
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 29 April 2024
Sec. Agro-Food Safety
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1406579
This article is part of the Research TopicEnsuring Food Safety And Quality Throughout The Supply ChainView all 15 articles
 Paul-Corneliu Boișteanu1
Paul-Corneliu Boișteanu1 Elena-Iuliana Flocea1
Elena-Iuliana Flocea1 Bianca-Georgiana Anchidin1
Bianca-Georgiana Anchidin1 Bianca-Maria Mădescu1
Bianca-Maria Mădescu1 Mădălina Matei1
Mădălina Matei1 Otilia Cristina Murariu2
Otilia Cristina Murariu2 Gabriela Frunză2
Gabriela Frunză2 Alina Narcisa Postolache3*
Alina Narcisa Postolache3* Marius-Mihai Ciobanu2*
Marius-Mihai Ciobanu2*Introduction: The level of essential minerals in meat is an important factor in human nutrition and health. Meat from responsibly managed wildlife is an alternative raw material with considerable nutritional benefits. Meat from hunted animals has essential and non-essential elements for the human body. It is important to carefully monitor the levels of heavy metals accumulated in the tissues of hunted animals in polluted areas to ensure food safety and environmental contamination. High levels of heavy metals in food and the environment can pose a danger to human health.
Methods: The study aims to investigate the levels of essential mineral elements and heavy metals in the muscle tissue and organs of wild boar harvested through the herd density control plan over the last decade in north-eastern Romania.
Results and discussion: The statistical analysis indicates that the age of the animals had a significant impact on Fe, Cu, and Zn levels in Longissimus lumborum. In the kidney a highly significant difference in Fe content by sex, with males showing higher values than females. The sex was shown to significantly influence the Mg levels. However, there are concerns about the accumulation of heavy metals such as Lead (Pb) and Cadmium (Cd), which may hurt the health of game meat consumers in the study area. Cd level it shows significant differences according to both age and sex, with higher concentrations in adults and males. Statistical analysis shows a negative correlation between Fe and Zn concentrations in muscle samples, while a positive correlation was found between Fe and Mn in kidney samples. There was also a positive association between Zn and Cu in muscle samples, but a negative association in kidney samples. Principal component analysis shows significant variation in essential element and heavy metals data between muscle and kidney samples. The loading plot shows a direct correlation between Pb and Cu and between Pb and Cd. However, an opposite correlation also is observed between Cu and Mg, Cd and Mg, and Pb and Mg. HQ (Hazard Quotient) for children compared to adults indicates a potentially higher risk associated with meat consumption among children because children are more vulnerable than adults. We report for the first time, to the best authors’ knowledge, various levels of essential minerals and exceeded maximum admitted level of heavy metals in the muscle tissues and kidneys of Sus scrofa ferus from Romania intended for human consumption, moreover, our findings highlight the need for strict monitoring and implementation of appropriate corrective measures, given the significant percentages of muscle and kidney samples exceeding the allowable limits for two of the most common toxic metals in the environment.
The recent studies focused on consumer perceptions and on their attitudes toward game meat have confirmed the growing interest in the product consumption and its positive characteristics, which meet consumers ‘need for healthy foods with a rich nutritional profile. Furthermore, the game meat contains bioactive compounds that directly influence the formation of a rich reservoir of flavor precursors that in turns result in specific sensory properties (Ciobanu et al., 2023). Game meat has a complete nutritional profile (Corradini et al., 2022; Frunză et al., 2023). In his study (Quaresma et al., 2011) it was found that an increased level of vitamin E in Sus scrofa muscle provides antioxidant protection for maintaining oxidative stability of the meat post-mortem. The outstanding value of wild boar meat seems to be its mineral content, especially iron (Fe) and zinc (Zn; Skobrák et al., 2011). Some nutrient deficiencies can affect the proper functioning of the whole body (Bourre, 2006). It is known that essential elements with well-defined physiological functions, including Magnesium (Mg), Manganese (Mn), Zinc (Zn), Copper (Cu), and Iron (Fe), are much more bioavailable in meat than in plant resources. The concentration of minerals in animal tissue can vary according to species, age, diet, and other environmental factors (Ribeiro et al., 2019; Murariu et al., 2023). Moreover, the absorption of a particular element may depend on the presence of minerals in the body. In particular, Zn and Cu can interact with each other in the absorption process (Dervilly-Pinel et al., 2017). Mg functions as a cofactor or activator for many critical enzymes involved in adenosine triphosphate (ATP) synthesis reactions, thus providing the energy required in all major metabolic pathways (Guo et al., 2003). Mg exhibits immune functions of the major histocompatibility complex (Tam et al., 2003; Alghamdi et al., 2023). Mn is an essential nutrient involved in intracellular activities, acting as a co-factor for various enzymes, including arginase, glutamine synthase (GS) and superoxide dismutase (SOD). Through these metalloproteins, manganese plays a crucial role in several processes like development, digestion, reproduction, antioxidant defense, energy production, immune response, and regulation of neuronal activities (Chen et al., 2018). Zn performs multiple roles throughout the body, acting as a regulator of molecular structure for various subcellular constituents and participating in the activity of various enzymes (Mehri, 2020). Fe is considered the essential element in the composition of myoglobin, a protein that supplies oxygen to muscles. It is indispensable for regular cell growth, development, and of hormones function, and connective tissue (Al-Fartusie and Mohssan, 2017). Haem forms covalent bonds with the protein globin to form hemoglobin, the main oxygen-carrying protein in mammalian red blood cells (Bhattacharya et al., 2016). Cu absorbed from food freely binds to plasma albumin and blood amino acids and next, in the liver, is incorporated in the copper-containing protein ceruloplasmin. Copper absorption occurs by active transport at lower levels of dietary copper and passive diffusion at high levels of dietary copper (Mehri, 2020).
Both humans and animals are subject to similar environmental pressures (Pereira and Vicente, 2013). As a result of the increased disruption of natural ecosystem functions, recent policy linkages have focused on the correlations between environmental conditions and food security on the welfare of animal resources, the quality of production offered and consumer safety (Matei et al., 2023). The heavy metals, such as Lead (Pb) and Cadmium (Cd) in particular, are often transported over long distances as fine particles by wind (Ciobanu et al., 2020). These toxic elements can enter the body via three different routes: ingestion, inhalation, and dermal absorption (Bodor et al., 2021). The number of reports on the chemical toxicology of heavy metals released into the environment from natural and anthropogenic sources has been constantly growing. These metals produce behavioral changes by altering the metabolism of neurotransmitters, especially catecholamines (Shukla and Singhal, 1984). Cadmium and Lead interact with the thiol group (mercapto-), chelating structures containing this ligand in biomolecules. Enzymes with a dithiol configuration in their active site are sensitive to the action of these heavy metals. The mercapto ligand appears to manifest a preference for polarizable metal ions, playing a role in the evolved defense mechanisms against these heavy metals. Glutathione, a ubiquitous tripeptide, offers potent protection for intracellular structures by chelating and sequestering metal ions. Similarly, the metallothionein protein, which contains a significant amount of cysteine, facilitates efficient metal sequestration within the cell, thereby contributing to the evolution of cellular tolerance to toxic metal ions (Kaczor-Kamińska et al., 2020). Numerous studies have reported that game meat may be contaminated with heavy metals and metalloids if harvested animals live in anthropogenically polluted areas or if the ammunition used damages the carcass (Srebočan et al., 2011; Taggart et al., 2011; Florijancic et al., 2015; Roslewska et al., 2016; Gerofke et al., 2019). Emerging research methods based on indicator species for heavy metals detection provide the most reliable data for monitoring the biological environment. In this respect, it has been reported that some species of Cervus elaphus L. and Sus scrofa harvested from a mine vicinity were found to contain more Pb in their bones than in their livers, indicating long-term exposure to Pb (Reglero et al., 2009). Some authors suggest that the kidneys are the most suitable target organs for testing accumulated heavy metals from the environment. Kidneys during harvesting are less likely to be contaminated or destroyed by bullets as opposed to the liver (Gall et al., 2015).
This work aimed to establish the level of the main mineral elements and heavy metals in the Longissimus lumborum muscle and kidneys from Sus scrofa ferus hunted between 2010 and 2021 in North-Eastern Romania, by controlling the environment and food resources. The animals were not strictly intended for the study, by the annual responsible wildlife management plan, the hunting fund administrator authorized the collection of samples for scientific purposes from deceased animals obtained in accordance with Romanian hunting regulations.
In order to control the herd density in the hunting region of Frasin District, Suceava County, located in North-Eastern Romania (Figure 1), a total of 124 wild boars (Sus scrofa ferus; 16 females and 108 males) were harvested during the 2010–2021 (winter) hunting seasons, in accordance with the national game, hunting, and wildlife management in Romania (Legea Vânătorii și a Protecției Fondului Cinegetic nr. 407/2006, 2010). The growth of wild boar was related to environmental conditions and food availability during the year (Postolache et al., 2015). Due to the limited feed supply during the cold winter months, supplementary feed was provided as concentrates and succulent forms (cereal grains, seeds, fresh fruit, red beets, turnips, potatoes, and carrots) in an average amount of 85.5 kg/animal/year between 1 November and 31 March each year.
Figure 1. The geographical origin of the samples used in the study depicting Suceava County, Frasin hunting area.
Animals were shot in the morning, and the health inspection was carried out in the afternoon in open environment (at the evisceration control point) at an average outside temperature between −5°C and − 15°C. Those shots in the upper right thigh were eliminated from the study. Animal age was calculated using dental eruption, which takes over 26 months to complete, replace, and wear patterns (Sáez-Royuela et al., 1989). After health inspection (Regulation (EC) no. 853/2004 of the European Parliament and of the Council, 2004) within the first 24 h postmortem, muscle, and kidney samples identified by sex and age class were collected, sealed in sterile bags, and transported to the laboratory under refrigerated conditions (0–4°C), cut from connective and adipose tissues, vacuum packed, and frozen at −20°C until analysis.
The processing of the samples and the method of determining the mineral elements were performed in accordance with the legislation (Standard European, 2002; European Committee for Standardization, 2003; European Commission, 2006). After cooling, ultrapure water and hydrochloric acid 6 N (Merck, Darmstadt, Germany) were added over the ash to dissolve it. The final residue was dissolved with 1 mL HCl 6 M and 10 mL ultrapure water. The obtained solution was filtered through a filter paper Whatman no. 1 and quantitatively transferred into a 50 mL plastic flask.
The calibration curves for the metals and essential minerals were:
The concentrations were determined by AA-6300 atomic absorption spectrophotometer (manufacturer: Shimadzu Corporation, Kyoto, Japan; Nunes et al., 2011) as follows: the obtained solutions were pulverized in the air-acetylene flame and the absorbance was registered at the characteristic wavelength for each studied element. The final results were calculated considering the dilution made and the initial amount of sample and were expressed in mg w.w. (Gimou et al., 2014; Babicz and Kasprzyk, 2019).
Through the process of assessing and managing the risk associated with the consumption of wild boar meat in mountain areas in terms of the presence of toxic metals, the Estimated Daily Intake (EDI) and Hazard Quotient (HQ) have been calculated. These tools provide a useful framework for estimating and assessing exposure to heavy metals and the risk to human health associated with the consumption of wild boar meat. By applying these methods, the aim is to identify and assess potential health risks to consumers and to develop appropriate management and prevention strategies (Doménech and Martorell, 2024; Zeng et al., 2024).
EDI calculation was performed taking into account the element content (g) found in meat/kidney samples (C), the exposure frequency (days/year), using the Eq. [1]:
was taken into consideration the following aspects: where C is the element content (_g/g), EF is the exposure frequency (days/year), ED is the duration of exposure (70 years for adults, 6 years for children), LC is the liver consumption (g), BW is the average body weight (70 kg for adults, 20 kg for children), and T is the average exposure time (365_ED; Kasprzyk et al., 2020; Pogurschi et al., 2023).
After the EDI calculation, the Hazard Quotient (HQ) was estimated using the calculation formula:
The risk values were calculated using the Eq. [2]:
where EDI is the Estimated Daily Intake (mg/kg b. w./day) and Rf D is the Reference Dose (Mohamed et al., 2017; Pilarczyk et al., 2020).
According to the frequency they consumed meat or kidney, the customers were divided into the following three main categories: those who consume wild boar meat or kidney regularly (90 times a year), those who consume on an infrequent basis (12 times a year), and individuals who consume meat or kidney on an as-needed basis (two times a year). It was predicted that an adult would consume 138.4 g of meat or kidney while young would consume 111.2 g. Based on the concentrations of certain substances that were found in the meat and kidney, an evaluation of the possible health risk (HQ) to consumers was carried out (Kasprzyk et al., 2020).
The hazard index (HI) is determined by adding up the Hazard Quotient (HQ) values. If the HQ value is below 1, it suggests that the exposed population is unlikely to face any adverse health risks. However, if the HQ surpasses 1, there could be potential concerns regarding non-cancerous effects (Pogurschi et al., 2023).
Statistical analyses were performed using the SPSS v.20 software package (SPSS Inc., Chicago, IL, United States). Using a specific test, the normality of the data was verified. Two-way analysis of variance (ANOVA) was used to investigate the effects of age and gender and their interaction on the traits analyzed. Principal component analysis (PCA) to extract significant components and associated loadings. Spearman’s correlation was also used to analyze the association between variables.
This investigation is the first comprehensive 10-year assessment of the mineral content in wild boars hunted in a mountainous region of North-Eastern Romania. In Table 1, the level of each element (mg w. w.) obtained from muscle tissue (Longissimus lumborum) for each gender and age category are described.
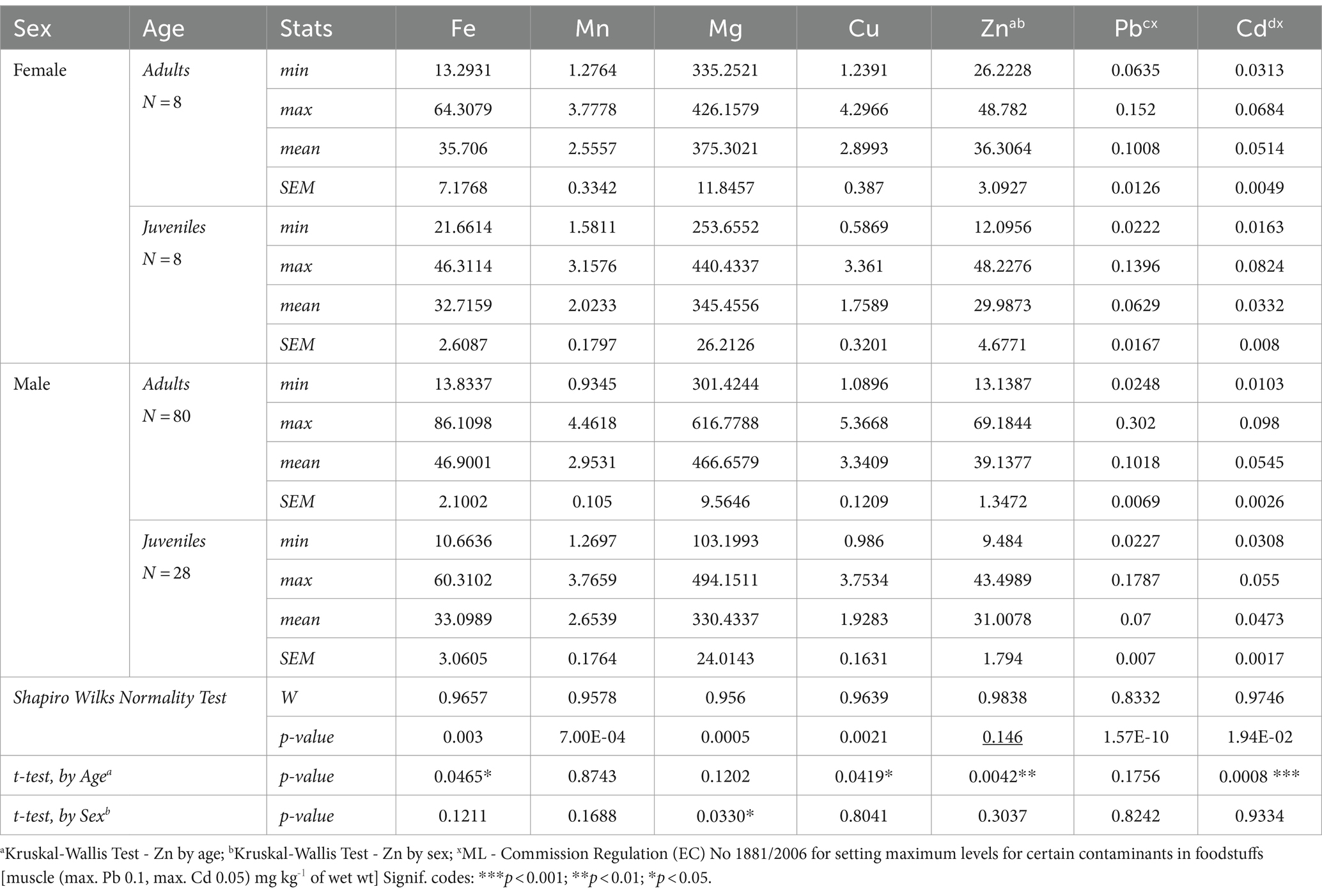
Table 1. Level of essential minerals and heavy metals (mg g-1 w.w.) in muscle tissue (Longissimus lumborum) from wild boars.
According to Table 1 data, a detailed picture of the mineral content in wild boar carcasses is described, in a direct correlation manner to the age-and sex-specific variations. The statistical analysis indicates that the age of the animals had a significant impact on Fe (*p < 0.05), Cu (*p < 0.05), and Zn (**p < 0.01) level. However, the adult and juvenile males showed higher levels of Fe, Cu, and Zn when compared to females. Additionally, no significant effect of age on Mn level was noted, although there was a trend toward higher concentration in muscle samples of males. According to Draghi et al. (2024), adult wild boar showed higher levels of Mn. As regards Mg content by sex, significant differences were found (*p < 0.05). Regarding the Pb level, no statistically significant differences among the analyzed batches were found, although increased levels were found in male adults. It is noteworthy to mention that the maximum permissible level of Pb is 0.1 mg/kg wet weight, according to Commission Regulation (EC) (n.d.). As for Cd level, it shows significant differences according to both age and sex, with higher concentrations in adults and males. However, Cd levels are under the maximum admitted level. Malmsten et al. (2021) confirmed that age was significantly associated with Cd concentration.
Further, in Table 2, the level of each element (mg w.w.) determined from kidneys for each gender and age category is described.
Interestingly, according to the age, significant differences in the essential element levels monitored in the kidneys were observed for Fe (*p < 0.05), Mg (***p < 0.001), Cu (**p < 0.01), and Zn (*p < 0.05). Both females and adult males showed higher levels of Fe, Mn, Mg, Cu, and Zn compared to juvenile females and males. Moreover, a highly significant difference in Fe content by sex (***p < 0.001), with males showing higher values than females. Furthermore, sex was shown to significantly influence (*p < 0.05) the Mg levels. Statistical analysis showed that sex had no significant effect on Cu and Zn levels in the kidneys analyzed in the present study As for Mn, no influence of age and sex on content was observed. Concerning Cd level, a highly statistically significant difference (***p < 0.001) was recorded between the analyzed groups, with higher values in adults compared to juveniles. The study by Bąkowska et al. (2024) reported elevated Cd values in the organs of wild animals, which included wild boars, but notes that cadmium levels were found to vary by geographical location. Cadmium levels showed significant differences both according to age and sex, with higher concentrations in adults and males. No statistically significant differences in Pb content were observed between the batches analyzed, although higher values were recorded in adults.
Regarding the Pb content, 19.44% of muscle samples from juveniles exceed the admitted limits, compared to 31.81% samples coming from adult wild boar (Table 3). In the study by Lénárt et al. (2023) the consumption of wild boar muscle poses a risk to the consumer due to lead content above the legal tolerable limits. The highest percentage of kidney samples exceeding the maximum admitted lead limit was recorded in adults (57.95%; Table 3). Regarding the Cd level, 55.55% of muscle samples from juveniles exceed the admitted value. In adults, 54.27% of muscle samples exceed the Cd level, while 45.45% of kidney samples showed higher values than the admitted limits. Overall, 28.23% of muscle samples and 46.77% of kidney samples exceed the Pb limit. For Cd, 53.23% of muscle samples and 32.26% of kidney samples reached the maximum threshold. Considering these data, it can be stated that lead and cadmium levels in meat and kidney are significantly higher in adults than in juveniles.
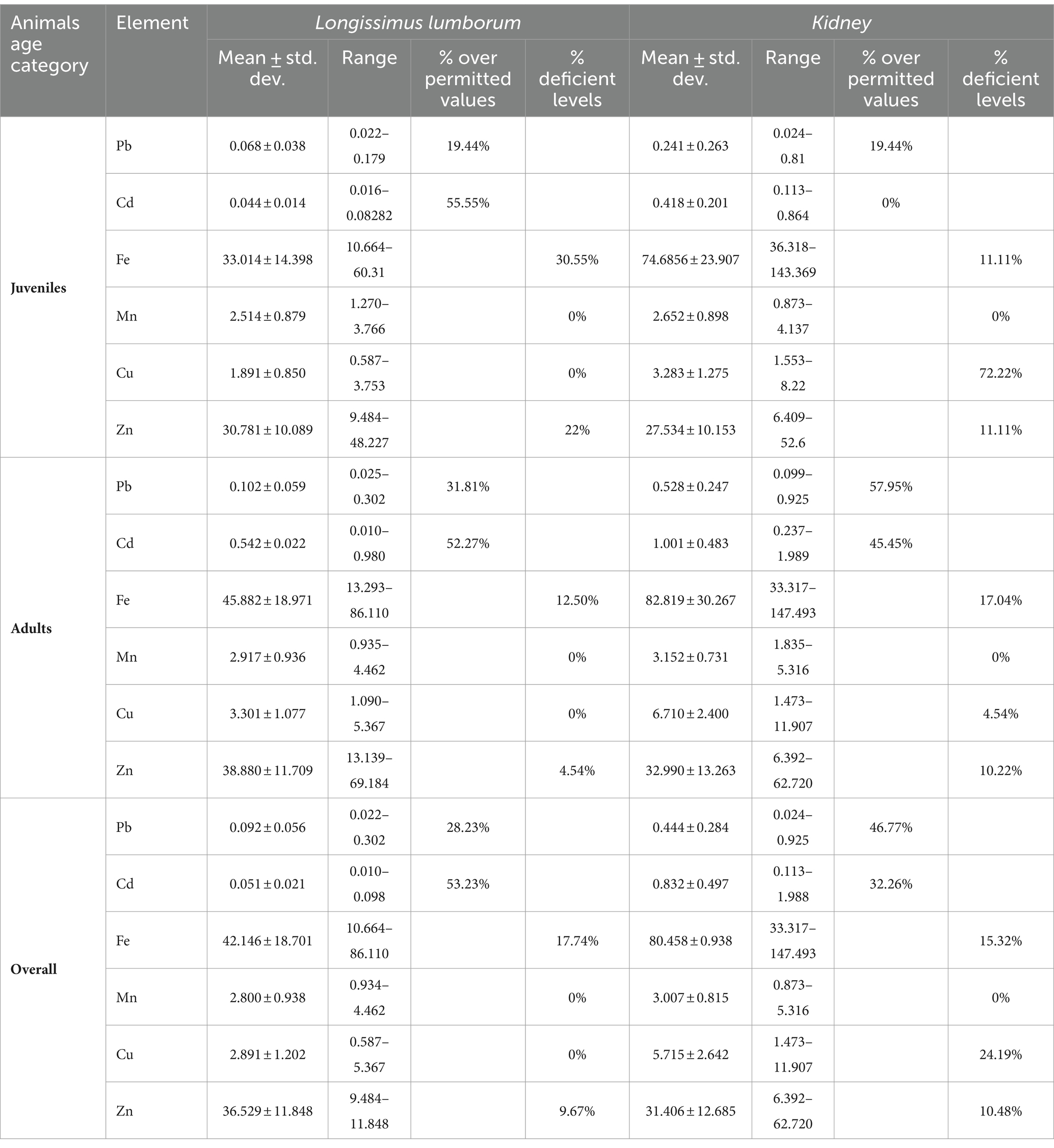
Table 3. Mean kidney/muscle concentrations (mg g-1 w.w.) of minerals Pb and Cd in Romanian wild boars and percentage of samples exceeding admitted maximum levels (ML for Pb and Cd) Mean ± std. dev.
In our research, we performed a correlation matrix analysis using Spearman correlation coefficient for Fe, Mn, Mg, Cu, Zn, Pb, and Cd variables in the studied samples as shown in Figure 2. With respect to, Fe-Zn levels, a significant negative correlation in kidney samples from juvenile males was identified. In multicellular organisms, Zn is predominantly found inside cells, with approximately 40% in the nucleus, 50% in the cytoplasm, organelles, and specialized vesicles, while the rest in is found in cell membranes (Stefanidou et al., 2006). In this research performed on muscle in juvenile females, a negative correlation between Fe and Zn content was observed. Studies have shown that increased iron uptake influences zinc transporter levels differently, impacting zinc availability in the body (Olivares et al., 2007). Hepcidin, an essential regulator of iron metabolism, functions to restrict access to iron stores in the body by sequestering it in macrophages. Evidence suggests that this systemic regulator of Fe homeostasis is also affected by Zn levels. A Zn deficiency is responsible for hepcidin over-expression, and Zn supplementation may reduce hepcidin production, leading to increased iron absorption (Bjørklund et al., 2017). Studies in Caco-2 cells have shown that their exposure to increased Zn concentrations significantly impairs Fe uptake (Iyengar et al., 2009).
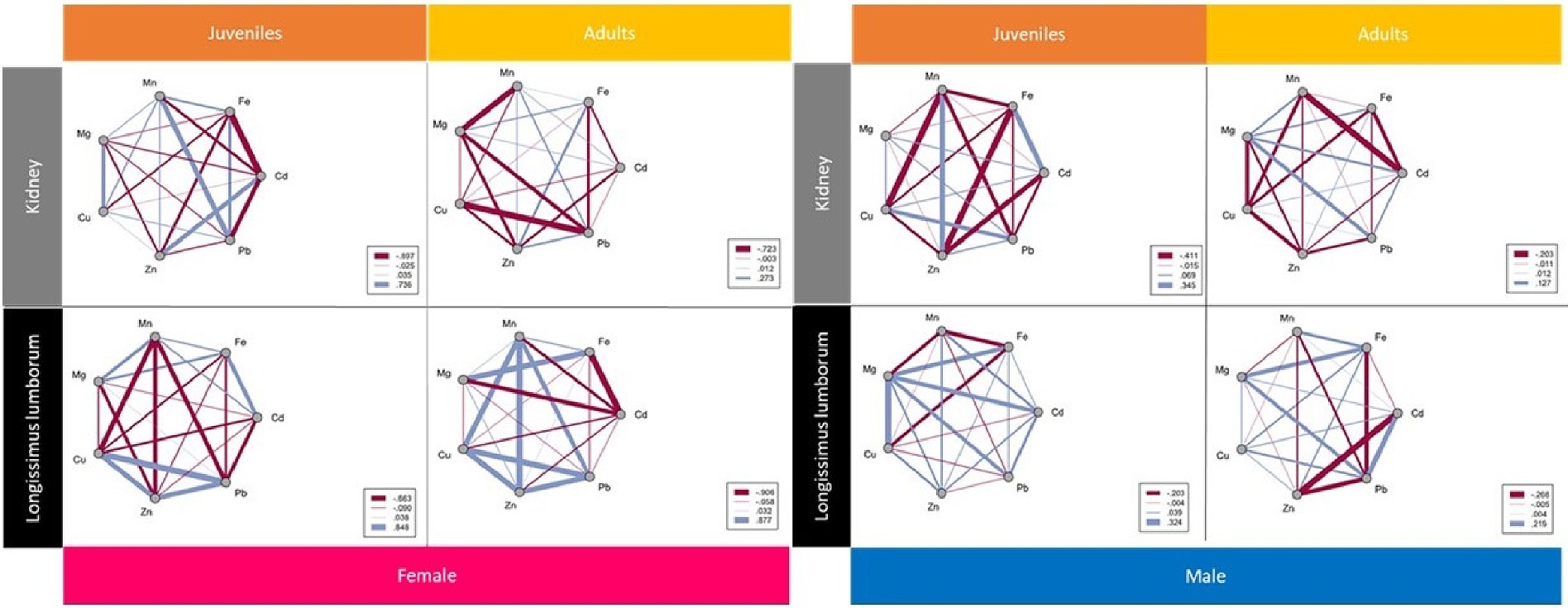
Figure 2. Correlation matrix analysis using Spearman’s correlation coefficient for the variables Fe, Mn, Mg, Cu, Zn, Pb and Cd in kidney samples from juvenile and adult females, juvenile and adult males, and from Longissimus lumborum samples from juvenile and adult females, juvenile and adult males.
In kidney samples from juvenile and adult females, a positive relationship between Fe and Mn content was demonstrated. This correlation is observed in females, while in the case of males, it was shown to be absent. Mn is an essential cofactor for various enzymes involved in cellular signal transduction (Roth and Garrick, 2003). In all muscle analyses from this study, both from females, adults, and juveniles, and from males, adults, and juveniles, a positive association between Zn and Cu content was observed, while in kidney the analysis revealed a negative association. It appears that the zinc-copper interaction most likely occurs at the level of the absorption process. According to Sandström (2001), within the usual range of dietary zinc intake, Cu is decreasing only slightly when higher zinc levels are occurring. Zinc, inducing intestinal metallothionein, inhibits the absorption of Cu from food and prevents reabsorption of endogenously secreted Cu, thus producing a negative Cu imbalance, as is seen in Wilson’s disease (Brewer et al., 1985). In recent years, the risk contamination of meat with heavy metals has been of great concern for both food safety and human health because of their toxicity, bioaccumulation and biomagnification in the food chain (Akele et al., 2022). Heavy metal (Cd, Pb) contamination of game meat is a potential hazard to human health and undermines global food security. In the field of toxicology, the assessment of exposure to heavy metals is predominantly carried out by analyzing the accumulation of these elements in tissues and organs of living organisms (Cygan-Szczegielniak and Stasiak, 2022). The bioaccumulation process of heavy metals can be passive or selective, and differences in this process can result in variations in absorption, digestion or both. In this study, the kidney samples from juvenile females showed a positive association between Pb and Fe. A similar correlation was noted in muscle tissue tests performed on juvenile males. A Fe deficiency leads to an increase in blood Pb concentration, while the excess of lead increases iron and copper excretion in the urine. Lead also inhibits the synthesis of ceruloplasmin, an aspect involved in Fe and Cu metabolism (Długaszek, 2019). The investigations performed on kidney samples from juvenile males demonstrated a negative correlation between Cd and Zn. Cadmium toxicity is historically associated with Zn homeostasis and oxidative stress in mammalian cells. This association is often explained by the protection provided by Zn against the harmful effects of Cd exposure (Moulis, 2010). Cadmium is absorbed in the small intestine by mechanisms similar to those of essential elements such as Ca, Fe, Cu, and Zn (Brzóska and Moniuszko-Jakoniuk, 2001).
Principal component analysis (PCA) is a useful tool for evaluation of the mineral and heavy metal content in the analyzed samples. The biplot obtained from this analysis illustrates the two dimensions of the PCA model, constructed based on concentrations of essential minerals and heavy metals in muscle tissue and kidneys from Sus scrofa ferus, as shown in Figure 3.
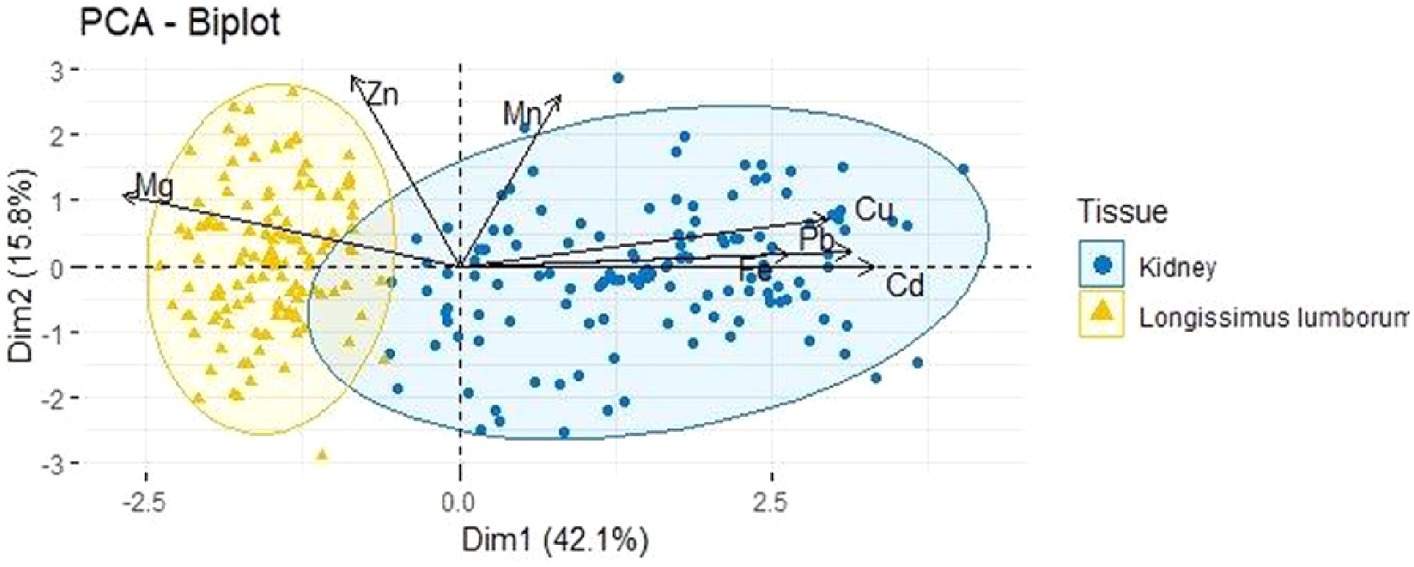
Figure 3. The PCA biplot represents the distribution of loadings for essential elements and heavy metals and the scores for the samples analyzed. This plot captures 57.9% of the variability in the dataset, with Dimension 1 contributing 42.1% and Dimension 2 15.8%.
Figure 3 highlights a distinct separation between the studied samples, where essential elements and associated heavy metals are represented as vectors. PCA showing a 57.9% of the variance in the essential element and heavy metals data between muscle and kidney samples is explained by the first two dimensions (dimension 1 and dimension 2). The biplots illustrate the relationship between PCA scores associated with muscle (marked in yellow) and kidney (marked in blue) samples. In detail, the variable loadings in the two dimensions of Figure 3 show that positive loadings on the dim1 ax-is are associated with Mg. The charge plot shows a direct correlation between Pb and Cu as well as between Pb and Cd. However, an opposite correlation is also observed between Cu and Mg, Cd and Mg, and Pb and Mg. Kidney samples (marked in blue) are grouped closer to the Pb and Cd variables, suggesting their higher concentrations in all samples. In contrast, muscle clusters are placed near the negative side of dimension 1 and close to the Mg variable, indicating higher Mg levels and lower Cd levels than recorded in most samples. Mg is an essential activator for the phosphate transfer enzymes myokinase, diphosphopyridine nucleotide kinase, and creatine kinase. Mg is absorbed in the intestines and then transported through the blood to cells and tissues (Soetan et al., 2010). For instance, the effects of low and high extracellular Mg in C2C12 myogenic differentiation have been studied. Non-physiological Mg concentrations induce oxidative stress in myoblasts. The increase in reactive oxygen species, which occurs early in the differentiation process, inhibits myoblast membrane fusion, affecting myogenesis. Therefore, proper Mg homeostasis, maintained by correct dietary intake, is essential for ensuring the regenerative capacity of skeletal muscle fibers (Zocchi et al., 2021). Dietary supplementation with magnesium oxide (MgO) can improve parameters of muscle energy metabolism, Ca2+ uptake, and meat quality (Lahucky et al., 2004). Wildlife can ingest substantial amounts of soil during feeding. Soil ingestion also is known to be important for animals as a nutrient supply (Beyer et al., 1994; Topa et al., 2021). Mineral components in the animal body exhibit different grades of synergistic or antagonistic interactions to maintain homeostasis (Lall and Kaushik, 2021).
The highest HQ values were observed in the “HI” rows for adults and children as follow: 0.248611 (90 times/year) for adult meat consumption, while for children; 0.43507 (90 times/year) for adult meat consumption (Tables 4, 5).
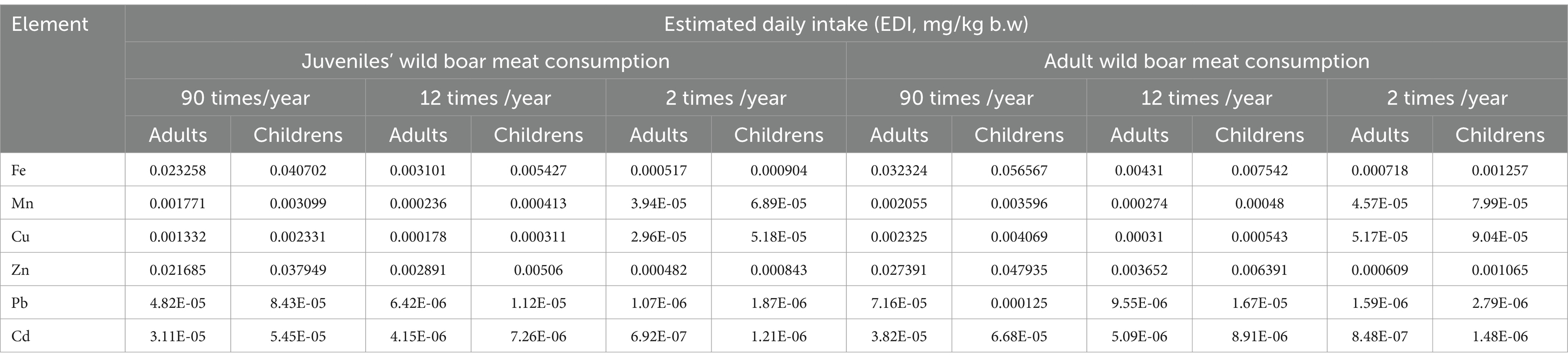
Table 4. Human estimated daily intake (EDI, mg kg-1 b.w.) of elements associated with wild boar meat consumption (Longissimus lumborum).
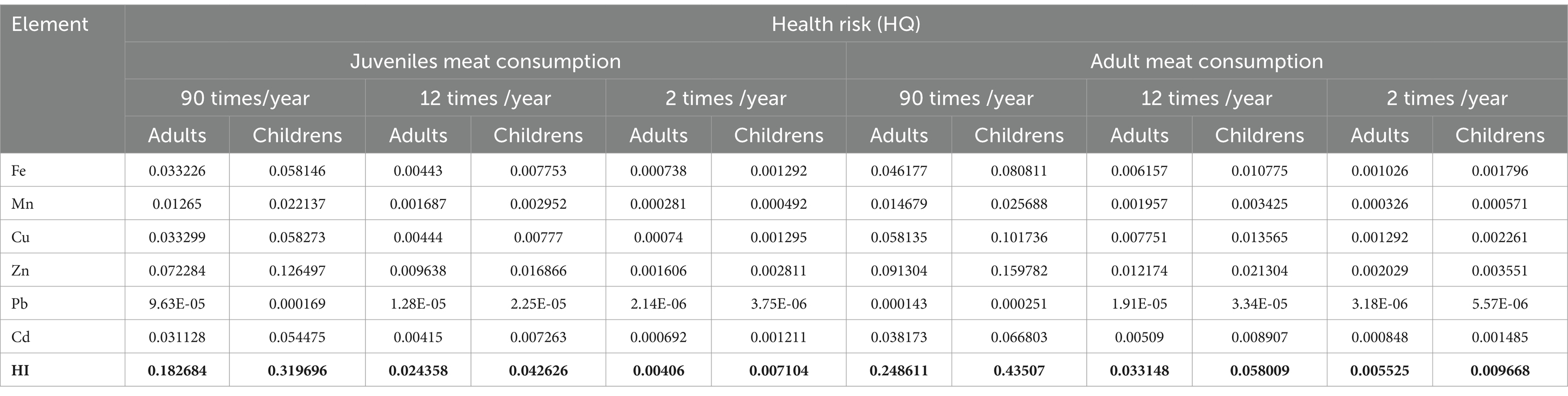
Table 5. Risk (HQ) and hazard index (HI) associated with wild boar meat consumption (Longissimus lumborum).
These values indicate the level of potential hazard associated with meat consumption and are expressed as a ratio of the concentration of the chemical (in this case, metals) to the reference level for that chemical. Higher values indicate a higher risk. Children are more vulnerable than adults to the acute and chronic effects of heavy metal intake because they consume twice as much food per unit of body weight (Zeinali et al., 2019). Fe concentrations are higher in adults compared to children, for all levels of meat consumption. Mn shows a similar pattern, with higher values in adults. Cu concentrations are also higher in adults for all levels of meat consumption. Zn concentrations follow the same pattern, with higher levels in adults. Pb concentrations are low, but slightly higher in adults. Cd concentrations are higher in adults compared to children. HI values increase with higher meat consumption and are generally higher for adults compared to children. Overall, health risk (HI) tends to be higher for adults across all elements and frequency of meat consumption. Consumption of these heavy metals through edible organs and meat over a long period might lead to toxicity due to the accumulative characteristics of these metals inside the body. Therefore, it is necessary to monitor these heavy metal residues in the meat, liver, and kidney of these food animals (Zeinali et al., 2019). Cd toxicity occurs mainly through ingestion of contaminated food and results in acute gastrointestinal disorder with diarrhea and vomiting. Long-term exposure was associated with calcium disorders, and osteoporosis, especially in postmenopausal women (Aendo et al., 2019). Kasprzyk et al. (2020) found elevated levels of Cd and Pb in wild boar liver. According to Bilandžić et al. (2010) research, wild boars in Croatia roaming freely in hunting areas were confirmed to be contaminated with Cd and Pb, highlighting the importance of identifying sources of contamination in animals and intensifying meat control as well. According to Pilarczyk et al. (2020), understanding the concentration of heavy metals in the organs of game animals allows indirect assessment of the contaminated environment. It also permits the determination of the potential risk to consumer health from consuming too high doses of some toxic elements, including Pb and Cd. Moreover, same authors (Pilarczyk et al., 2020) also observed exceedances of the permissible limits for Cd in wild boar muscle, but none of the hazard or health impact indicators (HQ or HI) exceeded 1, indicating a low probability of adverse effects associated with game meat consumption.
Various authors such as Babicz and Kasprzyk (2019) and Strazdiņa et al. (2013), together with Macháčková et al. (2021), confirm that wild boar meat can be a nutritionally excellent option, being rich in proteins, vitamins, and minerals essential for overall health to obtain the nutritional benefits of wild boar meat and to reduce the risk of exposure to heavy metals, consumers should choose reliable wild meat sources and include variety in their diet without over-consuming wild boar meat or any other type of meat.
Meat from the managed game is an alternative food source with considerable nutritional benefits. Careful monitoring of heavy metals content in meat, including wild boar meat, is essential to ensure food safety. Heavy metals such as lead and cadmium may contaminate the meat, posing a significant risk to human health. These metals have the potential to cause harmful effects on the nervous system, kidneys, and other vital organs. The research results provide a detailed insight into the mineral content of wild boar carcasses, highlighting age-and sex-specific variations. Statistical analyses reveal the significant impact of animal age on Fe, Cu, and Zn content in muscle samples, as for the other minerals in kidney samples. Regarding heavy metals content, the results indicate a significant concern. The high percentages of muscle and kidney samples exceeding the permitted limits for these metals underline the need for strict monitoring and appropriate corrective measures. Higher HQ (Hazard Quotient) for children compared to adults indicates a potentially higher risk associated with meat consumption among children. These findings underline the need to pay more attention to the impact of meat consumption on children’s health, as well as on adults, and the need to apply measures to minimize their exposure to hazardous chemicals that may be present in meat. It is essential to develop and implement safe and healthy food policies and practices to protect the health and well-being of consumers. It is recommended that transparent and accessible information on food safety and guidelines for the consumption of wild boar meat from polluted areas be provided. Extensive research and consumer education campaigns are needed on the benefits and risks associated with the consumption of wild boar meat and ways to reduce exposure to toxic substances.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The requirement of ethical approval was waived by Iași University of Life Sciences Practice hunting fund and application activities Frasin, Suceava for the studies involving animals because The study material included wild boars (Sus scrofa ferus) harvested during collective hunting by Romanian hunters during the 2010–2021 hunting seasons (winter), with the purpose of stock density control of Frasin District hunting area. In accordance with the Romanian law, male wild boar hunting is permitted throughout the year, while female wild boar hunting is permitted only between August 1st and February 15th. The animals were not strictly for study purposes, the manager approved the collection of samples for scientific purposes in the annual responsible wildlife management plan for the game reserve. On the basis of these Minister’s Orders, 124 wild boars (Sus scrofa ferus; 16 females and 108 males). After the sanitary inspection (at the evisceration checkpoint), in the first 24 h postmortem, muscle samples were collected, identified according to gender and age-class, sealed in sterile bags, and transported to the laboratory under refrigerated conditions, trimmed of connective and adipose tissues, vacuum packed, and frozen at −20°C until analysis. Since the entire study and sampling were performed on dead animals harvested in accordance with the Hunting and Game Fund Protection Law No 407/2006 and O.U.G. No 102/2010 and the National Management of Game, Hunting and Wildlife in Romania, such research did not require the consent of the Ethics Committee. Research protocol and procedures are unlikely to cause significant offense to readers when the results are published. Replacement of animals with alternatives whenever possible—no animals could be replaced to achieve the study objectives. Reducing the number of animals used—experimental groups will have 16 females and 108 males per decade of study, the lowest number of individuals considered representative for such studies (male wild boar hunting is permitted throughout the year, while female wild boar hunting is permitted only between August 1st and February 15th). Improve experimental conditions and procedures to minimize harm. Feed was supplemented in concentrate and succulent form (cereals, seeds, fruits, fresh, red beets, turnips, potatoes, and carrots) at an average of 85.5 kg/animal/year between November 1 and March 31 each year due to limited feed supply during the cold winter months. The studies were conducted in accordance with the local legislation and institutional requirements.
P-CB: Writing – original draft, Validation, Supervision, Project administration, Conceptualization. E-IF: Writing – original draft, Conceptualization. B-GA: Writing – review & editing, Formal analysis. B-MM: Writing – review & editing, Investigation. MM: Writing – review & editing, Investigation. OM: Writing – review & editing, Investigation. GF: Writing – review & editing, Investigation. AP: Writing – original draft, Validation, Software, Formal analysis, Data curation. M-MC: Writing – original draft, Visualization, Resources, Methodology, Funding acquisition.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the “Ion Ionescu de la Brad” Iași University of Life Sciences.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2024.1406579/full#supplementary-material
Aendo, P., Thongyuan, S., Songserm, T., and Tulayakul, P. (2019). Carcinogenic and non-carcinogenic risk assessment of heavy metals contamination in duck eggs and meat as a warning scenario in Thailand. Sci. Total Environ. 689, 215–222. doi: 10.1016/j.scitotenv.2019.06.414
Akele, M. L., Desalegn, S. K., Asfaw, T. B., Assefa, A. G., Alemu, A. K., and de Oliveira, R. R. (2022). Heavy metal contents in bovine tissues (kidney, liver and muscle) from Central Gondar zone, Ethiopia. Heliyon. 8:e12416. doi: 10.1016/j.heliyon.2022.e12416
Al-Fartusie, F. S., and Mohssan, S. N. (2017). Essential trace elements and their vital roles in human body. Indian J Adv Chem Sci. 5, 127–136. doi: 10.22607/IJACS.2017.503003
Alghamdi, M., Gutierrez, J., and Komarnytsky, S. (2023). Essential minerals and metabolic adaptation of immune cells. Nutrients 15:123. doi: 10.3390/nu15010123
Babicz, M., and Kasprzyk, A. (2019). Comparative analysis of the mineral composition in the meat of wild boar and domestic pig. Ital. J. Anim. Sci. 18, 1013–1020. doi: 10.1080/1828051X.2019.1610337
Bąkowska, M., Pilarczyk, B., Tomza-Marciniak, A., Pilarczyk, R., and Udała, J. (2024). Cadmium in selected organs of game animals from areas with different degrees of industrialisation and its intake by human consumers. Animals 14:305. doi: 10.3390/ani14020305
Beyer, W. N., Connor, E. E., and Gerould, S. (1994). Estimates of soil ingestion by wildlife. J. Wildl. Manag. 58, 375–382. doi: 10.2307/3809405
Bhattacharya, P. T., Misra, S. R., and Hussain, M. (2016). Nutritional aspects of essential trace elements in oral health and disease: an extensive review. Scientifica. 5464373. doi: 10.1155/2016/5464373
Bilandžić, N., Sedak, M., Đokić, M., and Šimić, B. (2010). Wild boar tissue levels of cadmium, lead and mercury in seven regions of continental Croatia. Bull. Environ. Contam. Toxicol. 84, 738–743. doi: 10.1007/s00128-010-9999-7
Bjørklund, G., Aaseth, J., Skalny, A. V., Suliburska, J., Skalnaya, M. G., Nikonorov, A. A., et al. (2017). Interactions of iron with manganese, zinc, chromium, and selenium as related to prophylaxis and treatment of iron deficiency. J. Trace Elem. Med. Biol. 41, 41–53. doi: 10.1016/j.jtemb.2017.02.005
Bodor, Z., Szép, A., and Szép, R. (2021). Human health impact assessment and temporal distribution of trace elements in Copșa Mică-Romania. Sci. Rep. 11:7049. doi: 10.1038/s41598-021-86488-5
Bourre, J. M. (2006). Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 2: macronutrients. J. Nutr. Health Aging 10, 386–399.
Brewer, G. J., Hill, G. M., Dick, R. D., Prasad, A. S., and Cossack, Z. T. (1985). Interactions of trace elements: clinical significance. J. Am. Coll. Nutr. 4, 33–38. doi: 10.1080/07315724.1985.10720064
Brzóska, M. M., and Moniuszko-Jakoniuk, J. (2001). Interactions between cadmium and zinc in the organism. Food Chem. Toxicol. 39, 967–980. doi: 10.1016/s0278-6915(01)00048-5
Chen, P., Bornhorst, J., and Aschner, M. A. (2018). Manganese metabolism in humans. Front. Biosci. 23, 1655–1679. doi: 10.2741/4665
Ciobanu, M.-M., Manoliu, D.-R., Ciobotaru, M.-C., Anchidin, B.-G., Matei, M., Munteanu, M., et al. (2023). The influence of sensory characteristics of game meat on consumer Neuroperception: a narrative review. Food Secur. 12:1341. doi: 10.3390/foods12061341
Ciobanu, M. M., Munteanu, M., Postolache, A. N., and Boișteanu, P. C. (2020). Toxic heavy metals content in wild boar and venison meat: a brief review. Scientific Papers. Series D. Animal Science. 63, 435–441.
Commission Regulation (EC) . No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Journal of the European Union (OJEU). Retrieved 15 January 2024. 365, 5–24. Available at: https://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF
Corradini, A., Marescotti, M. E., Demartini, E., and Gaviglio, A. (2022). Consumers' perceptions and attitudes toward hunted wild game meat in the modern world: a literature review. Meat Sci. 194:108955. doi: 10.1016/j.meatsci.2022.108955
Cygan-Szczegielniak, D., and Stasiak, K. (2022). Effects of age and sex on the content of heavy metals in the hair, liver and the longissimus lumborum muscle of roe deer Capreolus capreolus L. Environ. Sci. Pollut. Res. 29, 10782–10790. doi: 10.1007/s11356-021-16425-6
Dervilly-Pinel, G., Guérin, T., Minvielle, B., Travel, A., Normand, J., Bourin, M., et al. (2017). Micropollutants and chemical residues in organic and conventional meat. Food Chem. 232, 218–228. doi: 10.1016/j.foodchem.2017.04.013
Długaszek, M. (2019). Studies on relationships between essential and toxic elements in selected body fluids, cells and tissues. Chem. Biol. Interact. 297, 57–66. doi: 10.1016/j.cbi.2018.10.011
Doménech, E., and Martorell, S. (2024). Review of the terminology, approaches, and formulations used in the guidelines on quantitative risk assessment of chemical hazards in food. Food Secur. 13:714. doi: 10.3390/foods13050714
Draghi, S., Spinelli, M., Fontanarosa, C., Curone, G., Amoresano, A., Pignoli, E., et al. (2024). Evaluation of the difference in the content of essential and non-essential elements in wild boar and swine tissues sampled in the same area of northern Italy. Animals 14:827. doi: 10.3390/ani14060827
European Commission . (2006). Commission regulation (EC) no 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union, L 364/5. Available at: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32006R1881 (Accessed on 15 January 2024).
European Committee for Standardization (2003). EN 14082:2003: Foodstuffs-polymerase chain reaction (PCR) for the detection of food-borne pathogens - general requirements and definitions. Brussels: European Committee for Standardization.
Florijancic, T., Ozimec, S., Jelkic, D., Vuksic, N., Bilandzic, N., Gross Boskovic, A., et al. (2015). Assessment of heavy metal content in wild boar (Sus scrofa L.) hunted in eastern Croatia. J. Environ. Prot. Ecol. 16, 630–636.
Frunză, G., Murariu, O. C., Ciobanu, M.-M., Radu-Rusu, R.-M., Simeanu, D., and Boișteanu, P.-C. (2023). Meat quality in rabbit (Oryctolagus cuniculus) and hare (Lepus europaeus Pallas)—a nutritional and technological perspective. Agriculture 13:126. doi: 10.3390/agriculture13010126
Gall, J. E., Boyd, R. S., and Rajakaruna, N. (2015). Transfer of heavy metals through terrestrial food webs: a review. Environ. Monit. Assess. 187:201. doi: 10.1007/s10661-015-4436-3
Gerofke, A., Martin, A., Schlichting, D., Gremse, C., and Müller-Graf, C. (2019). Heavy metals in game meat. Chemical hazards in foods of animal origin. 7, 435–439. doi: 10.3920/978-90-8686-877-3_24
Gimou, M. M., Charrondière, U. R., Leblanc, J. C., Pouillot, R., Noël, L., and Guérin, T. (2014). Concentration data for 25 elements in foodstuffs in Yaounde: the Cameroonian Total diet study. J. Food Compos. Anal. 34, 39–55. doi: 10.1016/j.jfca.2014.02.005
Guo, Y., Zhang, G., Yuan, J., and Nie, W. (2003). Effects of source and level of magnesium and vitamin E on prevention of hepatic peroxidation and oxidative deterioration of broiler meat. Anim. Feed Sci. Technol. 107, 143–150. doi: 10.1016/S0377-8401(03)00116-0
Iyengar, V., Pullakhandam, R., and Nair, K. M. (2009). Iron-zinc interaction during uptake in human intestinal Caco-2 cell line: kinetic analyses and possible mechanism. Indian J. Biochem. Biophys. 46, 299–306.
Kaczor-Kamińska, M., Sura, P., and Wróbel, M. (2020). Multidirectional changes in parameters related to sulfur metabolism in frog tissues exposed to heavy metal-related stress. Biomol. Ther. 10:574. doi: 10.3390/biom10040574
Kasprzyk, A., Kilar, J., Chwil, S., and Rudaś, M. (2020). Content of selected macro-and microelements in the liver of free-living wild boars (Sus scrofa L.) from agricultural areas and health risks associated with consumption of liver. Animals 10:1519. doi: 10.3390/ani10091519
Lahucky, R., Küchenmeister, U., Bahelka, I., Vasicek, D., Liptaj, T., and Ender, K. (2004). The effect of dietary magnesium oxide supplementation on postmortem 31P NMR spectroscopy parameters, rate of Ca2+ uptake and ATPase activity of M. longissimus dorsi and meat quality of heterozygous and normal on malignant hyperthermia pigs. Meat Sci. 67, 365–370. doi: 10.1016/j.meatsci.2003.11.010
Lall, S. P., and Kaushik, S. J. (2021). Nutrition and metabolism of minerals in fish. Animals 11:2711. doi: 10.3390/ani11092711
Legea Vânătorii și a Protecției Fondului Cinegetic nr. 407/2006 . (2010). O.U.G. nr. 102/2010 Pentru Modificarea și Completarea Legii Vânătorii și a Protecției Fondului Cinegetic nr. 407/2006. Available at: https://legislatie.just.ro/Public/DetaliiDocument/77053 (Accessed on 10 January 2024). (In Romanian).
Lénárt, Z., Bartha, A., Abonyi-Tóth, Z., and Lehel, J. (2023). Monitoring of metal content in the tissues of wild boar (Sus scrofa) and its food safety aspect. Environ. Sci. Pollut. Res. 30, 15899–15910. doi: 10.1007/s11356-022-23329-6
Macháčková, K., Zelený, J., Lang, D., and Vinš, Z. (2021). Wild boar meat as a sustainable substitute for pork: a mixed methods approach. Sustain. For. 13:2490. doi: 10.3390/su13052490
Malmsten, A., Dalin, A. M., Pettersson, J., and Persson, S. (2021). Concentrations of cadmium, lead, arsenic, and some essential metals in wild boar from Sweden. Eur. J. Wildl. Res. 67, 1–8. doi: 10.1007/s10344-021-01460-y
Matei, M., Zaharia, R., Petrescu, S.-I., Radu-Rusu, C. G., Simeanu, D., Mierliță, D., et al. (2023). Persistent organic pollutants (POPs): a review focused on occurrence and incidence in animal feed and cow Milk. Agriculture 13:873. doi: 10.3390/agriculture13040873
Mehri, A. (2020). Trace elements in human nutrition (II)–an update. Int. J. Prev. Med. 11:2. doi: 10.4103/ijpvm.IJPVM_48_19
Mohamed, H., Haris, P. I., and Brima, E. I. (2017). Estimated dietary intakes of toxic elements from four staple foods in Najran city, Saudi Arabia. Int. J. Environ. Res. Public Health 14:1575. doi: 10.3390/ijerph14121575
Moulis, J. M. (2010). Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals 23, 877–896. doi: 10.1007/s10534-010-9336-y
Murariu, O. C., Murariu, F., Frunză, G., Ciobanu, M. M., and Boișteanu, P. C. (2023). Fatty acid indices and the nutritional properties of karakul sheep meat. Nutrients 15:1061. doi: 10.3390/nu15041061
Nunes, A. M., Acunha, T. S., Oreste, E. Q., Lepri, F. G., Vieira, M. A., Curtius, A. J., et al. (2011). Determination of ca, cu, Fe and mg in fresh and processed meat treated with tetramethylammonium hydroxide by atomic absorption spectrometry. J. Braz. Chem. Soc. 22, 1850–1857. doi: 10.1590/S0103-50532011001000004
Olivares, M., Hertrampf, E., and Uauy, R. (2007). Copper and zinc interactions in anemia: a public health perspective. Nutritional Anemia. 1, 99–109.
Pereira, P. M. D. C. C., and Vicente, A. F. D. R. B. (2013). Meat nutritional composition and nutritive role in the human diet. Meat Sci. 93, 586–592. doi: 10.1016/j.meatsci.2012.09.018
Pilarczyk, B., Tomza-Marciniak, A., Pilarczyk, R., Udała, J., Kruzhel, B., and Ligocki, M. (2020). Content of essential and non-essential elements in wild animals from western Ukraine and the health risks associated with meat and liver consumption. Chemosphere 244:125506. doi: 10.1016/j.chemosphere.2019.125506
Pogurschi, E. N., Grigore, D.-M., Ianitchi, D., Bahaciu, G., Popa, D. C., Dragomir, N., et al. (2023). Screening and detection of antibiotic residues on broiler meat based on trade system variations, seasonal differences, and the impact on final consumer safety in Romania. Front. Sustain. Food Syst. 7:1198411. doi: 10.3389/fsufs.2023.1198411
Postolache, A. N., Ciobanu, M. M., and Boișteanu, P. C. (2015). Selected biometric characteristics of wild boar (Sus scrofa Ferus) in north-East Romania. Food Science and Technology. 72, 137–138. doi: 10.15835/buasvmcn-fst:11062
Quaresma, M. A. G., Alves, S. P., Trigo-Rodrigues, I., Pereira-Silva, R., Santos, N., Lemos, J. P. C., et al. (2011). Nutritional evaluation of the lipid fraction of feral wild boar (Sus scrofa scrofa) meat. Meat Sci. 89, 457–461. doi: 10.1016/j.meatsci.2011.05.005
Reglero, M. M., Taggart, M. A., Monsalve-González, L., and Mateo, R. (2009). Heavy metal exposure in large game from a lead mining area: effects on oxidative stress and fatty acid composition in liver. Environ. Pollut. 157, 1388–1395. doi: 10.1016/j.envpol.2008.11.036
Regulation (EC) no. 853/2004 of the European Parliament and of the Council . Lay. Down Specif. Hyg. Rules Hyg. Foodst. (2004). 4, 30. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R0853&from=RO (Accessed on 15 January 2024).
Ribeiro, D. M., Mourato, M. P., and Almeida, A. M. (2019). Assessing mineral status in edible tissues of domestic and game animals: a review with a special emphasis in tropical regions. Trop. Anim. Health Prod. 51, 1019–1032. doi: 10.1007/s11250-019-01848-8
Roslewska, A., Stanek, M., Janicki, B., Cygan-Szczegielniak, D., Stasiak, K., and Buzala, M. (2016). Effect of sex on the content of elements in meat from wild boars (Sus scrofa L.) originating from the province of Podkarpacie (South-Eastern Poland). J. Elem. 21, 823–832. doi: 10.5601/jelem.2015.20.2.943
Roth, J. A., and Garrick, M. D. (2003). Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem. Pharmacol. 66, 1–13. doi: 10.1016/s0006-2952(03)00145-x
Sáez-Royuela, C., Gomariz, R. P., and Tellería, J. L. (1989). Age determination of European wild boar. Wildl. Soc. Bull. 17, 326–329.
Sandström, B. (2001). Micronutrient interactions: effects on absorption and bioavailability. Br. J. Nutr. 85, S181–S185. doi: 10.1079/BJN2000312
Shukla, G. S., and Singhal, R. L. (1984). The present status of biological effects of toxic metals in the environment: lead, cadmium, and manganese. Can. J. Physiol. Pharmacol. 62, 1015–1031. doi: 10.1139/y84-171
Skobrák, E. B., Bodnár, K., Jónás, E. M., Gundel, J., and Jávor, A. (2011). The comparison analysis of the main chemical composition parameters of wild boar meat and pork. Scientific Papers Animal Sci. Biotechnol. 44:105.
Soetan, K. O., Olaiya, C. O., and Oyewole, O. E. (2010). The importance of mineral elements for humans, domestic animals and plants: a review. Afr. J. Food Sci. 4, 200–222.
Srebočan, E., Crnić, A. P., Ekert-Kabalin, A. M., Lazarus, M., Jurasović, J., Tomljanović, K., et al. (2011). Cadmium, lead, and mercury concentrations in tissues of roe deer (Capreolus capreolus L.) and wild boar (Sus scrofa L.) from lowland Croatia. Czech J. Food Sci. 29, 624–633. doi: 10.17221/249/2010-CJFS
Standard European (2002). EN 13804:2002: Foodstuffs-determination of trace elements - pressure digestion. Brussels: European Committee for Standardization.
Stefanidou, M., Maravelias, C., Dona, A., and Spiliopoulou, C. (2006). Zinc: a multipurpose trace element. Arch. Toxicol. 80, 1–9. doi: 10.1007/s00204-005-0009-5
Strazdiņa, V., Jemeļjanovs, A., and Šterna, V. (2013). Nutrition value of wild animal meat. Proceedings of the Latvian Academy of Sciences. Section B. Natural, exact, and applied sciences 67, 373–377. doi: 10.2478/prolas-2013-0074
Taggart, M. A., Reglero, M. M., Camarero, P. R., and Mateo, R. (2011). Should legislation regarding maximum Pb and cd levels in human food also cover large game meat? Environ. Int. 37, 18–25. doi: 10.1016/j.envint.2010.06.007
Tam, M., Gómez, S., González-Gross, M., and Marcos, A. (2003). Possible roles of magnesium on the immune system. Eur. J. Clin. Nutr. 57, 1193–1197. doi: 10.1038/sj.ejcn.1601689
Topa, D., Cara, I. G., and Jităreanu, G. (2021). Long term impact of different tillage systems on carbon pools and stocks, soil bulk density, aggregation and nutrients: a field meta-analysis. Catena 199:105102. doi: 10.1016/j.catena.2020.105102
Zeinali, T., Salmani, F., and Naseri, K. (2019). Dietary intake of cadmium, chromium, copper, nickel, and Lead through the consumption of meat, liver, and kidney and assessment of human health risk in Birjand, southeast of Iran. Biol. Trace Elem. Res. 191, 338–347. doi: 10.1007/s12011-019-1637-6
Zeng, H. X., Man, Y. B., Wong, M. H., and Cheng, Z. (2024). Hair heavy metals and food consumption in residents of Chengdu: factors, food contribution, and health risk assessment. Biol. Trace Elem. Res. 202, 1503–1516. doi: 10.1007/s12011-023-03785-y
Keywords: microminerals, macrominerals, game meat, heavy metals, health risk
Citation: Boișteanu P-C, Flocea E-I, Anchidin B-G, Mădescu B-M, Matei M, Murariu OC, Frunză G, Postolache AN and Ciobanu M-M (2024) Essential and toxic elements analysis of wild boar tissues from north-eastern Romania and health risk implications. Front. Sustain. Food Syst. 8:1406579. doi: 10.3389/fsufs.2024.1406579
Received: 25 March 2024; Accepted: 16 April 2024;
Published: 29 April 2024.
Edited by:
Hamada Imtara, Arab American University, PalestineReviewed by:
Asmae Baghouz, Sidi Mohammed Ben Abdellah University, MoroccoCopyright © 2024 Boișteanu, Flocea, Anchidin, Mădescu, Matei, Murariu, Frunză, Postolache and Ciobanu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marius-Mihai Ciobanu, bWFyaXVzLmNpb2JhbnVAaXVscy5ybw==; Alina Narcisa Postolache, bmFyY2lzYS5wb3N0b2xhY2hlQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.