
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst., 01 July 2024
Sec. Crop Biology and Sustainability
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1368423
This article is part of the Research TopicNovel Ways and Methodologies to Improve Crop YieldView all 20 articles
Introduction: Potatoes are one of the world’s most important agricultural crops, with potential for making a major contribution to global food security. This study shows how a biostimulant derived from a plant extract can improve potato crop yield and global food supply. Successful potato production currently requires significant levels of inputs including fertiliser, pesticides and irrigation, however non-microbial plant biostimulants or mixtures of biostimulants with synergistic actions, have the capacity to reduce inputs and improve the sustainability of intensive agriculture.
Methods: A complex biostimulant containing a number of flavonoids including protocatechuic acid, quercetin, chlorogenic acid, coumaroyl quinic acid and gentistic acid was tested against three potato varieties for its efficacy in improving plant growth characteristics and tuber production in controlled and field environments.
Results: In containers, complex biostimulant treatment enhanced photosynthetic ability, with elevated levels of chlorophyll, higher specific leaf areas and significantly larger leaf assimilation areas. Treatment also significantly increased tuber yield by an average of 33% in tuber weight across three potato varieties and shifted tuber production toward larger sized tubers. The biostimulant derived from flavonoids was also assessed in 6 commercial potato crops and consistently increased total yield (average 5.2%) and marketable yield, representing an increase in margins of UK£700 per hectare across the 6 crops. Similar increases in yield were seen when comparing chitted and unchitted seed potatoes and both types of seed responded positively to biostimulant application. Biostimulant treatment had no significant effects on tuber specific gravity, dry matter percentage and starch content, except at one location where these quality characteristics were higher in the control plants.
Conclusion: The flavonoid based complex biostimulant produced significant effects on potato yield and quality in both container experiments and in field trials indicating its potential for contributing to sustainable potato production.
Potato (Solanum tuberosum L.) is one of the most important agricultural crops, and its production makes a significant contribution to global food security. This is mainly due to its relative ease of production and high potential yields (Wijesinha-Bettoni and Mouillé, 2019). Potato cultivation occupies an area of 17.6 Mha producing 365 Mt of tubers, with 80% of the crop grown in Europe and Asia (FAO, 2021).
Potato growers and researchers have developed a range of methods to maximise yields and optimise quality parameters including marketable tuber size, shape, absence of defects, skin finish, flavour, colour, nutritional composition (e.g., vitamin C, protein) and the texture of cooked or processed tubers (Stark et al., 2020). Many of these tuber quality parameters (e.g., tuber texture and colour of cooked and processed potato products) are affected by starch, sugar, phenolics and vitamin C content, so the ability to manage potato physiology and metabolism (e.g., carbohydrate and nitrogen metabolism) is key to managing crop condition.
Abiotic stress, in particular drought, salinity and extreme temperatures, is the primary cause of crop loss worldwide, reducing average yields for most major crop plants by more than 50% (Wang et al., 2003). These abiotic stresses are expected to have an increasingly negative impact worldwide, posing serious concerns for crop productivity, and thus food security. To help overcome this, the application of non-microbial and microbial biostimulants has been highlighted as one of the most promising and efficient drivers for sustaining crop yields (Rouphael et al., 2018; Rouphael and Colla, 2018). Two of the main factors which influence potato yield and quality are the supply and availability of soil nutrients (Naumann et al., 2020). Increasing soil nutrient availability and nutrient use efficiency is therefore fundamental in sustainable potato production, helping avoid an excessive use of external inputs (Guzman, 2018) without compromising crop performance. As with many other crops, evidence is growing to support the view that plant biostimulants can play a significant role in enhancing potato production (Caradonia et al., 2022).
In 2022 EC fertilising products Regulation (EU) 2019/1009 was implemented, providing a standard framework for regulating microbial and non-microbial biostimulants in Europe. The regulation uses a functional definition of plant biostimulants, i.e., materials able to stimulate plant nutrition processes (regardless of the nutrient content), with the aim of improving nutrient use efficiency, tolerance to abiotic stress, quality traits and the availability of confined nutrients in the soil or rhizosphere. Non-microbial plant biostimulants include humic and fulvic acids (HFA), animal and vegetal protein hydrolysates (PHs), chitosan (Chi), seaweed extracts (SWE), plant extracts (PE) and silicon (Si) whereas microbial plant biostimulants consist of fungal and bacterial strains (Caradonia et al., 2019). Notably, plant biostimulants do not include actives which act as plant protection products (e.g., fungicides, insecticides, nematicides).
When growing crops farmers must have a profitable and sustainable production system in place. Therefore, it is important to deliver an economic gain when adding additional inputs such as biostimulants. This can be realised in many different forms, such as reduced use of fertilisers and other agrochemical inputs, more efficient use of water, more efficient land use, increased crop yield, improved crop quality, better disease tolerance, faster recovery from stressful events, shorter time to harvest and market, greater homogeneity of germination and crop, and improved processing efficiencies or a combination of these.
Li et al. (2022) presented a meta-analysis of biostimulant yield effectiveness in field trials. This demonstrated that crop responses to biostimulant application depended on the biostimulant type, application method, crop category, soil properties and climate. In general, the greatest responses were observed after application of biostimulants based on plant extracts and in crops growing in sub-optimal conditions.
Several biostimulants can be combined to produce more complex biostimulant formulations which may elicit a synergistic response in the target crop (Rouphael and Colla, 2018; Asif et al., 2023). These can be non-microbial or microbial combinations and may include mixtures of single source biostimulants along with bioactive compounds from other sources. In the case of non-microbial/microbial mixtures, care must be taken to ensure that the microbes remain viable when exposed to varying concentrations of the other complex biostimulant components. Several biostimulants can be combined to produce more complex biostimulant formulations which may elicit a synergistic response in the target crop (Rouphael and Colla, 2018; Asif et al., 2023). These can be non-microbial or microbial combinations and may include mixtures of single source biostimulants along with bioactive compounds from other sources. In the case of non-microbial/microbial mixtures, care must be taken to ensure that the microbes remain viable when exposed to varying concentrations of the other complex biostimulant components. Campobenedetto et al. (2021) discussed the use of complex combinations of bioactive components and their effects on root architecture and salinity tolerance in tomato plants. They recognised that biostimulant composition can range from single compounds to complex combinations.
Wise et al. (2024) gained further insights into the actions of complex biostimulants and their individual components when they investigated the effects of a biostimulant complex (BC) on cannabis root development, root architecture and nutrient utilisation.
Flavonoids, the most abundant polyphenols produced by plants, are products of secondary metabolism and participate in multiple metabolic processes. Flavonoids control major features of the plant phenotype through actions such as modulation of protein activity (acting as transcription regulators), influencing the plant auxin/cytokinin balance and determining whether apical or lateral growth predominates. Many plant flavonoids exhibit antioxidant activity, protecting plant tissues from damage by reactive oxygen species (ROS) or oxidative distress. Other flavonoids act as allelo-chemicals, participating in plant defence against microbial infection, herbivorous insects and nematodes. These allelochemicals can also influence plant to plant interactions, impacting on the establishment and growth of neighbouring plants and reducing competition (Kong et al., 2019). Over 4,000 different plant flavonoid molecules have been identified and many have the potential to be highly active plant biostimulants.
Much of the published research regarding flavonoid use has been conducted to induce plant defence systems under unfavourable biotic and abiotic conditions (Nakabayashi et al., 2014; Davies et al., 2018). Significantly, Shah et al. (2022), investigating the biostimulant effects of a flavonoid extract, found that foliar applications could improve soybean (Glycine max) and canola (Brassica napus) growth.
The biostimulant MX42SEK (Maxstim Ltd., Elstead, Surrey, UK) is a plant extract rich in flavonoids and containing vegetal protein hydrolysates, organic acids, plant derived amino acids and seaweed (Ascophyllum nodosum) extract. Tests of this complex biostimulant have shown positive responses in a wide range of plants. Here we report on the response of potato (Solanum tuberosum) to MX42SEK in a series of container-based tests and commercial field trials.
A liquid suspension biostimulant, MX42SEK, comprising of bioflavonoids including protocatechuic acid, quercetin, chlorogenic acid, coumaroyl quinic acid and gentistic acid, seaweed extract, plant based amino acids, carbohydrates and trace elements was supplied by Maxstim Ltd., Elstead, Surrey, UK.
During 2022, the response of 3 potato varieties (Maris Piper, Navan, Lady Rosetta) to MX42SEK was assessed in a replicated (x5) container experiment. Each replicate comprised 3 untreated control plants and 3 treated with MX42SEK. Tubers (Maris Piper 40–50 mm diameter) were planted in 30 litre containers containing a 70:30 v/v compost/sand mixed with 45 g of OSMOCOTE Pro TE 3-4Ms (17:11:10). Plants were grown in fully randomised blocks in the open in full sunlight and watered as required. No fungicides were applied during the experiment.
In treated containers, 20 mL of 5% (w/w) MX42SEK was applied to the soil surface as a root drench at planting, with further applications at 20 days (full emergence of shoots), 40 days and 72 days post planting. Control plants were sprayed with 20 mL water on these dates.
80 days after first emergence, one replicate from each treatment was selected and leaf chlorophyll levels (Soil Plant Analysis Development - SPAD), leaf assimilation area and specific leaf area measured (Wadas and Kalinowski, 2017). The chlorophyll content was estimated using a portable chlorophyll metre SPAD-502 (Minolta, Osaka, Japan). The leaf greenness index (SPAD) reading provides a good estimate of the chlorophyll content of the leaf blade and are highly correlated (r = 0.97) with the analytical measurements of the chlorophyll content (Vos and Bom, 1993). The measurements were made on the youngest fully expanded leaf (i.e., the fourth or fifth leaf from the top).
134 days after planting when haulms in the remaining plants had fully senesced, tubers were collected, washed, size graded, counted and weighed.
The effect of MX42SEK on potato (variety Maris Piper) was assessed in 6 commercial potato crops at 5 locations in the United Kingdom during the 2022 season (details of locations, soil types, agronomic information including planting and harvest dates are shown in Table 1). Field preparation, pre-plant fertilisation, planting (26,000 seed tubers per ha) and all subsequent agronomic procedures were performed by the growers following their standard farm practises. The trials at one location included a comparison of chitted and unchitted seed potatoes. In order to encourage rapid sprout emergence after planting, seed potatoes are commonly physiologically aged {chitted) by incubating tubers under carefully managed temperature and light conditions (Dines, 2021). The use of chitted seed potatoes can be used to achieve an early harvest.
Biostimulant was applied by each grower following the schedule outlined in Table 2. Four applications of 10 litres ha−1 MX42SEK were applied in 200 litres water during the season using 36 m boom sprayers and flat fan nozzles with a double overlap spray pattern. The following treatments were applied: The first treatment was applied to the seed potatoes immediately after planting; the second treatment was applied to the plants after 20–30 cm full emergence; the third treatment was applied when the adjacent canopies met in the middle; and the fourth application went on the crop at flowering.
For assessment, in each crop, a randomised block experimental design (6 replicate plots of 20m2 per treatment) was used in each of the crops and with plots chosen using a randomiser (Haahr, 2023). At harvest, tubers in the assessment plots were collected and removed for washing, size grading, counting and weighing.
For each size grade, a Weltech Digital Hydrometer (Pot/Hyd-PW4) was used to calculate the dry matter content, starch percentage, underwater weight and specific gravity of the harvested potatoes using the weight in water method (Kleinkopf et al., 1987; United States Department of Agriculture, 1997; Yildrim and Tokusoglu, 2005; Mohammed, 2016).
Two-way ANOVA followed by Šídák’s multiple comparisons test was performed using GraphPad Prism version 10.1.0 for Windows, GraphPad Software, Boston, Massachusetts USA, www.graphpad.com (RRID:SCR_002798).
The biostimulant treatment significantly increased tuber yield (average 33% in tuber weight) in all 3 potato varieties tested – [ANOVA analysis Variety F (2, 18) = 100.1, p < 0.001]. Biostimulant [F (1, 18) = 83.25, p < 0.001]. Maris Piper exhibited the largest yield increase and Lady Rosetta the lowest yield increase (Figure 1). The biostimulant treatment also increased average tuber number per plant by 10.4% [Variety F (2, 18) = 66.95, p < 0.001; Biostimulant F (1, 18) = 6.18, p = 0.023; Interaction F (2, 18) = 0.599, p = 0.560] (Figure 2).
In all three varieties, based on both tuber weight and tuber number, tuber size shifted toward the larger grades when plants were treated with the biostimulant (Tables 3, 4). This was most pronounced in sizes 45–65 mm and 65–85 mm which comprise the marketable grades for most potato growers (Table 3).
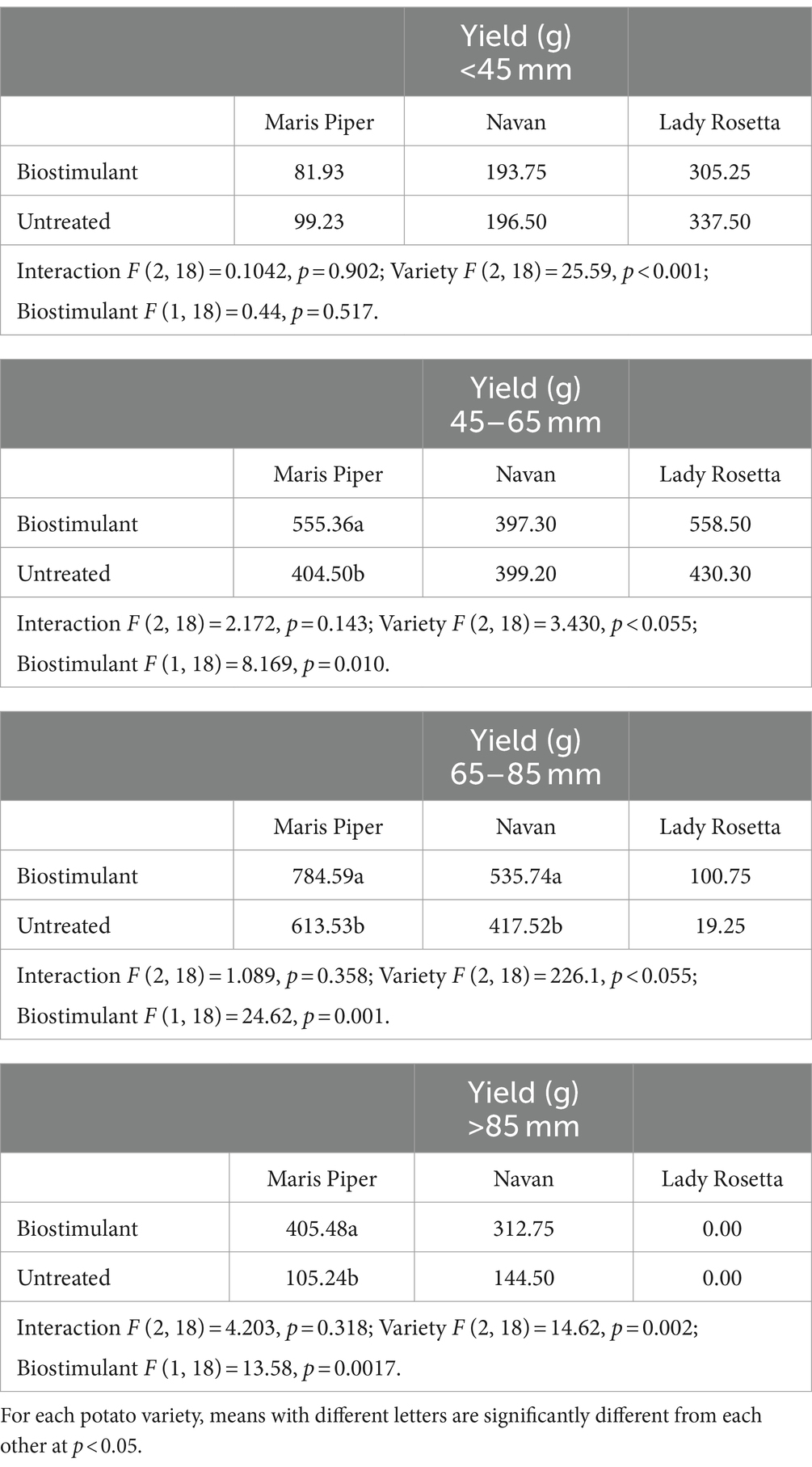
Table 3. Mean yield (g) per plant for different tuber size classes in three potato varieties treated with biostimulant (MX42SEK).
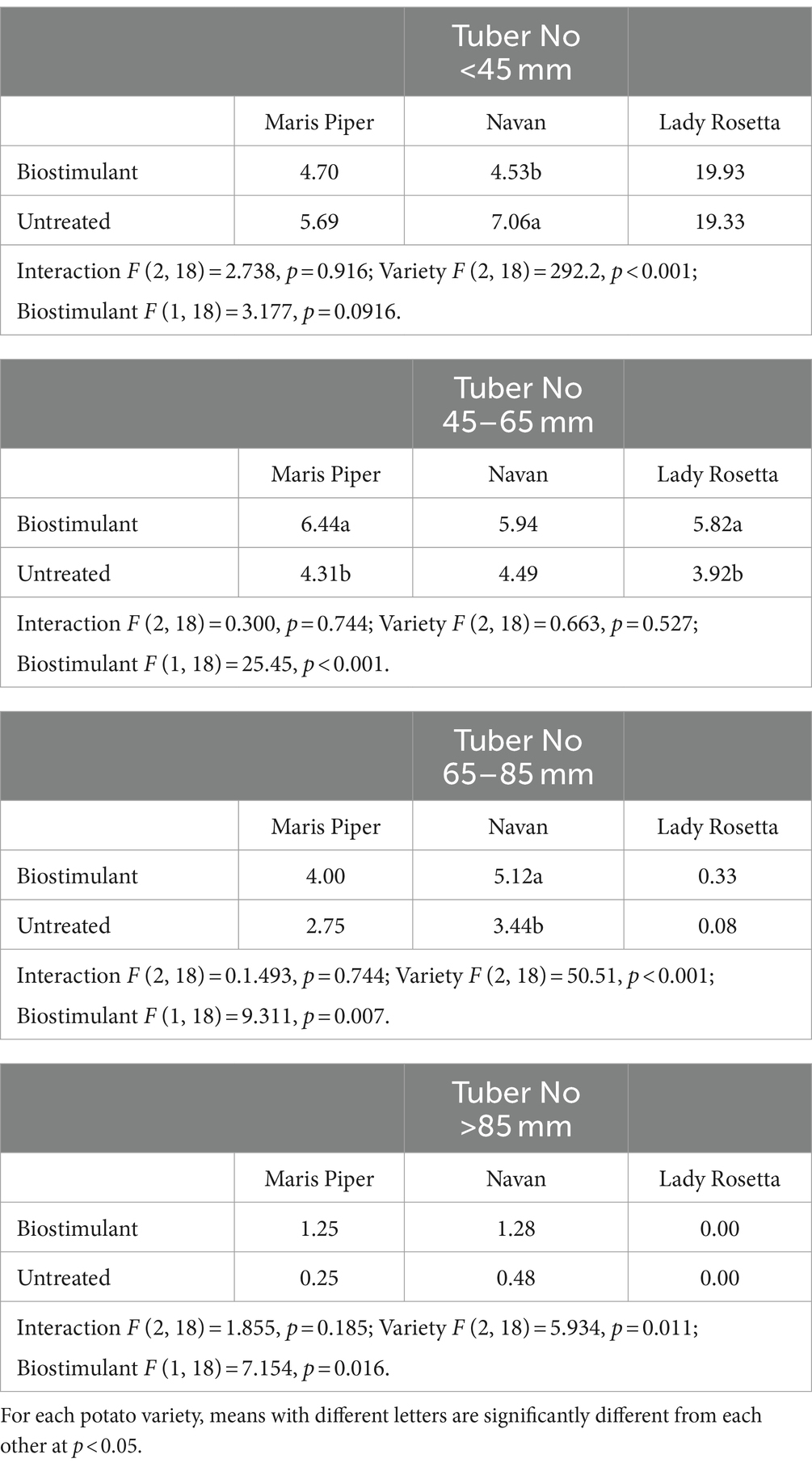
Table 4. Tuber number per plant for different size classes in three potato varieties treated with biostimulant (MX42SEK).
Leaf analyses provided an indication of some of the physiological effects of biostimulant treatment. Mean chlorophyll levels (SPAD) were higher (average 4%) in all 3 varieties, with a p = 0.055, just outside statistical significance (Table 5).
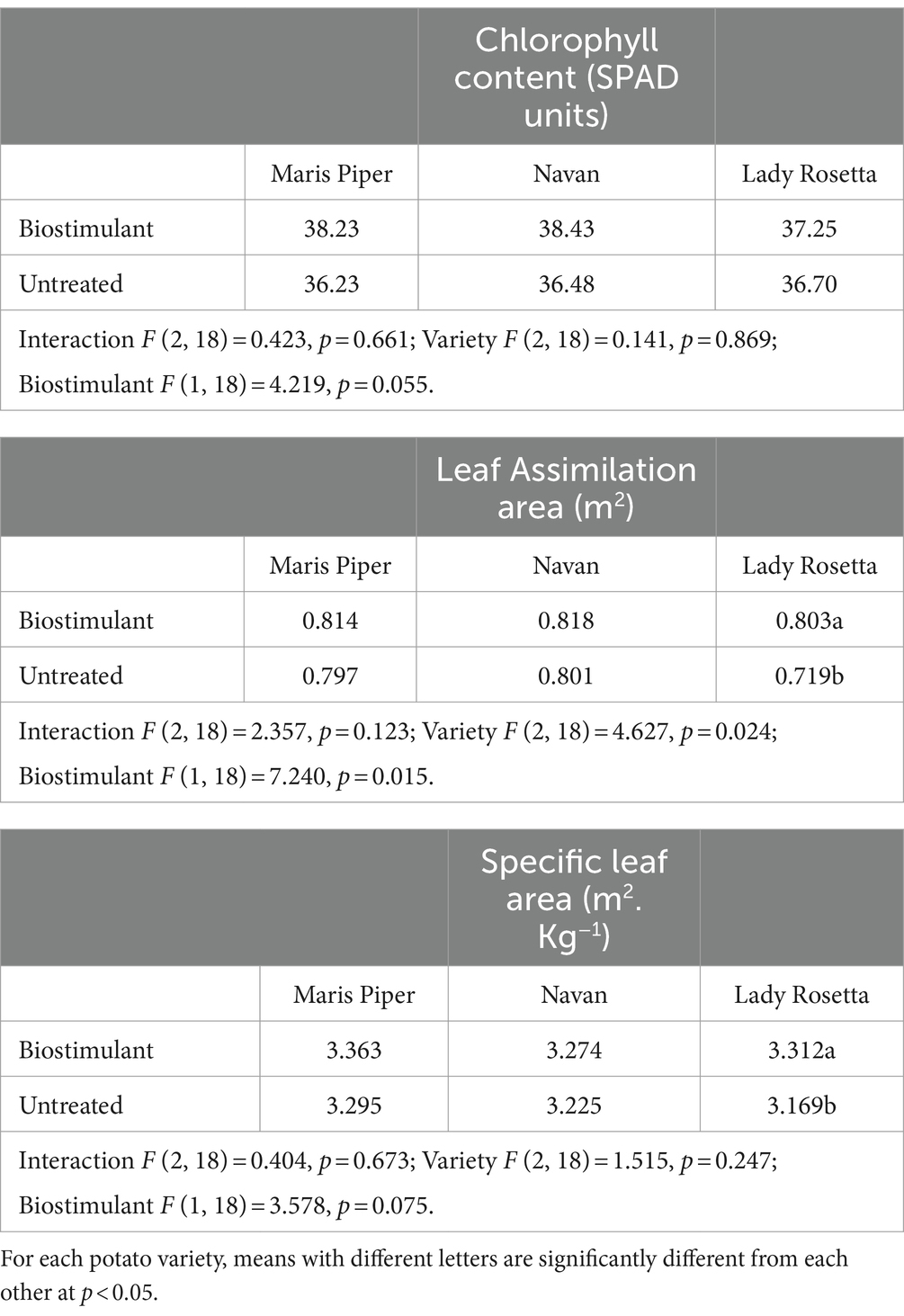
Table 5. Effects of biostimulant treatment on chlorophyll levels (SPAD), leaf assimilation area and specific leaf area in three potato varieties.
Similarly, mean leaf assimilation area and mean specific leaf area were both higher in biostimulant treated plants for the varieties tested. This increase was statistically significant for leaf assimilation area (p = 0.015).
At all six commercial Maris Piper potato trial locations, potato yields between 56 and 79 t/ha were observed. There were significant differences in between locations with the highest yield recorded at Lodge and the lowest at Ramsey. The crops showed a consistent response to biostimulant treatment, with total yields increasing by an average of 5.2% (Figure 3). A positive response to biostimulant treatment was also apparent in the marketable yield (45–85 mm grades) at all the locations (Figure 4). The trials at the Danehill LO6 location tested crops planted with chitted and unchitted seed potatoes. No significant differences in total yield or marketable yield were found between crops planted with chitted and unchitted Maris Piper seed, with or without biostimulant.
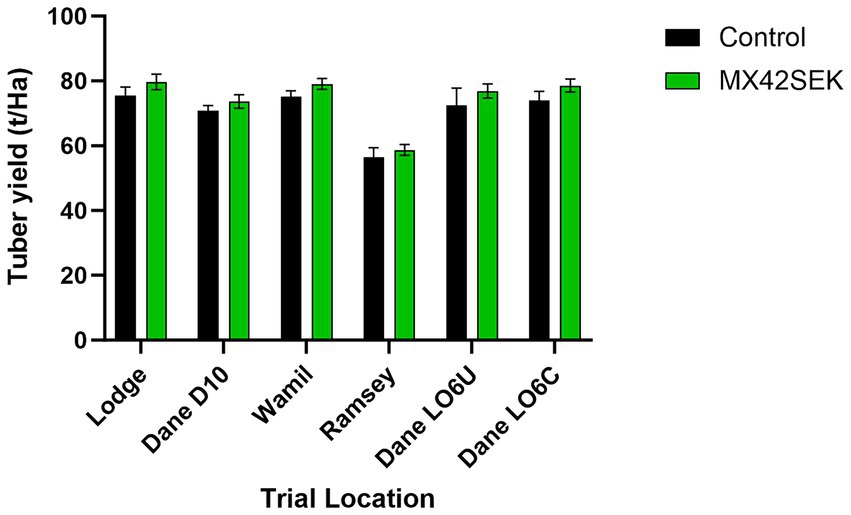
Figure 3. Effects of MX42SEK biostimulant on total tuber yield in six commercial Maris Piper potato crops. Interaction F (5, 60) = 0.069, p = 0.996; Trial F (5, 60) = 17.09, p < 0.001; Biostimulant F (1, 60) = 6.010, p = 0.0172.
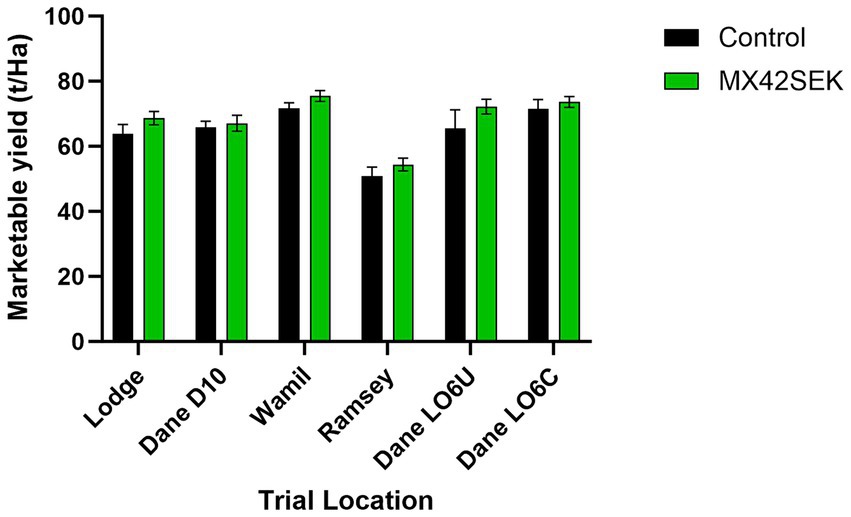
Figure 4. Effects of MX42SEK biostimulant on marketable tuber yield in six commercial Maris Piper potato crops. Interaction F (5, 60) = 0.264, p = 0.931; Trial F (5, 60) = 15.68, p < 0.001; Biostimulant F (1, 60) = 5.613, p = 0.0211.
Mean tuber weight was higher in biostimulant treated plots in 5 out of 6 crops but differences were not statistically significant. Lowest mean tuber weights were found at the Ramsey site (Table 6). There was no evidence of a significant biostimulant effect on tuber size distribution in the commercial crops (Tables 6, 7).
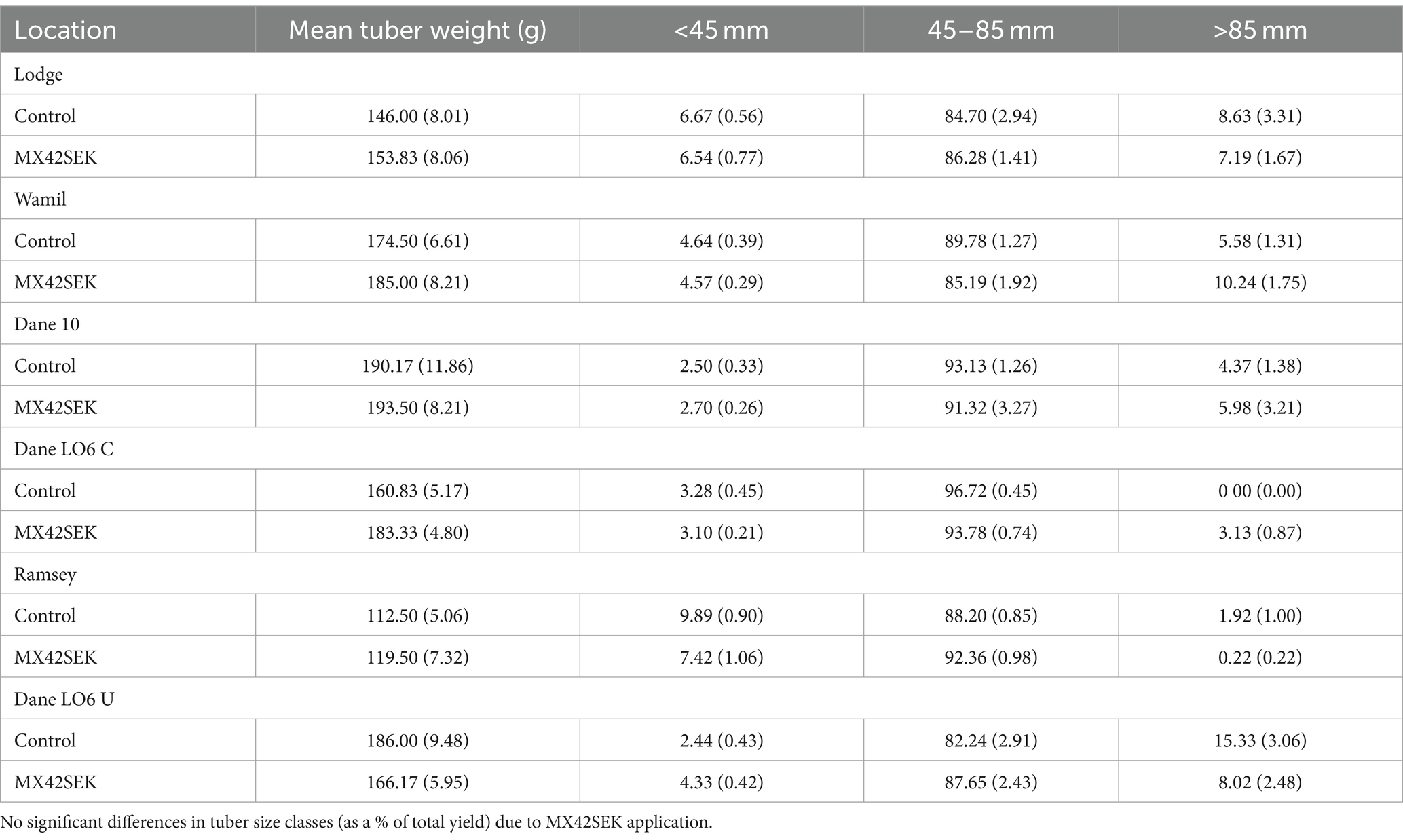
Table 6. Mean tuber weight and mean tuber yields (as a % of total yield) for different tuber size classes for biostimulant treated and untreated tubers from six commercial potato crops (standard error in brackets).
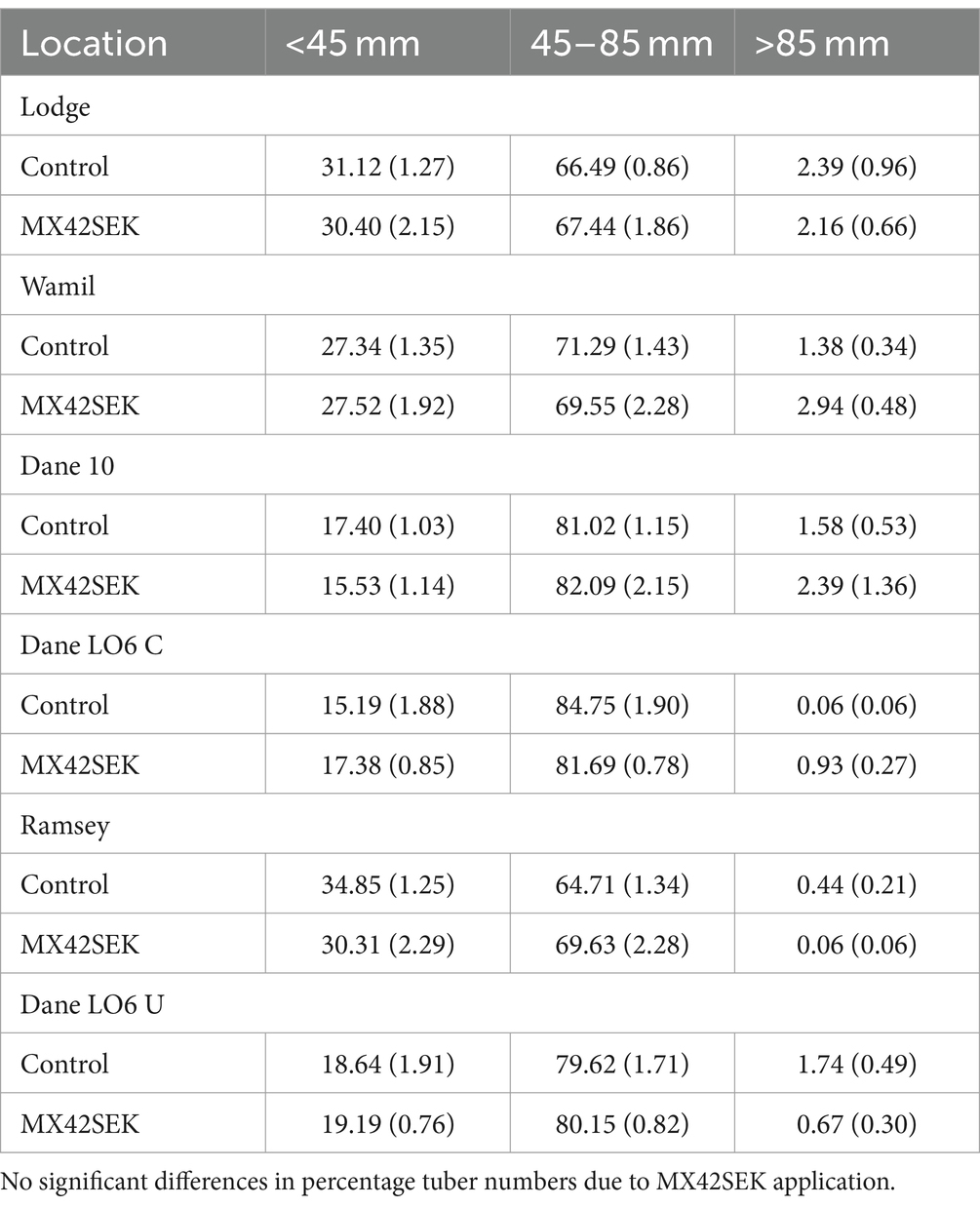
Table 7. Mean percentage (with standard error) tuber number in different size classes for biostimulant treated and untreated tubers from six commercial potato crops.
At each of the six locations, levels for specific gravity, dry matter and starch content in harvested tubers were within the normal range for Maris Piper. Comparing biostimulant treated and untreated potatoes there were no significant differences (p = 0.05) in specific gravity, dry matter and starch content at 5 of the locations (Table 8). At a single site, Ramsey, untreated tubers had significantly higher specific gravity, dry matter percentage and starch content.
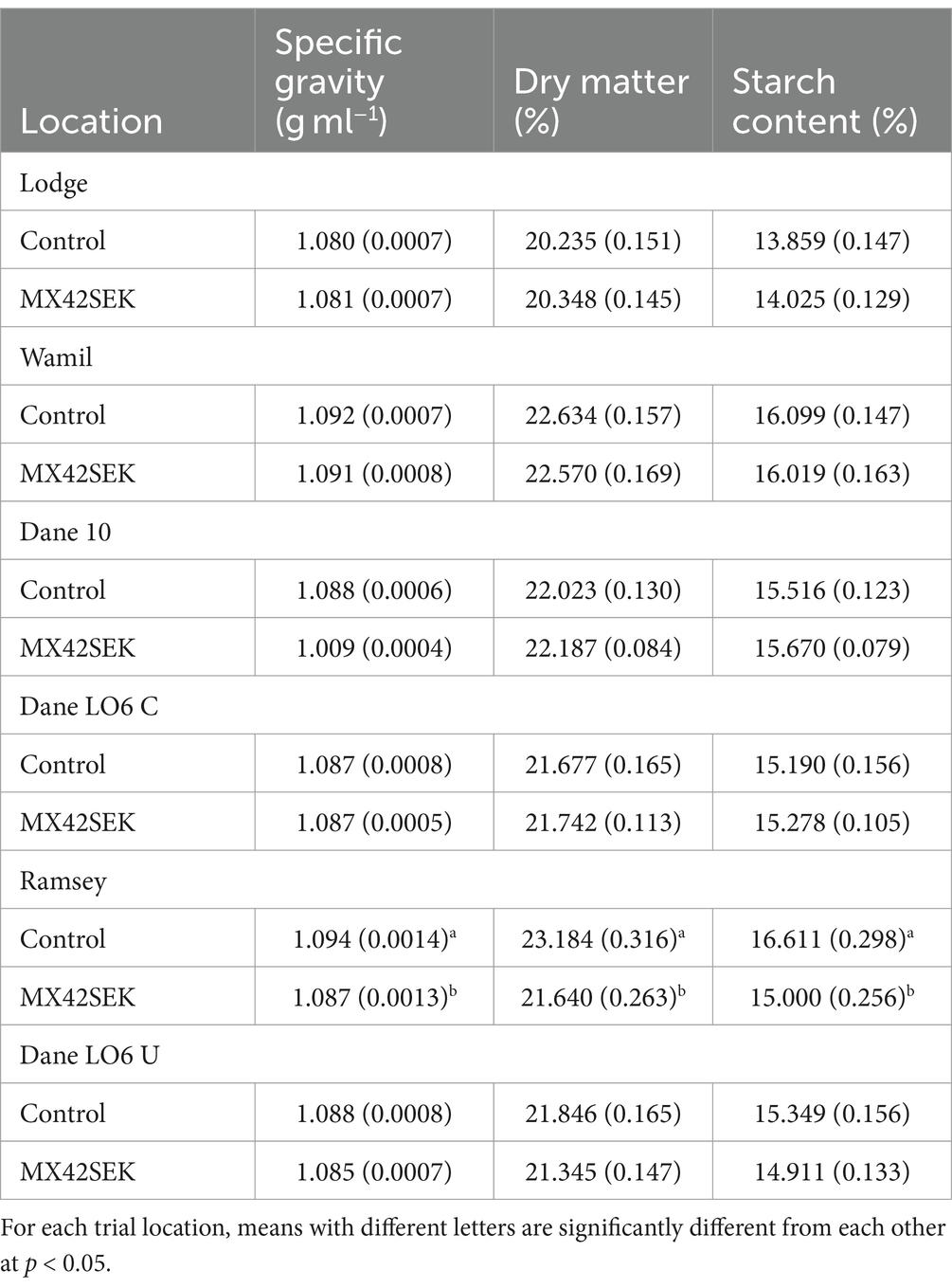
Table 8. Mean (with standard error) specific gravity, starch content and dry matter values for biostimulant treated and untreated tubers from six commercial potato crops.
Whilst previous studies Colla et al. (2017), Savvas and Ntatsi (2015), Sible et al. (2021) have examined the effects of biostimulants such as humic acid, hydrolysed proteins/amino acids, silicon and microalgae/seaweed extracts, the results presented here have demonstrated that treatment with MX42SEK, a flavonoid based complex biostimulant, can induce significant effects in the growth of early and maincrop potatoes, in both container experiments and in commercially grown field crops.
Meyling and Bodlaender (1981) examined the interactions between growth, development and tuber production across 6 potato varieties. They noted that variation in yields were associated with genetic differences affecting the rate of stem growth and development, as well as in photosynthetic characteristics such as leaf efficiency and leaf area. Growth was also strongly influenced by physiological differences, for example “earliness” of a particular potato variety, and by abiotic factors such as nitrogen availability and weather. Many of these yield and growth characteristics can also be influenced by plant biostimulants (Sanli et al., 2013; Sible et al., 2021). In addition, Caradonia et al. (2022), noted in their review that quality factors such as tuber size, specific gravity, dry matter, protein levels, vitamin C and starch content also respond to a wide variety of biostimulant treatments, including Humic substances, seaweed extracts, amino acids, hydrolysed proteins and microorganisms.
The container experiments in this study were designed to assess the response of 3 potato varieties to the application of MX42SEK. Maris Piper is a medium yielding maincrop variety with low to medium resistance to drought stress. Navan is a medium yielding maincrop variety (bred from Maris Piper) with good drought tolerance, whilst Lady Rosetta is a moderately early crisping variety which delivers medium yields and shows good drought tolerance.
The container trials showed that MX42SEK increased tuber yield per plant by 33% across the 3 varieties. Varietal differences were apparent, with the response strongest in Maris Piper which showed a 50% increase in yield. Despite its close genetic affinity with Maris Piper, the yield response in Navan was more similar to Lady Rosetta. Głosek-Sobieraj et al. (2018) also noted varietal differences in the response to seaweed extracts based biostimulants. In the present study the three varieties also showed an increased tuber number per plant which in part contributed to the yield increases. Yield increases in all 3 varieties were also helped by a shift toward larger and more commercially desirable tuber sizes (Zarzyńska and Boguszewska-Mańkowska, 2024). This contrasts with the investigation of Röder et al. (2018) who showed that tuber yield gains after a foliar spray containing sugarcane fermented broth [with 30% (w/v) of the amino acid L-glutamic acid] occurred due to the better growth of tubers, not due to an increase in the number of tubers.
The factors and metabolism involved in tuber production are complex (Fernie and Willmitzer, 2001; Kloosterman et al., 2005). For example, tuber initiation usually occurs over a short 2 week period, and is directed by environmental factors such as photoperiod, temperature, soil moisture and nitrogen availability, with environment and plant interactions affected by planting period and climate (Thornton, 2020). Plant hormones are also involved in tuber initiation. Gibberellic acid (GA) is a growth promoter, and at high levels in the plant it promotes shoot growth whilst delaying tuber growth. Abscisic acid (ABA), is a growth inhibitor that will slow shoot growth whilst promoting tuber growth. A high GA/ ABA ratio favours shoot growth and delays tuber growth. A low GA/ABA ratio will promote tuber growth (Thornton, 2020). Potato tuber bulking is also affected by plant hormones, as well as the metabolic pathways controlling cell division and expansion, and carbohydrate and amino acid metabolism (Aksenova et al., 2012; Augustin et al., 2012). Biostimulants will influence many of these pathways therefore predicting how a particular potato genotype will respond to a biostimulant applied at a specific time and within a particular environment is difficult.
The capacity of a potato plant to photosynthesize is a major factor in driving vegetative growth and tuber production. Tuber bulking is the process of dry matter or biomass accumulation in tubers resulting from the translocation of carbohydrates produced by photosynthesis in the leaves. Thornton (2020) noted that over 90% of the dry matter in tubers (i.e., yield) at harvest is directly tied to the output from photosynthesis during this growth.
Leaf efficiency, leaf area, and the leaf area duration (the length of time the leaves are photosynthesising) are parameters which influence plant productivity (Meyling and Bodlaender, 1981). Consequently, evaluating photosynthetic capacity can be an effective tool for assessing plant productivity. This can be achieved by measuring the SPAD value (Soil Plant Analysis Development, Konica Minolta Optics) which determines the relative chlorophyll content in plant leaves. However the SPAD value does not always imply a higher potato yield (Su et al., 2007). They do give a reliable indication of chlorophyll levels and plant nutrient status, especially that of nitrogen (Chen et al., 2018). Plants treated with MX42SEK consistently displayed elevated levels of chlorophyll, as well as larger leaf assimilation and specific leaf areas. The increases were statistically significant for leaf assimilation area and differences were larger in the early cropping variety (Lady Rosetta), which senesced well before the 2 maincrop varieties. The increase in photosynthetic ability and potato plant growth are consistent with the effects of MX42SEK observed on other dicotyledonous and monocotyledonous plants (R. Salvage PhD unpublished) and confirm the efficacy of flavonoid based plant extracts as effective plant biostimulants.
The effects of MX42SEK on the photosynthetic tissues of potato also support the earlier observations of Wadas and Dziugiel (2019), Wadas and Dziugieł (2020a) who found treatment with humic acid, fulvic acid and seaweed based biostimulants increased the leaf assimilation area, although they found no significant changes in specific leaf area (SLA) or leaf chlorophyll content (SPAD value). They found that the SPAD value depended to a greater extent on the cultivar and weather or on the soil conditions during potato growth. Selim et al. (2012) also reported that humic acid applications raised chlorophyll levels. Sarhan (2011) also increased potato leaf chlorophyll content by up to 11% after applying 2 seaweed extracts, with the largest increase resulting from mixing the two products, raising the possibility of a synergistic effect of these two biostimulants. Cordeiro et al. (2022) improved tuber yields and chlorophyll levels using a biostimulant derived from microalgae, as did Mystkowska (2022) and Wadas (2021) using a silicon based biostimulant.
Along with rice, wheat, soybean and maize, potato is in the top 5 most important agricultural crops and a key part of securing global food security. Average potato yields worldwide are around 19 t Ha−1 but in efficient production systems, with good agronomy, significantly higher yields are attained. For example, in the UK, commercial potato yields in excess of 50 t Ha−1 are achieved regularly (AHDB, 2020) but Haverkort and Struik (2015) reported that with optimal conditions, yields of above 120 Mg ha−1 are feasible.
Plant biostimulants have a significant role to play in meeting these challenging targets within a sustainable production system (Rouphael et al., 2018; Rouphael and Colla, 2018). However, in many regions the lack of good quality data demonstrating the economic benefits and efficacy of these tools within a commercial production system has restricted their uptake by the industry. In the case of complex flavonoid based biostimulants, field data is very scarce and demonstrating the value of MX42SEK to a commercial grower requires high quality trial data.
The 6 commercial potato crops in this study were grown in a range of soil types in the southeast of the UK and produced between 56 and 79 t/ha. These yields are typical for well performing potato crops in this region (AHDB, 2020). Treatment with MX42SEK consistently increased yields (average 5.2%) in these crops. These % increases are lower than many of those reported in previous trials, however a review of the literature suggests that the larger biostimulant induced % yield increases occurred in low yielding crops and in smaller plot trials (see references in Figure 5).
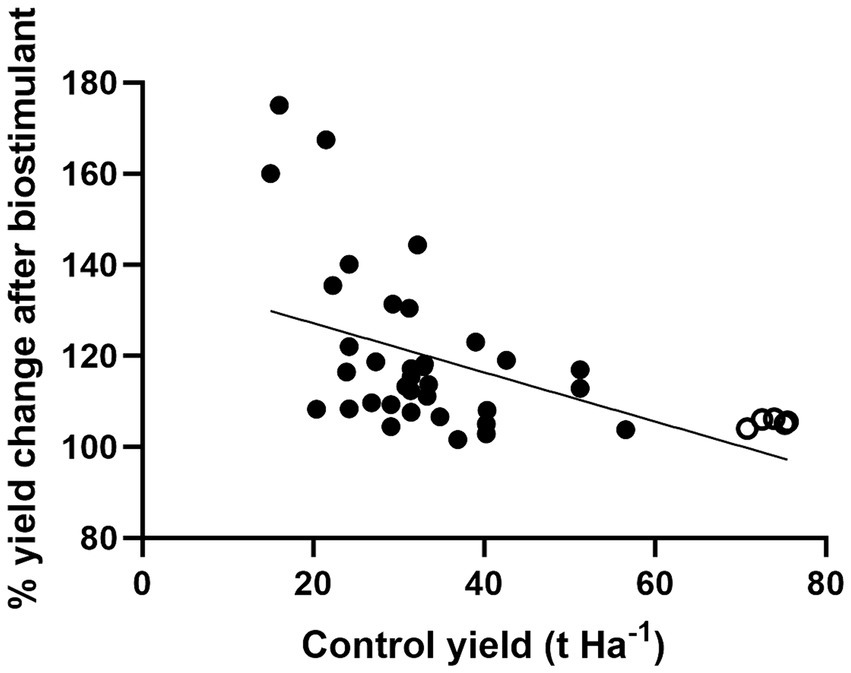
Figure 5. Reported percentage potato tuber yield increases after non-microbial biostimulant treatment. Data from Alenazi et al. (2016), Andrejiová et al. (2023), Cordeiro et al. (2022), Dziugieł and Wadas (2020), El-Zohiri and Asfour (2009), Ginter et al. (2022), Karak et al. (2023), Kołodziejczyk and Gwóźdź (2022), Kumar et al. (2018), Prajapati et al. (2016), Pramanick et al. (2017), Röder et al. (2018), Sanli et al. (2013), Sarhan (2011), Selim et al. (2012), Selladurai and Purakayastha (2016), Souza et al. (2019), Suh et al. (2014), Verlinden et al. (2009), Wadas and Dziugieł (2020b), Wierzbowska et al. (2015), Zarzecka et al. (2020). Open circles are data points from the current study.
Figure 5 shows the reported yield increases in trials involving non-microbial biostimulants, including the six commercial potato crops from the current study. Most of the previously published reports were based on small scale plot trials using a wide range of potato varieties and subjected to different climates, as well as varying levels of nutrients, abiotic and biotic stresses. This analysis suggests that biostimulants usually elicit the largest yield increases in underperforming potato crops. The current trials using the next generation biostimulant delivered significant yield increases in potato crops already producing high tuber yields. Importantly, the yield increases following MX42SEK treatment were also apparent in the marketable yields.
Economically, even a relatively small increase in a highly productive crop can have a large positive effect on the grower’s margins. In the present study, and based on prices at time of harvest, margins increased on average UK£700 per hectare across the 6 potato crops.
An important step in commercial potato production is the management of seed tuber sprout development (Olsen and Kleinkopf, 2020). Chitting or physiological ageing is a process in which dormant buds are encouraged to develop sprouts by manipulating temperature and light conditions. The resulting early emergence after planting accelerates crop development and reduces the impact of weeds (Dines, 2021). Chitting also influences other aspects of crop growth. For example, Varis (1973) found that in addition to early faster growth, chitting decreased the number of stems, reduced the weight of the haulms and roots and decreased the number of tubers whilst increasing overall yield, especially that of large tubers.
The Danehill trial location was used to assess the impact of MX42SEK on both chitted and unchitted seed potatoes. No significant differences were found in yield when comparing treated chitted and treated unchitted seed and both types of seed responded positively to biostimulant application. Growers incur a cost in preparing chitted seed so a biostimulant treatment which allows the use of unchitted tubers would improve economic margins.
The ability to manage quality characteristics such as tuber biomass content, specific gravity and starch content is essential for producing crops intended for specific markets (e.g., fresh, processing, crisping) (Stark et al., 2020). For example, potatoes produced for processing must meet closely monitored specifications for starch and sugar content. Whilst variety selection is usually the major factor in producing a crop with the required quality attributes, the final quality of potatoes can be modified in the field by managing irrigation, nutrition and plant growth. Here abiotic factors influencing tuber maturity, cultivar- and season-variability can have a significant impact on the final quality (Alamar et al., 2017). Specific gravity will tend to be higher if potato tubers are able to complete their full potential growth cycle without periods of stress. Mature tubers will have higher specific gravities than immature tubers and anything that shortens or interrupts the tuber growth cycle will reduce tuber specific gravity. It is likely that biostimulants may also play a role in this management process (Stark et al., 2020).
As potato plants begin to senesce, tubers mature and their specific gravity (dry matter) increases, improving tuber quality for both processing and fresh market consumption. Free sugars are converted to starch, which leads to better quality frying and crisping. In storage, well matured tubers have lower respiration rates, remain dormant longer, sprout later and have greater resistance to pathogens. If tubers remain too long in the soil after the plants die back, starch may convert back to sugar, and the specific gravity declines. These over-mature tubers often have higher respiration rates in storage, will sprout earlier, and are more susceptible to bacterial rots (Thornton, 2020). It has been shown that biostimulants can delay senescence in potato (Röder et al., 2018), a process which is likely to produce significant changes in tuber composition.
The impact of biostimulant treatment on tuber quality parameters has been examined in a number of studies producing a range of outcomes. Maciejewski et al. (2007) found no differences in tuber dry matter and starch content after applying a nitrophenol-based biostimulant to potato (varieties Satina, Bila and Ditta). Similarly, Wadas and Dziugieł (2020b) using foliar applications of extracts from the seaweeds Ascophyllum nodosum and Ecklonia maxima, as well as humic and fulvic acids, did not observe any changes in dry matter, protein, total sugars, monosaccharides and sucrose or L-ascorbic acid content in tubers of early potato varieties. Kołodziejczyk and Gwóźdź (2022) reported that Ecklonia seaweed and amino acid biostimulants increased starch content in potato (variety Vineta), though the change was not statistically significant. Al-Bayati and Al-Quraishi (2019) also used seaweed biostimulants (A. nodosum extracts) and did record a significant increased dry matter content in mature tubers of early (‘Arizona’, ‘Riviera’) and medium-early (‘Agria’) potato varieties. Andrejiová et al. (2023) applying humic biostimulant, increased potato (a medium early variety Spinela) yield, as well as dry matter, starch and vitamin C content. Selim et al. (2012) found higher starch content in the tubers of those plants which were treated with humic acid derived biostimulant. Murashev et al. (2020) found that treatment with an amino acid biostimulant accelerated potato plant development (reducing the growing season by 5 to 10 days) and increased yield. At the same time, they recorded significant increases (15–17%) in starch content. The variation in recorded responses suggests a complex interaction between different potato genotypes, environment and biostimulant types.
In the MX42SEK Maris Piper trials, there were no quality differences found between treated and untreated tubers, except at the Ramsey site, where untreated tubers had significantly elevated specific gravity, dry matter percentage and starch content compared to Ramsey MX42SEK treated tubers and tubers from the other locations. This may be due to the in furrow initial biostimulant application which is different to all the other locations. This would need to be verified with further studies. Given that Maris Piper was used in all the trials, potato genotype can be discounted as contributing to the quality differences found at Ramsey. Seed potato physiological age is another possible variable. The Ramsey trial used unchitted seed potatoes but treated and control tubers from the other site using unchitted seed (Danehill LO6U) showed no differences in dry matter, specific gravity or starch content.
Ramsey was distinct from the other locations in one respect. All the other crops were fully irrigated whilst the Ramsey crop only received irrigation at the start of the growing season, during which, significant drought stress was experienced. Good potato yields are achieved when soil moisture levels are optimal and adequate nitrogen is available to the plant (Thornton, 2020). A sufficient level of nitrogen is necessary to maintain a high growth rate of the potato plant, leading to increased tuber yield but decreased tuber specific gravity. Insufficient nitrogen levels result in reduced leaf area and tuber size due to early leaf drop, whilst excessive nitrogen content in the soil leads to an increase in plant dry matter content and a decrease in the duration of tuber growth (Akkamis and Caliskan, 2023).
It is likely that interactions between abiotic stress, biostimulant treatments and the potato plant’s water and nitrogen management metabolism can result in significant tuber compositional changes. However further work on the response of drought stressed potato to MX42SEK is required in order to confirm the factors that may be involved in producing the tuber quality differences detected at Ramsey.
The confirmation that MX42SEK can enhance potato yields in well performing potato crops whilst maintaining tuber quality, indicates the potential for flavonoid based complex biostimulants in potato production. Li et al. (2022) reviewed the available biostimulant field data and found tuber crops to be least responsive to biostimulants. They also noted that biostimulant effects were most obvious in crops growing in sub-optimal conditions. MX42SEK was able to deliver consistent and significant yield increases in a number of well managed potato crops. The significantly greater yield response to MX42SEK in container experiments indicates the potential of the biostimulant to maintain yield stability in highly stressed crops, which are likely to be more frequent as climate and water stress impact more and more on potato production (Adekanmbi et al., 2023). The container experiments also suggested that responses to MX4SEK may be subject to varietal differences, so field trials using a number of different potato varieties would be important to confirm efficacy across potato varieties.
The current study shows that a complex biostimulant containing flavonoids can significantly increase the yield of potato, one of the world’s most important crop species. This may help mitigate the growing global food shortage and contribute to delivering global food security.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
RS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. TC: Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing. PK: Investigation, Methodology, Supervision, Validation, Writing – review & editing. FL: Supervision, Writing – review & editing. CF: Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to thank all the commercial potato growers for their assistance in establishing, maintaining and assessing the field trials.
RS, TC, PK, and CF was employed by the company Maxstim Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adekanmbi, T., Wang, X., Basheer, S., Nawaz, R. A., Pang, T., Hu, Y., et al. (2023). Assessing future climate change impacts on potato yields—a case study for Prince Edward Island, Canada. Food Secur. 12:1176. doi: 10.3390/foods12061176
AHDB (2020) AHDB Potato Lifting Report Report 2 Week Ending – 20 October 2020. Available at:https://adas.co.uk/news/ahdb-second-potato-lifting-report-2020/. Accessed on 12 December 2023
Akkamis, M., and Caliskan, S. (2023). Responses of yield, quality and water use efficiency of potato grown under different drip irrigation and nitrogen levels. Sci. Rep. 13:9911. doi: 10.1038/s41598-023-36934-3
Aksenova, N. P., Konstantinova, T. N., Golyanovskaya, S. A., Sergeeva, L. I., and Romanov, G. A. (2012). Hormonal regulation of tuber formation in potato plants. Russ. J. Plant Physiol. 59, 451–466. doi: 10.1134/S1021443712040024
Alamar, M. C., Tosetti, R., Landahl, S., Bermejo, A., and Terry, L. A. (2017). Assuring potato tuber quality during storage: a future perspective. Front. Plant Sci. 8:2034. doi: 10.3389/fpls.2017.02034
Al-Bayati, H. J. M., and Al-Quraishi, G. M. A. (2019). Response of three potato varieties to seaweed extracts. Kufa J. Agric. Sci. 11, 36–48.
Alenazi, M., Wahb-Allah, M. A., Abdel-Razzak, H. S., Ibrahim, A. A., and Alsadon, A. (2016). Water regimes and humic acid application influences potato growth, yield, tuber quality and water use efficiency. Am. J. Potato Res. 93, 463–473. doi: 10.1007/s12230-016-9523-7
Andrejiová, A., Adamec, S., Hegedűsová, A., Hegedűs, O., and Rosa, R. (2023). Verification of the humic substances and PGPB biostimulants beneficial effects on the potato yield and bioactive substances content. Potravinarstvo Slovak J. Food Sci. 17, 1–15. doi: 10.5219/1805
Asif, A., Ali, M., Qadir, M., Karthikeyan, R., Singh, Z., Khangura, R., et al. (2023). Enhancing crop resilience by harnessing the synergistic effects of biostimulants against abiotic stress. Front. Plant Sci. 14:1276117. doi: 10.3389/fpls.2023.1276117
Augustin, L., Milach, S., Bisognin, D. A., and Suzin, M. (2012). Genotype x environment interaction of agronomic and processing quality traits in potato. Hortic. Bras. 30, 84–90. doi: 10.1590/S0102-05362012000100015
Campobenedetto, C., Mannino, G., Beekwilder, J., Contartese, V., Karlova, R., and Bertea, C. M. (2021). The application of a biostimulant based on tannins affects root architecture and improves tolerance to salinity in tomato plants. Sci. Rep. 11:354. doi: 10.1038/s41598-020-79770-5
Caradonia, F., Battaglia, V., Righi, L., Pascali, G., and La Torre, A. (2019). Plant biostimulant regulatory framework: prospects in Europe and current situation at international level. J. Plant Growth Reg. 38, 438–448. doi: 10.1007/s00344-018-9853-4
Caradonia, F., Ronga, D., Tava, A., and Francia, E. (2022). Plant biostimulants in sustainable potato production: an overview. Potato Res. 65, 83–104. doi: 10.1007/s11540-021-09510-3
Chen, G., Wang, L., Fabrice, M. R., Tian, Y., Qi, K., Chen, Q., et al. (2018). Physiological and nutritional responses of pear seedlings to nitrate concentrations. Front. Plant Sci. 9:1679. doi: 10.3389/fpls.2018.01679
Colla, G., Hoagland, L., Ruzzi, M., Cardarelli, M., Bonini, P., Canaguier, R., et al. (2017). Biostimulant action of protein hydrolysates: unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 8:2202. doi: 10.3389/fpls.2017.02202
Cordeiro, E. C. N., Mógor, Á. F., Amatussi, J. O., Mógor, G., Marques, H. M. C., and de Lara, G. B. (2022). Microalga biofertilizer improves potato growth and yield, stimulating amino acid metabolism. J. Appl. Phycol. 34, 385–394. doi: 10.1007/s10811-021-02656-0
Davies, K. M., Albert, N. W., Zhou, Y., and Schwinn, K. E. (2018). Functions of flavonoid and betalain pigments in abiotic stress tolerance in plants. Annu. Plant Rev. Online 1, 21–62. doi: 10.1002/9781119312994.apr0604
Dines, L. J. (2021). “Arable cropping” in The agricultural notebook. eds. R. J. Soffe and M. Lobley. 21st ed (Chichester, UK: John Wiley and Sons Ltd. UK), 71–151.
Dziugieł, T., and Wadas, W. (2020). Possibility of increasing early crop potato yield with foliar application of seaweed extracts and humic acids. J. Central Europ. Agric. 21, 300–310. doi: 10.5513/JCEA01/21.2.2576
El-Zohiri, S. S. M., and Asfour, Y. M. (2009). Effect of some organic compounds on growth and productivity of some potato cultivars. Ann. Agric. Sci. Moshtohor. 47, 403–415.
FAO (2021). Food and agriculture data. Available at:www.fao.org/faostat/en/. Accessed on 2 July 2023
Fernie, A. R., and Willmitzer, L. (2001). Molecular and biochemical triggers of potato tuber development. Plant Physiol. 127, 1459–1465. doi: 10.1104/pp.010764
Ginter, A., Zarzecka, K., and Gugała, M. (2022). Effect of herbicide and biostimulants on production and economic results of edible potato. Agronomy 12:1409. doi: 10.3390/agronomy12061409
Głosek-Sobieraj, M., Cwalina-Ambroziak, B., and Hamouz, K. (2018). The effect of growth regulators and a biostimulator on the health status, yield and yield components of potatoes (Solanum tuberosum L.). Gesunde Pflanzen 70, 1–11. doi: 10.1007/s10343-017-0407-7
Guzman, V. V. B. (2018). Pesticide use: Crop management, yield and environmental impact on potato fields in the Netherlands. MSc thesis. Wageningen: Wageningen University.
Haahr, M. (2023). RANDOM.ORG: true Random number service. Available at:https://www.random.org Accessed on 18 December 2023.
Haverkort, A. J., and Struik, P. C. (2015). Yield levels of potato crops: recent achievements and future prospects. Field Crop Res. 182, 76–85. doi: 10.1016/j.fcr.2015.06.002
Karak, S., Thapa, U., and Hansda, N. N. (2023). Impact of biostimulant on growth, yield and quality of potato (Solanum tuberosum L.). Biol. Forum 15, 297–302. doi: 10.13140/RG.2.2.16849.92007
Kleinkopf, G. E., Westermann, D. T., Wille, M. J., and Kleinschmidt, G. D. (1987). Specific gravity of russet Burbank potatoes. Am. Potato J. 64, 579–587. doi: 10.1007/BF02853760
Kloosterman, B., Vorst, O., Hall, R. D., Visser, R. G., and Bachem, C. W. (2005). Tuber on a chip: differential gene expression during potato tuber development. Plant Biotechnol. J. 3, 505–519. doi: 10.1111/j.1467-7652.2005.00141.x
Kołodziejczyk, M., and Gwóźdź, K. (2022). Effect of plant growth regulators on potato tuber yield and quality. Plant Soil Environ. 68, 375–381. doi: 10.17221/215/2022-PSE
Kong, C. H., Xuan, T. D., Khanh, T. D., Tran, H. D., and Trung, N. T. (2019). Allelochemicals and signalling chemicals in plants. Molecules 24:2737. doi: 10.3390/molecules24152737
Kumar, V., Singh, S. P., and Raha, P. (2018). Organic sources use of amino acids based biostimulants and irrigation schedule on yield: water use efficiency relationship on potato tuber. J. Pharmacogn. Phytochem. 7, 1255–1259.
Li, J., Van Gerrewey, T., and Geelen, D. (2022). A Meta-analysis of biostimulant yield effectiveness in field trials. Front. Plant Sci. 13:836702. doi: 10.3389/fpls.2022.836702
Maciejewski, T., Szukała, J., and Jarosz, A. (2007). Influence of biostymulator Asahi sl i atonik sl on qualitative tubers of potatoes. J. Res. Appl. Agric. Eng. 52, 109–112.
Meyling, H. G., and Bodlaender, K. B. A. (1981). Varietal differences in growth, development and tuber production of potatoes. Neth. J. Agri. Sci. 29, 113–127. doi: 10.18174/njas.v29i2.17012
Mohammed, W. (2016). Specific gravity, dry matter content, and starch content of potato (Solanum tuberosum L.) varieties cultivated in eastern Ethiopia. East Afr. J. Sci. 10, 87–102.
Murashev, S. V., Kiru, S. D., Verzhuk, V. G., and Pavlov, A. V. (2020). Potato plant growth acceleration and yield increase after treatment with an amino acid growth stimulant. Agron. Res. 18, 494–506. doi: 10.15159/AR.20.036
Mystkowska, I. (2022). The effect of biostimulants on the chlorophyll content and height of Solanum tuberosum L. plants. J. Ecol. Eng 23, 72–77. doi: 10.12911/22998993/151713
Nakabayashi, R., Yonekura-Sakakibara, K., Urano, K., Suzuki, M., Yamada, Y., Nishizawa, T., et al. (2014). Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 77, 367–379. doi: 10.1111/tpj.12388
Naumann, M., Koch, M., Thiel, H., Gransee, A., and Pawelzik, E. (2020). The importance of nutrient management for potato production part II: plant nutrition and tuber quality. Potato Res. 63, 121–137. doi: 10.1007/s11540-019-09430-3
Olsen, N., and Kleinkopf, G. (2020). “Storage Management” in Potato production systems. eds. J. Stark, M. Thornton, and P. Nolte (Cham: Springer), 523–545.
Prajapati, A., Patel, C., Singh, N., Jain, S., Chongtham, S., Maheshwari, M., et al. (2016). Evaluation of seaweed extract on growth and yield of potato. Environ. Ecol. 34, 605–608.
Pramanick, B., Brahmachari, K., Mahapatra, B. S., Ghosh, A., Ghosh, D., and Kar, S. (2017). Growth, yield and quality improvement of potato tubers through the application of seaweed sap derived from the marine alga Kappaphycus alvarezii. J. Appl. Phycol. 29, 3253–3260. doi: 10.1007/s10811-017-1189-0
Röder, C., Mógor, A. F., Szilagyi-Zecchin, V. J., Gemin, L. G., and Mógor, G. (2018). Potato yield and metabolic changes by use of biofertilizer containing L-glutamic acid. Comun. Sci. 9, 211–218. doi: 10.14295/cs.v9i2.2564
Rouphael, Y., and Colla, G. (2018). Synergistic biostimulatory action: designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 9:1655. doi: 10.3389/fpls.2018.01655
Rouphael, Y., Spíchal, L., Panzarová, K., Casa, R., and Colla, G. (2018). High-throughput plant phenotyping for developing novel biostimulants: from lab to field or from field to lab? Front. Plant Sci. 9:1197. doi: 10.3389/fpls.2018.01197
Sanli, A., Karadogan, T., and Tonguc, M. (2013). Effect of leonardite applications on yield and some quality parameters of potatoes (Solanum tuberosum L.). Turk. J. Field Crops 18, 20–26.
Sarhan, T. Z. E. (2011). Effect of humic acid and seaweed extracts on growth and yield of potato plant (Solanum tuberosum L.) Desiree cv. Mesop. J. Agric. 39, 19–25.
Savvas, D., and Ntatsi, G. (2015). Biostimulant activity of silicon in horticulture. Sci. Hortic. 196, 66–81. doi: 10.1016/j.scienta.2015.09.010
Selim, E. M., Shaymaa, I. S., Asaad, F. F., and El-Neklawy, A. S. (2012). Interactive effects of humic acid and water stress on chlorophyll and mineral nutrient contents of potato plants. J. Appl. Sci. Res. 8, 531–537.
Selladurai, R., and Purakayastha, T. J. (2016). Effect of humic acid multinutrient fertilizers on yield and nutrient use efficiency of potato. J. Plant Nutr. 39, 949–956. doi: 10.1080/01904167.2015.1109106
Shah, A., Subramanian, S., and Smith, D. L. (2022). Flavonoids and Devosia sp SL43 cell-free supernatant increase early plant growth under salt stress and optimal growth conditions. Front. Plant Sci. 13:1030985. doi: 10.3389/fpls.2022.1030985
Sible, C. N., Seebauer, J. R., and Below, F. E. (2021). Plant biostimulants: a categorical review, their implications for row crop production, and relation to soil health indicators. Agron. 11:1297. doi: 10.3390/agronomy11071297
Souza, E. F., Rosen, C. J., and Venterea, R. T. (2019). Contrasting effects of inhibitors and biostimulants on agronomic performance and reactive nitrogen losses during irrigated potato production. Field Crop Res. 240, 143–153. doi: 10.1016/j.fcr.2019.05.001
Stark, J. C., Love, S. L., and Knowles, N. R. (2020). “Tuber quality” in Potato production systems. eds. J. Stark, M. Thornton, and P. Nolte (Cham: Springer), 479–498.
Su, Y., Guo, H., and Chen, Y. (2007). Relationship between SPAD readings chlorophyll contents and yield of potato (Solanum tubersosum L.). Southwest China J. Agric. Sci. 20, 690–693.
Suh, H. Y., Yoo, K. S., and Suh, S. G. (2014). Tuber growth and quality of potato (Solanum tuberosum L.) as affected by foliar or soil application of fulvic and humic acids. Hortic. Environ. Biotechnol. 55, 183–189. doi: 10.1007/s13580-014-0005-x
Thornton, M. (2020). “Potato growth and development” in Potato production systems. eds. J. Stark, M. Thornton, and P. Nolte (Cham: Springer), 19–34.
United States Department of Agriculture (1997). United States standards for grades of potatoes for processing. Washington: U.S. Department of Agriculture.
Varis, E. (1973). The effects of tuber size and chitting method on the growth and yield of Amyla and Barima potatoes. Agric. Food Sci. 45, 297–318. doi: 10.23986/afsci.72949
Verlinden, G., Pycke, J., Mertens, J., Debersaques, F., Verheyen, K., Baert, G., et al. (2009). Application of humic substances results in consistent increase in crop yield and nutrient uptake. J. Plant Nutr. 32, 1407–1426. doi: 10.1080/01904160903092630
Vos, J., and Bom, M. (1993). Hand-held chlorophyll meter: a promising tool to assess the nitrogen status of potato foliage. Potato Res. 36, 301–308. doi: 10.1007/BF02361796
Wadas, W. (2021). Potato (Solanum tuberosum L.) growth in response to foliar silicon application. Agronomy 11:2423. doi: 10.3390/agronomy11122423
Wadas, W., and Dziugiel, T. (2019). Growth and marketable potato (Solanum tuberosum L.) tuber yield in response to foliar application of seaweed extract and humic acids. Appl. Ecol. Environ. Res. 17, 13219–13230. doi: 10.15666/aeer/1706_1321913230
Wadas, W., and Dziugieł, T. (2020a). Changes in assimilation area and chlorophyll content of very early potato (Solanum tuberosum L.) cultivars as influenced by biostimulants. Agronomy 10:387. doi: 10.3390/agronomy10030387
Wadas, W., and Dziugieł, T. (2020b). Quality of new potatoes (Solanum tuberosum L.) in response to plant biostimulants application. Agriculture 10:265. doi: 10.3390/agriculture10070265
Wadas, W., and Kalinowski, K. (2017). Effect of titanium on assimilation leaf area and chlorophyll content of very early-maturing potato cultivars. Acta Sci. Pol., Agric. 16, 87–98.
Wang, W., Vinocur, B., and Altman, A. (2003). Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1–14. doi: 10.1007/s00425-003-1105-5
Wierzbowska, J., Cwalina-Ambroziak, B., Głosek-Sobieraj, M., and Sienkiewicz, S. (2015). Effect of biostimulators on yield and selected chemical properties of potato tubers. J. Elem. 20, 757–768. doi: 10.5601/jelem.2014.19.4.799
Wijesinha-Bettoni, R., and Mouillé, B. (2019). The contribution of potatoes to global food security, nutrition and healthy diets. Am. J. Potato Res. 96, 139–149. doi: 10.1007/s12230-018-09697-1
Wise, K., Selby-Pham, J., Chai, X., Simovich, T., Gupta, S., and Gill, H. (2024). Fertiliser supplementation with a biostimulant complex of fish hydrolysate, Aloe vera extract, and kelp alters cannabis root architecture to enhance nutrient uptake. Sci. Hortic. 323:112483. doi: 10.1016/j.scienta.2023.112483
Yildrim, Z., and Tokusoglu, Ö. (2005). Some analytical quality characteristics of potato (Solanum tuberosum L.) Minitubers (cv. Nif) developed via in-vitro cultivation. Electron. J. Environ. Agric. Food Chem. 4, 916–925.
Zarzecka, K., Gugała, M., Sikorska, A., Grzywacz, K., and Niewęgłowski, M. (2020). Marketable yield of potato and its quantitative parameters after application of herbicides and biostimulants. Agriculture 10:49. doi: 10.3390/agriculture10020049
Keywords: potato, complex biostimulant, flavonoid, yield, quality, nutrient use efficiency, plant biostimulant, global food supply
Citation: Salvage R, Cannon T, Kingsmill P, Liu F and Fleming CC (2024) A complex biostimulant based on plant flavonoids enhances potato growth and commercial yields. Front. Sustain. Food Syst. 8:1368423. doi: 10.3389/fsufs.2024.1368423
Received: 10 January 2024; Accepted: 29 May 2024;
Published: 01 July 2024.
Edited by:
Miroslav Zoric, LoginEKO Research and Development Center, SerbiaReviewed by:
Markus Weinmann, University of Hohenheim, GermanyCopyright © 2024 Salvage, Cannon, Kingsmill, Liu and Fleming. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard Salvage, cnNhbHZhZ2UwMUBxdWIuYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.