chem. 57, 7039–7047. doi: 10.1021/jf803669x
Langa, S. (2018). Effects of edible coatings and moringa extracts on postharvest quality of papaya fruits (doctoral dissertation). South Africa: University of KwaZulu-Natal, Pietermaritzburg.
Le, K. H., Nguyen, M. D.-B., Dai Tran, L., Thi, H. P. N., Van Tran, C., Van Tran, K., et al. (2021). A novel antimicrobial ZnO nanoparticles-added polysaccharide edible coating for the preservation of postharvest avocado under ambient conditions. Prog. Org. Coat. 158:106339. doi: 10.1016/j.porgcoat.2021.106339
Liu, X., Sievert, J., Arpaia, M. L., and Madore, M. A. (2002). Postulated physiological roles of the seven-carbon sugars, mannoheptulose, and perseitol in avocado. J. Am. Soc. Hortic. Sci. 127, 108–114. doi: 10.21273/JASHS.127.1.108
Liu, W., Zhang, M., and Bhandari, B. (2020). Nanotechnology–a shelf life extension strategy for fruits and vegetables. Crit. Rev. Food Sci. Nutr. 60, 1706–1721. doi: 10.1080/10408398.2019.1589415
Maftoonazad, N., and Ramaswamy, H. (2005). Postharvest shelf-life extension of avocados using methyl cellulose-based coating. LWT Food Sci. Technol. 38, 617–624. doi: 10.1016/j.lwt.2004.08.007
Magwaza, L. S., and Tesfay, S. Z. (2015). A review of destructive and non-destructive methods for determining avocado fruit maturity. Food Bioprocess Technol. 8, 1995–2011. doi: 10.1007/s11947-015-1568-y
Maringgal, B., Hashim, N., Tawakkal, I. S. M. A., and Mohamed, M. T. M. (2020). Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 96, 253–267. doi: 10.1016/j.tifs.2019.12.024
Milbury, P. E., Chen, C.-Y., Dolnikowski, G. G., and Blumberg, J. B. (2006). Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 54, 5027–5033. doi: 10.1021/jf0603937
Mwelase, S., Mditshwa, A., Magwaza, L. S., and Tesfay, S. Z. (2022). Maturity indexing and postharvest performance of newly developed ‘lamb Hass’ avocado fruit. Int. J. Fruit Sci. 22, 453–470. doi: 10.1080/15538362.2022.2054906
Nair, M. S., Saxena, A., and Kaur, C. (2018). Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 240, 245–252. doi: 10.1016/j.foodchem.2017.07.122
Obeng, E., Kpodo, F., Tettey, C., Essuman, E., and Adzinyo, O. (2020). Antioxidant, total phenols and proximate constituents of four tropical leafy vegetables. Sci. Afr. 7:e00227. doi: 10.1016/j.sciaf.2019.e00227
Panahirad, S., Dadpour, M., Peighambardoust, S. H., Soltanzadeh, M., Gullón, B., Alirezalu, K., et al. (2021). Applications of carboxymethyl cellulose-and pectin-based active edible coatings in preservation of fruits and vegetables: a review. Trends Food Sci. Technol. 110, 663–673. doi: 10.1016/j.tifs.2021.02.025
Panahirad, S., Naghshiband-Hassani, R., Ghanbarzadeh, B., Zaare-Nahandi, F., and Mahna, N. (2019). Shelf life quality of plum fruits (Prunus domestica L.) improves with carboxymethylcellulose-based edible coating. HortScience 54, 505–510. doi: 10.21273/HORTSCI13751-18
Paniagua, A., East, A., Hindmarsh, J., and Heyes, J. (2013). Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 79, 13–19. doi: 10.1016/j.postharvbio.2012.12.016
Payasi, A., Mishra, N. N., Chaves, A. L. S., and Singh, R. (2009). Biochemistry of fruit softening: an overview. Physiol. Mol. Biol. Plants 15, 103–113. doi: 10.1007/s12298-009-0012-z
Peretto, G., Du, W.-X., Avena-Bustillos, R., Berrios, J. D. J., Sambo, P., and McHugh, T. (2017). Electrostatic and conventional spraying of alginate-based edible coating with natural antimicrobials for preserving fresh strawberry quality. Food Bioprocess Technol. 10, 165–174. doi: 10.1007/s11947-016-1808-9
Riaz, A., Aadil, R. M., Amoussa, A. M. O., Bashari, M., Abid, M., and Hashim, M. M. (2021). Application of chitosan‐based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassacv Hongyan) fruit. J. Food Proces. Preserv. 45:e15018. doi: 10.1111/jfpp.15018
Rivera, S. A., Ferreyra, R., Robledo, P., Selles, G., Arpaia, M. L., Saavedra, J., et al. (2017). Identification of preharvest factors determining postharvest ripening behaviors in ‘Hass’ avocado under long term storage. Sci. Hortic. 216, 29–37. doi: 10.1016/j.scienta.2016.12.024
Romanazzi, G., Feliziani, E., and Sivakumar, D. (2018). Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: eliciting, antimicrobial and film-forming properties. Front. Microbiol. 9:2745. doi: 10.3389/fmicb.2018.02745
Sharma, L., Saini, C. S., Sharma, H. K., and Sandhu, K. S. (2019). Biocomposite edible coatings based on cross linked-sesame protein and mango puree for the shelf life stability of fresh-cut mango fruit. J. Food Process Eng. 42:e12938. doi: 10.1111/jfpe.12938
Shezi, S., Magwaza, L. S., Tesfay, S. Z., and Mditshwa, A. (2020). Simple and multiple linear regression models for predicting maturity of ‘Mendez# 1’and ‘Hass’ avocado fruit harvested from inside and outside tree canopy positions. Int. J. Fruit Sci. 20, S1969–S1983. doi: 10.1080/15538362.2020.1839626
Singh, P., Magalhães, S., Alves, L., Antunes, F., Miguel, M., Lindman, B., et al. (2019). Cellulose-based edible films for probiotic entrapment. Food Hydrocoll. 88, 68–74. doi: 10.1016/j.foodhyd.2018.08.057
Sivakumar, D., and Bautista-Baños, S. (2014). A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 64, 27–37. doi: 10.1016/j.cropro.2014.05.012
Sivakumar, D., Tuna Gunes, N., and Romanazzi, G. (2021). A comprehensive review on the impact of edible coatings, essential oils, and their nano formulations on postharvest decay anthracnose of avocados, mangoes, and papayas. Front. Microbiol. 12:711092. doi: 10.3389/fmicb.2021.711092
Tesfay, S. Z., and Magwaza, L. S. (2017). Evaluating the efficacy of moringa leaf extract, chitosan and carboxymethyl cellulose as edible coatings for enhancing quality and extending postharvest life of avocado (Persea americana mill.) fruit. Food Packag. Shelf Life 11, 40–48. doi: 10.1016/j.fpsl.2016.12.001
Tesfay, S. Z., Magwaza, L. S., Mbili, N., and Mditshwa, A. (2017). Carboxyl methylcellulose (CMC) containing moringa plant extracts as new postharvest organic edible coating for avocado (Persea americana mill.) fruit. Sci. Hortic. 226, 201–207. doi: 10.1016/j.scienta.2017.08.047
Tesfay, S. Z., Mathe, S., Modi, A. T., and Mabhaudhi, T. (2016). A comparative study on antioxidant potential of selected African and exotic leafy vegetables. HortScience 51, 1529–1536. doi: 10.21273/HORTSCI11161-16
Thakur, R., Pristijono, P., Golding, J., Stathopoulos, C. E., Scarlett, C., Bowyer, M., et al. (2018). Development and application of rice starch based edible coating to improve the postharvest storage potential and quality of plum fruit (Prunus salicina). Sci. Hortic. 237, 59–66. doi: 10.1016/j.scienta.2018.04.005
Wang, S. Y., and Gao, H. (2013). Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.). LWT Food Sci. Technol. 52, 71–79. doi: 10.1016/j.lwt.2012.05.003
Wolstenholme, B. N. (2013). Developments in the world avocado industry, and their relevance to the South African and African industries. Acta Hortic. 1007, 865–871. doi: 10.17660/ActaHortic.2013.1007.103
Wróblewska-Krepsztul, J., Rydzkowski, T., Borowski, G., Szczypiński, M., Klepka, T., and Thakur, V. K. (2018). Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int. J. Polym. Anal. Charact. 23, 383–395. doi: 10.1080/1023666X.2018.1455382
Yaman, Ö., and Bayoιndιrlι, L. (2002). Effects of an edible coating and cold storage on shelf-life and quality of cherries. LWT Food Sci. Technol. 35, 146–150. doi: 10.1006/fstl.2001.0827
Zahedi, S. M., Hosseini, M. S., Karimi, M., and Ebrahimzadeh, A. (2019). Effects of postharvest polyamine application and edible coating on maintaining quality of mango (Mangifera indica L.) cv. Langra during cold storage. Food Sci. Nutr. 7, 433–441. doi: 10.1002/fsn3.802
Keywords: postharvest losses, fruit quality, edible coatings, shelf life, nutritional compounds
Citation: Ngubane S, Tesfay SZ, Magwaza LS and Mditshwa A (2024) The effect of composite edible coatings on the postharvest quality of “Hass” avocado fruit treated at different harvest maturities. Front. Sustain. Food Syst. 8:1473731. doi: 10.3389/fsufs.2024.1473731
Edited by:
Laurent Dufossé, Université de la Réunion, FranceReviewed by:
Rajinder Kumar Dhall, Punjab Agricultural University, IndiaGhulam Khaliq, Lasbela University of Agriculture, Water and Marine Sciences, Pakistan
Copyright © 2024 Ngubane, Tesfay, Magwaza and Mditshwa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Asanda Mditshwa, bWRpdHNod2FhQHVrem4uYWMuemE=
†ORCID: Sibonelo Ngubane, orcid.org/0000-0002-4251-9324
 Sibonelo Ngubane1†
Sibonelo Ngubane1† Samson Z. Tesfay
Samson Z. Tesfay Lembe S. Magwaza
Lembe S. Magwaza Asanda Mditshwa
Asanda Mditshwa