
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 30 May 2024
Sec. Aquatic Foods
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1396693
This article is part of the Research Topic Detection, Risk Analysis and Monitoring of Chemical Contaminants from Agro-Aqua Food Production and Processing: Implications on the One Health Triad View all 9 articles
Human norovirus can accumulate in shellfish in contaminated waters through their filter-feeding mechanism, and they can retain the virus for extended periods. It is important to note that this bioaccumulation can pose a risk to human health if the shellfish are consumed raw or undercooked. Jeotgal is a salted fermented food made from various types of seafood and is consumed in Korea and certain Asian countries. However, jeotgal is not sterilized during preparation and is typically consumed raw after fermentation. Bivalve shellfish, such as oysters, mussels, and clams, are considered high-risk foods for HuNoV transmission due to the potential for contaminated water to lead to the accumulation of HuNoV in their digestive tissues. Other foods may also contribute to HuNoV transmission, but bivalve shellfish are particularly susceptible. This study investigated the effect of high pressure processing (HPP) on the inactivation of HuNoV GII.4, in clam jeotgal. After HPP treatment, HuNoV GII.4 was quantified using RT-qPCR and combined with Propidium monoazide (PMA) + Sarkosyl, a pre-treatment agent, before RT-qPCR. As a result of this treatment HuNoV GII.4 was significantly (p < 0.05) reduced to 0.27–1.38 log copy number/μL. Compared to the RT-qPCR, the reduction in HuNoV was significantly greater (p < 0.05) (0.24 log, 43%) log copy number/μL in PMA + Sarkosyl/RT-qPCR when clam jeotgal was treated at 200–600 MPa of HPP. The Hunter “L” and “a” and Hunter “b” values increased and decreased significantly (p < 0.05), respectively, as the pressure of the HPP increased. Although the sensorial color significantly (p < 0.05) decreased as the pressure of the HPP increased, most of the sensory parameters (smell, taste, appearance, and overall acceptability) and the pH were not significantly (p < 0.05) different between non-HPP treated and HPP treated samples. Therefore, HPP pressure in excess of 400 MPa for 5 min appeared to be effective to viably reduce HuNoV levels by ≥90% without significant changes in the overall quality (pH, and most sensory parameters) of clam jeotgal.
Human Norovirus (HuNoV) belongs to the genus Norovirus of the family Caliciviridae. It is a small, non-enveloped, icosahedral symmetric virus with a positive-sense, single-stranded RNA genome organized in three open reading frames (ORFs) (Hall et al., 2013). HuNoV has a low estimated infectious dose of ~18 viral particles (Teunis et al., 2008) and is excreted in high concentrations (4–9 log genome copies/g) in feces for at least 2–3 weeks in both symptomatic and asymptomatic cases (Teunis et al., 2015; Sabria et al., 2016). HuNoV bioaccumulates in bivalve molluscs in contaminated waters through their filter-feeding mechanism and they retain the virus for long periods of time (Le Guyader et al., 2012).
Jeotgal is a salted fermented food consumed in Korea and some Asian countries, made from various types of seafood such as shrimps, oysters, shellfish, fish eggs and fish guts. Traditionally, the Jeotgal is processed with 20–30% salt as the main ingredient and is prized for its distinctive flavor and high nutritional content. In Korea, fresh jogaejeotgal (jogae, meaning clam; jeotgal, meaning salted seafood) is prepared by adding 10–20% salt and seasonings (such as sesame oil, minced garlic, ginger and finely chopped spring onions). Jeotgal is usually eaten raw after fermentation and is not sterilized during this process. Although many foods could be involved in the transmission of HuNoV, bivalve molluscs such as oysters, mussels and clams are considered relatively high-risk foods because contaminated water can lead to the accumulation of HuNoV in the digestive tissues of these organisms (Lees, 2000; Bellou et al., 2013).
Real-time quantitative polymerase chain reaction (RT-qPCR) is a methodology that accurately quantifies nucleic acid through monitoring the entire PCR process by utilizing a real-time fluorescence quantifier. However, although RT-qPCR is a very sensitive technique, it has certain limitations in the analysis of viruses in food, as it cannot clearly differentiate between infectious and non-infectious viruses and therefore does not allow efficient risk assessment (Hassard et al., 2017). As a rapid tool for the detection and quantification of viable cells of different microorganisms in various food matrices, qPCR tests combined with nucleic acid intercalating dyes based on propidium monoazide (PMA) have been developed (Liu et al., 2018; Laidlaw et al., 2019). PMA can be used as a pre-treatment prior to RT-qPCR. It helps to distinguish between live and dead cells (Nocker et al., 2006). Analytical applications based on the PMA treatment technique may benefit from its excellent and unique properties, such as high-water solubility, high foam stability and strong sorption capacity to proteins (Zi et al., 2018). Since the PMA dye only penetrates cells with damaged membranes and intercalates DNA/RNA, the PMA RT-qPCR technique is based on the integrity of the cell membrane (Fittipaldi et al., 2012; Elizaquível et al., 2013). Sodium lauroyl sarcosinate, also known as sarkosyl, is an anionic surfactant that is derived from sarcosine. The anionic detergent can help PMA penetrate damaged cells and improve the discrimination between dead and live cells (Lee et al., 2018).
High-pressure processing (HPP) has been identified as a promising form of nonthermal intervention to treat food with a high risk of viral contamination without significantly changing the nature of the food (López-Caballero et al., 2000). HPP technology entails hermetically sealing food in a flexible container and applying high-pressure of 100–600 MPa at room temperature. A liquid (typically water) is used as the pressure transfer medium to subject the interior and surface of the food to an even amount of pressure to achieve pasteurization (Balasubramaniam et al., 2015). In recent years, HPP has been used to treat high-risk foods, including fruit and vegetable products (e.g., salsa, apple sauce, and various fruit blends and purees) and shellfish (e.g., oysters and clams) for virus contamination. HPP disrupts living organisms by either destroying or deactivating microbial cells using a combination of physiological and biochemical effects on the microorganisms (San Martin et al., 2002). Treatment of oyster homogenate contaminated by inoculation with HuNoV genogroup II.4 at 300 MPa for 5 min at 6°C resulted in a 3.51 log reduction in the HuNoV genome (Ye et al., 2014). HPP at 600 MPa for 5 min at 6°C, but not at 400 MPa (at 6 or 25°C), completely inactivated HuNoV in seeded oysters, as reported by Leon et al. (2011).
Therefore, the objective of our study is to assess the efficiency of HPP treatment against HuNoV GII.4 infectivity of jeotgal assessed by PMA + Sarkosyl pretreatment combined with RT-qPCR. We also aimed to determine whether higher pressure levels (100–600 MPa) of HPP treatment would adversely affect the pH value and Hunter color attributes of jeotgal.
A fecal specimen from a patient displaying signs of gastroenteritis was obtained at the Gyeonggi Institute of Health and Environment (GIHE, Gyeonggido, Korea) in 2012. The presence of the HuNoV GII.4 Sydney variant as confirmed by Won et al. (2013) was verified in the sample analyzed for this study. The complete nucleotide sequence of the GII.4 Sydney variant was analyzed by Park et al. (2015). HuNoV GII.4 was purchased from WAVA in 500 μL phosphate-buffered saline (PBS) with a pH of 7.2. The sample was transported to the laboratory within a dry ice box and stored at −80°C until use.
Jeotgal samples (3 g) were inoculated with 20 μL of HuNuV GII.4 placed in a biological safety cabinet (CHC Lab Co., Daejeon, Korea) for 1 h to facilitate virus attachment. Approximately 3 g Jeotgal samples underwent HPP, using a WIP-L60-50-200 warm isostatic press pressure treatment system (WIP-L60-50-200, Ilshin Autoclave, Inc., Daejeon, Korea). The pressure chamber temperature was preserved at 18°C throughout the process. A warm isostatic press is an apparatus that applies isostatic pressure via water as the pressure medium, dispensing with the use of heat or gas. The apparatus comprises a high-pressure vessel, a high-pressure pump, a reservoir tank, a safety mechanism, an alert system, and a control mechanism. The samples were placed into polythene bags and sealed using a vacuum packing machine (chamber-type vacuum package, DP-901, Dew Pack Korea Machinery Co., Seoul, Korea). The packaged samples were subjected to a pressure of 100, 200, 300, 400, 500, and 600 MPa at 18°C for 5 min. The data presented here represent the mean ± standard deviation of the results of three assays.
For PMA treatment, the samples infected by virus were mixed straightaway with 200 μM PMA (Biotium, Hayward, CA, United States). The mixture was then kept in dark at room temperature for 5 min to allow the dye to penetrate. The samples were then exposed to 40 W LED light (Dynebio, Seongnam, Korea) at a wavelength of 460 nm for 20 min at room temperature to photoactivate both dyes. To determine whether dye treatment interfered with virus detection, a control was included that was neither treated with PMA nor exposed to halogen light. To optimise the sarkosyl concentration and test its effect on HuNoV, sodium lauroyl sarcosinate (Sigma-Aldrich, St. Louis, MO, United States) was evaluated at 1.0% (w/v). For detergent treatment, sarkosyl solution was added to the PMA mixture at the same time. Samples treated with PMA + Sarkosyl were incubated for 10 min in the dark at room temperature. To assess the ability of the PMA + Sarkosyl treatment to interfere with HuNoV detection, an untreated control was included that was not exposed to LED light. Finally, viral samples were subjected to the optimized PMA and sarkosyl pretreatments prior to RT-qPCR assays for the discriminatory detection of potentially infectious and non-infectious viral particles of HuNoV.
Virus RNA was extracted and purified with RNeasy Mini-Kit (Qiagen, Hilden, Germany) to a final volume of 60 μL according to the manufacturer’s instructions. This was done by adding 3 mL of a Proteinase K solution (Sigma, United States) at a final concentration of 0.1 mg/mL to an equal volume of sample (3 g) prepared as described above. The sample was then incubated for 1 h at 37°C with shaking (290 rpm). Next, the soluble homogenate (approximately 3.0 mL) was collected by centrifugation at 5,400 × g for 10 min (SUPRA22K, Hanil Science Industrial Co., Korea) after the samples were transferred to a water bath (60°C) for 15 min, and the pellet was discarded. The supernatant was collected in a sterile conical tube and stored at −80°C until needed. The extracted RNA was subjected to RT-qPCR assays for the detection and quantification of HuNoV GII.4.
Reverse transcription for cDNA synthesis was performed as described by Kageyama et al. (2003). To amplify the HuNoV GII.4 gene, we added 1 μL of enzyme mix (5 units/μL), 5 μL of 5X RT-PCR buffer, 1 μL of 10 mM dNTP, 0.25 μL of RNase inhibitor (5 units/μL), 1 μL of 10 μM primer (Forward and Reverse), 5 μL of RNA we extracted, and RNase-free water to make a final volume of 25 μL. RT-qPCR was carried out using a TP800-Thermal Cycler Dice Real-Time System (TaKaRa) as follows: initial denaturation at 95°C for 10 min, then 45 cycles of amplification at 53°C for 25 s and 62°C for 70 s. We improved the tools to increase the ability to detect and distinguish between the overlapping parts of ORF1 and ORF2. The forward and backward tool sequences are COG2F: 5’-CAR GAR BCN ATG TTY AGR TGG ATG AG–3′ and COG2R: 5’-TCG ACG CCA TCT TCA TTC ACA-3′, respectively. The TaqMan probe was RING2: 5′-TGG GAG GGC GAT CGC AAT CT-3′ marked with the reporter fluorophore 5’-FAM and the quencher fluorophore 3′-TAMRA. The positive control used was HuNoV RNA (TaKaRa, Shiga, Japan), while RNase-free water served as the negative control.
After FE-DBD plasma treatment, the color of jeotgal samples was measured using a colorimeter (UltraScan PRO, Hunterlab, United States) calibrated with the original value from a standard plate (‘L’ = 98.48, ‘a’ = 0.14, and ‘b’ = 0.41). Color was measured through a 6 mm aperture of the colorimeter using a D65 illuminant. The values are expressed in terms of three coordinates, namely ‘L’ (lightness+, darkness−), ‘a’ (redness+, greenness−), and ‘b’ (yellowness−, blueness−), according to Hunter colors.
For pH measurement, 90 mL of distilled water was added to 10 g of Jeotgal, stirred for 5 min at room temperature, and through a Whatman paper filter (Whatman Inc., Piscataway, New Jersey, United States). The liquid on top was checked three times using a pH meter (A211, Thermo Orion, Benchtop, MI, United States).
Thirty untrained panelists who studied in the Graduate School of Sea Food Science and Technology at the Gyeongsang University evaluated the sensory quality of treated samples. The samples underwent evaluation for their color, flavor, taste, texture and overall acceptability utilizing the hedonic scale. The quality was evaluated using the seven-point hedonic scale, with values ranging from “1” indicating extreme disliking and not being acceptable, “4” indicating neither liking nor disliking and a lower limit of the acceptable range, and “7” indicating the extreme liking of samples that are completely free of defects and preserve their original quality. A rating higher than 4 indicated greater acceptability of the food product. The plasma treated samples without viral inoculation were used for the sensory analysis. To prevent any bias in the sensory evaluation outcomes, the panelists assessed the samples impartially in an identical setting. Furthermore, individual samples were provided for each white cup and subsequently assigned a random three-digit code.
The data are presented as the mean ± standard deviation of the three determinations. Results were subjected to one-way analysis of variance (ANOVA) using SPSS (SPSS Inc., Chicago, IL, United States). HuNoV GII.4, with or without PMA + Sarkosyl treatment, was analyzed using logarithmic functions, pH, and Hunter color analysis. Differences between means were compared using Duncan’s multiple range test. One-way ANOVA by t-test was performed to evaluate the statistical significance of differences between non-PMA and PMA + Sarkosyl/RT-qPCR samples using SPSS software. The mean values represent the average of three replicate samples and were considered to be significantly different at a level of p < 0.05.
To evaluate the treatment-related reduction of HuNoV GII.4 in jeotgal, samples were subjected to HPP at 100–600 MPa for 5 min. The initial HuNoV viral RNA recovery from jeotgal without HPP was 3.19 log copies/μL (Table 1). All results can largely be described in two parts. First, the experiments to determine the extent to which HPP-treated HuNoV GII.4 in jeotgal influence on the presence or absence of PMA + sarkosyl yielded the following results. The overall amount of HuNoV GII.4 viral RNA in jeotgal significantly decreased (p < 0.05) following exposure to 100–600 MPa in the two groups comprising of the non-PMA + Sarkosyl treated and PMA + Sarkosyl treated HuNoV. For the non-PMA + Sarkosyl treated jeotgal, the HuNoV GII.4 viral RNA (log copies/μL) was: 2.92 (100 MPa), 2.71 (200 MPa), 2.52 (300 MPa), 2.40 (400 MPa), 2.20 (500 MPa), and 1.81 (600 MPa). In the PMA + Sarkosyl treated samples, the HuNoV GII.4 viral RNA (log copies/μL) was lowered to: 2.79 (100 MPa), 2.51 (200 MPa), 2.23 (300 MPa), 2.10 (400 MPa), 2.00 (500 MPa), and 1.57 (600 MPa). The mean viral RNA of HuNoV GII.4 (inoculated in jeotgal) was observed to decrease significantly (p < 0.05) with an increase in the experimental duration of both Non-PMA and PMA + Sarkosyl treated samples.

Table 1. Changes in HuNoV GII.4 titers in Korean fermented clam jeotgal resulting from high-pressure processing treatments by non-PMA + Sarkosyl/RT-qPCR and PMA + Sarkosyl/RT-qPCR.
The difference in HuNoV reduction, according to viral RNA, was significant between the samples treated with non-PMA + Sarkosyl and those treated with PMA + Sarkosyl, after being subjected to HPP treatment at 200–600 MPa for 5 min (p < 0.05) (Figure 1). The average viral RNA of HuNoV decreased by over 0.24 logarithmic units [= (0.68–0.47) + (0.95–0.67) + (1.08–0.79) + (1.19–0.99) + ((1.62–1.38) log-reduction)/5 (=42.5% reduction)] in samples treated with PMA + Sarkosyl, while non-PMA + Sarkosyl treated samples showed no similar reduction. Therefore, these results confirmed the necessity of employing sarkosyl to enhance the permeability of damaged viral particles for PMA when treated using HPP.
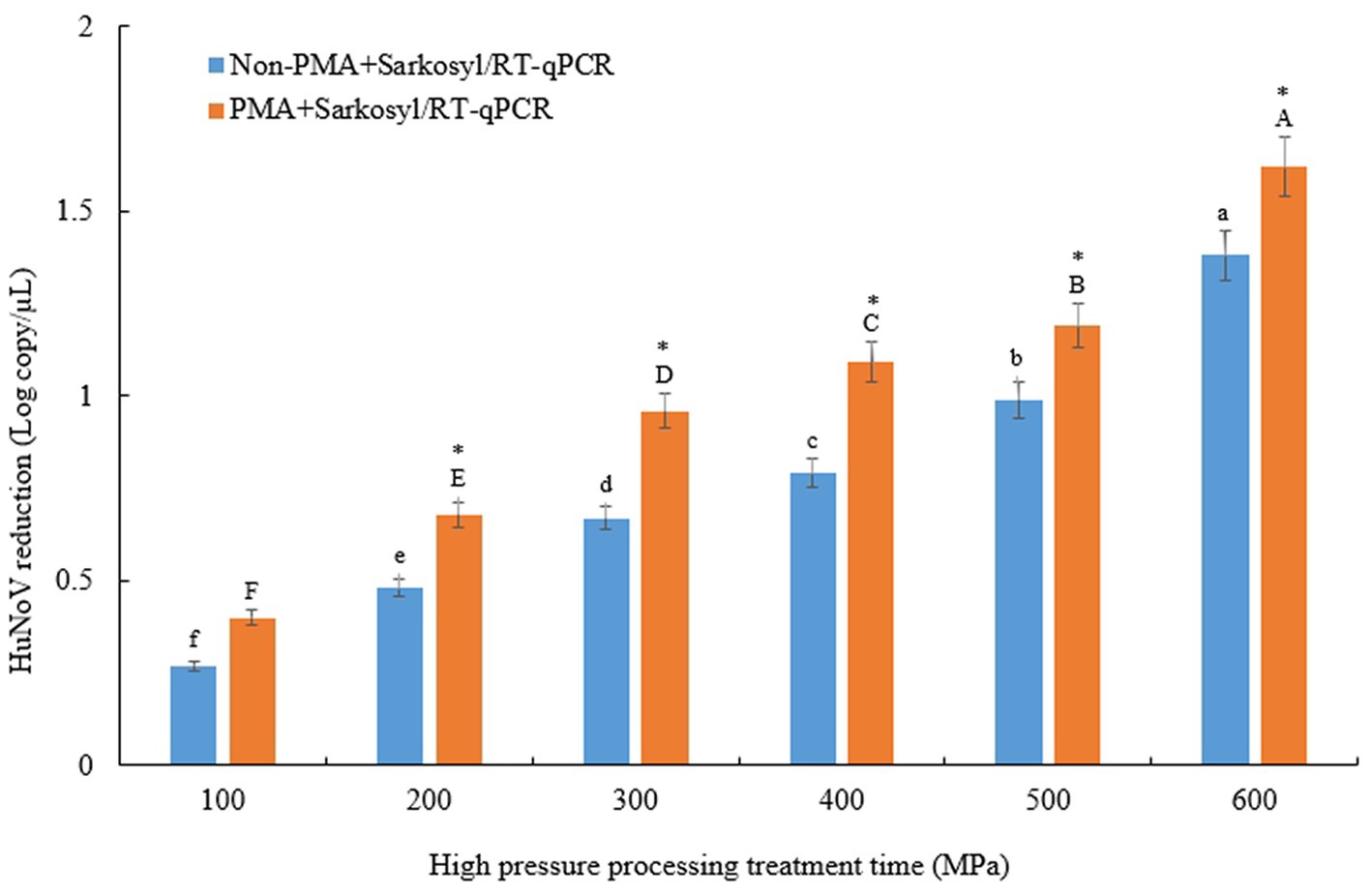
Figure 1. Comparison of log reduction values of HuNoV GII.4 between non-PMA + Sarkosyl and PMA + Sarkosyl Korean fermented clam jeotgal were treated with HPP at 100–600 MPa for 5 min. Within each treatment time, log reduction means with different letters a-f for non-PMA + Sarkosyl/RT-qPCR, A-F for PMA + Sarkosyl/RT-qPCR indicate a significant difference (p < 0.05) between non-PMA + Sarkosyl treated and PMA + Sarkosyl treated samples by Duncan’s multiple range test. Asterisk (*) also indicates a significant difference between non-PMA + Sarkosyl and PMA + Sarkosyl were treated with HPP at 200–600 MPa for 5 min by t-test.
Any potential mechanical color differences caused by HPP treatment were identified by quantifying the Hunter color “L” (lightness), “a” (redness), and “b” (yellowness) values (Table 2). Significant variations (p < 0.05) were detected in all Hunter colors across all samples when elevated hydrostatic pressures were employed to process the samples. The Hunter color “L”– values significantly (p < 0.05) decreased with stepwise increments in the experimental pressure (i.e., 0–600 MPa). In addition, the Hunter color “a”- and “b”-values significantly (p < 0.05) increased with the stepwise increments in experimental pressure (i.e., 0–600 MPa). Thus, the jeotgal samples treated at higher pressure were darker, with more red and yellow hues compared with those treated by applying lower pressure. Additionally, the differences in the pH between the control (0 MPa) and all HPP (100–600 MPa) treated jeotgal samples were insignificant (p > 0.05) (Table 2). The pH of the samples corresponded to the range 6.14–6.19.
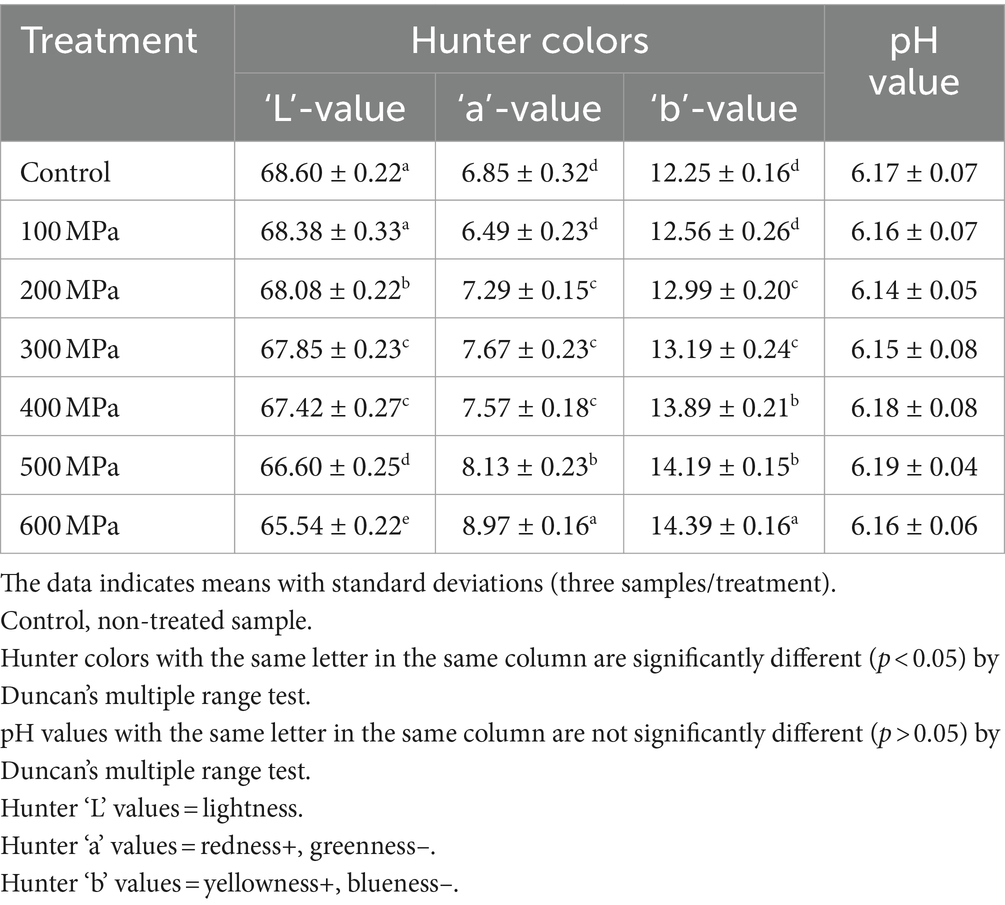
Table 2. Effects of high-pressure processing treatment on the Hunter color and pH value of Korean fermented clam jeotgal.
The effect of HPP treatment on the sensory qualities of jeotgal in terms of its color, smell, taste, appearance, and overall acceptability was evaluated by untrained panelists and the results appear in Table 3. The average general acceptability score for untreated controls was 5.50, whereas jeotgal treated at room temperature (18°C) scored 5.20, 5.10, 5.30, 5.30, 5.40, and 5.30 at applied pressure of 100, 200, 300, 400, 500, and 600 MPa, respectively. A significant preference (p < 0.05) for “Color” was observed between untreated controls and all pressure-treated samples.
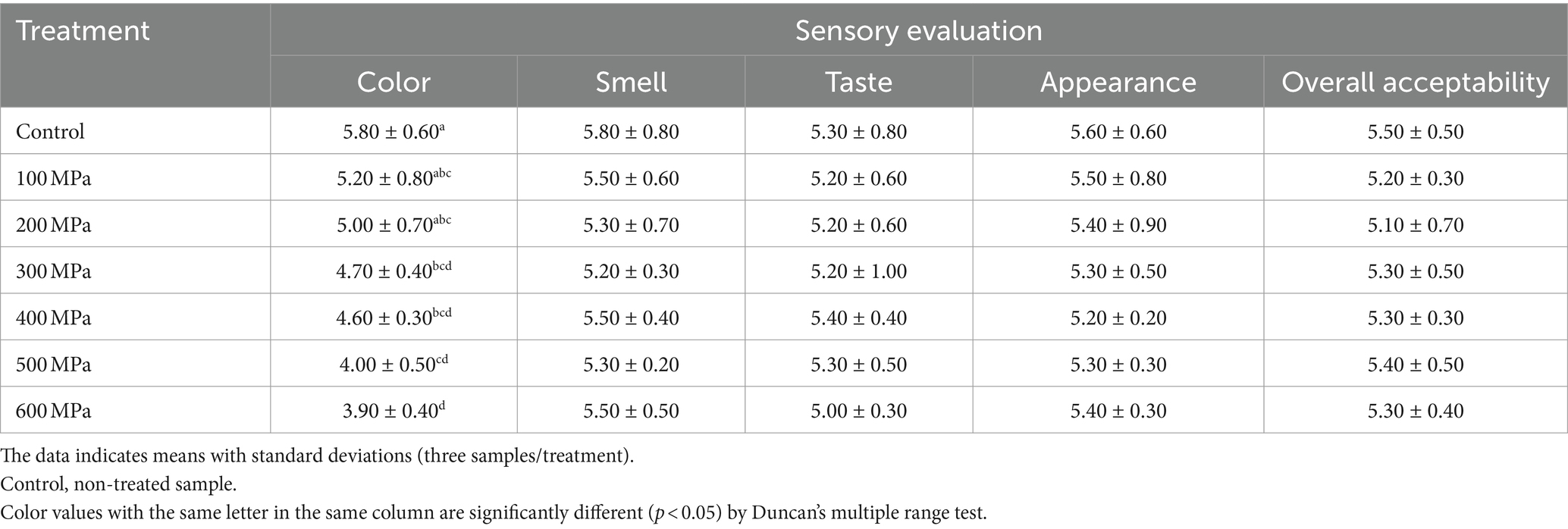
Table 3. Effects of high-pressure processing treatment on the sensory evaluation of Korean fermented clam jeotgal.
In the last 3 years, Korean fermented shellfish jeotgal, including clam jeotgal, have been identified as a cause of microbial food poisoning in Korea. Choi et al. (2018) reported that coliforms and Bacillus cereus were qualitatively detected in 17 (48.6%) and 20 (57.1%) of 35 seasoned jeotgals, respectively, and the total number of aerobic bacteria detected quantitatively in clam jeotgal was 5.9 log CFU/g. In 2018, oyster jeotgal and raw crab soy sauce jeotgal were found to contain HuNoV (Ministry of Food and Drug Safety (MFDS), 2018). The following year, hepatitis A virus (HAV) outbreaks were reported at specific restaurants in Busan, Korea, where 64 individuals who consumed clam jeotgal became infected with HAV (Anonymous, 2019). Therefore, the development of food processing technologies to deactivate pathogenic microorganisms mainly including HuNoV in potential high-risk jeotgal is urgently needed, especially in Korea. Contaminated shellfish are accountable for HuNoV outbreaks, and it is important to have competent techniques to detect and retrieve infectious HuNoV in shellfish. This study is involved on gauging the efficacy of high-pressure processing (HPP) treatment against HuNoV GII.4 infectivity in jeotgal by using RT-qPCR. To evaluate HPP-induced damage to the viral capsid proteins, the samples were subjected to proteinase K and PMA + Sarkosyl pretreatment prior to RT-qPCR assessment. Proteinase K was utilized to break down the protein capsid, facilitating the release of genomic RNA, which could then be degraded by RNase. The Proteinase K digestion methodology has undergone comprehensive evaluation and was included in the ISO 15216 standard procedure for the quantitative detection of Norovirus and hepatitis virus in 2017 (Anonymous, 2017). Recently, several studies have assessed the use of PMA intercalating dye pretreatment before RT-qPCR to distinguish between infectious and non-infectious viruses. Jeon et al. (2020) demonstrated a significant reduction in norovirus load in PMA treated crustacean samples compared to untreated samples using an optimized PMA treatment. Specifically, Jeong et al. (2017) reported a decrease in log copies of <1.5 for non-dye treated HuNoV on spinach, while spinach associated HuNoV was not detected after PMA treatment at 95°C for 2 min. Mohammad et al. (2015) reported that PMA/RT-PCR was able to differentiate between infectious and non-infectious murine norovirus following inactivation by chlorine. Although both of these viruses belong to the Caliciviridae family, the efficacy of PMA treatment may vary depending on the virus type, such as feline calicivirus (FCV) and murine norovirus (MNV-1). The differences in the ability of PMA treatment to distinguish between infectious and non-infectious viral particles may be due to the degree of damage to RNA or capsids caused by the various inactivation methods, including ultraviolet light, plasma, heat treatment, and gamma radiation. However, it is commonly known that intercalating dyes have a where the inactivation method may cause damage to the nucleic acid resulting in the loss of infectivity due to damaged nucleic acids, but the capsid remains intact (Leifels et al., 2015). Mohammad et al. (2015) reported that the addition of chlorine and exposure to heat can damage the viral capsid, allowing the genome to access the dye. Our findings indicate that there was no significant difference in the reduction of log copies between PMA pretreated and non-PMA pretreated samples subjected to HPP treatment, despite statistical differences between the two groups. This implies that, in these HPP conditions, the viral capsid may have remained partially intact or that the denatured capsid protein can safeguard a portion of the genome, preventing PMA binding.
Several studies have indicated the potential efficacy of surfactants, including triton-x-100, sodium deoxycholate, and sodium lauroyl sarcosinate (Sarkosyl), in improving the penetration of monoazide into slightly damaged capsids of HuNoV and HAV (Moreno et al., 2015; Fuster et al., 2016). In this study, the average difference between PMA/RT-qPCR and PMA + Sarkosyl/RT-qPCR was 0.14 log copies/μL (data not shown). Lee et al. (2018) reported that a combination of PMA and sarkosyl treatment of chlorine disinfected HuNoV GII.4 in water improved the discrimination between infectious and chlorine inactivated viruses by RT-qPCR. These observations were consistent with our findings, which revealed that HuNoV viability in the shellfish samples was more effectively assessed by PMA + Sarkosyl/RT-qPCT compared with the typical RT-qPCR sarkosyl enhanced permeability of dead or non-infectious particles to PMA. Taken together, the PMA and Sarkosyl pretreated method may indicate the infectivity of HuNoV in jeotgal following HPP treatment. As a result, it was utilized in this investigation.
High-pressure processing (HPP) has demonstrated potential as a non-thermal technology to enhance the safety and extend the shelf life of a wide variety of foods. This approach can maintain the organoleptic properties of food items whilst making them microbiologically safe with a prolonged shelf life (Patterson, 2005). The level of virus inactivation resulting from HPP treatment increases as the pressure and treatment duration increase. Nonetheless, the effectiveness of the treatment can significantly differ among various viruses. The potential of HPP treatment to inactivate HuNoV was investigated in preliminary studies Buckow et al. (2008) through the use of FCV and MNV-1 surrogate viruses. Tang et al. (2010) reported that application of pressure of 400 MPa for 5 min inactivated 8.22 log PFU of MNV-1. In other similar studies, FCV was reduced to undetectable levels in shellfish (4.21 and 3.83 log reductions in mussels and oysters, respectively) by applying pressure in excess of 300 MPa (Murchie et al., 2007). Kingsley et al. (2007) indicated that a temperature of 5°C, when 350 MPa pressure was applied, resulted in the greatest degree of inactivation, reducing the MNV-1 titer by 5.56 log, whereas warmer temperatures of 20 and 30°C yielded appreciably less inactivation of 1.79 and 1.15 log, respectively. In contrast, we found that treatment of HuNoV GII.4 at 600 MPa and 18°C resulted in a 1.62 log reduction in viral RNA using the PMA + Sarksoyl/RT-qPCR assay compared to a 1.38 log reduction at 20°C. In the study of Ye et al. (2014), HPP treatment of 300 and 600 MPa at 25°C resulted in 1.1–1.8 log reduction of HuNoV GII.4 and GI.1 in oysters. Li et al. (2013) reported that a reduction of 3.7 log RNA was achieved when oysters treated at 350 MPa for 2 min at 4°C were inoculated with HuNoV GII.4 strain, whereas a reduction of only 1.2 log RNA was observed when the virus inoculated oyster was treated by using 350 MPa for 2 min at 20°C. Our data are consistent with those of the above two studies indicating that HuNoV was more pressure resistant than FCV and MNV-1, a surrogate for the norovirus. This phenomenon may be attributed to various factors such as the innate properties of the virus, the size and structure of its particles, its high thermodynamic stability, discrepancies in the binding properties of the viral receptor, or differences in the protein structure and amino acid composition. Additionally, they concluded that a low initial sample temperature substantially increased HPP treatment of HuNoV compared with high temperature. Although the HPP-treated jeotgal sample was not subjected to cold temperatures (0–10°C) (a constant temperature of 18°C was used) in the current study, HuNoV inactivation could be speculated to occur at HPP of more than 600 MPa at cold temperatures. The mechanism underlying the HPP anti-HuNoV effects in foods including jeotgal has not been fully elucidated. However, typically, HPP treatment inactivates the virus through denaturation of the viral capsid proteins. This leads to the incapacitation of infection virions, preventing their attachment and penetration into host cells. Furthermore, variations in pH, salt concentration, fat composition, and protein composition have a significant impact on the sample matrix, resulting in pathogen inactivation. This has also been demonstrated to be true for viral inactivation (Buckow et al., 2008). In HPP treatment, salt in the food environment can provide protective benefits because it can stabilize viral capsid proteins. However, NaCl concentrations of up to 1% do not seem to offer significant protection to the virus. In contrast, higher concentrations of salt exhibit baro-protective characteristics (Grove et al., 2006; Kingsley et al., 2007; Kingsley and Chen, 2009). Comparing data from studies with varying treatment times, starting temperatures, and NaCl concentrations requires caution, as these factors can impact the extent of microbial inactivation.
The color, flavor, and texture are important quality characteristics of seafood and vegetables and are major factors that affect the sensory perception and consumer acceptance of foods. Many studies do not include a sensory evaluation or do not report whether HPP induced textural changes would have a detrimental effect on consumer acceptance of a product. In this experiment in which jeotgal was treated with HPP, the Hunter color values were darker, with more yellow and redness. This fact was possibly connected with the observation that the midgut gland of the shellfish was forced out as a result of the pressure. The pH value is widely regarded as a standard chemical indicator of alterations in the quality of foods. In our work, the pH value of the control (0 MPa) sample did not differ from that of all the HPP (100–600 MPa) treated jeotgal samples. These pH values were in accordance with those of a study by Jo (2019) who reported that the pH of jeotgal was 6.25. A few panelists mentioned that the jeotgal treated with HPP >500 MPa darkened in appearance whereas the non-treated jeotgal did not exhibit such color differences (data not shown). Few studies that investigated changes in the quality of seafood following HPP treatment have been reported, although both textural and visual changes to other bivalve shellfish following HPP treatment were reported. Arcangeli et al. (2012) found that subjecting clams to a pressure of 500 MPa for 1 minute at a temperature of 20°C is a viable method for MNV-1 activation, while leaving the appearance and texture of the clam unaffected. Ye et al. (2014) also reported that HPP (300–500 MPa) treatment for 2 min at 25 and 0°C did not significantly change the color or texture of oyster tissue. Similarly, López-Caballero et al. (2000) reported that HPP (400 MPa) treatment for 10 min at 7°C did not significantly change the appearance of oyster tissue. They concluded that HPP treatment has excellent antibacterial efficacy and that the sensual quality is preserved. HPP is therefore considered to be a potential and promising alternative to seafood conservation technology. However, additional HPP inactivation data for enteric viruses is necessary, across various treatment times and pressures, in both buffered systems and food matrices.
The research presented here establishes the efficacy of employing PMA + Sarkosyl pretreatment alongside RT-qPCR to assess the efficacy of HHP at 100–600 MPa for 5 min at ambient temperature against HuNoV GII.4 infection in jeotgal. The average viral RNA of HuNoV was reduced by around 43% (=0.24 log) in samples treated with PMA + Sarkosyl when compared to those not treated with PMA + Sarkosyl. To achieve a reduction in excess of one log in jeotgal treated with PMA + Sarkosyl, exposure to 400 MPa of HPP. The Hunter “L” color values decreased significantly (p < 0.05) with incremental increases in experimental pressure (0–600 MPa). Meanwhile, the Hunter “a” and “b” color values significantly increased (p < 0.05) as experimental pressure increased stepwise (0–600 MPa). The pH value ranged from 6.14 to 6.19. A statistically significant preference (p < 0.05) was found between untreated controls and all pressure-treated samples for the parameter “Color.” This outcome could potentially be explained by the midgut gland of the shellfish being extruded due to the applied pressure. These results suggest that RT-qPCR/PMA + Sarkosyl could be regarded as a tool to determine the viability of using HPP to treat Korean fermented shellfish product for HuNoV. Furthermore, our experiments have indicated that utilizing ≥400 MPa of HPP at 18°C for 5 min can be an effective means of reducing HuNoV by ≥90%. This process had no significant influence on the pH nor most sensorial qualities of jeotgal, with the exception of sensorial and mechanical colors.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
EBJ: Data curation, Validation, Writing – original draft. HSJ: Methodology, Writing – review & editing. SYP: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2021R1I1A3A04037468).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anonymous (2017). Microbiology of the food and animal feed. Horizontal method for determination of hepatitis a virus and norovirus using real-time RT-PCR. In: Part 1: Method for Quantification (ISO/TS 15216–1:2017). Geneva: International Organization for Standardization.
Anonymous (2019). South Korea: Hepatitis A outbreak linked to Busan restaurant. Available at: http://outbreaknewstoday.com/south-korea-hepatitis-a-outbreak-linked-to-busan-restaurant-34614 (accessed December 20, 2021).
Arcangeli, G., Terregino, C., Benedictis, P. D., Zecchin, B., Manfrin, A., Rossetti, E., et al. (2012). Effect of high hydrostatic pressure on murine norovirus in Manila clams. Lett. Appl. Microbiol. 54, 325–329. doi: 10.1111/j.1472-765x.2012.03211.x
Balasubramaniam, V. M., Martínez, M. S., and Gupta, R. (2015). Principles and application of high pressure based technologies in the food industry. Annu. Rev. Food Sci. Technol. 6, 435–462. doi: 10.1146/annurev-food-022814-015539
Bellou, M., Kokkinos, P., and Vantarakis, A. (2013). Shellfish-borne viral outbreaks: a systematic review. Food Environ. Virol. 5, 13–23. doi: 10.1007/s12560-012-9097-6
Buckow, R., Isbarn, S., Knorr, D., Hienz, V., and Lehmacher, A. (2008). Predictive model for inactivation of feline calcivirus, a norovirus surrogate, by heat and high hydrostatic pressure. Appl. Environ. Microbiol. 74, 1030–1038. doi: 10.1128/aem.01784-07
Choi, S. A., An, S. E., Jeong, H. G., Lee, S. H., Mun, K. H., and Kim, J. B. (2018). Evaluation of microbiological safety in commercial Jeotgal. Korean J. Food Preserv. 25, 270–278. doi: 10.11002/kjfp.2018.25.2.270
Elizaquível, P., Aznar, R., and Sánchez, G. (2013). Recent developments in the use of viability dyes and quantitative PCR in the food microbiology field. J. Appl. Microbiol. 116, 1–13. doi: 10.1111/jam.12365
Fittipaldi, M., Nocker, A., and Codony, F. (2012). Progress in understanding preferential detection of live cells using viability dyes in combination with DNA amplification. J. Microbiol. Methods 91, 276–289. doi: 10.1016/j.mimet.2012.08.007
Fuster, N., Pinto, R. M., Fuentes, C., Beguiristain, N., Bosch, A., and Guix, S. (2016). Propidium monoazide RT-qPCR assays for the assessment of hepatitis a inactivation and for a better estimation of the health risk of contaminated waters. Water Res. 101, 226–232. doi: 10.1016/j.watres.2016.05.086
Grove, S. F., Lee, A., Lewis, T., Stewart, C. M., Chen, H., and Hoover, D. G. (2006). Inactivation of foodborne viruses of significance by high pressure and other processes. J. Food Prot. 69, 957–968. doi: 10.4315/0362-028x-69.4.957
Hall, A. J., Lopman, B. A., Payne, D. C., Patel, M. M., Gastanaduy, P. A., Vinje, J., et al. (2013). Norovirus disease in the United States. Emerg. Infect. Dis. 19, 1198–1205. doi: 10.3201/eid1908.130465
Hassard, F., Sharp, J. H., Taft, H., LeVay, L., Harris, J. P., and McDonald, J. E. (2017). Critical review on the public health impact of norovirus contamination in shellfish and the environment: a UK perspective. Food Environ. Virol. 9, 123–141. doi: 10.1007/s12560-017-9279-3
Jeon, E. B., Choi, M. S., Kim, J. Y., Ha, K. S., Kwon, J. Y., Jeong, S. H., et al. (2020). Characterizing the effects of thermal treatment on human norovirus GII.4 viability using propidium monoazide combined with RT-qPCR and quality assessments in mussels. Food Control 109:106954. doi: 10.1016/j.foodcont.2019.106954
Jeong, M. I., Park, S. Y., and Ha, S. D. (2017). Thermal inactivation of human norovirus on spinach using propidium or ethidium monoazide combined with real-time quantitative reverse transcription-polymerase chain reaction. Food Control 78, 79–84. doi: 10.1016/j.foodcont.2017.02.026
Jo, J. Y. (2019). Application of chlorine dioxide and Electron beam irradiation for the reduction of norovirus in low-salted “Jogeajeotgal”, a traditional Korean salted and fermented clam. Seoul, Korea: University of Chung-Ang.
Kageyama, K., Komatsu, T., and Suga, H. (2003). Refined PCR protocol for detection of plant pathogens in soil. J. Gen. Plant Pathol. 69, 153–160. doi: 10.1007/s10327-002-0037-4
Kingsley, D. H., and Chen, H. (2009). Influence of pH, salt, and temperature on pressure inactivation of hepatitis a virus. Int. J. Food Microbiol. 130, 61–64. doi: 10.1016/j.ijfoodmicro.2009.01.004
Kingsley, D. H., Holliman, D. R., Calci, K. R., Chen, H., and Flick, G. J. (2007). Inactivation of a norovirus by high pressure processing. Appl. Environ. Microbiol. 73, 581–585. doi: 10.1128/aem.02117-06
Laidlaw, A. M., Gänzle, M. G., and Yang, X. (2019). Comparative assessment of qPCR enumeration methods that discriminate between live and dead Escherichia coli O157:H7 on beef. Food Microbiol. 79, 41–47. doi: 10.1016/j.fm.2018.11.002
Le Guyader, F. S., Atmar, R. L., and Le Pendu, J. (2012). Transmission of viruses through shellfish: when specific ligands come into play. Curr. Opin. Virol. 2, 103–110. doi: 10.1016/j.coviro.2011.10.029
Lee, H. W., Lee, H. M., Yoon, S. R., Kim, S. H., and Ha, J. H. (2018). Pretreatment with propidium monoazide/sodium lauroyl sarcosinate improves discrimination of infectious waterborne virus by RT-qPCR combined with magnetic separation. Environ. Pollut. 233, 306–314. doi: 10.1016/j.envpol.2017.10.081
Lees, D. N. (2000). Viruses and bivalve shellfish. Int. J. Food Microbiol. 59, 81–116. doi: 10.1016/s0168-1605(00)00248-8
Leifels, M., Jurzik, L., Wilhelm, M., and Hamza, I. A. (2015). Use of ethidium monoazide and propidium monoazide to determine viral infectivity upon inactivation by heat, UV- exposure and chlorine. Int. J. Hyg. Environ. Health 218, 686–693. doi: 10.1016/j.ijheh.2015.02.003
Leon, J. S., Kingsley, D. H., Montes, J. S., Richards, G. P., Lyon, G. M., Abdulhafid, G. M., et al. (2011). Randomized, double-blinded clinical trial for human norovirus inactivation in oysters by high hydrostatic pressure processing. Appl. Environ. Microbiol. 77, 5476–5482. doi: 10.1128/aem.02801-10
Li, X., Chen, H., and Kingsley, D. H. (2013). The influence of temperature, pH, and water immersion on the high hydrostatic pressure inactivation of GI.1 and GII.4 human noroviruses. Int. J. Food Microbiol. 167, 138–143. doi: 10.1016/j.ijfoodmicro.2013.08.020
Liu, Y., Zhong, Q., Wang, J., and Lei, S. (2018). Enumeration of Vibrio parahaemolyticus in VBNC state by PMA-combined real-time quantitative PCR coupled with confirmation of respiratory activity. Food Control 91, 85–91. doi: 10.1016/j.foodcont.2018.03.037
López-Caballero, M. E., Pérez-Mateos, M., Montero, P., and Borderías, A. J. (2000). Oyster preservation by high-pressure treatment. J. Food Prot. 63, 196–201. doi: 10.4315/0362-028x-63.2.196
Ministry of Food and Drug Safety (MFDS) (2018). A Report of Studies on Hazardous Microbiological Safety Management of Seafood. Available at: https://www.mfds.go.kr/eng/index.do (accessed December 10, 2022).
Mohammad, R. K., Fout, G. S., Johnsonc, C. H., Whitec, K. M., and Parshionikar, S. U. (2015). Propidium monoazide reverse transcriptase PCR and RT-qPCR for detecting infectious enterovirus and norovirus. J. Virol. Methods 219, 51–61. doi: 10.1016/j.jviromet.2015.02.020
Moreno, L., Aznar, R., and Sánchez, G. (2015). Application of viability PCR to discriminate the infectivity of hepatitis a virus in food samples. Int. J. Food Microbiol. 201, 1–6. doi: 10.1016/j.ijfoodmicro.2015.02.012
Murchie, W. L., Kellyc, A. L., Wiley, M., Adair, B. M., and Patterson, M. (2007). Inactivation of a calicivirus and enterovirus in shellfish by high pressure. Innov. Food Sci. Emerg. Technol. 8, 213–217. doi: 10.1016/j.ifset.2006.11.003
Nocker, A., Cheung, C. Y., and Camper, A. K. (2006). Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J. Microbiol. Methods 67, 310–320. doi: 10.1016/j.mimet.2006.04.015
Park, J. S., Lee, S. G., Jin, J. Y., Cho, H. G., Jeong, W. H., and Paik, S. Y. (2015). Complete nucleotide sequence analysis of the norovirus GII.4 Sydney variant in South Korea. Biomed. Res. Int. 7, 2015:374637. doi: 10.1155/2015/374637
Patterson, M. F. (2005). Microbiology of pressure-treated foods. J. Appl. Microbiol. 98, 1400–1409. doi: 10.1111/j.1365-2672.2005.02564.x
Sabria, A., Pinto, R. M., Bosch, A., Bartolome, R., Cornejo, T., and Torner, N. (2016). Norovirus shedding among food and healthcare workers exposed to the virus in outbreak settings. J. Clin. Virol. 82, 119–125. doi: 10.1016/j.jcv.2016.07.012
San Martin, M. F., Barbosa-Cánovas, G. V., and Swanson, B. G. (2002). Food processing by high hydrostatic pressure. Biosci. Biotechnol. Biochem. 42, 627–645. doi: 10.1080/20024091054274
Tang, O., Li, D., Xu, J., Wang, J., Zhao, Y., Li, Z., et al. (2010). Mechanism of inactivation of murine norovirus-1 by high pressure processing. Int. J. Food Microbiol. 137, 186–189. doi: 10.1016/j.ijfoodmicro.2009.10.033
Teunis, P. F., Moe, C. L., Liu, P., Miller, S. E., Lindesmith, L., and Baric, R. S. (2008). Norwalk virus: how infectious is it? J. Med. Virol. 80, 1468–1476. doi: 10.1002/jmv.21237
Teunis, P. F., Sukhrie, F. H., Vennema, H., Bogerman, J., Beersma, M. F., and Koopmans, M. P. (2015). Shedding of norovirus in symptomatic and asymptomatic infections. Epidemiol. Infect. 143, 1710–1717. doi: 10.1017/s095026881400274x
Won, Y. J., Park, J. W., and Han, S. H. (2013). Full-genomic analysis of a human norovirus recombinant GII.12/13 novel strain isolated from South Korea. PLoS One 8:e85063. doi: 10.1371/journal.pone.0085063
Ye, M., Li, X., Kingsley, D. H., Jiang, X., and Chen, H. (2014). Inactivation of human norovirus in contaminated oysters and clams by high hydrostatic pressure. Appl. Environ. Microbiol. 80, 2248–2253. doi: 10.1128/aem.04260-13
Keywords: human norovirus GII.4, clam jeotgal, propidium monoazide/sarkosyl, RT-qPCR, high pressure processing
Citation: Jeon EB, Jeong HS and Park SY (2024) Antiviral activity of high pressure processing of Korean fermented clam jeotgal against human norovirus GII.4 infectivity. Front. Sustain. Food Syst. 8:1396693. doi: 10.3389/fsufs.2024.1396693
Received: 06 March 2024; Accepted: 17 May 2024;
Published: 30 May 2024.
Edited by:
Sylvester Chibueze Izah, Bayelsa Medical University, NigeriaReviewed by:
Byron Brehm-Stecher, Iowa State University, United StatesCopyright © 2024 Jeon, Jeong and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shin Young Park, c3lwYXJrQGdudS5hYy5rcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.