- 1School of Management, Hefei University of Technology, Hefei, China
- 2School of Health Care Management, Anhui Medical University, Hefei, China
- 3Clinical Medical College, Anhui Medical University, Hefei, Anhui, China
- 4School of Pharmacy, Anhui Medical University, Hefei, Anhui, China
- 5College of Public Health, Anhui Medical University, Hefei, Anhui, China
- 6School of Law, Anhui Medical University, Hefei, China
- 7School of Marxism, Anhui Medical University, Hefei, China
Introduction: The use of the fungus Cordyceps sinensis (CS), which has a long history of use in China for its tonic properties, has sparked controversy based on the risk from high arsenic levels in CS.
Methods: This study collected all 24 online dispute cases related to CS from the China Legal Documents Network. Excel software was applied to fit the distribution of the sample data and analyze the trend of trial results of CS disputes and the tendency of professional claims trials. The analysis covers the trend of trial results of contract dispute cases involving CS products (CDCCS), and the tendency of the results of CDCCS, using correlation to analyze the factors influencing the results. We analyzed trial tendencies in CDCCS by the methods of complex network analysis (Gephi software) to explore each factor’s effect on the trial outcome of in these claims cases and to predict the trial outcomes of new cases. Simultaneously use KNN to train and predict CS cases.
Results: The peak number of trial cases was in 2018, and there has been a gradual increase in the rate of losing claims on the claimant. Correlation analysis was used to obtain that the likelihood of the defendant winning a claim is positively correlated with DR, FSS, IDP, and KSP, and negatively correlated with the adoption of JIFDD (3), JIFDD (15), and APL. Gephi software was used to conduct a complex network analysis of the factors influencing the tendency of trial results in CS disputes, showing that noncompliance with FSS, existence of KSP, and submission of IDP evidence by the buyer are the direct factors influencing the seller’s defeat. The seller’s “failure to perform DR” is the key factor in the seller’s defeat in cases. On this basis a probability function is developed to predict a trial win rate of 0.7803 for freshman cases, classifier to predict the remaining four samples and new cases, and get the prediction accuracy is 0.8, 95% CI: (0.2836, 0.9949), and the latter was higher than the former.
Discussion: It is concluded that the attribute of CS products is a scientific issue, the jurisdiction of CS disputes is confusing and needs to be clarified, and the application of the provisions in judicial trials is cannot adapt to the current environment and needs to be resolved. This study advocates that the government design policy from the top and establish a study on the boundary between administrative and judicial functions to provide a theoretical guarantee to circumvent lazy administration and unscientific law enforcement. The government can provide a legal and regulatory system to improve food quality and regulate management.
1. Introduction
Cordyceps sinensis (CS), a fungus of the genus Cordyceps of the ergot family (see Figure 1), is a valuable herbal medicine (Cordyceps sinensis, 2017). According to the Compendium of Materia Medica, CS possesses the effects of tonifying the kidney, benefiting the lungs, stopping bleeding, and resolving phlegm. It is mainly used for treating impotence and early ejaculation, soreness of the waist and knees, chronic cough and asthma, and coughing with phlegm and blood.
In 1999, Jin proposed the antitumor sterol utility of CS mycelium (Jin et al., 1999), and in 2000, Chiou explored the value of the hypotensive and vasodilatory activities of CS (Chiou et al., 2000). In 2001, based on the research of Chinese experts in related fields, the Department of Health of the Tibet Autonomous Region applied for the inclusion of CS in the list of dual-use substances for medicine and food [Health Law Supervision Food Convenience Letter (2001) No. 8] to further explore the pharmacological value of CS. In 2002, the Ministry of Health issued the Notice on Further Regulating the Management of Health Food Ingredients [Health Law Supervision (2002) No. 51], which restricted the use of CS in food for the purpose of protecting rare medicinal resources from indiscriminate harvesting and abuse leading to species extinction. In 2009, the Ministry of Health recognized CS as a valuable Chinese herbal medicine and informed that CS should not be used as a raw material for ordinary food. National medical experts performed in-depth research on the “precious Chinese herbal medicine CS,” clarifying that CS is abundant in health care, tonics, and other substances. Based on that research, the State Food and Drug Administration issued a “notice on the issuance of CS for health food pilot work program” [State Food and Drug Administration Baohua (2012) No. 225] in August 2012, forcing pilot enterprises to organize pilot-related work in accordance with certain requirements. Based on this national policy background, pilot and scientific research and development of CS were rapidly promoted.
The State Food and Drug Administration issued a consumer risk alert on February 4, 2016 after researchers discovered that CS pilot derivatives, powder tablets, and grass powders had exceeded the standard for “arsenic” elements after processing. On February 27, 2016, the Tibet Autonomous Region made a “Statement on the Application of CS “in response to the “Risk Alert of the State Food and Drug Administration,” suggesting that it is safe to use CS powder tablets and grass powder under the guidance of a doctor. On March 7, 2016, the State Food and Drug Administration for Beijing, Jiangxi, Hubei, Qinghai Province (city) Food and Drug Administration, and the General Administration of Health Food Evaluation Center issued a “Notice of the General Administration on the cessation of the pilot work of CS for health products.” This document strengthens the approval process for health food related to CS, but units that have completed the approval process are allowed to market CS products.
In China, the “Food Safety Law of the PRC (FSLPRC)” Article 38 clearly states that “no drugs may be added to food products, but substances that are traditionally both food and Chinese herbs may be added.” The term “traditionally both” refers to the directory of “both food and herbal substances” developed and published by the State Council administrative department of health in conjunction with the State Council food safety supervision and management departments. CS is a valuable Chinese herbal medicine and herbal medicines are the raw material for the preparation of many medicines, so whether CS is equivalent to medicine has become a controversial point. Also, whether other products derived from herbal medicines are classified as medicine is another controversial point. These two controversies have cultivated the soil for professional claims, and it is interesting to investigate where the resulting judicial disputes go from here. This is particularly relevant currently with maintaining legal rationality in case trials becoming a hot topic? As a result, it has been the key that mining the effectiveness of the administrative and judicial functions from the CS disputes in institutional governance of the business environment.
1.1. Literature review
Food safety follows a risk management system based on risk assessment, risk control, and risk communication (World Health Organization, 2006). The risk assessment of CS mainly focuses on physical factors (World Health Organization, 2008), such as the heavy metal arsenic content (State Food and Drug Administration, 2016). The Allergen Bureau in Australia issued thresholds for various sensitized foods in 2019 (Allergen Bureau, 2019). In the study of risk management, there are several typical food safety regulatory models, such as the Food and Drug Act of the United States (Barkan, 1985), the food safety regulations of the European Union (Henson and Caswell, 1999; Charlier and Valceschini, 2008), and the food safety law of China (Chen, 2022). The study of risk communication for CS mainly focuses on the pharmacological properties of CS (Chiou et al., 2000). CS not only has good nutritional functions but also has pharmacological properties and product development characteristics, but its use is limited by the current food safety law. There are risks such as unclear boundary and contradiction between administrative management and judicial governance. Taking CS products as an example, the analysis of the influencing factors and quantitative evaluation of judicial trials will help to improve the law enforcement level of judicial personnel and promote the revision of judicial provisions.
Complexity science is a new discipline (Ren and Meng, 2021). Complex network analysis methods derived from complexity science are applied to various fields (Yu et al., 2021), such as IoT technology (Deebak et al., 2022; Gadekallu et al., 2022), P2P networks (Wang et al., 2021), key node identification (Lalou et al., 2018), anomaly detection (Fernandes et al., 2019), mining key roles in complex systems (Bright et al., 2015), link prediction (Lim et al., 2019), and k-Nearest Neighbor (KNN) (Nugroho, 2021),. For example, Zhang proposed a complex network transitivity analysis method to obtain the network node transfer weight (Zhang, 2021). This study uses a complex network of networks to analyse trends in verdicts in CS, with the magnitude of the weights determining the degree of influence, which in turn leads to the main influencing factors affecting the success or failure of the case verdict. On this basis, the weight of each factor is used to replace the conditional probability of the case result, and the full probability formula is used to calculate the trial result of the pending case. Meanwhile, it was found that KNN algorithm was used in many cases of prediction, such as biological data prediction (Zhang, 2019; Fitriyadi and Muqorobin, 2021), application in the price prediction of agricultural products (Xu et al., 2014), and prediction of store sales (Ye, 2018), but there are few cases which are found to be predicted for trial results in judicial dispute cases. Judicial trials rely on the degree of influence of the evidence provided during the dispute process on the judge, which has an equivalent effect to the efficiency of sales influencing factors on sales. Then, it is believed that KNN can implement an effective prediction of trial outcomes in judicial dispute cases. Hence, the study simultaneously uses KNN to train and predict CS cases.
In 2013, Hall came up with the concept of professional claims, which mean that based on the Consumer Protection Law, FSLPRC, other Laws and Regulations of punitive damages to the operator to seek compensation for the purpose of profit, by the way of purchasing a product that you believe to be defective (such as labeling, additives, shelf life) (Hall and Osses, 2013), introduced into China in 1994, the formation of the CPLPRC, Article 49, “return one for one” punitive provisions, the professional claims represented by “Wang Hai” opened in China, the professional claims behavior objectively forced the improvement of the quality of goods, and played a positive role in the innovation of the concept of consumers and operators (Sun et al., 2021). With the revision of the CPLPRC, the FSLPRC and the introduction of food safety-related governance policies, the average annual growth of professional claims cases has increased by several figures, and while further improving food safety protection, the number of professional claims cases has increased tremendously, leading to a sharp increase in the number of complaints about professional claims from grass-roots administrative departments and professional claims disputes in the courts, which has resulted in a serious waste of administrative resources, and an imbalance between the administrative costs and the benefits of institutional governance (Sun et al., 2021). Based on the above analysis, we propose the following hypotheses:
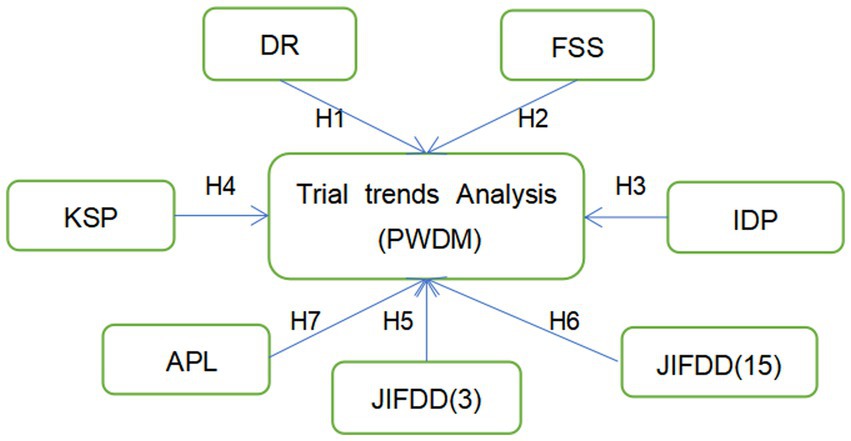
H1: DR is positively correlated with PWDM.
H2: FSS is positively correlated with PWDM.
H3: IDP is positively correlated with PWDM.
H4: KSP is positively correlated with PWDM.
H5: JIFDD (3) is negatively correlated with PWDM.
H6: JIFDD (15) is negatively correlated with PWDM.
H7: APL is negatively correlated with PWDM.
They form the conceptual model shown in Figure 2.
2. Materials and methods
2.1. Subjects
The study searched the China Legal Instruments Database for legal instrument data from 2011 to 2021. The China Legal Documents Network is a public database of court documents (Sun et al., 2021). The following keywords were used for the database search: the year (“2011,” “2012,” “2013,” “2014,” “2015,” “2016,” “2017,” “2018,” “2019,” “2020,” “2021”), civil cases, CS, sale and purchase contract disputes, and types of instruments (“judgments” and “rulings”). The criteria for eligibility are as follows: (a) ingredients containing “CS infusion” or “CS”; (b) the plaintiff is an individual actor. Exclusions: (a) cases filed in 2010 and closed in 2011; (b) cases filed in 2021 and closed in 2022; (c) cases where the plaintiff is an enterprise; (d) pharmaceutical products and over-the-counter medicines; (e) cordyceps tablets; and (f) cases where the defendant is not the first defendant.
2.2. Data extraction
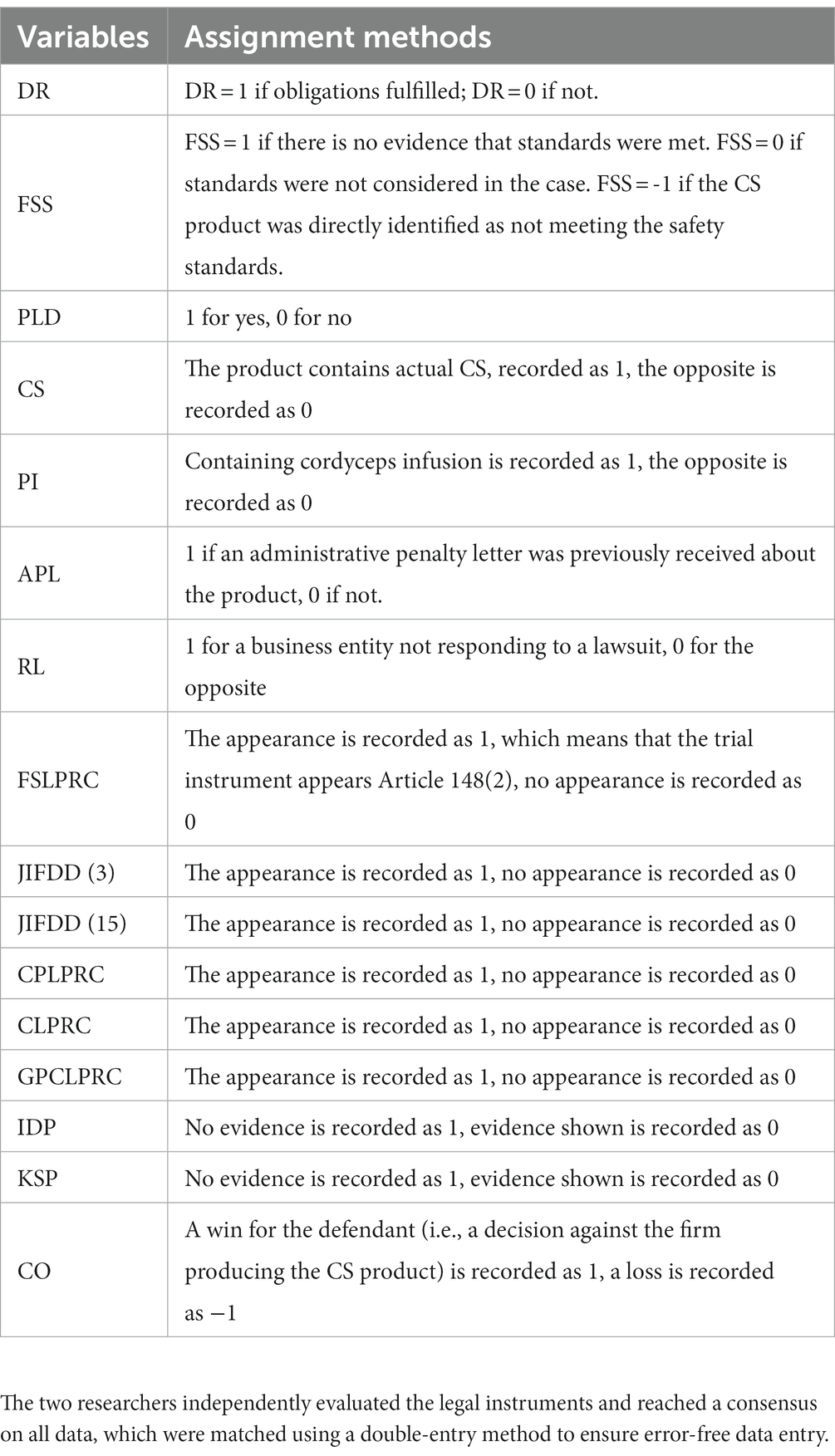
The data collected includes 24 judicial cases (multiple document on the same case, after multiple trials, calculated according to the final legally valid documents). The following data was extracted as a summary (Table 1):
2.3. Methods
We used textual analysis to explore the jurisprudential dispute points, fitting the distributional trend of the sample data by applying data trends in Excel software. The analysis covers the trend of trial results of contract dispute cases involving CS products (CDCCS), and the tendency of the results of CDCCS, using correlation to analyze the factors influencing the results. We analyzed trial tendencies in CDCCS by the methods of complex network analysis (Gephi software) to explore each factor’s effect on the trial outcome of in these claims cases and to predict the trial outcomes of new cases. Simultaneously use KNN to train and predict CS cases. The difference leading to our results is considered statistically significant at p < 0.05.
3. Results
3.1. Basic information of the CDCCS
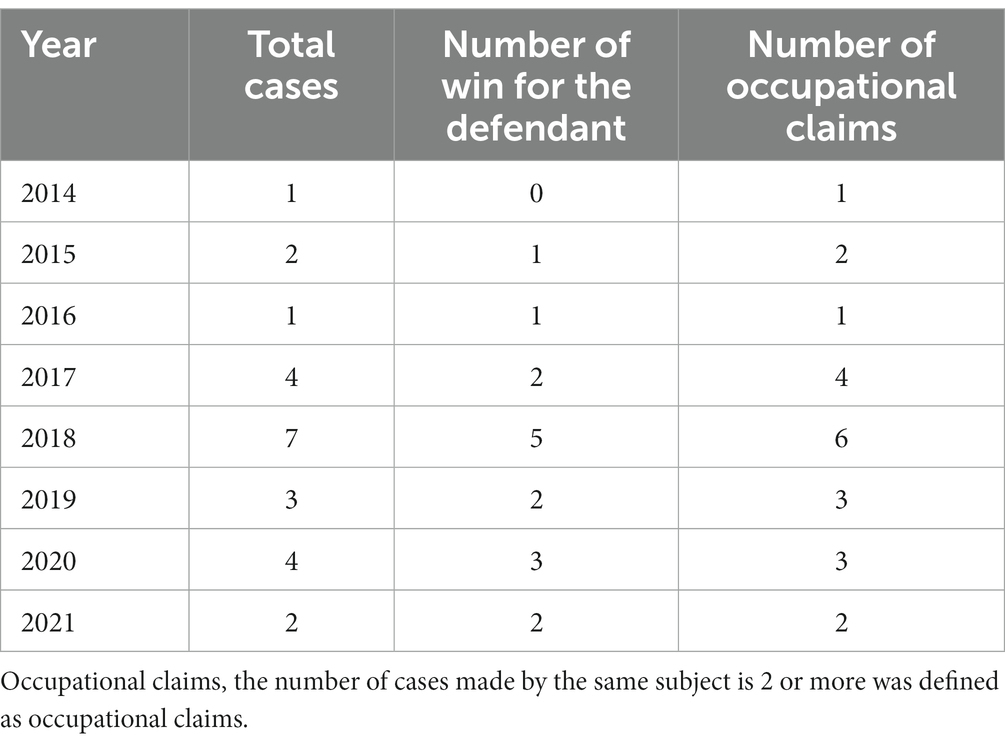
Through the data search, we obtained a total of 51 judgment cases. Using the principle of “combining multiple trials of the same case into one case,” the final trial cases included in this study numbered 24. The basic information is as follows (Table 2).
As can be seen from Table 2, the judicial hearings involving CDCCS steadily increased until 2018 and gradually decreased after 2018, reaching a peak in contract disputes in 2018.
3.2. Analysis of the rate of win for the defendant in CDCCS
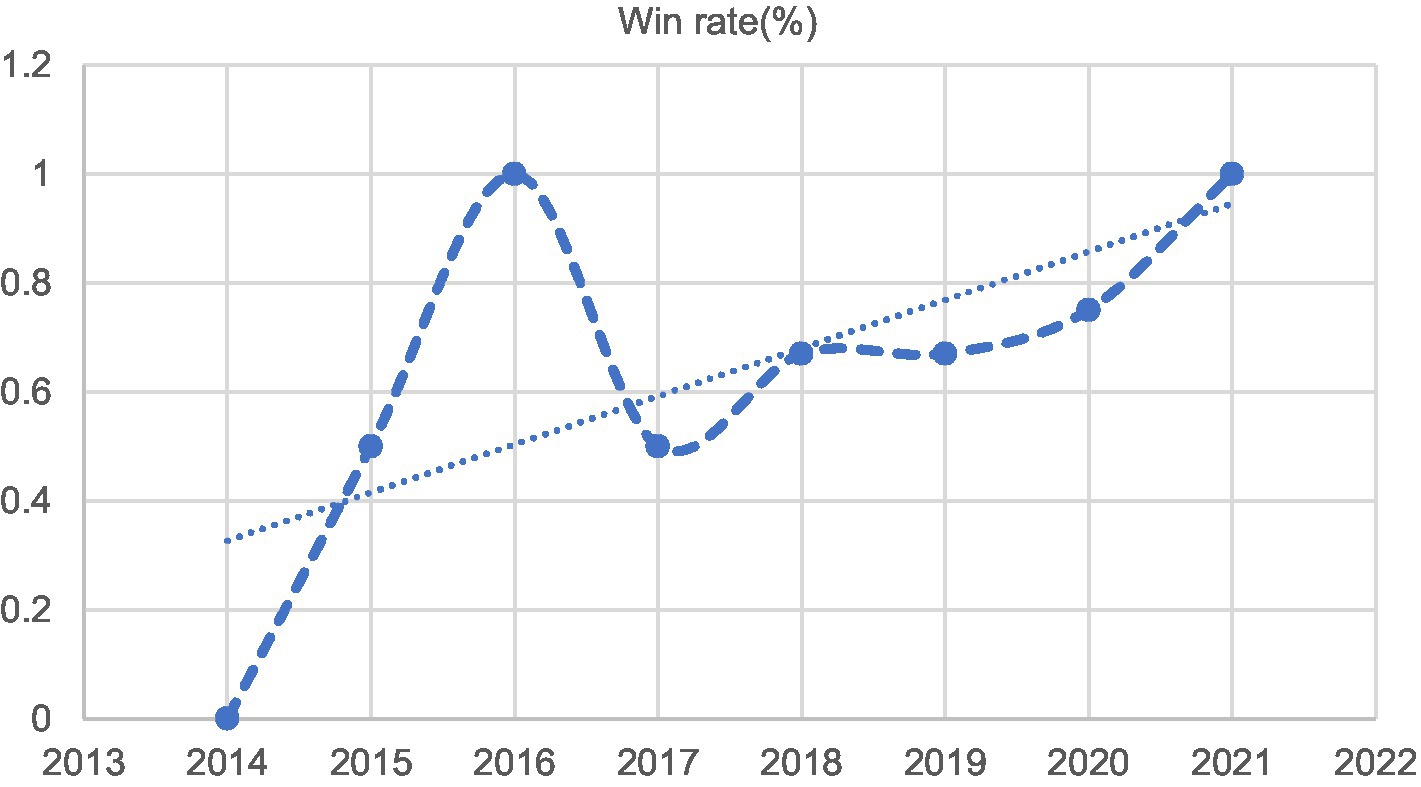
It can be found from Table 1 that a total of 22 of the 24 contracts under dispute were signed by professional claimant [23], accounting for 91.67%. Based on the perspective of the defendant, the judicial trial results of the studied cases were analyzed, and the annual trial win rates were obtained as shown in Figure 3.
As can be seen from Figure 3, the success rate of the defendant in contract disputes in the CS category steadily increased, and the corresponding risk of losing claims has been increasing. Especially since 2018, career claims benefit less than expected, and the risk of claims are all greater than 66.67%.
3.3. Correlation analysis of case trial tendencies
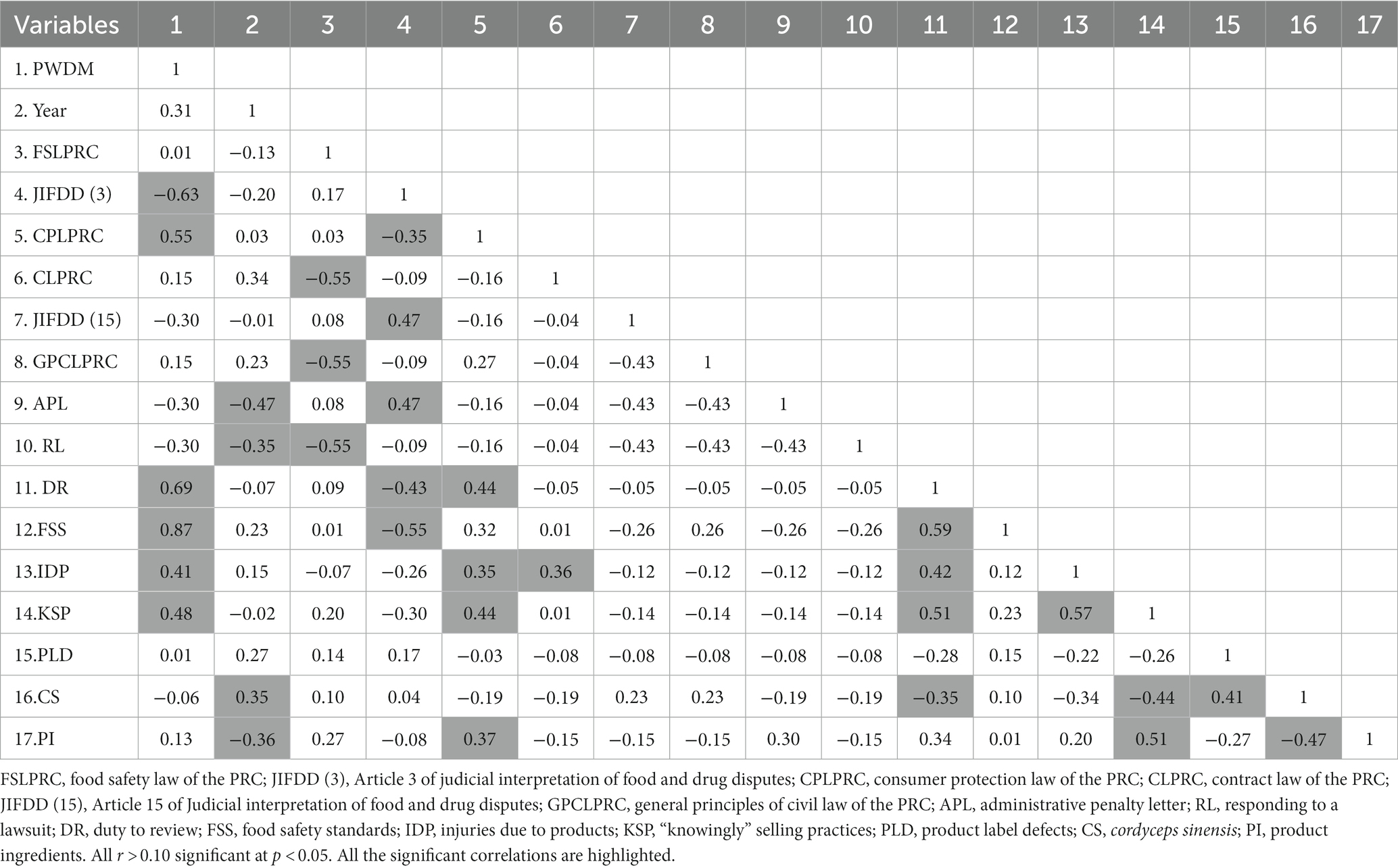
In order to understand the factors influencing the tendency of judicial trials of contract disputes for this type of product, we extracted the subject terms of the case trials and used correlation analysis to explore the correlations, and the results are shown in Table 3.
From Table 3, it can be seen that the propensity to win the dispute case is positively correlated with DR, FSS, IDP and KSP, positively correlated with the adoption of CPLPRC and negatively correlated with the adoption of JIFDD (3), these confirm H1, H2, H3, H4 and H5; the issuance of APL by the administrative department for products presents a negative correlation between CS and the year, and the response of the defendant to the lawsuit presents a negative correlation with the year; the adoption of CPLPRC by the instrument is negatively correlated with the simultaneous adoption of GPCLPRC and the adoption of CPLPRC is negatively correlated with the simultaneous adoption of GPCLPRC and RL; the adoption of JIFDD (3) is negatively correlated with the simultaneous adoption of CPLPRC and the willingness to determine that the products comply with FSS and fulfilled DR, and positively correlated with the adoption of JIFDD (15) and APL; the adoption of CPLPRC is positively correlated with the willingness to determine that no IDP, no KSP and fulfilled DR are provided; the adoption of CLPRC is positively correlated with the determination that no IDP is provided; determination of fulfilled DR was positively correlated with product compliance with FSS, no KSP, and negatively correlated with a product containing CS physical; determination of not providing IDP was positively correlated with no KSP; no KSP was negatively correlated with a product containing CS physical and positively correlated with PI; determination of CS physical did not comply with FSS and was negatively correlated with the determination of PI. All of the above correlations were statistically significant, while the correlations between the other variables were not statistically significant. JIFDD (15) and APL were not significantly correlated with PWDM. This result do not directly confirms the H6 and H7.
3.4. Case trial rationale and trends in judicial trials
We further used Gephi software to conduct a complex network analysis on the factors influencing the trial tendency of CDCCS to explore the degree of influence of each factor on the outcome of professional claims, and obtained the results shown in Figure 4.
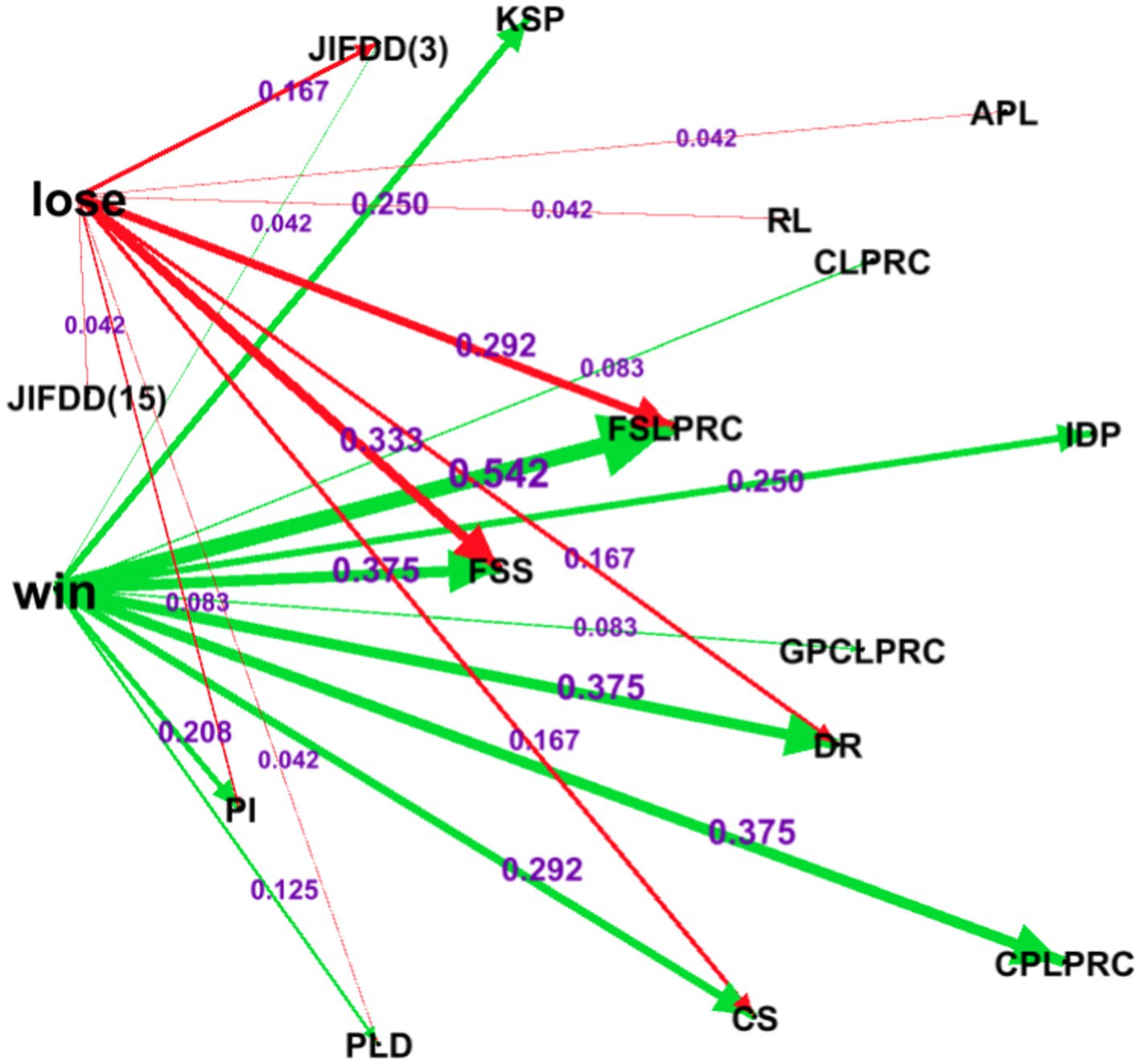
Figure 4. Complex network diagram of factors influencing trial tendency. The node meanings are the same as Table 2.
From Figure 4, we can see that “the product does not meet the quality and safety standards,” the seller’s “subjective sales behavior knowing that the product does not meet the quality and safety standards” and the buyer submitted evidence of IDP are the direct factors affecting the seller’s defeat; the seller’s “failure to fulfill the review obligation” is the key factor of the seller’s defeat, and the professional claims negatively affect the seller’s defeat.
4. Discussion
The results of the study showed a trend of increasing and then decreasing CS dispute cases, which peaked in 2018. This is largely consistent with the development trend of professional claims (Sun et al., 2021). One of the reasons may come from the fact that all CS disputes are professional claims; the second reason may be that professional claims were encouraged by the policy before 2016, the active promotion of the Consumer Protection Law, and the lure of “refunding one to compensate 10” led to the increase in the number of cases (András and Krisch, 2015; Su and Wang, 2020; Zhang et al., 2020); after 2016, they were gradually regulated, and the court trial cycle of “first trial, second trial, and retrial” led to the increase in the number of cases. The third reason may be the prolongation of the case cycle due to the active response of businessmen after the public attention to professional claims, for example, before 2016, many cases were finalized in the first trial, while after 2016, most of them were actively responded to the end. The results of the study further indicate a steadily increasing rate of lost occupational claims in the CS category. In particular, from 2018 onwards, occupational claims benefited less than expected, with all claims risk greater than 66.67%, which is in line with the study of literature (Sun et al., 2021). One of the possible reasons for this is that the motivation of professional claims is contrary to the original legislative intent of the Consumer Protection Law and the Food Safety Law ‘the prevailing norm of international legislation is to use ‘fraud’ as a premise for punitive damages’ (Drozen et al., 2011; European Commission, 2011). The second reason is that the harm of professional claims far exceeds the benefit, and has widely concerned the society. For example, “Hong Mingji,” a member of the National Committee of the Chinese People’s Political Consultative Conference (CPPCC), proposed a motion “Combat professional claims and optimize the business environment.” The National People’s Congress has suggested that the motive of “professional claims” is not to purify the market, but to use punitive damages for their profit or to take the opportunity to extort business, such behavior is a serious violation of the principle of good faith, disregard for judicial authority, a waste of judicial resources, should gradually curb the “professional claims” of profit-making counterfeiting behavior. Beijing TV and other major media actively publicize the spirit of “the General Office of the CPC Central Committee and the State Council calls for a severe crackdown on professional claimants” and calls for severe punishment of “professional claimants,” all of which indicate a negative attitude toward the behavior of “professional claimants.”
The results of the correlation study show that the propensity of the defendant to win lawsuits is positively correlated with DR, FSS, IDP, and KSP, the main core reason of which is the content of the judicial provisions, according to Article 1 of the General Provisions of the “Food Safety Law of the People’s Republic of China,” which states, “In order to ensure food safety and protect public health and life safety, this Law is enacted. “The original legislative intent is to protect public health and life safety, according to the Hengyang City Intermediate People’s Court of Hunan Province (2021) Xiang 04 Min Final 1,643 and Hangzhou Intermediate People’s Court of Zhejiang Province ruling (2017) Zhejiang 01 Min Shen 17 ruling were found: the application of the “Food Safety Law of the People’s Republic of China,” Article 148, paragraph 2 should meet two conditions: first “knowing” is not in line with food safety standards, but still sell food. Article 136 of the “Food Safety Law of the People’s Republic of China” provides that: food operators fulfill their obligations under this law to check the purchase of goods, and there is sufficient evidence to prove that they do not know that the food purchased does not meet food safety standards, and can truthfully explain the source of their purchases, can be exempt from punishment, resulting in personal, property or other damage, according to the law to bear the responsibility for compensation. On whether the seller “operating knowing that the food does not meet food safety standards,” the judgment, should be combined with the operator’s obligation to check the purchase of a comprehensive determination. Secondly, the “Food Safety Law of the People’s Republic of China” emphasizes food safety, and unsafe food poses a real or potential danger to those who consume it, which is what the “Food Safety Law of the People’s Republic of China” is supposed to provide for 10 times punitive damages. Therefore, the second prerequisite for obtaining 10 times compensation must be unsafe food. The professional claimant won the case with the adoption of JIFDD (3), JIFDD (15) and APL, which is positively correlated with the use of administrative penalties for unsafe food products, based on the use of judicial provisions “the production of food that does not meet food safety standards or the sale of food that is known to be not in compliance with safety standards, the consumer, in addition to requesting compensation for losses, according to The People’s Court shall support the claim for compensation from the producer or seller by the Food Safety Law and other laws and regulations,” which subjectively requires the operator to be in possession of all official documents concerning product quality. Ignoring the premise of “knowingly operating,” the court ruled that “10 times compensation” is a non-scientific act, which is consistent with the ruling of the Hangzhou Intermediate People’s Court of Zhejiang Province.
These results show that there is a positive correlation between the tendency of winning disputes and the adoption of CPLPRC, and a negative correlation between the adoption of CPLPRC and the simultaneous use of GPCLPRC and RL. To a certain extent, the concept of CDCCS trial is vague and the boundary is unclear. First of all, the main reason why there is little discussion on the “relationship between raw materials, ingredients and additives” is that most of the respondents or lawyers only analyze the case from the perspective of legal provisions, and few pay attention to the essence of the issue. According to the Baidu encyclopedia interpretation: white wine ingredients refer to the materials used in the blending process after the brewing of white wine. White wine ingredients generally refer to the raw materials used in the brewing process of white wine, such as grain, wine quill, etc. Raw materials can be ingredients, but ingredients may not be raw materials. FSLPRC and the “Food Safety National Standard for the Use of Food Additives GB2760-2011” gives a clear definition of raw materials and additives for food production, respectively. Until now, no laws and regulations have given clear restrictions on ingredients for food production, this provides evidence or defense material for the respondents or lawyers; another reason is that “food safety or not” is a scientific issue that requires scientific testing to uncover the truth, but scientific testing, in many cases, only focuses on the ingredients of the product and rarely gives a direct conclusion of “safety or not.” This also occurs because it is a scientific question and needs to be treated scientifically. 1 out of the 24 cases did the judge and the jury give their own opinion: the buyer of the product considered that the product was illegally added with non-food ingredients “CS,” which was essentially classified as “the buyer’s objection to the composition of the product,” and was not concerned with the intrinsic quality of the product. Therefore, the buyer is not concerned enough about whether the product is unsafe food; the objection should be handled by the relevant administrative agency, which is under administrative jurisdiction. This further illustrates that there is a blurred concept in the trial of CDCCS, and the boundary between administration and justice is unclear. Secondly, “CS” is confused with “CS infusion.” In the traditional Chinese production process, “extracting sugar from beets with water” and “extracting nutrients from herbs with base wine” is more common, and according to the Compendium of Materia Medica, CS has tonic effects. However, the traditional use of tonic health herbs in China has the custom of “dip” to extract nutrients, so the “dip” in “CS infusion “is only used as a material to absorb the tonic nutrients in the precious herbs, and it is not equivalent to the precious herbs “CS.” However, among the nine cases involving “CS infusion,” none of them pointed out the difference between the two. One of the possible reasons is that the lawyers are bound by rules and regulations, together with the fact that most of the lawyers are educated at the undergraduate level or below, and lack scientific knowledge. For example, in 2016, the State Food and Drug Administration issued a consumer risk alert “CS, the pure powder tablets of CS, powder products of CS arsenic content of 4.4–9.9 mg/Kg, more than the relevant expert analysis and research health food safety standards arsenic maximum limit value of 1 mg/Kg,” the respondent lacks the spirit of questioning, so he is unable to propose that the “arsenic content” of the nutrient solution “extracting the nutrients of CS” needs to be tested and identified in order to know the scientific nature of whether it exceeds the standard; the second reason is that the respondents are less educated and cannot distinguish the difference between the two, For example, the respondent does not understand the essential difference between “extracting the nutrients of CS” and “CS” as a traditional Chinese medicine, so it is impossible to conclude that the two are different materials; the third reason is that the differences mentioned in the pleadings are not accepted by the court, and the boundaries are misaligned, and the trial is not scientific and reasonable enough. Thirdly, the judicial trial does not take into account the origin of the product in the development of factors, “rigid” and “tough” use of legal provisions lack science and non-reasonable, such as from the 2001 Tibet Autonomous Region Department of Health request to include CS and other medicinal materials in the list of dual-use medicine and food, and to 2009, the Ministry of Health’s “Approval of Relevant Raw Materials in General Food,” to 2012, the State Food and Drug Administration issued the “Notice on Issuing the Pilot Program of Cordyceps for Health Food,” to 2016, the State Food and Drug Administration’s “Notice on Stopping the Pilot Program of CS for Health Food,” to February 2016, the State Food and Drug Administration’s “Consumer Tips on the products of CS,” to the Tibet Autonomous Region Food and Drug Administration’s “Explanation on the Use of CS” and a series of administrative approvals and developments. It can be seen that the state administration is still based on a scientific perspective, seeing that the precious Chinese herbal medicine CS is a research and development hotspot in the scientific community (Kai et al., 2018; Chen et al., 2021; Fang et al., 2021; Han et al., 2021; Li et al., 2021; Liu et al., 2021), and does not define the precious Chinese herbal medicine CS as a “toxic” substance, but actively researches and develops it, and the Tibet Autonomous Region, since the beginning, is still in the normal research and development stage. It is not in line with the scientific logic and the spirit of the national administrative documents to look at the “consumer risk alert issued by the State Food and Drug Administration in 2016″ in isolation. Besides, CS can be processed to form precious herbs the powder and powder tablets of CS, they are independent species, why cannot CS infusion be a new product developed through the technology? In the third classification, since CS is recognized as a precious herbal medicine, it has the basic conditions for research and development and innovation, because of this, the Tibet Autonomous Region has combined its advantages and recognized the series products of CS as innovative products belonging, also it is being used for precious Chinese herbal medicine. Therefore, the relevant state departments should not determine that CS is unsafe, let alone that CS infusion is unsafe, until there are scientific test results or evidence of toxicity.
The results of the complex network analysis showed that “the product complied the FSS” and the seller’s “subjective sales behavior of KSP” were the direct factors influencing the seller’s failure; the seller’s “failure to fulfill the review obligation” was the key factor influencing the seller’s failure, and the professional claims negatively influenced the seller’s failure. The possible reason for this finding, which is fully consistent with the results of the aforementioned correlation analysis, comes from the “judicial application” of court trials, which hardly consider the scientific nature of the issue, let alone the subjective extrapolation to explore the essence of the issue. In this way, judicial trials are favorable to relatively specialized professional claimants and unfavorable to individual businessmen and network sellers with low education and low financial capacity.
4.1. Empirical studies
4.1.1. Empirical analysis 1
The Jiangxi Juzhen Foodstuffs Trading Company and Changhe Feng network dispute began on July 1, 2020, when the nearly 57-year-old professional claimant started a “Changhe Feng v. Xunyang District Juzhen Foodstuffs Trading Company” network sale dispute over the purchase of CS wine, demanding “10 times the purchase price “compensation. After the first trial and second trial, the professional claimant lost the case and sued the High Court to carry out a retrial; the retrial is scheduled to be heard on March 25, 2022. The business entity made a written defense in the dimensions of “the product composition objection cannot be directly concluded that the product does not meet food safety standards,” the business entity has done its DR, and the relevant product documents and licenses can prove that the product is a safe product and the social attitude of the professional claimant, etc. Based on the results of the correlation analysis and complex network analysis model, we applied the full probability model with DR, FSS, KSP, and IDP independent variables.
Where is the event that the defendant wins the judicial dispute, is the obligation to have done DR, indicates that the defendant provides a copy of the relevant qualification of FSS, the defendant provides evidence of subjective intent without KSP, and the professional claimant does not submit evidence of IDP. From the results of the complex network model, the following conditional probabilities are obtained.
Based on the statistics of 24 cases, using the frequency of the event in question as a proxy for the probability of the event, we obtain:
Then
From the above results, we can find that the probability of winning the case of Juzhen Foodstuffs Trading Company is 0.7803. Based on the COVID-19 control, the business entity chose to write defense, the court ruled on 2 June 2022, ultimately finding in favor of Juzhen Foodstuffs Trading Company, further confirming the validity of the predictive model for CS occupational claims.
4.1.2. Empirical analysis 2
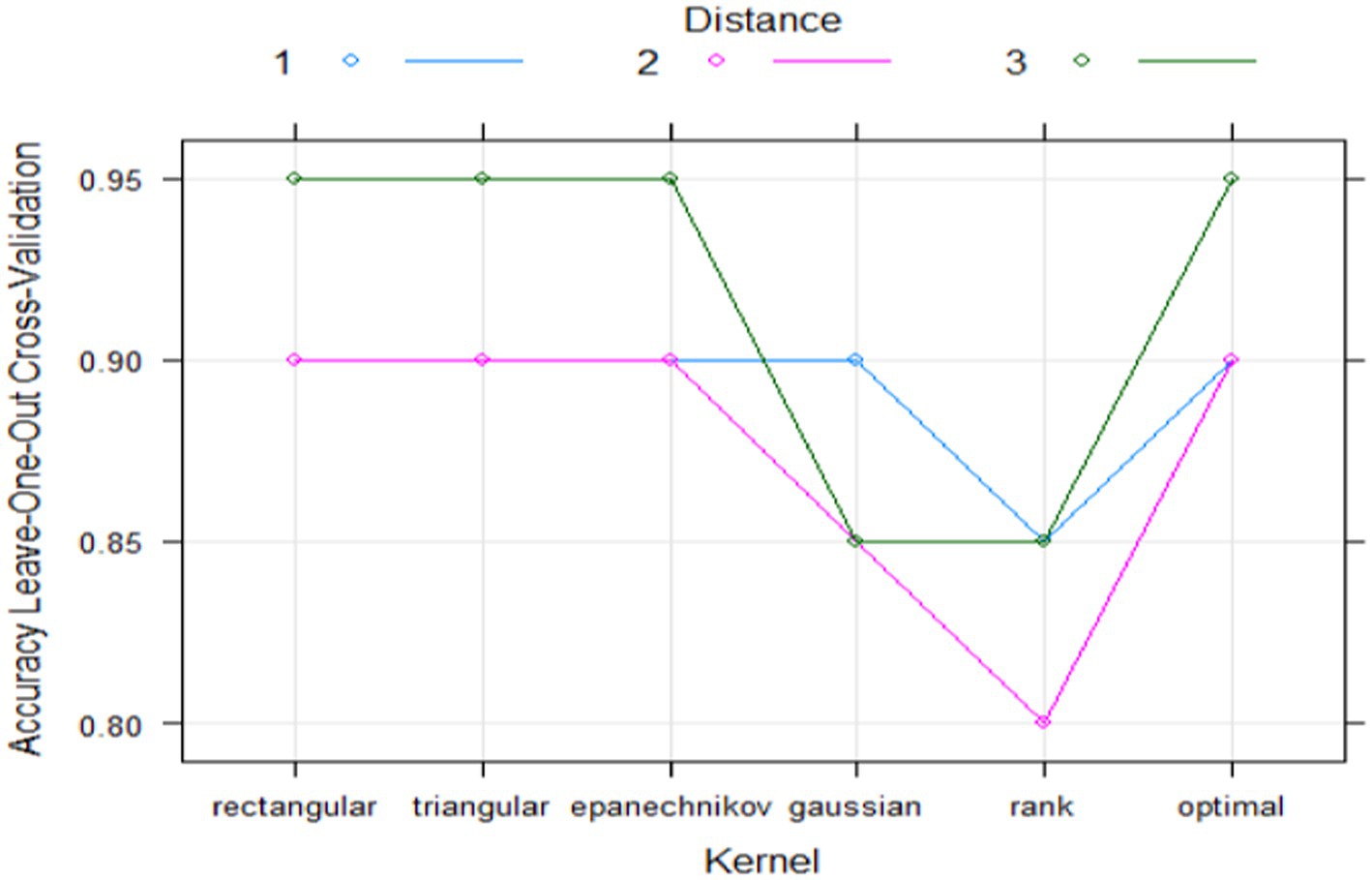
20 samples were randomly selected from 24 samples, and KNN was used to fit the classifier to the sample data. K is equal to 3 can be selected according to Figure 5.
Further, analyzed and obtained the accuracy of the classifier training data is 1, 95% CI: (0.8316, 1).
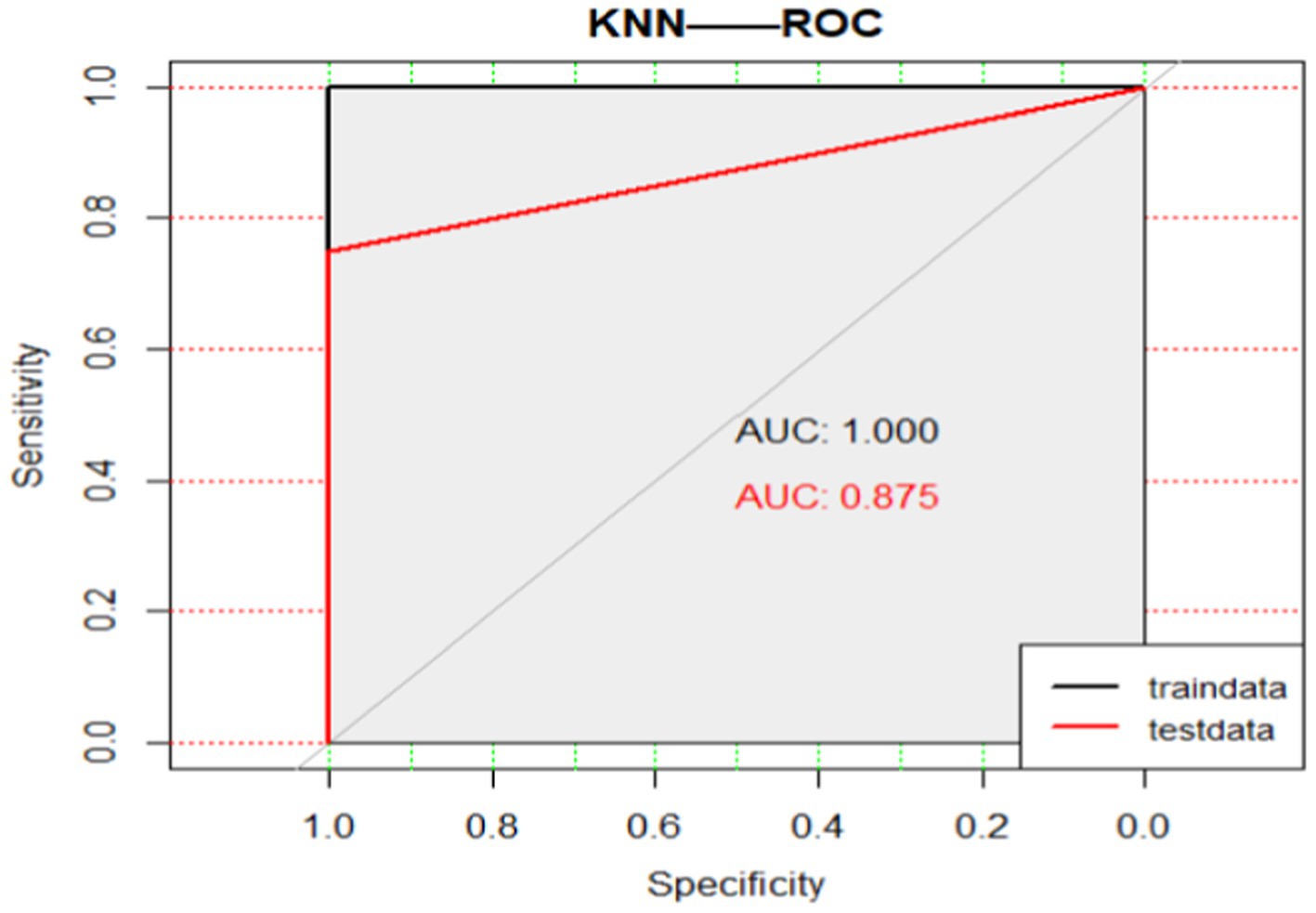
We use this classifier to predict the remaining four samples and new cases, and get the prediction accuracy is 0.8, 95% CI: (0.2836, 0.9949). KNN-ROC see Figure 6.
It is particularly worth emphasizing that the KNN classifier predicts that the trial result of the newborn case is the winner of the business, which is in perfect agreement with the actual result.
In summary, the two methods have good predictability for the trial prediction of Cordyceps sinensis series cases. In particular, the reasonable use of KNN classifier is conducive to the renewal of the breakthrough of the case by the judicial practitioners on the one hand, and on the other hand, it is also conducive to the judicial staff to improve the judicial system and provide a forward-looking perspective.
5. Conclusion
The jurisdiction of the CS dispute is confusing and needs to be clarified. The issue of product composition objection belongs to administrative jurisdiction, which should be assigned to the relevant administrative agency to deal with and clarify the division of functions; the people’s court should give a ruling based on the composition test results and the audit conclusion of the administrative department. Only in this way can the confusion between administrative and judicial functions be avoided, and only in this way can the people’s court judge the case scientifically and reasonably.
The attribution of the products of CS is a issue that needs to be returned to science. The state administration could issue a decree that the use of CS is to be restricted nationwide and used only for scientific research, with an effective date. On a specific date, any food or medicine with the word “CS” in it would be treated as “unsafe food.” This will facilitate the pharmacological development of the precious cordyceps, avoid unnecessary food disputes, reduce the waste of judicial resources, guide the defendant to operate in compliance, and combat professional claimants.
As a judge of the People’s Court, representing the symbol of public power, the image of justice, and the representative of justice, in the process of hearing cases, it is reasonable to play a medium degree of discretion and the pursuit of scientific supremacy, when accepting cases, should fully understand and use the concept of “administrative and judicial border demarcation.” At the trial of the case, the judge and jury are given full play to make a full understanding of the scientific and reasonable nature of what is involved in the case. In making the ruling, should take into full consideration the logic and pre-conditions of the judicial provisions of the evidence, and should not be taken out of context, to avoid the emergence of this collection of 2 rulings, the judge and jury the same, the case is similar, the verdict is different “capricious” event.
We call on the government to design from the top, establish a study on the boundary between administrative and judicial functions, and provide theoretical guarantees to avoid the administration and unscientific law enforcement, while calling on the administration and the judiciary to actively implement the regulation of professional claims, actively implement the spirit of relevant laws and regulations, and crack down on professional claims (Howard, 2004), to make due contributions to the purification of the business environment. It can provide a legal and regulatory system to improve food quality and regulate management.
This study analyzes the application of Chinese judicial provisions in the response to contract disputes and legal documents of CS, quantitatively analyzes the influencing factors of judicial trial, and provides a good response paradigm for lawyers to win professional claim cases. It provides practical data for promoting the reform of the judicial system. There is no denying that the sample size of this study is small because of the limitation of the research topic. In the follow-up research process, we can consider the application of Multiclass Model for Agriculture development using Multivariate Statistical method to analyze and model professional claims disputes on the basis of increasing the sample size, so as to make new contributions to expand the application of the theory. In addition, the research findings can be used by food retailers such as those selling CS to prevent and avoid disputes related to food quality and safety in online transactions. It reminds food retailers to save relevant evidences throughout the entire chain of “collection, management, and sale” of food, which objectively helps to improve the quality of food similar to CS. This is also beneficial for the effective connection of administrative management and judicial trial functions, enhancing the governance efficiency of the government, scientifically combating professional claimant behavior, and improving the business environment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
JS: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Writing – original draft, Writing – review & editing. LL: Data curation, Investigation, Software, Writing – review & editing. HL: Data curation, Investigation, Writing – review & editing. TL: Data curation, Investigation, Writing – review & editing. JD: Data curation, Investigation, Software, Methodology, Writing – review & editing. TZ: Data curation, Investigation, Formal analysis, Writing – review & editing. JL: Data curation, Investigation, Formal analysis, Writing – review & editing. JY: Data curation, Formal analysis, Methodology, Resources, Validation, Investigation, Writing – review & editing. LZ: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported in part by the NSF Center for Basic Science Project (No. 72188101), Open Program of Hospital Management Institute, Anhui Medical University (2023gykj09), and the Natural Science Foundation for the Higher Education Institutions of Anhui Province of China (No. KJ2021A0266, SK2020A0141 and No. KJ2021A1228). School-level offline courses (No. 2021xjkc13).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allergen Bureau. (2019). Vital scientific expert panel 2019 summary recommendations-the new allergen reference doses for vital®program version 3.0, Available at: http://allergenbureau.net/vital-scientific-expert-panel-2019-summary-recommendations-the-new-allergen-reference-doses-for-vital-program-version-3-0
András, P., and Krisch, J. (2015). Food safety in the public awareness. Analecta Technica Szegedinensia. 9, 25–30. doi: 10.14232/analecta.2015.1.25-30
Barkan, I. (1985). Industry invites regulation:the passage of the pure food and drug act of 1906. Am. J. Public Health 75, 18–26. doi: 10.2105/AJPH.75.1.18
Bright, D., Greenhill, C., Ritter, A., and Morselli, C. (2015). Networks within networks: using multiple link types to examine network structure and identify key actors in a drug trafficking operation. Global Crime 16, 219–237. doi: 10.1080/17440572.2015.1039164
Charlier, C., and Valceschini, E. (2008). Coordination for traceability in the food chain: a critical appraisal of european regulation. Eur. J. Law Econ. 25, 1–15. doi: 10.1007/s10657-007-9038-2
Chen, J. (2022). Leading edge development of food safety. Chin. J. Prevent. Med. 56, 545–548. doi: 10.3760/cma.j.cn112150-20220304-00206
Chen, S., Wang, J., Fang, Q., Dong, N., Fang, Q., Cui, S., et al. (2021). A polysaccharide from natural Cordyceps sinensis regulates the intestinal immunity and gut microbiota in mice with cyclophosphamide-induced intestinal injury. Food Funct. 12, 6271–6282. doi: 10.1039/D1FO00596K
Chiou, W., Chang, P., Chou, C., and Chen, C. (2000). Protein constituent contributes to the hypotensive and vasorelaxant activities of Cordyceps sinensis. Life Sci. 66, 1369–1376. doi: 10.1016/S0024-3205(00)00445-8
Cordyceps sinensis. (2017). Cordyceps sinensis (Berk.) Sacc. Available at: http://www.360doc.com/content/17/0504/18/13888283_651089407.shtml
Deebak, B., Memonet, F., Khowaja, S., Dev, K., and Wang, W. (2022). Lightweight Blockchain based remote mutual authentication for AI-empowered IoT sustainable computing systems. IEEE Internet Things J. doi: 10.1109/JIOT.2022.3152546
Drozen, M., Dubeck, J., Krasny, L., Mann, R., Nielsen, C., and Rath, M. (2011). Client alert: employer compliance guidance for whistleblower provisions under new Food safety modernization act. Available at: https://www.khlaw.com/4200
European Commission. (2011). Commission recommendation on the use of a harmonized methodology for classifying and reporting consumer complaints and enquiries (SEC (2010) 572). Available at: http://europy.cu/legislation_summaries/consumers/consumer information/co0014_cn.htm
Fernandes, R. G., Carvalho, J., FernandoAl-Muhtadi, L., and Proenca, J. J. M. (2019). A comprehensive survey on network anomaly detection. Telecommun. Syst. 70, 447–489. doi: 10.1007/s11235-018-0475-8
Fitriyadi, F., and Muqorobin, M. (2021). Prediction system for the spread of Corona virus in Central Java with K-nearest neighbor (KNN) method. Int. J. Coop. Inform. Syst. 2, 80–85. doi: 10.29040/IJCIS.V2I3.41
Fang, Q., Chen, S., Wang, J., Huang, X., and Nie, S. (2021). Fractions from natural cordyceps sinensis alleviated intestinal injury in cyclophosphamide-induced mice. Bioact. Carbohydr. Diet. Fibre 26:100271. doi: 10.1016/j.bcdf.2021.100271
Gadekallu, T., Huynh-The, T., Wang, W., Yenduri, G., Ranaweera, P., Pham, Q., et al., (2022). Blockchain for the metaverse: a review. Available at: https://arxiv.org/pdf/2203.09738.pdf
Henson, S., and Caswell, J. (1999). Food safety regulation: an overview of contemporary issues. Food Policy 24, 589–603. doi: 10.1016/S0306-9192(99)00072-X
Hall, C., and Osses, F. (2013). A review to inform understanding of the use of food safety messages on food labels. Int. J. Consum. Stud. 37, 422–432. doi: 10.1111/ijcs.12010
Han, S., Ahn, Y., Lee, H., Suh, H., and Jo, K. (2021). Antioxidant and immunostimulatory activities of a submerged culture of cordyceps sinensis using spent coffee. Foods 10:1697. doi: 10.3390/foods10081697
Howard, M. (2004). Food hygiene regulation and enforcement policy in the Uk: the underlying philosophy and comparisons with occupational health and safety law. Food Serv Technol. 4, 69–73. doi: 10.1111/j.1471-5740.2004.00091.x
Jin, W., Lermer, L., Chilton, J., Klingeman, H., and Towers, G. (1999). Antitumor sterols from mycelia of cordyceps sinensis. Phytochemistry 51, 891–898. doi: 10.1016/S0031-9422(99)00128-4
Kai, Z., Gao, Q., Zong, C., Ge, L., and Liu, J. (2018). Cordyceps sinensis prevents contrast-induced nephropathy in diabetic rats: its underlying mechanism. Int. J. Clin. Exp. Pathol. 11, 5571–5580.
Lalou, M., Tahraoui, M., and Kheddouci, H. (2018). The critical node detection problem in networks: a survey. Comp. Sci. Rev. 28, 92–117. doi: 10.1016/j.cosrev.2018.02.002
Lim, M., Abdullah, A., Jhanjhi, N., and Khan, M. (2019). Link prediction in time-evolving criminal network with deep reinforcement learning technique. IEEE Access 7, 184797–184807, doi: 10.1109/ACCESS.2019.2958873
Li, Y., Wang, L., Xu, B., Zhao, L., and Zhan, H. (2021). Based on network pharmacology tools to investigate the molecular mechanism of cordyceps sinensis on the treatment of diabetic nephropathy. J. Diabetes Res. 2021, 1–12. doi: 10.1155/2021/8891093
Liu, Y., Xiao, K., Wang, Z., Wang, S., and Xu, F. (2021). Comparison of metabolism substances in cordyceps sinensis and cordyceps militaris cultivated with tussah pupa based on lc-ms. J. Food Biochem. 45:e13735. doi: 10.1111/jfbc.13735
Nugroho, A. (2021). A decision guidance for solving success rate political campaign using distance weighted knn in nassi-shneiderman framework. Int. J. Intellig. Eng. Syst. 14, 410–420. doi: 10.22266/ijies2021.0430.37
Ren, X., and Meng, K. (2021). Vector complex network and its application in complex system modeling. Comp. Syst. App. 30, 309–316. doi: 10.15888/j.cnki.csa.008116
State Food and Drug Administration. (2016). Circular of the general Administration on stopping the pilot work of Cordyceps sinensis for health food. Available at: http://www.hnr.cn/news/jctj/201603/t20160305_2348509.html
Sun, J., Gao, Y., Jiang, X., Li, Y., and You, J. (2021). Research on business environment risk governance based on occupational claims: 1784 cases of food safety disputes. Complexity 2021, 1–8. doi: 10.1155/2021/6320387
Su, M., and Wang, Y. (2020). New countermeasures and thoughts on the issue of regulating occupational claims in Shanghai. Bus. Econ. Rev. 21, 44–52.
World Health Organization. (2006). Food and agriculture Organization of the United Nations. Food safety risk analysis: a guide for National Food Safety Authorities, Rome, FAO and WHO. Available at: https://apps.who.int/iris/handle/10665/43718
World Health Organization (2008). Food and agriculture Organization of the United Nations, codex Alimentarius commission procedure manual. 18th Edn Available at: https://www.fao.org/3/i0505c/i0505c.pdf.
Wang, W., Xu, H., Alazab, M., Gadekallu, T., Han, Z., and Su, C. (2021). Blockchain-based reliable and efficient Certificateless signature for IIoT devices. IEEE Trans. Industr. Inform. 18, 7059–7067. doi: 10.1109/TII.2021.3084753
Xu, Q. G., Liu, M. J., and Li, H. (2014). Agricultural product Price forecasting model based on improved KNN algorithm. J. Univ. Jinan. 28, 114–117.
Yu, W., Zhu, M., Li, H., Yu, N., Li, X., and Yang, X. (2021). Empirical research on event detection based on dynamic complex network analysis method. J. Intelligence 40, 108–114.
Ye, Z. (2018). Research on store sales forecast based on BP neural network and KNN. AnHui Univ. Sci. Technol. 2018, 1–76.
Zhang, S. (2021). A method for analyzing transmission characteristics of complex networks. Audio Eng. 45, 31–33. doi: 10.16311/j.audioe.2021.04.009
Zhang, A. (2019). Study on prediction of gene mutation and disease-free survival of lung squamous cell carcinoma based on digital pathological images. Univ. Sci. and Technol. China 2019, 1–46.
Zhang, C., Liu, R., Zhang, L., and Dong, L. (2020). Investigation and research on food safety knowledge, attitude and behavior of university students in Kunming based on big data analysis. J. Phys. Conf. Ser. 1648:022032. doi: 10.1088/1742-6596/1648/2/022032
Glossary
Keywords: Cordyceps sinensis, professional claims, dispute cases, administrative, judicial
Citation: Sun J, Li L, Li H, Liu T, Deng J, Zhang T, Li J, You J and Zhang L (2023) Governance of the business environment based on food safety disputes: empirical analysis based on the case of “Cordyceps sinensis”. Front. Sustain. Food Syst. 7:1273468. doi: 10.3389/fsufs.2023.1273468
Edited by:
Thanwadee Chinda, Thammasat University, ThailandReviewed by:
Babatunde Ajayi, National Astronomical Research Institute of Thailand, ThailandSuthathip Suanmali, Thammasat University, Thailand
Nuchjarin Intalar, Thammasat University, Thailand
Copyright © 2023 Sun, Li, Li, Liu, Deng, Zhang, Li, You and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangjie Sun, c3VuamlhbmdqaWVAYWhtdS5lZHUuY24=; Jinliang You, ODQxNDUwMjAxQHFxLmNvbQ==; Liping Zhang, emhhbmdsaXBpbmdAYWhtdS5lZHUuY24=
 Jiangjie Sun
Jiangjie Sun Limin Li2
Limin Li2 Hui Li
Hui Li Liping Zhang
Liping Zhang