- ICAR-Central Inland Fisheries Research Institute, Barrackpore, Kolkata, India
Introduction: Proteins and amino acids, as vital biomolecules, are not only part of key metabolic pathways in the body but are also essential for tissue repair, enzyme function, and hormone regulation. They also serve as building blocks for the formation of signaling molecules, such as neurotransmitters and catecholamines. Additionally, amino acids are the primary building components of proteins. Fish serves as a vital source of high-quality animal protein and amino acids, playing a crucial role in supporting human nutrition.
Methods: High-performance liquid chromatography (HPLC) using fluorescence detector was employed to investigated the amino acid content of eight food fishes from diverse aquatic habitats.
Results: The study revealed that the small indigenous fish (SIF) Systomus sarana and the marine fish Sardinella melanura were rich sources of all essential amino acids. Furthermore, estuarine fish like Pisodonophis boro can be recommended for specific amino acids like arginine, histidine, leucine, and valine, while Setipinna phasa is suggested for cysteine.
Discussion: These recommendations rely on the possible contribution of these fishes to the Recommended Dietary Allowance (RDA) regarding each nutrient. The insights gained from this study could be utilized as recommendations to meet amino acid requirement using fish as a natural supplement.
1 Introduction
Amino acids, integral to body protein construction, are primarily derived from dietary proteins and are crucial for cellular growth, repair, and maintenance (Basumatary et al., 2017). Amino acids supply nitrogen, hydrocarbon skeletons, and sulfur, essential components for various functions in the body. They cannot be substituted by other nutrients like carbohydrates or lipids because the body does not produce nitrogen or sulfur. Moreover, they serve as essential precursors for synthesizing proteins, peptides, and various vital substances, including glutathione, dopamine, nitric oxide, serotonin, creatine, and nucleic acids, holding immense physiological significance (Wu, 2009; Wu et al., 2009; San Gabriel and Uneyama, 2013; Chandel, 2021).
There are 20 standard amino acids responsible for the synthesis of proteins that are known as proteinogenic amino acids (Li et al., 2021). Among the 20 amino acids, animals typically possess seven conditionally essential amino acids (CEAAs), which they can synthesize and are often not required in their diets. However, these amino acids become essential for specific populations unable to synthesize them adequately. Conversely, four amino acids are categorized as “nonessential” (NEAAs) as the body can synthesize them, and therefore, are not obligatory in the diet. The remaining nine amino acids, termed “essential” (EAAs), must be obtained from the diet. While most bacteria and plants can synthesize all 20 amino acids, it is commonly understood that amino acids play a crucial role not only in protein synthesis but also in generating ATP, glucose, and fatty acids. Traditionally, for fish, birds, and mammals, amino acids are categorized as CEAAs, NEAAs, or EAAs according to demand, growth, or nitrogen balance. Recent studies highlight the roles of functional amino acids (FAAs) from both EAAs and NEAAs in various physiological processes. The primary metabolic pathways that improve an organism’s growth, health, development, reproduction, and survival are regulated by FAAs. In human nutrition, arginine, aspartate, cysteine, methionine, leucine, tryptophan, glutamic acid, tyrosine, glycine, taurine, and proline are classified as FAAs (Wu, 2010, 2013) (Table 1).
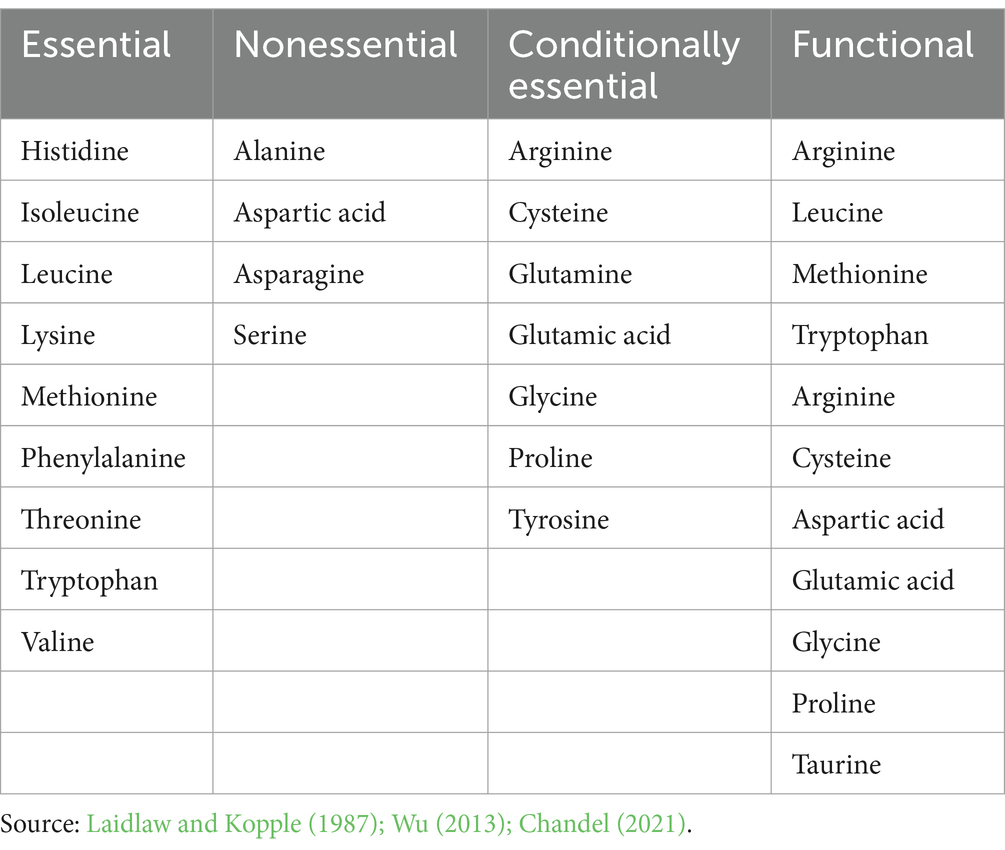
Table 1. Essential, nonessential, conditionally essential and functional amino acids in the human diet.
Amino acids are mostly obtained from proteins in the diet, and the quality of these proteins is evaluated based on the proportion of amino acids that are essential vs. the nonessential amino acids (WHO, 2007). Both plant and animal proteins from lentils, eggs, fish, and meat upon digestion, yield the amino acids necessary for intracellular protein synthesis (Chandel, 2021). The amino acid content of foods displays considerable variation (Raw, 2015), and lysine and the sulfur-containing amino acids (cystine and methionine), which are limiting amino acids, have particular importance as protein synthesis is restricted, if the limiting amino acids are not adequately present (Baker, 2009; Stipanuk et al., 2009). Cereals like wheat and rice exhibit notably lower lysine levels compared to animal-derived foods. Similarly, leguminous plants like soybeans and chickpeas have significantly lower concentrations of methionine than animal-based foods (Raw, 2015). Consequently, the nutritional value of protein varies greatly among diets, making animal-derived foods considered to be “high quality” protein sources in contrast to proteins derived from plants (Young and El-Khoury, 1996; Singh, 2002). Moreover, the digestibility of proteins in food also plays a significant role, representing the portion of dietary intake that remains accessible to the body following breakdown and assimilation (Moughan, 2005).
According to several suggestions, in contrast to terrestrial animal protein, aquatic animal protein enriched with essential amino acids and several peptides has a higher digestibility and amino acid bioavailability (Tacon and Metian, 2013; Cashion et al., 2017). Fish regarded as an excellent source of several nutrients, including protein, contains approximately 18–20 percent protein (Balachandran, 2012) and provides every necessary amino acid required for the well-being of humans. In particular, considerable amounts of limiting essential amino acids, mainly lysine, which is scarce in cereals, are also present in fish (FAO, 2005). Furthermore, since it has less connective tissue than other animal proteins, fish muscle is easier to digest (Suganthi et al., 2015). High-quality proteins with higher digestibility provide essential amino acids in amounts that meet human needs (Mohanty et al., 2014). Animal nutritionists prioritize dietary protein digestibility due to its significance in tissue growth and costliness in feed (Li et al., 2021). Thus, the knowledge of the nutrient composition within fish muscles is essential for realizing the health benefits for consumers (Sarma et al., 2013; Mohanty et al., 2014). In this context, a crucial role can be played by fish, as it is a vital and economical source of high-quality proteins.
India harbors more than 10% of the world’s fish diversity (Ayyappan and Diwan, 2007). With a yearly production of about 17.54 million metric tons, the country ranks third globally in fish production, accounting for 8.92% of the total global fish production (Goi, 2022). However, insufficient knowledge about the nutritional composition of most fish and how nutrient yields differ among fisheries has greatly hindered the formulation of policies necessary to effectively utilize fisheries for ensuring food as well as nutritional security (Golden et al., 2016; Hicks et al., 2019). Rivers serve as primary habitat for various species of fish. The Ganga River system, the largest river system in the Indian subcontinent, with its immense cultural and ecological significance, serves as a vital habitat for a diverse array of aquatic species, including numerous fish varieties (Dudgeon et al., 2006). A major portion of the lower stretch of the Ganges River flows across West Bengal before its discharge into the Bay of Bengal. The Ganges enters West Bengal as the Bhagirathi River. The Ganga River then diverges and descends into the Hooghly estuary, creating one of its first branches in the delta. Serving as a crucial connection between the Hooghly–Bhagirathi River basin and the Bay of Bengal, the Hooghly estuary has become a major section of the Ganges-Brahmaputra-Meghna (GBM), the biggest fluvio-marine delta in the world, situated within India’s geographical boundaries. The Hooghly estuary receives water from major and minor rivers such as the Falgu, Ajay, Churni, and Jalangi to the north, and the Mayurakshi, Rupnarayan, Haldi, and Damodar to the south (Das, 2015; Maheshvaran et al., 2019). River Matla is also one of the major rivers of the Sundarbans that supports numerous fish species (Mahapatra et al., 2014). Thus, due to its extensive distribution throughout the nation, including West Bengal, the Ganges River, with its diverse array of fish species, serves as an important protein source for millions of people residing in the Ganga basin (Maurya et al., 2019). Therefore, understanding the amino acid profiles of indigenous fish species inhabiting different stretches of the Ganges River is essential to evaluate their nutritional adequacy.
Humans need sufficient food and proper nutrition to sustain and maintain an active and fruitful lifestyle (Sharma et al., 2021). Food is composed of food ingredients, which (say, fish) are composed of several nutrients. The proportion of a nutrient that a healthy individual must obtain from food to fulfill his/her physiological needs is called nutrient requirement (Sharma et al., 2021). The Recommended Dietary Allowance (RDA) is designed for healthy individuals and can be recommended to fulfill the nutritional requirements for particular nutrients based on age, gender, and life stage. It aims to minimize the likelihood of insufficient nutrient intake (Indian Council of Medical Research, 2020). While recommendations are often in terms of protein intake, the true biological need is for amino acids (National Research Council, 1989). The Indian Council of Medical Research (ICMR) adopts the FAO/WHO/UNU guidelines as the basis for establishing the RDA, making minor adjustments as needed. RDAs for nutrients are provided in standardized units to facilitate easy calculation of individual needs based on basal metabolic rate and/or body weight.
In the past few decades, the rise in global population has also increased the demand for fish as a vital protein source due to its high nutritional value. Fish constitutes a substantial portion of human protein intake, with at least 20% consumed by a third of the global populace, particularly pronounced in developing nations (Béné et al., 2007). Furthermore, amino acids are concerned with health, and inadequacies in these essential nutrients can contribute to various diseases. Therefore, understanding the composition of amino acids forms the foundation for determining the potential nutritional value of foods (Williams, 2005). Hence, there is a necessity to produce and record nutritional data on several varieties of fish species that are available for consumption as it is a vital source of protein and essential nutrients, understanding the composition of amino acids is imperative for assessing their nutritional value and potential health benefits (Gatlin et al., 2007). In this context, the main objective of the research was to assess the composition of amino acids of eight selected fish species collected from various zones of the Ganges River. Additionally, it aimed to determine their potential contribution (expressed as daily value %) to the recommended dietary allowance (RDA), thereby enhancing their nutritional utility for humans.
2 Materials and methods
2.1 Ethical statement
All research conducted adheres to the ethical standards and guidelines and complies with the legal regulations of the nation where the study was conducted and was in accordance with Institute Animal Ethics Committee (No. CIFRI/IAEC-22-23/01).
2.2 Sample collection and processing
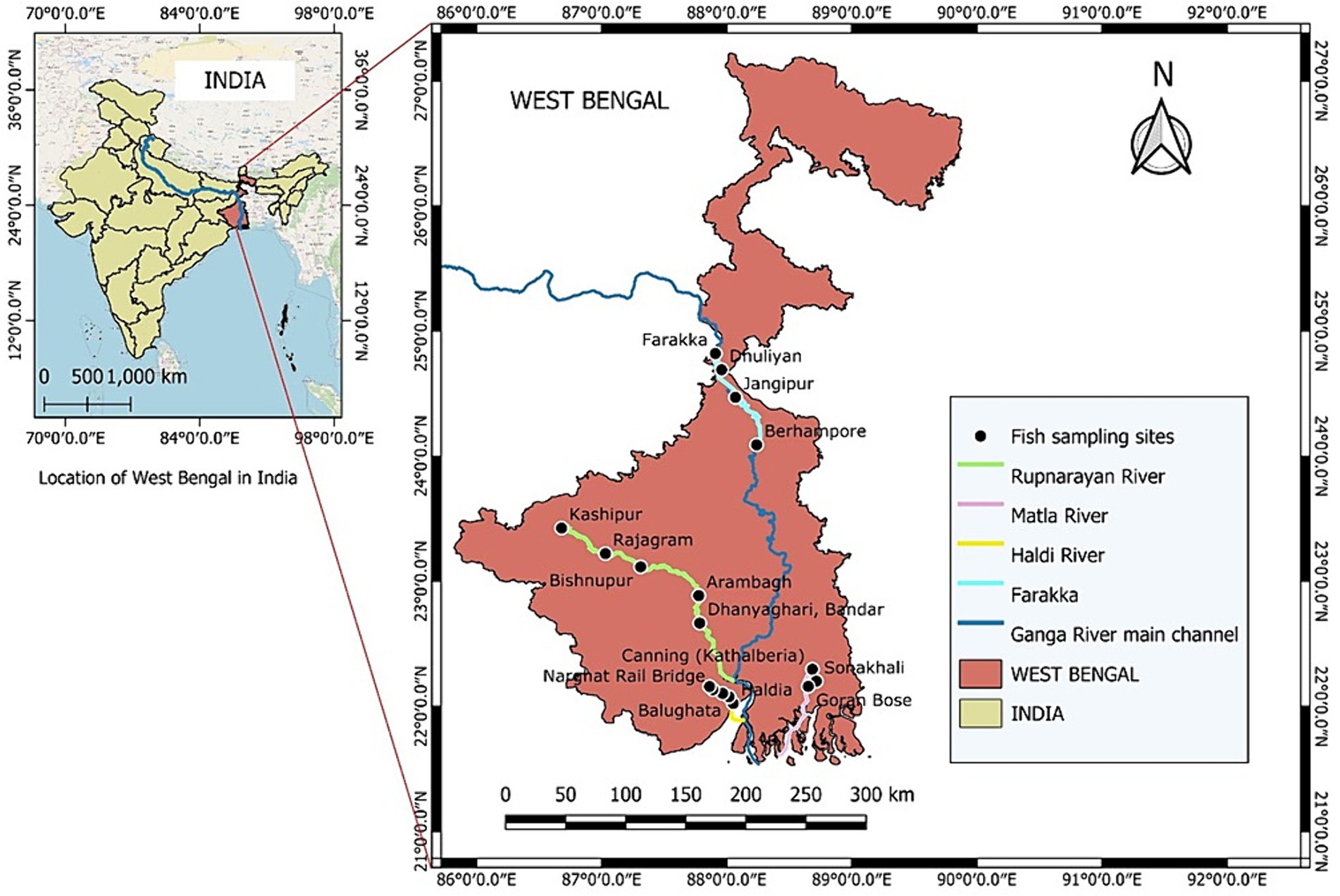
From the landing sites, freshly caught fish were collected and transported in ice to the laboratory. The eight fish species that were included in the amino acid profiling were the small indigenous fish (SIF) Systomus sarana (Rupnarayan), the catfishes Mystus bleekeri (Farraka), M. gulio (Haldi River) (all freshwater fishes), the estuarine fishes Setipinna phasa, (Haldi River), Otolithoides pama (Haldi River), and Pisodonophis boro (Matla River), the anadromous fish, Hilsa keele (Matla River), and the marine fish Sardinella melanura (Farrraka) (Figure 1). The species included in the present study are all highly preferred food fishes. The fish that are considered SIFs, attains a maximum of 20 cm length at maturity or the adult stage (Roos et al., 2003). After being washed, descaled, and degutted, the remaining edible portion of the SIFs (parts of bones and head) were gathered. Three pooled samples were prepared of each fish species containing 50 individual fish. All the pooled fish samples (n = 3) were kept at −40°C until they were analyzed.
2.3 Amino acid analysis
The amino acid composition was estimated as per Ishida et al. (1981). Fish muscle samples (50 mg) were hydrolyzed for 24 h at 110°C in anaerobic conditions using 5 mL of 6N hydrochloric acid. After neutralizing the resultant hydrolysates with 6N NaOH, an AccQ-Fluor Reagent kit (WAT052880, Waters) was used to derivatize them. Samples that had been derivatized were injected into a high-performance liquid chromatography (HPLC) system (1525, Waters) that had a fluorescence detector (2475, Waters) and a C18 RP column. By comparing the measured and standard retention times and peak areas (WAT088122, Waters), amino acids were identified.
2.4 Statistical analysis
The data are depicted as mean ± standard deviation and analyzed using MS Excel, 2010. SPSS 16.0 was employed to do a one-way ANOVA (p < 0.05), followed by a Tukey’s post hoc to evaluate significant differences between different fish species considering each amino acid.
2.5 Computation of DV% and potential contribution to RDA
The assessment of the richness of a fish species for a specific amino acid depends on its potential contribution (expressed as DV %) to the RDA for that specific amino acid. Based on the RDA for an adult male weighing 60 kg, the potential contribution of fish to the daily value of meals (DV%) was computed (WHO, 2007; Indian Council of Medical Research, 2020). For instance, the RDA for histidine is 10 mg/kg of an individual body weight when expressed as intake/kg body weight. This implies that a 60 kg individual would require 600 mg (10 × 60) of histidine per day. With this RDA in mind, a 60 kg individual would get 77.23% of their daily histidine need from eating 50 g of fish (Hilsa keele), which has 463.4 mg of histidine. This represents the histidine DV% of 50 g of Hilsa keele. Likewise, the DV% for each amino acid was computed by taking into account the requirement and content of each amino acid in the chosen fish.
3 Results
The composition of amino acids of the eight studied food fishes from the various sections of the Ganga River is shown in Table 2.
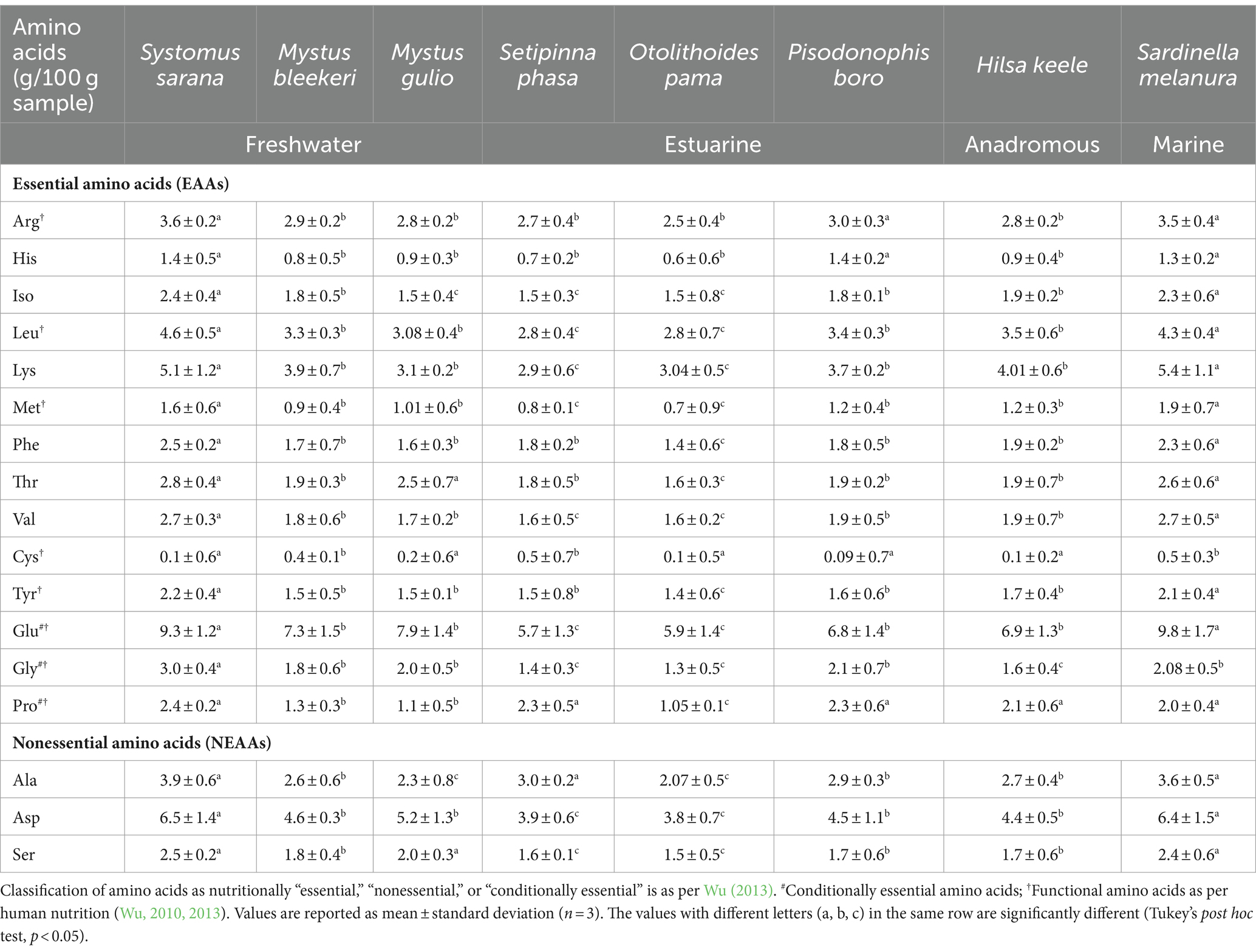
Table 2. Amino acid composition of eight food fishes from different stretches of the Ganga River in and around Kolkata, West Bengal, India.
Arginine levels were found to be high in SIF Systomus sarana (3.62 ± 0.2 g/100 g), marine fish Sardinella melanura, and estuarine fish Pisodonophis boro. Similarly, the histidine content was notably high in both the SIF S. sarana (1.4 ± 0.5 g/100 g) and the estuarine fish P. boro (1.4 ± 0.2 g/100 g).
Among the fishes examined, the SIF S. sarana (2.4 ± 0.4 g/100 g) contained the highest concentration of isoleucine. High leucine content was observed in the SIF S. sarana (4.6 ± 0.5 g/100 g), marine fish S. melanura (4.3 ± 0.4 g/100 g), and anadromous fish Hilsa keele (3.5 ± 0.6 g/100 g). The content of lysine was found to be high in the marine fish S. melanura (5.4 ± 1.1 g/100 g) and the SIF S. sarana (5.1 ± 1.2 g/100 g). Furthermore, the marine fish S. melanura contained the highest amount of methionine (1.9 ± 0.7 g/100 g) among all the fishes studied.
The highest amount of phenylalanine (2.5 ± 0.2 g/100 g) and threonine (2.8 ± 0.4 g/100 g) were found in the SIF S. sarana. Additionally, the highest glutamate concentrations were observed in the marine fish S. melanura (9.8 ± 1.7 g/100 g) and the SIF S. sarana (9.3 ± 1.2 g/100 g). The aspartic acid content was also found to be high in the SIF S. sarana (6.5 ± 1.4 g/100 g), followed by S. melanura (6.4 ± 1.5 g/100 g).
Figure 2 illustrates the potential contributions of various fish species (expressed as DV %) to the RDAs for important amino acids, applicable to an adult male weighing 60 kg. The fish’s wet weight is used to determine these values. The marine fish Sardinella melanura, provides 159.96% of the daily methionine requirement in a 50 g serving. Similarly, the estuarine fish, Setipinna phasa, supplies 99.50% of the daily cysteine requirement in a 50 g serving (Figure 2). Thus, these fishes (and similarly the other fishes with high DV% for a particular amino acid) are found to be rich sources of methionine and cysteine.
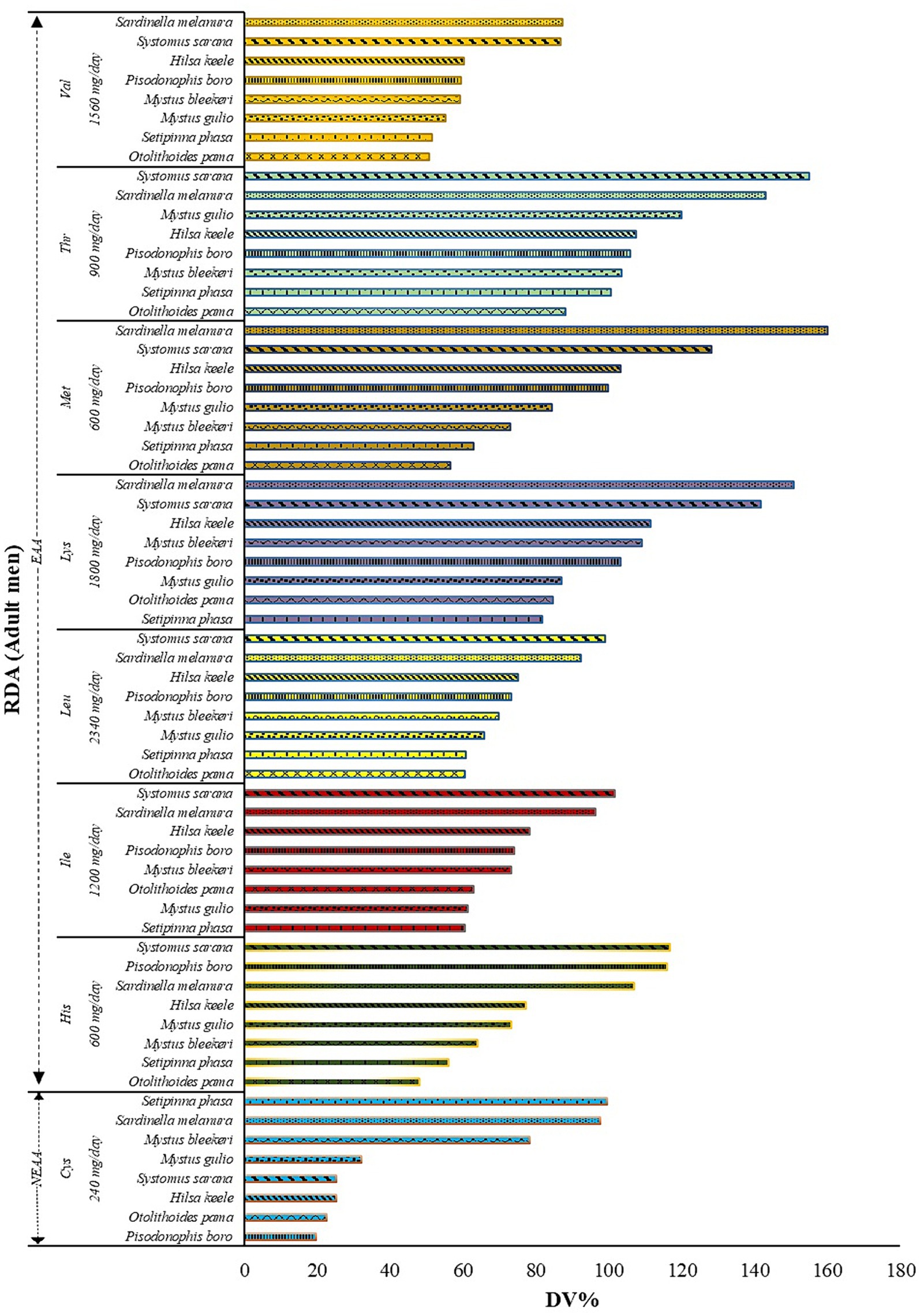
Figure 2. The recommended daily allowance (RDA) recommended for adult men is provided along the Y-axis. Accordingly, the potential contribution of fish (Daily value, DV %) is plotted along the X-axis. DV% was calculated using the analyzed values and the RDA recommended for adult men weighing 60 kg for Indians (WHO, 2007; Indian Council of Medical Research, 2020) with an appraisal of FAO/WHO recommendations. For the calculation of DV%, each serving was considered to be 50 g of fish.
4 Discussion
Amino acids, integral to body protein, are primarily derived from dietary proteins and are crucial for cellular growth, repair, and maintenance (Basumatary et al., 2017). Additionally, they serve as essential precursors for synthesizing proteins, peptides, and various vital substances, including glutathione, dopamine, nitric oxide, serotonin, creatine, and nucleic acids, holding immense physiological significance (Wu, 2009; San Gabriel and Uneyama, 2013). The quality of proteins is evaluated based on the proportion of amino acids that are essential vs. the nonessential amino acids (WHO, 2007). Also, the speed at which proteins are synthesized is directly influenced by the availability of amino acids in cells and plasma (Li et al., 2021). In this context, a crucial role can be played by fish, as it is a vital and economical source of high-quality proteins and amino acids. The present study included food fishes that are highly favored and have strong preferences by consumers.
Arginine exhibits diverse physiological functions and is regarded as a versatile amino acid. It participates in immune responses, ureagenesis, defense against antioxidants, regulation of the somatotropic axis, detoxification of ammonia, and stress response. In the present study, arginine levels were found to be high in SIF Systomus sarana, which is higher than Puntius sophore (0.1 ± 0.03 g/100 g sample) as reported by Mohanty et al. (2014).
Histidine, a precursor to histamine commonly found in hemoglobin, serves as a carbon source for purine synthesis. Furthermore, it is regarded as an essential amino acid crucial for several metabolic processes, including histamine production from certain pathways, inflammatory responses, tissue formation, and repair. Additionally, it contributes to osmoregulation, energy production, and adaptation to stress (Liao et al., 2013). In this study, the histidine content of the SIF S. sarana, and the estuarine fish P. boro was high. Previous studies revealed that the marine fish Rastrelliger kanagurta (Mohanty et al., 2014) and the SIF P. sophore (Mahanty et al., 2014) had high amounts of histidine.
The branched-chain amino acid isoleucine participates in the development of muscle by regulating protein synthesis and degradation and thus promotes growth (Braverman, 2003; Charlton, 2006). The SIF S. sarana contained the highest concentration of isoleucine among the fishes examined.
Leucine, regarded as an anabolic amino acid that activates the mammalian target of rapamycin (mTOR) signaling pathway in human skeletal muscle, is the sole amino acid that stimulates muscle protein synthesis and deposition and inhibits proteolysis (Drummond and Rasmussen, 2008). The leucine content of the SIF S. sarana was highest among all the fishes studied followed by S. melanura and Hilsa keele.
Lysine is an essential amino acid and must be consumed in sufficient amounts to support protein synthesis and maintain a positive nitrogen balance in human (Matthews, 2020). The content of lysine of the marine fish S. melanura was found to be substantially high among all the fishes studied followed by S. sarana.
Methionine is considered a crucial amino acid, essential due to its sulfur content, involved in cysteine synthesis and regulate the production of antioxidant, glutathione (Elango, 2020). Methionine has even been linked in humans to lower incidences of rectal cancer, risk of ovarian cancer in women, and also proximal colon cancer in men. A diet low in methionine affects the growth of tumor cells in stomach. In vitro, methionine deficit increases the rate of apoptosis and reduces cell-to-cell adhesion and migration, which is linked to both elevated gene expression and reduced E-cadherin promoter methylation (Schnekenburger and Diederich, 2015). Among all the fishes studied, the marine fish S. melanura contained the highest amount of methionine, higher than that reported in Sardinella longiceps (0.3 g/100 g) (Mohanty et al., 2014).
Phenylalanine is crucial for proper functioning of the central nervous system, associated with the formation of neurotransmitters like dopamine, epinephrine, and nor-epinephrine. It was found to be useful in the treating vitiligo in children as well as in adults. It also imparts weight loss benefits by the regulating the discharge of cholecystokinin hormone that regulates satiety after eating (Akram et al., 2020). The SIF S. sarana contained the highest amount of phenylalanine among all the fishes studied.
Threonine is an essential amino acid that regulates cell cycle transition from G1 to S phase and was found to be crucial for proliferation of human embryonic stem cells (Canfield and Bradshaw, 2019). The SIF S. sarana contained the highest amount of threonine among all the fishes studied, higher than in SIF P. sophore (0.3 g/100 g) (Mohanty et al., 2014).
Nutritionally, NEAA, synthesized adequately within the organism, though, need not be supplied in the diet, regulate cell signaling, gene expression, nutrient absorption, protein and DNA synthesis, metabolism, acid–base balance, fertility, antioxidative responses, neurotransmission, detoxification, and immunity (Hou et al., 2015). Arginine, glycine, glutamine, and proline play a significant role in collagen synthesis and extracellular matrix remodeling (Wu, 2021). Non-essential amino acids like tyrosine are essential for pigmentation in skin, hair, and eyes (Sturm, 2009). Aspartic acid (FAA) serves as the precursor for amino acids threonine, methionine, lysine, and isoleucine, while also governing the secretion of vital hormones (Mohanty et al., 2014). Emerging evidence suggests the dietary importance of NEAA for realizing genetic potential in growth, reproduction, and disease resistance in animals and humans (Hou et al., 2015). The content of aspartic acid considering the SIF S. sarana, was substantially high among the fishes studied, followed by S. melanura.
Glutamate is a non-essential amino acid for human but plays a significant role as an excitatory neurotransmitter in the brain. It is also essential for learning and memory and an optimal level of glutamate is vital for minimizing neuronal injury in neurodegenerative disorders like Alzheimer’s disease (Canfield and Bradshaw, 2019). The marine fish S. melanura had the highest glutamate concentration among all the fish studied.
The potential contribution (DV%) of the fishes studied to the respective RDA of individual amino acids revealed that among the fishes studied, S. sarana and S. melanura showed the highest contribution to the RDA of threonine, leucine, isoleucine, histidine and valine, methionine, and lysine, respectively. In the present study, the RDA was calculated for adult man; however, women and children will have different RDA for these amino acids as their requirement differs for any particular amino acid.
5 Conclusion
Fish is a significantly important source of proteins. Compared to other sources of animal proteins, consumers are provided with a broad range of choices for fish in terms of affordability, especially in tropical countries, where many varieties and species of fish are available (Mohanty, 2010). However, the nutrient composition of fish is affected by several variables, including species, habitat, and feeding habits. Therefore, we conducted a study to ascertain the amino acid composition of eight Indian food fish species, enriching our knowledge base on nutrient composition. This data are valuable for increasing consumers’ awareness about fish’s nutritional significance and enhancing their utility in human nutrition. Additionally, healthcare professionals can use this information as a reference point to prescribe specific fish for meeting particular dietary needs. Our findings indicate that the small indigenous fish Systomus sarana and the marine fish Sardinella melanura were rich sources of all essential amino acids. Furthermore, estuarine fish like Pisodonophis boro can be recommended for specific amino acids like arginine, histidine, leucine, and valine, while Setipinna phasa is suggested for cysteine. These suggestions rely on the potential contribution of these fishes to the Recommended Dietary Allowance (RDA) for each nutrient. However, further studies on these SIFs can ensure their inclusion as a vital source of amino acids in human nutrition.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by CIFRI Institute Animal Ethics Committee (IAEC). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
BD: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft. KK: Formal analysis, Writing – original draft. SG: Data curation, Investigation, Methodology, Writing – review & editing. AT: Data curation, Formal analysis, Methodology, Writing – original draft. AR: Data curation, Writing – review & editing. SD: Formal analysis, Methodology, Writing – review & editing. BB: Formal analysis, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The financial support for the research was funded by Ministry of Jal Shakti, National Mission for Clean Ganga (NMCG), under the project Fish Conservation and Stock Enhancement of Fishery of Ganga River Basin project (Ad-35012/1/2023-NMCG-NMCG).
Acknowledgments
The authors greatly acknowledge the help rendered by the fishermen of river Ganga during fish sampling.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akram, M., Daniyal, M., Ali, A., Zainab, R., Shah, S. M. A., Munir, N., et al. (2020). “Role of phenylalanine and its metabolites in health and neurological disorders” in Synucleins—Biochemistry and Role in Diseases. ed. A. Surguchov (London, UK: IntechOpen).
Ayyappan, S., and Diwan, A. D. (2007). National Fisheries Development Board and Fisheries Policy in Indian Fisheries: A Progressive Outlook. Cochin: CMFRI, 1–11.
Baker, D. H. (2009). Advances in protein–amino acid nutrition of poultry. Amino Acids 37, 29–41. doi: 10.1007/s00726-008-0198-3
Balachandran, K. K. (2012). “Biochemistry and Nutrition” in Post-harvest Technology of Fish and Fish Products (New Delhi: Daya Publishing House), 1–28.
Basumatary, S., Sarkar, A. P., and Das, S. (2017). Amino acid composition of ten fish species from Hel River, north East India. Asian J. Chem. 29, 2163–2166. doi: 10.14233/ajchem.2017.20666
Béné, C., Macfayden, G., and Allison, E. H. (2007). Increasing the contribution of small-scale fisheries to poverty alleviation and food security. FAO fisheries technical paper no. 481, FAO, Rome.
Braverman, E. R. (2003). “Branched-chain amino acids” in The Healing Nutrients Within: Facts, Findings, and New Research on Amino Acids. ed. C. Hirsch . 3rd ed (California, US: Basic Health Publications, Inc.), 243–260.
Canfield, C. A., and Bradshaw, P. C. (2019). Amino acids in the regulation of aging and aging-related diseases. Transl. Med. Aging 3, 70–89. doi: 10.1016/j.tma.2019.09.001
Cashion, T., Le Manach, F., Zeller, D., and Pauly, D. (2017). Most fish destined for fishmeal production are food-grade fish. Fish Fish 18, 837–844. doi: 10.1111/faf.12209
Chandel, N. S. (2021). Amino acid metabolism. Cold Spring Harb. Perspect. Biol. 13:a040584. doi: 10.1101/cshperspect.a040584
Charlton, M. (2006). Branched-chain amino acid enriched supplements as therapy for liver disease. J. Nutr. 136, 295S–298S. doi: 10.1093/jn/136.1.295S
Das, M. K. (2015). Estuarine dynamics, processes and sediment transport: A case study from the Hooghly estuary of the Ganges Delta. Doctoral dissertation. IIT Kharagpur.
Drummond, M. J., and Rasmussen, B. B. (2008). Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr. Opin. Clin. Nutr. Metab. Care 11, 222–226. doi: 10.1097/MCO.0b013e3282fa17fb
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z. I., Knowler, D. J., Lévêque, C., et al. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182. doi: 10.1017/S1464793105006950
Elango, R. (2020). Methionine nutrition and metabolism: insights from animal studies to inform human nutrition. J. Nutr. 150, 2518S–2523S. doi: 10.1093/jn/nxaa155
Gatlin, D. M. III, Barrows, F. T., Brown, P., Dabrowski, K., Gaylord, T. G., Hardy, R. W., et al. (2007). Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac. Res. 38, 551–579. doi: 10.1111/j.1365-2109.2007.01704.x
Goi, D. (2022). “Handbook of fisheries statistics” in Department of Fisheries, Ministry of Fisheries, Animal Husbandry and Dairying (New Delhi: Government of India).
Golden, C., Allison, E., Cheung, W., Dey, M. M., Halpern, B. S., McCauley, D. J., et al. (2016). Nutrition: fall in fish catch threatens human health. Nature 534, 317–320. doi: 10.1038/534317a
Hicks, C. C., Cohen, P. J., Graham, N. A. J., Nash, K. L., Allison, E. H., D’Lima, C., et al. (2019). Harnessing global fisheries to tackle micronutrient deficiencies. Nature 574, 95–98. doi: 10.1038/s41586-019-1592-6
Hou, Y., Yin, Y., and Wu, G. (2015). Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp. Biol. Med. 240, 997–1007. doi: 10.1177/1535370215587913
Indian Council of Medical Research (2020). Expert Group. Nutrient requirements and recommended dietary allowances for Indians: A report of the expert Group of the Indian Council of medical research. Indian Council of Medical Research.
Ishida, Y., Fujita, T., and Asai, K. (1981). New detection and separation method for amino acids by high-performance liquid chromatography. J. Chromatogr. A 204, 143–148. doi: 10.1016/S0021-9673(00)81650-7
Laidlaw, S. A., and Kopple, J. D. (1987). Newer concepts of the indispensable amino acids. Am. J. Clin. Nutr. 46, 593–605. doi: 10.1093/ajcn/46.4.593
Li, X., Zheng, S., and Wu, G. (2021). Nutrition and functions of amino acids in fish. Adv. Exp. Med. Biol. 1285, 133–168. doi: 10.1007/978-3-030-54462-1_8
Liao, S. M., Du, Q. S., Meng, J. Z., Pang, Z. W., and Huang, R. B. (2013). The multiple roles of histidine in protein interactions. Chem. Cent. J. 7:44. doi: 10.1186/1752-153X-7-44
Mahanty, A., Ganguly, S., Verma, A., Sahoo, S., Mitra, P., Paria, P., et al. (2014). Nutrient profile of small indigenous fish Puntius sophore: proximate composition, amino acid, fatty acid and micronutrient profiles. Natl. Acad. Sci. Lett. 37, 39–44. doi: 10.1007/S40009-013-0186-3
Mahapatra, B. K., Sarkar, U. K., and Lakra, W. S. (2014). A review on status, potentials, threats and challenges of the fish biodiversity of West Bengal. J. Biodivers. Biopros. Dev. 2, 2376–0214. doi: 10.4172/2376-0214.1000140
Maheshvaran, V., Murali, K., Sundar, V., and Chitra, K. (2019). Study on maintenance dredging for navigable depth assurance in the macro-tidal hooghly estuary. Lect. Notes Civ. Eng. 23, 353–367. doi: 10.1007/978-981-13-3134-3_27
Matthews, D. W. (2020). Review of lysine metabolism with a focus on humans. J. Nutr. 150, 2548S–2555S. doi: 10.1093/jn/nxaa224
Maurya, P. K., Malik, D. S., Yadav, K. K., Kumar, A., Kumar, S., and Kamyab, H. (2019). Bioaccumulation and potential sources of heavy metal contamination in fish species in river ganga basin: possible human health risks evaluation. Toxicol. Rep. 6, 472–481. doi: 10.1016/j.toxrep.2019.05.012
Mohanty, B., Mahanty, A., Ganguly, S., Sankar, T. V., Chakraborty, K., and Rangasamy, A. (2014). Amino acid compositions of 27 food fishes and their importance in clinical nutrition. J. Amino Acids 2014, 1–7. doi: 10.1155/2014/269797
Moughan, P. J. (2005). Dietary protein quality in humans—an overview. J. AOAC Int. 88, 874–876. doi: 10.1093/jaoac/88.3.874
National Research Council (1989). Commission on Life Sciences and Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances. National Academies Press.
Raw, P. (2015). Composition of foods Raw, processed, prepared USDA National Nutrient Database for standard reference, release 28 (2015) documentation and user guide. United States.
Roos, N., Islam, M. N., and Thilsted, S. H. (2003). Small indigenous fish species in Bangladesh: contribution to vitamin a, calcium and iron intakes. J. Nutr. 133, 4021S–4026S. doi: 10.1093/jn/133.11.4021S
San Gabriel, A., and Uneyama, H. (2013). Amino acid sensing in the gastrointestinal tract. Amino Acids 45, 451–461. doi: 10.1007/s00726-012-1371-2
Sarma, D., Akhtar, M. S., Das, P., Das, P., Shahi, N., Ciji, A., et al. (2013). Nutritional quality in terms of amino acid and fatty acid of five Coldwater fish species: implications to human health. Natl. Acad. Sci. Lett. 36, 385–391. doi: 10.1007/s40009-013-0151-1
Schnekenburger, M., and Diederich, M. (2015). “Chapter 18—nutritional epigenetic regulators in the field of cancer: new avenues for chemopreventive approaches” in Epigenetic Cancer Therapy. ed. S. G. Gray (London, UK: Academic Press), 393–425.
Sharma, S., Anuradha, K. P., Shivalingesh, K. K., and Thakar, S. (2021). “Recommended dietary allowances” in Advances in Nutrition. ed. P. Arya (Delhi, India: AkiiNik Publications), 95–107.
Singh, B. K. (2002). Amino acids and nutritional quality of plant products. Amino Acids 22:215. doi: 10.1007/s007260200009
Stipanuk, M. H., Ueki, I., Dominy, J. E., Simmons, C. R., and Hirschberger, L. (2009). Cysteine dioxygenase: a robust system for regulation of cellular cysteine levels. Amino Acids 37, 55–63. doi: 10.1007/s00726-008-0202-y
Sturm, R. A. (2009). Molecular genetics of human pigmentation diversity. Hum. Mol. Genet. 18, R9–R17. doi: 10.1093/hmg/ddp003
Suganthi, A., Venkatraman, C., and Chezhian, Y. (2015). Proximate composition of different fish species collected from Muthupet mangroves. Int. J. Fish. Aquat. Stud. 2, 420–423.
Tacon, A. G., and Metian, M. (2013). Fish matters: importance of aquatic foods in human nutrition and global food supply. Rev. Fish. Sci. 21, 22–38. doi: 10.1080/10641262.2012.753405
WHO (2007). Protein and amino acid requirements in human nutrition. World Health Organ. Tech. Rep. Ser. 935, 1–265.
Williams, M. H. (2005). Dietary supplements and sports performance: minerals. J. Int. Soc. Sports Nutr. 2, 43–49. doi: 10.1186/1550-2783-2-1-43
Wu, G. (2009). Amino acids: metabolism, functions, and nutrition. Amino Acids 37, 1–17. doi: 10.1007/s00726-009-0269-0
Wu, G. (2010). Functional amino acids in growth, reproduction, and health. Adv. Nutr. 1, 31–37. doi: 10.3945/an.110.1008
Wu, G. (2013). Functional amino acids in nutrition and health. Amino Acids 45, 407–411. doi: 10.1007/s00726-013-1500-6
Wu, G. (2021). “Amino acids” in Biochemistry and Nutrition. 2nd ed (Boca Raton, Florida, United States: CRC Press).
Wu, G., Bazer, F. W., Davis, T. A., Kim, S. W., Li, P., Marc Rhoads, J., et al. (2009). Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37, 153–168. doi: 10.1007/s00726-008-0210-y
Keywords: amino acids, proteins, fish, Ganga River, recommended dietary allowance
Citation: Das BK, Kumari K, Ganguly S, Talukder AK, Ray A, Dutta S and Baisakhi B (2024) Unlocking the nutritional potential: amino acid profile of eight Indian food fishes and their role in meeting recommended dietary allowances. Front. Sustain. Food Syst. 8:1432034. doi: 10.3389/fsufs.2024.1432034
Edited by:
Kathleen L. Hefferon, Cornell University, United StatesReviewed by:
Marie-Annick Moreau, University College London, United KingdomDiah Ayu Hartini, Poltekkes Kemenkes Palu, Indonesia
Copyright © 2024 Das, Kumari, Ganguly, Talukder, Ray, Dutta and Baisakhi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Basanta Kumar Das, YmFzYW50YWt1bWFyZEBnbWFpbC5jb20=
 Basanta Kumar Das
Basanta Kumar Das Kajal Kumari
Kajal Kumari Satabdi Ganguly
Satabdi Ganguly Anjon Kumar Talukder
Anjon Kumar Talukder Archisman Ray
Archisman Ray Subhamoy Dutta
Subhamoy Dutta Barsha Baisakhi
Barsha Baisakhi