- 1Department of Medical Laboratory Sciences, Addis Ababa University, Addis Ababa, Ethiopia
- 2Department of Biomedical Science, Asrat Woldeyes Health Science Campus, Debre Berhan University, Debre Birhan, Ethiopia
Background: Around the world, food-borne illnesses are still a frequent and significant hazard to public health. Human infection outbreaks brought on by eating raw fruits have happened more frequently. Fruits have been connected in recent years to a number of outbreaks of food-borne illness around the globe, including in Ethiopia.
Objective: To assess bacterial and parasitic contamination of fruits gathered from specific local markets in Addis Ababa, Ethiopia.
Methods: A cross-sectional study was carried out using fruits gathered from Addis Ababa local marketplaces. Convenient sampling were applied. With the assumption that each vendor provided 30 samples, a total of 120 fruit samples were gathered. Fruit samples were gathered in a plastic bag that had been sanitized and then brought to the lab for bacterial and parasitological investigation. All of the samples were checked for intestinal parasites and bacterial contamination. SPSS software version 25 was used to analyze the data. The Pearson's Chi-square test was used to assess categorical variables. The student's t-test was utilized to compare continuous variables, which were represented as the mean ± standard deviation. Using both univariate and multivariate analysis, odds ratios (OR) with 95% confidence intervals (CI) were computed. Statistical significance was defined as a P < 0.05.
Results: There was a 100 (83.3%) level of bacterial contamination. While Salmonella and Shigella species were not recovered, S. aureus isolates 16 (13.3%) were the most common bacterial contamination, followed by E. coli isolates 8 (6.7%). The samples of bananas, mangos, papayas, and avocados from all four sites had total coliform (TCC) bacteria ranging from 2.1 × 103 to 3.2 × 104, 8.3x 102 to 1.8x 105CFU/g, 1.6x 102 to 3.7 x 104, and 1.2x 102 to 3.8 x 104 CFU/g, in that order. No parasites were found in this investigation.
Conclusion and recommendation: It is usually recommended that customers in this research region wash and handle infected fruits properly to prevent bacterial illnesses. In addition, fruit dealers should have their bacterial contaminations routinely inspected. Large-scale research is advised to support this discovery.
Introduction
The World Health Organization's definition of fruits is more consistent and unified than its definition of vegetables when considering the plant origin, fleshy portion around seeds, sweet flavor, use as a snack or dessert, and typical consumption in a raw state (Keller and Tukuitonga, 2005). Certain microorganisms in agricultural settings can pose risks if proper precautions are not taken. Some contributing factors include: use of untreated human or animal waste as fertilizer, Contamination from bird droppings in growing fields, Poor personal hygiene practices by agricultural workers, contaminated irrigation or processing water sources, Use of unclean harvesting equipment, containers, or storage facilities. Fortunately, thoroughly washing produce in clean, safe water before consumption can help remove any potentially harmful microbes on the surface. This simple step can significantly reduce the risks associated with these microbial contaminants in the food supply (World Health Organization, 2012).
Worldwide, intestinal bacterial and parasite illnesses are common and pose a risk to public health. Living in poverty, having contaminated water, living in cramped quarters, and lacking in sanitation and hygiene are all strongly associated with infections with bacteria and medically significant parasites (intestinal helminths, protozoa). Consuming tainted, raw, or improperly cleaned fruits and vegetables can lead to human contraction of parasites (Adeyeba and Akinbo, 2002). When contamination occurs due to various interconnected issues during planting, harvesting, transportation, display, and mishandling at home, fruits and vegetables can become sources of parasite transmission (Omowaye and Audu, 2012).
Some parasites can exclusively spread through food, such as trematodes carried by fish. Others that enter the food chain through water or soil and can contaminate fresh produce include Ascaris lumbricoides, Cryptosporidium spp., Entamoeba histolytica, Enterobius vermicularis, Fasciola spp., Giardia intestinalis, Hookworm spp., Hymenolepis spp., Taenia spp., Trichuris trichiura, and ToxoCara spp (Idahosa, 2011).
Enterohaemorrhagic, Campylobacter, and Salmonella are the most frequently occurring bacterial species that cause contamination in fruit and food. Foodborne infections such as Escherichia coli can cause serious and often fatal consequences, affecting millions of people every year. Frequent symptoms include diarrhea, vomiting, headaches, fever, and stomach pain. Products derived from animals, such as eggs and poultry, can harbor salmonellosis. Drinking water, raw or undercooked poultry, and raw milk are the main sources of campylobacter. Fresh produce, undercooked meat, and unpasteurized milk are known to have enterohaemorrhagic Escherichia coli. Listeria and Vibrio cholera are two more types of bacterial species that may be the source of illness. Listeria can grow at refrigerator temperatures and is present in unpasteurized dairy products and other ready-to-eat foods, while Vibrio cholera is spread by contaminated water or food (Balali et al., 2020; Mostafidi et al., 2020).
Every bite we consume carries the potential risk of contracting a disease due to microbial contamination. Safer food practices quite literally save lives. To address this, we must build an adequate physical infrastructure for food storage. This includes proper temperature control, humidity regulation, and pest management to prevent spoilage and contamination. Design regulatory standards for food storage areas. These standards should cover cleanliness, sanitation, and worker hygiene protocols to ensure food safety. Use clean, safe water to wash and process fruits, vegetables, and other produce before they are ready for consumption or further processing. This helps remove harmful microbes. Follow good food production and handling procedures. This includes the appropriate and responsible use of agricultural chemicals, such as pesticides and fertilizers, to minimize the risk of contamination. Implementing these measures helps reduce the possibility of fruits, vegetables, and other food items becoming contaminated with harmful microbes. Prioritizing food safety at every step of the supply chain is critical for protecting public health.
Every year, millions of people get sick and millions of people die from eating contaminated food, putting billions of people at risk. In wealthier societies, there has been a sharp rise in food safety concerns. But the underdeveloped world is where the true tragedy of foodborne illnesses is being experienced. Human illness is mostly caused by intestinal parasites, which account for a considerable amount of morbidity and mortality. 350 million individuals are sick from these illnesses, out of an estimated 3.5 billion afflicted people, most of them are children (Bekele et al., 2017; Akoachere et al., 2018).
The WHO Foodborne Disease Burden Epidemiology Reference Group (FERG) produced the report, which demonstrated the WHO Initiative to Estimate the Global Burden of Foodborne Diseases. It offers the first estimates of the incidence, mortality, and disease burden of foodborne diseases worldwide in terms of Disability Adjusted Life Years (DALYs). Thirty-one foodborne risks that cause 32 diseases are included in the worldwide estimates. These include 11 agents of diarrheal disease (one virus, seven bacteria, and three protozoa), 7 agents of invasive infectious disease (one virus, five bacteria, and one protozoon), 10 helminths, and 3 chemicals (Idahosa, 2011).
In 2010, there were 420,000 (95% UI 310,000–600,000) deaths and 600 (95% uncertainty interval [UI] 420–960) million foodborne illnesses as a result of the 31 global risks combined. In 2010, the 31 hazards that cause foodborne illness accounted for 33 (95% UI 25–46) million DALYs worldwide; children under the age of five accounted for 40% of the foodborne illness burden. Entero-pathogenic Escherichia coli (EPEC) and NTS in particular were found to be the primary causes of foodborne diarrhea illness, accounting for 18 (95% UI 12–25) million DALYs globally.
Fishella typhimurium and Taenia solium were two more foodborne pathogens that significantly increased the worldwide burden. People of all ages are susceptible to foodborne illnesses, but those under five and those residing in low-income areas of the world are more at risk than the general population (World Health Organization, 2015; dos Santos Correia, 2018).
The annual incidence of foodborne illness in the United States is estimated to be 48 million cases, with an associated economic impact ranging from $51.0 to 77.7 billion (World Health Organization, 2015). Foodborne illness incidence is estimated to be between 3.1 and 5.0 million cases per year in Canada and 5.4 million cases per year, costing $1.2 billion, in Australia (Soulsby, 1982).
Foodborne or waterborne microbial pathogens are the main causes of illness in developing nations, with an estimated 1.9 million deaths worldwide each year. However, these diseases do not just affect developing nations; they also affect developed nations, with an estimated 1/3 of the population suffering from microbiological foodborne illnesses annually (Newman et al., 2015; Keerthirathne et al., 2016).
Many instances of a foodborne illness associated with fresh fruit and vegetable consumption have surfaced recently, particularly in poor nations. Numerous countries across the world have reported on issues related to infections in fresh produce, along with the resulting consequences for public health and trade (Andargie et al., 2008). The primary cause of these foodborne illnesses, which have a major negative impact on health and the economy, is the parasites Helminthes and Protozoan (Nyarango et al., 2008).
These estimates of loads are conservative; additional research is required to fill up all the gaps in the field. Apart from offering worldwide and local approximations, the Initiative aimed to encourage national initiatives. It is evident that foodborne illness has a significant worldwide impact on people of all ages. By incorporating these estimates into the development of national and regional policies, all stakeholders may help to improve food safety across the food chain. This included promoting the use of burden data in the formulation of evidence-based policy and developing capacity through national foodborne disease burden studies.
Justification of the study
Fruits are an important part of a healthy diet, providing essential vitamins, minerals, and other beneficial nutrients. However, fruits can also serve as vehicles for the transmission of harmful bacteria and parasites, posing a significant public health risk, especially in areas with poor sanitation and food safety practices. In developing countries like Ethiopia, local markets are important sources of fresh produce for many consumers, but these markets may lack adequate infrastructure and oversight to ensure the safety of the food sold.
Contaminated fruits can harbor a variety of pathogenic bacteria, such as Salmonella, Escherichia coli, and Listeria, as well as parasites like Giardia and Cryptosporidium. Consumption of these contaminated fruits can lead to foodborne illnesses, which can have serious health consequences, especially for vulnerable populations like young children, the elderly, and those with compromised immune systems. While food safety issues are a concern in many parts of the world, there is limited research on the extent of bacterial and parasitic contamination of fruits sold in local markets in Addis Ababa, Ethiopia. This study would help fill this knowledge gap and provide valuable insights into the food safety situation in the region. Understanding the level of contamination in these markets can inform targeted interventions to improve food safety and protect public health.
The findings of this study can be used to raise awareness among consumers, policymakers, and market authorities about the importance of food safety. This information can then be used to develop and implement effective strategies to improve the handling, storage, and sale of fruits in local markets, ultimately reducing the risk of foodborne illnesses and improving public health outcomes.
Improving food safety is a priority for the Ethiopian government, as well as global organizations like the World Health Organization (WHO) and the Food and Agriculture Organization (FAO). This study would contribute to the ongoing efforts to address food safety challenges and align with national and international development goals.
Methodology
Study area
Addis Ketema sub-city, Addis Ababa, Ethiopia, was the study's location. With 126 Woredas, the city is divided into 10 sub-cities. The estimated population of Addis Ababa is 3.4 million, and its estimated size is 540.14 square kilometers, of which 18.2 km2 are rural, according to the Federal Democratic Republic of Ethiopia Central Statistics Agency 2021 report. With 144,954 men and 152,839 women, the Addis Ketema sub-city is home to 297,793 people in all. The district is situated not far from the city's center in the northwest section of the city. It shares boundaries with the districts of Arada in the east, Lideta in the south, Kolfe Keranio in the west, and Gullele in the north. Addis Ketema is home to Africa's largest outdoor bazaar, Addis Mercato. It includes 9% of Addis Ababa's total population. The sub-city is divided into 10 woredas, 28 sub-woredas, 84 refers, and 303 blocks, with an estimated total land area of 863.85 hectares, or 1.66% of Addis Ababa's total land area.
Study period and design: between April 1 and April 30, 2021 G.C., a cross-sectional survey was carried out in the Addis Ketema sub-city local market in Addis Ababa, Ethiopia.
Source population: all local marketplaces in Addis Ababa's Addis Ketema sub-city.
Study population: fruit vendors from the Addis Ketema sub-city's local market were chosen for the study.
Inclusion criteria: ready to use/sale items include avocado, papaya, mango, and banana. Vendors offer their time to take part and gather the fruits.
Exclusion criteria: fruit that has been cleaned and treated is placed in a filthy container. Imagined eating raw fruits rather than fruits that had been preserved, as well as rotting fruits and vegetables.
Dependent variables
Salmonella species, Escherichia coli, Klebsiella species, Shigella species, Salmonella profiles, Staphylococcus aureus, and amount of parasitic.
Independent variable
Vendor's sociodemographic status: market type, fruit display type, storage conditions, and hygienic conditions.
Sample size determination
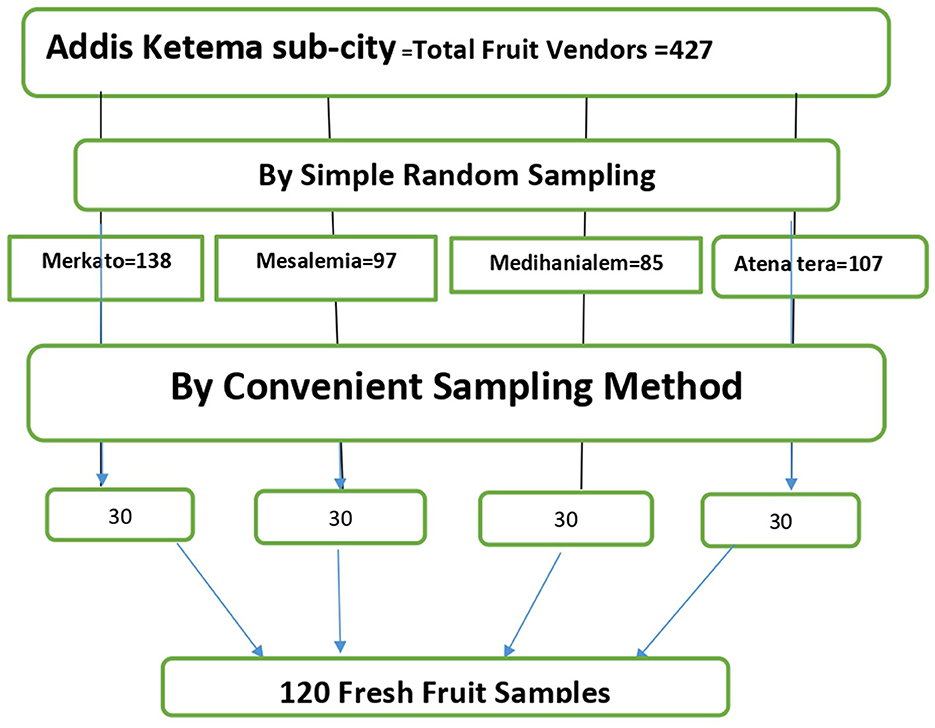
One hundred twenty (120) fruit samples altogether, representing four different types (banana, papaya, mango, and avocado), were gathered from four different locales. From a single vendor, four samples of each kind of fruit were gathered. That means that 30 samples of each kind of local market were gathered. It is found that 120 carefully chosen fruits constitute the minimal sample size needed for the investigation (Figure 1).
Sampling method and techniques
Convenient sampling technique was used to gather data. Four fruit varieties—banana, mango, papaya, and avocado—were bought from four (Omowaye and Audu, 2012) easily chosen local stores. The four marketplaces were chosen using a straightforward random selection method. The Addis Ketema sub-city's “Mesalemia,” “Merkato,” “Medanialem,” and “Atana tera” marketplaces are the ones that were chosen. From the chosen markets, equal numbers of samples (30 from each, for a total of 120 samples) were gathered.
Data collection procedure
After discussing the study's goal with the vendor, a consent document was obtained prior to data collection, and the structured questionnaire was then completed. In order to get accurate answers, the questionnaire includes sociodemographic information as well as a fruit delivery system that was created in English and translated into Amharic, the local tongue. Trained data collectors gathered the data. Every sample was put into a different plastic bag, marked with a special number, the date of collection, the name of the local market, and the location of the transfer to the Ethiopian Public Health Institution for bacteriological and parasitological examination.
Laboratory analysis
For parasitological analysis
Microscopic examination: a beaker filled with 500 mL of normal saline (0.9% NaCl) was used to cut about 200 g of each fruit sample. The samples were then rinsed and soaked for 30 min, and for an additional 15 min, the beaker was placed on a shaker to ensure proper washing. In order to ensure that the parasite stages were adequately sediment, the sample was taken out of the beaker and the washing solution was incubated for 12 h. The remaining roughly 10 mL of sediment was transferred to a centrifuge tube using a sieve to eliminate unwanted material after removing a solution from the incubator and discarding the supernatant. For 5 min, the material was centrifuged at 3,000 revolutions per minute. The sediment was then gently stirred by hand (tapping the tube's bottom) to re-suspend it after the supernatant had been carefully decanted without shaking (Williams, 2000; Kolhatkar et al., 2008). Lastly, a direct-mount light microscope was used to study the sediment in order to look for parasite ova, cysts, and larvae. The objectives were 10x and 40x.
For bacteriological examination
Fruits were treated with the serial dilution method to isolate microorganisms. To make a suspension, the fruits were crushed into five sterilized test tubes labeled 10-1, 10-2, 10-3, 10-4, and 10-5. Each test tube had nine milliliters of distilled water. The fruits were then mixed with distilled water in a pre-sterile crusher and mortar. The samples were appropriately diluted and homogenized (Serial Dilution in Microbiology, 2022; Serial Dilutions Plating, 2022). Making use of pipette measurement a 10-1 labeled test tube with 9 ml of alkaline peptone water inside was filled with 1 ml of the homogenized sample solution, and the tube was thoroughly shaken. To prevent further contamination, measure one milliliter of sample solution from test tube 10-1 once more, transfer it to test tube 10-2, shake it well, then transfer one milliliter from test tube 10-2 to test tube 3 again, one milliliter from test tube 3 to test tube 4 again, and finally one milliliter from test tube 5 to test tube inside the laminar hood (American Public Health Association, 1926; Huq et al., 2012).
Likewise, 100 milliliters of distilled water were used to weigh and measure the powdered selective media of interest. It was then dissolved in 500 milliliters of measuring flask and heated to 500 degrees Celsius to ensure full dissolution. Additionally, five sterile Petri plates with labels reading 10-1 through 10-5 were placed inside the hood. Subsequently, 1 milliliter of the sample solution was measured and aseptically poured into a 10-1 labeled Petri plate; 1 milliliter of the sample solution was poured into a 10-2 Petri plate; 1 milliliter of the sample solution was poured into a 10-3 labeled Petri plate; 1 milliliter of the sample solution was poured into a 10-4 labeled Petri plate; and lastly, 1 milliliter of the sample solution was aseptically poured into a 10-5 labeled Petri plate. The colony-forming unit formula (CFU) was utilized to ascertain the colonies of those pathogens because they were all enumerated from 10 to 5 serially diluted samples of fruit suspension (Batty-Smith, 1942).
CFU = (Number of colonies)/(Volume of dilution factor) x Dilution factor
Total Coliform Count (TCC): 15 ml of violet red bile agar medium (previously sterile and kept in a water bath at 45°C) was poured, swirled, and then incubated at 37°C for 24 to 48 h from each sample of the previously prepared serial dilution. One milliliter was transferred into sterile Petri dishes. Using a digital colony counter, purple-red colonies with a diameter of 0.5 mm or more and encircled by a zone of precipitated bile acids were counted.
Procedure
Placed 1 ml of a liquid sample or 10-1 homogenate into each of two Petri dishes; repeated with each dilution prepared. To each plate add 15 ml of molten violet red bile agar cooled to 44–47°C.
Mixed carefully and allowed to set. Overlaid each plate with a further 4–5 ml of molten, cooled VRBA. Allowed to set. Incubated the plates at 30°C or 37°C for 24 ± 2 h (22–26 h). Selected dishes that contain no more than 150 colonies and count purplish red. Colonies that have a diameter of 0.5 mm or greater, usually surrounded by precipitated bile acids were counted. Calculate the count per g or ml.
Standard Plate Count (SPC): from each of 120 local markets was representative portion was homogenized, serially diluted with alkaline peptone water and inoculated on Nutrient agar, Violet red bile agar, and Mannitol salt agar using pour plate method and incubated at 37°C for 24–48 h to determine Total Viable Count, Total Coliform Count, and Total Staphylococcal count respectively (American Public Health Association, 1926; Batty-Smith, 1942; Williams, 2000).
Staphylococcal Count (SCC): 15 ml of Mannitol salt agar (MSA) medium that had been previously sterile and kept in a water bath at 45°C was poured, swirled, and then incubated at 37°C for 24–48 h from each sample of previously prepared serial dilution. After an overnight sub-culturing in nutrient agar plates, colonies of yellow and orange color, encircled by yellow zones resulting from mannitol fermentation, were counted and subjected to further testing using the coagulase test.
Microscopic examination: microscopic investigation for Gram reaction and morphological features of the suspected colony was determined using the standard method of Gram's staining.
Detection of Salmonella: Each fruit sample was aseptically transferred in 0.5 ml volumes to a sterile bottle of nutrient broth, and it was then incubated for 24 h at 37°C. Next, 10 ml of selenite broth that had been heated to 37°C was filled with 1.0 ml of each pre-enriched sample. For 48 h, it was incubated at 37°C. Following incubation, each enrichment bottle was inoculated with one Petri-dish of Xylose lysine deoxy cholate agar (XLD), which was then incubated at 37°C for 24–48 h. The dish was then checked for characteristic Salmonella colonies.
Detection of Escherichia coli: A 0.5 ml of each fruit sample was inoculated on replicate MacConkey agar by spread plate method and incubated at 37°C 45°C for 24 h to identify thermos tolerant E. coli.
Biochemical tests: the suspicious colonies were sub-cultured into the sterile nutrient broth and incubated until the broth took on a murky appearance in order to identify Enterobacteriaceae from the initial culture on XLD and MacConkey agar. Sub-cultures of the organisms were then used for several biochemical assays, including the Indole test, the Motility test medium, the Triple Sugar Iron Agar, the Simmons citrate agar, the Lysine Iron agar, and the Urea broth. A conventional technique for detecting coliforms in water was employed. In order to assess the water quality before preparing fresh fruit, 100 milliliters of water were aseptically collected in a sterile falcon tube. Five 10 ml and one 50 ml portions of 100 ml alkaline peptone water samples were each given out in a tube containing sterile double strength MacConkey broth that included lactose and a pH indicator. The number of bottles exhibiting lactose fermentation, acid, and gas production was tallied following a 24-h incubation period at 44°C. The coliforms in the water fermented the lactose. The most likely quantity of coliforms in the 100 ml water sample was calculated using probability tables (Cheesbrough, 2006).
PH measurement: as soon as the samples were collected, the pH of each undiluted sample was determined using a pH meter. Before starting a microbiological analysis, it is crucial to ascertain the pH of the food sample because it can affect the organisms and colony count that are being looked for. Pathogens are generally not expected to thrive in foods with a pH of < 4.5; the only organisms that may be present would be yeasts, molds, and a few bacteria that can withstand acidic environments. Foods with a pH higher than 4.5 need to be thoroughly microbiologically analyzed.
Data quality control
The principal investigator coded and verified the completed checklist and questionnaire for accuracy and consistency. To ensure uniformity and correctness, data cleaning was done. Any mistake found was fixed right away. Additionally, all laboratory operations were conducted strictly in accordance with standard operating procedures, and the quality of the results was maintained in accordance with the laboratory quality control requirements of the Ethiopian Public Health Institution (EPHI).
Pre-analytical
Before being used, all culture media and fluids (0.9% Normal Saline) were visually inspected for contamination, notable physical flaws (such as uneven distributions, varying volumes of a medium in Petri dishes, tubes, or bottles, color, or noticeable surface deformation), and expiration dates. When media beyond their expiration date exhibit signs of deterioration such as drying, cracking, discoloration, or microbiological infection, they are thrown (not used). The samples were gathered using the sterile plastic bag. Before being used, American Type Culture Collection (ATCC) control strains were used to apply quality control for commercially generated microbiological culture media. Fill out the incubator's daily temperature monitoring checklist.
Analytical
The method's diagnostic efficacy will be determined by its imprecision and inaccuracy, hence the data generated had to be reliable. Based on the EPHI laboratory system, SOPs were closely adhered to. To ensure the proper detection, isolation, and identification of microorganisms, quality control was carried out on all materials and equipment. This covers media, stains, test reagents for biochemistry, and apparatus like autoclaves and incubators. Before being used, each fresh lot was subjected to quality control testing using standard strains of E. Coli ATCC. Control strains (E. Coli ATCC 25922 and Staphylococcus aureus ATCC 25923) were used to assess the medium's quality and the antibiotic's potency. To guarantee that no bacterial contamination is introduced by hand contact or contact with non-sterile surfaces or objects, samples were prepared aseptically. The dehydrated culture medium was kept in a dry, dark area. 15–25°C (59–77 °F) was the ideal storage temperature. Hygroscopic substance was added to the microscope so that the containers would always be placed back in the stage after use to stop moisture from entering and growing fungus.
Post-analytical
SOP served as the foundation for every result record. 25% glycerol was used to preserve the isolated bacteria, which were then kept at −200°C. Version 25 of the IBM SPSS program (Chicago, IL, USA) was used to enter the raw data, which was then treated carefully and securely.
Data analysis and interpretation
Data were modified, cleansed, verified for accuracy, and sorted according to fruit kind and the location of the nearby market. SPSS software version 25 (IBM, Chicago, IL, USA) was used for data analysis. The mean aerobic bacterial count differences between different vegetable varieties were compared using the ANOVA test. The means of the total coliform counts, fecal coliform population, and aerobic bacterial counts of vegetables from different sites were compared using the Bonferroni corrected post hoc t-test. By calculating the odds ratio (OR) and 95% confidence interval (CI), which indicate the strength of the link between infection and associated factors, logistic regression was used to make an internal comparison and ascertain the impact of independent variables. Bivariate and multivariable logistic regression analysis were used to calculate the crude and adjusted ORs, respectively. To determine statistical significance, a P < 0.05 and an adjusted OR with 95% CI were employed.
Ethical considerations
The Department of Medical Laboratory Sciences, College of Health Science, and Departmental Research and Ethics Review Committee (DRERC) of Addis Ababa University provided ethical clearance. Before any study participant was enrolled, they were asked to provide written informed consent, and the study's confidentiality was upheld at all times. An official letter of approval will also be obtained from the Addis Ababa City Administration Health office, Food and Nutrition Security Unit.
Results
Socio-demographic characteristics of study population
Banana, papaya, mango, and avocado were the four fruit varieties from which 120 (one hundred twenty) fruit samples were gathered for this investigation. Demographic information was requested from all four marketplaces' chosen fruit retail stores as well as ready-made juice vendors. The gender distribution of them was 86 (71.7%) female and 34 (28.3%) male. A mean of 37.4 years old is shared by all study participants, who range in age from 25 to 50. 101 (84.2%) study participants were urban dwellers, making up the majority of the sample (Table 1).
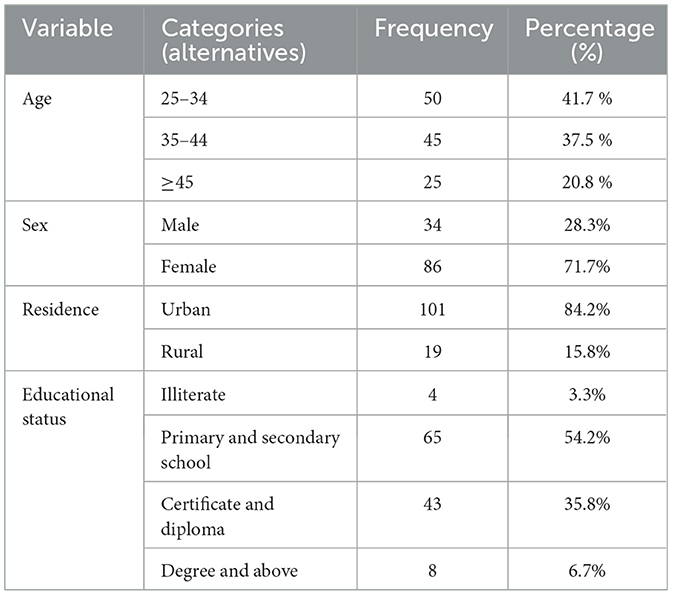
Table 1. Distributions of socio-demographic characteristics of the study participants from fruits retailers in the selected market places in Addis Ketema sub city, Addis Ababa, Ethiopia 2021.
Bacteriological contamination status of fresh avocado, papaya mango, and banana
Despite differences in the degree of contamination, every sample that was examined turned out to be tainted. According to this investigation, 20 fruits (16.7%) tested negative for aerobic plate count, while a total of 100 fruits (83.3%) had bacterial contamination. Mango (Table 2) was found to have the highest amount of contamination, with mean values of 3.5 × 105 (TAMC), 2.1 × 105 (TCC), and 4.1 × 104 (TFC), respectively. Avocado has the least amount of bacterial contamination, with mean values of 2.5 × 104 (TAMC), 1.5 × 103 (TCC), and 1.8 × 103 (TFC), respectively. Mango, banana, avocado, and papaya samples from all four sites had total aerobic mesophilic count (TAMC) bacterial counts that ranged from 3.5 × 105 to 7.5 × 103 CFU/g on average, as shown in Table 2. The papaya and avocado TAMC values that were lowest were found at Medihanialem Marketplace (2.2 × 102 CFU/g) and Atena Tera (2.7 × 104 CFU/g), respective. At each of the four market locations, there were statistically significant differences in the TAMC levels on mango (p = 0.04). Merkato and Mesalemia's TAMC levels were compared multiple times for mango samples, and the results were statistically significant (P = 0.02). This demonstrates that samples from Merkato and Mesalemia had significant TAMC contamination, whereas samples from Atena Tera and Medihanielem had TAMC contamination that was nearly identical. Site-by-site multiple comparison samples between Merkato and Atena Tera for bananas showed statistical significance (P = 0.02) in terms of TAMC level. Nonetheless, there was no statistically significant difference in the TAMC level between the Merkato and Medihanielem (P = 0.07 > 0.05) and Atena Tera and Mesalemia (P = 0.9 > 0.05) sampling sites. This demonstrates that samples from Merkato were significantly polluted with TAMC, whereas samples from AtenaTera and Medihanielm had TAMC levels that were almost identical.

Table 2. Mean values of total aerobic mesophilic count, total coliforms count, and total fecal coliform counts from fruits collected in the selected market places in Addis Ketema sub-city.
The samples of bananas, mangos, papayas, and avocados from all four sites had total coliform (TCC) bacteria ranging from 2.1 × 103 to 3.2 × 104, 8.3 × 102 to 1.8 × 105CFU/g, 1.6 × 102 to 3.7 × 104, and 1.2 × 102 to 3.8 × 104 CFU/g.
In banana, mango, papaya, and avocado, the mean values of fecal coliform (TFC) bacterial contamination varied amongst the three sites, ranging from 3.7 × 102 to 1.1 × 105 CFU/g, 3.3 × 102 to 4.6 × 106 CFU/g, 1.1 × 102 to 3.4 × 105, and 1.5 × 102 to 6.2 × 105.
Pathogenic bacterial isolates and intestinal parasites
Mango, banana, papaya, and avocado contamination with Escherichia coli bacteria ranged in mean values from 4.0 × 102 to 2.3 × 104 CFU/g, 1.9 × 102 to 2.6 × 103 CFU/g, 2.8 × 102 to 1.2 × 103 CFU/g, and 2.1 × 102 to 6.7 × 103 CFU/g, respectively, across all four sites (Figure 2; Table 3).
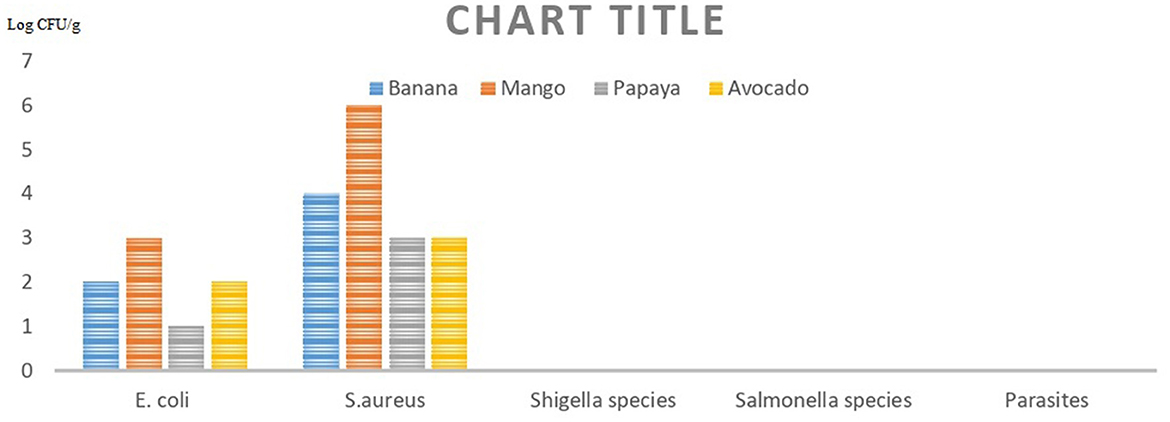
Figure 2. Magnitude contamination of selected pathogens in street marketplaces in Addis Sub-city of Addis Ababa, Ethiopia.
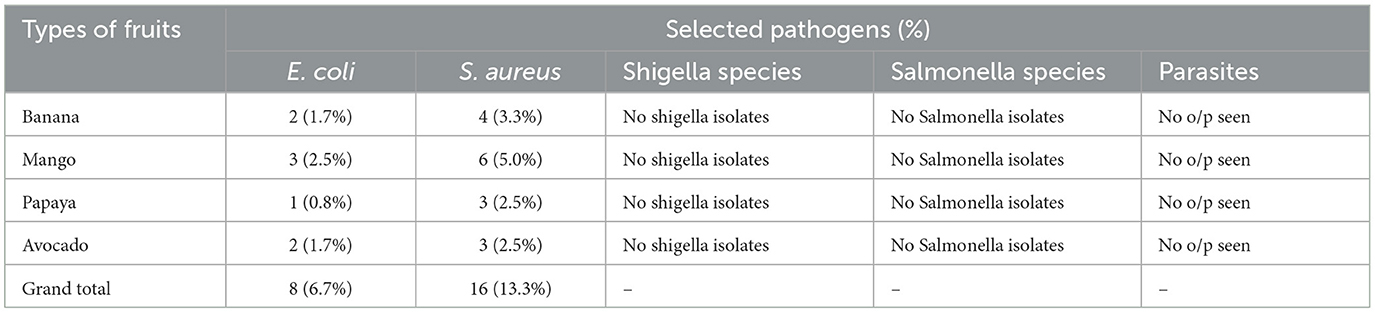
Table 3. The pathogenic bacterial species and intestinal parasites associated in fruits in selected markets of Addis ketema sub-city, Addis Ababa.
Associated risk factors related to bacterial contamination
Bivariable and multivariable logistic regression were used to examine associated risk variables for bacterial contamination of fresh fruit. Not a single variable was statistically linked to the contamination of fresh fruits that were ready to eat. Crude odds ratio and adjusted odds ratio analyses are shown in the Table 4. Nonetheless, the findings show that the p-value for the potential causes of fruit contamination is higher than 0.05, and the analysis's confidence interval contains a value of 1, indicating that the relationship is statistically insignificant.

Table 4. The magnitude of bacterial contamination of fruits collected and associated demographic factors.
Characteristics of respondents
Washing produce before selling was not a practice, according to 74 (61.7%) of the respondents. Less than three days are present in about 98 (81.7%) of the fruits on the juice house's shelf. Ninety-three percent (94.2%) of the respondents threw out their rubbish in open fields. In order to shield fruits from contamination, it is imperative that the hygienic conditions in the market be thoroughly addressed. As a result of moistening unsold fruits and vegetables with water instead of utilizing refrigeration, all responders (100%) extended the shelf life of these items.
Discussion
Fruits can serve as a hospitable environment for a range of microorganisms, including bacteria and parasites, allowing them to thrive and propagate. Fruits may become contaminated at various stages of the supply chain, such as during production, harvesting, transportation, preparation, or processing. This contamination can ultimately lead to health issues in humans. Similarly, vegetables are also susceptible to contamination by common pollutants, including bacteria and parasites (Park et al., 2013).
The study found that mango had the highest amount of contamination, with mean values of 3.1 × 105 (TCC), 4.1 × 104 (TFC), and 3.5 × 105 (TAMC). The results of this study are comparatively greater than those of a similar study carried out in Hossana town, Southern Ethiopia, which found that the total coliform count was 4.8 x 103 and the average aerobic mesophilic bacterial counts (CFU/ml) of mangos were 1.3 x 104 (Abisso et al., 2018). However, this study was found to be lower than a study done in Gondar (1.1 × 1010TAMC, and 4.9 × 104 CFU/ml TCC)from mango (Gultie and Sahile, 2013), from retail outlets in Qatar (1.3 × 105 CFU/ml TAMC) (Al-Jedah and Robinson, 2002). This may be due to many factors such as seasonal variation, time of sample collection, hygiene of the environment, handling practices, and incubation time.
Avocado had the lowest mean value of bacterial contamination, measuring 2.5 × 104 (TAMC), 1.5 × 103 (TCC), and 1.8 × 103 (TFC), respectively. This result is consistent with a study using fresh fruit juices in Hossana town, Southern Ethiopia, which found that avocados had an average aerobic mesophilic bacterial count of 2.4 × 104 CFU/ml (TAMC), which was higher than the total coliform count of 2.6 × 104 CFU/ml. Conversely, this outcome was less than that of a Gondar investigation on fruit in town marketplaces (8.9 × 109 CFU/ml TAMC), 1.1 × 104 CFU/ml TCC) (Gultie and Sahile, 2013), from Shewarobit town, Amhara, Ethiopia, about 2.2 × 105 CFU/ml TAMC was reported (Fufa and Liben, 2018), in Hawassa, Ethiopia 2.8 × 105 CFU/ml (Eromo et al., 2016), from retail outlets in Qatar 4.9 × 106 CFU/ml TAMC, 9.3 × 103 CFU/ml TCC (Al-Jedah and Robinson, 2002). This difference may be due to handling practice of fruits and general hygienic condition of the environment where fruits were displayed for sell in the marketplace.
In this investigation, the bacterial contamination level of banana fruit was determined to be 2.0 × 103 fecal coliform, 3.4 × 102 CFU/ml TCC, and 2.1 × 105 CFU/ml TAMC. This study is consistent with one conducted in Pakistan's Karachi metropolis. 2.8 × 103 fecal coliform, 3.2 × 102 CFU/ml TCC, and 2.5 × 105 CFU/ml TAMC (Alamgir et al., 2015). However, this finding is lower than from a similar study conducted in north-west Ethiopia, which shows 2.5 × 1, 010 CFU/ml of TAMC and 6.6 × 103 CFU/ml of TCC (Gultie and Sahile, 2013), from Hawassa town of southern Ethiopia, 1.82 × 107 of CFU/mlTAMC and 1.17 × 107 CFU/ml of TCC respectively (Dobo, 2019), from retail outlets in Qatar 2.2 × 106 CFU/ml of TAMC and 3.2 × 103 CFU/ml TCC (Al-Jedah and Robinson, 2002). On the other hand, this study is relatively higher than a study done from the Main markets of Multan, Pakistan, 1.5 × 104 CFU/ml TAMC (Rida and Deeba, 2018). This may be due to seasonal differences and the capacity of their laboratory setup to carryout difference analysis methods to detect microorganisms.
The total aerobic mesophilic bacteria in papaya were determined to be 1.3 × 104 CFU/ml of TFC, 2.3 × 103 CFU/ml of TCC, and 7.5 × 103 CFU/ml in this investigation. This study yielded lower results than one from Hossana town in southern Ethiopia, which found 2.8 × 104 CFU/ml of aerobic mesophilic bacteria overall (Abisso et al., 2018), from Arba Minch town, Ethiopia (1.9 × 106 CFU/mlTAMC) and (2.09 × 105 CFU/ml TCC) (Wedajo and Kadire, 2019), from Shewarobit town, Amhara, Ethiopia (1.9 × 105 CFU/mlTAMC), and (1.7 × 105 CFU/ml TCC) (Fufa and Liben, 2018).
In the four markets of Addis Ketema Sub-City, Addis Ababa, Ethiopia, a total of 8 (6.7%) cases of E. coli bacterial contamination were found. A total of 2 (1.7%), 3 (2.5%), 1 (0.8%), and 2 (1.7%) E. coli were found in avocado, papaya, mango, and banana, in that order. This study's findings were found to be consistent with research conducted in Debre-Markos Town, North Western Ethiopia, which revealed that ~3 (7.5%) and 1 (2.5%) E. coli were found in fresh avocado and mango fruits, respectively (Geta et al., 2018), from a study carried out in Karachi city, Pakistan 1 E. coli was detected from banana fruits (Alamgir et al., 2015). However, this study was lower than from a study conducted on street foods in Hawassa, Ethiopia, that reported 5 (23.8%) E. coli bacteria from fresh avocado fruit (Eromo et al., 2016), According to additional research, there were 7 (33.3%) E. coli-positive samples from avocado juice at Addis Ababa's fruit juice shops. However, compared to a similar study conducted in fresh fruit juice shops in Addis Ababa, where 2 (9.5%) E. coli positive samples from mangos were found, this study's result is greater (Kechero et al., 2019).
Similarly, from the chosen fruit samples, 16 (or 13.3%) S. aurues bacterial contamination was recovered. In this study, 4 (3.3%), as the following table illustrates. Bacteria belonging to the Staphylococcus aureus species were found in bananas, mangoes, avocados, and 3 (2.5%), 5 (5.0%), and 3 (2.5%), respectively. This result was less than that of a study conducted in Gondar, North Western Ethiopia, which found that 10 (62.5%), 13 (81.25%), and 7 (43.75%) of the Staphylococcus aureus bacteria were found in avocado, mango, and banana fruits, respectively (Gultie and Sahile, 2013), from Debre-Markos Town, North Western Ethiopia5 (12.5%) of Staphylococcus aureus were found from avocado juice (Geta et al., 2018). This study, however, is comparatively more extensive than one that looked at freshly squeezed fruit sold on the street in Karachi, Pakistan, and found that one Staphylococcus aureus was found in banana and mango juice (Alamgir et al., 2015), from avocado juice in Hawassa, Ethiopia, 2 (28.6) (Eromo et al., 2016), from banana, mango, papaya and avocado in Hossana Town, Southern Ethiopia, 1 Staphylococcus aureus was found in each fruit (Abisso et al., 2018) 74 (61.7%) of the respondents reported washing fruits and vegetables ahead of selling was not practiced. To keep fruits in the market free from infection, hygienic conditions must be effectively enhanced. The findings demonstrated that, instead of utilizing refrigeration, all respondents (100%) increased the shelf life of unsold fruits and vegetables by moistening them with water. The spread of harmful germs is aided by consumers and merchants handling food in contaminated markets and by misting fruits and vegetables with tainted water (Beuchat, 1996). Moistening fruits and vegetables with contaminated water and handling of produce by infected vendors and consumers in the marketplace promote the spread of pathogenic microorganisms (Beuchat, 1996). Unhygienic surroundings, improper waste disposal system, and inadequate water supply attract houseflies or fruit flies, probably further increasing food contamination (Chumber et al., 2007). Contaminated hands of vendors and perhaps lack of knowledge of hygienic practices and safety of food products are the main contributing factors of contamination (Fang et al., 2003). Animal excrement, including that of humans and donkeys, was also seen in close proximity to the exhibition locations. Fly swarms were also a regular sight. According to the observation, every store weighed various fruits using a single, shared balance, which could lead to cross-contamination. They did not follow personal hygiene rules and did not follow hygienic measures. It was discovered that the fruit and vegetable display area was unhygienic (Tefera et al., 2014; Alemu et al., 2018; Sahile and Legesse, 2019).
Conclusion
The findings of this study suggest that to reduce the growth or survival of pathogenic bacteria, fruits must be handled with care and using hygienic practices, and processed using effective methods. The development of fruit preservation techniques has been driven by the goal of extending shelf life.
Overall, the study indicates that these fruit juices are not produced with adequate hygiene, and consumers face a risk of contracting foodborne illnesses. Government health agencies should take steps to enact regulations that properly govern street food vending and educate vendors about food safety and hygiene standards. Further research is warranted to investigate other fruit varieties from various juice shops and sellers, as well as to identify additional viruses. Additionally, it is advisable to utilize processing technology to prepare pasteurized juices, in order to prevent food-related contamination.
Limitation of the study
Due to financial constraints and a lack of specialized equipment, we were unable to perform sero-grouping or species identification on the isolated microorganisms. This limited the scope of our investigation. We also did not conduct any molecular identification or confirmation of the bacterial strains.
Recommendation
It is wise to thoroughly wash raw fruits in clean water before consuming them. This helps reduce the density of microbiological contaminants on the surface. Government involvement is also necessary to preserve produce quality and protect consumers. Additionally, educating the public about the importance of properly washing fruits and vegetables in clean water before consumption is critical to prevent microbial and parasitic diseases.
To prevent future outbreaks of bacterial pathogens, regular quality monitoring of fruits, vegetables, and their products for human consumption must be implemented. Fruit juice businesses should not only focus on profits, but also provide training and address other safety-related matters to ensure proper food safety practices.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DM: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft. KD: Investigation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to express our gratitude to Addis Ababa University for providing me with the research opportunity. The data collectors and all staff members who worked on this project willingly and without reservation are appreciated by the writers.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abisso, T. G., Gugero, B., and Fissuh, Y. (2018). Physical quality and microbiological safety of some fruit juices served in cafes/juice houses: the case of hossana town, southern Ethiopia. J. Nutr. Food Sci. 8, 1–5. doi: 10.4172/2155-9600.1000689
Adeyeba, O., and Akinbo, J. (2002). Pathogenic intestinal parasites and bacterial agents in solid wastes. East Afr. Med. J. 79, 604–610. doi: 10.4314/eamj.v79i11.8807
Akoachere, J.-F. T. K., Tatsinkou, B. F., and Nkengfack, J. M. (2018). Bacterial and parasitic contaminants of salad vegetables sold in markets in Fako Division, Cameroon and evaluation of hygiene and handling practices of vendors. BMC Res. Notes. 11, 1–7. doi: 10.1186/s13104-018-3175-2
Alamgir, A., Fatima, N., Khan, M. A., and Shaukat, S. S. (2015). Microbiological assessment of street vended fresh fruit juices available in the Karachi city. Int. J. Biol. Biotechnol. 12, 505–509. doi: 10.1016/j.envpol.2015.02.009
Alemu, G., Mama, M., and Siraj, M. (2018). Bacterial contamination of vegetables sold in Arba Minch town, Southern Ethiopia. BMC Res. Notes. 11, 1–5. doi: 10.1186/s13104-018-3889-1
Al-Jedah, J., and Robinson, R. (2002). Nutritional value and microbiological safety of fresh fruit juices sold through retail outlets in Qatar. Pak. J. Nutr. 1, 79–81. doi: 10.3923/pjn.2002.79.81
American Public Health Association (1926). Standard Methods for the Examination of Water and Wastewater. Washington, DC: American Public Health Association.
Andargie, G., Kassu, A., Moges, F., Tiruneh, M., and Huruy, K. (2008). Prevalence of bacteria and intestinal parasites among food-handlers in Gondar town, northwest Ethiopia. J. Health Popul. Nutr. 26:451. doi: 10.3329/jhpn.v26i4.1887
Balali, G. I., Yar, D. D., Afua Dela, V. G., and Adjei-Kusi, P. (2020), Microbial contamination, an increasing threat to the consumption of fresh fruits vegetables in today's world. Int. J. Microbiol. 2020:3029295. doi: 10.1155/2020/3029295
Batty-Smith, C. (1942). The Eijkman test for faecal coli in the bacteriological examination of water supplies1: A survey and discussion of the experimental work from 1929 to the present day with a study of 104 water samples and of 602 cultures. Epidemiol. Infect. 42, 55–98. doi: 10.1017/S0022172400012638
Bekele, F., Tefera, T., Biresaw, G., and Yohannes, T. (2017). Parasitic contamination of raw vegetables and fruits collected from selected local markets in Arba Minch town, Southern Ethiopia. Infect. Dis. Pover. 6, 1–7. doi: 10.1186/s40249-016-0226-6
Beuchat, L. R. (1996). Pathogenic microorganisms associated with fresh produce. J. Food Prot. 59, 204–216. doi: 10.4315/0362-028X-59.2.204
Cheesbrough, M. (2006). District Laboratory Practice in Tropical Countries Part 2. New York: Cambridge University Press, 300–301. doi: 10.1017/CBO9780511543470
Chumber, S. K., Kaushik, K., and Savy, S. (2007). Bacteriological analysis of street foods in Pune. Indian J. Public Health. 51, 114–116.
Dobo, B. (2019). Fungal and bacterial contamination of fresh fruits and vegetables sold in hawassa town of Southern Ethiopia. GSJ 7:888. doi: 10.24294/th.v0i0.888
dos Santos Correia, M. (2018). Building Safe Food Chains in Developing Countries: Implications of a Case Study. Mozambique: Universidade de Lisboa (Portugal).
Eromo, T., Tassew, H., Daka, D., and Kibru, G. (2016). Bacteriological quality of street foods and antimicrobial resistance of isolates in Hawassa, Ethiopia. Ethiop. J. Health Sci. 26, 533–542. doi: 10.4314/ejhs.v26i6.5
Fang, T. J., Wei, Q. K., Liao, C. W., Hung, M. J., and Wang, T. H. (2003). Microbiological quality of 18 C ready-to-eat food products sold in Taiwan. Int. J. Food Microbiol. 80, 241–250. doi: 10.1016/S0168-1605(02)00172-1
Fufa, B. K., and Liben, M. D. (2018). Microbiological quality of fruit juices sold in cafes and restaurants of Shewarobit town, Amhara, Ethiopia. Afr. J. Microbiol. Res. 12, 623–628. doi: 10.5897/AJMR2018.8868
Geta, K., Kebede, A., and Chemedissa, M. (2018). Microbiological Safety of Fruit Juices Consumed in Cafes and Restaurants of Debre-Markos Town, North Western Ethiopia.
Gultie, A., and Sahile, S. (2013). Microbial spectrum of fruit in Gondar town markets, North Western Ethiopia. J. Microbiol. Res. 3, 1–10.
Huq, A., Haley, B. J., Taviani, E., Chen, A., Hasan, N. A., Colwell, R. R., et al. (2012). Detection, isolation, and identification of Vibrio cholerae from the environment. Curr. Prot. Microbiol. 26:6A. doi: 10.1002/9780471729259.mc06a05s26
Idahosa, O. T. (2011). Parasitic contamination of fresh vegetables sold in Jos markets. Global J. Med. Res. 11, 21–25.
Kechero, F. K., Baye, K., Tefera, A. T., and Tessema, T. S. (2019). Bacteriological quality of commonly consumed fruit juices and vegetable salads sold in some fruit juice houses in Addis Ababa, Ethiopia. J. Food Saf. 39:e12563. doi: 10.1111/jfs.12563
Keerthirathne, T. P., Ross, K., Fallowfield, H., and Whiley, H. A. (2016). review of temperature, pH, and other factors that influence the survival of Salmonella in mayonnaise and other raw egg products. Pathogens 5:63. doi: 10.3390/pathogens5040063
Keller, I., and Tukuitonga, C. (2005). “The WHO/FAO fruit and vegetable promotion initiative,” in International Symposium on Human Health Effects of Fruits and Vegetables, 744.
Kolhatkar, A., Ochei, J., and McGraw, T. (2008). Medical Laboratory Science: Theory and Practice. New York, NY: Tata Mcgraw Hill.
Mostafidi, M., Sanjabi, M. R., Shirkhan, F., and Zahedi, M. T. (2020). A review of recent trends in the development of the microbial safety of fruits and vegetables. Trends Food Sci. Technol. 103, 321–332. doi: 10.1016/j.tifs.2020.07.009
Newman, K., Leon, J., Rebolledo, P., and Scallan, E. (2015). The impact of socioeconomic status on foodborne illness in high-income countries: a systematic review. Epidemiol. Infect. 143, 2473–2485. doi: 10.1017/S0950268814003847
Nyarango, R. M., Aloo, P. A., Kabiru, E. W., and Nyanchongi, B. O. (2008). The risk of pathogenic intestinal parasite infections in Kisii Municipality, Kenya. BMC Public Health 8, 1–6. doi: 10.1186/1471-2458-8-237
Omowaye, O., and Audu, P. (2012). Parasites contamination and distribution on fruits and vegetables in Kogi, Nigeria. Cibtech J. Bio-Prot. 1, 44–47.
Park, S., Navratil, S., Gregory, A., Bauer, A., Srinath, I., Jun, M., et al. (2013). Generic Escherichia coli contamination of spinach at the preharvest stage: effects of farm management and environmental factors. Appl Environ Microbiol. 79, 4347–4358. doi: 10.1128/AEM.00474-13
Rida, B., and Deeba, F. (2018). Microbiological safety assessment of fresh fruits and vegetables collected from main markets of Multan, Pakistan. J. Biores. Manage. 5:1. doi: 10.35691/JBM.8102.0085
Sahile, S., and Legesse, T. (2019). Bacteriological quality assessment of fresh lettuce and tomato from local markets of Gondar, Ethiopia. J. Acad. Indust. Res. 8:1. doi: 10.1016/j.aej.2018.11.003
Serial Dilution in Microbiology (2022). Serial Dilution in Microbiology: Calculation, Method and Technique - Video and Lesson Transcript | Study.com.
Serial Dilutions and Plating (2022). Serial Dilutions and Plating: Microbial Enumeration | Microbiology.
Soulsby, E. (1982). Helminths, Arthropods and Protozoa of Domesticated Animals. London: e English Linguar Book Society and Bailliere Tindal.
Tefera, T., Biruksew, A., Mekonnen, Z., and Eshetu, T. (2014). Parasitic contamination of fruits and vegetables collected from selected local markets of Jimma Town, Southwest Ethiopia. Int. Schol. Res. Notices 2014:382715. doi: 10.1155/2014/382715
Wedajo, B., and Kadire, A. (2019). Assessment of bacterial load of some fresh and packed fruit juices in Arba Minch Town, Ethiopia. J. Nutr. Food Sci. 9, 1–7. doi: 10.35248/2155-9600.19.9.759
Williams, J. (2000). District Laboratory Practice in Tropical Countries. Part 1. Cambridgeshire: Tropical Health Technology.
World Health Organization (2012). Five Keys to Growing Safer Fruits and Vegetables: Promoting Health by Decreasing Microbial Contamination. Geneva: World Health Organization.
Keywords: parasitic, bacterial, contamination, market, Addis Ababa, parasite, Ethiopia, bacteria
Citation: Demisie KN and Melese DM (2024) Assessment of bacterial and parasitic contamination of fruits gathered from specific local markets in Addis Ababa, Ethiopia. Front. Sustain. Food Syst. 8:1402898. doi: 10.3389/fsufs.2024.1402898
Received: 17 April 2024; Accepted: 26 June 2024;
Published: 15 July 2024.
Edited by:
Dike Ukuku, Agricultural Research Service (USDA), United StatesReviewed by:
Agnes Kilonzo-Nthenge, Tennessee State University, United StatesWalid Janati, Sidi Mohamed Ben Abdellah University, Morocco
Abir El-araby, Sidi Mohamed Ben Abdellah University, Morocco
Copyright © 2024 Demisie and Melese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Molla Melese, ZGFuaWVsbW9sbGE1NEBnbWFpbC5jb20=
 Kelelaw Nigusie Demisie
Kelelaw Nigusie Demisie Daniel Molla Melese
Daniel Molla Melese