- 1Animal and Human Health, International Livestock Research Institute, Nairobi, Kenya
- 2Department of Veterinary Pathology, Microbiology and Parasitology, University of Nairobi, Nairobi, Kenya
- 3Department of Biomedical Science, School of Public Health and Community Development, Maseno University, Kisumu, Kenya
- 4Department of Food and Markets, Natural Resources Institute, Kent, United Kingdom
- 5Department of Animal and Veterinary Sciences, University of Copenhagen, Frederiksberg C, Denmark
Introduction: Approximately 70% of diarrheal cases in Kenya are attributed to ingestion of contaminated food and water and costs an estimated $ 1 billion USD due to morbidity and cost of treatment. This study aimed to assess the levels of microbiological contamination of meat sold in selected butcheries in Nairobi and the handling practices of butcher shop attendants.
Methods: A cross-sectional study design was used during which 200 meat samples were collected, and meat handling practices were observed. Total coliforms and Escherichia coli were enumerated using 3M™ Petrifilm® count plates. Additionally, quantification of tetracycline- and cefotaxime-resistant Enterobacteriaceae was done on agar plates containing the respective antibiotics. Bacterial species were confirmed by Matrix-Assisted Laser Desorption/Ionization-Time of Flight mass spectrometry.
Results and discussion: Seventy two percent and 84% of the samples had E. coli and total coliforms respectively above the acceptable regulatory limits (i.e. E. coli >100 CFU/g, Total coliforms >361 CFU/g,) respectively as per the Kenya Bureau of Standards South African microbiological standards the European Union. Enterobacteriaceae resistant to tetracycline and cefotaxime were detected in 35% and 9.5% of the samples respectively. Eighty-five percent of the butcher shop attendants neither washed their hands before nor after handling the meat, 91% handled money while selling meat concurrently, and 99% did not wear gloves while handling meat. These poor meat handling practices coupled with the presence of microbial loads above the regulatory acceptable limits imply an increased risk of foodborne illness to consumers. Therefore, there is an urgent need for education of butcher shop attendants on appropriate handling of meat, highlighting the importance of good hygienic practices and their relationship to food safety, and provision of incentives for behavior change. This study is important and serves to inform policymakers in the identification of key control points for designing meat safety intervention(s).
1 Introduction
Animal source foods (ASF) play an important role in nutrition, health, and economic development. These foods include meat, dairy products, eggs, and fish (Murphy and Allen, 2002). In Kenya, the average consumption of red meat is estimated at 15–16 kg per capita and a national total of approximately 600,000 metric tons annually; with beef accounting for 75–80% (Bergevoet and Van Engelen, 2014). The per capita consumption in Nairobi is the highest, estimated at 17 kg (KMT, 2019). The need and demand for proteins in low- and middle-income settlements is immense, especially among vulnerable groups such as young children, pregnant and lactating mothers, the elderly, and immunocompromised individuals. The gross Domestic Product (GDP) per capita is projected to increase by over 140 percent by 2050 (FAO, 2019); consequently, the demand for ASFs is expected to increase exponentially, with projections indicating an increase in consumption to 8.5 million tons by 2050 (OECD, 2021).
Meat is prone to contamination by spoilage bacteria and pathogenic bacteria; among which the most important are toxigenic Escherichia coli, Salmonella spp., and Campylobacter jejuni (Dhama et al., 2013; Pal et al., 2018). Unhygienic slaughtering, storage, transportation, distribution, and processing of meat, as well as poor personal hygiene of meat handlers, have been identified as important sources of meat contamination (Getenesh et al., 2020). ASFs have been linked to approximately 40% of the global burden of foodborne disease (Li et al., 2019). This burden is higher in low-and-middle-income countries (LMICs) and puts an added strain on an already fragile health system. Smallholder livestock production and informal food marketing systems predominate in LMICs but are poorly regulated and inadequately equipped (Jabbar and Grace, 2012).
In Kenya, regulation of meat safety is guided by the Meat Control Act, the Public Health Act, and the Chemical and Substance Act that promote public health and foster economic development (NFSP Draft, 2021). Additionally, standards from the Codex Alimentarius, and the International Standards Organization also guide the country’s meat sector (Sirma et al., 2023). Despite the presence of regulations, they are poorly enforced due to resources constraints particularly in the informal food chain which are common and serves the majority of people in Kenya (Kang’ethe et al., 2020). Previous studies identified structural vulnerabilities in the meat value chain in Nairobi, highlighting inadequate facilities, little to no quality control, unhygienic food handling practices, all of which contribute to contamination of meat and increase food safety risks (Alarcon et al., 2017; Gathura et al., 2020).
Foodborne diseases are prevalent and considered to cause most cases of diarrhea (NFSP Draft, 2021); however, the source attribution of cases is not clear. In addition, value chain actors in poor-resourced neighborhoods of Nairobi are not properly equipped with the infrastructure to ensure the safe processing and handling of meat (Gathura et al., 2020). Lastly, little is known about the status of meat quality and current meat handling practices, especially in Nairobi butcheries, which is where most consumers source their meat (Alarcon et al., 2017). There is a need for evidence-based measures that will address the meat safety challenges in a contextualized manner. Our investigation focused on evaluating the microbiological safety of beef in selected butcher shops across various wards in Nairobi. Furthermore, we documented the observed meat handling practices among the attendants in these specific butcher shops.
2 Materials and methods
2.1 Ethical approval for the study
Ethical approvals were obtained from the International Livestock Research Institute’s Research Ethics Committee (IREC); ILRI-IREC2021-62 and the National Commission for Science, Technology, and Innovation (NACOSTI); Ref No: 259467 and the Nairobi County; REF: EOP/NMS/HS/132. Approval was also obtained from the Faculty of Veterinary Medicine Biosafety, Animal Use and Ethics Committee, University of Nairobi; REF: FVM BAUEC/2022/416. More importantly, consent was sought from all participating butchery attendants/owners during the study.
2.2 Study area and butcher shop selection
This cross-sectional study was conducted between May and October 2022 in Nairobi County and specifically focused on four wards namely Kawangware, Kangemi, Huruma, and Waithaka (Figure 1). These sites were purposively selected based on their high concentration of butchery outlets and because most residents are low and middle-income class (Owuor et al., 2017). The study unit was the butchery, which was randomly selected, visited only once, and only one person from the outlet was interviewed, i.e., either the owner or the attendant, depending on who was available and directly involved in handling and selling meat. Butcheries selling beef and having meat available at the time of the study were included. The sample size was estimated in Stata® 15, for comparison of two proportions: p1 = estimate of the proportion of meat samples with unacceptable coliform counts, in the control group, and p2 = proportion of meat with unacceptable coliform counts, in the intervention group. Using p1 = 0.65, p2 = 0.50, alpha = 0.05, and power = 0.80, the minimum sample size required to detect a difference of 15% is 170 samples per group which translated to enrolling 340 butcheries. A correction for the finite population was done (as a prior mapping activity had established that there were 430 butcheries in the study areas). Considering possible withdrawals/ refusal to participate, a total of 200 butcheries were included in the study (100 butcheries per group).
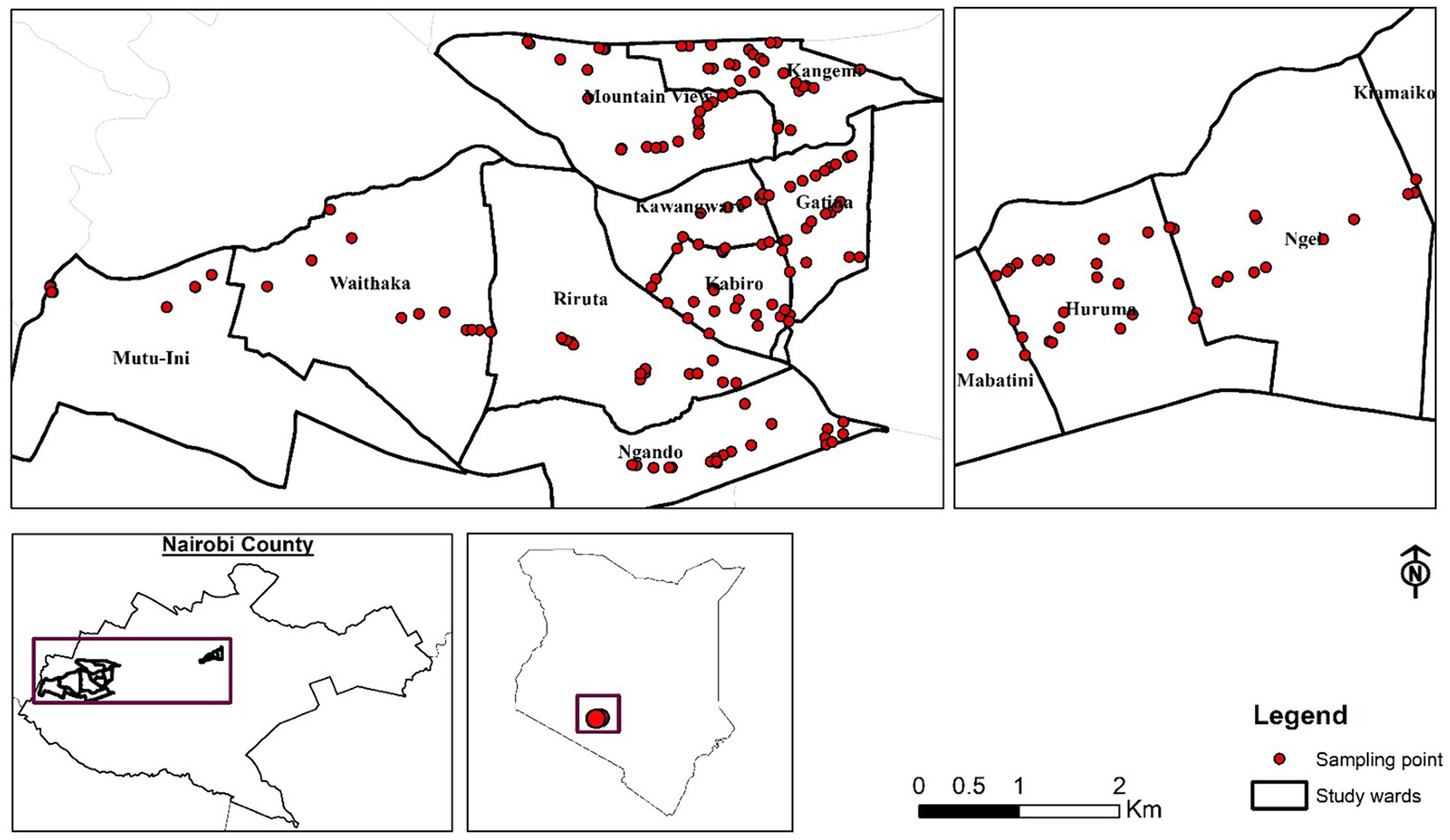
Figure 1. Spatial distribution of the selected 200 butcheries in the four study sites in Nairobi; Kawangware, Kangemi, Waithaka, and Huruma.
2.3 Sample collection and observation of meat handling practices
2.3.1 Meat sample collection
A 100 g of beef filet was purchased from each of the 200 butcher shops. The beef meat purchased came from the carcass that was being sold to customers at the time of sampling. The meat was selected from random parts of the carcass, at the attendant’s discretion. The attendant was further asked to cut the meat into smaller cubes of approximately 1 cm x 1 cm, a practice which is normally requested by customers, place it directly into a pre-labeled Ziplock bag, and transported on ice to the laboratory at the International Livestock Research Institute (ILRI) for processing on the same day. Each visit took between 30 and 45 min and included both the observations and collection of the sample. There was approximately 5 h between sample collection and arrival of the sample in the laboratory for immediate processing.
2.3.2 Observed meat handling practices
Additionally, a tool was developed based on the Codex Alimentarius Commission’s code of hygiene for meat (CAC, 2005) to document observed meat handling practices in each butcher shop, and key elements such as the cleanliness of the butchery, cleanliness of the butchery attendant, use of protective clothes, handling of money, presence of running water/ hand washing station, separation of offal from the meat being sold, whether ‘ready-to-eat food was also sold, cleanliness of the meat preparation surface, ambient temperature and humidity inside the butchery as measured using a hygrometer.
2.4 Total coliform and Escherichia coli counts
The meat was processed to evaluate the levels of E. coli and other coliforms using the 3M™ Petrifilm® E. coli/coliform count plate following the manufacturer’s instructions. Ten grams of the beef cubes were weighed ensuring that different surfaces of the meat were included and placed into a stomacher bag together with 90 mL of phosphate buffered saline and homogenized using a Stomacher® 400 circulator lab blender (United Kingdom) for 5 min at 300 rpm. This formed the 10−1 dilution, which was then further serially diluted to 10−4, giving four dilutions in total. One ml from each dilution was plated on the 3M™ Petrifilm® and incubated at 35°C for 24 h. No replicates were included. After incubation, the plates were evaluated according to the interpretation guidelines from the manufacturer. The colonies were counted and classified as either E. coli (blue colonies with bubbles) or total coliforms (blue and red colonies with and without bubbles; 3M Food Safety, 2017). From each Petrifilm with presumptive E. coli, one colony was selected and sub-cultured on MacConkey agar, and the bacterial species was confirmed by Matrix-Assisted Laser Desorption/Ionization-Time of Flight Mass spectrometry (MALDI-TOF MS) on the Bruker Biotype system (Bruker, Bremen, Germany).
2.5 Quantification of tetracycline and cefotaxime resistant Enterobacteriaceae
Twenty microliters from each dilution from 2.4 were spotted on MacConkey agar plates supplemented with either 8 mg/L tetracycline hydrochloride (TET; Carl ROTH, Karlsruhe Germany) or 0.25 mg/L cefotaxime (CTX; Sigma-Aldrich Darmstadt, Germany,) and incubated aerobically overnight. The concentrations chosen are above the epidemiological cut-off values for each antibiotic (CTX = 0.25 μg/mL and TET = 8 μg/mL).1 After overnight incubation, colonies were counted on each dilution and the colony-forming units (CFU/g) were calculated. A single reddish/pink colony from each plate (i.e., lactose fermenters such as E. coli, Klebsiella, Enterobacter and Citrobacter) was further sub-cultured on nutrient agar and stored at -80°C for bacterial species confirmation by MALDI-TOF MS. These were chosen because tetracycline is the most used antibiotic in livestock production (WOAH, 2022) thus was used as an indicator of resistance most likely originating from livestock. Cefotaxime, which is a critically important antibiotic in human health, is registered for use in livestock but is rarely used in Africa (WOAH, 2022). Moreover, 3rd generation cephalosporin resistant Enterobacteriaceae are identified as a critical priority group in the WHO priority pathogen list (WHO, 2024) hence, we investigated the presence of these clinically relevant bacteria in meat.
2.6 Statistical analysis
Descriptive statistics were used to analyze the detection of selected microbiological determinants, CFU counts, and frequencies of meat handling practices. A non-parametric test was used to compare the mean colony-forming units of different study sites and a pairwise comparison of the mean colony-forming units of the different sites was calculated using Dunn’s test. Statistical significance was determined using a p-value at the critical probability of p < 0.05.
3 Results
3.1 Distribution of sampled butcheries
Most of the participants were male (96.9%), aged between 20 to 30 years old (42.7%), had a secondary level of education (50%), and had >5 years working in meat selling (51.6%). Two hundred meat samples were collected which included 59 from Kawangware, 47 from Kangemi, 38 from Huruma, and 56 from Waithaka. Correspondingly, observation data were collected from all the butcheries, however, the data from one butchery was lost during the process of data cleaning.
3.2 Total coliform and Escherichia coli counts
Coliforms were present in 98% (n = 196) of samples; the counts ranged between 1.0 log10–8.0 log10 CFU/g, with a mean of 3.8 log10 CFU/g (Figure 2). E. coli were present in 78% (n = 156) of the samples, and the counts ranged between 1.0 log10–8.0 log10 CFU/g with a mean of 2.3 log10 CFU/g (Figure 3). According to Kenyan standards, 72% of the samples were contaminated with E. coli above the accepted level (>100 CFU/g). Eighty-four percent of the samples had mean total coliform (>316 coliform CFU/g) as the South African and European Commission cut offs, with variations between the four study areas. There were significant differences between the means of total coliform counts from the four study sites (p-value = 0.003). The mean total coliform CFU/g for samples between Kawangware and Waithaka was statistically different compared to those from Huruma and Kangemi, respectively (Figure 2). Likewise, there was a significant difference between the E. coli counts (p-value = 0.0003) from the different study sites. There was a significant difference between the mean E. coli CFU counts for Kawangware and Kangemi and between Huruma and Waithaka. Meat samples from Huruma had relatively higher contamination by E. coli with all the samples having counts above the mean (Figure 3). The meat samples from Waithaka had relatively low counts of both E. coli and other coliforms.
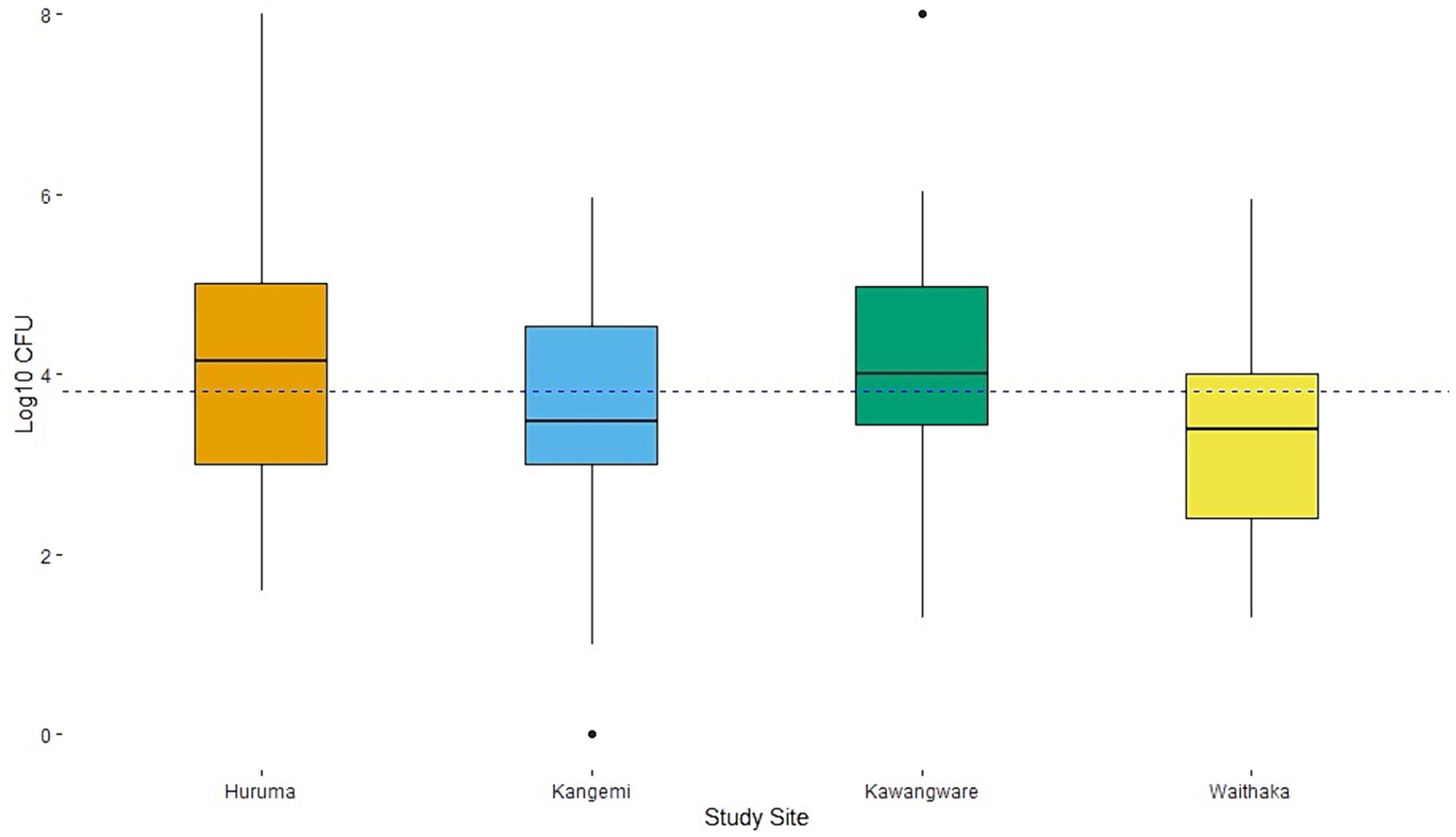
Figure 2. Total coliform counts (TCC) and distribution among the different study sites. The dotted line shows the overall mean of 3.8 log10 CFU/g and a standard deviation of 1.3. The acceptable threshold is 2.5 log10 CFU/g.
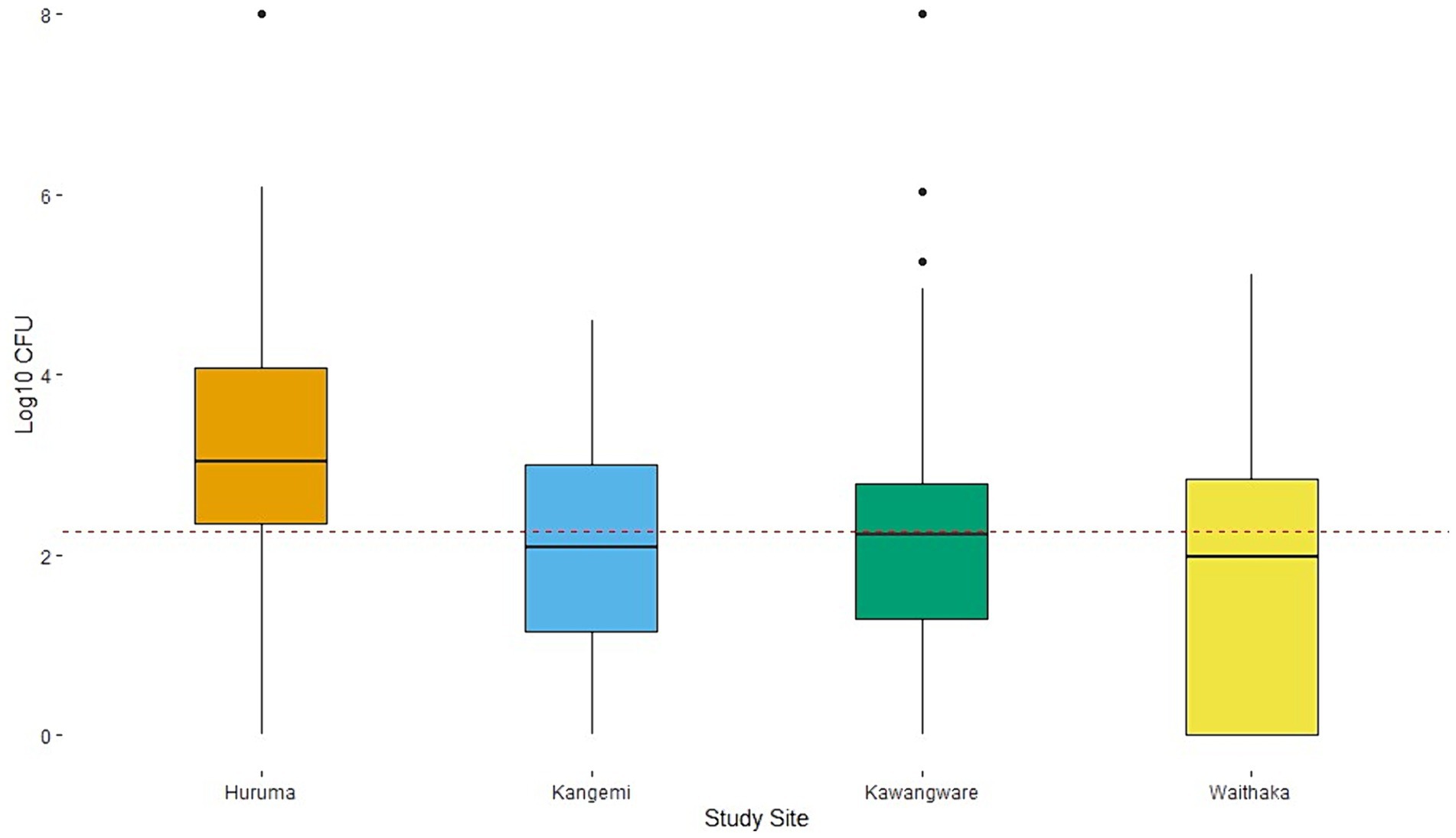
Figure 3. E. coli counts and distribution among the different study sites. The dotted line shows the overall mean of 2.3 log10 CFU/g and a standard deviation of 1.6. The acceptable threshold is 2 log100 CFU/g.
3.3 Quantification of TET-resistant and CTX-resistant Enterobacteriaceae
Of the 70 meat samples (35%) that had reddish-pink colonies on MacConkey agar plates containing TET, were identified as E. coli (51%), C. braakii (3%), C. freundii (34%), Moellerella wisconsensis (6%), Raoultella ornithinolytica (4%) and Lelliottia amnigena (2%). Nineteen (9.5%) meat samples had growth on CTX-containing MacConkey plates and were identified as E. coli (22%), A. veronii (6%), C. freundii (39%), Kluyvera cryocrescens (21%), Acinetobacter johnsonii (6%) and E. cloacae (6%). TET counts were between 1.0–4.8 log10 CFU/g while CTX counts were between 1.0–4.6 log10 CFU/g.
3.4 Meat handling practices
Forty-three percent of butcheries (n = 86) also sold in the same outlet, ready-to-eat meat, i.e., meat that has either boiled, fried or roasted. The mean temperature and humidity inside the butcher shops were 24 0c and 46%, respectively. Table 1 and Figures 4, 5 show the frequencies of the observed handling practices. Only 28% (56/199) of butcheries had hand-washing facilities, either a permanently placed water dispenser, e.g., a sink or a mobile water container, and half of these butcheries were found in Waithaka, while the remaining butcheries were scattered among the other three study sites. Seventy-five percent (n = 150) of the butcher attendants did not wash their hands before handling the raw meat and 85% (n = 170) did not wash their hands after handling the raw meat. Ninety-one percent handled money while selling meat simultaneously and neither washed nor wiped their hands after handling money. Almost all the butchery attendants did not wear gloves while handling meat, and 36% had either dirty hands (i.e., had visible dirt and/or meat debris) or long untrimmed nails. Fifty-eight percent of attendants did not use an apron while selling meat, 32% wore jewelry (such as rings, watches, and or bracelets) and only 39% covered their hair with a hair cover.
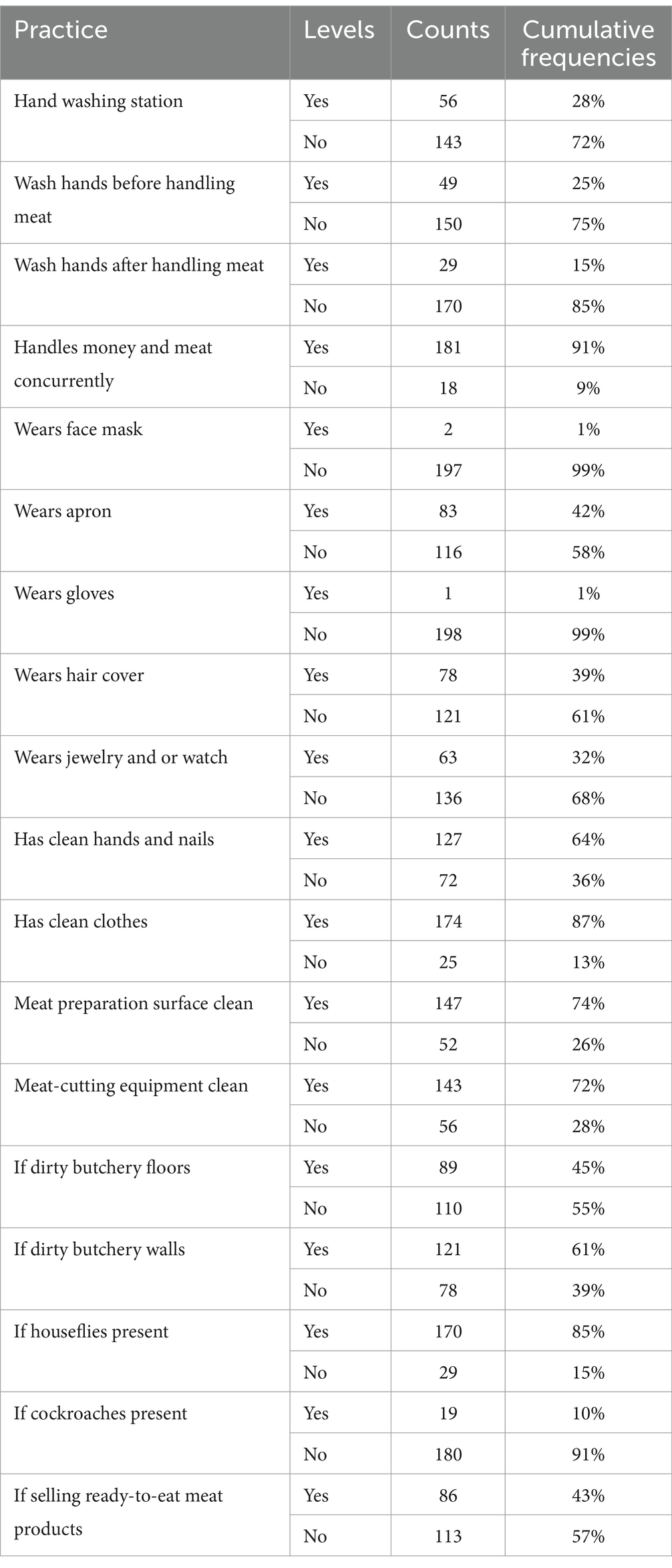
Table 1. Counts and cumulative frequencies of the observed meat-handling practices in peri-urban Nairobi butcheries.
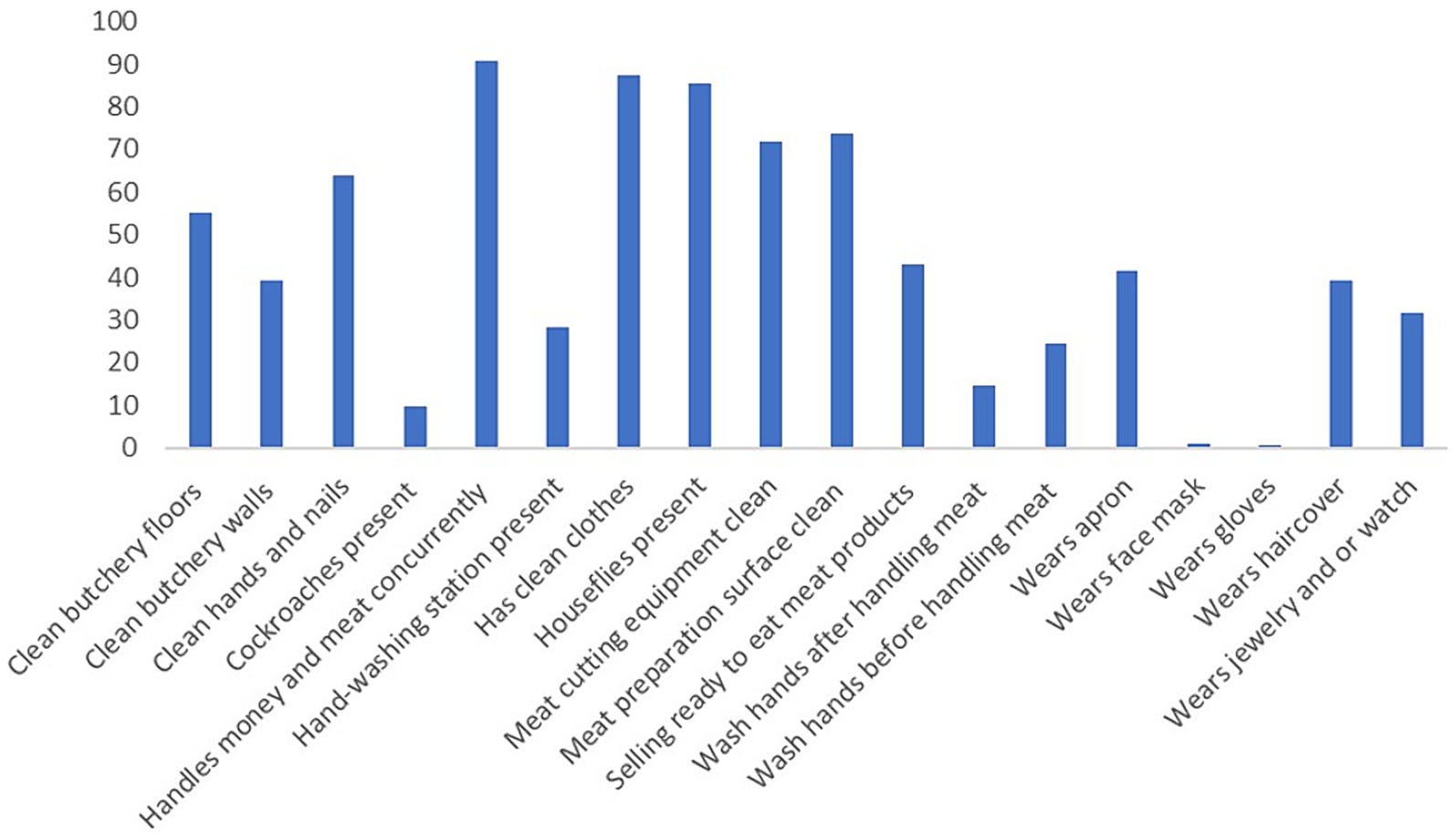
Figure 4. The percentage positive to the observed meat handling practice (n = 199) in Nairobi butcheries.

Figure 5. (A) Meat from different livestock species displayed together including offal, (B) storage of different meat types and offal in the same freezer space.
We observed flies inside the butcher shops and/ or on the meat surface in 170 (85%) of the butcheries. Moreover, live cockroaches were observed in 19 (10%) butcheries during the visit. More than half (55%) of butcheries had clean floors, i.e., free of dust and/or gross dirt while only 39% had clean walls, i.e., free of gross dirt and or blood. Most meat preparation surfaces (74%) were clean and free from blood, dust, and or meat debris. However, 26% (n = 52) of butcher shops had very dirty surfaces (i.e., blood and dried meat debris). In some butcheries, meat from different species was sold and displayed together, while in some, the meat types were mixed and placed in the same freezer together with offal (Figure 5). There was no significant association between any of the observed handling practices and microbial loads. Lastly, we observed that most of the butcheries in Kawangware were located along an open sewer line compared to the other study sites. And Huruma butcheries sourced their meat from multiple suppliers, combining them in their butcher shop.
4 Discussion
4.1 Discussion
In this study, we assessed the microbiological contamination of raw beef purchased from selected butcheries and observed the handling practices to identify the critical points of bacterial contamination. Butcher shops play a key role in the food chain and are critical control points to improve food safety and reduce the burden of foodborne diseases. Hygiene practices during the handling, storage, and sale of meat can influence the level of contamination of the meat (Wambui et al., 2017).
Out of the 200 meat samples, 196 (98%) were contaminated with bacteria at various levels. We found that 84% of meat samples had total coliform counts above the acceptable limit of 316 CFU/g (Commission Regulation, 2005; NDVQPH, 2010). Additionally, almost three quarters of our samples (72%) harbored E. coli at concentrations exceeding the acceptable levels (100 CFU/g) according to the Kenya Bureau of Standards (KEBS; KEBS, 2020). Meat above the acceptable microbiological limits for either coliforms or E. coli can have several negative consequences including foodborne diseases, especially if contaminated with known food-borne pathogens such as Salmonella, E. coli, Campylobacter, and Listeria. High bacterial loads accelerate meat spoilage and reduce shelf life resulting in economic losses to the butchery owners. The presence of E. coli and other coliforms in beef has been documented in previous studies in Kenya; E. coli loads found in this study were nearly the same as those found by Chepkemei et al. (2022) but higher than those isolated by Catherine et al. (2021). These findings are also like those of other studies where the bacteria found in meat exceeded acceptable levels (Jaja et al., 2018; Kassem et al., 2020; Zelalem et al., 2022).
Meat sold in Huruma and Kawangware was relatively more contaminated than other sites. It was observed next to the butcher shops in Kawangware, untreated sewage flowed openly along the streets. It is unclear if this directly had an impact on meat contamination but open sewage can increase vermin activity, and potentially can contaminate local water sources. If this water is used for washing meat, workers’ hands, shop surfaces or equipment, it can lead to contamination of meat with foodborne pathogens. Moreover, we frequently observed flies and cockroaches inside the butcher shops, which are known to carry a variety of pathogens and play a role in meat contamination. Studies have shown that flies can be vectors of foodborne pathogens such as E. coli and Salmonella, which can contaminate meat and cause food poisoning if consumed (Barreiro et al., 2013; Heilmann et al., 2017). Similar findings were found in other low- and middle-income areas of Nairobi (Kariuki, 2018; Maina et al., 2021). Lastly, beef from Huruma had relatively higher loads of E. coli as compared to the other sites. Here we noted that butcher shops sourced their meat from different abattoirs, which can affect contamination levels as it depends on factors such as hygiene practices of the abattoirs, transport conditions, and how meat is stored upon arrival. If meat from different abattoirs is mixed during storage, it can lead to cross-contamination (Rani et al., 2023).
Antibiotics are frequently used in livestock farms and antibiotic-resistant bacteria can be transferred to humans indirectly through ASFs. Between 9 and 35% of samples were contaminated with TET and CTX-resistant Enterobacteriaceae, respectively. We found more TET-resistant coliforms, which is expected as tetracycline is the most used antibiotic in animal production (WOAH, 2022; Mulchandani et al., 2023). Third generation cephalosporins are critically important antibiotics for humans and are also licensed for veterinary use (e.g., ceftiofur), but they are rarely used in animals in Africa (WOAH, 2022). The presence of CTX-resistant coliforms in meat is of clinical relevance, and could have originated from cattle or a result of human or environmental contamination during the slaughter, transport and storage processes (Mitman et al., 2022). Third generation cephalosporin resistant Enterobacterales is a critical group on the WHO global priority pathogens list of antibiotic-resistant bacteria as they can cause severe and often fatal infections (Shrivastava et al., 2018; WHO, 2024).
Close contact between the meat and the meat handler during retail operations highlights the importance of hand washing to prevent cross-contamination. Despite the existence of food safety regulations in Kenya, which include the requirement to have handwashing facilities (Kang’ethe et al., 2020; NFSP Draft, 2021; Sirma et al., 2023), most butcheries (72%) did not have hand-washing stations which could explain why most of the butcher attendants (85.4%) neither washed their hands before nor after handling raw meat.
Previous studies have shown various bacteria found under the fingernails of food handlers (Nel et al., 2004), reinforcing proper hand washing (Montville et al., 2002). We also noted that some meat handlers had visibly dirty hands and long, untrimmed fingernails, and did not wear gloves while handling meat. Mirembe (2002) found that hand-washing facilities were present in 76.7% of surveyed butcher shops in Uganda and Nepal showing that only 19% of the meat sellers washed their hands before and after handling meat (Bhattarai et al., 2017).
Personal protective equipment (PPE) including aprons, hairnets, and gloves is essential to prevent cross-contamination and the spread of pathogens while handling raw meat. Most attendants in our study did not wear PPE despite it being a requirement in Kenyan regulations (Government of Kenya, 2016). In addition, some attendants wore jewelry and watches while selling meat. Wearing jewelry during meat handling operations not only increases the presence of bacteria on hands but also reduces the effectiveness of hand washing (Lombar M et al., 2016). The proportion of butchery attendants who wore jewelry and who did not wear gloves while selling meat was comparable to the study by Siluma et al. (2023) and Azuamah et al. (2018). Moreover, handling money while selling meat using the same uncleaned hands was observed in most of the butcheries (91%) comparable to what (Chepkemoi et al., 2015; Subedi et al., 2022) found. Money is noxious for being contaminated with bacteria (Kuria et al., 2009) and concurrent handling of money and meat also poses a high risk of cross-contamination.
The Kenya Meat Control Act (chapter 256) stipulates that all equipment for preparing, handling, or storing meat should be kept clean (Government of Kenya, 2016). While most of the butcheries had clean equipment and meat preparation surfaces, in some butcher shops, meat was prepared on dirty surfaces, and others reused dirty equipment for cutting meat for subsequent customers. Meat preparation surfaces and equipment can harbor bacteria that can contaminate meat (Carrascosa et al., 2021). Therefore, ensuring the cleanliness of the meat preparation surfaces and the cutting equipment is key to improving meat microbial safety in butcheries.
Our study had some limitations such as: restricting sampling to beef filets only. Beef was found to be the dominant meat sold in butcher shops during the initial mapping exercise which was conducted before the study. We requested that the beef be cut into smaller pieces to mimic the usual consumer practice. However, butcher shops sold meat from other livestock species including offal, which were displayed side by side, and therefore contamination may have come from these other meat types. Offals can easily contaminated meat with Enterobacteriaceae from the gastrointestinal tract during slaughtering, dressing, evisceration and transportation and because of their nutritional content they promote the multiplication of these bacteria. Hence, selling of both offals and meat can give rise to cross-contamination in butcher shops especially if they are placed together or the same equipment is used (Fatena et al., 2013).
We found that 98% of the raw meat sold in peri-urban areas of Nairobi is contaminated with coliforms and E. coli above the accepted regulatory levels. We also observed poor meat handling practices among the butchery attendants which are known to affect the microbiological quality of meat. Despite the existence of food safety regulations, they appear to be poorly implemented. Public health authorities need to enforce the existing regulations to improve food safety in the beef supply chain.
Our study faced limitations. Initially, our sampling was confined to beef fillets, given that beef was the predominant meat available in the surveyed butcheries, as determined by the pre-study mapping exercise. Furthermore, the study specified cutting the meat into smaller pieces, mirroring customer practices. However, the display also featured meat from other species and offal alongside beef. The observed contamination might have originated from these additional meat types. Additionally, the potential sources of contamination include the cutting equipment, surfaces, water used in preparation, and the handlers’ hands.
Secondly, certain butcheries were observed storing leftover meat in the freezer overnight. Notably, some establishments practiced mixing different types of meat and offal during storage, potentially leading to cross-contamination. Limited resources also constrained the assessment of other potentially present bacteria. Finally, the antimicrobial susceptibility test was conducted to only two antibiotics, reflecting resource limitations.
4.2 Conclusion
This study found that most of the meat sold in peri-urban areas of Nairobi is contaminated with coliforms and E. coli above the accepted regulatory levels. The presence of cefotaxime-resistant Enterobacteriaceae albeit at low levels in raw meat, poses a threat to public health, as 3rd generation cephalosporins are critically important in human health. Poor meat handling practices were observed among the butchery attendants in peri-urban areas of Nairobi.
4.3 Recommendations
The appropriate authorities in the government enforce the application of Hazard analysis and critical control points (HACCP) principles along the beef supply chain. Regular basic and continuous meat hygiene training should be provided to the new and experienced meat handlers, respectively. More research should be done along the entire beef supply chain to determine the most likely sources of meat contamination.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by International Livestock Research Institute’s Research Ethics Committee (IREC), National Commission for Science, Technology, and Innovation (NACOSTI). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by International Livestock Research Institute’s Research Ethics Committee (IREC), National Commission for Science, Technology, and Innovation (NACOSTI), Faculty of Veterinary Medicine Biosafety, Animal Use and Ethics Committee, University of Nairobi. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
PK: Conceptualization, Writing – original draft, Visualization, Validation, Methodology, Investigation, Data curation. WO: Writing – review & editing, Investigation, Data curation, Conceptualization. LO: Writing – review & editing, Supervision. DG: Methodology, Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization. GG: Writing – review & editing, Supervision. LB: Writing – review & editing, Supervision. MK: Writing – review & editing, Software. FM: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization. AM: Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research study was funded by the German Federal Ministry for Economic Cooperation and Development (BMZ) through the One Health Research, Education, and Outreach Centre in Africa (OHRECA) led by ILRI.
Acknowledgments
We acknowledge CGIAR Fund Donors: https://www.cgiar.org/funders/. We also thank Nairobi County authorities and the study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Alarcon, P., Fèvre, E. M., Murungi, M. K., Muinde, P., Akoko, J., Dominguez-Salas, P., et al. (2017). Mapping of beef, sheep and goat food systems in Nairobi — a framework for policy making and the identification of structural vulnerabilities and deficiencies. Agric. Syst. 152, 1–17. doi: 10.1016/j.agsy.2016.12.005
Azuamah, Y., Amadi, A. N., and Iro, O. (2018). International journal of health sciences and research bacteriological qualities of red meat (beef) and meat hygiene practices among meat handlers in aba Metropolis. Nigeria. 8, 41–49.
Barreiro, C., Albano, H., Silva, J., and Teixeira, P. (2013). Role of flies as vectors of foodborne pathogens in rural areas. ISRN Microbiol. 2013, 1–7. doi: 10.1155/2013/718780
Bergevoet, R., and Van Engelen, A. (2014). The Kenyan meat sector; opportunities for Dutch agribusiness, 63. LEI.
Bhattarai, J., Badhu, A., Shah, T., and Niraula, S. (2017). Meat hygiene practices among meat sellers in Dharan municipality of eastern Nepal. Birat J. Health Sci. 2, 184–190. doi: 10.3126/bjhs.v2i2.18524
Carrascosa, C., Raheem, D., Ramos, F., Saraiva, A., and Raposo, A. (2021). Microbial biofilms in the food industry—a comprehensive review. Int. J. Environ. Res. Public Health 18, 1–31. doi: 10.3390/ijerph18042014
Catherine, K., David, E. K., and Grace, W. (2021). Meat quality status and postharvest handling practices along the meat value chain in Kenya. Afr. J. Food Sci. 15, 272–280. doi: 10.5897/ajfs2021.2084
Chepkemei, A., Mwaniki, J., Nyerere, A., and Kiiru, J. (2022). Phenotypic and genotypic characterisation of antibiotic resistance in Escherichia coli; Klebsiella spp., and Listeria monocytogenes; isolates from raw meat sold in Nairobi. Adv. Microbiol. 12, 603–620. doi: 10.4236/aim.2022.1211042
Chepkemoi, S., Lamuka, P. O., Abong, G. O., and Matofari, J. (2015). Sanitation and hygiene meat handling practices in small and medium Enterprise butcheries in Kenya -case study of Nairobi and Isiolo counties. Internet. J. Food Saf. 17, 64–74.
Commission Regulation (2005). No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 32, 1–26.
Dhama, K., Rajagunalan, S., Chakraborty, S., Verma, A. K., Kumar, A., Tiwari, R., et al. (2013). Food-borne pathogens of animal origin-diagnosis, prevention, control and their zoonotic significance: a review. Pak. J. Biol. Sci. 16, 1076–1085. doi: 10.3923/pjbs.2013.1076.1085
FAO (2019). The future of livestock in Kenya. Opportunities and challenges in the face of uncertainty. Licence: CC BY-NC-SA 3.0 IGO. Rome: Food and Agriculture Organization of the United Nations (FAO), 56 p.
Fatena, S. H., Amani, M. S., Mervat, S. H., and Gaafar, MH. (2013) Enterobacteriaceae in edible offal. Benha Vet. Med. J. 25:77–87.
Gathura, P. B., Obura, B., Muthuma, E., Mariach, N., Mkanga, B., Koigi, R., et al. Situational analysis of safety of animal-source foods, fruits and vegetables in Kenya. (2020).
Getenesh, T., Zerihun, A., and Abdi, K. (2020). Assessment of microbial quality status of raw beef around Addis Ababa city. Ethiopia. African J. Food Sci. 14, 209–214. doi: 10.5897/ajfs2019.1844
Heilmann, M., Roesel, K., Grace, D., Bauer, B., and Clausen, P. (2017). The impact of insecticide-treated material to reduce flies among pork outlets in Kampala, Uganda. Parasitol Res. 116, 1617–1626. doi: 10.1007/s00436-017-5450-x
Jabbar, M. A., and Grace, D. (2012). Regulations for safety of animal source foods in selected sub-Saharan African countries: current status and their implications. Prepared for The Safe Food, Fair Food Project International Livestock Research Institute, Nairobi, Kenya. doi: 10.22004/ag.econ.181867
Jaja, I. F., Green, E., and Muchenje, V. (2018). Aerobic mesophilic, coliform, Escherichia coli, and Staphylococcus aureus counts of raw meat from the formal and informal meat sectors in South Africa. Int. J. Environ. Res. Public Health 15, 1–13. doi: 10.3390/ijerph15040819
Kang’ethe, E, Mutua, F, Roesel, K, and Grace, D. National food safety architecture in Kenya. (2020).
Kariuki, (2018). Bacteriological safety of street food and factors associated with food contamination among street food vendors in Githurai and Gikomba markets. Kenya: Jomo Kenyatta University of Science and Technology, Emmah.
Kassem, I. I., Nasser, N. A., and Salibi, J. (2020). Prevalence and loads of fecal pollution indicators and the antibiotic resistance phenotypes of Escherichia coli in raw minced beef in Lebanon. Food Secur. 9, 1–13. doi: 10.3390/foods9111543
KEBS. Kenya standard meat grades and meat cuts-specification part 1: beef grades and cuts. Kenya Bureau of Standards. This is a statutory body setting National standards. (2020).
Kuria, J. K. N., Wahome, R. G., Jobalamin, M., and Kariuki, S. M. (2009). Profile of bacteria and fungi on money coins. East Afr. Med. J. 86, 151–155. doi: 10.4314/eamj.v86i4.46943
Li, M., Havelaar, A. H., Hoffmann, S., Hald, T., Kirk, M. D., Torgerson, P. R., et al. (2019). Global disease burden of pathogens in animal source foods, 2010. PLoS One 14. doi: 10.1371/journal.pone.0216545
Lombar M, M., NM, G., I, C., MA, L., and JI, A. (2016). Accessories of food handlers and restaurant staff as a source for food contamination. J. Food: Microbiol., Safety & Hygiene. 1, 1–5. doi: 10.4172/2476-2059.1000105
Maina, J., Ndung’u, P., Muigai, A., and Kiiru, J. (2021). Antimicrobial resistance profiles and genetic basis of resistance among non-fastidious gram-negative bacteria recovered from ready-to-eat foods in Kibera informal housing in Nairobi, Kenya. Access Microbiol. 3. doi: 10.1099/acmi.0.000236
Mitman, S. L., Amato, H. K., Saraiva-Garcia, C., Loayza, F., Salinas, L., Kurowski, K., et al. (2022). Risk factors for third-generation cephalosporin-resistant and extended-spectrum β-lactamase-producing Escherichia coli carriage in domestic animals of semirural parishes east of Quito, Ecuador. PLOS Global Public Health 2:e0000206. doi: 10.1371/journal.pgph.0000206
Montville, R., Chen, Y., and Schaffner, D. W. (2002). Risk assessment of hand washing efficacy using literature and experimental data. Int. J. Food Microbiol. 73, 305–313. doi: 10.1016/S0168-1605(01)00666-3
Mulchandani, R., Wang, Y., Gilbert, M., and Van Boeckel, T. P. (2023). Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Global Public Health. 3:e0001305. doi: 10.1371/journal.pgph.0001305
Murphy, S., and Allen, L. (2002). “Nutritional importance of animal source foods” in Animal source foods to improve micronutrient nutrition and human function in developing countries, Elsevier. 3927S–3931S.
NDVQPH (2010). Standard for the microbiological monitoring of meat, process hygiene and cleaning. Vpn/15/2010–01., 2–24.
Nel, S., Lues, J. F. R., Buys, E. M., and Venter, P. (2004). The personal and general hygiene practices in the deboning room of a high throughput red meat abattoir. Food Control 15, 571–578. doi: 10.1016/j.foodcont.2003.09.004
NFSP Draft. (2021) Kenya National Food Policy-2021. Kenyan Government. https://kilimo.go.ke/wp-content/uploads/2022/02/Draft-Food-Safety-Policy-2021.pdf
Owuor, S, Brown, A, and Wagner, J. (2017) The urban food system of Nairobi Kenya. Hungry Cities Partnership African Centre for Cities, University of Cape Town, South Africa, and Wilfrid Laurier University/Balsillie School of International Affairs, Waterloo, Canada.
Pal, M., Ayele, Y., Patel, A. S., and Dulo, F. (2018) Microbiological and hygienic quality of Meat and Meat Products. Beverage Food World, 45, 21–27.
Rani, Z. T., Mhlongo, L. C., and Hugo, A. (2023). Microbial profiles of meat at different stages of the distribution chain from the abattoir to retail outlets. Int. J. Environ. Res. Public Health 20, 1–12. doi: 10.3390/ijerph20031986
Shrivastava, S. R., Shrivastava, P. S., and Ramasamy, J. (2018). World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. JMS - J. Medical Society. 32, 76–77. doi: 10.4103/jms.jms_25_17
Siluma, B. J., Kgatla, E. T., Nethathe, B., and Ramashia, S. E. (2023). Evaluation of meat safety practices and hygiene among different butcheries and supermarkets in Vhembe District, Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 20:2230. doi: 10.3390/ijerph20032230
Sirma, A., Muthuma, E., Kariuki, J., Maina, A., Thaiya, J., and Njagi, O. (2023). Using a value chain framework for food safety assessment of edible offals in Nairobi, Kenya. Front Sustain Food Syst. 7:7. doi: 10.3389/fsufs.2023.1059058
Subedi, D., Bhattarai, T., and Acharya, D. R. (2022). Meat handling practices among retail meat shops in Dharan sub-Metropolitan City. Tribhuvan University J. Food Sci. Technol. 1, 1–8. doi: 10.3126/tujfst.v1i1.49930
Wambui, J., Karuri, E., Lamuka, P., and Matofari, J. (2017). Good hygiene practices among meat handlers in small and medium enterprise slaughterhouses in Kenya. Food Control 81, 34–39. doi: 10.1016/j.foodcont.2017.05.036
WHO. (2024) World Health Organization: Suggested citation “WHO Bacterial Priority Pathogens List, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. Geneva: World Health Organization.
Keywords: microbial contamination, E. coli , coliforms, food safety, LMIC
Citation: Koech PC, Ogutu WA, Ochieng L, Grace D, Gitao G, Bebora L, Korir M, Mutua F and Moodley A (2024) Evaluating microbiological safety and associated handling practices of butchery-sold meat in Nairobi, Kenya. Front. Sustain. Food Syst. 8:1386003. doi: 10.3389/fsufs.2024.1386003
Edited by:
Chunhong Yuan, Iwate University, JapanReviewed by:
Agnes Kilonzo-Nthenge, Tennessee State University, United StatesSaharuetai Jeamsripong, Chulalongkorn University, Thailand
Copyright © 2024 Koech, Ogutu, Ochieng, Grace, Gitao, Bebora, Korir, Mutua and Moodley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patricia Cherotich Koech, cGF0Y2hlcm90aWNoMjJAZ21haWwuY29t; Arshnee Moodley, YS5tb29kbGV5QGNnaWFyLm9yZw==; Florence Mutua, Zi5tdXR1YUBjZ2lhci5vcmc=
 Patricia Cherotich Koech
Patricia Cherotich Koech Winnie Aketch Ogutu
Winnie Aketch Ogutu Linnet Ochieng
Linnet Ochieng Delia Grace
Delia Grace George Gitao2
George Gitao2 Max Korir
Max Korir Florence Mutua
Florence Mutua Arshnee Moodley
Arshnee Moodley