
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sustain. Food Syst. , 01 February 2024
Sec. Waste Management in Agroecosystems
Volume 8 - 2024 | https://doi.org/10.3389/fsufs.2024.1321353
Farming is responsible for 30% of global anthropogenic emissions. A novel technology, aligning with current regulations of covering slurry stores, has been developed for processing anaerobic digestate liquor, which is obtained from the screw press stage. Instead of using hazardous chemicals, such as sulfuric acid, to capture ammonia and greenhouse gases, the artifact contains calcium chloride as deliquescent salt. Preliminary simulations in Aspen Plus® v12 showed that the absorption of NH3 and carbon dioxide during the solid–liquid separation was feasible and the resulting clean brine could be used as chemical amendment of untreated slurry. The characterization of the performance of the artifact was organized following a project-based learning active teaching method, for a group of 3 students (17-year-olds) of secondary education, as part of the In2ScienceUK program. The collection of empirical data allowed the contents of the curriculum to be contextualized, enhancing environmental awareness of participants. A titrimetric method was employed to determine the content of NH3 and CO2 in the brine, and the granulation of the dewatered liquor with the fibers of anaerobic digestate was investigated by determining the compression strength required to break the pellets. The operation capacity of the prototype was found to be 250 m3 of liquor per year, at a cost of £1/m3. The use of the filtered brine as chemical amendment also represented an additional 5.60 grams of ammoniacal nitrogen and 0.96 grams of inorganic carbon per cubic meter of unprocessed slurry. The concentrated liquor was a good binding agent that provided the pellets with a compressive strength of 207.00 ± 26.36 N, which was above the threshold value for commercialization purposes (50 N). The advances in the development of the prototype enhanced the suitability of the technology for industrial scale applications but hindered the adoption of subsequent editions of this pedagogic tool.
Farming is responsible for 30% of global anthropogenic emissions (Lal, 2021). In the UK, the Clean Air Strategy (UK Government, 2019) is an essential element of the Agricultural Transition Plan, which has been established as roadmap for the period 2021 to 2024 (DEFRA, 2020, p. 55). These regulations include a slurry investment scheme, which grants funding for new slurry stores and equipment to protect the environment by covering slurry and digestate stores or using slurry bags & using low emissions techniques for spreading slurries and digestate on land (for example, by injection, trailing shoe or trailing hose) (UK Government, 2019, p. 68). Many farms are opting for the CATNAP (Cheapest Available Technology Narrowly Avoiding Prosecution) approach that is not fully regulated (e.g., use of chemical amendments of slurry) and likely not very cost-effective (Lanigan et al., 2018; Buckley et al., 2020). This opens the possibility of developing and commercializing new technologies to enhance the rate of adoption by stakeholders (Hou et al., 2018). Some of these slurry additives claim to reduce gaseous emissions despite being cocktails of microbes that promote the fermentation of slurry (Kavanagh et al., 2021).
A modification of the static chamber method for measuring gaseous emissions from organic manures (Van der Stelt et al., 2007; Whelan et al., 2010) has been developed, taking into account all the possible synergies with the existing farm infrastructure, to minimize the cost of processing and land application of the slurry and keep the expenditure below £10/m3 (Moure Abelenda and Amaechi, 2022). The technology meets current regulations and corresponding subsidies available for farmers to seal their slurry stores (UK DEFRA, 2020) and process the manure in a closed environment which allows the saturation of the atmosphere, minimizes the emission of greenhouse gases, and maximizes the retention of nitrogen in the organic fertilizer. This technology combines the deliquescence property of the calcium chloride to enable the solid–liquid separation of slurry (Hjorth et al., 2009) and the formation of a chemical amendment (Husted et al., 1991; Vandré and Clemens, 1996). The maximum performance of the prototype is attained by optimizing this physicochemical exothermic reaction that takes place in the tank of condensation and brine formation (Figure 1). To ensure the best contact of the anhydrous salt with the moisturized air coming from the stabilization tank, this deliquescent material will be suspended in a net/tray in the condensation tank. By increasing the power consumption of the heating circuit in the stabilization tank, tubular fan for greater turbulence and mass transfer in headspace, or even cooling in the condensation tank (APROVIS Energy Systems Gmbh, 2023), it might be possible to attain the processing capacity specified in the guidelines published by the UK Government (2023) on eligibility of slurry processing items: Screw press slurry separator, capable of processing a minimum of 10 m3/h, with a resulting solid fraction of 30% dry matter according to the model’s theoretical capability. All other forms of slurry separation are not eligible under this item. However, it is important to highlight that the design of the novel artifact considers the current logistics of managing organic manures with the aim of minimizing the power consumption, which for commercial solid–liquid separation equipment is often around 3.0 to 7.5 kW (CRI-MAN S.p.A, n.d.), while still allowing the complete dehydration of the 3% of the total organic slurries (with >95% moisture) produced in farms.

Figure 1. Schematic of the artifact for solid–liquid separation of organic slurry and subsequent stabilization of the solid granular fertilizer with the production of a brine of CaCl2 rich in ammonia and carbon dioxide. Adapted from Moure Abelenda et al. (2023b) with the permission of the journal Environmental Technology and Innovation (Elsevier).
Additionally, unlike the use of hazardous chemicals to fix the NH3 of manures, such as sulfuric acid (Ndegwa et al., 2009; Guštin and Marinšek-Logar, 2011; Liu et al., 2015; Costamagna et al., 2020), it is possible to use a brine of sodium chloride, as in the Solvay process (Steinhauser, 2008). A similar strategy was already proposed by Fujioka and Ito (2020), but still the use of H2SO4 could not be avoided to recover NH3. The novelty of the prototype of Moure Abelenda et al. (2023b) for manure treatment offers, thereby, safer and more convenient processing conditions. A preliminary investigation was conducted in the process simulation package Aspen Plus® v12 to evaluate the likelihood of absorption and trapping CO2 and NH3 in brines of NaCl and CaCl2 (Equations 1, 2). For these simulations it was assumed that CO2 and NH3 were emitted at a rate of 132 mg/h and up to 5.1–510 mg/h, respectively, during the storage of manure at 25°C (Moure Abelenda, 2023a). The estimation of the most representative conditions to model the operation of the prototype (Figure 1) was based on the average content of CO2 and NH3 in the anaerobic digestate (~3 g/L CO2 and ~ 2 g/L NH3; Akhiar et al., 2017) and the operating capacity that was experimentally found: dehydrating 5 L anaerobic digestate liquor every 24 h. A simulation with the flash tank as calculation block, implemented ELECNRTL property method to model the liquid–gas mixture CO2-NH3-H2O with the above composition (995 g H2O, 3 g CO2, and 2 g NH3), resulted in no emission of volatile compounds (Moure Abelenda et al., 2023a). Therefore, the sensitivity analysis when using the anhydrous CaCl2 deliquescent salt to trap CO2 and NH3, was applied to the minimum volatilization conditions, as it is widely reported that there are gaseous emissions from organic manures (Kavanagh et al., 2021; Lal, 2021). From the fertilization point of view, it was considered that the most valuable compound that needed to be recovered was NH3. Importantly, it was found that the limiting factor to enable Equation (2) was the rate of volatilization of NH3, that is why this parameter was studied in more detail than the rate of volatilization of CO2 (Supplementary Figures 1–3). Similarly, the variability of the amount of CaCl2 was also investigated and the results of the simulation showed that the first formation of NH4Cl could be seen when the prototype operates with >5 kg CaCl2 and volatilization rates >100 mg/h NH3 (Supplementary Figure 3).
Depending on the extent of the deliquescence phenomenon, a liquid film over the solid CaCl2 could be more relevant in the condensation tank (i.e., earlier in the 24-h operation) than the liquid brine (i.e., aqueous solution), as the latter prevailed at more advanced stages of the dehydration of the anaerobic digestate liquor. The supersaturated solution of 0.5 kg H2O and 10 kg anhydrous salt was considered to account for any possible absorption in the liquid film that is generated during the deliquescence phenomenon (Supplementary Figure 1). Only in the case of using CaCl2 as dehydrating agent in the prototype, the simulation predicted the absorption of CO2 and NH3 plus the formation of calcium carbonate (Supplementary Figure 2) and ammonium chloride (Supplementary Figure 3), as per the stoichiometry of Equation (2). On the other hand, the extents of formation of sodium bicarbonate, according to Equation (1), and even ammonium bicarbonate or ammonium carbamate as described in a previous investigation (Moure Abelenda et al., 2023a) were found to be negligible under the conditions that the prototype operates. The greater capacity of the CaCl2, compared to NaCl, to absorb the NH3 can be seen by comparing the stoichiometries of Equations (1, 2). Another reason for the performance differences of the salts is more acid being required to neutralize the basicity of calcium oxide than that of sodium oxide; hence, the CaCl2 “contains” more hydrochloric acid than the NaCl. The formation of precipitates was validated with the empirical data available in the literature for the different solid forms of CaCl2∙xH2O (x being equal to 0, 1, 2, 4, and 6; Supplementary Figure 4), CaCO3 and calcium hydroxide (Supplementary Figure 5), and NaCl, NH4Cl, and NaHCO3 (Supplementary Figure 6).
It is necessary to experimentally validate the results of the simulation and confirm that the absorption of CO2 and NH3 in the forming brine of a deliquescent anhydrous salt is feasible. As described previously (Moure Abelenda et al., 2023b), since the functioning of the prototype relies on basic science (e.g., acid–base reactions, Ohm’s law, Watt’s law, etc.), its development could be also considered as part of a didactic proposal (i.e., project-based learning as active teaching-learning method) to address the educational needs of K-16 students (e.g., lack of contextualization of the contents of the curriculum, excess of propaedeutic approach, classroom not representing the most suitable learning environment, etc.). It is clear that the type of egg drop projects (Houpt, 2004), which are purely focused on scientific aspects of the design of the artifact, does not help students to understand their role in society and to acquire the skills to become active citizens promoting sustainable development. The present investigation describes the outcomes of a flexible model to support the knowledge transfer and the commercialization of this technology, where the artifact was developed to address the needs of the potential clients (i.e., stakeholders of the agroindustry). For the development of the prototype that addresses the current needs of agroindustry, the design relied on basic science because the emphasis was placed on searching for synergistic solutions that would demand the minimum consumption of energy and resources. Sufficient background was provided to secondary education students for them to play an active role in this research activity, which aimed to generate a significant contribution to manure processing technology. At the same time, the suitability of the pedagogic tool and didactic units that compose the different hands-on sessions of the project-based learning (PBL) were tested for addressing the educational needs of Advanced level (A-level) students in the UK. The preliminary SWOT (Strengths, Weakness, Opportunities, and Threats) analysis of the didactic proposal estimates that the outreach event will constitute a transversal experience for A-level students (Moure Abelenda et al., 2023b).
The didactic proposal (Make it happen!) of Moure Abelenda et al. (2023b) was adapted to fit the activities for a week of placements under the In2ScienceUK (2023a) program. A group of 3 students (17-year-old), selected following the criteria of In2ScienceUK (2023a), was hosted at the School of Engineering of Lancaster University for 5 days, and supported to advance the development of the prototype from its original incarnation (Figure 1). By accepting their place on the program, students confirmed that they have read, understood, and agree to abide by the code of conduct to In2ScienceUK (2023b), where is explicitly written: Be fully engaged in all tasks and actively participate in discussions. Additionally, the parents/guardians were asked for photo/video consent (or lack thereof) for the students, recordings consent (in case of online workshops and meetings involving students for safeguarding purposes), and travel consent for the students to be allowed to travel to and from their placements. This PBL activity (Make it happen!) provided them with experience of an entrepreneurial project beyond academic research. Table 1 details the items that were made available for students to replicate and improve the design of the artifact, with a total cost of approximately £700.
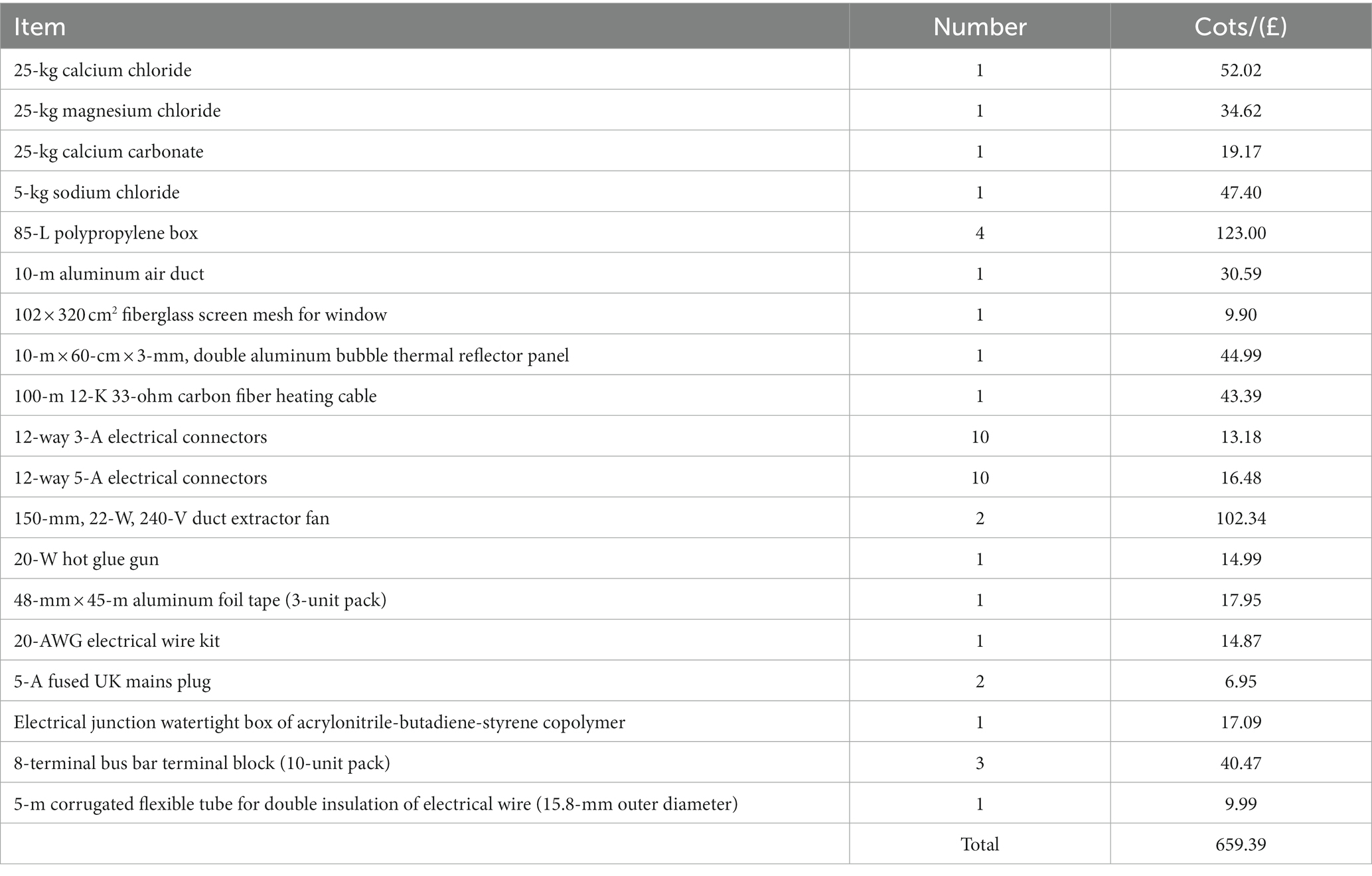
Table 1. List of components that were purchased to allow the 17-year-old students to replicate the artifact and propose improvements.
To evaluate the technical feasibility of the novel technology, it was necessary to collect empirical data that would provide insights several of parameters: rate of solid–liquid separation, power consumption, fixation of CO2 and NH3 during the stabilization process, and suitability of the isolated organic matter for granulation purposes. Among the whole chain of physicochemical effects on which the novel technology relies, the bottleneck of the process was considered to be the kinetics of the hydration of the calcium chloride. Therefore, the operating conditions of the artifact needed to be optimized for this slow stage, which indicated providing the condensation chamber with a sufficient amount of saturated vapor. Standard Operating Procedure, Risk Assessment, and form of COSHH (Control of Substances Hazardous to Health) were completed in line with the standard safety requirements, in addition to safeguarding of children (any person under the age of 18 years). As described in Table 2, the morning sessions ran from 10:00 am to 12:30 and the afternoon sessions went from 13:30 to 16:00.
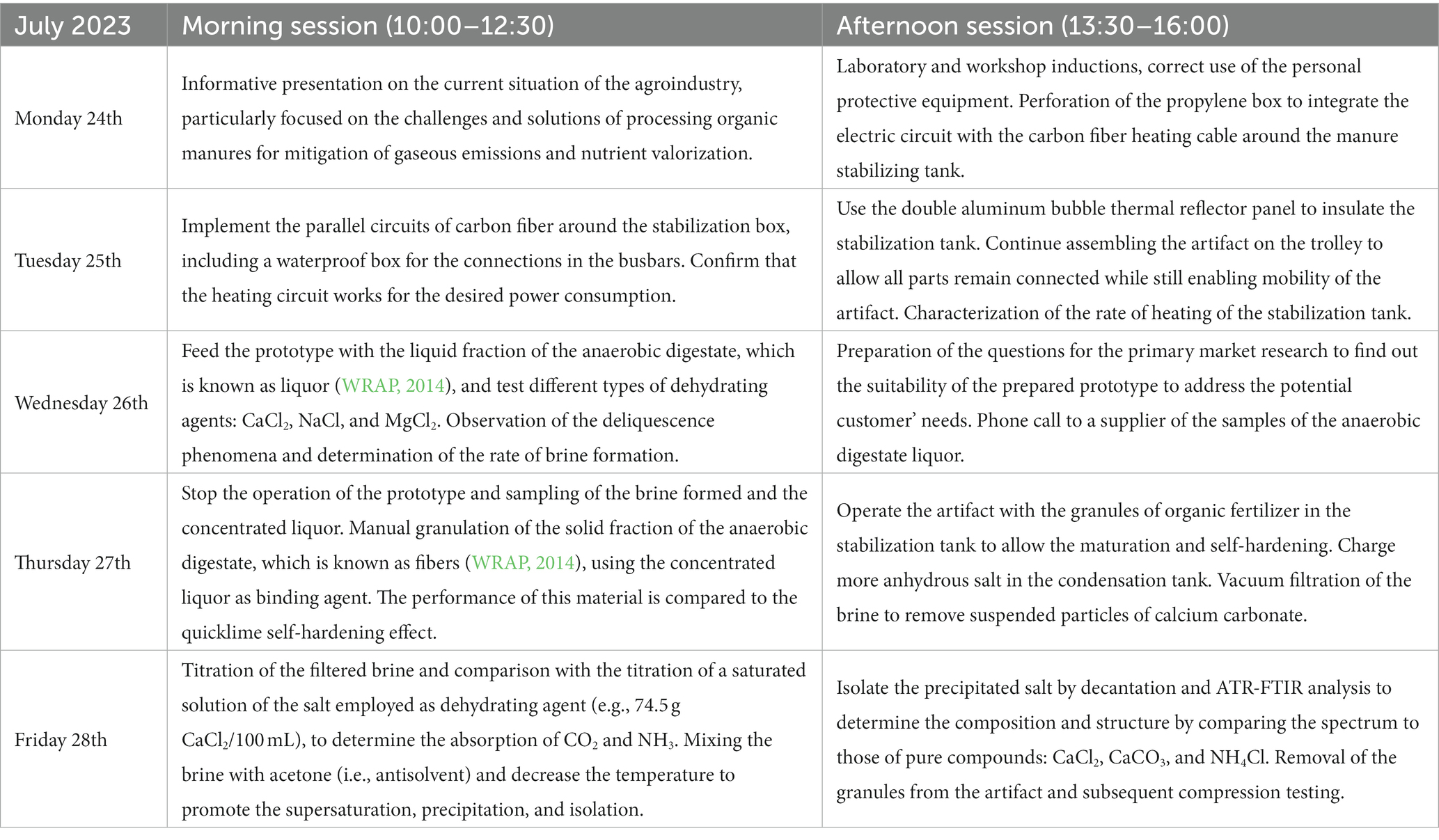
Table 2. Details of the different sessions in which was divided the work for the In2ScienceUK placements at Lancaster University.
The first morning session consisted of a presentation on the background of the project and students were able to visualize the traditional processing technological solutions (e.g., screw press, membrane treatment, etc.) available on the market to address the problem of handling organic slurry. An overview of the suitability of the operating conditions (e.g., processing capacity) and inconveniences (e.g., high power consumption and/or low efficiency solid–liquid separation) were described. On the first afternoon session, the students observed a functioning version of the prototype that they were tasked with replicating and improving (e.g., the addition of a heating circuit for the stabilization tank) with the materials that they were provided. The second day was devoted to assembling the pieces of the artifact that were already prepared in advance, due to the time constraint during the week of placements (e.g., 2 perforations of 150-mm diameter in each lid of the boxes to connect them with the aluminum tubes as described in Figure 1), and to adding new features. For example, the parallel heating circuits with the carbon fiber cable was entirely designed and implemented by the students during the second day, to enhance the dewatering of the slurry in the stabilization tank while maintaining a moderate power consumption of approximately 80 W. During the morning of the third day, the operation of the prototype was initiated (Figure 2) in the workshop of the School of Engineering, where 5 L of pasteurized anaerobic digestate liquor with >95% moisture content were charged in the stabilization tank and 2 kg of anhydrous salt were placed in the cradle of the tank of condensation. Figure 2 describes the methodology followed to operate the artifact and to analyze the products. The anhydrous salts of CaCl2, MgCl2, and NaCl were charged separately (i.e., in different experiments) in the condensation tank and the convective movement of the moisturized air coming from the stabilization tank with organic slurry was forced with an in-line tubular fan (Figure 1). The CO2 and NH3 released was absorbed in the forming brine as described by Equations (1), (2) and as the organic material was getting drier, the gaseous emissions increased.
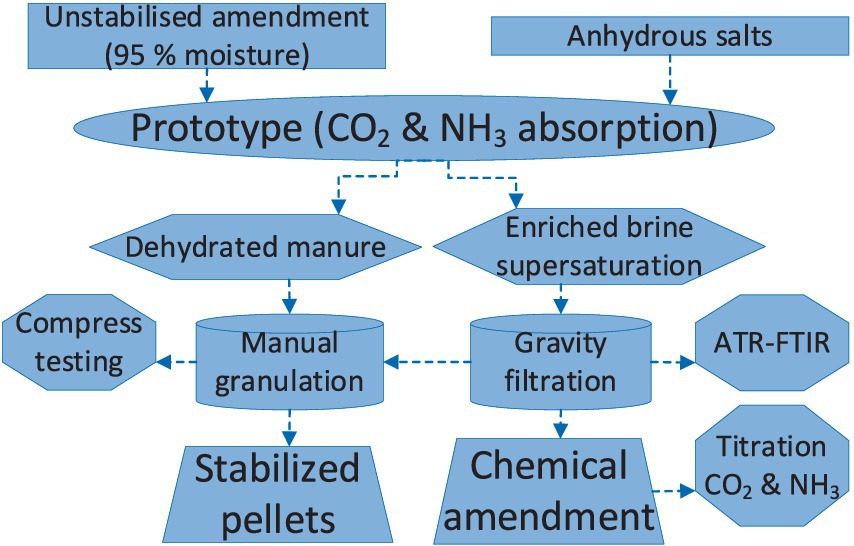
Figure 2. Methodology for processing the organic manure with the prototype of Figure 1. (I) Dewatering the slurry by means of convective movement. (II) Characterization of the precipitated salts. (III) Acid–base titration. (IV) Granulation of the scales of concentrated liquor (Supplementary Figure 7), before stabilization in the prototype and compression testing.
Since each batch operation took 24 h to attain complete dewatering of the liquor placed in the stabilization tank, the afternoon session of the third day was spent preparing and organizing the questions for the stakeholders. The spirit of the primary market research was illustrated with a video (MScTE48, 2014) for better understanding of how to enquire about the suitability of the designed artifact to meet the demands of potential clients. The coordination and cooperative learning of the group members was meant to be promoted with the use of an adapted business model canvas template, which includes the Rumsfeld matrix to enable their metacognition and to improve the action lines that the groups of students would be drafting to address the problems of the potential clients (Moure Abelenda et al., 2023b). On the morning session of the next day, the operation of the prototype was stopped and the solid paste of dewatered liquor that remained in the stabilization tank was used as binding agent, to enable the granulation of the fibers of anaerobic digestate. The self-hardening effect of concentrated liquor was compared to that of quicklime, as the CaO was also used to enable the granulation of the solid fibers of the anaerobic digestate (Pesonen et al., 2016). The stabilization tank can also be used to enhance the maturation of pellets with quasi-spherical geometry, using different binding agents (e.g., lime, concentrated liquor, etc.). Before feeding the granules to the stabilization tank of the prototype, to allow sufficient maturation and solidification for the compression testing the following day, the brine generated in the previous run was sampled and fresh anhydrous salt was placed in the shelf of the condensation tank. In the afternoon of the fourth day, the vacuum filtration of the brine was carried out in the lab to eliminate all the suspended particles of calcium carbonate, which otherwise would disturb the titration for determination of the NH3 absorbed. The filter paper employed for this purpose was QL100 Fisherbrand, with a pore size of 8 μm. The presence of CO2 and NH3 in the brine was determined using the titration methodology described in a previous investigation (Moure Abelenda et al., 2023a), using HCl and sodium hydroxide to prepare the aqueous solutions of titrants and NH4Cl and CaCO3 to prepare the model titration curves. Based on preliminary investigations, the concentration of the titrant solutions were chosen as 0.06 mol HCl/L with a pH of 1.13 at 21.1°C and 0.12 mol NaOH/L with a pH of 13.19 at 20.9°C, to avoid excessive dilution of the titrate during the data collection.
On the morning session of the last day, saturated solutions of 344.25 ± 19.21 g CaCl2/L (Supplementary Figure 4) were prepared and analyzed to have a blank titration curve, where no absorption of NH3 or CO2 can be accounted. The approximate concentration of the brine collected in the storage tank of the prototype (expressed in grams of CaCl2 per liter of aqueous solution) was calculated by performing an extrapolation of the calibration curve elaborated with the density of pure water and that of a supersaturated solution of 761.30 g CaCl2/L (20°C) (Haynes, 2010, pp. 8–122; Manivasakam, 2011, Table XVI; Solvay, 2023, p. 6). While preparing the aqueous solutions, students were encouraged to look at the temperature increase to appreciate the exothermic reaction of hydration of CaCl2 and to optimize the deliquescence phenomenon by preventing the increase of the temperature of the condensation tank. Besides the titration of the brine, an attempt to isolate the NH4Cl was done by promoting the supersaturation of que aqueous solution via addition of acetone as an antisolvent and cooling down. The precipitated salt was subsequently isolated, allowing the settling, decantation, and drying at room temperature in the fume hood. During the afternoon session, the attenuated total reflectance (ATR) Fourier-transform infrared spectroscopy (FTIR) analysis was performed for the precipitated salt and other chemicals of known composition: CaCl2, NH4Cl, and CaCO3, to compare the spectra and elucidate the structure of the complex mineral matrix isolated from the brine. The equipment used for this purpose was the Agilent Technologies Cary 630 FTIR Spectrometer, with an ATR sampling module for reflection mode measurement. Intra-Laboratories Ltd. supplied various grades of chemicals including CaCl2 (food/pharmaceutical grade), MgCl2 (cosmetic grade), CaCO3 (animal feed grade), and Fisher Scientific UK Ltd. supplied reagent grade HCl, NaOH, NaCl, and CaO. The mechanical compression testing of the pellets prepared using different binding agents was conducted with the Instron® 3,345 equipment, with a load cell capable of delivering up to 5 kN of compression force. Given the timeframe of the didactic activity, the granules with lime were prepared in advance (10 days before the preparation of the granules with the concentrated liquor), to allow the pellets to sufficiently self-harden before compression testing. Both the granules of lime and those of the concentrated liquor had a diameter of approximately 5 cm but the binder-to-fiber ratio was 5 times greater when using the concentrated liquor (2.07 ± 0.93), compared to that of lime (0.41 ± 0.20). Descriptive statistics (average values and standard deviations) were considered to identify whether the granule prepared with CaO had greater compressive strength than those prepared with CaCO3.
Additionally, student experience was characterized by formative and summative assessments in the middle (day 3) and at the end (day 5) of the placements, following the advice provided by National Co-ordinating Centre for Public Engagement (2017). Both surveys, presented in the Supplementary material, were designed to give information on whether the didactic proposal was being applied successfully, to capture the main benefits for students and any aspects that needed to be further developed in future editions of the PBL activity (Make it happen!). Filling out the surveys was considered a metacognitive exercise for students that allowed them to evaluate their own performance during the learning process. The questionnaires gave them clues on what skills they were meant to be developing (entrepreneurial spirit, teamwork, environmental awareness, etc.) and how they could apply these when facing another real-world project (Moure Abelenda et al., 2023c).
The 22-W duct fan (Table 1) that promotes the turbulence and gas exchange between the chambers (Figure 1) was complemented with 2 parallel heating circuits around the stabilization tank connected to power supplies of up to 3 A and 15 V, representing a total power consumption of around 100 W. The design of the heating circuits considered that the carbon fiber heating cable had a resistance of 13 Ω/m and the maximum heat dissipated could be attained when the current intensity is more relevant than the resistance. Therefore, the design of the parallel circuit relies on understanding the practical application of Ohm’s law and Watt’s law. The implementation of the heating circuit around the stabilization tank of the prototype enhanced the rate of dewatering of the organic slurry and the rate of brine formation in the condensation tank. The artifact was able to dehydrate 5 L of pasteurized anaerobic digestate liquor (> 95% moisture) within a 24-h operation. The overall mass balance was the production of 8.5 kg of brine in the condensation chamber (Supplementary Figure 8) and 0.5 kg of solid with a moisture content below 50% remaining in the stabilization tank (Supplementary Figure 7). Most of the moisture of the fresh organic slurry was trapped in the condensation tank (Figure 1), along with some CO2 and NH3. Based on the mass balance, the brine obtained should have a composition of 457.14 g CaCl2/L (resulting from mixing 4 kg CaCl2 and 4.5 kg H2O) but the concentration measured gravimetrically and volumetrically in the filtrated brine was virtually 915.84 ± 9.57 g CaCl2/L. This value of concentration was significantly higher than the saturation limit of 515.30 ± 200.02 g CaCl2/L that is reported in literature (Haynes, 2010, pp. 8–122; Manivasakam, 2011, Table XVI; Solvay, 2023, p. 6), despite this solution has been filtered. Therefore, it was inferred that the aqueous solution should contain other compounds different from CaCl2, which also contribute to increase the density of this liquid.
Figure 3A shows the titration curves of filtered brine collected from the prototype, blank saturated solution (761.30 g CaCl2/L), and blank diluted solution (344.25 g CaCl2/L). The pH of the filtered brine resulting from the dewatering of the organic slurry and that of the saturated and diluted CaCl2 solutions without the addition of any titration agent (i.e., acid dose of 0 Equivalents/L) were 7.86 ± 0.07, 9.07 ± 0.07, and 9.41 ± 0.15, respectively (Figure 3). Since most of the CaCO3 has been removed during the filtration stage, these differences in the pH were mainly given by the presence of NH4Cl in the brine coming from the prototype and the different ionic strengths of the aqueous solutions of CaCl2. The salt NH4Cl has acidic behavior, and the scale of pH shifts toward lower values, as the ionic strength increases (Kennedy, 1990; Samuelsen et al., 2019): blank diluted solution (9.31 M), blank saturated solution (20.58 M), and filtrated brine from the prototype (24.76 M). Under a sufficient flow of NH3 or CO2, these gases can affect the chemistry of an aqueous solution in intimate contact, but when the flow rate is limited, the existing chemistry of the aqueous solution determines the form under which NH3 and CO2 are stored in the liquid phase. In this way, both inorganic compounds prevent the change of the pH of the aqueous solution and a flatter titration curve is indicative of a greater content of inorganic nitrogen and carbon in the brine, because it takes an increased amount of the titrant agent to modify the pH. In the conditions of the prototype, the most stable ionic compounds for allocating the gaseous stream of inorganic nitrogen and inorganic carbon were NH4Cl and CaCO3. Both acidic and basic titrants were used to evaluate the buffer capacity of the aqueous solutions, as this is a proxy measurement of the content of NH3 and CO2, corresponding to 0.85 and 0.44 g/L, respectively. The dose of titrant in the X axis of Figure 3 is expressed in terms of the equivalents of acid (HCl) and the negative values correspond to the addition of basic titrant (NaOH), since both compounds have the same valence. Previous investigations have shown that this is the most effective and reliable way of expressing the dose of titrants (Regueiro et al., 2016; Moure Abelenda et al., 2021b, 2022a,c). Model aqueous solutions of 0.83 g NH3-NH4Cl/L, 0.47 g CO2-CaCO3/L, 0.88 g NH3-NH4Cl/L and 0.43 g CO2-CaCO3/L, and 0 g NH3-NH4Cl/L and 0.43 g CO2-CaCO3/L were prepared, and the titration curves were compared to that of the brine recovered from the condensation tank of the artifact.
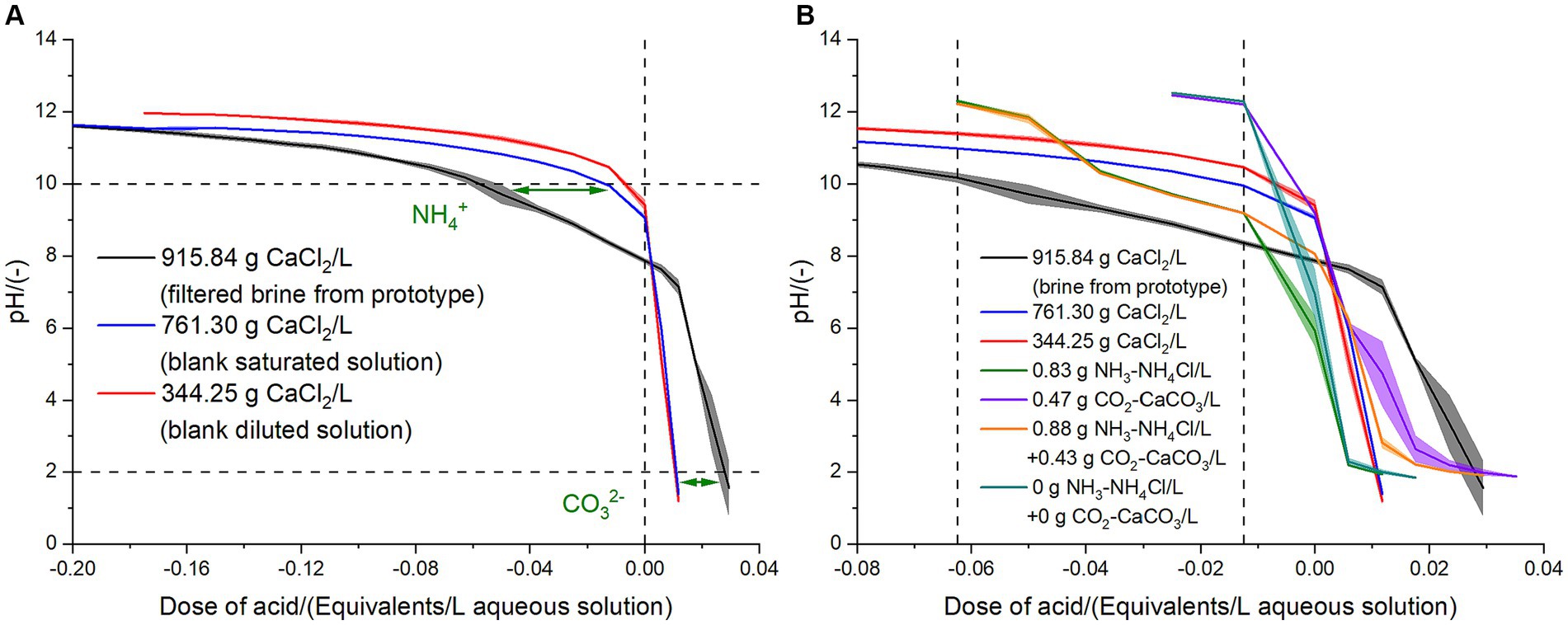
Figure 3. (A) Titration curves at 20°C of the brine generated during the dewatering of the pasteurized anaerobic digestate liquor with >95% moisture content (black line and error band; n = 4) and those of a model saturated solution of 761.30 g CaCl2/L H2O (blue line and error band; n = 4) and a model diluted solution of 344.25 g CaCl2/L H2O (blue line and error band; n = 4), which were used as blanks to calculate the amount of CO2 and NH3 absorbed (green arrows). The vertical dashed line marks the initial pH of both aqueous solutions (no titrant added). The horizontal dashed lines mark the titration endpoints of 2 and 10. (B) Comparison of the titration curves of the CaCl2 brines (including blanks) to those of the model solutions of 0.83 g NH3-NH4Cl/L, 0.47 g CO2-CaCO3/L, 0.88 g NH3-NH4Cl/L and 0.43 g CO2-CaCO3/L, and 0 g NH3-NH4Cl/L and 0.43 g CO2-CaCO3/L. The vertical dashed lines mark the beginning and the end of the straight line with constant slow given by the buffer equilibrium NH4+/NH3.
A conservative approach of comparing the titration curves of the filtrated brine to that of the saturated model solution indicated that approximately 2.5 times more equivalents of NH3 were available in each liter of brine than the equivalents of CO2 in the same volume (Figure 3A). This is in agreement with the fact that the CaCO3 (Supplementary Figure 4) is less soluble than the NH4Cl (Supplementary Figure 5), hence more amount of ammoniacal nitrogen (NH4+-N) remained in the aqueous solution after the filtration stage, compared to the amount of inorganic carbon (CO32−-C). The 761.30 g CaCl2/L saturated solution was, therefore, regarded as a blank measurement for the content of NH4Cl and CaCO3. The differences in the consumption of acidic and basic titrants by the filtered brine and the 761.30 g CaCl2/L solution were measured at the titration endpoints. According to Husted et al. (1991), at an ionic strength of 0.25 M, the mixed acidity constants (pKa values) for CO2/HCO3−, HCO3−/CO32− and NH4+/NH3 are 6.21, 9.90 and 9.46, respectively. However, it should be noted that the model solution of 1.08 g CaCO3/L (which can be expressed as 0.47 g CO2-CaCO3/L) did not show any buffer effect when alkalinizing above the initial pH of 9.19 ± 0.07 (Figure 3B), which implied that the CaCO3 added to the distilled water immediately dissociated to enable the formation of the bicarbonate: CO32− + H2O → HCO3− + OH−. Thereby, the titration endpoints were the pHs at which there were the greatest difference in the titrant consumption by the filtered brine and the saturated solution, when depleting the acidity of NH4+ (pH above 10) and the alkalinity of CO32− (pH below 2). These titration endpoints were also the end of the constant slope that define the rate of consumption of titrant under a particular buffer agent. In the case of pH above 10, both the filtered brine and the saturated solution ended at a similar pH of 11.61 ± 0.05 and 11.64 ± 0.01, respectively. This could not be considered as titration endpoint because the consumption of the basic titrant is not entirely associated to the conversion of NH4+ to NH3. Since both aqueous solutions have similar ionic strength, above pH 10, the basic titrant is consumed by the ions Ca2+ (pKa of 11.6) to produce CaOH+. Therefore, since the beginning of the smoother variation of the slope, above pH 10, the consumption of alkali is not considered as part of the NH4+ buffer effect. Figure 3B shows that the slope of the alkalinization curves (between −0.04 and − 0.01 equivalents acid/L) of the solution 0.83 g NH3-NH4Cl/L was the same as that of the titration curve of the filtered brine from the prototype but the intercept of the straight line of the model solution was higher due to its lower ionic strength. Also, there was a change in the titration curves of the solution and that of the brine from the prototype above a pH of 10. Finally, the profile of the solution 0.88 g NH3-NH4Cl/L & 0.43 g CO2-CaCO3/L follows a combination of the buffer trends of the solution 0.83 g NH3-NH4Cl/L and the solution 0.47 g CO2-CaCO3/L. The non-buffer parts of the titration curves of these solutions match the profile of the blank (0 g NH3-NH4Cl/L & 0 g CO2-CaCO3/L). The alkalinization of all model solutions (0.83 g NH3-NH4Cl/L, 0.47 g CO2-CaCO3/L, 0.88 g NH3-NH4Cl/L & 0.43 g CO2-CaCO3/L, and 0 g NH3-NH4Cl/L & 0 g CO2-CaCO3/L) reaches pH above 12 due to the negligible content of Ca2+. However, the purified water employed to prepare the model solutions contains some residual alkalinity, as can be seen in Figure 3B, and this resulted in the consumption of some 0.015 ± 0.004 Equivalents of acid/L of titrate to reach a pH below 2.
In order to attain the acid titration endpoint pH < 2 in a liter of brine from dewatering slurry, approximately 0.02 equivalents of acid were required more than when acidifying the fresh 761.30 g/L solution of CaCl2 (Figure 3A). Since the valence of HCl (employed as acidic titrant) is half of that of CaCO3, the moles of monovalent acid titrant should be twice the moles of carbonate per liter of brine. On the other hand, the alkalinization of a liter of brine obtained from dewatering the anaerobic digestate liquor, required 0.05 equivalents of NaOH more than what was found necessary for the alkalinization of the fresh saturated 761.30 g CaCl2/L to reach the titration endpoint of 10. Since the valence of NH4Cl is 1 and is the same as that of NaOH, the moles of ammonium per liter of brine were around 0.05. Based on this analysis, the contents of NH3 and CO2 in the brine generated in the artifact after 1 day of operation were 0.85 and 0.44 g/L, respectively. If all the volatilized NH3, CO2, and H2O in the 24-h operation ended up in the brine, the rate of release of these compounds during the dewatering of the anaerobic digestate liquor was around 230 mg NH3/h, 119 mg CO2/h, and 200 g H2O/h, which are comparable mass flow rates (i.e., in the same order of magnitude) to the values employed to run the initial simulations in Aspen Plus® v12 (Supplementary Figures 1–3). The artifact developed (Figure 1) is therefore a useful measurement tool of gaseous emissions from manure, avoiding the requirement for expensive analytical equipment since it can be easily coupled with a titration method.
The valorization of the dewatered liquor was investigated as binding agent to enable the granulation of the fibers (i.e., solid fraction) obtained from the screw press separator. The performance of the concentrated liquor as binding agent was compared against that of lime, which is often used as additive to promote the self-hardening of the granules upon sufficient maturation (Moure Abelenda and Amaechi, 2022). The results shown in Figure 4A indicate that 50% of the granules with lime showed high compression resistance: 111.84 ± 15.44 N of force and 6.09 ± 1.43 mm of size reduction before reaching mechanical failure. The other 50% of the lime granules showed a profile of low compression resistance: 50.20 ± 3.11 N of force and 10.32 ± 1.20 mm of oncoming platens of the testing machine, which account the deformation of the specimen. The reproducibility of the high compression strength granules prepared with the concentrated liquor as binding agent was lower than that of lime because only 25% of the pellets supported 207.00 ± 26.36 N of force and 4.07 ± 1.33 mm of size reduction before fracturing. The 75% of the pellets of concentrated liquor fell in the range of 45.54 ± 8.22 N and 5.92 ± 1.98 mm (Figure 4B). It is important to highlight that the pellets of lime were subjected to 10 days of maturation time under the fume hood, while the pellets prepared with concentrated liquor only underwent drying in the stabilization tank of the artifact for 24 h. Another difference is that more binding agent was employed in the case of pellets prepared with concentrated liquor than in the preparation of the lime pellets. Generally, Figure 4 shows that the high-compression-resistance pellets of the concentrated liquor withstood greater compression forces than the pellets prepared with lime and the shape of the concentrated liquor pellets was also less affected.
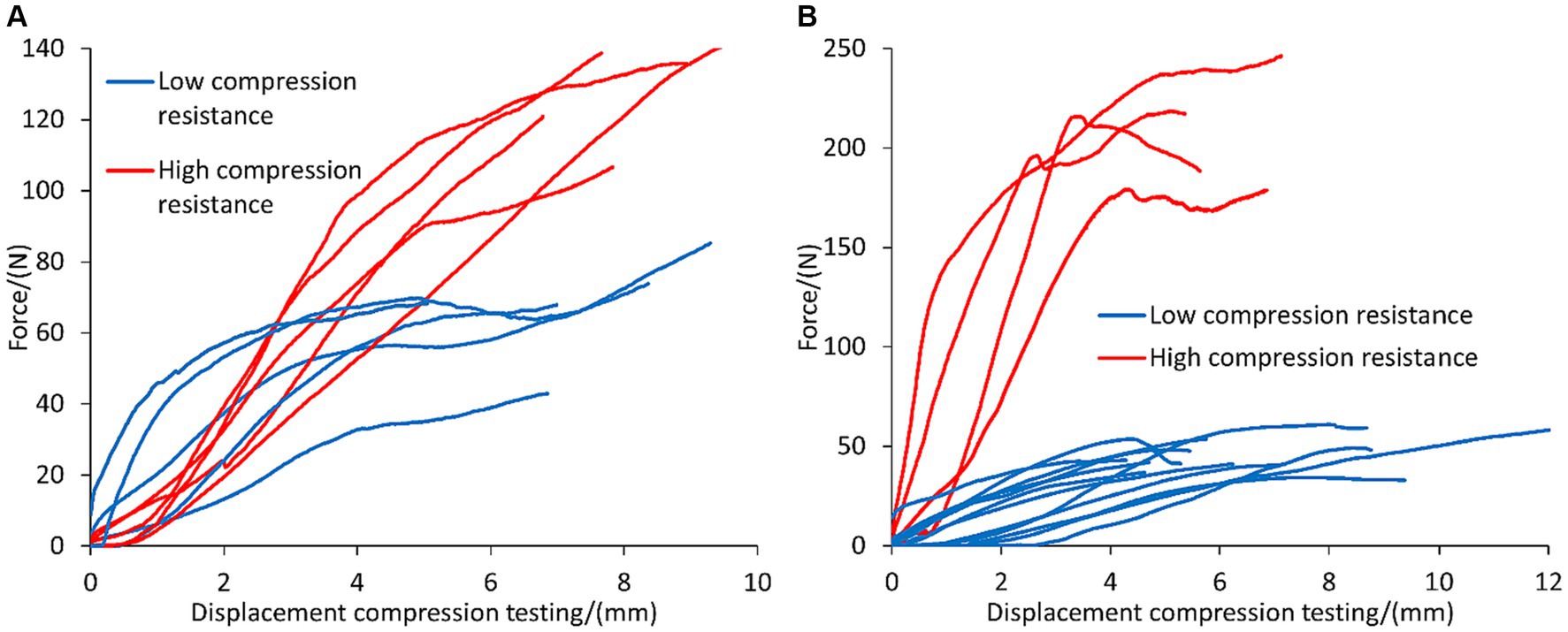
Figure 4. Profile of compression testing of the granules (approximately 5-cm diameter) of the fibers of anaerobic digestate that were produced manually, employing different binding agents to enable agglomeration and promote self-hardening: (A) 10 granules prepared with lime and allowing 1 week maturation, and (B) 16 granules of concentrated liquor with 24 h maturation. Profiles of low and high compression resistance of the pellets were highlighted with blue and red colors.
The success rate of employing the prototype as a didactic tool for secondary education students was quantified in the middle of the engagement activity (formative assessment in day 3; Figure 5A) and at the end (summative assessment in day 5; Figure 5B). The students were happy with the freedom that they were given to develop their own initiative and entrepreneurial spirit. Due to the limited time to conduct the activity, most of the formalities (e.g., preparation of the risk assessment and COSHH form) were done before the arrival of the students, and they have highlighted that his part might be missing for developing their project management skills. In line with the freedom to use different materials, the students have highlighted that this didactic activity gave them the opportunity of making mistakes, rectifying, and reflecting on their own learning process to have a superior understanding of their capabilities (i.e., metacognition). However, it could be interesting to include specific tasks to force their cognitive conflict. As indicated by In2ScienceUK (2023a), the preference of students is to work in small groups of 3 members that give them the chance of listening and being heard by others. In general, the physical activities proved more engaging than the cognitive work. Particularly, the students were observed to be very active and participative during the elaboration of the electric heating circuit. The complaint made by the students regarding the cognitive exercise of analyzing the results, was the difficulty of abstraction required for quantifying the content of NH3 and CO2 that ended up in the brine and provided buffer capacity (Figure 3). Thereby, more time should be devoted to the explanation of the titration method in the next edition of the didactic proposal because these concepts might fall outside of students’ curriculum content. Overcoming intrinsic difficulties associated to the analytical method could be achieved, for example, by simplifying the units of the dose of titrants, since the students might find the use of the term “equivalents” ambiguous, when expressing the amount of acid or base.
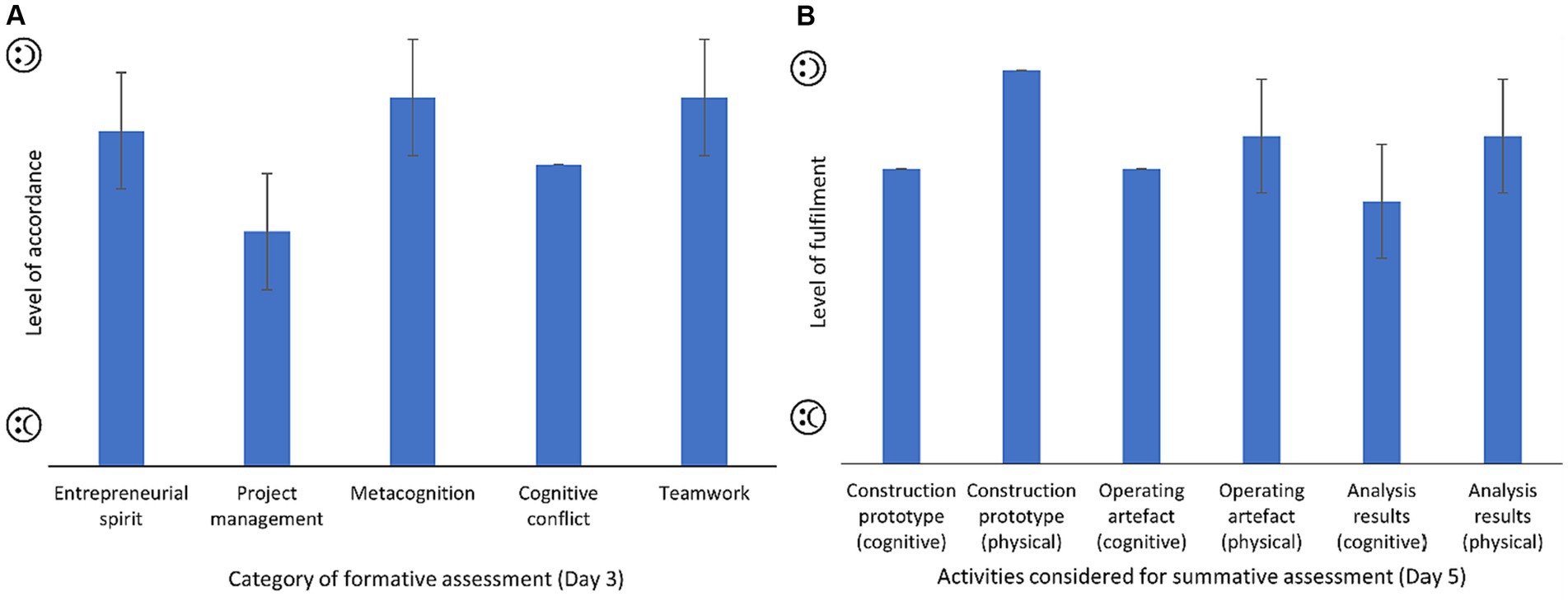
Figure 5. Results of the assessments to determine whether the didactic proposal addressed the educational needs of secondary education students (Moure Abelenda, 2023b): (A) formative evaluation on the third day and (B) summative evaluation on the fifth day of placements.
The comparison of the performance of the initial prototype (i.e., without the heating circuit and a total energy consumption of 22 W) allowed to characterize better the artifact developed by the students, where the heating was implemented. The relation between the power consumption of the artifact and rate of dehydration was not exactly direct: Doubling of power consumption increased the rate of dehydration of the slurry slightly less 2 times. Therefore, this needs to be taken into account when planning the next designs for expanding the processing capacity of the equipment to dehydrate larger quantities of organic slurry. Since the brine generated can be employed as chemical amendment (and additional source of NH4+-N) for the untreated slurry (Husted et al., 1991; Vandré and Clemens, 1996) that does not pass through the artifact, it could be also considered that this system exceeds the expectations of slurry processing for its energy consumption. The brine produced in the condensation tank of the prototype had a composition of approximately 10 M CaCl2 whereas typical concentrations employed for soil extraction are around 0.01 M CaCl2 (Houba et al., 2000) and for chemical stabilization of raw slurry range from 0.025 M (Vandré and Clemens, 1996) to 0.08 M (Husted et al., 1991). Since the prototype is able to produce around 2 tonnes of brine per year, consuming 0.9 MWh at £80 and 1 t of CaCl2 at £150, the total cost of adding the chemical amendment to 250 t of untreated slurry is £1/t, which is a competitive cost compared to other products available on the market. This way of preparing the chemical amendment by dehydrating 1% of the total organic slurry produced in the agribusiness is in line with the business model of the Bokashi composting method for the production of Effective Microorganisms (Lactobacillus), which can be applied to improve the management of the organic slurry upon suitable dilution (Aryantha and Guest, 1999; International Nature Farming Research Center (INFRC), 1999; Bastami et al., 2016; San Martin Ruiz et al., 2022).
Attaining a concentration of 0.08 M CaCl2 in the organic slurry should have the same impact on the pH as adding 0.16 equivalents of HCl per liter. It is noteworthy to mention that the acidic effect of the CaCl2 can only be seen in suspension where most of the H+ ions remain attached to solid particles. These protons are release upon a small addition of CaCl2 that provides Ca2+ to replace some of them in the surfaces of the suspended solids, hence the H+ ions remain free in the bulk solution (Department of Primary Industries and Regional Development. Government of Western Australia, 2018; BACTO, 2021). In fact, the material safety data sheet provided by the manufacturer of the solid flakes of CaCl2 indicates that a solution of 100 g/L at 20°C should have pH between 9.0 and 10.5 (Solvay, 2023). The use of this material is sustainable because it is a byproduct of Solvay’s Process for Soda-Ash production. According to the manufacturer, their product is free of materials that are harmful to the environment and contains a very low level of heavy metals and organic compounds. Total alkali chlorides (e.g., NaCl and KCl) are the main impurities of commercial calcium chloride. Since much more alkali than acid titrant was required to increase the pH of the aqueous solutions (Figure 3), above pH 10 almost flat titrations curves were found due to the progressive dilution effect, in addition to the content of Ca2+ in the titrate. Up to 8 mL of basic titration were added to 5 mL of brine to reach a pH close to 12, while it only took the addition of 3 mL (or less) of acid titrant to reach a pH below 2 in the titrate (Figure 3).
Unlike the NaCl, which showed very limited deliquescence, the MgCl2 provided sufficient brine formation for capturing NH3 and CO2, and the performance of this material should be compared to that of CaCl2. The treatment of the gases coming out of the stabilizing slurry can be improved by including a filter of activated carbon to trap the H2S (Yan et al., 2002; Coppola and Papurello, 2018). As with the static chamber method or any other procedure employing a sulfuric acid solution as a trap of NH3 (including bubbling systems), calibration should be performed to determine the efficiency of absorbing gases in the device (Ndegwa et al., 2009) and the air quality that remains in the headspace of the artifact by the end of the operation. This could be done by using a model solution of NH4Cl or NH4OH of known composition from where emissions are promoted by increasing the pH or the temperature and following the increase of the concentration of NH4+ in the H2SO4 trap. This further characterization of the performance of the artifact can be implemented as part of additional didactic units, which allow the determination of the absorption efficiency and compare this to that of traditional static chamber or closed chamber methods. A benefit of this methodology, compared to the closed chamber, is that it allows quantification of the emissions of NH3 and CO2 with titrations of the brine because, unlike the pH of the H2SO4 solution, the pH of the brine does change considerably due to the absorption of NH3 and CO2, which makes possible to avoid specialized analytical equipment (e.g., Autoanalyzer segmented flow colorimetry or Total Nitrogen machine). Interestingly, the absorption of NH3 in H2SO4 solution implies, as in the brine of the anhydrous salt, a decrease in pH (Moure Abelenda et al., 2021a), due to the formation of the acidic salt (NH4)2SO4, similarly to the developed artifact, which stores the NH3 as NH4Cl and decreases the pH. The range of pH of the CaCl2 brine is more suitable to detect low amounts of NH3 absorbed in the liquid, compared to the very low pH of the acid H2SO4 solutions. In a previous investigation, the initial pH of 10-mL solutions of H2SO4 (with concentrations ranging from 0.1 to 1 M) placed in the trap of the closed chamber method was 0.92 ± 0.005. Due to the absorption of NH3 released from the incubation of 3.22 ± 1.43 g of whole agrowaste anaerobic digestate (AWD) at pH 10.40 ± 0.46 for 60 h at 100 rpm at 20°C, the pH of the H2SO4 solutions decreased 0.25 ± 0.02 units (Moure Abelenda et al., 2021a). Assuming that all the NH3 released ended in the H2SO4 trap, the rates of volatilization were: 20.15 ± 8.22 mg/h in an incubation with wood fly ash (WFA) for 60 h (Moure Abelenda et al., 2021a), 22.65 ± 6.08 mg/h in an incubation with WFA for 7 h, 2.03 ± 0.71 mg/h in a incubation of the AWD alone for 7 h at pH 8.32 ± 0.12 (Moure Abelenda et al., 2021c), and 3.65 ± 1.83 mg/h in an incubation of the AWD for 48 h at pH 7.55 ± 0.40 (Moure Abelenda et al., 2022b). All these values of NH3 volatilization rate are lower than the value found for the artifact with the anhydrous salt (approximately 230 mg NH3/h), although for a fair comparison is necessary to account the differences between the treatments, besides the dehydration effect, temperature, length of incubation, the type of organic material and any additive to tune its pH. However, it is still possible to infer the greater performance of the artifact in terms of capturing NH3 than the classical closed chamber method. The present method with the formation of the brine might be also more convenient for isolating the ammonium as NH4Cl rather than as (NH4)2SO4, as the brine might prevent the absorption of impurities such as cyclohexene (Ukwuani and Tao, 2016).
Another inconvenience of the traditional static chamber method is that to measure the release of CO2, it would be necessary to include another trap with a solution of NaOH. The information available in the literature regarding the NaOH trap, describes the decrease of the pH in the aqueous solution (Yoo et al., 2013; Kang et al., 2017; Krauβ and Rzehak, 2017) as result of CO2 absorption. The use of basic titrant is therefore required for quantification of NH3 and CO2 absorbed, and the differentiation of these compounds should be given by the type of trap that would be characterized. Figure 6 shows that the main compounds in the naturally precipitated salts from the brine and those promoted by means of adding an antisolvent (acetone) and cooling were CaCl2 and CaCO3. Generally, it is considered that the fingerprint region of the ATR-FTIR spectra goes from 1,000 to 2,000 cm−1, with characteristic profile of the type of compound that allows the identification. The broad peak that can be seen for the precipitated salt and the CaCl2, between the frequencies of 3,000 and 3,500 cm−1, is associated with the O-H single bond stretch due to the presence of moisture in these hygroscopic samples. The N-H single bond stretch could be seen for the NH4Cl between 2,750 and 3,250 cm−1. The most relevant peak of the ATR-FTIR spectrum of the precipitated salt in relation to the structure of the NH4Cl was found at 1,380 cm−1, and this bending vibration was marked with a vertical dashed line in Figure 6. This agrees with the results of the preliminary simulation (Supplementary Figure 1), in relation to the lower likelihood of the formation of NH4Cl (Supplementary Figure 3), compared to that of the CaCO3 (Supplementary Figure 2). Since the isolation of the NH4Cl was found to be very complicated, the use of the filtered brine is more convenient as chemical amendment of untreated organic slurries, which also represent an additional supply of NH4+-N. The results of the titration of the brine showed that 0.85 g NH3 and 0.44 g CO2 are trapped in each liter of brine and, since 8 L of filtered brine are meant to be applied to each tonne of untreated slurry, this represents a recirculation rate of 5.60 g NH4+-N and 0.96 g CO32−-C per tonne of slurry. According to Lukehurst et al. (2010), the content of NH4+-N in different types of organic slurry (i.e., dairy cow slurry, pig slurry, and anaerobic digestate) is 3 orders of magnitude greater than the supply derived from the CaCl2 brine. Thereby, the addition of this chemical amendment for stabilization purposes should not prone NH3 emissions that would be consequence of an excess of inorganic nitrogen in the slurry, but enrich the fertilization capacity of the organic slurry.
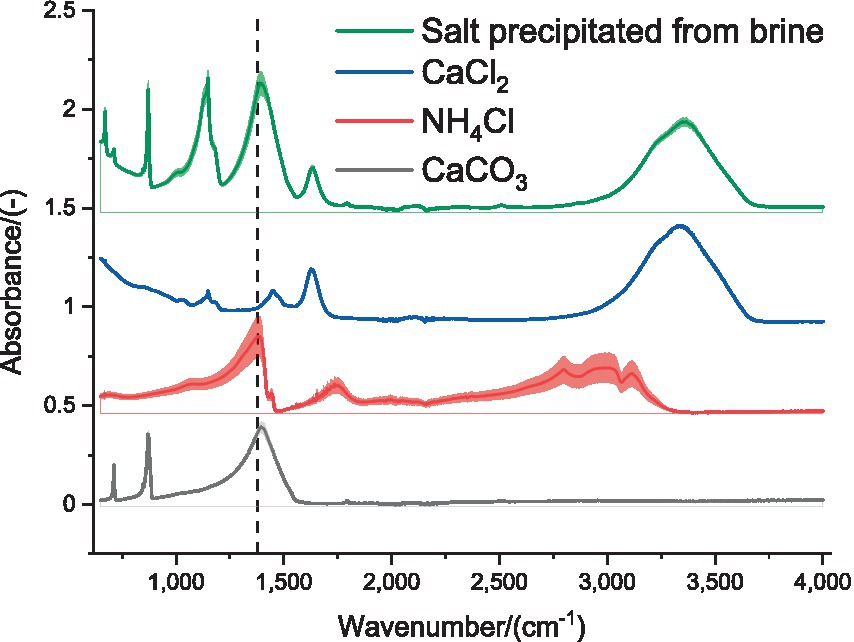
Figure 6. Identification of the structure of the salt naturally precipitated from the brine. Comparison of the ATR-FTIR spectra of several reagent grade salts. Vertical dashed line at 1,380 cm−1 to compare the peaks.
Another important outcome of the present investigation is to question the need for the solid–liquid separation via screw-press, if the granulation of the isolated solid fraction is implemented as part of the downstream processing. This is because very promising results were found for the use of the concentrated liquor as binding agent (Figure 4). The next step in the development of this processing route would be to determine whether drying the whole anaerobic digestate (which includes the liquor and the fibers) is sufficient to elaborate granules with the right mechanical properties or whether an addition of concentrated liquor is required to further increase the liquor-to-fibers ratio. While this interpretation is novel, the processing of the liquor alone to produce granular fertilizer has been already considered (Fivelman, 2013), hence the proposed strategy represents an enhancement of the valorization of the solid fraction that would come out of the screw-press. In a complex matrix with minerals, such as anaerobic digestate, there are many reactions that can be responsible of the self-hardening phenomena (Steenari and Lindqvist, 1997). The ratio of inorganic elements to organic matter is an important factor to optimize the mechanical properties of the pellets because the organic material acts as a sponge soaking the water that enables the reactions of self-hardening (Wróbel et al., 2016; Mudryk et al., 2018). Using lime as binding agent to benchmark the performance of the concentrated liquor is convenient since this material is employed to enhance the compressive strength of granules. Additionally, processing of whole digestate, fibers or liquor can be done by adding lime in the stabilization chamber to keep the pH above 12 for at least 2 h, to better control the biological activity in agreement with the regulations of the UK Government (2018). According to Pesonen et al. (2016), the main strength-providing reaction is the hydration of quicklime (CaO) to give slaked lime (Ca(OH)2), while the subsequent carbonation gives limestone (CaCO3) and provides further strength but this last reaction is very slow due to the limiting step of diffusion and absorption of CO2 from the surrounding environment, depending on the conditions of storage and maturation of the pellets.
The recyclability of calcium is very high and these reactions can be easily reverted by incineration or calcination at sufficiently high temperatures (above 600°C) to enable the restoration of the oxide form, due to the poor stability of the limestone and slaked lime that release CO2 and H2O (Sakthivel et al., 2012; Galan et al., 2013; Mor et al., 2016; Al-Mallahi et al., 2020). It is also important to highlight that the oxides are more alkaline than the hydroxides, and the latter compounds are also more alkaline than the carbonates. Thereby, aiming to increase the mechanical strength at shorter maturation times can harm the organic material by promoting hydrolysis, NH3 volatilization, or even pH shock in soil and burning of crop tissues (Steenari and Lindqvist, 1997; Demeyer et al., 2001; Huotari et al., 2015; Pesonen et al., 2016; Belviso, 2018; Voshell et al., 2018; Bachmaier et al., 2021). For this reason, cured materials, which are less reactive, are often preferred for the formulation of granular fertilizers. As part of the present investigation, a granule was prepared with CaO as binding agent, and this specimen did not provide significantly different compressive strength to that of granules prepared directly with CaCO3. This is likely because significant differences can only be seen at very short times of maturation for which the quicklime might be more suitable (e.g., 24 h), and these lime pellets were left drying in the fume hood for 10 days before the compression test. In any case, both the pellets of concentrated liquor and those of lime successfully achieved the minimum strength for granular fertilizer, which is around 50 N (Walker et al., 1997; Pesonen et al., 2016). Expressing the results of the compression test in N rather than in MPa is more convenient for granular fertilizers because the cross-sectional area does not remain constant in a sphere. In order to confirm the reproducibility of the mechanical properties found for the pellets prepared with the concentrated liquor, the procedure was repeated but using crystals of NH4HCO3 rather than the solid fiber, giving more homogenous granules, and subsequent allowing maturation for 4 weeks (Supplementary Figure 9). The results of the compression testing of these 2-cm diameter pellets of concentrated liquor and crystals of ammonium bicarbonate were (n = 8) 198.57 ± 58.43 N and 0.94 ± 0.75 mm, which confirms the superior mechanical properties of the concentrated liquor as binding agent.
The organization of the activities was successful for the level of understanding of the A-level students. However, the briefing that was given to them at the beginning of the day was not as effective in generating an enthusiastic response as it was the opportunity of participating in the hands-on activities. For this reason, for the next edition of the didactic proposal, explanations will be spread throughout the day, just before and after carrying out the physical exercises. The team of students worked well with 3 members, but they could have taken more initiative in developing their intellectual property if they were competing against other groups. The students requested to expand the primary market research to have the chance of interviewing more potential clients, to hear the point of view of more stakeholders of agroindustry, and to further clarify the current situation. This pedagogic intervention was useful to develop the skills of students that were not initially planned, such as some aspects of their environmental competence (Al-Balushi and Al-Aamri, 2014). The students were reluctant to perform the manual granulation (Supplementary Figure 9), giving the opportunity for discussion about the composition of the soil, which is also composed by a large extent of stabilized organic matter, as they could observe in a visit that was made to the nearest field. Also, the technique of manual granulation was explained to them as the traditional preparation of seed balls, which was developed by Masanobu Fukuoka for no-till planting (Helliwell, 1980; Paudel and Belo, 2017; Hannur, 2019; Tamilarasan et al., 2021; Parmar, 2022), and how this can be improved with the inoculation of microbial species (biofertilizers) during the granulation process (Jewiarz et al., 2018) to further enhance germination and sprouts. This conscientization process that every citizen should undergo, in terms of comprehending the role of organic matter and even some of the organic wastes that they generate throughout their daily life, is therefore ameliorated with this didactic activity, which is in line with other outreach events promoted by the British Society of Soil Science (2022), for example, Dirty matters: The soil game. The present didactic intervention could be improved by providing the students with a glossary in the first session, which would define all strange and ambiguous terms that are not part of the normal vocabulary (e.g., liquor, equivalents of acid, etc.). This would allow to minimize extrinsic difficulties of language, which are associated with the way in which the activities are presented to secondary education students. Nevertheless, what made this educational tool more effective in reaching the target audience was the observation of the physical phenomena of deliquescence of the anhydrous salts and the self-hardening of the pellets (Supplementary Figure 9). Intrinsic difficulties are inherent to the topic but could be solved with, for example, modeling with the kinetic molecular theory (Stern et al., 2008) the transfer of moisture from the stabilization tank to the condenser. In this way, students could have an interpretation of the important role of temperature and turbulence for the gas in the headspace to be in touch with solid and liquid phases of the 2 chambers of the artifact.
This investigation on the performance of the novel technology for processing organic slurry concluded that the operation capacity of the prototype was 5 L of liquor every 24 h or 2 t of slurry per year. Since approximately 2 t of CaCl2 brine are produced per year, with a composition of around 10 M and the use of this fluid as chemical amendment requires 0.08 M CaCl2 upon mixture with the slurry, a single prototype was deemed suitable for treating 250 t at a cost of £1/t of slurry. The results of the titration of the brine showed that 0.85 g NH3 and 0.44 g CO2 are trapped in each liter of filtered brine and, since 8 L of brine are meant to be applied to each tonne of untreated slurry, this represents a recirculation of additional 5.60 g N-NH4+ and 0.96 g C-CO2 per tonne of slurry.
This study questions whether the solid–liquid separation via screw press should be disregarded as best practice for handling organic slurry, if the subsequent processing of the fibers would be the granulation step. The use of the concentrated liquor as binding agent gave very promising results for the granulation of the fiber, since the liquor granules attained 207.00 ± 26.36 N, which was more than the lime granules (111.84 ± 15.44 N) and more than the threshold value that is generally required for commercialization of the pellets (50 N). A lower degree of deformation of the granules before fracture formation was also observed in the liquor granules (4.07 ± 1.33 mm), compared to the lime granules (6.09 ± 1.43 mm).
The flexible model for the commercialization of the environmental technology in the farming and educational sectors was considered suitable for this first edition of the PBL activity (Make it happen!). The advances in the development of the prototype and the expansion of the network of contacts enhanced the adoption of the technology in industry but hindered the application of subsequent editions of this PBL activity on slurry management in secondary educational institutions. This is because the future activities planned for the different sessions are hard to be conceived as a way of contextualizing the syllabus, aiming to improve the very basic foundations of science and environmental awareness that are required to be an active member of society. For example, the students found difficult to understand the titrimetric method to determine the amount of NH3 and CO2 absorbed in the brine, hence establishing the calibration curve to determine the efficiency of the absorption process in the artifact and compare it with the traditional static chamber method needs to be carefully planned.
The datasets generated for this study can be found in the ZENODO repository: https://doi.org/10.5281/zenodo.8427906, https://doi.org/10.5281/zenodo.10535943.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the [patients/ participants OR patients/participants legal guardian/next of kin] was not required to participate in this study in accordance with the national legislation and the institutional requirements.
AMA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JR: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This investigation received the support of the Engagement Fund of the Faculty of Science and Technology of Lancaster University and the Doctoral Prize of the Engineering and Physical Sciences Research Council (EPSRC) of the United Kingdom (Award ref. 1945857).
The authors would like to acknowledge the support received by In2ScienceUK to partly organize this outreaching event and the participation of the students in the activities for developing the artifact and fostering a wider adoption rate by the stakeholders of the agroindustry.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2024.1321353/full#supplementary-material
ATR, Attenuated total reflectance; A-level, Advanced level; CATNAP, Cheapest technology narrowly avoiding prosecution.; COSHH, Control of Substances Hazardous to Health; CO32−-C, Inorganic carbon; Fiber, Solid phase obtained from the screw-press separation; FTIR, Fourier-transform infrared spectroscopy; Liquor, Liquid phase obtained from the screw-press separation; NH4+-N, Ammoniacal nitrogen; PBL, Project-based learning; SWOT, Strengths, weaknesses, opportunities, and threats.
Akhiar, A., Battimelli, A., Torrijos, M., and Carrere, H. (2017). Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag. 59, 118–128. doi: 10.1016/j.wasman.2016.11.005
Al-Balushi, S. M., and Al-Aamri, S. S. (2014). The effect of environmental science projects on students’ environmental knowledge and science attitudes. Int. Res. Geogr. Environ. Educ. 23, 213–227. doi: 10.1080/10382046.2014.927167
Al-Mallahi, J., Sürmeli, R. Ö., and Çalli, B. (2020). Recovery of phosphorus from liquid digestate using waste magnesite dust. J. Clean. Prod. 272:122616. doi: 10.1016/j.jclepro.2020.122616
APROVIS Energy Systems Gmbh. (2023). APROVIS FriCon. PRO Available at: https://www.aprovis.com/en/products-en/aprovis-gas-technologie/aprovis-fricon/ (Accessed October 8, 2023).
Aryantha, N. P., and Guest, D. (1999). Bokashi (EM) as a bio-control agent to suppress the growth of Phytophthora Cinnamomi Rands. International conference on Kyusei nature farming. Available at: https://www.researchgate.net/publication/267795255_Bokashi_EM_as_a_Bio-control_Agent_to_Suppress_the_Growth_of_Phytophthora_Cinnamomi_Rands
Bachmaier, H., Kuptz, D., and Hartmann, H. (2021). Wood ashes from grate-fired heat and power plants: evaluation of nutrient and heavy metal contents. Sustainability (Switzerland) 13:5482. doi: 10.3390/su13105482
BACTO (2021). Measurement of pH in soil. Available at: https://bacto.com.au/measurement-of-ph-in-soil/ (Accessed July 13, 2021).
Bastami, M. S. B., Jones, D. L., and Chadwick, D. R. (2016). Reduction of methane emission during slurry storage by the addition of effective microorganisms and excessive carbon source from brewing sugar. J. Environ. Qual. 45, 2016–2022. doi: 10.2134/jeq2015.11.0568
Belviso, C. (2018). State-of-the-art applications of fly ash from coal and biomass: a focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 65, 109–135. doi: 10.1016/j.pecs.2017.10.004
British Society of Soil Science (2022). Dirty matters: The soil game. Available at: https://soils.org.uk/dirtymatters/ (Accessed September 16, 2023).
Buckley, C., Krol, D., Lanigan, G., Donnellan, T., Spink, J., Hanrahan, K., et al. (2020). An analysis of the cost of the abatement of ammonia emissions in Irish agriculture to 2030. Available at: https://www.teagasc.ie/media/website/publications/2020/NH3-Ammonia-MACC.pdf.
Coppola, G., and Papurello, D. (2018). Biogas cleaning: activated carbon regeneration for H2S removal. Clean Technol. 1, 40–57. doi: 10.3390/cleantechnol1010004
Costamagna, P., Delucchi, M., Busca, G., and Giordano, A. (2020). System for ammonia removal from anaerobic digestion and associated ammonium sulfate production: simulation and design considerations. Process Saf. Environ. Prot. 144, 133–142. doi: 10.1016/j.psep.2020.05.055
CRI-MAN S.p.A. (n.d.). Screw press separators. Available at: https://www.cri-man.com/products/screw-press-separators (Accessed September 15, 2023).
DEFRA (2020). The Path to Sustainable Farming: An Agricultural Transition Plan 2021 to 2024. Available at: https://www.gov.uk/government/publications/agricultural-transition-plan-2021-to-2024 (Accessed March 14, 2023).
Demeyer, A., Voundi Nkana, J. C., and Verloo, M. G. (2001). Characteristics of wood ash and influence on soil properties and nutrient uptake: an overview. Bioresour. Technol. 77, 287–295. doi: 10.1016/S0960-8524(00)00043-2
Department of Primary Industries and Regional Development. Government of Western Australia (2018). Soil pH. Available at: https://www.agric.wa.gov.au/soil-acidity/soil-ph?nopaging=1 (Accessed July 13, 2021).
Fivelman, Q. (2013). Granular fertiliser from anaerobic Digestate liquor. Benefits of ADFerTech. Available at: http://www.adfertech.com/ (Accessed January 4, 2022).
Fujioka, M., and Ito, R. (2020). Development of separation process of soluble nutrients from synthetic dairy slurry by modified Solvay process. Sanitat. Value Chain 4, 17–26. doi: 10.34416/svc.00016
Galan, I., Glasser, F. P., and Andrade, C. (2013). Calcium carbonate decomposition. J. Therm. Anal. Calorim. 111, 1197–1202. doi: 10.1007/s10973-012-2290-x
Guštin, S., and Marinšek-Logar, R. (2011). Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 89, 61–66. doi: 10.1016/j.psep.2010.11.001
Hannur, V. S. (2019). Interdependence in horticultural research. J. Horticult. Sci. 14, i–ii. doi: 10.24154/jhs.v14i1.719
Haynes, W. M. (2010). CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data. 91st, ed. Raton, Fla: CRC Press.
Helliwell, R. (1980). The one-straw revolution, by Masanobu Fukuoka. English translation published by Rodale press, Emmaus, Pennsylvania: xxviii + 181 pp., illustr., 21 × 13.5 × 2.5 cm, £4.75, 1978. Environ. Conserv. 7:83. doi: 10.1017/S0376892900006925
Hjorth, M., Christensen, K. V., Christensen, M. L., and Sommer, S. G. (2009). Solid-liquid separation of animal slurry in theory and practice. Sustain. Agric. 2, 953–986. doi: 10.1007/978-94-007-0394-0_43
Hou, Y., Velthof, G. L., Case, S. D. C., Oelofse, M., Grignani, C., Balsari, P., et al. (2018). Stakeholder perceptions of manure treatment technologies in Denmark, Italy, the Netherlands and Spain. J. Clean. Prod. 172, 1620–1630. doi: 10.1016/j.jclepro.2016.10.162
Houba, V. J. G., Temminghoff, E. J. M., Gaikhorst, G. A., and van Vark, W. (2000). Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 31, 1299–1396. doi: 10.1080/00103620009370514
Houpt, S. (2004). Egg drop competition involving only toothpicks and glue. Phys. Teach. 42, 205–207. doi: 10.1119/1.1696586
Huotari, N., Tillman-Sutela, E., Moilanen, M., and Laiho, R. (2015). Recycling of ash - for the good of the environment? For. Ecol. Manag. 348, 226–240. doi: 10.1016/j.foreco.2015.03.008
Husted, S., Jensen, L. S., and Jørgensen, S. S. (1991). Reducing ammonia loss from cattle slurry by the use of acidifying additives: the role of the buffer system. J. Sci. Food Agric. 57, 335–349. doi: 10.1002/jsfa.2740570305
In2ScienceUK (2023a). Promoting social mobility and diversity in science, Technology, Engineering and Maths. Available at: https://in2scienceuk.org/ (Accessed January 19, 2024).
In2ScienceUK (2023b). Student Code of Conduct. Available at: https://in2scienceuk.org/student-code-of-conduct/ (Accessed December 10, 2023).
International Nature Farming Research Center (INFRC) (1999). 6th International Conference on Kyusei Nature Farming. Available at: https://www.infrc.or.jp/knf/6th_Conf.html (Accessed September 22, 2023).
Jewiarz, M., Wróbel, M., Frączek, J., Mudryk, K., and Dziedzic, K. (2018). Digestate, ash and Trichoderm based fertilizer – production line concept design. MATEC Web of Conf. 168:04004. doi: 10.1051/matecconf/201816804004
Kang, J., Kwon, G., Nam, J. H., Kim, Y. O., and Jahng, D. (2017). Carbon dioxide stripping from anaerobic digestate of food waste using two types of aerators. Int. J. Environ. Sci. Technol. 14, 1397–1408. doi: 10.1007/s13762-017-1250-1
Kavanagh, I., Fenton, O., Healy, M. G., Burchill, W., Lanigan, G. J., and Krol, D. J. (2021). Mitigating ammonia and greenhouse gas emissions from stored cattle slurry using agricultural waste, commercially available products and a chemical acidifier. J. Clean. Prod. 294:126251. doi: 10.1016/j.jclepro.2021.126251
Kennedy, C. (1990). Ionic strength and the dissociation of acids. Biochem. Educ. 18, 35–40. doi: 10.1016/0307-4412(90)90017-I
Krauβ, M., and Rzehak, R. (2017). Reactive absorption of CO2 in NaOH: detailed study of enhancement factor models. Chem. Eng. Sci. 166, 193–209. doi: 10.1016/j.ces.2017.03.029
Lal, R. (2021). “Climate change and agriculture” in Climate change, observed impacts on planet earth. ed. T. M. Letcher (Amsterdam: Elsevier B.V), 661–686.
Lanigan, G. J., Donnellan, T., Lanigan, G. J., Hanrahan, K., Paul, C., Shalloo, L., et al. (2018). An Analysis of Abatement Potential of Greenhouse Gas Emissions in Irish Agriculture 2021-2030. Available at: https://www.teagasc.ie/media/website/publications/2018/An-Analysis-of-Abatement-Potential-of-Greenhouse-Gas-Emissions-in-Irish-Agriculture-2021-2030.pdf (Accessed January 19, 2024).
Liu, L., Pang, C., Wu, S., and Dong, R. (2015). Optimization and evaluation of an air-recirculated stripping for ammonia removal from the anaerobic digestate of pig manure. Process Saf. Environ. Prot. 94, 350–357. doi: 10.1016/j.psep.2014.08.006
Lukehurst, C., Frost, P., and Seadi, T.Al (2010). Utilisation of digestate from biogas plants as biofertiliser. Available at: https://task37.ieabioenergy.com/wp-content/uploads/sites/32/2022/02/Digestate_Brochure_Revised_12-2010.pdf (Accessed January 19, 2024).
Manivasakam, N. (2011). Practical boiler water treatment handbook. Chelsea, MA: Chemical Publishing.
Mor, S., Chhoden, K., and Ravindra, K. (2016). Application of agro-waste rice husk ash for the removal of phosphate from the wastewater. J. Clean. Prod. 129, 673–680. doi: 10.1016/j.jclepro.2016.03.088
Moure Abelenda, A. (2023a). Aspen plus® v12 models for design a project-based learning on developing a novel technology for slurry management.
Moure Abelenda, A. (2023b). Report of engagement activity: “Make it happen! Project-based learning on developing an environmental technology of slurry processing to support local students from disadvantaged backgrounds and farming.” Lancaster, UK Available at: https://www.research.lancs.ac.uk/portal/files/393186018/Report_of_engagement_activity.pdf (Accessed January 19, 2024).
Moure Abelenda, A., Aggidis, G., and Aiouache, F. (2023a). Modelling of amino acid fermentations and stabilization of anaerobic Digestates by extracting ammonium bicarbonate. Fermentation 9:750. doi: 10.3390/fermentation9080750
Moure Abelenda, A., Aiouache, F., and Moreno-Mediavilla, D. (2023b). Adapted business model canvas template and primary market research for project-based learning on management of slurry. Environ. Technol. Innov. 30:103106. doi: 10.1016/j.eti.2023.103106
Moure Abelenda, A., Aiouache, F., Roberts, J., and Sayer, E. (2023c). Make it happen! Project-based learning on developing an environmental technology of slurry processing to support local students from disadvantaged backgrounds and farming. Lancaster. Available at: https://www.research.lancs.ac.uk/portal/en/activities/make-it-happen-projectbased-learning-on-developing-an-environmental-technology-of-slurry-processing-to-support-local-students-from-disadvantaged-backgrounds-and-farming(0417b3d0-41cc-4b1c-bd32-87f644a5b561) (Accessed January 19, 2024).
Moure Abelenda, A., and Amaechi, C. V. (2022). Manufacturing of a granular fertilizer based on organic slurry and hardening agent. Inventions 7:26. doi: 10.3390/inventions7010026
Moure Abelenda, A., Semple, K. T., Aggidis, G., and Aiouache, F. (2022a). Circularity of bioenergy residues: acidification of anaerobic Digestate prior to addition of wood ash. Sustainability 14:3127. doi: 10.3390/su14053127
Moure Abelenda, A., Semple, K. T., Herbert, B. M. J., Aggidis, G., and Aiouache, F. (2022b). Valorization of agrowaste digestate via addition of wood ash, acidification, and nitrification. Environ. Technol. Innov. 28:102632. doi: 10.1016/j.eti.2022.102632
Moure Abelenda, A., Semple, K. T., Lag-Brotons, A. J., Herbert, B. M., Aggidis, G., and Aiouache, F. (2021a). Alkaline wood ash, turbulence, and traps with excess of sulfuric acid do not strip completely the Ammonia off an agro-waste Digestate. Edelweiss Chem. Sci. J. 4, 19–24. doi: 10.33805/2641-7383.127
Moure Abelenda, A., Semple, K. T., Lag-Brotons, A. J., Herbert, B. M. J., Aggidis, G., and Aiouache, F. (2021b). Impact of sulphuric, hydrochloric, nitric, and lactic acids in the preparation of a blend of agro-industrial digestate and wood ash to produce a novel fertilizer. J. Environ. Chem. Eng. 9:105021. doi: 10.1016/j.jece.2020.105021
Moure Abelenda, A., Semple, K. T., Lag-Brotons, A. J., Herbert, B. M. J., Aggidis, G., and Aiouache, F. (2021c). Kinetic study of the stabilization of an agro-industrial digestate by adding wood fly ash. Chem. Eng. J. Adv. 7:100127. doi: 10.1016/j.ceja.2021.100127
Moure Abelenda, A., Semple, K. T., Lag-Brotons, A. J., Herbert, B. M. J., Aggidis, G., and Aiouache, F. (2022c). Strategies for the production of a stable blended fertilizer of anaerobic digestates and wood ashes. Nat. Based Solut. 2:100014. doi: 10.1016/j.nbsj.2022.100014
MScTE48 (2014). The Mom Test. YouTube. Available at: https://www.youtube.com/watch?v=Hla1jzhan78 (Accessed September 24, 2023).
Mudryk, K., Frączek, J., Wróbel, M., Jewiarz, M., and Dziedzic, K. (2018). “Agglomeration of ash-based fertilizer mixtures from biomass combustion and Digestate” in Renewable energy sources: Engineering, technology Innovation. ed. M. Krzysztof (Sebastian (Springer): Springer)
National Co-ordinating Centre for Public Engagement (2017). How to evaluate public engagement projects and programmes. Guidance on how to evaluate your public engagement programme. Available at: https://www.publicengagement.ac.uk/resources/guide/how-evaluate-public-engagement-projects-and-programmes (Accessed October 10, 2023).
Ndegwa, P. M., Vaddella, V. K., Hristov, A. N., and Joo, H. S. (2009). Measuring concentrations of Ammonia in ambient air or exhaust air stream using acid traps. J. Environ. Qual. 38, 647–653. doi: 10.2134/jeq2008.0211
Parmar, S. (2022). Seed ball campaign: an effective implementation tool against global warming and deforestation. J. Environ. Eng. Stud. 1, 1–11.
Paudel, S. K., and Belo, A. C. (2017). Fukuoka technique seed ball planting. East Timor Available at: https://www.undp.org/sites/g/files/zskgke326/files/migration/tl/DARDC-Fukuoka-Technique_English-compressed.pdf (Accessed January 19, 2024).
Pesonen, J., Kuokkanen, V., Kuokkanen, T., and Illikainen, M. (2016). Co-granulation of bio-ash with sewage sludge and lime for fertilizer use. J. Environ. Chem. Eng. 4, 4817–4821. doi: 10.1016/j.jece.2015.12.035
Regueiro, I., Coutinho, J., and Fangueiro, D. (2016). Alternatives to sulfuric acid for slurry acidification: impact on slurry composition and ammonia emissions during storage. J. Clean. Prod. 131, 296–307. doi: 10.1016/j.jclepro.2016.05.032
Sakthivel, S. R., Tilley, E., and Udert, K. M. (2012). Wood ash as a magnesium source for phosphorus recovery from source-separated urine. Sci. Total Environ. 419, 68–75. doi: 10.1016/j.scitotenv.2011.12.065
Samuelsen, L., Holm, R., Lathuile, A., and Schönbeck, C. (2019). Buffer solutions in drug formulation and processing: how pKa values depend on temperature, pressure and ionic strength. Int. J. Pharm. 560, 357–364. doi: 10.1016/j.ijpharm.2019.02.019
San Martin Ruiz, M., González Puelles, J. E., Herra Bogantes, J., Rivera-Méndez, W., Reiser, M., and Kranert, M. (2022). Methane, nitrous oxide, and Ammonia emissions on dairy farms in Spain with or without bio-activator treatment. Atmosphere (Basel) 13:893. doi: 10.3390/atmos13060893
Solvay (2023). CASO® TECHNICAL FLAKES. Available at: https://www.solvay.com/en/product/caso-technical-flakes (Accessed September 18, 2023).
Steenari, B. M., and Lindqvist, O. (1997). Stabilisation of biofuel ashes for recycling to forest soil. Biomass Bioenergy 13, 39–50. doi: 10.1016/S0961-9534(97)00024-X
Steinhauser, G. (2008). Cleaner production in the Solvay process: general strategies and recent developments. J. Clean. Prod. 16, 833–841. doi: 10.1016/j.jclepro.2007.04.005
Stern, L., Barnea, N., and Shauli, S. (2008). The effect of a computerized simulation on middle school students’ understanding of the kinetic molecular theory. J. Sci. Educ. Technol. 17, 305–315. doi: 10.1007/s10956-008-9100-z
Tamilarasan, C., Jerlin, R., and Raja, K. (2021). Seed ball technique for enhancing the establishment of subabul (Leucaena leucocephala) under varied habitats. J. Trop. For. Sci. 33, 349–355. doi: 10.26525/jtfs2021.33.3.349
UK DEFRA (2020). Farming is Changing. Available at: https://www.gov.uk/government/publications/future-farming-changes-to-farming-in-england. (Accessed January 19, 2024).
UK Government (2018). Sewage sludge in agriculture: Code of practice for England, Wales and Northern Ireland. Guidance. Available at: https://www.gov.uk/government/publications/sewage-sludge-in-agriculture-code-of-practice/sewage-sludge-in-agriculture-code-of-practice-for-england-wales-and-northern-ireland (Accessed September 25, 2023).
UK Government (2019). Clean air strategy 2019. Available at: https://www.gov.uk/government/publications/clean-air-strategy-2019 (Accessed January 19, 2024).
UK Government (2023). Annex 3: FETF 2023 productivity and slurry eligible items. Guidance. Available at: https://www.gov.uk/government/publications/farming-equipment-and-technology-fund-fetf-2023/annex-3-fetf-2023-productivity-and-slurry-eligible-items (Accessed March 31, 2023).
Ukwuani, A. T., and Tao, W. (2016). Developing a vacuum thermal stripping – acid absorption process for ammonia recovery from anaerobic digester effluent. Water Res. 106, 108–115. doi: 10.1016/j.watres.2016.09.054
Van der Stelt, B., Temminghoff, E. J. M., Van Vliet, P. C. J., and Van Riemsdijk, W. H. (2007). Volatilization of ammonia from manure as affected by manure additives, temperature and mixing. Bioresour. Technol. 98, 3449–3455. doi: 10.1016/j.biortech.2006.11.004
Vandré, R., and Clemens, J. (1996). Studies on the relationship between slurry pH, volatilization processes and the influence of acidifying additives. Nutr. Cycl. Agroecosyst. 47, 157–165. doi: 10.1007/BF01991547
Voshell, S., Mäkelä, M., and Dahl, O. (2018). A review of biomass ash properties towards treatment and recycling. Renew. Sust. Energ. Rev. 96, 479–486. doi: 10.1016/j.rser.2018.07.025
Walker, G. M., Magee, T. R. A., Holland, C. R., Ahmad, M. N., Fox, N., and Moffatt, N. A. (1997). Compression testing of granular NPK fertilizers. Nutr. Cycl. Agroecosyst. 48, 231–234. doi: 10.1023/A:1009714306464
Whelan, M. J., Everitt, T., and Villa, R. (2010). A mass transfer model of ammonia volatilisation from anaerobic digestate. Waste Manag. 30, 1808–1812. doi: 10.1016/j.wasman.2009.08.012
WRAP (2014). BSI PAS 110:2014 Specification for whole digestate, separated liquor and separated fibre derived from the anaerobic digestion of source-segregated biodegradable materials. Available at: https://wrap.org.uk/sites/default/files/2021-03/PAS110_2014.pdf. (Accessed January 19, 2024).
Wróbel, M., Frączek, J., Jewiarz, M., Mudryk, K., and Dziedzic, K. (2016). Impact of selected properties of raw material on quality features of granular fertilizers obtained from Digestates and ASH mixtures. Agric. Eng. 20, 207–217. doi: 10.1515/agriceng-2016-0078
Yan, R., Liang, D. T., Tsen, L., and Tay, J. H. (2002). Kinetics and mechanisms of H 2 S adsorption by alkaline activated carbon. Environ. Sci. Technol. 36, 4460–4466. doi: 10.1021/es0205840
Keywords: ammonia, greenhouse gas emissions, mitigation technology, solid–liquid separation, modified static chamber method, circular economy, active teaching-learning method, curriculum content contextualization
Citation: Moure Abelenda A and Roberts J (2024) Developing a novel technology for slurry management by project-based learning. Front. Sustain. Food Syst. 8:1321353. doi: 10.3389/fsufs.2024.1321353
Received: 13 October 2023; Accepted: 12 January 2024;
Published: 01 February 2024.
Edited by:
José Martinez, Institut National de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), FranceReviewed by:
Marga Lopez, Universitat Politecnica de Catalunya, SpainCopyright © 2024 Moure Abelenda and Roberts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alejandro Moure Abelenda, YS5tb3VyZWFiZWxlbmRhQGxhbmNhc3Rlci5hYy51aw==; YWxlamFuZHJvLm1vdXJlQHVzYy5lcw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.