
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 14 June 2024
Sec. Agro-Food Safety
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1407497
This article is part of the Research TopicEnsuring Food Safety And Quality Throughout The Supply ChainView all 15 articles
This study aimed to identify and examine the prevalence of Aspergillus species in three types of feed collected from specialized dairy farms and local markets in Chiro town, Dire Dawa, and Harar cities in eastern Ethiopia. A total of 180 dairy feed samples were collected and sown, initially on YES agar and then sub-cultured to AFPA to identify Aspergillus species based on colony color, conidia, vesicle, and phialide features. Additionally, the aflatoxigenic potential of the colonies was tested using CAM-based UV fluorescence. The study revealed that the prevalence of Aspergillus species was 80.6% in dairy feeds with a mean count of 3.04 log10cfu/g. Among the identified species, A. flavus was found to be highly prevalent (80%) in the feed with a mean of 2.73 log10cfu/g (7.45 × 103 cfu/g). Meanwhile, A. parasiticus and A. niger were observed in 73.3% (mean 2.43 log10cfu/g) and 58.3% (mean 1.68 log10cfu/g) of feeds, respectively. Furthermore, the prevalence rates of all Aspergillus species in feeds were not significant (p > 0.05) among the study sites and feed sources. However, the mean count of total Aspergillus (3.47 ± 1.34 log10cfu/g), A. flavus (3.20 ± 1.27 log10cfu/g), and A. parasiticus (2.82 ± 1.41 log10cfu/g) was significantly higher in feeds from Dire Dawa city. Additionally, both the prevalence rates and mean counts of total Aspergillus (37.9% and 3.65 ± 1.16 log10cfu/g), A. flavus (38.2% and 3.26 ± 1.12 log10cfu/g), A. parasiticus (38.6% and 2.98 ± 1.34 log10cfu/g), and A. niger (37.1% and 2.11 ± 1.57 log10cfu/g) in total mixed ration were significantly higher (p < 0.05) than in other feed types. Out of the screened Aspergillus colonies, 81.42% were found to be aflatoxigenic, with 58.32% belonging to A. flavus and 41.68% to A. parasiticus. Therefore, widespread contamination of Aspergillus species in dairy feeds across the study sites raises food safety and public health concerns, which highlights the urgent need for stringent measures in feed quality control to curb its prevalence and the risk of aflatoxin exposure.
Dairy sector is vital to millions of people around the world by providing a wholesome food and a means of sustenance (FAO, 2019). However, the quality and safety of dairy cattle feed are essential to safeguard the wellbeing of the animals and safety of their products for human consumption. Thus, fungal contamination and its toxic metabolites adversely affect feed safety and quality, threatening the safety of dairy products and public health. Aspergillus, Penicillium, and Fusarium are among the fungal genera that frequently contaminate dairy feeds and feed ingredients globally (Adelusi et al., 2022). A. flavus, A. parasiticus, and A. niger are among the genera of Aspergillus species that are predominantly contaminate feedstuffs destined to dairy cattle (Fusseini et al., 2016; Gherbawy et al., 2020; Nleya et al., 2021). In particular, A. flavus and A. parasiticus are a primary producer of aflatoxins, such as AFB1, AFB2, AFG1, and AFG2 (Variane et al., 2018; Bouti et al., 2020), while certain strains of A. niger produces Ochratoxin A (El-Hamaky et al., 2016). Thus, Aspergillus fungus and the subsequent aflatoxins contamination in animal feeds poses serious problems for the dairy industries and public health worldwide.
Aspergillus fungus can contaminate a variety of agricultural commodities and animal feeds such as maize, wheat, oilseeds, peanuts, and others (Bayman and Baker, 2006; Richard, 2007; Adejumo and Adejoro, 2014; Chaisri et al., 2017). The contamination of feedstuff and their ingredients with Aspergillus fungus and aflatoxins can happen during the various stages of cultivation, harvesting, storage and transportation under different environmental conditions (Saleemi et al., 2017). Thus, the growth and proliferation of Aspergillus fungus in food and feed are determined by various climatic conditions such as ambient temperatures, relative humidity, precipitation, and others. Consequently, the hotter temperatures ranging from 25°C to 35°C and relative humidity above 70% foster the growth and proliferation of Aspergillus fungi, particularly, A. flavus and A. parasiticus (Awuchi et al., 2022). Additionally, Aspergillus fungi can proliferate in the feeds and feed ingredients under storage conditions with high air moisture and lacking air outlets (Iheanacho et al., 2014). Therefore, understanding the prevalence of Aspergillus species and aflatoxin production capacity under different geographical locations and climatic factors are vital, to designing of the appropriate mitigation strategies.
The prevalence of Aspergillus species in dairy feeds across several nations has been reported in numerous research studies (Omeiza et al., 2018; Claudious et al., 2019; Rangel-Muñoz et al., 2020; Adelusi et al., 2022). For instance, a study in South Africa reported that 63.6% of dairy feeds have been contaminated by Aspergillus species with colony counts of 4 × 104 cfu/g (Iheanacho et al., 2014). Similarly, Omeiza et al. (2019) found that Aspergillus species were present in 59.7% of dairy feeds in Fulani dairies in Northern Nigeria, of which 33.3% belonged to A. flavus and 8.3% to A. parasiticus. Similarly, Adelusi et al. (2022) reported that 80.0% of dairy cattle feeds collected from the smallholder dairy farmers in South Africa was contaminated by Aspergillus species. On the other hand, a contamination A. flavus (85.96%) and A. parasiticus (24.16%) in animal feed were reported (Rajarajan et al., 2021). Also, in grain feeds collected from Dhaka, Bangladesh, a colony count of A. flavus ranging from 2.8 × 102 to 3.8 × 102 cfu/g was found (Fakruddin et al., 2015).
Moreover, various types of animal feed and feed ingredients are frequently targeted by Aspergillus fungus all over the world. Accordingly, in the total mixed ration (TMR) of dairy cows from the provinces of Limpopo and Free State in South Africa, contamination rates of 48.6% for A. flavus and 40% for A. niger were revealed, with an average colony count of 7.1 × 105 cfu/g (Adelusi et al., 2022). Similarly, a study carried out in Aguascalientes, Mexico, found a 55.5% prevalence of total Aspergillus in TMR feed samples, with the majority of the samples exceeding 1 × 106 cfu/g colony counts (Álvarez-Días et al., 2022). Additionally, Rafik et al. (2022) found that 56% of maize samples from Dinajpur districts of Bangladesh was contaminated by Aspergillus fungus. Whereas, 100% of A. flavus contamination was reported in grain feed from Dhaka, Bangladesh (Fakruddin et al., 2015). Meanwhile, A. niger and A. flavus were found to be the most common contaminants in wheat bran from the Faisalabad district of Pakistan (Saleemi et al., 2017). Fungal count of 2.4 × 104 ± 1.3 × 104 log10cfu/g was reported in wheat bran, where 69.64% are Aspergillus species (Bouti et al., 2022).
Aspergillus fungus contamination in dairy feeds and aflatoxin in Ethiopia presents serious challenges for the dairy industry and public health (Abera, 2016; Mamo et al., 2020). A number of studies have demonstrated that aflatoxins specific to A. flavus and A. parasiticus, such as AFB1, AFB2, AFG1, and AFG2, are significantly abundant in dairy feeds, despite the fact that research on Aspergillus contamination in feeds is highly limited in Ethiopia. Research carried out in Ethiopia has demonstrated elevated levels of AFB1 contamination in a variety of dairy feeds, including wheat bran, oilseed cake, maize grains, and others, with concentrations ranging from 0 to 887.64 μg/kg (Dawit et al., 2016; Mulugeta, 2017; Rehrahie et al., 2018; Yohannes et al., 2018).
Furthermore, this study was carried out in the three major urban centers in eastern Ethiopia, which have a high number of specialized dairy farming that supplies the city’s rapidly increasing milk demand (Alemu, 2019). However, these specialized dairy producers mostly use a variety of concentrate feeds, such as wheat bran, maize feeds, total mixed rations, and brewer’s yeast byproducts to improve milk yield (Tegegn et al., 2017; Teshome et al., 2019; Zeleke, 2021). Fortunately, most of these feeds are highly susceptible to Aspergillus fungus and subsequent aflatoxins contamination (Dawit et al., 2016), which presents a serious risk to the dairy business and public health (Balina et al., 2018). Considering this reality, it is crucial to evaluate the Aspergillus fungi contamination in concentrate feeds to determine their prevalence and initiate appropriate mitigation strategies.
Additionally, it has been demonstrated that Aspergillus fungus growth and aflatoxin production is stimulated by hot ambient temperatures, high relative humidity, and precipitation (Awuchi et al., 2022; Chhaya et al., 2022). For this reason, the evaluation of Aspergillus fungi prevalence in dairy feeds under different geographical locations is essential to appreciate their significant effects and initiate appropriate mitigation strategies. Accordingly, the three main urban centers in eastern Ethiopia, which have different agro-climatic conditions were targeted for this study (Mohammed and De Waal, 2009; Brandsma et al., 2012; Ahmedin and Yesihak, 2020). Therefore, this study aimed to identify and examine the prevalence of Aspergillus species in three different feed types that were collected from dairy farms and local markets in Chiro town, Dire Dawa, and Harar cities in eastern Ethiopia.
Three major Eastern Ethiopian urban centers: Chiro town, Dire Dawa, and Harar cities (Figure 1) have been purposively selected for this study based on their potential in dairy production and their role as the primary milk marketing centers for the surrounding districts (Brandsma et al., 2012; Mengistu et al., 2016; Lemma et al., 2018). The selected urban centers are located in various agro-ecological zones: Dire Dawa city is situated in a lowland agro-ecological zone at an elevation of 1,170 m.a.s.l., while Chiro town has a semi-arid climate at 1,757 m.a.s.l. (Arabali and Amare, 2015; Abibeker et al., 2023). However, most parts of Harar city are located between 1900 and 2,200 meters above sea level in a midland agroecological zone (Biri et al., 2019).
A total of 180 concentrate feeds, including maize feeds (MF), total mixed ration (TMR), and wheat bran (WB), were collected for this investigation from two main feed sources: specialized dairy farms and local markets. The collection of feed samples was conducted between September 2021 and January 2022 in three specifically selected urban centers in Eastern Ethiopia, namely Chiro town, Dire Dawa, and Harar cities. In each of this urban centers, 10 samples of each feed type (MF: 10), (TMR: 10), and (WB: 10) were collected from the specialized dairy farms. Similarly, 10 samples from each of the three feed types (MF: 10), (TMR: 10), and (WB: 10) were gathered from local markets in each urban center. Consequently, 10x3x2x3 = 180 feed samples were collected from dairy cows and examined.
According to Daniel and Cross (2013), the sample size was calculated using a 5% level of precision and an expected prevalence (0.86) of Aspergillus species in feeds (Tahira et al., 2019). In-depth discussions with extension agents, livestock experts, and agricultural administrators of selected urban centers took place prior to the collection of feed samples. This was carried out to determine the sampling kebeles—the smallest administrative unit that has a comparatively higher number of specialized dairy farms and feed retailers and shops in the targeted urban centers. The Livestock Development Offices of respective urban centers were consulted to obtain the list of specialized dairy farms. Then, based on criteria such as milk output, lactating cows, feed utilized by the farms—wheat bran, maize feed, and total mixed ration, which are highly susceptible to Aspergillus contamination, the sampling dairy farms were identified. The dairy farms that were used for feed sample collection were then identified by random sampling technique. Subsequently, considering their feed retailers and shops that primarily sell feeds susceptible to Aspergillus fungus contamination, the local market centers were identified. Then, to gather commercial feed samples, the feed retailers and shops were identified using a systematic random sampling procedure, in collaboration with livestock extension workers. The feed samples were bought from the identified feed retailers and shops in each urban center.
Thus, an aggregated portion of the sample was created by taking a small amount of feed from different places of feed containers using the sampling spear. After the samples were well mixed, 0.5 kg of feed sample was taken from the aggregated sample and packed into a labeled sampling bag. Then, the feed samples were transported to Haramaya University’s Plant Pathology Laboratory, where the isolation and identification of Aspergillus species were performed.
Aspergillus species were isolated and identified from feed samples using two standard media, such as Yeast Extract Sucrose Agar (YES) and Aspergillus Flavus and Parasiticus Agar (AFPA; Variane et al., 2018). To prepare YES agar, the following ingredients were added: yeast extract (4 g/L), sucrose (20 g/L), potassium dihydrogen phosphate (1 g/L), magnesium sulfate (0.5 g/L), and agar (15 g/L). Meanwhile, 20 g/L of yeast extract, 10 g/L of bacteriological peptone, 0.5 g/L of ferric ammonium citrate, and 15 g/L of agar were used to prepare AFPA agar. Chloramphenicol was added to both culture media after they were autoclaved at 121°C and 15 psi for 15 min to inhibit bacterial growth.
Fungal isolation from the feed sample was conducted using the method outlined by Álvarez-Días et al. (2022) and Alsalabi et al. (2023) with some modifications. Briefly, 1 gram of ground feed samples to a particle size of 0.01 mm (Chincan, FW100, China), was weighed into a sterile test tube. Distilled water (9 mL) was added into 15 mL of sterilized falcon test tube and vortexed for 5 min. Serial dilutions of 10−1, 10−2, 10–3, and 10−4 were prepared, and 1 mL of the suspension was dispensed onto 90 mm petri plates containing YES agar medium. The plates were then incubated in the dark at 26°C for 5–7 days, following the method described by Variane et al. (2018). The primary identification of Aspergillus species were carried out using the sprouted cultures, following the method described by Klich (2002) and Samson et al. (2014). Colony counting was performed using a Gallenkamp, United Kingdom, colony counter. Subsequently, the colony count of Aspergillus species in feed samples was determined according to Adelusi et al. (2022) and expressed as colony-forming units per gram of sample (cfu/g).
Moreover, the contamination frequency (Fr) and relative density (RD) of the Aspergillus species colony were calculated as described in Vera et al. (2016).
To obtain pure cultures for morphological identification, the colonies of each species grown on YES agar were transferred to AFPA agar and incubated at 25°C in the dark for 5 days (Variane et al., 2018). Subsequently, the identification of Aspergillus species was carried out using their macroscopic and microscopic characteristics, as described in the keys provided by Klich (2002) and Samson et al. (2014). Macroscopic identification of Aspergillus species was based on colony color, while microscopic identification relied on the Lactophenol cotton blue slide staining technique, which examined conidia, vesicles, and phialides. Slide smears were viewed using a bright field compound microscope (Olympus CX2LI) under 10-x and 40-x magnification lenses, and photomicrographs of each species were taken.
From the identified Aspergillus species, the colony cultures of A. flavus and A. parasiticus, which can produce aflatoxins, were further sub-cultured on Coconut-Agar Medium (CAM). The preparation of CAM was based on the technique outlined by Ahmed et al. (2023) for testing of aflatoxigenic Aspergillus species using UV-fluorescence emission. To prepare CAM, 300 mL of hot distilled water and 100 g of coconut powder were mixed and passed through four layers of cheesecloth. Sodium hydroxide (Sigma-Adrich, India) was then added to adjust the pH to 7.0, and 20.0 g of agar was added to 1,000 mL of media as a solidifying agent. The culture media were autoclaved at 121°C and 15 psi for 15 min and chloramphenicol was added to inhibit bacterial growth. After incubating the A. flavus and A. parasiticus colony cultures in the dark at 26°C for 5–7 days, they were viewed under UV-fluorescence at a wavelength of 365 nm to test their aflatoxin-producing potential. The colonies producing aflatoxins displayed a blue-green fluorescence, while colonies that did not produce aflatoxin did not show such fluorescence (Abd El-Aziz et al., 2021).
The collected data was checked and entered into Microsoft Excel 2016 (MS Excel®) and then exported to SAS software (SAS Institute, Cary, NC, United States) for analysis. The incidence, RF and RD of Aspergillus species were summarized and presented using graphs and frequency tables. The logarithmic function log10 (x + 1) was used to transform the fungal counts prior to data analysis. The data analyses were performed using analysis of variance (ANOVA). Duncan’s test was employed to compare the mean colony counts (log10cfu/g) of each Aspergillus species among the study locations, feed sources, and feed types (α = 0.05).
The assessment of Aspergillus species incidence in the analyzed dairy feeds is presented in Figure 2. The overall frequency of Aspergillus species contamination in feed samples was analyzed. Out of the 180 concentrate feed samples examined, 145 (80.60%) feeds were contaminated by Aspergillus species, with an overall mean count of 3.04 log10cfu/g (Figure 3). However, 35 (19.4%) feed samples did not yield any Aspergillus species isolates. Furthermore, the feed samples collected from Dire Dawa city showed a higher incidence of Aspergillus species (29.4%) compared to other study locations such as Harar city (26.1%) and Chiro town (25%). Additionally, the feed samples collected from the specialized dairy farms (42.2%) had a higher incidence of Aspergillus species than the feed samples from local markets (38.3%). There was also a higher incidence of Aspergillus species in TMR (30.6%) compared to other feed types such as MF (26.7%) and WB (23.3%).
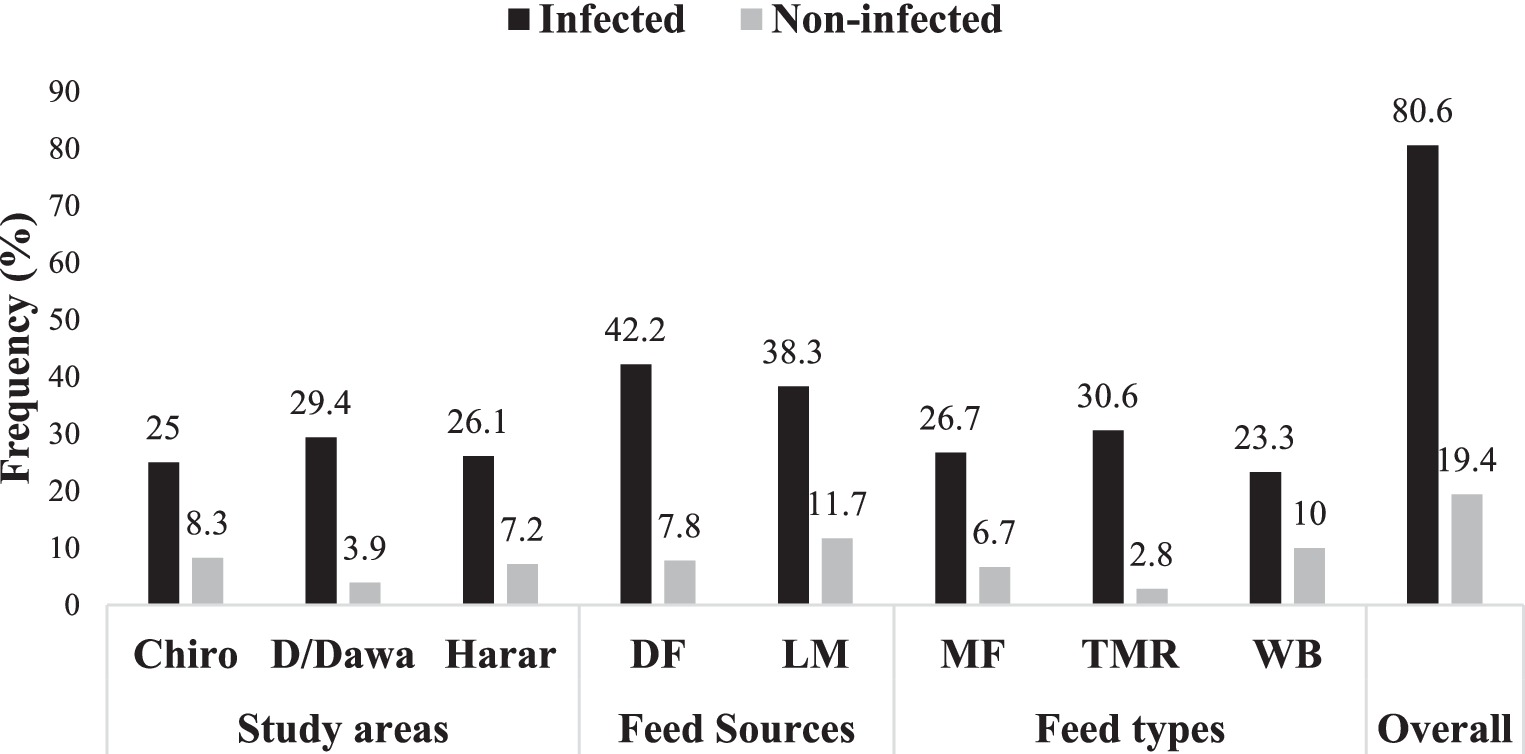
Figure 2. Incidence of Aspergillus species in feeds across study sites, feed sources, and feed types.
The figures below present the macroscopic and microscopic characterization of three different Aspergillus species. The isolates of A. flavus exhibited a yellowish-green color when viewed from the observe side and a cream to yellow hue when viewed from the reverse side (Figures 4A,B). Figures 5A,B depict colony isolates of A. parasiticus, which displayed a dark green color when viewed from the observe side and a creamy-yellow color from the reverse side. A. niger was observed with a creamy to creamy-yellow color from the reverse side and a black color from the observe side (Figures 6A,B).
Additionally, photomicrographs of each species were taken and examined to further identify the three Aspergillus species. For this purpose, slide smears from each pure culture were stained with lactophenol cotton blue and observed under a computer-mounted compound microscope. Consequently, A. flavus lactophenol-stained microscopic slide cultures displayed long hyphae with fertile vesicles on all sides (Figure 4C). On the other hand, A. parasiticus showed long, roughened hyphae with globose heads radiating from all surfaces (Figure 5C), and black A. niger revealed long hyphae with globose, blackish-brown fertile heads covering the entire surface (Figure 6C).
Moreover, Figure 3 illustrates the relative frequency, relative density, and mean count of Aspergillus species in dairy feeds. Among the identified species, A. flavus was the most dominant, accounting for 80% of the occurrence with a mean count of 2.73 log10cfu/g. A. parasiticus had a relative frequency of 73.3% with a mean count of 2.43 log10cfu/g, while A. niger had a frequency of 58.3% with a mean count of 1.68 log10cfu/g in dairy feeds. Similarly, in terms of relative density, A. flavus (53.8%) was the most abundant species, followed by A. parasiticus (27.74%) and A. niger (9.4%). Figure 3 also indicates the proportion of samples where the total count of Aspergillus species exceeded the good manufacturing practices (GMP) of >1 × 104 cfu/g in dairy feeds. Out of the total of 180 feed samples, 37.20% exceeded the GMP standard.
The occurrence of three identified Aspergillus species in dairy feeds was assessed and presented across the study sites, feed sources, and feed types (Table 1). The results showed that there was no significant difference (p > 0.05) in the occurrence of total Aspergillus, A. flavus, A. parasiticus, and A. niger between the study sites. However, there was a numerically higher occurrence of total Aspergillus species (36.6%), A. flavus (36.8%), A. parasiticus (37.1%), and A. niger (35.2%) in feed samples collected from Dire Dawa city compared to the other urban centers. In Harar city, the occurrence of total Aspergillus (31.7%), A. flavus (31.3%), A. parasiticus (32.6%), and A. niger (31.4%) was found, while in Chiro town, the occurrence of total Aspergillus (31.7%), A. flavus (31.9%), A. parasiticus (30.3%), and A. niger (33.3%) was observed.
Similarly, there was no significant difference (p > 0.05) in the occurrence of all Aspergillus species between the feed sources. The occurrence of total Aspergillus (52.4%), A. flavus (52.1%), A. parasiticus (50.7%), and A. niger (52.4%) was found in feed samples collected from dairy farms, and the occurrence of total Aspergillus (47.6%), A. flavus (47.9%), A. parasiticus (49.2%), and A. niger (47.6%) was observed in feed samples collected from local markets.
However, when analyzing the feed types, a significant difference (p < 0.05) was found in the occurrence of Aspergillus species, except for A. niger. The highest contamination of total Aspergillus (37.9%), A. flavus (38.2%), and A. parasiticus (38.6%) was observed in the total mixed ration (TMR), compared to maize feed (MF) and wheat bran (WB). The contamination rates of total Aspergillus (33.1%), A. flavus (33.3%), and A. parasiticus (32.6%) in MF were not significantly different from the contamination of total Aspergillus (29.0%), A. flavus (28.5%), and A. parasiticus (28.8%) in WB. Although there was no significant difference, a higher occurrence of A. niger was found in TMR (37.1%) compared to MF (32.4%) and WB (30.5%).
In this study, Tables 2, 3 present the log-transformed mean ± standard deviation, mean, and range of colony-forming units per gram (cfu/g) of Aspergillus species across the study sites, feed sources, and feed types. The results show a significantly different log10cfu/g mean of total Aspergillus species in feed samples between the study sites (p < 0.05) and feed types (p < 0.01). The feed samples collected from Dire Dawa city had a significantly higher mean count of 3.47 ± 1.34 log10cfu/g (1.09 × 104 cfu/g) for total Aspergillus, with a range of 1 × 103 to 3 × 104 cfu/g compared to the feeds from the other urban centers. However, there was no significant difference in the mean count of total Aspergillus species between feeds collected from Chiro town (2.77 ± 1.58 log10cfu/g, with a range of 3 × 102–1 × 104 cfu/g) and feed samples from Harar city (2.89 ± 1.64 log10cfu/g, with a range of 1.2 × 103–2.5 × 104 cfu/g).
Similarly, there was a significantly higher mean count of total Aspergillus species in total mixed ration (TMR) of 3.65 ± 1.16 log10cfu/g (1.18 × 104 cfu/g) with a range of 1 × 103 to 3 × 104 cfu/g compared to maize feed (MF) and wheat bran (WB). Additionally, the mean count of total Aspergillus species in MF (3.06 ± 1.57 log10cfu/g with a range of 1.2 × 102 to 2.5 × 104 cfu/g) was significantly higher than the mean count in WB (2.42 ± 1.63 log10cfu/g with a range of 1 × 103 to 1.4 × 104 cfu/g).
Furthermore, a highly significant (p < 0.01) log10cfu/g mean count of Aspergillus species was observed between the study sites, except for A. niger. The feed samples from Dire Dawa city had a significantly higher mean count of 3.20 ± 1.27 log10cfu/g (6.23 × 103 cfu/g) with a range of 3 × 102 to 1.7 × 104 cfu/g for A. flavus, and a mean count of 2.82 ± 1.41 log10cfu/g (3.91 × 103 cfu/g) with a range of 4 × 102 to 1.2 × 104 cfu/g for A. parasiticus than the other urban centers. However, a mean count of 2.53 ± 1.55 log10cfu/g (3.37 × 103 cfu/g) with a range of 3 × 102 to 1.2 × 104 cfu/g for A. flavus in feed samples from Harar city was not significant compared to a mean count of 2.47 ± 1.44 log10cfu/g (2.44 × 103 cfu/g) with a range of 3 × 102 to 1 × 104 cfu/g recovered in feed from Chiro town. Likewise, a mean count of 2.35 ± 1.54 log10cfu/g (2.46 × 103 cfu/g), with a range of 3 × 102 to 1 × 104 cfu/g for A. parasiticus in feed collected from Harar city was not significant compared to the mean count of 2.12 ± 1.55 log10cfu/g (1.84 × 103 cfu/g), with a range of 5 × 102 to 1 × 104 cfu/g in feed from Chiro town.
As for feed sources, there was no significant difference (p > 0.05) in the mean count of all Aspergillus species between feed sources. The feed from specialized dairy farms had mean counts of total Aspergillus (3.23 ± 1.45 log10cfu/g), A. flavus (2.89 ± 1.39 log10cfu/g), A. parasiticus (2.54 ± 1.55 log10cfu/g), and A. niger (1.79 ± 1.48 log10cfu/g). On the other hand, the mean counts of total Aspergillus (2.86 ± 1.63 log10cfu/g), A. flavus (2.58 ± 1.51 log10cfu/g), A. parasiticus (2.33 ± 1.50 log10cfu/g), and A. niger (1.57 ± 1.44 log10cfu/g) were found in the feed samples collected from local markets.
Moreover, the mean count of log10cfu/g of the three Aspergillus species in feed samples showed a highly significant difference (p < 0.01) between the analyzed feed types (Table 4). Specifically, A. flavus was found in TMR with a significantly higher mean count of 3.26 ± 1.12 log10cfu/g (5.83 × 103 cfu/g), ranging from 3 × 102 to 1.7 × 104 cfu/g compared to a mean count of 2.14 ± 1.52 log10cfu/g in WB. However, the mean count of A. flavus in MF was 2.80 ± 1.48 log10cfu/g (4.36 × 103 cfu/g), ranging from 4 × 102 to 1.3 × 103 cfu/g which was not significantly different from TMR. A mean count of 2.98 ± 1.34 log10cfu/g (4.62 × 103 cfu/g) within a range of 3 × 102 to 1.1 × 104 cfu/g for A. parasiticus and a mean count of 2.11 ± 1.57 log10cfu/g (1.33 × 103 cfu/g) with a range of 2 × 102 to 4 × 103 cfu/g for A. niger in TMR were found significantly higher compared to the other feed types. However, a mean count of 2.39 ± 1.55 log10cfu/g (2.50 × 103 cfu/g), with a range of 4 × 102 to 1.2 × 104 cfu/g of A. parasiticus in MF was not significant compared to a mean count of 1.92 ± 1.50 log10cfu/g (1.09 × 103 cfu/g) with a range of 5 × 102–1 × 104 cfu/g in WB. Also, a mean count of 1.63 ± 1.46 log10cfu/g (5.60 × 102 cfu/g) with a range of 3 × 102–3 × 103 cfu/g of A. niger in MF was not significant compared to a mean count of 1.30 ± 1.25 log10cfu/g (2.16 × 102 cfu/g) with a range of 1 × 102–3 × 103 cfu/g recovered from WB.
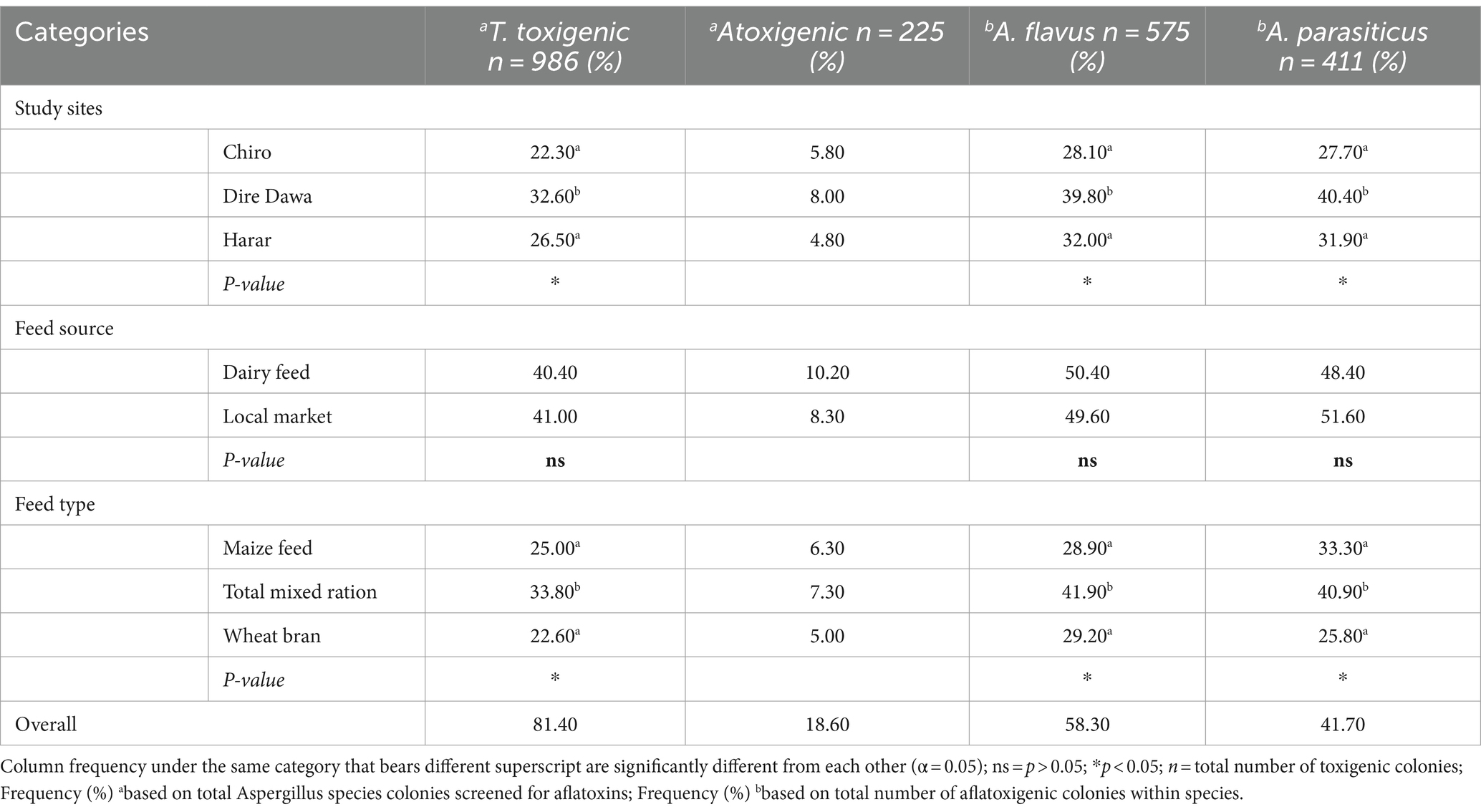
Table 4. Frequency of aflatoxigenic Aspergillus species colonies across feed types, feed sources, and study sites.
A CAM-based UV-fluorescence test was used to assess the aflatoxin-producing potential of A. flavus and A. parasiticus colonies across the different study sites, feed sources, and feed types (Table 4). Overall, 81.40% of the screened Aspergillus colonies were found to be aflatoxigenic, while 18.60% were non-aflatoxigenic. Among these toxigenic total Aspergillus colonies, 58.30% were A. flavus, and 41.70% were A. parasiticus. Therefore, the potential of aflatoxin-producing total Aspergillus colonies was significant (p < 0.05) between the study locations and feed types. The frequency of aflatoxigenic total Aspergillus colonies in feed samples from Dire Dawa city (32.60%) was significantly higher compared to the other study sites. However, there was no significant difference in the frequency of aflatoxigenic total Aspergillus between Chiro town (22.30%) and Harar city (26.50%). Similarly, the frequency of aflatoxigenic total Aspergillus colonies recovered in TMR (33.80%) was significantly higher compared to MF (25.0%) and WB (22.60%). There was no significant difference in the frequency of aflatoxigenic total Aspergillus colonies recovered from MF and WB.
The results revealed significant variation in the aflatoxigenic frequency of both species among the study sites (p < 0.01). Specifically, the frequency of aflatoxigenic A. flavus (39.8%) and A. parasiticus (40.40%) in feed samples collected from Dire Dawa city was statistically higher compared to the other study sites. In contrast, the aflatoxigenic frequencies of A. flavus (32.0%) and A. parasiticus (31.90%) colonies in feeds from Harar city were not significantly different from the frequency of A. flavus (28.10%) and A. parasiticus (27.70%) colonies in feeds from Chiro town. However, the frequency of aflatoxigenic A. flavus and A. parasiticus colonies was not significant (p > 0.05) between feed sources.
There was a significant difference (p < 0.01) in aflatoxin-producing A. flavus and A. parasiticus colonies between the feed types. Therefore, a higher frequency of aflatoxin-producing A. flavus (41.90%) and A. parasiticus (40.90%) isolates were found in TMR compared to other feed types. Additionally, the isolation frequency of aflatoxigenic A. flavus in MF (28.90%) was significantly higher than in WB (29.20%). However, the isolation frequency of aflatoxigenic A. parasiticus isolates in MF (33.30%) did not differ significantly from WB (25.80%).
Fungal contamination and related mycotoxins in animal feeds are a global issue because of their harmful effects on the health of humans and animals (Sarma et al., 2017). Similarly, fungal contamination of major crop produce poses a serious threat to food safety and security in Ethiopia (Ayelign and De Saeger, 2020; Mamo et al., 2020). This makes assessing the prevalence of Aspergillus fungus in dairy cattle feeds crucial. This study focused on the prevalence of Aspergillus species in different concentrate feeds collected from specialized dairy farms and local markets in three eastern Ethiopian urban centers.
The results of this study revealed that three distinct Aspergillus species were present in 80.60% (145/180) of the feeds that were examined, with variable proportions among study locations, feed sources, and feed types. Consistent with this finding, Motbaynor et al. (2021) reported a 72.5% incidence of Aspergillus in poultry feeds collected from Dire Dawa city in eastern Ethiopia. While research on fungal contamination in animal feeds is rather limited in Ethiopia, several studies have demonstrated a significant prevalence of Aspergillus species in cereal grains produced in various regions of the country. For instance, 94% of maize from Dire Dawa, Adama, and Ambo cities (Mamo et al., 2020), 70–100% of groundnut from eastern parts of Ethiopia (Mohammed et al., 2016), 80% of maize from South and Southwestern parts of Ethiopia (Getachew et al., 2018), and 47% of wheat grain from SNNP and Oromia regions were contaminated by Aspergillus fungi (Getahun et al., 2023).
Similar to the present findings, the incidence of Aspergillus species in dairy cattle feeds was reported at 80% (Adelusi et al., 2022) and 85% (El-Enbaawy et al., 2016) in South Africa and Giza governorate of Egypt, respectively. On the other hand, compared to the current findings, a relatively lower incidence of 63.6% (Iheanacho et al., 2014) and 33.3% (Omeiza et al., 2018) of Aspergillus species was reported in dairy feeds from South Africa and the Central State of Nigeria, respectively. In contrast, a higher incidence of Aspergillus species (100%) in dairy feeds from smallholder dairy farmers in Harare, Zimbabwe was reported (Claudious et al., 2019).
The isolation and identification of Aspergillus species were performed using both macroscopic and microscopic techniques. The macroscopic colony color was used to identify Aspergillus species. In addition, Aspergillus species were identified using microscopic characteristics such as conidiophore, vesicle, phialides, and conidia. Thus, in our investigation, three distinct Aspergillus species contaminating dairy cattle feeds were identified. Consistent with this finding, A. flavus, A. parasiticus, and A. niger were isolated and identified from animal feeds based on their morphological characteristics, including colony colors, conidia, vesicles, conidiophores, and phialides (Habib et al., 2015; Saleemi et al., 2017; Rajarajan et al., 2021).
A. flavus, which accounted for 80% of contamination in dairy feed, was the most prevalent Aspergillus species, followed by A. parasiticus, which had a prevalence rate of 73.3%. In contrast, feed samples had a comparatively lower level of A. niger contamination at 58.3%. These findings are consistent with earlier studies that found a contamination rate of 85.96% for A. flavus (Rajarajan et al., 2021) and 58.3% for A. niger (El-Enbaawy et al., 2016) in dairy feeds. On the other hand, lower contamination rates of A. flavus (65.6%), A. parasiticus (11.1%; Khalifa et al., 2022), and A. niger (24.16%; Rajarajan et al., 2021) were reported in feeds. Likewise, A. flavus was the most abundant species with a relative density of 53.8%, followed by A. parasiticus (27.74%) and A. niger (9.4%). The relative densities found in this study were in line with those found in dairy cow feeds: A. flavus (59.78%), A. parasiticus (19.4%), and A. niger (16.54%; Rosa et al., 2008; Makau et al., 2016; Rajarajan et al., 2021). However, it was shown that dairy feeds had lower relative densities of A. flavus (31.6%) and A. parasiticus (11.1%; Rosa et al., 2008; Khalifa et al., 2022).
Tables 1–3 shows the prevalence rate and mean log10cfu/g of the three identified Aspergillus species in dairy feeds. In this study, dairy feeds collected from Dire Dawa city had a slightly higher prevalence of total Aspergillus (36.6%), A. flavus (36.8%), A. parasiticus (37.1%), and A. niger (35.2%), however, the differences were not statistically significant (p > 0.05). It is difficult to find the prevalence data of Aspergillus fungi in animal feeds in Ethiopia. The results of this investigation are in line with a study carried out in a neighboring country, which found a non-significant (p > 0.05) Aspergillus contamination in maize from Nandi (73%) and Makueni (80%) counties of Kenya (Okoth et al., 2012). Similarly, Aspergillus species prevalence was shown to be non-significant (p > 0.05) in maize from different parts of Bangladesh (Rafik et al., 2022). However, Motbaynor et al. (2021) found that poultry feed from Dire Dawa city in Ethiopia had a greater prevalence of A. flavus (48.9%) and a lower prevalence of A. parasiticus (23.6%). The inconsistencies in the fungal prevalence may arise from the feed ingredients or feed processing methods used in chicken feeds. In contrast to this finding, a significantly different prevalence of A. flavus and A. parasiticus in maize from Nandi and Makueni counties was found in Kenya.
Furthermore, the results of this study indicate that the total Aspergillus species had an overall mean count of 3.04 log10cfu/g (7.45 × 103 cfu/g) with a range of 1 × 103 to 3 × 104 cfu/g. In line with this finding, Iheanacho et al. (2014) reported a 4 × 104 cfu/g of Aspergillus species population in compound feeds of dairy cattle from South Africa. However, a higher Aspergillus species count ranging from 1.4 × 103 to 7.3 × 105 cfu/g was found in the mixed feed of dairy cows from Rio de Janeiro state, Brazil (Keller et al., 2016). Similarly, Mirabile et al. (2019) reported a 7.91 log10cfu/g of Aspergillus species count in cattle feeds from Sicily, Italy, which was higher than the current finding. Whereas, a mean of 3.47 ± 1.34 log10cfu/g (1.09 × 104 cfu/g) of total Aspergillus count in the feeds collected from Dire Dawa city was significantly higher than in the feeds from other study sites. Consistent with the present study, high mean counts of Aspergillus species in maize feed collected from the Eastern region (1.09 ± 6.42 cfu/g; range of 0–9.0 cfu/g) compared to the Western region (0.82 ± 6.05 cfu/g, range of 0–7.33 cfu/g) of South Africa (Nji et al., 2022). On the other hand, a study conducted on poultry feed from five agroecological zones in Nigeria, which concurs with our findings, found a mean count of 3.56 log10cfu/g of total Aspergillus species, with A. flavus being the most prevalent species (Ezekiel et al., 2014).
On the other hand, there is a higher mean count of 2.73 log10cfu/g for A. flavus compared to the mean count of 2.43 log10cfu/g for A. parasiticus and 1.68 log10cfu/g for A. niger in dairy feed. Consistent with this, a higher mean count of A. flavus (2.12 log10cfu/g) compared to A. parasiticus (0.32 log10cfu/g) and A. niger (0.74 log10cfu/g) was found in dairy (Omeiza et al., 2019). Similarly, feed from the Iranian province of Basrah was found to contain a higher mean count of A. flavus (8 × 103 cfu/g) compared to A. parasiticus (0.3 × 103 cfu/g) and A. niger (6.6 × 103 cfu/g; Alkhursan et al., 2021). Moreover, the feed samples collected from Dire Dawa city had a higher mean count of 3.20 ± 1.27 log10cfu/g (6.23 × 103 cfu/g) with a range of 3 × 102 to 1.70 × 104 cfu/g for A. flavus and 2.82 ± 1.41 log10cfu/g (3.91 × 103 cfu/g) with a range of 4 × 102 to 1.2 × 104 cfu/g for A. parasiticus compared to the feed samples collected from the other urban centers. According to Udom et al. (2012), dairy feed from the Jos south area of Plateau state in Nigeria had a mean count of 3.4 log10cfu/g of A. flavus, which is consistent with our study. Similarly, commercial feeds from Nigeria had an average A. flavus population of 3.27 log10cfu/g (Ezekiel et al., 2014). However, Ghiasian and Maghsood (2011) found that dairy feeds from Hamadan, Iran had lower mean levels of A. flavus (7.25 × 102 cfu/g) and A. parasiticus (7.5 × 102 cfu/g). On the other hand, corn grain from North Sumatera in Indonesia showed a comparatively higher mean count of A. flavus (4.8 log10cfu/g; Nurtjahja et al., 2022).
The environmental factors, such as ambient temperatures and relative humidity, that promote fungal growth in animal feeds (Awuchi et al., 2022; Sissinto et al., 2023), may have been the underlying factors for the mean differences of Aspergillus fungi in feeds across the study sites. Consistent with this, Ráduly et al. (2020) reported that A. flavus (25–30°C), A. parasiticus (15–33°C) and A. niger (24–37°C) grow within a range of temperatures, whereas Shehu and Bello (2011) revealed that the growth of Aspergillus species increased linearly as relative humidity increased from 50.5 to 85.0 and 100%. Moreover, Mannaa and Kim (2018) revealed linear relationships, and a unit increase in temperature resulted in greater effects than that of relative humidity on fungal populations. In line with this, Dire Dawa city being located in a lowland agro-ecology and having higher ambient temperatures ranging from 19 to 32.8°C (Arabali and Amare, 2015) and (Dire Dawa Wikipedia, n.d.), may have contributed to a significantly higher mean count of total Aspergillus, A. flavus, and A. parasiticus than the other urban centers.
Furthermore, significantly different (p > 0.05) prevalence and mean count of total Aspergillus, A. flavus, and A. parasiticus were found between feed types (Tables 1–3). As a result, total mixed ration (TMR) had a significantly higher level of Aspergillus contamination (37.9%), A. flavus (38.2%), and A. parasiticus (38.6%) than maize feed (MF) and wheat bran (WB). This finding is consistent with a report by Omeiza et al. (2019), which found 33.3% A. flavus contamination in feeds for dairy cattle in South Africa. However, Davari et al. (2015) reported lower A. parasiticus (8.3%) contamination in TMR, compared to this study. Furthermore, the prevalence of total Aspergillus species, A. flavus, and A. parasiticus found in WB feed samples was not statistically significant when compared to their prevalence in MF. In line with our finding, the prevalence of A. flavus (20.0%) in WB (Ghaemmaghami et al., 2018) but higher in MF (52.5%; Ismael et al., 2019) was observed. On the other hand, a relatively lower prevalence of A. parasiticus in WB (16.67%) and MF (7.5%) was reported compared to the present finding (Saleemi et al., 2017; Ismael et al., 2019).
Moreover, a significantly higher mean count of 3.26 ± 1.12 log10cfu/g (5.83 × 103 cfu/g) of A. flavus was observed in TMR compared to WB feed samples. While a significantly higher mean count of 2.98 ± 1.34 log10cfu/g (4.62 × 103 cfu/g) of A. parasiticus and 2.11 ± 1.57 log10cfu/g (1.33 × 103 cfu/g) of A. niger in TMR was found compared to MF and WB feed samples. In contrast, a lower mean count of 2.12 log10cfu/g for A. flavus, 0.74 log10cfu/g for A. niger, and 0.32 log10cfu/g for A. parasiticus was reported in concentrate mix for dairy feeds collected from the Fulani province in South Africa (Omeiza et al., 2019). Similarly, in the present study, significantly higher mean counts of 2.80 ± 1.48 log10cfu/g (4.36 × 103 cfu/g) for A. flavus in MF were observed compared to WB. In contrast, commercial wheat grains obtained from the Brazilian states of Parana and São Paulo showed a lower mean count of 28.8 ± 7.32 cfu/g of A. flavus (Faria et al., 2017). However, the mean counts of A. parasiticus and A. niger were not significantly different between MF and WB feed samples. Contrary to the present finding, lower counts of A. flavus ranging from 2.8 × 102 to 3.8 × 102 cfu/g and A. parasiticus ranging from 0.8 × 101 to 1.2 × 102 cfu/g were reported in maize feeds (Fakruddin et al., 2015). Likewise, maize feed collected from Amman, Jordan, contains a lower count of A. flavus ranging from 0.7 × 101–1.05 × 102 cfu/g, and A. parasiticus ranging from 0.8 × 101–1.20 × 102 cfu/g (Al-Hmoud et al., 2012).
In addition to the impact of environmental factors, the type of substrate, nutrient composition, and moisture content may all play a critical role in the significant differences in fungal populations among various feed types. In line with this, Kos et al. (2023), noted that the degree of colonization of fungus in a given food or feedstuff depends on numerous factors, including the type of substrate, the availability of nutrients, humidity, and others. Moreover, Daou et al. (2021) noted that fungi may grow quickly on a substrate high in carbohydrates and rich in carbon and nitrogen. Accordingly, this may hold true specifically to our study since TMR (13%) has a greater level of crude fiber than MF (2.2%) and WB (8.2%; Kim et al., 2018).
Moreover, 37.20% of the samples greater than the GMP standard (1 × 104 cfu/g) in Aspergillus species isolates were found in this study. In agreement with the current finding, 37.5% of dairy cattle feed and 37.14% of beef cattle feed beyond the GMP fungal count were reported in the Markazi province of Iran (Rezaei et al., 2015). However, a higher sample proportion (48.4%) beyond 1 × 106 cfu/g fungal count was reported in dairy feeds from different provinces of Egypt (Khalifa et al., 2022). Moreover, Omeiza et al. (2019) reported that none of the mean counts of total Aspergillus species in dairy cattle feeds were beyond the maximum recommended limit for poor feed quality. Thus, comparing with this, the questions about feed safety fed to the dairy cattle without being checked, in the present study can be aroused.
On the other hand, there was no significant difference (p > 0.05) in the mean counts and occurrence of all Aspergillus species in the feed samples that were collected from dairy farms and local markets. However, compared to the occurrence (47.6%) and mean count (2.86 ± 1.63 log10cfu/g) in the feed samples from local markets, there was a numerically higher occurrence (52.4%) and mean counts (3.23 ± 1.45 log10cfu/g) of total Aspergillus in feeds from dairy farms. This may suggest that pre-harvest contamination of feed ingredients may contribute to the Aspergillus species contamination in the feedstuffs. In line with this, Assaye et al. (2016) reported both pre- and post-harvest contamination of Aspergillus fungi in cereal grain from the West Gojam zone of Ethiopia, where post-harvest had a higher fungal occurrence.
In Table 4, 81.40% of the screened Aspergillus colonies were found to be aflatoxigenic. Among these, 58.30% were identified as aflatoxigenic A. flavus colonies, while 41.70% were A. parasiticus colonies. Consistent with the present study, 81.08% of aflatoxigenic Aspergillus species were reported in grain feeds (Salisu et al., 2020). However, a lower frequency of aflatoxigenic Aspergillus species was reported in dairy feed (52%) from Katsina State of Nigeria, animal feed from Algeria (68.4%), and dairy feed (25%) obtained from the Fulani province in South Africa (Ghaemmaghami et al., 2016; Salisu et al., 2020). Moreover, a comparable aflatoxigenic Aspergillus species (70%) was found in poultry feeds collected from Tehran and Alborz provinces (Ghaemmaghami et al., 2018). In the present study, the frequency of aflatoxigenic Aspergillus colonies screened from the feed collected in Dire Dawa city (32.35%) was found to be significantly higher than in Chiro town (22.11%) and Harar city (26.29%). Similarly, significantly different aflatoxigenic Aspergillus species were found in maize from Makueni and Nandi counties of Kenya (Okoth et al., 2012). Furthermore, the frequency of aflatoxigenic A. flavus (56.9%) and A. parasiticus (52.7%) in the feeds collected from Dire Dawa city was found statistically higher than in the other study sites. Inconsistent with this 54% of A. flavus isolates were reported in dairy feed collected from the Northern Punjab of Pakistan (Khalid et al., 2018). Whereas, a higher aflatoxigenic A. parasiticus (75%) was reported in dairy feeds from Parana State of Brazil (Variane et al., 2018).
The high proportion of Aspergillus species with the potential to produce aflatoxins is a concern for the welfare of the dairy industry in the region, as this can affect animal health and pose a threat to public health. The variability in optimum temperatures among the urban centers may be one of the factors contributing to the significant difference in the frequency of aflatoxigenic A. flavus and A. parasiticus throughout the study sites. Thus, Ráduly et al. (2020) noted that the optimal temperature for A. flavus and A. parasiticus to produce aflatoxin is between 28 and 35°C. Accordingly, the higher ambient temperature in Dire Dawa city compared to the other urban centers may have contributed to the higher frequency of aflatoxigenic A. flavus and A. parasiticus colonies found in the feed from this urban center.
Likewise, a significantly higher frequency of aflatoxigenic A. flavus (42.82%) and A. parasiticus (38.21%) colonies was found in TMR compared to the other feed types. In contrast to this finding, a higher isolation frequency of aflatoxigenic A. flavus (70%) in mixed feeds was reported (Bhagya et al., 2019). Similarly, higher frequencies of aflatoxigenic A. flavus (43%) and A. parasiticus (67%) in maize, and A. flavus (50%) and A. parasiticus (80%) in wheat bran were reported (Saleemi et al., 2017). However, a lower frequency of A. parasiticus (12.85%) was reported in mixed feed (Makau et al., 2016). Additionally, ambient temperatures and availability of water were found to be important factors related to gene expression and aflatoxin biosynthesis of the fungus (Medina et al., 2014). Furthermore, complex sugar-containing substrates may be slower to digest and slow down aflatoxin production, while soluble sugar-containing substrates can readily digest and promote the production of more aflatoxins (Daou et al., 2021). Therefore, the higher proportion of aflatoxigenic Aspergillus species colonies in Dire Dawa city may have been promoted by both hotter climates and the substrates of TMR feed samples.
The analysis of the prevalence of Aspergillus species in dairy feeds under investigation revealed a worrisome level of feed contamination (80.60%), with an overall mean fungal load of 3.04 log10cfu/g. The identification and characterization of Aspergillus species highlighted the dominance of A. flavus (80.0%), followed by A. parasiticus (73.3%) and A. niger (58.3%). Thus, a significantly higher mean fungal load of total Aspergillus (3.47 ± 1.34 log10cfu/g), A. flavus (3.20 ± 1.27 log10cfu/g), and A. parasiticus (2.82 ± 1.41 log10cfu/g) was observed in samples from Dire Dawa city, highlighting climatic conditions as one of the main underlying factors for Aspergillus growth and proliferation. Moreover, both the occurrence and mean counts of fungal load of total Aspergillus (37.9% and 33.80 log10cfu/g), A. flavus (38.2% and 7.30 log10cfu/g), and A. parasiticus (38.6% and 41.90 log10cfu/g) were significantly higher in total mixed ration, suggesting a potential association between feed composition and fungal contamination. Additionally, 37.2% of examined feeds exceeded the GMP standard (>1 × 104 cfu/g or 4 log10cfu/g) of total fungal load for dairy feeds, raising serious safety and quality concerns of feed for dairy cattle.
Furthermore, the potency of Aspergillus species colonies to produce aflatoxins presents major worries about aflatoxin contamination in dairy feeds and signifies a public health risk associated with dairy products. To further understand its calamity to public health risk, research on aflatoxin occurrence in feed and dairy products is vital. Thus, the widespread Aspergillus species contamination in dairy feeds across the study sites raises food safety and public health concerns in study locations, highlighting the urgent need for stringent measures for feed quality control to curb the prevalence of Aspergillus species in feed and the risk of aflatoxin exposure in dairy products. Additionally, awareness creation and initiation of appropriate mitigation strategies are essential for dairy stakeholders to minimize Aspergillus species prevalence and safeguard public health.
The raw data supporting the conclusion of this article will be made available by the author, without undue reservation.
AT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. AM: Resources, Supervision, Validation, Writing – review & editing. MY: Supervision, Validation, Writing – review & editing. YY: Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
This study was made possible through the support of many esteemed individuals, community members, institutions, and others. Therefore, the author would like to acknowledge all parties contributed to completion of this research article. Specifically, the author would like to express gratitude to the FDRE Ministry of Education, Haramaya University, and Bule Hora University for their partial support of the research activities during the study period.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abd El-Aziz, A., Shehata, S. M., Hisham, S. M., and Alobathani, A. A. (2021). Molecular profile of aflatoxigenic and non-aflatoxigenic isolates of aspergillus flavus isolated from stored maize. Saudi J. Biol. Sci. 28, 1383–1391. doi: 10.1016/j.sjbs.2020.11.073
Abera, A. (2016). Review on the impact of Aflatoxine in dairy industry: occurrence and control the case of Ethiopia. Food Sci. Qual. Manag. 50, 56–64.
Abibeker, S. A., Mume, A. A., Mariye, M., Desalegn, D. G., and Furgasa, W. (2023). Causes of water pollution in Chiro River eastern Oromia. Ethiopia. Int. J. Sci. Res. Publ. 13, 111–119. doi: 10.29322/IJSRP.13.07.2023.p13912
Adejumo,, and Adejoro, (2014). Incidence of aflatoxins, fumonisins, trichothecenes and ochratoxins in Nigerian foods and possible intervention strategies. Food Sci. Qual. Manag. 31, 127–147.
Adelusi, O. A., Gbashi, S., Adebiyi, J. A., Makhuvele, R., Aasa, A. O., Oladeji, O. M., et al. (2022). Seasonal diversity and occurrence of filamentous Fungi in smallholder dairy cattle feeds and feedstuffs in South Africa. J. Fungi 8:1192. doi: 10.3390/jof8111192
Ahmed, M. Z., Alqahtani, A. S., Nasr, F. A., Rehman, M. T., Abdullah Alsufyani, S., AlAjmi, M. F., et al. (2023). Detection and isolation of aflatoxin producing aspergillus sp. in chewing and smokeless tobacco by microbial and molecular methods. Saudi. J. Biol. Sci. 103704:103704. doi: 10.1016/j.sjbs.2023.103704
Ahmedin, A., and Yesihak, Y. (2020). Milk production performance, challenges and opportunities of dairy cattle production in west Hararghe, Oromiya regional state. Open J. Anim. Sci. 10, 219–235. doi: 10.4236/ojas.2020.101012
Alemu, M. M. (2019). Urban and Peri-urban dairy cattle production in Ethiopia: a review. Online J. Anim. Feed Res. 9, 173–177.
Al-Hmoud, N., Ibrahim, M. A., Al-Rousan, H., and Alseyah, A. (2012). The prevalence of Aflatoxinogenic aspergillus parasiticus in Jordan. Int. J. Microbiol. 2012, 1–5. doi: 10.1155/2012/675361
Alkhursan, R. N., Khudor, M. H., and Abbas, B. A. (2021). Fungal contaminant of poultry feed in Basrah, Iraq. IOP Conf. Ser. Earth Environ. Sci. 761:012098. doi: 10.1088/1755-1315/761/1/012098
Alsalabi, F. A., Hassan, Z. U., Al-Thani, R. F., and Jaoua, S. (2023). Molecular identification and biocontrol of ochratoxigenic fungi and ochratoxin a in animal feed marketed in the state of Qatar. Heliyon 9:e12835. doi: 10.1016/j.heliyon.2023.e12835
Álvarez-Días, F., Torres-Parga, B., Valdivia-Flores, A. G., Quezada-Tristán, T., Alejos-De La Fuente, J. I., Sosa-Ramírez, J., et al. (2022). Aspergillus flavus and Total aflatoxins occurrence in dairy feed and aflatoxin M1 in bovine Milk in Aguascalientes, Mexico. Toxins (Basel). 14:292. doi: 10.3390/toxins14050292
Arabali, M., and Amare, E. G. (2015). A Cross sectional study on prevalence of Cephalopina titillator infection in camel (Camelus dromedaries) in Dire Dawa administrative region. Ethiopia. Adv. Biol. Res. (Rennes). 9, 225–229. doi: 10.5829/idosi.abr.2015.9.4.94209
Assaye, M., Gemeda, N., and Weledesemaya, G. (2016). Aspergillus species and aflatoxin contamination of pre and post- harvest maize grain in west Gojam. Ethiopia. Food Sci. Nutr. 2, 1–7. doi: 10.24966/FSN-1076/100013
Awuchi, C., Ondari, E., Eseoghene, I., Twinomuhwezi, H., Amagwula, I., and Morya, S. (2022). Fungal growth and mycotoxins production: types, toxicities, control strategies, and detoxification. Fungal Reproduction and Growth (IntechOpen), 1–20. doi: 10.5772/intechopen.100207
Ayelign, A., and De Saeger, S. (2020). Mycotoxins in Ethiopia: current status, implications to food safety and mitigation strategies. Food Control 113:107163. doi: 10.1016/j.foodcont.2020.107163
Balina, A., Kebede, A., and Tamiru, Y. (2018). Review on aflatoxin and its impacts on livestock. J. Dairy Vet. Sci. 6, 1–7. doi: 10.19080/JDVS.2018.06.555685
Bayman, P., and Baker, J. L. (2006). Ochratoxins: a global perspective. Mycopathologia 162, 215–223. doi: 10.1007/s11046-006-0055-4
Bhagya, A., Begum, S. R., Kiran, S., and Surekha, M. (2019). Incidence and Mycotoxigenic Fungi associated with cattle feeds in north Telangana region. India. Int. J. Curr. Microbiol. Appl. Sci. 8, 2247–2253. doi: 10.20546/ijcmas.2019.804.262
Biri, A., Ketema, K., Ayele, S., and Lule, D. (2019). Analysis of crop production constraints through participatory rural appraisal in Harari region, eastern Ethiopia; implications for Research and Development. J. Agric. Crop. 5, 209–217. doi: 10.32861/jac.510.209.217
Bouti, K., Mimoune, N. A., Mokrane, S., Djemouai, N., Vaessen, C. V., Mathieu, F., et al. (2022). Incidence of mycobiota and aflatoxin B1 in Algerian feed. Int. J. Postharvest Technol. Innov. 8, 125–144. doi: 10.1504/IJPTI.2022.121772
Bouti, K., Verheecke-Vaessen, C., Mokrane, S., Meklat, A., Djemouai, N., Sabaou, N., et al. (2020). Polyphasic characterization of aspergillus section Flavi isolated from animal feeds in Algeria. J. Food Saf. 40, 1–11. doi: 10.1111/jfs.12743
Brandsma, W., Mengistu, D., Kassa, B., Yohannes, M., and Lee, J.Van Der (2012). The major Ethiopian Milksheds ; Wageningen livestock research report 735, 245 blz. Lelystad, Netherlands.
Chaisri, W., Mongkon, W., Sugita-Konishi, Y., Van Dam, D., Huntley, I., and Suriyasathaporn, W. (2017). Feed and feed storage factors in relation to aflatoxin M1 contamination in bulk milk of smallholder dairy farms. Mycotoxins 67, 85–88. doi: 10.2520/myco.67_2_3
Chhaya, R. S., O’Brien, J., and Cummins, E. (2022). Feed to fork risk assessment of mycotoxins under climate change influences - recent developments. Trends Food Sci. Technol. 126, 126–141. doi: 10.1016/j.tifs.2021.07.040
Claudious, G., Sheperd, M., Charles, M. T., Jambwa, P., and Marumure, J. (2019). Isolation of AspergillusFlavusfrom dairy cattle feed And assessment of aflatoxin M1 in Milk from small dairy farms around Harare, Zimbabwe. Adv. Microbiol. Res. 3, 1–7. doi: 10.24966/AMR-694X/100009
Daniel, W. W., and Cross, C. L. (2013) in Biostatistics: A Foundation for Analysis in the health sciences. eds. C. L. Daniel and W. W. Cross. 10th ed (New York: John Wiley and Sons).
Daou, R., Joubrane, K., Maroun, R. G., Khabbaz, L. R., Ismail, A., and El Khoury, A. (2021). Mycotoxins: factors influencing production and control strategies. AIMS Agric. Food 6, 416–447. doi: 10.3934/AGRFOOD.2021025
Davari, E., Mohsenzadeh, M., Mohammadi, G., and Rezaeian-Doloei, R. (2015). Characterization of aflatoxigenic aspergillus flavus and A. parasiticus strain isolates from animal feedstuffs in northeastern Iran. Iran. J. Vet. Res. 16, 150–155
Dawit, G., Szonyi, B., Azage, T., Hanson, J., Grace, D., Gizachew, D., et al. (2016). Aflatoxin contamination of milk and dairy feeds in the greater Addis Ababa milk shed. Ethiopia. Food Control 59, 773–779. doi: 10.1016/j.foodcont.2015.06.060
Dire Dawa Wikipedia . (n.d.). Available at: https://en.wikipedia.org/wiki/Dire_Dawa [Accessed March 25, 2024].
El-Enbaawy, M., Soliman, M. M. H., and Ata, N. S. (2016). Frequency of fungal and aflatoxin B1 contaminants in cattle feed. Int. J. PharmTech Res. 9, 81–88.
El-Hamaky, A. M., Hassan, A. A., Yazeed, H. A.El, and Refai, M. (2016). Prevalence and detection of Toxigenic A. flavus, a. niger and A. ochraceus by traditional and molecular biology methods in feeds. Int. J. Curr. Res. 8, 25621–25633.
Ezekiel, C. N., Atehnkeng, J., Odebode, A. C., and Bandyopadhyay, R. (2014). Distribution of aflatoxigenic aspergillus section Flavi in commercial poultry feed in Nigeria. Int. J. Food Microbiol. 189, 18–25. doi: 10.1016/j.ijfoodmicro.2014.07.026
Fakruddin, M., Chowdhury, A., Hossain, M. N., and Ahmed, M. M. (2015). Characterization of aflatoxin producing aspergillus flavus from food and feed samples. Springerplus 4, 159–156. doi: 10.1186/s40064-015-0947-1
FAO (2019). The Global Dairy Sector: Facts. Food and Agriculture Organization of the United Nations, Rome, Italy.
Faria, C. B., Santos, F. C. Dos, Castro, F. F.De, Sutil, A. R., Sergio, L. M., Silva, M. V., et al. (2017). Occurrence of toxigenic aspergillus flavus in commercial bulgur wheat. Food Sci. Technol. 37, 103–111. doi: 10.1590/1678-457x.09316
Fusseini, I., Torkpo, S. K., and Afreh-Nuamah, K. (2016). Assessment of levels of temperature conditions on the effectiveness of multiple-layer hermetic bag for bio-rational management of aflatoxin in stored maize. AIMS Agric. Food 1, 342–353. doi: 10.3934/agrfood.2016.3.342
Getachew, A., Chala, A., Hofgaard, I. S., Brurberg, M. B., Sulyok, M., and Tronsmo, A.-M. (2018). Multimycotoxin and fungal analysis of maize grains from south and southwestern Ethiopia. Food Addit. Contam. Part B 11, 64–74. doi: 10.1080/19393210.2017.1408698
Getahun, M., Fininsa, C., Mohammed, A., Bekeko, Z., and Sulyok, M. (2023). Fungal species and multi-mycotoxin in bread wheat (Triticum aestivum L.) in Ethiopia. World Mycotoxin J. 16, 179–194. doi: 10.3920/WMJ2022.2820
Ghaemmaghami, S. S., Modirsaneii, M., Khosravi, A. R., and Razzaghi-Abyaneh, M. (2016). Study on mycoflora of poultry feed ingredients and fnished feed in Iran. Iran. J. Microbiol. 8, 47–54
Ghaemmaghami, S. S., Nowroozi, H., Nowroozi, H., and Tohidi Moghadam, M. (2018). Toxigenic fungal contamination for assessment of poultry feeds: mashed vs. Pellet. Iran. J. Toxicol. 12, 5–10. doi: 10.32598/ijt.12.5.534.1
Gherbawy, Y. A., Elhariry, H. M., Alamri, S. A., and El-Dawy, E. G. A. (2020). Molecular characterization of ochratoxigenic fungi associated with poultry feedstuffs in Saudi Arabia. Food Sci. Nutr. 8, 5298–5308. doi: 10.1002/fsn3.1827
Ghiasian, S. A., and Maghsood, A. H. (2011). Occurrence of aflatoxigenic fungi in cow feeds during the summer and winter season in Hamadan. Iran. African J. Microbiol. Res. 13, 1–6. doi: 10.5897/AJMR10.600
Habib, M. A., Abdu, P., Kwanashie, C. N., Kabir, J., and Negedu, A. (2015). Isolation and identification of aspergillus species from poultry feeds in Kaduna state. Nigeria. Microbiol. Res. Int. 3, 27–32.
Iheanacho, H. E., Njobeh, P. B., Dutton, F. M., Steenkamp, P. A., Steenkamp, L., Mthombeni, J. Q., et al. (2014). Morphological and molecular identification of filamentous aspergillus flavus and aspergillus parasiticus isolated from compound feeds in South Africa. Food Microbiol. 44, 180–184. doi: 10.1016/j.fm.2014.05.019
Ismael, M. A., Zaky, Z. M., Sharkawy, A. A., and Mohamed, A. M. S. (2019). Natural occurrence of some toxigenic fungi in some agricultural commodities used in animal feeds. Assiut Vet. Med. J. 65, 24–35.
Keller, L. A. M., Aronovich, M., Keller, K. M., Castagna, A. A., Cavaglieri, L. R., Rosa, C., et al. (2016). Incidence of mycotoxins (AFB1 and AFM1) in feeds and dairy farms from Rio de Janeiro state, Brazil. Vet. Med. - Open J 1, 29–35. doi: 10.17140/vmoj-1-106
Khalid, S., Hussain, N., and Imran, M. (2018). Detection of aflatoxigenicity of aspergillus flavus, based on potential gene marker, from food and feed samples. J. Food Saf. 38, 1–9. doi: 10.1111/jfs.12448
Khalifa, E., Mohesien, M. T., Mossa, M. I., Piekutowska, M., Alsuhaibani, A. M., Abdel-Wahab, B. A., et al. (2022). Diversity of toxigenic Fungi in livestock and poultry feedstuffs. Int. J. Environ. Res. Public Health 19:7250. doi: 10.3390/ijerph19127250
Kim, D. H., Choi, S. H., Park, S. K., Lee, S. S., and Choi, C. W. (2018). Effect of corn grain particle size on ruminal fermentation and blood metabolites of Holstein steers fed total mixed ration. Asian-Australasian J. Anim. Sci. 31, 80–85. doi: 10.5713/ajas.17.0069
Klich, M. A. (2002). Identification of common aspergillus species. The Netherlands: Centraalbureau voor Schimmelcultures.
Kos, J., Anic, M., Radic, B., Zadravec, M., Hajnal, E. J., and Pleadin, J. (2023). Climate change — a global threat resulting in increasing mycotoxin occurrence. Food Secur. 12, 1–18. doi: 10.3390/foods12142704
Lemma, S., Dagne, T., Gashaw, G., and Getu, A. (2018). Assessment of Cow’s Milk hygienic practices under small scale farmers in west Hararghe zone, Oromia National Regional State. Ethiopia. Adv. Life Sci. Technol. 68, 46–55.
Makau, C. M., Matofari, J. W., Muliro, P. S., and Bebe, B. O. (2016). Aflatoxin B1 and Deoxynivalenol contamination of dairy feeds and presence of aflatoxin M1 contamination in milk from smallholder dairy systems in Nakuru. Kenya. Int. J. Food Contam. 3:6. doi: 10.1186/s40550-016-0033-7
Mamo, F. T., Abate, B. A., Tesfaye, K., Nie, C., Wang, G., and Liu, Y. (2020). Mycotoxins in Ethiopia: a review on prevalence, economic and health impacts. Toxins (Basel). 12:648. doi: 10.3390/toxins12100648
Mannaa, M., and Kim, K. D. (2018). Effect of temperature and relative humidity on growth of aspergillus and Penicillium spp. and biocontrol activity of Pseudomonas protegens AS15 against Aflatoxigenic aspergillus flavus in stored Rice grains. Mycobiology 46, 287–295. doi: 10.1080/12298093.2018.1505247
Medina, A., Rodriguez, A., and Magan, N. (2014). Effect of climate change on aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 5, 1–7. doi: 10.3389/fmicb.2014.00348
Mengistu, K., Mohammed, A., Eyassu, S., Tarekegn, G., Estifanos, H., and Yonas, H. (2016). The dairy value chain and factors affecting choice of milk channels in Harar and Dire Dawa areas, eastern Ethiopia. Rev. Agric. Appl. Econ. 19, 10–18. doi: 10.15414/raae/2016.19.02.10-18
Mirabile, G., Bella, P., Conigliaro, G., Giambra, S., Alberto Vazquez, M., Davino, S., et al. (2019). Fungal contaminants in Sicilian livestock feeds and first studies on the enzymatic activity of aspergillus isolates. Cuba. J. Agric. Sci. 53, 373–386.
Mohammed, A., Chala, A., Dejene, M., Fininsa, C., Hoisington, D. A., Sobolev, V. S., et al. (2016). Aspergillus and aflatoxin in groundnut (Arachis hypogaea L.) and groundnut cake in eastern Ethiopia. Food Addit. Contam. Part B 9, 290–298. doi: 10.1080/19393210.2016.1216468
Mohammed, Y. K., and Waal, H. O.De. (2009). Herd management, milk production and reproduction of urban dairy farms in the Harar milk shed. Ethiop. J. Anim. Prod. 9, 57–75.
Motbaynor, A., Kassaye, D., Keffale, M., and Wasihun, P. (2021). Magnitude of Aflatoxigenic aspergillus species, level of aflatoxin B1, and associated factors in stored feed at poultry farms in Dire Dawa. Ethiopia. Vet. Med. Int. 2021, 1–11. doi: 10.1155/2021/6638083
Mulugeta, F . (2017). Study on level of aflatoxin in dairy cattle feeds and assess knowledge, attitude, and practice of feed producers, dairy farmers, and feed traders around Addis Ababa. Available at: https://projectng.com/topic/fo7183/.
Nji, N. Q., Christianah, A. M., Njie, A. C., and Mulunda, M. (2022). Biodiversity and distribution of aspergillus and their toxins in maize from Western and eastern regions of South Africa. Adv. Microbiol. 12, 121–149. doi: 10.4236/aim.2022.123011
Nleya, N., Ngoma, L., Adetunji, M. C., and Mwanza, M. (2021). Biodiversity of Aflatoxigenic aspergillus species in dairy feeds in Bulawayo. Zimbabwe. Front. Microbiol. 11, 1–10. doi: 10.3389/fmicb.2020.599605
Nurtjahja, K. Y., Bungsu, A., Esterina Silalahi, J., Simanullang, J., and Novi Lenta Gultom, B. (2022). Fungal contamination and characterization of aspergillus flavus on poultry feeds and their ingredients in north Sumatera. Curr. Appl. Sci. Technol. 23, 1–13. doi: 10.55003/cast.2022.01.23.004
Okoth, S., Nyongesa, B., Ayugi, V., Kangethe, E., Korhonen, H., and Joutsjoki, V. (2012). Toxigenic potential of aspergillus species occurring on maize kernels from two agro-ecological zones in Kenya. Toxins (Basel). 4, 991–1007. doi: 10.3390/toxins4110991
Omeiza, G. K., Kabir, J., Kwaga, J. K. P., Kwanashie, C. N., Mwanza, M., and Ngoma, L. (2018). A risk assessment study of the occurrence and distribution of aflatoxigenic aspergillus flavus and aflatoxin B1 in dairy cattle feeds in a central northern state. Nigeria. Toxicol. Reports 5, 846–856. doi: 10.1016/j.toxrep.2018.08.011
Omeiza, G. K., Kabir, J., Kwaga, J. K. P., Kwanashie, C. N., Mwanza, M., and Ngoma, L. (2019). Aflatoxin risk in dairy production: assessment of dairy cattle feed contamination by aspergillus Flavus and A. parasiticus in both conventional and traditional dairies. Glob. J. Med. Res. 19, 14–24.
Ráduly, Z., Szabó, L., Madar, A., Pócsi, I., and Csernoch, L. (2020). Toxicological and medical aspects of aspergillus-derived mycotoxins entering the feed and food chain. Front. Microbiol. 10, 1–23. doi: 10.3389/fmicb.2019.02908
Rafik, M., Afroz, F., and Rahman, M. (2022). Detection of aflatoxin-producing fungi in maize. Bangladesh Vet. 37, 27–35. doi: 10.3329/bvet.v37i1-2.59894
Rajarajan, P., Sylvia, K., Periasamy, M. P., and Subramanian, M. (2021). Detection of aflatoxin producing aspergillus flavus from animal feed in Karnataka. India. Environ. Anal. Heal. Toxicol. 36:e2021017. doi: 10.5620/eaht.2021017
Rangel-Muñoz, E. J., Valdivia-Flores, A. G., Moreno-Rico, O., Hernández-Delgado, S., Cruz-Vázquez, C., De-Luna-López, M. C., et al. (2020). Characterization of aspergillus flavus and quantification of aflatoxins in feed and raw milk of cows in Aguascalientes. Mexico. Rev. Mex. Ciencias Pecu. 11, 435–454. doi: 10.22319/rmcp.v11i2.5686
Rehrahie, M., Getnet, A., and Fassil, A. (2018). Determination of aflatoxin in dairy feeds and milk in some selected areas of Ethiopia. Food Environ. Saf. - XVII, 286–299.
Rezaei, M., Pourfard, I. M., Yahyaei, M., Gholamrezaei, M., Ghasemikhah, R., and Kazemi-Bonchenar, M. (2015). Evaluation of some dairy and beef cattle feed samples for fungal contamination in Markazi Province of Iran. Int.J.Curr.Microbiol.App.Sci 4, 1139–1146.
Richard, J. L. (2007). Some major mycotoxins and their mycotoxicoses-an overview. Int. J. Food Microbiol. 119, 3–10. doi: 10.1016/j.ijfoodmicro.2007.07.019
Rosa, C., Cavaglieri, L., Ribeiro, J., Keller, K., Alonso, V., Chiacchiera, S., et al. (2008). Mycobiota and naturally-occurring ochratoxin a in dairy cattle feed from Rio de Janeiro state. Brazil. World Mycotoxin J. 1, 195–201. doi: 10.3920/WMJ2008.1009
Saleemi, M. K., Khan, M. Z., Khan, A., Hameed, M. R., Khatoon, A., Abadin, Z. U., et al. (2017). Study of fungi and their toxigenic potential isolated from wheat and wheat bran. Toxin Rev. 36, 80–88. doi: 10.1080/15569543.2016.1233890
Salisu, B., Anua, S. M., Ishak, W. R. W., Mazlan, N., and Lawal, U. (2020). Incidence, distribution and phenotypic characterisation of aflatoxigenic fungi contaminating commonly consumed food grains in Katsina state. Nigeria. Malaysian J. Med. Heal. Sci. 16, 18–27.
Samson, R. A., Visagie, C. M., Houbraken, J., Hong, S. B., Hubka, V., Klaassen, C. H. W., et al. (2014). Phylogeny, identification and nomenclature of the genus aspergillus. Stud. Mycol. 78, 141–173. doi: 10.1016/j.simyco.2014.07.004
Sarma, U. P., Bhetaria, P. J., Devi, P., and Varma, A. (2017). Aflatoxins: implications on health. Indi JClin Biochem 32, 124–133. doi: 10.1007/s12291-017-0649-2
Shehu, K., and Bello, M. T. (2011). Effect of environmental factors on the growth of aspergillus species associated with stored millet grains in Sokoto. Niger. J. Basic Appl. Sci. 19, 218–223.
Sissinto, A. Y. C., Mintognisse, F. J. P., and Mawuton, A. H. U. (2023). Geographic distribution of aspergillus section Flavi subspecies isolated from crops, foods, and feedstuffs in Benin. Adv. Microbiol. 13, 361–372. doi: 10.4236/aim.2023.138023
Tahira, I., Sultana, N., Munir, A., Hasan, S. M., and Hanif, N. Q. (2019). Occurrence of aflatoxin M1 in raw and processed milk consumed in Pakistan. Pak. J. Pharm. Sci. 32, 1097–1101
Tegegn, A., Baudronb, F., and Wegary, D. (2017). Comparative performance of five maize varieties as livestock feed in the Rift Valley of Ethiopia. Acad. Res. J. Agric. Sci. Res. 2, 366–379. doi: 10.23880/oajar-16000143
Teshome, D., Fita, L., Feyissa, F., Kitaw, G., and Wondatir, Z. (2019). Effect of Total mixed ration on dry matter intake, Milk yield and composition of early lactating Jersey cows. J. Biol. Agric. Healthc. 7, 19–24.
Udom, I. E., Ezekiel, C. N., Fapohunda, S. O., Okoye, Z. S. C., and Kalu, C. A. (2012). Incidence of aspergillus section Flavi and concentration of aflatoxin in feed concentrates for cattle in Jos. Nigeria. J. Vet. Adv. 2, 39–46.
Variane, A. C. F., Dos, S. C. F., Castro, F. F.De, Barbosa-Tessmann, I. P., Santos, G. T. Dos, and Pozza, M. S. Dos S.,, et al. (2018). The occurrence of aflatoxigenic aspergillus spp. in dairy cattle feed in southern Brazil. Brazilian J. Microbiol. 49, 919–928. doi: 10.1016/j.bjm.2018.05.005
Vera, R., Arosemena, L., and Ángeles, M. (2016). Incidence of filamentous Fungi with toxigenic potential on samples of feed and raw materials for their manufacture. J. Microbiol. Biotechnol. Food Sci. 5, 599–601. doi: 10.15414/jmbfs.2016.5.6.599-601
Yohannes, B., Ayalew, W., and Getachew, A. (2018). Analysis to ascertain the determination for aflatoxin contamination of Milk and feeds from Gurage zone. Ethiopia. J. Agric. Res. 13, 1–11. doi: 10.3923/ijar.2018.1.11
Keywords: Aspergillus species, concentrate feeds, specialized dairy farms, commercial feed, local feed retailers, eastern Ethiopia
Citation: Tesfaye A, Mohammed A, Yusuf M and Yusuf Y (2024) Aspergillus species contamination in concentrate feeds collected from specialized dairy farms and local markets in selected urban centers of eastern Ethiopia. Front. Sustain. Food Syst. 8:1407497. doi: 10.3389/fsufs.2024.1407497
Received: 26 March 2024; Accepted: 20 May 2024;
Published: 14 June 2024.
Edited by:
Fatima Zahra Jawhari, Higher Institute of Nursing and Health Techniques, Fes Branch, MoroccoReviewed by:
Zineb Majbar, University Sidi Mohammed Ben Abdallah, MoroccoCopyright © 2024 Tesfaye, Mohammed, Yusuf and Yusuf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angassa Tesfaye, YW5nYXNzYXRlc2ZheWVAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.