- 1Research Center for Agroindustry, National Research and Innovation Agency, Bogor, Indonesia
- 2Research Center Estate Crop, National Research and Innovation Agency, Bogor, Indonesia
- 3Directorate of Food Safety and Quality Standards Formulation, National Food Agency (NFA), Jakarta, Indonesia
Indonesia’s vanilla industry requires continuous efforts to enhance its quality and safety to ensure its sustainability. The objective of this research is to identify the chemical composition, production constraints, and post-harvest practices impacting vanilla quality. This research investigates the quality of Indonesian vanilla through in-depth interviews with vanilla growers and exporters, as well as quality analysis in the laboratory. Purposive sampling targeted key producers across seven provinces from May to August 2023. Vanilla samples were collected for chemical analysis after a direct interview with each producer. The result shows that the physical and chemical qualities of vanilla products fulfill Indonesian standards (SNI). The vanillin content ranged from 1.21 to 3.50. Establishing universal standards requires considering various vanilla varieties and determining minimum quality attribute values. Due to the risk of theft, vanilla pods are often picked when they are less than 9 months old, before the fruit is fully ripe. This practice results in a drop in quality, particularly in vanillin concentration. Sustainable growth, stakeholder involvement, inclusive business models, education, and contract farming arrangements help navigate challenges like theft and premature harvesting. Advancements in processing methodologies and the selection of clonal materials reinforce the industry’s commitment to enhancing quality.
1 Introduction
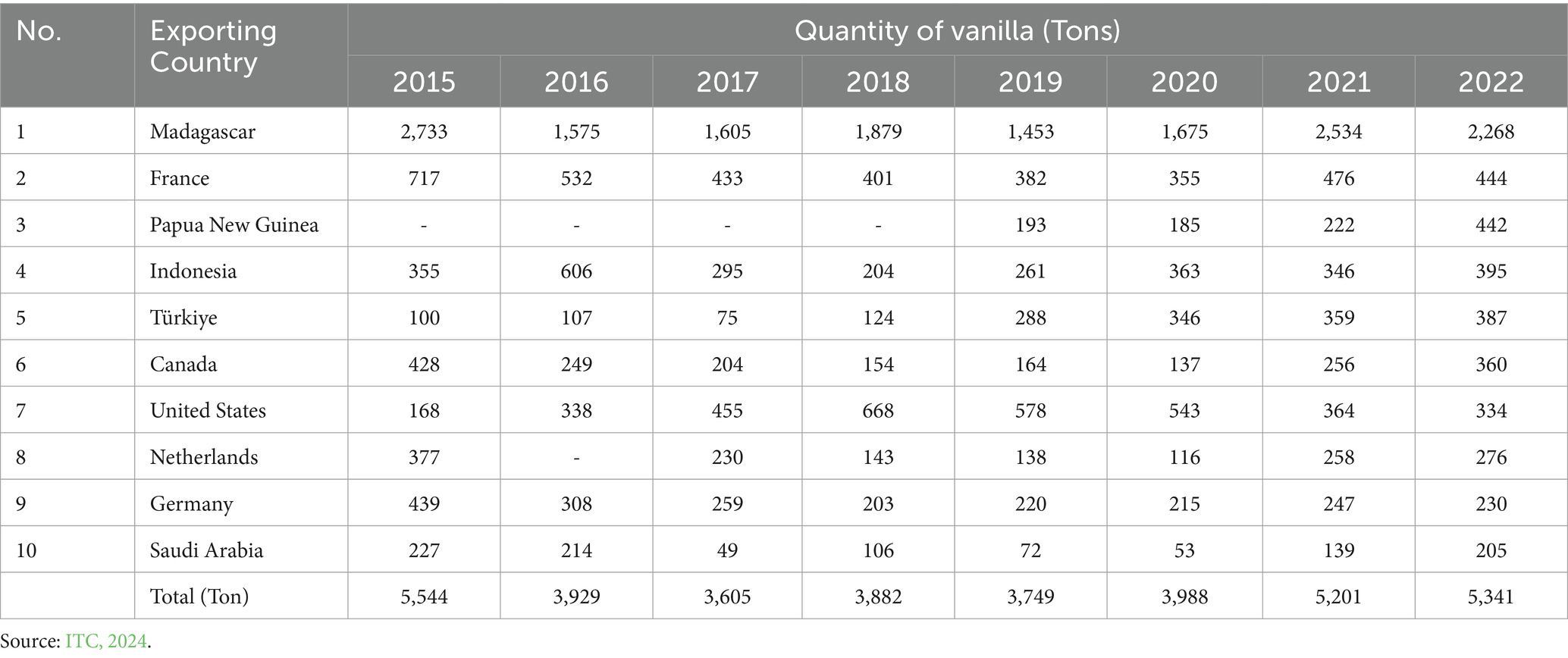
Vanilla (Vanilla planifolia Andrews) is the most important natural flavor used in industry, including the food, beverage, pharmaceutical, cosmetic, and tobacco industries. The main constituent of vanilla is vanillin; 4-hydroxy-3-methoxy benzaldehyde (Banerjee and Chattopadhyay, 2019). Indonesian vanilla has superior characteristics in the form of a strong and long-lasting aroma so that it has a high selling value and provides large profits for farmers and plantation managers (Udarno and Hadipoentyanti, 2009). The price of vanilla from Indonesia grade A is valued quite high, namely $140–$200/kg in 2022 which is still slightly lower than the price of vanilla on the global market with an average of EUR 270.40/kg for vanilla extract and EUR175.56/kg for whole vanilla in 2022 (Anwar, 2023). Indonesia is the fourth largest exporter in the world after Madagascar, France, and Papua New Guinea in 2022 which is presented in the Table 1.
The better quality of Indonesian vanilla, referred to as Java vanilla, surpasses that of Mexican vanilla (Guntoro and Fathoni, 2020). However, between 2005 and 2016, the popularity of vanilla decreased due to a decline in its quality caused by criminals who cheated it with iron sand and nails to increase its weight. They also mixed it with inferior vanilla, resulting in its blacklisting in the international market and a subsequent price decline. In 2017, there was a surge in demand for Indonesian vanilla in both the US and Europe, accompanied by a fall in production in key producing nations like Tahiti and Madagascar, and apprehensions that natural disasters would interrupt the supply of vanilla (Guntoro and Fathoni, 2020).
Efforts to increase vanilla production and marketing must be supported by appropriate vanilla cultivation and processing procedures so that chemical quality, especially vanillin content, is still high enough. The pattern of guidance and assistance to farmers needs to be carried out intensively, openly and providing appropriate prices. It is crucial to evaluate the suitability of cultivation methods through recommendations to prevent mistakes involving picking prematurely ripe vanilla. It is important to understand how farmers and exporters work together to source raw materials and complete the vanilla processing to spot processing mistakes and learn about the product’s quality.
The dynamics of international trade policy have been regulated by the WTO (World Trade Organization) by issuing Codex food quality standards. Codex Committee on Spices and Culinary Herbs (CCSCH) is one of the forums in CAC (Codex Alimentarius Commission) which is the world standards body under FAO/WHO for the category of dried and dehydrated spices and seasonings whether in whole, ground, crushed or crushed form. One of the standards under discussion in the forum is the Draft Standard for Vanilla. To establish this standard, current data on the quality of vanilla products manufactured by farmer-exporters in Indonesia are required. The objectives of this research are to identify the chemical quality attributes, manufacturing constraints, and post-production processes of vanilla. This information will be utilized to develop international vanilla standards and to formulate policy recommendations about the development of Indonesian vanilla.
2 Materials and methods
This study employed a survey method, collecting processed vanilla samples and chemically analyzing them in the laboratory. The research locations were chosen purposefully and using snowball sampling, considering the existence of main vanilla exporters as experts in several production centers. The criteria for the selected exporters were that they had more than 10 years of export experience, had a vanilla plantation and had fostered farmers in their area and were recognized by the local government. The snowball sampling technique was used to determine four farmers who were directly involved in working relationships with exporters because the total population of farmers was not known with certainty. Combining snowball and purposive sampling can provide a more complete picture of production patterns and identify key characteristics of interest.
The study was carried out between May and August 2023 in the provinces of North Sumatra, Central Java, East Java, East Nusa Tenggara (NTT), West Nusa Tenggara (NTB), Bali, and North Sulawesi. A total of 68 individuals, including 8 exporters and 60 farmers, were interviewed using a structured questionnaire. Selected exporter and farmer provided a vanilla sample, which was then assessed for quality. The number of vanilla samples for each quality class was four samples which were then statistically tested. Statistical data analysis was carried out with a one-way analysis of variance (ANOVA) using IBM SPSS 26. Significant differences among samples were compared using the Duncan test. Data were expressed as means ± SD and p < 0.05 represented a statistically significant difference.
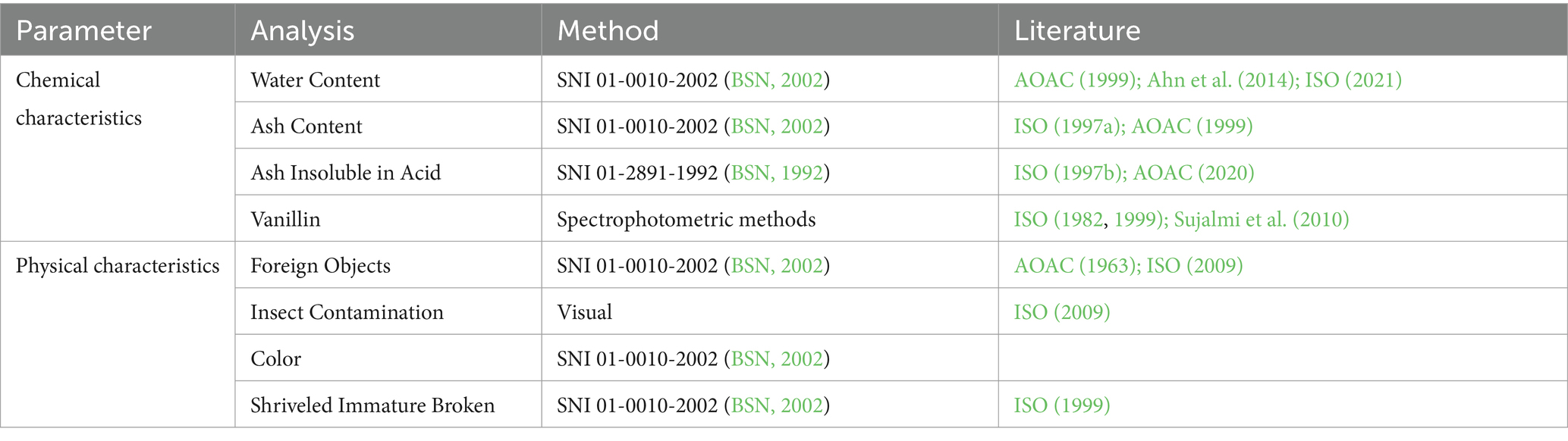
The acquired vanilla samples were subsequently examined in a certified laboratory. Laboratory tests are conducted to determine the chemical and physical quality requirements by the Indonesian National Standards (SNI) outlined in Table 2. The collected and analyzed data will be presented in a data table and described descriptively.
3 Results and discussion
3.1 Chemical and physical quality of Indonesian cured vanilla
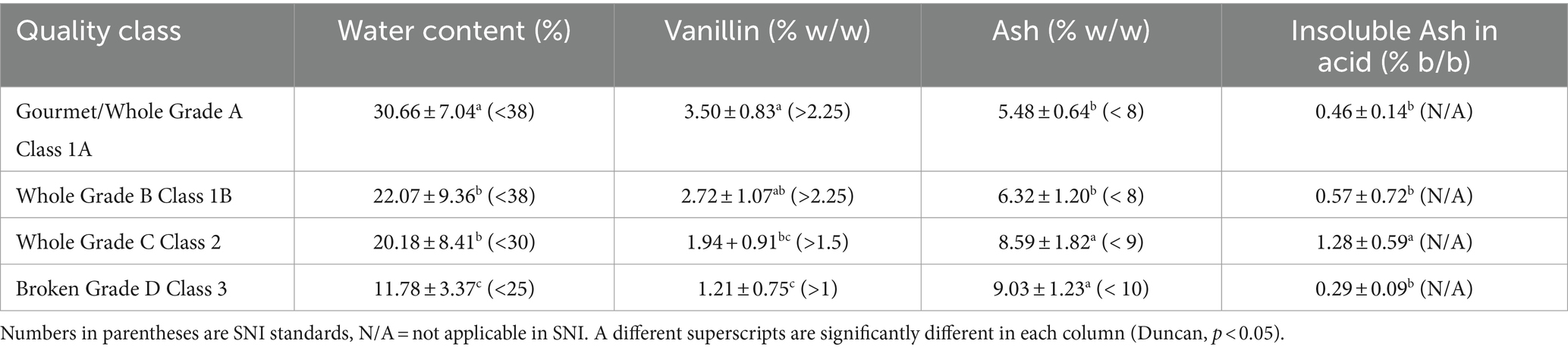
Indonesian vanilla (V. planifolia) generally have high levels of vanillin than other vanilla type, Vanillin accounts for 80% of total aroma compounds in V. planifolia and 50% of total aroma compounds in V. tahitensis (Brunschwig et al., 2009). Vanillin is the main aroma compound in vanilla and plays a significant role in its quality. Other non-volatile compounds that are frequently misidentified as vanillin when a UV spectrophotometer is employed include hydroxybenzoic acid, vanillic acid, and p-hydrozybenzaldehyde. These compounds, in addition to various volatile compounds, have an impact on the sensory attributes of vanilla (Havkin-Frenkel and Belanger, 2018). This higher vanillin content contributes to the intense, sweet, and slightly woody aroma associated with Indonesian vanilla. Water content also plays an important role in vanilla quality, affecting both its flavor and shelf life. Excessive moisture can lead to mold growth and deterioration of the beans, good quality vanilla beans should have a moisture content around 25–30% which facilitates enzymatic reactions during the curing process for converting glucovanillin (a tasteless precursor) into vanillin, Table 3 show chemical data from several classes of vanilla, namely water content, vanillin, ash, and insoluble ash in acid.
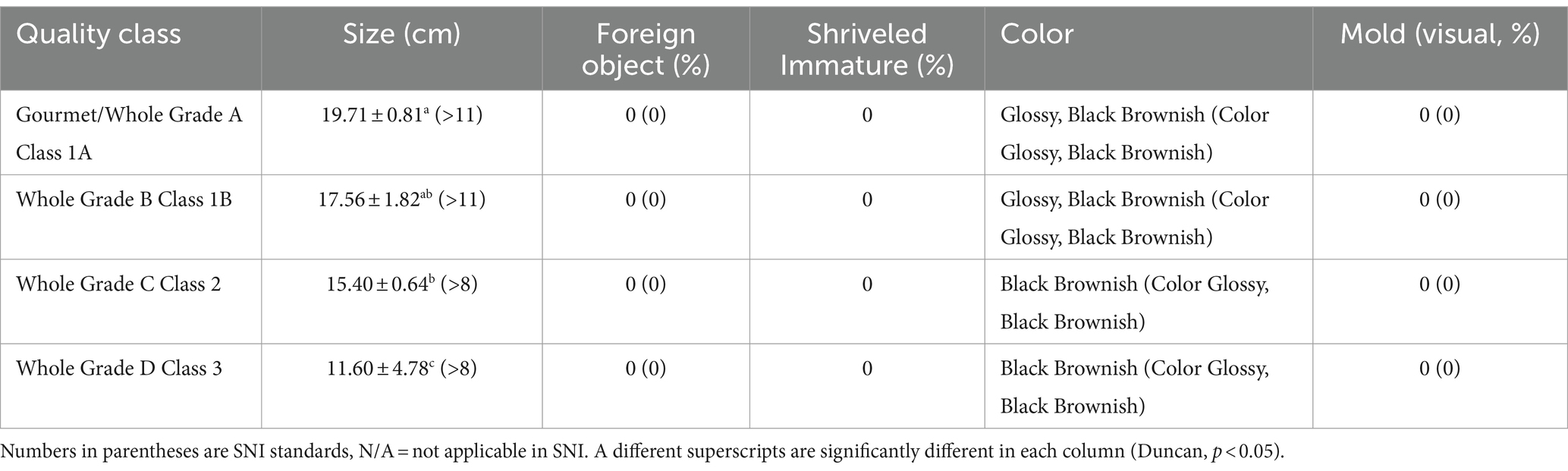
The water, vanillin, and ash content values of the samples in Table 3 meet the Indonesian standards (SNI). According to ISO 5565-1:2017 for Vanilla beans, the ash content should not exceed 7%, with a maximum of 1% for ash that is insoluble in acid, and any value below whole grade B is considered unsuitable. Ash concentration and insoluble ash in acid are both symptoms of possible adulteration and improper processing methods. Some collectors at the sub-district level still accept vanilla made by farmers without following the allowed technique, resulting in a high level of Insoluble Ash in the acid value. The SNI guidelines also control the physical attributes of vanilla like size, color, and mold percentage, but do not provide explicit regulations for Shriveled Immature and quantity of mold. Table 4 displays the physical features of various types of vanilla found in Indonesia.
The vanilla produced in Table 4 demonstrates that the best quality class is distinguished physically by longer vanilla fruit length and a higher vanillin content compared to the lower quality class. According to a study conducted by Adawiyah et al. (2022), it was observed that the aromatic characteristics of vanilla beans exhibited variations depending on their size and curing conditions. Notably, beans weighing more than 15 grams or measuring 19.6 cm in length demonstrated the maximum concentration of vanillin.
3.2 Vanilla cultivation performance
Vanilla belongs to the same family as orchids (Orchidaceae), which have a high value in commerce. Cultivation strategies such as adopting superior cultivars, adhering to cultivation standard operating procedures, fertilizing, and controlling plant insect species have a significant impact on vanilla production and quality.
The Indonesian Ministry of Agriculture introduced three national varieties to the vanilla community: Vania 1, Vania 2, and Alor. Apart from that, two new types have been released: Sovania Agribun and Hivania Agribun. Most farmers know the names of the cultivars they plant, although a minority of farmers (37.5%) are unfamiliar of the names of the types they plant. Farmers in Central Java, Bali, and North Sumatra have cultivated the Vania 1 and Vania 2 cultivars. In the eastern region, farmer groups purchase certified seeds from Central Java farmers. However, seed gardens in Southeastern Minahasa (North Sulawesi) that are officially certified by the Ministry of Agriculture offer farmers an alternative source of seeds.
The vanilla plant is a monocot with a main root that branches out at the base of the stem and spreads into the topsoil layer. The stems are gnarled, sinuous, fragile, and infrequently bifurcate. The leaves are single, oblong, and elongated, measuring around 2 to 25 cm in length and 2 to 8 cm in width. The blooms are organized in succession, usually consisting of 6 to 15 blossoms. Human interaction is essential for achieving optimal results in the important mating process of vanilla growth. The stigma of the vanilla flower is obscured by the labellum, which obstructs natural pollination. Additionally, the anther, which harbors two granules of pollen, is elevated above the stigma. A distinctive characteristic of the vanilla flower is the presence of an adhesive liquid on the stigma; this liquid enables pollen to adhere to the stigma immediately, facilitating pollination.
Approximately 71.4% of farms utilize organic fertilizer, whereas 14.3% do not use any fertilizer. Various types of organic fertilizer are used, including cattle compost and a blend of dried gamal leaves, animal manure, bamboo, and coconut fiber. Farmers utilize a fertilizer dose of 0.5–1 gram per tree. Farmers favor using organic compost due to its plant-friendly nature, lack of burning impact, easy accessibility from the garden, longer-lasting effects compared to chemical fertilizers, and alignment with purchasers’ preferences. Some NTB and North Sulawesi farmers possess organic certifications from local and government certifications.
Farmers’ vanilla planting patterns are generally monoculture. This planting strategy runs the risk of lowering farmers’ income levels because, in times of low prices for vanilla, farmers are often less motivated to maintain it. As a result, climbing trees go unpruned, and wild plants proliferate so that the microclimate changes, especially the light intensity and humidity factors (Rosman, 2010). Low light intensity will impair flowering; vanilla requires 35–55% light intensity. High humidity promotes the establishment of stem rot disease.
Fusarium oxysporum sp. vanillae is the pathogen that causes vanilla stem rot disease. Farmers utilize biological materials sprayed on the plants (42.8%) and fungicides (14.4%) to control the assault, while other farmers use mechanical action by chopping off the afflicted sections. During low disease outbreaks, farmers kill plants and replace them with healthy plants without changing the planting area.
Vanilla harvesting occurs when the plants reach 9 months of age when the color changes to faded green and yellow tips and the fruit size has reached its maximum. Generally, farmers know that the quality of vanilla is determined by the age at which the fruit is harvested. However, the obstacle faced by farmers in harvesting vanilla plants is the theft of vanilla pods, so some farmers choose to harvest younger vanilla plants at 7 months. The impulse to collect vanilla while it is still young is particularly strong since farmers need money to feed their families. Respondents reported that the age of the fruit during harvest ranged from less than 7 months (14.29%), 7 months (25.80%), to 9 months (57%). The impact of harvesting in less than 7 months is that the quality of the vanilla fruit produced is not good because the vanilla content is still low. Similar to berries and various other fruits, vanilla stops to ripen as soon as it is harvested. Some farmers get around planting in the forest and guard it 24 h a day on the land and place it on a tree/high climbing pole so that thieves will have difficulty harvesting vanilla pods. Figure 1 shows Vanilla harvesting from farmers and cultivation in the shade house.
3.3 Vanilla processing performance
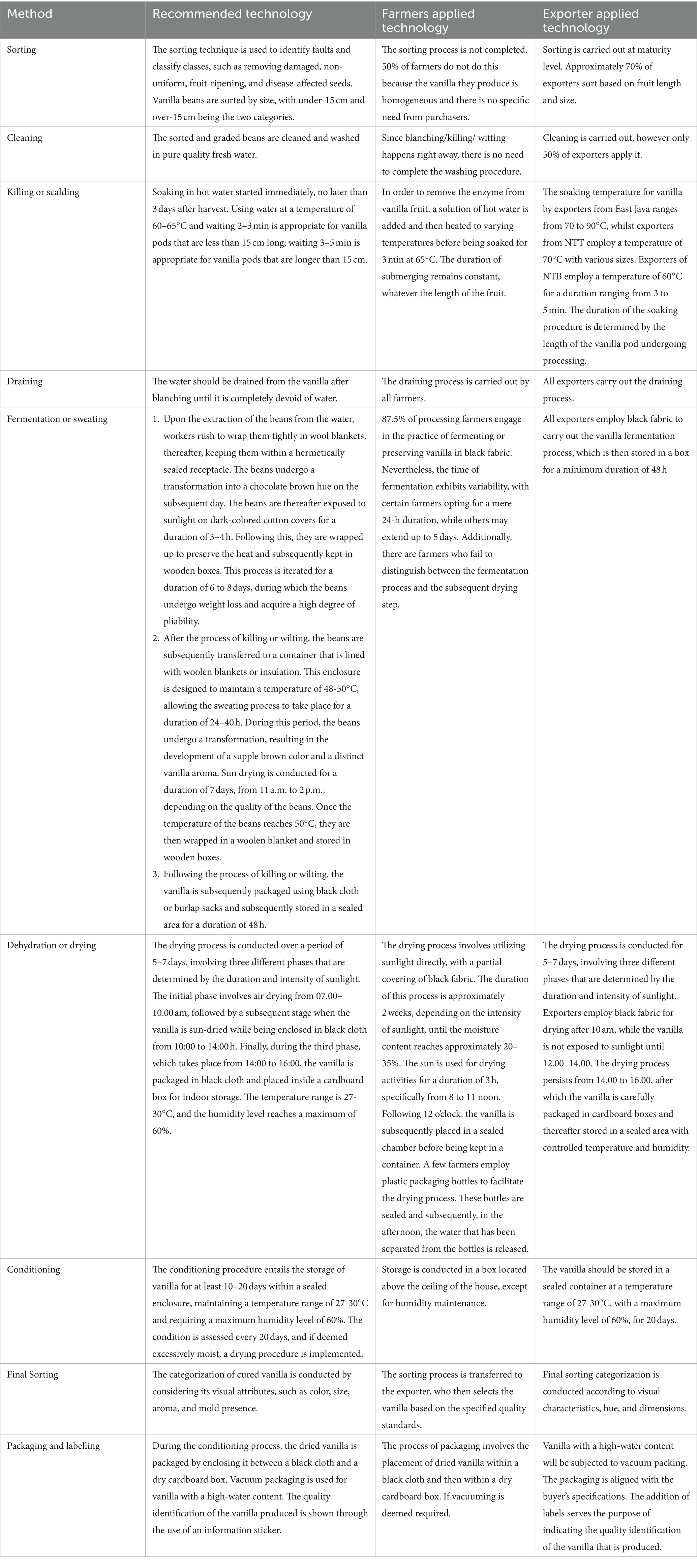
The vanilla raw material used by both farmers and exporters is obtained domestically from the Vanilla planifolia Andrews type. Vanilla raw materials are gathered from their own gardens (25%), farmers in their group (37.5%), and farmers outside their group (37.5%). Exporters occasionally bring in Vanilla tahitensis from Papua New Guinea. Aside from the type and variety of vanilla, the process of curing vanilla affects the quality of the vanilla produced. Hot water treatment on curing bean produced brown, good aroma and flavour than the other procedures used to kill the beans, such as freezing and scratching (Krishnakumar et al., 2007; Kumar and Balamohan, 2013). According to profiling studies on vanilla beans, a mild hot water blanching technique followed by sweating at 35–45°C and rapid drying results in cured beans with an attractive appearance and pleasing aroma (Van Dyk et al., 2010). Vanilla curing technology was identified by comparing recommended curing technology steps to establish its application at the farmer and exporter levels. Table 5 shows the various curing technologies utilized by farmers and exporters in Indonesia.
In general, the curing process has four stages: scalding, sweating/fermentation, drying, and conditioning. Figure 2 shows scalding, curing and drying vanilla in Indonesia. Traditional curing methods’ efficiency in vanillin extraction is influenced by processing temperatures, weather conditions, and aromatic compound loss, resulting in lower-quality vanilla extract (Pardío et al., 2018). Following the scalding procedure, the vanilla bean will reach a high temperature and subsequently be wrapped in cloth packing within a black box for an extended period to achieve adequate heat, an event referred to as the sweating/fermentation process. Glucovanillin (GV) and glucovanillic acid (GVA) under conventional curing conditions proceeded hydrolysis, resulting in the formation of vanillin and vanillic acid (Dignum et al., 2002). Vanilla bean curing involves β-glucosidase activity and vanillin concentration, reaching maximum levels after 10 cycles of drying and sweating (Peña-Barrientos et al., 2023). To optimize the glucovanillin hydrolysis process, pectinase and glucosidase enzymes were used to increase vanillin concentration (Marc, 1992). The hydrolysis of glucovanillin in vanilla beans is governed by the processes of cellular compartmentation and tissue degradation. Up to 50% of vanillin that is entrapped in cellulose can be extracted through enzymatic pretreatment using cellulytic enzymes (Waliszewski et al., 2007).
Dry matter content is strongly correlated with vanillin fragrance content, although lower dry matter and total soluble sugars in green fruit influence minor component concentration such as p-hydroxybenzoic acid, vanillic acid and p-hydroxybenzaldehyde (Sánchez-Galindo et al., 2018). Sánchez-Galindo study also discovered that harvesting 252-day-old fruit at a lower temperature affected aromatic balance. Similar to that, freezing and chilling green vanilla has been observed to lower β-glucosidase activity, which in turn lowers vanillin synthesis (Dignum et al., 2001). The reason for the decrease in vanillin concentration during the 96-h curing process is considered to be caused by the presence of peroxidase (POD) activity, which can use vanillin as a substrate under conditions similar to hydrolysis at 50°C and pH 5.0 (Pardío et al., 2018). This explains why vanillin concentration was not complete at the end of the process.
3.4 Prospective policies that support growth in the Indonesian vanilla industry
Sustainable growth, long-term development, and active involvement of stakeholders are the key priorities for the Indonesian vanilla industry. The Indonesian vanilla industry faces challenges such as theft and premature harvesting, which compromises the quality of beans and disrupts the curing process. To combat this, an inclusive business model, education and training initiatives, contract farming arrangements, and strict enforcement measures are essential. The primary objective of contract farming arrangements (CFA) in the Madagascar vanilla chain is to meet customer demands and handle societal issues. Major firms prioritize risk management and cooperative support in combating theft (Ralandison, 2021). In Indonesia, integrating middlemen/exporters and farmers into the value chain in the provinces of North Sumatra, North Sulawesi and West Nusa Tenggara (NTB) encourages direct involvement such as implementing education and training programs for farmers. The role of exporters and industry can improve efficiency, productivity, and profitability while maintaining the integrity of Indonesian vanilla. Local government policy through the establishment of self-supporting rural agricultural training center (P4S) institutions in Central Java Province supports the vanilla industry by providing training programs for farmers, establishing cooperative institutions, and investing in processing facilities. Boxy et al. (2020) proposed three supply chain sustainability policies, with a focus on empowering and expanding agribusiness, revitalizing curing processes, and price stability.
The lack of effective extension services and the slow acceptance of new technology are two of the biggest challenges that Indonesian vanilla producers confront. The integrated extension system for vanilla agribusiness development is expected to encourage the implementation of GAP and processing that maintains quality and build a sustainable market (Wulandari and Ardana, 2021). The system should be designed based on knowledge support services and reinforced by agribusiness support services, policy, market opportunities, and industry organizations. Recently, to close this gap, exporters have helped farmers from the cultivation process to harvest and have supported farmers financially so that harvest time can be optimal. The vanilla processing process is a crucial aspect of the responsibilities undertaken by exporters and farmer associations, as it involves facilitating the sale of vanilla between farmers and overseas customers. Long-term sustainability of Indonesia’s vanilla sector can be ensured by exporters’ endorsement of sustainable agricultural methods and promotion of collaboration.
Vanilla processing stands as a critical phase in the production chain, influencing the quality and value of the final product. In the vanilla processing process in Indonesia, both farmers and exporters employ the bourbon method, which involves immersing vanilla beans in hot water at a temperature of 63–65°C for a duration of 3–5 min. Subsequently, the boiling water is swiftly drained, and the beans are then placed in a wooden container that is covered with a black fabric covering. While the processing technique remains consistent, there may be variations in processing capacities among different manufacturers. Standardizing processing techniques is essential for ensuring consistent quality throughout the business. This needs the presence of suitable equipment and infrastructure and is made easier with government assistance.
Global demand and price volatility pose substantial dangers and opportunities for Indonesian vanilla producer. Indonesia is one of the most important producers of vanilla in the world. Indonesian vanilla production has the same type/species as vanilla from Madagascar, namely Vanilla planifolia Andrews. Meanwhile, Papua New Guinea produces vanilla from the Vanilla tahitensis species. The different types of vanilla cause each producer to have different market segments. Madagascar’s supply and price issues affect Indonesia’s demand, as seen in Cyclone Gamane and the November 2023 general election, which sometimes led to price increases despite market oversupply. Prices in the vanilla market can fluctuate dramatically, with worldwide prices capable of tripling in a short period of time due to a range of variables such as supply shortages and speculative trading (Neimark et al., 2019). During price spikes, farmers’ economic power is diminished as they become more reliant on collectors and dealers who set market conditions (Wulandari, 2021). One potential technique for reducing price volatility is to convert vanilla products into certified or branded goods. The digital marketing and e-commerce can help stabilize prices by eliminating uncertainty and increasing product value (Wahyudi et al., 2021).
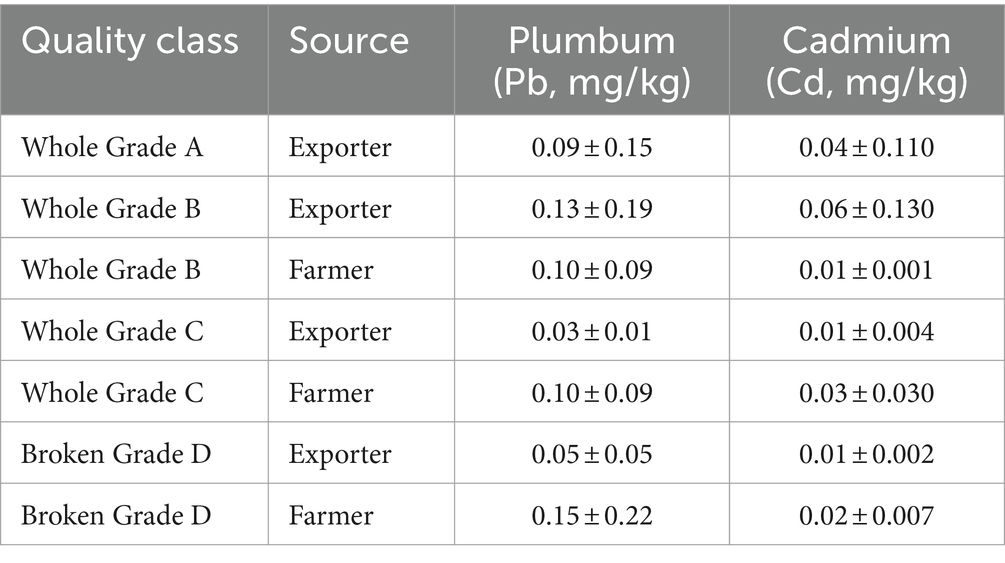
Dewan Vanili Indonesia (Indonesian Vanilla Council-IVC) is an organization that can act as an intermediary to help and facilitate collaboration, communication, and required actions. In partnership with the Ministry of Agriculture, smallholder farmers can receive help through training and consulting if there are concerns with their agribusiness activities. Indonesian exporters may set themselves out with their products as authentic, distinct, and different by leveraging the “clean-green-safety” paradigm. Indonesian vanilla (Vanilla planifolia) is known to have high levels of vanillin, had unique aroma and safety from the use of pesticides according to the test results in Table 6.
The Indonesian vanilla industry prioritizes selecting better and uniform clonal material for its production. Since 2018, three superior varieties have been released, namely Vania (Indonesian Vanilla) 1, Vania 2 and Vania Alor. The characteristics of the Vania 1 variety are that it has branched fruit bunches so that it has a wet pod production of 6.53–8.91 tons/ha and a dry pod production of 1.83–2.56 tons/ha with a vanillin content of 2.81–3.25%. While Vania 2 has a single bunch with a wet pod production of 5.37–8.29 tons/ha and a dry pod production of 1.54–2.19 tons/ha with a vanillin content of 2.98–3.16% (Tjahjana et al., 2011). The Alor variety has the characteristics of single and branched bunches and is a landrace of the Bali population with a wet pod production of 3.55–4.81 tons / ha with a dry pod production of 0.6–0.7 tons / ha with a vanillin content of 2.32–2, 85%. The superior variety has spread to almost all production center areas. In East Java and Central Java, many Vania 1 varieties are planted, in NTB there are Vania 1 and Vania 2, in NTT almost all farmers use the Alor variety because there are many seed source gardens for the Alor variety there, in North Sulawesi Vania 2 is widely developed, while in North Sumatra Vania 2 and local varieties are widely planted.
World vanilla production’s slow growth is attributed to stem rot diseases caused by fusarium oxysporum sp. fungus, which is difficult to control due to its ground presence (Hernández-Hernández, 2010). This stem rot disease is very easily transmitted to another plant if climate conditions allow (high humidity). The three varieties that have spread in Indonesia are not resistant to stem rot disease. In 2022, Indonesian researchers developed new superior varieties of Sovania Agribun and Hivania Agribun with mutase induction technology and hybridization. The advantages of both varieties are resistant to stem rot disease (Pribadi et al., 2021). However, this new type has not been introduced in vanilla production centers due to the licensing process. Farmers must emphasize using premium vanilla seeds and make sure they are free of pests and diseases in order to produce high-quality vanilla. To guarantee the production of premium vanilla, they should also enhance growing practices, such as appropriate fertilizer, insect control, and light and humidity levels. According to Wahyudi et al. (2023), shade houses offer several benefits for sustainable vanilla farming, such as increased productivity and quality, reduced risk of theft and microenvironmental hazards, lower maintenance costs, and simpler disease control.
4 Conclusion
The Indonesian vanilla industry is known for its high-quality vanilla, with high levels of vanillin contributing to their intense aroma. The widespread distribution of the Vanilla planifolia species in Indonesia leads to higher vanillin concentration than other vanilla species such as Vanilla tahitensis. Water content affects the flavor of vanilla beans, with an optimal range of 25–30% required to avoid mold growth and promote the enzymatic conversion of glucovanillin to vanillin during the curing process. Physically, the best quality vanilla is identified by longer pod lengths and a glossy, black-brownish appearance. Variations in curing techniques between farmers and exporters, including differences in blanching temperatures, fermentation times, and drying processes, impact the final quality of vanilla. The Indonesian vanilla industry faces challenges, such as theft, premature harvesting, and diseases like stem rot caused by Fusarium oxysporum. Sustainable growth of the industry requires improved cultivation practices, adoption of superior cultivars, effective disease management, and robust processing techniques. Policies to support the industry include promoting contract farming, providing education and training for farmers, and enhancing processing infrastructure. The involvement of industry stakeholders and local government initiatives, such as the establishment of self-supporting rural agricultural training centers, are crucial for sustaining the quality and market competitiveness of Indonesian vanilla.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The patients/participants [legal guardian/next of kin] provided written informed consent to participate in this study.
Author contributions
SM: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. YR: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. NS: Data curation, Formal analysis, Investigation, Resources, Validation, Writing – original draft. AA: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. EH: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – review & editing. PA: Investigation, Project administration, Resources, Validation, Writing – original draft. AS: Data curation, Investigation, Resources, Visualization, Writing – original draft. EK: Data curation, Formal analysis, Investigation, Resources, Validation, Writing – original draft. ML: Data curation, Investigation, Validation, Writing – original draft. SS: Conceptualization, Project administration, Resources, Supervision, Validation, Writing – review & editing, Data curation. YE: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing, Methodology, Resources. HE: Data curation, Validation, Writing – original draft, Formal analysis. MD: Investigation, Resources, Visualization, Writing – review & editing. ES: Data curation, Formal analysis, Project administration, Writing – original draft. LL: Formal analysis, Writing – original draft, Methodology, Supervision, Visualization. MH: Supervision, Validation, Writing – review & editing, Conceptualization, Data curation, Funding acquisition, Project administration.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project is supported by the Indonesian National Food Agency (NFA) in 2023 to support the draft international vanilla standards at the CAC (Codex Alimentarius Commission) forum. We would like to express our sincere gratitude to the NFA and The National Research and Innovation Agency (BRIN) for their funding assistance and close collaboration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adawiyah, D. R., Reri, P. Y. D., and Lioe, H. N. (2022). “The effect of bean size and curing process on aroma profile and vanillin/Glucovanillin content of Indonesian cured Vanilla beans.” in The 2nd SEAFAST international seminar (2nd SIS 2019) - facing future challenges: Sustainable food safety, quality and nutrition. SCITEPRESS – Science and Technology Publications, Lda. pp. 106–111.
Ahn, J. Y., Kil, D. Y., Kong, C., and Kim, B. G. (2014). Comparison of oven-drying methods for determination of moisture content in feed ingredients. Asian Australas. J. Anim. Sci. 27, 1615–1622. doi: 10.5713/ajas.2014.14305
Anwar, C. F. (2023). Ekspor Vanili Indonesia, Potensi Tumbuhan “Si Emas Hitam” yang Menjanjikan. Press Release Lembaga Pembiayaan Ekspor Indonesia (LPEI). Available at: https://www.indonesiaeximbank.go.id/public-information/ekspor-vanili-indonesia-potensi-tumbuhan-si-emas-hitam-yang-menjanjikan (Accessed April 17, 2024).
AOAC (1963). AOAC 960.37-1963, plant material (foreign) in vanilla extract. Association of Official Analytical Chemists.
AOAC (1999). Official methods of analysis. 16th edition, 5th revision Edn. Wasington DC: Association of Official Analytical Chemists.
AOAC (2020). Official methods of analysis. 17th Edn. Arlington, Virginia: Association of Official Analytical Chemists.
Banerjee, G., and Chattopadhyay, P. (2019). Vanillin biotechnology: the perspectives and future. J. Sci. Food Agric. 99, 499–506. doi: 10.1002/jsfa.9303
Boxy, M., Wuryandari, N. E., and Permana, D. (2020). “How to the Prospect of the supply chain performance stability and implication of the sustainability of Indonesia’s Vanilla origin commodities?” in Proceedings of the international conference on environmental and Technology of law, business and education on post Covid 19, ICETLAWBE 2020, 26 September 2020. Bandar Lampung.
Brunschwig, C., Collard, F. X., Bianchini, J.-P., and Raharivelomanana, P. (2009). Evaluation of chemical variability of cured Vanilla beans (Vanilla tahitensis and Vanilla planifolia). Nat. Prod. Commun. 4:1934578X0900401
Dignum, M. J. W., Kerler, J., and Verpoorte, R. (2001). β-Glucosidase and peroxidase stability in crude enzyme extracts from green beans of Vanilla planifolia Andrews. Phytochem. Anal. 12, 174–179. doi: 10.1002/pca.578
Dignum, M. J. W., Kerler, J., and Verpoorte, R. (2002). Vanilla curing under laboratory conditions. Food Chem. 79, 165–171. doi: 10.1016/S0308-8146(02)00125-5
Guntoro, T., and Fathoni, M. A. (2020). Teknik Terbaru Budidaya Vanili di Pekarangan Rumah dan Pot: Kawah Media, Jakarta Selatan.
Havkin-Frenkel, D., and Belanger, F. C. (2018). Handbook of vanilla science and technology. 2nd Edn. Hoboken, NJ, USA: John Wiley & Sons.
Hernández-Hernández, J. (2010) in Handbook of Vanilla science and technology. eds. D. Havkin-Frenkel and F. C. Belanger (Hoboken, New Jersey: John Wiley & Sons).
ISO (1999). ISO 5565-2:1999 Vanilla [Vanilla fragrans (Salisbury) Ames] part 2: Test methods. Geneva.
ISO (2009). ISO 927:2009 spices and condiments determination of extraneous matter and foreign matter content. Geneva.
ITC (2024). ITC trade map - trade statistics for international business development; list of exporters for the selected product: 0905 Vanilla. ITC Trade Map. Available at: https://www.trademap.org/Country_SelProduct_TS.
Krishnakumar, V., Bindumol, G. P., Potty, S. N., and Govindaraju, C. (2007). Processing of vanilla (Vanilla planifolia Andrews) beans - influence of storing fresh beans, killing temperature and duration of killing on quality parameters. J. Spices Aromat. Crops 16, 31–37.
Kumar, R. K., and Balamohan, T. (2013). Factors affecting the quality of Vanilla–a review. J. Agric. Allied Sci. 2, 37–41.
Marc, B. P. (1992). FR.Patent. 2680798A1. Paris: FR, Intitut National De La Propriete Industrielle. Procédé de obtention d’arôme naturel de vanille par traitement enzymatique des gousses de vanille vertes, arôme obtenu, 1–13.
Neimark, B., Osterhoudt, S., Alter, H., and Gradinar, A. (2019). A new sustainability model for measuring changes in power and access in global commodity chains: through a smallholder lens. Palgrave Commun. 5:1. doi: 10.1057/s41599-018-0199-0
Pardío, V. T., Flores, A., López, K. M., Martínez, D. I., Márquez, O., and Waliszewski, K. N. (2018). Effect of endogenous and exogenous enzymatic treatment of green vanilla beans on extraction of vanillin and main aromatic compounds. J. Food Sci. Technol. 55, 2059–2067. doi: 10.1007/s13197-018-3120-3
Peña-Barrientos, A., Perea-Flores, M. D. J., Martínez-Gutiérrez, H., Patrón-Soberano, O. A., González-Jiménez, F. E., Vega-Cuellar, M. Á., et al. (2023). Physicochemical, microbiological, and structural relationship of vanilla beans (Vanilla planifolia, Andrews) during traditional curing process and use of its waste. J. Appl. Res. Med. Aromat. Plants 32:100445. doi: 10.1016/j.jarmap.2022.100445
Pribadi, E. R., Poentiyanti, E. H., Wahyuni, S., Sukamto, S., Purwiyanti, S., Sirait, N., et al. (2021). Kelayakan ekonomi enam klon harapan untuk mendukung pelepasan varietas vanili tahan penyakit busuk batang. Jurnal Littri 27, 99–108. doi: 10.21082/littri.v27n2.2021.99-108
Ralandison, T. (2021). Exploring corporation-cooperative arrangements in agricultural value chains: the case of Madagascar Vanilla. Japanese. J. Agric. Econ. 23, 113–118. doi: 10.18480/jjae.23.0_113
Rosman, R. (2010). Inovasi teknologi budidaya vanili berbasis ekologi. Orasi pengukuhan Profesor Riset. Jakarta: Badan Penelitian dan Pengembangan Pertanian.
Sánchez-Galindo, M., Delgado-Alvarado, A., Herrera-Cabrera, B. E., and Osorio-García, C. (2018). Quality of green and cured vanilla (Vanilla planifolia jacks. Ex Andrews) fruit in relation to its age at harvest. Rev. Chapingo Ser. Hortic. 24:4. doi: 10.5154/r.rchsh.2018.02.004
Sujalmi, S., Suharso, S., Supriyanto, R., and Buchari, B. (2010). Determination of vanillin in vanilla (vanilla planifolia Andrews) from Lampung Indonesia by high performance liquid chromatography. Indian J. Chem. 5, 7–10. doi: 10.22146/ijc.21831
Tjahjana, B. E., Hadipoentyanti, E., and Udarno, L. (2011). Budidaya Tanaman Vania 1 Dan Vania 2. 1st Edn. Sukabumi: Balittri.
Udarno, L., and Hadipoentyanti, E. (2009). Panili budidaya dan kerabat liarnya. Pengembangan Tanaman Indust. 15, 20–28.
Van Dyk, S., McGlasson, W. B., Williams, M., and Gair, C. (2010). Influence of curing procedures on sensory quality of vanilla beans. Fruits 65, 387–399. doi: 10.1051/fruits/2010033
Wahyudi, A., Ermiati, E., and Sujianto, S. (2023). “Analysis of sustainability ranking of vanilla cultivation systems in West Java, Indonesia.” in IOP conference series: Earth and environmental science. p. 012062.
Wahyudi, A., Permadi, R. A., and Ermiati, (2021). “Technical risk control system of sustainable vanilli cultivation in Indonesia.” in E3S web of conferences. eds. Rubiyo and C. Indrawanto. p. 02036.
Waliszewski, K. N., Ovando, S. L., and Pardio, V. T. (2007). Effect of hydration and enzymatic pretreatment of vanilla beans on the kinetics of vanillin extraction. J. Food Eng. 78, 1267–1273. doi: 10.1016/j.jfoodeng.2006.01.029
Wulandari, S. (2021). “Investment risk management for vanilla agribusiness development in Indonesia.” in E3S Web of Conferences. eds. Juwaidah, P. Saiyut, M. M. Tjale, and Z. Rozaki. p. 02022.
Keywords: characteristics, handling, quality, safety, standard, vanillin
Citation: Munarso SJ, Rahardjo YP, Sjafrina N, Arianto A, Hadipoentyanti E, Astuti P, Setiadi A, Koeslulat EE, Lintang MMJ, Sulistyorini S, Egayanti Y, Elmatsani HM, Djafar MJ, Susetyo EB, Lanjar L and Hadipernata M (2024) From bean to market: exploring the chemical and production dynamics of high-quality Indonesian vanilla. Front. Sustain. Food Syst. 8:1425656. doi: 10.3389/fsufs.2024.1425656
Edited by:
Chunhong Yuan, Iwate University, JapanReviewed by:
Diego Fernando Roa-Acosta, University of Cauca, ColombiaXin Lu, Iwate University, Japan
Copyright © 2024 Munarso, Rahardjo, Sjafrina, Arianto, Hadipoentyanti, Astuti, Setiadi, Koeslulat, Lintang, Sulistyorini, Egayanti, Elmatsani, Djafar, Susetyo, Lanjar and Hadipernata. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Endang Hadipoentyanti, ZW5kYTA2NUBicmluLmdvLmlk; S. Joni Munarso, c2pvbjAwMUBicmluLmdvLmlk
 S. Joni Munarso1*
S. Joni Munarso1* Yogi P. Rahardjo
Yogi P. Rahardjo Noveria Sjafrina
Noveria Sjafrina Adi Setiadi
Adi Setiadi Ermi E. Koeslulat
Ermi E. Koeslulat Meivie M. J. Lintang
Meivie M. J. Lintang Huda M. Elmatsani
Huda M. Elmatsani