
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 12 April 2024
Sec. Agro-Food Safety
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1329100
This article is part of the Research Topic Ensuring Food Safety And Quality Throughout The Supply Chain View all 15 articles
 Asmae Baghouz1*
Asmae Baghouz1* Yassir Bouchelta2
Yassir Bouchelta2 Imane Es-safi3
Imane Es-safi3 Rajae El Brahimi3
Rajae El Brahimi3 Hamada Imtara4*
Hamada Imtara4* Mashail N. AlZain5
Mashail N. AlZain5 Omer M. Noman6
Omer M. Noman6 Abdelaaty A. Shahat6
Abdelaaty A. Shahat6 Raja Guemmouh1
Raja Guemmouh1Introduction: The post-harvest period of cowpea [Vigna unguiculata (L) Walp] is marked by substantial losses due to the insect pest Callosobruchus maculatus (Fabricius). The primary goal of the current study is to identify environmentally appropriate substitutes for synthetic pesticides in the management of stored seed pests. Thus, in a laboratory setting, the insecticidal activity of essential oils (EOs) from Ziziphora hispanica and Satureja calamintha against the cowpea weevil C. maculatus was assessed.
Methods: The fumigant effects of these two EOs were tested with concentrations (4, 8, 12, 16, and 20 μL L−1 of air per 10 g of cowpea seeds) on four biological parameters of C. maculatus: adult mortality, fecundity, fertility, and adult emergence, while concentrations of 4, 12, 16, and 20 μL/cm2 of air were used for the repulsion test.
Results and discussion: The fumigant effects of these two EOs were tested with concentrations (4, 8, 12, 16, and 20 μL L−1 of air per 10 g of cowpea seeds) on four biological parameters of C. maculatus: adult mortality, fecundity, fertility, and adult emergence, while concentrations of 4, 12, 16, and 20 μL/cm2 of air were used for the repulsion test. The results of fumigation tests showed a remarkable efficacy of both essential oils against adult C. maculatus after 24 h of exposure. Z. hispanica EO yielded a mortality rate of 80 ± 20%, with an LC50 of 2.77 μL L−1 for males and 66.66 ± 11.54% with an LC50 of 3.57 μL L-1 for females at 4 μL L−1 of air. However, the S. calamintha EO resulted in a mortality rate of 100% for males and 86.66 ± 23.09% with an LC50 of 2.17 μL L−1 for females at low doses. The fecundity was 1.33 ± 0.57 eggs per female. In contrast, this parameter was absent with S. calamintha EO at the low dose, while fertility and emerging adults were missing for both EOs. Furthermore, both EOs showed highly repellent activity towards C. maculatus adults, with 81.66% for Z. hispanica and 91.67% for S. calamintha EO. According to the results of the GC–MS analysis, the primary components of Z. hispanica EO were found to be pulegone (28.17%), alpha-naphtonitrite (10.77%), and 3-(3-thienyl) pro-2-enoic acid (10.62%). Similarly, the main constituents of S. calamintha EO were pulegone (21.48%), piperitenone oxide (17.71%), and eucalyptol (11.99%). Hence, these substances are regarded as the volatile compounds accountable for controlling C. maculatus activities. The study reports that Z. hispanica and S. calamintha show promising fumigant and repellent efficacy and offer new avenues for their potential use as an alternative to synthetic pesticides against stored seed pests.
Seed legumes occupy a considerable place in the world diet, particularly in developing countries, where they have a primary role among many populations in Africa, South America, and Asia (Singh and Singh, 1992). They are the most important source of protein for humans and are considered the second most important food source after cereals (Kouris-Blazos and Belski, 2016). Their incorporation in the diet, particularly in developing countries, can be a major contributor to combating malnutrition (Maphosa and Jideani, 2017). In addition to their nutritional value, legumes also have economic, cultural, physiological, and medicinal roles due to their presence of bioactive products beneficial to humans (Phillips, 1993; Bouchenak and Lamri-Senhadji, 2013; Remond and Walrand, 2017). Food legumes, such as soybeans, beans, cowpeas, broad beans, chickpeas, lentils, and peanuts, are consumed as protein-rich dry seeds. Humans cultivate them because of their key role in diversifying diets and ensuring global food security (Singh et al., 2022).
Cowpea (Vigna unguiculate L.), as known as black pea, is one of the most nutrient-rich native African legumes and is a great interest for food quality and nutritional health (Abebe and Alemayehu, 2022). Cowpea grains are rich in protein, carbohydrates, and fiber, while their leaves and pods contain numerous vitamins and minerals (Avanza et al., 2013).
Cowpea is known in Morocco; it is grown in small areas in the southern Atlas oases, and seeds are available in the souks and markets of the major cities, particularly in Fez (Bryssine, 1962). Currently, in Morocco, this legume is planted in the northwestern regions, especially in Fez, and is locally named Gnawas beans. Nowadays, the cultivation of cowpeas, as well as their preparation and consumption, has spread to other regions of Morocco. Although Moroccans widely consume cowpea, studies on this type of legume are still limited. In Morocco, insect pests cause significant damage to stored legumes. Despite the magnitude of losses caused by insect pests, only a limited number of studies have shed light on these pests in Africa.
Among legume beetles, the cowpea beetle, Callosobruchus maculatus (Fabricius), is the main culprit that causes severe damage to most legumes, especially cowpeas. This beetle is a member of the family Chrysomelidae and the subfamily Bruchinae (Kergoat et al., 2008). However, many stored legumes are affected by this beetle, which is commonly recognized as cowpea weevil or bean beetle (Onyido et al., 2011).
C. maculatus causes significant damage in seed storage areas, where females lay eggs on the seed coat following hatching. Larvae penetrate the seeds, develop by depleting the reserves of cotyledons, transform into pupae, and complete their life cycle by emerging as adult beetles, resulting in significant quantitative and qualitative losses and leading to damages that can reach 100% after a few months (Seri-Kouassi et al., 2004). Nevertheless, very few studies have been carried out in Morocco to quantify and evaluate the impact of this problem, although patchy results are available.
Due to the serious damage caused by C. maculatus to cowpea seeds, various approaches have been adopted to effectively control this pest (Kébé et al., 2020). Due to the serious damage caused by C. maculatus to cowpea seeds, various approaches have been adopted to effectively control this pest (Kébé et al., 2020). Several phytosanitary chemicals have been used to manage this pest during the seed storage period. However, the success of synthetic pesticides is hampered by several factors, including the high cost of their acquisition, the risks of environmental contamination, the toxicity of residues accumulated in food, pest resistance problems, the destruction of natural predators, and harmful effects on non-target organisms (Mohan et al., 2010; Ileke et al., 2020).
The valorization of plant products is an alternative approach to organic pesticides. These methods make it possible to extract active substances from various plant species offering different properties using appropriate devices, so that these bioproducts can be used to combat pests in stored foodstuffs (Aker et al., 2023).
The essential oils (EOs) derived from different plant species characterized by a pronounced odor and represent an attractive alternative to chemical products (Sarma et al., 2019). These natural products extracted from plants contain various bioactive compounds (Nisar et al., 2022). The EOs exert ovicidal and larvicidal effects on insects, impede their respiratory capacity, eliminate their oviposition mode, serve as both a repellent and attractant, decrease the emergence of adult insects, and prevent the recognition of the target plant (Rastegar et al., 2011). This research considers that Morocco has no data yet on the damage caused by the insect C. maculatus on cowpea.
There are several plant families that produce essential oils: Burseraceae, Compositae, Cupressaceae, Lamiaceae, Lauraceae, Myrtaceae, Piperaceae, Apiaceae, Asteraceae, Poaceae, Rutaceae, and Zingiberaceae (Chaubey, 2019; Matos et al., 2020; Teke and Mutlu, 2021; De Andrade Rodrigues et al., 2022). In this perspective, many species belonging to the Lamiaceae family have proved very effective for controlling various insect pests on stored foodstuffs (Mishra et al., 2012; Alkan, 2020).
This research has been carried out to examine the effectiveness of two EOs extracted from two plants of the Lamiaceae family and to determine their insecticidal fumigant and repellent effectiveness. Z. hispanica, as known by the by the Pennyroyal Mint of dry areas (Bellakhdar, 1997; Boullard, 2001), or named in Arabic Fliou, fliyou berri (Fennane and Rejdali, 2016; Najem et al., 2021), is a fragrant and highly aromatic plant. It’s odor is intense, pleasant, fresh, and penetrating, approaching those of the Pennyroyal Mint (Mentha pulegium). And S. calamintha or Sarriette, calament calamint (Lahsissene et al., 2009; Tibaldi et al., 2013) its Arabic name is manta, Nebta (Lahsissene et al., 2009), this plant has a scent of mint. These species are native to Europe and the Mediterranean Basin (Bellakhdar, 1997), and North Africa (Algeria, Morocco) (Boudjema et al., 2018; Redhouane et al., 2020). In Morocco, Z. hispanica and S. calamintha species grow spontaneously in the High Atlas, Middle Atlas, Eastern Plateaus, Eastern Mountains, and Rif regions (Fennane and Rejdali, 2016).
In the literature, no data are available on the fumigant and repellent effects of the EOs of Z. hispanica and S. calamintha on the cowpea insect pest C. maculatus (Fabricius). Indeed, the only study mentioning the insecticidal activity of Z. hispanica EO was carried out on Bruchus lentis, Bruchus pisorum, and Bruchus rufimanus (Coleoptera: Bruchidae) (Ainane et al., 2023). On the other hand, research conducted on the insecticidal potential of S. calamitha EO was only focused on Tribolium confusum (Duv.) (Coleoptera: Tenebrionidae), Rhyzopertha dominica (F.), and Sitophilus oryzae (L.) (Abbad et al., 2023). Therefore, the present study aimed to evaluate the toxicity and insecticidal power of EOs extracted from the two Moroccan medicinal and aromatic plants, on the biological parameters of C. maculatus under laboratory conditions and to profile the volatile compounds that are responsible for insecticidal activity.
The insect selection, C. maculatus males and females (Figure 1), was obtained from a local storage site and reared in glass jars containing approximately 500 g of Vigna unguiculata seeds.

Figure 1. Adult of C. maculatus: female (A) and male (B) (photographs taken by Baghouz et al., 2022).
The jars were preserved under conditions of the culture chamber (temperature at 27 ± 1°C, relative humidity at 70 ± 5%, and a photoperiod of 14 h of light and 10 h of dark) (Baghouz et al., 2022).
The aerial parts of Z. hispanica and S. calamintha were harvested in different regions of Morocco at the end of May and June 2021 (Figures 2, 3). The determination of the names of these two plants was carried out by the botanical professor, Amina Bari, who assigned them the voucher numbers (Table 1). The aerial parts (leaves and flowers) of these plants were cleaned and dried in the shade at room temperature (25–27°C during the day) for 15 days, then ground to a fine powder using an electric blender.

Figure 2. Ziziphora hispanica on its natural habitat in the Guigou region (Boulemane, Morocco, May 2021) (photo taken by Baghouz et al., 2022).

Figure 3. Satureja calamintha on its natural habitat in Jbabra region (Taounate, Morocco, June 2021) (photo taken by Baghouz et al., 2022).
In the present study, a Clevenger-type hydrodistillation apparatus was used to extract the essential oils for 3 to 4 h. One liter of distilled water was poured into a flask containing 100 g of dry leaf powder from each plants tested, and the whole was boiled until two phases appeared, one aqueous and other organic (essential oil). The essential oils were collected using a graduated burette, stored in airtight opaque tubes, and kept in the refrigerator at a temperature of around 4°C.
The essential oil content of the two plants was determined by applying the formula below (Piri et al., 2019):
R: yield of essential oils expressed in (%).
m 1: mass of essential oils in g.
m 2: mass of plant material in g.
The chemical composition of the essential oils was determined using a gas chromatograph-mass spectrophotometer (Brand Agilent Technologies Model 5,973 with an Agilent column 19091S-433 HP-5MS, 30 m long, 0.25 mm inside diameter, and 0.25 μm film thickness of the stationary phase, Helsinki, Finland) in positive mode by injection of 0.1 μL of the sample to be analyzed. Helium was used as the carrier gas, with a typical pressure range (psi) of 0.9 mL/s. The oven temperature program was set between 60 and 300°C for 10 min, then held at 300°C for 20 min. The detector temperature was set at 250°C and the injector at 260°C. Compounds were identified by comparing retention times with those of standards obtained from the NIST mass spectra library (Jawhari et al., 2020).
One-liter jars were used for the essential oil fumigation assay (Figure 4). Both EOs were tested in the vapor phase to evaluate their insecticidal activity. First, Whatman paper discs (3 cm2) were impregnated with different concentrations of the EOs (4, 8, 12, 16, and 20 μL/L of air) and attached to the inner surface of the jar lids to prevent direct contact with the insects. Next, 10 g of healthy cowpea seeds and five pairs of C. maculatus aged 0–48 h were introduced in each jar, whereas the control was performed without EO. Three replicates are performed for each assay. Then, the jars were kept in the culture chamber (Baghouz et al., 2022), and the biology of the cowpea beetle (C. maculatus) was observed and recorded regularly.
The mortality rate observed was corrected by the Abbott formula (Abbott, 1925):
Pc: Corrected mortality; Po: Observed mortality; Pt: Control mortality.
Female fecundity is the total number of eggs laid by C. maculatus females (hatched and unhatched).
Female fertility (Hatching rate) = (Number of eggs hatched/Number of eggs laid) × 100.
The repellency of the EOs on C. maculatus adults was studied by the preferential surface method on filter paper, following the technique described by McDonald et al. (1970). With Whatman No. 1 paper washers (8 cm2 in diameter), cut transversely into two identical parts, the first was treated with each essential oil evaluated at different concentrations (4, 12, 16, and 20 μL/cm2) diluted in 0.5 mL of acetone, and the second was treated exclusively with the same volume of acetone established as the control (McDonald et al., 1970). After air-drying both parts, five pairs of adult C. maculatus were placed in the center of each plate. After 30 min following the test, the insects found on both sides were counted. Three replicates are performed for each assay (Figure 5). The Percentage of repulsion (PR) was calculated using the following formula:
PR: Percentage of repulsion; N: Number of individuals present in part treated with acetone only; NT: Number of individuals present in part treated with the essential oil diluted in acetone.
To identify any significant differences between the effects of Z. hispanica and S. calamintha EOs on C. maculatus, an analysis of variance (two-way ANOVA) was performed, and significant means ± SD of the treatments were separated using Tukey’s multiple interval tests at p < 0.05 using GraphPad Prism 8. In the fumigation tests, the lethal concentrations (LC50 and LC90) and chi-square for each regression coefficient were determined by Probit analysis (Finney, 1971) using SPSS version 21 software. For repellency tests, counts of insects attracted to each concentration of product and control were compared to Tukey’s at p < 0.05, using graph pad prism 8 software.
The yield of Z. hispanica EO was 0.85% based on the dry matter, and GC/MS phytochemical analysis of the EO revealed 23 compounds, which represent 99.99% of the total composition of the oil. The detected volatiles were distinguished at 5.14 and 9.18 min. The EO was rich in oxygenated monoterpenes, which dominated its composition (34.97%). The major constituents that make up this class are pulegone (28.17%), trans-isopulegone (3.09%), and piperenone oxide (1.40%). Moreover, we also detected new compounds present in considerable proportions, including alpha-naphtonitrite (10.77%), 3-(3-thienyl) pro-2-enoic acid (10.62%), and + (3)-methyladipic acid (9.59%) (Table 2).
The yield of S. calamintha EO was 1.40% based on the dry matter. It was confirmed by GC/MS the presence of 24 compounds, forming 99.43%, which were shown between 4.414 and 9.622 min. S. calamintha EO has a large quantity of oxygenated monoterpenes, which constitute 75.46%, and the main constituents make up this class are pulegone (20.53%), piperenone oxide (17.05%), and 1,8-cineole (12.09%). At the same time, it’s also noted the presence of oxygenated sesquiterpenes with a content of 10.44% and composed mainly of spathulenol (9.14%) (Table 3).
The effects of Z. hispanica and S. calamintha EOs vapors on the mortality of adult males and females of C. maculatus, show that males are more sensitive than females to the EOs tested (Table 4).
Indeed, at the lowest concentration (4 μL/L), Z. hispanica EO causes 80 ± 20% mortality of C. maculatus males and 66.66 ± 11.54% of females after 24 h of exposure. Z. hispanica EO showed highly significant mortality with increasing concentrations (F = 805.64; df = 5.72; p < 0.0001) and exposure times (F = 49.16; df = 5.72; p < 0.0001) for males. Similarly, this mortality was significant for females at different concentrations (F = 1331.70; df = 5.72; p < 0.0001) and equivalent exposure durations (F = 94.20; df = 5.72; p < 0.0001).
At the same concentration (4 μL/L), S. calamintha EO causes 100% mortality of males and 86.66 ± 23.09% of females after 24 h. However, no mortality occurred in the control groups. The insecticidal potential of S. calamintha EO was manifested by very high and significant mortality in males, observed at various concentrations (F = 2048; df = 5.72; p < 0.0001) and exposure times (F = 102.80; df = 5.72; p < 0.0001). In parallel, significant mortality was observed in females at different concentrations (F = 805.64; df = 5.72; p < 0.0001) and exposure times (F = 49.16; df = 5.72; p < 0.0001).
At the highest concentration (20 μL/L), Z. hispanica and S. calamintha oils show significantly higher action than the control, causing 100% mortality, respectively, for both sexes.
Probit analysis of the two plant EOs showed variable efficacy against C. maculatus, with greater sensitivity among adult males than adult females.
The S. calamintha EO showed the highest fumigation activity against C. maculatus with an LC50 value of 2.17 μL/insect for females; on the other hand, the LC50 value of the Z. hispanica EO recorded is 3.57 μL/insect for the same sex after 24 h of exposure (Table 5).
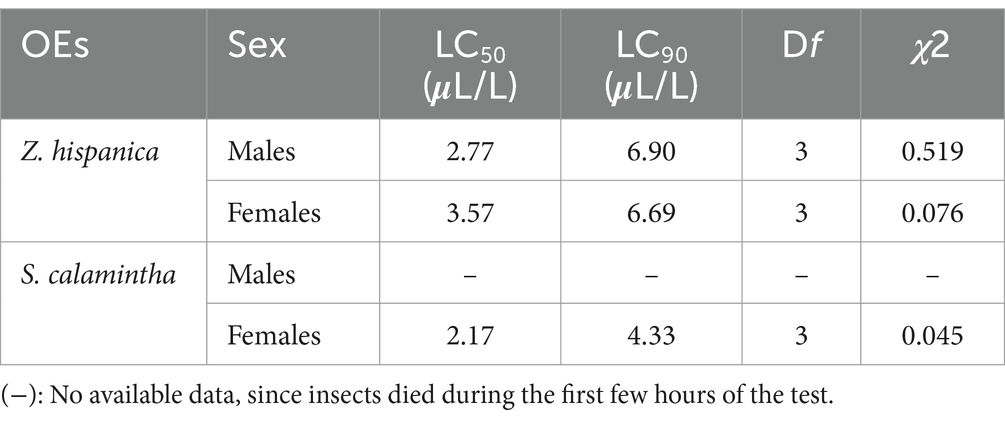
Table 5. Lethal concentrations (μL/L) and chi-square values (χ2) for EOs against adult C. maculatus.
The fumigation test with Z. hispanica and S. calamintha EOs induced a repressive effect on different biological parameters of C. maculatus.
Given the early mortality of C. maculatus adults, it is clear that low concentrations (4 μL, 8 μL) of EOs completely prevented egg-laying, fertility of females and inhibited the emergence of C. maculatus adults, as indicated in Figure 6.
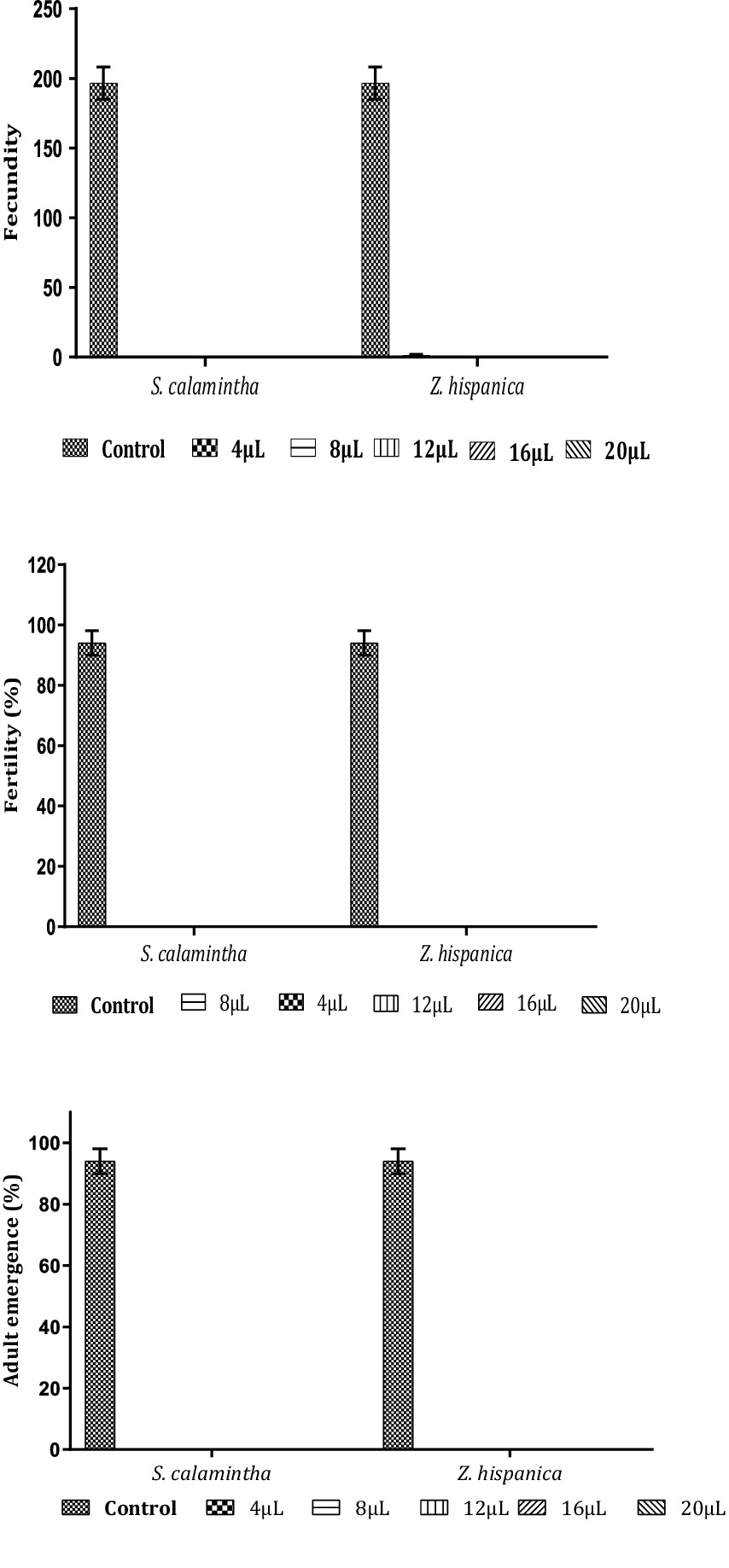
Figure 6. Impact of EOs of Z. hispanica and S. calamintha on biological parameters of C. maculatus (mean ± SD).
The repellent properties of Z. hispanica and S. calamintha oils were studied on C. maculatus adults at different concentrations (between 4 and 20 μL). The highest repellent activity (100%) was recorded at a concentration of 16 μL after 30 min for S. calamintha EO against C. maculatus adults, while (93.33%) was observed at the same concentration for Z. hispanica EO (Table 6). The repellency test revealed a highly significant toxicity caused by both EOs against C. maculatus, and this was dependent on concentration (F = 9.067; df = 3.8; p = 0.0059).
In this study, Z. hispanica and S. calamintha EOs isolated by Clevenger hydrodistillation yielded 0.85% for Z. hispanica and 1.40% for S. calamintha. Various studies have quantified the EO content of these species. Z. hispanica yields have been measured at 1.38, 0.53, and 1.01% (v/w), respectively, in some studies (Negueruela and Rico, 1986; Bekhechi et al., 2007; Rabah et al., 2013). The yields of S. calamintha obtained by different researchers are 1.3, 2.54, and 2.80% (v/w), respectively (Kerbouche et al., 2013; Bouzidi and Kemieg, 2021; El Brahimi et al., 2023). In fact, variations in EO content depend on various parameters, including soil type, geographical region, harvesting time, plant material qualification, and extraction process (Hussain et al., 2010; Gharib et al., 2020; Marques et al., 2023).
Results showed that the EOs extracted from two lamiacae plants have insecticidal activity against the males and females of C. maculatus. However, the toxicity varied according to the plant and exposure time. The plant species considered in this study are recognized for their environmentally friendly characteristics and safety for vertebrates, and they are also used in the medical field. The studies on the toxicity of Z. hispanica and S. calamintha on the cowpea pest are nonexistent, and no studies have been reported in the literature on the insecticidal activity of fumigants and the repellent power of EOs tested against stored product insects, especially C. maculatus. Our results showed that both EOs caused mortality of C. maculatus adults within 24 h of treatment. The EO of S. calamintha had the most significant insecticidal action, as the lowest concentration (4 μL/L), resulting in 100% mortality of males and 60.44 ± 3.35% of females. Several previous studies have demonstrated the effectiveness of Lamiaceae species EOs by fumigation on several insect pests, such as C. maculatus (Demirel and Erdogan, 2017; Baghouz et al., 2022; Yang et al., 2023). And Tribolium castaneum (Jaya et al., 2014). The insecticidal potential of S. calamintha EO against three species of stored grain insects (Tribolium confusum, Rhyzopertha dominica, and Sitophilus oryzae) was more pronounced than that observed with other plants (Alkan, 2020; Abbad et al., 2023). The LC50 values obtained by S. calamintha EO are considerably lower than those reported for several traditional insecticides used against insect pests (Yao et al., 2019; Attia et al., 2020).
This research shows that both EOs significantly reduce the number of eggs laid per female, fertility, and adult emergence of C. maculatus from the lowest concentration (4 μL). The mean number of eggs laid per female was 1.33 ± 0.577 eggs for Z. hispanica and 0 ± 0 for S. calamintha, compared with the control which had 196.66 ± 11.54 eggs. This indicates the presence of volatile components in EOs that obstruct egg-laying and larval development inside the seeds, thus preventing the emergence of adults. Our results concur with those of several researchers. Lolestani and Shayesteh (2009) studied the toxicity of Z. clinopodioides EO on the fecundity of C. maculatus females and reported 61.10% egg mortality at a concentration of 26.7 μL/L of air. In addition, Kheirkhah et al. (2015) found a strong ovicidal activity of Z. clinopodioides EO against the flour beetle E. kuehniella. Saeidi and Mirfakhraie (2017) determined that Mentha piperita EO has high repellent efficacy against C. maculatus, with a repellent percentage of 87.76 ± 0.96% at a dose of 360 μL/L of air after 24 h of exposure. Satongrod and Wanna (2020) proved that the EO of P. amboinicus showed the highest efficacy for repelling C. maculatus adults (87.50 ± 5%) at a concentration of 1 μL/L air after 48 h.
The male and female adults of C. maculatus showed a high sensitivity to the EOs, even at low concentrations and during short exposure times. Based on the results obtained, it is suggested that the insecticidal potential of both EOs could be attributed mainly to the presence of high quantities of the main monoterpene components (Abeywickrama et al., 2006; Abdelgaleil et al., 2009; Saeidi and Mirfakhraie, 2017; Romani et al., 2019; Abifarin et al., 2020; Boukraa et al., 2022). The EOs of many plants are rich in secondary metabolites, particularly monoterpenes, which confer insecticidal properties (Topuz et al., 2018; Hategekimana and Erler, 2020a; Gupta et al., 2023). These substances are highly volatile, resulting in potentially significant fumigant and repellent properties for controlling infestations of insect pests in stored products (Martynov et al., 2019). Consequently, these biochemical substances impact negatively on female fecundity and fertility, as well as on adult emergence (Saeidi and Mirfakhraie, 2017; Romani et al., 2019; Boukraa et al., 2022). The repellent effect resides in various behavioral responses of the insects transmitted by sensory hairs in their antennae that detect the chemically volatile organic compounds in the EOs (Romani et al., 2019; De Brito et al., 2021; Boukraa et al., 2022).
Pulegone, a monoterpenoid compound, constitutes the main component of Z. hispanica and S. calamintha EOs. Various studies have reported its insecticidal activity (Ainane et al., 2023). This compound is present in several Lamiaceae EOs and has a significant defensive role thanks to its repellent properties (Dancewicz et al., 2008). It can disrupt the reproduction, nutrition, growth, and embryonic development of certain insect species (Gunderson et al., 1985). Its toxic effect on various insects has been demonstrated and confirmed by Sousa et al. (2022).
Based on the present research findings, we suggest that the insecticidal activity observed against the different biological parameters of the cowpea pest C. maculatus, could be attributed to the high quantity of pulegone present in both EOs. Our observations confirm the findings of Božović and Ragno (2017), highlighting that pulegone is the most active compound among 20 monoterpenoids tested, causing a mortality rate of 100% in all five insect species tested (Sitophilus oryzae, Tribolium castaneum, Oryzaephilus surinamensis, Musca domestica, and Blattella germanica) at a dose of 50 mg/Lair. The pulegone exhibits a very high fumigation toxicity against adults of S. zeamais and T. castaneum (with LC50 = 3.47 and 11.56 mg cm−3, respectively) after 7 days of exposure; it proved more toxic than menthone, which presented LC50 values of 10.32 and 31.25 mg cm-3, respectively, against these pests (Koul et al., 2008; Liu et al., 2011). Pulegone appears as a potential useful element in the management of the corn weevil, S. zeamais, being the only compound that caused insect mortality among other bioactive compounds tested (Peschiutta et al., 2019). The fumigation toxicity of pulegone was higher than that of carvone and E-Z-ocimene against S. zeamais (Herrera et al., 2014).
The early mortality of C. maculatus adults, suggests that the action mode of the two EOs could be explained by the fumigant’s toxicity, which enters the insect’s body via its respiratory system. This can be explained as the fumigant and repellent properties of these EOs could concurrently influence different olfactory receptor neurons. Furthermore, monoterpenes are potent neurotoxins that could affect the inhibition of acetylcholinesterase enzyme in the central nervous system (Abdelgaleil et al., 2009; Abou-Taleb et al., 2016; Saad et al., 2018).
At the same time, various studies have confirmed that certain botanical pesticides interfere with the functioning of ion channels (“chloride channels” and “sodium channels” associated with glutamate) and block the action of acetylcholinesterase (AChE), leading to the suppression of neurons in insects and their eventual death. In some cases, insecticide toxicity results from the inhibition of mitochondrial activity by interruption of the protein phosphorylation process, and of chemosensory receptor cells via suppression of glucose and inositol stimulation (Rattan, 2010; Lengai et al., 2020; Rösner et al., 2020). Kiran and Prakash (2015) reported that inhibition of AChE function and changes in the antioxidant defense system, superoxide dismutase, catalase, glutathione, and oxidized glutathione, are related to the insecticidal potential exerted by Gaultheria procumbens L. EO. The work of Liao et al. (2016) notes the correlation between the insecticidal activity of Melaleuca alternifolia EO and changes in enzyme function (glutathione S-transferase, carboxylesterase, and acetylcholinesterase), including cytochrome P450, CarE, GST, and ATP-binding cassette transporters, against S. zeamais beetle. In accordance with some previous findings, certain EOs and monoterpenes have been described as AChE inhibitors or as positive allosteric modulators of GABA receptors (Miyazawa et al., 1997; Abdelgaleil et al., 2009; Abbad et al., 2023). Additionally, it was shown that the pulegone monoterpene has a high binding affinity for tyraminergic receptors, which play a crucial role in various vital insect physiological functions, including locomotion, reproduction, and response to pheromones (Ocampo et al., 2020).
It appears from the results obtained that the combination of components has increased efficacy, which could explain the effect of the minority compounds. The minor compounds in EOs play an important role in ensuring effective biological activity, reinforcing the results, and providing additional benefits (El-Shemy, 2018).
In fact, the minor constituents of EOs act synergistically, contributing to their effectiveness against insect pests (Linghu et al., 2016). Feng et al. (2020) pointed out that the minor components could play a crucial role in the toxicity of Valerianaceae species EOs against the red flour beetle Tribolium castaneum. Hategekimana and Erler (2020b) proved that the minor constituents of EOs played a significant role in insecticidal efficacy, highlighting the essential role of the complex composition of these oils in the expression of insecticidal properties.
The use of these EOs to combat insect pests in developing countries like Morocco, where plant biodiversity is rich and unique, is referred to as “green pesticides.” Their large-scale success would represent a veritable revolution in sustainable, environmentally friendly pest control. The development of EO-based bioinsecticides is booming, and expansion into existing and new markets is expected over the next decade (Isman, 2020). In this context, the successful use of EOs as biopesticides depends on a combination of factors: product availability, competitive pricing, and obtaining the necessary regulatory approvals (Isman, 2016).
The multitude of research projects dedicated to discovering new sources of insecticidal oils offer great potential, but the commercialization of new products remains uncertain and will depend on the ability to overcome challenges related to efficacy, safety, and production costs (Isman, 2017). This highlights the importance of close collaboration between researchers, developers, and industry to optimize the potential of essential oil-based bioinsecticides and meet the growing demand for sustainable pest management solutions.
The results of the present study suggest that the fumigant and repellent effects of Z. hispanica and S. calamintha EOs can affect the biological parameters of the cowpea insect pest C. maculatus. Our findings agree with the previous works, which indicate the success of plant EOs as bio-insecticides for the management of food products against the attacks of stored seed pests. The data revealed the high insecticidal efficacy of both EOs against adult males and females of C. maculatus. This could be attributed to its high volatility and its fumigant and repellent properties. Therefore, the EOs can serve as an ecological and efficient alternative for preserving stored food and reducing the issues associated with the use of synthetic insecticides. Further research is needed to deepen knowledge of these methods so that they can be used effectively and on a large scale, particularly in commercial applications. The formulation of these procedures is also essential. Additionally, their effectiveness in grain storage systems should be studied, and their odor should be fixed by appropriate methods. Finally, it is crucial to assess their potential toxicity to non-target organisms and humans to ensure their biosafety and enhance their credibility.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The animal study was approved by the Ethical Institutional Committee for the University Sidi Mohamed Ben Abdallah approved the protocol. All of the experimental proceedings using laboratory animals followed the Organization for Economic Cooperation and Development (OECD) guidelines 423. The study was conducted in accordance with the local legislation and institutional requirements.
AB: Conceptualization, Formal analysis, Methodology, Writing – original draft. YB: Conceptualization, Formal analysis, Methodology, Writing – review & editing. IE-s: Conceptualization, Writing – review & editing. RE: Data curation, Formal analysis, Writing – review & editing. HI: Data curation, Resources, Writing – review & editing. MA: Resources, Writing – review & editing. ON: Resources, Writing – review & editing. AS: Resources, Writing – review & editing. RG: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Princess Nourah bint Abdulrahman University, Researchers Supporting Project number (PNURSP2024R103), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Also funded by Researchers Supporting Project Number (RSPD2024R1057), King Saud University, Riyadh, Saudi Arabia.
The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2024R103), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors are also thankful to Researchers Supporting Project Number (RSPD2024R1057), King Saud University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2024.1329100/full#supplementary-material
Abbad, I., Soulaimani, B., and Abbad, A. (2023). Chemical composition, insecticidal and allelopathic properties of essential oils obtained from wild and cultivated Moroccan Satureja calamintha (L.). J. Nat. Pesticide Res. 3:100021. doi: 10.1016/j.napere.2023.100021
Abbott, W. S. (1925). A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18, 265–267. doi: 10.1093/jee/18.2.265a
Abdelgaleil, S. A., Mohamed, M. I., Badawy, M. E., and El-arami, S. A. (2009). Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 35, 518–525. doi: 10.1007/s10886-009-9635-3
Abebe, B. K., and Alemayehu, M. T. (2022). A review of the nutritional use of cowpea (Vigna unguiculata L. Walp) for human and animal diets. J. Agric. Food Res. 10:100383. doi: 10.1016/j.jafr.2022.100383
Abeywickrama, K., Adhikari, A. A. C. K., Paranagama, P., and Gamage, C. S. P. (2006). The efficacy of essential oil of Alpinia calcarata (Rosc.) and its major constituent, 1,8-cineole, as protectants of cowpea against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Can. J. Plant Sci. 86, 821–827. doi: 10.4141/P04-013
Abifarin, T. O., Otunola, G. A., and Afolayan, A. J. (2020). Chemical composition of essential oils obtained from Heteromorpha arborescens (Spreng.) cham. and schltdl leaves using two extraction methods. ScientificWorldJournal 2020:9232810. doi: 10.1155/2020/9232810
Abou-Taleb, H. K., Mohamed, M. I. E., Shawir, M. S., and Abdelgaleil, S. A. M. (2016). Insecticidal properties of essential oils against Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase and adenosine triphosphatases. Nat. Prod. Res. 30, 710–714. doi: 10.1080/14786419.2015.1038999
Ainane, T., Abdoul-Latif, F. M., Baghouz, A., El Montassir, Z., Attahar, W., Ainane, A., et al. (2023). Essential oils rich in pulegone for insecticide purpose against legume bruchus species: case of Ziziphora hispanica L. and Mentha pulegium L. AIMS Agric. Food 8, 105–118. doi: 10.3934/agrfood.2023005
Aker, O., Eser, F., and Yildirim, C. (2023). The laboratory evaluation of insecticidal activities and phytochemical analysis of Marrubium astracanicum Jacq. subsp. astracanicum Jacq. against Callosobruchus chinensis (L.) and Callosobruchus maculatus (F.). S. Afr. J. Bot. 160, 667–672. doi: 10.1016/j.sajb.2023.07.054
Alkan, M. (2020). Chemical composition and insecticidal potential of different Origanum spp.(Lamiaceae) essential oils against four stored product pests. Turk. J. Entomol. 44, 149–163. doi: 10.16970/entoted.620387
Attia, M. A., Wahba, T. F., Shaarawy, N., Moustafa, F. I., Guedes, R. N. C., and Dewer, Y. (2020). Stored grain pest prevalence and insecticide resistance in Egyptian populations of the red flour beetle Tribolium castaneum (Herbst) and the rice weevil Sitophilus oryzae (L.). J. Stored Prod. Res. 87:101611. doi: 10.1016/j.jspr.2020.101611
Avanza, M., Acevedo, B., Chaves, M., and Añón, M. (2013). Nutritional and anti-nutritional components of four cowpea varieties under thermal treatments: principal component analysis. LWT Food Sci. Technol. 51, 148–157. doi: 10.1016/j.lwt.2012.09.010
Baghouz, A., Bouchelta, Y., Es-safi, I., Bourhia, M., Abdelfattah, E. M., Alarfaj, A. A., et al. (2022). Identification of volatile compounds and insecticidal activity of essential oils from Origanum compactum Benth. and Rosmarinus officinalis L. against Callosobruchus maculatus (fab.). J. Chem. 2022, 1–9. doi: 10.1155/2022/7840409
Bekhechi, C., Bekkara, F. A., Abdelouahid, D. E., Liu, K., Casanova, J., and Tomi, F. (2007). Composition and antibacterial activity of the essential oil of Ziziphora hispanica (L.) from Algeria. J. Essent. Oil Bear. Plants 10, 318–323. doi: 10.1080/0972060X.2007.10643562
Bellakhdar, J. (1997). Contribution à l’étude de la pharmacopée traditionnelle au Maroc: la situation actuelle, les produits, les sources du savoir (enquête ethnopharmacologique de terrain réalisée de 1969 à 1992). Available at: https://hal.univ-lorraine.fr/tel-01752084
Bouchenak, M., and Lamri-Senhadji, M. (2013). Nutritional quality of legumes, and their role in Cardiometabolic risk prevention: a review. J. Med. Food 16, 185–198. doi: 10.1089/jmf.2011.0238
Boudjema, K., Bouanane, A., Gamgani, S., Djeziri, M., Abou Mustapha, M., and Fazouane, F. (2018). Phytochemical profile and antimicrobial properties of volatile compounds of Satureja calamintha (L) Scheel from northern Algeria. Trop. J. Pharm. Res. 17, 857–864. doi: 10.4314/tjpr.v17i5.16
Boukraa, N., Ladjel, S., Benlamoudi, W., Goudjil, M. B., Berrekbia, M., and Eddoud, A. (2022). Insecticidal and repellent activities of Artemisia herba-alba Asso, Juniperus phoenicea L and Rosmarinus officinalis L essential oils in synergized combinations against adults of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Biocatal. Agric. Biotechnol. 45:102513. doi: 10.1016/j.bcab.2022.102513
Bouzidi, N., and Kemieg, M. (2021). Antioxidant and antimicrobial activities of essential oil of Satureja calamintha ssp. nepeta (L.) Briq. from the northwest of Algeria. Agric. Conspec. Sci. 86, 349–356.
Božović, M., and Ragno, R. (2017). Calamintha nepeta (L.) Savi and its Main essential oil constituent Pulegone: biological activities and chemistry. Molecules 22:290. doi: 10.3390/molecules22020290
Bryssine, P. (1962). Comportament des varietes de Vigna sinens-Savi et possibilites de sa culture au Maroc. Al Awamia PRESS: Rabat 3, 1–56.
Chaubey, M. K. (2019). Essential oils as green pesticides of stored grain insects. Eur. J. Biol. Res. 9, 202–244. doi: 10.5281/zenodo.3528366
Dancewicz, K., Gabrys, B., Dams, I., and Wawrzeńczyk, C. (2008). Enantiospecific effect of pulegone and pulegone-derived lactones on Myzus persicae (Sulz.) settling and feeding. J. Chem. Ecol. 34, 530–538. doi: 10.1007/s10886-008-9448-9
De Andrade Rodrigues, R. M. B., Da Silva Fontes, L., De Carvalho Brito, R., Barbosa, D. R. E. S., Das Graças Lopes Citó, A.MDo Carmo, I. S., et al. (2022). A sustainable approach in the management of Callosobruchus maculatus: essential oil of Protium heptaphyllum and its major compound d-limonene as biopesticides. J. Plant Dis. Prot. 129, 831–841. doi: 10.1007/s41348-022-00617-4
De Brito, G. A., Rocha De Oliveira, P. F., De Andrade Silva, C. M., De Araújo Neto, M. F., Leite, F. H. A., Mesquita, P. R. R., et al. (2021). Identification of bioactive compounds against Aedes aegypti (Diptera: Culicidae) by bioassays and in silico assays. Chem. Biodivers. 18:e2100242. doi: 10.1002/cbdv.202100242
Demirel, N., and Erdogan, C. (2017). Insecticidal effects of essential oils from Labiatae and Lauraceae families against cowpea weevil, Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in stored pea seeds. Entomol. Appl. Sci. Lett 4, 13–19.
El Brahimi, R., El Barnossi, A., El Moussaoui, A., Chebaibi, M., Kachkoul, R., Baghouz, A., et al. (2023). Phytochemistry and biological activities of essential oils from Satureja calamintha Nepeta. Separations 10:344. doi: 10.3390/separations10060344
Feng, Y.-X., Wang, Y., Geng, Z.-F., Zhang, D., Almaz, B., and Du, S.-S. (2020). Contact toxicity and repellent efficacy of Valerianaceae spp. to three stored-product insects and synergistic interactions between two major compounds camphene and bornyl acetate. Ecotoxicol. Environ. Saf. 190:110106. doi: 10.1016/j.ecoenv.2019.110106
Fennane, M., and Rejdali, M. (2016). Aromatic and medicinal plants of Morocco: richness, diversity and threats.
Finney, D.J. (1971). Bulletin de l’Institut Scientifique, Rabat, Section Sciences de la Vie, 2016, n°, 38, 27–42.
Gharib, F. A., Mansour, K. H., Ahmed, E. Z., and Galal, T. M. (2020). Heavy metals concentration, and antioxidant activity of the essential oil of the wild mint (Mentha longifolia L.) in the Egyptian watercourses. Int. J. Phytoremediation 23, 1–11. doi: 10.1080/15226514.2020.1847035
Gunderson, C. A., Samuelian, J. H., Evans, C. K., and Brattsten, L. B. (1985). Effects of the mint monoterpene Pulegone on Spodoptera eridania (Lepidoptera: Noctuidae). Environ. Entomol. 14, 859–863. doi: 10.1093/ee/14.6.859
Gupta, H., Deeksha, U., and Reddy, S. E. (2023). Insecticidal and detoxification enzyme inhibition activities of essential oils for the control of pulse beetle, Callosobruchus maculatus (F.) and Callosobruchus chinensis (L.)(Coleoptera: Bruchidae). Molecules 28:492. doi: 10.3390/molecules28020492
Hategekimana, A., and Erler, F. (2020a). Fecundity and fertility inhibition effects of some plant essential oils and their major components against Acanthoscelides obtectus say (Coleoptera: Bruchidae). J. Plant. Dis. Prot. 127, 615–623. doi: 10.1007/s41348-020-00311-3
Hategekimana, A., and Erler, F. (2020b). Comparative repellent activity of single, binary and ternary combinations of plant essential oils and their major components against Sitophilus oryzae L.(Coleoptera: Curculionidae). J. Plant Diseases Prot. 127, 873–881. doi: 10.1007/s41348-020-00353-7
Herrera, J., Zunino, M., Massuh, Y., Pizzolitto, R., Dambolena, J., Gañan, N., et al. (2014). Fumigant toxicity of five essential oils rich in ketones against Sitophilus zeamais (Motschulsky). Agri Scientia 31, 35–41. doi: 10.31047/1668.298x.v31.n1.9839
Hussain, A. I., Anwar, F., Shahid, M., Ashraf, M., and Przybylski, R. (2010). Chemical composition, and antioxidant and antimicrobial activities of essential oil of spearmint (Mentha spicata L.) from Pakistan. J. Essent. Oil Res. 22, 78–84. doi: 10.1080/10412905.2010.9700269
Ileke, K. D., Idoko, J. E., Ojo, D. O., and Adesina, B. C. (2020). Evaluation of botanical powders and extracts from Nigerian plants as protectants of maize grains against maize weevil, Sitophilus zeamais (Motschulsky)[Coleoptera: Curculionidae]. Biocatal. Agric. Biotechnol. 27:101702. doi: 10.1016/j.bcab.2020.101702
Isman, M.B. (2016). Pesticides based on plant essential oils: phytochemical and practical considerations, In Jeliazkov Zheljazkov, V.D., C.L. Cantrell (Eds.), ACS symposium series. American Chemical Society: Washington, DC, 13–26
Isman, M. B. (2017). Bridging the gap: moving botanical insecticides from the laboratory to the farm. Ind. Crop. Prod. 110, 10–14. doi: 10.1016/j.indcrop.2017.07.012
Isman, M. B. (2020). Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 19, 235–241. doi: 10.1007/s11101-019-09653-9
Jawhari, F. Z., El Moussaoui, A., Bourhia, M., Imtara, H., Mechchate, H., Es-Safi, I., et al. (2020). Anacyclus pyrethrum (L): chemical composition, analgesic, anti-inflammatory, and wound healing properties. Molecules 25:5469. doi: 10.3390/molecules25225469
Jaya,, Singh, P., Prakash, B., and Dubey, N. K. (2014). Insecticidal activity of Ageratum conyzoides L., Coleus aromaticus Benth. And Hyptis suaveolens (L.) Poit essential oils as fumigant against storage grain insect Tribolium castaneum Herbst. J. Food Sci. Technol. 51, 2210–2215. doi: 10.1007/s13197-012-0698-8
Kébé, K., Alvarez, N., and Espíndola, A. (2020). Oviposition choice and larval development of the seed beetle Callosobruchus maculatus (F.)(Coleoptera: Chrysomelidae: Bruchinae) on three cowpea varieties. J. Stored Prod. Res. 86:101578. doi: 10.1016/j.jspr.2020.101578
Kerbouche, L., Hazzit, M., and Baaliouamer, A. (2013). Essential oil of Satureja calamintha subsp. nepeta (L.) Briq. From Algeria: analysis, antimicrobial and antioxidant activities. J. Biol. Act. Prod. Nat. 3, 266–272. doi: 10.1080/22311866.2013.833466
Kergoat, G. J., Delobel, A., Le Ru, B., and Silvain, J.-F. (2008). Seed beetles in the age of the molecule: recent advances on systematics and host-plant association patterns. Res. Chrysomelidae 1, 59–86.
Kheirkhah, M., Ghasemi, V., Yazdi, A. K., and Rahban, S. (2015). Chemical composition and insecticidal activity of essential oil from Ziziphora clinopodioides Lam. used against the Mediterranean flour moth, Ephestia kuehniella Zeller. J. Plant Protect. Res. 55, 260–265. doi: 10.1515/jppr-2015-0037
Kiran, S., and Prakash, B. (2015). Toxicity and biochemical efficacy of chemically characterized Rosmarinus officinalis essential oil against Sitophilus oryzae and Oryzaephilus surinamensis. Ind. Crop. Prod. 74, 817–823. doi: 10.1016/j.indcrop.2015.05.073
Koul, O., Walia, S., and Dhaliwal, G. S. (2008). Essential oils as green pesticides: potential and constraints. Biopesticides International, 4:63–84.
Kouris-Blazos, A., and Belski, R. (2016). Health benefits of legumes and pulses with a focus on Australian sweet lupins. Asia Pac. J. Clin. Nutr. 25, 1–17. doi: 10.6133/apjcn.2016.25.1.23
Lahsissene, H., Kahouadji, A., and Hseini, S. (2009). Catalogue des plantes médicinales utilisées dans la région de Zaër (Maroc Occidental). Lejeunia revue de botanique
Lengai, G. M., Muthomi, J. W., and Mbega, E. R. (2020). Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 7:e00239. doi: 10.1016/j.sciaf.2019.e00239
Liao, M., Xiao, J.-J., Zhou, L.-J., Liu, Y., Wu, X.-W., Hua, R.-M., et al. (2016). Insecticidal activity of Melaleuca alternifolia essential oil and RNA-Seq analysis of Sitophilus zeamais transcriptome in response to oil fumigation. PLoS One 11:e0167748. doi: 10.1371/journal.pone.0167748
Linghu, K., Lin, D., Yang, H., Xu, Y., Zhang, Y., Tao, L., et al. (2016). Ameliorating effects of 1,8-cineole on LPS-induced human umbilical vein endothelial cell injury by suppressing NF-κB signaling in vitro. Eur. J. Pharmacol. 789, 195–201. doi: 10.1016/j.ejphar.2016.07.039
Liu, Z. L., Chu, S. S., and Jiang, G. H. (2011). Toxicity of Schizonpeta multifida essential oil and its constituent compounds towards two grain storage insects. J. Sci. Food Agric. 91, 905–909. doi: 10.1002/jsfa.4263
Lolestani, F. A., and Shayesteh, N. (2009). Fumigant toxicity of Ziziphora clinopodioides (Boiss.) (Lamiaceae) against adults and eggs of Callosobruchus maculatus (Fab.) (Coleoptera: Bruchidae). J. Biol. Sci. 9, 92–95. doi: 10.3923/jbs.2009.92.95
Maphosa, Y., and Jideani, V. A. (2017). The role of legumes in human nutrition. Agric. Food Sci. Med. 10:66263. doi: 10.5772/intechopen.69127
Marques, S. De P.P.M., Pinheiro, R.O., Nascimento, R.A.Do, Andrade, E.H. De A., and De Faria, L.J.G. (2023). Effects of harvest time and Hydrodistillation time on yield, composition, and antioxidant activity of mint essential oil. Molecules 28:7583, doi: 10.3390/molecules28227583
Martynov, V. O., Titov, O. G., Kolombar, T. M., and Brygadyrenko, V. V. (2019). Influence of essential oils of plants on the migration activity of Tribolium confusum (Coleoptera, Tenebrionidae). Biosys. Divers. 27, 177–185. doi: 10.15421/011924
Matos, L. F., Barbosa, D. R. S., Lima, E. C., Dutra, K. A., Navarro, D. M. A. F., Alves, J. L. R., et al. (2020). Chemical composition and insecticidal effect of essential oils from Illicium verum and Eugenia caryophyllus on Callosobruchus maculatus in cowpea. Ind. Crop. Prod. 145:112088. doi: 10.1016/j.indcrop.2020.112088
McDonald, L. L., Guy, R. H., and Speirs, R. D. (1970). Preliminary evaluation of new candidate materials as toxicants, repellents, and attractants against stored-product insects US Agricultural Research Service.
Mishra, B. B., Tripathi, S. P., and Tripathi, C. P. M. (2012). Repellent effect of leaves essential oils from Eucalyptus globulus (Mirtaceae) and Ocimum basilicum (Lamiaceae) against two major stored grain insect pests of coleopterons. Nat. Sci. 10, 50–54.
Miyazawa, M., Watanabe, H., and Kameoka, H. (1997). Inhibition of acetylcholinesterase activity by monoterpenoids with ap-menthane skeleton. J. Agric. Food Chem. 45, 677–679. doi: 10.1021/jf960398b
Mohan, S., Pretheep-Kumar, P., and Balasubramanian, P. (2010). Insecticide resistance-stored-product insects. Dusseldorf: LAP Lambert Academic Publishing.
Najem, M., Nassiri, L., and Ibijbijen, J. (2021). Vernacular names of plants between diversity and potential risks of confusion: case of toxic plants used in medication in the central middle atlas, Morocco. J. Pharm. Pharmacogn. Res. 9, 222–250. doi: 10.56499/jppres20.950_9.2.222
Negueruela, A. V., and Rico, M. M. (1986). The volatile oil of Ziziphora hispanica L. Flav. Fragr. J 1, 111–113. doi: 10.1002/ffj.2730010304
Nisar, M. S., Ali, S., Hussain, T., Ramzan, H., Niaz, Y., Haq, I. U., et al. (2022). Toxic and repellent impacts of botanical oils against Callosobruchus maculatus (Bruchidae: Coleoptera) in stored cowpea [Vigna unguiculata (L.) Walp.]. PLoS One 17:e0267987. doi: 10.1371/journal.pone.0267987
Ocampo, A. B., Braza, M. K. E., and Nellas, R. B. (2020). The interaction and mechanism of monoterpenes with tyramine receptor (SoTyrR) of rice weevil (Sitophilus oryzae). SN Appl. Sci. 2, 1–9. doi: 10.1007/s42452-020-03395-6
Onyido, A. E., Zeibe, C. C., Okonkwo, N. J., Ezugbo-Nwobi, I. K., Egbuche, C. M., Udemezue, I. O., et al. (2011). Damage caused by the bean Bruchid, Callosobruchus maculatus (Fabricius) on different legume seeds on sale in Awka and Onitsha markets, Anambra state, South Eastern Nigeria. Afr. Res. Rev. 5:5. doi: 10.4314/afrrev.v5i4.69264
Peschiutta, M. L., Brito, V. D., Achimón, F., Zunino, M. P., Usseglio, V. L., and Zygadlo, J. A. (2019). New insecticide delivery method for the control of Sitophilus zeamais in stored maize. J. Stored Prod. Res. 83, 185–190. doi: 10.1016/j.jspr.2019.06.013
Phillips, R. D. (1993). Starchy legumes in human nutrition, health and culture. Plant Food Hum. Nutr. 44, 195–211. doi: 10.1007/BF01088314
Piri, E., Mahmoodi Sourestani, M., Khaleghi, E., Mottaghipisheh, J., Zomborszki, Z. P., Hohmann, J., et al. (2019). Chemo-diversity and antiradical potential of twelve Matricaria chamomilla L. populations from Iran: proof of ecological effects. Molecules 24:1315. doi: 10.3390/molecules24071315
Rabah, B., Lograda, T., Ramdani, M., Chalard, P., and Feguiredo, G. (2013). Chemical composition and antibacterial activity of essential oil of ziziphora hispanica l. Glob. J. Res. Med. Plants Indig. Med. 2:73.
Rastegar, F., Moharramipour, S., Shojai, M., and Abbasipour, H. (2011). Chemical composition and insecticidal activity of essential oil of Zataria multiflora Boiss.(Lamiaceae) against Callosobruchus maculatus (F.)(Coleoptera: Bruchidae) France: IOBC/WPRS.
Rattan, R. S. (2010). Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 29, 913–920. doi: 10.1016/j.cropro.2010.05.008
Redhouane, B., Khaled, B., Hadjira, B., Imene, I., Abderrezak, K., Fethia, F., et al. (2020). Acta Period. Technol, 87–102. doi: 10.2298/APT2051087R
Romani, R., Bedini, S., Salerno, G., Ascrizzi, R., Flamini, G., Echeverria, M. C., et al. (2019). Andean flora as a source of new repellents against insect pests: behavioral, morphological and electrophysiological studies on Sitophilus zeamais (coleoptera: Curculionidae). Insects 10:171. doi: 10.3390/insects10060171
Rösner, J., Wellmeyer, B., and Merzendorfer, H. (2020). Tribolium castaneum: a model for investigating the mode of action of insecticides and mechanisms of resistance. Curr. Pharm. Des. 26, 3554–3568. doi: 10.2174/1381612826666200513113140
Saad, M. M. G., Abou-Taleb, H. K., and Abdelgaleil, S. A. M. (2018). Insecticidal activities of monoterpenes and phenylpropenes against Sitophilus oryzae and their inhibitory effects on acetylcholinesterase and adenosine triphosphatases. Appl. Entomol. Zool. 53, 173–181. doi: 10.1007/s13355-017-0532-x
Saeidi, K., and Mirfakhraie, S. (2017). Chemical composition and insecticidal activity Mentha piperita L. essential oil against the cowpea seed beetle Callosobruchus maculatus F. (Coleoptera: Bruchidae). J. Entomol. Acarol. Res. 49:6769. doi: 10.4081/jear.2017.6769
Sarma, R., Adhikari, K., Mahanta, S., and Khanikor, B. (2019). Combinations of plant essential oil based terpene compounds as larvicidal and adulticidal agent against Aedes aegypti (Diptera: Culicidae). Sci. Rep. 9:9471. doi: 10.1038/s41598-019-45908-3
Satongrod, B., and Wanna, R. (2020). Chemical composition and bioactivity of essential oil from Indian borage (Plectranthus amboinicus (Lour.) Spreng) against Callosobruchus maculatus (F.). Int. J. Agric. Technol 16, 1243–1256.
Seri-Kouassi, B. P., Kanko, C., Aboua, L. R. N., Bekon, K. A., Glitho, A. I., Koukoua, G., et al. (2004). Action des huiles essentielles de deux plantes aromatiques de Côte-d’Ivoire sur Callosobruchus maculatus F. du niébé. C. R. Chim. 7, 1043–1046. doi: 10.1016/j.crci.2003.12.031
Singh, N., Jain, P., Ujinwal, M., and Langyan, S. (2022). Escalate protein plates from legumes for sustainable human nutrition. Front. Nutr. 9:977986. doi: 10.3389/fnut.2022.977986
Singh, U., and Singh, B. (1992). Tropical grain legumes as important human foods. Econ. Bot. 46, 310–321. doi: 10.1007/BF02866630
Sousa, P. A., Neto, J., Bastos, M. M., and Aguiar, A. A. (2022). Eugenol and Pulegone as potential biorational alternatives for Trioza erytreae (Hemiptera: Triozidae) control: preliminary results on nymphal toxicity and applicability on Citrus limon. J. Nat. Pesticide Res. 1:100004. doi: 10.1016/j.napere.2022.100004
Teke, M. A., and Mutlu, Ç. (2021). Insecticidal and behavioral effects of some plant essential oils against Sitophilus granarius L. and Tribolium castaneum (Herbst). J. Plant Dis. Prot. 128, 109–119. doi: 10.1007/s41348-020-00377-z
Tibaldi, G., Fontana, E., and Nicola, S. (2013). Postharvest management affects spearmint and calamint essential oils. J. Sci. Food Agric. 93, 580–586. doi: 10.1002/jsfa.5836
Topuz, E., Madanlar, N., and Erler, F. (2018). Chemical composition, toxic and development- and reproduction-inhibiting effects of some essential oils against Tetranychus urticae Koch (Acarina: Tetranychidae) as fumigants. J Plant Dis Prot 125, 377–387. doi: 10.1007/s41348-018-0161-9
Yang, X., Jin, C., Wu, Z., Han, H., Zhang, Z., Xie, Y., et al. (2023). Toxicity and physiological effects of nine Lamiaceae essential oils and their major compounds on Reticulitermes dabieshanensis. Molecules 28:2007. doi: 10.3390/molecules28052007
Yao, J., Chen, C., Wu, H., Chang, J., Silver, K., Campbell, J. F., et al. (2019). Differential susceptibilities of two closely-related stored product pests, the red flour beetle (Tribolium castaneum) and the confused flour beetle (Tribolium confusum), to five selected insecticides. J. Stored Prod. Res. 84:101524. doi: 10.1016/j.jspr.2019.101524
Keywords: cowpea, Callosobruchus maculatus , Ziziphora hispanica , Satureja calamintha , essential oils, biological parameters
Citation: Baghouz A, Bouchelta Y, Es-safi I, El Brahimi R, Imtara H, AlZain MN, Noman OM, Shahat AA and Guemmouh R (2024) Biocidal activity of Ziziphora hispanica L and Satureja calamintha Scheele L essential oils against the Callosobruchus maculatus (Fabricius) pest on cowpea seeds during storage. Front. Sustain. Food Syst. 8:1329100. doi: 10.3389/fsufs.2024.1329100
Received: 27 October 2023; Accepted: 25 March 2024;
Published: 12 April 2024.
Edited by:
Abhay K. Pandey, Tea Research Association, IndiaReviewed by:
Francimauro Morais, Instituto Federal de Educação, Ciência e Tecnologia do Amazonas (IFAM), BrazilCopyright © 2024 Baghouz, Bouchelta, Es-safi, El Brahimi, Imtara, AlZain, Noman, Shahat and Guemmouh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Asmae Baghouz, YXNtYWVfYmlvOTFAeWFob28uZnI=; Hamada Imtara, aGFtYWRhLnRhcmF5cmFoQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.