- 1Department of Zoology, University of Education, Lahore, Punjab, Pakistan
- 2Fish Nutrition Laboratory, Department of Zoology, Government College University Faisalabad, Faisalabad, Punjab, Pakistan
- 3Environmental Studies Department, University of California Santa Cruz, Santa Cruz, CA, United States
- 4Department of Environmental Sciences, Government College University, Faisalabad, Punjab, Pakistan
- 5Department of Biological Sciences and Technology, China Medical University, Taichung, Taiwan
- 6Department of Zoology, University of Sialkot, Sialkot, Punjab, Pakistan
- 7Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia
Introduction: Probiotics, one of functional feed additives (FFAs), have emerged as a potential supplement to strengthen fish health and mitigate oxidative stress. The main focus of our research was to explore the benefits of probiotics (protexin) on growth, digestibility, antioxidant enzyme activity, and blood indices of Cirrhinus mrigala fingerlings.
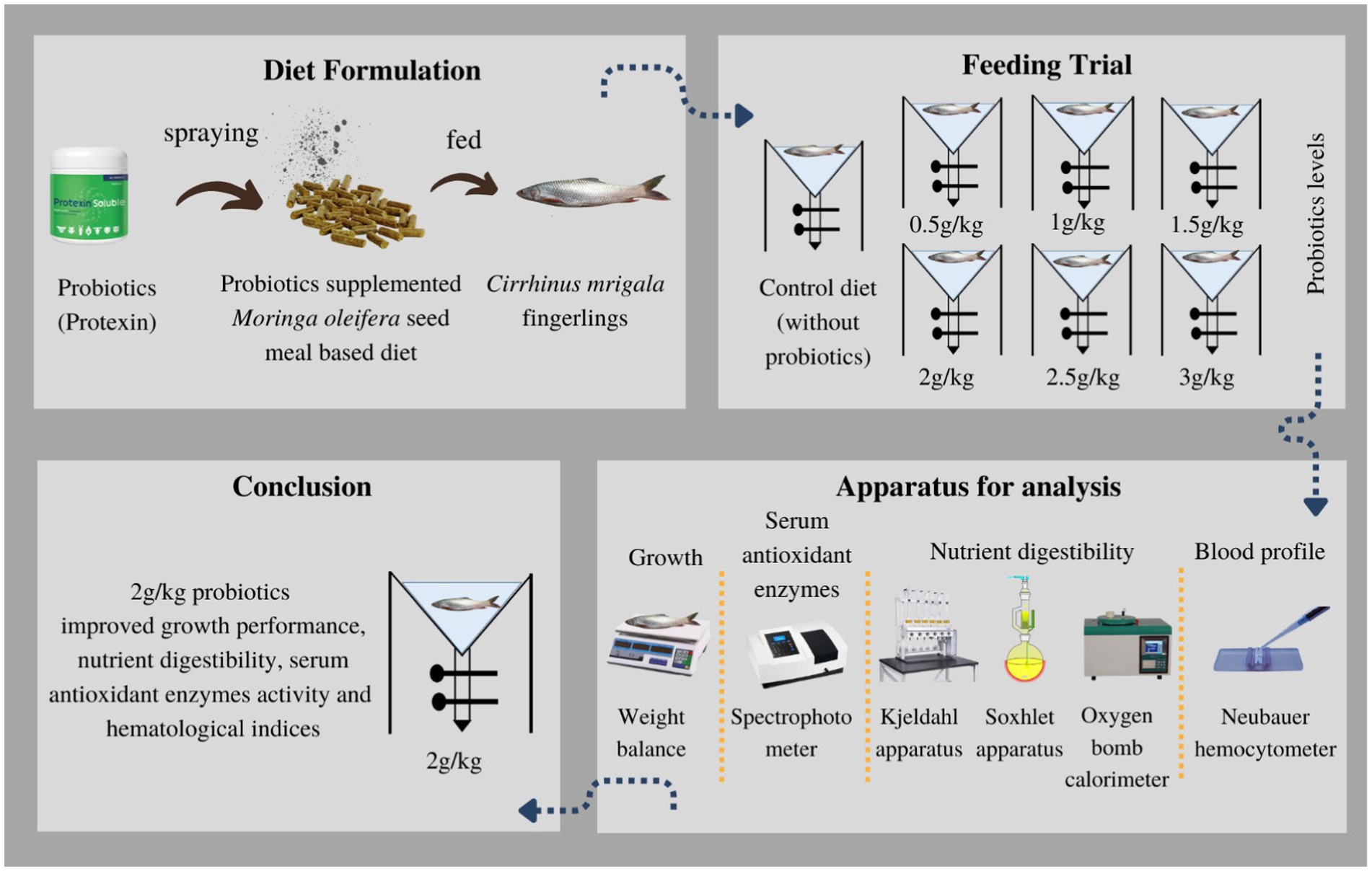
Methods: A total of 105 fish (7.42 ± 0.02 g/fish) were used in a 90-day feeding trial. Fish were fed two times a day, and diets were tested in triplicate tanks (15 fingerlings per tank). Moringa oleifera seed meal was chosen as a basal feed ingredient. Seven test diets were formulated: One was control (without supplement), and six diets had different concentrations of probiotics, such as 0.5, 1, 1.5, 2, 2.5, and 3 g kg−1.
Results: The results of growth and nutrient absorption were maximum at a probiotic concentration of 2 g kg−1. The optimal serum antioxidant enzyme activity and blood parameters were likewise obtained at a probiotic dose of 2 g kg−1.
Conclusions: Supplementing C. mrigala with 2 g kg−1 probiotics substantially improved their growth, nutrient digestibility, antioxidant enzyme activity, and hematological indices (p < 0.05).
1 Introduction
Aquaculture has now become a rapidly expanding food industry, which accounted for approximately 87.5 million tons of aquatic output in 2020 [Food and Agriculture Organization (FAO), 2022]. Cirrhinus mrigala, a valuable species of Indian major carp, is found in southeast Asia and is valued for its taste, low-cost, and commercial significance in polyculture [Food and Agriculture Organization (FAO), 2014]. It is an omnivorous species with a tendency toward herbivory. It feeds mostly on decaying matter and phytoplankton, followed by zooplankton and insects (Soni and Ujjania, 2018).
While fishmeal (FM) has been the gold standard for balanced diets in industrial fish farming due to its high-quality protein, digestibility, and essential nutrients (Guedes et al., 2015), its scarcity and increasing cost pose challenges for large-scale aquaculture (Ahmad et al., 2021; Oliva-Teles et al., 2022). To address this, researchers are intensifying their efforts to develop innovative and functional feeds as alternatives to FM, with plant by-products being a promising area of exploration (Caruso, 2015).
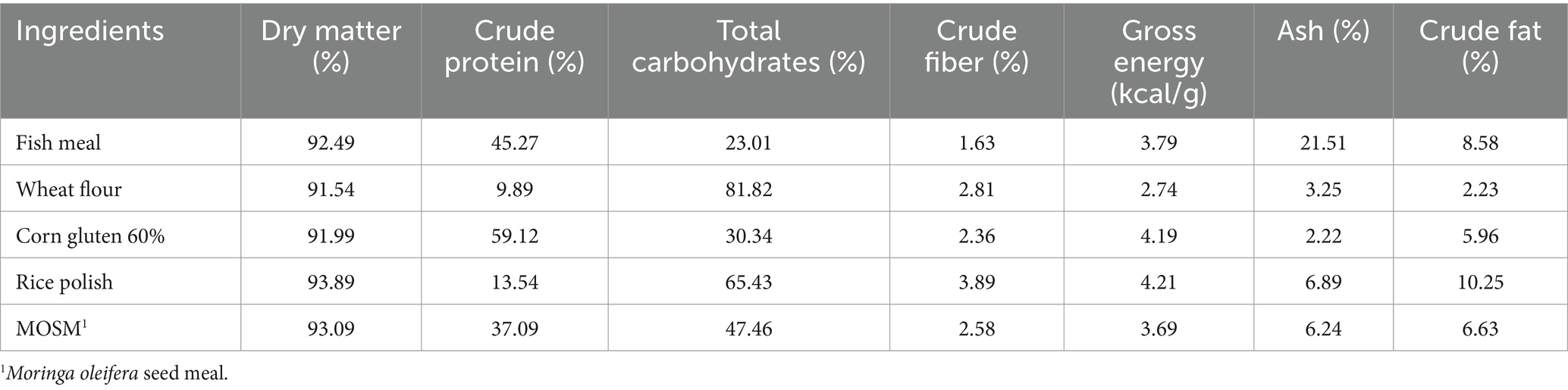
Moringa oleifera seed meal (MOSM) emerges as a potential fish feed alternative due to its high crude protein content, reaching up to 40.34% (Liang et al., 2019). Also named the “Miracle Tree” for its diverse uses in medicine, nutrition, and the economic value it holds (Gopalakrishnan et al., 2016). However, anti-nutritional factors such as tannins, alkaloids, and phytates (Abdel-Latif et al., 2022) in MOSM can hinder nutrient absorption, digestion, and metabolism in fish. Current research explores the use of functional feed additives (FFAs) such as probiotics to counteract these negative effects. FFAs can improve digestive enzyme activity, nutrient uptake, and energy utilization, ultimately promoting optimal growth, digestion, and overall fish performance (Hosain and Liangyi, 2020).
Probiotics, live and non-pathogenic microbes, are gaining significant recognition for their diverse health benefits in hosts, including improved gut health and a balanced gut microbiome (Hu et al., 2017). Multi-strain probiotics, like the commercially available protexin, offer advantages over single-strain options due to their combination of lactic acid bacteria, yeast, and fungi (Firouzbakhsh et al., 2011; Hossain et al., 2022). These microbes are essential for digestion, nutrient uptake, immunity, and disease resistance (Diwan et al., 2022). Probiotics enhance digestive enzyme efficacy, resulting in improved nutrient absorption and digestion in fish. They can also increase intestinal villi height, maximizing the surface area for nutrient uptake (Tong et al., 2023). Furthermore, probiotics upregulate antioxidant enzymes, strengthening the body’s defense system against stress (Gobi et al., 2018). Since Cirrhinus mrigala, an agastric fish, experiences extended food retention in its small intestine, probiotics can be particularly beneficial. By detoxifying harmful microbes and boosting digestive enzymes, probiotics can significantly improve nutrient absorption (Ntakirutimana et al., 2023). Therefore, this study aims to develop a new aquaculture feed formula containing varying levels of probiotics and assess its impact on growth, digestibility, antioxidant enzyme activity, and blood parameters in C. mrigala.
2 Materials and methods
The investigation was conducted at the Fish Nutrition Laboratory in the Department of Zoology at Government College University, Faisalabad.
2.1 Ethical statement
All experimental protocols were approved by the guidelines provided by the “Ethics Review Committee of the Government College University Faisalabad.” This research was performed in an ethical and moral way. In this study, no toxins were administered to fish that could harm them, and a minimum number of fish were employed with proper care in accordance with the standards of the Ethical Committee.
2.2 Preparation of fish feeding trial
In order to culture C. mrigala, they were procured from Fish Hatchery, which is located on Satiana Road, Faisalabad, Punjab, Pakistan. For a period of two weeks, these fingerlings were kept in V-shaped tanks (70-liter per tenk) at the location of the experiment to acclimatize to their surroundings and were supplied with fresh, oxygenated water daily. Before the trial began, they were dipped in 5 g of NaCl (saline solution) to prevent infection from ecto-parasites (Rowland, 1991). Consistent estimations were made within the ranges of 24.8°C–28.6°C, 7.4–8.5, and 5.8–7.4 milligrams per liter for the temperature, pH, and dissolved oxygen (DO), respectively.
2.3 Formulation of test diets
Before the MOSM-based diet preparation, all products were bought locally and seeds were de-fatted by drying and pressing method. After crushing and sieving with 0.3 mm mesh, MOSM was formed. The inert marker chromic oxide (Cr2O3) was also used as a diet ingredient to evaluate digestibility (Divakaran et al., 2002). Before making diets, feed components were analyzed chemically (Table 1). First, all ingredients were finely ground to pass through a 0.5 mm sieve and mixed for 5 min in an electric mixer. In addition, fish oil was provided to adjust fat content as required. Then, 10% to 15% of water was mixed to form a homogeneous paste that was pelletized via an electric extruder (sieve size 2 mm) to form feed pellets. Following oven drying, M. oleifera was used to produce seven distinct diets. One diet served as a control with no probiotics, while the remaining six diets were supplemented with varying levels of probiotics: 0.5, 1, 1.5, 2, 2.5, or 3 g kg−1. Probiotics were sprayed over the diets at required concentrations after mixing with 100 mL distilled water (Yao et al., 2021; Eissa et al., 2022; Table 2). Protexin (Protexin® CFU g−1 = 2 × 109; A multi-strain Probiotics, Probiotics International Ltd., England) contains Lactiplantibacillus plantarum subsp. plantarum, Lactobacillus acidophilus, L. delbrueckii subsp. bulgaricus, Bifidobacterium bifidum, Aspergillus oryzae, Candida printolopesi, and Streptococcus faecium. This was done because the change in temperature during processing affects the viability and functionality of probiotics. At the end, experimental diets were subjected to desiccation in an oven at 105°C for 24 h and subsequently stored at a temperature of-4°C until they were consumed.
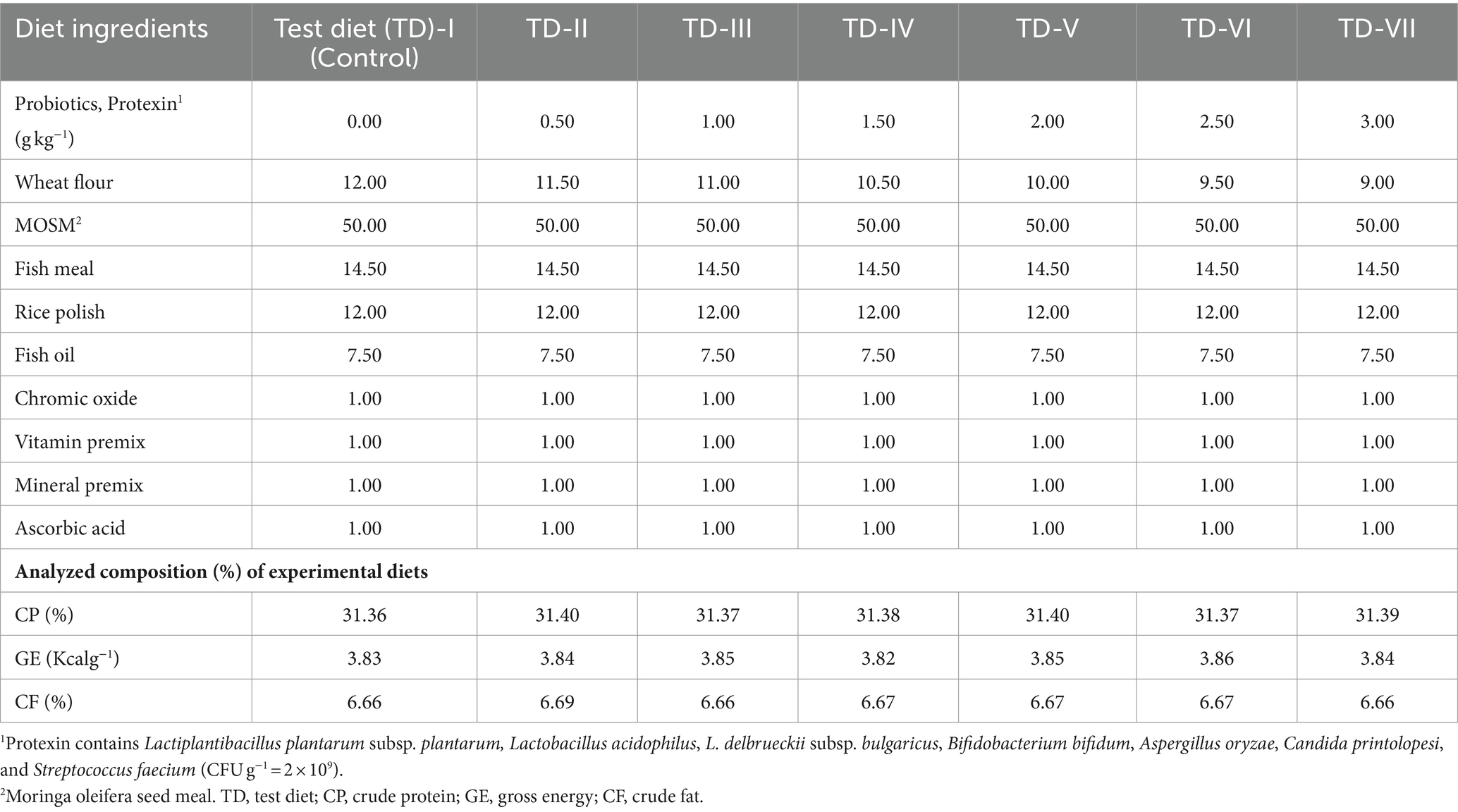
Table 2. Ingredients and analyzed composition of experimental diets (%) supplemented with probiotics.
2.4 The schedule of feeding and sample collection
The 60-day experiment involved feeding fingerlings a designated diet equivalent to 4% of their live body weight, administered twice daily. Three replicates of each diet were set up in V-shaped tanks with 15 fish per tank (a total of 21 tanks). At the beginning, the initial mass of each fingerling was noted. An hour after feeding, the remaining food was gathered, and the tank was thoroughly cleaned to eliminate any stray diet particles and replenished with water. Fecal matter was subsequently collected from each tank by opening the valves of the fecal collection system and taking extra precautions to minimize nutrient loss in the water system. The feces were dried at a temperature of 65°C and preserved for future chemical analysis.
2.5 Growth analysis
Growth of fingerlings was evaluated by comparing their initial (average initial weight: 7.42 ± 0.02 g) and final weights of the experiment. To evaluate the productivity of C. mrigala, the following standard arithmetic formulae were used as indicated by Ahmad et al. (2021) and Shahzad et al. (2022).
2.6 Nutrient digestibility analysis
For the purpose of the nutritional analysis (ADC%), the feed and fecal samples were pulverized in a high-speed grinder [Association of Official Analytical Chemists—International (AOAC), 2005]. To conduct an accurate analysis of the moisture, the samples were subjected to drying in an oven at 105°C for a period of 12 h. The Kjeldahl apparatus and a Soxtec device (utilizing petroleum ether extraction method) were used for the quantitative determination of crude protein (CP, N* 6.25) and crude fat (CF), respectively. An oxygen bomb calorimeter was employed to calculate the total gross energy (GE) by measuring the heat of combustion. Chromic oxide content in the diets was quantified using perchloric acid oxidation followed by spectrophotometric measurement of absorbance at 350 nm (Divakaran et al., 2002). The digestibility coefficient (ADC%) of diets was determined by using the formula, as defined in National Research Council (NRC) (2011).
2.7 Serum antioxidant enzymes
The blood samples were collected from the caudal vein of each fish using 1 mL heparinized syringe and transferred into Eppendorf tubes without additional anticoagulant solutions. The blood samples were kept at 4°C overnight. Following centrifugation at 3,000 g for 10 minutes, the serum was isolated and stored at -80°C for subsequent analysis. The levels of antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were subsequently determined using a spectrophotometer following established protocols (McCord and Fridovich, 1969; Habig et al., 1974; Aebi, 1984).
2.8 Hematology
Upon completion of the feeding trial, three fish from each tank were anesthetized for 15 min using a solution of 150 mg of tricaine methane sulfonate, following the method of Wagner et al. (1997). Blood samples were then collected from the caudal vein using heparinized syringes and placed in Eppendorf tubes containing EDTA as an anticoagulant. The mean cell volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and mean corpuscular hemoglobin (MCH) were calculated using formulas described by Shahzad et al. (2022). White blood cell (WBC) count, platelet (PLT) count, hemoglobin (Hb) concentration, and red blood cell (RBC) count were determined using established protocols outlined by Blaxhall and Daisley (1973) and Wedemeyer and Yasutake (1977).
2.9 Statistical analyses
One-way analysis of variance was conducted by using the computer application Co-Stat, and significant differences were analyzed by using Tukey’s honest significant difference test (Snedecor and Cochran, 1991). Graphs were created by using Microsoft Excel.
3 Results
3.1 Growth performance
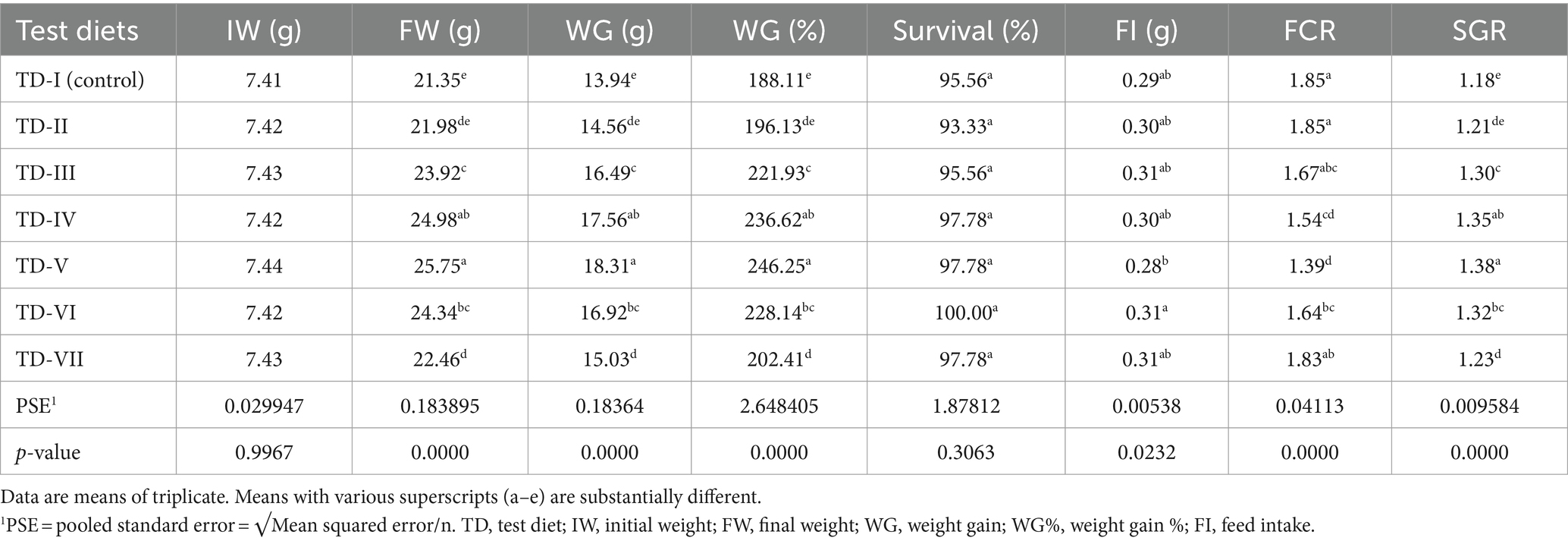
The results indicated that all growth parameters were improved significantly when fish were fed probiotic-supplemented diets with respect to control diet, as shown in Table 3 (p < 0.05). The best weight gain (WG, 18.31 g), specific growth rate (SGR, 1.38%), feed conversion ratio (FCR, 1.39), and WG% (246%) were noticed in C. mrigala-fed 2 g kg−1 protexin probiotic-supplemented diets (test diet-V). The lowest WG (14 g) and WG % (188%) were noted in fish fed the control diet. This evidence shows that 2 g kg−1 level of protexin probiotic allows to promote the maximum growth performance of C. mrigala. Quadratic regression analysis showed that optimum survival rate %, WG %, FCR, and SGR of C. mrigala-fed test diets were found at 5.25, 1.74, 1.68, and 1.74 g kg−1 level, respectively (Figure 1).
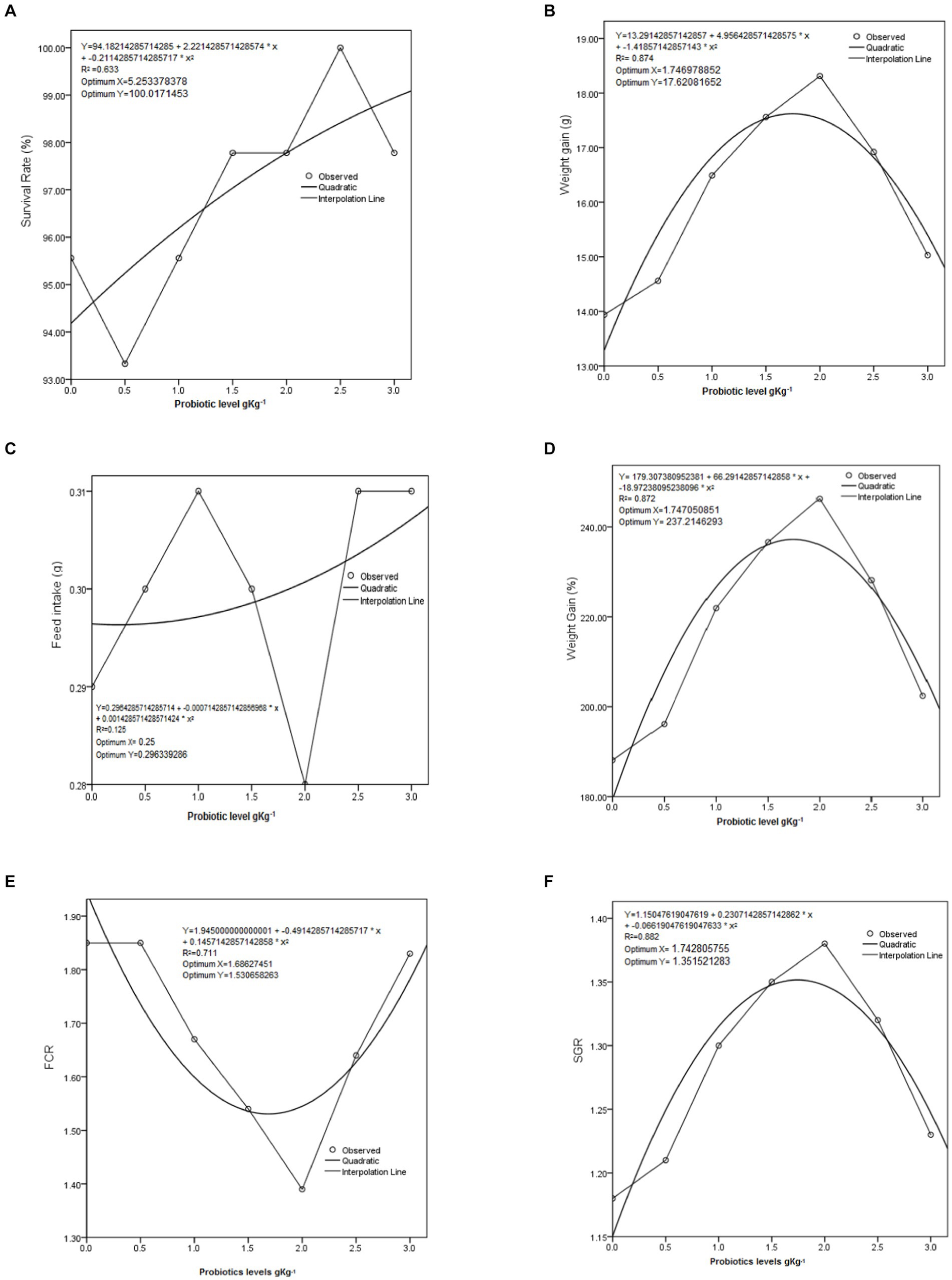
Figure 1. Quadratic regression of growth performance in Cirrhinus mrigala having probiotic-supplemented diets: (A) survival rate, % (B) weight gain, g (C) feed intake, g (D) weight gain, % (E) feed conversion ratio, % (F) specific growth rate.
3.2 Nutrient digestibility
ADC% (CP, CF, and GE) of C. mrigala-fed protexin-supplemented diets is shown in Table 4. These results indicated that highest ADC% of nutrients were recorded when fingerlings were fed test diet-V containing 2 g kg−1 of probiotics. The outcomes of CP, GE, and CF were considerably higher than the control diet. 2 g kg−1 supplementation of probiotics showed maximum CP (70%), GE (71%), and CF (75%), followed by the supplementation level of 2.5 g kg−1 (CP 69%, GE 70%, and CF 68%). Quadratic regression analysis showed that optimum apparent ND (CP, GE, and EE) of C. mrigala having MOSM-based test diets occurred at probiotic levels of 2.53, 2.04, and 1.99 g kg−1, respectively (Figure 2).
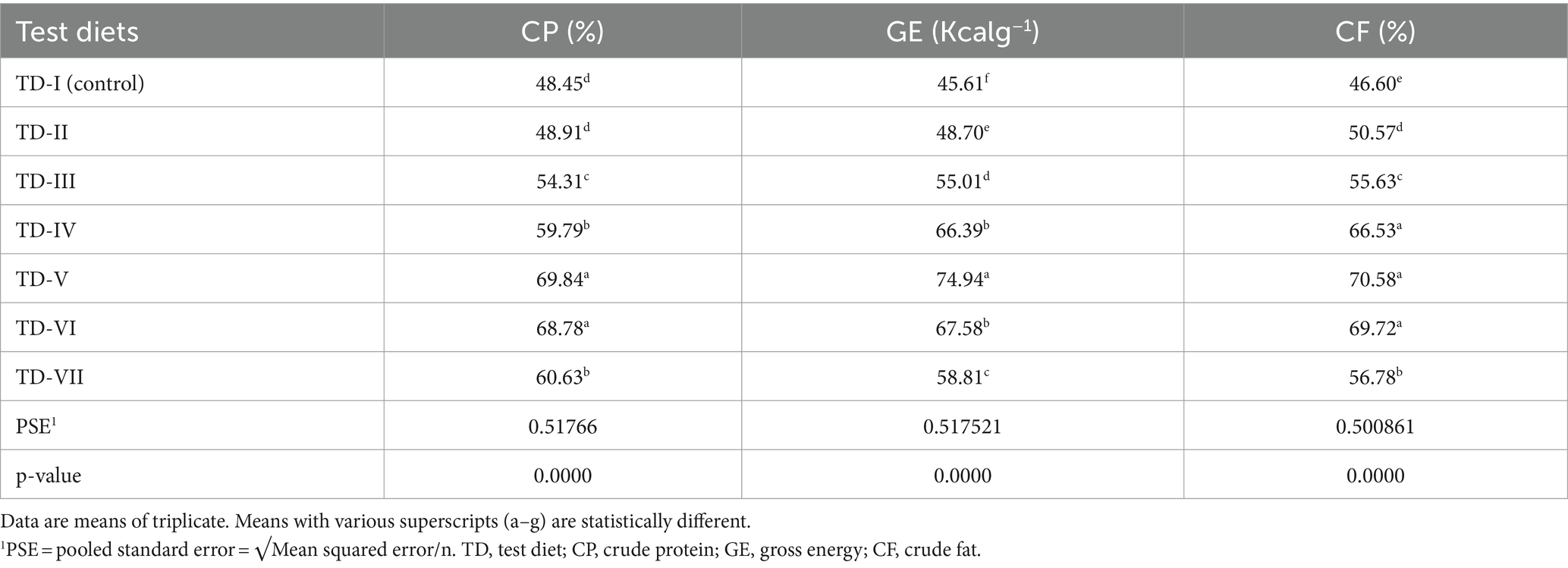
Table 4. Apparent digestibility analysis (%) of apparent CP, GE, and CF in Cirrhinus mrigala having probiotic-supplemented diets.
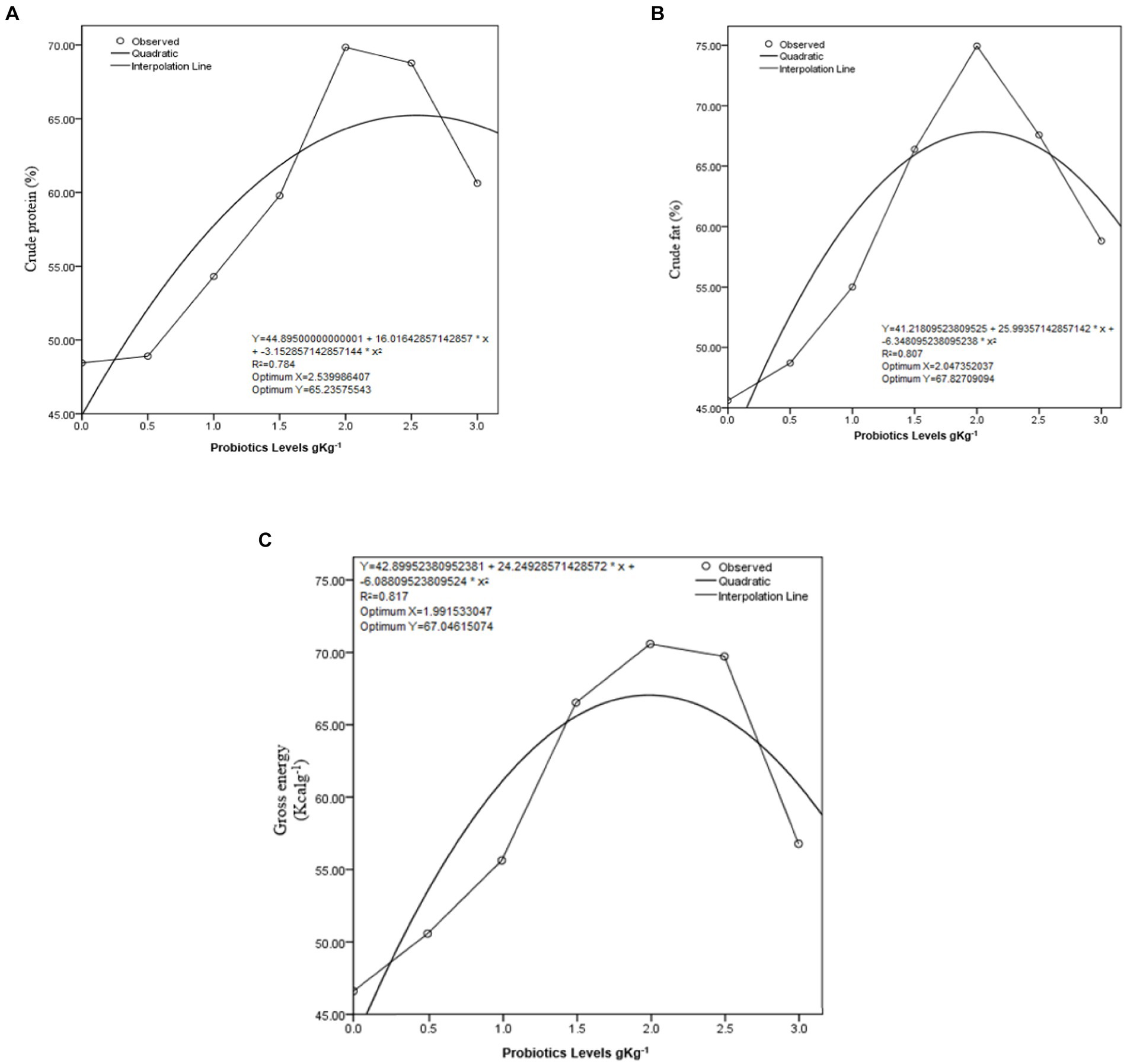
Figure 2. Quadratic regression of digestibility in Cirrhinus mrigala having probiotic-supplemented diets (A) crude protein, (B) crude fat, and (C) gross energy.
3.3 Antioxidant enzyme activity
Among all the probiotic levels, only test diet-V (2 g kg−1) indicated significant increase in SOD, CAT, and GPx activities, as illustrated in Figure 3 (p < 0.05). All the probiotic levels significantly enhanced (p < 0.05) the antioxidant enzyme activity to some extent with respect to control diet. However, test diets II, III, IV, and VII showed non- significant differences (p > 0.05) regarding CAT activity.
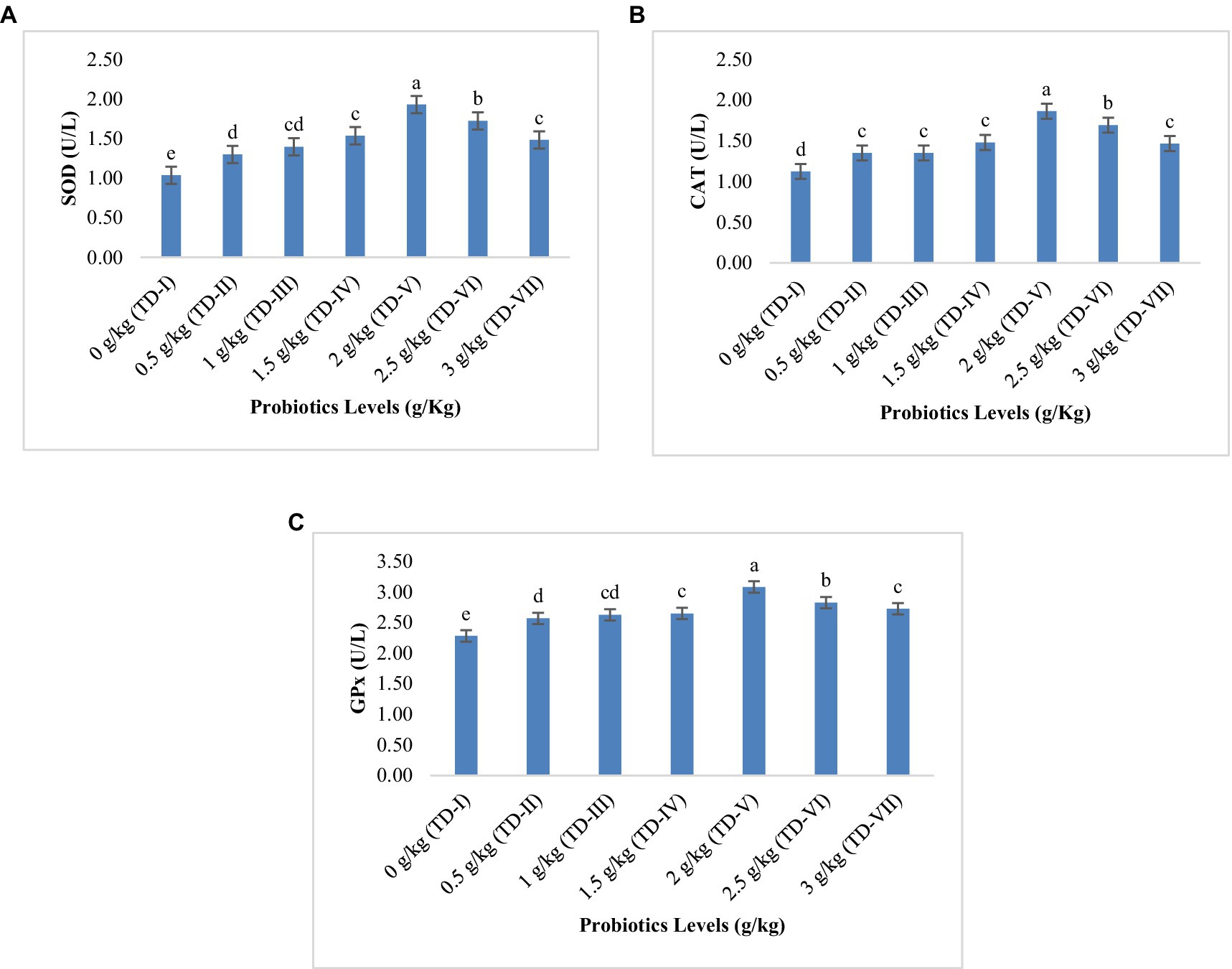
Figure 3. Antioxidant enzyme activity of Cirrhinus mrigala-fed diets supplemented with probiotics: (A) SOD (superoxide dismutase, UL−1), (B) CAT (catalase, UL−1), and (C) GPx (glutathione peroxidase, UL−1).
3.4 Hematology
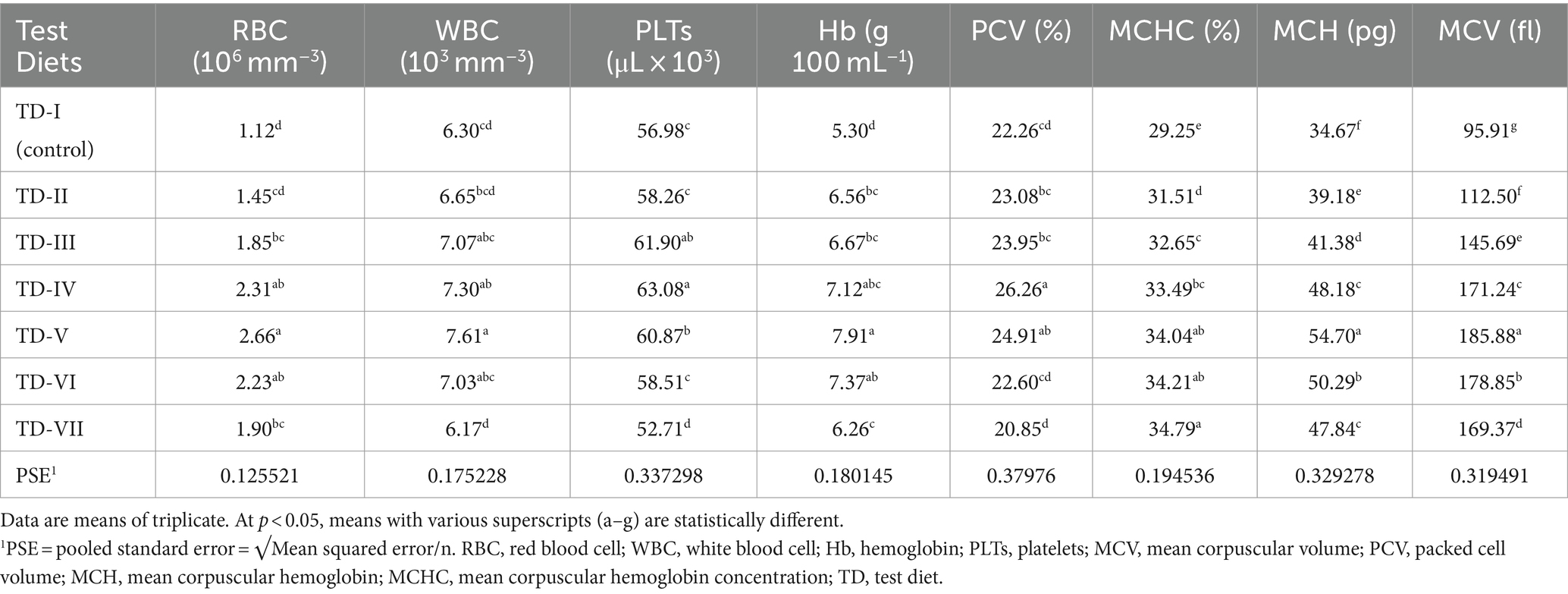
Blood indices of C. mrigala fingerlings showed that supplementation of protexin probiotics played a prominent role in the amelioration of blood parameters compared with the control diet (Table 5). The greater amounts of WBCs (7.61 × 103 mm−3), RBCs (2.66 × 106 mm−3), and Hb (7.91 g 100 mL−1) were recorded in the diet containing 2 g kg−1 of protexin. Accordingly, the lowest RBCs and Hb values were found in fish fed the control diet, while minimum WBCs (6.17 × 103 mm−3) and PLT (53 μL × 103) were recorded when fish were fed the 3 g kg−1 probiotic-supplemented diet. However, the maximum value of PCV (26.26%) was recorded for fingerlings that were fed the 1.5 g kg−1 probiotic level (test diet-IV). Moreover, optimum values of MCH (54.70 pg) and MCV (186 fl) were associated with the test diet-V (2 g kg−1 level), followed by MCH (50.29 pg) and MCV (179 fl) measured at a probiotic level of 2.5 g kg−1. The blood indices improved to their greatest values with a diet based on a level of 2 g kg−1 of probiotics. Quadratic regression analysis indicated that maximum RBCs, Hb, WBCs, and PLT of fish fed MOSM-based test diets occurred at 1.96, 1.79, 1.56, and 1.36 g Kg-1, respectively (Figure 4).

Figure 4. Quadratic regression of hematological parameters in Cirrhinus mrigala fingerlings having probiotic-supplemented diets: (A) red blood cells, (B) white blood cells, (C) platelets, and (D) hemoglobin.
4 Discussion
For the sustainability and integrity of aquaculture, probiotics are the best option because bacterial diseases are becoming more resistant to antibiotics (Hosain and Liangyi, 2020). Moreover, probiotics are a promising dietary feed supplement as they promote general health and growth performance of fish. They are also eco-friendlier and less expensive supplement as compared to other supplements (Rohani et al., 2022). In our study, it is concluded that 2 g kg−1 probiotics (protexin) increase the growth performance, nutrient absorption, antioxidant enzymes activities, and blood indices of C. mrigala fingerlings.
4.1 Growth parameters
The current findings demonstrated that 2 g kg−1 level of multi-strain probiotic gave the best growth performance results (WG, 18.31 g; SGR, 1.38%; FCR, 1.39; and WG%, 246) in C. mrigala. Because the MOSM-based diet contains anti-nutritional elements which affect the nutrient absorption, probiotics enhance the production of digestive enzymes, thereby facilitating the breakdown of recalcitrant nutrients in fish, which in turn improves FCR. The increased enzymatic activity stimulates appetite and boosts feed intake, ultimately leading to enhanced growth performance in fish. According to Opiyo et al. (2019), when two probiotic strains (Bacillus subtilis and Saccharomyces cerevisiae) were fed to tilapia, they produced different results. S. cerevisiae promoted the better growth parameters at 4 g kg−1, whereas B. subtilis gave similar result at 10 g kg−1. Another study found that probiotics at 1 × 108 CFU g−1 feed increased the growth in Oreochromis niloticus (Xia et al., 2020). In our study, a 2 g kg−1 probiotic level showed the maximum SGR (1.38%), WG (18.31 g), and WG% (246%) and minimum FCR (1.39) in C. mrigala fingerlings Similarly, Niu et al. (2019) demonstrated that multi-strain probiotics showed increased growth parameters (WG% 759, FCR 0.77) in olive flounder. Our outcomes are also in agreement with the study by Hasan et al. (2021), who demonstrated that 0.2% probiotic supplement improved the growth (WG, 16.19 g; WG%, 981; SGR, 3.87%) in O. niloticus.
It should be noted that the present study appears to be inconsistent with a previous research by Firouzbakhsh et al. (2011), which revealed that growth responses were enhanced by a protexin probiotic level of 0.15 g kg−1 in Oscar fish. These apparently contrasting results should be interpreted in light of the multi- and mono-species nature of the probiotics used because protexin is a multi-species probiotic and thus should be considered more effective and beneficial than a mono-species probiotic (Hossain et al., 2022). On the contrary, our study is in line with Giannenas et al. (2015), who evaluated that dietary supplementation of multi-strain probiotics (B. subtilis, Pediococcus acidilactici, Enterococcus faecium, and Limosilactobacillus reuteri) improved the growth of rainbow trout at a probiotic level of 1 g kg−1.
4.2 Nutrient digestibility
Our findings revealed that 2 g kg−1 level of probiotics resulted in the highest ADC% in C. mrigala fingerlings. Maximum digestibility levels of CP% (70%), GE% (71%), and CF% (75%) were reported at 2 g kg−1 probiotic level when fish were fed with MOSM-based diet. Moreover, best apparent digestibility in feces was also observed in TD-V (CP, 10.07%; CF, 1.24%; and GE, 1.82%). This might be related to the fact that C. mrigala is an agastric fish species, which implies that the feed shows longer retention time in the small intestine, thus increasing nutrient absorption via the gut (Zandonà et al., 2015; Soni and Ujjania, 2018). Probiotics boost the synthesis of digestive enzymes, detoxify the toxic ones, and lower the pH of the gut, improving nutrient digestion. Moreover, probiotics increase villi height, which ameliorates absorption of nutrients from intestine (Tong et al., 2023). The study of Munir et al. (2016) demonstrated that Channa striata fingerlings, when supplemented with probiotics (L. acidophilus) supplement, showed enhanced nutrient digestibility as compared to prebiotics because probiotics directly influence the production of microbial bioactive compounds in gastrointestinal tract. According to Niu et al. (2019), the probiotic-supplemented diet was the best in nutrient utilization (protein retention, 37.9; lipid retention, 51.9). In a related study, the inclusion of probiotics elevated the activity of numerous enzymes, increasing the nutritional digestibility of Siberian sturgeon (Shekarabi et al., 2022).
4.3 Serum antioxidant enzymes
SOD, CAT, and GPx are the common endogenous enzymatic antioxidants which prevent the body from oxidative damage through reactive oxygen species (ROS; Ighodaro and Akinloye, 2018). In the present research, serum antioxidant enzyme activity was significantly improved by the addition of 2 g kg−1 multi-strain probiotic level in MOSM-based diet in which optimum SOD (1.72 UL−1), CAT (1.69 UL−1), and GPx (2.82 UL−1) were observed. According to Gobi et al. (2018), tilapia when fed with 107 CFU g−1 of B. licheniformis Dahb1 probiotics improved the antioxidant enzyme activity. Stress triggers the production of ROS [hydroxyl radical (OḤ), superoxide anion (O2−), and hydrogen peroxide (H2O2)] that impair the defense mechanisms (Pisoschi et al., 2021). Enzymatic antioxidants are those produced naturally inside a living organism that neutralize harmful free radicals and reduce stress (Krishnamurthy and Wadhwani, 2012). Arghideh et al. (2022) demonstrated that C. carpio, when fed with Streptomyces chartreusis probiotics, did not show significant improvements in terms of SOD, CAT, and GPx activities. The differences among published results might be reasonably explained considering the differences among fish species or probiotics used, for example because C. mrigala is a more sensitive fish with more susceptibility to stress than C. carpio. Therefore, the supplementation of probiotics in the case of C. mrigala might better enhance the activity of enzymatic antioxidants to overcome oxidative stress. Other researchers observed similar behaviors. According to Tanveer et al. (2024), for example, supplementation with a multi-strain probiotic comprising B. subtilis, E. faecalis, and B. licheniformis, at a dose of up to 2.0 g/kg, led to significant improvements in antioxidant enzyme activity on Clarias batrachus fingerlings.
4.4 Hematology
The outcomes of this research found an optimal probiotic level of 2 g kg−1 for enhancing the blood indices (Hb 7.91 g 100 mL−1; WBCs 7.61 × 103 mm−3; RBCs 2.66 × 106 mm−3; MCH 54.70 pg.; MCHC 34.04%; MCV 185.88 fl; and PLTs 60.87 μL × 103). Hematology is required for the diagnosis of overall health of fish. More Hb and RBC levels demonstrate that the blood has a high oxygen content (Yaqub et al., 2023). Our results are in agreement with those of Hasan et al. (2021), who discovered that dietary probiotic supplementation of 0.2% enhanced the hematological parameters (Hb 5.70 g DL−1, RBCs 1.19 m μL−1, and WBCs 10.89 × 104 cumm−1) in O. niloticus. Similarly, Adorian et al. (2019) demonstrated that 1 × 106 CFU g−1 probiotics significantly improved the blood parameters (RBCs, WBCs, and Hb) of Asian sea bass as compared to control. In contrast to the aforementioned and the present studies, Sayed Hassani et al. (2020) exhibited no significant effects of a probiotic-containing diet on RBCs, WBCs, MCH, MCV, and Hb when compared to a diet without probiotic supplementation. Similarly, Adorian et al. (2019) demonstrated that 1 × 106 CFU g−1 probiotics significantly improved the blood parameters (RBCs, WBCs, and Hb) of Asian sea bass as compared to control. In addition, Tanveer et al. (2024) stated that supplementing with 2.0 g/kg multi-strain probiotics (B. subtilis, E. faecalis, and B. licheniformis) showed improvement in hematological indices of C. batrachus.
5 Conclusion
Conclusively, protexin probiotics have shown to be a better supplement for the long-term sustainability and viability of aquaculture sector. The current results demonstrate that feeding C. mrigala fingerlings with MOSM along with 2 g kg−1 probiotic supplementation as FFA improves the growth performance, nutrient digestibility, antioxidant enzymes activities, and hematological indices. These findings therefore suggest that probiotic supplement can be used without compromising animal health and production.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All experimental protocols were approved by the guidelines provided by the “Ethics Review Committee of the Government College University Faisalabad.” This study was performed in an ethical and moral way. In this study, no toxins were administered to fish that could harm them, and minimum number of fish were employed with proper care and husbandry in accordance with the standards of the Ethical Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
DR: Formal analysis, Methodology, Writing – original draft. SMH: Conceptualization, Data curation, Supervision, Writing – review & editing. PS: Formal analysis, Investigation, Writing – review & editing. SA: Formal analysis, Investigation, Writing – review & editing. AN: Data curation, Writing – review & editing. EN: Data curation, Writing – review & editing. NN: Data curation, Writing – review & editing. KMA-A: Data curation, Writing – review & editing. MAF: Data curation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study received funding from the University of California Santa Cruz. This work was supported by the Researchers Supporting Project number (RSP2024R154), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The study was supported by HEC Pakistan for which the authors are grateful. Moreover, we thank the Cynthia and George Mitchell Foundation for partially supporting the work. We also thank the University of California Santa Cruz, Dean of Social Sciences and Executive Vice Chancellor. The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP2024R154), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Latif, H. M., Abdel-Daim, M. M., Shukry, M., Nowosad, J., and Kucharczyk, D. (2022). Benefits and applications of Moringa oleifera as a plant protein source in Aquafeed: a review. Aquaculture 547:737369. doi: 10.1016/j.aquaculture.2021.737369
Adorian, T. J., Jamali, H., Farsani, H. G., Darvishi, P., Hasanpour, S., Bagheri, T., et al. (2019). Effects of probiotic bacteria bacillus on growth performance, digestive enzyme activity, and hematological parameters of Asian sea bass, Lates calcarifer (Bloch). Probio. Antimicrob. Prot. 11, 248–255. doi: 10.1007/s12602-018-9393-z
Aebi, H. (1984). Catalase in vitro. Methods Enzymol. 105, 121–126. doi: 10.1016/S0076-6879(84)05016-3
Ahmad, B., Hussain, S. M., Ali, S., Arsalan, M. Z. U. H., Tabassum, S., and Sharif, A. (2021). Efficacy of acidified phytase supplemented cottonseed meal based diets on growth performance and proximate composition of Labeo rohita fingerlings. Braz. J. Biol. 83:e247791. doi: 10.1590/1519-6984.247791
Arghideh, M., Hoseinifar, S. H., Ghorbani Nasrabadi, R., Mazandarani, M., El-Haroun, E., and Van Doan, H. (2022). Evaluation of soil-derived Streptomyces chartreusis KU324443 effects as probiotic on growth performance, antioxidant enzyme activity, mucosal and serum immune parameters, and related gene expression in common carp (Cyprinus carpio) fingerlings. Aquacult. Nutr. 2022, 1–9. doi: 10.1155/2022/2278130
Association of Official Analytical Chemists—International (AOAC) (2005). Official methods of analysis. 18th Edn. Gaithersburg, MD, USA: AOAC.
Blaxhall, P. C., and Daisley, K. W. (1973). Routine haematological methods for use with fish blood. J. Fish Biol. 5, 771–781. doi: 10.1111/j.1095-8649.1973.tb04510.x
Caruso, G. (2015). Use of plant products as candidate fish meal substitutes: an emerging issue in aquaculture productions. Fish. Aqua. J. 6:1000e123. doi: 10.4172/2150-3508.1000e123
Divakaran, S., Obaldo, L. G., and Forster, I. P. (2002). Note on the methods for determination of chromic oxide in shrimp feeds. J. Agric. Food Chem. 50, 464–467. doi: 10.1021/jf011112s
Diwan, A. D., Harke, S. N., Gopalkrishna, S., and Panche, A. N. (2022). Aquaculture industry prospective from gut microbiome of fish and shellfish: an overview. Anim Physiol Nutr 106, 441–469. doi: 10.1111/jpn.13619
Eissa, E. S. H., Baghdady, E. S., Gaafar, A. Y., El-Badawi, A. A., Bazina, W. K., Abd Al-Kareem, O. M., et al. (2022). Assessing the influence of dietary Pediococcus acidilactici probiotic supplementation in the feed of European sea bass (Dicentrarchus labrax L.) (Linnaeus, 1758) on farm water quality, growth, feed utilization, survival rate, body composition, blood biochemical parameters, and intestinal histology. Aquacult. Nutr. 2022, 1–11. doi: 10.1155/2022/5841220
Firouzbakhsh, F., Noori, F., Khalesi, M. K., and Jani-Khalili, K. (2011). Effects of a probiotic, protexin, on the growth performance and hematological parameters in the Oscar (Astronotus ocellatus) fingerlings. Fish Physiol. Biochem. 37, 833–842. doi: 10.1007/s10695-011-9481-4
Food and Agriculture Organization (FAO) (2014). The state of food and agriculture. Rome, Italy: FAO.
Food and Agriculture Organization (FAO) (2022). The world fisheries and aquaculture – Towards blue transformation. Rome, Italy: FAO.
Giannenas, I., Karamaligas, I., Margaroni, M., Pappas, I., Mayer, E., Encarnação, P., et al. (2015). Effect of dietary incorporation of a multi-strain probiotic on growth performance and health status in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 41, 119–128. doi: 10.1007/s10695-014-0010-0
Gobi, N., Vaseeharan, B., Chen, J. C., Rekha, R., Vijayakumar, S., Anjugam, M., et al. (2018). Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol. 74, 501–508. doi: 10.1016/j.fsi.2017.12.066
Gopalakrishnan, L., Doriya, K., and Kumar, D. S. (2016). Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci. Human Wellness 5, 49–56. doi: 10.1016/j.fshw.2016.04.001
Guedes, A. C., Sousa-Pinto, I., and Malcata, F. X. (2015). “Application of microalgae protein to aquafeed” in Handbook of marine microalgae. ed. S. K. Kim (Busan, South Korea: Academic Press). 93–125.
Habig, W. H., Pabst, M. J., and Jakoby, W. B. (1974). Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139. doi: 10.1016/S0021-9258(19)42083-8
Hasan, R., Hossain, M. A., Islam, M. R., and Iqbal, M. M. (2021). Does commercial probiotics improve the growth performance and hematological parameters of Nile tilapia, Oreochromis niloticus? Aquacult. Res. 4, 160–168. doi: 10.3153/AR21013
Hosain, M. A., and Liangyi, X. (2020). Impacts of probiotics on feeding technology and its application in aquaculture (2020). J. Aqua. Fish. Fish Sci. 3, 174–185. doi: 10.25177/JAFFS.3.1.RA.622
Hossain, M. K., Islam, S. M., Rafiquzzaman, S. M., Nuruzzaman, M., Hossain, M. T., and Shahjahan, M. (2022). Multi-species probiotics enhance growth of Nile tilapia (Oreochromis niloticus) through upgrading gut, liver and muscle health. Aquacult. Res. 53, 5710–5719. doi: 10.1111/are.16052
Hu, S., Wang, L., and Jiang, Z. (2017). Dietary additive probiotics modulation of the intestinal microbiota. Protein Pept. Lett. 24, 382–387. doi: 10.2174/0929866524666170223143615
Ighodaro, O. M., and Akinloye, O. A. (2018). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 54, 287–293. doi: 10.1016/j.ajme.2017.09.001
Krishnamurthy, P., and Wadhwani, A. (2012). Antioxidant enzymes and human health. Antioxidant. Enzyme 1, 3–18. doi: 10.5772/48109
Liang, L., Wang, C., Li, S., Chu, X., and Sun, K. (2019). Nutritional compositions of Indian Moringa oleifera seed and antioxidant activity of its polypeptides. Food Sci. Nutr. 7, 1754–1760. doi: 10.1002/fsn3.1015
McCord, J. M., and Fridovich, I. (1969). Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244, 6049–6055. doi: 10.1016/S0021-9258(18)63504-5
Munir, M. B., Hashim, R., Chai, Y. H., Marsh, T. L., and Nor, S. A. M. (2016). Dietary prebiotics and probiotics influence growth performance, nutrient digestibility and the expression of immune regulatory genes in snakehead (Channa striata) fingerlings. Aquaculture 460, 59–68. doi: 10.1016/j.aquaculture.2016.03.041
National Research Council (NRC) (2011). Nutrient requirements of fish. Washington, DC, USA: National Academy Press.
Niu, K. M., Khosravi, S., Kothari, D., Lee, W. D., Lim, J. M., Lee, B. J., et al. (2019). Effects of dietary multi-strain probiotics supplementation in a low fishmeal diet on growth performance, nutrient utilization, proximate composition, immune parameters, and gut microbiota of juvenile olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 93, 258–268. doi: 10.1016/j.fsi.2019.07.056
Ntakirutimana, R., Syanya, F. J., and Mwangi, P. (2023). Exploring the impact of probiotics on the gut ecosystem and morpho-histology in fish: current knowledge of tilapia. Asian J. Fish. Aqua. Res 25, 93–112. doi: 10.9734/ajfar/2023/v25i3670
Oliva-Teles, A., Enes, P., Couto, A., and Peres, H. (2022). Replacing fish meal and fish oil in industrial fish feeds. Feed Feeding Pract. Aqua 2022, 231–268. doi: 10.1016/B978-0-12-821598-2.00011-4
Opiyo, M. A., Jumbe, J., Ngugi, C. C., and Charo-Karisa, H. (2019). Different levels of probiotics affect growth, survival and body composition of Nile tilapia (Oreochromis niloticus) cultured in low input ponds. Scient. Africa. 4:e00103. doi: 10.1016/j.sciaf.2019.e00103
Pisoschi, A. M., Pop, A., Iordache, F., Stanca, L., Predoi, G., and Serban, A. I. (2021). Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 209:112891. doi: 10.1016/j.ejmech.2020.112891
Rohani, M. F., Islam, S. M., Hossain, M. K., Ferdous, Z., Siddik, M. A., Nuruzzaman, M., et al. (2022). Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish Immunol. 120, 569–589. doi: 10.1016/j.fsi.2021.12.037
Rowland, S. J. (1991). Diseases of australian native freshwater fishes with particular emphasis on the ectoparasitic and fungal diseases of Murray cod (Maccullochella peeli), golden perch (Macquaria ambigua) and silver perch (Bidyanus bidyanus). Sydney, NSW, Australia: NSW Agriculture, 21–23.
Sayed Hassani, M. H., Jourdehi, A. Y., Zelti, A. H., Masouleh, A. S., and Lakani, F. B. (2020). Effects of commercial superzist probiotic on growth performance and hematological and immune indices in fingerlings Acipenser baerii. Aquac. Int. 28, 377–387. doi: 10.1007/s10499-019-00468-1
Shahzad, M. M., Rashid, H., Hussain, S. M., Bashir, S., Khalid, F., and Ahmad, N. (2022). Improvement in body composition and blood parameters of Catla catla fingerlings by supplementing rapeseed meal based diet with probiotics. Pak. J. Zool. 55, 1–9. doi: 10.17582/journal.pjz/20211011061010
Shekarabi, S. P. H., Ghodrati, M., Dawood, M. A., Masouleh, A. S., and Roudbaraki, A. F. (2022). The multi-enzymes and probiotics mixture improves the growth performance, digestibility, intestinal health, and immune response of Siberian sturgeon (Acipenser baerii). Annal. Anim. Sci. 22, 1063–1072. doi: 10.2478/aoas-2022-0006
Snedecor, G. W., and Cochran, W. G. (1991). Statistical methods. 8th Edn. Ames, IA, USA: Iowa State University Press.
Soni, N., and Ujjania, N. C. (2018). Gut contents analysis and preponderance index based study on feeding habit of Cirrhinus mrigala from Ukai dam. J. Fish. Life Sci. 3, 19–21.
Tanveer, A., Khan, N., Fatima, M., Ali, W., Nazir, S., Bano, S., et al. (2024). Effect of multi-strain probiotics on the growth, hematological profile, blood biochemistry, antioxidant capacity, and physiological responses of Clarias batrachus fingerlings. Aquacul. Inter 32, 1817–1833. doi: 10.1007/s10499-023-01245-x
Tong, D. Q., Lu, Z. J., Zeng, N., Wang, X. Q., Yan, H. C., and Gao, C. Q. (2023). Dietary supplementation with probiotics increases growth performance, improves the intestinal mucosal barrier and activates the Wnt/β-catenin pathway activity in chicks. J. Sci. Food Agric. 103, 4649–4659. doi: 10.1002/jsfa.12562
Wagner, A. D., Gabrieli, J. D., and Verfaellie, M. (1997). Dissociations between familiarity processes in explicit recognition and implicit perceptual memory. J. Exp. Psychol. Learn. Mem. Cogn. 23, 305–323. doi: 10.1037/0278-7393.23.2.305
Wedemeyer, G. A., and Yasutake, W. T. (1977). Clinical methods for the assessment of the effects of environmental stress on fish health. Washington, DC, USA: US Fish and Wildlife Service.
Xia, Y., Wang, M., Gao, F., Lu, M., and Chen, G. (2020). Effects of dietary probiotic supplementation on the growth, gut health and disease resistance of juvenile Nile tilapia (Oreochromis niloticus). Anim. Nut. 6, 69–79. doi: 10.1016/j.aninu.2019.07.002
Yao, W., Li, X., Zhang, C., Wang, J., Cai, Y., and Leng, X. (2021). Effects of dietary synbiotics supplementation methods on growth, intestinal health, non-specific immunity and disease resistance of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 112, 46–55. doi: 10.1016/j.fsi.2021.02.011
Yaqub, A., Nasir, M., Kamran, M., Majeed, I., and Arif, A. (2023). Immunomodulation, fish health and resistance to Staphylococcus aureus of Nile tilapia (Oreochromis niloticus) fed diet supplemented with zinc oxide nanoparticles and zinc acetate. Biol. Trace Elem. Res. 201, 4912–4925. doi: 10.1007/s12011-023-03571-w
Keywords: functional diet, probiotics, functional feed additive, oxidative stress, antioxidant enzyme
Citation: Riaz D, Hussain SM, Sarker PK, Ali S, Naeem A, Naeem E, Nazish N, Al-Anazi KM and Farah MA (2024) Use of protexin as a probiotic-supplemented feed additive: assessment of growth, digestibility, serum antioxidant enzyme activity, and blood profile in Cirrhinus mrigala. Front. Sustain. Food Syst. 8:1449325. doi: 10.3389/fsufs.2024.1449325
Edited by:
Roberto Anedda, Porto Conte Ricerche, ItalyReviewed by:
Malgorzata Ziarno, Warsaw University of Life Sciences, PolandMd. Nur Hossain, Bangladesh Council of Scientific and Industrial Research, Bangladesh
Jidan Ye, Jimei University, China
Copyright © 2024 Riaz, Hussain, Sarker, Ali, Naeem, Naeem, Nazish, Al-Anazi and Farah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Syed Makhdoom Hussain, ZHJtYWtoZG9vbWh1c3NhaW5AZ2N1Zi5lZHUucGs=; Pallab K. Sarker, cHNhcmtlckB1Y3NjLmVkdQ==; Shafaqat Ali, c2hhZmFxYXRhbGlnaWxsQGdjdWYuZWR1LnBr
 Danish Riaz
Danish Riaz Syed Makhdoom Hussain
Syed Makhdoom Hussain Pallab K. Sarker
Pallab K. Sarker Shafaqat Ali
Shafaqat Ali Adan Naeem2
Adan Naeem2 Khalid Mashay Al-Anazi
Khalid Mashay Al-Anazi Mohammad Abul Farah
Mohammad Abul Farah