- Department of Plant Agriculture, University of Guelph, Guelph, ON, Canada
Millions of small scale legume farmers lack access to rhizobia bacterial inoculants that improve crop protein and yield, and minimize fossil-fuel based nitrogen fertilizers, through biological nitrogen fixation (BNF). BNF converts atmospheric nitrogen gas into ammonia, required to synthesize chlorophyll and amino acids. BNF is catalyzed by rhizobia that inhabit nodule organs in legume roots (e.g., soybean, cowpea, chickpeas, lentil, fava, peas, beans). Rhizobia inoculant access in remote communities is limited by centralized facilities to grow bacteria, inadequate transportation networks and refrigeration. Recently, we proposed that rhizobia inoculants can be diffused by farmers themselves, simply, by crushing nodules onto seeds, and demonstrated its efficacy under field conditions. A concern was whether nodules remain viable between growing seasons. Here we provide preliminary evidence that bean nodules, dried and stored at room temperature after 6 months, retain nodulation potential. We discuss: (1) the feasibility, constraints and risks of nodule crushing; (2) scaling up strategies; (3) entrepreneurship that benefits women farmers (e.g., nodule-selling microenterprises); and (4) empowering farmers to directly select, evolve and indigenize rhizobia for the first time. Finally, we prioritize research questions and encourage the formation of a global participatory research network, with the goal of decentralizing and democratizing rhizobia inoculants.
Introduction
Two-thirds of severe global malnutrition occurs in rural and peri-urban areas, which are dominated by small scale farms of area <2 Ha (FAO, IFAD, UNICEF, WFP and WHO 2023; Nature Editorial 2020; Laborde et al., 2020). One billion people worldwide suffer from protein deficiency, mainly in Central Africa and South Asia, where 30% of children are afflicted (Wu et al., 2014; FSIN, 2022). Protein deficiency in humans is connected to soil nitrogen (N), as nitrogen is the building block for amino acids (Gutiérrez-Preciado et al., 2010). Excess rainfall in the Tropics leaches N-fertilizers, while insufficient soil organic matter in the Subtropics prevents N-fertilizer retention (Lal, 2021). As N is required to synthesize chlorophyll and amino acids, crop yield and grain nitrogen content, respectively, are hampered by the limitation of soil-N (Lal, 2020). When fossil fuel prices increase, such as in 2007–2008 and 2022, the price of synthetic fertilizer also increases, making it unaffordable for small scale farmers (UN 2011; FSIN, 2022). As a result, the more than 2 billion people who rely on the world’s 475 million small scale farms, primarily in Asia and Africa (Lowder et al., 2016), suffer from lower agricultural returns. There is a need to improve the resiliency of smallholders, who are already being impacted by climate change (Doso Jnr, 2014; Müller et al., 2011).
Grain legume crops, including soybeans, common bean, lentil, chickpea, cowpea and fava bean, represent solutions to the nitrogen fertilizer and protein deficiency challenges of the world’s poorest farmers. This is because legumes recruit symbiotic rhizobia bacteria from the soil into specialized root organs called nodules, where the rhizobia convert atmospheric nitrogen gas (N2) into ammonia (NH3) which is required for amino acid biosynthesis (Wagner 2011). The process is termed biological nitrogen fixation (BNF). BNF enhances the protein content of legume grain, as well as shoots for livestock fodder (Kebede, 2021). However, as of 2020, average legume yields in Africa were only 1.1 t/ha compared to 2.6 t/ha in North America (FAOSTAT, 2022). Legumes not only fix N for self-use but also enrich soil following decomposition to fertilize non-legumes (e.g., corn/maize, rice, wheat) as intercrops or rotation crops (Rao et al., 2019; Awika, 2011; Peoples et al., 2009).
BNF decreases the need for fossil fuel derived N-fertilizer and hence the carbon footprint of farms (Guo et al., 2022; Imran et al., 2021). Furthermore, rhizobia bacteria deposit fixed nitrogen directly inside root systems, avoiding leaching (especially on sandy soils) and volatilization, to reduce on-farm greenhouse gas emissions such as N2O (Syers et al., 1996, Menegat et al., 2022).
Legumes do not transfer rhizobia through seeds inter-generationally and hence rely on native soil rhizobia. Actual N-fixation can be limited by sub-optimal rhizobia strains in the soil (e.g., when a crop species is new to a region), host-bacteria incompatibility, competition from non-rhizobia strains and poor soil health (Thilakarathna and Raizada, 2016). One approach to overcome the existing yield gap of legumes in Africa and Asia is through the use of elite rhizobia bacterial inoculants (Santos et al., 2019; Vanlauwe et al., 2019; Chibeba et al., 2018; van Heerwaarden et al., 2018). Inoculants are living microorganisms deliberately introduced by humans to benefit host plants.
In India and Brazil, private-public sector partnerships have improved rhizobia access (Singh et al., 2014; Rao et al., 2019; Santos et al., 2019). However, commercial inoculants, whether exotic or local, are not readily available in many developing nations including in South Asia (Rao et al., 2019) and especially across Africa (Vanlauwe et al., 2019; Raimi et al., 2021). This is because of the remoteness of many small scale farms, compounded by poor distribution systems and lack of refrigeration which is required to maintain rhizobia viability during shipment and storage (Vanlauwe et al., 2019). Additional major problems of rhizobia inoculants include contamination during shipping and a short shelf life (Vanlauwe et al., 2019). Consequently, many commercial rhizobia arrive with few viable cells (Woomer et al., 2014). Viable inoculant may suffer from low environmental stress tolerance (e.g., soil temperature, pH, drought), as well as competition from local strains (Thilakarathna and Raizada, 2016; Furseth et al., 2012; Deaker et al., 2004). Therefore, elite rhizobia are not reaching millions of small scale farmers (Bala et al., 2011; Vanlauwe et al., 2019). A new strategy is needed that bypasses the limitations of shipping elite rhizobia strains from a centralized manufacturing facility, through a distribution network, to small scale farmers.
Nodule crushing: a simple new tool in the inoculant toolbox
We recently demonstrated that legume seeds can be inoculated with rhizobia simply by soaking them in crushed nodules (Pudasaini et al., 2023). We call this technique nodule crushing (Figure 1). The nodule crushing procedure involves harvesting nodules (Figure 1A), surface sterilizing and crushing them in sterile water then mixing the lysate with a local sticky agent and finally coating the mixture onto seeds (Figure 1B). In 10 independent experiments in Canada over 2 years, nodule crushing was effective in diverse legumes including those with determinate nodules (soybean, cowpea) and indeterminate nodules (pea, lentil) including soybean, lentil and cowpea under field conditions (Figures 1C–H; Pudasaini et al., 2023). Critically, in a virgin soil that had never seen cowpea, nodule crushing significantly increased nodulation and the fraction of active (pink) including soybean, lentil and cowpea under field conditions (Pudasaini et al., 2023), mimicking introduction of a new legume species to a farm. Soybean is being adopted across Africa (Vanlauwe et al., 2019; FAOSTAT, 2022; Foyer et al., 2019), and in this context, we demonstrated that Bradyrhizobium japonicum (USDA 110), an elite strain shown to improve soybean yields in Africa (Chibeba et al., 2018; van Heerwaarden et al., 2018), could be effectively introduced using nodule crushing (Pudasaini et al., 2023).

Figure 1. The nodule crushing technique and its effectiveness. (A) Overview of biological nitrogen fixation. (B) The nodule crushing technique. (C) Example of the efficacy of nodule crushing on shoot growth. Shown are soybeans growing on sterile sand: plants inoculated using nodule crushing (left), pure inoculant (middle) and buffer control (right). (D–F) Example of the efficacy of nodule crushing on root nodulation. Soybean roots grown on sterilized sand, after seeds were treated with: (D) nodule crushing (E) pure culture (positive control), and (F) the buffer control. (G,H) Example of the efficacy of nodule crushing under field conditions. Cowpea roots harvested from the field, after seeds were treated with (G) nodule crushing, and (H) the buffer control. (I) Dry nodules of common bean stored for 6 months. (J) Rehydration of stored dry nodules of common bean after soaking in water for 1 h. (K,L) Rhizobia colonies on a YM plate, cultured from rehydrated nodules after being dried and stored, after being surface sterilized for different durations: (K) 3 min in 2% sodium hypochlorite versus (L) 5 min in 2% sodium hypochlorite. (M–O) Experiment to test the viability of using common bean nodules that had been dried, stored for 6 months and rehydrated, as an inoculant via nodule crushing: (M) Plant growth system using sterile sand in a 4 L jar. (N) example of successful nodulation in a root system derived from seeds treated with nodule crushing using dried, stored, rehydrated nodules. (O) Example of a negative control root system derived from seeds treated with buffer, showing no nodules. (P) Proposed steps for farmers to utilize nodule crushing.
We proposed that nodule crushing bypasses the need for smallholders to purchase/acquire elite rhizobia from distant sources (Pudasaini et al., 2023). Instead, after distribution of an elite strain to a few farmers, we proposed they can then distribute nodules regionally. However, since farmers typically grow a legume crop only one season per year, we noted the challenge of long-term rhizobia viability in stored nodules (Pudasaini et al., 2023). During the review process of that publication, one anonymous reviewer, subsequently revealed to be Prof. John Maingi (Kenyatta University, Kenya), suggested we attempt to store dried nodules. Accordingly, we recently harvested and rinsed common bean nodules, placed them on paper towels to dry at room temperature for 2–3 days, then stored them in paper bags for 6 months at room temperature. After 6 months, the nodules appeared dry and shriveled (Figure 1I). However, within 1 h of adding water, the nodules rehydrated to their original size/shape and had a brownish-pinkish color (Figure 1J); upon squeezing, they were firm, without evidence of decomposition. Upon surface sterilizing for 3 min in 2% sodium hypochlorite, rhizobia could successfully grow on YM plates after incubating for 3 days at 30°C (Figure 1K). However, surface sterilizing for 5 min under the same conditions notably lowered the colony count (Figure 1L), suggesting high sensitivity of dry nodules to surface sterilization. Later, the bacterial identity of a purified colony was confirmed by 16S rRNA sequencing (Genbank accession PQ150388) to match (100%) the original inoculant (Rhizobium tropici CIAT 899). When healthy-looking, rehydrated nodules were crushed onto common bean seeds, our preliminary results demonstrated they retained nodulation capacity (Figures 1M,N) compared to the buffer control which showed no authentic nodulation (Figure 1O).
Therefore, we propose that after collecting nodules, farmers can air dry them at room temperature (sun may be damaging), and store them for the next growing season in a cool/dry location (Figures 1P, 2A,B). Before the start of the next growing season, to multiply donor nodules, farmers can crush the stored nodules onto a small number of seeds in a pre-season nursery. To overcome the potential challenge of low titer rhizobia in stored nodules being outcompeted by soil rhizobia after planting, we propose farmers at the pre-season nursery stage can use instead sow into pure sand (mixed with well decomposed manure/compost for mineral nutrients). The discovery that dried common bean nodules retain viability after months of storage means that farmers can now share, trade/sell nodule inoculant in local markets, similar to how seeds have been shared for thousands of years.
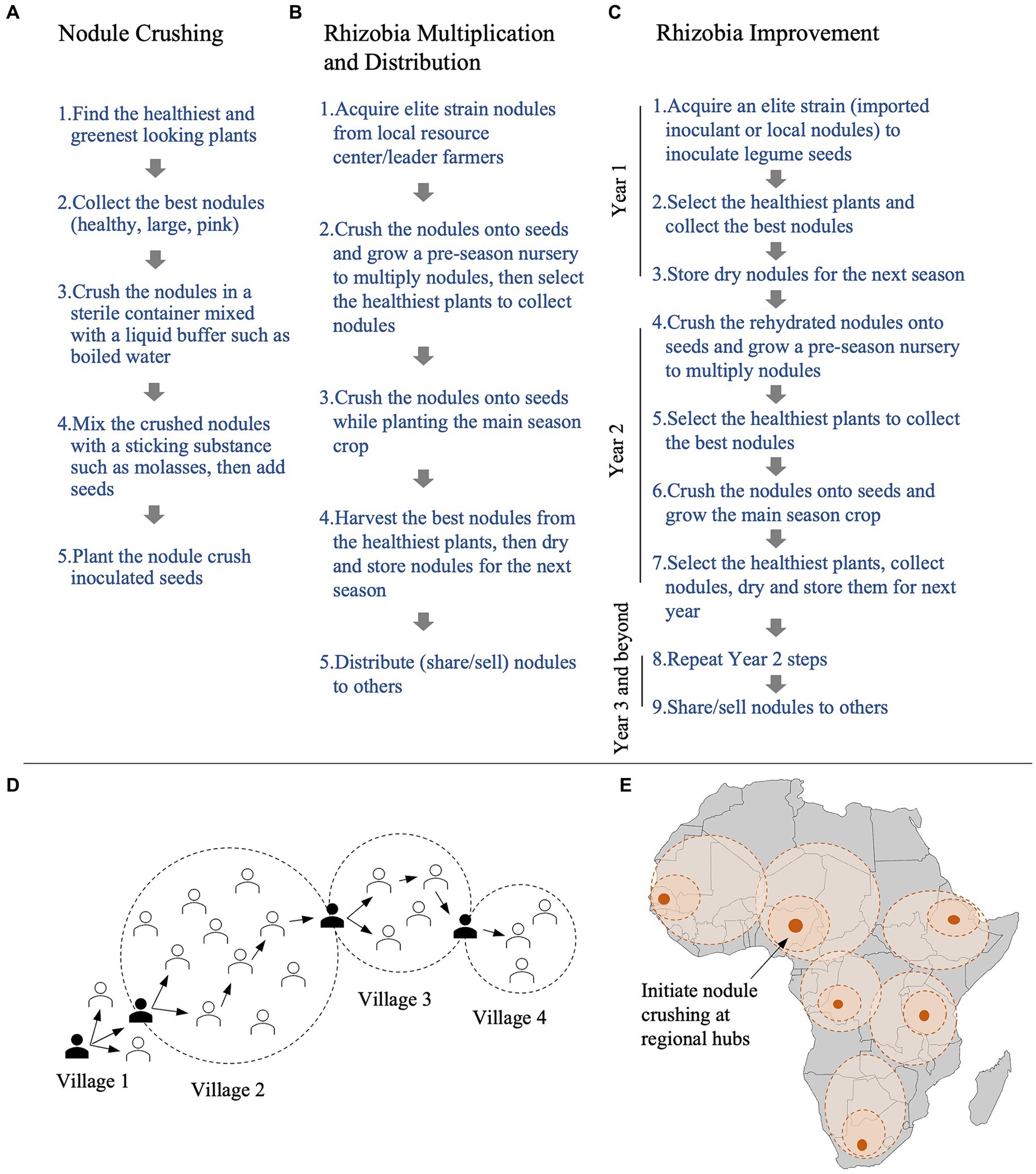
Figure 2. Schematics to summarize processes by which nodule crushing can be used to multiply, distribute, improve and scale up rhizobia inoculants. (A–C) Flow charts of steps required for: (A) the nodule crushing technique, (B) nodule-crushing based rhizobia multiplication and distribution by individual farmers, and (C) rhizobia improvement using repeated cycles of nodule crushing. (D,E) Proposed diffusion of nodules containing elite and improved rhizobia, (D) from farmer-to-farmer at the local level, and (E) regional level in Africa. The map in panel (E) was adapted from: Andreas 06, Wikimedia Commons.
Farmer selection and evolution of rhizobia by nodule crushing
However, inoculation with elite rhizobia may not necessarily improve nitrogen fixation (Thilakarathna and Raizada, 2016). Exotic inoculants may not adapt to the local soil pH, temperature and/or predators, compete with local strains, and/or be compatible with local legume species/cultivars (Thilakarathna and Raizada, 2016; Vanlauwe et al., 2019; Mendoza-Suárez et al., 2021; Nyaga and Njeru, 2020; Thilakarathna et al., 2019). Rather, many studies have revealed that re-inoculating soybean seeds with indigenous rhizobia can result in greater yield than exotic elite strains under field conditions (Nyaga and Njeru, 2020; Hungria et al., 2006). There is a need to indigenize (adapt) elite strains or improve indigenous strains. However, as legume seeds do not effectively transmit rhizobia inter-generationally (Chartrel et al., 2021; Moroenyane et al., 2021), crop selection by farmers does not directly improve rhizobia.
We proposed that smallholders can be self-empowered to improve rhizobia through repeated cycles of plant and nodule selection (Pudasaini et al., 2023). In this strategy (Figure 2C), at pre-harvest (Step 1), prior to late-season nodule senescence, farmers can select the healthiest, greenest legume plants from their field (inoculated or not). In Step 2, from those plants, farmers can then select the largest pink nodules; large nodules have greater rhizobia purity than small nodules (Hossain et al., 2023), while pink nodules are more likely to contain compatible, active rhizobia rather than non-rhizobial cheaters (Westhoek et al., 2021; Mendoza-Suárez et al., 2021). Farmers can then dry/store the nodules for the next growing season (Step 3), and crush rehydrated nodules onto pre-season nursery seeds (Step 4). Then, after collecting large/pink nodules from the healthiest nursery plants, at Step 5, farmers can inoculate their field using nodule crushing. At Step 6, before harvest, farmers can select the best plants/nodules and repeat the selection cycles. By this process, farmers can simultaneously select against pathogens and cheaters (Sachs et al., 2010). Finally, in Step 7, farmers can share, trade or sell dried nodules containing improved rhizobia. Small scale farmers may also co-breed both their crops and rhizobia by simultaneously screening the best local legume genotype as a high-nodulating host.
Nodule crushing based selection has been used successfully in Mimosa plants on laboratory-based gel media (Guan et al., 2013; Remigi et al., 2014; Marchetti et al., 2017). In preliminary experiments, we have extended this approach to common bean, employing real-world field soil: shoot yield, chlorophyll and nodule number/biomass improved after 4 cycles of nodule-crush based selection indoors (Pudasaini, 2024). These results suggest the potential of nodule crushing based improvement of rhizobia by farmers.
Discussion
Adoption and scaling up of nodule crushing by small scale farmers: feasibility, constraints, risks
We are optimistic that nodule crushing will be feasible for smallholders when combined with guidance and locally available and inexpensive materials. In some legume crops, nodules are visible to the naked eye; however, other legumes have small nodules—for those, a low cost magnifying glass/sheet can be used. Since a single healthy soybean nodule is sufficient to inoculate 50 seeds (Pudasaini et al., 2023), and a single legume plant can produce a few hundred nodules, a few healthy pre-season nursery plants should provide sufficient inoculum for a smallholder. Vanlauwe et al. (2019) estimated that to provide 60 kg of protein per year for a rural African family of seven, a smallholder would only need to grow ~30 m2 of soybean or ~ 60 m2 of common bean. Boiled water can be used to sterilize required tools (e.g., small pot container and metal crushing utensil), and after cooling, could be used as buffer for crushing nodules. Locally available sticky agents (molasses, Gum Arabic, etc.) can be used to bind inoculant to seeds (Somasegaran and Hoben, 1994). Furthermore, nodule crushing with rehydrated nodules makes repeat inoculation of rhizobia across multiple seasons feasible; repeat inoculation has been shown to improve nodule occupancy and nitrogen fixation (Obaton et al., 2002).
We can imagine one risk from this technology for farmers: pathogens within the nodule microbiome. Conversely, though commercial inoculants are theoretically pure, in practice, they are well known to suffer from pathogen contamination (Vanlauwe et al., 2019) as already noted. By contrast, farmer selection for healthy donor nodules from healthy plants should avoid nodule-derived pathogens—a significant advantage. An exception might be the ability of farmers to separate small nodules from root debris which may support plant pathogens. In our experience, root debris separates easily from dried nodules, and a quick shake causes the debris to settle to the bottom of a container. Relying on only the naked eye, and by selecting fresh or dried nodules derived from healthy donor plants, we have never observed nodule crushing to result in a single diseased plant after >20 independent experiments spanning 5 legume species, across multiple indoor trials and 2 years of field trials. However, we had the advantage of starting with disease-free seed.
Nevertheless, nodule crushing is a completely new concept that needs to be taught to farmers. Fortunately, due to its simplicity, even low-literacy farmers should easily be able to learn the technique from demonstrations, Farmer Field Schools (FFS), picture-based lessons, videos, smartphones and television (Bentley et al., 2022; van den Berg et al., 2020; Devkota et al., 2020; Raizada and Smith, 2016; Davis et al., 2012). Farmers need to be taught the basic principles of biological nitrogen fixation, and associating shoot color and vigor with nodule size, number and color, so that they understand the reasons for selecting large, pink nodules. As farmers grow multiple legume crops, they need to be taught that nodule donors from each crop species should be kept separate. Additionally, households need to be taught techniques pertaining to pathogen-avoidance, nodule drying/storage and early-season nursery nodule multiplication. We recommend that farmers try nodule crushing on small plots side-by-side with their traditional practice, to evaluate the technology.
Farmer-to-farmer training is an effective and sustainable extension approach (Franzel et al., 2015), and critically, farmers trust the opinion of other farmers when a technology or practice is novel (Ssemakula and Mutimba, 2011). Therefore, after early adopting farmers acquire an elite rhizobia strain, their on-farm trials may convince other farmers within a village to adopt nodule crushing. Farmers can then distribute nodules to their network of family and friends, eventually diffusing the technology regionally (Figures 2D,E).
Finally, to enable entrepreneurs to translate local nodule breeding successes to improve legume yields on commercial farms, low interest infrastructure loans could enable the purchase of small microbial bioreactors, refrigerators and electrical generators.
Maximizing benefits to women farmers
The best approach to lifting a family out of poverty is to empower women (Lumet et al., 2022) who often suffer from lower literacy than men (UNESCO, 2018). Women could benefit from nodule crushing in home gardens where they keep profits (e.g., green peas, vegetable bean/cowpea pods); however men are more likely to receive income from field grains (Fischer et al., 2017; Njuki et al., 2019). We note that women are more likely to keep income generated from small scale value addition activities such as cooking and packaging (e.g., roasted legume snackfood) and if they are organized into women’s groups (Smale et al., 2022; ActionAid, 2024). Therefore, women farmers can be organized into farmers’ organizations such as cooperatives that facilitate visual-based learning, value chain development (e.g., post-harvest value addition/processing, linkages to markets, marketing, creating consumer demand, access to micro-finance) to ensure they gain benefits from increased legume production (Adlam 2023; Bizikova et al., 2020). Women farmers could sell the resulting nodules as for-profit microenterprises, similar to the practice by indigenous peoples of selling local seeds (Vernooy et al., 2015).
The need for more research: a call to scientists and farmer groups
Nodule crushing is promising, but there are some unknowns. Urgently, on-farm participatory testing with smallholders is needed, after which the protocol may need modification. There is a need to test the effectiveness of nodule crushing across diverse crops and agro-ecosystems including when a large bank of competitor rhizobia exist in the soil (Mwenda et al., 2023; Dowlin and Broughton 1986). The optimal age of donor plants and optimal nodule size need to be determined for different crops, including any differences between determinate and indeterminate nodules (Hirsch, 1992), and amide versus ureide exporters (Andrews et al., 1984). For the nodule crushing procedure itself, the optimal nodule:seed ratio for different crops needs to be determined, the duration/condition of nodule lysate incubation with seeds, as well as the requirement for seed sterilization. An important question is how long nodules can be stored in dry form, the optimal storage conditions and the resulting rhizobia viability for different legume crops, and whether specific rehydration conditions can improve this viability. Careful on-farm studies are needed to measure and prevent pathogen spread on small scale farms associated with nodule crushing and long-term nodule storage. Studies are also needed to determine the extent to which nodule crushing based selection can reduce cheater nodule bacteria (Sachs et al., 2010) and promote helper bacteria (Martínez-Hidalgo and Hirsch 2017; Hossain et al., 2023). Strategies are needed to maintain evolved nodules at the village level (e.g., similar to a community seed bank) and protect the resulting intellectual property rights of smallholders. Finally, research is needed to evaluate the effectiveness of using nodule crushing to indigenize introduced, elite rhizobia strains compared to starting with indigenous strains. As noted earlier, since introduced rhizobia are allochthonous to the new soil environment, they may face barriers including competition from autochthonous soil microbes and predators (Thilakarathna and Raizada, 2016; Mendoza-Suárez et al., 2021). However, the current global strategy for rhizobia inoculants is based on elite rhizobia strains, with major investments, commercial infrastructure and success (Santos et al., 2019; van Heerwaarden et al., 2018). To answer these questions, we encourage the formation of a global network of researchers and farmers to test and make recommendations.
Conclusion
After nearly a century, conventional rhizobia inoculants remain inaccessible to many smallholders. Over the last 10,000 years, illiterate, subsistence farmers were able to domesticate and improve crops (Hufford et al., 2019). Nodule crushing may now empower farmers to directly select and evolve beneficial microbes for the first time—to adapt and indigenize promising rhizobia. Nodule crushing can also be used to improve nitrogen fixation in orphan legumes (e.g., Bambara groundnut in Africa; Tan et al., 2020) and landraces, that are often ignored by companies and governments (Li et al., 2020; Popoola et al., 2022). Ultimately, nodule crushing has the potential to decentralize rhizobia inoculant technologies; however more research is needed.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: PQ150388 (Genbank).
Author contributions
RP: Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. MR: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. RP is a recipient of the Arrell Graduate Scholarship (University of Guelph). Funding was provided by grants to MR from the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) and the Natural Sciences and Engineering Research Council (NSERC) of Canada. None of the funders were involved in the design, interpretation or writing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adlam, H. (2023). Supporting female farming cooperatives: the smart choice for the African development bank. JPIA. 34. Available at: https://jpia.princeton.edu/news/supporting-female-farming-cooperatives-smart-choice-african-development-bank (Accessed on 26 November, 2023).
ActionAid. (2024). Women’s empowerment and value chains. Available at: https://acfid.asn.au/sites/site.acfid/files/resource_document/WEVC%20report%20_%20fnl.pdf (Accessed on 26 November, 2023)
Andrews, M., Sutherland, J. M., Thomas, R. J., and Sprent, J. I. (1984). Distribution of nitrate reductase activity in six legumes: the importance of the stem. New Phytol. 98, 301–310. doi: 10.1111/j.1469-8137.1984.tb02740.x
Awika, J. M. (2011). “Major cereal grains production and use around the world” in Advances in cereal science: implications to food processing and health promotion. eds. J. M. Awaika, V. Piironen, and S. Bean, ACS symposium series, vol. 2011 (Washington, DC: American Chemical Society), 15–30.
Bala, A., Karanja, N., Murwira, M., Lwimbi, L., Abaidoo, R., and Giller, K. (2011). Production and use of rhizobial inoculants in Africa. Available at: www.N2Africa.org (Accessed on 15 August, 2023).
Bentley, J., van Mele, P., Chadare, F., and Chander, M. (2022). Videos on agroecology for a global audience of farmers: an online survey of access agriculture. Int. J. Agric. Sustain. 20, 1100–1116. doi: 10.1080/14735903.2022.2057641
Bizikova, L., Nkonya, E., Minah, M., Hanisch, M., Turaga, R. M. R., Speranza, C. I., et al. (2020). A scoping review of the contributions of farmers’ organizations to smallholder agriculture. Nat. Food. 1, 620–630. doi: 10.1038/s43016-020-00164-x
Chartrel, V., Dugat-Bony, E., Sarthou, A. S., Huchette, S., Bonnarme, P., and Irlinger, F. (2021). The microbial community associated with pea seeds (Pisum sativum) of different geographical origins. Plant Soil 462, 405–427. doi: 10.1007/s11104-021-04856-6
Chibeba, A. M., Kyei-Boahen, S., and Guimarães, M. (2018). Feasibility of transference of inoculation-related technologies: a case study of evaluation of soybean rhizobial strains under the agro-climatic conditions of Brazil and Mozambique. Agric. Ecosyst. Environ. 261, 230–240. doi: 10.1016/j.agee.2017.06.037
Davis, K., Nkonya, E., Kato, E., Mekonnen, D. A., Odendo, M., Miiro, R., et al. (2012). Impact of farmer field schools on agricultural productivity and poverty in East Africa. World Dev. 40, 402–413. doi: 10.1016/j.worlddev.2011.05.019
Deaker, R., Roughley, R. J., and Kennedy, I. R. (2004). Legume seed inoculation technology - a review. Soil Biol. Biochem. 36, 1275–1288. doi: 10.1016/j.soilbio.2004.04.009
Devkota, R., Odame, H. H., Fitzsimons, J., Pudasaini, R., and Raizada, M. N. (2020). Evaluating the effectiveness of picture-based agricultural extension lessons developed using participatory testing and editing with smallholder women farmers in Nepal. Sustain. For. 12:9699. doi: 10.3390/su12229699
Doso Jnr, S. (2014). Land degradation and agriculture in the Sahel of Africa: causes, impacts and recommendations. J. Agric. Sci. Appl. 3, 67–73. doi: 10.14511/jasa.2014.030303
Dowlin, D. N., and Broughton, W. J. (1986). Competition for nodulation of legumes. Ann. Rev. Microbiol. 40, 131–157. doi: 10.1146/annurev.mi.40.100186.001023
FAO, IFAD, UNICEF, WFP and WHO (2023). The state of food security and nutrition in the world 2023. Urbanization, agrifood systems transformation and healthy diets across the rural–urban continuum. Rome: FAO.
FAOSTAT. (2022). Crops and livestock products. Food and agriculture Organization of the United Nations, Rome. Available at: https://www.fao.org/faostat/en/#data (Accessed on 17 June 2022)
Fischer, G., Gramzow, A., and Laizer, A. (2017). Gender, vegetable value chains, income distribution and access to resources: insights from surveys in Tanzania. Eur. J. Hortic. Sci. 82, 319–327. doi: 10.17660/eJHS.2017/82.6.7
Foyer, C. H., Siddique, K. H. M., Tai, A. P. K., Anders, S., Fodor, N., Wong, F. L., et al. (2019). Modelling predicts that soybean is poised to dominate crop production across Africa. Plant Cell Environ. 42, 373–385. doi: 10.1111/pce.13466
Franzel, S., Degrande, A., Kiptot, E., Kirui, J., Kugonza, J., et al. (2015). “Farmer-to-farmer extension” in Note 7. GFRAS good practice notes for extension and advisory services (Lindau, Switzerland: GFRAS).
FSIN (2022). Global report on food crisis 2022. Food security information Network. Available at: https://www.wfp.org/publications/global-report-food-crises-2022 (Accessed on 27 June 2022)
Furseth, B. J., Conley, S. P., and Ané, J. (2012). Soybean response to soil rhizobia and seed-applied rhizobia inoculants in Wisconsin. Crop Sci. 52, 339–344. doi: 10.2135/cropsci2011.01.0041
Guan, S. H., Gris, C., Cruveiller, S., Pouzet, C., Tasse, L., Leru, A., et al. (2013). Experimental evolution of nodule intracellular infection in legume symbionts. ISME J. 7, 1367–1377. doi: 10.1038/ismej.2013.24
Guo, K., Yang, J., Yu, N., Luo, L., and Wang, E. (2022). Biological nitrogen fixation in cereal crops: progress, strategies, and perspectives. Plant Commun. 4:d100499. doi: 10.1016/j.xplc.2022.100499
Gutiérrez-Preciado, A., Romero, H., and Peimbert, M. (2010). An evolutionary perspective on amino acids. Nat. Educ. 3:29.
Hirsch, A. M. (1992). Developmental biology of legume nodulation. New Phytol. 122, 211–237. doi: 10.1111/j.1469-8137.1992.tb04227.x
Hossain, M. S., DeLaune, P. B., and Gentry, T. J. (2023). Microbiome analysis revealed distinct microbial communities occupying different sized nodules in field-grown peanut. Front. Microbiol. 14:1075575. doi: 10.3389/fmicb.2023.1075575
Hufford, M. B., Berny Mier, Y., Teran, J. C., and Gepts, P. (2019). Crop biodiversity: an unfinished magnum opus of nature. Annu. Rev. Plant Biol. 70, 727–751. doi: 10.1146/annurev-arplant-042817-040240
Hungria, M., Franchini, J. C., Campo, R. J., Crispino, C. C., Moraes, J. Z., Sibaldelli, R. N. R., et al. (2006). Nitrogen nutrition of soybean in Brazil: contributions of biological N2 fixation and N fertilizer to grain yield. Can. J. Plant Sci. 86, 927–939. doi: 10.4141/P05-098
Imran, A., Hakim, S., Tariq, M., Nawaz, M. S., Laraib, I., Gulzar, U., et al. (2021). Diazotrophs for lowering nitrogen pollution crises: looking deep into the roots. Front. Microbiol. 12:637815. doi: 10.3389/fmicb.2021.637815
Kebede, E. (2021). Contribution, utilization, and improvement of legumes-driven biological nitrogen fixation in agricultural systems. Front. Sustain. Food Syst. 5:767998. doi: 10.3389/fsufs.2021.767998
Laborde, D., Murphy, S., Parent, M., Porciello, J., and Smaller, C. (2020). Ceres2030: Sustainable solutions to end hunger - summary report. US: Cornell University, IFPRI and IISD.
Lal, R. (2020). “Effects of fertilizers on soil quality and functionality” in Soil and fertilizers. eds. R. Lal and B. A. Stewart (Boca Raton, FL: CRC Press), 1–10.
Lal, R. (2021). “Soil health and human nutrition” in The soil-human health Nexus. ed. R. Lal (Boca Raton, FL: CRC Press), 315–326.
Li, X., Yadav, R., and Siddique, K. H. M. (2020). Neglected and underutilized crop species: the key to improving dietary diversity and fighting hunger and malnutrition in Asia and the Pacific. Front. Nutr. Frontiers Media S.A. 7:593711. doi: 10.3389/fnut.2020.593711
Lowder, S. K., Skoet, J., and Raney, T. (2016). The number, size, and distribution of farms, smallholder farms, and family farms worldwide. World Dev. 87, 16–29. doi: 10.1016/j.worlddev.2015.10.041
Lumet, K., Gitau, R., and Owuor, G. (2022). The influence of women’s empowerment on poverty reduction: a case of smallholder sugarcane farmers in western Kenya. Afric. J. Agric. Res. Econ. 17, 255–271. doi: 10.53936/AFJARE.2022.17(3).17
Marchetti, M., Clerissi, C., Yousfi, Y., Gris, C., Bouchez, O., Rocha, E., et al. (2017). Experimental evolution of rhizobia may lead to either extra-or intracellular symbiotic adaptation depending on the selection regime. Mol. Ecol. 26, 1818–1831. doi: 10.1111/mec.13895
Martínez-Hidalgo, P., and Hirsch, A. M. (2017). The nodule microbiome: N2 fixing rhizobia do not live alone. Phytobiomes J. 1, 70–82. doi: 10.1094/PBIOMES-12-16-0019-RVW
Mendoza-Suárez, M., Andersen, S. U., Poole, P. S., and Sánchez-Cañizares, C. (2021). Competition, nodule occupancy, and persistence of inoculant strains: key factors in the rhizobium-legume symbioses. Front. Plant Sci. 12:690567. doi: 10.3389/fpls.2021.690567
Menegat, S., Ledo, A., and Tirado, R. (2022). Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 12:14490. doi: 10.1038/s41598-022-18773-w
Moroenyane, I., Tremblay, J., and Yergeau, E. (2021). Soybean microbiome recovery after disruption is modulated by the seed and not the soil microbiome. Phytobiomes J. 5, 418–431. doi: 10.1094/PBIOMES-01-21-0008-R
Müller, C., Cramer, W., Hare, W. L., and Lotze-Campen, H. (2011). Climate change risks for African agriculture. PNAS 108, 4313–4315. doi: 10.1073/pnas.1015078108
Mwenda, G. M., Hill, Y. J., O’Hara, G. W., Reeve, W. G., Howieson, J. G., and Terpolilli, J. J. (2023). Competition in the Phaseolus vulgaris-Rhizobium symbiosis and the role of resident soil rhizobia in determining the outcomes of inoculation. Plant Soil 487, 61–77. doi: 10.1007/s11104-023-05903-0
Nature Editorial. (2020). To end hunger, science must change its focus. Nature 586. Available at: https://www.nature.com/articles/d41586-020-02849-6.pdf (Accessed on 28 December, 2023)
Njuki, J., Doss, C. R., and Boote, S. (2019). “Women’s control over income: implications for women’s empowerment and the agricultural sector” in 2019 annual trends and outlook report: Gender equality in rural Africa: From commitments to outcomes. eds. A. R. Quisumbing, R. S. Meinze-Dick, and J. Njuki (Washington, DC: International Food Policy Research Institute (IFPRI)), 149–175.
Nyaga, J. W., and Njeru, E. M. (2020). Potential of native rhizobia to improve cowpea growth and production in semiarid regions of Kenya. Front. Agron. 2:606293. doi: 10.3389/fagro.2020.606293
Obaton, M., Bouniols, A., Piva, G., and Vadez, V. (2002). Are Bradyrhizobium japonicum stable during a long stay in soil? Plant Soil 245, 315–326. doi: 10.1023/A:1020447928911
Peoples, M., Herridge, D., Alves, R., Urquiaga, S., Boddey, R., et al. (2009). The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 48, 1–17. doi: 10.1007/BF03179980
Popoola, J. O., Aworunse, O. S., Ojuederie, O. B., Adewale, B. D., Ajani, O. C., Oyatomi, O. A., et al. (2022). The exploitation of orphan legumes for food, income, and nutrition security in sub-Saharan Africa. Front. Plant Sci. 13:782140. doi: 10.3389/fpls.2022.782140
Pudasaini, R. (2024). White Fonio phenotyping and legume nodule crushing to assist smallholder farmers. PhD Thesis, University of Guelph, Canada.
Pudasaini, R., Hewedy, O. A., and Raizada, M. N. (2023). Improving field legume nodulation by crushing nodules onto seeds: implications for small-scale farmers. Front. Agron. 5:1161978. doi: 10.3389/fagro.2023.1161978
Raimi, A., Roopnarain, A., and Adeleke, R. (2021). Biofertilizer production in Africa: current status, factors impeding adoption and strategies for success. Sci. Afric. 11:e00694. doi: 10.1016/j.sciaf.2021.e00694
Raizada, M. N., and Smith, L. (2016). A picture book of best practices for subsistence farmers: Edition: Sub Saharan Africa/Carribbean. Canada: University of Guelph Sustainable Agriculture Kit (SAK) Project, Guelph.
Rao, D. L. N., Adhya, T. K., and Saxena, A. K. (2019). Agricultural microbiology research progress in India in the new millennium. Proc. Indian Natn. Sci. Acad. 85, 925–947. doi: 10.16943/ptinsa/2019/49718
Remigi, P., Capela, D., Clerissi, C., Tasse, L., Torchet, R., Bouchez, O., et al. (2014). Transient hypermutagenesis accelerates the evolution of legume endosymbionts following horizontal gene transfer. PLoS Biol. 12:e1001942. doi: 10.1371/journal.pbio.1001942
Sachs, J. L., Ehinger, M. O., and Simms, E. L. (2010). Origins of cheating and loss of symbiosis in wild Bradyrhizobium. J. Evol. Biol. 23, 1075–1089. doi: 10.1111/j.1420-9101.2010.01980.x
Santos, M. S., Nogueira, M. A., and Hungria, M. (2019). Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 9:205. doi: 10.1186/s13568-019-0932-0
Singh, S., Gupta, G., Khare, E., Behal, K. K., and Arora, N. K. (2014). Effect of enrichment material on the shelf life and field efficiency of bioformulation of Rhizobium sp. and P-solubilizing Pseudomonas fluorescens. Sci. Res. Report. 4, 44–50.
Smale, M., Theriault, V., Allen, A., and Sissoko, M. (2022). Is cowpea a ‘women’s crop’ in Mali? Implications for value chain development. Afric. J. Agric. Res. Econ. 17, 157–170. doi: 10.53936/afjare.2022.17(2).11
Somasegaran, P., and Hoben, H. J. (1994). “Testing the survival of rhizobia on inoculated seeds” in Handbook for rhizobia (New York: Springer).
Ssemakula, E., and Mutimba, J. K. (2011). Effectiveness of the farmer-to-farmer extension model in increasing technology uptake in Masaka and Tororo districts of Uganda. S. Afr. J. Agric. Ext. 39, 30–46.
Syers, J. K., Lingard, J., Pieri, C., Ezcurra, E., and Faure, G. (1996). Sustainable land management for the semiarid and subhumid tropics. Ambio 25, 484–491.
Tan, X. L., Azam-Ali, S., Mustafa, M., Chai, H. H., Ho, W. K., Mayes, S., et al. (2020). Bambara groundnut: an underutilized leguminous crop for global food security and nutrition. Front. Nutr. 7:601496. doi: 10.3389/fnut.2020.601496
Thilakarathna, M. S., Chapagain, T., Ghimire, B., Pudasaini, R., Tamang, B. B., Gurung, K., et al. (2019). Evaluating the effectiveness of Rhizobium inoculants and micronutrients as technologies for Nepalese common bean smallholder farmers in the real-world context of highly variable hillside environments and indigenous farming practices. Agric. (Switz.) 9:20. doi: 10.3390/agriculture9010020
Thilakarathna, M. S., and Raizada, M. N. (2016). A meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil Biol. Biochem. 105, 177–196. doi: 10.1016/j.soilbio.2016.11.022
UN. (2011). The global social crisis: Report on the world social situation, Department of Economics and Social Affairs, United Nations, New York, 61–73. Available at: https://www.un.org/esa/socdev/rwss/docs/2011/rwss2011.pdf (Accessed on December 5, 2023)
UNESCO (2018). Global education monitoring report gender review. Paris: United Nations Educational, Scientific and Cultural Organization.
van den Berg, H., Phillips, S., Dicke, M., and Fredrix, M. (2020). Impacts of farmer field schools in the human, social, natural and financial domain: a qualitative review. Food Secur. 12, 1443–1459. doi: 10.1007/s12571-020-01046-7
van Heerwaarden, J., Baijukya, F., Kyei-Boahen, S., Adjei-Nsiah, S., Ebanyat, P., Kamai, N., et al. (2018). Soyabean response to rhizobium inoculation across sub-Saharan Africa: patterns of variation and the role of promiscuity. Agric. Ecosyst. Environ. 261, 211–218. doi: 10.1016/j.agee.2017.08.016
Vanlauwe, B., Hungria, M., Kanampiu, F., and Giller, K. E. (2019). The role of legumes in the sustainable intensification of African smallholder agriculture: lessons learnt and challenges for the future. Agric. Ecosyst. Environ. 284:106583. doi: 10.1016/j.agee.2019.106583
Vernooy, R., Shrestha, P., and Sthapit, B. (2015). Community seed banks: Origins, evolution and prospects. New York: Routledge.
Westhoek, A., Clark, L. J., Culbert, M., Dalchau, N., Griffiths, M., Jorrin, B., et al. (2021). Conditional sanctioning in a legume-Rhizobium mutualism. PNAS 118:e2025760118. doi: 10.1073/pnas.2025760118
Woomer, P., Huising, J., Giller, K., Baijukya, F., and Kantengwa, S. (2014). N2Africa final report of the first phase 2009–2013, N2Africa, Nairobi, Kenya. 138 pp.
Keywords: rhizobium, symbiosis, biological nitrogen fixation, smallholder, nodule, Africa, legume, inoculant
Citation: Pudasaini R and Raizada MN (2024) Nodule crushing: a novel technique to decentralize rhizobia inoculant technology and empower small-scale farmers to enhance legume production and income. Front. Sustain. Food Syst. 8:1423997. doi: 10.3389/fsufs.2024.1423997
Edited by:
P. V. Vara Prasad, Kansas State University, United StatesReviewed by:
John M. Maingi, Kenyatta University, KenyaCopyright © 2024 Pudasaini and Raizada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manish N. Raizada, cmFpemFkYUB1b2d1ZWxwaC5jYQ==
 Roshan Pudasaini
Roshan Pudasaini Manish N. Raizada
Manish N. Raizada