- 1Fish Nutrition Laboratory, Department of Zoology, Government College University Faisalabad, Faisalabad, Pakistan
- 2Environmental Studies Department, University of California Santa Cruz, Santa Cruz, CA, United States
- 3Department of Environmental Sciences and Engineering, Government College University, Faisalabad, Punjab, Pakistan
- 4Department of Biological Sciences and Technology, China Medical University, Taichung, Taiwan
- 5Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia
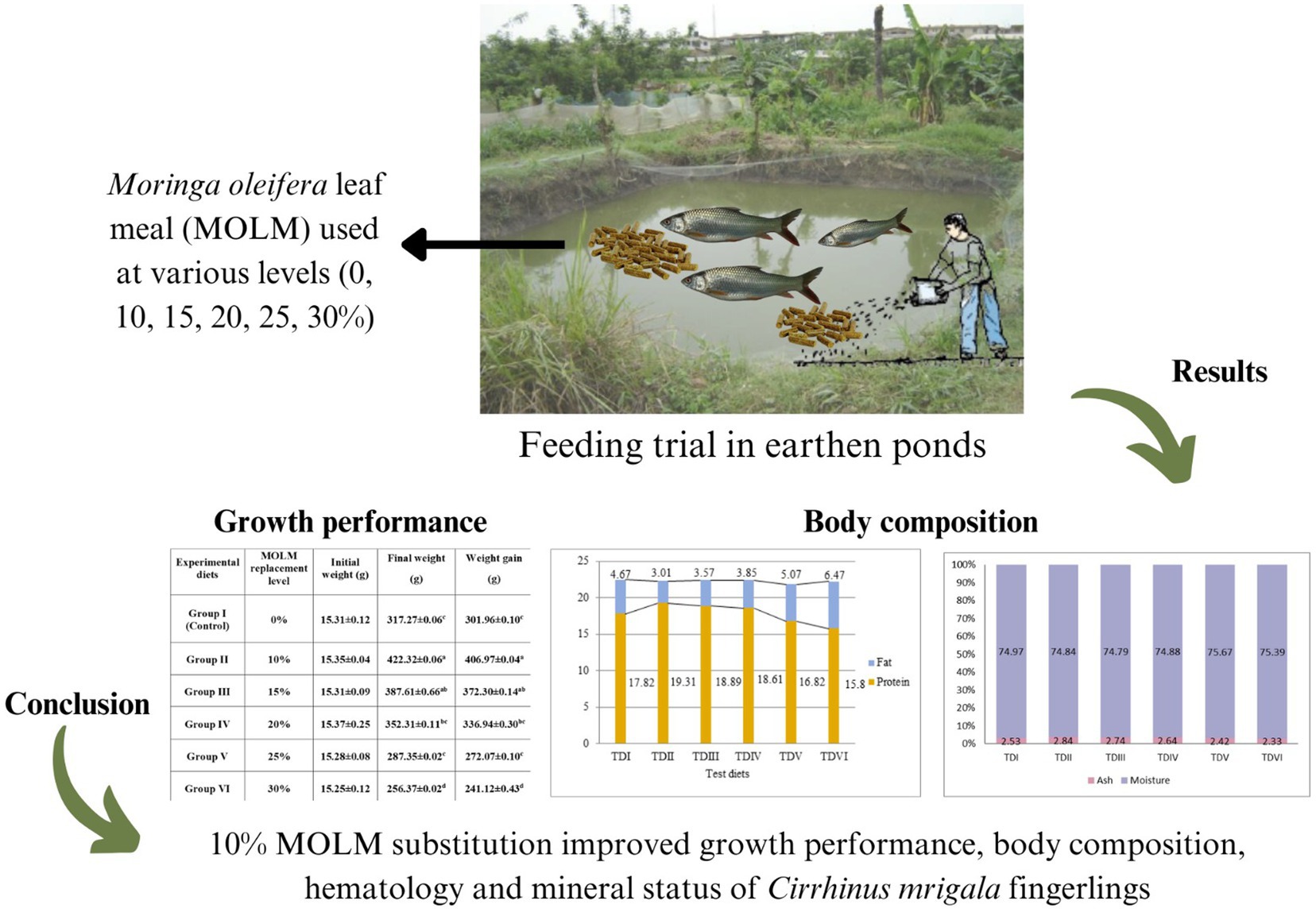
Introduction: In the current study, Cirrhinus mrigala was used as an animal model to investigate the impacts of Moringa oleifera leaf meal (MOLM) on their overall performance reared in six earthen ponds.
Methods: In this study, fishmeal (FM) was substituted with MOLM at various levels in the diet: 0% (control), 10, 15, 20, 25, and 30%, for a six-month feeding trial. A total of 270 fish with 15 fingerlings (15.31 ± 0.12 g/fish) stocked in each of the six earthen ponds in triplicates.
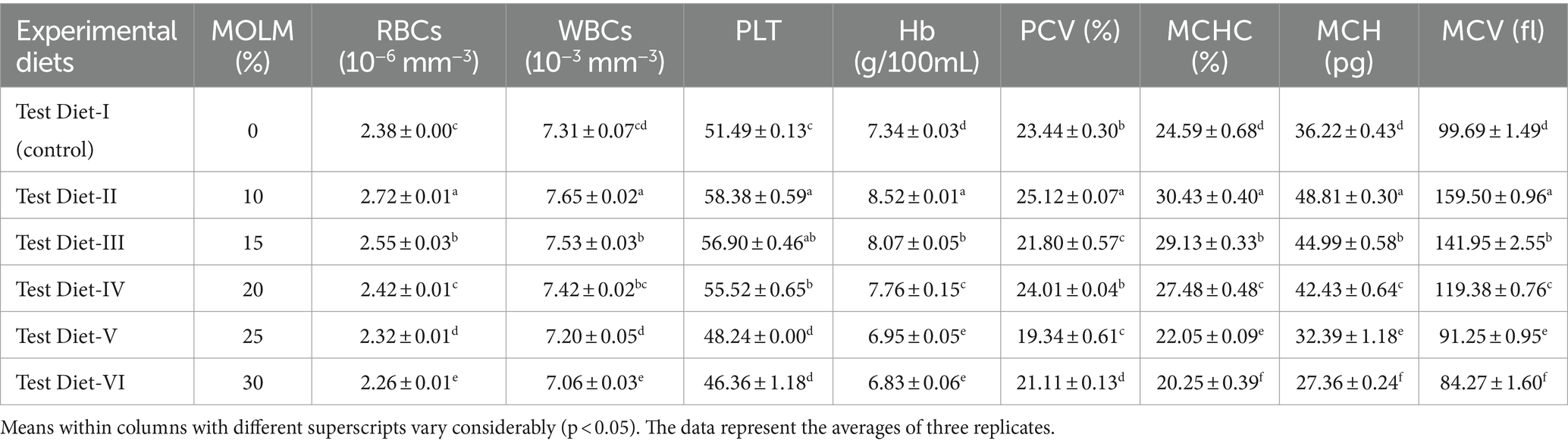
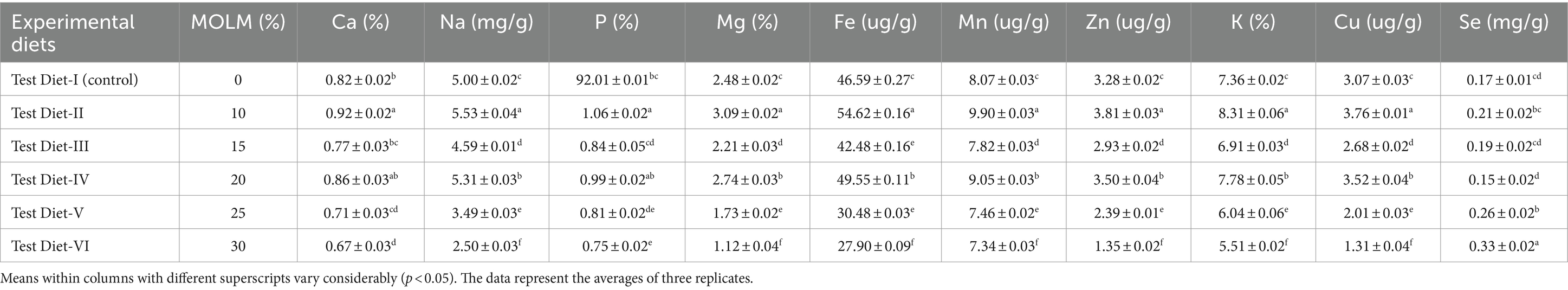
Results: When compared to other experimental and control groups, analyses revealed that fish given a diet having 10% MOLM had the highest growth performance (final weight: 422.32 g, weight gain: 406.97 g). Fingerlings fed a diet which substituted with 10% of FM with MOLM showed the greatest results for body composition (crude protein: 19.31%; crude fat: 3.01%). The results of hematology, i.e., WBCs: 7.65 × 103 mm−3, PLT: 58.38, hemoglobin level: 8.52 g 100 mL−1, PCV: 25.12%, MCHC: 30.45%, MCH: 48.81 pg., MCV: 159.50 fl, also showed that 10% MOLM was the optimum level for FM substitution. Furthermore, the outcomes of body mineralization (P, Ca, Na, Mn, Fe, Mg, Zn, K, and Cu, 1.06, 0.92%, 5.53 mg/g, 9.90 ug/g, 54.62 ug/g, 3.09%, 3.81 ug/g, 8.31%, and 3.76 ug/g respectively) also indicated that 10% level was the best.
Conclusion: Conclusively, the current study found that substituting 10% of FM with MOLM in the diet of C. mrigala fingerlings improved growth performance, carcass, hematology, and mineral status.
1 Introduction
Aquaculture aims to provide the largest quantity of nutritious food that is high in protein in order to meet the global needs for nutrition of consumers (Dawood, 2021). A healthy diet is necessary for all living organisms, including fish, to thrive, produce offspring, and sustain their health (Adebayo et al., 2020). Fish is a great source of several vitamins and minerals as well as superior-quality and readily digestible protein (Gore et al., 2021). Aquaculture sector supplies 50% of all global food fish and is expanding at the high rate as compared to any other food processing sector (FAO, 2020). This industry is significantly developing at a rate of about 5.8% annually (FAO, 2020). Its accomplishments and involvements are predicted to lessen hunger and poverty while simultaneously providing protein for the majority of the world’s food requirements (Dawood, 2021).
Fishmeal (FM) is considered a premium nutritional ingredient for fish feed, because of its majority of vitamins, good digestibility, balanced amino acid profile, growth-promoting and attracting qualities (Allam et al., 2020). Fish diet contributes about 40–60% of production cost in aquaculture (Hassaan et al., 2019; Nasr et al., 2021). Therefore, for the purpose to reduce the cost of aqua-diets, it is necessary to look for affordable, sustainable, and locally accessible protein sources (Mansour et al., 2021). Many researchers have used plant-based feeds to replace FM (Davies et al., 2019; Elumalai et al., 2021). In most developing nations, plant proteins are seen to be the most practical choice for the commercial production of fish as they are quite affordable, more environment friendly, highly sustainable, and easily available (Hardy, 2010; Hassaan et al., 2018).
Moringa oleifera leaves stand out as an excellent choice and alternate source of feed in the fish sector due to their higher concentrations of minerals, vitamins, and proteins. Native to northern India, M. oleifera is a fast-growing member of the moringaceae family that can thrive in deserts (Islam et al., 2021). This versatile herb has been included in diets of ruminants, fishes, hens, rabbits, and rats during the last several years to assess its impacts on growth and reproductive efficiency (Momin and Memiş, 2023). Possibly more than any other tropical vegetable, moringa leaf thought to be a rich source of proteins, vitamins, and minerals with a variety of therapeutic benefits (Ebuka et al., 2021). Moreover, Moringa leaves have been noticed to contain higher ratio of iron, potassium, calcium, and vitamins A and C, as well as protein (25–32%), than other dietary items such as bananas, oranges, milk, yoghurt, and carrots (Gopalakrishnan et al., 2016). Additionally, its leaves contain more than 16–19 amino acids, ten of which are essential (Moyo et al., 2014). When its leaves are dehydrated, they have high crude protein content, 5.9% crude fibre, 7.09% crude fat and 7.6–12% ash (Su and Chen, 2020). However, Moringa leaves also possess some of the anti-nutritional elements such as saponins, phytates, tannins and phenols (Tacon, 1985; Wee and Wang, 1987). They bind with minerals like zinc and phosphorus, and have a bitter taste. They are hazardous in large amounts and can disrupt metabolic and digestive enzymes, which may impede nutritional absorption (Gemede and Ratta, 2014). M. oleifera leaf meal (MOLM) has been used by several researchers to partially substitute soybean meal and other plant protein sources in the diets of Oreochromis niloticus and Clarias gariepinus (Elabd et al., 2019; Ebuka et al., 2021).
One of the renowned Indian major carp, Cirrhinus mrigala, accounts for around 20–25% of all major carp’s production in India (Kumar et al., 2018). C. mrigala, sometimes referred to as “Mori,” is extensively found in Pakistan’s freshwater reservoirs and has a high market value in addition to its economic significance. Consumers consider it more appealing due to its excellent flavor and potent nutritional content (Tabassum et al., 2021). Earthen ponds, reservoirs to culture fish species in a natural aquatic environment, are cheaper to maintain and have a relatively higher stocking capacity than concrete ponds, which are constructed without preserving natural habitat (Marywil Farms, 2022). Due to the deficit of research on this species in earthen ponds and its usage in higher proportion on commercial scale, it is very significant to carry out a comprehensive research on this species. The main objective of this study was to assess the potency of MOLM as a substitute protein source for C. mrigala fingerlings reared in earthen ponds and its impact on their growth and other body parameters.
2 Materials and methods
2.1 Experimental site and animals
The C. mrigala fingerlings (15.31 ± 0.12 g/fish) were bought from Government Fish Hatchery, Faisalabad and transported to experimental site. After that, fingerlings were placed in a cemented pond (length: 10 feet, width: 7 feet) for 15 days under lab conditions for acclimatization. Then fingerlings were transferred to the Fisheries Research Farms, Department of Zoology, University of Agriculture Faisalabad. At the Fisheries Research Farms, a semi-intensive rearing technique was used for a six-month feeding trial. It was carried out in 18 earthen ponds between February and August 2021. Each pond had a rectangular form. An adjacent deep tube well was used to irrigate the ponds using groundwater.
2.2 Controlled conditions
Before the feeding experiment began, the fingerlings were treated with NaCl (5 g/L) to eradicate any ectoparasites and infections (Rowland and Ingram, 1991). The air was provided by recirculating water to keep up the level of dissolved oxygen (DO). Physicochemical variables were monitored, including pond water temperature, pH and DO, on daily basis, by using multiprobe water checker (U-10 model, Horiba, Tokyo, Japan) dipped in 20–25 cm below water. They were kept at 25.5–27.7°C, 7.5–8.1, and 5–6 mg/L, respectively, throughout experimental period and fed with basal diet two times a day (9:00 am and 4:00 pm).
2.3 Moringa oleifera leaf processing to prepare experimental diets
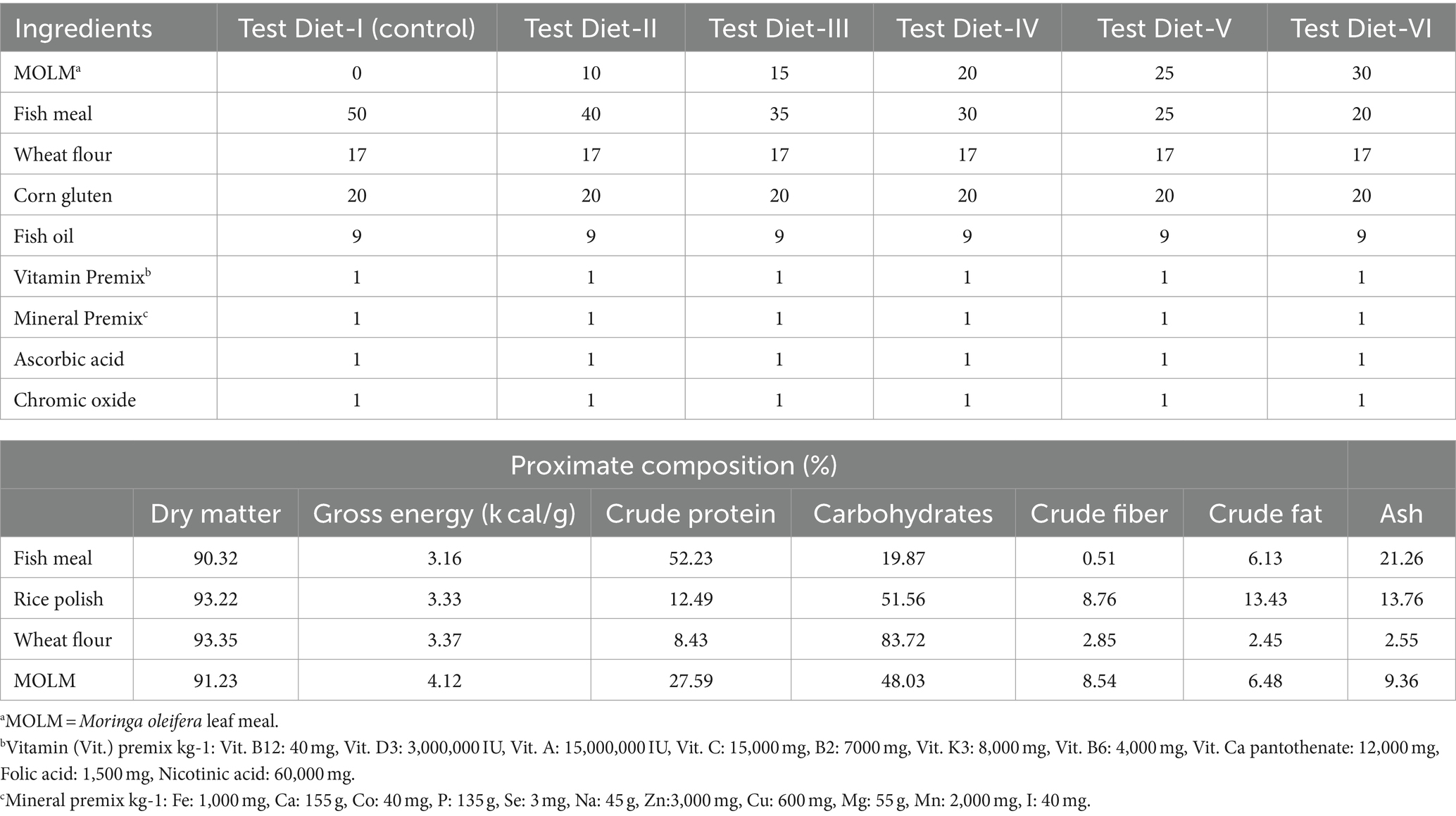
MOLM was employed as a test element to create experimental diets. Fingerlings were fed with varying levels of MOLM diet (Test Diet-I: 0%, Test Diet-II: 10%, Test Diet-III: 15%, Test Diet-IV: 20%, Test Diet-V: 25%, and Test Diet-VI: 30%), where control group was simply fed with basal diet and other five trial diets with varying amount of substitute (Table 1; Javid et al., 2018). Fifteen fingerlings were placed in each earthen pond in triplicate (n = 270), and each day they were fed at a ratio of 5 % of their biomass.
The ingredients were brought from market and moringa leaves are taken from Multan (city of Punjab). Initially, the pulverized leaves were soaked for three days at room temperature in tap water. They were soaked for three days to lessen the quantities of anti-nutritional elements like saponins (Tacon, 1985; Wee and Wang, 1987). After completing the soaking period, the leaves were dehydrated to lessen the quantity of water in it. Chemical composition of ground ingredients was also checked prior to mixing in basal diet [AOAC (Association of Official Analytical Chemists), 2005a].
In order to pass the feed ingredients through a 0.5 mm sieve, they were properly crushed. Chrome oxide was used as an inert marker in the test diets. Fish oil was added gradually then allowed them to mix for 5 min in a mixer. Moreover, 10–15% water was added to create a dough with the homogenous texture. This dough was then passed through an electric extruder to form pellets (Lovell, 1989).
2.4 Proximate analyses
Before being subjected to the normal process for analyses, the fish diet and whole body samples were separately homogenized with a motor and pestle. The laboratory analyses were done at Zoology Department, Government College University Faisalabad, Pakistan. Moisture content was evaluated through oven drying at 105°C for 12 h, protein was indicated by using a micro Kjeldahl instrument, ash was determined by using an electric furnace ignition for 12 h (650°C) (Eyela-TMF 3100) and fat was determined using a petroleum ether extraction process by Soxtec HT2 1,045 system. By using an atomic absorption spectrophotometer, the mineral content of fingerlings was determined [AOAC (Association of Official Analytical Chemists), 2005b].
2.5 Study of growth
Growth parameters of fingerlings were measured at the start and end of a period of six months. The maximum number of fingerlings samples from each pond were taken. During this experiment, the following growth parameters were examined by following formulae.
2.6 Hematological parameters
Blood was drawn from sample fingerlings’ caudal vein, an anticoagulated syringe was used for this purpose and then these samples were taken to analysis. A hemocytometer with a certified Neubauer counting chamber was used to count RBCs (Red Blood Cells), Platelets and WBCs (White Blood Cells) (Blaxhall and Daisley, 1973). The micro-hematocrit technique was used to evaluate hematocrit, capillary tubes were used for this purpose (Brown, 1988). The concentration of Hb (hemoglobin) was determined by the methods stated by Wedemeyer and Yasutake (1977). Measurement of MCHC (mean corpuscular hemoglobin concentration), MCH (mean corpuscular hemoglobin), and MCV (mean corpuscular volume) was done by using standard formulae (mean cell volume) described by Tabassum et al. (2021).
2.7 Statistical analysis
To statistically investigate the effects of varying concentrations of MOLM on growth, carcass, hematology and mineralization, ANOVA (One-way analysis of variance) was used (Steel et al., 1996). In order to verify data normality, the Bartlett test of homogeneity of variance was performed. To analyze the differences between the means, ‘Tukey’s Honest Significant Difference’ Test was employed, and p < 0.05 was considered significant (Snedecor and Cochran, 1991). Values were presented as mean ± standard error (SD), with significant differences observed at p < 0.05. The Co-Stat computer program (Version 6.303, PMB 320, Monterey, CA, 93940 United States) was implemented for the statistical analysis.
3 Results
3.1 Growth studies
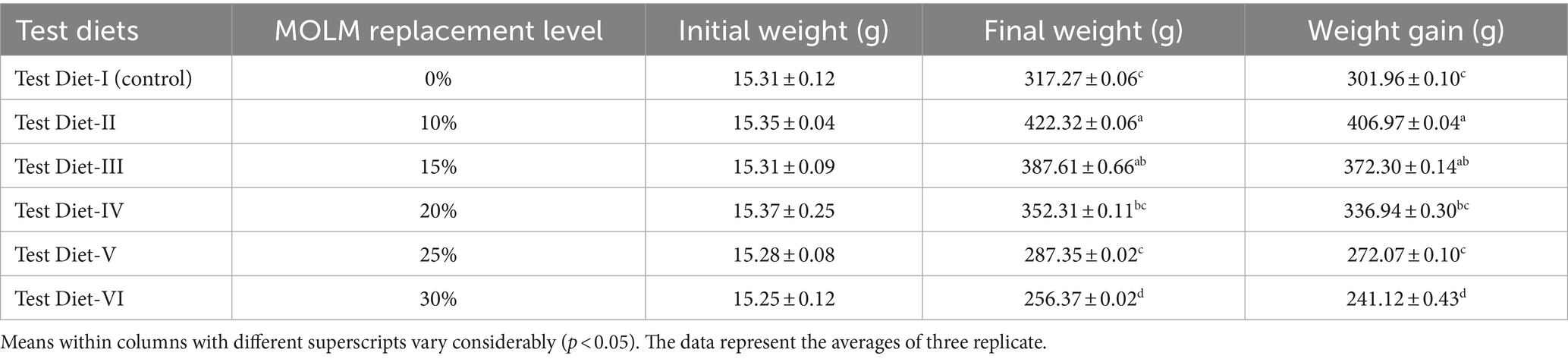
Table 2 displays the results of growth performance of fish given diets based on MOLM. Fish fed with 10% MOLM-based diets showed considerably (p < 0.05) enhanced growth in terms of FW and WG. The fish fed a 10% MOLM-based diet showed the maximum value of FW (422.32 g), and WG (406.97 g) while the fish fed with 30% MOLM-based diet showed the minimum value of FW (256.37 g) and WG (241.12 g).
3.2 Body composition
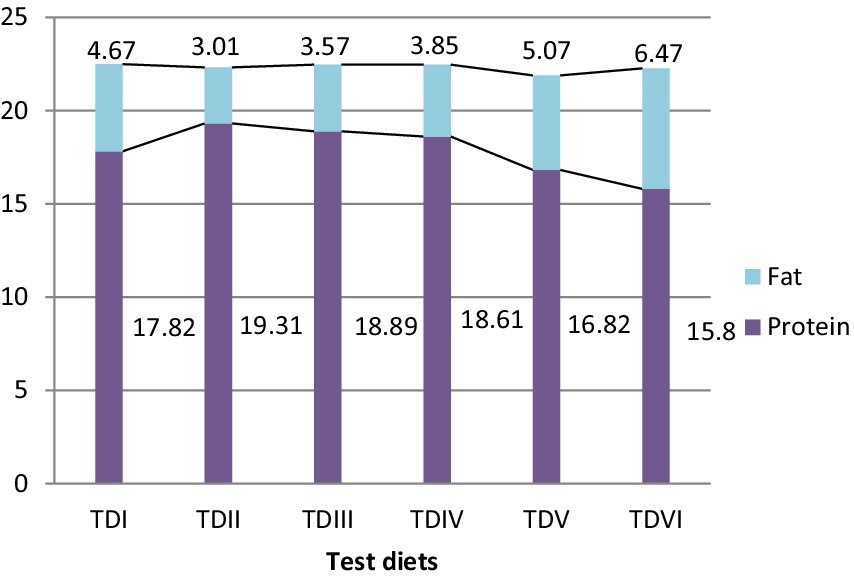
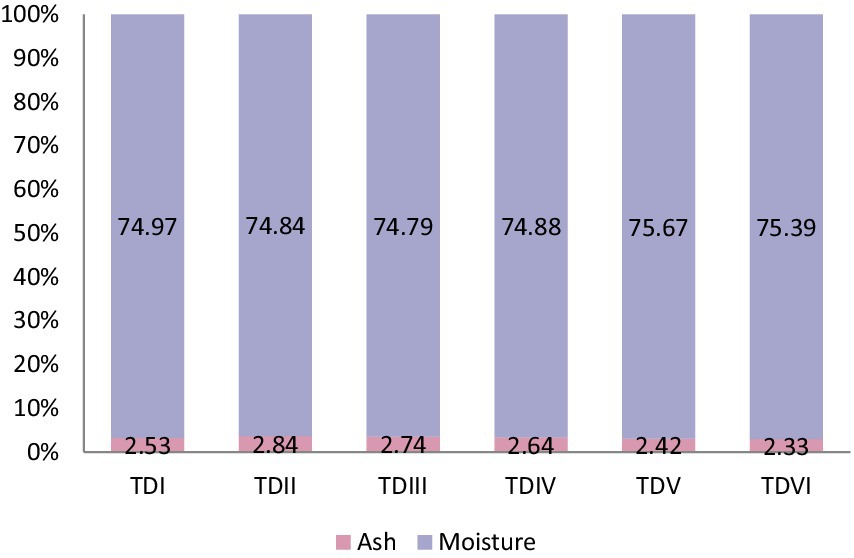
The whole body proximate of fish varied significantly (p < 0.05) (Figures 1, 2). Fish fed with 10% MOLM had the highest protein content (19.31%) and lowest fat value (3.01%) while, fish fed with 50% MOLM had the highest proportion of fat (6.47%) and the lowest value of protein (15.80%). Test diet-II exhibited the highest ash content value (2.84%), whereas test diet-VI had the lowest ash content value (2.33%). Furthermore, the body composition of fish would not be significantly affected by adding 25% or 30% MOLM in their diet. Test diet-V fish had the highest moisture content (75.67%) while, test diet-III had the lowest moisture value (74.79%).
3.3 Hematological parameters
Table 3 displays the results of the hematological parameters of C. mrigala fed diets based on MOLM. Fish given a 10% level of MOLM had the highest value of RBCs (2.72 × 106 mm−3). Furthermore, Test diet-II reported the highest value of PLT value (58.38), WBCs (7.65 × 103 mm−3) and hemoglobin level (8.52 g/100 mL). MCHC, MCV, MCH, and PCV, levels were optimum at 30.45%, 159.50 fl, 48.81 pg. and 25.12%, respectively, in fish given a 10% inclusion of a diet based on MOLM. Research revealed that no improvement of hematological parameters was noticed in the diet of fish at 30% level of MOLM.
3.4 Body mineralization
Table 4 displays the findings of total body mineralization in fingerlings. In test diet-II (10% MOLM), fingerlings had the highest concentrations of P, Ca, Na, Mn, Fe, Mg, Zn, K, and Cu (1.06, 0.92%, 5.53 mg/g, 9.90 ug/g, 54.62 ug/g, 3.09%, 3.81 ug/g, 8.31%, and 3.76 ug/g, respectively) in their body. The fish fed diet with 30% substitution of MOLM had the lowest levels of Ca (0.67%), Na (2.50 mg/g), P (0.75%), Mg (1.12%), Fe (27.90 ug/g), Mn (7.34 ug/g), Zn (1.35 ug/g), K (5.51%), and Cu (1.31 ug/g) in their body. The fish given with test diet-VI (30% MOLM level) had the maximum Se concentration (0.33 mg/g), whereas test diet-I (control) had the lowest Se value (0.15 mg/g).
4 Discussion
Plant-based proteins are a practical option for commercially producing fish due to their low cost and easy accessibility. Furthermore, using plant-based ingredients as a substitute for FM has significantly improved the performance of fish, resulting in reduced discharge of nutrients such as phosphorous and nitrogen (Hardy, 2010; Chakraborty et al., 2019). Hence, the current research was carried out to evaluate the efficacy of varying levels of MOLM on growth parameters, nutritional composition, hematology, and mineral composition of C. mrigala.
4.1 Growth performance
In this study, it was investigated that how six different MOLM-based levels affected the growth of C. mrigala fingerlings. Present findings are in line with those of Monir et al. (2020) who examined the impacts of adding moringa leaf extract on Nile tilapia diets and found that growth indices were significantly improved. Due to high nutritional content (proteins, minerals, and vitamins) of MOLM, the overall performance and growth of fish improve. Our findings are matched with those of Doctolero and Bartolome (2019), who fed O. niloticus with diets containing up to 20% MOLM, and showed that this increased the growth of fingerlings. Additionally, this research revealed that O. niloticus flourished significantly when fed up to 20% meal based on MOLM. Similarly, our findings also align with those of Elabd et al. (2019) who fed O. niloticus diets based on MOLM and noted a considerable improvement in fish growth performance. The findings of Hassaan et al. (2018) had shown that adding 10–20% MOLM to the diets of L. rohita boosted the WG substantially, but adding more MOLM to the diet did not improve the fish growth performance. Additionally, Tabassum (2017) noted that adding 10% MOLM to the diet of Mozambique tilapia fingerlings enhanced the growth performance. Additionally, Yuangsoi and Charoenwattanasak (2011) recommended the incorporation of 10% moringa leaves to diet of Nile tilapia. However, Kasiga and Lochmann (2014) noticed that it is feasible to substitute up to 30% protein source in soybean meal with MOLM without having any negative impact on growth of Nile Tilapia. It has been claimed that moringa leaves can be used in place of 10% of FM in Asian seabass to increase growth performance (Ganzon-Naret, 2014). Another research contradicted previous findings that adding M. oleifera in the diet inhibits fish growth, this might be due to negative effects of anti-nutrients (saponins, phytates, tannins and phenols) (Mehdi et al., 2016). The reason for the opposite outcome might be because the experimental duration, size of fingerlings, and diet content were all different.
4.2 Body composition
The findings of this investigation were parallel to those of Arsalan et al. (2016), who found that 10% substitution of MOLM showed the maximum CP and 40% inclusion level resulted in the minimum CP when compared with a control group. It was also shown that a substantial protein substitution occurs when FM was substituted by10% MOLM. This might be due to activities of antioxidant and phenolic compounds in MOLM playing hypolipidemic role (Imoru, 2019). Similarly, Ganzon-Naret (2014) found that CP at 10% was significantly higher than that of other MOLM diets when examining the carcass composition of Asian sea bass fed on these diets at different inclusion levels. The results of Ayotunde Ezekiel et al. (2016), examined the body composition of C. gariepinus to determine whether the partial FM substitution with a diet based on MOLM had positive effects on fish. The greatest value was recorded in the group that was fed a diet including 30% MOLM. This group also exhibited a rising pattern of fat contents with increased addition levels of MOLM-based diet, while the lowest value showed a decline in fat content. Consistent with recent research, the fish’s CF increased as the quantity of MOLM-based diet incorporation increased (Ganzon-Naret, 2014). Ganzon-Naret (2014) reported similar findings, that when MOLM’s proportion in the fish diet increased (10–20%), the ash content of Asian sea bass decreased.
4.3 Hematological indices
Hematological markers are becoming more significant aspects of aquaculture because they represent fish growth and well-being (Fazio, 2019). According to Ayotunde Ezekiel et al. (2016), when leaves of moringa were added in the feed, the hematological indices of C. garipenus, such as the RBC count, PCV, Hb, and WBC count, improved dramatically. Ahmed et al. (2014) exhibited that feeding moringa leaves to Nile tilapia resulted in appreciable increase in their hematological parameters. When moringa leaves were fed to Cyprinus carpio more often, blood parameters including RBC, WBC, Hb, and PCV improved (Adeshina et al., 2018). According to Arsalan et al. (2016), L. rohita exhibited the greatest Hb value and RBC count when 10% of the FM was substituted with moringa leaves. According to studies on catfish, adding 10% more moringa leaves to the meal had no detrimental effects on the hematological indices or blood enzymes (Dienye and Olumuji, 2014). The results of this research are comparable to those of Tabassum et al. (2021), who reported that a rise in MOLM led to a reduction in hematological parameters in C. mrigala. They additionally recommended that a 10% substitution of MOLM in the feed improved fish hematology. Similarly, according to Billah et al. (2020), feeding MOLM to fish inhibited O. niloticus’s ability to enhance its RBC and WBC counts. Current study results showed contradiction with the results of Abd El-Gawad et al. (2020) who found that when fish were fed 1.5% MOLM, WBCs greatly improved, while O. niloticus Hb and RBCs levels did not significantly change. It could be caused by the fish’s size, diet concentration, variety, and overall health.
4.4 Mineralization
Minerals are inorganic compounds that are found naturally and are necessary for the fish body to work normally. Our findings concurred with those of Shahzad et al. (2021) who found that feeding common carp with MOLM increased the amount of minerals in the body of fish. The current study found that adding 10% MOLM to the diets of C. mrigala significantly increased the amount of minerals. More study is recommended as less literature is available regarding the effects of MOLM on fish species reared in earthen ponds.
5 Conclusion
The performance of C. mrigala fingerlings was shown to significantly increase when 10% FM was replaced with MOLM in their diet; however, increasing the percentage of MOLM in their diets had no noticeable impacts. Moreover, MOLM might substitute FM by 10 to 20% without compromising the performance of fingerlings. Hence, according to current study, 10% replacement of FM by MOLM is an excellent alternative source of protein in the diet of fingerlings. This research also suggested the formulation of MOLM based diet in earthen ponds that is economical as well as environment friendly. Further research is required to assess the impacts of MOLM on different fish species in earthen ponds. Moreover, future research should fully understand the mechanisms that mediate the M. oleifera effects on fish health.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by in this study, none of the authors used human beings as research subjects. This study has been performed in a responsible and ethical manner. It is in full consent with the relevant codes of experimentation and legislations. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
MF: Writing – original draft, Methodology. SMH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. PKS: Conceptualization, Funding acquisition, Investigation, Writing – review & editing. SA: Conceptualization, Investigation, Writing – review & editing. KAAG: Data curation, Formal analysis, Investigation, Writing – review & editing. ZY: Data curation, Formal analysis, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by University of California, Santa Cruz, United States.
Acknowledgments
The study was funded by HEC Pakistan Projects No. 20-4892/NRPU/R&D/HEC/14/1145. The authors extend their gratitude to the Researchers Supporting Project Number (RSP2024R48), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd El-Gawad, E. A., El Asely, A. M., Soror, E. I., Abbass, A. A., and Austin, B. (2020). Effect of dietary Moringa oleifera leaf on the immune response and control of Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus) fry. Aquac. Int. 28, 389–402. doi: 10.1007/s10499-019-00469-0
Adebayo, I. A., Akin-Obasola, B. J., and Abe, B. A. (2020). Growth and haematological responses of Clarias gariepinus juveniles fed diets containing varying digestible lipids of plant origin. Int. J. Fish. Aquac. 6, 10–23. doi: 10.37745/ijfar.15
Adeshina, I., Sani, R. A., Adewale, Y. A., Tiamiyu, L. O., and Umma, S. B. (2018). Effects of dietary leaf meal as a replacement for soybean meal on growth, body composition and health status in juveniles. Croat. J. Fish. 76, 174–182. doi: 10.2478/cjf-2018-0021
Ahmed, H. S., Adel, M., and Adel, E. (2014). Incorporation of Moringa oleifera leaf in Nile tilapia Oreochromis niloticus diet and its effect on growth performance and immune status. J. Vet. Sci. 1, 8–6.
Allam, B. W., Khalil, H. S., Mansour, A. T., Srour, T. M., Omar, E. A., and Nour, A. A. M. (2020). Impact of substitution of fish meal by high protein distillers dried grains on growth performance, plasma protein and economic benefit of striped catfish (Pangasianodon hypophthalmus). Aquaculture 517:734792. doi: 10.1016/j.aquaculture.2019.734792
AOAC (Association of Official Analytical Chemists) (2005a). Official methods of analysis. 18th Edn. Gaithersburg, Maryland: AOAC.
AOAC (Association of Official Analytical Chemists) (2005b). Official methods of analysis. 18th Edn. Arlington, VA: Association of official analytical chemists.
Arsalan, M. Z. H., Hussain, S. M., Asrar, M., Anwar, H., Rehan, M. M. H., Shahzad, M. M., et al. (2016). Effects of Moringa oleifera leaf meal (MOLM) based diets on carcass composition and hematology of Labeo rohita fingerlings. J. Environ. Sci. 9, 214–223.
Ayotunde Ezekiel, O., Ada Fidelis, B., and Udeh Grace, N. (2016). Effect of partial replacement of fishmeal with Moringa oleifera leaf meal on the haematology, carcass composition and growth perfomance of Clarias gariepinus (Burchell 1822) fingerlings. Int. J. Fish. Aquat. Stud. 4, 307–311.
Billah, M. B., Haque, M. E., Sarkar, S., Hossain, M. M., and Dey, S. K. (2020). Growth performance, hematological disorder and bacterial challenge on nile tilapia (Oreochromis niloticus) using Moringa oleifera plant leaf as feed supplement. Bangladesh J. Zool. 48, 151–166. doi: 10.3329/bjz.v48i1.47884
Blaxhall, P. C., and Daisley, K. W. (1973). Routine haematological methods for use with fish blood. J. Fish Biol. 5, 771–781. doi: 10.1111/j.1095-8649.1973.tb04510.x
Chakraborty, P., Mallik, A., Sarang, N., and Lingam, S. S. (2019). A review on alternative plant protein sources available for future sustainable aqua feed production. Int. J. Chem. Stud 7, 1399–1404.
Davies, S. J., Laporte, J., Gouveia, A., Salim, H. S., Woodgate, S. M., Hassaan, M. S., et al. (2019). Validation of processed animal proteins (mono-PAPS) in experimental diets for juvenile gilthead sea bream (Sparus aurata L.) as primary fish meal replacers within a European perspective. Aquac. Nutr. 25, 225–238. doi: 10.1111/anu.12846
Dawood, M. A. (2021). Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev. Aquac. 13, 642–663. doi: 10.1111/raq.12492
Dienye, H. E., and Olumuji, O. K. (2014). Growth performance and haematological responses of African mud catfish Clarias gariepinus fed dietary levels of Moringa oleifera leaf meal. Net J. Agric. Sci. 2, 79–88.
Doctolero, J. S., and Bartolome, R. M. (2019). Utilization of horseradish (Moringa oleifera) as an alternative protein-source feed ingredient on the diet of red Nile tilapia (Oreochromis niloticus). Int. J. Fish. Aquat. Stud. 7, 94–97.
Ebuka, I. A., Adogbeji, E. P., Oghenebrorhie, O., Lydia, A. M., Ejovwokoghene, O. J., and Ufuoma, A. E. (2021). Moringa oleifera leaf meal as partial replacement of soybean meal in diets of Clarias gariepinus juveniles. Livest. Res. Rural. Dev. 33:8.
Elabd, H., Soror, E., El-Asely, A., Abd El-Gawad, E., and Abbass, A. (2019). Dietary supplementation of Moringa leaf meal for Nile tilapia Oreochromis niloticus: effect on growth and stress indices. Egypt. J. Aquat. Res. 45, 265–271. doi: 10.1016/j.ejar.2019.05.009
Elumalai, P., Kurian, A., Lakshmi, S., Musthafa, M. S., Ringo, E., and Faggio, C. (2021). Effect of Leucas aspera against Aeromonas hydrophila in nile tilapia (Oreochromis niloticus): immunity and gene expression evaluation. Turk. J. Fish. Aquat. Sci. 22:TRJFAS19802. doi: 10.4194/TRJFAS19802
Fazio, F. (2019). Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture 500, 237–242. doi: 10.1016/j.aquaculture.2018.10.030
Ganzon-Naret, E. S. (2014). Utilization of Moringa oleifera leaf meals as plant protein sources at different inclusion levels in fish meal based diets fed to Lates calcarifer. Anim. Biol. Anim. Husb. 6, 158–167.
Gemede, H. F., and Ratta, N. (2014). Antinutritional factors in plant foods: potential health benefits and adverse effects. Int. J. Nutr. Food Sci. 3, 284–289. doi: 10.11648/j.ijnfs.20140304.18
Gopalakrishnan, L., Doriya, K., and Kumar, D. S. (2016). Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci. Human Wellness 5, 49–56. doi: 10.1016/j.fshw.2016.04.001
Gore, S. B., Balange, A. K., Nayak, B. B., Kumar, H. S., Tandale, A. T., and Xavier, K. M. (2021). Comparative analysis of unwashed and single washed mince gel from Indian major carps. J. Food Sci. 59, 377–387. doi: 10.1007/s13197-021-05024-5
Hardy, R. W. (2010). Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquac. Res. 41, 770–776. doi: 10.1111/j.1365-2109.2009.02349.x
Hassaan, M. S., El-Sayed, A. I. M., Soltan, M. A., Iraqi, M. M., Goda, A. M., Davies, S. J., et al. (2019). Partial dietary fish meal replacement with cotton seed meal and supplementation with exogenous protease alters growth, feed performance, hematological indices and associated gene expression markers (GH, IGF-I) for Nile tilapia, Oreochromis niloticus. Aquaculture 503, 282–292. doi: 10.1016/j.aquaculture.2019.01.009
Hassaan, M. S., Soltan, M. A., Mohammady, E. Y., Elashry, M. A., El-Haroun, E. R., and Davies, S. J. (2018). Growth and physiological responses of Nile tilapia, Oreochromis niloticus fed dietary fermented sunflower meal inoculated with Saccharomyces cerevisiae and Bacillus subtilis. Aquaculture 495, 592–601. doi: 10.1016/j.aquaculture.2018.06.018
Imoru, A. (2019). Effect of dietary supplementation of Moringa oleifera leaf meal on the carcass characteristics and meat quality of broiler chickens. J. Anim. Sci. Vet. Med 4, 151–156. doi: 10.31248/JASVM2019.154
Islam, Z., Islam, S. M., Hossen, F., Mahtab-ul-Islam, K., Hasan, M. R., and Karim, R. (2021). Moringa oleifera is a prominent source of nutrients with potential health benefits. Int. J. Food Sci. 2021, 1–11. doi: 10.1155/2021/6627265
Javid, A., Hussain, A. I., Aslam, N., Ali, Q., Hussain, M., Khalid, A., et al. (2018). Replacement of fish meal with Moringa oleifera leaf meal (MOLM) and its effect on growth performance and nutrient digestibility in Labeo rohita fingerlings. Pak. J. Zool. 50, 1815–1823. doi: 10.17582/journal.pjz/2018.50.5.1815.1823
Kasiga, T., and Lochmann, R. (2014). Nutrient digestibility of reduced-soybean-meal diets containing Moringa or Leucaena leaf meals for nile tilapia Oreochromis niloticus. J. World Aquac. Soc. 45, 183–191. doi: 10.1111/jwas.12102
Kumar, P., Jain, K. K., Sardar, P., Jayant, M., and Tok, N. C. (2018). Effect of dietary synbiotic on growth performance, body composition, digestive enzyme activity and gut microbiota in Cirrhinus mrigala (ham.) fingerlings. Aquac. Nutr. 24, 921–929. doi: 10.1111/anu.12628
Mansour, A. T., Allam, B. W., Srour, T. M., Omar, E. A., Nour, A. A. M., and Khalil, H. S. (2021). The feasibility of monoculture and polyculture of striped catfish and Nile tilapia in different proportions and their effects on growth performance, productivity, and financial revenue. J. Mar. Sci. Eng. 9:586. doi: 10.3390/jmse9060586
Marywil Farms. (2022). Advantages and disadvantages of earthen pond you should consider in fish farming. Available at: https://marywilfarms.com.ng/2022/09/12/advantages-and-disadvantages-ofearthen-pond-you-should-consider-infish-farming/ (accessed: 04 November, 2022)
Mehdi, H., Khan, N., Iqbal, K. J., Rasool, F., Chaudhry, M. S., and Khan, K. J. (2016). Effect of Moringa oleifera meal on the growth, body composition and nutrient digestibility of Labeo rohita. Int. J. Biosci. 8, 11–17. doi: 10.12692/ijb/8.4.11-17
Momin, M., and Memiş, D. (2023). Dietary Moringa oleifera leaves to male rainbow trout (Oncorhynchus mykiss) broodstock: effects on sperm quality and reproductive performance. Aquaculture 577:739991. doi: 10.1016/j.aquaculture.2023.739991
Monir, W., Abdel-Rahman, M. A., Hassan, S. E. D., and Awad, S. M. (2020). Pomegranate peel and moringa-based diets enhanced biochemical and immune parameters of Nile tilapia against bacterial infection by Aeromonas hydrophila. Microb. Pathog. 145:104202. doi: 10.1016/j.micpath.2020.104202
Moyo, B., Masika, P. J., and Muchenje, V. (2014). Effect of feeding Moringa (Moringa oleifera) leaf meal on the physico-chemical characteristics and sensory properties of goat meat. S. Afr. J. Anim. Sci. 44, 64–70. doi: 10.4314/sajas.v44i1.9
Nasr, M. A., Reda, R. M., Ismail, T. A., and Moustafa, A. (2021). Growth, hemato-biochemical parameters, body composition, and myostatin gene expression of Clarias gariepinus fed by replacing fishmeal with plant protein. Animal 11:889. doi: 10.3390/ani11030889
Rowland, S. J., and Ingram, B. A. (1991). Diseases of Australian native fishes. Fish. Bull. 4, 21–23.
Shahzad, M. M., Rafique, T., Hussain, S. M., Hussain, Z., Zahoor, M. Y., Hussain, M., et al. (2021). Effect of phytase supplemented Moringa by-products based diets on the performance of Oreochromis niloticus fingerlings. J. Anim. Plant Sci 31, 288–295. doi: 10.36899/JAPS.2021.1.0216
Snedecor, G. W., and Cochran, W. G. (1991). Statistical methods. 8th Edn. Americans USA: Iowa State University. Press.
Steel, R. G. D., Torrie, J. H., and Dickey, D. A. (1996). Principles and procedures of statistics. 3rd Edn. New York. USA: McGraw Hill international Book Co. Inc.
Su, B., and Chen, X. (2020). Current status and potential of Moringa oleifera leaf as an alternative protein source for animal feeds. Front. Vet. Sci. 7:53. doi: 10.3389/fvets.2020.00053
Tabassum, S. (2017). Effect of Moringa oleifera leaves on growth performance and survival rate of Mozambique tilapia fingerlings (doctoral dissertation).
Tabassum, S., Hussain, S. M., Ali, S., Arsalan, M., Ahmad, B., Asrar, M., et al. (2021). Partial replacement of fish meal with Moringa oleifera leaf meal in practical diets of Cirrhinus mrigala fingerlings. Braz. J. Biol. 83:e246333. doi: 10.1590/1519-6984.246333
Tacon, A. G. J. (1985). Utilization of conventional and unconventional protein sources in practical fish feed. A review. Nutr. Feed. Fish 119-145:10014553332.
Wedemeyer, G. A., and Yasutake, W. T. (1977). Clinical methods for the assessment of the effects of environmental stress on fish health, vol. 89: Department of the Interior, Fish and Wildlife Service.
Wee, K. L., and Wang, S. S. (1987). Nutritive value of Leucaena leaf meal in pelleted feed for Nile tilapia. Aquaculture 62, 97–108. doi: 10.1016/0044-8486(87)90314-0
Yuangsoi, B., and Charoenwattanasak, S. (2011). “Utilization of moringa (Moringa oleifera lam.) leaf on growth performance and protein digestibility in Tilapia (Oreochromis niloticus L.)” in Proceedings of the 49th Kasetsart University annual conference, Kasetsart University (Thailand: Kasetsart University).
Keywords: moringa leaf meal, growth performance, body composition, hematology, mineral status
Citation: Faisal M, Hussain SM, Sarker PK, Ali S, Al-Ghanim KA and Yousaf Z (2024) Utilization of Moringa oleifera leaf meal as a protein source in diets for Cirrhinus mrigala: effects on growth, body composition, and hematology. Front. Sustain. Food Syst. 8:1405614. doi: 10.3389/fsufs.2024.1405614
Edited by:
Roberto Anedda, Parco Scientifico e Tecnologico della Sardegna, ItalyReviewed by:
Kenneth Prudence Abasubong, University of South Bohemia, CzechiaPeng Tan, Marine Fishery Institute of Zhejiang Province, China
Copyright © 2024 Faisal, Hussain, Sarker, Ali, Al-Ghanim and Yousaf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Syed Makhdoom Hussain, ZHJtYWtoZG9vbWh1c3NhaW5AZ2N1Zi5lZHUucGs=; Pallab K. Sarker, cHNhcmtlckB1Y3NjLmVkdQ==
 Muhammad Faisal1
Muhammad Faisal1 Syed Makhdoom Hussain
Syed Makhdoom Hussain Pallab K. Sarker
Pallab K. Sarker Shafaqat Ali
Shafaqat Ali Khalid A. Al-Ghanim
Khalid A. Al-Ghanim