- 1Professor Jayashankar Telangana State Agricultural University, Hyderabad, India
- 2International Crops Research Institute for the Semi-Arid Tropics, Patancheruvu, India
Storage is an important aspect of groundnut, as the in-shell and shelled kernels are prone to infestation by insects, pests, and fungi. Among several storage pests, the groundnut bruchid, Caryedon serratus, causes serious losses. Farmers often resort to different management practices, including hermetic storage, to control it. The moisture content of the commodity plays an important role in insect infestation during storage. Drying to safe moisture levels before storage is known to prevent the activity of various living organisms, such as storage pests. However, drying to low levels of moisture may not be economical for farmers, as they may not have access to devices to accurately check product moisture. In this regard, we wanted to demonstrate the efficacy of triple-layer hermetic storage bags in preventing the damage caused by C. serratus when the groundnuts are stored at intermediate (10%) and high (14%) levels of moisture compared to traditionally used bags such as polypropylene bags and jute bags. Groundnut pods at 10% moisture content and 14% moisture content were separately inoculated with adult bruchids and a toxigenic strain of Aspergillus flavus fungal inoculum before storing them for 6 months. Results from groundnut samples taken at two-month intervals indicated that groundnut pods stored in triple-layer hermetic bags were completely free from infestation by C. serratus by recording a zero number of eggs laid, number of pupae, adult emergence, percentage of loss, and percentage of damage up to 6 months of storage, by creating low oxygen (hypoxia) and high carbon dioxide (hypercarbia) conditions. Results also indicate no loss of pod weight stored in triple-layer bags, but a slight reduction in germination percentage was recorded due to a slight increase in fungal activity, but the reduction was significantly less in triple-layer plastic bags compared to other bag types. Similarly, biochemical constituents such as oil and protein content were slightly reduced in triple-layer plastic bags when pods were stored at a 10% moisture level, but a higher reduction was observed at a 14% moisture level. However, the reduction was very high and significant in other bag types at both 10 and 14% moisture levels.
1 Introduction
Groundnut is an important food legume and an oilseed crop worldwide. In India, it is cultivated in an area of 4.91 million ha with an annual production of 8.36 million tons and productivity of 1758 kg ha−1, which makes India the second largest producer after China (Indiastat, 2022). Groundnut is a semi-perishable commodity and contains 44–56% oil and 22–30% protein on a dry-seed basis. Nutritionally, groundnut is a very important crop as it is a rich source of minerals (phosphorus, calcium, magnesium, and potassium) and vitamins (E, K, and B) (Arya et al., 2016). Groundnut is usually stored as pods (unshelled) and in kernels (shelled) for different uses. Generally, the harvested produce is stored by farmers, processors, seed entrepreneurs, and other oil extraction units for about 6–9 months before final use (Baoua et al., 2015). The quality and quantity of the groundnut crop are reduced during storage by several insect pests, such as the groundnut bruchid, Caryedon serratus (Olivier); the pod sucking bug, Elasmolomus sordidus (Fabricius); and the red flour beetle, Tribolium castaneum (Herbst). Among these storage pests, the groundnut bruchid is an important one. The adult is brown in color and lays small, translucent, milky white eggs on the pod wall. The larva burrows through the pod wall and starts eating the seed. The larvae bore through groundnut hulls and facilitate attack by secondary pests. At the same time, they favor the spread of Aspergillus flavus (Link) (Sembene, 2006). Fully grown larvae often leave the storage bag and pupate in large numbers at the bottom of the sack pile. The total life cycle varies from 65 to 80 days at 28 ± 2°C and 70 ± 5% RH. Adult longevity for male bruchids is 20 days and 17 days for females. The pre-oviposition period is 2 days, the oviposition period is 7 days, the post-oviposition period is 9 days, and the average fecundity per female is 67 eggs (Behera et al., 2016). Apart from insect pests, different mycoflora belonging to storage fungi, such as A. flavus and A. parasiticus, also reduce quality by producing secondary metabolites known as aflatoxins. In India, groundnut storage losses range between 10 and 15% despite the use of chemicals, including fumigants (Rao et al., 2010).
For groundnut storage, jute (gunny) and woven polypropylene bags are widely used (Bulaong and Dharmaputra, 2002; Baributsa et al., 2017). Pod storage in jute bags, both at the farm and at retail levels, is frequently infested with bruchids and mold growth, especially with Aspergillus flavus fungi. Since jute bags are highly porous and can easily absorb moisture, the chances of rapid growth and multiplication of these aflatoxigenic molds are high. Polypropylene bags are non-absorbent but tend to trap heat inside (Mutegi et al., 2013). In contrast to these traditional storage methods, hermetic storage offers a promising solution and is also a sustainable practice. It works on the principle of creating an airtight seal in which oxygen levels are reduced by insect, fungal, and seed respiration (Murdock et al., 2012). Although extensive research has been done in the field of storage entomology, the generated technologies have not shown a remarkable reduction in storage losses, with the exception of relatively high-cost technologies such as “silos,” whose adoption is difficult and not economically feasible for smallholder farmers.
The “Purdue Improved Crop Storage” (PICS) bags, which work on the principle of hermetic storage, have recently been made available to farmers in several countries around the world. Extensive research has been done on the efficacy of these bags in preventing storage losses primarily incited by insect pests in crops such as cowpea (Murdock et al., 2003; Baoua et al., 2012), maize (Baoua et al., 2014), pigeonpea (Vales et al., 2014), and sorghum (Waongo et al., 2019). In an initial attempt, these bags were evaluated for their efficacy in groundnut storage and were found to be very effective in containing both bruchid infestation and aflatoxin contamination (Sudini et al., 2015). Subsequently, another study was conducted in Niger on unshelled and shelled groundnuts while evaluating the performance of PICS bags and found that they could safely store shelled and unshelled groundnuts (Baributsa et al., 2017). However, properly dried groundnuts with safe moisture levels (around 8% moisture content) were used in all these studies. Drying crop commodities to low and safe moisture levels may not be economical for smallholder farmers (Weinberg et al., 2008). Moreover, at the smallholder farmer level, no tool or device is available to accurately measure the moisture content of groundnut pods, so they may end up storing pods with a moisture content of approximately 10% (intermediate moisture level) or up to 14% (high moisture level). In such a case, it is important to know what would happen if the most cost-effective hermetic storage technology was used. With this in mind, the efficacy of triple-layer hermetic bags on groundnut pod quality with intermediate (10%) and high moisture (14%) content was determined and compared to traditional storage bags such as woven polypropylene and jute bags.
2 Materials and methods
2.1 Source of groundnuts and preparation for storage
Laboratory experiments were carried out for 6 months at the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT, India). Approximately 800 kg of freshly harvested groundnut pods of variety ICGV 02266 were collected from the Groundnut Breeding Unit at ICRISAT. The groundnut plants were harvested by hand and dried in the windrows until the pod moisture dropped to 15%, which was ideal for hand threshing. The pods were then shade-dried to safe moisture levels (8% moisture) for storage. The average oil and protein content is 54 and 30%, respectively. Though the initial content of the aflatoxin levels was 1.0 μg/kg as estimated by an indirect competitive enzyme-linked immunosorbent assay, the groundnut pods chosen for the study were apparently healthy. The groundnut pods were conditioned with distilled water and held for 24–48 h at room temperature to reach the desired levels of 10 and 14% moisture (wet basis). After achieving the desired moisture content, the pods were stored in different types of storage bags for up to 6 months.
2.2 Storage bags and description
Four different types of storage bags, viz., (i) triple-layer hermetic storage bags, (ii) polypropylene bags, (iii) jute bags treated with Spinosad, and (iv) jute bags, were used to evaluate their efficacy in managing groundnut bruchids. Brand-new jute bags and polypropylene bags were purchased from a local market. Untreated jute bags and polypropylene bags were used as is for storing groundnut pods, while the treated jute bags were turned inside out before being sprayed with the insecticide Spinosad (Tracer 45% SC) at 1 ppm. Treated jute bags were shade-dried and used for the experiment. The triple-layer hermetic bags were manufactured locally at Sri Mahalakshmi Woven Sacks Pvt. Ltd., Hyderabad, as per the technical specifications developed by Purdue University, USA. These bags consist of three layers: the inner and middle layers consist of 80-micron-thick high-density polyethylene (HDPE) material and do not allow diffusion of gases (oxygen and carbon dioxide), while the outermost layer is a normal woven sack made of polypropylene and provides strength for handling. Overall, the storage capacity of the bag is 50 kg. The length and width of the outer layer (woven sack) are 115 cm and 60 cm, respectively, whereas the two inner layers each have a length of 120 cm and a width of 60 cm.
2.3 Bruchid culture and maintenance
The culture of groundnut bruchid, C. serratus (Olivier), was collected from naturally infested pods stored in the godowns of the groundnut breeding unit at ICRISAT, Patancheru. The bruchid population was then multiplied under laboratory conditions at a temperature of 25 ± 2°C and 70% relative humidity using pods of the ICGV 02266 variety. The Bruchid population was maintained in plastic jars (15 cm × 10 cm in diameter) fitted with fine mesh lids and exposed to the ambient air. Freshly emerged 1-day-old males and females were separated by sexing and used for the experiment (Deshmukh, 2022).
2.4 Sexing of test insects
Since female insects are bigger than males, the sexing of adult bruchids was done by observing the last visible segments of the abdomen. In males, the pygidium, or fifth visible tergite, projects downward and is hidden by the elytra. The fifth visible sternite is deeply incurved, and the seventh tergite projects between the fifth sternite and the pygidium. In females, the pygidium can be seen in a dorsal view, projecting beyond the elytra. The fifth sternite is fully extended, and the ventral surface is more or less flat. The seventh tergite was not observed in female bruchids (Bandara and Saxena, 1995).
2.5 Aspergillus flavus (Link ex Fr, Teleomorph: Petromyces flavus) inoculum
A toxigenic strain of A. flavus (AF11-4) was obtained from the pure culture collection of the Groundnut Pathology Laboratory at ICRISAT, Patancheru, India. The inoculum of AF 11–4 was aseptically sub-cultured onto potato dextrose agar (PDA) petri plates, then sealed with parafilm and incubated at room temperature (25°C). Two weeks later, a profusely developed culture of A. flavus was observed on the PDA, and conidia were harvested in sterile distilled water (SDW). The concentration was adjusted to 5 × 105 CFU/mL by following a serial dilution technique and a hemocytometer (Sudini et al., 2015).
2.6 Experimental setup and sampling
All treatment bags were filled with 10 kg of groundnut pods and grouped into two sets. In one set, pods with 10% moisture were filled, and in the other set, pods containing 14% moisture were filled. The bags were infested with 30 pairs of adult bruchids, infested pods with eggs (Harish et al., 2014), and spore suspension of the A. flavus toxigenic strain (AF 11–4) at 15 mL/bag (Sudini et al., 2015). The bags were then moved gently upside down for uniform mixing of A. flavus spore suspension and adult bruchids before closing them. The storage bags (one layer at a time starting with the innermost layer in the case of triple-layer hermetic bags) were then tied manually by twisting the loose end of the bag and folding it over, then tying it tightly at the base of the twist and around the folded loop using a strong thread. Each of the four bags used for the experiment was replicated three times for each moisture level. Hence, a total of 24 storage bags were formed as a batch. Three such batches were formed, which were tested for bruchid development and fungal growth after an interval of 2, 4, and 6 months of storage (72 bags total).
Sampling was done every 2 months to determine insect damage, seed properties, and aflatoxin contents. A sample was drawn randomly covering the upper, middle, and bottom portions of each bag (approximately 1 kg total) and inspected for adult bruchid population, which was finally represented as the average number of adult bruchids per kg of the sample for each bag. The number of eggs was also recorded by counting the round white- to pale yellow-colored eggs adhered to 100 randomly selected pods obtained from a representative sample drawn from three different portions of a bag. Similarly, the number of damaged pods (a pod was considered “damaged” if one or more holes were observed) was also recorded by selecting 100 pods randomly from the sample. A representative sample of approximately 100 g of groundnut kernels was collected from each treatment to quantify aflatoxins using indirect competitive ELISA (Reddy et al., 2001). Another 100-g sample was used to obtain the oil and fatty acid composition by using Near Infrared Reflectance Spectroscopy (NIRS; model XDS RCA, FOSS Analytical AB, Denmark).
The percentage of damage and percentage of weight loss were calculated using formulas given by Lale and Igwebuike (2002):
a = initial weight of stored produce before starting the experiment.
b = Final weight of stored produce after terminating the experiment.
2.7 Moisture content determination
Moisture estimation was done by the oven dry method (American Society of Agricultural Engineers, 1989) using the SANYO Electric Drying Oven (SANYO Electric Co., Ltd., Japan, Model: MOV-212). The randomly selected 100-g groundnut pods were pre-weighed to determine the initial weight. The selected 100-g pods were then placed in an aluminum dish of known weight. The pods were then dried in an oven at 105°C for 17 h, and the sample was later cooled and weighed (ASABE Standards, 2010).
2.8 Temperature and relative humidity
Temperature and relative humidity within the sealed bags were automatically recorded every hour from the beginning of the experiment until the end of the experiment over a period of 6 months by placing the programmed data loggers (model EL-USB-2, Lascar Electronics, Wiltshire, UK) inside the treatment bags. One data logger was kept in the storage room to record the ambient conditions of the experimental room.
2.9 Gas composition in storage bags
Oxygen (O2) and carbon dioxide (CO2) levels in different storage bags were measured at the beginning of the experiment and at weekly intervals using the Mocon PAC Check® 183 model 325 headspace analyzer (Mocon, Minneapolis, MN, USA) (Sudini et al., 2015).
2.10 Total protein estimation and fatty acid profiling
Total protein content and fatty acid composition were estimated using near-infrared reflectance spectroscopy (NIRS) (model XDS RCA, FOSS Analytical AB, Sweden, Denmark), a non-destructive method for estimating biochemical constituents (Sundaram et al., 2010). Approximately 70–100 g of groundnut kernels were scanned in a rectangular cup. The scanned sample was then analyzed by the equipment. Data for total oil content and compositions of different fatty acids such as palmitic acid, stearic acid, oleic acid, linoleic acid, and protein content were displayed on the equipment’s monitor and recorded.
2.11 Statistical analysis
This experimental trial was laid out in a completely randomized design. The data had multiple measurements of the response variable on the same experimental unit. Data were recorded at 0 (initiation of the experiment), 2, 4, and 6 months. For each response variable, data were analyzed by repeated measures of analysis of variance (Hao et al., 2004) using the Statistical Analysis System (SAS) Mixed Procedure (SAS Institute Inc, 2018, SAS V 9.4). Here, treatments and moisture levels were between-subject factors, time was a within-subject factor, and all factors were considered fixed effects. A first-order auto-regressive [AR (1)] covariance structure was used for these data, which was selected based on the AIC (Akaike Information Criteria). BLUEs (Best Linear Unbiased Estimates) and pairwise comparisons (for significant effects) were calculated for all effects from the analysis of variance.
3 Results
3.1 Effect of seed moisture content and storage duration on important seed parameters, insect damage, and aflatoxin development in groundnut pods stored in different storage bags
Storage of groundnut pods/kernels at inappropriate storage levels impacted the most important seed characteristic, i.e., seed germination, and the impact aggravated as the duration of the storage period extended (Table 1). The percentage of seed germination was comparatively lower in groundnut pods stored with a high moisture percentage (14%) than in those stored with a low moisture level (10%). The percentage of seed germination was recorded differently in different types of bags. It was in the range of 75–53.3% for the pods with 10% moisture content that were stored in hermetic storage bags for a period of 6 months, while it was
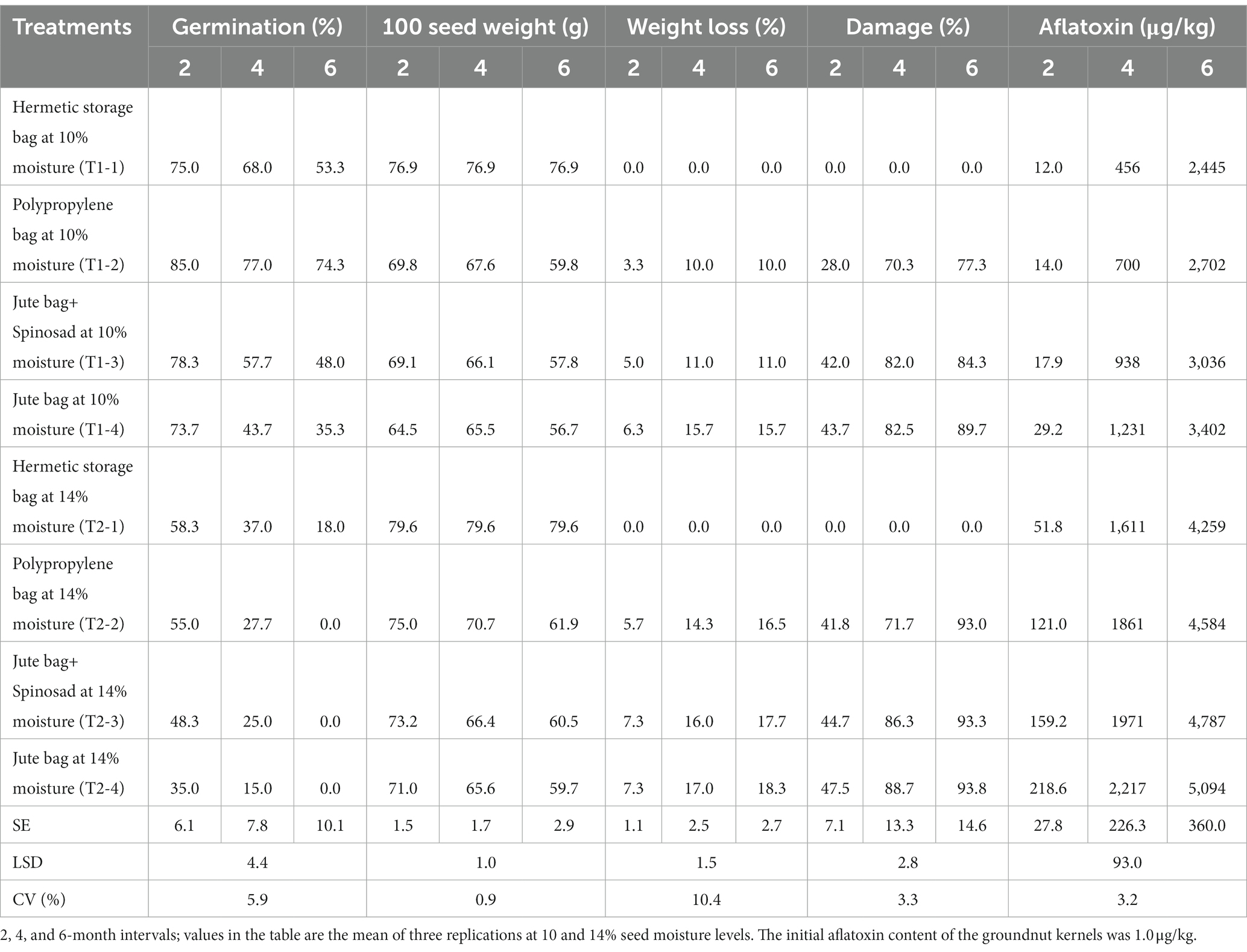
Table 1. Effect of seed moisture levels and storage intervals on important seed parameters, seed damage due to insect infestation, and aflatoxin content of groundnut pods stored in different types of storage bags.
comparatively lower and in the range of 58.3–18% for the pods stored at 14% moisture (Table 1). Among the four different types of bags used, polypropylene bags conserved seed germination for a longer duration when pods were stored at a lower moisture content (10%), which was in the range of 85–74.3%. However, the seed germination drastically reduced and was in the range of 55–0% when the pods were stored at 14% moisture. Similar results were also observed for the pods stored in jute bags and jute bags treated with Spinosad. Interestingly, hermetic storage bags recorded a slightly lower percentage of seed germination when pods with 10% moisture were stored. However, the bags recorded a comparatively better seed germination percentage even after an extended period of time when the pods were stored at a higher (14%) moisture percentage. The seed weight was initially comparatively higher in pods stored at 14% moisture, irrespective of the bags in which they were stored. However, it started declining rapidly with the duration of storage. The decline was higher in jute bags (73.2 g to 60.5 g) and jute bags treated with Spinosad (71.0 g to 59.7 g) within a span of 6 months, while it was considerably moderate in polypropylene bags (75.0 g to 61.9 g). The seed weight remained unchanged in the hermetic storage bags throughout the duration of the experiment (Table 1). Similarly, the pods stored in hermetic storage bags recorded no loss in weight or damage for a period of 6 months, irrespective of high or low moisture content, as there was no moisture loss. The initial infestation did not survive, as the adult bruchids were dead in a short period of time (within two months) due to the non-availability of oxygen. The weight loss was higher in jute bags, ranging from 7.3 to 18.3% (at 14% moisture) and 6.3 to 15.7% (at 10% moisture), and also in jute bags treated with Spinosad, ranging from 7.3 to 17.7% (at 14% moisture) and 5.0 to 11.0% (at 10% moisture). The highest percentage of pod damage, reaching 93.8, 93.3, and 93.0%, respectively, was observed after 6 months of storage at 14% moisture in jute bags, jute bags treated with Spinosad, and polypropylene bags, respectively. The pod damage was low and was in the range of 89.7–77.3% in the same jute bags, jute bags treated with Spinosad, and polypropylene bags when pods were stored at 10% moisture (Table 1). Aflatoxin content, which primarily depends on the moisture content of the stored produce and infestation by bruchid and available microclimatic conditions, including oxygen, was found to be higher in pods that were stored at 14% moisture content than those stored at 10% moisture. However, total aflatoxins (B1 + B2 + G1 + G2) quantified were very low in pods stored in hermetic storage bags, 12 μg/kg, but increased with an increase in the duration of the storage period and were found to be 2,445 μg/kg at the end of the 6 months. They were almost four times higher (51.8 μg/kg) when pods were stored at 14% moisture in the same triple-layer bags and reached a maximum of 4,259 μg/kg by the end of 6 months. The pods stored in polypropylene bags or jute bags recorded a comparatively high aflatoxin content, ranging from 2,702 to 3,402 μg/kg when the pods were stored at 10% moisture. Additionally, they recorded a very high aflatoxin content in the range of 4,584–5,094 μg/kg when stored at 14% moisture (Table 1).
The data obtained in Table 1 clearly show that the percentage of moisture during storage, the storage interval, and the type of bag used for storage impact seed germination, seed weight, seed damage, and aflatoxin content of the stored seed. Statistical analysis shows that a strong negative correlation (−0.72) exists between the moisture content of the pods used for storage and the percentage of seed germination (Table 2). A similar negative correlation (−0.55) was observed with respect to the duration of storage, showing a reduction in the percentage of seed germination with increasing duration of storage. A significant negative correlation (−0.27) on seed germination was also found with respect to the type of bag used (Table 2). Interpretation of data from Table 1 showed that hermetic storage bags recorded comparatively fewer percentage reductions in seed germination as compared to polypropylene bags and jute bags, except for groundnuts stored at 10% moisture level in polypropylene bags.
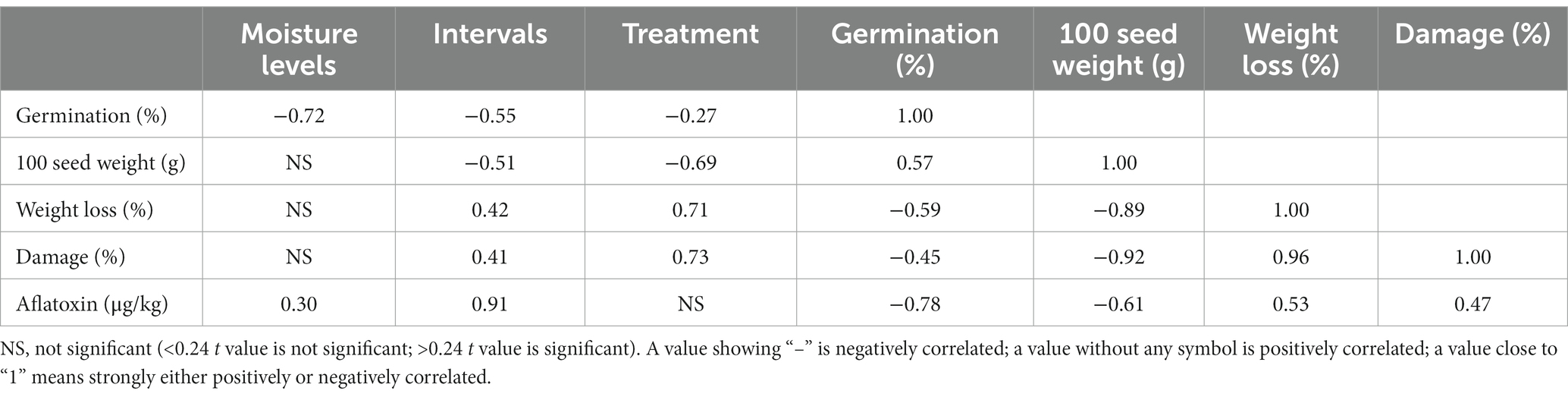
Table 2. Correlation between seed moisture levels, storage intervals, and bag type on the percentage of seed germination, seed weight, weight loss, percentage of damage, and aflatoxin content of stored groundnut pods.
Seed weight showed no significant correlation with moisture content (10% or 14%) of the stored seed but recorded a significant negative correlation (−0.51) with respect to the duration of the storage period and type of storage bag used (−0.69), confirming the result expressed in Table 1 that more reduction in seed weight occurred in polypropylene and jute bags compared to triple-layer hermetic storage bags. A healthy seed obviously results in a high germination percentage, which was demonstrated with a positive correlation of 0.57. The percentage of weight loss and percentage of damage showed no significant correlation with respect to the moisture content of the seed used for storage but showed a positive correlation (0.42 and 0.41, respectively) with the duration of storage. Similarly, a strong positive correlation with a value of 0.71 for weight loss and 0.73 for percentage of damage was observed for the type of storage bag used. The type of bag used showed no significant impact on the development of aflatoxin content, as it developed in all types of bags, but the development was low when hermetic storage bags were used for storage (Table 1). Reduction in test weight of seeds is the clear articulation of higher percentage damage in polypropylene bags and jute bags.
3.2 Effect of seed moisture content and storage duration on gas composition, respiratory physiology, survival, and reproduction of Caryedon serratus (Olivier) on groundnut pods stored in different bags
The results regarding the effect of storage bags and seed moisture content presented in Table 3 showed that in all the bags, except for hermetic storage bags, the survival and reproduction of bruchids continued to increase with increasing storage duration. In the hermetic storage bags, the reproductive and survival parameters such as eggs/100 pods, pupae/100 pods, emergence holes/100 pods, live adults, and dead adults were found to be absolute zero both at both 10 and 14% moisture levels up to 6 months. However, CO2 and O2, which govern the respiratory physiology of the bruchids, were found to change with increasing duration. Carbon dioxide concentrations increased from 10.5 to 10.9% by the end of the fourth month and reached 12.1% by the end of the sixth month, while oxygen concentrations declined from 1.8 to 1.5% and then decreased to 1.3% by the end of the sixth month in the hermetic storage bags containing 10% moisture content groundnut pods. Similar observations were recorded when 14% moisture content pods were stored, where the concentration of CO2 increased from 10.8 to 12.7% and O2 decreased from 1.7 to 1.4% (Table 3).
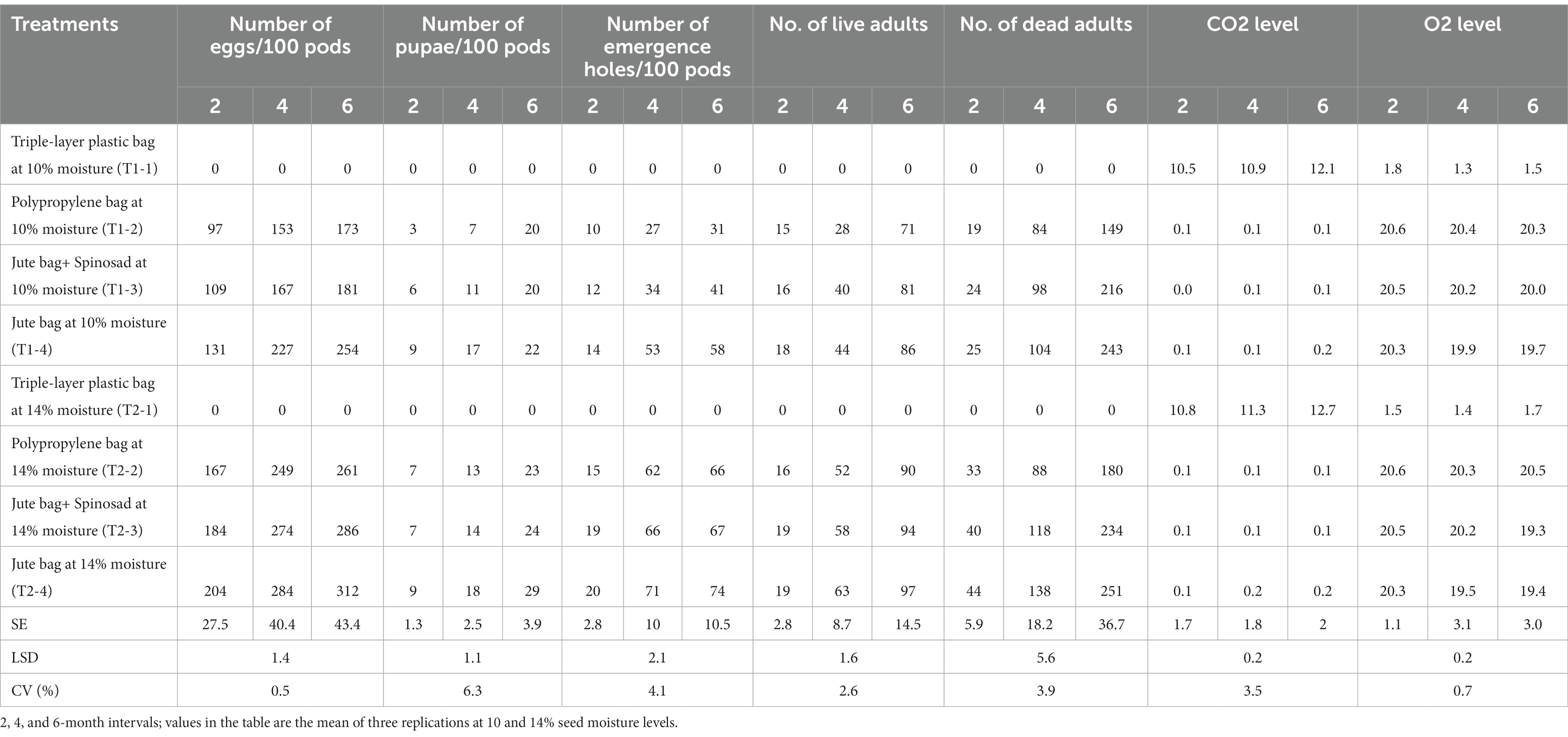
Table 3. Effect of storage bag, storage interval, and seed moisture levels on respiratory physiology, survival, and reproduction of the groundnut bruchid.
Polypropylene bags, jute bags, and jute bags treated with Spinosad favored insect growth, survival, and reproduction with an increasing duration of the experiment. In addition to the type of bag used, the percentage of pod moisture also influenced the insect infestation. However, among the three bags, polypropylene bags with 10% moisture favored less growth and survival as compared to jute bags and jute bags treated with Spinosad. This is evident from the observations where a lower number of infestations was recorded in the polypropylene bags, i.e., 173 eggs, 20 pupae, 31 emergence holes/100 pods, 71 live adults, and 149 dead adults at the end of the 6 months, compared to that recorded in the jute bags and the jute bags treated with Spinosad (Table 3). Untreated jute bags that contained 14% moisture pods favored insect growth and survival as they recorded the highest number of eggs (312), pupae (29), and emergence holes (74) per 100 pods, along with the highest number of live adults (97) and dead adults (251) after 6 months (Table 3). Carbon dioxide and oxygen concentrations were found to be in the range of 0.1–0.2 and 19.3–20.6% in these bags, which was in accordance with the normal atmospheric levels.
The data on the effect of storage bags, storage moisture, and storage duration on the survival and reproduction of bruchids show that except for egg laying (0.29) and adult emergence (0.28), i.e., reproductive and developmental biology, pod moisture played no significant role in the survival of the insects (Table 4). As the duration of the storage increased, bruchid survival (0.63) and reproduction increased (0.28) and were positively correlated. The type of storage bag influenced the survival, growth, and reproduction of the bruchid, as evidenced by the positive correlation parameters that ranged between 0.56 and 0.77 (Table 4). The concentration of CO2 showed a significant negative correlation with the survival and reproduction of the bruchid population, as evidenced by the data that ranged between (−) 0.59 and (−) 0.85. Similarly, oxygen concentration favored the survival and growth of the bruchid, with a strong positive correlation ranging from 0.57 to 0.84 (Table 4).
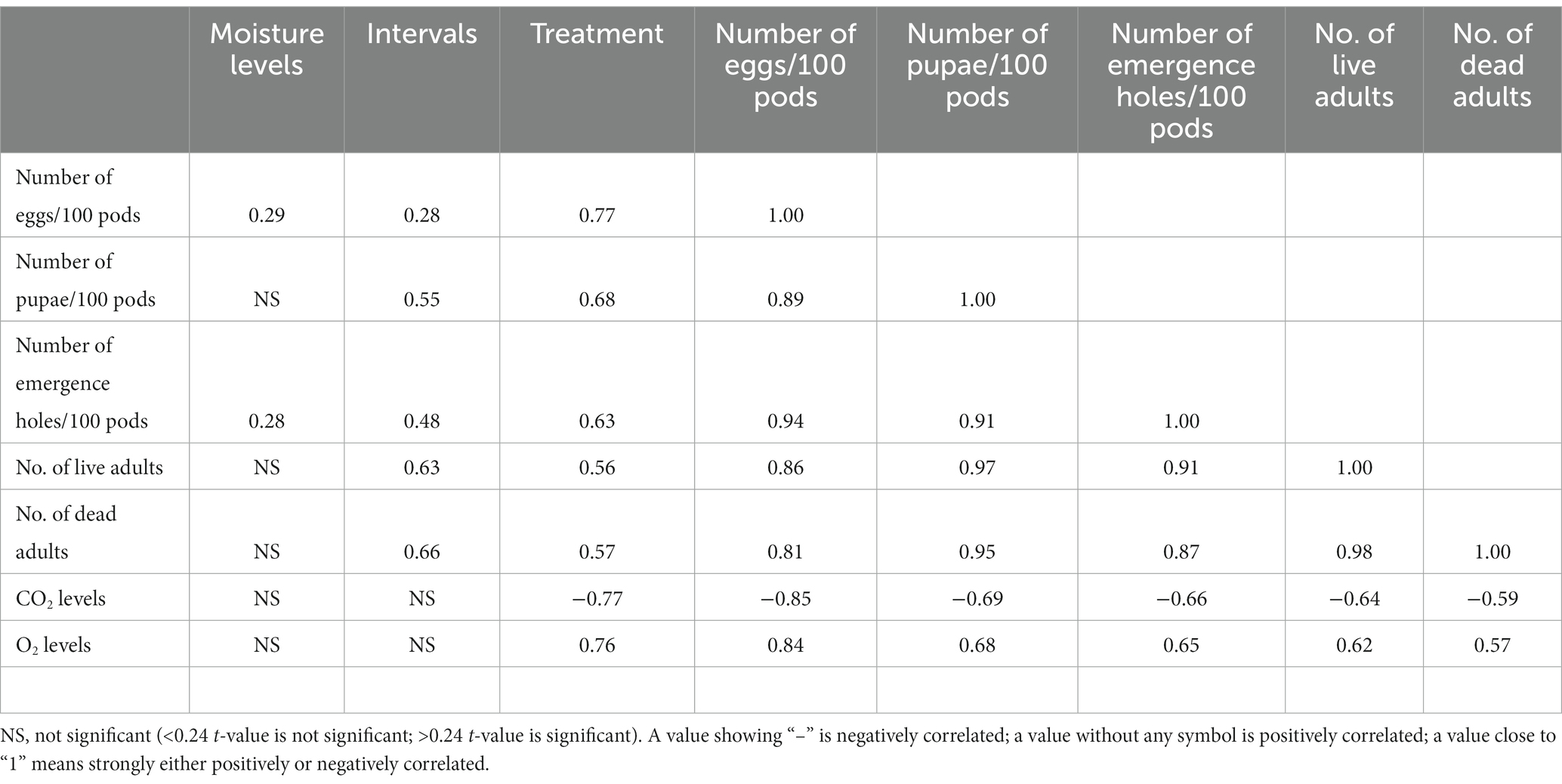
Table 4. Correlation between seed moisture levels, storage interval, and storage bag on respiratory physiology, survival, and reproduction of the groundnut bruchid.
3.3 Effect of seed moisture content and storage duration on biochemical parameters of groundnut seeds stored in different storage bags
3.3.1 Oil content
Results concerning the effect of storage moisture and storage duration showed a reduction in the percentage oil content of the pods with increasing time in both pods stored at 10 and 14% moisture contents. The initial oil content of the pods was found to be 54%; however, the pods stored at lower moisture content recorded little reduction in oil content compared to those stored at 14% moisture content. Pods with a 10% moisture content that were stored in hermetic storage bags recorded an oil content of 52.6% at the end of the 6 months, while the pods with a 14% moisture content recorded a value of 49.6% oil content (Table 5). Polypropylene bags with 10 and 14% moisture content have less oil content compared to hermetic storage bags, which were in the range of 52.7–52.1% and 51.6–48.6%, respectively (Table 5). Similar results with lower oil content were also recorded in jute bags, which ranged from 52.4 to 50.8% when pods were stored at 10% moisture content and from 50.7 to 47.4% when pods were stored at 14% moisture. Jute bags treated with Spinosad also had similar low oil content, ranging from 52.6 to 50.8% when stored at 10% moisture and from 51.1 to 47.4% when stored at 14% moisture (Table 5).
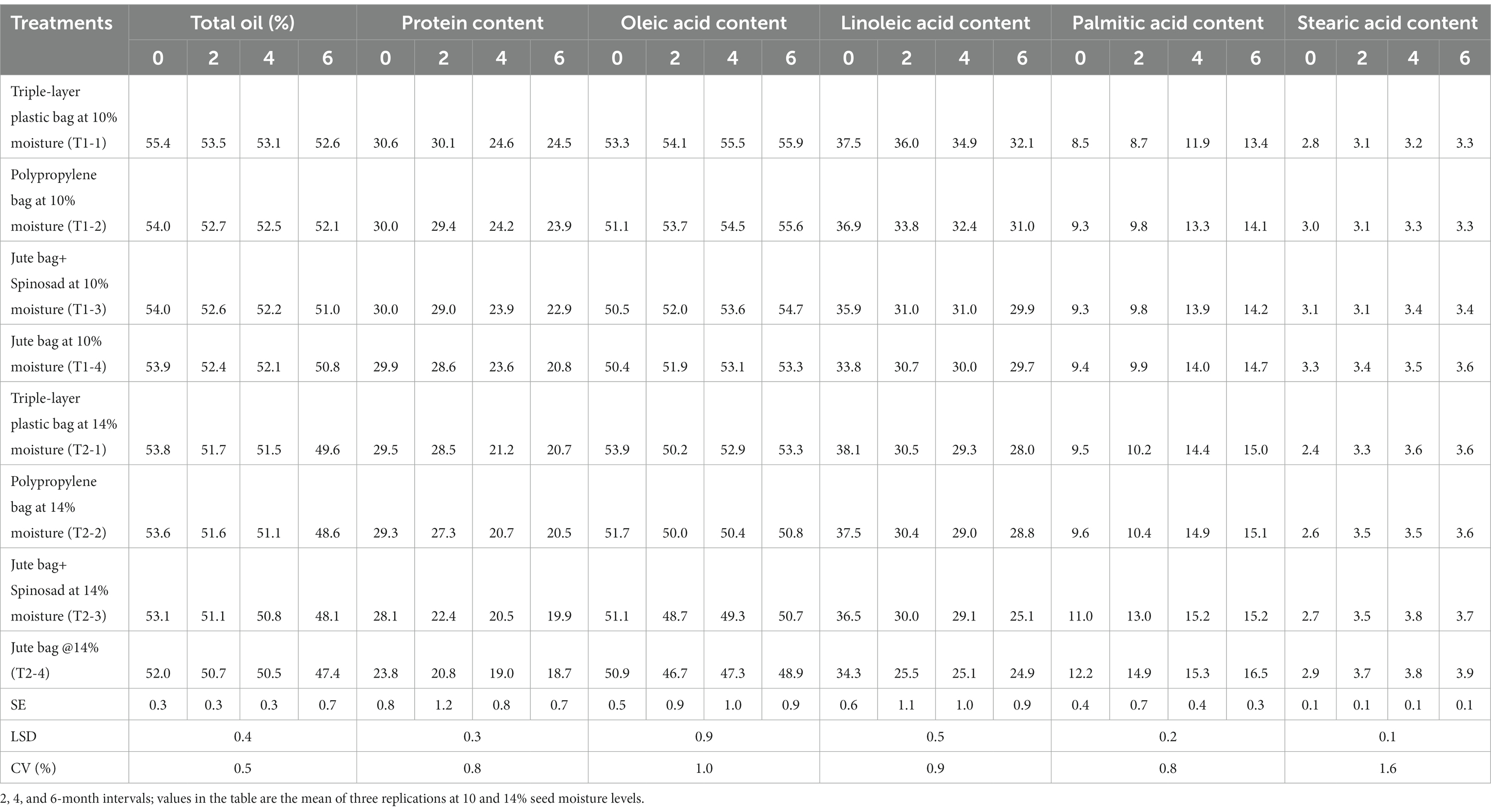
Table 5. Effect of seed moisture levels and storage intervals on biochemical parameters of groundnut seed stored in different storage bags.
3.3.2 Protein content
The protein content, which was 30% before storage, was reduced to 18.7% after 6 months when the pods were stored in jute bags at 14% moisture. A similar reduction (20.8%) was also observed when pods were stored at 10% moisture, but the reduction was lower when compared to pods that were stored at 14% moisture. The reduction in protein content was much less (24.5%) after 6 months when pods were stored in hermetic storage bags at 10% moisture. However, the reduction in protein content was slightly higher (20.7%) when the pods were stored for 6 months at 14% moisture content. The reduction in protein content increased with storage duration and pod moisture. Among the different bags tested, the maximum loss of protein content was observed in jute bags, followed by polypropylene bags and hermetic bags (Table 5).
3.3.3 Oleic acid content
The oleic acid content either increased slightly from 53.3 to 55.9% or remained constant in all the bags with increasing duration in pods stored at 10% moisture content. Contrary to the above findings, oleic acid showed a gradual reduction in all bags when pods were stored at 14% moisture. However, hermetic storage bags showed no change in oleic acid content with increasing duration, even when the pods were stored at 14% moisture (Table 5). The polypropylene bag with pods stored at 14% moisture recorded a decrease in oleic acid content that was found to be 50.8% at the end of the 6 months (Table 5). Similarly, the jute bags also recorded a reduction in oleic acid content, which was higher in the initial period (53.3–48.7%) and later recovered to 50.7% in the jute bags treated with Spinosad and 46.7–48.9% in the normal jute bags (Table 5).
3.3.4 Linoleic acid content
At the start of the experiment, linoleic acid content was 37.5%, but unlike oleic acid, linoleic acid content decreased with the increase in storage duration. However, the reduction was greater in pods stored at 14% moisture than in pods stored at 10% moisture. Among the different bags used for storage, less reduction was observed in hermetic storage bags compared to polypropylene and jute bags. Linoleic acid decreased from an initial content of 37.5–36.0% after 2 months, then to 34.9%, and finally reached 32.1% by the end of the 6 months. Comparatively, a higher reduction in linoleic acid was observed, ranging from 37.5 to 28.0%, when pods were stored at 14% moisture in the same hermetic storage bags (Table 5). Polypropylene bags recorded a slight reduction in linoleic acid content, which ranged from 37.5 to 31.0% and 37.5 to 28.8%, compared to hermetic storage bags when pods were stored in them at 10 and 14% moisture, respectively (Table 5). Jute bags recorded the maximum reduction in linoleic acid content, which reached up to 29.7% at the end of the 6 months when pods were stored at 10% moisture, while it was still reduced to 24.9% in pods stored at 14% moisture.
3.3.5 Palmitic acid content
Palmitic acid was found to be 9.3% initially but recorded an increase in concentration with the increase in storage period. It was also observed that pods stored with moisture content favored increased levels of palmitic acid with the increase in storage duration. However, lower levels of palmitic acid were observed in hermetic storage bags that contained pods stored at a 10% moisture level, which ranged from 9.3% initially to 13.4% by the end of the 6 months. Similar increases were also observed in the same bags at a 14% moisture content, but the increases were higher and ranged between 9.3 and 15.0% (Table 5). Jute bags with 14% moisture recorded a maximum increase in palmitic acid content that ranged from 9.3 to 16.5%. Polypropylene bags were intermediary and displayed a moderate increase in palmitic acid from 9.3 to 14.1% when pods were stored at 10% moisture content and increased up to 15.1% when pods were stored at 14% moisture.
3.3.6 Stearic acid content
Stearic acid content was found to be 2.8% initially, but it increased with the time of storage. It was also observed that not only the duration of storage but also the high moisture content influenced the increase in the stearic acid concentration. The increase was lower in hermetic storage bags as well as polypropylene bags with pods stored at 10 and 14% moisture after 6 months of storage. In bags that contained pods with 10% moisture, the increase ranged from 2.8 to 3.3%, while the pods that were stored at 14% moisture content had a higher increase, ranging between 2.8 and 3.6% (Table 5). Polypropylene bags recorded slightly higher levels of stearic acid both at 10 and 14% moisture during the second and fourth months of the storage period compared to hermetic bags. Jute bags recorded higher stearic acid content compared to hermetic bags and polypropylene bags that ranged from 2.8–3.6% and 2.8–3.9%, respectively, when pods with 10 and 14% moisture content were stored (Table 5).
The correlation clearly suggests that seed moisture levels have a negative impact on total oil content, protein content, oleic acid, and linoleic acid contents, with values of (−) 0.52, (−) 0.44, (−) 0.61, and (−) 0.37, respectively. Similarly, the duration of the storage period also showed an impact on the biochemical parameters of the pods, with a significant reduction in oil (−0.72) and protein (−0.77) contents with an increase in storage duration. Similar reductions were also observed for linoleic acid content, with an increase in the storage period with a value of (−) 0.70. However, certain biochemical parameters, such as palmitic acid and stearic acid recorded a positive correlation with values of 0.82 and 0.70, respectively, as their contents increased with prolonged storage duration (Table 6). The type of bag used also showed a certain impact on the biochemical parameters of the pods stored in them, which included a significant reduction in oleic acid content with a (−) 0.52 value (Table 6).
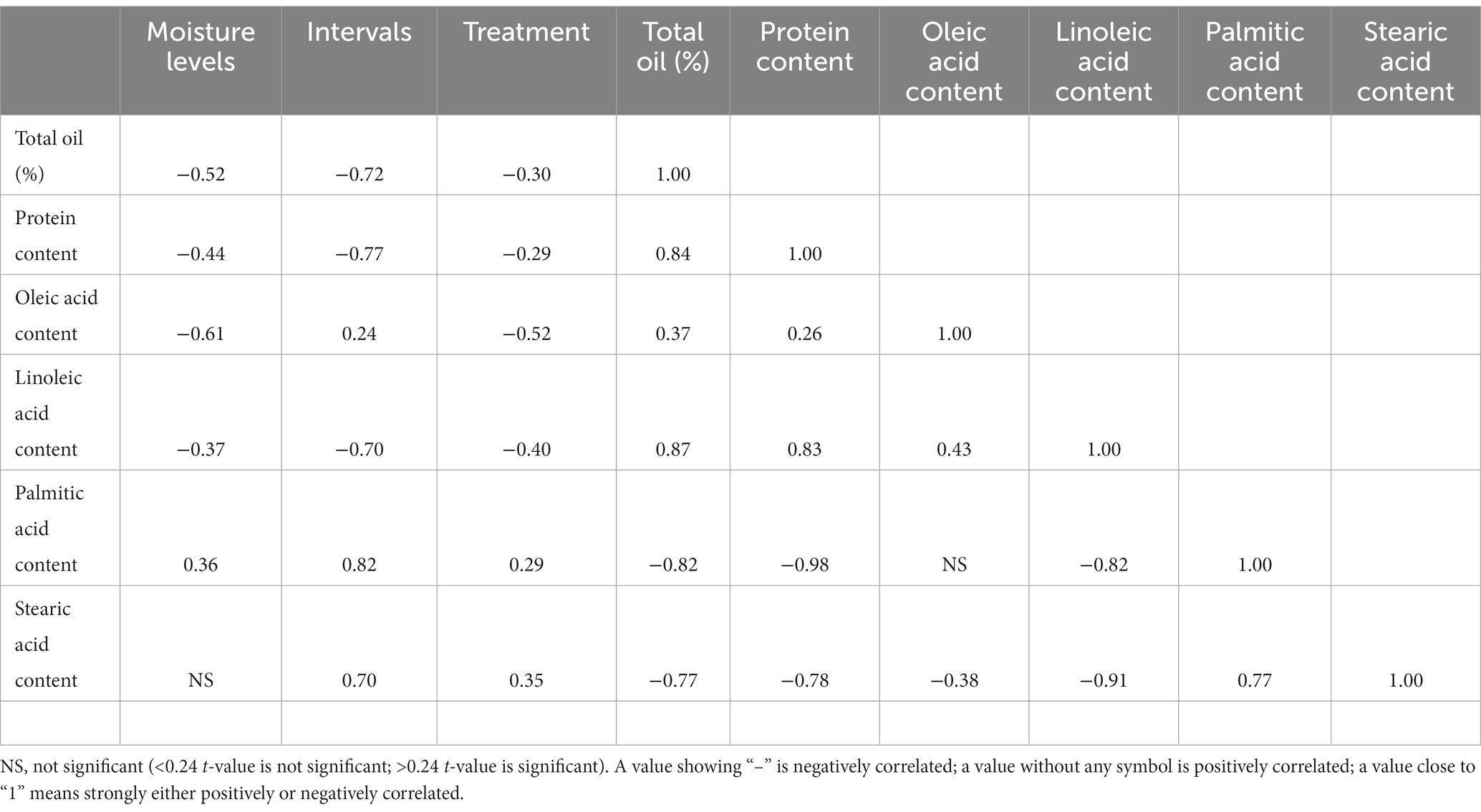
Table 6. Correlation between seed moisture levels and storage intervals on biochemical parameters of groundnut pods stored in different storage bags.
3.4 Storage temperature, relative humidity, and moisture levels in different bags and storage periods
The data loggers measured temperature and relative humidity inside each treatment combination at different moisture levels of 10 and 14% every 24 h. However, we presented the data and plotted them based on bimonthly intervals of averages at 10 and 14% moisture. The comparison does not have statistical power and should only be considered in terms of trends (Table 7).
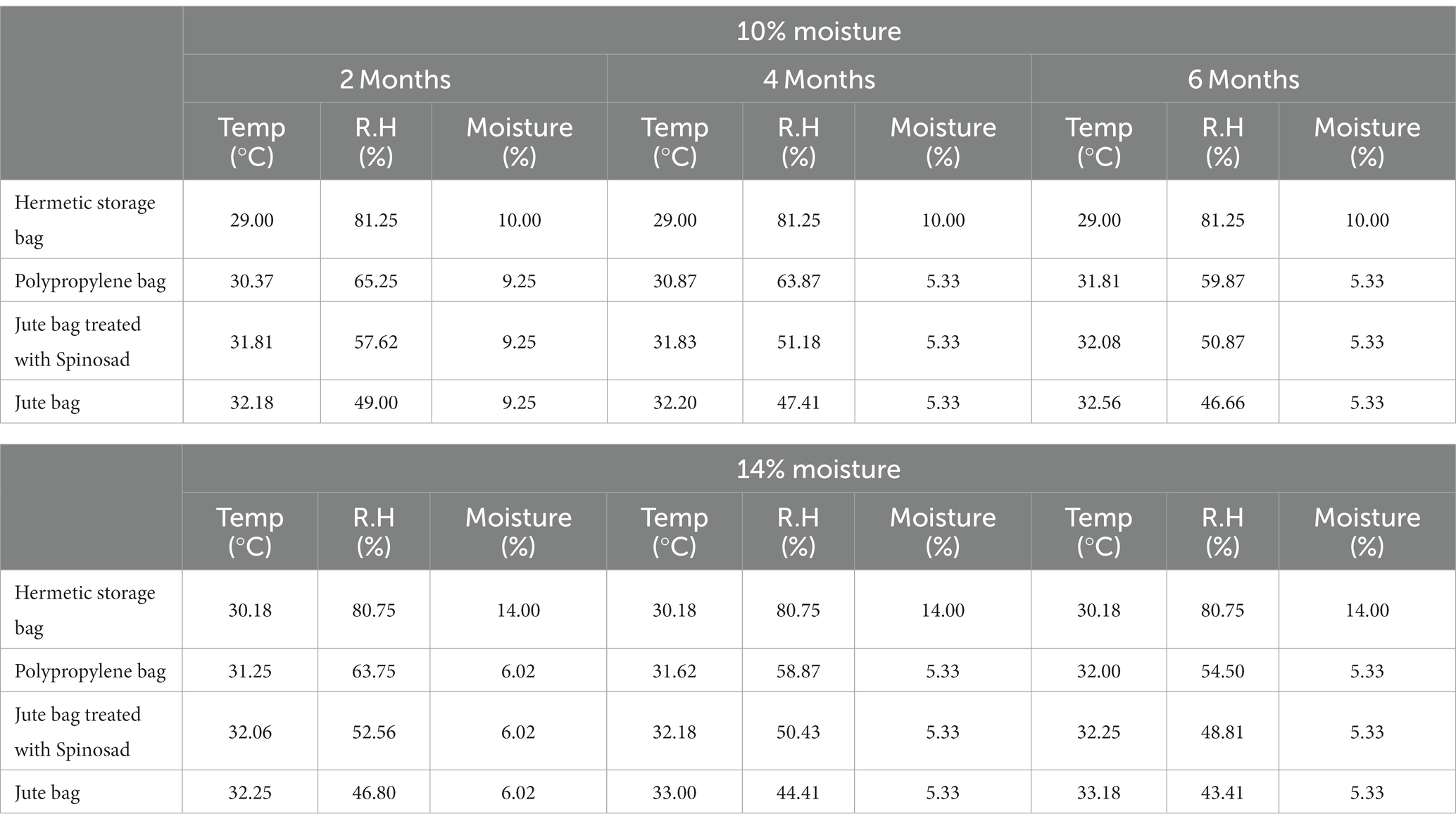
Table 7. Observed mean temperature, RH, and moisture percentage in different storage bags at different storage intervals.
The data loggers recorded higher temperatures and low relative humidity in jute bags where pods were stored with 10 and 14% moisture, respectively. After 2 months, a temperature of 32.25°C and 46.8% RH were recorded in jute bags with 14% moisture pods, while the temperature was 32.18°C, and RH was 49% in jute bags with pods at a 10% moisture content. After 4 months, the temperature was 33°C and RH was 44.41% in bags with a 14% moisture content, and 32.2°C and 47.41% RH in bags with a 10% moisture content; after 6 months, the temperature was 33.18°C and RH was 43.41% in bags with pods stored at 14% moisture, and 32.56°C and 46.66% RH in bags with pods stored at 10% moisture (Table 7). The minimum temperature and maximum relative humidity recorded in the hermetic storage bag at 10% (29°C, 81.25%) moisture in comparison to 14% (30.18°C, 80.75%) did not change at 2-, 4-, and 6-months’ storage (Table 7).
Maximum temperature and minimum relative humidity were recorded in jute bag followed by a jute bag treated with Spinosad and polypropylene bag at 14% moisture in comparison to 10% moisture after 6 months’ storage followed by 4 and 2 months’ storage.
A maximum decrease in moisture content was observed in jute bags with an initial 14% moisture content compared to 10% moisture (Table 7). However, the moisture content was constant in hermetic storage bags after 2, 4, and 6 months of storage. After 2 months, jute bags, polypropylene bags, and jute bags treated with Spinosad recorded minimum changes in moisture content of 10% (reduced to 9.25%) compared to 14% (reduced to 6.02%). After 4 and 6 months, jute bags, jute bags treated with Spinosad, and polypropylene bags did not differ significantly but differed at 10 and 14% moisture levels (Table 7). At 10% moisture levels, the moisture reduction was 5.33%, and at 14%, it was 4% due to a higher rate of growth and multiplication of insects and fungi.
3.5 Interaction of seed moisture content, storage duration, and bag type on certain important seed characteristics, insect and fungal incidence, and biochemical parameters of groundnut pods
The interactive effects of all three variables, such as seed moisture content, storage duration, and type of storage bag, clearly show that these variables significantly impact certain important seed characteristics, which include germination percentage and seed weight. Among the three tested variables, a significant impact was observed on the type of bag used, moisture content, and duration of storage interval on the germination percentage of seed, with F-values for variables and their combinations ranging between 4.1 and − 1830.1 (Figure 1A). Seed weight was impacted significantly by all three variables individually (Table 8), but the interaction of the three variables did not show a considerable impact (Figure 1B). Aflatoxin growth and development were found to be significantly impacted by all three variables, such as seed moisture content, storage duration, and type of storage bag, and their interactions (Figure 1C). Survival, growth, and development of bruchid populations were significantly impacted by all three variables, with F-values ranging from 9.8 to 12560.5 (Figure 1D). Oxygen and carbon dioxide concentrations were highly impacted by the type of bag used (Figures 1E,F), with F-values of 127,414 and 107,820, respectively. The moisture content of the pods and duration of the storage period impacted the oil content of the pods majorly when compared to the type of bag used for storage, as witnessed by the variables showing F-values of 94.3 for moisture content, 54.8 for duration of storage, and 41.2 for the type of storage bag used. Similarly, protein content was primarily impacted by the duration of the storage period with an F-value of 6893.8, and moisture content with an F-value of 3573.0. The bag type also influenced the protein content of the pods stored, with an F-value of 1756.8 (Table 8).
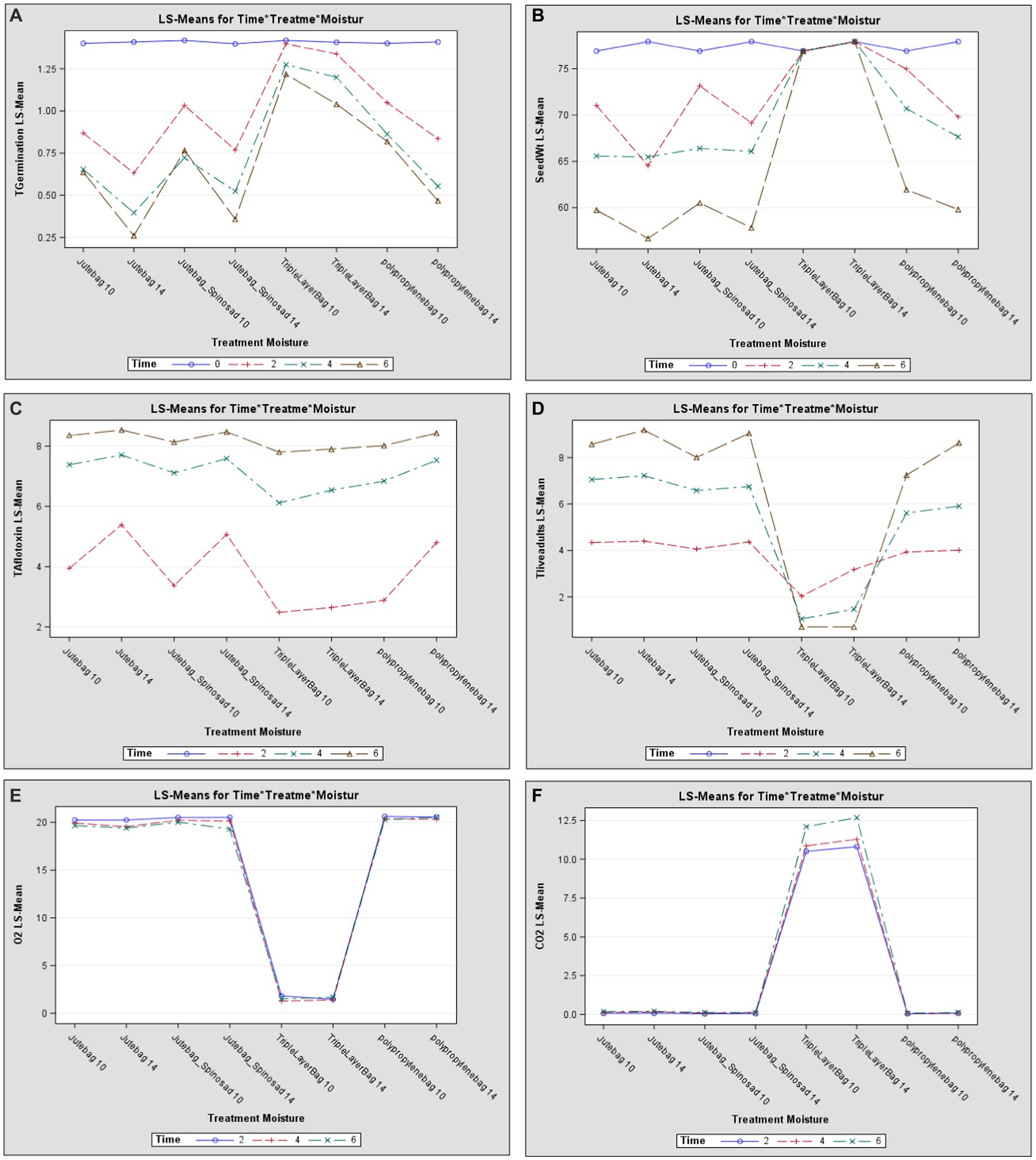
Figure 1. (A–F) Interactive effect of seed moisture content, storage intervals, and bag type on certain important seed characteristics, insect, and fungal incidence parameters of groundnut pods following F-3 test analysis.
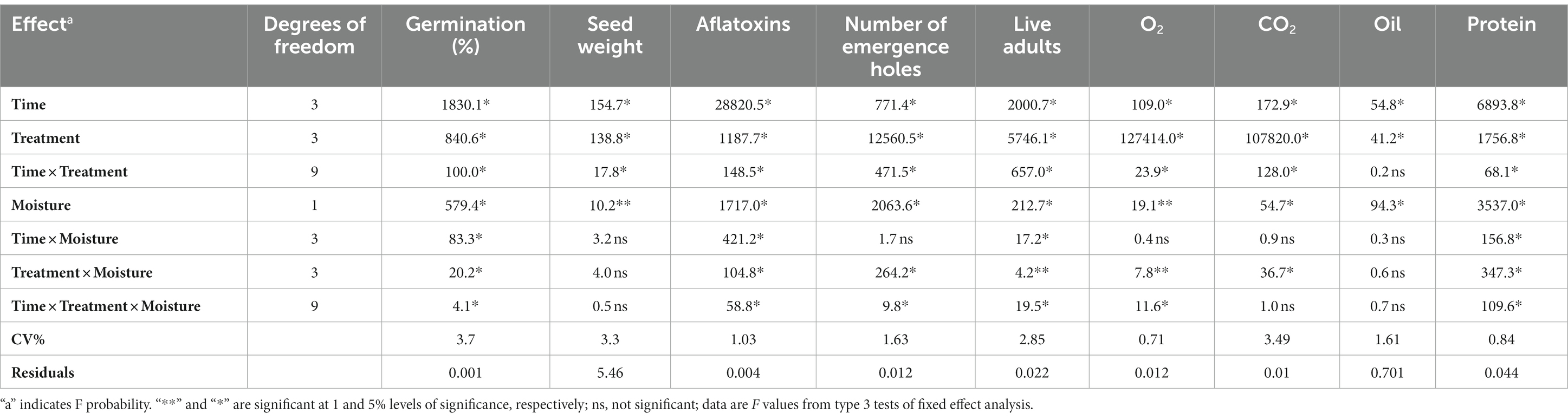
Table 8. Interactive effects of seed moisture content, storage interval, and bag type on certain important seed characteristics, insect and fungal infestation, and biochemical parameters of groundnut pods.
4 Discussion
Hermetic storage technologies prevent quantitative and qualitative losses of agricultural commodities caused by insect pests and molds during storage. This has been proven in several crops using hermetic storage bags based on Purdue Improved Crop Storage (PICS) technology, which works on the hermetic (airtight) principle. Our previous work demonstrated that hermetic bags are superior to conventional/non-hermetic bags in protecting groundnut pods from insects during storage, mold development, and subsequent accumulation of aflatoxin contents (Sudini et al., 2015). In contrast, we wanted to confirm the performance of the hermetic bags in the case that the farmers store their produce without properly drying it to the required level of moisture.
During the study, it was established that certain important seed quality parameters, such as seed weight and germination percentage, were not affected by storage in hermetic bags. Insect count in terms of number of eggs, pupae, emergence holes, and live adults was lower in hermetic bags compared to conventional /non-hermetic storage bags.
The percentage of damage and percentage of weight loss were lower in hermetic storage bags as compared to other bags. In a similar study conducted on maize seed storage, weight loss of up to 41.2 and 48.5% was reported in the polypropylene and jute bags, respectively, while only 1% damage was observed in maize seeds stored in hermetic storage bags (Nganga et al., 2016a). The decrease in insect population can be attributed to the reduced levels of O2 and increased levels of CO2 inside the hermetic bags (Sudini et al., 2015; Baributsa et al., 2017). The primary reason for the formation of low oxygen conditions was due to the usage of a large amount of oxygen by the groundnut bruchid, C. serratus (Olivier), for its survival (Swathi et al., 2017). The large size of the bruchid requires higher quantities of oxygen for its general metabolism; hence, the initial population quickly uses the available oxygen, which later results in elevated CO2 conditions due to the non-diffusion of oxygen from the external environment into the hermetic storage bags due to their impermeability to gases (Sudini et al., 2015; Baributsa et al., 2017). Similarly, the emission of CO2 by the insects during their metabolic activities might have increased the CO2 concentrations (Bert et al., 2022).
The lethal effect of CO2 increases the solubility of body fluids, which subsequently lowers the pH. A drop in pH creates lesions in the cell membrane of larvae and adult insects, which compromises cell integrity and ultimately results in the death of insects (Nielsen, 2001; Murdock et al., 2012). Pod weight loss and damage were higher in jute bags due to the inherent nature of the bag, which promoted congenial conditions for insect growth and reproduction. Similar results were observed in jute bags treated with Spinosad, which failed to stop the multiplication of C. serratus on groundnut. The reason may be that a single application of the insecticide may not be sufficient for long-term efficacy, as re-infestation and cross-infestation can occur at any time during storage (Dasbak et al., 2009).
The initial release of C. serratus (Olivier) in various bags played a crucial role in the percentage of damage and weight loss, as the number of adults multiplied over time in different bags. The higher the level of adult emergence, the higher the pest infestation is (Harish et al., 2012). The jute bags with greater permeability to the external environment have plenty of available oxygen, which was used by the insects for their multiplication. Njoroge et al. (2014) reported an increase in the moisture content of maize stored in polypropylene bags for 6 months and attributed this to a heavy insect infestation.
In the present context, insect damage paved the way for fungal infestation, as pods broken by the insects were more prone to attack by A. flavus infection. There was a positive correlation observed where insect-damaged pods had a huge infestation of A. flavus in jute bags, followed by jute bags treated with Spinosad, polypropylene bags, and triple-layer bags. Not only oxygen permeability, but moisture content of pods, in addition to the moisture absorbed by pods due to storage in jute and polypropylene bags, also played a major role in fungal multiplication (Williams et al., 2014). Improper maintenance of moisture leads to fungal development, which was also evident in triple-layer bags (Nganga et al., 2016b). However, compared to jute bags and polypropylene bags, aflatoxin development was very low and within acceptable limits. An increase of less than 5% in aflatoxin content in maize seeds stored in hermetic storage bags was realized by Walker et al. (2018). The results of their work suggested the comparative effectiveness of hermetic storage bags in protecting seed quality when maize seeds were stored at a higher moisture percentage than recommended for a period of6 6 months.
In the present study, the lowest accumulation of aflatoxin was recorded in hermetic storage bags due to increased CO2 levels, reduced O2 levels, and less insect activity inside the bags. All these factors may have proved to be detrimental to fungal growth and aflatoxin production. However, significant levels of aflatoxin were observed in pods with 14% moisture compared to pods with 10% moisture content. Although the recommended pre-storage moisture content for groundnut is ≤8%, the pods stored at 10% moisture content still accumulated only 12 μg/kg of aflatoxin after 2 months of storage. This is still within the allowable limits set by the regulatory bodies of many countries, for example, India (15 μg/kg), the USA (20 μg/kg), etc. However, when the storage period was increased to 4 and 6 months, even the hermetic storage bags did not provide resistance against aflatoxin accumulation, as the levels observed were higher than the permissible limit. This finding means that groundnut pods with sub-optimal moisture levels can be stored in hermetic storage bags for shorter periods, such as up to 2 months. Subsequently, the accumulation of aflatoxin was relatively low in hermetic storage bags compared to conventional bags as the storage period progressed to 4 and 6 months. Moisture content, along with the microclimate inside the bags, including relative humidity and temperature, plays an important role in mold growth and aflatoxin production. Insect damage can lead to the multiplication of fungi (Harish et al., 2014). Therefore, aflatoxin levels were higher in conventional bags compared to hermetic bags.
Groundnut seeds are a costly input for farmers, and yet many smallholder farmers have been unable to store their own seeds, even though groundnut is a self-pollinating crop and seeds can be stored and utilized year after year (Ntare et al., 2008). A cost-effective storage tool that helps maintain seed germination is of immense benefit to smallholder farmers. In this regard, seed germination and 100 seed weight are important parameters to be observed in groundnut storage. Seed germination and 100 seed weight were found to be good in hermetic storage bags as compared to other storage bags studied. Although oxygen was drastically reduced in hermetic storage bags due to consumption by the insects for their respiration and regular metabolism (as mentioned, the insect is very big in size, unlike other storage pests), it did not have any measurable impact on seed germination. Groundnut seeds stored as pods must have received sufficient oxygen, and its utilization by the seeds could be a probable reason for maintaining a high percentage of seed germination. However, the requirement of oxygen for an embryo of any seed will be very low to negligible; hence, the availability of a negligible amount of oxygen in a given environment will be sufficient for the respiration of the seed and its viability. The safety and viability of the seeds during storage in the triple-layer plastic bags were also reported by Omondi et al. (2011), who observed that the seeds stored in the triple-layer plastic bags maintained the germination percentage of up to 85% when stored for a period of 9 months, compared to the traditionally used gunny (jute) bags, where the germination percentage was reduced to 14.76% within 3 months. The slight reduction in germination percentage in triple-layer plastic bags in the present study can be attributed to the storage of seeds with moderately high moisture content (14%), which attracted mold growth. However, the germination percentage was much higher when compared to both jute and polypropylene bags. A similar study by Lahari and Sudha Rani (2022) also established the safety of the seed viability of different seeds, such as pigeonpea, which was found to lose a marginal 3% of germination when stored for a period of 4 months in triple-layer storage bags, whereas the loss rose to 7.5% when storage happened in gunny bags. The authors also reported similar results in mung bean, where they found only a 1% loss of germination, while in groundnut seeds they found a loss of 4.1% germination when stored for 4 months in triple-layer storage bags. Previous studies conducted on the efficacy of hermetic storage bags for groundnut storage have confirmed the safety and viability of the seeds (Sudini et al., 2015; Baributsa et al., 2017).
In addition to seed viability, the biochemical constituents, such as oil content and protein content of seeds, are key nutritional factors that need to be considered and tracked during storage. It is well known that prolonged storage reduces the nutritional composition of seeds. However, certain other factors that also contribute to a rapid decline in the nutritional composition of seeds are the storage of seeds with high moisture content in addition to the availability of oxygen to seeds during storage. The findings of Singh et al. (2022) clearly indicate a significant increase in moisture content, free fatty acids, headspace concentration of CO2, and aflatoxins and a decrease in oil content, protein content, and headspace concentration of O2 in groundnut seeds stored in gunny bags and high-density polyethylene (HDPE) bags except for vacuum packaging. This finding clearly suggests that the permeability of HDPE bags and gunny bags increases the scope for an increase in moisture content, which favors insect multiplication and aflatoxin production. A similar finding was also reported by Vijayalakshmi and Malabasari (2018), who recorded the highest germination (83.67%), oil content (47.39), and protein content (27.52) with the lowest moisture content (5.03%) in groundnut seeds stored in hermetic storage bags. On the other hand, seeds stored in gunny bags deteriorated rapidly in all the seed quality parameters and recorded the lowest germination (54%), oil content (45.01%), and protein content (25.32%) with a fluctuatingly higher moisture content (12.03%) at the end of 8 months of storage.
Storage in hermetic bags prevented the survival of C. serratus and resulted in less damage to groundnut pods. It is evident from our study that the number of eggs, pupae, and emergence holes was lower on groundnut pods stored in hermetic bags. The absence of live adults after 6 months of storage in hermetic bags is due to decreased oxygen levels and increased concentrations of carbon dioxide, which may have shown a synergistic effect on insect mortality (Banks and Annis, 1990; Murdock et al., 2012). In other storage bags, they were more due to their permeable nature, which favored the exchange of gases and diffusion of oxygen into the bags and carbon dioxide out of the bags, and also moisture present in the air absorbed by pods through the perforations in the bags (Mutungi et al., 2014). The traditional jute bags, the jute bags treated with Spinosad, and the polypropylene bags recorded maximum changes in temperature and relative humidity due to the porosity of their structure, providing only a slight restriction to O2 movement across the bag walls. Access to oxygen in these bags allowed the insects to breathe freely and led to the multiplication of insects and mold; also, the material of the bags, which allowed moisture to be expelled during the drier winter months, produced significantly more heat and reduced levels of relative humidity. These results are similar to those found in rice storage (Martin et al., 2015) and pigeonpea storage (Vales et al., 2014).
Unlike triple-layer hermetic storage bags, jute bags and polypropylene bags are permeable to oxygen and water vapor. The permeability of a polypropylene bag for oxygen is 0.25 ± 0.03 g m−2 day−1 Pa−1 and 5.1 × 10−5 g (m day Pa)−1 for water vapor, whereas in the case of a jute bag, the oxygen permeability is 0.40 ± 0.10 g m−2 day−1 Pa−1 and the water vapor permeability is 5.9 × 10−4 g (m day Pa) −1 at 28° C and 75% RH (Omodara et al., 2021). Changes in oxygen and water vapor permeability occur in different bags as considerable amounts of moisture get transferred from the ambient air in warehouses to the stored seeds as the environmental conditions change, depending on the initial moisture content of the grains. However, hermetic bags provide a good barrier against fluctuations in grain moisture content, either by loss or absorption of moisture by the seeds stored in them, whereas moisture content of grain stored in Polypropylene bags loose moisture while jute bags absorb moisture from the external environment. The moisture content of maize stored in polypropylene and jute bags gradually decreased during storage owing to the warm and dry conditions (Nganga et al., 2016a), while an increase in the moisture content of maize stored in polypropylene bags for 6 months was observed and attributed to heavy insect infestation (Njoroge et al., 2014). Such changes have implications for the quality and safety of stored produce and the saleable weight.
Similarly, in our study, the lower moisture content in jute and polypropylene bags, as opposed to hermetic storage bags, could be due to the lower ambient relative humidity levels of the air during the drier winter months. These investigations are similar to previous studies on rice storage by Martin et al. (2015). Hermetic storage bags appeared to retain the initial pod moisture of 10 and 14% and preserved it against changes related to seasonal variations in the external environment, due to the HDPE liners in the triple-layer hermetic bag. They are essentially watertight and retain the existing water present in the bag when it is tied shut. By contrast, the jute bag and other bag types are essentially open to the ambient air and will slowly balance with the outside environment over time. These results are in agreement with previous investigations on maize storage by Ognakossan et al. (2013).
Groundnut are considered an important source of protein and vegetable oil for millions of people around the world. Our study clearly revealed that there was a significant reduction in protein and oil content when the seeds were stored in jute bags, jute bags treated with Spinosad, and polypropylene bags for any period of storage (2, 4, and 6 months) as compared to hermetic storage bags. Furthermore, it was observed that the reduction was higher when groundnut pods were stored with higher levels of moisture (14%). The reasons could be heavy insect infestation, insect feeding, and fungal infestation under improper storage conditions (Bhattacharya and Raha, 2002; Rani et al., 2013).
Overall, our study reveals that storing groundnut pods in hermetic bags keeps quantitative and qualitative losses in check compared to other traditional bags. Moisture plays a vital role in the development of mold and also in the survival and multiplication of insects, whereas groundnut pods stored below 10% moisture content have been shown to avoid the development of mold. In this study, germination, 100 seed weight, insect count, percentage of damage, and percentage of weight loss were lower in hermetic storage bags as compared to traditional bags, even though mold multiplication and aflatoxin content were observed in hermetic bags mainly due to moisture content, i.e., 10 and 14%. Therefore, storage of pods below 10% moisture content in hermetic bags is recommended.
All traditional storage bags recorded a maximum number of live insects at 14% moisture compared to 10% moisture. The high moisture content created heat and a favorable environment for insect development (Harish et al., 2014). Bruchid multiplication was higher in traditional storage bags due to their permeability for gas exchange and diffusion of oxygen into jute bags, carbon dioxide out of the bags, and moisture present in the air through the perforations in the bags.
Hermetic bags efficiently managed groundnut bruchid infestation and, to some extent, mycotoxin-producing fungi compared to traditional storage bags when the pods were properly dried. Groundnut pods stored in traditional storage bags resulted in high insect multiplication, pod damage, and weight loss. Pods stored in hermetic and traditional bags were not suitable for human consumption after 4 months as they were completely damaged. The study revealed that hermetic bags maintained a good germination percentage of the stored seeds and could represent the best alternative to traditional storage bags for short- and medium-term storage, provided the produce is sufficiently dried (<10%) before storage. Hermetic bags may serve better as an alternative to jute bags. Further research should focus on increasing farmer/public awareness of timely drying and safe storage of the produce by adapting hermetic storage technologies such as PICS bags.
5 Conclusion
Groundnut bruchid infestation and mycotoxin-producing fungi were effectively controlled by PICS technology-based triple-layer plastic bags compared to traditional storage bags when the pods were properly dried compared to pods stored with slightly higher and/or sub-optimal moisture levels. Groundnut pods stored in traditional storage bags resulted in high insect multiplication, pod damage, and weight loss. Pods stored in traditional bags were not suitable for human consumption after 6 months of storage because they were highly damaged. The study also revealed that triple-layer plastic bags maintained a good germination percentage of stored seeds and could be the best alternative to traditional storage bags for short- and medium- term storage, provided the produce is sufficiently dried (<10%). The triple-layer bags may serve better as an alternative to jute bags. Further research should be aimed at increasing farmer/public awareness of the safe storage of their produce by implementing hermetic storage technologies such as triple-layer bags.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YS: Investigation, Writing – original draft. PR: Writing – review & editing. SJ: Writing – review & editing. UM: Investigation, Writing – review & editing. GA: Investigation, Writing – review & editing. VA: Data curation, Formal analysis, Writing – review & editing. HS: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work has been undertaken as part of the CGIAR Research Program-Grain Legumes and Dryland Cereals (CRP-GLDC) which was led by ICRISAT.
Acknowledgments
The authors are grateful to ICRISAT for the financial support provided to undertake this study through its CGIAR Research Program on Grain Legumes and Dryland Cereals (CRP-GLDC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
American Society of Agricultural Engineers (1989). American Society of Agricultural Engineers (ASAE) Standards. 36th Edn, S352.1. Moisture measurement-grain and seeds. St. Joseph, Mich: ASAE.
Arya, S. S., Salve, A. R., and Chauhan, S. (2016). Peanuts as functional food: a review. J. Food Sci. Technol. 53, 31–41. doi: 10.1007/s13197-015-2007-9
Bandara, K. A. N. P., and Saxena, R. C. (1995). A technique for handling and sexing Callosobruchus maculatus (F.) adults (Coleoptera: Bruchidae). J. Stored Prod. Res. 31, 97–100. doi: 10.1016/0022-474X(94)00030-W
Banks, H. J., and Annis, P. C. (1990). “Comparative advantage of high CO2 and low O2 of controlled atmospheres for grain storage” in Food preservation by modified atmospheres. eds. M. Calderon and R. Barkai-Golan (Boca Raton: CRS Press), 93–119.
Baoua, I. B., Amadou, L., Abdourahmane, M., Bakoye, O., Baributsa, D., and Murdock, L. L. (2015). Grain storage and insect pests of stored grain in rural Niger. J. Stored Prod. Res. 64, 8–12. doi: 10.1016/j.jspr.2015.04.007
Baoua, I. B., Amadou, L., Ousmane, B., Baributsa, D., and Murdock, L. L. (2014). PICS bags for post-harvest storage of maize grain in West Africa. J. Stored Prod. Res. 58, 20–28. doi: 10.1016/j.jspr.2014.03.001
Baoua, I. B., Margam, V., Amadou, L., and Murdock, L. L. (2012). Performance of triple bagging hermetic technology for postharvest storage of cowpea grain in Niger. J. Stored Prod. Res. 51, 81–85. doi: 10.1016/j.jspr.2012.07.003
Baributsa, D., Baoua, I. B., Bakoye, O. N., Amadou, L., and Murdock, L. L. (2017). PICS bags safely store unshelled and shelled groundnuts in Niger. J. Stored Prod. Res. 72, 54–58. doi: 10.1016/j.jspr.2017.03.007
Behera, P., Mohanty, A., Patra, D., and Kar, D. S. (2016). Life cycle of ground nut seed beetle (Caryedon Serratus). Paripex Indian J. Res. 5, 4–6.
Bert, D., Jan, B., and Martijntje, V. (2022). Hermetic bags for the storage of maize: perspectives on economics, food security and greenhouse gas emissions in different sub-Saharan African countries. Front. Sustain Food Systems 6:767089. doi: 10.3389/fsufs.2022.767089
Bhattacharya, K., and Raha, S. (2002). Deteriorative changes of maize, groundnut, and soybean seeds by fungi in storage. Mycopathologia 155, 135–141. doi: 10.1023/A:1020475411125
Bulaong, S. S. P., and Dharmaputra, O. S. (2002). Fungal population, aflatoxin and free fatty acid contents of peanuts packed in different bag types. Biotropica 19, 1–25. doi: 10.11598/btb.2002.0.19.229
Dasbak, M. A., Echezona, B. C., and Asiegbu, J. E. (2009). Post-harvest bruchid richness and residual activity of pirimiphos-methyl on Callosobruchus maculatus (F.) infested pigeon pea (Cajanus cajan L. Millsp.) in storage. Afr. J. Biotechnol. 8, 311–315.
Deshmukh, Poonam, mane, P.N., Undirwade, D. B., and Sonalkar, V. U. (2022). Biology of groundnut bruchid Caryedon Serratus (Olivier). Indian J. Entomol. 84, 700–701. doi: 10.55446/IJE.2021.314
Hao, L., Constance, L. W., Thomas, V. G., Marilyn, L. G., and Arnold, J. S. (2004). Analysis of oligonucleotide array experiments with repeated measures using mixed models. BMC Bioinformatics 5:209. doi: 10.1186/1471-2105-5-209
Harish, G., Nataraja, M. V., Ajay, B. C., Holajjer, P., Savaliya, S. D., and Gedia, M. V. (2014). Comparative efficacy of storage bags, storability and damage potential of bruchid beetle. J. Food Sci. Technol. 51, 4047–4053. doi: 10.1007/s13197-013-0964-4
Harish, G., Prasanna, H., Savaliya, S. D., and Gedia, M. V. (2012). Population density on damage of groundnut by Caryedon serratus. Ann. Plant. Protect. Sci. 20, 217–219.
Indiastat (2022). India statistical database. Available at: https://www.indiastat.com
Lahari, K., and Sudha Rani, K. (2022). Evaluation of triple layer hermetic bags for safe storage of seeds by the small holding farmers in Anantapuramu District of Andhra Pradesh. Biological forum – an. Int. J. 14, 1587–1590.
Lale, N. E. S., and Igwebuike, J. U. (2002). Field infestation of Faidherbia (Acacia) albida (Del.) A. Chew. Pods by stored product Coleoptera in the Nigeria savanna and effect of infestation on nutrient quality. J. Arid Environ. 51, 103–112. doi: 10.1006/jare.2001.0898
Martin, D. T., Baribusta, D., Huesing, J. E., Williams, S. B., and Murdock, L. L. (2015). PICS bags protect wheat grain, Triticum aestivum (L.), against rice weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae). J. Stored Prod. Res. 63, 22–30. doi: 10.1016/j.jspr.2015.05.001
Murdock, L. L., Margam, V., Baoua, I., Balfe, S., and Shade, R. E. (2012). Death by desiccation: effects of hermetic storage on cowpea bruchids. J. Stored Prod. Res. 49, 166–170. doi: 10.1016/j.jspr.2012.01.002
Murdock, L. L., Seck, D., Ntoukam, G., Kitch, L., and Shade, R. E. (2003). Preservation of cowpea grain in sub-Saharan Africa-bean/cowpea CRSP contributions. Field Crop Res. 82, 169–178. doi: 10.1016/S0378-4290(03)00036-4
Mutegi, C. K., Wagacha, J. M., Christie, M. E., Kimani, J., and Karanja, L. (2013). Effect of storage conditions on quality and aflatoxin contamination of peanuts (Arachis hypogaea L.). International. J. Agric. Sci. 3, 746–758.
Mutungi, C. M., Affognon, H., Njoroge, A. W., Baributsa, D., and Murdock, L. L. (2014). Storage of mung bean (Vigna radiata (L.) Wilczek) and pigeonpea grains (Cajanus cajan (L.) Millsp) in hermetic triple-layer bags stops losses caused by Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J. Stored Prod. Res. 58, 39–47. doi: 10.1016/j.jspr.2014.03.004
Nganga, J., Mutungi, C., Imathiu, S., and Affognon, H. (2016a). Effect of triple-layer hermetic bagging on mould infection and aflatoxin contamination of maize during multi-month on-farm storage in Kenya. J. Stored Prod. Res. 69, 119–128, doi: 10.1016/j.jspr.2016.07.005
Nganga, J., Mutungi, C., Samuel, M., Imathiu, S., and Affognon, H. (2016b). Low permeability triple-layer plastic bags prevent losses of maize caused by insects in rural on-farm stores. Food Security. 8, 621–633. doi: 10.1007/s12571-016-0567-9
Nielsen, P. S. (2001). The effect of carbon dioxide under pressure against eggs of Ephestia kuehniella Zeller and adults of Stegobium paniceum (L.) and Oryzaephilus surinamensis (L). J. Pest. Sci. 74, 85–88.
Njoroge, A. W., Affognon, H. D., Mutungi, C. M., Manono, J., Lamuka, P. O., and Murdock, L. L. (2014). Triple bag hermetic storage delivers a lethal punch to Prostephanus truncatus (horn) (coleoptera: bostrichidae) in stored maize. J. Stored Prod. Res. 58, 12–19. doi: 10.1016/j.jspr.2014.02.005
Ntare, B. R., Diallo, A. T., Ndjeunga, J, and Waliyar, F (2008). Groundnut seed production manual. Patancheru, Andhra Pradesh, India: International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) 20
Ognakossan, E. K., Tounou, A. K., Yendouban, L., and Kerstin, H. (2013). Post-harvest insect infestation in maize grain stored in woven polypropylene and in hermetic bags. Int. J. Tropical Insect Sci. 33, 71–81. doi: 10.1017/S1742758412000458
Omodara, M. A., Samuel, G., Neill, M., and Montross, M. D. (2021). Water vapor permeability of bag materials used for corn storage. Agric. Engin. Int. 23, 329–340.
Omondi, B. A., Jiang, N., Den Berg, J. V., and Schulthess, F. (2011). The flight activity of Prostephanus truncatus (horn) (Coleoptera: Bostrichidae) and Teretrius nigrescens Lewis (Coleoptera: Histeridae) in Kenya. J. Stored Prod. Res. 47, 13–19. doi: 10.1016/j.jspr.2010.08.002
Rani, P. R., Chelladurai, V., Jayas, D. S., White, N. D. G., and Kavitha-Abirami, C. V. (2013). Storage studies on pinto beans under different moisture contents and temperature regimes. J. Stored Prod. Res. 52, 78–85. doi: 10.1016/j.jspr.2012.11.003
Rao, G. V. R., Rao, V. R., and Nigam, S. N., (2010). Postharvest insect pests of groundnut and their management. International Crops Research Institute for the Semi-Arid Tropics: Patancheru, Andhra Pradesh, India, 20.
Reddy, S. V., Mayi, D. K., Reddy, M. U., Devi, K. T., and Reddy, D. V. R. (2001). Aflatoxin B1 in different grades of chillies (Capsicum annum) as determined by indirect competitive-ELISA. Food Addit. Contam. 18, 553–558. doi: 10.1080/02652030119491
Sembene, M. (2006). The origin of groundnut infestation by the seed beetle Caryedon serratus (Olivier) (Coleoptera: Bruchidae): results from cytochrome B and ITS1 gene sequences. J. Stored Prod. Res. 42, 97–111. doi: 10.1016/j.jspr.2004.12.002
Singh, R., Kaur, G., and Kaur, G. (2022). Shelf-life prolongation of spring groundnut pods (Arachis hypogaea L.) using packaging systems. J. Sci. Industrial Res. 81, 393–340.
Sudini, H., Rao, G. V. R., Gowda, C. L. L., Chandrika, R., Margam, V., Rathore, A., et al. (2015). Purdue improved crop storage (PICS) bags for safe storage of groundnuts. J. Stored Prod. Res. 64, 133–138. doi: 10.1016/j.jspr.2014.09.002
Sundaram, J., Kandala, C. V., Holser, R. A., Butts, C. L., and Windham, W. R. (2010). Determination of in-shell peanut oil and fatty acid composition using near infrared reflectance spectroscopy. J. Am. Oil Chem. Soc. 87, 1103–1114. doi: 10.1007/s11746-010-1589-7
Swathi, Y., Rajanikanth, P., and Satyanarayana, J. (2017). Hermetic storage negatively affects the respiratory metabolism of groundnut bruchid Caryedon serratus (Olivier) and thereby it’s control. Int. J. Pure Appl. Biosci. 5, 832–837. doi: 10.18782/2320-7051.5510
Vales, M. I., Rao, G. V. R., Sudini, H., Patil, S. B., and Murdock, L. L. (2014). Effective and economic storage of pigeonpea seed in triple layer plastic bags. J. Stored Prod. Res. 58, 29–38. doi: 10.1016/j.jspr.2014.01.004
Vijayalakshmi, N., and Malabasari, T. A. (2018). Effect of packaging materials, desiccant on longevity of summer groundnut (Arachis hypogaea L.) cv. G2-52 stored both in the form of pod and kernel international journal of pure and applied. Bioscience 6, 1661–1667.
Walker, S., Jaime, R., Kagot, V., and Probst, C. (2018). Comparative effects of hermetic and traditional storage devices on maize grain: mycotoxin development, insect infestation and grain quality. J. Stored Prod. Res. 77, 33–44. doi: 10.1016/j.jspr.2018.02.002
Waongo, A., Traore, F., Malick, N. B., Dabire-Binso, C., Murdock, L. L., Baributsa, D., et al. (2019). Effects of PICS bags on insect pests of sorghum during long-term storage in Burkina Faso. J. Stored Prod. Res. 83, 261–266. doi: 10.1016/j.jspr.2019.07.010
Weinberg, Z. G., Yan, Y., Chen, Y., Finkelman, S., Ashbell, G., and Navarro, S. (2008). The effect of moisture level on high-moisture maize (Zea mays L.) under hermetic storage conditions-in vitro studies. J. Stored Prod. Res. 44, 136–144. doi: 10.1016/j.jspr.2007.08.006
Keywords: groundnut, triple-layer hermetic storage bags, aflatoxin, bruchids, sub-optimal moisture
Citation: Swathi Y, Rajanikanth P, Jella SN, Mangala UN, Adithya G, Anilkumar V and Sudini HK (2024) Effect of sub-optimal moisture levels on the quality of groundnut (Arachis hypogaea L.) during storage in triple-layer hermetic storage bags. Front. Sustain. Food Syst. 7:1275133. doi: 10.3389/fsufs.2023.1275133
Edited by:
Célia Quintas, University of Algarve, PortugalReviewed by:
Francisco Bueno-Pallero, University of Algarve, PortugalWasiu Awoyale, Kwara State University, Nigeria
Copyright © 2024 Swathi, Rajanikanth, Jella, Mangala, Adithya, Anilkumar and Sudini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hari Kishan Sudini, aGFyaWtpc2hhbi5zdWRpbmlAaWNyaXNhdC5vcmc=
 Y. Swathi1
Y. Swathi1 P. Rajanikanth
P. Rajanikanth Uppala N. Mangala
Uppala N. Mangala Vemula Anilkumar
Vemula Anilkumar Hari Kishan Sudini
Hari Kishan Sudini