- 1ICAR-Directorate of Onion and Garlic Research, Pune, India
- 2ICAR-Directorate of Floriculture Research, Pune, India
- 3ICAR-Indian Institute of Horticultural Research, Bengaluru, India
- 4Agricultural Scientist Recruitment Board, New Delhi, India
The use of beneficial microbes is hitherto known and constantly increasing in agriculture due to their positive impact on crop growth and yield, and their minimal negative impact on the environment. The objective of this study was to evaluate the impact of eight Trichoderma strains of diverse origin on crop growth and yield of onion under field conditions. The identity of the strains used in the current study was confirmed by ITS and Tef1 gene sequencing. Field experiments were conducted in the Rabi season for 2 years (2020–21, and 2022–23) to evaluate the effect of the application of eight different Trichoderma strains that were applied individually and separately as eight different treatments (T1–T8) in experimental plots. In the plant growth promotion assay conducted in vitro, all strains showed the ability to produce IAA (indole-3-acetic acid), with levels ranging from 23.52 μg/mL (T6) to 45.54 μg/mL (T3). Our results revealed that Trichoderma treated experimental plots displayed better growth indices (plant height, pseudostem diameter), RWC (Relative water content), leaf chlorophyll content, and yield-attributing features like biomass (bulb and root dry mass), bulb diameter, and harvested bulb yield compared to the untreated control plants. In terms of yield, the T2 strain exhibited the highest bulb yield consistently for both the years (2020–21 and 2022–23) followed by T3 being statistically at par with T5. Among all the evaluated Trichoderma strains, the strain T2 (OGRDT2) and T3 (GRDT1), taxonomically identified as Trichoderma longibrachiatum, registered bulb yield of 32.24 t/ha and 30.76 t/ha, respectively while T5 (GRDT3), identified as Trichoderma asperellum, registered 30.55 t/ha average yield for 2 years compared to 24.08 t/ha average yield recorded for untreated control plants with an increase of 34, 28 and 27%, respectively. Based on our findings, it is concluded that the T. longibrachiatum strains OGRDT2 (T2) and GRDT1 (T3), T. asperellum strain GRDT3 (T5) are the best inducers of the onion crop growth and yield in the Rabi season and would be explored further for its commercial application in onion farming.
Introduction
Onion (Allium cepa L.) cultivation plays a pivotal role in India’s agricultural landscape, contributing significantly to food security, rural livelihoods, and economic growth (Kale et al., 2024). Although India is now first among the leading onion producers globally, onion cultivation still faces several challenges including low productivity, poor bulb quality, and susceptibility to pests and diseases. Moreover, the erratic weather conditions, high input costs (seed, fertilizers, pesticides, labour), post-harvest losses, and fluctuating market prices make onion cultivation less remunerative for farmers. Various research efforts have been made to improve onion productivity including improved practice packages (Abdelrasheed et al., 2021), precision agriculture (Zude-Sasse et al., 2016; Hahn et al., 2024), the development of improved onion varieties (Khosa et al., 2016; Mahajan et al., 2018), and genomic interventions employing modern biotechnological tools (Mainkar et al., 2023). However, all such efforts have met partial success due to the limitations relating to longer times required for tangible outputs (varietal development), or the complexity of location-specific responses and multiple factors determining the yield response.
Growing awareness about the detrimental effects of chemical fertilizers on the environment and ecosystems has led to an increasing adoption of alternative eco-friendly practices which primarily includes the use of beneficial microbes in agriculture (Sridevi and Ramakrishnan, 2010). The use of friendly microbes such as Pseudomonas (Novello et al., 2021), Bacillus (Younes et al., 2023), Azotobacter (Kurrey et al., 2018), Rhizobium (Siswanto et al., 2023), Mycorrhizae (Ramadan, 2019), and Trichoderma spp. (Younes et al., 2023) has been explored as an economically viable and sustainable solution to improve the growth, yield, and overall health of the onion crop. Such beneficial microbes (bacteria/fungi) may colonize the root zone and help the plants in nutrient acquisition, hormone production, stress tolerance, and disease suppression (Soni and Keharia, 2021; Cao et al., 2023). Using microbes to promote plant growth offers numerous advantages over chemical fertilizers and pesticides, as it is a sustainable and environment-friendly approach that enhances soil health and reduces dependence on chemical inputs. This method can lead to higher crop yields and increased profits for farmers. Nevertheless, the success of this approach relies on several factors, including the type of microbe used, crop variety, and environmental conditions. Hence, selecting and applying beneficial microbes correctly is critical for achieving optimal plant growth promotion.
Trichoderma is a genus of soil-borne fungi that are widely recognized for their plant growth promotion potential and plant disease suppression (Tyskiewicz et al., 2022). The use of Trichoderma spp. has emerged as a viable option for plant growth promotion and disease control in many crops including rice (Singh et al., 2023), tomato (Sehim et al., 2023), cucumber (Lian et al., 2023), maize (Syamsiyah et al., 2023), and wheat (Saadaoui et al., 2023). Trichoderma effectively colonize the rhizoplane, rhizosphere, and plant roots, and produce several metabolites with biostimulating (phytohormones, phytoregulators) and anti-microbial (cell wall degrading enzymes, antibiotics, volatile, and non-volatile compounds) features (Tyskiewicz et al., 2022). These metabolites can enhance nutrient uptake and assimilation by the plant, resulting in improved root and shoot growth. Trichoderma species can also induce plant systemic resistance against biotic and abiotic stresses. Due to its eco-friendly nature, Trichoderma is recommended for sustainable agriculture practices and integrated pest management (Monte, 2023). The Trichoderma application to agricultural field crops has been done using various methods that include seed treatment (Abdolmaleki et al., 2021), bulb treatment (Younes et al., 2023) soil drenching (Abdel-Kader et al., 2023), foliar spray (Mishra, 2019), and drip irrigation (Bonini et al., 2019; Vojnović et al., 2023). Whereas the method of seed treatment with Trichoderma spp. improves seedling vigor and reduces seedling diseases, and the soil drenching method improves root growth and nutrient uptake. Similarly, foliar spray with Trichoderma spp. increases plant resistance to pathogens and pests, and drip irrigation with Trichoderma spp. can deliver the biocontrol agent directly to the roots, resulting in improved root colonization and disease control. Moreover, species such as T. viride are compatible with arbuscular mycorrhizal (AM) fungi and positively influence various growth parameters and pigment content in onion plants, and have been proposed as potential alternatives to chemical fertilizers (Metwally and Al-Amri, 2020).
Trichoderma application to crops has been demonstrated to positively influence plant growth and development. In tomato plants, T. harzianum application resulted in increased plant height, stem diameter, and leaf area, as well as increased fruit yield and quality (Uddin et al., 2018). In cucumber plants, T. harzianum application resulted in increased plant height, root length, and shoot weight, as well as increased fruit yield and quality (Lian et al., 2023). In maize plants, T. harzianum T22 application resulted in increased root and shoot growth, as well as increased grain yield (Akladious and Abbas, 2012). Although common for radish, tomato, carrot, or head cabbage seeds, the technique has been less popular for onion-like crops. The onion crop is grown in Rabi, Kharif, and late Kharif season in India, and the maximum production of onion comes from the harvest of the Rabi season. However, the onion crop confronts many biotic and abiotic challenges during different growing seasons that primarily include high input costs due to high pest occurrence and disease infestations resulting in loss of crop and low yield. The use of Trichoderma spp. is seen as a viable, sustainable, and eco-friendly option to combat the diseases and the consequent yield losses. Moreover, field application of Trichoderma to an onion crop may lead to better crop growth and bulb yield. In a study conducted by Ortega-García et al. (2015), the application of T. asperellum resulted in increased bulb biomass and phenolic content in onion. However, most of these studies have been conducted under controlled conditions in the laboratory or greenhouse, and there is limited information on the field application of Trichoderma-based formulations in onion cultivation.
Therefore, the present study investigated the effects of field application of eight Trichoderma strains (T1–T8) on Rabi onion growth, final yield, and bulb quality under natural environmental conditions over 2 years (2020–21, 2022–23). Based on the findings of this study, we propose that the application of strains OGRDT2 (T2), GRDT1 (T3), and GRDT3 (T5) significantly increased the onion crop yield compared to the untreated control. These results suggest that Trichoderma-based bio-formulations could be further explored for their potential to sustainably and eco-friendly enhance onion yield.
Materials and methods
Isolation, identification, and phylogenetic analyses of Trichoderma strains
The Trichoderma strains used in the current study were originally isolated from soil samples collected from experimental farms of ICAR-Directorate of Onion and Garlic Research (DOGR), Pune, and ICAR-Directorate of Groundnut Research (DGR), Junagadh, Gujarat, India using the method as described in Rai et al. (2016). After an initial screening of 12 strains, eight strains were finally chosen based on their superior biocontrol potential. Total genomic DNA was extracted from fresh mycelium harvested from PDA (Potato Dextrose Agar) plates after 4 days with cetyl-trimethylammonium bromide (CTAB) method as described by Kumar et al. (2013). The internal transcribed spacer (ITS) and translation elongation factor alpha (tef 1) fragments were amplified using the primers pairs (5′-TCCGTAGGTGAACCTGCGG-3′ and 5′-TCCTCCGCTTATTGATATGC-3′) and (5′-CATCGAGAAGTTCGAGAAGG-3′ and 5′-TACTTGAAGGAACCCTTACC-3′), respectively. The PCR reactions were carried out in 20 μL reaction mixture containing 10× PCR buffer, 50 ng DNA template, 1.5 mM MgCl2, 0.25 mM dNTP mixture, and 0.25 μM each of primer, and one unit of DreamTaq DNA Polymerase (Thermo Scientific, India). PCR reactions were run in Applied Biosystems Thermocycler, USA with the following settings: Denaturation at 94°C (5 min); then 35 cycles of 94°C (30 s), 56°C (45 s), and 72°C (90 s). The final extension was done at 72°C for 10 min. The ITS and Tef1 gene sequences obtained were BLAST searched against the NCBI GenBank database to confirm the identity of the strains. All the ITS and Tef1 gene sequences have been submitted to NCBI GenBank with accessions, ITS1 (OR048743-OR048747, OR048750, OR048751, OR048753) and Tef1 (OR102865-OR102869, OR102872, OR102873, OR102875). The phylogenetic analysis of the Trichoderma strains used in this study and reference sequences of the same species obtained from GenBank was carried out using MEGA software (Tamura et al., 2021). The aligned Tef1 sequences were used to construct the maximum likelihood tree with 500 replicates. The reference strains details are provided in Supplementary Table S1.
Plant growth promotion assay (PGPA) screening of Trichoderma strains
Screening for IAA production
To determine the production of indole-3-acetic acid (IAA) by the eight Trichoderma strains used in this study, 1 mL of a spore suspension containing 108 spores/mL from each strain was individually added to 100 mL of potato dextrose broth media. The media was supplemented with 100 mg/L of L-tryptophan as a precursor for IAA synthesis. The fermentation broth was incubated in a rotary shaker at 180 rpm min− 1 for 5 days at 28°C, centrifuged at 10,000g for 20 min at 4°C, and the culture was filtrated through a Whatman’s paper No.3 followed by filtration through a 0.22 μm Millipore membrane. IAA concentration was quantified using the Salkowski reagent assay by measuring the absorbance at 530 nm of the pink-colored complex formed through the reaction of IAA with the Salkowski reagent (Glickmann and Dessaux, 1995).
Screening for siderophore production
Siderophore production by the Trichoderma strains was determined using the CAS (chrome-azurol S- agar) method (Louden et al., 2011). A 5 mm mycelial agar disc taken from the edge of an actively growing fungus was incubated on CAS agar plates. The strains exhibiting the development of an orange halo on Petri plates after incubation at 30 ± 1°C for 5 days were considered as siderophore producers. For quantitative estimation of siderophore production, a 10 mL aliquot of supernatant from a liquid culture of each fungus grown in potato dextrose broth media incubated at 25 ± 1°C was mixed with 10 mL CAS assay solution prepared according to Schwyn and Neilands (1987). A reference was prepared with the same medium used for growing the fungi, but uninoculated. The sample (s) and reference (r) absorbances at 630 nm were read after 2 h of incubation at room temperature using UV/Vis spectrophotometer. The “siderophore units %” was calculated using the following formula (Machuca and Milagres, 2003);
Where; Ar = Absorbance of reference As = Absorbance of sample.
In vitro P-solubilization assay
The eight Trichoderma strains used in the current study were evaluated for their capacity to solubilize P in vitro using Modified Pikovskaya’s Agar (MPA) as described in Promwee et al. (2014). The development of a clear halo around the fungal colonies was considered as evidence of their capacity to solubilize the phosphate. A 5 mm dia mycelial disc was taken from an actively-growing Trichoderma colony and placed in the centre of a Pikovskaya’s Agar plate and incubated at room temperature for 3 days. The diameter of the clear halo zone around the colony was used to quantitatively assess the phosphate solubilization index (PSI) of each Trichoderma strain using the formula below:
Zinc solubilization assay
Trichoderma isolates were screened for their zinc-solubilizing ability on basal medium (glucose-10.0 g/L; ammonium sulphate1.0 g/L; potassium chloride-0.2 g/L; dipotassium hydrogen phosphate-0.1 g/L; magnesium sulphate-0.2 g/L; pH 7.0) supplemented with 0.2% ZnO (Sharma et al., 2012). Briefly, actively growing Trichoderma strains (~5 mm in diameter) were inoculated on Petri plates containing medium separately amended with zinc oxide and incubated at 25 ± 1°C for 5 days to observe the formation of a clear halo zone around colonies. Subsequently, the colony diameter and the diameter of the halo zone (mm) formed around the colony were measured after 5 days of inoculation and Zn solubilization was calculated using the following formula:
Multiplication of Trichoderma strains for field application
Each Trichoderma strain was grown in potato dextrose broth at 25 ± 2°C for 36 h, and 100 mL of liquid culture adjusted to 108 cfu/mL was mixed thoroughly in 50 kg of FYM (Farm Yard Manure) of non-defined but of near uniform composition (ensured through vigorous mixing) in a rectangular pit of 100 cm × 100 cm × 100 cm (l × b × h). The pit was covered with a polythene cover to allow Trichoderma strains to multiply in the FYM for 12 days.
Experimental site and climatic conditions
The field experiments were conducted during the Rabi season of 2 years viz., 2020–21 and 2022–23, at the experimental research farm of ICAR-DOGR, Pune, Maharashtra, India located at Rajgurunagar having 18.50350 N (latitude) and 73.53050 E (longitude) at 611 m msl (mean sea level) with a temperature range of 5.5–42.0°C and annual mean rainfall of 669 mm. The experiment site is situated in the Deccan Plateau’s agro-ecological region where 91% of rainfall occurs between June and December. The weather data for the 2020–21 and 2022–23 growing seasons were obtained from Automatic Weather Station, ICAR-DOGR, located 50 m apart from the experimental site and are given in Supplementary Table S2. The soil of the experimental field was black with a texture composition of 55.2% sand, 8.5% silt, and 36.3% clay and had a pH of 8.3; and EC of 0.23 dS/m.
Field experiments, experimental design, and Trichoderma application
The field experiments were arranged in a completely randomized block design with nine treatments T1–T8 eight Trichoderma strains and one untreated control (T9). Each treatment was replicated three times and treatment blocks measured 1 × 8 m. The Trichoderma enriched FYM was applied to each plot at a rate of 250 g/plot (8 sqm), equivalent to 250 kg FYM/ha as a soil amendment before transplanting of onion seedlings, then at 35 DAT (days after transplantation), 65 DAT and 95 DAT, while similarly processed FYM with no inoculum added to it, was applied at the same rate to the control plot (T9). FYM in both the treated and control plots was broadcasted and then irrigated through a drip to maintain the moisture required for Trichoderma multiplication. The seedlings of the “Bhima Shakti” variety of onion were initially raised in the nursery and were transplanted in the main field after the application of Trichoderma enriched FYM in each block. The basal dose of fertilizers and standard recommended practices of crop cultivation were followed during the crop cycle.
Measurement of crop growth and yield attributes
Plant height, pseudostem diameter, and number of leaves
For the measurement of plant growth parameters, five randomly selected onion plants were selected from each replicate plot, and crop growth parameters, plant height, number of leaves per plant, pseudostem diameter (mm), and number of leaves were measured at weekly intervals, with the initial reading taken at 27 DAT. Owing to the measurable thickness only achieved after 45 DAT, the first reading of the pseudostem diameter of onion plants was recorded at 48 DAT.
Measurement of relative water content (RWC) and chlorophyll content
To determine the RWC, 1 g leaf sample (fresh weight) was picked from a randomly selected plant at 60 DAT from each replicate plot and kept in ordinary water to obtain the turgid weight, the leaves were then dried at 70°C in a hot air oven until they reached a constant dry weight. The RWC content of the leaves was calculated using the formula mentioned below.
The chlorophyll content of the randomly picked plant leaves at 60 DAT from each treatment plot was measured spectrophotometrically using the method described by Lichtenthaler (1987). Spectroscopic readings were used to calculate the chlorophyll content using the formula.
Biomass determination
To determine the effects of Trichoderma application on biomass production, the shoot, root, and bulb were excised from randomly selected representative plant taken from each replicate of the experimental plot at 60 DAT to calculate their individual fresh and dry weight per plant basis. To determine the final dry weight of the shoot, root, and bulb, the excised samples were dried in an oven set at 60°C until they reached a constant weight. The difference in initial fresh weight was normalized by expressing the final biomass as a percentage of the initial biomass using the equation below.
Bulb grades and bulb yield
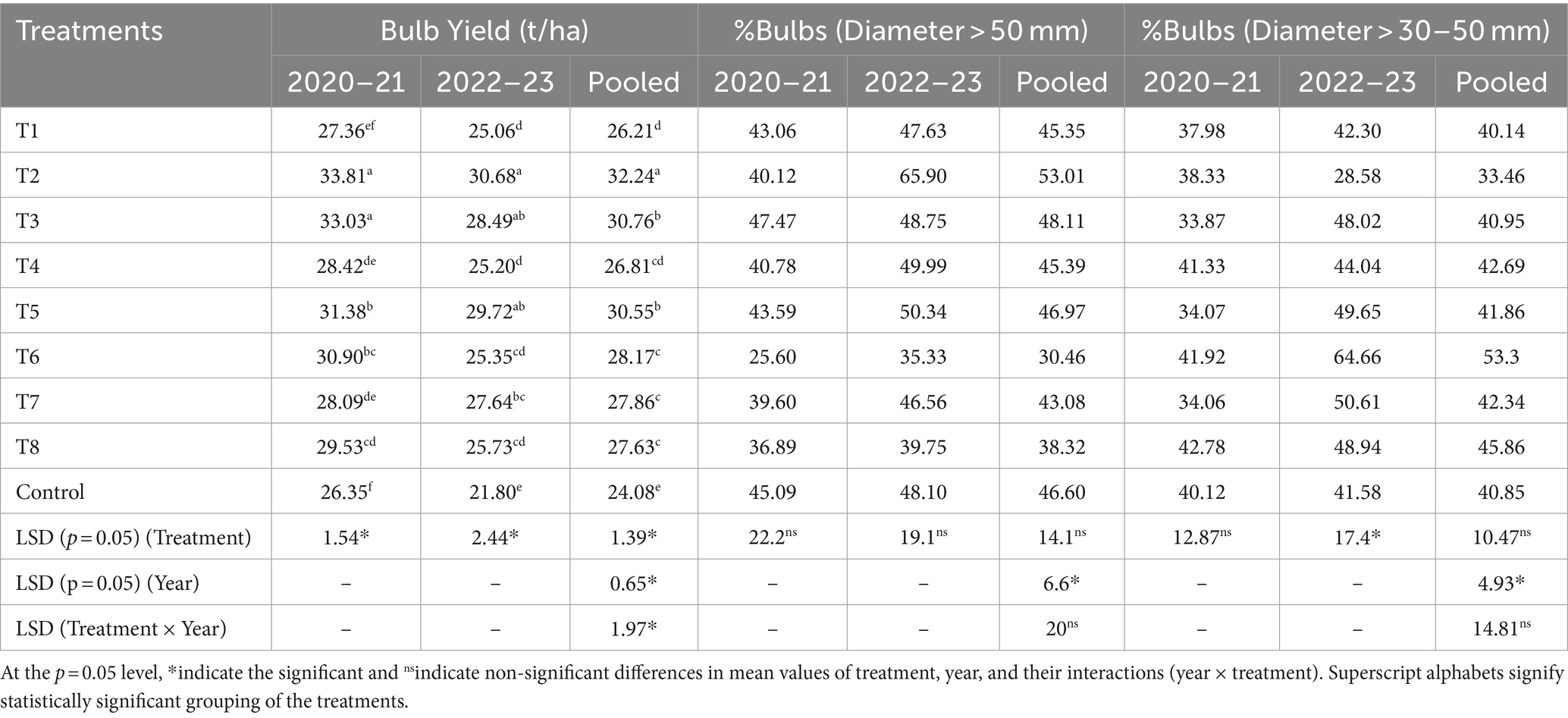
The mature harvested bulbs were size sorted based on the bulb diameter as superior quality bulbs (> 50 mm diameter) and inferior quality bulbs (>30–50 mm diameter). The bulbs that were less than 30 mm were classified as non-grade bulbs. The percentage of bulbs in each category was expressed with reference to total bulbs harvested from each experimental plot. The bulb yield per plot obtained was expressed in t/ha.
Statistical analysis
All the data and their derived variables from the experiments were statistically analyzed using IBM-SPSS version 26 to study the effect of different Trichoderma strains on various crop growth and yield parameters recorded for 2 years (2020–21, and 2022–23). For each variable measured, the data was analyzed by one-way ANOVA (analysis of variance) using Fisher’s least significant difference (p = 0.05) to compare the treatment means. Two-way ANOVA was used to compare the effects of year and Trichoderma application on onion bulb yield. Test of the homogeneity of error variances was evaluated using Bartlett’s chi-square test for both years’ data. The pooled analysis was conducted, depending on the identification of homogeneity in error variances. In instances of heterogeneous data, a data transformation was applied using the square root of the mean squared error (MSE) for the corresponding year before performing the pooled analysis. The Fit ANOVA Model was used to assess the interactions between treatments and years using the equation.
Y = Y is the dependent variable (plant growth); μ = Overall mean; Treatment and Year are categorical variables representing treatments and years, respectively; Treatment × Year is the interaction term; ε is the error term.
After fitting the ANOVA model, the significance of the interaction term (Treatment × Year) was examined using an F-test.
Results
The eight fungal strains used in the current study were originally isolated from the soil of agricultural fields of ICAR-DGR, Junagadh, Gujarat, India, and ICAR-DOGR, Pune, India. The pure cultures of the isolated strains were identified as Trichoderma spp. using ITS and Tef1 gene sequencing (Supplementary Table S3). Based on the BLAST search results of ITS and Tef1 gene sequences against NCBI GenBank database, the five strains viz., OGRDT1, OGRDT2, GRDT1, GRDT5 and GRDT8 were identified as T. longibrachiatum and the strains (GRDT3, GRDT6) and GRDT2 were identified as T. asperellum and T. harzianum, respectively. The phylogeny of the strains used in the current study was inferred based on the phylogenetic tree constructed based on the Tef1 gene (Figure 1).
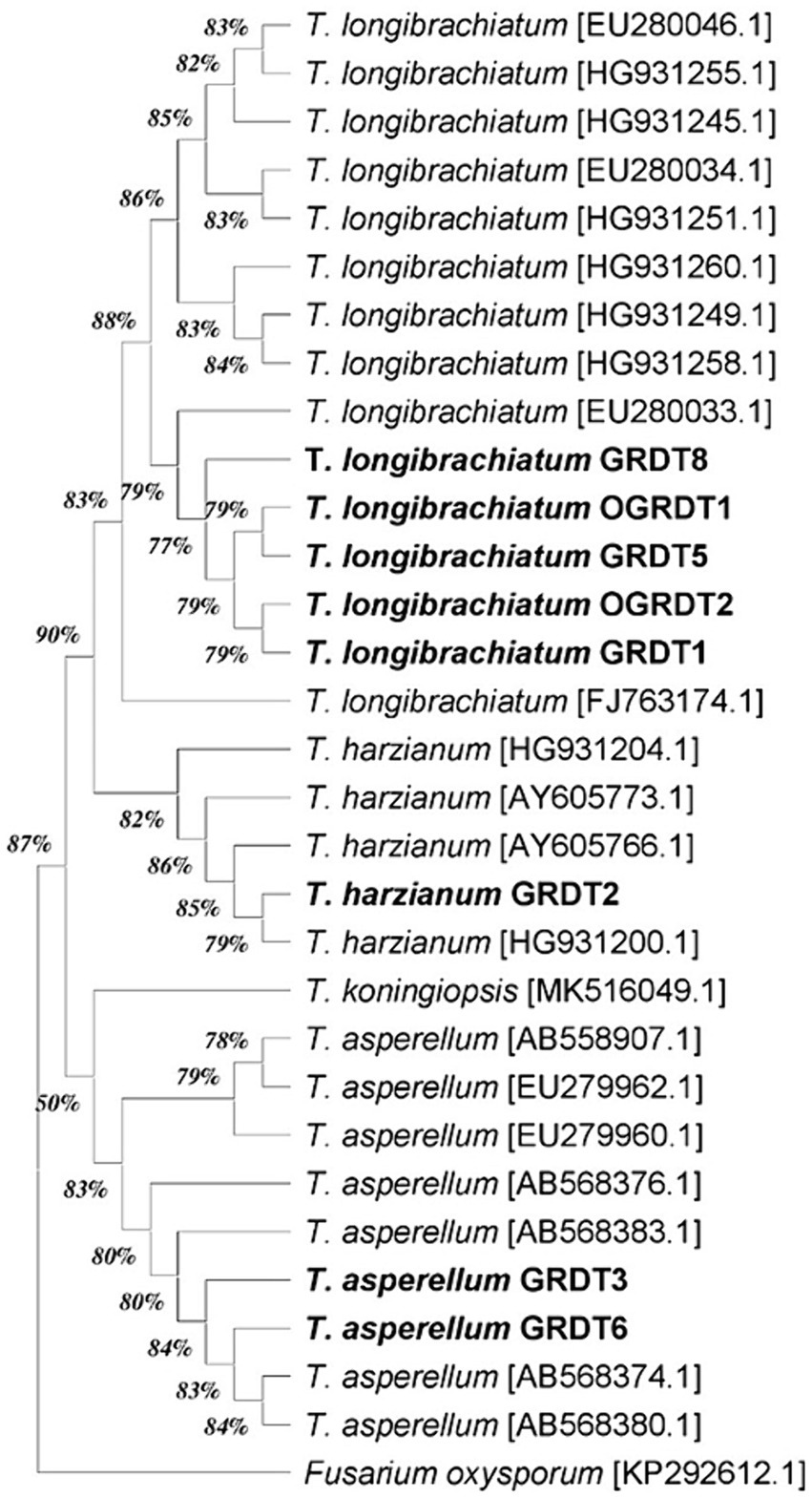
Figure 1. The phylogenetic tree inferred using Tef1 gene sequences of 8 Trichoderma strains and 23 reference strains using the Maximum Likelihood method and Tamura-Nei model (bootstrap values are indicated at each node).
PGPA screening of Trichoderma strains
The eight Trichoderma strains used in the study were screened for their plant growth promotion activity (PGPA) viz., IAA production, siderophore formation, phosphate solubilization, and zinc solubilization. All eight Trichoderma strains produced IAA ranging between 23.52 (T6) to 45.54 μg/mL (T3). Trichoderma strains in T3 (GRDT1) and T2 (OGRDT2) produced the highest level of indole-3-acetic acid (IAA), with IAA concentrations of 45.54 μg/mL and 38.45 μg/mL, respectively (Table 1). Similarly, all eight strains showed evidence of siderophore production. Strains in T7, T3, T4, and T2 were hyper-siderophore producers displaying siderophore production percentages of 39.4, 39.2, 38.51, and 38.43%, respectively. In addition, strains in T1, and T8 were able to solubilize phosphate (Table 1); Strain in T1 demonstrated the highest levels of phosphate solubilization (3.10 mm). All strains were positive for zinc solubilization potential but strains in T7 (5.6 mm) and T5 (4.75 mm), showed the highest zinc solubilization. These data indicate that all eight Trichoderma isolates could be used as multifunctional biological stimulants to enhance nutrient availability for plant growth and development.
Effect of Trichoderma application on crop growth parameters
Canopy traits: plant height, number of leaves, and pseudostem diameter
In this study, the impact of Trichoderma application on onion plants was assessed by measuring various growth parameters of randomly selected plants from each treatment plot on weekly basis from transplant to crop maturity. A clear positive effect of Trichoderma application was noted on plant height compared to the untreated control for both years. In 2020–21, the maximum height of the onion plants reached 48.46 (±0.7) cm for T1 compared to 39 (±0.8) cm for control plants (Figure 2A). Strain T1 also performed best in second year (2022–23) with onion plants height reaching up to 43.9 (±0.42) cm again compared to 38.9 (±0.77) cm for control plants. Analysis of pooled (2-year) data showed a statistically significant effect of Trichoderma application on plant height with T1 strain performing best with a mean height of 46.21 (±1.08) cm compared to the control height of 38.5 (±1.2) cm.
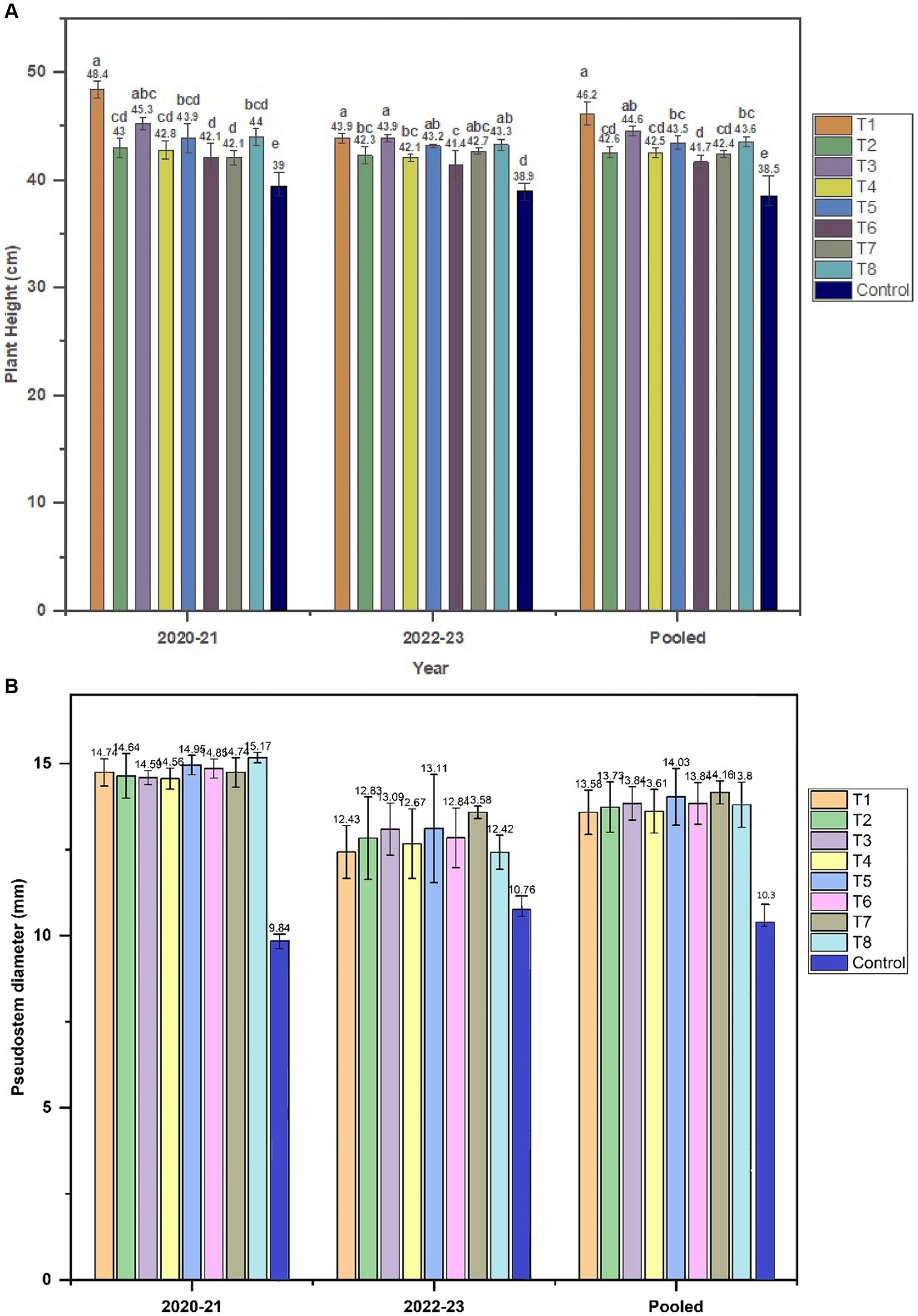
Figure 2. Effect of Trichoderma application on plant growth parameters of onion crop. (A) Plant height. (B) pseudo-stem diameter recorded for 2 years (2020–21 and 2022–23).
Differences were observed in the number of leaves produced by Trichoderma-treated and untreated control plants for either of the study years. Each plant developed a maximum of 9–10 leaves. Trichoderma had a significant effect on pseudostem diameter with strains in T7 and T8 inducing the greatest growth response in both years, with an increase of 60.20% (15.7 mm compared to the control, 9.8 mm) and 26.07% (13.56 mm against 10.76 mm for control) in 2020–21 and 2022–23, respectively (Figure 2B).
Relative water content (RWC) and chlorophyll content
The RWC content in onion leaves ranged from a minimum of 65% for control plants to a maximum of 71.31% for T1 treated plants in 2020–21 whereas the RWC in 2022–23 ranged from a minimum of 54.21% (control plants) to 58.2% for T6 and T7 (Figure 3A). RWC values were higher in all Trichoderma treatments compared to the control plants in both years. However, the overall RWC values recorded in 2022–23 were comparatively low compared to those recorded in 2020–21. Comparing cumulative RWC values for both years, the Trichoderma application enhanced the relative water content in leaves by up to 8.2% was noted for treatments T2, T6, T7, and T8. Significant differences were noted in leaf chlorophyll content and significantly higher “total leaf chlorophyll” levels of 4.21 μg/mL, and 4.19 μg/mL were observed for T3 and T1 plants, respectively, compared to 1.74 μg/mL “total leaf chlorophyll” in the control plants (Figure 3B). All the Trichoderma treatments (except T4 and T5) induced >2 fold increase in the “total chlorophyll content” indicating a clear positive influence of Trichoderma on leaf chlorophyll content. There was a clear relationship between the “total chlorophyll content” and chlorophyll “a” content but the chlorophyll “b” content did not follow the similar trend.
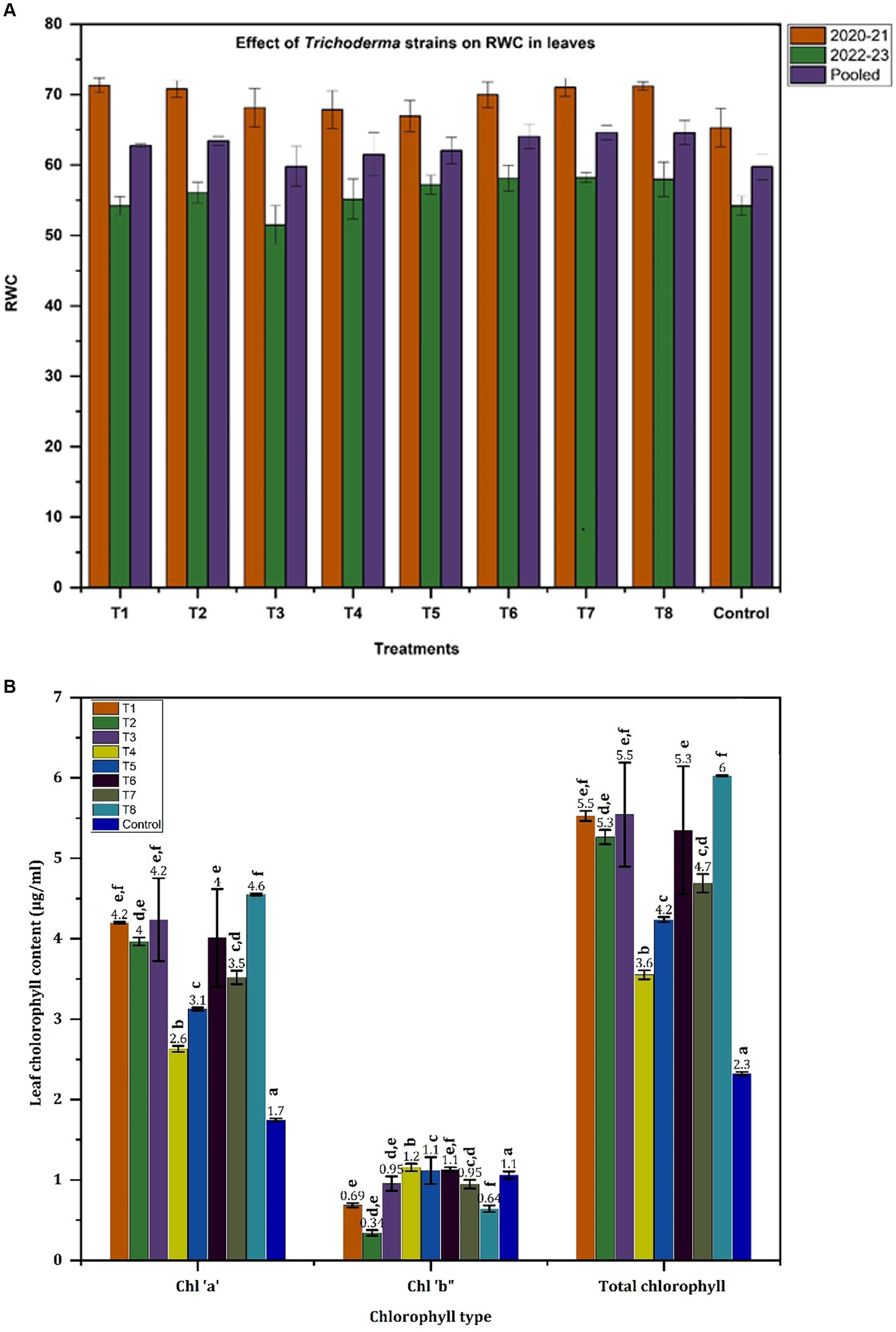
Figure 3. Effect of Trichoderma application on RWC and chlorophyll content of onion crop. (A) Relative water content. (B) Chlorophyll content.
Root, shoot, bulb, and total biomass accumulation
Significant differences were detected in shoot, root, and bulb biomass between treated and untreated plants in both trial years, with a notable increase in biomass in Trichoderma-treated vs. control plots. Effect of Trichoderma application was more pronounced on the root biomass compared to the shoot biomass. The highest biomass was recorded for T2 with a dry weight that was equivalent to 11.9% of fresh weight of roots whereas the control plants had an average biomass weight equivalent to 8.64% of the fresh weight. Strains in T1, T7, and T8 performed statistically at par with the T2 strain in terms of root biomass accumulation (Table 2). The highest average biomass accumulation for root and bulb tissue in both years was obtained in plants treated with T2 (Table 2). A two-way ANOVA revealed a significant interaction between ‘year’ and ‘treatment’ and the biomass accumulated during 2022–23 was higher than 2020–21 for most treatments.
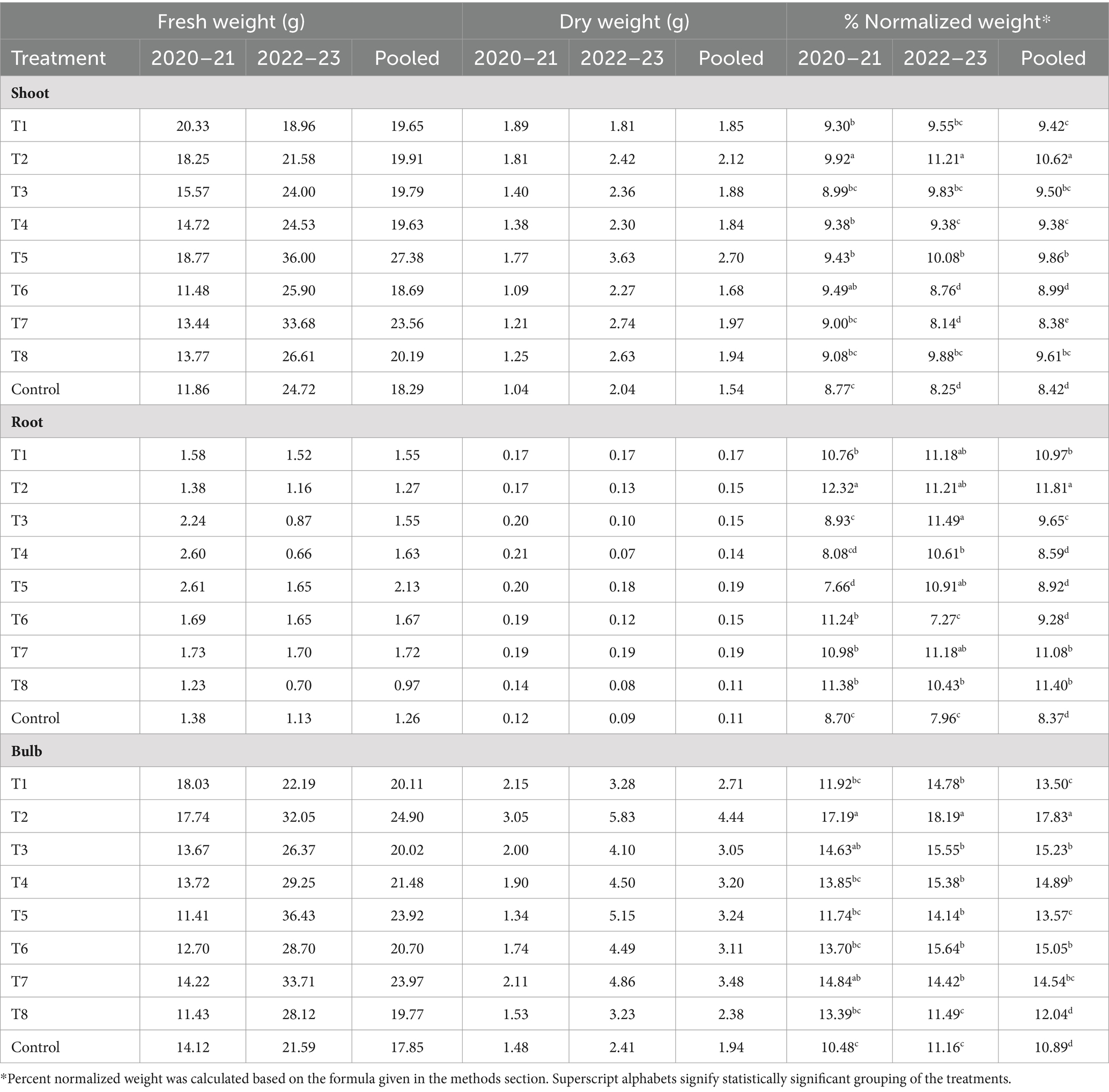
Table 2. Effect of application of different Trichoderma strains on shoot, root and bulb biomass of onion plants.
Effect of treatment on yield
The different Trichoderma strains influenced the bulb yield to a varying extent. Significant effects on bulb yield were seen in both experimental years. The treatment component p-value was 0.016 in 2020–21 and thus the LSD multiple comparison test was performed to evaluate means (Table 3). The highest yield was recorded from plots treated with the T2 strain (33.81 t/ha) and T3 (33.03 t/ha) was found statistically (p = 0.05) at par with T2 in the year 2020–21. In 2022–23, the highest bulb yields were again recorded from plants treated with T2 (30.68 t/ha). Yields from plants treated with T3 and T5 were not statistically (p = 0.05) different from those obtained from plants treated with T2. The F test conducted to check the homogeneity of the variance (p value of the F test = 0.35) showed the results are non-significant and the error MS of both the years were homogenous and hence the yield data from both experimental years were pooled and analyzed The analysis showed that yields were significantly higher from plots treated with strain T2 (32.24 t/ha) and was significantly higher than that obtained under all other Trichoderma treatments (Table 3).
Trichoderma application effect on onion bulb diameter
The highest number of superior quality bulbs (bulb diameter > 50 mm) were obtained from plots treated with T2 and T3, with the total percentage of bulbs reaching the desired diameter making up 48 and 53% of total harvested in 2020–21 and 2022–23, respectively (Table 3). The lowest number of inferior quality bulbs (bulb diameter 30–50 mm) was also harvested from plots treated with T2, and made up an average of 33% of total harvest for both trial years.
Discussion
This study included eight Trichoderma strains, originally isolated from agricultural fields of ICAR-DOGR, Pune, Maharashtra and ICAR-DGR, Junagadh, Gujarat. Trials were done to evaluate their relative efficacy for promoting onion plant growth and yield. Trichoderma spp. are well-known for their biocontrol and plant growth promotion potential (Tyskiewicz et al., 2022; Kredics et al., 2024). These fungi are versatile as opportunistic symbionts in plants (Abdelrahman et al., 2016) and act as microbial biostimulants, activating signaling networks, biosynthetic pathways, and hormonal interactions upon entering plant tissues (Younes et al., 2023). The identity of the Trichoderma strains used in this study was confirmed by ITS and Tef1 gene sequencing, with five of them specifically identified as Trichoderma longibrachiatum, a species known for its potential to mitigate onion plant damage induced by abiotic stress and also to the infection by Sclerotium cepivorum (Camacho-Luna et al., 2023).
The investigations on PGP potential of these taxonomically related strains assumed that even taxonomically related isolates may differ substantially in their ecological function in soil ecosystem and may impact the plant growth differently. The stimulatory effect of Trichoderma on plants is probably related to their involvement in the crosstalk between the growth hormones synthesized by these fungi and the defense hormones induced by them in the plant (Tyskiewicz et al., 2022). Important traits like auxin phytohormone production are strain dependent and influenced by external environmental factors (Nieto-Jacobo et al., 2017). Therefore, it is now believed that Trichoderma-mediated plant growth promotion is strain specific rather than species-specific and individual isolates may exhibit considerable variation in plant-growth promotion effects, and even plant specificity (Stewart and Hill, 2014). Therefore, the present study also evaluated the PGP potential of eight Trichoderma isolates, notably for their capacity to produce IAA, siderophore formation, phosphate solubilization and zinc solubilization. Although five of isolates classified as Trichoderma longibrachiatum, there was considerable variation in IAA production affirming that PGPA traits are strain specific. The findings highlight the importance of strain selection for desired effects on the crop of interest (Pedrero-Mendez et al., 2021).
In our study, onion plants treated with Trichoderma exhibited enhanced growth characteristics compared to untreated control plants. The beneficial outcome of applying Trichoderma was particularly notable in growth characteristics such as plant height (Figure 2A) and pseudostems diameter (Figure 2B) which are crucial indicators of plant health and vigor. Our findings are consistent with those of Metwally and Al-Amri (2020), wherein the application of T. viride resulted in a significant increase in onion plant height and neck diameter, with improvements of 52.7 and 17.8%, respectively, compared to uninoculated control plants. The increase in the height of Trichoderma treated plants can be ascribed to the ability of the strains to produce indole-3-acetic acid, a vital plant growth regulator that plays a central role in cell elongation and division. Trichoderma strains T2 and T3 which produced highest level of IAA in the in vitro assays also induced the greatest increase in the height of onion plants compared to the other Trichoderma strains tested. Moreover, it has been reported that even the same Trichoderma strain may produce variable amounts of IAA under different environmental conditions (Nieto-Jacobo et al., 2017). Our results were consistent with those of Ortega-García et al. (2015) where the authors reported a corresponding higher vigor of onion plants by the application of a high IAA producing T. asperellum strain. IAA is also well-known for its role in cell elongation, softening the plant cell walls, thus allowing cells to expand and elongate, leading to increased plant height and size (Reetha et al, 2014). IAA also promotes root growth which is an essential trait for better nutrient and water uptake from the soil (Stewart and Hill, 2014). The positive impact of OGRDT2 (T2) and GRDT1(T3) strains may be partly attributed to their ability to produce high levels of IAA, as well as other compounds such as siderophores. The strains OGRDT2 (T2) and GRDT1(T3) siderophores compared to the other strains. Higher siderophore production can lead to enhanced iron uptake by the plants because siderophores released by Trichoderma can in turn chelate iron ions from the soil, forming complexes that are readily taken up by colonized roots. Trichoderma siderophores have been shown to promote plant growth in vegetable crops like bean (Phaseolus vulgaris L.) (Hoyos-Carvajal et al., 2009) and cucumber (Qi and Zhao, 2013). Although, the test isolates were screened for their ability to solubilize phosphate, isolates OGRDT1 (T1) and GRDT8 (T8) showed the highest phosphate solubilization potential. They did not induce higher growth or yield potential. Similarly, isolates GRDT6 (T7) and GRDT3 (T5) with highest zinc solubilization potential, they did not promote plant growth or enhance yield.
The present study also recorded canopy related traits like number of leaves, pseudostem diameter, relative water content (RWC) and chlorophyll content. Traits like “number of leaves” were not affected by application of Trichoderma; this is a genetic trait that only differs with the variety or cultivar used. Trichoderma applications improved RWC in onion leaves, to varying degrees across the different treatments but differences were not statistically significant. Similarly, leaf chlorophyll content was positively influenced by the Trichoderma treatments, with leaves containing higher levels of chlorophyll “a” and total chlorophyll in compared to untreated control plants. Leaf chlorophyll and accessory pigments are integral components of the photosynthetic machinery in plants playing a vital role in the absorption of light energy and thus driving the plant’s photosynthetic activity which in turn translates into enhanced plant biomass production (Simkin et al., 2022). Our study showed that Trichoderma boosted photosynthetic pigment levels and resulted in better growth of onion under field conditions. Our results agree with earlier reports where the application of T. asperellum to onion plants improved photochemical performance compared with that of uninoculated plants; and the chlorophyll and carotenoid contents were 130 and 40% higher, respectively (Rodriguez-Hernandez et al., 2023). The positive impact of Trichoderma application on onion leaf chlorophyll has also been reported previously by Metwally and Al-Amri (2020). Furthermore, the application of Trichoderma has been reported to boost the enzymatic superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and glutathione peroxidase (GPX) and non-enzymatic antioxidants like phenolics and flavonoids (Ortega-García et al., 2015; Vojnović et al., 2024) in onion plants, ensuring reduced production of Reactive Oxygen Species(ROS) in the growing plants which may otherwise damage thylakoid membranes and thus decrease the photosynthetic activity due to the oxidative damage. Rodriguez-Hernandez et al. (2023) have reported a two-fold increase in phenolic content and 15–70% higher antioxidant activity in plants treated with T. asperellum compared to the uninoculated plants.
Furthermore, the study investigated Trichoderma’s influence on plant biomass and bulb yield. Trichoderma-treated plants produced higher total biomass compared to the control, particularly in plants treated with isolates T2, T1 and T3. Biomass is an important measure of treatment effect on crop production and productivity. The use of bio-stimulants has been reported to increase the biomass of onions cultivated under hot conditions in Serbia (Vojnović et al., 2023). In this study, we investigated the effects of Trichoderma application on both belowground and above-ground tissues of onion plants. Isolate OGRDT2 (T2) had a greatest effect on root biomass. The beneficial effect of T2 on onion bulb yield, we believe to be principally mediated through effects on the root system. The present study confirmed the beneficial effect of Trichoderma isolate T2 on bulb yield both the experimental years with an average of 32.24 t/ha yield recorded against 24.08 t/ha from the control plants. Bulb yield is the most important parameter given that it is this part of the plant that is consumed but bulb shape is an important consumer trait, with preference for bulbs with a perfect spherical shape and a diameter of more than 50 mm are preferred. Isolate T2 was notable in its effects on the important trait and an average of 53% of bulbs harvested from T2-treated plots were > 50 mm is diameter, compared to 46.6% of bulbs from the control. Moreover, the Trichoderma treated plants also registered a lower percentage of inferior size bulbs, with a diameter < 30 mm (33%) with 40% of the bulbs harvested from the control plots considered as C-grade bulbs. The impact of Trichoderma inoculation on the bulb sphericity and size has been reported recently by Rodriguez-Hernandez et al. (2023).
Our results underscore the diverse positive effects of applying Trichoderma on onion plant growth and development. The study underscores the potential of Trichoderma as a valuable biological stimulant for bolstering onion growth and productivity. Based on the results obtained in this study, we infer that Trichoderma strains OGRDT2 (T2) and GRDT1 (T3) showed the greatest potential to improve onion production.
Conclusion
The current study evaluated the effects of eight different Trichoderma isolates on plant growth and yield parameters of Rabi onion crops under field conditions over 2 years. Our results suggest that despite their phylogenetic similarity, the eight Trichoderma isolates differed in their plant growth promotion potential as assessed by in vitro assays on IAA production, siderophore formation, phosphate solubilization and zinc solubilization. As a result, the eight Trichoderma isolates had variable effects on plant growth promotion and yield attributes of onions. Still, each Trichoderma isolate evaluated promoted onion growth and productivity compared to the untreated control reaffirming the plant growth promoting potential of this beneficial fungus. Our results showed that the Trichoderma longibrachiatum isolates OGRDT2 (T2), GRDT1 (T3) and GRDT3 (T5) outperformed all other Trichoderma isolates tested and increased bulb yield of 34, 28 and 27%, respectively, over the untreated control. These isolates could be explored further for their commercial application in onion farming.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
RD: Conceptualization, Project administration, Supervision, Writing – review & editing. SK: Formal analysis, Writing – original draft, Writing – review & editing. KJ: Data curation, Investigation, Methodology, Visualization, Writing – review & editing. AR: Data curation, Formal analysis, Software, Validation, Writing – review & editing. KB: Data curation, Investigation, Writing – review & editing. DM: Visualization, Methodology, Writing – review & editing. VK: Visualization, Writing – review & editing. HB: Visualization, Writing – review & editing. RB: Visualization, Writing – review & editing. VG: Visualization, Writing – review & editing. VM: Visualization, Writing – review & editing. MS: Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Indian Council of Agricultural Research (ICAR), through ICAR-DOGR project “Development, refinement and validation of management strategies for major fungal diseases of Onion-Garlic” grant number IXX16074.
Acknowledgments
The authors gratefully acknowledge the Indian Council of Agricultural Research and ICAR-DOGR, Pune for funding support and providing the necessary facilities to conduct the research. Authors are also grateful to those who directly or indirectly supported in this work and development of manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2024.1427303/full#supplementary-material
References
Abdel-Kader, M. M., El-Mougy, N. S., Khalil, M. S. A., El-Gamal, N. G., and Attia, M. (2023). Soil drenching and foliar spray with bioagents for reducing wheat leaf diseases under natural field conditions. J. Plant Dis. Prot. 130, 279–291. doi: 10.1007/s41348-023-00705-z
Abdelrahman, M., Abdel-Motaal, F., El-Sayed, M., Jogaiah, S., Shigyo, M., Ito, S. I., et al. (2016). Dissection of Trichoderma longibrachiatum-induced defense in onion (Allium cepa L.) against fusarium oxysporum f. sp. cepa by target metabolite profiling. Plant Sci. 246, 128–138. doi: 10.1016/j.plantsci.2016.02.008
Abdelrasheed, K. G., Mazrou, Y., Omara, A. E. D., Osman, H. S., Nehela, Y., Hafez, E. M., et al. (2021). Soil amendment using biochar and application of K-humate enhance the growth, productivity, and nutritional value of onion (Allium cepa L.) under deficit irrigation conditions. Plants 10:2598. doi: 10.3390/plants10122598
Abdolmaleki, A., Tohidloo, G., and Shahbazi, S. (2021). Study of onion (Allium cepa) seed coating whit disinfection, GA3 and Trichoderma on germination traits. Iranian J. Seed Sci. Technol. 9, 123–128.
Akladious, S. A., and Abbas, S. M. (2012). Application of Trichoderma harziunum T22 as a biofertilizer supporting maize growth. Afr. J. Biotechnol. 11, 8672–8683. doi: 10.5897/AJB11.4323
Bonini, P., Rouphael, Y., Cardarelli, M., Ceccarelli, A. V., and Colla, G. (2019). Effectiveness of Trichoderma application through drip-irrigation to reduce Sclerotinia disease incidence and improve the growth performance of greenhouse lettuce. In XI international symposium on protected cultivation in mild winter climates and I international symposium on nettings and 1268 (pp. 199–204).
Camacho-Luna, V., Pizar-Quiroz, A. M., Rodríguez-Hernández, A. A., Rodríguez-Monroy, M., and Sepúlveda-Jiménez, G. (2023). Trichoderma longibrachiatum, a biological control agent of Sclerotium cepivorum on onion plants under salt stress. Biol. Control 180:105168. doi: 10.1016/j.biocontrol.2023.105168
Cao, M., Narayanan, M., Shi, X., Chen, X., Li, Z., and Ma, Y. (2023). Optimistic contributions of plant growth-promoting bacteria for sustainable agriculture and climate stress alleviation. Environ. Res. 217:114924. doi: 10.1016/j.envres.2022.114924
Glickmann, E., and Dessaux, Y. (1995). A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 61, 793–796. doi: 10.1128/aem.61.2.793-796.1995
Hahn, L., Kurtz, C., de Paula, B. V., Feltrim, A. L., Higashikawa, F. S., Moreira, C., et al. (2024). Feature-specific nutrient management of onion (Allium cepa) using machine learning and compositional methods. Sci. Rep. 14:6034. doi: 10.1038/s41598-024-55647-9
Hoyos-Carvajal, L., Orduz, S., and Bissett, J. (2009). Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol. Control 51, 409–416. doi: 10.1016/j.biocontrol.2009.07.018
Kale, R. B., Gavhane, A. D., Thorat, V. S., Gadge, S. S., Wayal, S. M., Gaikwad, S. Y., et al. (2024). Efficiency dynamics among onion growers in Maharashtra: a comparative analysis of drip irrigation adopters and non-adopters. BMC Plant Biol. 24:237. doi: 10.1186/s12870-024-04875-2
Khosa, J. S., McCallum, J., Dhatt, A. S., and Macknight, R. C. (2016). Enhancing onion breeding using molecular tools. Plant Breed. 135, 9–20. doi: 10.1111/pbr.12330
Kredics, L., Büchner, R., Balázs, D., Allaga, H., Kedves, O., Racić, G., et al. (2024). Recent advances in the use of Trichoderma-containing multicomponent microbial inoculants for pathogen control and plant growth promotion. World J. Microbiol. Biotechnol. 40:162. doi: 10.1007/s11274-024-03965-5
Kumar, S., Rai, S., Maurya, D. K., Kashyap, P. L., Srivastava, A. K., and Anandaraj, M. (2013). Cross-species transferability of microsatellite markers from fusarium oxysporum for the assessment of genetic diversity in fusarium udum. Phytoparasitica 41, 615–622. doi: 10.1007/s12600-013-0324-y
Kurrey, D. K., Lahre, M. K., and Pagire, G. S. (2018). Effect of Azotobacter on growth and yield of onion (Allium cepa L.). J. Pharmaco. Phytochem 7, 1171–1175.
Lian, H., Li, R., Ma, G., Zhao, Z., Zhang, T., and Li, M. (2023). The effect of Trichoderma harzianum agents on physiological-biochemical characteristics of cucumber and the control effect against fusarium wilt. Sci. Rep. 13:17606. doi: 10.1038/s41598-023-44296-z
Lichtenthaler, H. K. (1987). “Chlorophylls and carotenoids: pigments of photosynthetic biomembranes” in Methods in enzymology (vol. 148). eds. L. Packer and R. Douce (New York: Academic Press), 350–382.
Louden, B. C., Haarmann, D., and Lynne, A. M. (2011). Use of blue agar CAS assay for siderophore detection. J. Microbiol. Biol. Educ. 12, 51–53. doi: 10.1128/jmbe.v12i1.249
Machuca, A., and Milagres, A. M. F. (2003). Use of CAS-agar plate modified to study the effect of different variables on the siderophore production by aspergillus. Lett. Appl. Microbiol. 36, 177–181. doi: 10.1046/j.1472-765X.2003.01290.x
Mahajan, V., Gupta, A. J., Lawande, K. E., and Singh, M. (2018). Onion improvement in India. J. Allium Res. 1, 7–20.
Mainkar, P., Manape, T. K., Satheesh, V., and Anandhan, S. (2023). CRISPR/Cas9-mediated editing of PHYTOENE DESATURASE gene in onion (Allium cepa L.). Front. Plant Sci. 14:1226911. doi: 10.3389/fpls.2023.1226911
Metwally, R. A., and Al-Amri, S. M. (2020). Individual and interactive role of Trichoderma viride and arbuscular mycorrhizal fungi on growth and pigment content of onion plants. Lett. Appl. Microbiol. 70, 79–86. doi: 10.1111/lam.13246
Mishra, S. (2019). Disease controlling potential of Trichoderma harzianum and Trichoderma viride against purple blotch of onion. J. Pharmacogn. Phytochem. 8, 368–371.
Monte, E. (2023). The sophisticated evolution of Trichoderma to control insect pests. Proc. Natl. Acad. Sci. 120:e2301971120. doi: 10.1073/pnas.2301971120
Nieto-Jacobo, M. F., Steyaert, J. M., Salazar-Badillo, F. B., Nguyen, D. V., Rostás, M., Braithwaite, M., et al. (2017). Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant. Sci. 8:102. doi: 10.3389/fpls.2017.00102
Novello, G., Cesaro, P., Bona, E., Massa, N., Gosetti, F., Scarafoni, A., et al. (2021). The effects of plant growth-promoting bacteria with biostimulant features on the growth of a local onion cultivar and a commercial zucchini variety. Agronomy 11:888. doi: 10.3390/agronomy11050888
Ortega-García, J. G., Montes-Belmont, R., Rodríguez-Monroy, M., Ramírez-Trujillo, J. A., Suárez-Rodríguez, R., and Sepúlveda-Jiménez, G. (2015). Effect of Trichoderma asperellum applications and mineral fertilization on growth promotion and the content of phenolic compounds and flavonoids in onions. Scientia Horti. 195, 8–16. doi: 10.1016/j.scienta.2015.08.027
Pedrero-Mendez, A., Insuasti, H. C., Neagu, T., Illescas, M., Rubio, M. B., Monte, E., et al. (2021). Why is the correct selection of Trichoderma strains important? The case of wheat endophytic strains of T. harzianum and T. simmonsii. J. Fungi 7:1087. doi: 10.3390/jof7121087
Promwee, A., Issarakraisila, M., Intana, W., Chamswarng, C., and Yenjit, P. (2014). Phosphate solubilization and growth promotion of rubber tree (Hevea brasiliensis Muell. Arg.) by Trichoderma strains. J. Agril. Sci. 6:8. doi: 10.5539/jas.v6n9p8
Qi, W., and Zhao, L. (2013). Study of the siderophore-producing Trichoderma asperellum Q1 on cucumber growth promotion under salt stress. J. Basic Microbiol. 53, 355–364. doi: 10.1002/jobm.201200031
Rai, S., Kashyap, P. L., Kumar, S., Srivastava, A. K., and Ramteke, P. W. (2016). Identification, characterization and phylogenetic analysis of antifungal Trichoderma from tomato rhizosphere. Springerplus 5:1939. doi: 10.1186/s40064-016-3657-4
Ramadan, M. S. (2019). Mycorrhizal inoculation and phosphorus fertilizers to improve onion productivity in saline soil. Acta Sci. Pol. Hortorum Cultus 18, 57–66. doi: 10.24326/asphc.2019.1.6
Reetha, S., Bhuvaneswari, G., Thamizhiniyan, P., and Mycin, T. R. (2014). Isolation of indole acetic acid (IAA) producing rhizobacteria of pseudomonas fluorescens and Bacillus subtilis and enhance growth of onion (Allium cepa L.). Int. J. Curr. Microbiol. Appl. Sci. 3, 568–574.
Rodriguez-Hernandez, A. A., Herrera-Alvarez, M., Zapata-Sarmiento, D. H., Becerra-Martínez, E., Rodríguez-Monroy, M., and Sepúlveda-Jiménez, G. (2023). Trichoderma asperellum promotes the development and antioxidant activity of white onion (Allium cepa L.) plants. Hortic. Environ. Biotechnol. 64, 25–39. doi: 10.1007/s13580-022-00467-x
Saadaoui, M., Faize, M., Bonhomme, L., Benyoussef, N. O., Kharrat, M., Chaar, H., et al. (2023). Assessment of Tunisian Trichoderma isolates on wheat seed germination, seedling growth and fusarium seedling blight suppression. Microorganisms 11:1512. doi: 10.3390/microorganisms11061512
Schwyn, B., and Neilands, J. B. (1987). Universal chemical assay for the detection and determination of siderophore. Anal. Biochem. 160, 47–56. doi: 10.1016/0003-2697(87)90612-9
Sehim, A. E., Hewedy, O. A., Altammar, K. A., Alhumaidi, M. S., and Abd Elghaffar, R. Y. (2023). Trichoderma asperellum empowers tomato plants and suppresses fusarium oxysporum through priming responses. Front. Microbiol. 14:1140378. doi: 10.3389/fmicb.2023.1140378
Sharma, S. K., Sharma, M. P., Ramesh, A., and Joshi, O. P. (2012). Characterization of zinc solubilizing Bacillus isolates and their potential to influence zinc assimilation in soybean seeds. J. Microbiol. Biotechnol. 22, 352–359. doi: 10.4014/jmb.1106.05063
Simkin, A. J., Kapoor, L., Doss, C. G. P., Hofmann, T. A., Lawson, T., and Ramamoorthy, S. (2022). The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 152, 23–42.
Singh, P., Singh, R., Madhu, G. S., and Singh, V. P. (2023). Seed biopriming with Trichoderma Harzianum for growth promotion and drought tolerance in rice (Oryza Sativus). Agric. Res. 12, 154–162. doi: 10.1007/s40003-022-00641-8
Siswanto, Y., Sumartono, I., and Ilman, M. (2023). Effectiveness of eco enzyme administration and rhizobium isolation against the growth and production of onions red (Allium ascolonicum L). World J. Adv. Res. Rev. 17, 688–705. doi: 10.30574/wjarr.2023.17.3.0450
Soni, R., and Keharia, H. (2021). Phytostimulation and biocontrol potential of gram-positive endospore-forming Bacilli. Planta 254:49. doi: 10.1007/s00425-021-03695-0
Sridevi, S., and Ramakrishnan, K. (2010). Effects of combined inoculation of AM fungi and Azospirillum on the growth and yield of onion (Allium cepa L.). J. Phytology 2, 88–90.
Stewart, A., and Hill, R. (2014). “Applications of Trichoderma in plant growth promotion” in Biotechnology and biology of Trichoderma. eds. V. K. Gupta, M. Schmoll, A. Herrera-Estrella, R. S. Upadhyay, I. Druzhinina, and M. G. Tuohy (Amsterdam: Elsevier), 415–428.
Syamsiyah, J., Herdiansyah, G., and Hartati, S. (2023) Use of Trichoderma as an effort to increase growth and productivity of maize plants. In IOP conference series: Earth and environmental science (Vol. 1165), 012020, IOP Publishing, doi: 10.1088/1755-1315/1165/1/012020
Tamura, K., Stecher, G., and Kumar, S. (2021). MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027. doi: 10.1093/molbev/msab120
Tyskiewicz, R., Nowak, A., Ozimek, E., and Jaroszuk-Ściseł, J. (2022). Trichoderma: the current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 23:2329. doi: 10.3390/ijms23042329
Uddin, M. N., Rahman, U. U., Khan, W., Uddin, N., and Muhammad, M. (2018). Effect of Trichoderma harzianum on tomato plant growth and its antagonistic activity against Phythium ultimum and Phytopthora capsici. Egypt. J. Biol. Pest Control 28, 1–6. doi: 10.1186/s41938-018-0032-5
Vojnović, Đ., Maksimović, I., Horecki, A. T., Žunić, D., Adamović, B., Milić, A., et al. (2023). Biostimulants affect differently biomass and antioxidant status of onion (Allium cepa) depending on production method. Horticulturae 9:1345. doi: 10.3390/horticulturae9121345
Vojnović, Đ., Maksimović, I., Tepić Horecki, A., Milić, A., Šumić, Z., Žunić, D., et al. (2024). Biostimulants improve bulb yield, concomitantly affecting the Total Phenolics, flavonoids, and antioxidant capacity of onion (Allium cepa). Horticulturae 10:391. doi: 10.3390/horticulturae10040391
Younes, N. A., Anik, T. R., Rahman, M. M., Wardany, A. A., Dawood, M. F., Tran, L. S. P., et al. (2023). Effects of microbial biostimulants (Trichoderma album and Bacillus megaterium) on growth, quality attributes, and yield of onion under field conditions. Heliyon 9:e14203. doi: 10.1016/j.heliyon.2023.e14203
Keywords: onion, Trichoderma , T. asperellum , T. longibrachiatum , bulb yield, bioformulation, PGPA screening, soil amendments
Citation: Dutta R, Kumar S, Jayalakshmi K, Radhakrishna A, Bhagat K, Manjunatha Gowda DC, Karuppaiah V, Bhandari HR, Bomble R, Gurav V, Mahajan V and Singh M (2024) Potential of Trichoderma strains to positively modulate plant growth processes and bulb yield in Rabi onion. Front. Sustain. Food Syst. 8:1427303. doi: 10.3389/fsufs.2024.1427303
Edited by:
Surendra K. Dara, Oregon State University, United StatesReviewed by:
Chris Greer, University of California Agriculture and Natural Resources, United StatesMichael Brownbridge, BioWorks Inc., United States
Copyright © 2024 Dutta, Kumar, Jayalakshmi, Radhakrishna, Bhagat, Manjunatha Gowda, Karuppaiah, Bhandari, Bomble, Gurav, Mahajan and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ram Dutta, cmR1dHRhLmljYXJAZ21haWwuY29t; Satish Kumar, c2F0aXNoLmt1bWFyMTBAaWNhci5nb3YuaW4=; K. Jayalakshmi, amF5YWxha3NobWlwYXRAZ21haWwuY29t; A. Radhakrishna, YXIua3Jpc2huYUBpY2FyLmdvdi5pbg==; Vijay Mahajan dmlqYXkubWFoYWphbkBpY2FyLmdvdi5pbg==
 Ram Dutta
Ram Dutta Satish Kumar
Satish Kumar K. Jayalakshmi
K. Jayalakshmi A. Radhakrishna1*
A. Radhakrishna1* D. C. Manjunatha Gowda
D. C. Manjunatha Gowda V. Karuppaiah
V. Karuppaiah