
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 27 June 2024
Sec. Nutrition and Sustainable Diets
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1430146
 Kangkang Xie1
Kangkang Xie1 Jiayi Ling2
Jiayi Ling2 Awdhesh Kumar Mishra3*
Awdhesh Kumar Mishra3* Muhammad Adil Farooq4*
Muhammad Adil Farooq4* Samreen Ahsan4
Samreen Ahsan4 Sadia Hassan5
Sadia Hassan5 Ahmed M. El-Sherbeeny6
Ahmed M. El-Sherbeeny6 Mostafa R. Abukhadra7
Mostafa R. Abukhadra7 Shan He2,8*
Shan He2,8* Huaxia Liu9
Huaxia Liu9 Shengle Zheng10
Shengle Zheng10 Nabeel Ahmad11
Nabeel Ahmad11The development of baby spinach as a vehicle to transfer Lactobacillus plantarum 299v (LP299v) and Lactobacillus rhamnosus GG (LGG) is quite promising and may address the research regarding the absence of suitable whole vegetable carriers in the current probiotic food industry. The objective of this study was to observe the effects of food storage and preparation on Lp299v and LGG viability in baby spinach before consumption. The strains were sequentially introduced into baby spinach by dipping leaves in probiotic suspension to achieve an attachment of approximately 8 log 10 CFU/g spinach. Then, probiotic viability was tested using serial dilutions. Furthermore, data processing and ANOVA during 7-day storage, with or without salad dressing, were performed using Tukey’s test. In the 7-day storage trials, LP299v and LGG viability on baby spinach declined after 7 days with significant differences by 0.19 and 0.39 log10 CFU/g, respectively. In salad dressing trials, LP299v (p value = 0.79 > 0.05) and LGG (p value = 0.58 > 0.05) survivability on baby spinach after the addition of salad dressing fluctuated approximately 8.27 and 8.40 log10 CFU/g with no statistically significant difference, respectively. LP299V and LGG viability on baby spinach in both trials was greater than 8 log10 CFU/g and close to FDA requirements, showing that food storage and preparation do not affect their viability and can be used commercially.
Currently, consumer awareness and interest in the relationship between diet and health has increased significantly, and it has gradually become one of the main enablers for food marketing positioning. Functional foods with additional health-promoting properties due to bioactive components are of great importance as an alternative to medical interventions due to their ability to prevent chronic health conditions. It is estimated that approximately 60–70% of the total functional food market is probiotic food, and global sales of probiotic products totaled over US$54 billion in 2020 (Pereira et al., 2011; Freire et al., 2017).
The main structure of this huge probiotic food industry is for dairy-based products, such as yogurt and milk-based beverages, which are unsuitable for vegetarians (Martins et al., 2013), lactose intolerance, milk protein allergies, and cholesterol-restricted diets (Fisberg and Machado, 2015). Other carriers may be fruit and vegetable beverages, grains, meat, and soy products (Granato et al., 2010; Thakur et al., 2015; Ranadheera et al., 2017; Min et al., 2019; Ho and Turner, 2020). The increasing demand for fruits and vegetables worldwide (Sarron et al., 2021) has made fruits and vegetables an excellent carrier option for delivering probiotics.
Whole fruits or vegetables differ from current fruit and vegetable beverage carriers, representing a potential research gap with few scientific publications. In addition to basic nutrients, whole vegetable probiotic food can convey additional benefits to gut health, improve vegetable consumption, prevent pathogen growth, and maintain shelf-life (Wan et al., 2019; Guo et al., 2024). Lactobacillus plantarum 299v (LP299v) and Lactobacillus rhamnosus GG (LGG) represent a new approach from whole fruit or vegetable to provide health-beneficial probiotics and facilitate as potential probiotic carriers. The study evaluated the viability of LP299V and LGG on baby spinach before consumption. The findings of this study may provide information about the ability of baby spinach to act as a carrier to transfer LP299V and LGG.
The objective of this research was to determine the role of baby spinach as a carrier of probiotics (LP299V and LGG) before human consumption, as determined by the effect of storage time and different salad dressings such as balsamic salad dressing, French salad dressing, and Italian salad dressing. Furthermore, the study also explores the ability of baby spinach to stably carry probiotics before consumption.
The findings of this research were expected to identify the role of baby spinach as a carrier of LG299v and LGG in the context of food storage and preparation, providing commercial feasibility for bagged probiotic baby spinach salad. This could potentially attract industry attention, as the development of new types of probiotic food could generate consumer interest and huge industrial benefits, provided the relevant health claims are validated by regulatory bodies.
This research was based on the control fraction method to compare the probiotic plate count of baby spinach with or without different salad dressings during a storage time of 7 days and in triplicate. In experimental trials, probiotics (LP299V and LGG) were obtained from the Food Microbiology Laboratory culture collection of the University of Queensland. Preparation of probiotic glycerol 167 stocks of LP299V and LGG (Figure. 1) was performed after 37 days of culture using the 16-streak plate method on MRS (Oxoid, Basingstoke, 169 United Kingdom) agar (1.4% agar) plates to isolate a 170 single colony at 37°C anaerobic incubation for 18–20h.
The baby spinach was purchased in pre-packaged form (Coles, Australia) and kept refrigerated at 4°C. Commercial salad dressings were purchased from Price (NSW, Australia) and refrigerated at 4°C after opening, i.e., balsamic salad dressing, French salad dressing, and Italian salad dressing.
The preparation of probiotic (LP299V or LGG) inoculation of baby spinach consisted of two parts: preparation of a 150-mL probiotic solution and probiotic inoculation into baby spinach. The preparation of control baby spinach was generally the same as that of probiotic baby spinach, except that 150 mL of saline (0.85% NaCl solution) solution was used instead of 150 mL of probiotic solution. The process was the same for both LP299V and LGG, and LP299V is taken as an example for brief illustration.
The preparation of the 150 mL LP299V solution (Figure 2) began with the LP299V glycerol stock. To obtain the stationary phase, 10 μL LP299V glycerol stock was inoculated into each of six tubes containing 50 mL MRS broth for overnight 37°C anaerobic incubation. After 22 h, the washed LP299V pellets were re-suspended in 150 mL of saline solution using a refrigerated centrifuge (Allegra) to obtain a 150-mL LP299V probiotic solution.
The refrigerated centrifuge was operated in advance to reach the desirable 4°C condition for the centrifugation step. At each centrifugation, the top solution was removed and an appropriate amount of saline was added to wash the probiotic cells. The same procedure was followed in the preparation step of 150 mL of LGG probiotic solution. It is noteworthy that 150 mL of probiotic solution was used to inoculate 100 g of baby spinach.
The inoculation was carried out in a class II biological safety cabinet (BSCII). Tap-washed 100 g of baby spinach was first dried by a salad spinner for 2 min and was placed in a PET bag in a laminar flow cabinet and stored at 4°C (Figure 3). Then, 100 g of baby spinach was immersed in 500 mL of LP299V suspended solution (150 mL of LP299V probiotic solution with 350 mL of saline) for 5 min. After collecting and drying for 2 min in a spinner, the LP299V-inoculated baby spinach was weighed into five bags of baby spinach (20 g per bag) and stored at 4°C for 7-day storage and salad dressing trials to be done. The inoculation step for 100 g of the LGG sample followed the same procedure as for the LP299V sample.
In 7-day storage trials (Section “Methods”), control baby spinach was also required (Figure 3) and both the control and probiotic inoculated samples were subjected to the same procedure at BSCII, except that the control sample was completely. It was soaked in salt water containing 500 mL. 5 min instead of 500 mL of probiotic suspended solution. Differently, in the salad dressing trials, only probiotic inoculated baby spinach was required and is in Section 3.4. in working order.
After preparing the control and probiotic samples, 7-day storage tests were conducted for LP299V or LGG vaccinated baby spinach using the same method. Four bags of 20 g of control samples and four bags of 20 g of LP299V or LGG inoculated samples (Figure 4) were labeled as day 0, day 3, day 7, and pH samples, respectively, for a maximum of 7 days. They were refrigerated at 4°C to observe probiotic survival.
During storage on days 0, 3, and 7, 180 mL of 0.1% peptone water (Bacteriological Peptone, Oxoid) was added to each bag of samples and mixed using a stirrer for 1 min. Subsequently, 10-fold serial dilutions up to 10–6 were made for spread plating on MRS agar plates, and these plates were incubated using anaerobic pouches and jars at 37°C for 24 h to obtain probiotic counts. The pH value of the control and probiotic inoculated baby spinach was determined using a pH meter from pH samples on day 7.
A set of 7-day storage tests included control and corresponding probiotic sample data, and specifically, the 7-day storage tests were performed in triplicate. Overall, these tests generated three sets of data.
In salad dressing trials, five bags of 20 g each of LP299V or LGG inoculated baby spinach (Figure 5) were refrigerated for 1 h at 4°C. Then, after shaking well, 5 mL of three different salad dressings was added to three bags of probiotic baby spinach, respectively. The remaining two bags of probiotic samples served as the control group: one was added with 5 mL of sterilized water, while the other remained intact without any additional additions. In total, five bags of baby spinach samples, namely, control, water sample, balsamic sample, French sample, and Italian sample, were prepared.
After that five bags of samples were maintained at room temperature for 15 min. Each bag of samples was serially diluted using 0.1% peptone water and spread on MRS agar plates. Probiotic counts were obtained by incubating these agar plates anaerobically at 37°C for 24 h. The pH values of various salad dressings were determined and recorded. Salad dressing trials were conducted in triplicate, following the procedures outlined in Figure 5.
Probiotic colonies from triplicate samples of two tests were manually counted and recorded. CFU/g of samples was calculated by dividing the total number of probiotics by the dilution factor of the spread plate volume result. The detection limit of probiotics was 100 CFU/g, and the probiotic count of value 0 was expressed as less than 100 CFU/g. Then, base 10 logarithmic notation was applied to the CFU/g values to effectively represent probiotic log reduction, i.e., log 10 (CFU/g). Then, all log10 (CFU/g) data were plotted in a column chart with error bars for comparison.
The statistical software Minitab ExpressTM (version 1.5.0, Minitab Inc., United States) was used to optimize the statistical analyses using ANOVA. One-way ANOVA was used to assess the statistical significance and Tukey’s test was used for comparison across groups.
In the 7-day storage trials, baby spinach leaves were dipped in probiotics, LP299V and LGG, respectively, to compare the survival over 7 days with respect to control samples. The results obtained from these experimental procedures are presented below with explanations in the form of graphs and tables.
The survival rate of LP299V on young spinach leaves (Figure 6) showed a slight decrease from 8.35 log10 CFU/g on day 0 to 8.20 log10 CFU/g on day 3 and 8.16 log10 CFU/g on day 7. Studies reported that probiotic survival rate can be affected by the physicochemical characteristics of green leafy vegetables. For example, the microbes present on the surface of the spinach leaves and antimicrobial compounds can affect the viability of probiotics such as LP299V (Dudzicz et al., 2018; Axling et al., 2020). The p-value of 0.04 (<0.05) between the three LP299V samples with different storage periods suggested that there was a significant difference in the viability of LP299V on baby spinach at 7 days. As can be seen in Table 1, the only p-value (<0.05) from the Tukey test was the p-value for the day 7 sample compared to the day 0 sample (0.04), suggesting a decline of 0.19 log10 CFU/kg from day 0 to 7 with no statistically significant difference in survival of LP299V on baby spinach during storage. Even though P299V viability declined with a significant margin to 0.19 log 10 CFU/g after 7-day storage, the initial and final survival rates were still more than 8 log10 CFU/g, meaning that the 7-day storage did not impact the role of baby spinach in delivering the LP299V. The decrease in viability was due to the storage temperature, oxygen, and water activity of the material (Ardanareswari et al., 2017). Moreover, probiotics have a higher survival rate, and changes in the optimal storage conditions can lead to a gradual decrease in their availability.
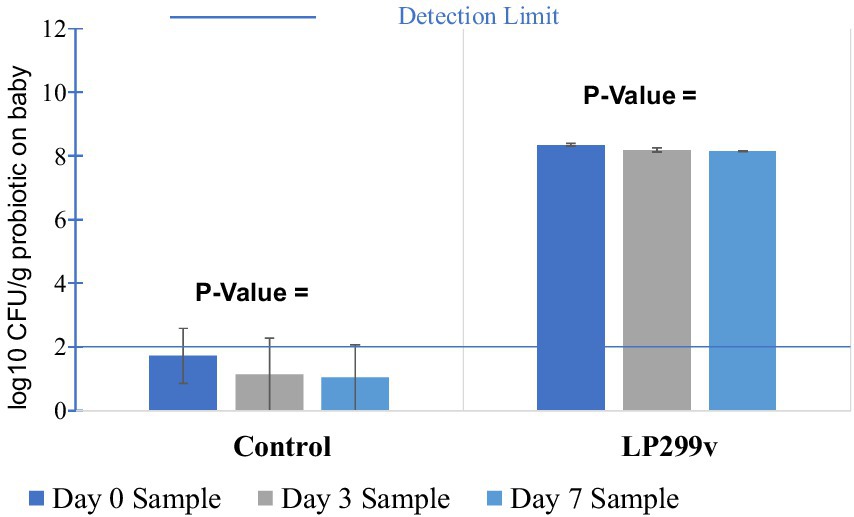
Figure 6. Survival rate of LP299v and control on baby spinach in 7-day storage trials. Control: Baby spinach dipped in saline.
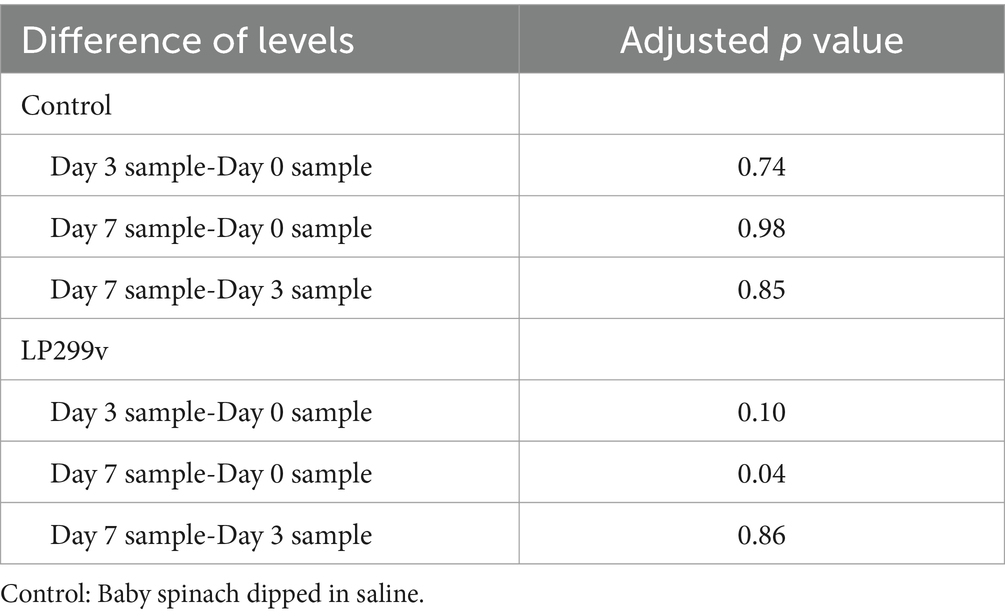
Table 1. Differences of means (p value) of LP299v and control survivability in 7-day storage trials from Tukey’s simultaneous tests.
For the probiotic LGG, the viability of baby spinach (Figure 7) decreased slightly from 8.55 log10 CFU/g on day 0 to 8.32 log10 CFU/g on day 3 and 8.16 log10 CFU/g on day 7. A gradual decrease in the viability is due to the persistent metabolic activity and cellular stress responses (Segers and Lebeer, 2014). LGG viability results on baby spinach were 0.01 (<0.05) with an increase of 7 days, which suggests the presence of statistically significant differences among the three LGG samples with different storage periods. From Table 2, it can be seen that the p values for both the day 3 sample compared to the day 0 sample (0.03) as well as the day 7 sample compared to the day 0 sample (0.01) were less than 0.05. This suggests that a decrease of 0.23 log10 CFU/g could be observed in LGG survival on baby spinach during storage, with a statistically significant difference from day 0 to day 3.
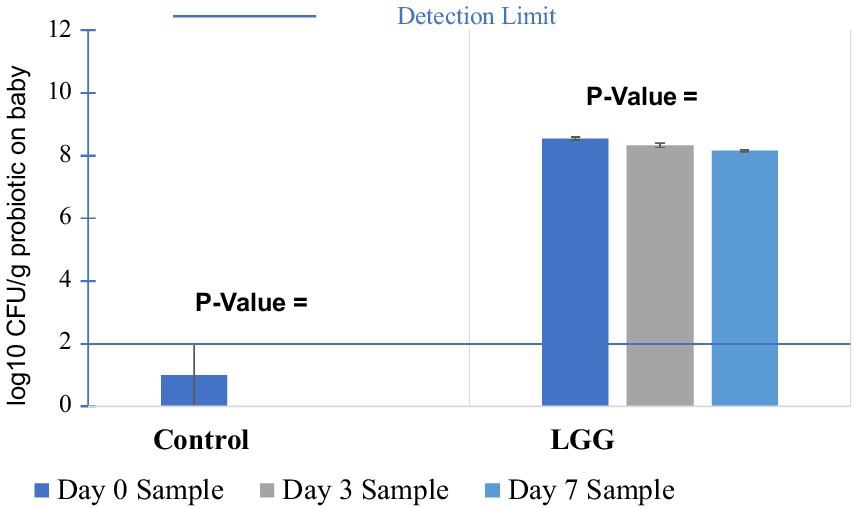
Figure 7. Survival rate of LGG and control on baby spinach in 7-day storage trials. Control: Baby spinach dipped in saline.
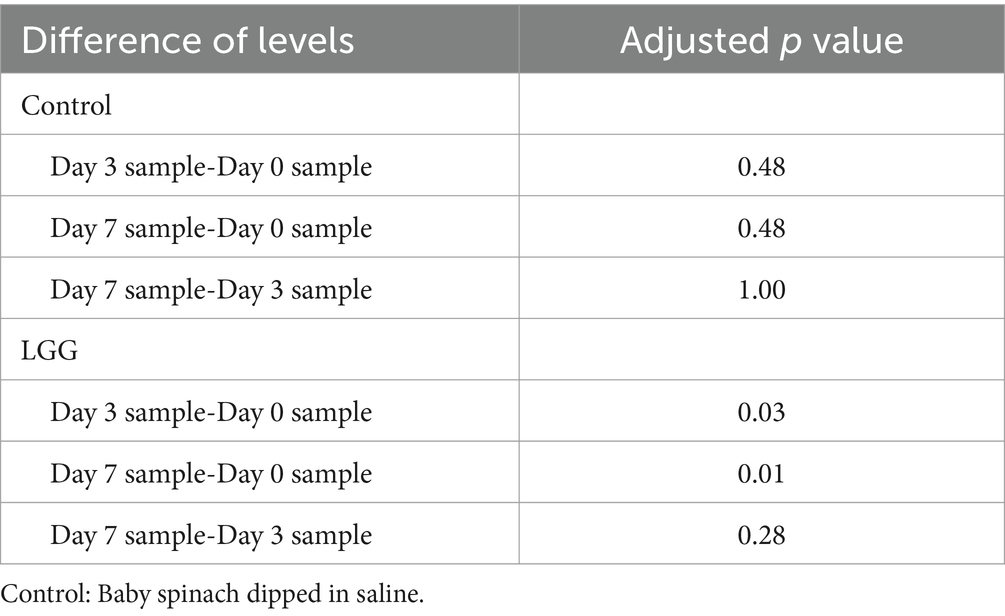
Table 2. Differences of means (p value) of LGG and control survivability in 7-day storage trials from Tukey’s simultaneous tests.
Similar to LP299V, although the survival of LGG showed a decrease of 0.39 log10 CFU/g with a statistically significant difference after 7-day storage, the initial and final viability were still higher than 8 log10 CFU/g, indicating that even 7-day storage. This finding was consistent with the study reported by Campos et al. (2019).
When comparing the survival of LP299V (Figure 6) and LGG (Figure 7), the survival of LGG (0.19 log10 CFU/g) was 0.20 log10 CFU compared to LP299V (0.39 log10 CFU/g) after 7-day storage. A significant decrease was observed. The survival conditions for LP299V were pH 2.4–8.8 and 12–40°C, while for LGG; they were pH 3–7 and 2.7–52°C or 6–41°C. The pH of both the LP299V (Table 3) and LGG (Table 4) samples were in the range of the appropriate pH required for growth, approximately 6.40–7.00, while the pH of the probiotic samples was statistically lower than the control samples. A significant difference is probably attributed to their slower secretion of acidic substances under low temperatures (Bermudez-Brito et al., 2012). The difference in viability changes of LP299V and LGG is consistent with the fact that strain differences can substantially affect the phenotype. The greater decline in the survival of LGG may be a result of its greater tolerance to low temperatures compared to LP299v.
As regulated by the FDA (2016), probiotic LP299V should be present up to 1,011 CFU/serving and probiotic LGG usage levels should range from 108 to 1,010 CFU/serving. Typically, the size of one serving of baby spinach salad is approximately 70 g based on the packaging information for pre-packed baby spinach. The Lp299V levels in 70 g of baby spinach are close to 10.00– 10.20 log10’ CFU. The FDA requirement is (11 log10 CFU), while the LGG levels (10.00–10.40 log10 CFU) approached the upper limit of the FDA-required use level range (108–1,010 CFU). Therefore, these results and findings indicate the commercial feasibility of baby spinach as a carrier of Lp299V and LGG in the context of 7-day storage at the regulatory body and food industry level. It may be possible for regulatory bodies to make health claims for the GRAS probiotic LP299V based on established clinical trial studies and credible scientific publications. These implications could be very important in the development of a new type of probiotic food, bagged probiotic baby spinach salad, which could lead to consumer interest and huge industrial profits.
In salad dressing trials, probiotic-inoculated baby spinach was subjected to various salad dressings and compared according to probiotic survival with reference to pure probiotic baby spinach or probiotic baby spinach with sterilized water. The results along with the discussion are presented below.
The survival rates of LP299V and LGG on baby spinach (Figure 8), under five different preparations without water and with different salad dressings, fluctuated slightly around 8.27 and 8.40 log10 CFU/g, respectively. There was an increase. The p value of LP299V samples (0.79) and the p value of LGG samples (0.58) were greater than 0.05 with reference to Table 5, indicating that no statistically significant difference was present in the results. To conclude, the viability of LP299V and LGG in the presence of balsamic, French, and Italian salad dressings compared to control and water samples did not change with a statistically significant difference and was more than 8 log10 CFU/g. The reason behind this is that both LP299 and LGG are known for their robustness and ability to survive in harsh conditions, such as different pH levels and food matrices. Moreover, LP299v can survive in acidic environments or salad dressings like balsamic and French. Moreover, vinegar as a common ingredient present in salad dressing may restrict the growth of harmful bacteria and allow probiotics such as LP 299v and LGG to thrive (Montel et al., 2014).
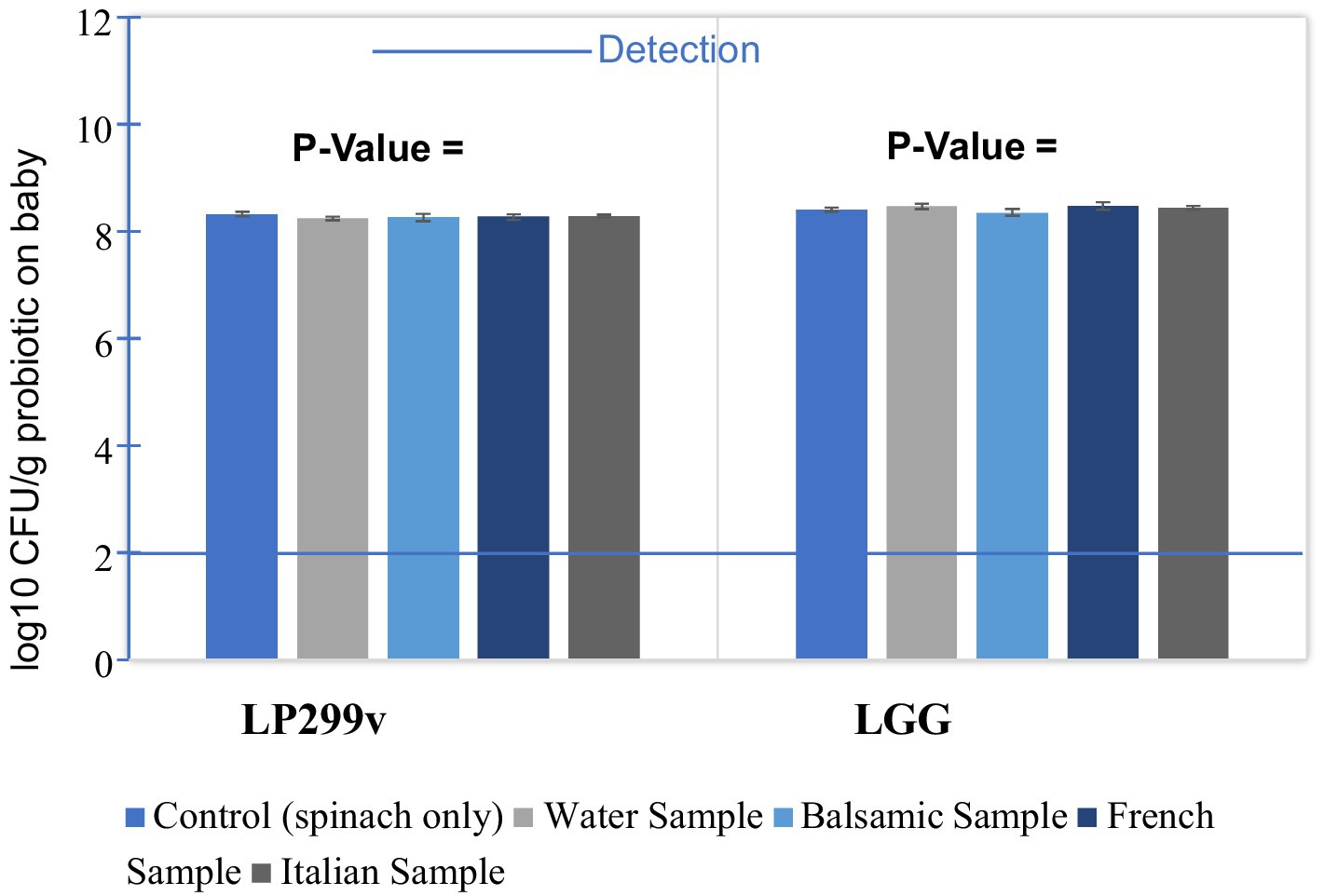
Figure 8. Survivability of probiotics (LP299v and LGG) on baby spinach with the addition of three types of salad dressings.
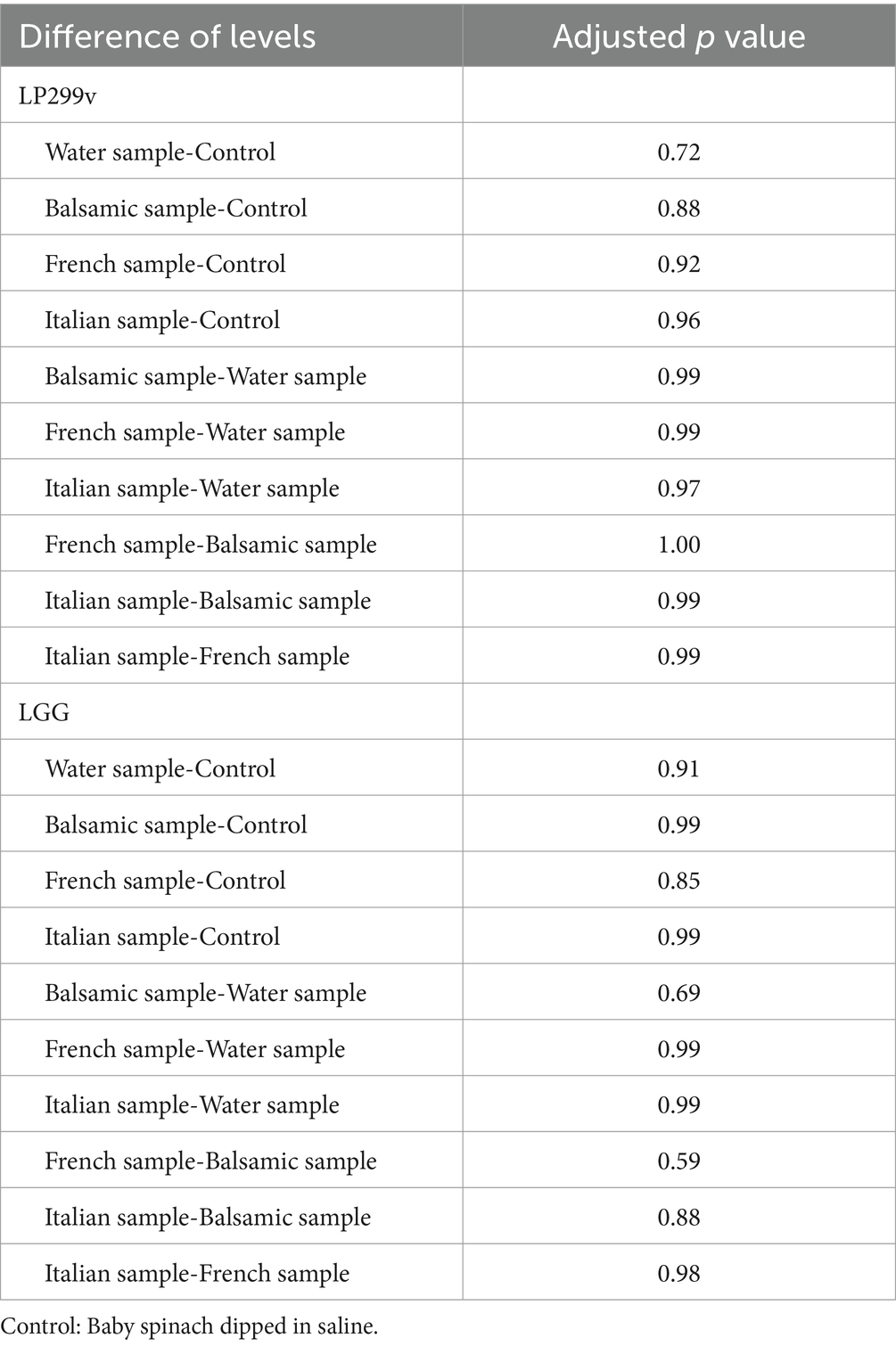
Table 5. Differences of means (p value) of LP299v and LGG survivability in salad dressing trials from Tukey’s simultaneous tests.
That is to say, the salad dressing trials did not negatively impact the role of baby spinach in providing probiotic LP299V and LGG, and this result was expected in Hypothesis II. Adding probiotic LP299V and LGG baby spinach to different salad dressings before consumption did not disintegrate probiotic cells or reduce probiotic survival rates. The underlying reason for this of salad dressings. The normal pH of salad dressings is approximately 4.4; as a result, salad dressings are quite acidic. It can be seen from Table 6, that the pH values of balsamic, French, and Italian salad dressings are 3.25, 3.32, and 3.04, respectively, and are in the range of reasonable survival pH of LP299V (2.4–8.8) and LGG (3–8.8.7). Therefore, adding acidic salad dressings poses no threat to LP299V and LGG.
After adding various salad dressings, the levels of LP299 in 70 g of baby spinach (10.11–10.13 log10 CFU) and LGG in 70 g of baby spinach (10.21–10.33 log10 CFU) are all close to the FDA requirement, which ensures the feasibility of delivering LP299V and LGG in baby spinach for food preparation. As a result, regulatory bodies may be able to make health claims, and the food industry may profit from this new product.
Baby spinach as a vehicle for LP299V and LGG fills the research gap lacking a complete vegetable carrier in current probiotic diets and related established scientific publications. This research investigated the effects of storage time and the addition of different salad dressings on the survival levels of LP299V and LGG in baby spinach. The general findings of this research are summarized based on two experimental tests, namely 7-day storage tests and salad dressing tests.
In the 7-day storage trials, a slight decline in the survival levels of both LP299V and LGG was observed on baby spinach. Considering the viability higher than 8 log10 CFU/g, baby spinach could be a suitable carrier for LP299V and LGG under 7-day storage. In salad dressing trials, the survival rates of both LP299V and LGG on baby spinach remained the same with or without different salad dressings, and adding salad dressing did not differentiate LP299V and LGG cells. With viability greater than 8 log10 CFU/g, baby spinach can be a suitable medium for LP299V and LGG under salad dressing.
In conclusion, baby spinach shows promise as a medium for delivering GRAS probiotics LP299V and LGG in the context of food storage and preparation with salad dressings. LP299V and LGG levels in two trials close to FDA requirements showed potential for commercialization in bagged baby spinach probiotic products. This professional capacity must also manage health claims for regulatory bodies.
In the future, research subjects could engage in human digestion trials to understand the effects of harsh environments on the role of baby spinach in carrying LP299V and LGG. Furthermore, sensory tests are needed to gain better knowledge about the sensory properties and nutritional quality of probiotic baby spinach during storage. Surveys on consumer interests and industrial trials are necessary to obtain probiotic baby spinach on a large scale for the commercialization of probiotic baby spinach. In addition, clinical data about LP299V and LGG should be researched in the future to provide enough evidence to make health claims on the package for better advertising effects. Finally, further studies regarding the effects of other salad dressings on probiotic viability and sensory properties of probiotic-enriched spinach may be very important in order to commercialize the product. This study provides a way forward to explore more diverse options in order to fulfill the dietary and nutritional needs of an individual by investigating the use of other whole vegetables. In the industrial sector, it will provide promising opportunities to producers for the development of innovative probiotic food products. Moreover, the consumption of probiotic baby spinach will help to reduce the risk of chronic disease and inflammation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
KX: Writing – review & editing, Writing – original draft, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. JL: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. AM: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Project administration, Data curation, Conceptualization. AE: Writing – review & editing, Data curation, Resources, Software, Visualization. MA: Writing – review & editing, Data curation, Resources, Software, Visualization. MF: Resources, Software, Validation, Visualization, Writing – review & editing. SA: Writing – review & editing, Visualization, Validation, Software, Resources, Data curation. SHa: Writing – review & editing, Visualization, Validation, Software, Resources, Data curation. NA: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Data curation, Conceptualization. SHe: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Data curation, Conceptualization. HL: Writing – review & editing, Visualization, Validation, Software, Resources, Methodology, Data curation. SZ: Writing – review & editing, Visualization, Software, Resources, Methodology, Investigation.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors extend their appreciation to King Saud University for funding this study through the Researchers Supporting Project (number: RSP2024R133), King Saud University, Riyadh, Saudi Arabia; and the Guangzhou Philosophy and Social Science Development “14th Five-Year Plan” 2022 Joint Project “Research on Industrial Upgrading of Guangzhou High-end Equipment Manufacturing Industry Based on Dual Value Chain Perspective” (Project No.: 2022GZGJ79).
HL was employed by Presafe (Qingyuan) Phosphor Chemical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ardanareswari, K., Utami, T., and Rahayu, E. S. (2017). Effect of heat adaptation and pH adjustment on the survival of spray-dried Lactobacillus paracasei SNP2. Br. Food J. 119, 2267–2276. doi: 10.1108/BFJ-10-2016-0519
Axling, U., Önning, G., Combs, M. A., Bogale, A., Högström, M., and Svensson, M. (2020). The effect of Lactobacillus plantarum 299v on iron status and physical performance in female iron-deficient athletes: a randomized controlled trial. Nutrients 12:1279. doi: 10.3390/nu12051279
Bermudez-Brito, M., Plaza-Díaz, J., Muñoz-Quezada, S., Gómez-Llorente, C., and Gil, A.. (2012). Probiotic mechanisms of action. Ann Nutr Metab 61:160–74. doi: 10.1159/000342079
Campos, P. A., Martins, E. M. F., Martins, M. L., de Oliveira Martins, A. D., de Castro Leite Júnior, B. R., da Silva, R. R., et al. (2019). In vitro resistance of Lactobacillus plantarum LP299v or Lactobacillus rhamnosus GG carried by vegetable appetizer. LWT 116:108512. doi: 10.1016/j.lwt.2019.108512
Dudzicz, S., Kujawa-Szewieczek, A., Kwiecień, K., Więcek, A., and Adamczak, M. (2018). Lactobacillus plantarum 299v reduces the incidence of Clostridium difficile infection in nephrology and transplantation Ward—results of one year extended study. Nutrients 10:1574. doi: 10.3390/nu10111574
Fisberg, M., and Machado, R. (2015). History of yogurt and current patterns of consumption. Nutr. Rev. 73, 4–7. doi: 10.1093/nutrit/nuv020
Freire, A. L., Ramos, C. L., da Costa Souza, P. N., Cardoso, M. G. B., and Schwan, R. F. (2017). Nondairy beverage produced by controlled fermentation with potential probiotic starter cultures of lactic acid bacteria and yeast. Int. J. Food Microbiol. 248, 39–46. doi: 10.1016/j.ijfoodmicro.2017.02.011
Granato, D., Branco, G. F., Nazzaro, F., Cruz, A. G., and Faria, J. A. F. (2010). Functional foods and nondairy probiotic food development: trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 9, 292–302. doi: 10.1111/j.1541-4337.2010.00110.x
Guo, Q., Cui, B., Yuan, C., Guo, L., Li, Z., Chai, Q., et al. (2024). Fabrication of dry S/O/W microcapsule and its probiotic protection against different stresses. J Sci Food Agric, 104, 2842–2850. doi: 10.1002/jsfa.13175
Ho, V. T. T., and Turner, M. S. (2020). Future probiotic foods. Microbiology 41, 58–60. doi: 10.1071/MA20017
Martins, E. M. F., Ramos, A. M., Vanzela, E. S. L., Stringheta, P. C., de Oliveira Pinto, C. L., and Martins, J. M. (2013). Products of vegetable origin: a new alternative for the consumption of probiotic bacteria. Food Res. Int. 51, 764–770. doi: 10.1016/j.foodres.2013.01.047
Min, M., Bunt, C. R., Mason, S. L., and Hussain, M. A. (2019). Non-dairy probiotic food products: an emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 59, 2626–2641. doi: 10.1080/10408398.2018.1462760
Montel, M.-C., Buchin, S., Mallet, A., Delbes-Paus, C., Vuitton, D. A., Desmasures, N., et al. (2014). Traditional cheeses: rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 177, 136–154. doi: 10.1016/j.ijfoodmicro.2014.02.019
Pereira, A. L. F., Maciel, T. C., and Rodrigues, S. (2011). Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Res. Int. 44, 1276–1283. doi: 10.1016/j.foodres.2010.11.035
Ranadheera, C. S., Vidanarachchi, J. K., Rocha, R. S., Cruz, A. G., and Ajlouni, S. (2017). Probiotic delivery through fermentation: dairy vs. non-dairy beverages. Fermentation 3:67. doi: 10.3390/fermentation3040067
Sarron, E., Gadonna-Widehem, P., and Aussenac, T. (2021). Ozone treatments for preserving fresh vegetables quality: A critical review. Foods 10, 605. doi: 10.3390/foods10030605
Segers, M. E., and Lebeer, S. (2014). Towards a better understanding of Lactobacillus rhamnosus GG—host interactions. Microb. Cell Factories 13:S7. doi: 10.1186/1475-2859-13-S1-S7
Thakur, M., Satwadhar, P., and Deshpande, H. W. (2015). Exploiting fruits and vegetables for development of probiotic products. Trends Biosci. 8, 6039–6046.
Keywords: spinach, probiotics, consumption, Lactobacillus plantarum, Lactobacillus rhamnosus
Citation: Xie K, Ling J, Mishra AK, Farooq MA, Ahsan S, Hassan S, El-Sherbeeny AM, Abukhadra MR, He S, Liu H, Zheng S and Ahmad N (2024) Impact of baby spinach as a carrier for the development of sustainable probiotics prior to consumption. Front. Sustain. Food Syst. 8:1430146. doi: 10.3389/fsufs.2024.1430146
Received: 09 May 2024; Accepted: 28 May 2024;
Published: 27 June 2024.
Edited by:
Anwar Ali, Hong Kong Polytechnic University, Hong Kong SAR, ChinaReviewed by:
Mohammad Aslam, Donald Danforth Plant Science Center, United StatesCopyright © 2024 Xie, Ling, Mishra, Farooq, Ahsan, Hassan, El-Sherbeeny, Abukhadra, He, Liu, Zheng and Ahmad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shan He, aGUwMDkxQGdtYWlsLmNvbQ==; : Awdhesh Kumar Mishra, YXdkaGVzaEB5bnUuYWMua3I=; : Muhammad Adil Farooq, YWRpbGZhcm9vcTkxNTZAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.