- 1Department of Fisheries and Marine Science, Noakhali Science and Technology University, Noakhali, Bangladesh
- 2Department of Environmental Studies, University of California, Santa Cruz, Santa Cruz, CA, United States
- 3Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia
- 4Environmental and Life Sciences Programme, Faculty of Science, Universiti Brunei Darussalam, Bandar Seri Begawan, Brunei
- 5School of Engineering and Built Environment, Griffith University, Brisbane, QLD, Australia
Evaluating the breeding performance of cultivable fish using gonadotropin-releasing hormone (GnRH) analogues is crucial for optimizing reproductive efficiency and enhancing the sustainability of aquaculture practices. However, there is a lack of understanding regarding which GnRH analogue performs best under hatchery conditions for stinging catfish. Therefore, this study was conducted to assess the breeding performance of stinging catfish (Heteropneustes fossilis) using three commercially available GnRH analogs: Wova-FH, Ovaprim, and Easy-spawn. For this purpose, an experiment was set up in a commercial hatchery by dividing the samples into three groups, each treated with one of the analogs: Wova-FH, Ovaprim, or Easy-spawn. In the experiment, a ratio of 1.5:1 male to female was maintained, and 1 mL syringes were used to inject the analogs. The results showed that the spawning induction efficiency differed among the three inducing agents. Compared to Ovaprim and Easy-spawn, Wova-FH-treated H. fossilis exhibited higher breeding success in all measured aspects. Specifically, Wova-FH achieved a latency period of 8 h, an ovulation rate of 87.25%, a fertilization rate of 89.33%, a hatching rate of 88.85%, and an incubation period of 16 h. In comparison, Ovaprim and Easy-spawn had incubation periods of 18 h and 24 h, respectively. In Ovaprim-induced individuals, the latency period was approximately 8.30 h, with an ovulation rate of 82.08%, a fertilization rate of 86.75%, and a hatching rate of 85.97%. In contrast, the Easy-spawn-induced individuals had a latency period of 9 h, an ovulation rate of 27.50%, a fertilization rate of 27.10%, and a hatching rate of 26.15%. Significant differences (p < 0.05) were observed among the treatments in both the ovulation rate, fertilization rate, latency period incubation period, and hatching rate. The findings suggest that Wova-FH is a superior alternative to Ovaprim and Easy-spawn for GnRH analogs in the induced breeding programs of H. fossilis, offering optimal yield.
1 Introduction
The stinging catfish (Heteropneustes fossilis) is a highly prized freshwater fish species native to South and Southeast Asia. It belongs to the Siluriformes order and the Heteropneustidae family (Marx and Chakraborty, 2007). It inhabits freshwater bodies such as rivers, lakes, ponds, and marshes. H. fossilis is an omnivorous species. Its fry is planktivorous, juveniles feed on crustaceans, plants, miscellaneous matter, and insects, while the adults consume insects, detritus, and plant matter (Hossain et al., 2021). The fish possesses a special respiratory organ called labyrinth, which allows it to breathe atmospheric air. This adaptation enables the fish to survive in hypoxic conditions and withstand high stocking densities in aquaculture (Samad et al., 2017). The spawning season of this catfish typically occurs from March to August, with a peak in May to June. The size at sexual maturity for males and females is estimated to be 15.5 cm in total length (Parvin et al., 2022). The culture of stinging catfish in Bangladesh has been optimized through both homestead tank and earthen pond systems. Larger stocking density in tanks (Nabi et al., 2020; Ahamed et al., 2023), and a moderate stocking density in ponds (Ahamed et al., 2017; Hossain et al., 2021) are recommended for better growth and economic returns. The fish is known for its low-fat content, minimal spine, and high digestibility (Marx and Chakraborty, 2007). Its medicinal value is often recommended for malaria patients due to its energizing properties (Mahalder et al., 2023). The high demand for stinging catfish in local markets, particularly in Bangladesh, has led to its economic significance. The culture systems have shown net benefits ranging from BDT 63,677 to BDT 1,517,771 per hectare, depending on the stocking density and management practices (Ali et al., 2018).
The stinging catfish, H. fossilis is a promising species for aquaculture due to its unique air-breathing apparatus, hardiness, tolerance to high stocking densities, and ability to withstand low oxygen levels (Ali et al., 2018). It can be successfully stocked on both small and large scales, yielding several times more than traditional carp species. As H. fossilis populations are gradually decreasing in the wild, induced breeding in hatcheries is essential for enhancing fish production and ensuring the sustainability of this valuable species (Hossain et al., 2021). This breeding technique employs synthetic hormones known as gonadotropin-releasing hormone (GnRH) analogs to stimulate breeding in H. fossilis, which cannot reproduce naturally in captivity (Rather et al., 2013; Elakkanai et al., 2015). These analogs are used in over 40 farmed fish species due to their convenience, potency, and extended shelf life (Mylonas and Zohar, 2001). GnRH analogs are neuropeptides that stimulate the release of gonadotropins from the pituitary gland, triggering physiological events such as final oocyte maturation, ovulation, and spawning (Rather et al., 2013). Commercial preparations like Wova-FH and Ovaprim contain a GnRH analog and a dopamine antagonist (Acharjee et al., 2017). The dopamine antagonist enhances the effectiveness of the GnRH analog by blocking its inhibitory effects on gonadotropin secretion (Elakkanai et al., 2015). Minor variations in natural and synthetic analogs can affect their receptor binding efficiencies, and consequently affecting their potency (Quiniou et al., 2014). Improper use of GnRH can lead to stress, immune and endocrine disequilibrium, and reproductive failure. Rapid clearance from fish bodies can limit their effectiveness, requiring multiple injections, which may further stress the fish (Rather et al., 2013).
Induced breeding methods in H. fossilis have been documented by several authors (Khan and Mukhopadhyay, 1976; Sarkar et al., 1979; Saha, 1986; Marimuthu et al., 2000; Nayak et al., 2001). Ovulatory changes and embryonic development in stinging catfish using GnRH analogs such as Ovaprim (Marx and Chakraborty, 2007; Christopher et al., 2011; Rahman et al., 2013; Das et al., 2016), WOVA-FH (Marx and Chakraborty, 2007; Das et al., 2016), and Easy-spawn (Araf et al., 2021) have also been investigated. However, there is a lack of comprehensive studies on the comparative efficacy of these synthetic hormones to ensure the effective breeding of H. fossilis. Assessing the efficacy of three GnRH analogs in the induced breeding of stinging catfish (Heteropneustes fossilis) aids in identifying the most effective breeding method, leading to higher reproductive success and optimized hatchery production. This supports the sustainable growth of fish farming, enhancing food security and boosting economic development in countries like Bangladesh. Additionally, increased breeding efficiency decreases dependency on wild fish stocks, fostering more sustainable and eco-friendly aquaculture practices. Therefore, this study aimed to evaluate the breeding performance of stinging catfish using three GnRH analogs, WOVA-FH, Ovaprim, and Easy-spawn, from different pharmaceutical companies. The result will help increase fish production, and make fish more accessible and affordable, contributing to better nutrition and health outcomes for communities.
2 Materials and methods
2.1 Study design
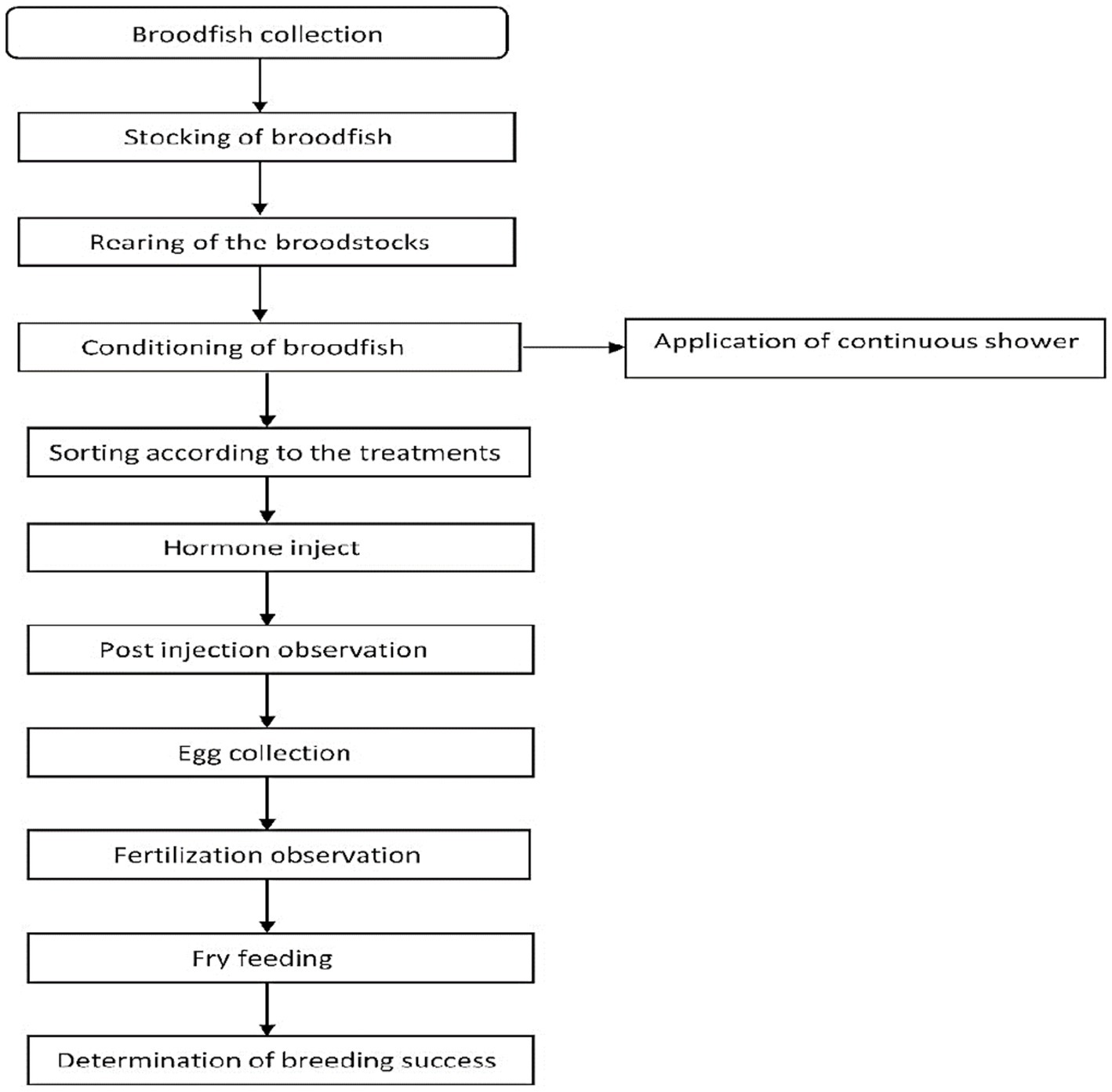
The study site of this research was in the Rakamary Matsha Khamar in Feni district, Bangladesh. It is an established, popular, and renowned hatchery, and the only catfish hatchery in the greater Noakhali region. The period of this study was April to August. Strong, mature, hardy, and disease-free broods were collected. A total of 10 brooders in each batch were stocked. They were reared for 1 week with a quality feed at the ratio of 10% body weight which was prepared with 20% fish meal, 10% soybean meal, 10% wheat flour, 20% rice bran, 20% rice polish, and 20% mastered oil cake and trace amounts (~1–2%) of vitamins and minerals. The crude protein level was 28–30%. Feed was given 2 times daily, once in the morning and another in the afternoon as they were nocturnal in habit. Di calcium phosphate was given daily to prevent organ deformity of Broods. Probiotics (e.g., pseudomonas) were also used to enhance the water quality. Additionally, animal manure (at 1,250 kg/hectare), and inorganic fertilizers: Urea (at 50 kg/hectare), and Triple Super Phosphate (at 25 kg/hectare) were applied in the water (Rahman et al., 2013). Based on secondary sexual characteristics as mentioned in Haniffa et al. (2008), broods were collected from the brood fish ponds using a cast net in the afternoon between 4:00 and 5:00 pm before 15 h of the hormone injecting trials and immediately transferred to circular tanks for conditioning (Figure 1). Males have an elongated, pointed genital papilla and are generally more slender, while females are bulkier, especially around the abdomen, and possess a round, blunt genital papilla. The males and females were kept in separate tanks with continuous water flow (at 10 L/min). However, no supplementary feed was provided throughout the conditioning period.
When selecting broodstock for induced breeding of stinging catfish (Heteropneustes fossilis), mature and healthy individuals with good body condition, free from any visible signs of disease or deformities, are chosen to improve the chances of successful fertilization and higher hatching rates. The whole process was divided into three treatments and marked as T1, T2, and T3. Each treatment was conducted with three batches having 10-piece broods in each batch. The broods under treatment T1, T2, and T3 were treated with wova-FH, ovaprim and easy-spawn, respectively. Various sizes and weights of air sac catfish were used in the study, as uniformity in biological elements cannot be achieved. In each treatment, the length and weight of fish are shown in Table 1.
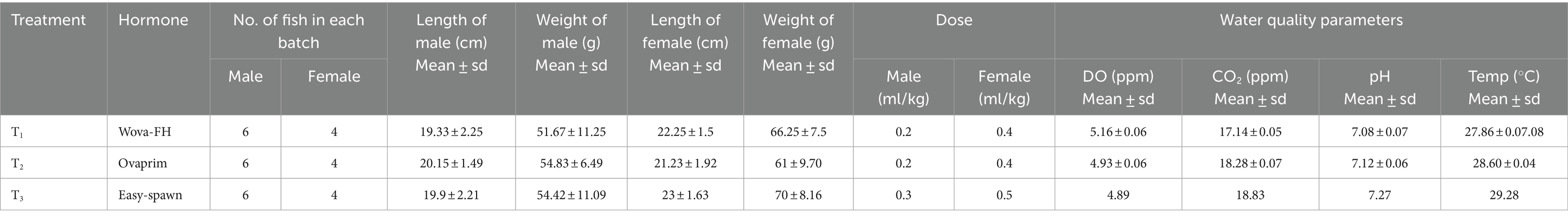
Table 1. Number of male and female fish, their length and weight, doses of different GnRH analogues, and water quality parameters in each treatment for induced breeding of Shing (Heteropneustes fossilis).
The size for a broodfish pond was between 500 and 700 m2 with a depth of 1.0 meter. Circular tanks had a diameter of four meters and a depth of 1.2 meters.
2.2 Hormonal dose and induced breeding
In the experiment, three types of GnRH (wova-FH, ovaprim, and easy-spawn) were produced by different pharmaceutical companies. Wova-FH was manufactured and marketed by “USV Ltd” and “Biostadt India Ltd” respectively while Ovaprim was manufactured by “Mac Mohan Pharma Ltd.” The third GnRH, easy-spawn was manufactured by “Srimpex Biotech Services Pvt. Ltd.” The male and female ratio was 1.5:1, as the maximum egg will be found in this ratio. The maturity of the female fish was assessed using external morphological characteristics such as body length, weight, and the presence of a distended abdomen, indicating readiness for spawning. Additionally, we determined whether the female stinging catfish were ready for hormone injection by observing external signs, including a swollen, soft abdomen and a reddish, protruding genital papilla, which signals egg readiness. After the selection of broodfish, GnRH analogs were used to induce them to breed. A single dose was used for both males and females. The hormone was injected intra-muscularly near the dorsal fin and above the lateral line with the 1 mL syringe, as applied by Ali et al. (2014). The female was treated with 0.4 mL/kg body weight of fish and the male was treated with 0.2 mL/kg body weight of fish with both Wova-FH and Ovaprim. In the case of easy-spawn, the female was treated with 0.5 mL/kg body weight of fish, and the male was treated with 0.3 mL/kg body weight of fish. During the present study water quality parameters, i.e., DO, CO2, pH, and Temperature etc. was observed, and monitored regularly using Hach HQ40d Portable Multi-Parameter Meter (Hach, USA).
After injection, the males and females under each treatment were kept together in indoor cemented circular tanks with continuous showering. The shower was given through the perforated PVC pipe to ensure the maximum oxygen supply. During the daytime, the dark condition was created by falling the shatter, and fish were left without any disturbance. After 8–9 h of injection, fish spawned naturally and eggs were gathered in the center of the cistern. The fertilized eggs settled down on the bottom of the cistern, and they were collected from the bottom through siphoning using a small pipe into a plastic bowl. Then the collected fertilized eggs were kept in the small cistern where 8–10 cm water depth was maintained. Temperature was kept at about 27–31°C, dissolved oxygen at 5 mg/L, and the pH was maintained between 7 and 8. Around 22–24 h were required for the hatching of fertilized eggs. The recently hatched larvae had a light brown color and were transparent. Their bodies were laterally compressed, and they did not have distinct mouths and fins. The hatchlings also had unpigmented eyes. Due to the small size of their heads, it was difficult to distinguish them from the yolk sac. The first feeding was given to ensure the availability of food when the yolk sac was absorbed. They were cannibalistic and that is why feed was given at 3 times daily. Ground small tubificid (Tubifex tubifex) worm mixed with water was used as feed and used up to satiation level. Feed was given once in the morning, another in the afternoon, and lastly at night. After 3–5 days of rearing, the Shing fry was transferred into the nursery pond.
2.3 Assays of egg amount, ovulation, fertilization, and hatching rate
To estimate the number of eggs released into each tray using volumetric methods (Smith et al., 2015), a systematic procedure was followed. In this method, a representative sample of eggs was collected from each tray a pipette or dropper, and handled carefully to avoid loss or damage. The volume occupied by the eggs was measured using a graduated cylinder or volumetric flask. The number of eggs in a small aliquot was counted with a microscope, and this count was extrapolated to estimate the total number of eggs in each tray. The process was repeated three times and the average number of eggs per 5 mL was estimated. Finally, the total numbers of eggs were calculated by multiplying the average egg counts with the total volume of the eggs.
Ovulation rate, fertilization, and hatching rates were recorded to determine the effectiveness of hormone and breeding performance using the following (Equations 1, 2 and 3) (Bhuiyan et al., 2013):
2.4 Statistical analysis
To analyze data on the reproductive performance of stinging catfish (Heteropneustes fossilis) using three hormone analogs, descriptive statistics were initially performed to summarize the mean, median, standard deviation, and range of reproductive metrics such as spawning efficacy, fertilization rates, and hatching success. ANOVA was used to determine statistically significant differences in reproductive performance among the three hormone treatments. Following ANOVA, post-hoc tests like Tukey’s HSD (Honestly Significant Difference) were conducted to identify specific group differences. Additionally, regression analyses assessed the relationship between hormone dosages and reproductive outcomes, identifying optimal dose–response relationships. Data analysis was conducted using Microsoft Excel 2019, while statistical software SPSS version 10.0 was utilized with a significance level set at p < 0.05.
3 Results and discussion
3.1 Ovulation status
The ovulation rates of stinging catfish for different hormones are presented in Table 2. ANOVA revealed significant differences (p < 0.05) in ovulation rates among the treatments, indicating that the hormones had distinct effects on ovulation rates (Table 2). Females responded differently to the treatments, with varying ovulation rates and egg maturity levels. Among the treatments, the female fish exhibited particularly a strong response to the “wova-FH” hormone, resulting in profuse ovulation. They produced a high number of ripe eggs with an ovulation rate of 87.25 + 1.39%. Similarly, the females responded well to the “ovaprim” treatment, showing considerable ovulation and yielding both a sufficient number of ripe eggs and a considerable number of unripe eggs (Table 3). The ovulation rate for fish injected with ovaprim was recorded as 82.08 + 0.87%. On the other hand, the females exhibited a less pronounced response to the “easy-spawn” treatment, with ovulation occurring with a preponderance of unripe eggs. The ovulation rate for fish injected with “easy-spawn” was recorded as 27.50% (Table 2). These differences could be because of GnRH analogs types; although they all are synthetic; their content is different. Such as ovaprim and wova-FH contain Salmon GnRH, dopamine inhibitors, and propylene glycol, but easy-spawn contains GnRH and domperidone. Several studies have explored the efficacy of GnRH analogs from various sources in H. fossilis. In this study, H. fossilis was injected with ovaprim at a dose of 0.5 mL/kg body weight and showed 92% ovulation. Another study found higher ovulation rates than this study (Rahman et al., 2013). They found ovulation rates was highest (93.77%) when Ovaprim was used at a rate of 0.5 mL/kg body weight, and (90%) ovulation was found when the dose was applied at a rate of 0.3 mL/kg body weight. The authors suggested that the differences in ovulation rates was due to the variations in purity, potency, and pharmacokinetic properties of the GnRH analogs produced by different companies. It is speculated that the differences in ovulation rates are the results of variations in the structural and functional characteristics of the GnRH analogs, which can influence their binding affinity to GnRH receptors and subsequent signaling flow in the fish. Hyper-stimulation of ovarian functions with other hormonal treatments results in rapid oocyte maturation (Kucharczyk et al., 2020).
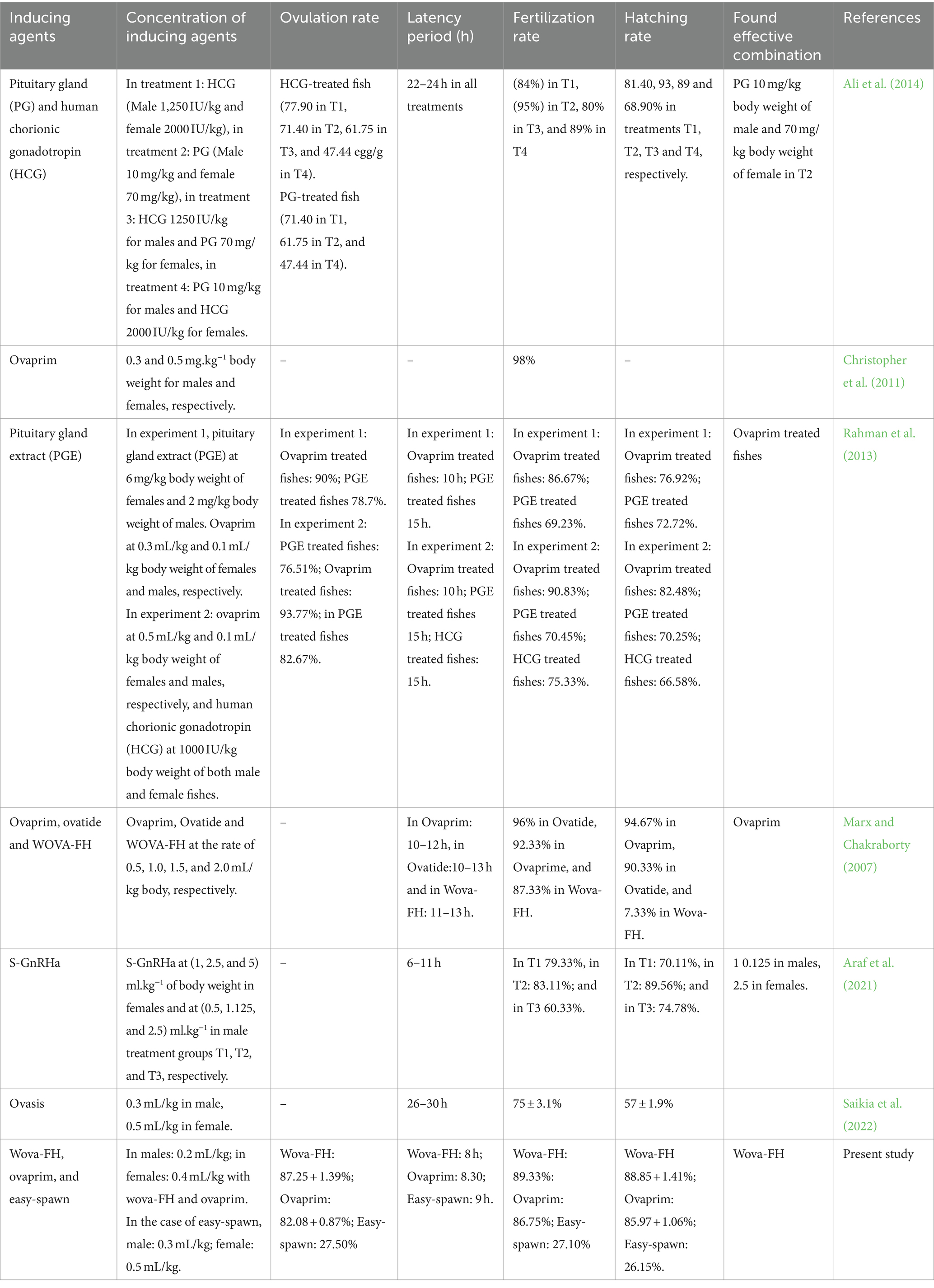
Table 2. Effect of different doses of GnRH Analogues on breeding performances of Stinging Catfish, Heteropneustes fossilis.
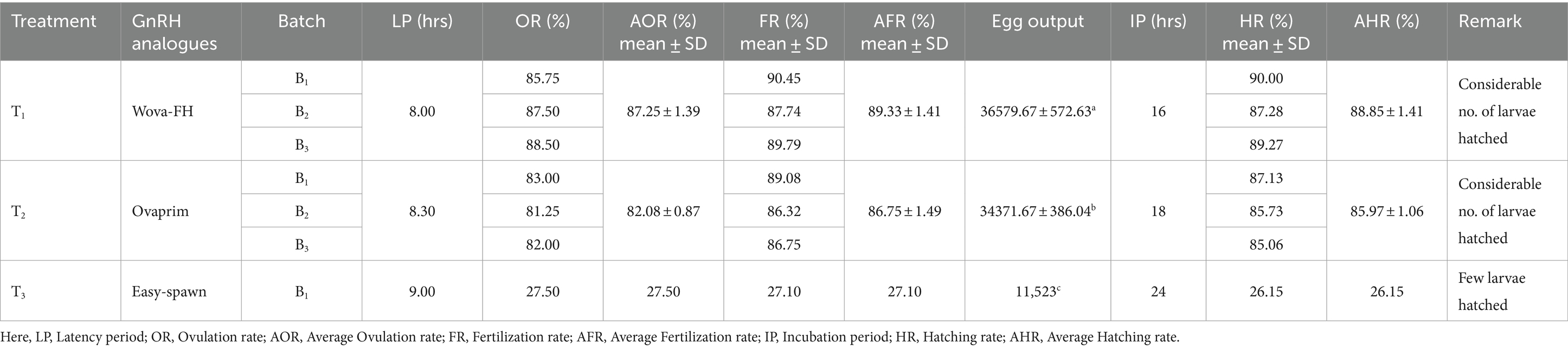
Table 3. Effect of GnRH analogues on the ovulation of females, fertilization, and hatching of eggs of shing (Heteropneustes fossilis).
However, it is difficult to identify the precise reason for such differing results because a group of factors is likely to influence biological experiments particularly those involving hormones (Gheyas et al., 2002). These compared studies highlight the importance of carefully evaluating and selecting the appropriate GnRH analog from different sources to optimize ovulation and breeding success in Heteropneustes fossilis and other commercially important fish species. The choice of GnRH analog can significantly impact the overall efficiency and reliability of induced breeding protocols in aquaculture.
3.2 Latency period
The spawning latency period is a critical factor in the success of induced breeding. In this study, a single dose of wova-FH showed induced spawning within approximately 8 h, which was notably quicker than the 8.30 and 9 h observed with “ovaprim” and “easy-spawn” respectively. The fertility by ovaprim and easy-spawn was found less than wova-FH (Table 2). This could be attributed to the more potent and targeted action of the GnRH analog wova-FH, which might be better optimized for H. fossilis. The latency period followed the following decreasing order: easy-spawn ˃ ovaprim ˃ wova-FH. The p-value showed (<0.05) significant difference among the latency periods of different treatments. The observed decreasing order of latency periods in stinging catfish can be attributed to the interaction of various factors affecting oocyte maturation. Oocyte size plays a significant role, as larger oocytes generally require more time to mature and respond to hormonal stimuli compared to smaller ones (Khan et al., 2021). This variation in size can influence how effectively different hormonal treatments induce ovulation. Water quality parameters such as pH, dissolved oxygen, and salinity are also crucial; suboptimal water conditions can stress the fish, impairing their physiological responses and potentially lengthening the latency period (Nguyen et al., 2022). Temperature is another critical factor, as it affects metabolic rates and hormonal activities. Higher temperatures typically accelerate these processes, potentially reducing the latency period, whereas lower temperatures can slow down maturation, extending the latency period (Jin et al., 2023). Each hormonal induction method—easy-spawn, ovaprim, and wova-FH—interacts differently with these environmental and physiological factors. For example, easy-spawn might be less effective in less-than-ideal conditions, resulting in a longer latency period (Smith and Liu, 2024). In contrast, wova-FH may be more adaptable to varying conditions, leading to shorter latency periods. The combined effects of oocyte size, water quality, and temperature with each hormonal treatment method ultimately determine the observed latency periods. Comparative studies in the past have also highlighted variations in the effectiveness of these treatments. The present study found less latency period than in the study of Marx and Chakraborty (2007), Puvaneswari et al. (2009), and Nayak et al. (2001) where they found, H. fossilis injected with ovaprim at 0.5 mL/kg, 0.4 mL /kg, and 0.5 mL/kg body weight, respectively, all ovulated at 10–12 h. Rahman et al. (2013) also observed a latency period of 10 h in H. fossilis treated with ovaprim at 0.3 mL/kg and 0.5 mL/kg. Using the same dose of ovaprim, a much longer latency period (18–24) was recorded by Kather Haniffa and Sridhar (2002) and Singh Kohli and Goswami (1987). On the other hand, (Marx and Chakraborty, 2007) observed that WOVA-FH injected fish at 0.5 mL/kg showed 13 h of latency period, which is much higher than this study. It is challenging to explain the conducive factors for the observed variances. This difference in the latency period could be the reason for brood quality, environmental conditions, and for quality of hormones. Besides, the maturity of fish also regulates this latency period. If a fish becomes fully matured then a relatively small amount of GnRH analogs is sufficient to ovulate the brood. If the hormone dose is higher, then the seed quality will deteriorate (Zohar, 1994). The operation weather also plays a major role in spawning, such as high ambient temperature takes relatively less time for ovulation, shortens the incubation period, and consequently affects the survival rate and hatching rate of the eggs, but a day with higher humidity takes more time for ovulation (Zadmajid et al., 2017).
3.3 Fertilization rate
Results obtained from wova-FH-treated fish (0.4 mL/kg), showed the highest fertilization rate (89.33%) whereas 86.75 and 27.10% were observed in the case of ovaprim and easy-spawn-treated ovulated eggs, respectively, (Figure 2). The fertilization rate among different treatments showed significant differences (p < 0.05). Rahman et al. (2013) found 86.67 and 90.83% fertilization rate of H. fossilistreated with ovaprim at 0.3 mL/kg and 0.5 mL/kg respectively, which is similar to the present study. Nandeesha et al. (1990) and More et al. (2010) observed different results, where ovaprim treatments showed higher fertilization rates (89 and 93% at 0.4 and 0.5 mL/kg respectively). Kather Haniffa and Sridhar (2002) observed a lower fertilization rate than this study (70 and 75%) of H. fossilistreated with ovaprim at 0.3 mL/kg and 0.5 mL/kg, respectively. Marx and Chakraborty (2007) observed that WOVA-FH injected fish at 0.5 mL/kg showed 87.33% fertilization rate, which is much lower than this study. Such deviations in the fertilization rate can be ascribed to the differences in hormonal doses, and size of the brood fish. Additionally, seasonal variation (Gheyas et al., 2002; Kather Haniffa and Sridhar, 2002; Nwokoye et al., 2007), environmental factors, water quality parameters including alkalinity, DO, pH, hardness, etc. (Khan et al., 2006) are also influencing factors. When hormones were administered at higher doses in H. fossilis a regular decline in the fertilization rate was observed (Marx and Chakraborty, 2007).
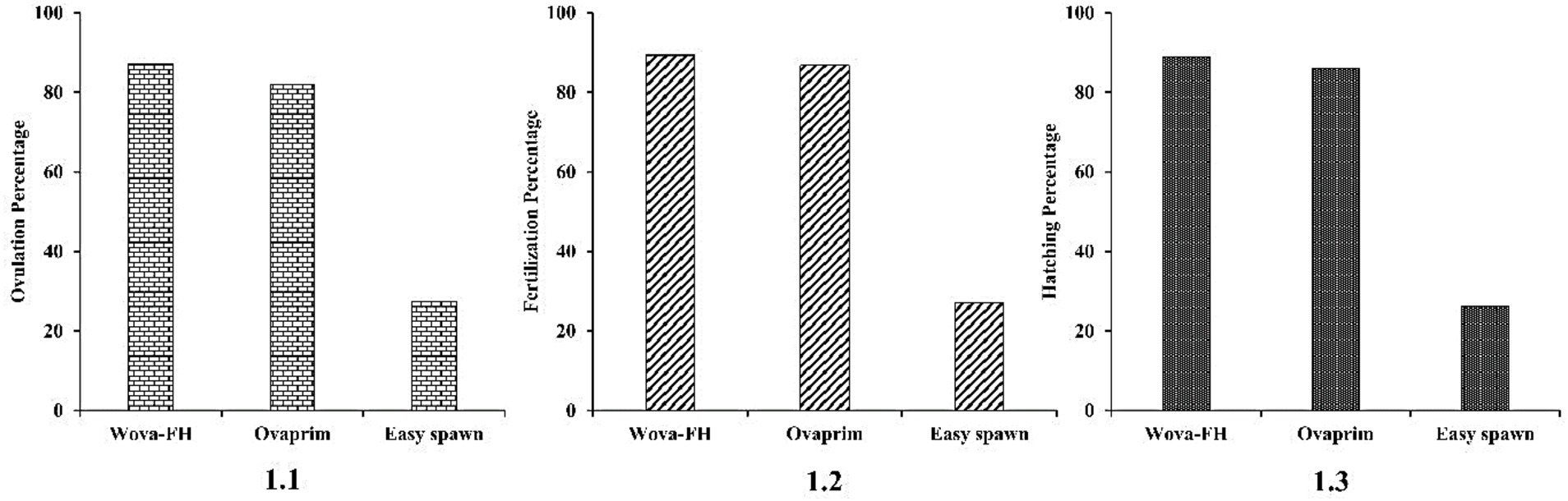
Figure 2. Comparison of different hormones on ovulation of females (1.1), on average fertilization rate (1.2), and hatching of fertilized eggs (1.3) (Heteropneustes fossilis).
3.4 Hatching rate
In the present study, the hatching rates showed the following decreasing order: (88.85 ± 1.41%) from wova-FH treated eggs ˃ (85.97 ± 1.06%) from ovaprim treated eggs ˃ (26.15%) from easy-spawn treated eggs (Figure 3). ANOVA analysis showed a significant difference (p < 0.05) between the treatments and hatching rate, indicating the different effects of hormones on hatching rates. The incubation period for eggs was high in the wova-FH-treated fish (16 h), which was followed by ovaprim treated fish (18 h) and easy-spawn-treated fish (24 h). Rahman et al. (2013) observed hatching rates of 76.92 and 82.48% for eggs in the ovaprim-treated fishes when ovaprim was used at 0.3 and 0.5 mL/kg body weight, respectively. This finding was lower than the present study. Marx and Chakraborty (2007) also observed a lower hatching rate (at 0.5 mL/kg showed 77.33%) in WOVA-FH injected fish. Kather Haniffa and Sridhar (2002) showed lower hatching rates (50.5 and 60%) for H. fossilis injected with ovaprim at a rate of 0.3 and 0.5 mL/kg, respectively. 100% fertilization and hatching rate was not achieved in this work which could be due to hypoxia, unripe conditions among the eggs, and fertilization (Olufeagba et al., 2015).
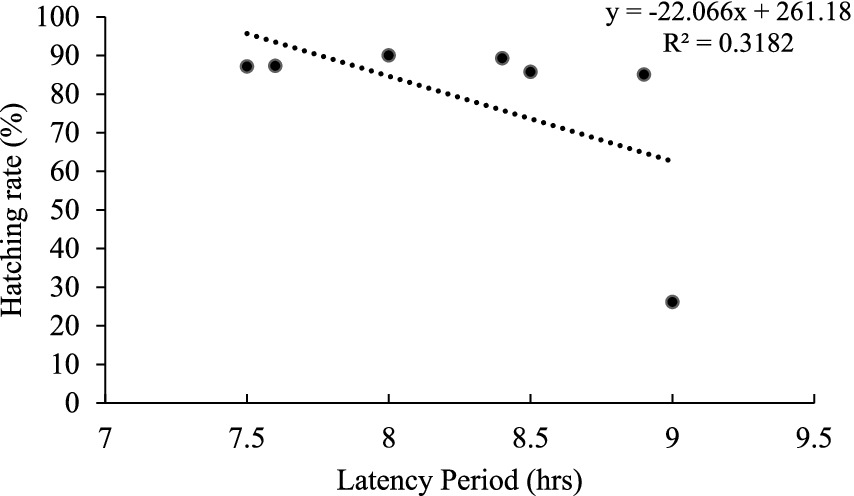
Figure 3. Relationship between latency time and hatching rates in the artificial spawning of stinging catfish (Heteropneustes fossilis) using three different hormonal treatments in captive conditions.
3.5 Variables on spawning success
The linear relationship between latency period, and hatching rates suggests a negative correlation between latency time and hatching rates, as represented by the linear equation y = −22.066x + 261.18. This means that in the present study, the longer latency period resulted in decreasing hatching rates. However, the coefficient of determination (R2 = 0.3182) indicates that only about 31.82% of the variability in the hatching rates can be explained by the latency period. This suggests that other factors such as ovulation rate, fertilization rate, and environmental parameters might potentially be influencing the rates of hatching. Besides, variations in the hormonal treatments and the inherent variety in the biological responses of the stinging catfish can also influence this variation. These findings are consistent with previous studies on the artificial spawning of stinging catfish. For example, Alok et al. (1993) found that synthetic human Kisspeptin1 (hKiss1) and catfish Kisspeptin2 (cfKiss2) significantly influenced the hypothalamic–pituitary-ovarian (HPO) axis, final oocyte maturation, and ovulation in pre-spawning female stinging catfish. Similarly, Mahalder et al. (2023) found that climate and water quality characteristics had a substantial impact on the embryonic and larval development of stinging catfish.
No mortality was observed after the injection in trials indicating a high degree of safety for all the fishes used in this study. Poor water quality, egg binding during the induction spawning procedure, and administration route can cause mortality (Hill et al., 2009). Nandeesha et al. (1990), and More et al. (2010), showed that ovaprim-treated fish yield better results than PGE-treated fish, but this study suggests that ovaprim creates serious tissue damage in the injecting place. In India, most of the breeders have preferred ovaprim, as a survey showed that only 10–15% of fish breeders use carp pituitary extract due to its complexity of technique (Tiwana and Raman, 2012). It is also true for Bangladesh, where ovaprim is used widely instead of PG and HCG. Ovaprim and Wova-FH are effective for induced spawning as they contain Salmon GnRH, a native peptide commonly found in most teleosts. Easy-Spawn encountered issues with hormone spoilage and leakage upon injection, necessitating a higher dosage for the fish. The present results suggested that Wova-FH might be a superior GnRH analog compared to Ovaprim and Easy-Spawn for induced breeding.
4 Conclusion
The evaluation of GnRH analogs (Wova-FH, Ovaprim, and Easy-spawn) revealed that ovulation status, latency period, fertilization rate, and hatching rate followed the following decreasing order: wova-FH ˃ ovaprim ˃ easy-spawn in all treatments. Notably, the injection of ovaprim resulted in major tissue damage at the injection site, raising concerns about its potential impact on fish reproductive health. It is imperative to conduct further advanced research to ascertain the underlying reasons for this tissue damage and explore potential strategies to mitigate this issue. Furthermore, it was observed that the easy-spawn hormone rapidly dispersed from the injection site, leading to a substantial loss of the hormone and significantly impacting ovulation. This highlights the need for extensive research to determine the optimal hormone delivery method and dosage. Future research efforts should focus on establishing a reliable hormone and dosage regimen that has been successfully validated in other fish species, such as Ruhu and Catla. It is suggested that wova-FH could be used in the induced breeding of the catfish, Heteropneustes fossilis, under hatchery conditions for large-scale fry production. This study will provide valuable guidance to fish farmers, alleviating their uncertainties regarding the selection of a suitable GnRH analog for H. fossilis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RY: Data curation, Software, Writing – original draft. MR: Conceptualization, Methodology, Writing – original draft. SC: Investigation, Writing – original draft. BS: Supervision, Writing – original draft. MB: Investigation, Writing – original draft. PS: Funding acquisition, Resources, Writing – review & editing. MA: Data curation, Funding acquisition, Writing – review & editing. TA: Funding acquisition, Writing – review & editing. MH: Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Researchers Supporting Project Number (RSP2024R436), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
Thanks are extended to the Researchers Supporting Project Number (RSP2024R436), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acharjee, A., Chaube, R., and Joy, K. P. (2017). Ovaprim, a commercial spawning inducer, stimulates gonadotropin subunit gene transcriptional activity: a study correlated with plasma steroid profile, ovulation and fertilization in the catfish Heteropneustes fossilis. Gen. Comp. Endocrinol. 251, 66–73. doi: 10.1016/j.ygcen.2016.10.001
Ahamed, S., Hasan, K. R., Hossain, M., Mahmud, Y., and Rahman, M. K. (2017). Adaptability of polyculture of stinging catfish (Heteropneustes fossilis) in seasonal water bodies of greater northern region, Bangladesh. Int.J. Fisheries Aquatic Stu. 5, 433–439.
Ahamed, M. T., Siddique, M. A. B., Akter, S., Nahiduzzaman, M., Rahman, M. H., and Haque, M. A. (2023). Culture suitability of stinging catfish Heteropneustes fossilis in homestead tank: selection of suitable stocking size. Arch. Agric. Environ. Sci. 8, 319–324. doi: 10.26832/24566632.2023.080307
Ali, A., Rahman, M. R., Alam, M. J., Nishat, A. A., Rabbi, M. F., Haque, M. A., et al. (2018). Production of stinging catfish (Heteropneustes fossilis) in different stocking densities with GIFT (Oreochromis niloticus) and Thai Sharpunti (Barbonymusgonionotus) in ponds. J. Fisheries Life Sci. 3, 9–15.
Ali, M. F., Rahman, M. M., Bashar, M. K., Rahmatullah, R., Hadiuzzaman, M., and Amin, M. (2014). Comparative study on induced breeding of shing, Heteropneustes fossilis (Bloch) between HCG and PG with different combination. Int. J. Fisheries Aquatic Stu. 2, 104–108.
Alok, D., Krishnan, T., Talwar, G. P., and Garg, L. C. (1993). Induced spawning of catfish, Heteropneustes fossilis (Bloch), using D-Lys6 salmon gonadotropin-releasing hormone analog. Aquaculture 115, 159–167. doi: 10.1016/0044-8486(93)90366-7
Araf, T., Hossen, M. A., Chowdhury, G., Hossain, M. A., Rahman, M. A., and Iqbal, M. M. (2021). Artificial propagation and embryonic growth of stinging catfish, Heteropneustes fossilis (Bloch, 1794) using S-GnRHa (Salmon gonadotropin releasing hormone analogue). J. Trop. Life Sci. 11, 141–149. doi: 10.11594/jtls.11.02.03
Bhuiyan, A. S., Akhter, J., and Mushahida-Al-Noor, S. (2013). Efficacy of two inducing agents, PG and DOM+ SGNRH on the induced breeding of the major carp, Kalibaus (Labeocalbasu). Our Nat. 11, 17–24. doi: 10.3126/on.v11i1.8239
Christopher, J. G., Murugesan, A. G., and Sukumaran, N. (2011). Optimization of artificial fertilization in the stinging catfish Heteropneustes fossilis (Bloch). Zygote 19, 63–66. doi: 10.1017/S0967199410000122
Das, P., Behera, B. K., Meena, D. K., Singh, S. K., Mandal, S. C., Das, S. S., et al. (2016). Comparative efficacy of different inducing agents on breeding performance of a near threatened cyprinid Osteobrama belangeri in captivity. Aquaculture Rep. 4, 178–182. doi: 10.1016/j.aqrep.2016.11.001
Elakkanai, P., Francis, T., Ahilan, B., Jawahar, P., Padmavathy, P., Jayakumar, N., et al. (2015). Role of GnRH, HCG and Kisspeptin on reproduction of fishes. Indian J. Sci. Technol. 8, 1–10.
Gheyas, A. A., Islam, M. S., Mollah, M. F. A., and Hussain, M. G. (2002). A comparative study on the embryonic development of gynogen, triploid, haploid and normal diploid embryos of stinging catfish, Heteropneustes fossilis. J. Inland Fisheries Soc. India 6, 107–115.
Haniffa, M. A., Dhanaraj, M., Muthu Ramakrishnan, C., Sethuramalingam, T. A., Arun Singh, S. V., Ananth Kumar, Y., et al. (2008). Threatened fishes of the world: Heteropneustes fossilis (Bloch, 1794) (Cypriniformes: Saccobranchidae). Environ. Biol. Fish 82, 203–204. doi: 10.1007/s10641-007-9314-6
Hill, J. E., Kilgore, K. H., Pouder, D. B., Powell, J. F., Watson, C. A., and Yanong, R. P. (2009). Survey of ovaprim use as a spawning aid in ornamental fishes in the United States as administered through the University of Florida Tropical Aquaculture Laboratory. N. Am. J. Aquac. 71, 206–209. doi: 10.1577/A08-020.1
Hossain, M. A., Nabi, S. N., Rashid, M. H. U., Hossain, M. A., and Sikandar, M. A. (2021). Optimum stocking density of stinging catfish (Heteropneustes fossilis) based carp polyculture in homestead ponds in a drought prone area of Bangladesh. Bangladesh J. Fisheries 33, 177–186. doi: 10.52168/bjf.2021.33.20
Jin, J., Zhang, Y., and Wang, L. (2023). Temperature effects on oocyte maturation and hormonal induction in aquatic species. Mar. Biol. 170, 215–226.
Kather Haniffa, M. A., and Sridhar, S. (2002). Induced spawning of spotted murrel (Channa punctatus) and catfish (Heteropneustes fossilis) using human chorionic gonadotropin and synthetic hormone (ovaprim). Veterinarskiarhiv. 72, 51–56.
Khan, M. A., Hossain, M. S., and Ahmed, M. M. (2021). Effects of oocyte size on the efficiency of hormonal induction in fish. Aquac. Res. 52, 1543–1552.
Khan, H. A., and Mukhopadhyay, S. K. (1976). Production of stocking material of some air-breathing fishes by hypophysation. J. Inland Fisher. Soc. India 7, 156–161.
Khan, A. M., Shakir, H. A., Ashraf, M. U. H. A. M. M. A. D., and Ahmad, Z. (2006). Induced spawning of Labeorohita using synthetic hormones. Punjab Univ. J. Zool. 21, 67–72.
Kucharczyk, D., Nowosad, J., Wyszomirska, E., Cejko, B. I., Arciuch-Rutkowska, M., Juchno, D., et al. (2020). Comparison of artificial spawning effectiveness of hCG, CPH and GnRHa in combination with dopamine inhibitors in a wild strain of ide Leuciscus idus (L.) in hatchery conditions. Anim. Reprod. Sci. 221:106543. doi: 10.1016/j.anireprosci.2020.106543
Mahalder, B., Haque, M. M., Siddique, M. A. B., Hasan, N. A., Alam, M. M., Talukdar, M. M. N., et al. (2023). Embryonic and larval development of stinging catfish, heteropneustes fossilis, in relation to climatic and water quality parameters. Life 13:583. doi: 10.3390/life13020583
Marimuthu, K., Muruganandam, M., Haniffa, M. A., and Arockiaraj, J. (2000). Induced spawning of the Indian catfish Heteropneustes fossilis (Singhi) using a synthetic hormone, Ovatide. Fishing Chimes. 19, 105–106.
Marx, K. K., and Chakraborty, R. (2007). A comparative study on the induced breeding and in vitro fertilization performance by various inducing agents in catfish, Heteropneustes fossilis (Bloch). Bangladesh J. Res. 11, 1–5.
Mylonas, C. C., and Zohar, Y. (2001). Endocrine regulation and artificial induction of oocyte maturation and spermiation in basses of the genus Morone. Aquac. 202, 205–220.
More, P. R., Bhandare, R. Y., Shinde, S. E., Pathan, T. S., and Sonawane, D. L. (2010). Comparative study of synthetic hormones Ovaprim and carp pituitary extract used in induced breeding of Indian major carps. Libyan Agric. Res. Cen. J. Intl. 1, 288–295.
Nabi, S. N., Hossain, M. A., Alam, M. M., Harun-Ur-Rashid, M., and Hossain, M. A. (2020). Effect of carp species combination on production and economics of stinging catfish, Heteropneustes fossilis based polyculture in homestead ponds under drought prone area of Bangladesh. J. Fisheries. 8, 920–927. doi: 10.17017/j.fish.282
Nandeesha, M. C., Rao, K. G., Jayanna, R., Parker, N. C., Varghese, T. J., Keshavanath, P., et al. (1990). “Induced spawning of Indian major carps through single application of ovaprim” in The second Asian fisheries forum, Asian fisheries society, Manila, Phillipines (Manila: Asian Fisheries Society), 581–585.
Nayak, P. K., Mishra, T. K., Singh, B. N., Pandey, A. K., and Das, R. C. (2001). Induced maturation and ovulation in Heteropneustes fossilis by using LHRHa, pimozide and ovaprim for production of quality eggs and larvae. Indian J. Fish. 48, 269–275.
Nguyen, T. T., Pham, V. H., and Le, D. T. (2022). Impact of water quality on hormonal induction efficacy in aquaculture. J. Fish Biol. 100, 1684–1699.
Nwokoye, C. O., Nwuba, L. A., and Eyo, J. E. (2007). Induced propagation of African clariid catfish, Heterobranchus bidorsalis (Geoffrey saint Hillarie, 1809) using synthetic and homoplastic hormones. Afr. J. Biotechnol. 6:13.
Olufeagba, S. O., Raji, A., Majumda, K. C., Ravinda, K., and Okomoda, V. T. (2015). Induced breeding and early development of stinging catfish, Heteropneustesfossilis (Bloch) (Siluridae). Int. J. Aquac. 5:13. doi: 10.5376/ija.2015.05.0013
Parvin, M. F., Hossain, M. Y., Sarmin, M. S., Rahman, O., Tanjin, S., Samad, M. A., et al. (2022). Reproductive performance of Asian stinging catfish Heteropneustesfossilis (Bloch 1794) in the Ganges River (NW Bangladesh) in relation to environmental factors. Environ. Sci. Pollut. Res. 29, 42822–42836. doi: 10.1007/s11356-022-18816-9
Puvaneswari, S., Marimuthu, K., Karuppasamy, R., and Haniffa, M. A. (2009). Early embryonic and larval development of Indian catfish, Heteropneustes fossilis. Eurasia J Biosci. 3, 84–96.
Quiniou, S. M., Bosworth, B., Chatakondi, N., and Oberle, D. (2014). Evaluation of a gonadotropin releasing hormone analog of cGnRH II as a spawning aid for Channel Catfish versus analogs of mGnRH I and sGnRH III. N. Am. J. Aquac. 76, 281–288.
Rahman, M. M., Hossain, M. Y., Hossain, M. I., Provhat, S. J., Islam, M. S., and Hossain, M. B. (2013). Induced breeding of the stinging catfish, Heteropneustes fossilis: comparison among different inducing agents. Turk. J. Fish. Aquat. Sci. 13:3.
Rather, M. A., Sharma, R., Gupta, S., Ferosekhan, S., Ramya, V. L., and Jadhao, S. B. (2013). Chitosan-nanoconjugated hormone nanoparticles for sustained surge of gonadotropins and enhanced reproductive output in female fish. PloS one, 8:e57094.
Saha, M. R. (1986). Effects of various doses of ovaprim for breeding of Clarias spp. Tripura JIFSI 28, 75–84.
Saikia, C., Singh, M. K., and Sonowal, S. (2022). Captive breeding of the stinging catfish, Heteropneustes fossilis (Bloch, 1794) found in Brahmaputra River, Assam, India using inducing agent ovasis and its early embryogenesis. Egypt. J. Aquatic Biol. Fisher. 26, 161–173. doi: 10.21608/ejabf.2022.258922
Samad, M. A., Nahiduzzama, M., Ashrafuzzaman, M., Rashid, M. A., and Akter, M. (2017). Culture of indigenous catfish Shingi, Heteropneustes fossilis (Bloch, 1794), with available low cost formulated feed in earthen ponds of Bangladesh. J. Coastal Life Med. 5, 288–292. doi: 10.12980/jclm.5.2017J7-86
Sarkar, P. K., Chaudhuri, A., and Mandal, R. K. (1979). Developmental biology of catfish Heteropneustesfossilis Bloch: changes in nucleic & protein content & effects of some inhibitors during embryonic development. Indian J. Exp. Biol. 17, 1012–1015.
Singh Kohli, M. P., and Goswami, U. C. (1987). Spawning behaviour of a freshwater air-breathing Indian catfish Heteropneustes fossilis (Bloch). Matsya 12, 180–183.
Smith, J., Brown, R., and Green, A. (2015). Quantitative analysis of aquatic eggs using volumetric methods. J. Aquatic Res. 7, 29–36. doi: 10.1016/j.jare.2015.01.006
Smith, J. A., and Liu, X. (2024). Comparative efficacy of hormonal induction methods in fish reproduction. Aquac. Int. 32, 75–90.
Tiwana, G. S., and Raman, S. (2012). An economically viable approach for induced breeding of Labeorohita by ovatide, ovaprim and carp pituitary extract. J. Agric. Vet. Sci. 1, 30–32.
Zadmajid, V., Mirzaee, R., Hoseinpour, H., Vahedi, N., and Butts, I. A. E. (2017). Hormonal induction of ovulation using Ovaprim™[(D-Arg6, Pro9NEt)-sGnRH+ domperidone] and its impact on embryonic development of wild-caught Longspine scraper, Capoeta trutta (Heckel, 1843). Anim. Reprod. Sci. 187, 79–90. doi: 10.1016/j.anireprosci.2017.10.009
Keywords: stinging catfish, GnRH hormones, induced breeding, sustainable aquaculture, performance
Citation: Yasmin R, Rahman MM, Chakraborty S, Sarker BS, Bappy MMM, Sarker PK, Albeshr MF, Arai T and Hossain MB (2024) Comparative evaluation of the efficacy of three GnRH analogues in induced breeding of stinging catfish, Heteropneustes fossilis under hatchery conditions. Front. Sustain. Food Syst. 8:1445760. doi: 10.3389/fsufs.2024.1445760
Edited by:
Neaz A. Hasan, Bangabandhu Sheikh Mujibur Rahman Science and Technology University, BangladeshReviewed by:
Domitila Kyule, Kenya Marine and Fisheries Research Institute, KenyaKailasam Muniyandi, Central Institute of Brackishwater Aquaculture (ICAR), India
Copyright © 2024 Yasmin, Rahman, Chakraborty, Sarker, Bappy, Sarker, Albeshr, Arai and Hossain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pallab Kumer Sarker, cHNhcmtlckB1Y3NjLmVkdQ==; M. Belal Hossain, bWJobnN0dUBnbWFpbC5jb20=
 Rahima Yasmin1
Rahima Yasmin1 Md. Mofizur Rahman
Md. Mofizur Rahman Pallab Kumer Sarker
Pallab Kumer Sarker Mohammed Fahad Albeshr
Mohammed Fahad Albeshr Takaomi Arai
Takaomi Arai M. Belal Hossain
M. Belal Hossain